Abstract
Background
Renal cell carcinoma (RCC) is one of the most lethal urological malignancies, and because early-stage RCC is asymptomatic, many patients present metastatic diseases at first diagnosis. With the development of immunotherapy, the treatment of RCC has entered a new stage and has made a series of progress. This study mainly outlines the knowledge map and detects the potential research hotspots by using bibliometric analysis.
Methods
Publications concerning RCC immunotherapy from 2002 to 2021 in the Web of Science Core Collection were collected. Visualization and statistical analysis were mainly performed by freeware tools VOSviewer, CiteSpace, R software, and Microsoft Office Excel 2019.
Results
A total of 3,432 papers were collected in this study, and the annual number of papers and citations showed a steady growth trend. The United States is the leading country with the most high-quality publications and is also the country with the most international cooperation. The University of Texas MD Anderson Cancer Center is the most productive organization. The Journal of Clinical Oncology is the highest co-cited journal, and Brian I. Rini is both the most prolific author and the author with the largest centrality. The current research hotspots may be focused on “immune checkpoint inhibitors (ICIs),” “PD-1,” and “mammalian target of rapamycin.”
Conclusion
Immunotherapy has a bright future in the field of RCC treatment, among which ICIs are one of the most important research hotspots. The main future research directions of ICI-based immunotherapy may focus on combination therapy, ICI monotherapy, and the development of new predictive biomarkers.
Keywords: immunotherapy, renal cell carcinoma, immune checkpoint inhibitors, bibliometric, visualization
Introduction
Renal cell carcinoma (RCC) is a common malignant tumor in the genitourinary system and one of the top 10 most common cancers in the world (1–3). More than 60% of patients with RCC suffer from localized tumors (4, 5). However, more than 15% of them may develop locally advanced progression. In addition, although early-stage RCC is curable, it is usually hard to detect, as patients are usually asymptomatic or mildly symptomatic. Many studies demonstrated that 16% of patients presented metastatic diseases at first diagnosis (6). Currently, surgical removal remains the mainstay of the treatment of RCC at an early stage, and after effective surgical treatment, the 5-year overall survival (OS) can exceed 90% (7). Unfortunately, the 5-year OS will decrease to 12% once metastatic disease occurs (8). Moreover, RCC is poorly responsive to conventional chemotherapy and radiotherapy, which leads to poor prognosis in patients with metastatic disease (9–11).
The emergence and development of immunotherapy provide more therapeutic methods for patients with RCC. Immunotherapy, as a potentially beneficial addition to conventional treatments for cancers, can modulate and enhance the host’s immune system to eliminate tumor cells and prevent tumor recurrence so as to prolong the survival times of patients (12). The emergence of treatment targeting immune checkpoints signaled the arrival of the era of immunotherapy (13). After a long period of development, cancer immunotherapy has revolutionized oncology and provides new treatment options for many refractory tumors. At present, the most common cancer immunotherapy methods are adoptive T-cell therapy and chimeric antigen receptor T-cell immunotherapy (CAR-T) (14). Findings from previous studies showed that CAR-T has been widely applied in the treatment of multiple tumors, including B-cell acute lymphoblastic leukemia, and has achieved therapeutic effects. However, no significant therapeutic effects were found on solid tumors (15–17).
Over the past few decades, benefiting from the development of vascular endothelial growth factor (VEGF) tyrosine kinase inhibitors and immune checkpoint inhibitors (ICIs), immunotherapy has been widely used in the treatment of RCC (18–20). As of 2018, ipilimumab plus nivolumab was the only ICI drug treatment approved by the Food and Drug Administration (FDA) for the treatment of RCC (21). Currently, a variety of ICIs have been approved by the FDA, and the combination of ICIs and tyrosine kinase inhibitors is the first-line treatment of clear cell RCC (ccRCC) (22). Moreover, ICI monotherapy is currently undergoing clinical trials and is expected to become a promising alternative therapeutic method for patients with combination therapy intolerance (23–25). The research focus in the field of RCC immunotherapy is constantly changing with the effect of drugs, indicating that the research topics related to cancer immunotherapy are being updated rapidly, and it is necessary to monitor the research progress. Therefore, determining the current research hotspots and future research trends in this field may contribute to understanding the latest research directions.
Bibliometrics uses the citation data from database to evaluate the published research and to systematically study and visualize the knowledge structure and development trend of a certain scientific field by qualitatively and quantitatively analyzing the cooperation, co-occurrence, or co-citation of publications (26–29). It is a powerful tool to investigate the progress of research on different topics and to assess future research trends. Currently, bibliometric analysis has been applied in many fields (30, 31), but there is no specific bibliometric study in the field of RCC immunotherapy. The purpose of this study is to create a comprehensive summary of existing publications on RCC immunotherapy research in the past 20 years through bibliometrics, aiming to perform knowledge mapping to explore the hotspots or frontiers in this field.
Methods
Database and study collection
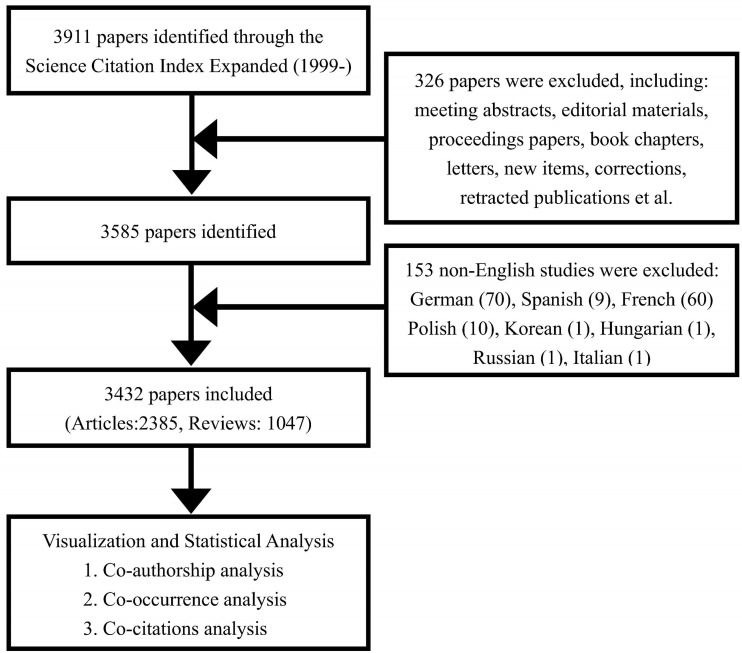
The Web of Science Core Collection (WoSCC) is an optimal database with more than 10,000 high-quality journals and is also the most commonly used database in previous bibliometric studies (32, 33). In this study, the Science Citation Index-Expanded of the WoSCC was selected as our database. To avoid data bias, two researchers independently conducted the literature search for original articles and reviews on May 1, 2022. The searching terms are as follows: #1: Topic (TS)=(Immunotherapy OR Immunotherapies OR immunotherapeutic) OR Author Keywords (AK)=(Immunotherapy OR Immunotherapies OR immunotherapeutic); #2: TS=(renal OR kidney) NEAR/2 (cancer* OR tumor* OR tumour* OR oncology OR neoplasm* OR carcinoma*) OR AK=(renal OR kidney) NEAR/2 (cancer* OR tumor* OR tumour* OR oncology OR neoplasm* OR carcinoma*); Final data source: #1 AND #2. The period of study was from 1st January, 2002 to 31th December, 2021 and the language was restricted to English. The study flowchart is shown in Figure 1 .
Figure 1.
Flowchart of the literature searching and screening in the study.
Visualization and statistical analysis
Microsoft Office Excel 2019 (Microsoft, Redmond, WA, USA) was the main software used to analyze the data from the WoSCC and to construct a polynomial regression model to predict the number of publications and total citations in 2022. In addition, the indicators, which included the Hirsch index (H-index), impact factor (IF), and quartile in the category of journals, were also collected and analyzed through Microsoft Office Excel 2019. The H-index, proposed by Jorge Hirsch (34), is a mixed quantitative index, and it can be used to evaluate the number and level of academic output of researchers. The higher a researcher’s H-index, the greater the influence of his/her article. Furthermore, in many previous studies, the H-index was also applied to evaluate the productivity and academic status of countries, organizations, or journals (35–37). The Bibliometrix package in R software (Version 4.0.3) and an online bibliometric analysis platform (http://bibliometric.com/) were used to perform the collaboration among countries/regions. Visualization was mainly performed through VOSviewer and CiteSpace.
VOSviewer is widely used bibliometric visualization software to conduct network visualization maps and knowledge structure (38). The network visualization map, overlay visualization map, and density visualization map are the three main visual maps that VOSviewer provides. In this study, VOSviewer (Version 1.6.16) was utilized to conduct co-authorship analysis of country/author/institution, co-citation analysis of journal, and author keyword co-occurrence analysis. The options and settings of VOSviewer are displayed in Supplementary Table S1 .
