Abstract
BACKGROUND
Chronic abdominal pain is the most common cause for gastroenterology consultation and is frequently associated with functional gastrointestinal disorders including irritable bowel syndrome and inflammatory bowel disease. These disorders present similar brain/gut/microbiota trialogue alterations, associated with abnormal intestinal permeability, intestinal dysbiosis and colonic hypersensitivity (CHS). Intestinal dysbiosis can alter colon homeostasis leading to abnormal activation of the innate immunity that promotes CHS, perhaps involving the toll-like receptors (TLRs), which play a central role in innate immunity.
AIM
To understand the mechanisms between early life event paradigm on intestinal permeability, fecal microbiota composition and CHS development in mice with TLRs expression in colonocytes.
METHODS
Maternal separation model (NMS) CHS model, which mimics deleterious events in childhood that can induce a wide range of chronic disorders during adulthood were used. Colonic sensitivity of NMS mice was evaluated by colorectal distension (CRD) coupled with intracolonic pressure variation (IPV) measurement. Fecal microbiota composition was analyzed by 16S rRNA sequencing from weaning to CRD periods. TLR mRNA expression was evaluated in colonocytes. Additionally, the effect of acute intrarectal instillation of the TLR5 agonist flagellin (FliC) on CHS in adult naive wildtype mice was analyzed.
RESULTS
Around 50% of NMS mice exhibited increased intestinal permeability and CHS associated with intestinal dysbiosis, characterized by a significant decrease of species richness, an alteration of the core fecal microbiota and a specific increased relative abundance of flagellated bacteria. Only TLR5 mRNA expression was increased in colonocytes of NMS mice with CHS. Acute intrarectal instillation of FliC induced transient increase of IPV, reflecting transient CHS appearance.
CONCLUSION
Altogether, these data suggest a pathophysiological continuum between intestinal dysbiosis and CHS, with a role for TLR5.
Keywords: Chronic abdominal pain, Colonic hypersensitivity, Toll-like receptors, Intestinal microbiota, Early life events
Core Tip: Neonatal maternal separation (NMS) model mimic deleterious events in childhood, which can induce a wide range of chronic disorders during adulthood. Herein, around 50% of NMS mice exhibited increased intestinal permeability and colonic hypersensitivity (CHS) associated with intestinal dysbiosis. Only toll-like receptor 5 (TLR5) mRNA expression was increased in colonocytes of NMS mice with CHS and acute intrarectal instillation of flagellin transiently increased intracolonic pressure variations, reflecting transient CHS appearance. Together, those findings suggest a pathophysiological continuum between intestinal permeability, intestinal dysbiosis and CHS, with a previously undescribed role for TLR5 in CHS.
INTRODUCTION
Irritable bowel syndrome (IBS) is one of the major chronic gastrointestinal disorders, strongly related to stress. It is characterized by abdominal pain, changes in bowel habits and increased intestinal permeability without macroscopic organic alterations. Such changes has been hypothesized to trigger impairment of life’s quality and the development of comorbidities such as anxiety and depression[1]. A worldwide prevalence of 3%-5% has been reported and today, efficient pharmacological treatments are limited to relieve symptoms[2]. Colonic hypersensitivity (CHS), frequently associated with abdominal pain, has been described as the main cause of medical consultation in IBS patients with a prevalence ranging from 33% to 90%[3]. This symptom is defined by an altered sensation in response to colorectal stimuli and is clinically revealed by enhanced perception of mechanical triggers applied to the bowel. The common hypothesis is that CHS may result from colonic homeostasis changes and/or alterations of the brain-gut connection. In fact, the brain-gut axis has been shown to be impacted by inflammation and immunological factors, psychological factors, dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, abnormal activation of the vagus nerve and the enteric nervous system and intestinal dysbiosis[4].
Qualitative and/or quantitative alterations of the intestinal microbiota has been characterized in most of the functional gastrointestinal disorders including IBS[5]. Despite the numerous studies carried out, data on specific bacterial groups altered in IBS patients are still inconclusive. However, Enterobacteriaceae family, Lactobacillaceae family, and Bacteroides genus seem to be increased in patients with IBS compared with controls, whereas uncultured Clostridiales I, Faecalibacterium and Bifidobacterium genus were decreased in IBS patients[6]. Furthermore, it has been described that some IBS patients with chronic abdominal pain present specific intestinal microbiota dysbiosis, allowing considerations of the gut microbiota as a potential therapeutic target[7].
In healthy conditions, the interaction between gut microbiota and pattern recognition receptors (PRRs), especially local toll-like receptors (TLRs), allow maintenance of intestinal barrier in a homeostatic state. Indeed, TLRs, mostly present on the membrane of immune and epithelial cells, identify pathogen-associated molecular patterns (PAMPs) and induce intracellular signaling cascade resulting in the production of cytokines and chemokines important for colonic homeostasis. The mammalian TLRs family consists of 13 members (TLR1-10 in humans, TLR1-9 and TLR11-13 in mice) and each TLR responds to distinct PAMPs leading to the activation of specific signaling pathway. For example, TLR4 recognizes lipopolysaccharide (LPS) and TLR5, which is expressed in the basolateral membrane of the intestinal epithelium, detects flagellin (FliC)[8]. In a dysbiotic state, alterations in the signature of microbial molecules sensed by the host can lead to abnormal activation state of the immune system and induce a low-grade intestinal inflammation[9].
The breakdown of the symbiotic relationship between TLRs and gut microbiota could contribute to the development of various multifactorial intestinal diseases, such as IBS. Previous studies have reported modifications of TLRs expression and activation in intestinal biopsies of IBS patients[10-15]. Furthermore, a preclinical study assessed the effect of neonatal maternal separation (NMS) in rats on TLRs expression, showing an upregulation of TLRs in colonic mucosa[16]. In this context, because of correlation between IBS and early life adverse events[17], this study investigated the impact of NMS paradigm on intestinal permeability, fecal microbiota composition and CHS development in mice as well as the association with TLRs expression.
MATERIALS AND METHODS
Animals
Seven-week-old wild type C57Bl/6J males and females mice were purchased from Janvier Laboratories (Le Genest Saint Isle, France). They were mated to obtain male pups for the NMS protocol. After birth, wild-type C57Bl/AJ pups were isolated from their mother from post-natal days P2 to P14, three hours per day (from 9:00 a.m. to 12:00 p.m.). These mice were named NMS mice. Pups were then left with their mothers up to weaning (P21) (Figure 1A). Control wild-type C57Bl/AJ pups were co-housed in the animal facility and were called non-handled (NH) mice. In addition, ten-week-old wild type C57Bl/6J males were purchased from Janvier Laboratories and used for FliC intrarectal instillation experiment. Animals were given access to food and water ad libitum and housed with a 12 h light-dark cycle. All experiments were performed on twelve-week-old male mice and were performed according to the ethical guidelines set out by the International Association for the Study of Pain (IASP), complied with the European Union regulation and were approved by ethics committees: The local committees C2EA-02 of Clermont-Ferrand (approvals CE110-12 and CE111-12).
