Abstract
Clinical studies have shown a significant positive correlation between age and the likelihood of being infected with SARS-CoV-2. This increased susceptibility is positively correlated with chronic inflammation and compromised neurocognitive functions. Postmortem analyses suggest that acute lung injury (ALI)/acute respiratory distress syndrome (ARDS), with systemic and lung hyperinflammation, can cause significant morbidity and mortality in COVID-19 patients. Supraphysiological supplemental oxygen, also known as hyperoxia, is commonly used to treat decreased blood oxygen saturation in COVID-19 patients. However, prolonged exposure to hyperoxia alone can cause oxygen toxicity, due to an excessive increase in the levels of reactive oxygen species (ROS), which can overwhelm the cellular antioxidant capacity. Subsequently, this causes oxidative cellular damage and increased levels of aging biomarkers, such as telomere shortening and inflammaging. The oxidative stress in the lungs and brain can compromise innate immunity, resulting in an increased susceptibility to secondary lung infections, impaired neurocognitive functions, and dysregulated hyperinflammation, which can lead to ALI/ARDS, and even death. Studies indicate that lung inflammation is regulated by the central nervous system, notably, the cholinergic anti-inflammatory pathway (CAIP), which is innervated by the vagus nerve and α7 nicotinic acetylcholine receptors (α7nAChRs) on lung cells, particularly lung macrophages. The activation of α7nAChRs attenuates oxygen toxicity in the lungs and improves clinical outcomes by restoring hyperoxia-compromised innate immunity. Mechanistically, α7nAChR agonist (e.g., GAT 107 and GTS-21) can regulate redox signaling by 1) activating Nrf2, a master regulator of the antioxidant response and a cytoprotective defense system, which can decrease cellular damage caused by ROS and 2) inhibiting the activation of the NF-κB-mediated inflammatory response. Notably, GTS-21 has been shown to be safe and it improves neurocognitive functions in humans. Therefore, targeting the α7nAChR may represent a viable therapeutic approach for attenuating dysregulated hyperinflammation-mediated ARDS and sepsis in COVID-19 patients receiving prolonged oxygen therapy.
Keywords: COVID-19, Acute respiratory distress syndrome (ARDS), Inflammation, Supplemental oxygen therapy, Aging, Mitochondria, Cholinergic anti-inflammatory pathway, α7 nicotinic acetylcholine receptor
Graphical abstract
Abbreviations
- α7nAChR
α7 nicotinic acetylcholine receptor
- ACE2
angiotensin converting enzyme 2
- ACh
acetylcholine
- AD
Alzheimer's disease
- ALI
acute lung injury
- ALT
alanine aminotransferase
- ARDS
acute respiratory distress syndrome
- AST
aspartate aminotransferase
- BBB
blood-brain barrier
- CAIP
cholinergic anti-inflammatory pathway
- cCFR
corrected case fatality ratio
- CFR
case fatality rate
- cIFR
corrected infection fatality ratio
- CNS
central nervous system
- COPD
chronic obstructive pulmonary disease
- COVID-19
Coronavirus disease 2019
- CT
computer-assisted tomography
- CYP
children and young people
- ETC
electron transport chain
- FiO2
fraction of inspired oxygen
- GAT107
(+)-enantiomer of racemic 4-(4-bromophenyl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline-8-sulfonamide
- GTS-21
DMXBA
- H2O2
hydrogen peroxide
- HMGB1
high mobility group box protein 1
- ICU
intensive care units
- IL:
interleukins
- LPS
lipopolysaccharide
- mtDNA
mitochondrial DNA
- mtROS
mitochondrial reactive oxygen species
- NF-κB
nuclear factor kappa B
- Nrf2
nuclear factor E2-related factor 2
- O2
oxygen
- O2·−
superoxide
- PaO2
arterial oxygen
- PhenoAgeAccel
PhenoAge acceleration
- PNS
peripheral nervous system
- ROS
reactive oxygen species
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus-2
- SOD
superoxide dismutase
- TNF
tumor necrosis factor
1. Introduction
It is our great honor to contribute to this special issue celebrating the scientific achievements of Dr. Bruce Ames in the redox field. Although we have not had the honor of collaborating with him, some of us have attended talks/seminars given by Dr. Ames. His talks were always scientifically enlightening and contained elements of his delightful humor and wit. Dr. Ames has made significant contributions to the field of redox biology and medicine in the areas of mitochondrial aging, the triage theory of vitamins and micronutrients in longevity, and the role of oxidative stress in the pathogenesis of human diseases. In addition, Dr. Ames has provided insightful comments and interpretations about the failure of many clinical trials using antioxidants, which opened new vistas for scientists to conduct research in the field of redox medicine. The profound love for his fellow citizens is indicated by his pioneering work in areas that have positively impacted public health. His pursuit of a new field every 10–15 years is indicative of his never-ending scientific curiosity. We believe the redox community has benefited significantly from many scientists, exemplified by Dr. Ames, who is brilliant, insightful, visionary, and generous. We believe that Dr. Ames' significant contributions to the aging and redox research areas will no doubt help us elucidate the pathogenesis underlying the susceptibility to SARS-CoV-2 infection and the progression of COVID-19-induced disease.
The current coronavirus pandemic (COVID-19) is caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which is an enveloped, positive-strand RNA virus [1,2]. Over the past 2 years, the SARS-CoV-2 virus has spread globally and, consequently declared a pandemic by the World Health Organization (WHO), on March 11, 2020. COVID-19 has caused more than 580 million infections and more than 6.4 million deaths globally. Fortunately, as a result of unprecedented global collaborations among scientists, medical professionals and public health workers, SARS-CoV-2 RNA was sequenced and vaccines for the SARS-CoV-2 were rapidly developed [[3], [4], [5], [6], [7]]. Despite this great success, we are still facing unprecedented challenges as: 1) a significant percentage of the world's population is not vaccinated and 2) the emergence of new variants of SARS-CoV-2, such as Omicron (notably the BA.4 and BA.5 variants), which are more contagious than alpha and delta, causing breakthrough infections, even in vaccinated individuals and 3) an estimated 10–30% infected with SARS-CoV-2 develop the post-acute sequelae of SARS-CoV-2 infection (also known as Long COVID, among other designations), which greatly affects the health and quality of life [[8], [9], [10], [11]]. The significant impact of SARS-CoV-2 infection on global health, economy and healthcare resources has created an urgent need to identify 1) the risk factors associated with its prevalence and 2) the underlying mechanisms that increase the incidence of severe and fatal outcomes in order to develop efficacious treatments for patients with COVID-19.
COVID-19 is diagnosed primarily based on clinical symptomatology, computer-assisted tomography (CT) scans, and the detection of specific SARS-CoV-2 antigens [1,12,13]. Data obtained by the United States Center for Disease Control (CDC) indicates that 81% of COVID-19 patients experience mild-to-moderate symptoms, including cough, fatigue, diarrhea, headache, muscle pain, shortness of breath, and mild forms of pneumonia. However, 14% and 5% of patients diagnosed with COVID-19 have severe to life-threatening symptoms, respectively, which include fever, dyspnea, hypoxia, respiratory failure, and multiple organ failure [1,14]. COVID-19 can produce acute and chronic dysfunctions in the lung, brain, renal, musculoskeletal and gastrointestinal tissues [15,16].
During the early stages of the COVID-19 pandemic, clinical data indicated that 14% of all hospitalized patients had bilateral ground-glass opacities in the lung, and 75% were diagnosed with pneumonia [17]. The primary cause of death in patients with COVID-19 is the acute respiratory distress syndrome (ARDS), a severe form of acute lung injury (ALI), which is characterized by dysregulated hyperinflammation in the lung and circulation [[18], [19], [20]]. The mortality rate reached 39–72% in the critically severe COVID-19 patients admitted to intensive care units (ICU), even though the ICU patients are routinely on mechanical ventilation with supraphysiological concentrations of supplemental oxygen (hyperoxia) [[6], [19], [21]]. This high mortality rate could be attributed to prolonged exposure to hyperoxia, which itself can produce oxygen toxicity, causing ALI/ARDS [22].
Oxygen (O2) is imperative for the survival of the vast majority of organisms on earth [23]. Since its discovery in the atmosphere in 1771 by Carl Wilhelm Scheele and the recognition of its value in medical practice in the 19th century by Antonie Lavoisier, O2 has been known as an essential component in the medical management of certain diseases because of the metabolic needs of aerobic organisms [24]. The appreciation of the vital role of sufficient blood oxygen saturation in our survival has become more apparent during this unprecedented COVID-19 pandemic. The presentation of hypoxemic respiratory failure is one of the most common problems in hospitalized COVID-19 patients [25]. Mechanical ventilation with hyperoxia has been a vital tool for maintaining adequate oxygenation in patients with severely impaired lung functions, in order to sustain the life of patients diagnosed with severe and critical cases of COVID-19 [25,26].
Although it is a life-saving intervention, hyperoxia, whether it is given by noninvasive means or invasive mechanical ventilation, can be deleterious as it can produce oxygen toxicity [25,[27], [28], [29], [30], [31], [32], [33]]. Upon prolonged exposure to hyperoxia, excessive and dysregulated inflammation occurs in the lungs, increasing the incidence of secondary infections, leading to ventilator-associated pneumonia, ALI/ARDS, sepsis and even death [27,34,35]. Therefore, although oxygen therapy with supplemental oxygen is a critical part of the treatment of hypoxemia, the exposure of patients to hyperoxia can induce oxygen toxicity after prolonged exposure [25,36].
There are thousands of ongoing clinical trials being conducted to identify efficacious treatments for SARS-CoV-2 infections. Currently, the only FDA-approved treatment for COVID-19 in adults and children who are 12 years of age or older is the antiviral drug, remdesivir, an inhibitor of the enzyme, RNA-dependent RNA polymerase, which decreases SARS-CoV-2 RNA synthesis [37]. Although remdesivir has been shown to accelerate the recovery rate if administered during the early phases of COVID-19, its overall efficacy is limited by the absence of a significant survival benefit, particularly in the more severe cases of COVID-19 [[37], [38]]. Thus, novel therapeutic strategies that are safe and efficacious for treating severe COVID-19 are urgently needed.
In this review, we will discuss the potential effects of oxygen toxicity on the deleterious clinical outcome of patients with COVID and some novel strategies, based on data indicating the important role that neuroimmunomodulation plays in modulating lung inflammation, to prevent dysregulated hyperinflammation-induced ALI/ARDS. This review highlights: 1) Aging as the primary risk factor in the susceptibility to SARS-CoV-2 infection and mortality due to COVID-19; 2) The contribution of oxygen toxicity to the pathogenesis of ALI/ARDS and to the positive correlation of oxygen toxicity with aging biomarkers and 3) Targeting alpha7-nicotinic cholinergic receptors (α7nAChR), an essential component of the cholinergic anti-inflammatory pathway (CAIP)/the inflammatory reflex, to attenuate or prevent dysregulated hyperinflammation-mediated ALI/ARDS.
2. Aging is the primary risk factor in the susceptibility to SARS-CoV-2 infection and COVID-19 mortality
Due to the high rate of SARS-CoV-2 infection and the resulting morbidity and mortality, the identification of risk factors contributing to the fatal outcome has been of great importance. Early epidemiological data indicated that the COVID-19 pandemic affects people of different ages at distinct rates, although SARS-CoV-2 can infect people of all ages [39,40].
2.1. The elderly are significantly more susceptible to SARS-CoV-2 infection and have a higher risk of developing severe COVID-19, compared to individuals that are young
Russell et al. [41] conducted one of the first studies to investigate the correlation between age and the prevalence of COVID-19. They evaluated the infection rate and case fatality rate of COVID-19, using data obtained from passengers aboard the cruise ship, Diamond Princess [41]. As reported by the National Institute of Infectious Diseases, by February 20, 2020, there were 619 confirmed COVID-19-positive passengers aboard the Diamond Princess [42]. Of the 619 confirmed cases, 318 were asymptomatic and 301 were symptomatic [42]. A total of 3063 PCR tests were conducted, beginning with the elderly passengers and then all others, descending by age [42]. The all-age corrected infection fatality ratio (cIFR) on the cruise ship was estimated to be 1.3% (95% CI: 0.38–3.6), with an estimated corrected case fatality ratio (cCFR) of 2.6% (95% CI: 0.89–6.7) [41]. For individuals aged 70 and older, the age-stratified estimates for cIFR and cCFR were 6.4% (95% CI: 2.6–13) and 13% (95% CI: 5.2–26), respectively. The [41] investigation suggests that age does contribute to the susceptibility to SARS-CoV-2 infection and case fatality rates in COVID-19 [41].
The disparity in age-related vulnerability to SARS-CoV-2 infection was also shown in a study by Davies et al. [43]. In this study, the age disparity was evaluated by fitting an age-structured mathematical model to data, obtained from China, Italy, Japan, Singapore, Canada, and South Korea [43]. The investigations compared the susceptibility to SARS-CoV-2 infection in individuals <20 years of age with that of people >70 years old. Although the susceptibility to SARS-CoV-2 infection in individuals <20 years of age is approximately half of that of adults >20 years of age, the prevalence of symptomatic COVID-19 was 21% (95% credible interval: 12–31%) in 10–19 years old and increased to 69% (57–82%) in people >70 years old. These results suggest that age was a factor in the susceptibility to SARS-CoV-2 infection and in the risk of developing clinical symptoms. Furthermore, there was a significantly greater risk of developing severe disease in older individuals [43]. These clinical observations led to the hypothesis that the prevalence of COVID-19 increases with age.
To further examine, quantify and analyze factors that may be significantly correlated with COVID-19 mortality, Williamson et al. [44] conducted a large cohort study (n = 17,278,392 subjects) in England in April 2020 [44]. OpenSAFELY, a novel data analytics platform, was used to determine factors significantly correlated with COVID-19-related mortality. Of the 17,278,392 subjects enrolled in the study, 10,926 were linked to COVID-19. The overall incidence of COVID-19-associated deaths within a 90-day period was <0.01% in 18–39 years old, whereas the mortality rate in men or women, 80 years old or older, was 0.67% and 0.44%, respectively. The Williamson et al. study provided an early insight into factors that were significantly correlated with disease progression, suggesting that age is a major contributing cause to COVID mortality [44].
Moreover, Verity et al. [40] sought to determine the case fatality rate (CFR) that accounted for censoring and ascertainment biases [40]. This was done retrospectively in 1023 patients who died from COVID-19 in China in February of 2020. They analyzed the demographics and mortality of patients at every 10-year age interval. The results indicate a 3-fold higher CFR for patients >60 years old (n = 151, CFR: 4.5%, [1·8–11]), compared to patients <60 years old (n = 360, CFR: 1.4%, [0·4–3·5]). In addition, the mortality rate increased at every 10-year age interval. These results indicate that age is a risk factor for COVID-19 mortality.
Due to the limited information available on the presentation and outcome of severe COVID-19 patients, Richardson et al. [39] conducted a case study on sequentially hospitalized patients with confirmed COVID-19 [39]. A total of 5700 confirmed COVID-19 patients (median age = 63 years old), that were admitted to 12 Northwell Health System hospitals between March 1 and April 4, 2020, were enrolled in this study [39]. This study found that the mortality rate was 0% for both male and female patients younger than 20 years old; however, the mortality rates were higher in those older than 20 years old. Notably, this study found that mechanical ventilation with hyperoxia augmented the mortality rates. Specifically, the mortality rate for patients who received mechanical ventilation in the 18–65 years old group was 76.4%, whereas those who received no mechanical ventilation in this group had a mortality rate of 1.98%. Moreover, patients in the older than 65 group who received mechanical ventilation had a mortality rate of 97.2%, whereas the mortality rate of patients in this group that were not mechanically ventilated was 26.6% [39]. Furthermore, the readmission rates for the discharged patients increased progressively in older patients, compared to the younger group of patients, as older patient groups had higher readmission rates than that of younger groups. This study further confirms the findings in the studies discussed above, which indicating that there is a positive correlation between age and mortality rate in COVID-19 patients and that mechanical ventilation increases the mortality rate.