CiteSpace V (Version 5.8.R3) is another popular visualization tool that was developed by Professor Chaomei Chen (39–41). It was mainly applied to perform the visualization map of co-citation analysis of references/authors and to detect the keywords/references with the strongest citation bursts in this study. In addition, a dual-map overlay of journals was also created by CiteSpace V. The parameters included in CiteSpace were as follows: time span (2002–2021), year per slice (1 year), node type (reference, cited author, and cited journal), selection criteria (top 50 per slice), and pruning methods (minimum spanning tree (MST) and pruning sliced networks).
Results
Analysis of annual publications and citation trends
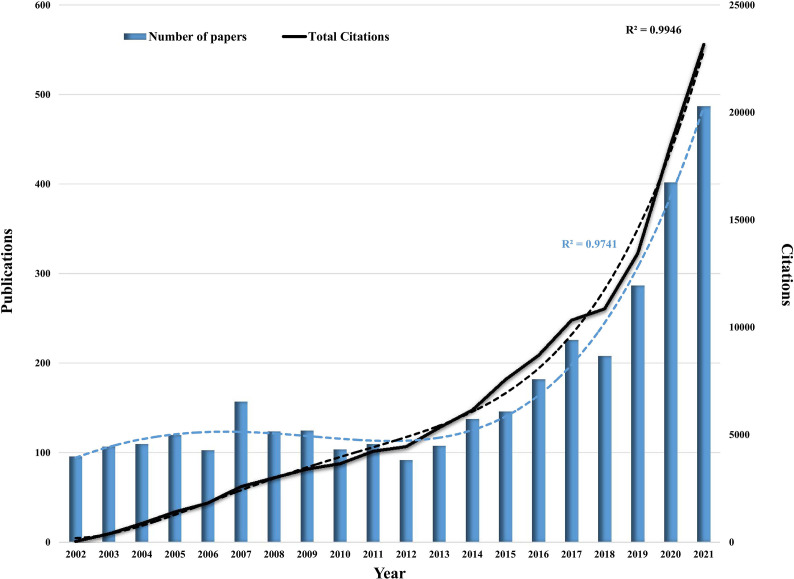
A total of 3,432 publications in the field of RCC immunotherapy were collected after a thorough search. Regarding the search data, the sum of the times that all publications were cited was 135,782, and the average number of citations per item (ACI) was 39.56. The H-index of all papers was 147. As shown in Figure 2 , between 2002 and 2012, the growth rate of the annual number of papers in RCC immunotherapy varied. After 2012, the annual number of relevant papers grew rapidly, reaching a peak in 2021 (487 papers). Through data fitting, a statistically significant relationship between publications and the published year (correlation coefficient R2 = 0.9741) became clear. Correspondingly, the curve of annual citations had shown a steady increase since 2002 and reached a peak in 2021 with 23,169 citations (R2 = 0.9946). According to the fitting curve in Figure 2 , the annual number of publications and citations concerning RCC immunotherapy will be 611 and 28,688, respectively, in 2022.
Figure 2.
Global trend of publications and total citations on RCC immunotherapy from 2002 to 2021. The blue and black dotted lines represent the trend-fitted curves using polynomial regression model. The correlation coefficients (R2) are displayed in the figure.
Contribution of active countries/regions
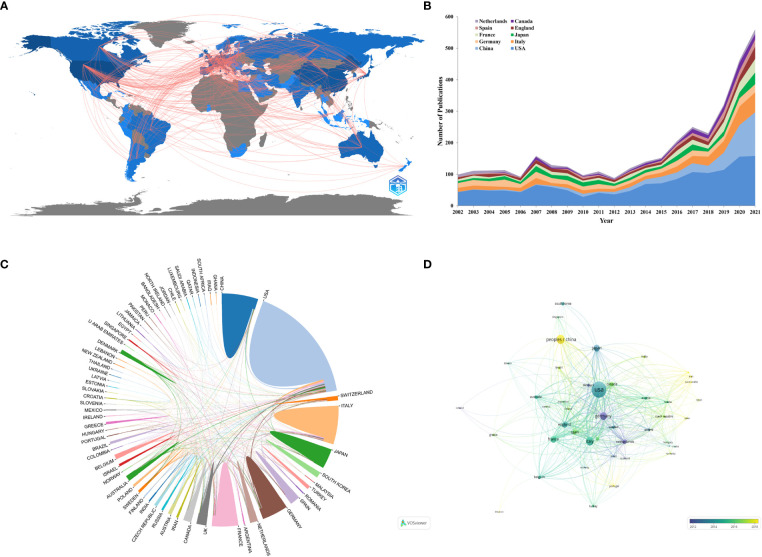
A total of 73 countries/regions were included in the study. Figure 3A shows the geographical distribution map of the RCC immunotherapy study. It can be observed that studies about RCC immunotherapy were mainly reported from the countries in North America, Europe, and Asia. The annual number of publications of the top 10 productive countries/regions is displayed in Figure 3B , showing that the number of publications concerning RCC immunotherapy retains a swift growth. The top 10 productive countries/regions as regards the number of publications are listed in Table 1 . Among them, the United States is the leading country in this field with 1,431 papers, accounting for more than 41% of all papers included. It is noteworthy that of the top 10 productive countries, China is the only country with an ACI lower than 20 even though China ranks second in the number of publications.
Figure 3.
(A) Geographic distribution map based on the total publications of different countries/regions. (B) The changing trend of the annual publication quantity in the top 10 countries/regions from 2002 to 2021. (C) The international collaborations visualization map of countries/regions. (D) The countries/regions citation overlay visualization map generated by using VOSviewer. Each node means a country/region, and the size of node indicates the number of publications. The connection between the nodes represents the citation relationship, and the thickness of the connection lines indicates citation strength.
Table 1.
Top 10 productive countries/regions related to RCC immunotherapy research.
| Rank | Country | Counts | % of 3432 | H-index | ACI | TLS |
|---|---|---|---|---|---|---|
| 1 | USA | 1431 | 41.696 | 131 | 61.78 | 748 |
| 2 | China | 444 | 12.937 | 40 | 19.01 | 114 |
| 3 | Italy | 399 | 11.626 | 50 | 25.13 | 417 |
| 4 | Germany | 324 | 9.441 | 58 | 39.95 | 363 |
| 5 | Japan | 255 | 7.43 | 47 | 27.73 | 166 |
| 6 | France | 241 | 7.022 | 56 | 50.68 | 380 |
| 7 | England | 201 | 5.857 | 46 | 39.88 | 322 |
| 8 | Canada | 127 | 3.7 | 38 | 49.71 | 251 |
| 9 | Spain | 127 | 3.7 | 33 | 30.15 | 293 |
| 10 | Netherlands | 125 | 3.642 | 41 | 47.09 | 202 |
ACI, Average Citations per Item; TLS, Total Link Strength.
For the collaboration analysis of countries/regions, an online bibliometric platform and VOSviewer were utilized to construct the co-authorship network map of countries. The international cooperation map indicated the extensive cooperation among countries ( Figure 3C ). The United States is the cooperation center in this field, having the closest relationship with Italy, France, and China. As shown in Figure 3D , the top three countries with the highest total link strength (TLS) are ranked as follows: the United States (TLS = 748), Italy (TLS = 417), and France (TLS = 380). Germany was the first to study RCC immunotherapy, with an average publication year of 2011.89. It is noteworthy that China has become active in recent years with an average publication year of 2018.15, which started much later than most productive countries.
