Abstract
BACKGROUND
The incidence of hypertriglyceridemic acute pancreatitis (HTG-AP) has increased yearly, but updated population-based estimates on the incidence of HTG-AP are lacking. Reducing serum triglyceride (TG) levels quickly is crucial in the early treatment of HTG-AP. Decreased serum TG levels are treated by non-invasive methods, which include anti-lipidemic agents, heparin, low-molecular weight heparin, and insulin, and invasive methods, such as blood purification including hemoperfusion (HP), plasmapheresis, and continuous renal replacement therapy. However, authoritative guidelines have not been established. Early selection of appropriate treatment is important and beneficial in controlling the development of HTG-AP.
AIM
To evaluate the effect between patients treated with intravenous insulin (INS) and HP to guide clinical treatment.
METHODS
We retrospectively reviewed 371 patients with HTG-AP enrolled in the Department of Fujian Provincial Hospital form April 2012 to March 2021. The inpatient medical and radiologic records were reviewed to determine clinical features, severity, complications, mortality, recurrence rate, and treatment. Multivariate logistic regression analyses were used to analyze risk factors for severe HTG-AP. Propensity score matching was used to compare the clinical outcomes of INS and HP.
RESULTS
A total of 371 patients met the HTG-AP criteria. The incidence of HTG-AP was increased by approximately 2.6 times during the 10 years (8.4% in April 2012-March 2013 and 22.3% in April 2020-March 2021). The highest incidence rate of acute pancreatitis was observed for men in the age group of 30-39 years. The amylase level was elevated in 80.1% of patients but was only three times the normal value in 46.9% of patients. The frequency of severe acute pancreatitis (26.9%), organ failure (31.5%), rate of recurrence (32.9%), and mortality (3.0%) of HTG-AP was high. Improved Marshall score, modified computed tomography severity index score, baseline TG, baseline amylase, C-reactive protein (CRP), albumin, aspartate aminotransferase, low-density lipoprotein cholesterol, urea nitrogen, creatinine, calcium, hemoglobin, free triiodothyronine, admission to intensive care unit, and mortality were significantly different between patients with different grades of severity (P < 0.050). Multivariate logistic regression analysis confirmed that high CRP [P = 0.005, odds ratio (OR) = 1.011, 95%CI: 1.003-1.019], low calcium (P = 0.003, OR = 0.016, 95%CI: 0.001-0.239), and low albumin (P = 0.023, OR = 0.821, 95%CI: 0.693-0.973) were risk factors of severe HTG-AP. After propensity score matching adjusted by sex, age, severity of HTG-AP, and baseline TG, the serum TG significantly decreased in patients treated with INS (P < 0.000) and HP (P < 0.000) within 48 h. However, the clearance rate of TG (57.24 ± 33.70% vs 56.38 ± 33.61%, P = 0.927) and length of stay (13.04 ± 7.92 d vs 12.35 ± 6.40 d, P = 0.730) did not differ between the two groups.
CONCLUSION
The incidence of HTG-AP exhibited a significant increase, remarkable severity, and recurrent trend. Patients with mild and moderately severe acute pancreatitis can be treated effectively with INS safely and effectively without HP.
Keywords: Hypertriglyceridemic acute pancreatitis, Triglyceride, Improved Marshall score, Severity of acute pancreatitis, Intravenous insulin, Hemoperfusion
Core Tip: We assessed the clinical characteristics of hypertriglyceridemic acute pancreatitis, determined factors related to grades of severity, and evaluated differences in clinical outcomes between patients treated with intravenous insulin and hemoperfusion to guide clinical diagnosis and treatment.
INTRODUCTION
Acute pancreatitis (AP) is an inflammatory condition of the pancreas that originates within the pancreatic acinar cells and causes pancreatic necrosis, systemic inflammatory response syndrome, and multiple organ failure[1], with a mortality rate for severe cases as high as 20%-25%[2]. The great majority of AP is driven by gallstones (40%-70%) and alcohol (25%-35%)[3]. With the change of people’s diet structure and lifestyle, the incidence and mortality of hypertriglyceridemic (HTG)-AP are increasing year by year and has surpassed alcohol as the second leading cause of AP in China[4]. The standardized incidence rate of HTG-AP increased from 0.7 to 1.7 per 100000 person-years from 2008 to 2019 in Denmark[5], and the incidence has increased by 2.4 times in 10 years. However, the increasing number of HTG-AP incidence remains unclear in China.
The mechanism by which severe HTG precipitates to AP remains unknown. Studies have shown that pancreatic lipase hydrolyses excess triglyceride (TG) with accumulation of free fatty acids, thereby inducing the production of acinar cell and pancreatic capillary injury; chylomicrons lead to increased blood viscosity and local tissue ischemia[6,7]. Therefore, early detection of serum TG and active treatment measures to reduce serum TG are crucial for the prognosis of AP. Some scholars believe that rapid reduction of serum triglyceride levels within 48 h before the onset of HTG-AP is the key to treatment[8]. Effective treatments of reducing serum TG levels include anti-lipidemic agents, insulin, heparin, low-molecular weight heparin, and blood purification, such as hemoperfusion (HP), plasmapheresis (PE), and continuous renal replacement therapy[6,9]; however, no HTG-AP treatment guideline has been established. At present, selecting routine treatment or blood purification for patients with HTG-AP after admission remains controversial.
In view of the increasing incidence of HTG-AP in recent years and its short and long-term harmful effects on patients, families, and society, scholars have focused on preventing and effectively blocking HTG-AP as well as on its diagnosis and treatment. However, the causes remain unknown[10-12]; the low elevation of amylase (AMY) levels[13] and other characteristics[14] lead to the early misdiagnosis of HTG-AP. In addition, HTG-AP is prone to young age of onset[10], many complications[15], higher chance of systemic inflammatory response syndrome and cardiopulmonary and renal insufficiency[16], severe tendency[17], and lack of unified clinical treatment standards, which bring some difficulties to clinical treatment.
This study aims to improve the clinical diagnosis rate of HTG-AP by summarizing the clinical characteristics of HTG-AP and developing appropriate and cost-effective treatments for patients with HTG-AP.
MATERIALS AND METHODS
Patients
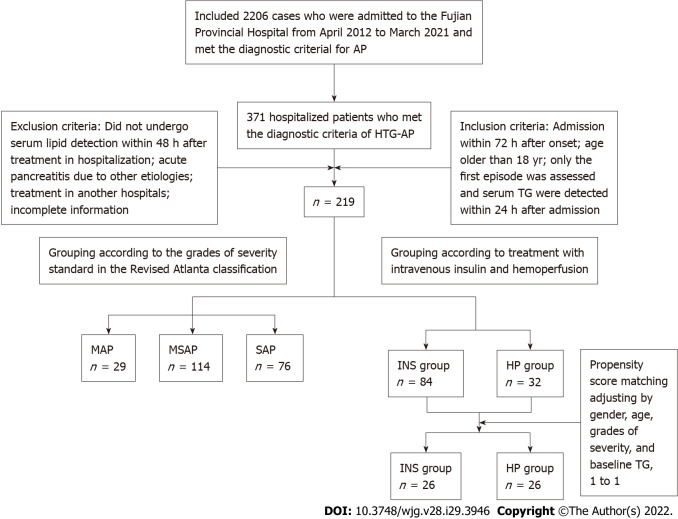
From April 2012 to March 2021, AP was diagnosed in 2206 patients in the Fujian Provincial Hospital. A total of 371 hospitalized patients who met the diagnostic criteria of HTG-AP were retrospectively studied, and 219 patients met the inclusion and exclusion criteria that were used to explore risk factors for severe HTG-AP. Fifty-two patients were included after propensity score matching (PSM) was adjusted by sex, age, grades of severity, and baseline TG. Clinical outcomes were compared between the 52 patients treated with intravenous insulin (INS) and HP (Figure 1). The Ethics Committee of Fujian Provincial Hospital approved the study (K2021-02-007).
