Abstract
The production of type 8 capsular polysaccharide (CP8) in Staphylococcus aureus is regulated in response to a variety of environmental factors. The cap8 genes required for the CP8 production in strain Becker are transcribed as a single large transcript by a primary promoter located within a 0.45-kb region upstream of the first gene of the cap8 gene cluster. In this study, we analyzed the primary cap8 promoter region in detail. We determined the transcription initiation site of the primary transcript by primer extension and identified the potential promoter sequences. We found several inverted and direct repeats upstream of the promoter. Deletion analysis and site-directed mutagenesis showed that a 10-bp inverted repeat of one of the repeats was required for promoter activity. We showed that the distance but not the specific sequences between the inverted repeat and the promoter was critical to the promoter activity. However, insertion of a DNA sequence with two or four helix turns in this intervening region had a slight effect on promoter activity. To demonstrate the biological significance of the 10-bp inverted repeat, we constructed a strain with a mutation in the repeat in the S. aureus Becker chromosome and showed that the repeat affected CP8 production mostly at the transcriptional level. By gel mobility shift assay, we demonstrated that strain Becker produced at least one protein capable of specific binding to the 10-bp inverted repeat, indicating that the repeat serves as a positive regulatory protein binding site. In addition, reporter gene fusion analysis showed that the cap8 promoter activity was influenced by various growth media and affected most by yeast extract. Our results suggest that yeast extract may exert its profound inhibitory effect on cap8 gene expression through the 10-bp inverted repeat element.
Staphylococcus aureus is a common human pathogen that causes a variety of diseases. The organism has the ability to produce a number of virulence factors, including capsular polysaccharide (CP). Although 11 serotypes of CP have been identified in S. aureus strains, more than 80% of the clinical isolates produce either type 5 CP (CP5) or type 8 CP (CP8) (1, 3, 18, 20, 21, 39, 48). These strains produce a small amount of CP and are hence referred to as microencapsulated (52). In contrast, strains elaborating either type 1 CP (CP1) or type 2 CP (CP2) produce a large amount of capsule and manifest a mucoid phenotype on agar. While CP1 and CP2 have been shown to confer virulence to S. aureus (26, 29, 32) by evading phagocytes (38), the role of CP5 and CP8 in virulence has been controversial (2, 4, 22, 35, 54). Recent reports, however, showed that antibodies against CP5 and CP8 were protective against S. aureus infections when immunized mice were challenged intraperitoneally (11) and that CP5 was an important virulence factor of S. aureus in a murine septic arthritis model (36).
The cap8 and cap5 gene clusters, involved in the synthesis of CP8 and CP5, respectively, have been cloned and sequenced (28, 44–46). Each gene cluster consists of 16 genes which are tightly clustered and transcribed in one direction. The two gene clusters share 12 genes, while the remaining 4 genes in each cluster are type specific. The two gene clusters have the same organization in that the 4 type-specific genes in the central region are flanked by the common genes. The fact that they have genes in common explains why CP5 and CP8 are almost identical trisaccharide-repeated polysaccharides that differ only in the location of O-acetylation and the position linking monosaccharides (12, 34). Molecular characterization of the cap8 locus showed that all 16 genes were transcribed as a large transcript from a primary promoter upstream of the first gene, cap8A. However, several internal promoters within the cap8 locus were also identified by genetic complementation and reporter gene fusion studies (46). The cap1 gene cluster, required for CP1 synthesis, has also been cloned, sequenced, and molecularly characterized in our laboratory (25, 29, 37). The cap1 locus consists of 13 closely linked genes that are transcribed as one major transcript with several weak internal promoters, similar to the cap8 gene cluster. The cap5 and cap8 loci are allelic, whereas the cap1 locus mapped at a different location (44–46).
A number of studies have shown that the production of CP5 and CP8 is influenced by various environmental factors. Lee et al. (27) showed that low iron concentration, growth in vivo, or growth on solid medium enhanced CP8 production. Similarly, production of CP5 was affected by medium, pH, oxygen tension, carbon dioxide, and growth phase (8, 17, 50). In the presence of milk, increased production of microcapsules had been observed in mastitis isolates of S. aureus (31, 51). Recently, a global regulatory locus, agr (41), was shown to positively regulate CP5 production (9). Taken together, these studies suggest that microcapsules are highly regulated. However, regulation of staphylococcal capsule production has not been studied at the molecular level. As a first step in such studies, we analyzed the primary promoter region by deletion analysis and site-directed mutagenesis. We showed that a cis-acting element was required for cap8 promoter activity and CP8 production. The cis-acting element may serve as a positive regulatory protein binding site.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
Escherichia coli HB101 and XL1-Blue, which were used for plasmid constructions and transformations, were cultivated in Luria broth or agar (Difco Laboratories). Trypticase soy broth (TSB) and Columbia broth (CB) were obtained from Difco Laboratories. Tryptone-glucose-NaCl broth (TDNB) contains 1% Tryptone, 0.25% dextrose, and 0.5% NaCl. Low-phosphate medium (LPM) was prepared as described by Wunschel et al. (53) except it contains 0.32 mM phosphate (0.2 mM K2HPO4 · H2O and 0.12 mM KH2PO4) and 0.1 M Tris-HCl (pH 7.5). High-phosphate medium (HPM) is the same as LPM except that the total phosphate concentration is 50 mM (33.3 mM K2HPO4 · H2O and 16.7 mM KH2PO4). S. aureus RN4220 (24) was used as the recipient in electroporations of plasmids. Electroporation was carried out by the procedure of Kraemer and Iandolo (23). Transduction was carried out as described by Shafer and Iandolo (47), using bacteriophage 52A (25). Quantitation of CP8 was performed by rocket immunoelectrophoresis (RIE) as described previously (44).
DNA manipulations.
Standard DNA manipulations were performed as described by Sambrook et al. (43). Plasmid DNA was purified by using a plasmid purification kit (Qiagen). Rapid small-scale plasmid DNA purification from E. coli was done by the method of Holmes and Quigley (19). Small-scale plasmid DNA isolation from S. aureus was performed by using a Wizard Plus Minipreps kit (Promega). Bulk chromosomal DNA from S. aureus was purified as described by Dyer and Iandolo (10). Transfer of DNA to nitrocellulose membranes was done by the method of Southern (49). Conditions used for hybridization analysis have been described previously (44). DNA sequencing was carried out with an automatic DNA sequencer (LICOR 4000L).
Primer extension and RNA manipulations.