Contribution of productive organizations and funding agencies
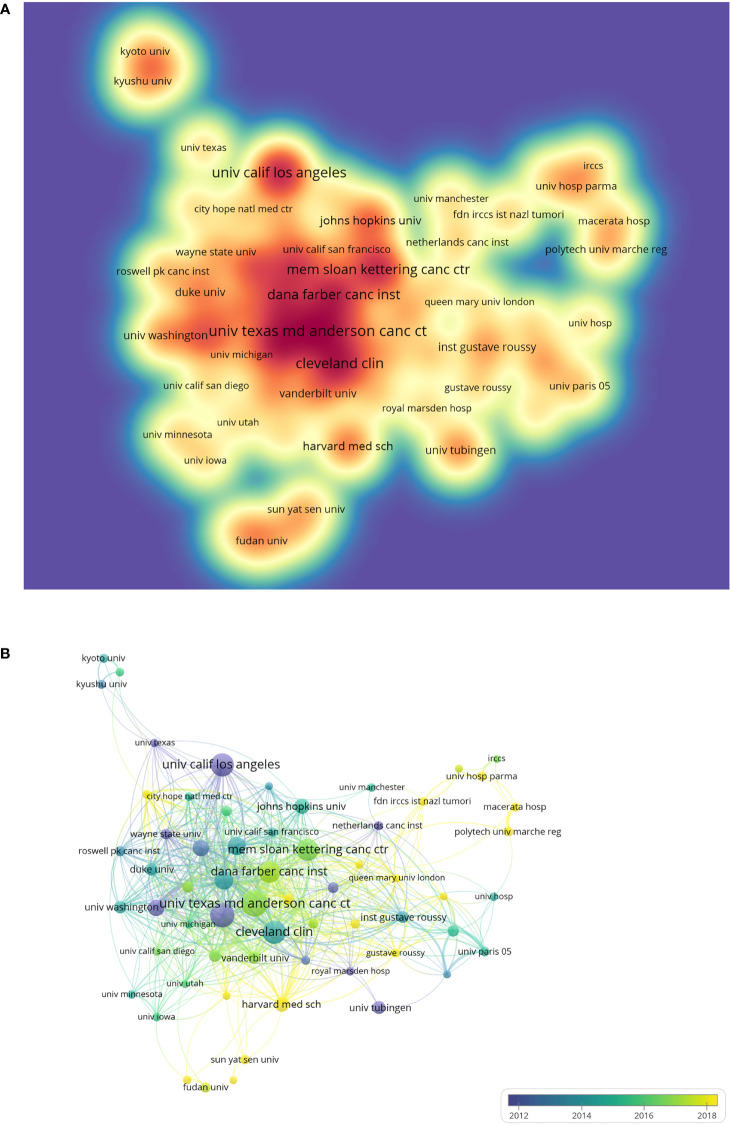
A total of 3,756 organizations have participated in the RCC immunotherapy study. From the results of Table 2 , it can be observed that the top 10 productive organizations are all from the United States. The University of Texas MD Anderson Cancer Center ranks first in terms of the number of papers (N = 100), followed by the National Cancer Institute (N = 91), Cleveland Clinic (N = 84), and University of California, Los Angeles (N = 84). Total citations is an important index for measuring the international influence of institutions. As displayed in Table 2 , the Memorial Sloan Kettering Cancer Center, Dana-Farber Cancer Institute, and Beth Israel Deaconess Medical Center are the top three organizations with the highest total citations. Only organizations with a minimum of 20 papers were included and visualized in the spectral density map ( Figure 4A ). The top three organizations with the highest TLS are the Dana-Farber Cancer Institute, Beth Israel Deaconess Medical Center, and Memorial Sloan Kettering Cancer Center. The overlay visualization map of organizations’ collaboration is shown in Figure 4B . Organizations in the United States or Germany began RCC immunotherapy studies earlier than those in China. Organizations in China, such as Sun Yat-sen University and Fudan University, have become active in recent years and have published more important papers in this field.
Table 2.
The top 10 most productive organizations and funding agencies related to RCC immunotherapy research.
| Rank | Organizations | Countries | Counts | TLS | Total Citations | Funding Agencies | Countries | Counts |
|---|---|---|---|---|---|---|---|---|
| 1 | University of Texas MD Anderson Cancer Center | USA | 100 | 370 | 9202 | United States Department of Health Human Services | USA | 476 |
| 2 | National Cancer Institute | USA | 91 | 226 | 7074 | National Institutes of Health | USA | 475 |
| 3 | Cleveland Clinic | USA | 84 | 352 | 5313 | National Cancer Institute | USA | 376 |
| 4 | University of California, Los Angeles | USA | 84 | 154 | 7158 | National Natural Science Foundation of China | China | 192 |
| 5 | Memorial Sloan Kettering Cancer Center | USA | 79 | 371 | 15206 | Bristol Myers Squibb | USA | 98 |
| 6 | Dana-Farber Cancer Institute | USA | 75 | 479 | 14436 | Pfizer | USA | 75 |
| 7 | Beth Israel Deaconess Medical Center | USA | 64 | 402 | 12719 | Novartis | Switzerland | 67 |
| 8 | Mayo Clinic | USA | 61 | 185 | 9463 | European Commission | European Commission | 66 |
| 9 | Harvard University | USA | 53 | 197 | 6113 | Ministry of Education Culture Sports Science And Technology | Japan | 58 |
| 10 | University of Pittsburgh | USA | 53 | 176 | 2828 | Roche Holding | Switzerland | 44 |
TLS, Total Link Strength.
Figure 4.
(A) The spectral density map of organizations was performed with VOSviewer. The deeper the color of the node, the more documents the organization published. (B) The overlay visualization map of organizations’ collaborations based on VOSviewer. The purple nodes represented the early institutions that participated in the research in this field, while the yellow nodes reflected the later organizations.
The top 10 funding agencies are also summarized in Table 2 . Among them, half of the total funding agencies are from the United States, showing the United States’ strong economic foundation and support for scientific study. The United States Department of Health and Human Services ranked first with 476 papers, followed by the National Institutes of Health (N = 475) and the National Cancer Institute (N = 376).
Contribution of active authors
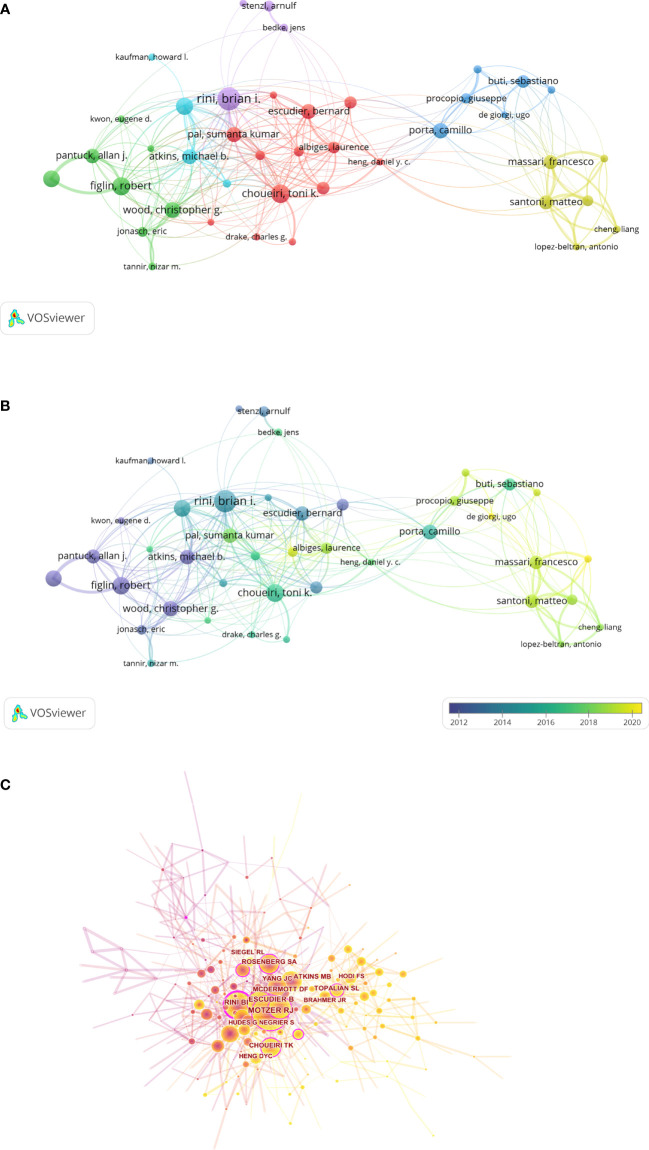
Of all authors who participated in the studies on RCC immunotherapy, the 10 most productive authors and the top 10 authors with the largest centrality are summarized in Table 3 . It is easy to deduce that eight of the top 10 authors are from the United States, and the two remaining authors are from Italy and France. Among them, the top three authors with the most papers are Brian I. Rini (N = 60), Toni K. Choueiri (N = 47), and Robert Figlin (N = 46). It is noteworthy that Brian I. Rini is also the top co-cited author with the largest centrality of 0.32. The authors with a minimum number of 15 documents are shown in Figure 5A , and when combined with the overlay visualization map of author co-authorship analysis ( Figure 5B ), it can be observed that the authors in the green cluster are considered pioneers in the field of RCC immunotherapy, whereas the authors in the blue and yellow clusters began to publish papers in recent years. In addition, close collaboration and communication among different clusters are lacking. As shown in the map of co-cited authors ( Figure 5C ), Brian I. Rini, Robert J. Motzer, and Toni K. Choueiri are the top three co-cited authors in this analysis, showing their dominance in this field.
Table 3.