Figure 1.
Research sample screening and grouping process. HTG-AP: Hypertriglyceridemic acute pancreatitis; MAP: Mild acute pancreatitis; MSAP: Moderately severe acute pancreatitis; SAP: Severe acute pancreatitis; TG: Triglyceride; INS: Insulin; HP: Hemoperfusion; AP: Acute pancreatitis.
The inclusion criteria were as follows: (1) Diagnosis of HTG-AP; (2) Admission within 72 h after onset; (3) Age older than 18 years; (4) Assessment of the first episode (for patients with multiple episodes of AP); and (5) Serum TG detected within 24 h after admission.
The exclusion criteria were as follows: (1) Did not undergo serum lipid detection within 48 h after treatment upon hospitalization; (2) AP due to other etiologies (including gallstones, alcohol, autoimmune, drug-induced, hypercalcemia, hyperparathyroidism, pancreatic tumor-related etiology of AP); (3) Treatment in another hospital; and (4) Incomplete information.
Grouping methods
According to the grades of severity standard in the Revised Atlanta Classification, patients were classified into the mild acute pancreatitis (MAP) group, moderately severe acute pancreatitis (MSAP) group, and severe acute pancreatitis (SAP) groups.
Patients were divided into the INS group and HP group according to treatment method. All patients were given basic support treatment including abrosia, gastrointestinal decompression, enema, fluid resuscitation, water maintenance, electrolyte and acid-base balance, lactulose to improve intestinal function, low molecular weight heparin, proton pump inhibitors for gastric acid secretion, and somatostatin/octreotide inhibitor pancreatic secretion. The INS group was given INS. The HP group was treated with HP. Blood access was established by puncture of the femoral vein or internal jugular vein. HP was performed with a resin irrigator (HA330, Zhuhai Lizhu Group, Biological Material Co, Ltd., China) for 2 h every 24 h with a blood flow of 150-250 mL/min. During the procedure, heparin was used to flush the infusion tube. After the procedure, coagulation markers were monitored. For those with prolonged coagulation times, 10-15 mg protamine was given to neutralize the effect of heparin. For patients with bleeding tendency, low molecular weight heparin was chosen, or the dose of heparin was appropriately reduced.
Definition
The diagnosis of AP was in accordance with the Revised Atlanta Definitions[18]. AP was diagnosed when two of the following three characteristics were met: (1) Abdominal pain consistent with AP (acute onset of a persistent, severe, epigastric pain often radiating to the back); (2) At least three times higher levels of AMY and/or lipase above the upper limit of the normal value; and (3) Abdominal imaging (including computed tomography, magnetic resonance imaging, or transabdominal ultrasonography) consistent with changes in AP.
HTG-AP was considered in patients with AP when the level of serum TG was: (1) Over 1000 mg/dL; and (2) Between 500 and 1000 mg/dL with lactescent serum at admission[19].
The severity of AP was graded according to the Revised Atlanta Definitions[18]: MAP, MSAP, and SAP. MAP was defined by the absence of organ failure and the absence of local or systemic complications. MSAP was defined by the presence of transient (< 48 h) organ failure or local or systemic complications in the absence of persistent organ failure. SAP was defined by persistent (> 48 h) organ failure.
Organ failure was determined according to the improved Marshall score standard in the Revised Atlanta classification[18]. Three organ systems were assessed to define organ failure: respiratory, cardiovascular, and renal. For respiratory (PaO2/FiO2), 301 mmHg ≤ PaO2/FiO2 ≤ 400 mmHg was scored as 1 point, 201 mmHg ≤ PaO2/FiO2 ≤ 300 mmHg was scored as 2 points, 101 mmHg ≤ PaO2/FiO2 ≤ 200 mmHg was scored as 3 points, and PaO2/FiO2 ≤ 101 mmHg was scored as 4 points. For renal serum creatinine (SCR), 134 μmol/L ≤ SCR ≤ 169 μmol/L was scored as 1 point, 170 μmol/L ≤ SCR ≤ 310 μmol/L was scored as 2 points, 311 μmol/L ≤ SCR ≤ 439 μmol/L was scored as 3 points, and SCR > 439 μmol/L was scored as 4 points. For cardiovascular systolic blood pressure (BP), BP < 90 mmHg and fluid responsive was scored as 1 point, BP < 90 mmHg without fluid responsive was scored as 2 points, BP < 90 mmHg and pH < 7.3 was scored as 3 points, and BP < 90 mmHg and pH < 7.2 was scored as 4 points. A score of 2 or more in any system defined the presence of organ failure.
Data collection
Data were obtained from the patients’ medical records and hospital electronic database records. Sex, age, and comorbidities were collected. Clinical manifestations, improved Marshall score[18], modified computed tomography severity index (MCTSI) score[20], organ failure[18], laboratory and imaging data, treatments, intensive care unit admission, length of stay, and prognosis during hospitalization were recorded. All laboratory data were measured within 24 h after admission. Baseline TG and serum AMY were measured using the first tested values since onset. Serum lipids were reviewed within 48 h after administering lipid-lowering treatment.
Statistical analysis
SPSS 25.0 (IBM Corp., Armonk, NY, United States) was used for data analysis, and GraphPad Prism7.0 was used for mapping. Measurement data in normal distribution were expressed as mean ± SD and analyzed with Student’s t-test or analysis of variance. Otherwise, variables were described as medians and interquartile ranges and analyzed by Mann-Whitney U test or Kruskal-Wallis test. Categorical variables were presented as absolute numbers and proportions and tested by χ2 or Fisher’s exact test. Paired t test was used for continuous variables before and after treatment. Multivariate logistic regression analysis was used to identify independent risk factors with odds ratios (ORs) and 95%CIs. In addition, 1-1 PSM was performed, followed by univariate analysis. P < 0.05 was considered statistically significant.
RESULTS
Trends in incidence of HTG-AP
For nearly a decade, 371 patients were diagnosed with HTG-AP in Fujian Provincial Hospital. The total number of patients with HTG-AP in our hospital increased, and the incidence of HTG-AP was increased by approximately 2.6 times over the past 10 years and ranged from 8.4% to 22.3% (Figure 2).
Figure 2.
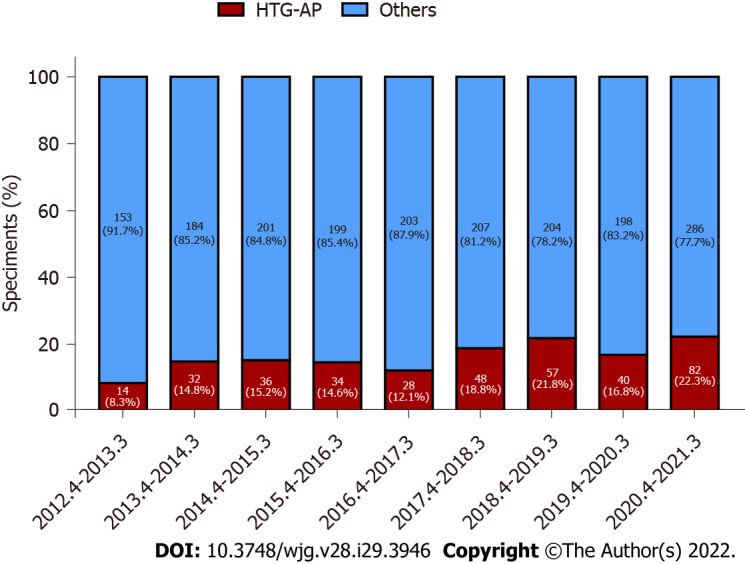
The total number of patients with hypertriglyceridemic acute pancreatitis and other acute pancreatitis in Fujian Provincial Hospital increased significantly during nearly 10 years. HTG-AP: Hypertriglyceridemic acute pancreatitis.