To obtain additional data on how SARS-CoV-2 infection affects children and young people (CYP), who are <18 years old, Smith et al. [45] conducted a clinical review using the Linked Mandatory Child Death Reporting data and quantified the mortality risk of all deaths in CYP in England following SARS-CoV-2 infection. The primary objective of the study was to identify subjects where SARS-CoV-2 infection contributed to mortality [45]. Data were obtained during the first pandemic year from March 2020 to February 2021 to differentiate between those who died of SARS-CoV-2 infection and those who were SARS-CoV-2 positive but died from other causes. The results indicated that 3105 CYP died within the designated time interval, whereas 25 CYP died directly due to SARS-CoV-2 infection. While the mortality rate was 0.0002% in children and young people, the mortality rate of those >18 years old was 3.2% during the same period of time in England [45]. In summary, these studies indicated that the elderly, compared to younger age groups, are significantly more susceptible to SARS-CoV-2 infection and there is a greater probability of more severe disease and mortality in older compared to younger patients.
2.2. Medical co-morbidity significantly increases the likelihood of SARS-CoV-2 infection and COVID-19 disease severity
Based on the discussion above, SARS-CoV-2 infection affects the elderly at disproportionate rates, which increased probabilities of fatal outcome. In contrast, children and young people are typically asymptomatic or only experience mild symptoms following infection with SARS-CoV-2 [46,47]. Thus, the elderly are significantly more vulnerable to SARS-CoV-2 infection and are at a greater risk of developing severe disease and mortality than young individuals. However, it remains to be determined as to why some children became infected with SARS-CoV-2 and died from COVID-19.
To ascertain the underlying health conditions that may be contributing to COVID-19 mortality in addition to chronological age, Smith et al. [45] analyzed the mortality data in children and young people who died of COVID-19, collected in England between March 1st, 2020, and February 28th, 2021 [45]. It was reported that 64% (n = 16) of the 25 children and young people who died of SARS-CoV-2 infection presented with comorbidities in two or more organ systems, compared to 45% (n = 1373) in children and young people (n = 3080), who died of other causes [45]. Furthermore, neurological conditions were the most prevalent comorbidity in children and young people who died of COVID-19 (52%), compared with children and young people who died from all other causes (40%). Overall, these data suggest that medical comorbidities, particularly neurological comorbidities, are a major risk factor in the fatal outcomes in young patients infected with SARS-CoV-2.
To determine if preexisting chronic illness or medical comorbidity also contributes to a higher incidence of COVID-19 mortality in elderly patients, [48] evaluated the potential risk factors associated with fatal outcomes due to COVID-19 in a retrospective study of a cohort of 1590 hospitalized subjects in China. The results indicated a greater incidence of more severe cases and mortality in the elderly (≥65 years old), especially those ≥75 years old compared to patients <65 years old, a finding consistent with the clinical studies discussed above. More importantly, there was a greater rate of severe cases and mortality in patients with preexisting comorbidities, including cardiovascular and cerebrovascular diseases, such as hypertension and diabetes. This study suggests that preexisting illness is an independent risk factor in COVID-19-induced mortality in the elderly [49].
The identification of medical comorbidities as an independent risk factor in the progression and mortality of COVID-19 is critical in determining the underlying pathogenesis of the disease. The critical contribution of medical comorbidity to COVID-19 was further confirmed by Nandy et al. [50]; who conducted a meta-analysis of data derived from 3994 patients. Based on data from 16 studies, this meta-analysis indicated that the presence of chronic obstructive pulmonary disease (COPD), chronic kidney disease, hypertension, diabetes, and cardiovascular disease, significantly increased the probability of COVID-19 disease progression. These results further confirmed that medical comorbidities play a major role in COVID-19 prevalence and increase the likelihood of developing severe COVID-19.
The investigations that were conducted to compare COVID-19 pathogenesis in the elderly with that in younger age groups suggest that medical comorbidities produce a higher prevalence and greater COVID-19 severity. Interestingly, age-related diseases are among the most important medical comorbidities that contribute to the progression of COVID-19. In a study of 5700 patients hospitalized with COVID-19 in the New York City area, it was reported that hypertension (56.6%), obesity (41.7%) and diabetes (33.8%) were the most prevalent comorbidities among these hospitalized COVID-19 patients [39]. These findings were congruent with a study conducted by Williamson et al. [44]; which reported that diabetes, severe respiratory disease, chronic heart disease, liver disease, stroke, and dementia (all age-related diseases) increased the risk of death from COVID-19 [44].
Furthermore, Santesmasses et al. [51] reported that although COVID-19 mortality in people with no medical comorbidity increased with age, COVID-19 mortality in patients with Alzheimer's disease and dementia increased exponentially with age, particularly in patients >60 years old [51]. The important role of neurocognitive impairment in COVID-19 mortality was consistent with the findings reported by [45] in children and young people with neurological comorbidities. Results from this study indicate that young patients who died of COVID-19 had complex neurological impairments due to a combination of underlying genetic or metabolic conditions, hypoxic ischemic events or prematurity [45]. It is noteworthy that chronic inflammation is often present in these aforementioned preexisting medical comorbidities [52].
In summary, these studies suggest that the presence of inflammatory comorbidity (such as age-related diseases) significantly contributes to COVID-19 disease progression in both young and elderly patients infected with SARS-CoV-2. Furthermore, neurocognitive impairment due to chronic inflammation (discussed in later sections) may play a critical role in the increased severity and probability of death in COVID-19 patients. Therefore, studies on characterizing the contribution of aging and medical comorbidities to the pathogenesis of COVID-19 indicate that inflammation and inflammation-induced downstream events are risk factors to COVID-19 susceptibility and disease progression.
2.3. Biological age as a predictor for COVID-19 severity and mortality
The identification of age and medical comorbidities as risk factors that contribute to the prevalence and the progression of COVID-19 has brought attention to the concept known as biological age. Chronological age is defined as the amount of time that has passed from birth to a specific date, whereas biological age considers several factors, including the chronological age, diseases and other conditions, such as genetics, nutrition, and lifestyle [53]. In June of 2020, Blagosklonny hypothesized that chronological age and preexisting age-related medical conditions are factors that affect biological age. Thus, biological age is determined by an increase in chronological age and an increase in the presence of age-related diseases, such as hypertension, cardiovascular disease, arthritis, type II diabetes, atherosclerosis and cancer [53]. Furthermore, the vulnerability to SARS-CoV-2 infection and the clinical manifestations of COVID-19 are positively correlated with biological age, as opposed to chronological age.
To confirm if a patient's biological age plays a significant role in the severity of COVID-19, Kuo et al. [54] analyzed 6100 samples collected between March 16, 2020, and May 31, 2020, from 445,875 participants in England [54]. They used PhenoAge, a supervised machine learning model that uses biomarkers (i.e., albumin, alkaline phosphatase, creatinine, log C-reactive protein, glucose, lymphocyte percentage, mean corpuscular volume, red blood cell distribution width, and white blood cell count), to predict all-cause mortality. Their results indicated that 1273 (20.9%) people were SARS-CoV-2 positive and survived the infection, whereas 197 (3.2%) tested positive and died of COVID-19. PhenoAge acceleration (PhenoAgeAccel) uses four logistical models to predict COVID-19 severity outcomes by comparing individual's chronological age to their measured PhenoAge. A higher PhenoAgeAccel value, an indicator of a higher biological age, was positively correlated with COVID-19 severity [54]. The results from this study suggest that people with lower/younger biological ages are less likely to have age-related diseases and therefore, are less likely to develop more severe cases of COVID-19.
The accelerated aging in elderly populations and those with preexisting conditions is most likely due to chronic inflammation. This is based on the biomarker profiles and the shared correlation between PhenoAge and multiple diseases with COVID-19 severity [54]. It is possible that the correlation of COVID-19 severity with certain preexisting diseases results from a dysregulation of specific immune-related pathways [54]. For example, age-related diseases caused by mitochondrial dysfunction can increase disease progression and inflammation [55]. The release of mitochondrial DNA (mtDNA) has been shown to increase with age and the levels of proinflammatory cytokines [56]. Specifically, the levels of mtDNA are significantly increased in individuals >50 years old and this increase is positively correlated with increased levels of proinflammatory cytokines [56]. Moreover, the activation of inflammasomes can damage mitochondria, which can release mitochondrial proteins and mtDNA from damaged mitochondria, further increasing inflammasome activation [55]. Furthermore, SARS-CoV-2 infection can increase the activation of inflammasomes, which can exacerbate the risk of hyperinflammation, particularly in the elderly [57].
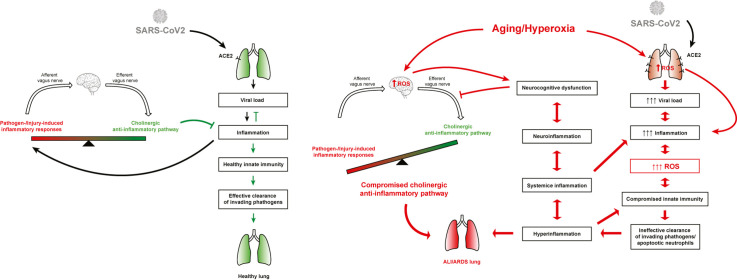
Overall, these studies reported a significant positive correlation between the magnitude of preexisting chronic inflammation in biologically aged individuals and the susceptibility to SARS-CoV-2 infection and the progression of COVID-19 disease. Mechanistically, the preexisting inflammation in the biologically elderly can be augmented by infection with SARS-CoV-2 and exacerbated by oxygen toxicity due to prolonged exposure to supplemental oxygen. This can result in unregulated hyperinflammation in the lungs and the circulation, which may lead to neuro-inflammation, ALI/ARDS, and the progression of severe COVID-19 (Fig. 1 ). Thus, understanding the mechanisms underlying the dysregulated inflammatory homeostasis in the lung and the circulation may provide important insight for developing therapeutic approaches to decrease morbidity and mortality in COVID-19 patients and improve clinical outcomes in these patients.
Fig. 1.
Aging and age-related diseases are the major risk factors that are correlated with the susceptibility and severity of COVID-19. Exacerbated symptoms of COVID-19 in patients are correlated with age and medical comorbidities, such as pneumonia, dementia, COPD, diabetes, obesity, cardiovascular diseases. Patients with increasingly severe COVID-19 symptoms are significantly more likely to have higher and sustained systemic and local levels of pro-inflammatory mediators. This pro-inflammatory phenotype increases the production of reactive oxygen species (ROS), producing oxidative stress, which contributes to chronic inflammation. The treatment of COVID-19 patients with supraphysiological supplemental oxygen therapy (≥21% O2) for prolonged periods of time induces oxidative stress, which alters immune cell functions and the inflammatory response that further exacerbates unresolved chronic inflammation. SARS-CoV-2-related viral pathology and the presence of oxidative stress associated with chronic inflammation make the biologically elderly more susceptible to severe COVID-19 and mortality. The size of the box indicates the magnitude of the inflammatory responses.
3. Supplemental oxygen-induced oxygen toxicity is characterized by an increased inflammatory response, aging biomarkers and acute lung injury
As previously discussed, low blood oxygen saturation and hypoxemic respiratory failure are the most common problems in hospitalized COVID-19 patients and supplemental oxygen is routinely used to treat patients with COVID-19, specifically those with ALI/ARDS. However, oxygen toxicity, produced by prolonged exposure to hyperoxia, may contribute to the deleterious clinical outcome in patients diagnosed with severe or critical cases of COVID-19. Prolonged exposure to hyperoxia can produce a pronounced inflammatory response and an increase in other aging biomarkers.
3.1. Oxygen toxicity produced by prolonged exposure to hyperoxia is characterized by increased levels of reactive oxygen species (ROS), cellular damage and acute inflammatory lung injury
Given the importance of maintaining sufficient O2 levels in circulation and tissues, supplemental oxygen is frequently administered to patients to treat tissue hypoxia and hypoxemia associated with various acute and chronic diseases, including COVID-19, characterized by low oxygen saturation [27,36,[58], [59], [60]]. Consequently, oxygen therapy with supplemental oxygen, especially mechanical ventilation, has served as a vital tool to sustain the life of patients diagnosed with ARDS, as in many critical cases of COVID-19 [26]. During oxygen therapy, the patient is administered supplemental oxygen at a concentration greater than 21%, the concentration of oxygen in ambient air at sea level, producing hyperoxia [23,27,36]. Hyperoxia can occur after the administration of supplemental oxygen via “non-invasive” or “invasive” methods. For example, nasal cannula, which delivers 24–50% fraction of inspired oxygen (FiO2), are used in patients with both type I and II respiratory failure [23]. An oxygen face mask can deliver variable concentrations of O2 (typically 35–60%) in patients with type I respiratory failure, whereas a reservoir mask can deliver O2 (60–80% or higher) for a defined period of time to critically ill patients [23]. Mechanical ventilation, an invasive tool and a life-sustaining therapy, is used to maintain adequate oxygenation in patients with severely impaired lung function and respiratory failure [25,61]. Despite the significant benefits of O2 in human health, “oxygen toxicity” is a foreign concept for the majority of the general public, especially given that “free radicals” is a popular phrase because of their role in producing inflammation to neutralize infections.
Oxygen toxicity, initially characterized by tracheobronchitis, was first reported as the Lorrain Smith effect by J. Lorrain Smith more than a century ago [62]. The pathological effects of hyperoxia are due to an increase in oxygen tension [27]. The magnitude of oxygen toxicity is time- and concentration-dependent, contingent upon the partial pressures of arterial oxygen (PaO2), the duration and the frequency of the exposure to hyperoxia and the health status of the individual [34]. Prolonged exposure to hyperoxia can damage cells, including endothelial cells, epithelial cells, alveolar macrophages, platelets, neurons and neuroglia and cause cell death [25,29,35,[63], [64], [65]]. Subsequently, the injury and the loss of these cells in the lung, the pulmonary microvasculature, and brain tissues can produce tissue damage, causing impairment in gas exchange, surfactant production, and neurocognitive dysfunction [25,[27], [28], [29],31,35]. Animals exposed to hyperoxia are frequently used as model systems to study the pathophysiology of ALI/ARDS [29]. Hyperoxia-induced acute lung injury can produce a significant inflammatory response, characterized by leukocyte infiltration and accumulation, pulmonary edema, microthrombi, impaired gas exchange and ultimately respiratory failure [25,59,60,66,67]. Similar to hyperoxia-induced ALI, ARDS is characterized by proteinaceous pulmonary edema, a hyperinflammatory response, and an increase in the permeability of the alveolar-capillary barrier [68,69].
3.2. Hyperoxia increases the levels of biomarkers indicative of aging
Published studies have addressed the question of whether prolonged exposure to hyperoxia increases biomarkers correlated with the aging phenotype, such as increased levels of reactive oxygen species (ROS), loss of mitochondrial integrity, senescent cell and lipofuscin accumulation, telomere shortening, and inflammation. The findings support the concept that hyperoxia can increase several biomarkers correlated with the aging phenotype. The persistent increase in the levels of these biomarkers has been observed in cells or animals subjected to prolonged exposure to hyperoxic conditions.
Hyperoxia-induced cell injury is initiated and mediated by increased levels of reactive oxygen species (ROS), especially superoxide [70]. Under normal physiological conditions, oxygen is primarily consumed by the complex IV of the electron transport chain (ETC) during oxidative phosphorylation, producing ATP in mitochondria [71,72]. ROS are produced as a byproduct of cellular respiration and approximately 0.2–2% of the electrons in the ETC leak and interact with oxygen to produce superoxide, O2 ·− [59,72]. Under hyperoxic conditions, increased production of ROS was first reported in the 1980s. In 1981, Freeman and Crapo reported that exposure to hyperoxia increased ROS production in rat lungs [73]. In addition, the production of H2O2 and O2 − by complexes I and III of ETC can be increased as oxygen tension increases [74] and increased levels of H2O2 and O2 − were present in mitochondria isolated from the lungs of animals exposed to hyperoxia [74,75]. An increase in mitochondrial ROS is positively correlated with the acceleration of the loss of mitochondrial integrity, an age-associated phenotype [76,77]. The increased levels of ROS, especially mtROS, in lung cells exposed to hyperoxia, can cause damage to mitochondria [78,79]. Notably, the mitochondria in lung capillary endothelial cells exposed to hyperoxia were reported larger than those remained in room air, and altered cristae and inclusion bodies have been identified in these cells [74,80,81]. Data indicating mitochondrial damage, as a consequence of oxidative stress, can be identified in nearly every age-related disease [82].
