Abstract
Pemetrexed is a folate analog metabolic inhibitor used in treatment of locally advanced or metastatic nonsquamous non-small cell lung cancer and for mesothelioma.
Intra-lot and inter-lot variability in the spectra of ALIMTA® was detected in the Drug Quality Study (DQS) using Fourier transform near-infrared spectrometry (FTNIR). One vial of 12 (8%) sampled from lot S20I013A appeared 3.0 multidimensional SDs from the other vials, suggesting that it represents a different material. Consequently, additional spectra from other lots were analyzed.
Spectra of 147 vials from 23 lots in the spectral library contained 14 vials that were outside the main group (26.4 SDs using a subcluster detection test), suggesting that the 14 library vials (9.5% of the total) also contain differing materials.
Introduction
The University of Kentucky’s (UK) Drug Quality Study was established in August of 2019 to engage in consumer-level quality assurance screening for drugs used within UK HealthCare’s pharmacies. DQS currently screens medications using FTNIR and Raman spectroscopy for potential quality defects indicated by variability in absorbance peak intensities and locations. Through years of continuous monitoring, DQS has assembled a spectral library containing medications typically used in a health system setting. Statistical analyses using DQS’ spectral library are performed to identify potential intra-lot and inter-lot variability in medications under review. Using MedWatch, DQS reports its findings in an effort to hold manufacturers accountable for GMP requirements and to improve patient outcomes by providing information on quality to augment the information on price that is already available. The increasing transparency is designed to improve the pharmaceutical supply chain. At all levels, DQS staff are committed to achieving service excellence by pursuing compliance with the standards set forth by our patients and broad GxP requirements (Isaacs, 2022a).
Drug Product
Pemetrexed (ALIMTA®, Lilly) is supplied as a sterile lyophilized powder for intravenous infusion available in single-dose vials. The product is a white to either light yellow or green-yellow lyophilized solid. Each 100-mg or 500-mg vial of ALIMTA contains pemetrexed disodium equivalent to 100 mg pemetrexed and 106 mg mannitol or 500 mg pemetrexed and 500 mg mannitol, respectively. Hydrochloric acid and/or sodium hydroxide may have been added to adjust pH. Figure 1 is a photo of the drug product.
Figure 1.
Vials of pemetrexed for injection. The drug appears as a white freeze-dried powder in the vial on the right. The vials were scanned in the near-infrared spectral region through the bottom of the vials.
The lot numbers making up the spectral library were D184933D, D211218J, S19I021A, S19L018A, S19L025A, S20A008A, S20D015A, S20F002A, S20F014A, S20F017A, S20G011A, S20I005A, S20I013A, S20I014A, S20I024A, S20I029A, S20I041A, S20L039A, S20M006A, S20M021A, S21A015A, S21A016A, and S21A018A.
FDA Medwatch
An FDA Form 3500 Medwatch describing the findings of this Rapid Communication was filed on June 29, 2022 (FDA Form 3500, 2022).
Methods
FTNIR (Fourier Transform Near-Infrared) Spectrometry
Using nondestructive analytical techniques, FTNIR spectra were collected for inventory belonging to lots S20I013A and S20I014A as part of routine medication quality screening. A representative sample of 12 individual vials were selected for screening from lots S20I013A and S20I014A and noted to be stored under the conditions required by the manufacturer in their original packaging. FTNIR spectra were collected noninvasively and nondestructively through the bottom of the vials using a Thermo Scientific Antaris II FTNIR Analyzer (Waltham, MA, USA)(Isaacs, 2022b).
Multiplicative Scatter Correction (MSC)
Multiplicative scatter correction (MSC) is a widely used spectrometric normalization technique. Its purpose is to correct spectra in such a way that they are as close as possible to a reference spectrum, generally the mean of the data set, by changing the scale and the offset of the spectra (Isaksson, 1988).