Clinical characteristics of HTG-AP
A total of 371 patients were diagnosed with HTG-AP, the mean age of the patients with HTG-AP was 39.86 ± 10.20 years, and most of the patients (approximately 93.8%) were young and middle-aged individuals, with a male/female ratio of 2.0 (247/124). The highest incidence rate of AP was observed for men in the age group of 30-39 years (Figure 3).
Figure 3.
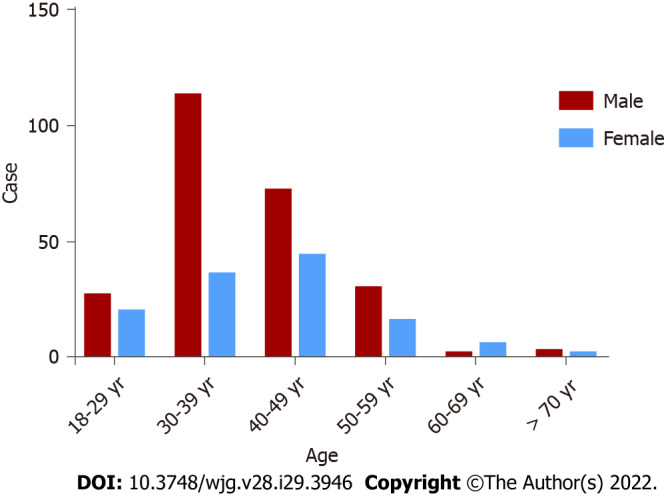
Incidence rates of hypertriglyceridemic acute pancreatitis stratified by age and sex.
The mean serum baseline TG values in HTG-AP were significantly high (2544.59 ± 2305.37 mg/dL). The serum AMY elevated levels were higher than normal in 80.1% of the patients with HTG-AP but only three times greater than normal in 46.9% of patients (Table 1).
Table 1.
Clinical characteristics of hypertriglyceridemic acute pancreatitis
|
Characteristic
|
All (n = 371)
|
| Sex, n (%) | |
| Male | 247 (66.6) |
| Female | 124 (33.4) |
| Age, yr | 39.86 ± 10.20 |
| BMI (kg/m2) | 25.99 ± 3.18 |
| Causes, n (%) | 203 (54.7) |
| Diet (high fatty acid) | 127 (34.2) |
| Drinking (beer) | 55 (14.8) |
| Mixed | 21 (5.7) |
| Complications, n (%) | |
| Diabetes mellitus | 115 (31.0) |
| Hypertension | 62 (16.7) |
| Fatty liver disease | 336 (90.6) |
| Pregnancy | 11 (3.0) |
| Recurrence, n (%) | 122 (32.9) |
| Grades of severity, n (%) | |
| MAP | 63 (17.0) |
| MSAP | 208 (56.1) |
| SAP | 100 (26.9) |
| Improved Marshall score | 1.30 ± 1.77 |
| Organ failure, n (%) | 117 (31.5) |
| MCTSI score | 5.00 ± 1.83 |
| Lipid-lowering treatment, n (%) | |
| Intravenous insulin | 144 (38.8) |
| HP | 32 (8.6) |
| CRRT/HP + CRRT | 77 (20.8) |
| Only anti-lipemic | 118 (31.8) |
| Baseline TG, mg/dL | 2544.59 ± 2305.37 |
| Baseline AMY (nUNL) | 5.00 ± 6.47 |
| > UNL, n (%) | 297 (80.1) |
| ≥ 3UNL, n (%) | 174 (46.9) |
| Admission to ICU, n (%) | 126 (34.0) |
| Death, n (%) | 11 (3.0) |
BMI: Body mass index; MAP: Mild acute pancreatitis; MSAP: Moderately severe acute pancreatitis; SAP: Severe acute pancreatitis; MCTSI: Modified computed tomography severity index; HP: Hemoperfusion; CRRT: Continuous renal replacement therapy; TG: Triglyceride; AMY: Amylase; UNL: Upper limit of normal; ICU: Intensive care unit.
About 90.6% of patients with HTG-AP had comorbidity with fatty liver disease, and 11 women (3.0%) had an HTG-AP attack during pregnancy. About 54.7% of the cases were related to diet (high fatty acid) and/or drinking (beer). Patients with HTG-AP had a high frequency of SAP (100, 26.9%), organ failure (117, 31.5%), recurrence (122, 32.9%), and high MCTSI score (5.00 ± 1.83). Eleven patients (3.0%) died during hospitalization (Table 1).
Comparisons of different grades of severity of HTG-AP
A total of 219 patients met the inclusion and exclusion criteria divided into the MAP group (n = 29), MSAP group (n = 114), and SAP group (n = 76).
Table 2 shows that the more severe HTG-AP was, the more frequent blood purification was used. Improved Marshall score (P < 0.000), MCTSI score (P < 0.000), baseline TG (P = 0.035), baseline AMY (P < 0.000), CRP (P < 0.000), albumin (P < 0.000), aspartate aminotransferase (P < 0.000), low-density lipoprotein-cholesterol (P = 0.003), urea nitrogen (P < 0.000), creatinine (P < 0.000), calcium (P < 0.000), hemoglobin (P = 0.010), free triiodothyronine (P = 0.018), admission to the intensive care unit (P < 0.000), and mortality (P < 0.000) were significantly different between patients with different grades of severity.
Table 2.
Comparisons of clinical characteristics and laboratory parameters with different grades of severity of hypertriglyceridemic acute pancreatitis
|
Characteristic
|
All (n = 219)
|
MAP group (n = 29)
|
MSAP group (n = 114)
|
SAP group (n = 76)
|
P
value
|
| Sex, n (%) | 0.111 | ||||
| Male | 140 (63.9) | 23 (79.3) | 67 (58.8) | 50 (65.8) | |
| Female | 79 (36.1) | 6 (20.7) | 47 (41.2) | 26 (34.2) | |
| Age, yr | 38.92 ± 10.02 | 38.66 ± 9.73 | 38.96 ± 10.12 | 38.96 ± 10.12 | 0.941 |
| BMI (kg/m2) | 26.13 ± 3.30 | 26.20 ± 1.56 | 25.58 ± 3.54 | 28.51 ± 2.97 | 0.097 |
| Complications, n (%) | |||||
| Diabetes mellitus | 109 (49.8) | 14 (48.3) | 58 (50.9) | 37 (48.7) | 0.982 |
| Fatty liver disease | 205 (93.6) | 25 (86.2) | 106 (93.0) | 74 (97.4) | 0.112 |
| Lipid-lowering treatment, n (%) | < 0.001 | ||||
| Intravenous insulin | 84 (38.4) | 14 (48.3) | 69 (60.5) | 1 (1.3) | |
| HP | 32 (14.6) | 1 (3.4) | 26 (22.8) | 5 (6.6) | |
| CRRT/HP + CRRT | 60 (27.4) | 0 (0.0) | 1 (0.9) | 59 (77.6) | |
| Only anti-lipemic | 43 (19.6) | 14 (48.3) | 18 (15.8) | 11 (14.5) | |