S. aureus Becker was grown on Trypticase soy agar overnight at 37°C, and total RNA was isolated by the procedure of Cheung et al. (7), using a FastRNA kit from BIO 101. RNA dot blot was carried out as described by Sambrook et al. (43). Primer extension analysis was performed by using an avian myeloblastosis virus reverse transcriptase primer extension kit as recommended by the supplier (Promega), with modifications. Briefly, about 30 μg of total RNA was ethanol precipitated along with an end-labeled primer (5′TTAATTCTAATGTACTTTCC3′) complementary to the N-terminal coding sequence of cap8A. After washing with 70% ethanol, the pellet was suspended in 7 μl of 250 mM KCl and incubated at 42°C for 3 h. The reaction mixture was then treated with avian myeloblastosis virus reverse transcriptase. The reaction was stopped with the addition of 10 μl of sequencing stop buffer, denatured, and resolved in a 6% polyacrylamide gel. A sequencing reaction using the same primer was run simultaneously as a molecular weight standard.
Construction of transcriptional fusion plasmids.
The promoterless xylE gene from Pseudomonas putida was used as the reporter gene for transcriptional fusions. All plasmids, unless mentioned specifically, were constructed by ligating various PCR-generated DNA fragments of the cap8 promoter region to the EcoRI and HindIII sites of pLC4 (40). Plasmid pCL7816, with an 5.7-kb insert which contains the cap8 promoter region, was used as the template in PCR amplification for constructing mutations shown in Fig. 2 and 3 (except for pCL8265). PCR amplifications were achieved by pairing various forward primers containing appropriate changes and a reverse primer, Pp8ar, listed in Table 1. The amplified fragments were first cloned in pGEM-T (Promega), verified by sequencing, and then cloned into the reporter vector. Plasmids pCL8239 and pCL8240 were constructed by replacing the SnaBI-HindIII fragment of pCL8218 with the respective PCR fragments. Plasmids pCL8284, pCL8308, pCL8312, and pCL8321 were constructed by ligating the phosphorylated annealed complementary primers, P21merF (5′GCATATTAAGGATCCTAATCG3′) and P21merR (5′CGATTAGGATCCTTAATATGC3′) to the SnaBI site of pCL8218 and verified by sequencing. Plasmid pCL8265 contains an insert equivalent to that of plasmid pCL8074 except that the insert was PCR amplified from plasmid pCL8226, described below. All plasmids were first electroporated into S. aureus RN4220 and then transduced into strain Becker by bacteriophage 52A. The catechol 2,3-dioxygenase (the gene product of xylE) activities of the strains containing various fusions were assayed as previously described (55).
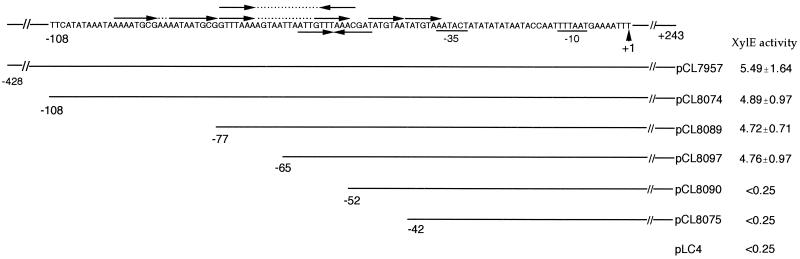
FIG. 2.
Deletion analysis of the region upstream of the cap8 promoter. The relevant DNA sequence is shown at the top; arrows indicate direct and inverted repeats. The numbers at the end of the sequence indicate the endpoints of the fragments with respect to the transcription start site, +1; the −10 sequence and the tentative −35 sequence of the promoter are also shown. XylE activities of the fusion plasmids are expressed in milliunits per milligram of protein as means ± standard deviations of three independent tests.
FIG. 3.
Site-directed mutagenesis of the region upstream of the cap8 promoter. The altered nucleotides are outlined. Other symbols and descriptions are as described in the legend to Fig. 2.
TABLE 1.
Primers for PCR
| Primera | Sequence |
|---|---|
| Forward | |
| Pp8af1 (pCL8074) | 5′GAATTCATATAAATAAAAATGCGAAAAT3′ |
| Pp8af4 (pCL8089) | 5′GAATTCGTTTAAAAGTAATTAATTGTTTA3′ |
| Pp8af6 (pCL8097) | 5′GAATTCTTAATTGTTTAAACGATATGT3′ |
| Pp8af5 (pCL8090) | 5′GAATTCGATATGTAATATGTAAATACT3′ |
| Pp8af2 (pCL8075) | 5′GAATTCTATGTAAATACTATATATATAA3′ |
| Pp8af9 (pCL8116) | 5′GAATTCTTACATGTTTAAACGATATGTAAT3′ |
| Pp8af11 (pCL8118) | 5′GAATTCTTAATCGTTTAAACGATATGT3′ |
| Pp8af12 (pCL8160) | 5′GAATTCTTAATTTTTTAAACGATATGT3′ |
| Pp8af10 (pCL8117) | 5′GAATTCTTAATTGCATAAACGATATGTAAT3′ |
| Pp8af13 (pCL8161) | 5′GAATTCTTAATTGTTCAAACGATATGT3′ |
| Pp8af15 (pCL8204) | 5′GAATTCTTAATTGTTTCGACGATATGT3′ |
| Pp8af16 (pCL8195) | 5′GAATTCTTAATTGTTTAACGGATATGT3′ |
| Pp8af26 (pCL8263) | 5′GAATTCTTAATTGTTTAAAAGATATGTAATATG3′ |
| Pp8af14 (pCL8162) | 5′GAATTCTTAATTGTTTAAACAATATGT3′ |
| Pp8af27 (pCL8264) | 5′GAATTCTTAATTGTTTAAACGGTATGTAATATG3′ |
| Pp8af17 (pCL8218) | 5′GAATTCTTAATTGTTTAAACGATACGTAATATGT3′ |
| Pp8af28 (pCL8279) | 5′GAATTCTTAATTGTTTAAACGATATGTGATATCTAAATACTA3′ |
| Pp8af18 (pCL8219) | 5′GAATTCTTAATTGTTTAAACGATATAATATGTAAATACTA3′ |
| Pp8af30 (pCL8331) | 5′GAATTCTTAATTGTTTAAACGATACATATCTACTTATTCTAACTATGTAATATGTAAATACTATATA3′ |
| Pp8af20 (pCL8239) | 5′TACGTAATATGTAAGGCCTATATATATAA3′ |
| Pp8af21 (pCL8240) | 5′TACGTAATATGTATTGACAATATATATAATAC3′ |
| Pp8af1 (pCL8265) | 5′GAATTCATATAAATAAAAATGCGAAAAT3′ |
| Reverse | |
| Pp8ar | 5′AAGCTTGTATTTACAAGTTGAATATTAC3′ |
Plasmids containing the inserts amplified by the corresponding forward primers are shown in parentheses.
Mutagenesis of the inverted repeat on the chromosome.