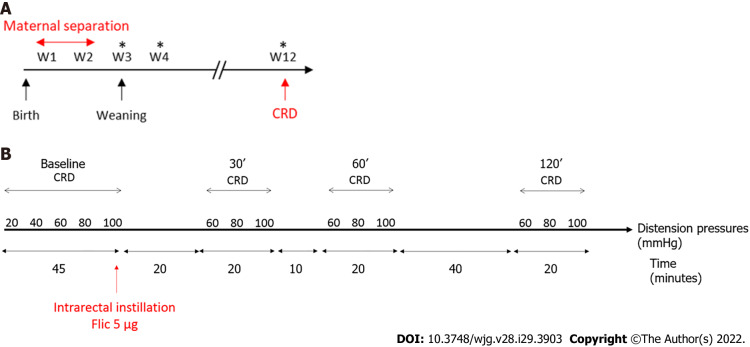
Figure 1.
Time course protocols used in this study. A: Time course protocol for neonatal maternal separation experiment; B: Time course protocol for flagellin intrarectal instillation experiment. *Feces sample collection for Next Generation Sequencing; CRD: Colorectal distention test.
Colorectal distension test
Colorectal distension test (CRD) was performed using the non-invasive manometric method described[18]. A miniaturized pressure transducer catheter (Mikro-Tip SPR-254; Millar Instruments, Houston, TX, United States) equipped with a custom-made balloon (length: 1.5 cm) prepared from a polyethylene plastic bag which avoid any colonic compliance effect. On the day of the experiment, the mice were accustomed to the holding device for 1 h before the CRD. Then, under mild anesthetic (2.5% isoflurane), the balloon was inserted into the rectum such that the distal end of the balloon was 5 mm from the anal margin. Subsequently, the animals were placed in the holding device and allowed to recover for 30 min prior to CRD. The balloon was connected to an electronic barostat (Distender Series II, G&J Electronics, Toronto, Canada) and a preamplifier (PCU-2000 Dual Channel Pressure Control Unit, Millar Instruments, Houston, TX, United States) connected to the PowerLab interface (AD Instruments, Dunedin, New Zealand). The barostat enabled the control of the balloon pressure. The distension protocol consisted of a set of increasing distension pressures (20, 40, 60, 80 and 100 mmHg), each of which was repeated twice, which was applied for 20 s with a 4 min inter-pressure interval. The signal was acquired and analyzed using LabChart 7 software (ADInstruments, Dunedin, New Zealand). After intracolonic pressure recording for each animals along the CRD protocols and signal treatment as previously described[18], intracolonic pressure variation (IPV), reflecting the colonic sensitivity, was calculated as previously described[19] for each distension pressure. Briefly, IPV was calculated by subtracting the integral (area under the curve) of the treated signal corresponding to the 20 s preceding the CRD from the integral (area under the curve) of the treated signal during the 20 s of CRD stimulation. Therefore, two groups of NMS mice were defined: NMS non-sensitized (NMS NS) and NMS sensitized (NMS S) mice. The NMS S animals are distinguished according to the area under the curve (AUC) value in response to the distention pressures from 60 to 100 mmHg during CRD procedure[20]. Briefly, if this value is higher than the average AUC of the NH control animals plus twice the SEM value (AUCNMS S ≥ AUCNH + 2 × SEMNH), this mouse is considered as hypersensitive and are placed in the NMS S group. Others are considered as NMS NS. For FliC intrarectal instillation experiment, the distension protocol was the same before intrarectal instillation and, only a set of distension pressure 60, 80 and 100 mmHg was used 30 min, 60 min and 120 min after intrarectal instillation.
In vivo intestinal permeability
In vivo intestinal permeability was assessed using fluorescein dextran (FITC- dextran 3000-5000 Da, TdB Consultancy AB, Uppsala, Sweden) as previously described[21]. Briefly, before CRD, NMS and NH mice were orally gavaged with 0.6 g/g body weight of FITC-dextran and blood samples were obtained from the retro-orbital venous plexus 3 h after this administration. Plasma FITC levels were determined by fluorometry at 488 nm using a microplate reader (Tecan, Lyon, France).
Fecal pellets collection, DNA extraction and microbiota sequencing
Fecal pellets were collected from mice at week 3, 4 and 12 and stored at -80 °C prior to DNA extraction. Bacterial DNA was extracted from fecal bacteria following the protocol of NucleoSpin® Soil kit (Macherey-Nagel, Düren, Germany). DNA concentrations and purity were then assessed using Take3 micro-volume plate and Epoch Microplate Spectrophotometer (BioTek, Winooski, VT, United States). The 16S rRNA gene V4 variable region polymerase chain reaction (PCR) primers 515/806 with barcode on the forward primer were used in a 30 cycles PCR using the HotStarTaq Plus Master Mix Kit (Qiagen®, Germantown, MD, United States). Next generation sequencing (NGS) was performed at Molecular Research DNA (MR DNA - Shallowater, TX, United States) on a MiSeq following the manufacturer’s guidelines. Sequences data analysis was performed using the quantitative insights into microbial ecology pipeline (QIIME)[22]. The analysis was carried out on the core microbiota i.e. the operational taxonomic units (OTUs) present in the fecal microbiota of 90% of the mice.
FliC intrarectal instillation
FliC from wildtype Salmonella enterica serovar typhimurium (SL3201, fljB−) was provided by Pr. A. Gewirtz (Center for Inflammation, Georgia State University, Atlanta, GA, United States). Briefly, FliC was purified through sequential cation- and anion-exchange chromatography and purity was verified as described previously[8]. Intrarectal instillation was performed under mild anesthetic (2.5% isoflurane) using orogastric feeding tube and inserted 2.5 cm up the colon (Figure 1B). At this point, 50 μL of FliC diluted in PBS, corresponding to 5 µg was slowly administered over 30 s while pressure was applied to the anal area to prevent leakage. Following the injection of the solution, the tube was slowly removed and the rectal pressure was maintained for a further 30 s.
Colonocytes extraction
Following mice euthanasia, fragments of colon (3-4 cm) were flushed and opened longitudinally along the mesentery and homogenized in cold PBS to remove feces. Then, these fragments were incubated into HBSS containing EDTA solution (2 mmol/L) 30 min at 37 °C with strong agitation every 10 min. After HBSS/EDTA incubation, colons were removed and samples were centrifuged at 2000 g for 10 min. Then, HBSS/EDTA was removed and colonocytes were deep-frozen in liquid nitrogen and stored at -80 °C for further analysis.
RNA extraction, reverse transcription and quantitative PCR
Total RNA from mice colonocytes was extracted using the RNeasy Plus Mini Kit (Qiagen®, Germantown, MD, United States) according to the manufacturer's protocol. After RNA extraction, reverse transcription was performed with the High Capacity cDNA RT Kit (Applied Biosystems, Foster City, CA, United States) with 500 ng of RNA, followed by a qPCR using LightCycler FastStart DNA Master SYBR Green Kit (Roche Applied Science, Penzberg, Germany). The primers used for TLRs expression analysis are described in Table 1. All results were normalized to the HPRT gene. Samples were tested in duplicate, and the average values were used for quantification by using 2-ΔΔCt method.
Table 1.