| Improved Marshall score | 1.00 (0.00, 3.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 1.00) | 3.00 (3.00, 4.75) | < 0.001 |
| MCTSI score | 5.11 ± 1.70 | 2.14 ± 0.92 | 5.14 ± 1.06 | 6.18 ± 1.35 | < 0.001 |
| Baseline TG, mg/dL | 2713.82 ± 2458.65 | 1895.15 ± 1685.17 | 2480.80 ± 2040.458 | 3386.29 ± 3081.51 | 0.035 |
| Baseline AMY (nUNL) | 2.92 (1.43, 6.41) | 2.81 (1.47, 5.21) | 2.22 (0.98, 5.08) | 5.28 (2.21, 8.66) | < 0.001 |
| CRP, mg/L | 196.36 ± 121.97 | 143.17 ± 97.51 | 178.65 ± 103.25 | 247.12 ± 143.73 | < 0.001 |
| Albumin, g/L | 32.66 ± 5.99 | 36.48 ± 4.96 | 34.46 ± 5.16 | 28.49 ± 5.16 | < 0.001 |
| TBIL, mmol/L | 16.60 (10.80, 22.90) | 18.91 (13.02, 23.68) | 15.35 (10.69, 22.46) | 16.57 (10.60, 24.92) | 0.511 |
| ALT, U/L | 20.00 (13.60, 30.70) | 21.30 (14.90, 35.75) | 20.25 (13.00, 31.93) | 19.75 (14.03, 27.30) | 0.648 |
| AST, U/L | 25.00 (17.50, 39.50) | 21.30 (17.00, 28.05) | 20.00 (16.08, 30.63) | 36.55 (25.00, 56.80) | < 0.001 |
| Total cholesterol, mmol/L | 8.58 ± 4.56 | 7.47 ± 2.96 | 8.82 ± 4.53 | 8.64 ± 5.06 | 0.522 |
| HDL-C, mmol/L | 0.82 (0.61, 1.10) | 0.88 (0.75, 1.17) | 0.86 (0.66, 1.16) | 0.69 (0.52, 0.99) | 0.241 |
| LDL-C, mmol/L | 2.92 ± 1.55 | 3.77 ± 1.41 | 2.91 ± 1.58 | 2.62 ± 1.46 | 0.003 |
| Glucose, mmol/L | 10.50 ± 3.64 | 9.47 ± 3.70 | 10.34 ± 3.54 | 11.13 ± 3.69 | 0.100 |
| Urea nitrogen, mmol/L | 4.73 ± 3.31 | 4.56 ± 1.63 | 3.66 ± 1.76 | 6.40 ± 4.64 | < 0.001 |
| Creatinine, μmol/L | 83.03 ± 66.48 | 71.90 ± 18.03 | 65.46 ± 19.11 | 113.63 ± 103.46 | < 0.001 |
| Calcium, mmol/L | 1.93 ± 0.31 | 2.16 ± 0.18 | 2.00 ± 0.21 | 1.75 ± 0.37 | < 0.001 |
| WBC, × 109/L | 11.67 ± 4.10 | 11.22 ± 3.64 | 11.99 ± 4.06 | 11.37 ± 4.33 | 0.351 |
| PLT, × 109/L | 200.19 ± 69.19 | 194.69 ± 73.58 | 208.67 ± 65.17 | 189.57 ± 72.51 | 0.159 |
| Hb, g/L | 141.11 ± 24.52 | 143.69 ± 19.12 | 136.87 ± 22.76 | 146.49 ± 27.78 | 0.010 |
| STSH, mIU/L | 0.28 (0.16, 0.65) | 0.49 (0.28, 0.98) | 0.28 (0.14, 0.66) | 0.24 (0.16, 0.62) | 0.188 |
| FT3, pmol/L | 2.29 ± 1.00 | 2.90 ± 0.77 | 2.36 ± 0.86 | 2.89 ± 1.18 | 0.018 |
| FT4, pmol/L | 12.56 ± 3.70 | 12.83 ± 2.10 | 12.88 ± 3.95 | 12.11 ± 3.68 | 0.302 |
| Admission to ICU, n (%) | 98 (44.7) | 1 (3.4) | 27 (23.7) | 70 (92.1) | < 0.001 |
| Death, n (%) | 9 (4.1) | 0 (0.0) | 0 (0.0) | 9 (11.8) | < 0.001 |
MAP: Mild acute pancreatitis; MSAP: Moderately severe acute pancreatitis; SAP: Severe acute pancreatitis; BMI: Body mass index; HP: Hemoperfusion; CRRT: Continuous renal replacement therapy; MCTSI: Modified computed tomography severity index; TG: Triglyceride; AMY: Amylase; UNL: Upper limit of normal; CRP: C-reactive protein; TBIL: Total bilirubin; ALT: Alanine aminotransferase; AST: Aspartate transaminase; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; WBC: White blood cell; PLT: Blood platelet; Hb: Hemoglobin; STSH: Sensitive thyrotropin; FT3: Free triiodothyronine; FT4: Free thyroxine; ICU: Intensive care unit.
Multivariate logistic regression analysis confirmed that high CRP (P = 0.005, OR = 1.011, 95%CI: 1.003-1.019), low calcium (P = 0.003, OR = 0.016, 95%CI: 0.001-0.239), and low albumin (P = 0.023, OR = 0.821, 95%CI: 0.693-0.973) were risk factors of severe HTG-AP (Table 3).
Table 3.
Factors associated with severe hypertriglyceridemic acute pancreatitis according to multivariate logistic regression analysis
|
Variable
|
B
|
OR
|
OR (95%CI)
|
P
value
|
| Baseline TG | 0.023 | 1.023 | (0.991-1.056) | 0.159 |
| Baseline AMY | 0.345 | 1.412 | (0.916-2.175) | 0.118 |
| CRP | 0.110 | 1.011 | (1.003-1.019) | 0.005 |
| Albumin | -0.197 | 0.821 | (0.693-0.973) | 0.023 |
| AST | 0.018 | 1.018 | (0.975-1.063) | 0.423 |
| LDL-C | -0.173 | 0.814 | (0.556-1.272) | 0.412 |
| Urea nitrogen | -0.334 | 0.709 | (0.462-1.086) | 0.114 |
| Creatinine | 0.025 | 1.026 | (0.988-1.065) | 0.186 |
| Calcium | -4.152 | 0.016 | (0.001-0.239) | 0.003 |
| Hb | 0.008 | 1.008 | (0.975-1.043) | 0.621 |
| FT3 | -1.324 | 0.266 | (0.055-1.281) | 0.099 |
OR: Odds ratio; TG: Triglyceride; AMY: Amylase; CRP: C-reactive protein; AST: Aspartate transaminase; LDL-C: Low-density lipoprotein cholesterol; Hb: Hemoglobin; FT3: Free triiodothyronine.
Comparisons between INS and HP treatments
Of the 219 patients, 84 patients were treated with INS in the INS group and 32 patients were treated with HP in the HP group. The entire cohort showed that the grades of severity of HTG-AP (P = 0.002) and baseline TG (P = 0.037) were significantly different between patients treated with INS and HP (Table 4).
Table 4.