BEST (Bootstrap Error-Adjusted Single-sample Technique)
The BEST calculates distances in multidimensional, asymmetric, nonparametric central 68% confidence intervals in spectral hyperspace (roughly equivalent to standard deviations)(Dempsey, 1996). The BEST metric can be thought of as a "rubber yardstick" with a nail at the center (the mean). The stretch of the yardstick in one direction is therefore independent of the stretch in the other direction. This independence enables the BEST metric to describe odd shapes in spectral hyperspace (spectral point clusters that are not multivariate normal, such as the calibration spectra of many biological systems). BEST distances can be correlated to sample composition to produce a quantitative calibration, or simply used to identify similar regions in a spectral image. The BEST automatically detects samples and situations unlike any encountered in the original calibration, making it more accurate in chemical investigation than typical regression approaches to near-IR analysis. The BEST produces accurate distances even when the number of calibration samples is less than the number of wavelengths used in calibration, in contrast to other metrics that require matrix factorization. The BEST is much faster to calculate as well (O(n) instead of the O(n3) required by matrix factorization.)
Principal Components (PCs)
Principal component analysis is the process of computing the principal components of a dataset and using them to execute a change of basis (change of coordinate system) on the data, usually employing only the first few principal components and disregarding the rest (Joliffe, 2016). PCA is used in exploratory data analysis and in constructing predictive models. PCA is commonly utilized for dimensionality reduction by projecting each data point onto only the first few principal components to obtain lower-dimensional data while preserving as much of the original variation in the data as possible. The first principal component is the direction that maximizes the variance of the projected data. The second principal component is the direction of the largest variance orthogonal to the first principal component. Decomposition of the variance typically continues orthogonally in this manner until some residual variance criterion is met. Plots of PC scores help reveal underlying structure in data.
Subcluster Detection
In typical near-infrared multivariate statistical analyses, samples with similar spectra produce points that cluster in a certain region of spectral hyperspace. These dusters can vary significantly in shape and size due to variation in sample packings, particle-size distributions, component concentrations, and drift with time. These factors, when combined with discriminant analysis using simple distance metrics, produce a test in which a result that places a particular point inside a particular cluster does not necessarily mean that the point is actually a member of the cluster. Instead, the point may be a member of a new, slightly different cluster that overlaps the first. A new cluster can be created by factors like low-level contamination, moisture uptake, or instrumental drift. An extension added to part of the BEST, called FSOB (Fast Son of BEST) can be used to set nonparametric probability-density contours inside spectral clusters as well as outside (Lodder, 1988), and when multiple points begin to appear in a certain region of cluster-hyperspace the perturbation of these density contours can be detected at an assigned significance level using r values, and visualized using quantile-quantile (QQ) plots. The detection of unusual samples both within and beyond 3 SDs of the center of the training set is possible with this method. Within the ordinary 3 SD limit, however, multiple instances are needed to detect unusual samples with statistical significance.
Results and Discussion
Intralot Analysis
Different lots of a drug often cluster in slightly different regions of near-IR spectral hyperspace. There can be a little drift in the manufacturing process from batch to batch that slightly alters the composition of the drug yet still represents the approved product. This drift is consequently reflected in the spectra. However, it is unusual for spectra of drugs from the same lot number to vary dramatically. A process that is in control generally produces the same drug over and over again, and as a result, the spectra are very similar.
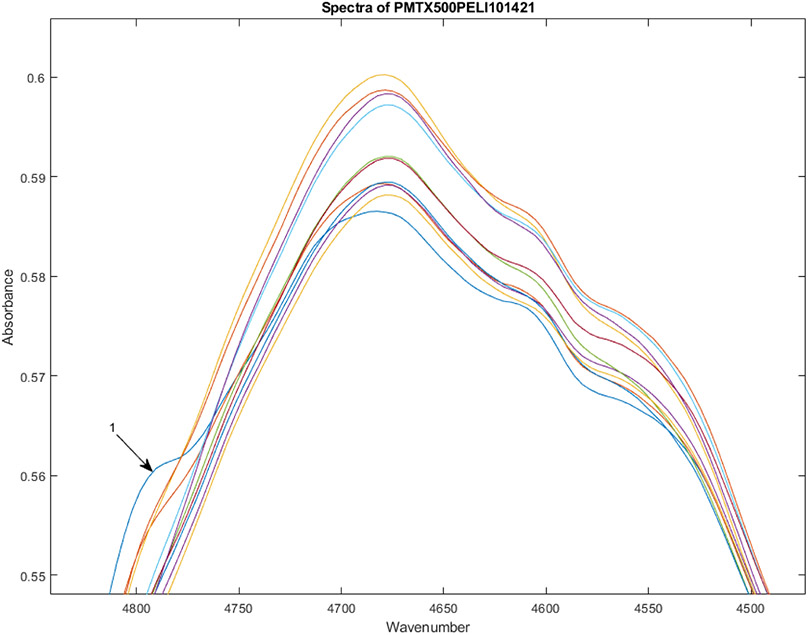
Figure 2 shows the spectra of 12 vials from Lot S20I013A and S20I014A from 4450-4850 cm−1. Two spectra show a small peak near 4790 cm−1 These two spectra represent vials 1 and 2. In the full spectrum in absorbance space, vial 1 is 3.0 multidimensional SDs from the rest of the vials.