The biodegradation of mitochondria and their subsequent accumulation is a known characteristic of the aging process [83]. Damage to mitochondrial DNA and the loss of mitochondrial integrity and function, such as decreased ATP formation, and mitochondrial swelling, was identified in cells exposed to hyperoxia and was likely caused by oxidative stress [[83], [84], [85]]. In addition, lysosome clearance, mediated by the autophagocytotic processes, is critical in the removal of degraded mitochondria. Blocking lysosome clearance can increase the accumulation of not only biodegraded and dysfunctional mitochondria, but also the unbiodegradable molecule lipofuscin, another well-known biomarker of aging, which is inversely related to longevity [84,86]. Due to the lack of biodegradation, lipofuscin can continue to accumulate within lysosomes and further limit their autophagocytotic functions, resulting in abnormal mitochondrial biodegradation, additional mitochondrial damage due to increased ROS production and decreased ATP production [84].
Leonard Hayflick was the first scientist to report phenotypic changes in cultured human diploid cells. It was observed that generation time, waste accumulation, and cellular degeneration increased alongside a decrease in mitotic activity, as the cultured cells multiplied and aged for approximately one year [87]. These results reported by Hayflick established that, despite alterations in the cell culturing procedure, cultured cells are subjected to the innate characteristic of a finite lifetime due to their expression of aging or senescence [87]. von Ziglinicki et al. [88] analyzed the rate of senescence development in cells exposed to hyperoxia. Biomarkers of aging, such as lipofuscin accumulation and irreversible inhibition of proliferation, increased proportionally with aging, based on the comparison between young and aging fibroblasts [88]. Interestingly, the young hyperoxia-exposed cells exhibited the same morphology and lipofuscin levels as in senescent cells [88]. Furthermore, fibroblasts exposed to long-term hyperoxia were also irreversibly blocked from undergoing cell proliferation, similar to senescent cells [88]. These results suggest that hyperoxia exposure produces an increase in the aging phenotype in young fibroblast cells to a level comparable to aged, senescent cells.
Lipofuscin is commonly referred to as an “age-pigment” because its content in cells is age-dependent and it accumulates within cells over time at different rates, depending on different factors, such as species type, type of cells and levels of oxidative stress [89]. The accumulation of lipofuscin structures was reported to be greater and more complex at 12 days of age in vitro, compared to 8- or 3-day-old mouse cardiac myocyte cell cultures, congruent with the previously reported age-dependent accumulation of lipofuscin [90]. Under 40% hyperoxia, lipofuscin levels were increased (89%) in cultured mouse cardiac myocytes when the cells were allowed to age between 7 and 12 days in vitro [90]. The accumulation of lipofuscin increased progressively in an oxygen concentration-dependent manner, suggesting that the degree of oxidative stress under varying hyperoxic conditions and the age of the cell culture plays a critical role in the levels of accumulation of lipofuscin [90]. The increased accumulation of lipofuscin in aged cells due to hyperoxia produces adverse consequences, as previously mentioned.
Telomeres are part of the chromatin structure and are responsible for capping and protecting the ends of the chromosomes from DNA damage responses [91]. In mice and humans, telomeres begin to shorten and deteriorate as age increases [92,93]. Allsopp et al. [92] reported that in fibroblast cells obtained from various aged donors, the mean telomere length throughout the lifespan of humans will decrease by approximately 1.5 kb [92]. Shorter telomere length has been shown to increase the risk of humans developing certain diseases, such as idiopathic pulmonary fibrosis [94]. To determine whether hyperoxia exposure alters telomere length, von Zglinicki et al. [88] analyzed DNA samples from human fibroblasts that were cultured in either room air or hyperoxia [88]. Results from this study indicated a significant decrease in telomere length in human fibroblast cells exposed to hyperoxia for two weeks, compared to cells exposed to room air. The length of the telomeres in the hyperoxia-exposed cells was similar or equal in length to the telomeres in senescent cells [88], suggesting that hyperoxia exposure in fibroblast cells can affect telomere length, until a senescent-like morphology is induced.
3.3. Exposure to hyperoxia increases the expression of the protein, angiotensin converting enzyme 2 (ACE2)
The binding of the SARS-CoV-2 virus spike protein to Angiotensin converting enzyme 2 (ACE2) is a point of entry of the SARS-CoV-2 virus into human cells [95]. It has been hypothesized that the expression level of ACE2 plays a critical role in the susceptibility to SARS-CoV-2 infection [96]. ACE2, an enzyme that catalyzes the hydrolysis of angiotensin II to angiotensin (1–7), is present in the lungs, heart, intestines, kidneys and brain of humans [97,98].
Interestingly, the levels of ACE2 expression in airway epithelial cells are age-dependent [99]. Yee et al. [99] compared the expression levels of ACE2 in young (2 months old), middle aged (12 months old) and old (24 months old) mice. They found that the levels of ACE2 in the lungs of mice were lowest at 2 months of age, compared to that of both 12- or 24-month-old mice, and were significantly greater in 24-month-old mice, compared to 12-month-old mice. These results, provided they can be extrapolated to humans, suggest that ACE2 expression increases with age and could explain, in part, the increased susceptibility of the elderly to SARS-CoV-2 infection. Intriguingly, the expression of ACE2 in the airway epithelial cells was increased in mice exposed to hyperoxia, compared to mice exposed to room air [99]. These results suggest that supplemental oxygen can increase the levels of ACE2 expression, which may further facilitate the entry of SARS-CoV-2 to the airways, thereby potentially increasing the aging phenotype.
3.4. Does hyperoxia contribute to inflammaging?
Inflammation is an integral part of the host defense of the innate immunity and essential for protecting the host against inflections and injuries [100]. Under normal conditions, inflammation is a local, temporary response to eliminate the initial causes of cell injury and gets resolved when the harmful stimuli is eliminated to restore physiological homeostasis. While too little inflammation can lead to tissue damage by the deleterious stimuli, excessive and/or chronic inflammation in the absence of initial overt infection or other stimuli can be detrimental to the host, leading to tissue damage and causing various inflammatory diseases, observed in the severe and critical cases of COVID-19. Prolonged excessive chronic inflammation can contribute to inflammaging, which is a chronic and low-grade inflammation developed with advanced age [101]. The concept of inflammaging was derived from the positive correlation between increased proinflammatory phenotypes with aging in mammals [102].
The key initiator for inflammation is proinflammatory cytokines which are produced and secreted mainly by immune cells especially macrophages [103].
Due to its key role in the production of cytokines, transcription factor, NF-κB, has been a major topic of research in the area of aging progression [104]. Experimental data suggests that the activity of NF-κB is age-specific and positively correlated with the expression of aging phenotypes. Thus, it is designated as one of the biomarkers of cellular aging [104,105]. Activation of NF-κB is often implicated as an essential contributor to inflammatory tissue injury including ALI [106]. Exposure of cultured macrophages to hyperoxia can activate NF-κB [[107], [108], [109], [110]]. Interestingly, NF-κB inhibitors, such as BAY 11–7082, can restore macrophage function compromised by hyperoxia [110] and decrease levels of ROS, markers of senescence and cellular damage in mouse cells subjected to aging [105]. Therefore, these results suggest that hyperoxia augments the aging phenotype associated with NF-κB activation and NF-κB inhibitors may represent a viable strategy to attenuate the consequences produced by aging and oxygen toxicity in immune cells.
Other aging biomarkers discussed above may also contribute to inflammaging. For example, increased levels of circulating mitochondrial DNA play a significant role in propagating this inflammatory response, because it is associated with the increased levels of proinflammatory cytokines [56]. Furthermore, mitochondrial DNA not only increases with age after the fifth decade of life, but it also maintains the proinflammatory phenotype reported in the elderly [56].
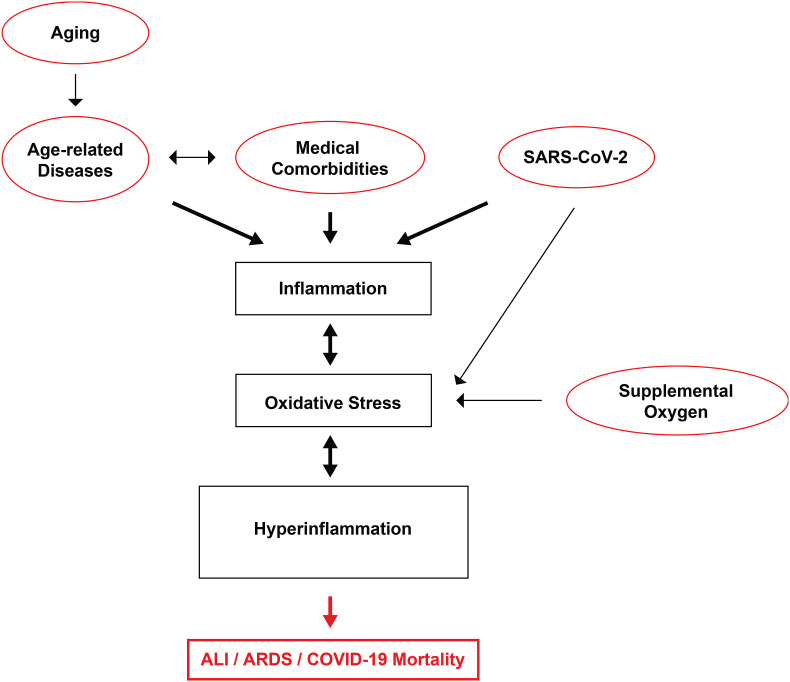
In summary, these studies suggest a positive correlation between an increase in the levels of aging biomarkers, indicative of biological aging, and the exposure to hyperoxia (Fig. 2 ). These biomarkers include an increase in levels of ROS, lipofuscin accumulation, senescent cells, and a decrease in mitochondrial integrity, exacerbated telomere shortening and the “inflammaging” response. If mitochondrial degradation is disrupted, cellular “waste” can accumulate, resulting in the increased presence of cellular toxicants, such as lipofuscin, which then continues this cycle, leading to inhibition of mitochondrial degradation. This increases the accumulation of more lipofuscin and further increases ROS production, until the cell becomes senescent or dies. The activation of “inflammaging”, specifically in tandem with the NF-κB-mediated downstream events, also facilitates the development of other age-related phenotypes. Furthermore, hyperoxia increases the expression of the ACE2 protein, facilitating the entry of SARS-CoV-2 into host cells, and increasing the risk of SARS-CoV-2 infection. The persistent increase in the levels of these aging biomarkers in cells exposed to hyperoxia can eventually produce several deleterious effects. The acceleration of aging appears to be positively correlated with an increase in the risk of developing various types of disease and the exacerbation of symptoms in preexisting medical conditions and an increase in the likelihood of mortality. If the health problems caused by hyperoxia are not addressed in a timely and decisive manner, the consequences of hyperoxia will accumulate and ultimately lead to mortality. Assuming that these findings may be applied to humans, targeting these mechanisms of aging may prove to be a feasible mode of treatment to address issues associated with aging, specifically the increase in susceptibility to other diseases. At least, if we can effectively attenuate the increase in aging biomarkers produced under hyperoxic conditions, it may be possible to develop strategies that can effectively improve hypoxemia with supplemental oxygen, while minimizing the effects of oxygen toxicity and thus, improve the overall clinical outcome.
Fig. 2.
Hyperoxia exacerbates the production of aging biomarkers that increase inflammaging and decrease health span. Prolonged exposure to hyperoxia (≥21% O2) increases the levels of reactive oxygen species (ROS). Subsequently, the increased ROS production can directly result in 1) increased expression of angiotensin-converting enzyme-2 (ACE2); 2) increased activation of NF-κB; 3) inflammation of the lungs; 4) accumulation of lipofuscin; 5) decreased length of telomeres; 6) accumulation of senescent cells, and 7) disrupted mitochondrial integrity. These effects can increase the susceptibility to other disease and disease severity. For example, the increased expression of ACE2 in the lungs may result in an increased susceptibility to SARS-CoV-2 infection. If oxygen therapy needed, neurological injury and lung inflammation can together contribute to systemic inflammation that triggers in a vicious cycle of inflammation and tissue injury, thereby increasing lung and brain dysfunction. An increase in the aging phenotype negatively affects inflammaging and health span, a measure of the length of a life without disease or injury, resulting in increased morbidity and mortality.
4. Neural modulation of inflammatory responses by the cholinergic anti-inflammatory pathway/the inflammatory reflex
As discussed above, chronic inflammation and excessive dysregulated inflammatory responses in the lungs and the circulation are major contributors to the susceptibility to SARS-CoV-2 infection and can worsen the clinical outcome of patients with COVID-19 [102,[111], [112], [113]]. In the last two and a half years, significant efforts have been made to identify effective treatments to dampen the unrestrained intemperate inflammatory responses in COVID-19. Although inflammatory responses initiated by innate immunity play an essential role in the clearance of pathogens and injured cells/debris, failure to modulate the extent of inflammation can result in unregulated robust production of proinflammatory cytokines, characteristics of excessive dysregulated and/or chronic inflammation [[114], [115], [116]]. The cholinergic anti-inflammatory pathway (CAIP), also known as the inflammatory reflex, was first discovered by Dr. Kevin J Tracy [117]. The CAIP, a neurocircuit, can modulate the magnitude of the inflammatory response in the visceral organs and the circulation [117]. Under physiological conditions, an excessive inflammatory response in the lungs or other visceral organs can activate the CAIP, which can significantly decrease the production of pro-inflammatory mediators [117]. The activation of the CAIP can maintain the inflammatory homeostasis to prevent prolonged dysregulated inflammation, which can produce tissues injury, such as ALI/ARDS, systemic inflammation, sepsis, multiple organ failure and death [33,118,119]. Numerous studies have shown that activation of the CAIP can significantly tame the overproduction of proinflammatory cytokines and attenuate lung injury in mouse models of ALI/ARDS-induced by hyperoxia, pneumonia or sepsis [109,[120], [121], [122], [123], [124]].
4.1. The cholinergic anti-inflammatory pathway modulates the lung inflammatory response by activating α7 nicotinic acetylcholine receptors
Inflammation is an initial host defense response of the innate immunity to infections or injuries [100]. The release of pro-inflammatory cytokines (including chemokines) from immune cells, notably the residential macrophages, is one of the early stages of the innate immune response that initiates inflammation to prevent damage to tissues [125,126]. However, the resulting infiltration and accumulation of leukocytes in local tissues can further increase the levels of pro-inflammatory mediators and exacerbate the proinflammatory response, causing inflammation-induced tissue injury if not properly modulated [33,117,127,128]. Thus, the modulation/regulation of local inflammatory responses is critically important in maintaining the functions of the visceral organs, including the lungs [117].
The CAIP is a cranial reflex, whereby the levels of proinflammatory cytokines in local tissues can be detected by the autonomic nervous system, which can activate significant responses to modulate local inflammation [117]. By signaling between the tissue immune responses and the brain, the CAIP plays a critical role in maintaining inflammatory homeostasis in the visceral organs [117]. The CAIP involves the vagus nerve, the 10th cranial nerve, which originates from the brainstem and innervates the magnitude of the inflammatory response in the visceral organs. Once this neuronal anti-inflammatory reflex is activated, it helps to maintain the physiological functions of these organs [117].