Comparisons of clinical characteristics and laboratory parameters with different treatment between intravenous insulin and hemoperfusion before and after propensity score matching
|
Characteristic
|
Entire cohort
|
P
value
|
PSM
|
P
value
|
||
|
INS group (n = 84)
|
HP Group (n = 32)
|
INS group (n = 26)
|
HP group (n = 26)
|
|||
| Sex, n (%) | 0.626 | 0.184 | ||||
| Male | 51 (60.7) | 21 (65.6) | 20 (76.9) | 16 (61.5) | ||
| Female | 33 (39.3) | 11 (34.4) | 6 (23.1) | 10 (38.5) | ||
| Age, yr | 39.99 ± 10.36 | 36.44 ± 11.60 | 0.965 | 36.81 ± 10.79 | 36.42 ± 10.10 | 0.895 |
| Grades of severity, n (%) | 0.002 | 0.755a | ||||
| MAP | 14 (16.7) | 1 (3.1) | 1 (3.8) | 1 (3.8) | ||
| MSAP | 69 (82.1) | 26 (81.3) | 25 (96.2) | 25 (96.2) | ||
| SAP | 1 (1.2) | 5 (15.6) | 0 (0.0) | 0 (0.0) | ||
| Improved Marshall score | 0.00 (0.00, 1.00) | 1.00 (1.00, 2.00) | < 0.001 | 0.00 (0.00, 1.00) | 1.00 (0.00, 1.00) | 0.186a |
| MCTSI score | 4.67 ± 1.59 | 5.75 ± 0.84 | < 0.001 | 5.15 ± 1.29 | 5.62 ± 0.80 | 0.127a |
| Baseline TG, mg/dL | 2493.20 ± 1958.06 | 3443.88 ± 1676.31 | 0.037 | 3484.64 ± 2275.248 | 3264.91 ± 2375.37 | 0.734a |
| Treated TG within 48 h, mg/dL | 946.25 ± 769.05 | 1145.60 ± 699.05 | 0.205 | 1108.39 ± 856.76 | 1040.16 ± 686.65 | 0.753 |
| Clearance rate of TG within 48 h, % | 47.65 ± 34.64 | 63.91 ± 23.32 | 0.005 | 57.24 ± 33.70 | 56.38 ± 33.61 | 0.927a |
| CRP, mg/L | 184.75 ± 99.84 | 198.43 ± 94.00 | 0.552 | 214.612 ± 119.22 | 198.28 ± 97.09 | 0.629 |
| Albumin, g/L | 34.05 ± 4.81 | 32.50 ± 6.39 | 0.219 | 34.56 ± 5.03 | 32.53 ± 6.58 | 0.218 |
| TBIL, mmol/L | 17.80 ± 8.96 | 19.83 ± 13.61 | 0.439 | 18.70 ± 9.38 | 20.87 ± 14.41 | 0.523 |
| ALT, U/L | 24.55 ± 17.77 | 23.15 ± 18.15 | 0.705 | 24.58 ± 16.69 | 21.81 ± 16.47 | 0.550 |
| AST, U/L | 25.70 ± 14.23 | 30.85 ± 26.87 | 0.187 | 24.84 ± 12.40 | 32.10 ± 29.44 | 0.252 |
| Total cholesterol, mmol/L | 8.38 ± 3.89 | 8.93 ± 4.37 | 0.511 | 9.84 ± 4.91 | 8.52 ± 4.58 | 0.318 |
| Glucose, mmol/L | 11.08 ± 3.65 | 10.83 ± 3.42 | 0.739 | 11.71 ± 2.74 | 10.07 ± 3.22 | 0.057 |
| Urea nitrogen, mmol/L | 4.02 ± 1.86 | 3.83 ± 2.27 | 0.652 | 3.54 ± 1.76 | 3.34 ± 1.94 | 0.688 |
| Creatinine, mmol/L | 63.73 ± 18.23 | 67.72 ± 24.01 | 0.338 | 65.00 ± 12.33 | 67.00 ± 23.36 | 0.702 |
| Calcium, mmol/L | 1.98 ± 0.25 | 1.90 ± 0.28 | 0.100 | 2.03 ± 0.23 | 1.89 ± 0.31 | 0.063 |
| WBC, × 109/L | 11.79 ± 4.20 | 12.33 ± 4.37 | 0.541 | 12.40 ± 4.00 | 12.51 ± 4.44 | 0.920 |
| PLT, × 109/L | 205.39 ± 68.44 | 204.34 ± 65.98 | 0.941 | 201.66 ± 59.77 | 197.38 ± 57.91 | 0.795 |
| Hb, g/L | 137.48 ± 23.67 | 138.12 ± 27.60 | 0.902 | 143.44 ± 19.50 | 135.38 ± 27.50 | 0.230 |
| Length of stay, d | 11.88 ± 6.37 | 11.93 ± 6.01 | 0.965 | 13.04 ± 7.92 | 12.35 ± 6.40 | 0.730 |
| Death, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
P value changed after propensity score matching.
PSM: Propensity score matching; INS: Intravenous insulin; HP: Hemoperfusion; MAP: Mild acute pancreatitis; MSAP: Moderately severe acute pancreatitis; SAP: Severe acute pancreatitis; MCTSI: Modified computed tomography severity index; TG: Triglyceride; CRP: C-reactive protein; TBIL: Total bilirubin; ALT: Alanine aminotransferase; AST: Aspartate transaminase; WBC: White blood cell; PLT: Blood platelet; Hb: Hemoglobin.
Given the large severity and baseline TG gap between the two groups, patients were selected for further analysis using PSM, adjusted by sex, age, grades of severity, and baseline TG. After matching, 26 patients were in the INS group and 26 patients were in the HP group (1:1, match tolerance = 0.02). No significant differences in sex (P = 0.184), age (P = 0.895), grades of severity (P = 0.755), improved Marshall score (P = 0.186), MCTSI score (P = 0.127), and baseline TG (P = 0.734) were found between the two groups (Table 4).
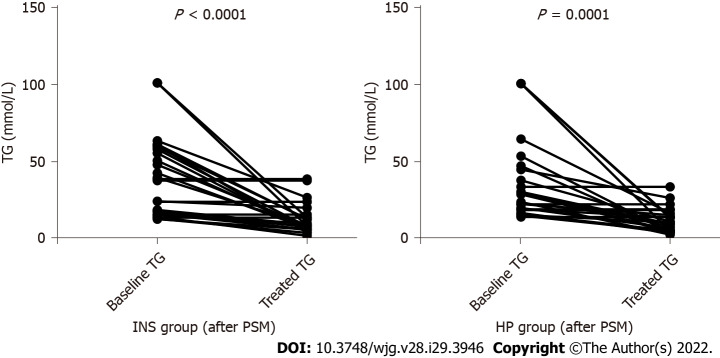
In patients with MAP and MSAP, the serum TG level significantly decreased in patients treated with INS (P < 0.000) and HP (P < 0.000) within 48 h (Figure 4). The clearance rates of TG were 57.24% ± 33.70% and 56.38% ± 33.61%, respectively (P = 0.927). However, the clearance rate of TG (P = 0.927) and length of stay (13.04 ± 7.92 d vs 12.35 ± 6.40 d, P = 0.730) did not differ between the two groups (Table 4).
Figure 4.
Changes of serum triglyceride levels in both groups before and after treatment. TG: Triglyceride; INS: Insulin; PSM: Propensity score matching; HP: Hemoperfusion.
DISCUSSION
Trends in incidence of HTG-AP
AP has many etiologies, and previous studies reported that HTG as an etiologic factor is between 1.3% and 6.9%[21,22]. However, HTG-AP increased at a fast rate in the Asian region during recent years. Taiwan reported that the frequency of HTG as an etiologic factor in patients with AP ranged from 6.3% to 12.3%[23]. A study reported that the incidence of HTG-AP reached 25.6% in 2013[14]. Zheng et al[24] retrospectively analyzed 2461 patients with AP in Beijing during a 5-year period and reported that the causes of AP included biliary (55.75%), alcoholism (10%), hypertriglyceridemia (10.36%), and others (23.89%); however, this work did not mention the rate of increase in HTG-AP.
The standardized incidence rate of HTG-AP increased from 0.7 to 1.7 per 100000 person-years from 2008 to 2019 in Denmark, and the incidence has increased by 2.4 times over the past 10 years[5]. Another study from Guangdong showed that the incidence of HTG-AP in 2000 to 2005 was 2.6 times higher than that in 1990 to 1994 (8.9% vs 3.4%, P < 0.05)[25]. Based on recent observations from China[26], the proportion of patients with HTG-AP increased from 14.0% to 34.0% during a 16-year period in a tertiary hospital setting. Our study found that the incidence of HTG-AP increased by approximately 2.6 times and ranged from 8.4% to 22.3% during nearly 10 years, with a significant increase detected after 2017. Our results are similar to previously reported data.
At present, updated population-based estimates on the incidence of HTG-AP are lacking. Some studies have shown that the incidence and mortality of HTG-AP were increasing year by year, which was related to the change of people’s diet structure and lifestyle[4]. At the same time, the availability of the detection of serum TG also improved the diagnosis of HTG-AP. For example, the emergency of our hospital began testing serum TG in 2017, and Figure 1 shows the incidence of HTG-AP has increased since 2017.