A 2.4-kb EcoRI-HindIII DNA fragment containing the upstream region of the cap8 locus, the cap8A coding region, and the 5′ portion of the cap8B coding region was cloned into the EcoRI and HindIII sites of plasmid pALTER-1 (Promega). The resulting plasmid was used for site-directed mutagenesis of the 10-bp inverted repeat, using mutagenic primer 5′TTAAAAGTAATTAATGGATCCAACGATATGTAATATG3′, which contains a BamHI site (underlined) within the repeat, according to the manufacturer’s protocol. The EcoRI-HindIII fragment containing the desired mutation, verified by sequencing, was subsequently cloned into the similarly digested temperature-sensitive plasmid vector pCL52.1 (29). The resultant plasmid, pCL8226, was electroporated into strain RN4220 and then transferred into strain Becker by phage 52A transduction. An allelic exchange procedure described previously (29) was used to generate mutant strain CYL6401. Southern blotting using BamHI-digested DNAs was used to confirm the construction (data not shown). Sequences of the promoter region in the chromosome of CYL6401 were amplified by PCR and verified by sequencing. No mutations other than the desired one were detected (data not shown).
DNA gel mobility shift assay.
S. aureus Becker cultures were grown in broth media at 37°C overnight. Cell extracts were made from lysostaphin-treated cells as described by Mahmood and Khan (30). A 63-bp DNA fragment containing either the wild-type 10-bp inverted repeat upstream of cap8A or the mutated repeat in the corresponding region was obtained by PCR amplification using a pair of primers, 5′GTATTTACATATTACATATC3′ and 5′AATGCGAAAATAATGCGG3′. The amplified DNA fragments were purified by a PCR purification kit (Qiagen) and end labeled with [32P]ATP, using T4 polynucleotide kinase (Gibco-BRL). The labeled DNA fragments were used in a gel mobility shift assay performed as described by Cheung et al. (6), with slight modifications. Briefly, 4 μl of Becker cell extracts was mixed with end-labeled DNA fragments and 1 μg of poly(dI-dC) in the assay buffer (10 mM Tris-HCl [pH 7.5], 1 mM EDTA, 50 mM NaCl, 5% glycerol). The mixtures (20 μl, final volume) were incubated at room temperature for 15 min, and the reaction was terminated by the addition of the stop buffer containing 0.1% bovine serum albumin, 50% glycerol, and 0.01% xylene cyanol. The samples were resolved in a 6% polyacrylamide gel in Tris borate-EDTA buffer for 2 h at 200 V. After electrophoresis, the gels were dried and autoradiographed.
RESULTS
Mapping of the transcription initiation site.
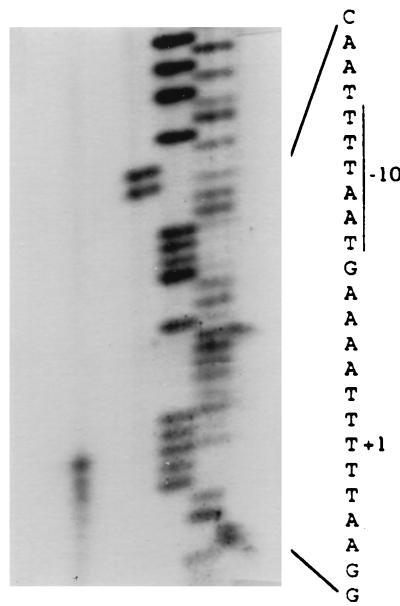
Previously, we reported that the 16 cap8 genes were organized as an operon transcribed in a 17-kb transcript from the primary promoter upstream of cap8A, the first gene of the cap8 operon (46). It is conceivable that the regulation of CP8 production in S. aureus is exerted at the primary promoter. To locate the promoter more precisely, the 5′ end of the 17-kb transcript was determined by primer extension analysis. As shown in Fig. 1, a major band corresponding to the T residue located 17 nucleotides upstream of the ATG codon of the cap8A gene was detected, though several smaller bands were also found. This result was reproducible, suggesting that there may be other starting sites near the major start site. The sequence 5′TTTAAT3′, with five matches to the consensus −10 promoter sequence of Bacillus and E. coli (16, 33), was found seven nucleotides upstream of the start site. However, no consensus −35 sequence was detected. Nevertheless, the sequence 5′AATACT3′, with only two matches to the consensus −35 promoter sequence, was found 17 nucleotides upstream of the −10 promoter sequence. For ease of discussion, we tentatively assigned this sequence as the potential −35 sequence (Fig. 2).
FIG. 1.
Mapping of the 5′ end of the cap8 primary transcript by primer extension. The position of the major initiation site is indicated by +1. The DNA sequence ladder was generated by using the same primer.
Requirement for a 10-bp inverted repeat for cap8 gene transcription.
Interestingly, just upstream of the tentative −35 sequence we found several direct repeats and inverted repeats which could serve as potential protein binding sites for regulators. To determine whether any of these repeats can act as a cis-acting regulatory element for cap8 gene expression, DNA fragments containing the primary cap8 promoter with various 5′-end deletions were constructed by PCR and transcriptionally fused to a promoterless xylE reporter gene in pLC4. The resultant plasmids were electroporated into strain RN4220 and moved into strain Becker by phage 52A transduction. The results in Fig. 2 showed that deletions up to nucleotide −65 did not affect promoter activity, whereas deletions beyond nucleotide −52 reduced promoter activity to the basal level. These results indicate that the 14-bp inverted repeat with sequence 5′ATTGTTTAAACGAT3′ may be required for cap8 genes transcription, while other repeats may not play a regulatory role under our experimental conditions.
To confirm that the 14-bp inverted repeat described above is required for promoter activity, site-directed mutations within the repeat were constructed by PCR using primers containing various changes (see Materials and Methods). The resultant fragments containing the mutations were again fused with the xylE reporter gene to assess promoter activities. The results (Fig. 3) showed that changing the first two nucleotides of the inverted repeat (pCL8116) as well as replacing nucleotide A located next to the last nucleotide of the repeat (pCL8264) did not affect promoter activity. These results suggest that the four nucleotides at the periphery of the inverted repeat are dispensable. However, mutations with one- or two-nucleotide replacement in the central eight nucleotides within the 14-bp inverted repeat decreased promoter activity to the basal level (pCL8160, pCL8117, pCL8161, pCL8204, pCL8195, and pCL8263), indicating that the central eight nucleotides are critical for transcription. The 14-bp repeat is an imperfect one, with two noncomplementary nucleotides, A and C, located at the third positions from the ends of the repeat. To determine whether mutations converting the repeat to a perfect match would affect promoter activity, we changed these two nucleotides to ones that are complementary to each other. Our results showed that while changing the left repeat to match the right repeat reduced promoter activity to the basal level (pCL8118), changing the right repeat to match the left repeat (pCL8162) reduced the activity only about threefold compared to the wild type. Taken together, these results indicate that the inner 10 nucleotides of the inverted repeat are crucial for enhancing cap8 promoter activity.