The 10 most productive authors and the top 10 authors with largest centrality in the field of RCC immunotherapy.
| Rank | Author | Country | Counts | Total Citations | H-index | TLS | Co-Cited Author | Country | Total Citations | TLS | Centrality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Rini, Brian I. | USA | 60 | 4474 | 26 | 550 | Rini, Brian I. | USA | 1868 | 132263 | 0.32 |
| 2 | Choueiri, Toni K. | USA | 47 | 1585 | 26 | 509 | Motzer Robert J | USA | 5065 | 300035 | 0.16 |
| 3 | Figlin, Robert | USA | 46 | 4519 | 28 | 374 | Choueiri Toni K | USA | 1235 | 83538 | 0.16 |
| 4 | Mcdermott, David F. | USA | 43 | 2589 | 26 | 435 | Mcdermott David F | USA | 872 | 62732 | 0.15 |
| 5 | Belldegrun, Arie S. | USA | 42 | 4102 | 29 | 326 | Powles T | UK | 371 | 30888 | 0.15 |
| 6 | Wood, Christopher G. | USA | 39 | 1752 | 20 | 303 | Amato Robert J | USA | 324 | 23158 | 0.15 |
| 7 | Porta, Camillo | Italy | 38 | 649 | 16 | 306 | Escudier, Bernard | France | 1546 | 99947 | 0.13 |
| 8 | Atkins, Michael B. | USA | 37 | 2828 | 23 | 355 | Rosenberg Steven A | USA | 945 | 87075 | 0.13 |
| 9 | Escudier, Bernard | France | 37 | 2750 | 25 | 352 | Simons JW | USA | 91 | 7776 | 0.12 |
| 10 | Pal, Sumanta Kumar | USA | 37 | 904 | 16 | 301 | Topalian Suzanne L | USA | 645 | 51850 | 0.11 |
TLS, Total Link Strength.
Figure 5.
The network visualization map (A) and overlay visualization map (B) of author co-authorship analysis conducted by VOSviewer. (C) The visualization map of co-cited authors carried on CiteSpace.
Analysis of influential journals and co-cited journals
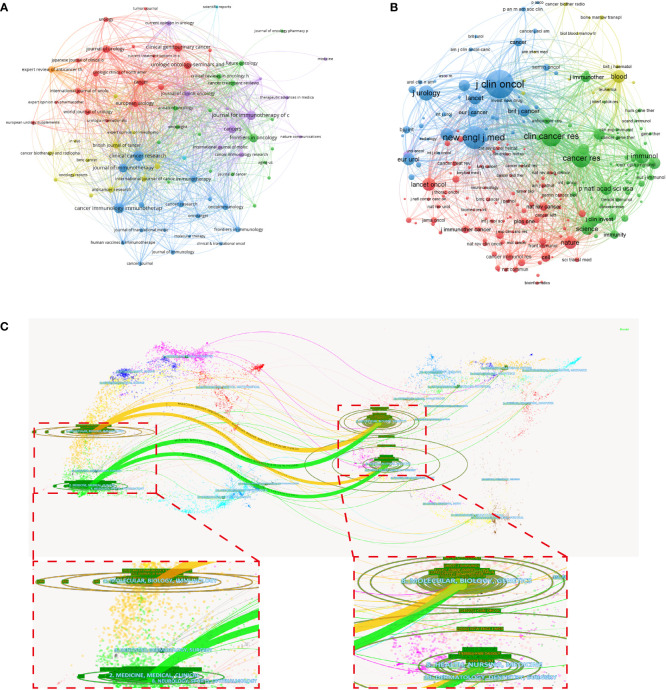
More than 700 journals were assessed in this study, with Cancer Immunology Immunotherapy (N = 102, IF = 6.968, Q1), Clinical Cancer Research (N = 99, IF = 12.531, Q1), and Journal for Immunotherapy of Cancer (N = 85, IF = 13.751, Q1) as the top three journals with most publications ( Table 4 ). Among the top 10 most productive journals, Clinical Cancer Research has the highest H-index (51) and total citations (8,585), whereas Journal for Immunotherapy of Cancer has the highest IF. In addition, all of the top 10 co-cited journals shown in Table 4 are cited more than 2,800 times, with Journal of Clinical Oncology (14,539 times) being the most cited. The network visualization maps of citing journals and co-cited journals were produced using by VOSviewer. As shown in Figures 6A , B , many journals co-occurred in both maps and have active citation relationships.
Table 4.
Top 10 productive journals and co-cited journals in the field of RCC immunotherapy.
| Rank | Journals | Country | Counts | IF(2020) | JCR(2020) | H-index | Total Citations | Co-cited journals | IF(2020) | JCR(2020) | Total citations |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cancer Immunology Immunotherapy | USA | 102 | 6.968 | Q1 | 35 | 3528 | Journal of Clinical Oncology | 44.544 | Q1 | 14539 |
| 2 | Clinical Cancer Research | USA | 99 | 12.531 | Q1 | 51 | 8585 | New England Journal of Medicine | 91.245 | Q1 | 9607 |
| 3 | Journal For Immunotherapy of Cancer | UK | 85 | 13.751 | Q1 | 28 | 3002 | Clinical Cancer Research | 12.531 | Q1 | 7090 |
| 4 | Clinical Genitourinary Cancer | USA | 72 | 2.872 | Q3/Q4 | 16 | 757 | Cancer Research | 12.701 | Q1 | 6273 |
| 5 | Frontiers in Oncology | Switzerland | 71 | 6.244 | Q2 | 12 | 753 | Journal of Urology | 7.45 | Q1 | 4188 |
| 6 | Journal of Immunotherapy | USA | 71 | 4.456 | Q2 | 30 | 3056 | Journal of Immunology | 5.422 | Q2 | 4008 |
| 7 | Cancers | Switzerland | 61 | 6.639 | Q1 | 11 | 428 | Blood | 22.113 | Q1 | 3076 |
| 8 | Urologic Oncology-Seminars and Original Investigations | Netherlands | 61 | 3.498 | Q2/Q3 | 17 | 864 | lancet oncology | 41.316 | Q1 | 3006 |
| 9 | Journal of Urology | USA | 56 | 7.45 | Q1 | 36 | 4114 | Annals of Oncology | 32.976 | Q1 | 2889 |
| 10 | Cancer | USA | 48 | 6.86 | Q1 | 29 | 3170 | Proceedings of The National Academy of Sciences of The United States of America | 11.205 | Q1 | 2851 |
IF, Impact Factor; JCR, Journal Citation Reports.
Figure 6.
The network visualization maps of citing journals (A) and co-cited journals (B) were produced by VOSviewer. (C) A dual-map overlap of journals on RCC immunotherapy carried out by CiteSpace.
A dual-map overlay of journals, generated by CiteSpace, was applied to portray the topic distribution of scientific journals. As displayed in Figure 6C , the citation connections between citing and co-cited journals were indicated by four main color lines. All of the paths indicated that the studies published in Molecular/Biology/Genetics and Health/Nursing/Medicine journals are usually cited by Molecular/Biology/Immunology journals or Medicine/Medical/Clinical journals.
Analysis of references and co-cited references
Reference analysis was conducted in this study to understand the development of RCC immunotherapy research. Therefore, the references with the most citations were analyzed, and CiteSpace was utilized to visualize the reference co-citation network. The top 10 cited and co-cited references are summarized in Tables 5 , 6 . The most cited reference is the article published by Suzanne L. Topalian (2012) (42) in the New England Journal of Medicine, with 8,208 citations, followed by Julie R. Brahmer (2012) (43) and Julie R. Brahmer (2010) (44). From the results in Table 6 , the top three co-cited references were all published by Robert J. Motzer (21, 45, 46).
Table 5.
Top 10 cited papers concerning the research of RCC immunotherapy.
| Title | Journals | First author | Year | Citations |
|---|---|---|---|---|
| Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer | New England Journal of Medicine | Topalian Suzanne L | 2012 | 8208 |
| Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer | New England Journal of Medicine | Brahmer Julie R | 2012 | 5217 |
| Phase I Study of Single-Agent Anti-Programmed Death-1 (MDX-1106) in Refractory Solid Tumors: Safety, Clinical Activity, Pharmacodynamics, and Immunologic Correlates | Journal of Clinical Oncology | Brahmer Julie R | 2010 | 2036 |
| Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy | Nature Reviews Cancer | Topalian Suzanne L | 2016 | 1332 |
| PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy | Molecular Cancer Therapeutics | Patel Sandip Pravin | 2015 | 1181 |
| Renal cell carcinoma | Lancet | Rini Brian I | 2009 | 1046 |
| Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma | Nature Medicine | Parsa Andrew T | 2007 | 970 |
| Cytokines in cancer pathogenesis and cancer therapy | Nature Reviews Cancer | Dranoff G | 2004 | 951 |
| Renal cell carcinoma | Nature Reviews Disease Primers | Hsieh James J | 2017 | 907 |
| The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy | Nature Reviews Cancer | Havel Jonathan J | 2019 | 901 |
Table 6.