Figure 2.
Spectra of 12 vials from Lot S20I013A and S20I014A from 4450-4850 cm−1. Two spectra (vials 1 and 2) show a small peak near 4790 cm−1. The spectrum with the most prominent of these peaks is marked 1 (vial 1), and is 3.0 multidimensional SDs from the rest of the vials.
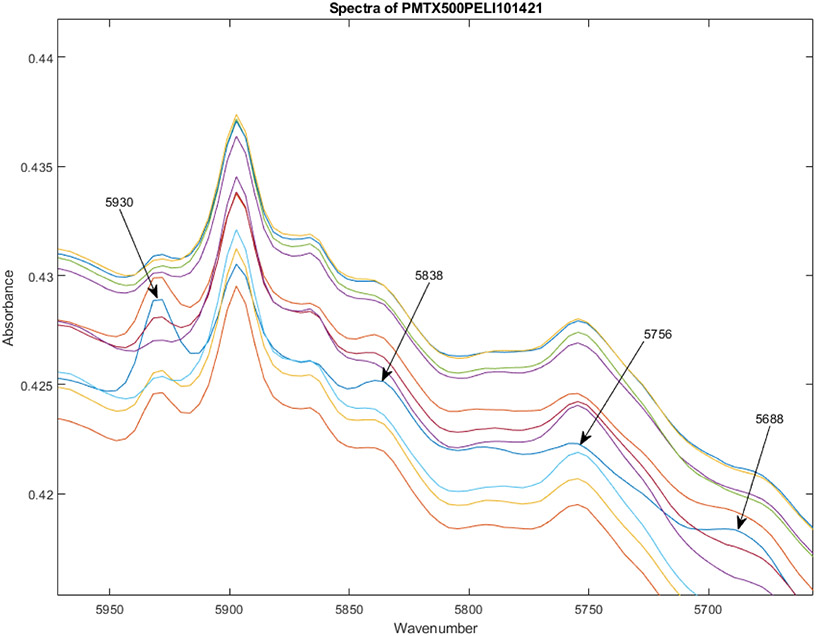
Figure 3 shows the spectra of the same 12 vials in Figure 2, but in the spectral region from 5650-5975 cm−1. Vial 1 is still the blue line, and the peaks at 5688, 5838, and 5930 cm−1 are larger than for the rest of the vials in the lot, while the peak at 5756 cm−1 is smaller. The peak at 5930 cm−1 has 2-3x the area of the median peak area for the lot.
Figure 3.
Spectra of the same 12 vials in Figure 2, but in the spectral region from 5650-5975 cm−1. Vial 1 is still the blue line, and the peaks at 5688, 5838, and 5930 cm−1 are larger than for the rest of the vials in the lot, while the peak at 5756 cm−1 is smaller.
Table 1 lists the principal components of the spectra of the 12 vials sampled from Lot S20I013A, the variation described by each PC, and the cumulative variation described by the PCs.
Table 1.