Similar to other reflexes, the CAIP is composed of the 1) afferent vagus nerve, the sensory branch, which can be activated by cytokines, such as HMGB1 and TNF-α, and 2) efferent vagus nerve, the motor branch, which sends signals to the residential macrophages, to decrease the excessive production of cytokines [116,117]. Specifically, in response to inflammatory mediators produced upon exposure to invading pathogens and tissue damage, the sensory/afferent fiber of the vagus nerve transmits signals from the visceral organs to the nucleus tractus solitaries in the medulla oblongata of the brainstem [129]. The sensory signals received from the afferent vagus nerve are integrated in the brainstem and then relayed by the motor/efferent vagal neurons in the medulla oblongata to the lungs (Fig. 3 ), heart, liver, kidneys and gastrointestinal tract [117]. The receptors that detect damage or invading pathogens on chemosensory cells in the vagal paraganglia are an integral part of the pathway, contributing to the transmission and the activation of the CAIP [130,131]. In the respiratory system, the vagus nerve innervates parasympathetic ganglia located within the airway walls [132]. The terminal efferent vagus nerve in the airway releases the neurotransmitter acetylcholine (ACh) to activate downstream signaling pathways in residential macrophages, the target cells [[133], [134], [135], [136]]. ACh can also be released from pulmonary neuroendocrine and immune cells from parasympathetic ganglia [137,138]. As an integral component of the CAIP, ACh can bind to the α7 nicotinic acetylcholine receptors (α7nAChRs) on different cells of the visceral organs to downregulate hyperinflammatory responses [139]. The ligand-gated α7nAChRs are homo-pentameric ligand-gated ion channels and play a major role in the homeostasis of local and systemic inflammation and in neurocognitive functions [140,141]. The activation of the α7nAChRs in residential macrophages, such as alveolar macrophages and microglia, can attenuate the release of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 [117,131].
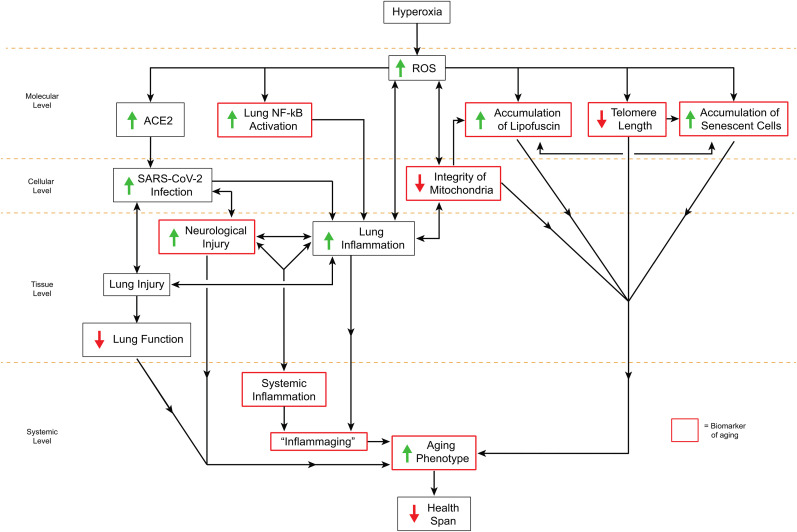
Fig. 3.
Proposed mechanisms by which α7nAChR agonists protect patients from hyperoxia-induced neurocognitive dysfunction and compromised innate immunity by activating the cholinergic anti-inflammatory pathway. Under normoxic conditions, lung inflammation in response to infections or injuries in healthy subjects is at least partially modulated by the activation of the cholinergic anti-inflammatory pathway (CAIP). The CAIP aids in the homeostatic maintenance of lung and brain inflammation, which influences healthy lung and cognitive functions. Under hyperoxic conditions and as a consequence of aging and neurodegenerative diseases, increased levels of reactive oxygen species (ROS) can impair macrophage functions in the lung and the neurons in the brain. ROS-induced damage to the cells in the central nervous system (CNS), peripheral nervous system (PNS), and blood-brain barrier (BBB) can result in cognitive dysfunction, which can cause impairment of the CAIP. The compromised CAIP can establish a pathological inflammatory cycle, resulting in dysregulated hyperinflammatory responses in the lung and the development of the acute lung injury (ALI)/acute respiratory distress syndrome (ARDS). Increased levels of ROS in the lung also activate NF-κB, which further increase the release of proinflammatory cytokines, exacerbating lung inflammation. Hyperinflammatory responses can then contribute to systemic inflammation and neuroinflammation and produce a further deterioration in cognitive function, thus establishing a vicious cycle of compromised neuro-immune modulation. α7 nicotinic acetylcholine receptor (α7nAChR) agonists, such as GTS-21 or GAT107, help re-establish the homeostatic inflammatory balance. The activation of α7nAChR by GTS-21 or GAT107 can down-regulate the excessive production/secretion of proinflammatory cytokines (including HMGB1), which is correlated with the attenuation of hyperoxia-compromised innate immunity and neurocognitive function. Therefore, α7nAChR agonists may have a protective role in hyperoxia-induced neurocognitive dysfunction and inflammatory lung diseases.
The efficacy of CAIP activation has been reported in animals and patients with inflammatory disorders. For example, the activation of the CAIP by α7nAChRs agonists can inhibit the excessive production of proinflammatory cytokines and improve clinical outcomes in animal models of sepsis and ALI/ARDS [109,115,121,123,142]. Electronic-based interventions, such as vagus nerve stimulation and electro-acupuncture points, can also decrease the levels of both proinflammatory cytokines, such as IL-4, IL-5, IL-6, IL-13, and inflammatory cell infiltration as seen in animal models of inflammatory diseases/disorders, including endotoxemia-induced ALI/sepsis [[143], [144], [145]]. Moreover, the vagus nerve stimulation facilitates the resolution of inflammation. In a mouse model of peritonitis, vagus nerve stimulation decreased the duration of inflammation resolution, by augmenting the levels of specific pro-resolving mediators, and the efferocytosis of infiltrated leukocytes [146]. Importantly, Koopman et al. [147] reported that vagal nerve stimulation can inhibit cytokine production, which attenuated disease severity in patients with rheumatoid arthritis [147]. However, when the CAIP-mediated immunomodulation is dysfunctional, the excessive levels of pro-inflammatory mediators are present in the visceral organs such as the lungs, which can reflux back to circulation, causing exaggerated systemic inflammation, which can activate neuroinflammation, producing neurocognitive dysfunction and inflammation-associated mortality [115,124,127,142,148]. Thus, under pathophysiological conditions, such as sepsis and neurodegenerative diseases, impaired vagus nerve innervation and function can disrupt the immunomodulation in the lungs, which is preceded by subsequent cell death and lung injury (Fig. 3) [115,142,149,150]. These studies indicate the importance of the CAIP in modulating the local inflammatory homeostasis to prevent tissue injuries caused by excessive inflammation.
4.2. Prolonged exposure to hyperoxia compromises innate immunity by impairing macrophage functions, causing an excessive inflammatory response and lung injury
As discussed above, functional CAIP is critical for pulmonary integrity and its physiological functions. Thus, the activation of α7nAChR in the CAIP is important, as this can decrease the levels of certain pro-inflammatory cytokines, thereby maintaining inflammatory homeostasis in the lungs [109,116,117,121,123,151]. The α7nAChRs are widely expressed in lung cells, and have been identified in airway epithelial cells, alveolar macrophages and epithelial cells [152], neutrophils [152], and B- and T-lymphocytes [153,154].
Alveolar and interstitial macrophages, the resident phagocytes in the lungs, are essential components of the innate immunity that maintain the functions of the lungs in response to infections and injuries [100]. Under normoxic conditions, the response to pulmonary infections and/or injuries by lung macrophages initiates an inflammatory response by secreting proinflammatory cytokines, to induce the infiltration of leukocytes, which phagocytose the invading pathogens, injured cells and debris [155,156]. In addition to phagocytosing invading pathogens, macrophages are critical in resolving inflammation by clearing cells that have undergone apoptosis, particularly neutrophils, from the lungs by efferocytosis, a process by which dying cells/debris are removed by phagocytes [157,158]. Therefore, the integrity and function of lung macrophages are essential in 1) the clearance of invading pathogens, injured cells and molecules that activate inflammation and 2) communicating with the brain via the vagus nerve, to modulate the level of the proinflammatory response by CAIP to prevent excessive inflammation and subsequent lung injury [[157], [158], [159]].
Following prolonged exposure to hyperoxia, however, lung macrophages are damaged and undergo cell death, which can lead to compromised innate immunity in the clearance of invading pathogens and injured cells and the removal of molecules or stimuli that activate inflammation [[30], [65],[159], [160]]. Subsequently, the compromised macrophages cannot effectively activate CAIP, leading to excessive dysregulated inflammatory responses [161,162]. Interestingly, unpublished data from our laboratory indicates that the expression of α7nAChR in macrophages exposed to hyperoxia (95% oxygen for 24 h) was significantly decreased, compared to macrophages exposed to normoxia. The failure to achieve normal CAIP activation under hyperoxic conditions may result in an excessive, dysregulated hyperinflammatory response, which can subsequently augment leukocyte infiltration and accumulation in the lungs, damaging the parenchyma cells in the lung [35,107]. This could explain data obtained from experiments in hyperoxic animals, where excessive levels of proinflammatory cytokines and leukocytes in the lungs increased alveolar permeability, which further increased leukocyte infiltration, producing acute lung injury [30,159,[163], [164], [165]].
Mechanistically, the excessive dysregulated inflammation can further increase hyperoxia-induced ROS production and cause mitochondrial damage to cells in the lungs and the vasculature, which can produce systemic inflammation [27,35]. Indeed, hyperoxia-induced macrophage dysfunction is positively correlated with an increase in the levels of ROS in the macrophages and mitochondrial lysates, which can be attenuated by antioxidants, such as superoxide dismutase (SOD) and vitamin C [30,108,159,160](Gauthier et al., unpublished data). The clearance or removal of invading pathogens, infected cells and apoptotic cells/debris requires a sustained and uncompromised phagocytic/efferocytotic activity in the macrophages that is dependent on the mitochondrial ATP production [166]. The disruption of mitochondrial integrity and function in macrophages can further compromise both innate and acquired immunity [167]. Therefore, oxidative stress due to increased ROS production in macrophages exposed to hyperoxia can compromise the ability of macrophages to clear invading pathogens and dying cells, and to activate the CAIP [30,110,159,160].
HMGB1, a nuclear damage-associated molecular pattern protein, is a proinflammatory cytokine following its release into the extracellular milieu [33,168,169]. In mice and cultured macrophages, hyperoxia can induce the release of nuclear HMGB1 into the airways and circulation via activating NF-κB [33,109,110,123,159,164]. Furthermore, extracellular HMGB1 can directly compromise macrophage phagocytosis, which compromises innate immunity by decreasing bacterial clearance and the removal of neutrophils from the lungs, causing severe inflammation in the lungs [33,111,159,164,170]. High levels of extracellular HMGB1 in the airways and circulation affects mitochondrial respiration and ATP synthesis, causing cell injury and loss of cellular functions [[171], [172], [173]]. The accumulation of extracellular HMGB1 in the airway and circulation can contribute to acute lung injury, which can further cause systemic inflammation and the subsequent neuroinflammation reported in patients diagnosed with sepsis [109,111,123,164,168,169]. Using mouse models of ALI-induced by hyperoxia alone or in combinaition with bacterial infections, we have reported that hyperoxia causes an HMGB1-mediated hyperinflammatory response and ALI [159,164,170].
Remarkably, similar to ALI-induced by proinflammatory responses under hyperoxic conditions, macrophages, NF-κB activation, and high levels of extracellular HMGB1 have been hypothesized to be involved in the pathogenesis of COVID-19. For example, macrophages have been purported to play a key role in causing severe disease in patients with COVID-19 by producing excessive systemic and lung inflammation, causing ARDS and pneumonia [174]. In addition, the spike protein of SARS-CoV can activate the NF-κB-mediated innate immune response in cultured human monocytes/macrophages [175]. Interestingly, HMGB1 can increase the expression of ACE2 (a protein involved in the cellular entry of SARS-CoV-2 in host cells) in cultured human lung epithelial cells, liver cells, intestine cells, and urinary bladder cells [176]. Furthermore, patients with severe COVID-19 have significantly higher serum levels of HMGB1, compared to patients diagnosed with mild cases [17,33,[177], [178], [179]]. Importantly, increased serum levels of HMGB1 are positively correlated with COVID-GRAM risk scores in hospitalized patients [48], and with the levels of cys-leukotrienes, D-dimer, AST and ALT [179]. HMGB1 levels greater than 125.4 ng/mL have been reported to be the cut off level that distinguishes patients that are at a high risk of death [179]. The serum levels of HMGB1 at the early inflammatory phase of the disease can have a predictive value for the pathogenic outcome, including the occurrence of the increased levels of cytokines and chemokines and the increased probability of death in these patients [179]. Notably, all patients in this study received some form of supplemental oxygen [179]. Thus, the serum level of HMGB1 may represent a prognostic biomarker for the risk of death in severe COVID-19, and a novel target for the treatment of patients diagnosed with severe COVID-19.
These observations suggest that patients diagnosed with severe or critical COVID-19 may suffer from a compromised CAIP response and prolonged exposure to supplemental oxygen may be involved. Thus, it has been hypothesized that the exogenous activation of CAIP could be a novel strategy in the treatment of ARDS with hyperinflammation in COVID-19 patients [151,180,181].
4.3. Agonists of the α7nAChR decrease inflammatory lung injury in models of hyperoxia-induced ALI/ARDS by attenuating the excessive inflammatory response and improving macrophage function
To test the hypothesis that hyperoxia-compromised CAIP can be restored by α7nAChR agonists, we treated mice subjected to prolonged exposure to hyperoxia with GTS-21, a selective partial α7nAChR agonist of α7nAChR [182,183]. The i. p. administration of 4 mg/kg of GTS-21 significantly reduced hyperoxia-induced acute inflammatory lung injury and the infiltration of inflammatory monocytes/macrophages and neutrophils into the lung tissue and airways [109,123]. In addition, GTS-21 can restore hyperoxia-impaired phagocytic activity in macrophages by inhibiting NF-κB-mediated HMGB1 release into the airways and the circulation [109,123]. Hyperoxia-induced ALI and secondary bacterial pneumonia can also be attenuated following the i. p. administration of 4 mg/kg GTS-21 [109,123]. GAT107, the (+)-enantiomer of racemic 4-(4-bromophenyl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline-8-sulfonamide, is a positive allosteric modulator and direct allosteric activator that augments/potentiates the response to orthosteric site ligands and activates the α7nAChR ion channel by binding to an allosteric site [[184], [185], [186], [187], [188]]. The α7nAChR can be rapidly desensitized by ACh, whereas in vitro, the combination of GAT107 with ACh produces a significant decrease in the ACh-induced desensitization of the receptor [185,187]. GAT107 (3.3 mg/kg i.p.) can restore the hyperoxia-induced impairment of bacterial clearance in mice by enhancing the phagocytic function of cultured murine macrophages [121] and increasing the activity and protein levels of the antioxidant enzyme, superoxide dismutase (SOD1). In addition to SOD1, GAT107 also increased the activation of transcription factor Nrf2 and its downstream effector, heme oxygenase-1 in cultured macrophages [121]. Thus, α7nAChR agonists can effectively maintain CAIP regulation of macrophage-mediated inflammatory responses and the integrity of the function of the innate immunity under hyperoxic conditions [109,121,123,127]. Together, we and others have shown that activation of α7nAChRs of the CAIP by α7nAChR agonists can enhance macrophage function and lung innate immunity and improve mortality outcomes in animal models of ALI, sepsis and ventilator-associated pneumonia [109,121,123,182,183,189].
4.4. The efficacy of α7nAChR agonists in increasing cognitive function of age-related disease
Aging has been shown to be a potential confounding factor for hyperoxia-induced oxygen toxicity [190,191]. For example, patients undergoing abdominal surgery were significantly more likely to die if they were treated with 80% oxygen supply, compared to patients randomly assigned to receive 30% oxygen [191]. Thus, the risk of perioperative hyperoxia may outweigh the benefits of supplemental oxygen in specific age groups [190,191]. It is possible that age-dependent vulnerability to oxygen toxicity may help to explain why the elderly are more likely to develop severe COVID-19 compared to younger patients. Consequently, by attenuating oxygen toxicity, α7nAChR agonists could provide clinical benefits to patients receiving supplemental oxygen.
