Abstract
Introduction:
For decades it has been recommended that children with sickle cell anemia (SCA) receive antibiotic prophylaxis to prevent serious infections and transcranial Doppler (TCD) screening to identify those at highest risk of overt stroke. We assessed recent temporal trends in antibiotic prophylaxis fills and TCD screening among children with SCA using validated quality measures.
Procedure:
Using validated claims-based definitions, we identified children with SCA who were enrolled in Michigan or New York State (NYS) Medicaid programs (2011–2018). Among recommended age groups, two outcomes were assessed yearly: (1) filling of ≥300 days of antibiotics, and (2) receipt of ≥1 TCD. The proportion of children with each outcome was calculated by state. Temporal trends in each preventive service were assessed using generalized linear models.
Results:
A total of 1,784 children were eligible for antibiotic prophylaxis (Michigan: 384; NYS: 1,400), contributing 3,322 person-years. Annual rates of filling ≥300 days of antibiotics ranged from 16% to 22% and were similar by state. There was no change in rates of antibiotic filling over time in Michigan (p-value: 0.10), but there was a decrease in NYS (p-value: 0.02). A total of 3 439 children with SCA were eligible for TCD screening (Michigan: 710; NYS: 2,729), contributing 10,012 person-years. Annual rates of TCD screening ranged from 39% to 45%, were similar by state, and did not change over time (p-values>0.05).
Conclusions:
Most children with SCA do not receive recommended antibiotic prophylaxis and/or TCD screening. New, sustainable, and coordinated interventions across preventive services are urgently needed.
Keywords: Epidemiology, quality of life, sickle cell anemia
Introduction
Sickle cell disease is a genetic disorder associated with substantial morbidity and early mortality.1 There are numerous subtypes of sickle cell disease; sickle cell anemia (SCA), consisting primarily of Hemoglobin (Hb) SS or HbSβ0 thalassemia, confers the highest risk for morbidities.1 If untreated and unscreened, children with SCA have 100 times the risk of serious encapsulated bacterial infections compared to those without SCA,2, 3 and 11% of children with SCA will experience an overt stroke before the age of 18.4, 5
While these risks are formidable, regular receipt of preventive services by children with SCA can markedly reduce the threat of both infection and stroke. Antibiotic prophylaxis has been demonstrated to reduce the risk of septicemia due to Streptococcus pneumoniae by over 80%.6 Similarly, transcranial Doppler (TCD) screening effectively identifies children at highest risk of overt stroke with a non-invasive outpatient ultrasound procedure. Initiation of chronic transfusions reduces stroke risk by over 90% in these high risk children.7, 8 The potential impact of these relatively low-cost and effective interventions has led the National Heart, Lung, and Blood Institute (NHLBI) to recommend that children with SCA receive antibiotic prophylaxis from 0 to 5 years of age since 1987, as well as to have an annual TCD screen from ages 2 to 16 years of age since 1997.9–12
The significance of these preventive services and their relation to improved outcomes among children with SCA is reflected in the endorsement of these two quality measures by the National Quality Forum (NQF) in 2016 and 2017.13, 14 NQF endorsement indicates that a measure has undergone a rigorous peer-review and testing process that demonstrates its importance, validity, and feasibility.15 These measures assess the proportion of children with SCA that receive antibiotic prophylaxis throughout the year, as well as those that receive at least one TCD screen annually. Historically, filling of antibiotic prophylaxis prescriptions and receipt of TCD screening among children with SCA has been consistently very low; fewer than 1 in 5 children had an appropriate filled amount of antibiotic prophylaxis (at least 300 days) and only 1 in 3 received TCD screening annually.16, 17 18 Gaps such as these have led the US Department of Health and Human Services and the Centers for Medicare & Medicaid Services (CMS) to identify the quality of care for those living with SCA as a national priority.19–22 However, there are no estimates of trends in antibiotic prophylaxis or TCD screening since the increased focus on preventive services among this vulnerable population at the national level. With those priorities in mind, this study aims to provide the most current data available on temporal trends in antibiotic prophylaxis prescription filling and TCD screening among children with SCA enrolled in two large state Medicaid programs.
Methods
Our study population consisted of children with SCA 3 months up to 16 years of age an individual is not included for a year in which he or she turns 16 years old) enrolled for at least one year from 2011–2018 in either the Michigan or New York State (NYS) Medicaid programs. National Medicaid data often has a significant lag; therefore, Medicaid administrative data directly from states is considered the most timely, accurate, and complete source of information regarding quality of care for these children. Among children in the study population, all Medicaid administrative claims, demographic, and enrollment data were obtained for each year. Children were continuously enrolled in Medicaid for each eligible year and could contribute multiple years of observation; these years could be sequential or non-sequential. Children were excluded if they were covered by any other form of health insurance within the calendar year to maximize the completeness of administrative claims capture among the study population.
Children with SCA were identified using validated administrative-claims based case definitions.14, 23,24 The ICD-9-CM case definition required ≥3 claims for SCA (282.61, 282.62) within each calendar year (2011-September 2015); this method had a sensitivity of 91% and a specificity of 80% compared to the gold standard of newborn screening.14 The ICD-10-CM case definition required ≥1 outpatient visit with a SCA-related diagnosis (D5700, D5701, D5702), or a non-specific sickle cell disease (D571) diagnosis code during the period October 2015–2018. The ICD-10 case definition had a sensitivity of 95% and a specificity of 92% as compared to newborn screening records.24 It is essential to note that this case definition identifies children with sickle cell anemia, as opposed to more generally those with sickle cell disease. This nuance is deliberate and important, as the recommendations for antibiotic prophylaxis and TCD screening are specific to children with this subtype.