Variation accounted for by each of the principal components of the spectra of Lot S20I013A
| PC Number | Variation in this PC | Cumulative PC Variation |
|---|---|---|
| 1 | 0.4701 | 0.4701 |
| 2 | 0.1864 | 0.6565 |
| 3 | 0.1272 | 0.7837 |
| 4 | 0.0756 | 0.8593 |
| 5 | 0.0621 | 0.9213 |
| 6 | 0.0289 | 0.9502 |
| 7 | 0.0151 | 0.9653 |
| 8 | 0.0127 | 0.9780 |
| 9 | 0.0089 | 0.9869 |
| 10 | 0.0068 | 0.9937 |
| 11 | 0.0063 | 1.0000 |
| 12 | 0.0000 | 1.0000 |
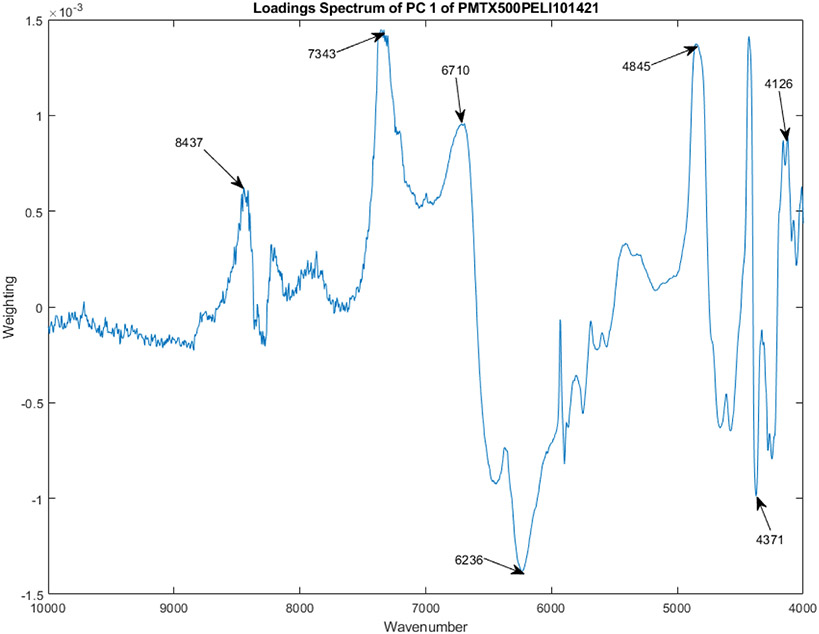
Figure 4 shows the PC loadings spectra of 12 vials from Lot S20I013A and S20I014A for PC 1. The wave numbers that contribute most strongly to the construction of PC 1 are marked in the figure. These wavenumbers are 4126, 4371, 4845, 6236, 6710, 7343, and 8437 cm−1.
Figure 4.
The PC loadings spectra of 12 vials from Lot S20I013A and S20I014A for PC 1.
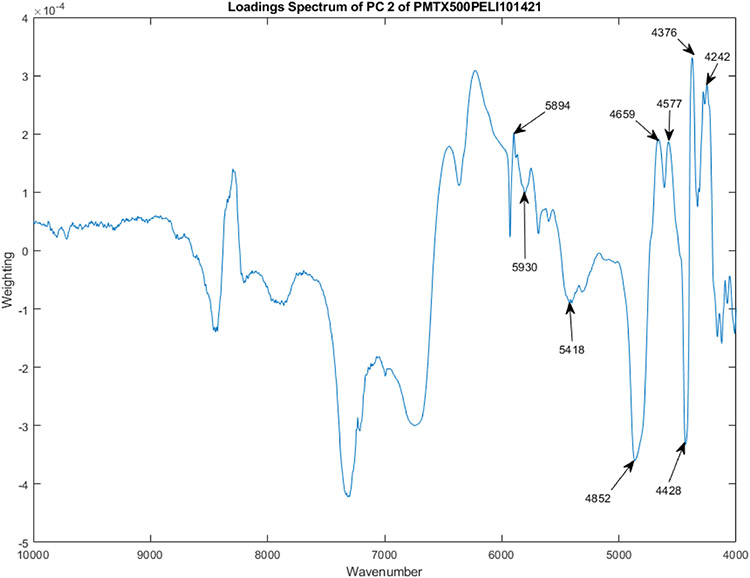
Figure 5 shows the PC loadings spectra of 12 vials from Lot S20I013A and S20I014A for PC 2.
Figure 5.
The PC loadings spectra of 12 vials from Lot S20I013A and S20I014A for PC 2.