Primers used for toll-like receptors expression analysis
|
Gene
|
5’-3’ Forward
|
5’-3’ Reverse
|
| tlr2 | ACCAAGATCCAGAAGAGCCA | CATCACCGGTCAGAAAACAA |
| tlr3 | GCGTTGCGAAGTGAAGAACT | TTCAAGAGGAGGGCGAATAA |
| tlr4 | TTCAGAACTTCAGTGGCTGG | TGTTAGTCCAGAGAAACTTCCTG |
| tlr5 | GCAGGATCATGGCATGTCAAC | ATCTGGGTGAGGTTACAGCCT |
| tlr9 | AACCGCCACTTCTATAACCAG | GTAAGACAGAGCAAGGCAGG |
| hprt | TTGCTGACCTGCTGGATTA | AGTTGAGAGATCATGTCCAC |
Fecal FliC and LPS load quantification
FliC and LPS were quantified using human embryonic kidney (HEK)-Blue-mTLR5 and HEK-Blue-mTLR4 cells, respectively (Invivogen, San Diego, California, United States). Fecal material was resuspended in PBS to a final concentration of 100 mg/mL and homogenized for 10 s using a Mini-Beadbeater-24 without the addition of beads to avoid bacteria disruption. The samples were then centrifuged at 8000 g for 2 min and the resulting supernatant was serially diluted and applied to mammalian cells. Purified Escherichia coli FliC and LPS (Sigma, St Louis, Missouri, United States) were used for standard curve determination using HEK-Blue-mTLR5 and HEK-Blue-mTLR4 cells, respectively. After 24 h of stimulation, cell culture supernatant was applied to QUANTI-Blue medium (Invivogen, San Diego, California, United States) and alkaline phosphatase activity was measured at 620 nm after 30 min.
Statistical analysis
Statistical analyses were performed with Prism 7 software (GraphPad, La Jolla, CA, United States). The Kolmogorov-Smirnov test has been used to check if data follow a normal distribution. One-way ANOVA, Kruskal-Wallis test or two-way ANOVA (more than two groups) were used for intergroup-comparisons with Tukey’s, Dunn’s and Dunnett’s test for the post-hoc analysis. Correlation was assessed using Pearson’s test. ANOSIM method followed by Monte-Carlo permutation test was performed to assess the significativity of beta-diversity analysis of fecal microbiota using the QIIME. A P value ≤ 0.05 was considered statistically significant.
RESULTS
NMS paradigm induces CHS and intestinal permeability increase in a subset of mice
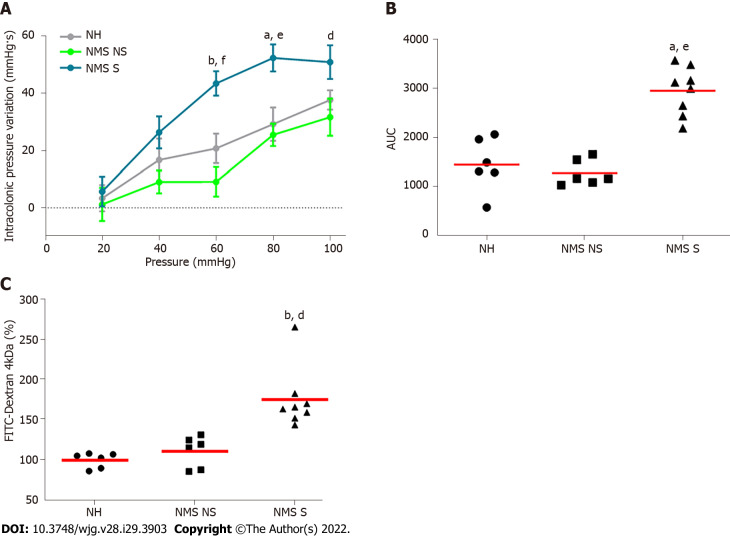
In order to evaluate colonic sensitivity, a CRD test was performed on twelve-week-old NH or NMS mice (Figure 1A). As previously described[23], among NMS mice only a subset developed CHS in comparison to NH mice Therefore, two groups of NMS mice were defined: NMS NS and NMS S mice. In fact, colorectal distension assessment revealed significant increase of IPV for the highest distension pressures 60, 80 and 100 mmHg in the NMS S group in comparison to NMS NS and NH groups (Figure 2A). Analysis of the areas under the curve (AUC) for each mouse confirmed this significant difference between NH, NMS NS and NMS S groups (Figure 2B). Intestinal permeability assessment revealed significant increase of FITC-Dextran plasma levels in the NMS S group compared to NH and NMS NS groups (Figure 2C).
Figure 2.
Neonatal maternal separation induces colonic hypersensitivity and increases intestinal permeability in mice. A: Intracolonic pressure variation (IPV) in response to colorectal distension in non-handled (NH), neonatal maternal separated non-sensitized (NMS NS) and neonatal maternal separated sensitized (NMS S) mice; B: Area under the curve (AUC) of the IPV relative to colorectal distension for each NH, NMS NS and NMS S mouse; C: FITC-dextran 4 kDa plasmatic concentrations, 3 h after oral gavage with 15 mg of FITC-dextran of NH, NMS NS and NMS S mice. Values are expressed as a percentage of FITC-dextran per mL of plasma in comparison to the NH group mean. NH: n = 6; NMS NS: n = 6; NMS S: n = 8. aP < 0.05 and bP < 0.01 vs NH group; and dP < 0.05, eP < 0.01 and fP < 0.001 vs NMS NS group. For IPV to CRD test, dots represent means and error bars represent SEM. For AUC and FITC-dextran, each dot represents one mouse and red lines represent means.