The intervening region and the tentative −35 sequence.
The fact that plasmid pCL8264, which contains a mutation between the 10-bp inverted repeat and the tentative −35 sequence, exhibits about the same XylE activity as plasmid pCL8097, which contains no mutation, suggests that specific sequences in the 14-bp intervening region may not play a role in cap8 gene activation. To test this notion, two plasmids containing mutations within the 14-bp intervening sequence (pCL8218 and pCL8279) were constructed. The results in Fig. 3 strongly suggest that specific sequences between the 10-bp inverted repeat and the tentative −35 sequence are not critical. Furthermore, these results indicate that the direct repeat found in the intervening region (Fig. 3) is not involved in cap8 gene expression. Interestingly, deletion of two nucleotides within the intervening sequence resulted in the abrogation of promoter activity (pCL8219). Taken together, these results imply that the distance between the inverted repeat and the tentative −35 sequence is crucial for promoter activation, though the exact sequences are not important. These results also suggest that maintaining the inverted repeat and the cap8 promoter in the same face of the DNA helix may be important for promoter activity. To test this hypothesis, we constructed insertions in the intervening region. Since plasmid pCL8218, which contains a SnaBI site, showed promoter activity similar to that of the control plasmid pCL8097, we took advantage of the SnaBI site and inserted sequences at this site. As shown in Fig. 3, plasmids with insertions of 21 bp (pCL8331) and 42 bp (pCL8308), which represent about two and four multiples of helix turn of 10.5 bp per turn, respectively, still exhibited significant promoter activity. On the other hand, an insertion of 17 bp (pCL8321), 20 bp (pCL8312), or 39 bp (pCL8284), which shifted the inverted repeat out of phase with respect to the promoter, reduced promoter activity to the basal level.
As stated above, upstream of the transcriptional start site we found no good match to the canonical −35 sequence. To define the promoter sequence more precisely, plasmid pCL8239, which contains three nucleotide changes in the tentative −35 region (Fig. 3), was constructed from pCL8218. The mutation resulted in no promoter activity, indicating that the tentative −35 sequence is crucial for promoter activity. Surprisingly, changing the tentative −35 sequence to match the canonical −35 sequence, 5′TTGACA3′, still resulted in no promoter activity (pCL8240). These results indicate that the tentative −35 sequence is likely a bona fide promoter element equivalent to the −35 sequence.
The 10-bp inverted repeat is required for CP8 production.
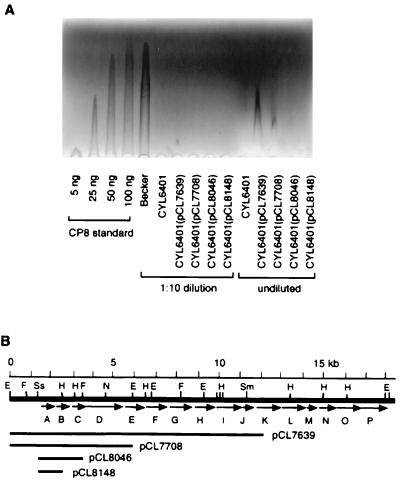
The above analyses were all performed by cloning the inserts into a multiple-copy plasmid vector and therefore were somewhat artificial. We wished to determine whether the inverted repeat has biological relevance with respect to capsule production in its native environment. To this end, we constructed plasmid pCL8226, with a 2.4-kb insert containing a mutation in the inverted repeat which changed the sequence to a BamHI recognition site. A PCR fragment containing this mutation site was recloned into pLC4 (pCL8265) and assayed for XylE activity. The result (Fig. 3) confirmed that the mutation abolished promoter activity. Plasmid pCL8226 was then used to construct a mutant strain (CYL6401) with a chromosomal mutation in the inverted repeat by allele replacement. The introduction of a BamHI recognition site allowed us to conveniently screen the desired mutant by Southern hybridization (not shown). The production of CP8 from strain CYL6401 was then quantitated and compared with that of the wild-type strain Becker, using RIE. The results in Fig. 4 show that the mutant strain CYL6401 produced undetectable CP8 even in undiluted samples, whereas the wild-type strain Becker produced approximately 100 ng at a 10-fold dilution. These results support the notion that the 10-bp inverted repeat is a cis-acting element for cap8 gene expression and thus required for the capsule production in S. aureus Becker.
FIG. 4.
(A) RIE analysis of CP8 production. Each well contains 5 μl of the extract. Strains used to prepare the extracts are shown below the wells. The samples were prepared from cultures standardized by optical density measurement. The samples were run either undiluted or in 1:10 dilution. Various amounts of purified CP8 were run as standards. (B) Plasmids used for complementing CYL6401. The cap8 genes are indicated by arrows and capital letters. Abbreviations for restriction sites: E, EcoRI; F, FspI; H, HindIII; N, NcoI; Sm, SmaI; Ss, SstI.
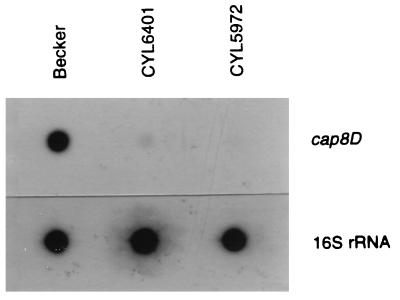
To determine whether the inverted repeat is required for the transcription of the cap8 genes, we compared, by RNA dot blot analysis, the amounts of RNA produced from wild-type strain Becker, strain CYL6401, and a negative control strain, CYL5972, a Becker derivative with a chromosomal deletion of most of the cap8 genes, including cap8D (15). The result (Fig. 5) showed that CYL6401 produced about 24-fold less mRNA than the wild-type strain, as determined by densitometric estimation of the messages, whereas CYL5972 produced no detectable message. This result indicates that the 10-bp inverted repeat affects CP8 production mostly at the transcriptional level and that the mutation in the inverted repeat greatly reduced the transcription.
FIG. 5.
RNA dot blot analysis. Total RNAs from strains indicated at the top were blotted to a nitrocellulose membrane and hybridized with a gene probe specific to cap8D. As a loading control, the RNAs were also hybridized with a probe specific to the 16S rRNA gene.
Internal promoters within the cap8 locus are functional.