Figure 1 showed the decrease in the incidence of HTG-AP from April 2016 to March 2017 and from April 2019 to March 2020, which coincided with the opening of the South Hospital of Fujian Provincial Hospital and the outbreak of coronavirus disease 2019 in China, leading to the diversion and reduction of the number of patients with AP and HTG-AP.
Clinical feature of HTG-AP
This study also found that the clinical manifestations of patients with HTG-AP included the most common abdominal pain and abdominal distension as well as nausea, vomiting, and anhelation without specificity. However, patients with HTG-AP have some clinical characteristics, such as serum TG level (2544.59 ± 2305.37 mg/dL) that was significantly higher than normal values at the onset of disease. Hence, serum TG level ≥ 1000 mg/dL is the most important characteristic of HTG-AP[19]. Thus, we can improve the diagnosis rate of HTG-AP by improving the early detection rate of serum TG in clinical work.
We found that the mean age of patients with HTG-AP was 39.86 ± 10.20 years. The highest incidence rate of AP was observed for men in the age group of 30-39 years, and the male/female ratio was 2.0 (247/124). Li et al[10] reported that patients with HTG-AP were younger (40 vs 51, P < 0.01) and were mostly males (214/91 vs 242/183, P < 0.01) compared with patients with biliary AP.
Sekimoto et al[27] stated that the average age for HTG-AP was lower than that for other causes. Zheng et al[24] reported a higher proportion of alcoholic and HTG-AP in men than in women and in patients younger than 50 years. Olesen et al[5] reported that the highest incidence rate of severe HTG was observed for men in the age group of 40-49 years, and severe HTG is a well-known risk factor for AP. Therefore, HTG-AP is becoming more prevalent among younger individuals.
AMY was elevated to levels higher than the normal value in 80.1% of patients but only three times higher than the normal in 46.9% of patients. About 50% of patients with HTG-AP showed no significant increase in serum and urine AMY levels[13], which may be due to the presence of AMY activity inhibitors in their plasma; these inhibitors can enter the urine through the kidneys and inhibit urinary AMY activity. In addition, increased TG levels directly affected the determination of AMY. Therefore, the early diagnosis of HTG-AP is more difficult. The diagnostic accuracy of lipase for HTG-AP was 91.83%, while that of AMY was only 40.38%[14]. Thus, we can improve the diagnosis rate of HTG-AP by combining serum AMY and lipase.
In this study, the frequency of SAP (26.9%), organ failure (31.5%), rate of recurrence (32.9%), and mortality (3.0%) of HTG-AP was high. A large multicenter study in China showed higher incidences of local complication (34.13% vs 15.72%, P < 0.000) and MSAP (28.85% vs 12.95%, P < 0.000) in patients with HTG-AP than in patients without HTG-AP[14]. HTG-AP varied in severity between mild (41%), moderate (26%), and severe (33%)[17]. A foreign study showed that patients with HTG-AP had significantly higher percentages of multiple organ dysfunction syndrome (24.1% vs 12.1%, P = 0.009) and cardiovascular failure (17.6% vs 4.6%, P < 0.001) compared with biliary AP[26].
Some reports showed a more severe course of AP induced by HTG compared with other causes, whereas other scholars seemed to favor no significant difference in disease severity[28]. Vipperla et al[17] reported that the risk of recurrent AP attacks was 32%, often in patients with poorly controlled diabetes, alcoholism, and TG levels. Our study showed that the recurrence rate of MSAP was higher than that of SAP, which may be due to insufficient attention and medical care. Different studies reported that the mortality rate of HTG-AP ranged from 0.48% to 7.9%[10,14,26], but the mortality rate for severe cases reached as high as 20%-25%[2].
The high frequency rates of fatty liver disease, diabetes mellitus, and hypertension were found in patients with HTG-AP, with values of 90.6%, 31.0%, and 16.7%, respectively. Patients with HTG-AP were often complicated with metabolic diseases such as diabetes mellitus and obesity, and patients with type 2 diabetes mellitus had an elevated risk of AP compared with patients without diabetes[15].
Comparisons of different grades of severity of HTG-AP
A comparison was conducted among patients with MAP, MSAP, and SAP to investigate the association of the severity of HTG-AP with clinical data and laboratory indicators. Table 2 showed that age and sex had no difference among the three groups (all P > 0.050). Improved Marshall score, MCTSI score, baseline TG, baseline AMY, CRP, albumin, aspartate aminotransferase, low-density lipoprotein-cholesterol, urea nitrogen, creatinine, calcium, hemoglobin, and free triiodothyronine were significantly different among patients with different grades of severity of HTG-AP (all P < 0.050). Multivariate logistic regression analysis confirmed that high CRP (P = 0.005, OR = 1.011, 95%CI: 1.003-1.019), low calcium (P = 0.003, OR = 0.016, 95%CI: 0.001-0.239), and low albumin (P = 0.023, OR = 0.821, 95%CI: 0.693-0.973) were risk factors of severe HTG-AP.
CRP, serum calcium, and serum albumin are well-known predictors of severe AP with non-HTG-AP and are widely used in its early detection. Experts suggested that CRP levels > 150 mg/L 48 h after the onset of symptoms have a high sensitivity for predicting the severity of AP[29]. Yu et al[30] reported that patients with HTG-AP had lower serum ionized calcium associated with a higher risk of developing SAP. Chen et al[31] confirmed that low serum albumin (P = 0.004, OR = 3.362, 95%CI: 1.492–8.823) and high CRP (P = 0.005, OR = 3.061, 95%CI: 1.407–6.659) were risk factors of moderately severe to severe HTG-AP.
Our study showed that the predictors of SAP with HTG-AP were similar to those of AP with other etiologies including alcoholic and biliary AP.
Comparisons among different treatments
Reducing serum TG levels quickly is crucial in the early treatment of HTG-AP. This method mainly includes two categories of routine treatment, and blood purification had been implemented for the patients with HTG-AP. Currently, the TG levels should be reduced to below 500 mg/dL as soon as possible; when follow-up TG levels were < 500 mg/dL, an associated reduction in the risk of clinical events and decrease in health care resource use and costs were observed[32]. However, selecting routine treatment or blood purification for patients with HTG-AP after admission to obtain economic cost effectiveness remains controversial. Routine treatments, such as insulin, heparin, and anti-HTG drugs, are effective in reducing TG and have the advantages of non-invasiveness and low cost.
Blood purification includes HP, PE, and continuous renal replacement therapy, which have the disadvantages of invasiveness and expensive. Compared with HP and PE, continuous renal replacement therapy can not only reduce TG rapidly but also remove inflammatory mediators and is more accurate for systemic inflammatory response syndrome control[9]. However, selecting routine treatment or blood purification for patients with HTG-AP after admission in order to obtain economic cost effectiveness remains controversial. Therefore, this study mainly compared the effect of INS and HP on lowering serum TG.
Among patients with MAP and MSAP, a significant decrease in serum TG was found in patients treated with INS (P < 0.000) and HP (P < 0.000) within 48 h. The clearance rates of TG were 57.24% ± 33.70% and 56.38% ± 33.61%, respectively (P = 0.927). This rate of decline was similar to a report[33] wherein 22 episodes of HTG-AP had a calculated fall in serum TG of 69.8% within 48 h by conservative management. This finding is also similar to that reported in an HP case series, which demonstrated 49%-80% reductions in serum TG after a single session[34,35]. These reports showed no difference in the rate of TG decline between patients managed with or without HP. This study also showed that length of stay (13.04 ± 7.92 d vs 12.35 ± 6.40 d, P = 0.730) did not differ between the two treatments. A large multicenter retrospective study collected 1159 patients with SAP, which included 30 patients with HTG-AP, and 10 patients treated with PE compared with 20 patients treated with routine therapy; no additional reduction in TG levels and no improvement in clinical outcomes were detected[36].