Fecal microbiota dysbiosis is associated with CHS in neonatal maternal separated mice
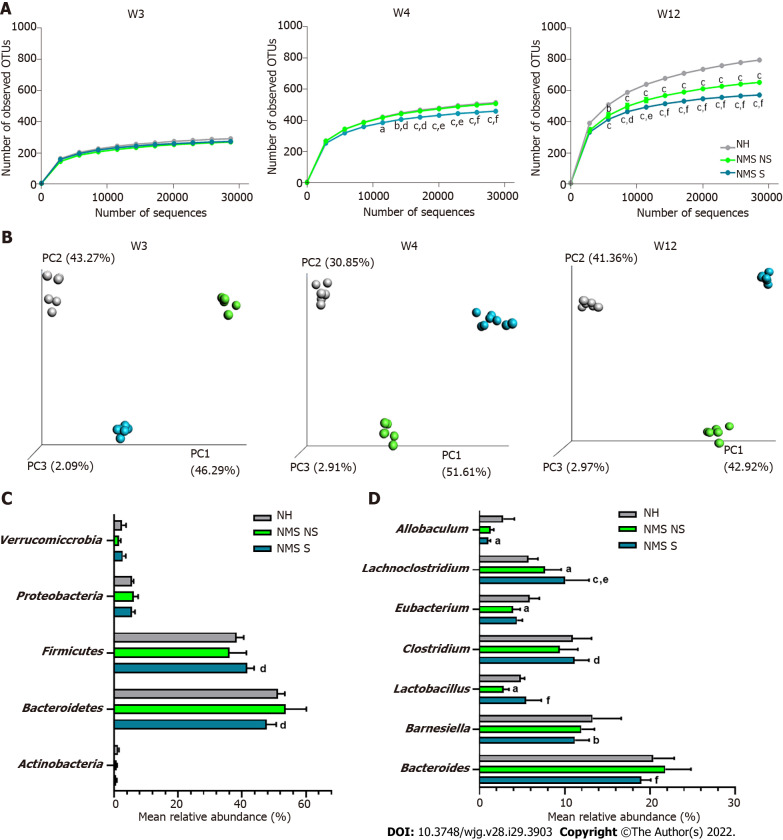
Illumina sequencing of the 16S rRNA gene was performed on fecal pellets DNA extracts from NH, NMS NS and NMS S mice at W3, W4 and W12 (just before the CRD test) according to the time course protocol for NMS experiment (Figure 1A). Alpha-diversity analysis (number of observed OTUs) of the core fecal microbiota revealed no statistical difference between NH, NMS NS and NMS S animals at week 3, before weaning (Figure 3A-left panel). However, a significant decrease of species richness appeared at W4 in NMS S mice in comparison to NH or NMS NS animals and persisted at adulthood (W12, time point of CRD test), even if NMS NS and NMS S mice were co-housed in the same cage during all the experiment (Figure 3A-middle and right panels). In addition, a significant decrease of the observed OTUs number was present in NMS NS at adulthood (W12) in comparison to NH mice. Principal coordinates analysis based on unweighted UniFrac distances confirmed the alteration of the core fecal microbiota. It enabled to significantly (ANOSIM method followed by the Monte-Carlo permutation test, P < 0.05) identify the three animals’ groups from W3 to W12 (Figure 3B). The taxonomic analysis of the fecal core microbiota composition in the NMS S group revealed in twelve weeks old mice a decreased relative abundance of bacteria belonging to the phylum Bacteroidetes and an increase in Firmicutes in comparison to the NMS NS group (Figure 3C). At lower taxonomic levels, NMS S mice were characterized by a decreased abundance of bacteria from the genera Allobaculum and Barnesiella compared to control NH mice, and a decreased abundance of bacteria from the genera Bacteroides compared to NMS NS mice. The relative abundances of Lachnoclostridium, Clostridium and Lactobacillus were increased in these NMS animals with CHS in comparison to NMS mice without CHS. Surprisingly, the relative abundance of Lactobacillus was decreased in NMS NS animals compared to NH group (Figure 3D).
Figure 3.
Neonatal maternal separation paradigm induces alterations of core fecal microbiota related to colonic hypersensitivity. A: Alpha-diversity analysis of the core microbiota. Number of observed operational taxonomic units according to the number of sequences per samples of fecal samples from non-handled (NH), neonatal maternal separated non-sensitized (NMS NS) and neonatal maternal separated sensitized (NMS S) at week 3 (W3), week 4 (W4) and week 12 (W12); B: Beta-diversity analysis of the core microbiota. Principal coordinates analysis (PCoA) of unweighted UniFrac distances of NH, NMS NS and NMS S mice at W3, W4 and W12; C and D: Mean relative abundances of bacterial phyla (C) and genera (D) significantly altered by the NMS paradigm between NH, NMS NS and NMS S mice at W12. NH: n = 6; NMS NS: n = 6; NMS S: n = 8. aP < 0.05, bP < 0.01 and cP < 0.001 vs NH or dP < 0.05, eP < 0.01 and fP < 0.001 vs NMS NS groups respectively. For alpha-diversity analysis, dots represent means and error bars represent SEM. For PCoA analysis each dot represents one mouse.
CHS induced by NMS exposure increased fecal level of FliC and is related to TLR5 overexpression in colonocytes
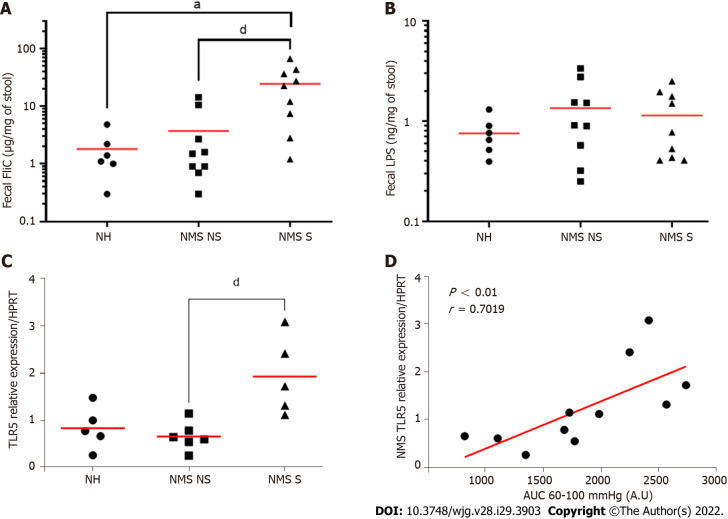
To understand potential mechanisms between fecal microbiota dysbiosis and CHS induced by NMS, quantification of two different PAMPs, FliC and LPS, was performed in feces from twelve-week-old NH, NM NS and NMS S mice. Exposure to NMS paradigm increased significantly fecal level of FliC (Figure 4A) rather than fecal LPS which is not significant better between different animal (Figure 4B).
Figure 4.
Neonatal maternal separation induced colonic hypersensitivity is associated with increased flagellin fecal content and colonocytes toll-like receptor 5 expression. A: Levels of fecal flagellin (FliC) assayed with toll-like receptor 5 (TLR5) reporter cells; B: Levels of fecal lipopolysaccharide assayed with TLR4 reporter cells; C: Colonocytes mRNA expression of TLR5 in non-handled (NH), neonatal maternal separated non-sensitized (NMS NS) and neonatal maternal separated sensitized (NMS S) mice at week 12. Values are expressed as relative expression of TLR5 mRNA compared to HPRT expression; D: Correlation between NMS colonocytes TLR5 expression and area under the curve (AUC) corresponding of the intracolonic pressure variation (IPV) for highest colorectal distension pressures (60, 80 and 100 mmHg). A and B: NH: n = 6; NMS NS: n = 9; NMS S: n = 9. aP < 0.05 vs NH group; and dP < 0.05 vs NMS NS group. C and D: NH: n = 5; NMS NS: n = 6; NMS S: n = 5. dP < 0.05 vs NMS NS. For FliC quantification TLR5 mRNA relative expression, each dot represents one mouse and red lines represent means and for correlation between TLR5 expression and AUC of IPV, each dot represents one mouse and red line represents the linear regression curve.
As TLRs are the main receptors of PAMPs, the TLRs mRNA expression in colonocytes from NH, NMS NS and NMS S mice was quantified in adult (W12) mice. As previously described, three mouse groups were defined, based on the CHS (Supplementary Figure 1A and B). In those mouse groups, the TLR2, 3, 4 and 9 mRNA were not modified between NH, NMS NS and NMS S animals (Supplementary Figure 1C), whereas TLR5 mRNA expression is significantly increased only in NMS S subgroup (NH: 0.836 ± 0.200, NMS NS: 0.662 ± 0.120, NMS S: 1.925 ± 0.363, P < 0.05 vs NMS NS) (Figure 4C). AUC corresponding to the IPV for highest colorectal distension pressures (60, 80 and 100 mmHg) significantly correlated with the mRNA expression level of TLR5 in colonocytes of NMS mice (P < 0.01) (Figure 4D).