Previously, we showed by genetic complementation tests that there were numerous internal promoters within the cap8 locus (46). We found that all cap8 genes except cap8D and possibly cap8L were transcribed by promoters upstream of the respective genes. These internal promoters were much weaker than the primary promoter located upstream of the cap8A gene, as determined by xylE reporter gene fusion analysis. To determine whether these internal promoters have any biological relevance, strain CYL6401 was complemented by various plasmids with inserts containing intact primary promoter but lacking different number of downstream genes in the cap8 operon. These plasmids were transduced by phage 52A into strain CYL6401, and the resultant strains were assayed for CP8 production by RIE. As shown in Fig. 4A, no CP8 was detected when CYL6401 was complemented either with pCL8046, which contains intact cap8A and cap8B, or with pCL8148, which contains only intact cap8A. On the other hand, CP8 production of CYL6401 could be partially complemented by pCL7639, which contains intact cap8A through cap8J, and by pCL7708, which contains intact cap8A through cap8D. The partial complementation results suggest that there are significantly active internal promoters downstream of the primary promoter that can transcribe, from the chromosome of CYL6401, the cap8 genes not present in pCL7639 or pCL7708. The fact that only very small amounts of CP8 could be detected in the transductants is consistent with our previous data showing that these internal promoters are very weak promoters and that the primary promoter is required for the full capsule production (46). Since we showed that there were also weak promoters just upstream of cap8B and cap8C and that their strengths were similar to those of other internal promoters, we were somewhat surprised that no CP8 was detected from CYL6401(pCL8046) and CYL6401(pCL8148). However, it is possible that RIE is not sensitive enough to detect small amounts of CP8.
An alternative to the above explanation of active internal promoters is to assume that at least a key gene required for the limiting step in CP8 synthesis is present in both pCL7639 and pCL7708 but not in pCL8046 and pCL8148. The overexpression of this plasmid-located key gene plus a low level of transcription from the mutated primary promoter would also result in a small amount of CP8 production. Because cap8D is the only gene present in both pCL7639 and pCL7708 and absent in pCL8046 and pCL8148, cap8D should be the limiting key gene. However, in our previous genetic study (44), derivatives of strain Becker with an intact primary promoter containing pCL7639 produced the same amount of CP8 as the wild-type Becker strain, indicating that cap8D, which is present in pCL7639, is not likely a limiting gene in CP8 synthesis. Furthermore, we detected no activity from the mutated primary promoter whereas activities from the internal promoters were readily detected (46), indicating that transcription from CYL6401 is most likely due to the internal promoters.
The inverted repeat serves as a protein binding site.
Since the 10-bp inverted repeat is required for cap8 promoter activity and for CP8 production, it likely serves as the binding site for a positive regulatory protein. To test this possibility, we performed gel mobility shift assays. A 32P-labeled 63-bp fragment containing the inverted repeat from −31 to −93 bp upstream of the promoter region was reacted with the cell extract prepared from the wild-type Becker strain grown in TSB. As shown in lane 3 of Fig. 6A, a prominent shifted band and a higher but much weaker band were detected. The intensity of the prominent shifted band was dramatically reduced (lane 4) when the DNA fragment containing a mutation in the inverted repeat (same mutation as in pCL8265 [Fig. 3]) was used in the reaction. In addition, the prominent shifted band could be competed away by the 63-bp nonlabeled wild-type DNA fragment but not by the 63-bp nonlabeled mutated fragment (lanes 5 and 6, respectively). These results indicate that there is at least one protein which can specifically bind to the 10-bp inverted repeat, thus activating the cap8 promoter.
FIG. 6.
(A) Results of a gel mobility shift assay performed with a 63-bp fragment (−93 to −31) upstream of cap8A containing the 10-bp inverted repeat. Lanes: 1, labeled fragment amplified from pCL8074 containing the wild-type inverted repeat; 2, labeled fragment amplified from pCL8265 containing a mutation in the inverted repeat; 3, labeled fragment with the wild-type inverted repeat reacted with the crude extract prepared from TSB-grown Becker cells; 4, labeled fragment with a mutation in the inverted repeat reacted with the crude extract prepared from TSB-grown Becker cells; 5, reaction in lane 3 with 100-fold excess of cold fragment containing the wild-type inverted repeat amplified from pCL8074 as competitor DNA; 6, reaction in lane 3 with 100-fold excess of cold fragment containing a mutation in the inverted repeat amplified from pCL8265 as competitor DNA. (B) Effect of YE on results of a gel mobility assay using the 63-bp fragment. Lanes: 1, labeled fragment with a mutation in the 10-bp inverted repeat amplified from pCL8265 reacted with the crude extract prepared from Becker cells grown in TSB; 2, labeled fragment with the wild-type 10-bp inverted repeat amplified from pCL8074 reacted with the crude extract prepared from Becker cells grown in TSB; 3, same as in lane 2 except that the crude extract was prepared from cells grown in TSB–0.5% YE.
Regulation by environmental factors.
To study the effects of some of the environmental factors on CP8 production, we measured the XylE activity of Becker(pCL7957) under various growth media. XylE activities, expressed as mean milliunits per milligram of protein ± standard deviations of three independent tests, were as follows: TSB, 5.49 ± 1.64; TSB–0.5% yeast extract (YE), <0.25; CB, 0.53 ± 0.10; TDNB, 12.93 ± 1.40; TDNB–0.5% YE, <0.25; LPM, 6.69 ± 0.82; LPM–0.5% YE, <0.25; HPM, 3.02 ± 0.69; and HPM–0.5% YE, <0.25. The finding that cap8 promoter activity varied in different media suggests that these growth conditions affect cap8 gene transcription. Of special note is that the addition of YE to all media drastically suppressed cap8 promoter activity. The strong inhibition by YE prompted us to further study its effect on cap8 gene transcription. Becker strains containing pCL8089 or pCL8097 (Fig. 2) were grown in TSB in the presence of 0.5% YE and tested for xylE reporter gene expression. Both strains showed no detectable promoter activity. Since plasmid pCL8097 contains a deletion just upstream of the 10-bp inverted repeat, we speculate that YE may exert its effect through the 10-bp inverted repeat. To test this, we performed a gel mobility shift experiment. The 32P-labeled 63-bp fragment containing the wild-type 10-bp inverted repeat described above was reacted with the extract prepared from cells grown in TSB with or without 0.5% YE. The results (Fig. 6B) showed that the intensity of the prominent shifted band detected in the reaction containing extract from TSB-grown cells (lane 2) was much lower than that in the reaction containing extract from cells grown in TSB with YE (lane 3). This inhibition of the shifted band by YE was also observed when the labeled DNA fragment containing a mutation in the 10-bp inverted repeat was reacted with extract from TSB-grown cells (lane 1). These results suggest that YE may inhibit cap8 transcription through the 10-bp inverted repeat.