The brain maintains a high level of metabolic function that accounts for 20% of the total oxygen consumption in the body [49,192]. To maintain normal mammalian brain function, oxygen pressure must be maintained at a level that sustains homeostasis [193]. Oxygen toxicity caused by prolonged exposure to hyperoxia may cause damage and death of neurons and various neuroglia cells, leading to the loss of integrity and function in aged brains [31,190]. Consequently, oxygen toxicity-induced injury and the death of neurons and neuroglial cells can result in a disruption of the structure and neurocognitive functions of the brain, producing neuro-degenerative disorders in aged brain [31,190]. Notably, this oxygen toxicity-induced neurocognitive decline is even more pronounced in mice with Alzheimer's disease (AD), compared to normal mice [194]. In these AD mice, prolonged exposure to hyperoxia caused a significant impairment in learning and memory, which was correlated with increased lipid peroxidation in the hippocampus and entorhinal cortex [194].
Remarkably, patients with age-related neurodegenerative diseases, such as AD, Parkinson's disease and multiple sclerosis, have been reported to have compromised vagus nerve function and unregulated neuroinflammation [[195], [196], [197]]. Interestingly, α7nAChRs are also highly expressed in the CNS and the PNS, including parasympathetic and sympathetic ganglia [198]. Furthermore, these receptors play a critical role in normal neurocognitive functions and the homeostasis of neuro and systemic inflammation [140,141]. In the brain, α7nAChRs are expressed in the hippocampus, the thalamic reticular nucleus and the lateral and medial geniculate nuclei [180]. In addition, α7nAChRs are also expressed by neuroglial cells, such as microglia and astrocytes [199]. These cells are involved in mediating neuroinflammation [200,201]. The α7nAChRs in murine-derived microglial cells are activated by ACh or nicotine, which can inhibit LPS-induced TNF-α release from cultured neuroglial cells [202], suggesting the inflammatory reflex plays a pivotal role in maintaining brain integrity and function. Clinical data indicates that patients with AD have a decreased rate of learning during conditioned responses [203], primarily due to their decreased cognitive abilities. The progression of AD is characterized, in part, by a decrease in the expression of cholinergic receptors in areas of the cerebral cortex and the hippocampus [195]. A histological examination of post-mortem brain tissues of patients with AD indicated a significant decrease in binding sites for ACh and nicotine, compared to the normal control group, suggesting presynaptic cholinergic damage in these patients [197]. The loss of cholinergic receptors supports the hypothesis that the loss of cholinergic neurons in certain brain areas is positively correlated with a decrease in overall cognitive function. Interestingly, cerebellar Purkinje cell loss, hippocampal cholinergic dysfunction and pyramidal cell loss increase with age and are significantly greater in AD patients compared to those without AD [[204], [205], [206], [207]]. In addition, ACh, which activates nicotinic cholinergic receptors [139], could also affect cognitive functions [208].
The importance of CAIP in normal cognitive function is further illustrated in patients treated with drugs that can enhance CAIP activation. Galantamine is a reversible, competitive cholinesterase inhibitor and a positive allosteric modulator of the nicotinic receptor, which can activate the CAIP. In a two year, multicenter, randomized, placebo-controlled study of 2045 elderly AD patients, it was reported that the mortality and the decline in cognition were significantly decreased in the patient group (1,024) treated with galantamine (8–24 mg/day oral extended-release capsules for 12 weeks), compared to patients treated with placebo [209]. Furthermore, the trial had to be stopped before its completion, because of the benefit of increased survival in the patients treated with galantamine. The results of this clinical trial further support the importance of CAIP activation in improving clinical outcomes in age-related neurodegenerative diseases.
Numerous studies have also tested the hypothesis that the activation of the α7nAChRs could represent a treatment for AD [210,211]. In the Woodruff-Pak et al. [212] study, the effects of DMXBA, also known as GTS-21, on improving neurocognitive functions were assessed using the eye-blink classical conditioning test in aged rabbits. In this study, rabbits were injected with varying doses of GTS-21 or saline 15 min before an 850-ms, 85 dB, 1-kHz tone that was used as the conditioned stimulus. A 100-ms, 3-psi corneal air puff, the unconditioned stimulus, was presented to the left eye approximately 750-ms after the conditioned stimulus, which produced a reflexive blink, the unconditioned response [212]. The investigators examined if the rabbits developed a conditioned response within nine consecutive trials. The density of nicotinic receptors in the older rabbits was determined three days after testing [212]. The older rabbits treated with 0.5 or 1.0 mg/kg s. c. of GTS-21 had faster rates of acquisition, assessed by measuring the interval between the conditioned stimulus and the unconditioned stimulus. When the training sessions were repeated in a group of untreated younger rabbits, the level of function achieved with the older group was not significantly different from that of the younger rabbits [212]. The 0.5 and 1.0 mg/kg doses of GTS-21 also activated α7nAChRs and decreased the acquisition rate in the older rabbits. Overall, these results indicated that GTS-21 increases overall cognitive function in aged rabbits. One property that makes GTS-21 a favorable drug candidate for the treatment of AD is that it readily crosses the blood-brain barrier and has a rapid onset of action. More recent studies have shown that α7nAChRs agonists, GTS-21 and GAT107, can decrease the level of pro-inflammatory mediators in cultured astrocytes and ameliorate cognitive impairment in a mouse model of Alzheimer's disease [213,214].
GTS-21 has been shown in humans to be safe and efficacious in improving cognitive functions [215,216]. Thus, these α7nAChR agonists could represent a viable treatment option for AD patients as they can increase overall cognitive function to improve activation of CAIP in the brain by activating α7nAChRs and decreasing neuronal inflammation (Fig. 3). Given the importance of α7nAChR activation in CAIP, neuroinflammation and cognitive function, α7nAChR may present a therapeutic target in maintaining the innate immunity against infections and cognitive functions in patients with COVID-19.
5. Conclusions
In conclusion, we have discussed studies suggesting that aging is a major contributing factor that increases susceptibility to SARS CoV-2 infection, due to impaired signaling between the brain and the lung that is required to maintain immune homeostasis in the lungs. The likelihood of developing severe COVID-19 disease is significantly greater in the elderly and in individuals with certain underlying co-morbidities, who were likely to have received prolonged therapy with supplemental oxygen. Although supplemental oxygen can be lifesaving, it can produce oxygen toxicity, which can cause lung injury and compromise neurocognitive functions, producing the impairment of CAIP neuroimmunomodulation of lung inflammation, resulting in excessive dysregulated inflammation with impaired innate immunity in the lungs and circulation. This could result in ARDS and sepsis in COVID-19 patients. Neurocognitive/neuropsychiatric disorders affect the lives of millions worldwide in a variety of ways, particularly in patients that have been diagnosed with the post-COVD-19 syndrome (also known as post-acute sequelae of SARS CoV-2 infection (PASC)). One prominent recurring problem in PASC is neurocognitive dysfunction. Data from preclinical studies, provided they can be translated to humans, suggest that compounds that activate α7nAChR, such as GTS-21 and GAT107, could improve cognitive functions and hyperoxia-induced inflammatory lung injury. However, appropriate clinical trials must be conducted to determine if α7nAChR agonists will be efficacious in improving/restoring the CAIP neuroimmunomodulation of the lung inflammatory homeostasis in the biologically elderly, to decrease the dysregulated inflammation that produces ALI/ARDS. Overall, we hypothesize that, just like “location, location, location”, are the three things that matter the most in real estate, “communication, communication, communication”, should be the phrase that is paramount regarding the development of treatments to improve our health span, with or without COVID-19.
Footnotes
This article is a contribution to the special issue entitled “Mitochondrial Redox Signaling in Aging-Related Diseases: In Honor of Bruce N. Ames: A Pioneer in Mitochondrial Redox Signaling in Aging Research” Guest Edited by Prof. Jiankang Liu & Prof. Douglas C. Wallace.
References
- 1.Han H., Ma Q., Li C., Liu R., Zhao L., Wang W., Zhang P., Liu X., Gao G., Liu F., Jiang Y., Cheng X., Zhu C., Xia Y. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microb. Infect. 2020;9:1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai X., Wang M., Qin C., Tan L., Ran L., Chen D., Zhang H., Shang K., Xia C., Wang S., Xu S., Wang W. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in wuhan, China. JAMA netw. Open. 2020;3:e209666. doi: 10.1001/jamanetworkopen.2020.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A.B., Flach B., Lin B.C., Doria-Rose N.A., O'Dell S., Schmidt S.D., Corbett K.S., Swanson P.A., Padilla M., Neuzil K.M., Bennett H., Leav B., Makowski M., Albert J., Cross K., Edara V.V., Floyd K., Suthar M.S., Martinez D.R., Baric R., Buchanan W., Luke C.J., Phadke V.K., Rostad C.A., Ledgerwood J.E., Graham B.S., Beigel J.H. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A., Flach B., Doria-Rose N.A., Corbett K.S., Morabito K.M., O'Dell S., Schmidt S.D., Swanson P.A., Padilla M., Mascola J.R., Neuzil K.M., Bennett H., Sun W., Peters E., Makowski M., Albert J., Cross K., Buchanan W., Pikaart-Tautges R., Ledgerwood J.E., Graham B.S., Beigel J.H. An mRNA vaccine against SARS-CoV-2 — preliminary report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang F., Kream R.M., Stefano G.B. An evidence based perspective on mRNA-SARS-CoV-2 vaccine development. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2020;26 doi: 10.12659/MSM.924700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C., Chen X., Cai Y., Xia J., Zhou Xing, Xu S., Huang H., Zhang L., Zhou Xia, Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou Xin, Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. Plus. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callard F., Perego E. How and why patients made. Long Covid. Soc. Sci. Med. 2021;268 doi: 10.1016/j.socscimed.2020.113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raveendran A.V., Jayadevan R., Sashidharan S. Long COVID: an overview. Diabetes Metab. Syndr. Clin. Res. Rev. 2021;15:869–875. doi: 10.1016/j.dsx.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Lancet Facing up to long COVID. Lancet. 2020;396(1861) doi: 10.1016/S0140-6736(20)32662-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yong S.J. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Inf. Disp. 2021;53:737–754. doi: 10.1080/23744235.2021.1924397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiskopf D., Schmitz K.S., Raadsen M.P., Grifoni A., Okba N.M.A., Endeman H., van den Akker J.P.C., Molenkamp R., Koopmans M.P.G., van Gorp E.C.M., Haagmans B.L., de Swart R.L., Sette A., de Vries R.D. Phenotype and kinetics of SARS-CoV-2–specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abd2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.To K.K.-W., Tsang O.T.-Y., Leung W.-S., Tam A.R., Wu T.-C., Lung D.C., Yip C.C.-Y., Cai J.-P., Chan J.M.-C., Chik T.S.-H., Lau D.P.-L., Choi C.Y.-C., Chen L.-L., Chan W.-M., Chan K.-H., Ip J.D., Ng A.C.-K., Poon R.W.-S., Luo C.-T., Cheng V.C.-C., Chan J.F.-W., Hung I.F.-N., Chen Z., Chen H., Yuen K.-Y. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Displays. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farsalinos K., Angelopoulou A., Alexandris N., Poulas K. COVID-19 and the nicotinic cholinergic system. Eur. Respir. J. 2020;56 doi: 10.1183/13993003.01589-2020. 2001589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J., Jiang Q., Xia X., Liu K., Yu Z., Tao W., Gong W., Han J.J. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell 19. 2020. [DOI] [PMC free article] [PubMed]
- 18.Guan W., Ni Z., Hu Yu, Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., Du B., Li L., Zeng G., Yuen K.-Y., Chen R., Tang C., Wang T., Chen P., Xiang J., Li S., Wang Jin-lin, Liang Z., Peng Y., Wei L., Liu Y., Hu Ya-hua, Peng P., Wang Jian-ming, Liu J., Chen Z., Li G., Zheng Z., Qiu S., Luo J., Ye C., Zhu S., Zhong N. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. NEJMoa2002032. 2020. [DOI] [PMC free article] [PubMed]
- 19.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y., Yuan M.-L., Zhang Y.-L., Dai F.-H., Liu Y., Wang Q.-M., Zheng J.-J., Xu L., Holmes E.C., Zhang Y.-Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. Plus. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hochberg C.H., Semler M.W., Brower R.G. Oxygen toxicity in critically ill adults. Am. J. Respir. Crit. Care Med. 2021;204:632–641. doi: 10.1164/rccm.202102-0417CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh V., Khatana S., Gupta P., Bhagol A. Supplemental oxygen therapy: important considerations in oral and maxillofacial surgery. Natl. J. Maxillofac. Surg. 2011;2(10) doi: 10.4103/0975-5950.85846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heffner J.E. The story of oxygen. Respir. Care. 2013;58:18–31. doi: 10.4187/respcare.01831. [DOI] [PubMed] [Google Scholar]
- 25.Hanidziar D., Robson S.C. Hyperoxia and modulation of pulmonary vascular and immune responses in COVID-19. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2021. 320, L12-L16. [DOI] [PMC free article] [PubMed]
- 26.Cronin J.N., Camporota L., Formenti F. Mechanical ventilation in COVID-19: a physiological perspective. Exp. Physiol. 2021 doi: 10.1113/EP089400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher A.B. Oxygen therapy: side effects and toxicity 1,2. Am. Rev. Respir. Displays. 1980;122:61–69. doi: 10.1164/arrd.1980.122.5P2.61. [DOI] [PubMed] [Google Scholar]
- 28.Gore A., Muralidhar M., Espey M.G., Degenhardt K., Mantell L.L. Hyperoxia sensing: from molecular mechanisms to significance in disease. J. Immunot. 2010;7:239–254. doi: 10.3109/1547691X.2010.492254. [DOI] [PubMed] [Google Scholar]
- 29.Matute-Bello G., Frevert C.W., Martin T.R. Animal models of acute lung injury. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2008. 295, L379-L399. [DOI] [PMC free article] [PubMed]
- 30.Morrow D.M.P., Entezari-Zaherab T., Romashko J., Azghani A.O., Javdan M., Ulloa L., Miller E.J., Mantell L.L. Antioxidants preserve macrophage phagocytosis of Pseudomonas aeruginosa during hyperoxia. Free Radic. Biol. Med. 2007;42:1338–1349. doi: 10.1016/j.freeradbiomed.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reich B., Hoeber D., Bendix I., Felderhoff-Mueser U. Hyperoxia and the. Immature Brain. Dev. Neurosci. 2016;38:311–330. doi: 10.1159/000454917. [DOI] [PubMed] [Google Scholar]