Outcomes
Antibiotic prophylaxis prescription fills were assessed annually among children 3 months up to 5 years of age, consistent with NHLBI published guidelines.11, 12 Appropriate antibiotic prophylaxis prescription fills were defined as having filled pharmacy claims for at least 300 days of penicillin, erythromycin, or amoxicillin within a calendar year, consistent with the methodology of the NQF-endorsed measure discussed above.14 The total number of days’ supply that antibiotic prophylaxis was filled per calendar year was determined by summing the days’ supply from each filled prescription within that calendar year.
Receipt of TCD screening was assessed annually among children 2 up to 16 years of age, consistent with NHLBI published guidelines.11, 12 Presence of a TCD screen was identified within each calendar year using claims (CPT codes 93886, 93888, 93890, 93892, and 93893) and classified dichotomously (yes/no), consistent with the methodology of the NQF-endorsed measure discussed above.13
Statistical Analysis
Demographics were summarized by state and year across the study period. The proportion of children 3 months up to 5 years of age receiving at least 300 days of antibiotic prophylaxis was calculated annually, as was the proportion of children 2 up to 16 years of age receiving at least one TCD screen. Both proportions were evaluated overall and by state each year. Temporal trends for receipt of each type of preventive service were assessed using generalized linear models.
Sensitivity Analyses.
We performed several sensitivity analyses to understand potential targets for intervention. First, we assessed geographic variation in rates of antibiotic prophylaxis and TCD. In Michigan and NYS, each child eligible to receive either preventive service (antibiotic prophylaxis or TCD screening) was assigned to Medicaid service regions in Michigan (know as prosperity regions) and region or borough in NYS in 2018. Performance scores with at least 50 children in the denominator were compared across prosperity region or region/borough, as is common in quality of care assessments within Medicaid programs.25 Second, we assessed summary statistics of days’ supply of antibiotic prophylaxis in Michigan in 2018 to compare to previous estimates from Sox et al (2003) for the time period 1995–1999 in two state Medicaid programs (Tennessee and Washington).26 In NYS, we calculated the proportion of children across the study period by days’ supply of antibiotics filled within a calendar year (0; 1–120; 121–149; and 150–300 days). Finally, we assessed TCD screening rates among different age groups. First, we assessed TCD screening rates for children ages 3 through 12 years using Michigan Medicaid data in 2018 and compared to the overall rate of TCD screening for children within the NHLBI-recommended age range (2 through 15 years old). Second, we calculated TCD screening rates for the following age groups in NYS: 2 through 5 years; 6 through 11 years; 12 through 15 years.
Results
Antibiotic prophylaxis
From 2011–2018, a total of 1 784 children 3 months to 5 years of age were eligible for antibiotic prophylaxis and contributed at least one year of observation (Children in Michigan: 384; Children in NYS: 1 400). Newborn screening results from each state indicate that there are approximately 3.5 times the number of newborns with SCA born in NYS as compared to Michigan; therefore, the ratio of children included in each state for this analysis is reasonable in the context of Medicaid enrollment and migration. Collectively, these children contributed a total of 3 322 person-years across the study period (Michigan: 697; NYS: 2 625) (Tables 1 and 3). Overall rates of appropriate antibiotic prophylaxis fills (Michigan and NYS combined) ranged from a minimum of 16% (2016, 2018) to a maximum of 22% (2011). These rates were similar by state (Fig. 1). There was a decrease in appropriate antibiotic fills over time in NYS (p-value: 0.02), although no change was observed in Michigan during the same period (p-value: 0.10). Our first sensitivity analysis explored geographic variation in rates; in Michigan, only one prosperity region located in southeast Michigan had a sufficiently large population of children with SCA eligible for antibiotic prophylaxis. In this region, 5% of children received at least 300 days of antibiotic prophylaxis in 2018. Assessment of days’ supply of antibiotics in 2018 in Michigan revealed that 12.6% of children had zero days’ supply; median supply was 116 days, with an IQR of 40 to 214 days. In NYS, only two regions/boroughs had a large enough population of children with SCA eligible for antibiotic prophylaxis; the performance scores for these regions were 16% and 19% in 2018. Among these children, days’ supply of antibiotics varied considerably; 7% had 0 days’; 27% had 1–120 days’; 7% had 121–149 days’; and 38% had 150–300 days’.
TABLE 1.
Demographic characteristics of children with sickle cell anemia ages 3 months – 5 years continuously enrolled in Michigan or New York State Medicaid for at least one year from 2011–2018, N = 1 784
| Michigan (n=384) | New York State (n=1 400) | ||
|---|---|---|---|
| Sex | Male | 203 | 655 |
| Female | 181 | 745 | |
| Race as recorded in Medicaid enrollment | Non-Hispanic Black | 320 | 852 |
| Non-Hispanic White | 19 | 35 | |
| Hispanic | 11 | 14 | |
| Unknown Race / Ethnicity | 29 | 237 | |
| Asian/Pacific Islander | <10 | 14 | |
| Other | 0 | 71 | |
| American Indian / Alaskan Native | <10 | 0 | |
| Birth Cohort | 2007–2009 | 88 | 301 |
| 2010–2011 | 65 | 284 | |
| 2012–2013 | 87 | 306 | |
| 2014–2015 | 83 | 318 | |
| 2016–2017 | 61 | 191 |
TABLE 3.