DISCUSSION
Staphylococcal microcapsules are highly regulated. Our previous characterization of the cap8 genes has located a primary promoter within a DNA fragment of 0.45 kb upstream of the first cap8 gene (46). In this work, we further studied the primary cap8 promoter at the molecular level. We first mapped the transcription start site by a primer extension experiment. By examining the sequence upstream of the transcription start site, we found a consensus −10 sequence but not a consensus −35 sequence. Based on the distance from −10, we tentatively assigned a 6-bp sequence with only two matches to the canonical sequence as the putative −35 sequence. Mutagenesis within this 6-bp sequence markedly reduced promoter activity. However, changing the 6-bp sequence to the canonical −35 sequence also decreased promoter activity to the basal level, suggesting that the original sequence in the −35 region is critical for promoter activity. The lack of a consensus −35 sequence has been found for a number of bacterial promoters that require additional activators for transcription and suggests to us that the primary cap8 promoter may require a regulator for transcription. The fact that there are several inverted repeats and direct repeats upstream of the promoter prompted us to test these repeats for possible cis-acting activity. Our data from deletion analysis and site-directed mutagenesis confirmed that a 10-bp inverted repeat located 14 bp upstream of the tentative −35 sequence was indeed required for cap8 promoter activity.
The 10-bp inverted repeat likely serves as a regulatory protein binding site. Indeed, our gel mobility shift assay results indicate that there exists at least one protein factor capable of binding to the repeat. This protein factor is most likely a positive regulator since mutations in the 10-bp inverted repeat abolished promoter activity. The fact that the sequence required for promoter activity is palindromic suggests that the regulatory protein may function as a dimer. However, the inverted repeat is imperfect, and changing either half site to make it a perfect inverted repeat did not increase promoter activity over the wild-type level. In comparison, lacI repressor binds about 10-fold more tightly when the right-half repeat is changed to match the left-half repeat (42). Thus, our results indicate that the regulator may not simply bind symmetrically as a homodimer or tetramer; instead, the binding of the regulator to each half site may be asymmetrical. Alternatively, the protein may bind as a monomer. Further studies by cloning and characterizing the regulatory gene, which are now under way, are required to elucidate the mechanism.
Although we have not replaced all of the sequences between the 10-bp inverted repeat and the tentative −35 sequence, our finding of several changes within the intervening sequences suggests that the specific sequences in this region are not important for cap8 promoter activity. On the other hand, the distance between the two sites is critical. Deletions or insertions of a nonintegral helical turn between the two sites reduced promoter activity drastically, whereas promoter activity was affected only slightly when the two sites were separated by two or four full turns of helix. Thus, the two DNA sites are required to be at the same face of the DNA helix for activation, suggesting that protein-protein interaction is required. These results are consistent with the hypothesis that the positive regulator binding at the 10-bp inverted repeat causes bending or looping of the DNA so that it can interact with the RNA polymerase as in the case of catabolite activator protein (CAP)-dependent promoters in E. coli (5). However, it is interesting that while insertions of 10, 21, and 31 bp in the spacer region of the E. coli melR promoter caused only reduced CAP-dependent activation, insertion of a 42-bp sequence totally abolished the activation. This is possibly due to the 42-bp insertion creating too great a distance between the CAP binding site and the RNA polymerase binding site (14). In contrast, we showed that a 42-bp insertion between the tentative −35 promoter sequence and the 10-bp inverted repeat resulted in only twofold reduction of cap8 promoter activity. This result indicates that a long intervening sequence has little effect on cap8 promoter activity, suggesting that binding of the regulator may induce extensive looping so that the regulator can still interact with the RNA polymerase properly.
The finding that a mutation within the 10-bp inverted repeat upstream of the cap8 promoter in the Becker chromosome abolished CP8 synthesis suggests strongly that the repeat is biologically significant in stimulating CP8 production. It supports the data obtained from the reporter gene study and therefore rules out the possibility that the reporter gene data are mere artifacts due to cloning in a multiple-copy plasmid vector. The effect of the inverted repeat on CP8 production is largely at the transcriptional level, since only about 4% of the cap8 message could be detected in the mutant by RNA dot blot analysis. However, RIE analysis showed that the mutation in the inverted repeat reduced CP8 synthesis to an undetectable level. It is possible that the discrepancy is due to different sensitivities of the two methods. Alternatively, we cannot rule out the possibility that cap8 gene expression is also controlled through the 10-bp inverted repeat at the translational level.
The mechanism by which environmental factors regulate S. aureus microcapsules has not been investigated. In this study, we found cap8 transcription was influenced by different growth media. Most strikingly, the addition of YE to several media greatly inhibited cap8 transcription. Results of the gel mobility shift assay (Fig. 6B) suggest that YE could inhibit transcription by preventing binding of a potential positive regulator to the 10-bp inverted repeat identified in this study, or YE may affect production of the regulatory protein. However, definitive proof requires cloning and characterization of the positive regulatory gene. The inhibitory effect of YE on CP5, a microcapsule similar to CP8, has been reported earlier (8). This similarity in regulation is consistent with the fact that the promoter regions of cap8 and cap5 are almost identical, with only one difference between positions −108 and +1 (45). However, in the earlier study, YE diffusate was used, which may explain why inhibition was observed at 2% YE, compared to 0.5% in this study. Recently, Fox et al. (13) reported that CP8 production in S. aureus was not affected by phosphate concentration. Their report is inconsistent with our finding that high phosphate concentration inhibited cap8 promoter activity by about 50% (see Results). However, it is not known whether the effect of phosphate detected at the transcriptional level in our study would reflect the level of CP8 production.
It is interesting that the weak internal promoters that we have previously identified by genetic complementation and reporter gene fusion (46) are biologically active (Fig. 4). These results are similar to those for the cap1 gene cluster in which the weak internal promoters are also biologically active (37). Since both the cap1 and cap8 gene clusters are transcribed by a primary promoter to form long transcripts, the distal gene may not be adequately expressed due to unstable long transcripts. Thus, active internal promoters, though weak, can ensure that the distal genes are expressed in amounts sufficient for optimum biogenesis of capsule.
Although transcription of the cap8 locus is similar to that of the cap1 locus, these two loci are apparently regulated differently. While an inverted repeat is required for activation of the cap8 genes, no requirement of such an inverted repeat was identified upstream of the cap1 gene cluster. In fact, 5′-end deletion up to the upstream boundary of the −35 sequence did not affect cap1 promoter activity as assayed by xylE fusion, suggesting that the cap1 genes may be constitutively expressed (37). Furthermore, the cap1 promoter is much stronger than the cap8 promoter, indicating that it is intrinsically a very strong promoter that requires no accessory factor for its transcription. These differences further support the notion that CP1 and CP8, representing mucoid capsule and microcapsule, respectively, contribute differently to the biology and the pathogenesis of S. aureus.