- 32.Rogers L.K., Cismowski M.J. Oxidative stress in the lung – the essential paradox. Curr. Opin. Toxicol. 2018;7:37–43. doi: 10.1016/j.cotox.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang M., Gauthier A., Daley L., Dial K., Wu J., Woo J., Lin M., Ashby C., Mantell L.L. The role of HMGB1, a nuclear damage-associated molecular pattern molecule, in the pathogenesis of lung diseases. Antioxid. Redox Signal. 2019;31:954–993. doi: 10.1089/ars.2019.7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asfar P., Singer M., Radermacher P. Understanding the benefits and harms of oxygen therapy. Intensive Care Med. 2015;41:1118–1121. doi: 10.1007/s00134-015-3670-z. [DOI] [PubMed] [Google Scholar]
- 35.Mantell L.L., Horowitz S., Davis J.M., Kazzaz J.A. Hyperoxia-induced cell death in the lung-the correlation of apoptosis, necrosis, and inflammation. Ann. N. Y. Acad. Sci. 1999;887:171–180. doi: 10.1111/j.1749-6632.1999.tb07931.x. [DOI] [PubMed] [Google Scholar]
- 36.Mach W.J., Thimmesch A.R., Pierce J.T., Pierce J.D. Consequences of hyperoxia and the toxicity of oxygen in the lung. Nurs. Res. Pract. 2011;2011:1–7. doi: 10.1155/2011/260482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubin D., Chan-Tack K., Farley J., Sherwat A. FDA approval of remdesivir — a step in the right direction. N. Engl. J. Med. 2020;383:2598–2600. doi: 10.1056/NEJMp2032369. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Yi, Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Lond. Englera. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., the Northwell COVID-19 Research Consortium. Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., Cookingham J., Coppa K., Diefenbach M.A., Dominello A.J., Duer-Hefele J., Falzon L., Gitlin J., Hajizadeh N., Harvin T.G., Hirschwerk D.A., Kim E.J., Kozel Z.M., Marrast L.M., Mogavero J.N., Osorio G.A., Qiu M., Zanos T.P. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323(2052) doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N., Cuomo-Dannenburg G., Thompson H., Walker P.G.T., Fu H., Dighe A., Griffin J.T., Baguelin M., Bhatia S., Boonyasiri A., Cori A., Cucunubá Z., FitzJohn R., Gaythorpe K., Green W., Hamlet A., Hinsley W., Laydon D., Nedjati-Gilani G., Riley S., van Elsland S., Volz E., Wang H., Wang Y., Xi X., Donnelly C.A., Ghani A.C., Ferguson N.M. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect. Displays. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell T.W., Hellewell J., Jarvis C.I., van Zandvoort K., Abbott S., Ratnayake R., Cmmid COVID-19 working group, Flasche S., Eggo R.M., Edmunds W.J., Kucharski A.J. Estimating the infection and case fatality ratio for coronavirus disease (COVID-19) using age-adjusted data from the outbreak on the Diamond Princess cruise ship, February 2020. Eurosurveillance 25. 2020. [DOI] [PMC free article] [PubMed]
- 42.Field Briefing: Diamond Princess COVID-19 Cases, 20 Feb Update . National Institute of Infectious Diseases; 2020. [Google Scholar]
- 43.Davies N.G., Klepac P., Liu Y., Prem K., Jit M., Cmmid COVID-19 working group, Pearson C.A.B., Quilty B.J., Kucharski A.J., Gibbs H., Clifford S., Gimma A., van Zandvoort K., Munday J.D., Diamond C., Edmunds W.J., Houben R.M.G.J., Hellewell J., Russell T.W., Abbott S., Funk S., Bosse N.I., Sun Y.F., Flasche S., Rosello A., Jarvis C.I., Eggo R.M. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. 2020;26:1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 44.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., Cockburn J., McDonald H.I., MacKenna B., Tomlinson L., Douglas I.J., Rentsch C.T., Mathur R., Wong A.Y.S., Grieve R., Harrison D., Forbes H., Schultze A., Croker R., Parry J., Hester F., Harper S., Perera R., Evans S.J.W., Smeeth L., Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith C., Odd D., Harwood R., Ward J., Linney M., Clark M., Hargreaves D., Ladhani S.N., Draper E., Davis P.J., Kenny S.E., Whittaker E., Luyt K., Viner R., Fraser L.K. Deaths in children and young people in England after SARS-CoV-2 infection during the first pandemic year. Nat. Med. 2021;28(1):185–192. doi: 10.1038/s41591-021-01578-1. [DOI] [PubMed] [Google Scholar]
- 46.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., Holden K.A., Read J.M., Dondelinger F., Carson G., Merson L., Lee J., Plotkin D., Sigfrid L., Halpin S., Jackson C., Gamble C., Horby P.W., Nguyen-Van-Tam J.S., Ho A., Russell C.D., Dunning J., Openshaw P.J., Baillie J.K., Semple M.G. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ m1985. 2020. [DOI] [PMC free article] [PubMed]
- 47.Viner R.M., Mytton O.T., Bonell C., Melendez-Torres G.J., Ward J., Hudson L., Waddington C., Thomas J., Russell S., van der Klis F., Koirala A., Ladhani S., Panovska-Griffiths J., Davies N.G., Booy R., Eggo R.M. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2021;175(143) doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L., Long X., Xu Q., Tan J., Wang G., Cao Y., Wei J., Luo H., Zhu H., Huang Liang, Meng F., Huang Lifang, Wang N., Zhou X., Zhao L., Chen X., Mao Z., Chen C., Li Z., Sun Z., Zhao J., Wang D., Huang G., Wang W., Zhou J. Elevated serum levels of S100A8/A9 and HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients. Cell. Mol. Immunol. 2020;17:992–994. doi: 10.1038/s41423-020-0492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nandy K., Salunke A., Pathak S.K., Pandey A., Doctor C., Puj K., Sharma M., Jain A., Warikoo V. Coronavirus disease (COVID-19): a systematic review and meta-analysis to evaluate the impact of various comorbidities on serious events. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:1017–1025. doi: 10.1016/j.dsx.2020.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santesmasses D., Castro J.P., Zenin A.A., Shindyapina A.V., Gerashchenko M.V., Zhang B., Kerepesi C., Yim S.H., Fedichev P.O., Gladyshev V.N. COVID-19 is an emergent disease of aging. Aging Cell 19. 2020. [DOI] [PMC free article] [PubMed]
- 52.Callender L.A., Curran M., Bates S.M., Mairesse M., Weigandt J., Betts C.J. The impact of pre-existing comorbidities and therapeutic interventions on COVID-19. Front. Immunol. 2020;11(1991) doi: 10.3389/fimmu.2020.01991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blagosklonny M.V. From causes of aging to death from COVID-19. Aging. 2020;12:10004–10021. doi: 10.18632/aging.103493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuo C.-L., Pilling L.C., Atkins J.L., Masoli J.A., Delgado J., Tignanelli C., Kuchel G.A., Melzer D., Beckman K.B., Levine M.E. COVID-19 severity is predicted by earlier evidence of accelerated aging (preprint). Epidemiology. 2020. [DOI]
- 55.Jang J.Y., Blum A., Liu J., Finkel T. The role of mitochondria in aging. J. Clin. Invest. 2018;128:3662–3670. doi: 10.1172/JCI120842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pinti M., Cevenini E., Nasi M., De Biasi S., Salvioli S., Monti D., Benatti S., Gibellini L., Cotichini R., Stazi M.A., Trenti T., Franceschi C., Cossarizza A. Circulating mitochondrial DNA increases with age and is a familiar trait: implications for “inflamm-aging”: innate immunity. Eur. J. Immunol. 2014;44:1552–1562. doi: 10.1002/eji.201343921. [DOI] [PubMed] [Google Scholar]
- 57.Shah A. Novel coronavirus-induced NLRP3 inflammasome activation: a potential drug target in the treatment of COVID-19. Front. Immunol. 2020;11(1021) doi: 10.3389/fimmu.2020.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bateman N.T., Leach R.M. ABC of Oxygen: acute oxygen therapy. BMJ. 1998;317:798–801. doi: 10.1136/bmj.317.7161.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kallet R.H., Matthay M.A. Hyperoxic acute lung injury. Respir. Care. 2013;58:123–141. doi: 10.4187/respcare.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vyas-Read S., Vance R.J., Wang W., Colvocoresses-Dodds J., Brown L.A., Koval M. Hyperoxia induces paracellular leak and alters claudin expression by neonatal alveolar epithelial cells. Pediatr. Pulmonol. 2018;53:17–27. doi: 10.1002/ppul.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slutsky A.S. History of mechanical ventilation. From vesalius to ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 2015;191:1106–1115. doi: 10.1164/rccm.201503-0421PP. [DOI] [PubMed] [Google Scholar]
- 62.Smith J.L. The pathological effects due to increase of oxygen tension in the air breathed. J. Physiol. 1899;24:19–35. doi: 10.1113/jphysiol.1899.sp000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mantell L.L., Shaffer T.H., Horowitz S., Foust R., 3rd, Wolfson M.R., Cox C., Khullar P., Zakeri Z., Lin L., Kazzaz J.A., Palaia T., Scott W., Davis J.M. Distinct patterns of apoptosis in the lung during liquid ventilation compared with gas ventilation. Am. J. Physiol. Cell. Mol. Physiol. 2002;283:L31–L41. doi: 10.1152/ajplung.00037.2001. [doi] [DOI] [PubMed] [Google Scholar]
- 64.Mantell L.L., Kazzaz J.A., Xu J., Palaia T.A., Piedboeuf B., Hall S., Rhodes G.C., Niu G., Fein A.F., Horowitz S. Unscheduled apoptosis during acute inflammatory lung injury. Cell Death Differ. 1997;4:600–607. doi: 10.1038/sj.cdd.4400278. [DOI] [PubMed] [Google Scholar]
- 65.Petrache I., Choi M.E., Otterbein L.E., Chin B.Y., Mantell L.L., Horowitz S., Choi A.M.K. Mitogen-activated protein kinase pathway mediates hyperoxia-induced apoptosis in cultured macrophage cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1999. 277, L589-L595. [DOI] [PubMed]
- 66.Jones R., Zapol W.M., Reid L. Pulmonary artery remodeling and pulmonary hypertension after exposure to hyperoxia for 7 days. A morphometric and hemodynamic study. Am. J. Pathol. 1984;117:273–285. [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar V.H.S., Chaker El Khoury J., Gronostajski R., Wang H., Nielsen L., Ryan R.M. Nfib hemizygous mice are protected from hyperoxic lung injury and death. Phys. Rep. 2017;5:e13398. doi: 10.14814/phy2.13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Galani V., Tatsaki E., Bai M., Kitsoulis P., Lekka M., Nakos G., Kanavaros P. The role of apoptosis in the pathophysiology of Acute Respiratory Distress Syndrome (ARDS): an up-to-date cell-specific review. Pathol. Res. Pract. 2010;206:145–150. doi: 10.1016/j.prp.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 69.Pugin J., Verghese G., Widmer M.-C., Matthay M.A. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome: Crit. Care Med. 1999;27:304–312. doi: 10.1097/00003246-199902000-00036. [DOI] [PubMed] [Google Scholar]
- 70.Chabot F., Mitchell J.A., Gutteridge J.M., Evans T.W. Reactive oxygen species in acute lung injury. Eur. Respir. J. 1998;11:745–757. [PubMed] [Google Scholar]
- 71.Chance B., Williams G.R. Respiratory enzymes in oxidative phosphorylation. J. Biol. Chem. 1955;217:395–407. doi: 10.1016/S0021-9258(19)57190-3. [DOI] [PubMed] [Google Scholar]
- 72.Zhao R., Jiang S., Zhang L., Yu Z. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019;44(1):3–15. doi: 10.3892/ijmm.2019.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Freeman B.A., Crapo J.D. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J. Biol. Chem. 1981;256:10986–10992. [PubMed] [Google Scholar]
- 74.Turrens J.F., Freeman B.A., Crapo J.D. Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Arch. Biochem. Biophys. 1982;217:411–421. doi: 10.1016/0003-9861(82)90519-7. [DOI] [PubMed] [Google Scholar]
- 75.Freeman B.A., Topolosky M.K., Crapo J.D. Hyperoxia increases oxygen radical production in rat lung homogenates. Arch. Biochem. Biophys. 1982;216:477–484. doi: 10.1016/0003-9861(82)90236-3. [DOI] [PubMed] [Google Scholar]
- 76.Beckman K.B., Ames B.N. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 77.Fleming J.E., Miquel J., Cottrell S.F., Yengoyan L.S., Economos A.C. Is cell aging caused by respiration-dependent injury to the mitochondrial genome? Gerontology. 1982;28:44–53. doi: 10.1159/000212510. [DOI] [PubMed] [Google Scholar]
- 78.Aravamudan B., Thompson M.A., Pabelick C.M., Prakash Y. Mitochondria in lung diseases. Expet Rev. Respir. Med. 2013;7:631–646. doi: 10.1586/17476348.2013.834252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xuefei Y., Xinyi Z., Qing C., Dan Z., Ziyun L., Hejuan Z., Xindong X., Jianhua F. Effects of hyperoxia on mitochondrial homeostasis: are mitochondria the hub for bronchopulmonary dysplasia? Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.642717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Crapo J.D., Barry B.E., Foscue H.A., Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am. Rev. Respir. Displays. 1980;122:123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- 81.Rosenbaum R.M., Wittner M., Lenger M. Mitochondrial and other ultrastructural changes in great alveolar cells of oxygen-adapted and poisoned rats. Lab. Investig. J. Tech. Methods Pathol. 1969;20:516–528. [PubMed] [Google Scholar]
- 82.Landis G.N., Abdueva D., Skvortsov D., Yang J., Rabin B.E., Carrick J., Tavare S., Tower J. Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2004;101:7663–7668. doi: 10.1073/pnas.0307605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cataldi A., Di Giulio C. “Oxygen supply” as modulator of aging processes: hypoxia and hyperoxia models for aging studies. Curr. Aging Sci. 2009;2:95–102. doi: 10.2174/1874609810902020095. [DOI] [PubMed] [Google Scholar]
- 84.Brunk U.T., Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic. Biol. Med. 2002;33:611–619. doi: 10.1016/S0891-5849(02)00959-0. [DOI] [PubMed] [Google Scholar]
- 85.Gray D.A., Woulfe J. Lipofuscin and aging: a matter of toxic waste. Sci. Aging Knowl. Environ. 2005;2005(5):1–5. doi: 10.1126/sageke.2005.5.re1. 2005, re1–re1. [DOI] [PubMed] [Google Scholar]
- 86.Brunk U.T., Terman A. The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur. J. Biochem. 2002;269:1996–2002. doi: 10.1046/j.1432-1033.2002.02869.x. [DOI] [PubMed] [Google Scholar]
- 87.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 88.von Zglinicki T., Saretzki G., Döcke W., Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp. Cell Res. 1995;220:186–193. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- 89.Terman A., Brunk U.T. Lipofuscin: mechanisms of formation and increase with age. APMIS. 1998;106:265–276. doi: 10.1111/j.1699-0463.1998.tb01346.x. [DOI] [PubMed] [Google Scholar]
- 90.Sohal R.S., Marzabadi M.R., Galaris D., Brunk U.T. Effect of ambient oxygen concentration on lipofuscin accumulation in cultured rat heart myocytes—a novel in vitro model of lipofuscinogenesis. Free Radic. Biol. Med. 1989;6:23–30. doi: 10.1016/0891-5849(89)90155-X. [DOI] [PubMed] [Google Scholar]
- 91.Chan S.W.-L., Blackburn E.H. New ways not to make ends meet: telomerase, DNA damage proteins and heterochromatin. Oncogene. 2002;21:553–563. doi: 10.1038/sj.onc.1205082. [DOI] [PubMed] [Google Scholar]
- 92.Allsopp R.C., Vaziri H., Patterson C., Goldstein S., Younglai E.V., Futcher A.B., Greider C.W., Harley C.B. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. USA. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blasco M.A. Telomere length, stem cells and aging. Nat. Chem. Biol. 2007;3:640–649. doi: 10.1038/nchembio.2007.38. [DOI] [PubMed] [Google Scholar]