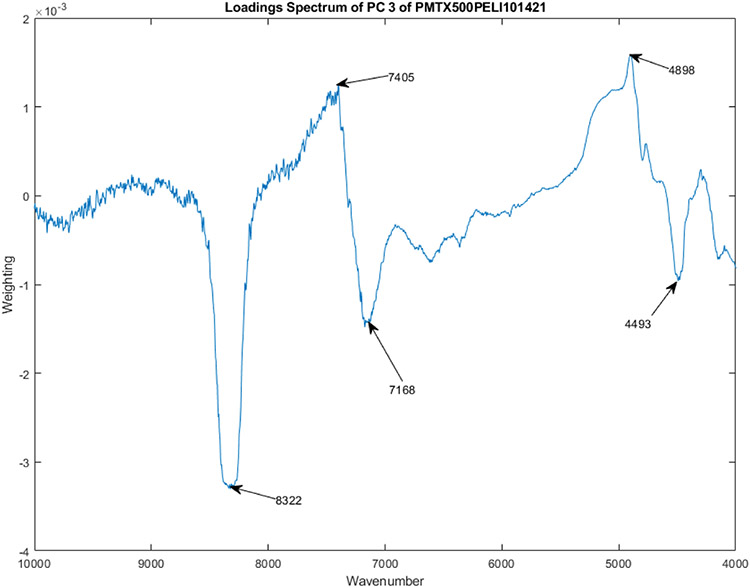
Figure 6 shows the PC loadings spectra of 12 vials from Lot S20I013A and S20I014A for PC 3. The wavenumbers that contribute most strongly to the construction of PC 1 are marked in the figure. These wavenumbers are 4493, 4898, 7168, 7405, and 8322 cm−1.
Figure 6.
The PC loadings spectra of 12 vials from Lot S20I013A and S20I014A for PC 3.
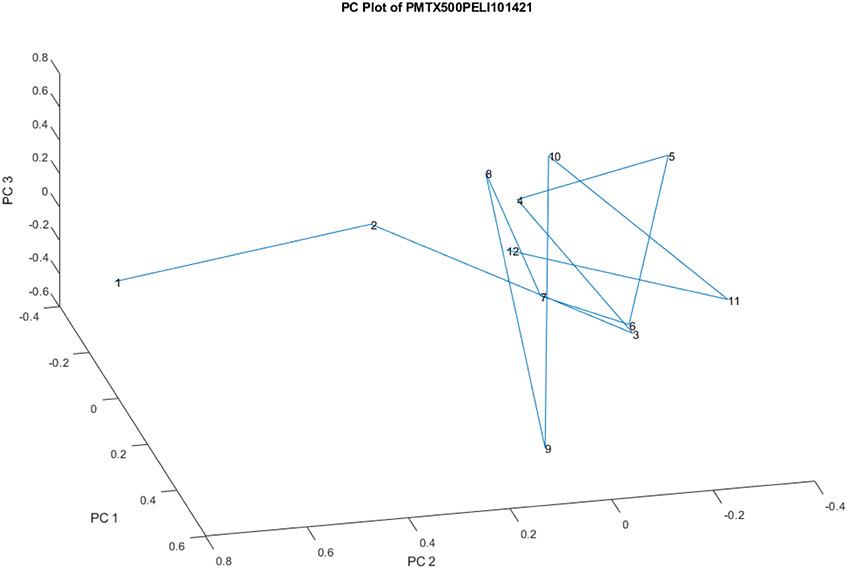
Figure 7 is a scatter plot of the 12 vials from Lot S20I013A and S20I014A for PC 1, 2, and 3. Most of the vials cluster together, with vial 2 being slightly displaced in the group and vial 1 being significantly replaced from the group (3.0 SDs). Most of the displacement projects on PC 2. The PC loadings spectrum for PC 2 is shown in Figure 5. In this view, vial number 12 appears near the middle of all of the vials, unlike in Figure 8.
Figure 7.
The PC scores scatter plot for 12 vials from Lot S20I013A and S20I014A for PC 1, 2, and 3.