ACKNOWLEDGMENT
This work was supported by grant R01AI37027 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Albus A, Fournier J M, Wolz C, Boutonnier A, Ranke M, Høiby N, Hochkeppel H, Döring G. Staphylococcus aureus capsular types and antibody response to lung infection in patients with cystic fibrosis. J Clin Microbiol. 1988;26:2205–2209. doi: 10.1128/jcm.26.12.2505-2509.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albus A, Arbeit R D, Lee J C. Virulence of Staphylococcus aureus mutants altered in type 5 capsule production. Infect Immun. 1991;59:1008–1014. doi: 10.1128/iai.59.3.1008-1014.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbeit R D, Karakawa W W, Vann W F, Robbins J B. Predominance of two newly described capsular polysaccharide types among clinical isolates of Staphylococcus aureus. Diagn Microbiol Infect Dis. 1984;2:85–91. doi: 10.1016/0732-8893(84)90002-6. [DOI] [PubMed] [Google Scholar]
- 4.Baddour L M, Lowrance C, Albus A, Lowrance J H, Anderson S K, Lee J C. Staphylococcus aureus microcapsule expression attenuates bacterial virulence in a rat model of experimental endocarditis. J Infect Dis. 1992;165:749–753. doi: 10.1093/infdis/165.4.749. [DOI] [PubMed] [Google Scholar]
- 5.Busby S, Ebright R H. Promoter structure, promoter recognition, and transcription activation in prokaryotes. Cell. 1994;79:743–746. doi: 10.1016/0092-8674(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 6.Cheung A L, Bayer M G, Heindrichs J H. sar genetic determinants necessary for transcription of RNAII and RNAIII in the agr locus of Staphylococcus aureus. J Bacteriol. 1997;179:3963–3971. doi: 10.1128/jb.179.12.3963-3971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung A L, Eberhardt K J, Fischetti V A. A method to isolate RNA from Gram-positive bacteria and mycobacteria. Anal Biochem. 1994;222:511–514. doi: 10.1006/abio.1994.1528. [DOI] [PubMed] [Google Scholar]
- 8.Dassy B, Stringfellow W T, Lieb M, Fournier J M. Production of type 5 capsular polysaccharide by Staphylococcus aureus grown in a semi-synthetic medium. J Gen Microbiol. 1991;137:1155–1162. doi: 10.1099/00221287-137-5-1155. [DOI] [PubMed] [Google Scholar]
- 9.Dassy B, Hogan T, Foster T J, Fournier J M. Involvement of the accessory gene regulator (agr) in expression of type 5 capsular polysaccharide by Staphylococcus aureus. J Gen Microbiol. 1993;139:1301–1306. doi: 10.1099/00221287-139-6-1301. [DOI] [PubMed] [Google Scholar]
- 10.Dyer D W, Iandolo J J. Rapid isolation of DNA from Staphylococcus aureus. Appl Environ Microbiol. 1983;46:283–285. doi: 10.1128/aem.46.1.283-285.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fattom A I, Sarwar J, Ortiz A, Naso R. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect Immun. 1996;64:1659–1665. doi: 10.1128/iai.64.5.1659-1665.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fournier J M, Vann W F, Karakawa W W. Purification and characterization of Staphylococcus aureus type 8 capsular polysaccharide. Infect Immun. 1984;45:87–93. doi: 10.1128/iai.45.1.87-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox K F, Stewart G C, Fox A. Synthesis of microcapsule by Staphylococcus aureus is not responsive to environmental phosphate concentrations. Infect Immun. 1998;66:4004–4007. doi: 10.1128/iai.66.8.4004-4007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaston K, Bell A, Kolb A, Buc H, Busby S. Stringent spacing requirements for transcription activation by CRP. Cell. 1990;62:733–743. doi: 10.1016/0092-8674(90)90118-x. [DOI] [PubMed] [Google Scholar]
- 15.Gillaspy A F, Lee C Y, Sau S, Cheung A L, Smeltzer M S. Factors affecting the collagen capacity of Staphylococcus aureus. Infect Immun. 1998;66:3170–3178. doi: 10.1128/iai.66.7.3170-3178.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawley D K, McClure W R. Compilation and analysis of Escherichia coli promoter DNA sequence. Nucleic Acids Res. 1983;11:2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbert S, Worlitzsch D, Dassy B, Boutonnier A, Fournier J M, Bellon G, Dalhoff A, Doring G. Regulation of Staphylococcus aureus capsular polysaccharide type 5: CO2 inhibition in vitro and in vivo. J Infect Dis. 1997;176:431–438. doi: 10.1086/514061. [DOI] [PubMed] [Google Scholar]
- 18.Hochkeppel H K, Braun D G, Vischer W, Imm A, Sutter S, Staeubli U, Guggenheim R, Kaplan E L, Boutonnier A, Fournier J M. Serotyping and electron microscopy studies of Staphylococcus aureus clinical isolates with monoclonal antibodies to capsular polysaccharide type 5 and type 8. J Clin Microbiol. 1987;25:526–530. doi: 10.1128/jcm.25.3.526-530.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmes D S, Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981;114:193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- 20.Karakawa W W, Vann W F. Capsular polysaccharides of S. aureus. Semin Infect Dis. 1982;4:285–293. [Google Scholar]
- 21.Karakawa W W, Fournier J M, Vann W F, Arbeit R, Schneerson R, Robbins J B. Method for the serological typing of the capsular polysaccharides of Staphylococcus aureus. J Clin Microbiol. 1985;22:445–447. doi: 10.1128/jcm.22.3.445-447.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karakawa W W, Sutton A, Schneerson R, Karpas A, Vann W F. Capsular antibodies induce type-specific phagocytosis of Staphylococcus aureus by human polymorphonuclear leukocytes. Infect Immun. 1988;56:1090–1095. doi: 10.1128/iai.56.5.1090-1095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraemer G R, Iandolo J J. High-frequency transformation of Staphylococcus aureus by electroporation. Curr Microbiol. 1990;21:373–376. [Google Scholar]
- 24.Kreiswirth B N, Lofdahl S, Betley M J, O’Reilly M, Shlievert P M, Bergdoll M S, Novick R P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature (London) 1983;305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 25.Lee C Y. Cloning of genes affecting capsule expression in Staphylococcus aureus strain M. Mol Microbiol. 1992;6:1515–1522. doi: 10.1111/j.1365-2958.1992.tb00872.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee J C, Betley M J, Hopkins C A, Perez N E, Pier G B. Virulence studies, in mice, of transposon-induced mutants of Staphylococcus aureus differing in capsule size. J Infect Dis. 1987;156:741–750. doi: 10.1093/infdis/156.5.741. [DOI] [PubMed] [Google Scholar]