Top 10 co-cited references involved in the research of RCC immunotherapy.
| Title | First author | Year | Citations | Journals | IF (2020) |
|---|---|---|---|---|---|
| Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma | Robert J Motzer | 2015 | 615 | New England Journal of Medicine | 91.245 |
| Sunitinib versus interferon alfa in metastatic renal-cell carcinoma | Robert J Motzer | 2007 | 461 | New England Journal of Medicine | 91.245 |
| Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma | Robert J Motzer | 2018 | 400 | New England Journal of Medicine | 91.245 |
| Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma | Gary Hudes | 2007 | 354 | New England Journal of Medicine | 91.245 |
| Safety, activity, and immune correlates of anti-PD-1 antibody in cancer | Suzanne L Topalian | 2012 | 340 | New England Journal of Medicine | 91.245 |
| Sorafenib in advanced clear-cell renal-cell carcinoma | Bernard Escudier | 2007 | 332 | New England Journal of Medicine | 91.245 |
| Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy | G Fyfe | 1995 | 310 | Journal of Clinical Oncology | 44.54 |
| Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma | Brian I Rini | 2019 | 298 | New England Journal of Medicine | 91.245 |
| Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer | R C Flanigan | 2001 | 282 | New England Journal of Medicine | 91.245 |
| Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial | G H Mickisch | 2001 | 260 | Lancet | 79.321 |
IF, impact factor.
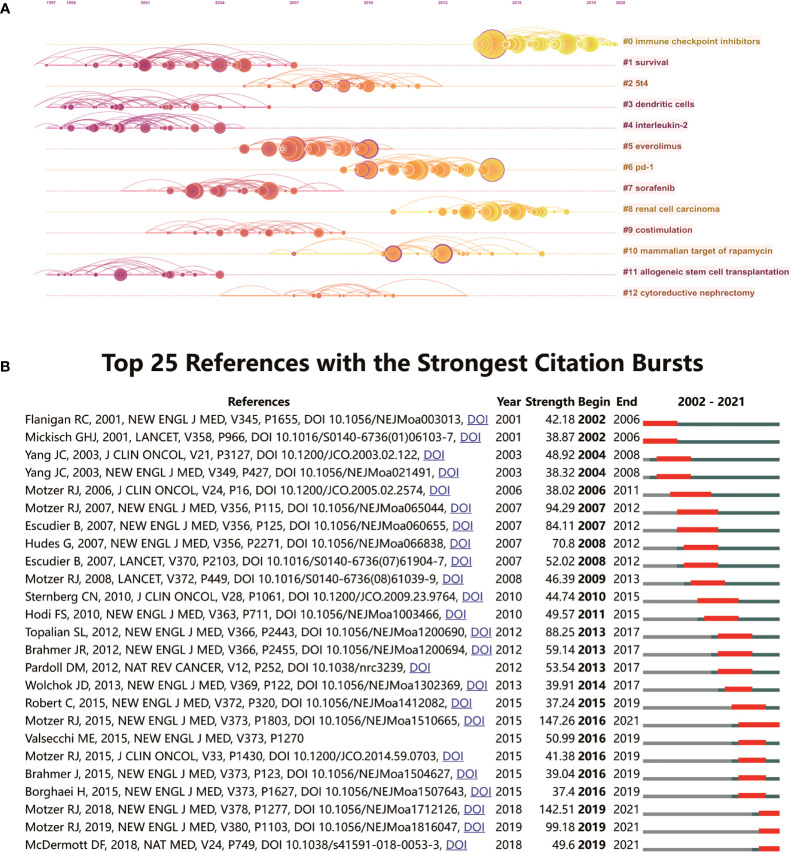
As shown in Supplementary Figure S1 , the reference co-citation network map is composed of 536 nodes, which can be grouped into 13 subclusters. The modularity Q and the mean silhouette S were higher than 0.75, showing a significant cluster result and a good homogeneity effect. Simultaneously, the timeline view of co-cited references visually shows the changing trend of research topics over time ( Figure 7A ). It can be observed that #1 survival, #3 dendritic cells, #4 interleukin-2, and #11 allogeneic stem cell transplantation are the early research topics in this field. Clusters #0 immune checkpoint inhibitors, #6 pd-1, #8 renal cell carcinoma, and #10 mammalian target of rapamycin are located at the line’s rightmost end, demonstrating the current new research foci in this field. Reference citation bursts were applied to show the popularity and importance over time of references in this field. From the results of Figure 7B , we can summarize that the publications of R. C. Flanigan (2001) (47) and G. H. J. Mickisch (2001) (48) are the references with the earliest citations bursts. Meanwhile, R. J. Motzer (2015) (45), R. J. Motzer (2018) (21), R. J. Motzer (2019) (49) and D. F. McDermott (2018) (50) have current emergence of strong citation references.
Figure 7.
(A) CiteSpace visualization map of timeline view of co-citation references analysis. (B) CiteSpace visualization map of top 25 references with the strongest citation bursts from 2002 to 2021.
Analysis of keyword co-occurrence
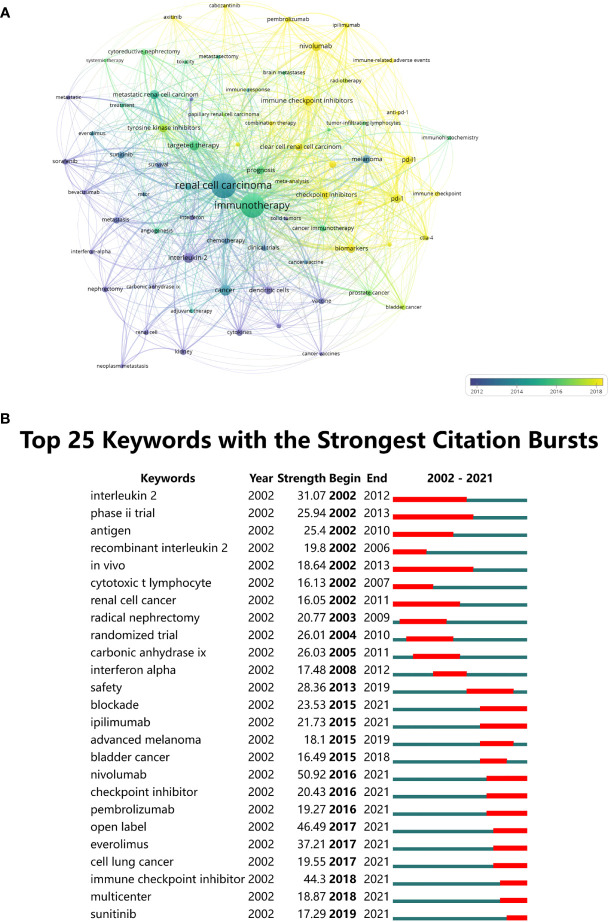
After the synonymous keywords were merged and meaningless keywords removed, VOSviewer software was applied to create the overlay visualization map of keywords. There were 4,539 keywords included, and 73 keywords emerged with a minimum of 20 occurrences ( Figure 8A ). Among them, the top 20 co-occurrence author keywords with the highest frequency in this study are listed in Table 7 . It can be observed that “immune checkpoint inhibitors”, “targeted therapy”, “pd-1”, “pd-l 1”, and “nivolumab” are the keywords that have occurred in recent years; in other words, these keywords seem to represent the current research frontiers ( Figure 8A ). The top 25 keywords with the strongest citation bursts were also detected through CiteSpace ( Figure 8B ), and when combined with the most frequent keywords in Figure 8A , the keywords related to RCC immunotherapy with ongoing citation bursts until 2021 were “blockade”, “ipilimumab”, “nivolumab”, “checkpoint inhibitor”, “pembrolizumab”, “open label”, “everolimus”, “immune checkpoint inhibitor”, “multicenter”, and “sunitinib”. These were the keywords that we mainly focused on because of their effect in identifying the frontiers of RCC immunotherapy research.
Figure 8.
(A) The time-overlay visualization map of the co-occurrence keywords generated by using VOSviewer. (B) CiteSpace visualization map of top 25 keywords with the strongest citation bursts of publications in the field of RCC immunotherapy from 2002 to 2021.
Table 7.
Top 20 co-occurrence keywords involved in the research of RCC immunotherapy.
| Rank | Keywords | Occurrences | TLS | Rank | Keywords | Occurrences | TLS |
|---|---|---|---|---|---|---|---|
| 1 | renal cell carcinoma | 1286 | 5641 | 11 | tyrosine kinase inhibitors | 123 | 715 |
| 2 | immunotherapy | 1200 | 5625 | 12 | biomarkers | 122 | 573 |
| 3 | cancer | 207 | 1021 | 13 | prognosis | 119 | 517 |
| 4 | interleukin-2 | 178 | 786 | 14 | melanoma | 116 | 617 |
| 5 | immune checkpoint inhibitors | 175 | 916 | 15 | checkpoint inhibitors | 104 | 605 |
| 6 | targeted therapy | 173 | 811 | 16 | dendritic cells | 98 | 592 |
| 7 | nivolumab | 154 | 851 | 17 | clear cell renal cell carcinoma | 98 | 536 |
| 8 | pd-1 | 147 | 878 | 18 | sunitinib | 83 | 433 |
| 9 | metastatic renal cell carcinoma | 146 | 663 | 19 | tumor microenvironment | 82 | 425 |
| 10 | pd-l1 | 128 | 793 | 20 | metastasis | 74 | 320 |
TLS, Total Link Strength.