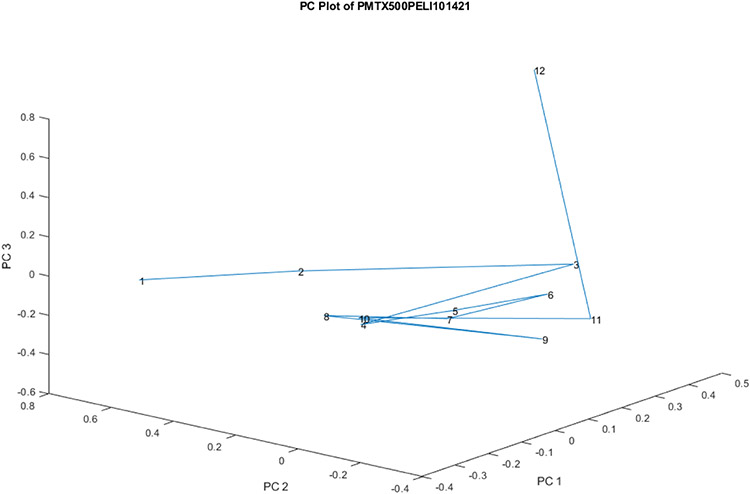
Figure 8.
Another view of the PC scores scatter plot for 12 vials from Lot S20I013A and S20I014A for PC 1, 2, and 3.
Figure 8 is a scatter plot of the 12 vials from Lot S20I013A and S20I014A for PC 1, 2, and 3. Figure 8 is a rotated view of Figure 7. Most of the vials cluster together, with vial 2 being slightly displaced in the group and vial 1 being significantly replaced from the group (3.0 SDs). Most of the displacement is on PC 2. The PC loadings spectrum for PC 2 is shown in Figure 5. In this view, vial number 12 appears as an outlier displaced orthogonally from the displacement of vial numbers 1 and 2. Most of the displacement of vial 12 occurs along PC 3. The loadings spectrum of PC 3 appears in Figure 6.
Interlot Analysis
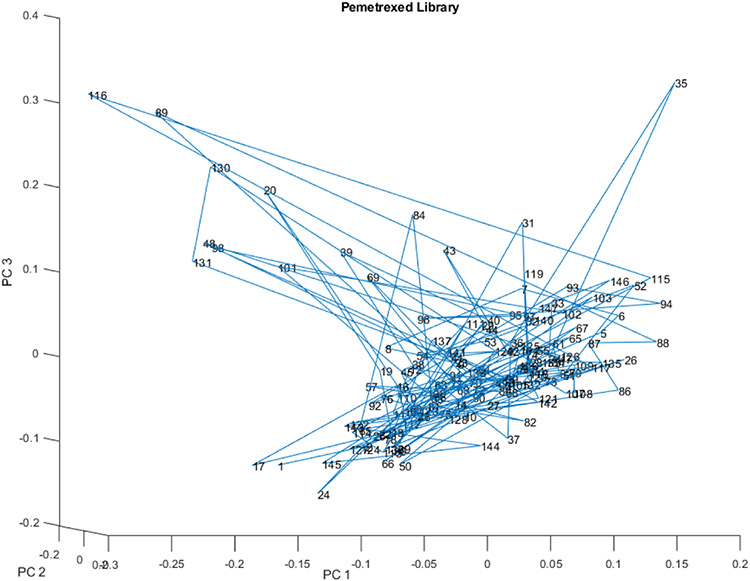
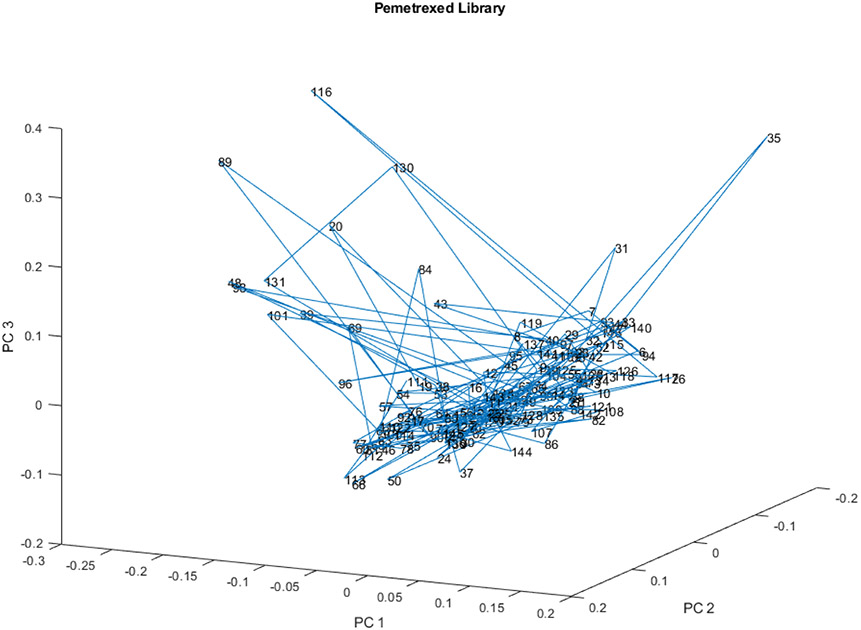
The PC scores for PCs 1, 2, and 3 of the complete spectral library of 147 vials appears in Figure 9. Displacements occur in two directions from the main spectral library. Vial 35 is displaced orthogonal to the displacement of vials 20, 39, 43, 48, 69, 84, 89, 96, 101, 116, 130, and 131.
Figure 9.
PC scores scatter plot for 147 vials in the spectral library from 23 lots for PC 1, 2, and 3.
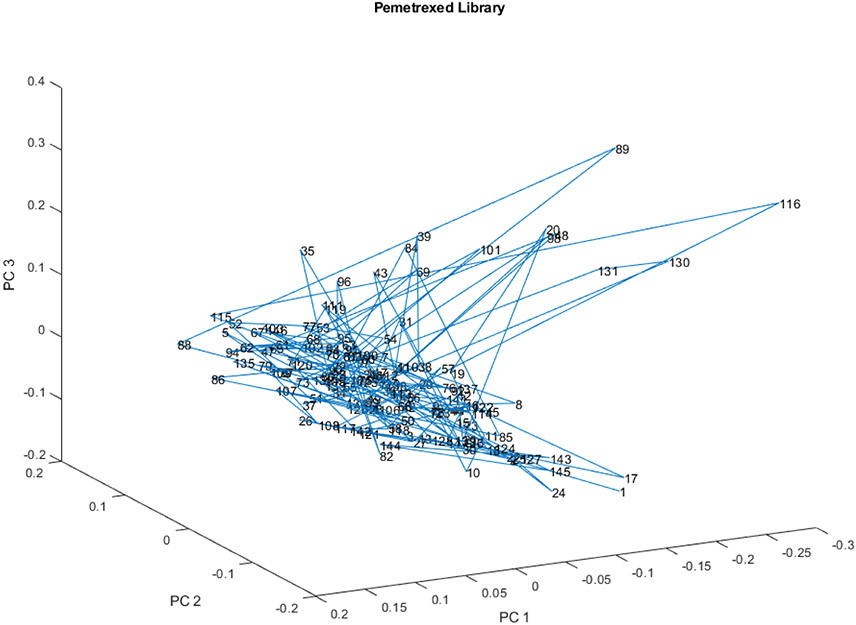
Figure 10 is a rotation of Figure 9 that shows the spread of the main group of outliers, and also shows that vial 31 is displaced a bit in the direction of vial 35.
Figure 10.
Another view of the PC scores scatter plot for 147 vials in the spectral library from 23 lots for PC 1, 2, and 3. Vial 35 is displaced orthogonally from vials 89 and 116. Vial 35 is 3.4 SDs from the center of the spectral library.
Figure 11 is a rotation of Figure 10 that shows that in some views, all of the outliers are displaced in the same direction.
Figure 11.
Another view of the PC scores scatter plot for 147 vials in the spectral library from 23 lots for PC 1, 2, and 3. Vial 116 is 4.5 SDs from the center of the spectral library, while vial 89 is 3.8 SDs away.