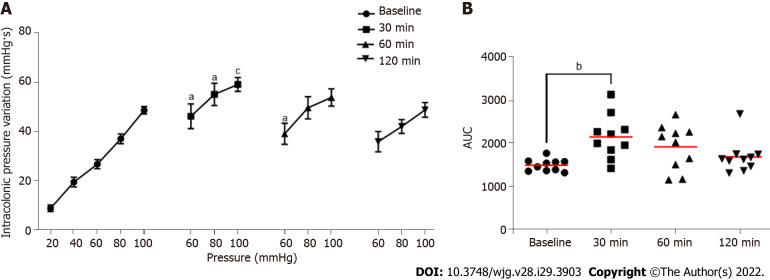
FliC intrarectal instillation is associated with a transient increase of colonic sensitivity
Intrarectal instillation of FliC, agonist of the receptor TLR5, significantly increased IPV at the 60, 80 and 100 mmHg distension pressure 30 min and 60 min post-instillation (Figure 5A). The increase in the response to CRD test was transient and did not persist 120 min after FliC instillation. AUC confirmed this significant increase of IPV 30 min after intrarectal instillation of FliC (Figure 5B).
Figure 5.
Evaluation of the impact of intrarectal instillation of flagellin on colonic sensitivity. A: Intracolonic pressure variation (IPV) in response to colorectal distension in males mice before (Baseline) and after (30, 60 and 120 min) intrarectal instillation of flagellin (5 µg); B: Area under the curve (AUC) of the IPV relative to highest colorectal distension pressures (60, 80 and 100 mmHg). For each mouse and each time point, n = 10. aP < 0.05, bP < 0.01 and cP < 0.001 respect to Baseline. For IPV to colorectal distension test, dots represent means and error bars represent SEM. For AUC, each dot represents one mouse and red lines represent means.
DISCUSSION
Abdominal pain, frequently associated with CHS, has been shown to be a common feature of IBS patients. It also strongly impacts on patient’s quality of life, leading to an important rate of consultation in Gastroenterology[24]. According to clinical studies, 33% to 90% of IBS patients exhibit CHS[3,25]. IBS presents a poorly first line treatment efficacy, especially regarding the treatment of abdominal pain[26]. Thus, in accordance with the aim of our study, a better characterization of mechanisms associated with CHS is important for the establishment of new potential pharmacological targets.
The etiology of this condition, resulting in various symptoms, remains unclear even if biological, psychological and social factors seem to be involved. Indeed, several studies reported an increased risk of IBS associated with early adverse life events[17,27-29]. These events refer to traumatic experience during childhood such as physical, sexual or emotional abuse as well as discordant relationship with primary caretaker. Using NMS stress animal model[30], our study demonstrates the impact of early adverse life events on colonic sensitivity of adult mice. Interestingly, only a subset of NMS mice presented CHS, revealed by CRD test, compared to control non-handled mice. These results were consistent with data obtained in previous studies carried out in both rats and mice[23,31,32].
Many studies reported an association between activation of the HPA axis, the major neuroendocrine system regulating various bodily processes in response to psychological or physical stressors, and intestinal permeability increase[33,34]. Furthermore, alteration of the intestinal barrier is a key clinical feature of IBS and it has been related to CHS[35]. In our study, assessment of intestinal permeability was carried out by measurement of FITC-dextran plasma level. Only NMS animals with CHS exhibited high plasmatic levels of FITC-dextran, suggesting that NMS paradigm induced CHS is associated with altered intestinal barrier. This result is in accordance with previous reports showing increased intestinal permeability following NMS paradigm or chronic stress exposure[34,36,37]. In addition, the link between the weakness of the intestinal mucosa barrier and CHS has been demonstrated in a mouse model of post-infectious IBS[20].
Consistent studies reported intestinal dysbiosis in IBS patients[6]. The main distinguishing feature of IBS patients compared to heathy volunteers is on one hand the increased abundance of bacteria belonging to the Firmicutes phylum and on the other hand, the decreases abundance of bacteria belongs to the Bacteroidetes phylum. Implication of the intestinal microbiota in CHS and associated chronic abdominal pain was also suggested[38]. In the present study, the characterization of the fecal microbiota composition using high-throughput sequencing of the 16S rRNA revealed the presence of a dysbiotic state making it possible to discriminate NH, NMS NS and NMS S mice. Indeed, the beta-diversity analyses showed that the composition of the fecal microbiota is different between NMS and NH control mice but also between NMS NS and NMS S mice while these animals came from the same litters and were co-housed. Changes in intestinal microbiota composition associated with NMS and CHS appeared very early, before weaning the animals (week 3) and persisted over time up to 12 wk. These alterations in the fecal microbiota composition were also characterized by a decreased bacterial richness in NMS S mice from week 4 to week 12. In general, this decrease was associated with a physiological disorder in the host, which seemed to be in agreement with the results obtained in this model[39]. A reduction in the bacterial diversity of the intestinal microbiota has notably been demonstrated in IBD and IBS patients but also in stress animal models[40-44]. Clostridium and Lachnoclostridium, flagellated bacteria, are among the genera whose abundance was increased in NMS S mice, at W12, the time of colonic sensitivity assessment, compared to NMS mice without CHS. Studies carried out in animals subjected to stress during the neonatal period have also shown an increase in the relative abundance of the Clostridium genus[43,45,46]. Furthermore and interestingly, Luna et al[47] highlighted an increased relative abundance of different species of Clostridium and Lachnoclostridium within the mucosa-associated microbiota in children with an autistic disorder associated with functional gastrointestinal disorders and in particular abdominal pain. These findings suggest an implication of the intestinal microbiota in the development of CHS in the NMS model.
In a dysbiotic state, particularly associated with an increase in intestinal permeability, alterations in the signature of microbial molecules sensed by the host can lead to a different activation state of the immune system[9]. Indeed, PAMPs, such as LPS or FliC, are sensed by PRRs including TLRs, which are expressed on the host cell surface or in the cytosolic compartment of numerous cell types. In this context, the aim of our study was to characterize the expression of different TLRs in colonocytes from our different animal subgroups after NMS paradigm. It is important to note that NMS paradigm is not associated with a modification of the intestinal inflammation status[23,36]. An increased TLR5 expression was observed only in animals presenting CHS after NMS paradigm, moreover, correlation between gene expression of TLR5 and AUC from 60 to 100 mmHg (corresponding to nociceptive stimulation) in NMS mice. These findings are in line with some reports showing upregulation of TLRs in IBS patient’s colonic biopsies[10-12,15]. An increased expression of some TLRs was also observed in NMS model but without association with visceral pain[11]. Few publications have indicated TLRs implication in animal pain model, especially inflammatory and neuropathic pain[48,49]. In visceral pain context, Tramullas et al[50] in 2014 demonstrated involvement of TLR4 in visceral sensitivity in a chronic stress model. Furthermore, Luczynski et al[51] demonstrated increased colonic sensitivity to colorectal distention in germ free mice, associated with an increase of TLRs expression in spinal cord. Finally, in 2018, a study published by Zhou et al[52] established TLR4 implication in inflammatory visceral pain in animals with high-fat diet. Following the demonstration of FliC increase in NMS S mice fecal content and the upregulation of TLR5 expression in the NMS S mouse colonocytes, the effect of FliC was assessed on visceral sensitivity in naïve animals. We highlighted a transient increase of colonic sensitivity between 30 min and 60 min after FliC intra-rectal instillation. These results are the first to demonstrate potential FliC and TLR5 involvement in CHS in a non-inflammatory IBS-like animal model. Indeed, only Das et al[53] have shown that TLR5 signaling mediates hypersensitivity in a model of allodynia and that sensitivity was reversed by blocking TLR5 with a specific antagonist. Moreover, Dlugosz et al[54] has found a significantly higher serum level of antibodies to FliC patients with IBS. Our data, associated with the results of previous studies suggest that TLR5, through its activation by FliC, could play a key role in CHS induced by dysbiosis related to the NMS paradigm and more generally, in the pathophysiology of IBS.
