Abstract
The gut barrier is a single cell layer that separates gut micro-organisms from the host, and gut permeability defects result in the translocation of microbial molecules from the gut into the blood. Despite the silent clinical manifestation, gut translocation of microbial molecules can induce systemic inflammation that might be an endogenous exacerbating factor of systemic lupus erythematosus. In contrast, circulatory immune-complex deposition and the effect of medications on the gut, an organ with an extremely large surface area, of patients with active lupus might cause gut translocation of microbial molecules, which worsens lupus severity. Likewise, the imbalance of gut microbiota may initiate lupus and/or interfere with gut integrity which results in microbial translocation and lupus exacerbation. Moreover, immune hyper-responsiveness of innate immune cells (macrophages and neutrophils) is demonstrated in a lupus model from the loss of inhibitory Fc gamma receptor IIb (FcgRIIb), which induces prominent responses through the cross-link between activating-FcgRs and innate immune receptors. The immune hyper-responsiveness can cause cell death, especially apoptosis and neutrophil extracellular traps (NETosis), which possibly exacerbates lupus, partly through the enhanced exposure of the self-antigens. Leaky gut monitoring and treatments (such as probiotics) might be beneficial in lupus. Here, we discuss the current information on leaky gut in lupus.
Keywords: leaky gut, innate immunity, systemic lupus erythematosus
1. Introduction
Systemic lupus erythematosus (SLE or lupus) is a common autoimmune disease with the involvement of multiple organs (skin, joints, and central nervous system) due to the deposition of the immune complexes between self-antigens and autoantibodies in the microcirculatory networks of several organs [1]. There is multifactorial pathogenesis of lupus consisting of the combination between genetic predispositions and environmental factors that trigger abnormal immune responses in the innate (antigen-presenting cells) and adaptive immunity (self-reactive T and B cells) [1]. While the hallmark of SLE is an abnormality in the adaptive immunity resulting in the elevation of autoantibodies (mostly against the nuclear antigens), the influence of innate immunity-induced inflammation in lupus disease progression is well known [1]. Although the gastrointestinal (GI) symptoms in lupus are not predominant, the immune complex deposition in the gut of patients and mice with lupus is mentioned, partly due to the large surface area of the gastrointestinal system [2]. Interestingly, the gut immune complex deposition induces inflammatory responses and possibly facilitates gastrointestinal permeability defect with the translocation of pathogen molecules from the gut into the blood circulation, referred to as “gut leakage or leaky gut” [2], because (i) the reactions against pathogen-associated molecular patterns (PAMPs) that are foreign to the host are usually more severe than the responses toward the host antigens [3], (ii) the presence of PAMPs in blood possibly induces a potent immune activation, especially innate immune responses [2], and (iii) acute and chronic inflammation is an exacerbating factor of lupus activity [4]. The translocation of PAMPs or viable organisms from the gut might be an endogenous factor to trigger inflammation or disease flare-ups in patients with lupus. Interestingly, a defect of gut permeability could be demonstrated without any symptoms or only subtle non-specific symptoms (fatigue, nausea, and bloating) [5]. Hence, the flare-up of lupus activity due to the inflammatory responses against a silent gut translocation of endogenous PAMPs might be responsible for the fluctuation of lupus disease activity in some patients without an obvious exposure to the exogenous exacerbating factors. However, the concerns about these silent exacerbation factors in lupus are still too few despite the established methods for the determination of gut leakage. It is interesting to note that patients with lupus are not only susceptible to the exogenous environmental factors (chemical substances, organisms, and PAMPs) [6], but also the stimulators from the endogenous factors due to gut translocation. In this review, we discuss the possible impacts of leaky gut in SLE focusing on the activation of innate immunity and proposed the possible treatment of gut leakage based on the improved understanding of this topic.
2. General Aspects of Gut Permeability Defects
In each person, the gastrointestinal epithelial lining approximately includes the surface area of a small room (32 m2), and not only separates the host from the external environments but functions as a first-line innate immune defense to safeguard the entry of foreign antigens [2]. Interestingly, this barrier consists of only a single layer of epithelial cells with cell junctional complexes and several humoral factors, including mucins, antimicrobial molecules, immunoglobulins, and cytokines [7,8]. Under normal conditions, the intestinal barrier acts as a barrier to select the entry of only some molecules mainly with two pathways: the intestinal cell paracellular space and a transcellular passage [2]. The translocation through the paracellular space is regulated by cell junctional complexes, including tight junctions (TJs), adherens junctions, and desmosomes [2]. The epithelial TJs, located at the apical end of lateral membranes, consist of four major protein complexes; occludin, claudin, junction-adhesion molecules (JAMs), and the coxsackievirus-adenovirus receptor (CAR) protein, which regulate the paracellular trafficking and allow the molecules that are smaller than 3.6 A° (or 0.6 kDa) to pass through the normal paracellular passage [2]. This size selectivity of the gut barrier prevents gut translocation of viable organisms and several PAMPs which are the drivers of systemic inflammation [8,9,10,11]. As such, Gram-negative bacteria and fungi (mostly Candida albicans) are the most and the second most prominent organisms in the human gut and are the main source of lipopolysaccharides (LPS) and (1→3)-β-d-glucan (BG), respectively, in the intestinal contents [2]. Both LPS, molecular weight (MW) ranging from 10 to >100 kDa, and BG (MW 6 to >600 kDa) are the major cell wall components of bacteria and fungi which might be the main PAMPs in gut contents with potent immune activation properties [8,9,10,11]. Moreover, the free nucleic acid from the microbial breakdown might be another pathogen molecule from the gut translocation that may activate immune responses. The microbial-free DNA is rapidly naturally broken down by several processes (depurination or deamination DNA) into smaller sizes of less than 100 bp (65 kDa) which can cross the gut barrier [12,13] even though the intact bacterial genome at a molecular size of 100 to 15,000 kbp (6.5 × 104–9.8 × 106 kDa) [14] cannot pass through the gut barrier. The detection of microbial-free deoxyribonucleic acid (DNA) in several conditions without positive bacteremia; such as coronavirus disease (COVID-19) and obesity, and the detection of intestinal bacterial ribosomal RNA (rRNA) in the lungs of patients with acute respiratory distress syndrome (ARDS), and leaky gut measurement in patients with the post-abdominal operation, imply gut translocation of microbial nucleic acids [9,15,16,17]. During gastrointestinal translocation, PAMPs could enter the body’s circulatory system through the lymphatics (mesenteric lymph nodes and thoracic lymph duct) and portal circulation (portal vein) [2]. Furthermore, the attachment of LPS to lipoproteins/chylomicrons from the gut during routine lipid absorption may physiologically enhance LPS levels in systemic circulation [18]. Because the gut barrier consists of only a single layer of enterocytes in a very large surface area, silent gut leakage without the detectable PAMPs in the blood circulation might be a normal physiologic condition [19]. Indeed, several host mechanisms regulate PAMPs from gut translocation, mostly from neutralization using innate immunity, for example, the LPS binding protein, soluble cluster of differentiation (CD)-14, cell surface receptors (toll-like receptor (TLR)-4, dectin, and scavenger receptors), complement receptor 3, and some enzymes such as acyl-oxy-acyl hydrolase (AOAH), alkaline phosphatase, and DNAase or RNAase [2,20,21]. Hence, the detection of PAMPs in blood indirectly indicates gut translocation of a significant abundance of PAMPs beyond the capacity of host neutralization mechanisms possibly due to the significant intestinal mucosal damage despite asymptomatic clinical presentation in some cases.
3. Gut Permeability Defects in Lupus
In lupus, the immune complex in the vascular networks is the main multi-organ pathogenesis that can deposit throughout the gut from mouth to rectum, due to the enormous surface area of the organ, in different intensities, including asymptomatic deposition, mucosal injury, or bowel ischemic vasculitis [22,23]. The autopsy and endoscopic studies exhibited abdominal injury in approximately 70% of patients with SLE, although only 10% of these patients show GI symptoms [24,25]. Indeed, the immune-complex deposition in the GI tract during the active lupus nephritis without the observed clinical GI abnormalities (diarrhea or weight loss) in the lupus rodent model from the deletion of Fc gamma receptor IIb (FcgRIIb-/- mice) [26,27] with the defects on intestinal tight junctions (Figure 1) are demonstrated. Notably, FcgRIIb is the only inhibitory receptor among the Fc gamma receptor (FcgR) family, and the loss of FcgRIIb facilitates immune responses resulting in the spontaneous development of full-blown lupus nephritis (serum anti-dsDNA, proteinuria, and uremia) after 6 months in FcgRIIb-/- mice.
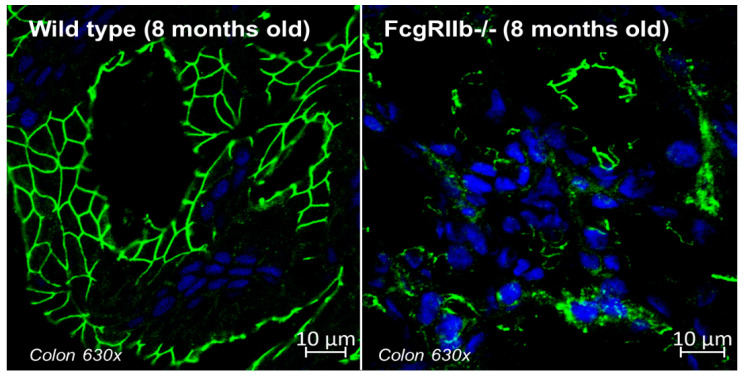
Figure 1.
The representative picture of zona occludens-1 (ZO-1, green fluorescent color), a tight junction protein, in enterocyte of the colon from 8-week-old mice of wild-type control and FcgRIIb-/- lupus mice are demonstrated (original magnification 630×) [26]. The blue color is the staining of the nucleus using DAPI (4′,6-diamidino-2-phenylindole), a blue-fluorescent DNA stain (Alexa Fluor 488, Abcam, Cambridge, MA, USA). The samples were prepared in Cryogel (Leica Biosystems, Richmond, IL, USA) and photographed by a ZEISS LSM 800 (Carl Zeiss, Germany). Notably, there is a well-defined green color border in wild type versus the unclear borders of ZO-1-stained colon in FcgRIIb-/- lupus mice at 8 weeks old (full-blown lupus).
Despite the non-GI symptoms, gut leakage during active lupus is indicated by the elevation of PAMPs in serum (LPS and BG) and the tight junction protein level in blood circulation in either patients or mice with lupus [26,28,29,30,31]. Moreover, gut permeability defects might be severe enough for the translocation of viable bacteria. As such, gut translocation of Enterococcus gallinarum, one of the pathobiont in some situations, to the liver and other organs trigger anti-dsDNA (the major autoantibody in lupus) through TLR-7/8 activation in New Zealand White (NZW)/F1 lupus mice (and also possibly in patients), which is attenuated by antibiotics, has been demonstrated [28]. Nevertheless, gut permeability damage in lupus is not only due to immune-complex deposition in the gut, but also from the adverse effects of some medications used in autoimmune diseases, including nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, and disease-modifying antirheumatic drugs (DMARDs) [32,33,34,35]. As such, the long-term use of NSAIDs induces GI lesions (in the stomach and intestines) [33,36,37] and disrupts gut integrity partly through cell apoptosis induction [34], which possibly exacerbates lupus progression in a lupus mouse model [27]. It is interesting to note that gut translocation of PAMPs after NSAIDs use possibly facilitates lupus activity through a significant systemic inflammation as indicated by increased serum cytokines, despite the anti-inflammatory property of NSAIDs [27]. Similarly, the induction of gut leakage by a low dose of dextran sulfate solution (direct damage to enterocyte tight junction) induces lupus disease activity without diarrhea [26], implying that the silent leaky gut without GI symptoms is a possible exacerbating factor of lupus. Additionally, infection, including intestinal and systemic infection, is a major complication in patients with lupus due to immunosuppressive drugs [38,39,40,41] or defects in the pathogen control from lupus itself, as the infection is a common cause of death even before the era of immunosuppressive drugs [42,43,44]. Perhaps a possible leaky gut during enterocolitis or diarrhea in patients with lupus should be a concern. While gut translocation of PAMPs in intestinal infection is easily explained through the enterocyte tight junction injury, leaky gut in systemic infection is possibly a result of systemic inflammation-induced enterocyte damage [2]. Indeed, diarrhea in some patients with systemic infection (primarily non-gastrointestinal infections) [45] and endotoxemia in severe viral infection [17,46] support a possibility of leaky gut during systemic infection. Although diarrhea is not a good biomarker for leaky gut, as diarrhea is not demonstrated in sepsis or asymptomatic Clostridium difficile infection in mouse models [47], diarrhea in lupus might be associated with leaky gut and, at least, a subtle systemic inflammation. Despite the well-known systemic inflammation during active lupus (especially endotoxemia without systemic infection), data on the consequence of gut leakage as an endogenous exacerbation factor of lupus is still lacking. Hence, all of the evidence mentioned above suggests that leaky gut in lupus is possible and might be clinically important.
4. Gut Dysbiosis in Lupus
Several studies demonstrated that the healthiness of intestinal mucosa [48,49] and the proper immune maturation [50,51,52,53] depended on gut microbiota [49,54,55]. Germ-free mice show decreased secreting IgA plasma cells with the thinner mucus layer and Peyer’s patches when compared with regular mice [56,57]. An increase in pathogenic organisms (pathobionts) in the gut, referred to as “gut dysbiosis”, is one of the possible causes of leaky gut [58] that are demonstrated in several diseases, including obesity, inflammatory bowel disease (IBD), and autoimmune diseases [59,60,61]. Notably, gut dysbiosis can be a cause or a consequence of gut leakage. As such, oral administration of bacteria or fungi caused leaky gut from an increase in pathobionts [62,63], while leaky gut by dextran sulfate induces dysbiosis [26]. Gut dysbiosis in SLE might be a result of a combination of (i) the genetic defects in the immune responses against some gut organisms that selectively allow some group of organisms to survive in the gut, together with (ii) the gut environmental factors from the host behaviors (diets, alcohol, smoking, and medications). For example, fecal transfer from triple congenic lupus-prone mice during active lupus to germ-free mice induces autoantibodies and autoimmune phenotypes [64]. Lupus caused by complement deficiencies (C1q, C2, C3, and C4) [65,66] might be associated with gut dysbiosis due to a defect in the control of some organisms as indicated by the dysbiosis in C3 deficient mice [67]. However, the environmental factors are more important than host genetics in the alteration of gut microbiota from a study of 1,046 healthy individuals [68].
Nevertheless, the increased Bacteroidetes with decreased Firmicutes was demonstrated in patients with lupus in different regions of the world [69,70,71,72], and in various lupus models [28,31,58,71,73,74]. Indeed, Firmicutes, mostly Gram-positive bacteria with obligate aerobes or facultative anaerobes, are the major gut microbes that alter complex carbohydrates into short-chain fatty acids (SCFA), particularly butyrate, which serve as growth factors for gut epithelium [75]. Meanwhile, Bacteroides, mostly Gram-negative anaerobes, are pathogenic bacteria in some situations [76]. Normal gut microbiota also maintains gut epithelial integrity using other substances. For example, tryptophan metabolites (indole and tryptamine) upregulate Muc2 (mucin production), through the pregnane X receptor (PXR) and aryl hydrocarbon receptor (AhR), and induce glucagon-like peptide 1 (GLP-1; an enterocyte growth factor) from enteroendocrine L-cells [77,78]. Leaky gut with reduced Muc2 in PXR-deficient mice has been demonstrated [79,80]. Although the Firmicutes/Bacteroides ratio is increased and decreased in IBD and obesity, respectively, the clinical use of this ratio, including in lupus, is still unclear. It is possible that inflammatory responses from leaky gut, possibly due to the reduced Firmicutes, might initiate or exacerbate lupus in some patients who are already prone to lupus, or gut inflammation during active lupus might decrease Firmicutes bacteria. Despite the unclear role of gut dysbiosis in lupus, healthy gut integrity prevents gut translocation of PAMPs that might possibly be helpful for the control of lupus exacerbation. Despite inconclusive pathogenesis, gut dysbiosis in lupus is well known and might be associated with leaky gut, which can be a cause or a consequence of lupus.
5. Leaky Gut-Induced Innate Immunity and Cell Death in Lupus
Innate immunity is the first-line recognition of PAMPs and damage-associated molecular patterns (DAMPs) from microbes and host cells, respectively. Although the abnormalities in the adaptive immunity are dominant in SLE as indicated by the immune-complex formation, lupus exacerbation by innate immunity-induced inflammation and innate immunity dysfunction in lupus is well known [1,81]. For example, the defect of macrophages in clearing apoptotic cell debris induces prolonged exposure to autoantigens [82,83] and abnormal adaptive immunity due to the antigen processing property of dendritic cells (DCs) in lupus [84,85,86]. Accordingly, LPS and BG from gut translocation mainly activate several innate immune cells through TLR-4 and dectin-1, respectively, and the co-presence of LPS and BG induces the synergy of pro-inflammatory responses. Additionally, there is a dramatic decrease in glomerular IgG deposition and mesangial cell proliferation with reduced autoantibody titers in TLR-2- or TLR-4-deficient MRL lymphoproliferation strain (MRL/lpr) lupus mice [87]. Despite a limited exploration of leaky gut in lupus models, gut dysbiosis and the correlation between gut microbiota and systemic inflammation in lupus is well established [58,88,89]. During the gut permeability defect in lupus, PAMPs and DAMPs enhance systemic inflammation and induce lupus disease progression via the activation of several innate immune cells including neutrophils, macrophages, and DCs. Despite the diverse pathogenesis of lupus, defect in inhibitory FcgRIIb is one of the causes of lupus and hypofunctional FcgRIIb polymorphisms are frequently reported in the population in Asia and East Asia partly due to the selection by malarial infection in the region [90]. Defect in inhibitory FcgRIIb causes hyper-responsiveness against malaria in these regions, while enhancing the incidence of lupus [90]. Because FcgRIIb is detectable in neutrophils, macrophages, DCs, and B cells (but not T cells), FcgRIIb-/- mice have been used to explore both innate and adaptive immune cells in lupus [91,92]. Because of the possible cross-talk between the Fc gamma receptor and TLR-4, which is a pattern recognition receptor against several ligands [46], both the endogenous human molecules (DAMPs; such as heat-shock protein, beta-amyloid, and cell-free DNA) and exogenous microbial or environmental molecules (such as LPS, mannan, and particle matter 2.5) [93], the co-presence of these TLR-4 ligands together with activation of the activating-FcgRs (non-FcgRIIb), perhaps by the natural immunoglobulin, might induce synergistic pro-inflammatory responses through the FcgR-TLR-4 cross-talk [94]. Although the FcgRIIb defect is only a part of the genetic abnormalities in lupus, FcgR-TLR-4 cross-talk might be responsible for the hyper-immune responses against several conditions in patients with lupus through macrophages and neutrophils, and the blockage of spleen-tyrosine-kinase (Syk, a shared downstream signaling molecule of both FcgR and TLR-4) attenuates lupus-induced inflammation both in mice and in patients [31,95]. This information supports the impact of innate immunity in lupus.
Furthermore, several processes of cell death, such as cell apoptosis and necrosis, are a common consequence of inflammation that could exacerbate lupus activity partly through the presentation of DAMPs, possibly due to inadequate clearance of these molecules during overwhelming cell death [96]. The hyper-inflammatory responses of immune cells (both innate and adaptive immunity) and the immune-induced cell injury in several organs by several stimuli (such as cytokines, PAMPs, and mitochondrial DNA) induce both apoptosis and necrosis of immune cells and parenchymal cells in lupus. Subsequently, the extracellular nucleic acids from host cells and mitochondria and nucleoproteins from the damaged cells might be the autoantigens that facilitate the autoantibody production and circulating immune-complex (CIC) deposition, which highlights the importance of cell death and DAMPs in lupus [97,98]. Although CIC deposition is a possible continuous process in lupus, an alteration in the abundance of CIC is possible which might be responsible for the variation of lupus activity (exacerbation versus inactivity) [99]. In lupus, the production of CIC and lupus exacerbation possibly depends on the increase in abundance of self-antigens from cell death [100,101] and autoantibody production, possibly through the non-specific activation of B cells (or plasma cells) by a viral infection, vaccination, or some medications [102,103]. For the enhanced self-antigen exposure, immune responses against pathogen molecules in blood during leaky gut might increase cell apoptosis as the spleen is responsible for the recognition of foreign molecules in the blood, and profound or chronic activation of some cells in the spleen (such as in the splenic marginal zone) may cause cell apoptosis [104]. For example, LPS during lupus-induced leaky gut might enhance spleen apoptosis, as demonstrated in LPS injection [105] and colitis mouse models [26]. The lupus exacerbation from the enhanced exposure to the self-antigens due to the increased apoptosis is also indicated in cancer treatment by anti-program cell death (PD)-1 (an apoptosis inhibitor) [106]. Moreover, a leaky gut also possibly facilitates autoantibody production. Accordingly, while infection and vaccination induce some specific clones of B cells using some growth factors, such as interleukin (IL)-2, and the pro-inflammatory processes (similar to the role of adjuvants), these factors might non-specifically stimulate self-reactive B cells clones in the host with lupus. Hence, the non-specific inflammatory responses against pathogen molecules during leaky gut might increase self-antigen exposure and accidentally facilitate autoantibodies.
Then, gut leakage that is severe enough to induce systemic inflammation might accelerate immune cell apoptosis, especially in spleens and livers, which exacerbates lupus activity [26] as demonstrated in the working hypothesis diagram (Figure 2). Likewise, cell death by neutrophil extracellular traps (NETs), referred to as “NETosis”, is a form of neutrophil death triggered by PAMPs (including LPS and BG) to release extracellular nuclear chromatin [107,108], which is a direct anti-organismal mechanism together with the stimulation of innate and adaptive immunities [101,109]. However, NETosis also induces the exposure of nuclear antigens that accelerate autoantibodies and lupus activity through several mechanisms, including (i) interferon (IFN) type I production from peripheral blood mononuclear cells and DCs through TLR-9 activation [109,110], (ii) the post-translational modifications (histone acetylation and citrullination) of some proteins, (iii) the impaired NET degradation through DNase deficiency [109,110], and (iv) the complex of anti-NET antibodies with NETs that block DNase and enhance the cells positive for TLR-9 and CD32 (DCs, monocytes, B cells, and GM-CSF-induced neutrophils) [96]. Because both LPS and BG from lupus-induced gut translocation induce NETosis that is possibly synergized by activating-FcgRs, gut leakage can enhance NETosis in lupus [111]. Moreover, pyroptosis [112], canonically dependent cell death through the plasma membrane pores using inflammasome-caspase 1-gasdermin-D that causes osmotic lysis, might also enhance the presentation of self-nucleic acids in lupus [113,114]. Pyroptosis also increases high-mobility group box 1 (HMGB1), a nuclear DNA binding protein ubiquitously expressed in eukaryotic cells, that increases in patients with SLE [115,116], and then, HMGB1 binding DNA complex can stimulate type I IFN release from DCs through TLR-9 [115,117] and activates B cells via the advanced glycation end-products (RAGE) receptor [118]. Hence, gut leakage in lupus possibly activates inflammatory responses in several types of immune cells severe enough to induce death of these immune cells and cells in several organs that are responsible for the exposure of self-antigens, autoantibody production, and lupus exacerbation.
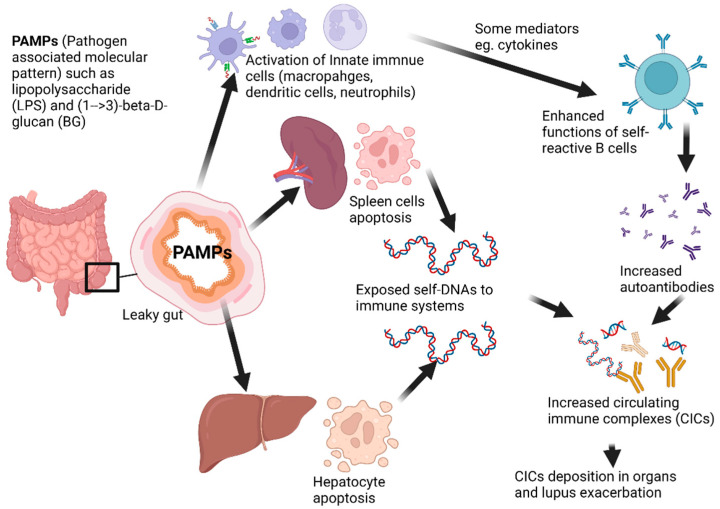
Figure 2.
The working hypothesis diagram demonstrates the possible mechanism of leaky gut-exacerbated lupus through the elevated circulating immune complexes, from (i) increased exposure to the self-antigens from overwhelming apoptosis (such as splenocytes and hepatocytes) and (ii) enhanced autoantibody production from inflammatory mediators, due to the activation by PAMPs from leaky gut.
6. Leaky Gut-Induced Inflammation and Molecular Mimicry in Lupus
Inflammation not only exacerbates lupus through the induction of cell death and the non-specifically enhanced autoantibody production but also mediates lupus activity through other pathways. Interestingly, some lupus-associated pathogenesis cytokines can be induced by pathogens or gut microbial translocation. For example, interferon (IFN) type I is an important cytokine that augments adaptive immunity through enhanced antigen presentation and promotes the high-affinity antigen-specific T and B cells in lupus pathogenesis [119]. It is also induced by CD11c positive DCs in the intestinal laminar propria during bacterial colitis [119,120,121,122] to prevent enterocyte disruption [123,124]. Additionally, extracellular DNAs exacerbate lupus activity through type I IFN activation from DCs in the intestinal laminar propria through TLR-9 [111]. Then, IFN type I during some bacterial colitis might be responsible for the lupus exacerbation [125]. Moreover, TNF-α and IL-6 are also important for lupus exacerbation as the potent pro-inflammatory cytokines in macrophage and neutrophil activation. As such, gene expression profiles in myeloid-derived macrophages from patients with SLE demonstrate increased gene expression of pro-inflammatory macrophages (M1 polarization), including signal transducer and activator of transcription (STAT)-1, suppressor of cytokine signaling (SOCS)-3, CD80, and CD86, and a decrease in the genes of alternatively anti-inflammatory macrophages (M2 polarization) (STAT-3, STAT-6, and CD163) [126,127,128]. Not surprisingly, studies in patients and lupus mice also exhibit an increasing baseline of TNF-α and IL-6 compared to healthy volunteers, which correlates with lupus disease severity [31,129,130]. In FcgRIIb-/- lupus mice, LPS from gut leakage in synergy with BG induces prominent M1 pro-inflammatory macrophages partly through the induction of TNF-α and IL-6 [131,132,133,134,135]. At the same time, TNF-α also directly induces programmed cell death including apoptosis and necroptosis, which accelerates the excess of auto-nuclear antigens [136]. Mice with a chronic overproduction of TNF-α (TNFdeltaARE mice) spontaneously develop Crohn’s disease-like inflammation in the small intestine [137], suggesting that TNF-α may directly induce leaky gut. Due to the possible dual impacts in lupus pathogenesis and the responses against gut organisms and leaky gut of some cytokines, these cytokines might be responsible for the correlation between lupus exacerbation and intestinal inflammation. Furthermore, molecular mimicry is a process that non-self-peptides, mainly from microbial origins, share sequence homology with self-peptides, eliciting cross-reactive recognition between foreign and self-antigens, leading to activation of autoreactive T cells in several autoimmune diseases, including lupus [138]. Both DCs and macrophages are well known as professional antigen-presenting cells (APC) which are found in many tissues, along with the gastrointestinal tract. Interactions between APC and T cells are a direct link between innate and adaptive immunity through the process of antigens on major histocompatibility complexes (MHC) class II. Thus, organismal molecules from gut translocation might enhance the autoantibody production in lupus due to the molecular mimicry between some microbial and self-antigens. As such, the intestinal expansion of Ruminococcus gnavus (RG2strain) is associated with lupus nephritis along with the detection of antibodies against lipoglycans (a molecule in the cell wall of R. gnavus) with increased anti-dsDNA (a specific lupus antibody) and the extract of R. gnavus RG2 cross-reacts with anti-dsDNA antibodies, suggesting the molecular mimicry between RG2 cell wall moieties and native DNA molecules [73]. Additionally, the antibody against Ro protein (Ro60, a 60 kDa self-antigens) that is commonly found in lupus, cross-reacts with the Ebstein–Barr virus nuclear antigen-1 (EBNA-1), and the ortholog Ro60-containing is demonstrated in Bacteroides thetaiotaomicron (gut commensal bacteria) in microbiome analysis from patients with SLE [138,139]. These data suggest that lupus humoral autoimmunity might be initiated via molecular mimicry between PAMPs from gut translocation and self-antigens highlighting another correlation between gut permeability defect and lupus exacerbation or initiation.
7. Monitoring of Gut Leakage in Lupus
Interestingly, several methods might be suitable for the detection and monitoring of leaky gut in lupus. The direct measurement of gut integrity is based on (i) the detection of non-absorbable carbohydrates or other probed molecules in urine (or in the blood) after an oral administration [19] and/or (ii) the recognition of PAMPs in blood without an obvious source of infection, such as endotoxemia and glucanemia, as an indirect biomarker of gut translocation of pathogen molecules with large MW [47]. Although there are several molecular probes for leaky gut, such as (i) LPS detection assays, including Limulus amebocyte lysate (LAL), endotoxin activity assay (EAA), and ELISAs, (ii) BG tests, and (iii) an oral administration of a non-absorbable carbohydrate (before detection of the carbohydrate in blood or in urine) with several benefits and limitations [140,141], the use of these strategies for monitoring leaky gut in lupus is less common. Notably, the detection of serum zonulin (a regulator of epithelial and endothelial barrier functions) [142] is another possible indicator of gut integrity damage; however, the association between gut barrier defect and serum zonulin is still inconclusive [143,144,145]. Due to (i) the clinical availability and the convenience of the indirect leaky gut parameters (serum LPS and/or BG), and (ii) the safety of a single oral carbohydrate administration (mannitol with lactulose or sucralose), we propose to use these parameters for leaky gut monitoring in patients with lupus.
8. Therapeutics Targeting Gut Leakage in Lupus
Because gut dysbiosis, gut leakage, and the translocation of PAMPs might be associated with lupus disease activity, gut dysbiosis attenuation, gut integrity improvement, and the neutralization of PAMPs are potential methods for slowing SLE progression in susceptible individuals. Among several proposed interventions, probiotic administration and fecal transplantation are frequently mentioned [146]. Accordingly, probiotics are beneficial microbes that colonize the GI tract and confer beneficial effects on the host through several mechanisms including competition, anti-bacterial effects, protection of the GI barrier, and modulation of immune responses. The clinical implication of probiotics with the diverse bacterial species was firstly described in 1954 [147] in several diseases [146]. In SLE, several beneficial probiotics, particularly Lactobacillus spp., have been reported (Table 1). However, probiotics with mixed organisms are usually used, despite an uncertain benefit, over the single strain probiotic products [148]. Hence, fecal transplantation, an administration of feces from healthy donors that consist of several organisms, might be even more beneficial than multi-bacterial probiotics. Although fecal transplantation is never been tested in patients with lupus, the fecal microbiota transplantation (FMT) of feces from patients with lupus into germ-free mice induces some lupus-like characteristics, including increased autoantibodies, cytokines, altered immune cell distribution (in mucosa and blood), and upregulated SLE-related genes [149]. Due to a possible causal role of aberrant gut microbiota in lupus pathogenesis, FMT of feces from healthy individuals might be beneficial in patients with lupus and a clinical trial of FMT in patients with active SLE effectively modified the gut microbiota [150]. More studies on this topic are ongoing [151,152,153,154]. On the other hand, the strategies of the direct attenuation of intestinal integrity by the protective molecules from probiotics (such as the epithelial cell growth factors and short-chain fatty acids) with neutralization of PAMPs from gut translocation are also interesting. Surprisingly, an alteration of free fatty acids (FFA) in serum and feces of patients with lupus is common and partially associated with gut dysbiosis [72]. The administration of butyrate in MRL/lpr lupus-prone mice ameliorates gut microbiota dysbiosis [155] which might be even more interesting than probiotics due to the possible easier preparation and storage of FFA as a chemical drug. Nevertheless, probiotics are the current strategy to improve gut integrity and gut dysbiosis with the most intensive studies with an adverse effect (systemic dissemination) in patients with potent immunosuppression [156]. However, the disseminated probiotics are easily eradicated by a simple antibiotic because of the facultative anaerobic nature of the organisms. Hence, the additional indicators of gut dysbiosis, gut leakage, and the monitoring biomarkers might be beneficial for lupus. More studies are warranted.
Table 1.
Beneficial probiotics in SLE.
| Probiotic | Effect To Attenuate Leaky Gut | Studying in SLE Human or Lupus Murine Model | Ref. | |
|---|---|---|---|---|
| Strain | Models/Observed Effect | |||
| Lactobacillus rhamnosus | GG ATCC 9595 | Pristine-induced murine model: anti-dsDNA ↓, IFNγ ↓, Th1-Th17 polarization ↓, Treg cell ↑ | [162,163] | |
| Patients with SLE: miR-181a ↓, miR155 ↓ | [154] | |||
| LMS 201 | MRL/lpr murine model: attenuate lupus nephritis, IL-6 ↓, IL-10 ↑, IgG2a ↓ | [58] | ||
| Lactobacillus delbrueckii |
|
PTCC 1743 | Pristine-induced murine model: anti-dsDNA ↓, IFNγ ↓, Th1-Th17 polarization ↓, Treg cell ↑ | [154,163] |
| Lactobacillus Plantarum | LP299v | NZB/W F1 murine model: anti-inflammatory phenotype of BM-DCs ↑, IL-10 ↑, IL-12 ↑ | [169] | |
| LC40 | NZB/W F1 murine model: improve gut dysbiosis, renal injury ↓, plasma LPS ↓, occludin and ZO-1 expression ↑, TNF-α ↓, Th1-Th17 polarization ↓, Treg cell ↓ | [74] | ||
| Lactobacillus reuteri | GMNL-263 | NZB/W F1 murine model: IL-6 ↓, TNF-α ↓, MMP-9 ↓, CRP ↓, TLR-4 ↓, TLR-7 ↓, TLR-9 ↓, Treg cell ↑ | [153,172] | |
| GMNL-89 | NZB/W F1 murine model: hepatic apoptosis ↓, IL-6 ↓, TNF-α ↓, MMP-9 ↓, CRP ↓ | [153] | ||
| DSM 17509 | NZB/W F1 murine model: survival rate ↑, anti-inflammatory phenotype of BM-DCs ↑, IL-10 ↑, IL-12 ↑ | [169] | ||
| Lactobacillus Casei | B255 | NZB/W F1 murine model: survival rate ↑, anti-inflammatory phenotype of BM-DCs ↑, IL-10 ↑, IL-12 ↑ | [169] | |
| shirota | MRL/lpr murine model: B220 positive T cell in spleen and MLN ↓, IL-6 expression in macrophage ↓ | [175] | ||
|
Lactobacillus
Paracasei |
|
GMNL-32 | NZB/W F1 murine model: cardiac apoptosis ↓, hepatic apoptosis ↓, IL-6 ↓, TNF-α ↓ | [153,177] |
9. Conclusions
The summary of our working hypothesis is presented in Figure 3. As such, gut leakage caused by immune-complex deposition, gut dysbiosis, infection, and medications might be a silent exacerbating factor of lupus activity. Gut translocation of PAMPs, especially LPS and BG, into blood circulation may induce systemic inflammation and enhance lupus activity through induction of several cytokines and several types of cell death from innate immune cells. Moreover, the molecular mimicry between gut pathogens and self-antigens might possibly increase the autoantibodies and lupus flare-ups. Because probiotics could attenuate gut dysbiosis and strengthen gut integrity without serious adverse effects, probiotic treatment in the selected patients with leaky gut in lupus using several biomarkers is interesting.
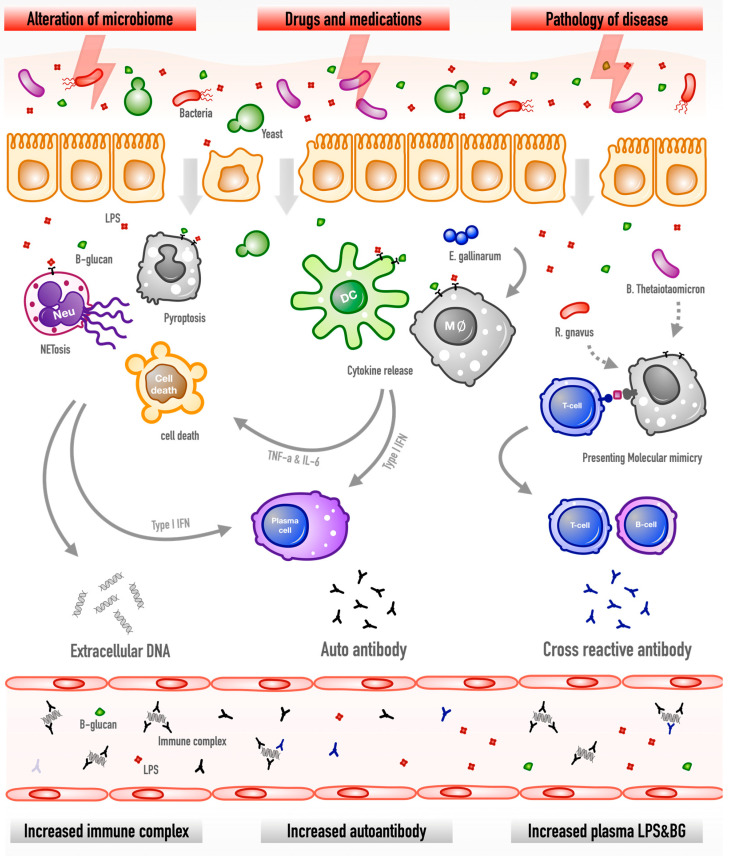
Figure 3.
The hypothesis diagram demonstrates gut permeability defects in lupus through several mechanisms (microbiome alteration, drugs, and lupus-induced inflammation) facilitate the translocation of microbial molecules that induce the death of immune cells (apoptosis, NETosis, and pyroptosis), the release of cytokines (type I IFN and IL-6), and molecular mimicry, results in extracellular DNA exposure, autoantibody production, and cross-reactive antibodies. Hence, leaky gut might enhance pathogen molecules in the blood, such as lipopolysaccharides (LPS) from Gram-negative bacteria and (1→3)-β-d-glucan (BG) from gut fungi, autoantibody production, and the circulating immune complex.
Author Contributions
Conceptualization, A.C. and A.L.; visualization, A.C. and K.S.-k.; writing—original draft preparation, A.C., K.S.-k. and A.L. all authors critically revised, discussed, and edited the article until it reached its current form. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by Chulalongkorn University through the Funding platform of research conveys academic education 2022, Fundamental Fund 65 [CUFRB65_hea (33) _040_30_21], the National Research Council of Thailand (grant number NRCT-N41A640076) and (811/2563) with NSRF via the Program Management Unit for Human Resources & Institutional Development, Research, and Innovation (B16F640175 and B05F640144).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Iwasaki A., Medzhitov R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amornphimoltham P., Yuen P.S.T., Star R.A., Leelahavanichkul A. Gut Leakage of Fungal-Derived Inflammatory Mediators: Part of a Gut-Liver-Kidney Axis in Bacterial Sepsis. Am. J. Dig. Dis. Sci. 2019;64:2416–2428. doi: 10.1007/s10620-019-05581-y. [DOI] [PubMed] [Google Scholar]
- 3.Eppensteiner J., Kwun J., Scheuermann U., Barbas A., Limkakeng A.T., Kuchibhatla M., Elster E., Kirk A.D., Lee J. Damage- and pathogen-associated molecular patterns play differential roles in late mortality after critical illness. JCI Insight. 2019;4:e127925. doi: 10.1172/jci.insight.127925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottschalk T., Etsantikos E., Hibbs M.L. Pathogenic Inflammation and Its Therapeutic Targeting in Systemic Lupus Erythematosus. Front. Immunol. 2015;6:550. doi: 10.3389/fimmu.2015.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park J.H., Park D.I., Kim H.J., Cho Y.K., Sohn C.I., Jeon W.K., Kim B.I., Won K.H., Park S.M. The Relationship between Small-Intestinal Bacterial Overgrowth and Intestinal Permeability in Patients with Irritable Bowel Syndrome. Gut Liver. 2009;3:174–179. doi: 10.5009/gnl.2009.3.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L.R., Barber C.E., Johnson A.S., Barnabe C. Invasive fungal disease in systemic lupus erythematosus: A systematic review of disease characteristics, risk factors, and prognosis. Semin. Arthritis Rheum. 2014;44:325–330. doi: 10.1016/j.semarthrit.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Chelakkot C., Ghim J., Ryu S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018;50:103. doi: 10.1038/s12276-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson L.W., Artis D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 9.Wischmeyer P.E. Glutamine: Role in gut protection in critical illness. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9:607–612. doi: 10.1097/01.mco.0000241672.09676.03. [DOI] [PubMed] [Google Scholar]
- 10.Fasano A., Shea-Donohue T. Mechanisms of disease: The role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005;2:416–422. doi: 10.1038/ncpgasthep0259. [DOI] [PubMed] [Google Scholar]
- 11.Jiminez J.A., Uwiera T.C., Douglas Inglis G., Uwiera R.R. Animal models to study acute and chronic intestinal inflammation in mammals. Gut Pathog. 2015;7:29. doi: 10.1186/s13099-015-0076-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellot P., Francés R., Such J. Pathological bacterial translocation in cirrhosis: Pathophysiology, diagnosis and clinical implications. Liver Int. 2013;33:31–39. doi: 10.1111/liv.12021. [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez-Laiz G.P., Zapater P., Melgar P., Alcázar C., Franco M., Giménez P., Pascual S., Bellot P., Palazón J.M., Rodríguez M., et al. Bacterial DNA translocation contributes to systemic inflammation and to minor changes in the clinical outcome of liver transplantation. Sci. Rep. 2019;9:835. doi: 10.1038/s41598-018-36904-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochman H., Caro-Quintero A. Genome Size and Structure, Bacterial. In: Kliman R.M., editor. Encyclopedia of Evolutionary Biology. Academic Press; Oxford, UK: 2016. pp. 179–185. [Google Scholar]
- 15.Quan Z.F., Yang C., Li N., Li J.S. Effect of glutamine on change in early postoperative intestinal permeability and its relation to systemic inflammatory response. World J. Gastroenterol. 2004;10:1992–1994. doi: 10.3748/wjg.v10.i13.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickson R.P., Singer B.H., Newstead M.W., Falkowski N.R., Erb-Downward J.R., Standiford T.J., Huffnagle G.B. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat. Microbiol. 2016;1:16113. doi: 10.1038/nmicrobiol.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirivongrangson P., Kulvichit W., Payungporn S., Pisitkun T., Chindamporn A., Peerapornratana S., Pisitkun P., Chitcharoen S., Sawaswong V., Worasilchai N., et al. Endotoxemia and circulating bacteriome in severe COVID-19 patients. Intensive Care Med. Exp. 2020;8:72. doi: 10.1186/s40635-020-00362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Deventer S.J., Cate J.W.T., Tytgat G.N. Intestinal endotoxemia. Clinical significance. Gastroenterology. 1988;94:825–831. doi: 10.1016/0016-5085(88)90261-2. [DOI] [PubMed] [Google Scholar]
- 19.Camilleri M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut. 2019;68:1516–1526. doi: 10.1136/gutjnl-2019-318427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu L., Li J., Moussaoui M., Boix E. Immune Modulation by Human Secreted RNases at the Extracellular Space. Front. Immunol. 2018;9:1012. doi: 10.3389/fimmu.2018.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soboleva S.E., Zakharova O.D., Sedykh S.E., Ivanisenko N.V., Buneva V.N., Nevinsky G.A. DNase and RNase activities of fresh cow milk lactoferrin. J. Mol. Recognit. 2019;32:e2777. doi: 10.1002/jmr.2777. [DOI] [PubMed] [Google Scholar]
- 22.Tian X.P., Zhang X. Gastrointestinal involvement in systemic lupus erythematosus: Insight into pathogenesis, diagnosis and treatment. World J. Gastroenterol. 2010;16:2971–2977. doi: 10.3748/wjg.v16.i24.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwok S.K., Seo S.H., Ju J.H., Park K.S., Yoon C.H., Kim W.U., Min J.K., Park S.H., Cho C.S., Kim H.Y. Lupus enteritis: Clinical characteristics, risk factor for relapse and association with anti-endothelial cell antibody. Lupus. 2007;16:803–809. doi: 10.1177/0961203307082383. [DOI] [PubMed] [Google Scholar]
- 24.Takeno M., Ishigatsubo Y. Intestinal manifestations in systemic lupus erythematosus. Intern. Med. 2006;45:41–42. doi: 10.2169/internalmedicine.45.0136. [DOI] [PubMed] [Google Scholar]
- 25.Endo H., Kondo Y., Kawagoe K., Ohya T.R., Yanagawa T., Asayama M., Hisatomi K., Teratani T., Yoneda M., Inamori M., et al. Lupus enteritis detected by capsule endoscopy. Intern. Med. 2007;46:1621–1622. doi: 10.2169/internalmedicine.46.0137. [DOI] [PubMed] [Google Scholar]
- 26.Thim-Uam A., Surawut S., Issara-Amphorn J., Jaroonwitchawan T., Hiengrach P., Chatthanathon P., Wilantho A., Somboonna N., Palaga T., Pisitkun P., et al. Leaky-gut enhanced lupus progression in the Fc gamma receptor-IIb deficient and pristane-induced mouse models of lupus. Sci Rep. 2020;10:777. doi: 10.1038/s41598-019-57275-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhunyakarnjanarat T., Udompornpitak K., Saisorn W., Chantraprapawat B., Visitchanakun P., Dang C.P., Issara-Amphorn J., Leelahavanichkul A. Prominent Indomethacin-Induced Enteropathy in Fcgriib Defi-cient lupus Mice: An Impact of Macrophage Responses and Immune Deposition in Gut. Int. J. Mol. Sci. 2021;22:1377. doi: 10.3390/ijms22031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manfredo Vieira S., Hiltensperger M., Kumar V., Zegarra-Ruiz D., Dehner C., Khan N., Costa F.R.C., Tiniakou E., Greiling T., Ruff W., et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359:1156–1161. doi: 10.1126/science.aar7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zegarra-Ruiz D.F., El Beidaq A., Iniguez A.J., Lubrano Di Ricco M., Manfredo Vieira S., Ruff W.E., Mubiru D., Fine R.L., Sterpka J., Greiling T.M., et al. A Diet-Sensitive Commensal Lactobacillus Strain Mediates TLR7-Dependent Systemic Autoimmunity. Cell Host. Microbe. 2019;25:113–127. doi: 10.1016/j.chom.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogunrinde E., Zhou Z., Luo Z., Alekseyenko A., Li Q.Z., Macedo D., Kamen D.L., Oates J.C., Gilkeson G.S., Jiang W. A Link Between Plasma Microbial Translocation, Microbiome, and Autoantibody Development in First-Degree Relatives of Systemic Lupus Erythematosus Patients. Arthritis Rheumatol. 2019;71:1858–1868. doi: 10.1002/art.40935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Issara-Amphorn J., Somboonna N., Pisitkun P., Hirankarn N., Leelahavanichkul A. Syk inhibitor attenuates inflammation in lupus mice from FcgRIIb deficiency but not in pristane induction: The influence of lupus pathogenesis on the therapeutic effect. Lupus. 2020;29:1248–1262. doi: 10.1177/0961203320941106. [DOI] [PubMed] [Google Scholar]
- 32.Ostensen M., Villiger P.M. Nonsteroidal anti-inflammatory drugs in systemic lupus erythematosus. Lupus. 2000;9:566–572. doi: 10.1191/096120300678828794. [DOI] [PubMed] [Google Scholar]
- 33.Sigthorsson G., Tibble J., Hayllar J., Menzies I., Macpherson A., Moots R., Scott D., Gumpel M.J., Bjarnason I. Intestinal permeability and inflammation in patients on NSAIDs. Gut. 1998;43:506–511. doi: 10.1136/gut.43.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhatt A.P., Gunasekara D.B., Speer J., Reed M.I., Pena A.N., Midkiff B.R., Magness S.T., Bultman S.J., Allbritton N.L., Redinbo M.R. Nonsteroidal Anti-Inflammatory Drug-Induced Leaky Gut Modeled Using Polarized Monolayers of Primary Human Intestinal Epithelial Cells. ACS Infect. Dis. 2018;4:46–52. doi: 10.1021/acsinfecdis.7b00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanas A., Chan F.K.L. Peptic ulcer disease. Lancet. 2017;390:613–624. doi: 10.1016/S0140-6736(16)32404-7. [DOI] [PubMed] [Google Scholar]
- 36.Price A.B. Pathology of drug-associated gastrointestinal disease. Br. J. Clin. Pharmacol. 2003;56:477–482. doi: 10.1046/j.1365-2125.2003.01980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein N.S., Cinenza A.N. The histopathology of nonsteroidal anti-inflammatory drug-associated colitis. Am. J. Clin. Pathol. 1998;110:622–628. doi: 10.1093/ajcp/110.5.622. [DOI] [PubMed] [Google Scholar]
- 38.Cervera R., Khamashta M.A., Font J., Sebastiani G.D., Gil A., Lavilla P., Mejia J.C., Aydintug A.O., Chwalinska-Sadowska H., de Ramon E., et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: A comparison of early and late manifestations in a cohort of 1000 patients. Medicine. 2003;82:299–308. doi: 10.1097/01.md.0000091181.93122.55. [DOI] [PubMed] [Google Scholar]
- 39.Kang K.Y., Kwok S.K., Ju J.H., Park K.S., Cho C.S., Kim H.Y., Park S.H. The causes of death in Korean patients with systemic lupus erythematosus over 11 years. Lupus. 2011;20:989–997. doi: 10.1177/0961203311402245. [DOI] [PubMed] [Google Scholar]
- 40.Ward M.M., Pyun E., Studenski S. Causes of death in systemic lupus erythematosus. Long-term followup of an inception cohort. Arthritis Rheum. 1995;38:1492–1499. doi: 10.1002/art.1780381016. [DOI] [PubMed] [Google Scholar]
- 41.Feng X., Zou Y., Pan W., Wang X., Wu M., Zhang M., Tao J., Zhang Y., Tan K., Li J., et al. Prognostic indicators of hospitalized patients with systemic lupus erythematosus: A large retrospective multicenter study in China. J. Rheumatol. 2011;38:1289–1295. doi: 10.3899/jrheum.101088. [DOI] [PubMed] [Google Scholar]
- 42.De Achaval S., Suarez-Almazor M.E. Treatment adherence to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and systemic lupus erythematosus. Int. J. Clin. Rheumtol. 2010;5:313–326. doi: 10.2217/ijr.10.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang E.Y., Inoue T., Leone V.A., Dalal S., Touw K., Wang Y., Musch M.W., Theriault B., Higuchi K., Donovan S., et al. Using corticosteroids to reshape the gut microbiome: Implications for inflammatory bowel diseases. Inflamm. Bowel Dis. 2015;21:963–972. doi: 10.1097/MIB.0000000000000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Danza A., Ruiz-Irastorza G. Infection risk in systemic lupus erythematosus patients: Susceptibility factors and preventive strategies. Lupus. 2013;22:1286–1294. doi: 10.1177/0961203313493032. [DOI] [PubMed] [Google Scholar]
- 45.Reisinger E.C., Fritzsche C., Krause R., Krejs G.J. Diarrhea caused by primarily non-gastrointestinal infections. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005;2:216–222. doi: 10.1038/ncpgasthep0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chancharoenthana W., Leelahavanichkul A., Ariyanon W., Vadcharavivad S., Phatcharophaswattanakul S., Kamolratanakul S., Leaungwutiwong P., Phumratanaprapin W., Wilairatana P. Leaky Gut Syndrome Is Associated with Endotoxemia and Serum (1 → 3)-β-D-Glucan in Severe Dengue Infection. Microorganisms. 2021;9:2390. doi: 10.3390/microorganisms9112390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leelahavanichkul A., Worasilchai N., Wannalerdsakun S., Jutivorakool K., Somparn P., Issara-Amphorn J., Tachaboon S., Srisawat N., Finkelman M., Chindamporn A. Gastrointestinal Leakage Detected by Serum (1 → 3)-β-D-Glucan in Mouse Models and a Pilot Study in Patients with Sepsis. Shock. 2016;46:506–518. doi: 10.1097/SHK.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 48.Lynch S.V., Pedersen O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 49.Pickard J.M., Zeng M.Y., Caruso R., Nunez G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017;279:70–89. doi: 10.1111/imr.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gill N., Finlay B.B. The gut microbiota: Challenging immunology. Nat. Rev. Immunol. 2011;11:636–637. doi: 10.1038/nri3061. [DOI] [PubMed] [Google Scholar]
- 51.Fukata M., Arditi M. The role of pattern recognition receptors in intestinal inflammation. Mucosal. Immunol. 2013;6:451–463. doi: 10.1038/mi.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi Y., Mu L. An expanding stage for commensal microbes in host immune regulation. Cell Mol. Immunol. 2017;14:339–348. doi: 10.1038/cmi.2016.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hooper L.V., Littman D.R., Macpherson A.J. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katz-Agranov N., Zandman-Goddard G. The microbiome and systemic lupus erythematosus. Immunol. Res. 2017;65:432–437. doi: 10.1007/s12026-017-8906-2. [DOI] [PubMed] [Google Scholar]
- 55.Rosser E.C., Mauri C. A clinical update on the significance of the gut microbiota in systemic autoimmunity. J. Autoimmun. 2016;74:85–93. doi: 10.1016/j.jaut.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 56.Tomkovich S., Jobin C. Microbiota and host immune responses: A love-hate relationship. Immunology. 2016;147:1–10. doi: 10.1111/imm.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jain N., Walker W.A. Diet and host-microbial crosstalk in postnatal intestinal immune homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2015;12:14–25. doi: 10.1038/nrgastro.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mu Q., Zhang H., Liao X., Lin K., Liu H., Edwards M.R., Ahmed S.A., Yuan R., Li L., Cecere T.E., et al. Control of lupus nephritis by changes of gut microbiota. Microbiome. 2017;5:73. doi: 10.1186/s40168-017-0300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walker A.W., Sanderson J.D., Churcher C., Parkes G.C., Hudspith B.N., Rayment N., Brostoff J., Parkhill J., Dougan G., Petrovska L. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011;11:7. doi: 10.1186/1471-2180-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tannock G.W. Molecular analysis of the intestinal microflora in IBD. Mucosal. Immunol. 2008;1((Suppl. 1)):S15–S18. doi: 10.1038/mi.2008.54. [DOI] [PubMed] [Google Scholar]
- 61.Turnbaugh P.J., Hamady M., Yatsunenko T., Cantarel B.L., Duncan A., Ley R.E., Sogin M.L., Jones W.J., Roe B.A., Affourtit J.P., et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Panpetch W., Somboonna N., Bulan D.E., Issara-Amphorn J., Finkelman M., Worasilchai N., Chindamporn A., Palaga T., Tumwasorn S., Leelahavanichkul A. Oral administration of live- or heat-killed Candida albicans worsened cecal ligation and puncture sepsis in a murine model possibly due to an increased serum (1 → 3)-β-D-glucan. PLoS ONE. 2017;12:e0181439. doi: 10.1371/journal.pone.0181439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Panpetch W., Phuengmaung P., Hiengrach P., Issara-Amphorn J., Cheibchalard T., Somboonna N., Tumwasorn S., Leelahavanichkul A. Candida Worsens Klebsiella pneumoniae Induced-Sepsis in a Mouse Model with Low Dose Dextran Sulfate Solution through Gut Dysbiosis and Enhanced Inflammation. Int. J. Mol. Sci. 2022;23:7050. doi: 10.3390/ijms23137050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi S.C., Brown J., Gong M., Ge Y., Zadeh M., Li W., Croker B.P., Michailidis G., Garrett T.J., Mohamadzadeh M., et al. Gut microbiota dysbiosis and altered tryptophan catabolism contribute to autoimmunity in lupus-susceptible mice. Sci. Transl. Med. 2020;12:eaax2220. doi: 10.1126/scitranslmed.aax2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Truedsson L., Bengtsson A.A., Sturfelt G. Complement deficiencies and systemic lupus erythematosus. Autoimmunity. 2007;40:560–566. doi: 10.1080/08916930701510673. [DOI] [PubMed] [Google Scholar]
- 66.Tsukamoto H., Horiuchi T., Kokuba H., Nagae S., Nishizaka H., Sawabe T., Harashima S., Himeji D., Koyama T., Otsuka J., et al. Molecular analysis of a novel hereditary C3 deficiency with systemic lupus erythematosus. Biochem. Biophys. Res. Commun. 2005;330:298–304. doi: 10.1016/j.bbrc.2005.02.159. [DOI] [PubMed] [Google Scholar]
- 67.Choi Y.J., Kim J.E., Lee S.J., Gong J.E., Son H.J., Hong J.T., Hwang D.Y. Dysbiosis of Fecal Microbiota From Complement 3 Knockout Mice With Constipation Phenotypes Contributes to Development of Defecation Delay. Front. Physiol. 2021;12:650789. doi: 10.3389/fphys.2021.650789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rothschild D., Weissbrod O., Barkan E., Kurilshikov A., Korem T., Zeevi D., Costea P.I., Godneva A., Kalka I.N., Bar N., et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 69.Hevia A., Milani C., Lopez P., Cuervo A., Arboleya S., Duranti S., Turroni F., Gonzalez S., Suarez A., Gueimonde M., et al. Intestinal dysbiosis associated with systemic lupus erythematosus. mBio. 2014;5:e01548-14. doi: 10.1128/mBio.01548-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He Z., Shao T., Li H., Xie Z., Wen C. Alterations of the gut microbiome in Chinese patients with systemic lupus erythematosus. Gut Pathog. 2016;8:64. doi: 10.1186/s13099-016-0146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Y., Wang H.F., Li X., Li H.X., Zhang Q., Zhou H.W., He Y., Li P., Fu C., Zhang X.H., et al. Disordered intestinal microbes are associated with the activity of Systemic Lupus Erythematosus. Clin. Sci. 2019;133:821–838. doi: 10.1042/CS20180841. [DOI] [PubMed] [Google Scholar]
- 72.Rodriguez-Carrio J., Lopez P., Sanchez B., Gonzalez S., Gueimonde M., Margolles A., de Los Reyes-Gavilan C.G., Suarez A. Intestinal Dysbiosis Is Associated with Altered Short-Chain Fatty Acids and Serum-Free Fatty Acids in Systemic Lupus Erythematosus. Front. Immunol. 2017;8:23. doi: 10.3389/fimmu.2017.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Azzouz D., Omarbekova A., Heguy A., Schwudke D., Gisch N., Rovin B.H., Caricchio R., Buyon J.P., Alekseyenko A.V., Silverman G.J. Lupus nephritis is linked to disease-activity associated expansions and immunity to a gut commensal. Ann. Rheum. Dis. 2019;78:947–956. doi: 10.1136/annrheumdis-2018-214856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Toral M., Robles-Vera I., Romero M., de la Visitacion N., Sanchez M., O’Valle F., Rodriguez-Nogales A., Galvez J., Duarte J., Jimenez R. Lactobacillus fermentum CECT5716: A novel alternative for the prevention of vascular disorders in a mouse model of systemic lupus erythematosus. FASEB J. 2019;33:10005–10018. doi: 10.1096/fj.201900545RR. [DOI] [PubMed] [Google Scholar]
- 75.Louis P., Young P., Holtrop G., Flint H.J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol. 2010;12:304–314. doi: 10.1111/j.1462-2920.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- 76.Zafar H., Saier M.H., Jr. Gut Bacteroides species in health and disease. Gut Microbes. 2021;13:1–20. doi: 10.1080/19490976.2020.1848158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roager H.M., Licht T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018;9:3294. doi: 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hubbard T.D., Murray I.A., Perdew G.H. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab. Dispos. 2015;43:1522–1535. doi: 10.1124/dmd.115.064246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Venkatesh M., Mukherjee S., Wang H., Li H., Sun K., Benechet A.P., Qiu Z., Maher L., Redinbo M.R., Phillips R.S., et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity. 2014;41:296–310. doi: 10.1016/j.immuni.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whitfield-Cargile C.M., Cohen N.D., Chapkin R.S., Weeks B.R., Davidson L.A., Goldsby J.S., Hunt C.L., Steinmeyer S.H., Menon R., Suchodolski J.S., et al. The microbiota-derived metabolite indole decreases mucosal inflammation and injury in a murine model of NSAID enteropathy. Gut Microbes. 2016;7:246–261. doi: 10.1080/19490976.2016.1156827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Herrada A.A., Escobedo N., Iruretagoyena M., Valenzuela R.A., Burgos P.I., Cuitino L., Llanos C. Innate Immune Cells’ Contribution to Systemic Lupus Erythematosus. Front. Immunol. 2019;10:772. doi: 10.3389/fimmu.2019.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bijl M., Reefman E., Horst G., Limburg P.C., Kallenberg C.G. Reduced uptake of apoptotic cells by macrophages in systemic lupus erythematosus: Correlates with decreased serum levels of complement. Ann. Rheum. Dis. 2006;65:57–63. doi: 10.1136/ard.2005.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tas S.W., Quartier P., Botto M., Fossati-Jimack L. Macrophages from patients with SLE and rheumatoid arthritis have defective adhesion in vitro, while only SLE macrophages have impaired uptake of apoptotic cells. Ann. Rheum. Dis. 2006;65:216–221. doi: 10.1136/ard.2005.037143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu Y.J. IPC: Professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 85.Mageed R.A., Isenberg D.A. Tumour necrosis factor alpha in systemic lupus erythematosus and anti-DNA autoantibody production. Lupus. 2002;11:850–855. doi: 10.1191/0961203302lu306rr. [DOI] [PubMed] [Google Scholar]
- 86.Ohl K., Tenbrock K. Inflammatory cytokines in systemic lupus erythematosus. J. Biomed. Biotechnol. 2011;2011:432595. doi: 10.1155/2011/432595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lartigue A., Colliou N., Calbo S., Francois A., Jacquot S., Arnoult C., Tron F., Gilbert D., Musette P. Critical role of TLR2 and TLR4 in autoantibody production and glomerulonephritis in lpr mutation-induced mouse lupus. J. Immunol. 2009;183:6207–6216. doi: 10.4049/jimmunol.0803219. [DOI] [PubMed] [Google Scholar]
- 88.Mu Q., Cabana-Puig X., Mao J., Swartwout B., Abdelhamid L., Cecere T.E., Wang H., Reilly C.M., Luo X.M. Pregnancy and lactation interfere with the response of autoimmunity to modulation of gut microbiota. Microbiome. 2019;7:105. doi: 10.1186/s40168-019-0720-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mu Q., Tavella V.J., Kirby J.L., Cecere T.E., Chung M., Lee J., Li S., Ahmed S.A., Eden K., Allen I.C., et al. Antibiotics ameliorate lupus-like symptoms in mice. Sci. Rep. 2017;7:13675. doi: 10.1038/s41598-017-14223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clatworthy M.R., Willcocks L., Urban B., Langhorne J., Williams T.N., Peshu N., Watkins N.A., Floto R.A., Smith K.G.C. Systemic lupus erythematosus-associated defects in the inhibitory receptor FcγRIIb reduce susceptibility to malaria. Proc. Natl. Acad. Sci. USA. 2007;104:7169–7174. doi: 10.1073/pnas.0608889104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ritprajak P., Kaewraemruaen C., Hirankarn N. Current Paradigms of Tolerogenic Dendritic Cells and Clinical Implications for Systemic Lupus Erythematosus. Cells. 2019;8:1291. doi: 10.3390/cells8101291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith K.G., Clatworthy M.R. FcgammaRIIB in autoimmunity and infection: Evolutionary and therapeutic implications. Nat. Rev. Immunol. 2010;10:328–343. doi: 10.1038/nri2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Molteni M., Gemma S., Rossetti C. The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediat. Inflamm. 2016;2016:6978936. doi: 10.1155/2016/6978936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vogelpoel L.T.C., Hansen I.S., Rispens T., Muller F.J.M., van Capel T.M.M., Turina M.C., Vos J.B., Baeten D.L.P., Kapsenberg M.L., de Jong E.C., et al. Fc gamma receptor-TLR cross-talk elicits pro-inflammatory cytokine production by human M2 macrophages. Nat. Commun. 2014;5:5444. doi: 10.1038/ncomms6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Deng G.-M., Kyttaris V.C., Tsokos G.C. Targeting Syk in Autoimmune Rheumatic Diseases. Front. Immunol. 2016:7. doi: 10.3389/fimmu.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mobarrez F., Svenungsson E., Pisetsky D.S. Microparticles as autoantigens in systemic lupus erythematosus. Eur. J. Clin. Invest. 2018;48:e13010. doi: 10.1111/eci.13010. [DOI] [PubMed] [Google Scholar]
- 97.Su K.Y., Pisetsky D.S. The role of extracellular DNA in autoimmunity in SLE. Scand. J. Immunol. 2009;70:175–183. doi: 10.1111/j.1365-3083.2009.02300.x. [DOI] [PubMed] [Google Scholar]
- 98.Lou H., Pickering M.C. Extracellular DNA and autoimmune diseases. Cell Mol. Immunol. 2018;15:746–755. doi: 10.1038/cmi.2017.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nagata S., Hanayama R., Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 100.Fenton K. The effect of cell death in the initiation of lupus nephritis. Clin. Exp. Immunol. 2015;179:11–16. doi: 10.1111/cei.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mistry P., Kaplan M.J. Cell death in the pathogenesis of systemic lupus erythematosus and lupus nephritis. Clin. Immunol. 2017;185:59–73. doi: 10.1016/j.clim.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Quaglia M., Merlotti G., De Andrea M., Borgogna C., Cantaluppi V. Viral Infections and Systemic Lupus Erythematosus: New Players in an Old Story. Viruses. 2021;13:277. doi: 10.3390/v13020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.He Y., Sawalha A.H. Drug-induced lupus erythematosus: An update on drugs and mechanisms. Curr. Opin. Rheumatol. 2018;30:490–497. doi: 10.1097/BOR.0000000000000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McGaha T.L., Karlsson M.C. Apoptotic cell responses in the splenic marginal zone: A paradigm for immunologic reactions to apoptotic antigens with implications for autoimmunity. Immunol. Rev. 2016;269:26–43. doi: 10.1111/imr.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nežić L., Amidžić L., Škrbić R., Gajanin R., Nepovimova E., Vališ M., Kuča K., Jaćević V. Simvastatin Inhibits Endotoxin-Induced Apoptosis in Liver and Spleen Through Up-Regulation of Survivin/NF-κB/p65 Expression. Front. Pharmacol. 2019;10:54. doi: 10.3389/fphar.2019.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kostine M., Richez C., Schaeverbeke T., Fhu A. Response to: ‘Drug-induced lupus erythematosus following immunotherapy with anti-programmed death-(ligand) 1′ by Michot et al and ‘Checkpoint inhibitor-associated immune arthritis’ by Arnaud et al. Ann. Rheum. Dis. 2019;78:e69. doi: 10.1136/annrheumdis-2018-213691. [DOI] [PubMed] [Google Scholar]
- 107.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 108.Brinkmann V., Zychlinsky A. Beneficial suicide: Why neutrophils die to make NETs. Nat. Rev. Microbiol. 2007;5:577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 109.Garcia-Romo G.S., Caielli S., Vega B., Connolly J., Allantaz F., Xu Z., Punaro M., Baisch J., Guiducci C., Coffman R.L., et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Denny M.F., Yalavarthi S., Zhao W., Thacker S.G., Anderson M., Sandy A.R., McCune W.J., Kaplan M.J. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 2010;184:3284–3297. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Saisorn W., Saithong S., Phuengmaung P., Udompornpitak K., Bhunyakarnjanarat T., Visitchanakun P., Chareonsappakit A., Pisitkun P., Chiewchengchol D., Leelahavanichkul A. Acute Kidney Injury Induced Lupus Exacerbation Through the Enhanced Neutrophil Extracellular Traps (and Apoptosis) in Fcgr2b Deficient Lupus Mice With Renal Ischemia Reperfusion Injury. Front. Immunol. 2021;12:669162. doi: 10.3389/fimmu.2021.669162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bergsbaken T., Fink S.L., Cookson B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fink S.L., Cookson B.T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 114.Miao E.A., Leaf I.A., Treuting P.M., Mao D.P., Dors M., Sarkar A., Warren S.E., Wewers M.D., Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ardoin S.P., Pisetsky D.S. The role of cell death in the pathogenesis of autoimmune disease: HMGB1 and microparticles as intercellular mediators of inflammation. Mod. Rheumatol. 2008;18:319–326. doi: 10.3109/s10165-008-0054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Popovic K., Ek M., Espinosa A., Padyukov L., Harris H.E., Wahren-Herlenius M., Nyberg F. Increased expression of the novel proinflammatory cytokine high mobility group box chromosomal protein 1 in skin lesions of patients with lupus erythematosus. Arthritis Rheum. 2005;52:3639–3645. doi: 10.1002/art.21398. [DOI] [PubMed] [Google Scholar]
- 117.Vande Walle L., Kanneganti T.D., Lamkanfi M. HMGB1 release by inflammasomes. Virulence. 2011;2:162–165. doi: 10.4161/viru.2.2.15480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tian J., Avalos A.M., Mao S.Y., Chen B., Senthil K., Wu H., Parroche P., Drabic S., Golenbock D., Sirois C., et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 119.Kole A., He J., Rivollier A., Silveira D.D., Kitamura K., Maloy K.J., Kelsall B.L. Type I IFNs regulate effector and regulatory T cell accumulation and anti-inflammatory cytokine production during T cell-mediated colitis. J. Immunol. 2013;191:2771–2779. doi: 10.4049/jimmunol.1301093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tschurtschenthaler M., Wang J., Fricke C., Fritz T.M., Niederreiter L., Adolph T.E., Sarcevic E., Kunzel S., Offner F.A., Kalinke U., et al. Type I interferon signalling in the intestinal epithelium affects Paneth cells, microbial ecology and epithelial regeneration. Gut. 2014;63:1921–1931. doi: 10.1136/gutjnl-2013-305863. [DOI] [PubMed] [Google Scholar]
- 121.Katlinskaya Y.V., Katlinski K.V., Lasri A., Li N., Beiting D.P., Durham A.C., Yang T., Pikarsky E., Lengner C.J., Johnson F.B., et al. Type I Interferons Control Proliferation and Function of the Intestinal Epithelium. Mol. Cell Biol. 2016;36:1124–1135. doi: 10.1128/MCB.00988-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mangan N.E., Fung K.Y. Type I interferons in regulation of mucosal immunity. Immunol. Cell Biol. 2012;90:510–519. doi: 10.1038/icb.2012.13. [DOI] [PubMed] [Google Scholar]
- 123.Mirpuri J., Brazil J.C., Berardinelli A.J., Nasr T.R., Cooper K., Schnoor M., Lin P.W., Parkos C.A., Louis N.A. Commensal Escherichia coli reduces epithelial apoptosis through IFN-alphaA-mediated induction of guanylate binding protein-1 in human and murine models of developing intestine. J. Immunol. 2010;184:7186–7195. doi: 10.4049/jimmunol.0903116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schnoor M., Betanzos A., Weber D.A., Parkos C.A. Guanylate-binding protein-1 is expressed at tight junctions of intestinal epithelial cells in response to interferon-gamma and regulates barrier function through effects on apoptosis. Mucosal. Immunol. 2009;2:33–42. doi: 10.1038/mi.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pan Q., Guo F., Huang Y., Li A., Chen S., Chen J., Liu H.-f., Pan Q. Gut Microbiota Dysbiosis in Systemic Lupus Erythematosus: Novel Insights into Mechanisms and Promising Therapeutic Strategies. Front. Immunol. 2021;12:799788. doi: 10.3389/fimmu.2021.799788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 127.Labonte A.C., Kegerreis B., Geraci N.S., Bachali P., Madamanchi S., Robl R., Catalina M.D., Lipsky P.E., Grammer A.C. Identification of alterations in macrophage activation associated with disease activity in systemic lupus erythematosus. PLoS ONE. 2018;13:e0208132. doi: 10.1371/journal.pone.0208132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mohammadi S., Saghaeian-Jazi M., Sedighi S., Memarian A. Immunomodulation in systemic lupus erythematosus: Induction of M2 population in monocyte-derived macrophages by pioglitazone. Lupus. 2017;26:1318–1327. doi: 10.1177/0961203317701842. [DOI] [PubMed] [Google Scholar]
- 129.Sabry A., Elbasyouni S.R., Sheashaa H.A., Alhusseini A.A., Mahmoud K., George S.K., Kaleek E.A., abo-Zena H., Kalil A.M., Mohsen T., et al. Correlation between levels of TNF-alpha and IL-6 and hematological involvement in SLE Egyptian patients with lupus nephritis. Int. Urol. Nephrol. 2006;38:731–737. doi: 10.1007/s11255-006-0047-9. [DOI] [PubMed] [Google Scholar]
- 130.Umare V., Pradhan V., Nadkar M., Rajadhyaksha A., Patwardhan M., Ghosh K.K., Nadkarni A.H. Effect of proinflammatory cytokines (IL-6, TNF-alpha, and IL-1beta) on clinical manifestations in Indian SLE patients. Mediat. Inflamm. 2014;2014:385297. doi: 10.1155/2014/385297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ferwerda G., Meyer-Wentrup F., Kullberg B.J., Netea M.G., Adema G.J. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 2008;10:2058–2066. doi: 10.1111/j.1462-5822.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 132.Dennehy K.M., Ferwerda G., Faro-Trindade I., Pyz E., Willment J.A., Taylor P.R., Kerrigan A., Tsoni S.V., Gordon S., Meyer-Wentrup F., et al. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur. J. Immunol. 2008;38:500–506. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Panpetch W., Somboonna N., Bulan D.E., Issara-Amphorn J., Worasilchai N., Finkelman M., Chindamporn A., Palaga T., Tumwasorn S., Leelahavanichkul A. Gastrointestinal Colonization of Candida Albicans Increases Serum (1-->3)-beta-D-Glucan, without Candidemia, and Worsens Cecal Ligation and Puncture Sepsis in Murine Model. Shock. 2018;49:62–70. doi: 10.1097/SHK.0000000000000896. [DOI] [PubMed] [Google Scholar]
- 134.Szymula A., Rosenthal J., Szczerba B.M., Bagavant H., Fu S.M., Deshmukh U.S. T cell epitope mimicry between Sjogren’s syndrome Antigen A (SSA)/Ro60 and oral, gut, skin and vaginal bacteria. Clin. Immunol. 2014;152:1–9. doi: 10.1016/j.clim.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eggimann P., Pittet D. Candida colonization index and subsequent infection in critically ill surgical patients: 20 years later. Intensive Care Med. 2014;40:1429–1448. doi: 10.1007/s00134-014-3355-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wajant H., Pfizenmaier K., Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 137.Kontoyiannis D., Pasparakis M., Pizarro T.T., Cominelli F., Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: Implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–398. doi: 10.1016/S1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 138.McClain M.T., Heinlen L.D., Dennis G.J., Roebuck J., Harley J.B., James J.A. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat. Med. 2005;11:85–89. doi: 10.1038/nm1167. [DOI] [PubMed] [Google Scholar]
- 139.Greiling T.M., Dehner C., Chen X., Hughes K., Iniguez A.J., Boccitto M., Ruiz D.Z., Renfroe S.C., Vieira S.M., Ruff W.E., et al. Commensal orthologs of the human autoantigen Ro60 as triggers of autoimmunity in lupus. Sci. Transl. Med. 2018;10:eaan2306. doi: 10.1126/scitranslmed.aan2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Su W., Ding X. Methods of Endotoxin Detection. SLAS Technol. 2015;20:354–364. doi: 10.1177/2211068215572136. [DOI] [PubMed] [Google Scholar]
- 141.Finkelman M.A. Specificity Influences in (1→3)-β-d-Glucan-Supported Diagnosis of Invasive Fungal Disease. J. Fungi. 2020;7:14. doi: 10.3390/jof7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Teshima C.W., Meddings J.B. The measurement and clinical significance of intestinal permeability. Curr. Gastroenterol. Rep. 2008;10:443–449. doi: 10.1007/s11894-008-0083-y. [DOI] [PubMed] [Google Scholar]
- 143.Power N., Turpin W., Espin-Garcia O., Smith M.I., Croitoru K. Serum Zonulin Measured by Commercial Kit Fails to Correlate With Physiologic Measures of Altered Gut Permeability in First Degree Relatives of Crohn’s Disease Patients. Front. Physiol. 2021;12:645303. doi: 10.3389/fphys.2021.645303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Aasbrenn M., Lydersen S., Farup P.G. Changes in serum zonulin in individuals with morbid obesity after weight-loss interventions: A prospective cohort study. BMC Endocr. Disord. 2020;20:108. doi: 10.1186/s12902-020-00594-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sturgeon C., Fasano A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016;4:e1251384. doi: 10.1080/21688370.2016.1251384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rao R.K., Samak G. Protection and Restitution of Gut Barrier by Probiotics: Nutritional and Clinical Implications. Curr. Nutr. Food Sci. 2013;9:99–107. doi: 10.2174/1573401311309020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gauhe A., György P., Hoover J.R.E., Kuhn R., Rose C.S., Ruelius H.W., Zilliken F. Bifidus factor. IV. Preparations obtained from human milk. Arch. Biochem. Biophys. 1954;48:214–224. doi: 10.1016/0003-9861(54)90326-4. [DOI] [PubMed] [Google Scholar]
- 148.Chapman C.M., Gibson G.R., Rowland I. Health benefits of probiotics: Are mixtures more effective than single strains? Eur. J. Nutr. 2011;50:1–17. doi: 10.1007/s00394-010-0166-z. [DOI] [PubMed] [Google Scholar]
- 149.Ma Y., Guo R., Sun Y., Li X., He L., Li Z., Silverman G.J., Chen G., Gao F., Yuan J., et al. Lupus gut microbiota transplants cause autoimmunity and inflammation. Clin. Immunol. 2021;233:108892. doi: 10.1016/j.clim.2021.108892. [DOI] [PubMed] [Google Scholar]
- 150.Huang C., Yi P., Zhu M., Zhou W., Zhang B., Yi X., Long H., Zhang G., Wu H., Tsokos G.C., et al. Safety and efficacy of fecal microbiota transplantation for treatment of systemic lupus erythematosus: An EXPLORER trial. J. Autoimmun. 2022;130:102844. doi: 10.1016/j.jaut.2022.102844. [DOI] [PubMed] [Google Scholar]
- 151.Khailova L., Dvorak K., Arganbright K.M., Halpern M.D., Kinouchi T., Yajima M., Dvorak B. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297:G940-9. doi: 10.1152/ajpgi.00141.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mennigen R., Nolte K., Rijcken E., Utech M., Loeffler B., Senninger N., Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G1140-9. doi: 10.1152/ajpgi.90534.2008. [DOI] [PubMed] [Google Scholar]
- 153.Hsu T.-C., Huang C.-Y., Liu C.-H., Hsu K.-C., Chen Y.-H., Tzang B.-S. Lactobacillus paracasei GMNL-32, Lactobacillus reuteri GMNL-89 and L. reuteri GMNL-263 ameliorate hepatic injuries in lupus-prone mice. Br. J. Nutr. 2017;117:1066–1074. doi: 10.1017/S0007114517001039. [DOI] [PubMed] [Google Scholar]
- 154.Vahidi Z., Samadi M., Mahmoudi M., RezaieYazdi Z., Sahebari M., Tabasi N., Esmaeili S.-A., Sahebkar A., Rastin M. Lactobacillus rhamnosus and Lactobacillus delbrueckii ameliorate the expression of miR-155 and miR-181a in SLE patients. J. Funct. Foods. 2018;48:228–233. doi: 10.1016/j.jff.2018.07.025. [DOI] [Google Scholar]
- 155.He H., Xu H., Xu J., Zhao H., Lin Q., Zhou Y., Nie Y. Sodium Butyrate Ameliorates Gut Microbiota Dysbiosis in Lupus-Like Mice. Front. Nutr. 2020;7:604283. doi: 10.3389/fnut.2020.604283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Costa R.L., Moreira J., Lorenzo A., Lamas C.C. Infectious complications following probiotic ingestion: A potentially underestimated problem? A systematic review of reports and case series. BMC Complement. Altern. Med. 2018;18:329. doi: 10.1186/s12906-018-2394-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Peng L., Li Z.R., Green R.S., Holzman I.R., