Transinnominate Impella 5.5 tunneled out through the right supraclavicular space.
Central Message.
Transinnominate Impella 5.5 insertion via ministernotomy is a viable short-term option for full circulatory support and left ventricular unloading in children with refractory cardiogenic shock.
Temporary mechanical circulatory support (MCS) is employed to stabilize patients with refractory heart failure as a bridge to decision, recovery, durable ventricular assist device (VAD), or transplantation. Currently, temporary MCS options in pediatrics are limited, with venoarterial extracorporeal membrane oxygenation (VA-ECMO) being a widely used strategy.1 VA-ECMO augments cardiac output and perfusion pressure to promote end-organ recovery; however, these advantages are countered by increased afterload and myocardial wall stress as well as excess morbidity from bleeding, stroke, and limb ischemia. The Impella (Abiomed) is a microaxial VAD with growing off-label use in pediatrics given its advantages over VA-ECMO, namely increased left ventricular (LV) unloading and decreased myocardial oxygen consumption.2 We present our technique of transinnominate Impella 5.5 insertion via ministernotomy in an adolescent as a bridge-to-transplantation.
Clinical Vignette
A 14-year-old male patient (75 kg) with propionic acidemia underwent liver transplantation in 2015 and developed dilated cardiomyopathy 2 years later. He presented in acute decompensated heart failure, requiring intubation and high-dose inotropes with progressive end-organ dysfunction. Transesophageal echocardiogram (TEE) demonstrated severe LV dysfunction and mild right ventricular dysfunction. Right heart catheterization revealed a pulmonary capillary wedge pressure (PCWP) of 32 mm Hg and cardiac index of 2.1 L/min/m2. Given his continued deterioration (Interagency Registry for Mechanically Assisted Circulatory Support 2), he was planned to undergo HeartMate 3 (Abbott) as bridge-to-transplantation; however, developed worsening pneumonia on the day of surgery. Given this clinical change, we opted for a temporary MCS strategy. Bilateral axillary arteries measured 4 mm by ultrasound, too small to accommodate a percutaneous device. Thus, the patient was taken to the hybrid operating room for Impella 5.5 insertion via the innominate artery.
Surgical Technique
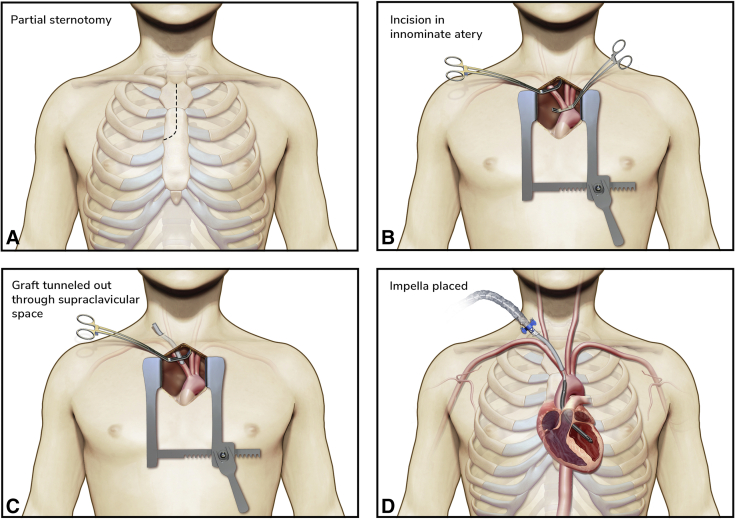
An upper partial sternotomy was performed into the third intercostal space (Figure 1, A). The innominate vein was retracted inferiorly to facilitate exposure of the innominate artery while leaving the pericardium intact. Care was taken to avoid injury to the right recurrent laryngeal nerve. After administration of 5000 units of intravenous heparin, the innominate artery was clamped and opened (Figure 1, B). A 10-mm Gelweave graft (Vascutek) was sewn end-to-side onto the artery using 5-0 PROLENE. The graft was tunneled out to the supraclavicular space (Figure 1, C) and the Impella was introduced through the graft over a guidewire and positioned into the LV under fluoroscopic and TEE guidance (Figure 1, D). Following TEE confirmation of inflow position ∼4.5 cm from the aortic valve annulus, the Impella was turned on and increased to a performance level of P7, providing 4 L/min of flow. Right heart catheterization demonstrated a reduction in PCWP to 17 mm Hg. The graft was trimmed and the silicon plug was placed at skin level. After securing the device to the graft with multiple silk ties, the covering sheath was advanced and secured to the graft and skin. A mediastinal chest tube was placed before chest closure.
Figure 1.
Procedural steps for transinnominate Impella 5.5 insertion. A, Upper partial sternotomy into the third intercostal space. B, The innominate artery is dissected and controlled proximally and distally before arteriotomy. Note that the pericardium is left completely intact. C, A Gelweave graft is sewn end-to-side onto the artery and then tunneled out to the right supraclavicular space. D, The Impella is introduced through the graft and positioned into the LV under fluoroscopic and TEE guidance, ensuring an inflow position ∼4.5 cm from the aortic valve annulus.
Postoperative Course
The patient was extubated 4 days later; however, he developed worsening volume overload. Impella support was increased to P9, which led to a reduction in PCWP from 25 mm Hg to 18 mm Hg, improved renal function, and weaning of inotropes. The patient was maintained on Impella 5.5 support for 21 days before undergoing a successful heart transplant. Sternal re-entry was uneventful and intrapericardial dissection was free of adhesions. The intraventricular aspect of the Impella was removed after crossclamp application and cardiectomy to reduce the risk of thromboembolism. The proximal catheter was removed after reperfusion by transiently reducing bypass flow. Finally, the innominate graft was ligated, oversewn, and buried in the subcutaneous tissue.
Comments
The Impella microaxial LVAD has emerged as a feasible alternative to ECMO in stabilizing patients with hemodynamic collapse.3,4 The Impella 5.5 provides >6 L/min of peak flow and can be inserted via a transaxillary or transaortic approach.5 The former is limited by vessel size, requiring an axillary artery diameter of ≥6 mm. While the transaortic approach is feasible in patients of all sizes, it requires a sternotomy and pericardiotomy, which may complicate future reentry. A transinnominate approach involves a small incision and leaves the pericardium intact, facilitating subsequent durable VAD or heart transplant. Moreover, the Impella 5.5 must enter the aorta at least 5 cm distal to the aortic valve; a transinnominate approach permits insertion over 2 cm more distal than through the ascending aorta, expanding its use in children and smaller adolescents. Notably, our patient was successfully bridged to transplantation without any vascular complications, stroke, or dialysis requirement. This technique is reproducible and may be a valuable addition to the armamentarium of cardiac surgeons and interventional cardiologists.
Footnotes
Disclosures: Illustrations provided by medical illustrator and service fees covered by Abiomed, Inc. The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Adachi I., Jaquiss R.D. Mechanical circulatory support in children. Curr Cardiol Rev. 2016;12:132–140. doi: 10.2174/1573403X12666151119165841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimas V.V., Morray B.H., Kim D.W., Almond C.S., Shahanavaz S., Tume S.C., et al. A multicenter study of the Impella device for mechanical support of the systemic circulation in pediatric and adolescent patients. Catheter Cardiovasc Interv. 2017;90:1249. doi: 10.1002/ccd.26973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lima B., Kale P., Gonzalez-Stawinski G.V., Kuiper J.J., Carey S., Hall S.A. Effectiveness and safety of the Impella 5.0 as a bridge to cardiac transplantation or durable left ventricular assist device. Am J Cardiol. 2016;117:1622–1628. doi: 10.1016/j.amjcard.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 4.Parekh D., Jeewa A., Tume S.C., Dreyer W.J., Pignatelli R., Horne D., et al. Percutaneous mechanical circulatory support using Impella devices for decompensated cardiogenic shock: a pediatric heart center experience. ASAIO J. 2018;64:98–104. doi: 10.1097/MAT.0000000000000581. [DOI] [PubMed] [Google Scholar]
- 5.Ramzy D., Soltesz E., Anderson M. New surgical circulatory support system outcomes. ASAIO J. 2020;66:746–752. doi: 10.1097/MAT.0000000000001194. [DOI] [PMC free article] [PubMed] [Google Scholar]