Study strengths and limitations
The strengths of this study are the high accuracy of data due to the strict inclusion and exclusion criteria and the use of PSM to avoid test errors. At present, few studies have reported on how to choose the treatment mode of HTG-AP, which is the innovation of this study.
Our study has some important limitations. This study adopted a single-center retrospective design. The incidence of HTG-AP is not universal and can only reflect the situation of our hospital. Data such as body mass index, urine AMY, blood lipase, and blood gas analysis were missing. The choice of treatment had selection bias.
CONCLUSION
The incidence of HTG-AP exhibited a significant increase, remarkable severity, and recurrent trend. By understanding the characteristics of HTG-AP, we can improve the clinical diagnosis rate and identify patients who are likely to develop severe disease early. Patients with MAP and MSAP can be treated with INS safely and effectively without HP. This work provides a basis for doctors to choose an appropriate treatment plan for patients.
ARTICLE HIGHLIGHTS
Research background
The incidence of hypertriglyceridemic acute pancreatitis (HTG-AP) has increased yearly, but updated population-based estimates on the incidence of HTG-AP are lacking. Reducing serum triglyceride (TG) levels quickly is crucial in the early treatment of HTG-AP. Currently, there are many treatments to reduce TG levels, but there is still a lack of authoritative guidelines.
Research motivation
We wanted to explore appropriate treatments to block the progression of HTG-AP.
Research objectives
To explore the clinical characteristics to reduce the missed diagnosis rate of HTG-AP and to identify the patients who would develop severe acute pancreatitis early. To compare the clinical outcomes of intravenous insulin (INS) and hemoperfusion (HP) and guide the choice of treatment for patients.
Research methods
We retrospectively reviewed the incidence and clinical characteristics of 371 patients with HTG-AP in our hospital from the past 10 years. Then, 219 patients who met the inclusion and exclusion criteria were further screened and divided to different groups according to grades of severity of HTG-AP and treatments. Multivariate logistic regression analyses were used to identify the independent risk factors for severe HTG-AP. Propensity score matching was used to compare the clinical outcomes of INS and HP.
Research results
The incidence of HTG-AP increased by approximately 2.6 times during the 10 years and ranged from 8.4% to 22.3% (8.4% in April 2012–March 2013 and 22.3% in April 2020–March 2021). Multivariate logistic regression analysis confirmed that high C-reactive protein [P = 0.005, odds ratio (OR) = 1.011, 95%CI: 1.003-1.019], low calcium (P = 0.003, OR = 0.016, 95%CI: 0.001-0.239), and low albumin (P = 0.023, OR = 0.821, 95%CI: 0.693-0.973) were risk factors of severe HTG-AP. After propensity score matching with sex, age, grades of severity, and baseline TG, there was a significant decrease in serum TG in patients treated with INS (P < 0.0001) and HP (P = 0.0001) within 48 h. However, the clearance rate of TG and length of stay did not differ between the two groups.
Research conclusions
The incidence of HTG-AP exhibited a significant increase. Patients with mild and moderately severe acute pancreatitis can be treated with INS safely and effectively without HP.
Research perspectives
Identifying patients with a severe tendency at the early stage of HTG-AP and choosing cost-effective treatments is the future direction of this research.
Footnotes
Institutional review board statement: The Ethics Committee of Fujian Provincial Hospital approved the study (K2021-02-007).
Informed consent statement: Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 6, 2021
First decision: April 16, 2022
Article in press: June 30, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cho E, South Korea; Fujimori N, Japan; Gupta R, India; Kitamura K, Japan; Trna J, Czech Republic S-Editor: Zhang H L-Editor: Filipodia CL P-Editor: Zhang H
Contributor Information
Xue-Yan Lin, Department of Gastroenterology, Fujian Provincial Hospital, Fujian Medical University Provincial of Clinical Medicine, Fuzhou 350001, Fujian Province, China.
Yi Zeng, Department of Gastrointestinal Surgical Oncology, Fujian Cancer Hospital, Fujian Medical University Cancer Hospital, Fuzhou 350014, Fujian Province, China.
Zheng-Chao Zhang, Department of Emergency Surgery, Fujian Provincial Hospital, Fujian Medical University Provincial of Clinical Medicine, Fuzhou 350001, Fujian Province, China.
Zhi-Hui Lin, Department of Gastroenterology, Fujian Provincial Hospital, Fujian Medical University Provincial of Clinical Medicine, Fuzhou 350001, Fujian Province, China.
Lu-Chuan Chen, Department of Gastrointestinal Surgical Oncology, Fujian Cancer Hospital, Fujian Medical University Cancer Hospital, Fuzhou 350014, Fujian Province, China.
Zai-Sheng Ye, Department of Gastrointestinal Surgical Oncology, Fujian Cancer Hospital, Fujian Medical University Cancer Hospital, Fuzhou 350014, Fujian Province, China. flyingengel@sina.cn.
Data sharing statement
Participants gave informed consent for data sharing.
References
- 1.Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology. 2018;154:1096–1101. doi: 10.1053/j.gastro.2018.01.032. [DOI] [PubMed] [Google Scholar]
- 2.Johnson CD, Abu-Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut. 2004;53:1340–1344. doi: 10.1136/gut.2004.039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khatua B, El-Kurdi B, Singh VP. Obesity and pancreatitis. Curr Opin Gastroenterol. 2017;33:374–382. doi: 10.1097/MOG.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carr RA, Rejowski BJ, Cote GA, Pitt HA, Zyromski NJ. Systematic review of hypertriglyceridemia-induced acute pancreatitis: A more virulent etiology? Pancreatology. 2016;16:469–476. doi: 10.1016/j.pan.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Olesen SS, Harakow A, Krogh K, Drewes AM, Handberg A, Christensen PA. Hypertriglyceridemia is often under recognized as an aetiologic risk factor for acute pancreatitis: A population-based cohort study. Pancreatology. 2021;21:334–341. doi: 10.1016/j.pan.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Tsuang W, Navaneethan U, Ruiz L, Palascak JB, Gelrud A. Hypertriglyceridemic pancreatitis: presentation and management. Am J Gastroenterol. 2009;104:984–991. doi: 10.1038/ajg.2009.27. [DOI] [PubMed] [Google Scholar]
- 7.Ewald N, Hardt PD, Kloer HU. Severe hypertriglyceridemia and pancreatitis: presentation and management. Curr Opin Lipidol. 2009;20:497–504. doi: 10.1097/MOL.0b013e3283319a1d. [DOI] [PubMed] [Google Scholar]
- 8.Dominguez-Muñoz JE, Malfertheiner P, Ditschuneit HH, Blanco-Chavez J, Uhl W, Büchler M, Ditschuneit H. Hyperlipidemia in acute pancreatitis. Relationship with etiology, onset, and severity of the disease. Int J Pancreatol. 1991;10:261–267. [PubMed] [Google Scholar]
- 9.Guo YY, Li HX, Zhang Y, He WH. Hypertriglyceridemia-induced acute pancreatitis: progress on disease mechanisms and treatment modalities. Discov Med. 2019;27:101–109. [PubMed] [Google Scholar]