Discussions
General information
Different from reviews or meta-analysis, bibliometric analysis has unique advantages in summarizing the development trend of specific research fields and detecting the research focus. This is the first study to perform a knowledge structure and to analyze the next potential research frontiers in the field of RCC immunotherapy study by using the bibliometric method.
RCC is one of the most common malignancies in both men and women, and its treatment is also a global concern health concern. In the past 20 years, as shown in Figure 2 , great progress has been made in the field of RCC immunotherapy. However, concerning the analysis of countries, there are only 73 countries/regions included in this study, and notably, less than half of the countries published more than 10 papers. It is noteworthy that although China has been participating actively in this field with more than 400 papers, its ACI is the lowest among the top 10 countries. Therefore, the two aspects that should be focused on to change the status quo are as follows: 1) increasing cooperation and exchange with other countries, especially the United States, Italy, and Germany, and 2) paying close attention to scientific innovations to improve the quality of publications.
The top 10 most productive organizations are from the United States. This might explain why the United States contributed the most to the study of RCC immunotherapy. These results implied an imbalance of global academic resources, and the establishment of world-class scientific organizations is the key foundation in promoting the national academic status. In addition, funding support also plays an important role in scientific research, which was also confirmed in this study ( Table 2 ).
Of the top 10 prolific authors, Brian I. Rini is the most prolific author with 60 papers and 4,474 citations in this field. Moreover, among the top co-cited authors, it is obvious that Brian I. Rini is also ranked first as regards centrality, followed by Robert J. Motzer and Toni K. Choueiri. Brian I. Rini is an oncologist at Vanderbilt University Medical Center, and he is famous for his contributions to exploring the treatment and immunotherapy mechanisms of RCC (51). Robert J. Motzer has led many clinical trials on patients with kidney carcinoma and has published many high-level articles in New England Journal of Medicine. Toni K. Choueiri published a well-known review titled “Systemic Therapy for Metastatic Renal-Cell Carcinoma” in New England Journal of Medicine, which systemically summarized the current first-line or second-line therapeutic schedules, as well as surgery strategies for RCC, and constructively put forward the future research directions for RCC treatments (52). Therefore, the aforementioned articles provided reliable reference value for researchers in this field.
Among the top 10 productive journals, Cancer Immunology Immunotherapy published the most papers related to RCC immunotherapy, showing its core role in this field, and more significant findings are more likely to be published in this journal. Except for Journal of Immunology, all of the co-cited journals in Table 4 are located in Q1, demonstrating the importance of RCC immunotherapy in future research.
Knowledge base
Co-citation analysis is an effective method to evaluate the degree of connection among papers (37, 53). It is generally believed that the higher the citation frequency of an article, the more meaningful it is in this field. The top 10 co-citation references shown in Table 6 are all well-known clinical trials published in top-ranked journals. These papers summarized the discovery and development of RCC immunotherapy from cytokines to target therapy and then to ICI-based immunotherapy.
Until 2004, the cytokines IL-2 and IFN-α were considered the only therapeutic drugs that target mRCC. A high-dose IL-2 regimen was also approved by the FDA in 1992 for the treatment of mRCC. Fyfe et al. (54) performed a clinical trial in 1995 to identify the safety and efficacy of high-dose IL-2 in 255 patients with mRCC. Although their results showed that patients with mRCC benefited from high-dose IL-2, severe complications and metastatic diseases still occurred. IFN-α is another cytokine used for treating mRCC and has less severe toxicities, although its overall treatment effect is unsatisfactory. Clinical studies conducted by Flanigan et al. (47) and Mickisch et al. (48) demonstrated that radical nephrectomy before IFN-α treatment can improve the OS and delay the time of disease progression in patients with mRCC. As demonstrated, the treatment options for mRCC are limited. Although IL-2 and IFN-α have suboptimal efficacy and high incidence of toxicity, before 2004, they were the only treatment for patients with mRCC. IL-2 and IFN-α were the beginning of RCC immunotherapy, promoting the development of targeted therapy and combination therapy.
With the deepening of the research on the mechanism of RCC, drugs targeting the pathogenesis of RCC, such as VEGF inhibitors or Mechanistic Target of Rapamycin Complex 1 (mTORC1) inhibitors, have also been continuously developed and applied. Sorafenib is the first VEGF inhibitor drug approved by the FDA in 2005 for treating RCC. Subsequently, sunitinib, pazopanib, and axitinib received FDA approval and are being widely used in the clinical setting. There have been many phase 3 clinical trials that determined the effects of targeted drugs on disease progression and OS in patients with advanced RCC. Motzer et al. (46) studied the curative effects of sunitinib and IFN-α in patients with mRCC, and their results showed that the median progression-free survival (11 vs. 5 months) and objective response rate (31% vs. 6%) were higher in patients with mRCC who were treated with sunitinib than in those who received IFN-α. At the same time, Escudier et al. (55) published a randomized, double-blind, phase 3 study on sorafenib in the treatment of advanced ccRCC, which demonstrated that sorafenib, compared with placebo, can improve the median progression-free survival of patients with advanced ccRCC. Nevertheless, they observed adverse reactions such as hypertension. A few months after the publication of Escudier et al., another well-known randomized clinical trial was published by Hudes et al. (56). Their results showed that compared with IFN-α, temsirolimus might improve the OS of patients with advanced RCC. The aforementioned studies confirmed that the VEGF receptor and mTOR are important targets for the treatment of RCC, and many targeted drugs have also been developed and applied in the clinical setting, improving the OS and progression-free survival of patients. Moreover, sunitinib has become the standard control drug in RCC clinical studies (3).
However, the application of these targeted therapies is often limited by drug resistance. In 2012, Topalian et al. (42) assessed the antitumor activity and safety of an anti-PD-1 antibody (BMS-936558) and preliminarily demonstrated the role of anti-PD-1 in the treatment of RCC. Three years later, nivolumab (PD-1 inhibitor) became the first checkpoint inhibitor to receive FDA approval for RCC treatment. Since then, many clinical studies have proved the safety and efficacy of different ICIs in patients with RCC. The CheckMate 025 study (45) compared the safety and efficacy of nivolumab with those of everolimus in 821 patients with advanced RCC. As their results showed, nivolumab can provide better median OS and objective response than everolimus. Motzer et al. (21, 57) conducted a phase 3 trial to compare the therapeutic efficacy of nivolumab plus ipilimumab with that of sunitinib, and they found that the dual ICI group achieved better OS and progression-free survival than the sunitinib group. This study was the first to demonstrate that dual-ICI combination therapy is more promising than the combination of VEGF and mTOR inhibitor, and nivolumab plus ipilimumab was approved by the FDA as the first-line treatment of mRCC. Since then, many combination therapies have been proposed and confirmed, providing more immunotherapy choices for RCC treatment (18, 49).
Emerging hotspots
The timeline view of co-cited references visualized the dynamic evolution and research hotspots of RCC immunotherapy. As shown in Figure 7A , the research focus has shifted from #1 survival, #3 dendritic cells, #4 interleukin-2, and #11 allogeneic stem cell transplantation to #2 5T4, #5 everolimus, #7 sorafenib, #9 costimulation, and #12 cytoreductive nephrectomy. Currently, clusters #0 immune checkpoint inhibitors, #6 pd-1, #8 renal cell carcinoma, and #10 mammalian target of rapamycin are the new research hotspots in this field.
Reference citation burst detection is a method to identify the references that are highly cited over a certain period. From the results presented in Figure 7B , there are four references with citation bursts to date. R. J. Motzer (2015) (45) is the reference with the strongest citation burst (burst strength 147.26, 2016–2021), followed by R. J. Motzer (2018) (21), R. J. Motzer (2019) (49) and D. F. McDermott (2018) (50). The rise and development of ICIs have brought RCC immunotherapy into a new stage. Nivolumab is the first ICI drug approved by the FDA for RCC treatment and has become one of the most representative drugs in RCC immunotherapy. In the following years, nivolumab or nivolumab-based combination therapy has been widely applied in many clinical studies. Currently, combination therapy may be one of the optimal choices for the immunotherapy of RCC. The first combination therapy implemented in this field was nivolumab-based combination therapy in 2012 (58, 59). R. J. Motzer (2018) (21), R. J. Motzer (2019) (49) and D. F. McDermott (2018) (50) conducted significant trials of combination immunotherapy for RCC, and these studies confirmed that ICI-based combination therapy was more effective than sunitinib for RCC treatment. Undoubtedly, combination therapy is the current research hotspot of RCC immunotherapy, showing good therapeutic potential. The purpose of combination therapy is to improve therapeutic efficacy without affecting safety. However, because combination therapy may create more adverse effects, some clinical trials of ICI monotherapy are also being carried out simultaneously, which hope to find a better ICI therapy regimen as an alternative treatment with fewer adverse effects. Based on the results of D. F. McDermott (2018) (50), atezolizumab (PD-L1 inhibitor) exhibited a high response rate and was well tolerated, showing its excellent potential in ICI monotherapy.