Person-years of enrollment for children with sickle cell anemia eligible for antibiotic prophylaxis or transcranial Doppler screening in Michigan and New York Medicaid programs, 2011–2018
| Antibiotic Prophylaxis (n=3 322 person-years) | Transcranial Doppler Screening (n=10 012 person-years) | ||||
|---|---|---|---|---|---|
| Michigan (n=697) | New York State (n=2625) | Michigan (n=2 150) | New York State (n=7 862) | ||
| Year Enrolled | 2011 | 85 | 290 | 237 | 819 |
| 2012 | 83 | 309 | 231 | 899 | |
| 2013 | 85 | 324 | 229 | 932 | |
| 2014 | 73 | 299 | 238 | 893 | |
| 2015 | 75 | 345 | 257 | 1 019 | |
| 2016 | 110 | 373 | 345 | 1 104 | |
| 2017 | 91 | 338 | 301 | 1 099 | |
| 2018 | 95 | 347 | 312 | 1 097 | |
Figure 1.
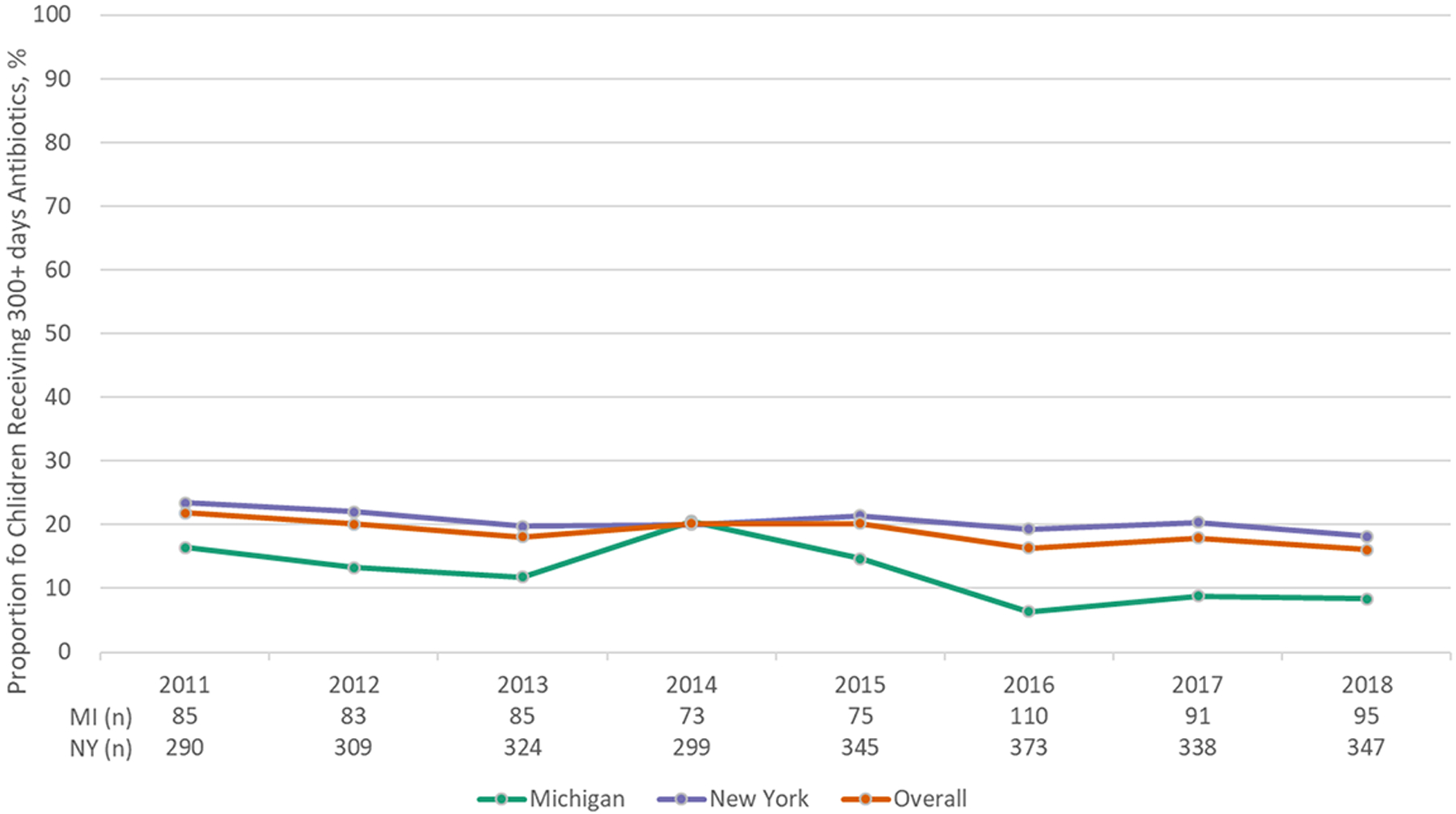
Proportion of children with sickle cell anemia in Medicaid receiving 300+ days of antibiotic prophylaxis
Transcranial Doppler Screening
From 2011–2018, a total of 3 439 children with SCA between 2 to 16 years of age were eligible for TCD screening and contributed at least one year of observation (Children in Michigan: 710; Children in NYS: 2 729). These children contributed a total of 10,012 person-years across the study period (Michigan: 2 150; NYS: 7 862) (Tables 2 and 3). Overall, rates of TCD screening ranged from 39% (2016) to 45% (2013, 2018) and were similar by state across the study period (Fig. 2). TCD screening rates did not change over time in either state (Michigan p-value: 0.11; NYS p-value: 0.28). Our sensitivity analysis focused on geographic variation in rates indicated that in the one Michigan prosperity region with at least 50 children eligible, 35% of children received a TCD screen. In NYS, TCD screening rates ranged from 36% to 70% across the seven regions/boroughs with at least 50 eligible children. Our sensitivity analysis examining the impact of age on receipt of TCD screening indicated that 45% of children ages three through twelve years received a TCD screen in Michigan in 2018; this proportion is not meaningfully different than the overall proportion of children receiving a TCD screen (43%). In NYS, 44% of children ages 2 through 5 years received a TCD screen compared to 43% for children 6 through 11 years and 37% for children 12 through 15 years.