- 94.Armanios M.Y., Chen J.J.-L., Cogan J.D., Alder J.K., Ingersoll R.G., Markin C., Lawson W.E., Xie M., Vulto I., Phillips J.A., Lansdorp P.M., Greider C.W., Loyd J.E. Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 95.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J. Infect. 2020;80:e14–e18. doi: 10.1016/j.jinf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hamming I., Timens W., Bulthuis M., Lely A., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Keidar S., Kaplan M., Gamliellazarovich A. ACE2 of the heart: from angiotensin I to angiotensin (1–7) Cardiovasc. Res. 2007;73:463–469. doi: 10.1016/j.cardiores.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 99.Yee M., David Cohen E., Haak J., Dylag A.M., O'Reilly M.A. Neonatal hyperoxia enhances age-dependent expression of SARS-CoV-2 receptors in mice. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-79595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 101.Alberro A., Iribarren-Lopez A., Sáenz-Cuesta M., Matheu A., Vergara I., Otaegui D. Inflammaging markers characteristic of advanced age show similar levels with frailty and dependency. Sci. Rep. 2021;11(4358) doi: 10.1038/s41598-021-83991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Franceschi C., Bonafè M., Valensin S., Olivieri F., De Luca M., Ottaviani E., De Benedictis G. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2006;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 103.Zhang J.-M., An J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Adler A.S., Sinha S., Kawahara T.L.A., Zhang J.Y., Segal E., Chang H.Y. Motif module map reveals enforcement of aging by continual NF-κB activity. Genes Dev. 2007;21 doi: 10.1101/gad.1588507. 000.1–000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tilstra J.S., Robinson A.R., Wang J., Gregg S.Q., Clauson C.L., Reay D.P., Nasto L.A., St Croix C.M., Usas A., Vo N., Huard J., Clemens P.R., Stolz D.B., Guttridge D.C., Watkins S.C., Garinis G.A., Wang Y., Niedernhofer L.J., Robbins P.D. NF-κB inhibition delays DNA damage–induced senescence and aging in mice. J. Clin. Invest. 2012;122:2601–2612. doi: 10.1172/JCI45785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xie K., Yu Y., Huang Y., Zheng L., Li J., Chen H., Han H., Hou L., Gong G., Wang G. Molecular hydrogen ameliorates lipopolysaccharide-induced acute lung injury in mice through reducing inflammation and apoptosis. Shock Augusta Ga. 2012;37:548–555. doi: 10.1097/SHK.0b013e31824ddc81. [DOI] [PubMed] [Google Scholar]
- 107.Mantell L.L., Lee P.J. Signal transduction pathways in hyperoxia-induced lung cell death. Mol. Genet. Metabol. 2000;71:359–370. doi: 10.1006/mgme.2000.3046. [DOI] [PubMed] [Google Scholar]
- 108.Patel V.S., Sampat V., Espey M.G., Sitapara R., Wang H., Yang X., Ashby C.R., Thomas D.D., Mantell L.L. Ascorbic acid attenuates hyperoxia-compromised host defense against pulmonary bacterial infection. Am. J. Respir. Cell Mol. Biol. 2016;55:511–520. doi: 10.1165/rcmb.2015-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sitapara R.A., Gauthier A.G., Patel V.S., Lin M., Zur M., Ashby C.R., Mantell L.L. The α7 nicotinic acetylcholine receptor agonist GTS-21 improves bacterial clearance in mice by restoring hyperoxia-compromised macrophage function. Mol. Med. 2020;26(98) doi: 10.1186/s10020-020-00224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang M., Gorasiya S., Antoine D.J., Sitapara R.A., Wu W., Sharma L., Yang H., Ashby C.R., Vasudevan D., Zur M., Thomas D.D., Mantell L.L. The compromise of macrophage functions by hyperoxia is attenuated by ethacrynic acid via inhibition of NF-κB–mediated release of high-mobility group box-1. Am. J. Respir. Cell Mol. Biol. 2015;52:171–182. doi: 10.1165/rcmb.2013-0544OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Andersson U., Ottestad W., Tracey K.J. Extracellular HMGB1: a therapeutic target in severe pulmonary inflammation including COVID-19? Mol. Med. 2020;26(42) doi: 10.1186/s10020-020-00172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Masso-Silva J.A., Moshensky A., Lam M.T.Y., Odish M.F., Patel A., Xu L., Hansen E., Trescott S., Nguyen C., Kim R., Perofsky K., Perera S., Ma L., Pham J., Rolfsen M., Olay J., Shin J., Dan J.M., Abbott R.K., Ramirez S., Alexander T.H., Lin G.Y., Fuentes A.L., Advani I., Gunge D., Pretorius V., Malhotra A., Sun X., Duran J., Hepokoski M., Crotty S., Coufal N.G., Meier A., Crotty Alexander L.E. Increased peripheral blood neutrophil activation phenotypes and neutrophil extracellular trap formation in critically ill coronavirus disease 2019 (COVID-19) patients: a case series and review of the literature. Clin. Infect. Dis. ciab437. 2021. [DOI] [PMC free article] [PubMed]
- 114.Pavlov V.A. Cholinergic modulation of inflammation. Int. J. Clin. Exp. Med. 2008;1:203–212. [PMC free article] [PubMed] [Google Scholar]
- 115.Pavlov V.A., Tracey K.J. The cholinergic anti-inflammatory pathway. Brain. Behav. Immunity. 2005;19:493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 116.Tracey K.J. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tracey K.J. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 118.Boomer J.S., To K., Chang K.C., Takasu O., Osborne D.F., Walton A.H., Bricker T.L., Jarman S.D., Kreisel D., Krupnick A.S., Srivastava A., Swanson P.E., Green J.M., Hotchkiss R.S. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306(2594) doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Engel O., Akyüz L., da Costa Goncalves A.C., Winek K., Dames C., Thielke M., Herold S., Böttcher C., Priller J., Volk H.D., Dirnagl U., Meisel C., Meisel A. Cholinergic pathway suppresses pulmonary innate immunity facilitating pneumonia after stroke. Stroke. 2015;46:3232–3240. doi: 10.1161/STROKEAHA.115.008989. [DOI] [PubMed] [Google Scholar]
- 121.Gauthier A.G., Wu J., Lin M., Sitapara R., Kulkarni A., Thakur G.A., Schmidt E.E., Perron J.C., Ashby C.R., Mantell L.L. The positive allosteric modulation of alpha7-nicotinic cholinergic receptors by GAT107 increases bacterial lung clearance in hyperoxic mice by decreasing oxidative stress in macrophages. Antioxidants. 2021;10(135) doi: 10.3390/antiox10010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Giebelen I.A.J., Leendertse M., Florquin S., van der Poll T. Stimulation of acetylcholine receptors impairs host defence during pneumococcal pneumonia. Eur. Respir. J. 2008;33:375–381. doi: 10.1183/09031936.00103408. [DOI] [PubMed] [Google Scholar]
- 123.Sitapara R.A., Gauthier A.G., Valdés-Ferrer S.I., Lin M., Patel V., Wang M., Martino A.T., Perron J.C., Ashby C.R., Tracey K.J., Pavlov V.A., Mantell L.L. The α7 nicotinic acetylcholine receptor agonist, GTS-21, attenuates hyperoxia-induced acute inflammatory lung injury by alleviating the accumulation of HMGB1 in the airways and the circulation. Mol. Med. 2020;26(63) doi: 10.1186/s10020-020-00177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.van Westerloo D.J., Giebelen I.A.J., Florquin S., Daalhuisen J., Bruno M.J., de Vos A.F., Tracey K.J., van der Poll T. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J. Infect. Dis. 2005;191:2138–2148. doi: 10.1086/430323. [DOI] [PubMed] [Google Scholar]
- 125.Turner M.D., Nedjai B., Hurst T., Pennington D.J. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta BBA - Mol. Cell Res. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 126.Unanue E.R., Beller D.I., Calderon J., Kiely J.M., Stadecker M.J. Regulation of immunity and inflammation by mediators from macrophages. Am. J. Pathol. 1976;85:465–478. [PMC free article] [PubMed] [Google Scholar]
- 127.Gauthier A.G., Lin M., Wu J., Kennedy T.P., Daley L.-A., Ashby C.R., Mantell L.L. From nicotine to the cholinergic anti-inflammatory reflex – can nicotine alleviate the dysregulated inflammation in COVID-19? J. Immunot. 2021;18:23–29. doi: 10.1080/1547691X.2021.1875085. [DOI] [PubMed] [Google Scholar]
- 128.Mantell L.L., Parrish W.R., Ulloa L. HMGB-1 as a therapeutic target for infectious and inflammatory disorders. Shock. 2006;25:4–11. doi: 10.1097/01.shk.0000188710.04777.9e. [DOI] [PubMed] [Google Scholar]
- 129.Pavlov V.A., Chavan S.S., Tracey K.J. Molecular and functional neuroscience in immunity. Annu. Rev. Immunol. 2018;36:783–812. doi: 10.1146/annurev-immunol-042617-053158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Goehler L.E., Gaykema R.P.A., Hansen M.K., Anderson K., Maier S.F., Watkins L.R. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton. Neurosci. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- 131.Pavlov V.A., Tracey K.J. The vagus nerve and the inflammatory reflex—linking immunity and metabolism. Nat. Rev. Endocrinol. 2012;8:743–754. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barnes P.J. Primer on the Autonomic Nervous System. Elsevier; 2012. Autonomic control of the lower airways; pp. 201–204. [DOI] [Google Scholar]
- 133.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hassert D.L., Miyashita T., Williams C.L. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav. Neurosciences. 2004;118:79–88. doi: 10.1037/0735-7044.118.1.79. [DOI] [PubMed] [Google Scholar]
- 135.Kenney M.J., Ganta C.K. In: Comprehensive Physiology. Terjung R., editor. Wiley; 2014. Autonomic Nervous System and Immune System Interactions; pp. 1177–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Roosevelt R.W., Smith D.C., Clough R.W., Jensen R.A., Browning R.A. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res. 2006;1119:124–132. doi: 10.1016/j.brainres.2006.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang Hong, Yu M., Ochani M., Amella C.A., Tanovic M., Susarla S., Li J.H., Wang Haichao, Yang H., Ulloa L., Al-Abed Y., Czura C.J., Tracey K.J. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 138.Wu H., Li L., Su X. Vagus nerve through α 7 nAChR modulates lung infection and inflammation: models, cells, and signals. BioMed Res. Int. 2014;2014:1–20. doi: 10.1155/2014/283525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pavlov V.A., Wang H., Czura C.J., Friedman S.G., Tracey K.J. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol. Med. 2003;9:125–134. [PMC free article] [PubMed] [Google Scholar]
- 140.Colovic M.B., Krstic D.Z., Lazarevic-Pasti T.D., Bondzic A.M., Vasic V.M. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr. Neuropharmacology. 2013;11:315–335. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sinkus M.L., Graw S., Freedman R., Ross R.G., Lester H.A., Leonard S. The human CHRNA7 and CHRFAM7A genes: a review of the genetics, regulation, and function. Neuropharmacology. 2015;96:274–288. doi: 10.1016/j.neuropharm.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rosas-Ballina M., Tracey K.J. Cholinergic control of inflammation. J. Intern. Med. 2009;265:663–679. doi: 10.1111/j.1365-2796.2009.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bosmans G., Appeltans I., Stakenborg N., Gomez-Pinilla P.J., Florens M.V., Aguilera-Lizarraga J., Matteoli G., Boeckxstaens G.E. Vagus nerve stimulation dampens intestinal inflammation in a murine model of experimental food allergy. Allergy. 2019;74:1748–1759. doi: 10.1111/all.13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Caravaca A.S., Gallina A.L., Tarnawski L., Tracey K.J., Pavlov V.A., Levine Y.A., Olofsson P.S. An effective method for acute vagus nerve stimulation in experimental inflammation. Front. Neurosciences. 2019;13(877) doi: 10.3389/fnins.2019.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jianbo Y., Shuan D., Xiaoqing L., Lirong G., Yuan Z., Man W., Xinshun C., Daquan L. Role of HO-1 in protective effect of electro-acupuncture against endotoxin shock-induced acute lung injury in rabbits. Exp. Biol. Med. 2013;238:705–712. doi: 10.1177/1535370213489487. [DOI] [PubMed] [Google Scholar]
- 146.Caravaca A.S., Gallina A.L., Tarnawski L., Shavva V.S., Colas R.A., Dalli J., Malin S.G., Hult H., Arnardottir H., Olofsson P.S. Vagus nerve stimulation promotes resolution of inflammation by a mechanism that involves Alox15 and requires the α7nAChR subunit. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2023285119. e2023285119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Koopman F.A., Chavan S.S., Miljko S., Grazio S., Sokolovic S., Schuurman P.R., Mehta A.D., Levine Y.A., Faltys M., Zitnik R., Tracey K.J., Tak P.P. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA. 2016;113:8284–8289. doi: 10.1073/pnas.1605635113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.van Westerloo D.J., Giebelen I.A., Florquin S., Bruno M.J., LaRosa G.J., Ulloa L., Tracey K.J., van der Poll T. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 149.Sifringer M., Bendix I., von Haefen C., Endesfelder S., Kalb A., Bührer C., Felderhoff-Mueser U., Spies C.D. Oxygen toxicity is reduced by acetylcholinesterase inhibition in the developing rat brain. Dev. Neurosci. 2013;35:255–264. doi: 10.1159/000346723. [DOI] [PubMed] [Google Scholar]
- 150.Toiber D., Berson A., Greenberg D., Melamed-Book N., Diamant S., Soreq H. N-Acetylcholinesterase-Induced apoptosis in Alzheimer's disease. PLoS One. 2008;3:e3108. doi: 10.1371/journal.pone.0003108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Andersson U. The cholinergic anti-inflammatory pathway alleviates acute lung injury. Mol. Med. 2020;26(64) doi: 10.1186/s10020-020-00184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Su X., Lee J.W., Matthay Z.A., Mednick G., Uchida T., Fang X., Gupta N., Matthay M.A. Activation of the α7 nAChR reduces acid-induced acute lung injury in mice and rats. Am. J. Respir. Cell Mol. Biol. 2007;37:186–192. doi: 10.1165/rcmb.2006-0240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fujii T., Mashimo M., Moriwaki Y., Misawa H., Ono S., Horiguchi K., Kawashima K. Physiological functions of the cholinergic system in immune cells. J. Pharmacol. Sci. 2017;134:1–21. doi: 10.1016/j.jphs.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 154.Skok M.V., Grailhe R., Agenes F., Changeux J.-P. The role of nicotinic receptors in B-lymphocyte development and activation. Life Sci. 2007;80:2334–2336. doi: 10.1016/j.lfs.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 155.Azuma Y., Shinohara M., Wang P.-L., Hidaka A., Ohura K. Histamine inhibits chemotaxis, phagocytosis, superoxide anion production, and the production of TNFα and IL-12 by macrophages via H2-receptors. Int. Immunopharm. 2001;1:1867–1875. doi: 10.1016/S1567-5769(01)00112-6. [DOI] [PubMed] [Google Scholar]
- 156.Shapouri-Moghaddam A., Mohammadian S., Vazini H., Taghadosi M., Esmaeili S., Mardani F., Seifi B., Mohammadi A., Afshari J.T., Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 157.Schett G., Neurath M.F. Resolution of chronic inflammatory disease: universal and tissue-specific concepts. Nat. Commun. 2018;9(3261) doi: 10.1038/s41467-018-05800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Serhan C.N., Chiang N., Dalli J. The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin. Immunol. 2015;27:200–215. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Patel V.S., Sitapara R.A., Gore A., Phan B., Sharma L., Sampat V., Li J.H., Yang H., Chavan S.S., Wang H., Tracey K.J., Mantell L.L. High mobility group box–1 mediates hyperoxia-induced impairment of Pseudomonas aeruginosa clearance and inflammatory lung injury in mice. Am. J. Respir. Cell Mol. Biol. 2013;48:280–287. doi: 10.1165/rcmb.2012-0279OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gore A., Gauthier A.G., Lin M., Patel V., Thomas D.D., Ashby C.R., Mantell L.L. The nitric oxide donor, (Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate (DETA-NONOate/D-NO), increases survival by attenuating hyperoxia-compromised innate immunity in bacterial clearance in a mouse model of ventilator-associated pneumonia. Biochem. Pharmacology. 2020;176 doi: 10.1016/j.bcp.2020.113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat. Rev. Drug Discov. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- 162.Wang H., Liao H., Ochani M., Justiniani M., Lin X., Yang L., Al-Abed Y., Wang H., Metz C., Miller E.J. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 163.Baleeiro C.E.O., Wilcoxen S.E., Morris S.B., Standiford T.J., Paine R. Sublethal hyperoxia impairs pulmonary innate immunity. J. Immunol. 2003;171:955–963. doi: 10.4049/jimmunol.171.2.955. [DOI] [PubMed] [Google Scholar]