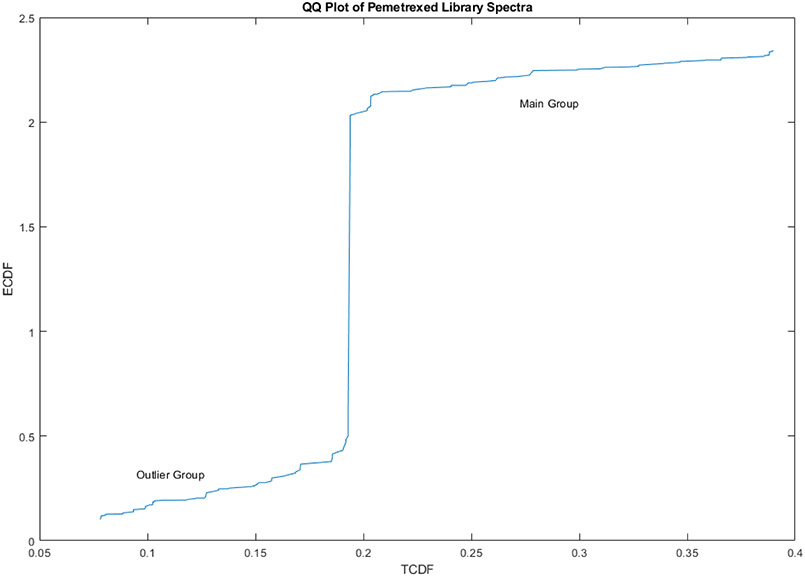
Figure 12 is a QQ plot from the subcluster detection test on PCs 1, 2 and 3 of the spectral library (rlim=0.98, rtst=0.87, p=0.02). The clusters are 26.4 SDs apart by the subcluster detection metric.
Figure 12.
QQ plot from the subcluster detection test on PCs 1, 2 and 3 of the spectral library. (rlim=0.98, rtst=0.87, p=0.02) The clusters are 26.4 SDs apart by the subcluster detection metric.
Fourteen vials were outside the main group (26.4 SDs using a subcluster detection test), suggesting that the 14 library vials (9.5% of the total) also contain differing materials.
Conclusions
Pemetrexed is a folate analog metabolic inhibitor used in treatment of locally advanced or metastatic nonsquamous non-small cell lung cancer and for mesothelioma. The authors are not aware of any recent quality problems with pemetrexed.
Intra-lot and inter-lot variability in the spectra of ALIMTA® was detected in the Drug Quality Study (DQS) using Fourier transform near-infrared spectrometry (FTNIR). One vial of 12 (8%) sampled from lot S20I013A appeared 3.0 multidimensional SDs from the other vials, suggesting that it represents a different material from the rest of the lot. Spectra of 147 vials from 23 lots in the spectral library contained 14 vials that were outside the main group (26.4 SDs using a subcluster detection test), suggesting that the 14 library vials (9.5% of the total) also contain differing materials.
Quality control is important in drug manufacturing. Good drugs lead to good patient outcomes. These FTNIR results do not prove an excess level of impurities or adulteration. However, they suggest that the manufacturing process may have been operating outside of a state of process control. Additional investigation is needed.
Acknowledgements
The project described was supported in part by NSF ACI-1053575 allocation number BIO170011 and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR001998. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Dempsey RJ, Davis DG, Buice RG Jr, & Lodder RA (1996). Biological and medical applications of near-infrared spectrometry. Applied Spectroscopy, 50(2), 18A–34A. [Google Scholar]
- FDA Form 3500 Medwatch, filed June 29, 2022.
- Isaacs JT, Almeter PJ, Henderson BS, Hunter AN, Platt TL, & Lodder RA (2022a). Variability in Content of Piperacillin and Tazobactam Injection. Contact in Context, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs JT, Almeter PJ, Henderson BS, Hunter AN, Platt TL, & Lodder RA (2022b). Lack of Content Uniformity in MMR Vaccine. Contact in context, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson T, & Næs T (1988). The effect of multiplicative scatter correction (MSC) and linearity improvement in NIR spectroscopy. Applied Spectroscopy, 42(7), 1273–1284. [Google Scholar]
- Jolliffe IT, & Cadima J (2016). Principal component analysis: a review and recent developments. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 374(2065), 20150202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder RA, & Hieftje GM (1988). Detection of subpopulations in near-infrared reflectance analysis. Applied spectroscopy, 42(8), 1500–1512. [Google Scholar]