TABLE 2.
Demographic characteristics of children with sickle cell anemia ages 2 – 16 years continuously enrolled in Michigan or New York State Medicaid for at least one year from 2011–2018, N = 3 439
| Michigan (n=710) | New York State (n=2 729) | ||
|---|---|---|---|
| Sex | Male | 348 | 1 312 |
| Female | 362 | 1 417 | |
| Race as recorded in Medicaid enrollment | Non-Hispanic Black | 622 | 1 652 |
| Non-Hispanic White | 28 | 70 | |
| Hispanic | 15 | 362 | |
| Unknown Race / Ethnicity | 42 | 458 | |
| Asian/Pacific Islander | <10 | 35 | |
| Other | 0 | 151 | |
| American Indian / Alaskan Native | <10 | <10 | |
| Birth Cohort | 1996–1998 | 68 | 218 |
| 1999–2001 | 80 | 343 | |
| 2002–2004 | 110 | 460 | |
| 2005–2007 | 126 | 470 | |
| 2008–2010 | 136 | 554 | |
| 2011–2013 | 137 | 468 | |
| 2014–2016 | 53 | 216 |
Figure 2.
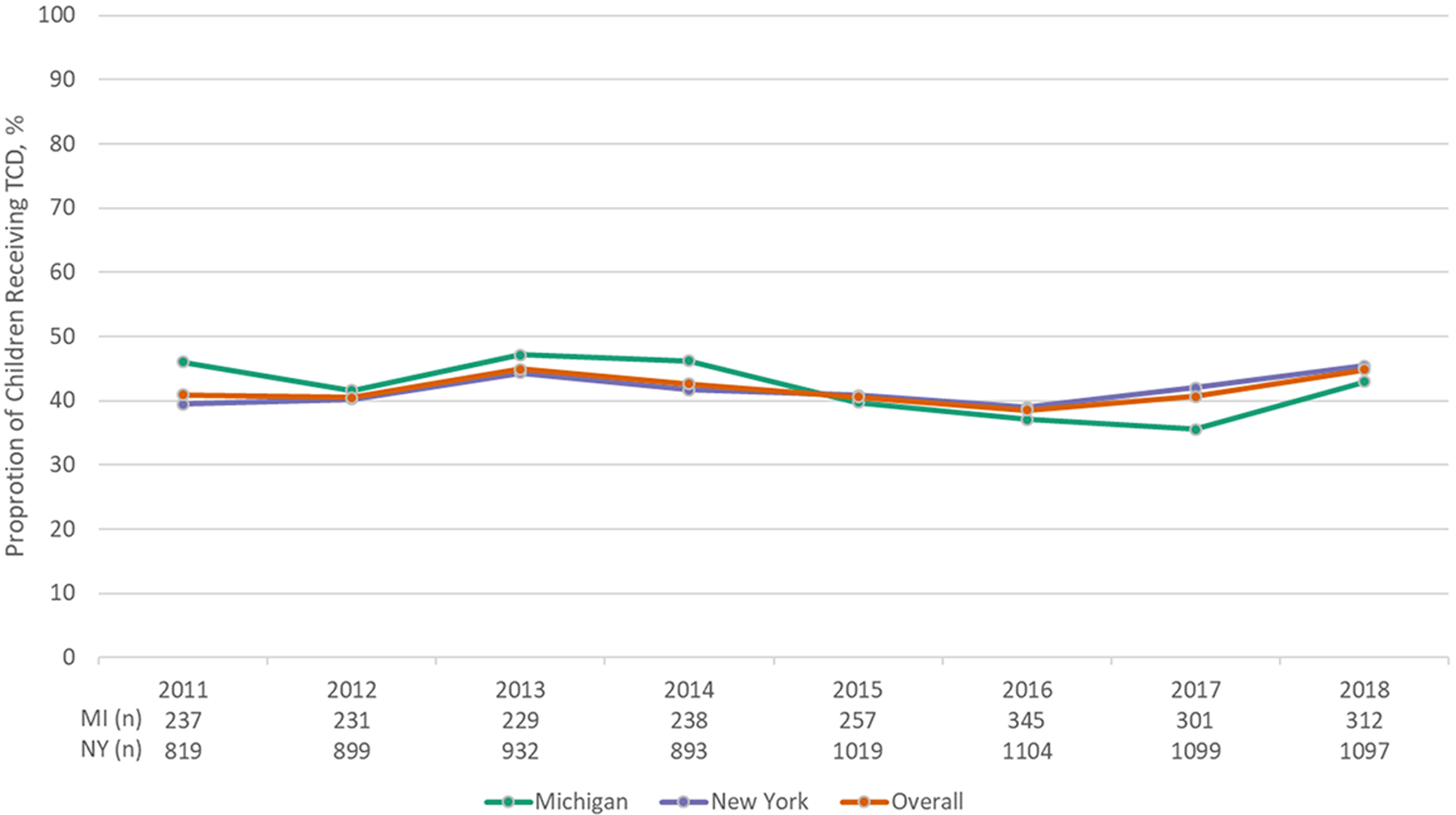
Proportion of children with sickle cell anemia in Medicaid receiving annual TCD screen
Discussion
We found that the majority of children with SCA enrolled in the Michigan and NYS Medicaid programs do not receive recommended preventive services. Despite the national focus on improving receipt of preventive services among those with SCA and overwhelming evidence of the efficacy of these services in preventing serious morbidity, low rates continue to persist. There is no evidence of improvement in the receipt of TCD screening or antibiotic prophylaxis among children with SCA across the decade of updated data included in this study.11, 12, 16, 17, 26–28 This is demonstrated by our findings for filled days of antibiotics within a year, which are nearly identical to those reported for the period 1995–1999.26 It is imperative that new and sustainable strategies to address these gaps in quality of care among children with SCA are developed and implemented. Absent such focused efforts, it is likely that improvements to the quality of care among children with SCA will continue to be hampered. Given the documented impact of these preventive services on incidence of invasive pneumococcal infection and stroke, increasing receipt of preventive services will decrease mortality and morbidity among this vulnerable population.29, 30
Prior studies have demonstrated high levels of outpatient service utilization among those eligible for antibiotic prophylaxis and TCD screening; on average, these children experience up to 13 outpatient office visits a year.14, 31 These encounters provide opportunities to intervene to improve rates of preventive services.27, 32, 33 However, the mechanisms driving these gaps in quality of care within the healthcare system are largely unknown, which limits the ability to develop and implement impactful interventions. For example, there are several potential steps in the process of obtaining antibiotic prophylaxis for a child with SCA, including 1) having access to a prescriber who is knowledgeable about SCA and the need for prophylactic antibiotics; and 2) obtaining the medication from the pharmacy. Obtaining