- 164.Entezari M., Javdan M., Antoine D.J., Morrow D.M.P., Sitapara R.A., Patel V., Wang M., Sharma L., Gorasiya S., Zur M., Wu W., Li J., Yang H., Ashby C.R., Thomas D., Wang H., Mantell L.L. Inhibition of extracellular HMGB1 attenuates hyperoxia-induced inflammatory acute lung injury. Redox Biol. 2014;2:314–322. doi: 10.1016/j.redox.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.O'Reilly P.J., Hickman-Davis J.M., Davis I.C., Matalon S. Hyperoxia impairs antibacterial function of macrophages through effects on actin. Am. J. Respir. Cell Mol. Biol. 2003;28:443–450. doi: 10.1165/rcmb.2002-0153OC. [DOI] [PubMed] [Google Scholar]
- 166.Ramond E., Jamet A., Coureuil M., Charbit A. Pivotal role of mitochondria in macrophage response to bacterial pathogens. Front. Immunol. 2019;10(2461) doi: 10.3389/fimmu.2019.02461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tur J., Pereira-Lopes S., Vico T., Marín E.A., Muñoz J.P., Hernández-Alvarez M., Cardona P.-J., Zorzano A., Lloberas J., Celada A. Mitofusin 2 in macrophages links mitochondrial ROS production, cytokine release, phagocytosis, autophagy, and bactericidal activity. Cell Rep. 2020;32 doi: 10.1016/j.celrep.2020.108079. [DOI] [PubMed] [Google Scholar]
- 168.Abraham E., Arcaroli J., Carmody A., Wang H., Tracey K.J. Cutting edge: HMG-1 as a mediator of acute lung inflammation. J. Immunol. 2000;165:2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 169.Wang H. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 170.Entezari M., Weiss D.J., Sitapara R., Whittaker L., Wargo M.J., Li J., Wang H., Yang H., Sharma L., Phan B.D., Javdan M., Chavan S.S., Miller E.J., Tracey K.J., Mantell L.L. Inhibition of high-mobility group box 1 protein (HMGB1) enhances bacterial clearance and protects against Pseudomonas aeruginosa pneumonia in cystic fibrosis. Mol. Med. 2012;18:477–485. doi: 10.2119/molmed.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol. Cell Biol. 1999;19:5237–5246. doi: 10.1128/mcb.19.8.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen G., Ward M.F., Sama A.E., Wang H. Extracellular HMGB1 as a proinflammatory cytokine. J. Interferon cytokine res. 2004. 24, 329-333. [DOI] [PubMed]
- 173.Tohme S., Yazdani H.O., Liu Y., Loughran P., van der Windt D.J., Huang H., Simmons R.L., Shiva S., Tai S., Tsung A. Hypoxia mediates mitochondrial biogenesis in hepatocellular carcinoma to promote tumor growth through HMGB1 and TLR9 interaction: tohme, Yazdani, et al. Hepatology. 2017;66:182–197. doi: 10.1002/hep.29184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chang Y.-S., Ko B.-H., Ju J.-C., Chang H.-H., Huang S.-H., Lin C.-W. SARS unique domain (SUD) of severe acute respiratory syndrome coronavirus induces NLRP3 inflammasome-dependent CXCL10-mediated pulmonary inflammation. Int. J. Mol. Sci. 2020;21(3179) doi: 10.3390/ijms21093179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chen R., Huang Y., Quan J., Liu J., Wang H., Billiar T.R., Lotze M.T., Zeh H.J., Kang R., Tang D. HMGB1 as a potential biomarker and therapeutic target for severe COVID-19. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen Y., Qin C., Huang J., Tang X., Liu C., Huang K., Xu J., Guo G., Tong A., Zhou L. The role of astrocytes in oxidative stress of central nervous system: a mixed blessing. Cell Prolif. 2020;53 doi: 10.1111/cpr.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sivakorn C., Dechsanga J., Jamjumrus L., Boonnak K., Schultz M.J., Dondorp A.M., Phumratanaprapin W., Ratanarat R., Naorungroj T., Wattanawinitchai P., Siripoon T., Duangdee C., Techarang T. High mobility group box 1 and interleukin 6 at intensive care unit admission as biomarkers in critically ill COVID-19 patients. Am. J. Trop. Med. Hyg. 2021;105:73–80. doi: 10.4269/ajtmh.21-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vicentino A.R.R., Fraga-Junior V. da S., Palazzo M., Tasmo N.R.A., Rodrigues D.A.S., Barroso S.P.C., Ferreira S.N., Borges A.C.N., Allonso D., Fantappié M.R., Scharfstein J., Oliveira A.C., Vianna-Jorge R., Vale A.M., Coutinho-Silva R., Savio L.E.B., Canetti C., Benjamim C.F. HMGB1 correlates with severity and death of COVID-19 patients (preprint) Infectious Diseases (except HIV/AIDS) 2022 doi: 10.1101/2022.05.26.22275611. In press. [DOI] [Google Scholar]
- 180.Farsalinos K., Eliopoulos E., Leonidas D.D., Papadopoulos G.E., Tzartos S., Poulas K. vol. 21. 2020. Nicotinic cholinergic system and COVID-19; p. 5807. (Silico Identification of an Interaction between SARS-CoV-2 and Nicotinic Receptors with Potential Therapeutic Targeting Implications. Int. J. Mol. Sci). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fudim M., Qadri Y.J., Ghadimi K., MacLeod D.B., Molinger J., Piccini J.P., Whittle J., Wischmeyer P.E., Patel M.R., Ulloa L. Implications for neuromodulation therapy to control inflammation and related organ dysfunction in COVID-19. J cardiovasc. Transl. Res. 2020;13:894–899. doi: 10.1007/s12265-020-10031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Giebelen I.A.J., van Westerloo D.J., LaRosa G.J., de Vos A.F., van der Poll T. Local stimulation of α7 cholinergic receptors inhibits LPS-induced TNF-α release in the mouse lung. Shock. 2007;28:700–703. doi: 10.1097/shk.0b013e318054dd89. [DOI] [PubMed] [Google Scholar]
- 183.Pavlov V.A., Ochani M., Yang L.-H., Gallowitsch-Puerta M., Ochani K., Lin X., Levi J., Parrish W.R., Rosas-Ballina M., Czura C.J., LaRosa G.J., Miller E.J., Tracey K.J., Al-Abed Y. Selective α7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis*: crit. Care Med. 2007. 35, 1139-1144. [DOI] [PubMed]
- 184.Bagdas D., Wilkerson J.L., Kulkarni A., Toma W., AlSharari S., Gul Z., Lichtman A.H., Papke R.L., Thakur G.A., Damaj M.I. The α7 nicotinic receptor dual allosteric agonist and positive allosteric modulator GAT107 reverses nociception in mouse models of inflammatory and neuropathic pain: α7 ago-PAM in inflammatory and neuropathic pain. Br. J. Pharmacol. 2016;173:2506–2520. doi: 10.1111/bph.13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Horenstein N.A., Papke R.L., Kulkarni A.R., Chaturbhuj G.U., Stokes C., Manther K., Thakur G.A. Critical molecular determinants of α7 nicotinic acetylcholine receptor allosteric activation: separation of direct allosteric activation and positive allosteric modulation. J. Biol. Chem. jbc.M115.692392. 2016. [DOI] [PMC free article] [PubMed]
- 186.Papke R.L., Stokes C., Damaj M.I., Thakur G.A., Manther K., Treinin M., Bagdas D., Kulkarni A.R., Horenstein N.A. Persistent activation of α7 nicotinic ACh receptors associated with stable induction of different desensitized states. Br. J. Pharmacol. 2018;175:1838–1854. doi: 10.1111/bph.13851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Papke R.L., Chojnacka K., Horenstein N.A. The minimal pharmacophore for silent agonism of the α7 nicotinic acetylcholine receptor. J. Pharmacol. Exp. Therapeut. 2014;350:665–680. doi: 10.1124/jpet.114.215236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Thakur G.A., Kulkarni A.R., Deschamps J.R., Papke R.L. Expeditious synthesis, enantiomeric resolution and enantiomer functional characterization of (4-(4-bromophenyl)-3a, 4, 5, 9b-tetrahydro-3H-cyclopenta[c]quinoline-8-sulfonamide (4BP-tqs): an allosteric agonist-positive allosteric modulator of α7 nicotinic acetylcholine receptors. J. Med. Chem. 2013;56:8943–8947. doi: 10.1021/jm401267t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kang R., Chen R., Zhang Q., Hou W., Wu S., Cao L., Huang J., Yu Y., Fan X., Yan Z., Sun X., Wang H., Wang Q., Tsung A., Billiar T.R., Zeh H.J., Lotze M.T., Tang D. HMGB1 in health and disease. Mol. Aspect. Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Habre W., Peták F. Perioperative use of oxygen: variabilities across age. Br. J. Anaesth. 2014;113:ii26–ii36. doi: 10.1093/bja/aeu380. [DOI] [PubMed] [Google Scholar]
- 191.Meyhoff C.S., Jorgensen L.N., Wetterslev J., Christensen K.B., Rasmussen L.S. Increased long-term mortality after a high perioperative inspiratory oxygen fraction during abdominal surgery: follow-up of a randomized clinical trial. Anesth. Analgesia. 2012;115:849–854. doi: 10.1213/ANE.0b013e3182652a51. [DOI] [PubMed] [Google Scholar]
- 192.Masamoto K., Tanishita K. Oxygen transport in brain tissue. J. Biomech. Eng. 2009;131 doi: 10.1115/1.3184694. 074002. [DOI] [PubMed] [Google Scholar]
- 193.Erecińska M., Silver I.A. Tissue oxygen tension and brain sensitivity to hypoxia. Respir. Physiol. 2001;128:263–276. doi: 10.1016/S0034-5687(01)00306-1. [DOI] [PubMed] [Google Scholar]
- 194.Arendash G.W., Cox A.A., Mori T., Cracchiolo J.R., Hensley K.L., Roberts L.J. Oxygen treatment triggers cognitive impairment in Alzheimer's transgenic mice. Neuroreport. 2009;20:1087–1092. doi: 10.1097/WNR.0b013e32832e6459. [DOI] [PubMed] [Google Scholar]
- 195.Chen X.-Q., Mobley W.C. Exploring the pathogenesis of alzheimer disease in basal forebrain cholinergic neurons: converging insights from alternative hypotheses. Front. Neurosciences. 2019;13(446) doi: 10.3389/fnins.2019.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Guzman-Martinez L., Maccioni R.B., Andrade V., Navarrete L.P., Pastor M.G., Ramos-Escobar N. Neuroinflammation as a common feature of neurodegenerative disorders. Front. Pharmacology. 2019;10(1008) doi: 10.3389/fphar.2019.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nordberg A., Winblad B. Reduced number of [3H]nicotine and [3H]acetylcholine binding sites in the frontal cortex of Alzheimer brains. Neurosci. Lett. 1986;72:115–120. doi: 10.1016/0304-3940(86)90629-4. [DOI] [PubMed] [Google Scholar]
- 198.Broide R.S., Winzer-Serhan U.H., Chen Y., Leslie F.M. Distribution of α7 nicotinic acetylcholine receptor subunit mRNA in the developing mouse. Front. Neuroanatomy. 2019;13(76) doi: 10.3389/fnana.2019.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cortes M., Cao M., Liu H.L., Burns P., Moore C., Fecteau G., Desrochers A., Barreiro L.B., Antel J.P., Frasch M.G. RNAseq profiling of primary microglia and astrocyte cultures in near-term ovine fetus: a glial in vivo-in vitro multi-hit paradigm in large mammalian brain. J. Neurosci. Methods. 2017;276:23–32. doi: 10.1016/j.jneumeth.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Papouin T., Dunphy J.M., Tolman M., Dineley K.T., Haydon P.G. Septal cholinergic neuromodulation tunes the astrocyte-dependent gating of hippocampal NMDA receptors to wakefulness. Neuron. 2017;94:840–854. doi: 10.1016/j.neuron.2017.04.021. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Suzuki T., Hide I., Matsubara A., Hama C., Harada K., Miyano K., Andrä M., Matsubayashi H., Sakai N., Kohsaka S., Inoue K., Nakata Y. Microglial α7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J. Neurosci. Res. 2006;83:1461–1470. doi: 10.1002/jnr.20850. [DOI] [PubMed] [Google Scholar]
- 202.Shytle R.D., Mori T., Townsend K., Vendrame M., Sun N., Zeng J., Ehrhart J., Silver A.A., Sanberg P.R., Tan J. Cholinergic modulation of microglial activation by α7 nicotinic receptors: cholinergic modulation of microglial activation. J. Neurochem. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 203.Kem W.R. The brain α7 nicotinic receptor may be an important therapeutic target for the treatment of Alzheimer's disease: studies with DMXBA (GTS-21). Behav. Brain Res. 2000;113:169–181. doi: 10.1016/S0166-4328(00)00211-4. [DOI] [PubMed] [Google Scholar]
- 204.Fukutani Y., Cairns N.J., Rossor M.N., Lantos P.L. Purkinje cell loss and astrocytosis in the cerebellum in familial and sporadic Alzheimer's disease. Neurosci. Lett. 1996;214:33–36. doi: 10.1016/0304-3940(96)12875-5. [DOI] [PubMed] [Google Scholar]
- 205.Mani R.B., Lohr J.B., Jeste D.V. Hippocampal pyramidal cells and aging in the human: a quantitative study of neuronal loss in sectors CA1 to CA4. Exp. Neurol. 1986;94:29–40. doi: 10.1016/0014-4886(86)90269-4. [DOI] [PubMed] [Google Scholar]
- 206.Mann D.M.A. Pyramidal nerve cell loss in Alzheimer's disease. Neurodegeneration. 1996;5:423–427. doi: 10.1006/neur.1996.0057. [DOI] [PubMed] [Google Scholar]
- 207.Schliebs R., Arendt T. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 2011;221:555–563. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 208.Whitehouse P.J., Martino A.M., Antuono P.G., Lowenstein P.R., Coyle J.T., Price D.L., Kellar K.J. Nicotinic acetylcholine binding sites in Alzheimer's disease. Brain Res. 1986;371:146–151. doi: 10.1016/0006-8993(86)90819-X. [DOI] [PubMed] [Google Scholar]
- 209.Baseman A., Hager K., Nye J.S., Brashear R., Han J., Sano M., Davis B., Richards H. Effects of galantamine in a 2-year, randomized, placebo-controlled study in Alzheimer's disease. Neuropsychiatric Dis. Treat. 2014;391 doi: 10.2147/NDT.S57909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Conejero-Goldberg C., Davies P., Ulloa L. Alpha7 nicotinic acetylcholine receptor: a link between inflammation and neurodegeneration. Neurosci. BioBehav. Rev. 2008;32:693–706. doi: 10.1016/j.neubiorev.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sadigh-Eteghad S., Talebi M., Mahmoudi J., Babri S., Shanehbandi D. Selective activation of α7 nicotinic acetylcholine receptor by PHA-543613 improves Aβ25–35-mediated cognitive deficits in mice. Neuroscience. 2015;298:81–93. doi: 10.1016/j.neuroscience.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 212.Woodruff-Pak D.S., Li Y.-T., Kem W.R. A nicotinic agonist (GTS-21), eyeblink classical conditioning and nicotinic receptor binding in rabbit brain. Brain Res. 1994;645:309–317. doi: 10.1016/0006-8993(94)91665-9. [DOI] [PubMed] [Google Scholar]
- 213.Patel H., McIntire J., Ryan S., Dunah A., Loring R. Anti-inflammatory effects of astroglial α7 nicotinic acetylcholine receptors are mediated by inhibition of the NF-κB pathway and activation of the Nrf2 pathway. J. Neuroinflammation 14. 2017. [DOI] [PMC free article] [PubMed]
- 214.Takata K., Amamiya T., Mizoguchi H., Kawanishi S., Kuroda E., Kitamura R., Ito A., Saito Y., Tawa M., Nagasawa T., Okamoto H., Sugino Y., Takegami S., Kitade T., Toda Y., Kem W.R., Kitamura Y., Shimohama S., Ashihara E. Alpha7 nicotinic acetylcholine receptor-specific agonist DMXBA (GTS-21) attenuates Aβ accumulation through suppression of neuronal γ-secretase activity and promotion of microglial amyloid-β phagocytosis and ameliorates cognitive impairment in a mouse model of Alzheimer's disease. Neurobiol. Aging. 2018;62:197–209. doi: 10.1016/j.neurobiolaging.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 215.Kitagawa H., Takenouchi T., Azuma R., Wesnes K.A., Kramer W.G., Clody D.E., Burnett A.L. Safety, pharmacokinetics, and effects on cognitive function of multiple doses of GTS-21 in healthy, male volunteers. Neuropsychopharmacology. 2003;28:542–551. doi: 10.1038/sj.npp.1300028. [DOI] [PubMed] [Google Scholar]
- 216.Olincy A., Blakeley-Smith A., Johnson L., Kem W.R., Freedman R. Brief report: initial trial of alpha7-nicotinic receptor stimulation in two adult patients with autism spectrum disorder. J. Autism Dev. Disord. 2016;46:3812–3817. doi: 10.1007/s10803-016-2890-6. [DOI] [PMC free article] [PubMed] [Google Scholar]