- 27.Lee J C, Takeda S, Livolsi P, Paoletti L C. Effects of in vitro and in vivo growth conditions on expression of type 8 capsular polysaccharide by Staphylococcus aureus. Infect Immun. 1993;61:1853–1858. doi: 10.1128/iai.61.5.1853-1858.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J C, Xu S, Albus A, Livolsi P J. Genetic analysis of type 5 capsular polysaccharide expression by Staphylococcus aureus. J Bacteriol. 1994;176:4883–4889. doi: 10.1128/jb.176.16.4883-4889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin W S, Cunneen T, Lee C Y. Sequence analysis and molecular characterization of genes required for the biosynthesis of type 1 capsular polysaccharide in Staphylococcus aureus. J Bacteriol. 1994;176:7005–7016. doi: 10.1128/jb.176.22.7005-7016.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahmood R, Khan S A. Role of upstream sequences in the expression of the staphylococcal enterotoxin B gene. J Biol Chem. 1990;265:4652–4656. [PubMed] [Google Scholar]
- 31.Mamo W, Rozgonyi F, Hjertén S, Wadström T. Effect of milk on surface properties of Staphylococcus aureus from bovine mastitis. FEMS Microbiol Lett. 1987;48:195–200. [Google Scholar]
- 32.Melly M A, Duke L J, Liau D F, Hash J H. Biological properties of the encapsulated Staphylococcus aureus M. Infect Immun. 1974;10:389–397. doi: 10.1128/iai.10.2.389-397.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moran C P, Jr, Lang N, LeGrice S F J, Lee G, Stephens M, Sonenshein A L, Pero J, Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186:339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- 34.Moreau M, Richards J C, Fournier J M, Byrd R A, Karakawa W W, Vann W F. Structure of the type-5 capsular polysaccharide of Staphylococcus aureus. Carbohydr Res. 1990;201:285–297. doi: 10.1016/0008-6215(90)84244-o. [DOI] [PubMed] [Google Scholar]
- 35.Nemeth J, Lee J C. Antibodies to capsular polysaccharides are not protective against experimental Staphylococcus aureus endocarditis. Infect Immun. 1995;63:375–380. doi: 10.1128/iai.63.2.375-380.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nilsson I-M, Lee J C, Bremell T, Rydén C, Tarkowski A. The role of staphylococcal polysaccharide microcapsule expression in septicemia and septic arthritis. Infect Immun. 1997;65:4216–4221. doi: 10.1128/iai.65.10.4216-4221.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouyang S, Lee C Y. Transcriptional analysis of type 1 capsule genes in Staphylococcus aureus. Mol Microbiol. 1997;23:473–482. doi: 10.1046/j.1365-2958.1997.d01-1865.x. [DOI] [PubMed] [Google Scholar]
- 38.Peterson P K, Wilkinson B J, Kim Y, Schmeling D, Quie P G. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1978;19:943–949. doi: 10.1128/iai.19.3.943-949.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poutrel B, Boutonnier A, Sutra L, Fournier J M. Prevalence of capsular polysaccharide types 5 and 8 among Staphylococcus aureus isolates from cow, goat, and ewe milk. J Clin Microbiol. 1988;26:38–40. doi: 10.1128/jcm.26.1.38-40.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ray C, Hay R E, Carter H J, Moran C P., Jr Mutations that affect utilization of a promoter in stationary-phase Bacillus subtilis. J Bacteriol. 1985;163:610–612. doi: 10.1128/jb.163.2.610-614.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Recsei P, Kreiswirth B, O’Reilly M, Schlievert P, Gruss A, Novick R. Regulation of exoprotein gene expression by agr. Mol Gen Genet. 1986;202:58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- 42.Sadler J R, Sasmor H, Betz J L. A perfectly symmetric lac operator binds the lac repressor very tightly. Proc Natl Acad Sci USA. 1983;80:6785–6789. doi: 10.1073/pnas.80.22.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 44.Sau S, Lee C Y. Cloning of type 8 capsule genes and analysis of gene clusters for the production of different capsular polysaccharides in Staphylococcus aureus. J Bacteriol. 1996;178:2118–2126. doi: 10.1128/jb.178.7.2118-2126.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sau S, Bhasin N, Wann E R, Lee J C, Foster T J, Lee C Y. The Staphylococcus aureus allelic genetic loci for serotype 5 and 8 capsule expression contain the type-specific genes flanked by common genes. Microbiology. 1997;143:2395–2405. doi: 10.1099/00221287-143-7-2395. [DOI] [PubMed] [Google Scholar]
- 46.Sau S, Sun J, Lee C Y. Molecular characterization and transcriptional analysis of type 8 genes in Staphylococcus aureus. J Bacteriol. 1997;179:1614–1621. doi: 10.1128/jb.179.5.1614-1621.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shafer M W, Iandolo J J. Genetics of staphylococcal enterotoxin B in methicillin-resistant isolates of Staphylococcus aureus. Infect Immun. 1979;25:902–911. doi: 10.1128/iai.25.3.902-911.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sompolinsky E, Samra Z, Karakawa W W, Vann W F, Schneerson R, Malik Z. Encapsulation and capsular types in isolates of Staphylococcus aureus from different sources and relationship to phage types. J Clin Microbiol. 1985;22:828–834. doi: 10.1128/jcm.22.5.828-834.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 50.Stringfellow W T, Dassy B, Lieb M, Fournier J M. Staphylococcus aureus growth and type 5 capsular polysaccharide production in synthetic media. Appl Environ Microbiol. 1991;57:618–621. doi: 10.1128/aem.57.2.618-621.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sutra L, Rainard C, Poutrel B. Phagocytosis of mastitis isolates of Staphylococcus aureus and expression of type 5 capsular polysaccharide are influenced by growth in the presence of milk. J Clin Microbiol. 1990;28:2253–2258. doi: 10.1128/jcm.28.10.2253-2258.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilkinson B J. Staphylococcal capsules and slime. In: Easmon C S G, Adlam G, editors. Staphylococci and staphylococcal infections. New York, N.Y: Academic Press; 1983. pp. 481–523. [Google Scholar]
- 53.Wunschel D S, Fox K F, Nagpal M L, Kim K K, Stewart G C, Shagholi M. Quantitative analysis of neutral and acidic sugars in whole bacterial cell hydrolysates using high performance anion exchange liquid chromatography elecrospray ionization tandem mass spectrometry. J Chromatogr A. 1997;776:205–219. doi: 10.1016/s0021-9673(97)00356-7. [DOI] [PubMed] [Google Scholar]
- 54.Xu S, Arbeit R D, Lee J C. Phagocytic killing of encapsulated and microencapsulated Staphylococcus aureus by human polymorphonuclear leukocytes. Infect Immun. 1992;60:1358–1362. doi: 10.1128/iai.60.4.1358-1362.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zukowski M M, Gaffney D F, Speck D, Kauffmann M, Findeli A, Wisecup A, Lecocq J P. Chromogenic identification of genetic regulatory signals in Bacillus subtilis based on expression of a cloned Pseudomonas gene. Proc Natl Acad Sci USA. 1983;80:1101–1105. doi: 10.1073/pnas.80.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]