medication from the pharmacy may be a particularly challenging barrier as families must refill these antibiotics frequently, typically twice monthly. TCD screenings may include additional appointments and travel to an offsite location to receive the screen, which is usually in addition to attending pediatric hematology appointments.34 Each of these barriers to completion of preventive services may impact overall quality of care. However, there are promising strategies to mitigate these barriers, including increasing primary care provider knowledge regarding antibiotic prophylaxis, identifying robust prescription delivery services, and co-locating TCD screens within sickle cell clinics to reduce travel barriers for patients. Other strategies include providing a patient navigator or case manager at the health system or health plan level, reminder letters to families regarding TCD screening and antibiotic prophylaxis, and providing health plan and/or provider-level incentives to reach performance benchmarks for the quality of care among children with SCA.35–39 However, these strategies have not been proven to work at the population-level and are in need of additional study. Given the low and unchanging rate of prophylaxis and screening observed in this study and the multiple barriers that may co-exist, it is likely that a combination of strategies will be needed to ensure that all children with SCA receive antibiotic prophylaxis and TCD screening.
Implementation of quality improvement strategies across health plans, health systems, and providers can be encouraged by extrinsic motivators such as consistent, annual reporting of the quality of care among children with SCA by state Medicaid programs. Such annual quality measurement reporting through the Core Set of Children’s Health Quality Measures has driven change in other quality measures by furnishing objective data upon which progress can be monitored. Importantly, the validated performance measures described in this study for antibiotic prophylaxis and TCD screening among children with SCA have been successfully tested for use by state Medicaid programs and health plans, culminating in their endorsement by NQF.13, 14 Inclusion of one or both of these measures in the Core Set could foster increased attention by state Medicaid programs on this largely underserved population, serving to underscore the need for interventions aimed to improve consistently inadequate care.40 Further, population-based strategies, such as inclusion in the Core Set, will include children that may not be associated with a specific health system or sickle cell center.41 This may be particularly true for regions of the country where children with SCA may be more dispersed across the state or live in rural areas. These children would not benefit from center-specific quality improvement activities; therefore, both population-based and center-specific strategies are necessary to move toward equitable receipt of quality of care for these vulnerable children.