Unfortunately, the efficacy of ICI-based therapy for solid tumors is still unsatisfactory (60–62). On the one hand, the response rate to ICI-based therapy is closely related to many factors such as the tumor mutational burden, indicating that ICI-based therapy may be not effective for most patients. On the other hand, ICI-based therapy may produce some toxic adverse effects. Moreover, there are currently no methods or ancillary tests to identify the precise group that can benefit from ICI-based therapy. Therefore, the development of new biomarkers for predicting the response rate to ICIs and selecting patients who can gain therapeutic benefits may be of great value for RCC immunotherapy.
“Keywords with citation bursts” were also analyzed through CiteSpace in this study. By combining Figure 7A with Figure 8B , one can observe that the bursting keywords were consistent with the current research hotspots analyzed above. Of these keywords, several bursting keywords such as ipilimumab, nivolumab, or pembrolizumab are designated as ICIs, which demonstrated that research on ICIs would still be the focus of this field in the future. It is noteworthy that everolimus and sunitinib were also bursting keywords, indicating that many clinical trials based on the combination therapy of ICIs and mTOR/VEGF inhibitors will be performed in the future. This type of combination therapy is expected to achieve better therapeutic effects and decreased toxic adverse effects through the combination of drugs with different mechanisms of action.
Limitations
Some limitations should be noted in this study. First, the data in this study were collected from the WoSCC database, which means some relevant papers in other data sources may have been excluded. Second, the focus was only on papers published in English, and as a result, high-quality articles published in other languages may have been ignored, leading to selection bias. Last, recently published high-quality papers may not appear in our analysis due to the low citations.
Conclusion
In summary, this is the first bibliometric analysis to outline the knowledge map of RCC immunotherapy from 2002 to 2021 and to predict the future research hotspots. In this analysis, the United States was the leading country with the most high-quality publications and was also the country with the most international communication and cooperation. The cooperation should be enhanced among organizations. Moreover, “immune checkpoint inhibitors” were the most important research hotspot, and the research on ICIs mainly included the following aspects, which may be the next research hotspots: combination therapy, ICI monotherapy, and the development of new predictive biomarkers. Research in this field will help us develop tailored treatment regimens and achieve precision medicine for specific patients with RCC.
Data availability statement
The original contributions presented in the study are included in the article/ supplementary material . Further inquiries can be directed to the corresponding authors.
Author contributions
ZS, JK and HW conceived the study. KL and SZ collected the data and KL wrote the manuscript. JL and YZ analyzed the data. JK, HW and ZS revised and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Xiamen Medical and Health Guidance Project (3502Z20209069).
Acknowledgements
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The handling editor YZ declared a shared affiliation with the authors JK and ZS at the time of review.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.969217/full#supplementary-material
References
- 1. Huang JJ, Hsieh JJ. The therapeutic landscape of renal cell carcinoma: From the dark age to the golden age. Semin Nephrol (2020) 40(1):28–41. doi: 10.1016/j.semnephrol.2019.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barata PC, Rini BI. Treatment of renal cell carcinoma: Current status and future directions. CA: Cancer J Clin (2017) 67(6):507–24. doi: 10.3322/caac.21411 [DOI] [PubMed] [Google Scholar]
- 3. Kathuria-Prakash N, Drolen C, Hannigan CA, Drakaki A. Immunotherapy and metastatic renal cell carcinoma: A review of new treatment approaches. Life (Basel) (2021) 12(1):24. doi: 10.3390/life12010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quhal F, Mori K, Bruchbacher A, Resch I, Mostafaei H, Pradere B, et al. First-line immunotherapy-based combinations for metastatic renal cell carcinoma: A systematic review and network meta-analysis. Eur Urol Oncol (2021) 4(5):755–65. doi: 10.1016/j.euo.2021.03.001 [DOI] [PubMed] [Google Scholar]
- 5. Porpiglia F, Checcucci E, Amparore D, Piramide F, Volpi G, Granato S, et al. Three-dimensional augmented reality robot-assisted partial nephrectomy in case of complex tumours (PADUA ≥10): A new intraoperative tool overcoming the ultrasound guidance. Eur Urol (2020) 78(2):229–38. doi: 10.1016/j.eururo.2019.11.024 [DOI] [PubMed] [Google Scholar]
- 6. Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, et al. Renal cell carcinoma. Nat Rev Dis Primers (2017) 3:17009. doi: 10.1038/nrdp.2017.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Narayan V, Puligandla M, Haas NB, Subramanian P, DiPaola RS, Uzzo R. Patterns of relapse and implications for post-nephrectomy surveillance in patients with high risk nonclear cell renal cell carcinoma: Subgroup analysis of the phase 3 ECOG-ACRIN E2805 trial. J Urol (2019) 201(1):62–8. doi: 10.1016/j.juro.2018.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graham J, Dudani S, Heng DYC. Prognostication in kidney cancer: Recent advances and future directions. J Clin Oncol: Off J Am Soc Clin Oncol (2018), Jco2018790147. doi: 10.1200/jco.2018.79.0147 [DOI] [PubMed] [Google Scholar]
- 9. Siva S, Kothari G, Muacevic A, Louie AV, Slotman BJ, Teh BS, et al. Radiotherapy for renal cell carcinoma: renaissance of an overlooked approach. Nat Rev Urol (2017) 14(9):549–63. doi: 10.1038/nrurol.2017.87 [DOI] [PubMed] [Google Scholar]
- 10. Bilen MA, Carlisle JW, Sonpavde G. The prospects for combination therapy with capecitabine in the rapidly evolving treatment landscape of renal cell carcinoma. Expert Opin Investigational Drugs (2018) 27(2):163–70. doi: 10.1080/13543784.2018.1427731 [DOI] [PubMed] [Google Scholar]
- 11. Gulati S, Vaishampayan U. Current state of systemic therapies for advanced renal cell carcinoma. Curr Oncol Rep (2020) 22(3):26. doi: 10.1007/s11912-020-0892-1 [DOI] [PubMed] [Google Scholar]
- 12. Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer (2017) 17(5):286–301. doi: 10.1038/nrc.2017.17 [DOI] [PubMed] [Google Scholar]
- 13. Zibelman M, Plimack ER. Integrating immunotherapy into the management of renal cell carcinoma. J Natl Compr Cancer Network: JNCCN (2017) 15(6):841–7. doi: 10.6004/jnccn.2017.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taefehshokr S, Parhizkar A, Hayati S, Mousapour M, Mahmoudpour A, Eleid L, et al. Cancer immunotherapy: Challenges and limitations. Pathol Res Pract (2022) 229:153723. doi: 10.1016/j.prp.2021.153723 [DOI] [PubMed] [Google Scholar]
- 15. You F, Jiang L, Zhang B, Lu Q, Zhou Q, Liao X, et al. Phase 1 clinical trial demonstrated that MUC1 positive metastatic seminal vesicle cancer can be effectively eradicated by modified anti-MUC1 chimeric antigen receptor transduced T cells. Sci China Life Sci (2016) 59(4):386–97. doi: 10.1007/s11427-016-5024-7 [DOI] [PubMed] [Google Scholar]
- 16. Kosti P, Maher J, Arnold JN. Perspectives on chimeric antigen receptor T-cell immunotherapy for solid tumors. Front Immunol (2018) 9:1104. doi: 10.3389/fimmu.2018.01104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Priceman SJ, Tilakawardane D, Jeang B, Aguilar B, Murad JP, Park AK, et al. Regional delivery of chimeric antigen receptor-engineered T cells effectively targets HER2+ breast cancer metastasis to the brain. Clin Cancer Res (2018) 24(1):95–105. doi: 10.1158/1078-0432.Ccr-17-2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med (2019) 380(12):1116–27. doi: 10.1056/NEJMoa1816714 [DOI] [PubMed] [Google Scholar]
- 19. Powles T, Plimack ER, Soulières D, Waddell T, Stus V, Gafanov R, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol (2020) 21(12):1563–73. doi: 10.1016/s1470-2045(20)30436-8 [DOI] [PubMed] [Google Scholar]
- 20. Motzer RJ, Porta C, Eto M, Powles T, Grünwald V, Hutson TE, et al. Phase 3 trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) or everolimus (EVE) versus sunitinib (SUN) monotherapy as a first-line treatment for patients (pts) with advanced renal cell carcinoma (RCC) (CLEAR study). J Clin Oncol (2021) 39(6_suppl):269. doi: 10.1200/JCO.2021.39.6_suppl.269 33275488 [DOI] [Google Scholar]