CONCLUSION
In conclusion, our results demonstrated the association of fecal dysbiosis, characterized especially by an increased abundance of flagellated bacteria, with impaired intestinal permeability, increased TLR5 expression and induced CHS. Taken together, TLR5 signaling upon recognition of FliC is relevant in visceral pain through both direct and indirect mechanisms, and application of TLR5-specific antagonists could potentially reversed CHS in non-inflammatory visceral pain context[23,36].
ARTICLE HIGHLIGHTS
Research background
Chronic abdominal pain associated to irritable bowel syndrome (IBS) is strongly related to stress and is the most common cause for gastroenterology consultation. Brain/gut/microbiota trialogue alterations are suspected to be involved in colonic hypersensitivity (CHS), responsible for chronic abdominal pain. It is also associated with abnormal intestinal permeability and intestinal dysbiosis, which can alter colon homeostasis leading to abnormal activation of the innate immunity that promotes CHS, perhaps involving the toll-like receptors (TLRs), which play a central role in innate immunity.
Research motivation
The breakdown of the relationship between TLRs and gut microbiota could contribute to the development of IBS. Thus, because of correlation between IBS and early life adverse events, our study investigated the impact of neonatal maternal separation (NMS) paradigm on intestinal homeostasis, fecal microbiota composition and CHS development in mice as well as the association with TLRs expression.
Research objectives
A better characterization of mechanisms associated with CHS is important for the establishment of new potential pharmacological targets.
Research methods
In our study, we used a referenced CHS animal model, the NMS paradigm, which mimics deleterious events in childhood that can induce a wide range of chronic disorders during adulthood. In addition, we have evaluated colonic sensitivity of NMS mice by colorectal distension (CRD) coupled with intracolonic pressure variation measurement. Fecal microbiota composition was analyzed by 16S rRNA sequencing from weaning to CRD periods. TLR mRNA expression was evaluated in colonocytes.
Research results
This study, based on the preclinical mouse model of NMS, demonstrated that around 50% of NMS mice exhibited increased intestinal permeability and CHS associated with intestinal dysbiosis. In particular, a significant increased amount of flagellated bacteria was observed in the NMS mice with CHS. In association, only tlr5 mRNA expression was increased in colonocytes of NMS mice with CHS.
Research conclusions
Taken together, our results suggest a pathophysiological continuum between intestinal dysbiosis and CHS, with a role for TLR5.
Research perspectives
TLR5 signaling upon recognition of flagellin is relevant in visceral pain through both direct and indirect mechanisms, and application of TLR5-specific antagonists could potentially reversed CHS in non-inflammatory visceral pain context.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Abdelkrim Alloui (Animal facilities) for animal care.
Footnotes
Institutional animal care and use committee statement: All experiments were performed according to the ethical guidelines set out by the International Association for the Study of Pain, complied with the European Union regulation and were approved by ethics committees: the local committees C2EA-02 of Clermont-Ferrand (approvals CE110-12 and CE111-12).
Conflict-of-interest statement: All authors have nothing to disclose.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 15, 2022
First decision: May 29, 2022
Article in press: July 5, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghannam WM, Egypt; Plaza MA, Spain S-Editor: Zhang H L-Editor: A P-Editor: Yu HG
Contributor Information
Geoffroy Mallaret, Department of Pharmacology, UMR 1107 NeuroDol, University of Clermont Auvergne, Clermont-Ferrand 63000, France.
Amandine Lashermes, Department of Microbiology, Université Paris-Saclay, National Research Institute for Agriculture, Food and the Environment, AgroParisTech, Micalis Institute, Jouy-en-Josas 78350, France.
Mathieu Meleine, Department of Pharmacology, UMR 1107 NeuroDol, University of Clermont Auvergne, Clermont-Ferrand 63000, France.
Ludivine Boudieu, Department of Pharmacology, UMR 1107 NeuroDol, University of Clermont Auvergne, Clermont-Ferrand 63000, France.
Julie Barbier, Department of Pharmacology, UMR 1107 NeuroDol, University of Clermont Auvergne, Clermont-Ferrand 63000, France.
Youssef Aissouni, Department of Pharmacology, UMR 1107 NeuroDol, University of Clermont Auvergne, Clermont-Ferrand 63000, France.
Agathe Gelot, Department of Pharmacology, UMR 1107 NeuroDol, University of Clermont Auvergne, Clermont-Ferrand 63000, France.
Benoit Chassaing, Team “Mucosal Microbiota in Chronic Inflammatory Diseases”, INSERM U1016, CNRS UMR 8104, Université Paris Cité, Paris 75014, France.
Andrew T Gewirtz, Center for Inflammation, Institute for Biomedical Sciences, Georgia State University, Atlanta, GA30033, United States.
Denis Ardid, Department of Pharmacology, UMR 1107 NeuroDol, University of Clermont Auvergne, Clermont-Ferrand 63000, France.
Frederic Antonio Carvalho, Department of Pharmacology, INSERM 1107 NeuroDOL/University of Clermont Auvergne, Clermont-Ferrand 63000, France. frederic.carvalho@inserm.fr.
Data sharing statement
All sequencing raw data have been deposited in European Nucleotide Archive (ENA) under accession number PRJEB50651.
References
- 1.Kopczyńska M, Mokros Ł, Pietras T, Małecka-Panas E. Quality of life and depression in patients with irritable bowel syndrome. Prz Gastroenterol. 2018;13:102–108. doi: 10.5114/pg.2018.75819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, Whitehead WE, Dumitrascu DL, Fang X, Fukudo S, Kellow J, Okeke E, Quigley EMM, Schmulson M, Whorwell P, Archampong T, Adibi P, Andresen V, Benninga MA, Bonaz B, Bor S, Fernandez LB, Choi SC, Corazziari ES, Francisconi C, Hani A, Lazebnik L, Lee YY, Mulak A, Rahman MM, Santos J, Setshedi M, Syam AF, Vanner S, Wong RK, Lopez-Colombo A, Costa V, Dickman R, Kanazawa M, Keshteli AH, Khatun R, Maleki I, Poitras P, Pratap N, Stefanyuk O, Thomson S, Zeevenhooven J, Palsson OS. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology. 2021;160:99–114.e3. doi: 10.1053/j.gastro.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Farzaei MH, Bahramsoltani R, Abdollahi M, Rahimi R. The Role of Visceral Hypersensitivity in Irritable Bowel Syndrome: Pharmacological Targets and Novel Treatments. J Neurogastroenterol Motil. 2016;22:558–574. doi: 10.5056/jnm16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe S, Radman-Livaja M, Rando OJ, Peterson CL. A histone acetylation switch regulates H2A.Z deposition by the SWR-C remodeling enzyme. Science. 2013;340:195–199. doi: 10.1126/science.1229758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enck P, Mazurak N. Dysbiosis in Functional Bowel Disorders. Ann Nutr Metab. 2018;72:296–306. doi: 10.1159/000488773. [DOI] [PubMed] [Google Scholar]
- 6.Pittayanon R, Lau JT, Yuan Y, Leontiadis GI, Tse F, Surette M, Moayyedi P. Gut Microbiota in Patients With Irritable Bowel Syndrome-A Systematic Review. Gastroenterology. 2019;157:97–108. doi: 10.1053/j.gastro.2019.03.049. [DOI] [PubMed] [Google Scholar]
- 7.Jalanka-Tuovinen J, Salonen A, Nikkilä J, Immonen O, Kekkonen R, Lahti L, Palva A, de Vos WM. Intestinal microbiota in healthy adults: temporal analysis reveals individual and common core and relation to intestinal symptoms. PLoS One. 2011;6:e23035. doi: 10.1371/journal.pone.0023035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 9.Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17:219–232. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- 10.Brint EK, MacSharry J, Fanning A, Shanahan F, Quigley EM. Differential expression of toll-like receptors in patients with irritable bowel syndrome. Am J Gastroenterol. 2011;106:329–336. doi: 10.1038/ajg.2010.438. [DOI] [PubMed] [Google Scholar]
- 11.McKernan DP, Gaszner G, Quigley EM, Cryan JF, Dinan TG. Altered peripheral toll-like receptor responses in the irritable bowel syndrome. Aliment Pharmacol Ther. 2011;33:1045–1052. doi: 10.1111/j.1365-2036.2011.04624.x. [DOI] [PubMed] [Google Scholar]
- 12.Belmonte L, Beutheu Youmba S, Bertiaux-Vandaële N, Antonietti M, Lecleire S, Zalar A, Gourcerol G, Leroi AM, Déchelotte P, Coëffier M, Ducrotté P. Role of toll like receptors in irritable bowel syndrome: differential mucosal immune activation according to the disease subtype. PLoS One. 2012;7:e42777. doi: 10.1371/journal.pone.0042777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dlugosz A, Zakikhany K, Acevedo N, D'Amato M, Lindberg G. Increased Expression of Toll-Like Receptors 4, 5, and 9 in Small Bowel Mucosa from Patients with Irritable Bowel Syndrome. Biomed Res Int. 2017;2017:9624702. doi: 10.1155/2017/9624702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koçak E, Akbal E, Köklü S, Ergül B, Can M. The Colonic Tissue Levels of TLR2, TLR4 and Nitric Oxide in Patients with Irritable Bowel Syndrome. Intern Med. 2016;55:1043–1048. doi: 10.2169/internalmedicine.55.5716. [DOI] [PubMed] [Google Scholar]
- 15.Shukla R, Ghoshal U, Ranjan P, Ghoshal UC. Expression of Toll-like Receptors, Pro-, and Anti-inflammatory Cytokines in Relation to Gut Microbiota in Irritable Bowel Syndrome: The Evidence for Its Micro-organic Basis. J Neurogastroenterol Motil. 2018;24:628–642. doi: 10.5056/jnm18130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKernan DP, Nolan A, Brint EK, O'Mahony SM, Hyland NP, Cryan JF, Dinan TG. Toll-like receptor mRNA expression is selectively increased in the colonic mucosa of two animal models relevant to irritable bowel syndrome. PLoS One. 2009;4:e8226. doi: 10.1371/journal.pone.0008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradford K, Shih W, Videlock EJ, Presson AP, Naliboff BD, Mayer EA, Chang L. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol. 2012;10:385–90.e1. doi: 10.1016/j.cgh.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larauche M, Gourcerol G, Million M, Adelson DW, Taché Y. Repeated psychological stress-induced alterations of visceral sensitivity and colonic motor functions in mice: influence of surgery and postoperative single housing on visceromotor responses. Stress. 2010;13:343–354. doi: 10.3109/10253891003664166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picard E, Carvalho FA, Agosti F, Bourinet E, Ardid D, Eschalier A, Daulhac L, Mallet C. Inhibition of Cav 3.2 calcium channels: A new target for colonic hypersensitivity associated with low-grade inflammation. Br J Pharmacol. 2019;176:950–963. doi: 10.1111/bph.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lashermes A, Boudieu L, Barbier J, Sion B, Gelot A, Barnich N, Ardid D, Carvalho FA. Adherent-Invasive E. coli enhances colonic hypersensitivity and P2X receptors expression during post-infectious period. Gut Microbes. 2018;9:26–37. doi: 10.1080/19490976.2017.1361091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, Scholz CC, Fraisl P, Lasitschka F, Mollenhauer M, Saunders SP, Maxwell PH, Carmeliet P, Fallon PG, Schneider M, Taylor CT. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology. 2010;139:2093–2101. doi: 10.1053/j.gastro.2010.06.068. [DOI] [PubMed] [Google Scholar]
- 22.Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics. 2010;26:266–267. doi: 10.1093/bioinformatics/btp636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meleine M, Boudieu L, Gelot A, Muller E, Lashermes A, Matricon J, Silberberg C, Theodorou V, Eschalier A, Ardid D, Carvalho FA. Comparative effects of α2δ-1 ligands in mouse models of colonic hypersensitivity. World J Gastroenterol. 2016;22:7111–7123. doi: 10.3748/wjg.v22.i31.7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:991–1000. doi: 10.1038/ajg.2012.131. [DOI] [PubMed] [Google Scholar]
- 25.Kanazawa M, Palsson OS, Thiwan SI, Turner MJ, van Tilburg MA, Gangarosa LM, Chitkara DK, Fukudo S, Drossman DA, Whitehead WE. Contributions of pain sensitivity and colonic motility to IBS symptom severity and predominant bowel habits. Am J Gastroenterol. 2008;103:2550–2561. doi: 10.1111/j.1572-0241.2008.02066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camilleri M, Boeckxstaens G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut. 2017;66:966–974. doi: 10.1136/gutjnl-2016-313425. [DOI] [PubMed] [Google Scholar]
- 27.Ju T, Naliboff BD, Shih W, Presson AP, Liu C, Gupta A, Mayer EA, Chang L. Risk and Protective Factors Related to Early Adverse Life Events in Irritable Bowel Syndrome. J Clin Gastroenterol. 2020;54:63–69. doi: 10.1097/MCG.0000000000001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SH, Videlock EJ, Shih W, Presson AP, Mayer EA, Chang L. Adverse childhood experiences are associated with irritable bowel syndrome and gastrointestinal symptom severity. Neurogastroenterol Motil. 2016;28:1252–1260. doi: 10.1111/nmo.12826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitehead WE, Crowell MD, Robinson JC, Heller BR, Schuster MM. Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut. 1992;33:825–830. doi: 10.1136/gut.33.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moloney RD, O'Mahony SM, Dinan TG, Cryan JF. Stress-induced visceral pain: toward animal models of irritable-bowel syndrome and associated comorbidities. Front Psychiatry. 2015;6:15. doi: 10.3389/fpsyt.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Botschuijver S, Welting O, Levin E, Maria-Ferreira D, Koch E, Montijn RC, Seppen J, Hakvoort TBM, Schuren FHJ, de Jonge WJ, van den Wijngaard RM. Reversal of visceral hypersensitivity in rat by Menthacarin®, a proprietary combination of essential oils from peppermint and caraway, coincides with mycobiome modulation. Neurogastroenterol Motil. 2018;30:e13299. doi: 10.1111/nmo.13299. [DOI] [PubMed] [Google Scholar]
- 32.Yi L, Zhang H, Sun H, Zhou L, Chen Y, Xuan L, Jiang Y, Xu S. Maternal Separation Induced Visceral Hypersensitivity from Childhood to Adulthood. J Neurogastroenterol Motil. 2017;23:306–315. doi: 10.5056/jnm16089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Punder K, Pruimboom L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front Immunol. 2015;6:223. doi: 10.3389/fimmu.2015.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24:503–512. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miquel S, Martín R, Lashermes A, Gillet M, Meleine M, Gelot A, Eschalier A, Ardid D, Bermúdez-Humarán LG, Sokol H, Thomas M, Theodorou V, Langella P, Carvalho FA. Anti-nociceptive effect of Faecalibacterium prausnitzii in non-inflammatory IBS-like models. Sci Rep. 2016;6:19399. doi: 10.1038/srep19399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rincel M, Olier M, Minni A, Monchaux de Oliveira C, Matime Y, Gaultier E, Grit I, Helbling JC, Costa AM, Lépinay A, Moisan MP, Layé S, Ferrier L, Parnet P, Theodorou V, Darnaudéry M. Pharmacological restoration of gut barrier function in stressed neonates partially reverses long-term alterations associated with maternal separation. Psychopharmacology (Berl) 2019;236:1583–1596. doi: 10.1007/s00213-019-05252-w. [DOI] [PubMed] [Google Scholar]
- 38.Moloney RD, Johnson AC, O'Mahony SM, Dinan TG, Greenwood-Van Meerveld B, Cryan JF. Stress and the Microbiota-Gut-Brain Axis in Visceral Pain: Relevance to Irritable Bowel Syndrome. CNS Neurosci Ther. 2016;22:102–117. doi: 10.1111/cns.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosca A, Leclerc M, Hugot JP. Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosystem? Front Microbiol. 2016;7:455. doi: 10.3389/fmicb.2016.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lepage P, Häsler R, Spehlmann ME, Rehman A, Zvirbliene A, Begun A, Ott S, Kupcinskas L, Doré J, Raedler A, Schreiber S. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology. 2011;141:227–236. doi: 10.1053/j.gastro.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Pozuelo M, Panda S, Santiago A, Mendez S, Accarino A, Santos J, Guarner F, Azpiroz F, Manichanh C. Reduction of butyrate- and methane-producing microorganisms in patients with Irritable Bowel Syndrome. Sci Rep. 2015;5:12693. doi: 10.1038/srep12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moussaoui N, Jacobs JP, Larauche M, Biraud M, Million M, Mayer E, Taché Y. Chronic Early-life Stress in Rat Pups Alters Basal Corticosterone, Intestinal Permeability, and Fecal Microbiota at Weaning: Influence of Sex. J Neurogastroenterol Motil. 2017;23:135–143. doi: 10.5056/jnm16105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tap J, Derrien M, Törnblom H, Brazeilles R, Cools-Portier S, Doré J, Störsrud S, Le Nevé B, Öhman L, Simrén M. Identification of an Intestinal Microbiota Signature Associated With Severity of Irritable Bowel Syndrome. Gastroenterology. 2017;152:111–123.e8. doi: 10.1053/j.gastro.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 45.Zhou XY, Li M, Li X, Long X, Zuo XL, Hou XH, Cong YZ, Li YQ. Visceral hypersensitive rats share common dysbiosis features with irritable bowel syndrome patients. World J Gastroenterol. 2016;22:5211–5227. doi: 10.3748/wjg.v22.i22.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murakami T, Kamada K, Mizushima K, Higashimura Y, Katada K, Uchiyama K, Handa O, Takagi T, Naito Y, Itoh Y. Changes in Intestinal Motility and Gut Microbiota Composition in a Rat Stress Model. Digestion. 2017;95:55–60. doi: 10.1159/000452364. [DOI] [PubMed] [Google Scholar]
- 47.Luna RA, Oezguen N, Balderas M, Venkatachalam A, Runge JK, Versalovic J, Veenstra-VanderWeele J, Anderson GM, Savidge T, Williams KC. Distinct Microbiome-Neuroimmune Signatures Correlate With Functional Abdominal Pain in Children With Autism Spectrum Disorder. Cell Mol Gastroenterol Hepatol. 2017;3:218–230. doi: 10.1016/j.jcmgh.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lacagnina MJ, Watkins LR, Grace PM. Toll-like receptors and their role in persistent pain. Pharmacol Ther. 2018;184:145–158. doi: 10.1016/j.pharmthera.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicotra L, Loram LC, Watkins LR, Hutchinson MR. Toll-like receptors in chronic pain. Exp Neurol. 2012;234:316–329. doi: 10.1016/j.expneurol.2011.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tramullas M, Finger BC, Moloney RD, Golubeva AV, Moloney G, Dinan TG, Cryan JF. Toll-like receptor 4 regulates chronic stress-induced visceral pain in mice. Biol Psychiatry. 2014;76:340–348. doi: 10.1016/j.biopsych.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Luczynski P, Tramullas M, Viola M, Shanahan F, Clarke G, O'Mahony S, Dinan TG, Cryan JF. Microbiota regulates visceral pain in the mouse. Elife. 2017;6:e25887. doi: 10.7554/eLife.25887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou SY, Gillilland M 3rd, Wu X, Leelasinjaroen P, Zhang G, Zhou H, Ye B, Lu Y, Owyang C. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. J Clin Invest. 2018;128:267–280. doi: 10.1172/JCI92390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Das N, Dewan V, Grace PM, Gunn RJ, Tamura R, Tzarum N, Watkins LR, Wilson IA, Yin H. HMGB1 Activates Proinflammatory Signaling via TLR5 Leading to Allodynia. Cell Rep. 2016;17:1128–1140. doi: 10.1016/j.celrep.2016.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dlugosz A, Nowak P, D'Amato M, Mohammadian Kermani G, Nyström J, Abdurahman S, Lindberg G. Increased serum levels of lipopolysaccharide and antiflagellin antibodies in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2015;27:1747–1754. doi: 10.1111/nmo.12670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All sequencing raw data have been deposited in European Nucleotide Archive (ENA) under accession number PRJEB50651.