Strategies to address low antibiotic and TCD screening rates could also serve to improve other aspects of clinical care. Other studies have demonstrated that improving care in specific areas may also spill over to improve overall quality of care.42–48 Mechanisms for this improvement may include increased clinic attendance as well as access to more knowledgeable providers. This suggests that efforts resulting in meaningful improvements in the provision of these basic preventive services could likewise result in enhancements in other important aspects of care for those living with SCA. Therefore, these results further support the need for a comprehensive approach to achieve high-quality medical care for those living with SCA. This comprehensive approach could be furthered by the development of a suite of SCA-related measures to identify opportunities for improvement in quality of care. For example, in addition to assessing appropriate antibiotic prophylaxis and TCD screening, measures could include adherence to other NHLBI recommendations, such as use of hydroxyurea, well-child visits with pediatricians, and twice a year visits with hematologists.12
There are limitations to this study that are important to consider. Although these results are reliant on the completeness and accuracy of administrative claims, the measures considered in this study have a high level of validity as reflected by extensive testing and NQF endorsement. Our case definition to identify children with SCA is also based upon administrative data; as such, this requires all individuals within our study population to have sickle cell-related healthcare utilization. Therefore, there may be a proportion of children without this utilization that we are not capturing; however, we expect this proportion to be small. Further, inclusion of these children with limited health services utilization would likely further lower rates of quality of care than reported within this study. Another limitation is that we were unable to determine if a child was prescribed, but did not fill an antibiotic or was referred to but did not complete TCD screening; or if there were additional uncommon factors that rendered a child ineligible for TCD screening such as a bone marrow transplant, technical limitations with conducting the TCD screen, or a previous overt stroke.49, 50 However, even assuming that 11% of children in the cohort had a stroke and would no longer qualify for routine TCD screening, the percentage of children who appropriately received TCD screening from 2011–2018 would still have been suboptimal. We do not know if the antibiotics that were filled from the pharmacy were administered to the child; therefore, our antibiotic results may be an overestimation of coverage among this population. Finally, we did not assess the number of bacteremia/sepsis or pediatric stroke cases due to lack of longitudinal follow-up data and difficulty assessing these outcomes in administrative claims.51
In conclusion, the findings of this study underscore the important opportunities that exist to improve care for children with SCA. We found that most children with SCA do not fill recommended antibiotic prophylaxis or receive TCD screening; this pattern is deep-rooted, having continued to persist over another decade. It is likely that meaningful improvements to the quality of care among children with SCA will require the development of multiple sustainable and well-coordinated interventions that can broadly improve clinical care. Looking ahead, ongoing evaluation of progress will require the implementation of validated quality measures, as well as the development of new measures as additional recommended services become available.
Abbreviation
- CMS
Centers for Medicare & Medicaid Services
- CPT
Current Procedural Terminology
- Hb
Hemoglobin
- NHLBI
National Heart, Lung, and Blood Institute
- NQF
National Quality Forum
- NYS
New York State
- SCA
Sickle cell anemia
- TCD
transcranial Doppler
Footnotes
Conflict of interest: The authors have no conflicts of interest to report.
Financial Disclosures: The authors have no financial disclosures to report.
Data availability statement:
Research data are not shared.
References
- 1.National Heart Lung and Blood Institute. Sickle Cell Disease. Accessed February 22, 2018, https://www.nhlbi.nih.gov/health/health-topics/topics/sca
- 2.Stuart MJ, Nagel RL. Sickle-cell disease. Lancet. Oct 9-15 2004;364(9442):1343–60. doi: 10.1016/s0140-6736(04)17192-4 [DOI] [PubMed] [Google Scholar]
- 3.Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. N Engl J Med. Nov 20 2008;359(21):2254–65. doi: 10.1056/NEJMra0804411 [DOI] [PubMed] [Google Scholar]
- 4.Verduzco LA, Nathan DG. Sickle cell disease and stroke. Blood. Dec 10 2009;114(25):5117–5125. [DOI] [PubMed] [Google Scholar]
- 5.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. Jan 1 1998;91(1):288–294. [PubMed] [Google Scholar]
- 6.Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med. Jun 19 1986;314(25):1593–9. doi: 10.1056/nejm198606193142501 [DOI] [PubMed] [Google Scholar]
- 7.Adams RJ, McKie VC, Brambilla D, et al. Stroke prevention trial in sickle cell anemia. Control Clin Trials. Feb 1998;19(1):110–129. [DOI] [PubMed] [Google Scholar]
- 8.Cherry M, Greenhalgh J, Osipenko L, et al. The Clinical Effectiveness and Cost-Effectiveness of Primary Stroke Prevention in Children with Sickle Cell Disease: A Systematic Review and Economic Evaluation. vol 16.43. NIHR Journals Library; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Heart Lung and Blood Institute. Sickle Cell Disease: Milestones and Clinical Progress. 2018:1–12. 10–7657. https://www.nhlbi.nih.gov/sites/default/files/publications/Sickle_Cell_Milestones_in_Research_508_Updated_0.pdf
- 10.National Heart Lung and Blood Institute. The Management of Sickle Cell Disease. Accessed December 10, 2019. https://www.nhlbi.nih.gov/files/docs/guidelines/sc_mngt.pdf
- 11.National Heart Lung and Blood Institute. The Management of Sickle Cell Disease. Accessed February 22, 2019, https://www.nhlbi.nih.gov/files/docs/guidelines/sc_mngt.pdf
- 12.National Heart Lung and Blood Institute. Evidence-Based Management of Sickle Cell Disease: Expert Panel Report, 2014. Accessed May 8, 2020, https://www.nhlbi.nih.gov/sites/default/files/media/docs/sickle-cell-disease-report%20020816_0.pdf
- 13.Transcranial Doppler ultrasonography screening for children with sickle cell disease (National Quality Measures Clearinghouse/Agency for Healthcare Research and Quality; ) [DOI] [PubMed] [Google Scholar]
- 14.Reeves S, Madden B, Shevrin C, McCormick J, Freed G, Dombkowski K. Antibiotic Prophylaxis Among Children with Sickle Cell Anemia. National Quality Forum (NQF). Accessed October 22, 2019. http://chear.org/sites/default/files/SCA_Antibiotic%20Measure%20Testing.pdf
- 15.National Quality Forum (NQF). Measure Evaluation Criteria. Accessed January 19, 2020. http://www.qualityforum.org/Measuring_Performance/Submitting_Standards/Measure_Evaluation_Criteria.aspx
- 16.Reeves SL, Madden B, Freed GL, Dombkowski KJ. Transcranial Doppler Screening Among Children and Adolescents With Sickle Cell Anemia. JAMA pediatrics. Jun 01 2016;170(6):550–6. doi: 10.1001/jamapediatrics.2015.4859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reeves SL, Tribble AC, Madden B, Freed GL, Dombkowski KJ. Antibiotic Prophylaxis for Children With Sickle Cell Anemia. Pediatrics. Mar 2018;141(3)doi: 10.1542/peds.2017-2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeves SL, Jary HK, Gondhi JP, Kleyn M, Wagner AL, Dombkowski KJ. Pneumococcal vaccination coverage among children with sickle cell anemia, sickle cell trait, and normal hemoglobin. Pediatr Blood Cancer. Oct 2018;65(10):e27282. doi: 10.1002/pbc.27282 [DOI] [PubMed] [Google Scholar]
- 19.Library of Congress. S.2465 - Sickle Cell Disease and Other Heritable Blood Disorders Research, Surveillance, Prevention, and Treatment Act of 2018. Accessed November 19, 2019. https://www.congress.gov/bill/115th-congress/senate-bill/2465
- 20.Agency for Healthcare Research and Quality (AHRQ). Pediatric Quality Measures Program (PQMP). Accessed January 19, 2020. https://www.ahrq.gov/pqmp/index.html
- 21.U.S. Department of Health and Human Services Office of Minority Health. About Sickle Cell Disease. Accessed November 19, 2019. https://www.minorityhealth.hhs.gov/sicklecell/index.html
- 22.Centers for Medicare & Medicaid Services. CMS Health Equity Blog- On the Path to Health Equity: Improving the Quality of Sickle Cell Disease Care. Accessed November 19, 2019. https://www.cms.gov/About-CMS/Agency-Information/OMH/about-cms-omh/blog/sickle-cell-disease-care.html
- 23.Reeves S, Garcia E, Kleyn M, et al. Identifying Sickle Cell Disease Cases Using Administrative Claims. Acad Pediatr. 9// 2014;14(5, Supplement):S61–S67. doi: 10.1016/j.acap.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reeves SL, Madden B, Wu M, et al. Performance of ICD-10-CM diagnosis codes for identifying children with Sickle Cell Anemia. Health Serv Res. Apr 2020;55(2):310–317. doi: 10.1111/1475-6773.13257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Health Services Advisory Group. 2019 HEDIS Aggregate Report for Michigan Medicaid. Michigan Department of Health and Human Services. Updated September 2019. Accessed January 18, 2021, https://www.michigan.gov/documents/mdhhs/MI2019_HEDIS-Aggregate_Report_rev_669299_7.pdf
- 26.Sox CM, Cooper WO, Koepsell TD, DiGiuseppe DL, Christakis DA. Provision of pneumococcal prophylaxis for publicly insured children with sickle cell disease. JAMA. 2003;290(8):1057–1061. doi: 10.1001/jama.290.8.1057 [DOI] [PubMed] [Google Scholar]
- 27.Eckrich MJ, Wang WC, Yang E, et al. Adherence to transcranial Doppler screening guidelines among children with sickle cell disease. Pediatr Blood Cancer. Feb 2013;60(2):270–4. doi: 10.1002/pbc.24240 [DOI] [PubMed] [Google Scholar]
- 28.Bundy DG, Abrams MT, Strouse JJ, Mueller CH, Miller MR, Casella JF. Transcranial Doppler screening of Medicaid-insured children with sickle cell disease. J Pediatr. Jan 2015;166(1):188–90. doi: 10.1016/j.jpeds.2014.09.018 [DOI] [PubMed] [Google Scholar]
- 29.Kwiatkowski JL, Voeks JH, Kanter J, et al. Ischemic stroke in children and young adults with sickle cell disease in the post-STOP era. Am J Hematol. Dec 2019;94(12):1335–1343. doi: 10.1002/ajh.25635 [DOI] [PubMed] [Google Scholar]
- 30.Armstrong-Wells J, Grimes B, Sidney S, et al. Utilization of TCD screening for primary stroke prevention in children with sickle cell disease. Neurology. Apr 14 2009;72(15):1316–1321. [DOI] [PubMed] [Google Scholar]
- 31.Reeves S, Madden B, Shevrin C, McCormick J, Freed G, Dombkowski K. Transcranial Doppler Screening Among Children with Sickle Cell Anemia. National Quality Forum (NQF). Accessed October 22, 2019. https://chear.org/sites/default/files/TranscranialDopplerScreeningMeasureSpecification.pdf
- 32.Weisman JK, Diamond CE, Kappa S, Nickel RS. Transcranial Doppler Screening Adherence among Children with Sickle Cell Anemia Seen in the Emergency Department. J Pediatr. Feb 2020;217:172–176.e1. doi: 10.1016/j.jpeds.2019.10.049 [DOI] [PubMed] [Google Scholar]
- 33.Lewen MO, Kavanagh PL, Sobota AE. A comment on improving transcranial Doppler ultrasonography screening in children with sickle cell anemia. Am J Hematol. 2017;92(6):E121–E122. doi:doi: 10.1002/ajh.24727 [DOI] [PubMed] [Google Scholar]
- 34.Bollinger LM, Nire KG, Rhodes MM, Chisolm DJ, O’Brien SH. Caregivers’ perspectives on barriers to transcranial Doppler screening in children with sickle-cell disease. Pediatr Blood Cancer. Jan 2011;56(1):99–102. doi: 10.1002/pbc.22780 [DOI] [PubMed] [Google Scholar]
- 35.Shook LM, Farrell CB, Kalinyak KA, et al. Translating sickle cell guidelines into practice for primary care providers with Project ECHO. Med Educ Online. 2016;21:33616. doi: 10.3402/meo.v21.33616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith D, Bach J, Lyon-Callo S, Young W. A Public Health Strategic Plan to Address Sickle Cell Disease Across the Lifespan. Michigan Department of Health and Human Services. Updated October 2015. Accessed April 30, 2020, https://www.michigan.gov/documents/mdhhs/MDHHS_Final_SCD_Strategic_Plan_504325_7.pdf
- 37.Meier ER, Janson IA, Hampton K, et al. Adherence to Quality of Care Indicators and Location of Sickle Cell Care Within Indiana. J Community Health. 2020;45(1):81–87. [DOI] [PubMed] [Google Scholar]
- 38.Iyengar RN, Balagere DS, Henderson RR, LeFrancois AL, Rabbitt RM, Frazee SG. Association between dispensing channel and medication adherence among medicare beneficiaries taking medications to treat diabetes, high blood pressure, or high blood cholesterol. J Manag Care Pharm. 2014;20(8):851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCarville MB, Goodin GS, Fortner G, et al. Evaluation of a comprehensive transcranial doppler screening program for children with sickle cell anemia. Pediatr Blood Cancer. Apr 2008;50(4):818–821. [DOI] [PubMed] [Google Scholar]
- 40.Freed GL. A Missed Opportunity to Address a National Shame: The Case of Sickle Cell Disease in the United States. JAMA pediatrics. Jun 17 2019;173(8):715–716. doi: 10.1001/jamapediatrics.2019.1536 [DOI] [PubMed] [Google Scholar]
- 41.Kanter J, Smith WR, Desai PC, et al. Building access to care in adult sickle cell disease: defining models of care, essential components, and economic aspects. Blood Adv. Aug 25 2020;4(16):3804–3813. doi: 10.1182/bloodadvances.2020001743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lloyd R Standardize Before You Improve. Accessed December 20, 2018. http://www.ihi.org/communities/blogs/standardize-before-you-improve
- 43.Batt RB H; Soltani M Quality Improvement Spillovers: Evidence from the Hospital Readmissions Reduction Program. Accessed 12/05, 2019. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3132770
- 44.Yue D, Pourat N, Chen X, et al. Enabling Services Improve Access To Care, Preventive Services, And Satisfaction Among Health Center Patients. Health Aff (Millwood). Sep 2019;38(9):1468–1474. doi: 10.1377/hlthaff.2018.05228 [DOI] [PubMed] [Google Scholar]
- 45.Galvin SL, Grandy R, Woodall T, Parlier AB, Thach S, Landis SE. Improved Utilization of Preventive Services Among Patients Following Team-Based Annual Wellness Visits. N C Med J. Sep-Oct 2017;78(5):287–295. doi: 10.18043/ncm.78.5.287 [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Huidobro D, Shippee N, Joseph-DiCaprio J, O’Brien JM, Svetaz MV. Effect of Patient-Centered Medical Home on Preventive Services for Adolescents and Young Adults. Pediatrics. Jun 2016;137(6)doi: 10.1542/peds.2015-3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Powers BJ, Olsen MK, Oddone EZ, Bosworth HB. The effect of a hypertension self-management intervention on diabetes and cholesterol control. Am J Med. Jul 2009;122(7):639–46. doi: 10.1016/j.amjmed.2008.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ibrahim AM, Nathan H, Thumma JR, Dimick JB. Impact of the Hospital Readmission Reduction Program on Surgical Readmissions Among Medicare Beneficiaries. Ann Surg. Oct 2017;266(4):617–624. doi: 10.1097/sla.0000000000002368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adams RJ, McKie VC, Carl EM, et al. Long-term stroke risk in children with sickle cell disease screened with transcranial Doppler. Ann Neurol. Nov 1997;42(5):699–704. [DOI] [PubMed] [Google Scholar]
- 50.Ashorobi D, Bhatt R. Bone Marrow Transplantation In Sickle Cell Disease. StatPearls. StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 51.Agrawal N, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Imaging data reveal a higher pediatric stroke incidence than prior US estimates. Stroke. Nov 2009;40(11):3415–21. doi: 10.1161/STROKEAHA.109.564633 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.