- 10.Li X, Ke L, Dong J, Ye B, Meng L, Mao W, Yang Q, Li W, Li J. Significantly different clinical features between hypertriglyceridemia and biliary acute pancreatitis: a retrospective study of 730 patients from a tertiary center. BMC Gastroenterol. 2018;18:89. doi: 10.1186/s12876-018-0821-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devlin JW, Lau AK, Tanios MA. Propofol-associated hypertriglyceridemia and pancreatitis in the intensive care unit: an analysis of frequency and risk factors. Pharmacotherapy. 2005;25:1348–1352. doi: 10.1592/phco.2005.25.10.1348. [DOI] [PubMed] [Google Scholar]
- 12.Durval A, Zamidei L, Bettocchi D, Luzzio MG, Consales G. Hyperlipidemic acute pancreatitis: a possible role of antiretroviral therapy with entecavir. Minerva Anestesiol. 2011;77:1018–1021. [PubMed] [Google Scholar]
- 13.Yadav D, Pitchumoni CS. Issues in hyperlipidemic pancreatitis. J Clin Gastroenterol. 2003;36:54–62. doi: 10.1097/00004836-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Yin G, Cang X, Yu G, Hu G, Ni J, Xiong J, Hu Y, Xing M, Chen C, Huang Y, Tang M, Zhao Y, Cheng G, Wan R, Wang S, Wang X. Different Clinical Presentations of Hyperlipidemic Acute Pancreatitis: A Retrospective Study. Pancreas. 2015;44:1105–1110. doi: 10.1097/MPA.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 15.Girman CJ, Kou TD, Cai B, Alexander CM, O'Neill EA, Williams-Herman DE, Katz L. Patients with type 2 diabetes mellitus have higher risk for acute pancreatitis compared with those without diabetes. Diabetes Obes Metab. 2010;12:766–771. doi: 10.1111/j.1463-1326.2010.01231.x. [DOI] [PubMed] [Google Scholar]
- 16.Bosques-Padilla FJ, Vázquez-Elizondo G, González-Santiago O, Del Follo-Martínez L, González OP, González-González JA, Maldonado-Garza HJ, Garza-González E. Hypertriglyceridemia-induced pancreatitis and risk of persistent systemic inflammatory response syndrome. Am J Med Sci. 2015;349:206–211. doi: 10.1097/MAJ.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 17.Vipperla K, Somerville C, Furlan A, Koutroumpakis E, Saul M, Chennat J, Rabinovitz M, Whitcomb DC, Slivka A, Papachristou GI, Yadav D. Clinical Profile and Natural Course in a Large Cohort of Patients With Hypertriglyceridemia and Pancreatitis. J Clin Gastroenterol. 2017;51:77–85. doi: 10.1097/MCG.0000000000000579. [DOI] [PubMed] [Google Scholar]
- 18.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Wang G, Qiu Z, He X, Liu C. Elevated Serum Triglycerides in the Prognostic Assessment of Acute Pancreatitis: A Systematic Review and Meta-Analysis of Observational Studies. J Clin Gastroenterol. 2017;51:586–593. doi: 10.1097/MCG.0000000000000846. [DOI] [PubMed] [Google Scholar]
- 20.Zhao K, Adam SZ, Keswani RN, Horowitz JM, Miller FH. Acute Pancreatitis: Revised Atlanta Classification and the Role of Cross-Sectional Imaging. AJR Am J Roentgenol. 2015;205:W32–W41. doi: 10.2214/AJR.14.14056. [DOI] [PubMed] [Google Scholar]
- 21.Fortson MR, Freedman SN, Webster PD 3rd. Clinical assessment of hyperlipidemic pancreatitis. Am J Gastroenterol. 1995;90:2134–2139. [PubMed] [Google Scholar]
- 22.Gullo L, Migliori M, Oláh A, Farkas G, Levy P, Arvanitakis C, Lankisch P, Beger H. Acute pancreatitis in five European countries: etiology and mortality. Pancreas. 2002;24:223–227. doi: 10.1097/00006676-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Chen CH, Dai CY, Hou NJ, Chen SC, Chuang WL, Yu ML. Etiology, severity and recurrence of acute pancreatitis in southern taiwan. J Formos Med Assoc. 2006;105:550–555. doi: 10.1016/S0929-6646(09)60149-2. [DOI] [PubMed] [Google Scholar]
- 24.Zheng Y, Zhou Z, Li H, Li J, Li A, Ma B, Zhang T, Liao Q, Ye Y, Zhang Z, Yang Y, Wang Z, Yang J, Li F. A multicenter study on etiology of acute pancreatitis in Beijing during 5 years. Pancreas. 2015;44:409–414. doi: 10.1097/MPA.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 25.Huang YX, Jia L, Jiang SM, Wang SB, Li MX, Yang BH. Incidence and clinical features of hyperlipidemic acute pancreatitis from Guangdong, China: a retrospective multicenter study. Pancreas. 2014;43:548–552. doi: 10.1097/MPA.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 26.Jin M, Bai X, Chen X, Zhang H, Lu B, Li Y, Lai Y, Qian J, Yang H. A 16-year trend of etiology in acute pancreatitis: The increasing proportion of hypertriglyceridemia-associated acute pancreatitis and its adverse effect on prognosis. J Clin Lipidol. 2019;13:947–953.e1. doi: 10.1016/j.jacl.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Sekimoto M, Takada T, Kawarada Y, Hirata K, Mayumi T, Yoshida M, Hirota M, Kimura Y, Takeda K, Isaji S, Koizumi M, Otsuki M, Matsuno S JPN. JPN Guidelines for the management of acute pancreatitis: epidemiology, etiology, natural history, and outcome predictors in acute pancreatitis. J Hepatobiliary Pancreat Surg. 2006;13:10–24. doi: 10.1007/s00534-005-1047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 2014;48:195–203. doi: 10.1097/01.mcg.0000436438.60145.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dervenis C. Assessments of severity and management of acute pancreatitis based on the Santorini Consensus Conference report. JOP. 2000;1:178–182. [PubMed] [Google Scholar]
- 30.Yu S, Wu D, Jin K, Yin L, Fu Y, Liu D, Zhang L, Yu X, Xu J. Low Serum Ionized Calcium, Elevated High-Sensitivity C-Reactive Protein, Neutrophil-Lymphocyte Ratio, and Body Mass Index (BMI) Are Risk Factors for Severe Acute Pancreatitis in Patients with Hypertriglyceridemia Pancreatitis. Med Sci Monit. 2019;25:6097–6103. doi: 10.12659/MSM.915526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Huang Y, Yu H, Pan K, Zhang Z, Man Y, Hu D. The association of parameters of body composition and laboratory markers with the severity of hypertriglyceridemia-induced pancreatitis. Lipids Health Dis. 2021;20:9. doi: 10.1186/s12944-021-01443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christian JB, Arondekar B, Buysman EK, Johnson SL, Seeger JD, Jacobson TA. Clinical and economic benefits observed when follow-up triglyceride levels are less than 500 mg/dL in patients with severe hypertriglyceridemia. J Clin Lipidol. 2012;6:450–461. doi: 10.1016/j.jacl.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Berberich AJ, Ziada A, Zou GY, Hegele RA. Conservative management in hypertriglyceridemia-associated pancreatitis. J Intern Med. 2019;286:644–650. doi: 10.1111/joim.12925. [DOI] [PubMed] [Google Scholar]
- 34.Galán Carrillo I, Demelo-Rodriguez P, Rodríguez Ferrero ML, Anaya F. Double filtration plasmapheresis in the treatment of pancreatitis due to severe hypertriglyceridemia. J Clin Lipidol. 2015;9:698–702. doi: 10.1016/j.jacl.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Valdivielso P, Ramírez-Bueno A, Ewald N. Current knowledge of hypertriglyceridemic pancreatitis. Eur J Intern Med. 2014;25:689–694. doi: 10.1016/j.ejim.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Miyamoto K, Horibe M, Sanui M, Sasaki M, Sugiyama D, Kato S, Yamashita T, Goto T, Iwasaki E, Shirai K, Oe K, Sawano H, Oda T, Yasuda H, Ogura Y, Hirose K, Kitamura K, Chiba N, Ozaki T, Oshima T, Yamamoto T, Nagata K, Mine T, Saito K, Sekino M, Furuya T, Matsuda N, Hayakawa M, Kanai T, Mayumi T. Plasmapheresis therapy has no triglyceride-lowering effect in patients with hypertriglyceridemic pancreatitis. Intensive Care Med. 2017;43:949–951. doi: 10.1007/s00134-017-4722-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Participants gave informed consent for data sharing.