- 21. Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med (2018) 378(14):1277–90. doi: 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nabi S, Kessler ER, Bernard B, Flaig TW, Lam ET. Renal cell carcinoma: a review of biology and pathophysiology. F1000Research (2018) 7:307. doi: 10.12688/f1000research.13179.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McDermott DF, Lee JL, Ziobro M, Suarez C, Langiewicz P, Matveev VB, et al. Open-label, single-arm, phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced non-clear cell renal cell carcinoma. J Clin Oncol: Off J Am Soc Clin Oncol (2021) 39(9):1029–39. doi: 10.1200/jco.20.02365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McKay RR, McGregor BA, Xie W, Braun DA, Wei X, Kyriakopoulos CE, et al. Optimized management of nivolumab and ipilimumab in advanced renal cell carcinoma: A response-based phase II study (OMNIVORE). J Clin Oncol: Off J Am Soc Clin Oncol (2020) 38(36):4240–8. doi: 10.1200/jco.20.02295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Atkins MB, Jegede OA, Haas NB, McDermott DF, Bilen MA, Stein M, et al. Phase II study of nivolumab and salvage Nivolumab/Ipilimumab in treatment-naive patients with advanced clear cell renal cell carcinoma (HCRN GU16-260-Cohort a). J Clin Oncol: Off J Am Soc Clin Oncol (2022), Jco2102938. doi: 10.1200/jco.21.02938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen C, Dubin R, Kim MC. Emerging trends and new developments in regenerative medicine: a scientometric update (2000 - 2014). Expert Opin Biol Ther (2014) 14(9):1295–317. doi: 10.1517/14712598.2014.920813 [DOI] [PubMed] [Google Scholar]
- 27. Shen J, Shen H, Ke L, Chen J, Dang X, Liu B, et al. Knowledge mapping of immunotherapy for hepatocellular carcinoma: A bibliometric study. Front Immunol (2022) 13:815575. doi: 10.3389/fimmu.2022.815575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shen Z, Wu H, Chen Z, Hu J, Pan J, Kong J, et al. The global research of artificial intelligence on prostate cancer: A 22-year bibliometric analysis. Front Oncol (2022) 12:843735. doi: 10.3389/fonc.2022.843735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yeung AWK, Kulnik ST, Parvanov ED, Fassl A, Eibensteiner F, Völkl-Kernstock S, et al. Research on digital technology use in cardiology: Bibliometric analysis. J Med Internet Res (2022) 24(5):e36086. doi: 10.2196/36086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma L, Ma J, Teng M, Li Y. Visual analysis of colorectal cancer immunotherapy: A bibliometric analysis from 2012 to 2021. Front Immunol (2022) 13:843106. doi: 10.3389/fimmu.2022.843106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ou Z, Qiu L, Rong H, Li B, Ren S, Kuang S, et al. Bibliometric analysis of chimeric antigen receptor-based immunotherapy in cancers from 2001 to 2021. Front Immunol (2022) 13:822004. doi: 10.3389/fimmu.2022.822004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li C, Wu H, Sun Z, Chen Z, Trampuz A. Global publication trends and research hotspots of revision hip and knee arthroplasty: A 21-year bibliometric approach. J Arthroplasty (2022) 37(5):974–84. doi: 10.1016/j.arth.2022.01.022 [DOI] [PubMed] [Google Scholar]
- 33. Ke L, Lu C, Shen R, Lu T, Ma B, Hua Y. Knowledge mapping of drug-induced liver injury: A scientometric investigation (2010-2019). Front Pharmacol (2020) 11:842. doi: 10.3389/fphar.2020.00842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci USA (2005) 102(46):16569–72. doi: 10.1073/pnas.0507655102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheng K, Guo Q, Shen Z, Yang W, Wang Y, Sun Z, et al. Bibliometric analysis of global research on cancer photodynamic therapy: Focus on nano-related research. Front Pharmacol (2022) 13:. doi: 10.3389/fphar.2022.927219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu H, Zhou Y, Xu L, Tong L, Wang Y, Liu B, et al. Mapping knowledge structure and research frontiers of ultrasound-induced blood-brain barrier opening: A scientometric study. Front Neurosci (2021) 15:706105. doi: 10.3389/fnins.2021.706105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu H, Tong L, Wang Y, Yan H, Sun Z. Bibliometric analysis of global research trends on ultrasound microbubble: A quickly developing field. Front Pharmacol (2021) 12:646626. doi: 10.3389/fphar.2021.646626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics (2010) 84(2):523–38. doi: 10.1007/s11192-009-0146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen C. CiteSpace II: Detecting and visualizing emerging trends. J Am Soc Inform Sci Technol (2006) 57(3):359–77. JJotASfIS, Technology. doi: 10.1002/asi.20317 [DOI] [Google Scholar]
- 40. Synnestvedt MB, Chen C, Holmes JH. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA Ann Symp Proc Arch (2005) 2005:724. JAASpASAS. [PMC free article] [PubMed] [Google Scholar]
- 41. Chen C. CiteSpace: A practical guide for mapping scientific literature. Nova Science Publishers; (2016). [Google Scholar]
- 42. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med (2012) 366(26):2443–54. doi: 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med (2012) 366(26):2455–65. doi: 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol: Off J Am Soc Clin Oncol (2010) 28(19):3167–75. doi: 10.1200/jco.2009.26.7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med (2015) 373(19):1803–13. doi: 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med (2007) 356(2):115–24. doi: 10.1056/NEJMoa065044 [DOI] [PubMed] [Google Scholar]
- 47. Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med (2001) 345(23):1655–9. doi: 10.1056/NEJMoa003013 [DOI] [PubMed] [Google Scholar]
- 48. Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: A randomised trial. Lancet (London England) (2001) 358(9286):966–70. doi: 10.1016/s0140-6736(01)06103-7 [DOI] [PubMed] [Google Scholar]
- 49. Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med (2019) 380(12):1103–15. doi: 10.1056/NEJMoa1816047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McDermott DF, Huseni MA, Atkins MB, Motzer RJ, Rini BI, Escudier B, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med (2018) 24(6):749–57. doi: 10.1038/s41591-018-0053-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet (London England) (2009) 373(9669):1119–32. doi: 10.1016/s0140-6736(09)60229-4 [DOI] [PubMed] [Google Scholar]
- 52. Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med (2017) 376(4):354–66. doi: 10.1056/NEJMra1601333 [DOI] [PubMed] [Google Scholar]
- 53. Small H. Co-Citation in the scientific literature: A new measure of the relationship between two documents. J Am Soc Inform Sci Technol (1973) 24(4):265–9. doi: 10.1002/asi.4630240406 [DOI] [Google Scholar]
- 54. Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol: Off J Am Soc Clin Oncol (1995) 13(3):688–96. doi: 10.1200/jco.1995.13.3.688 [DOI] [PubMed] [Google Scholar]
- 55. Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med (2007) 356(2):125–34. doi: 10.1056/NEJMoa060655 [DOI] [PubMed] [Google Scholar]
- 56. Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med (2007) 356(22):2271–81. doi: 10.1056/NEJMoa066838 [DOI] [PubMed] [Google Scholar]
- 57. Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthélémy P, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: Extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open (2020) 5(6):e001079. doi: 10.1136/esmoopen-2020-001079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hammers HJ, Plimack ER, Infante JR, Rini BI, McDermott DF, Lewis LD, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: The CheckMate 016 study. J Clin Oncol: Off J Am Soc Clin Oncol (2017) 35(34):3851–8. doi: 10.1200/jco.2016.72.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Amin A, Plimack ER, Ernstoff MS, Lewis LD, Bauer TM, McDermott DF, et al. Safety and efficacy of nivolumab in combination with sunitinib or pazopanib in advanced or metastatic renal cell carcinoma: the CheckMate 016 study. J Immunother Cancer (2018) 6(1):109. doi: 10.1186/s40425-018-0420-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tchou J, Zhao Y, Levine BL, Zhang PJ, Davis MM, Melenhorst JJ, et al. Safety and efficacy of intratumoral injections of chimeric antigen receptor (CAR) T cells in metastatic breast cancer. Cancer Immunol Res (2017) 5(12):1152–61. doi: 10.1158/2326-6066.Cir-17-0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chai LF, Prince E, Pillarisetty VG, Katz SC. Challenges in assessing solid tumor responses to immunotherapy. Cancer Gene Ther (2020) 27(7-8):528–38. doi: 10.1038/s41417-019-0155-1 [DOI] [PubMed] [Google Scholar]
- 62. Leko V, Rosenberg SA. Identifying and targeting human tumor antigens for T cell-based immunotherapy of solid tumors. Cancer Cell (2020) 38(4):454–72. doi: 10.1016/j.ccell.2020.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ supplementary material . Further inquiries can be directed to the corresponding authors.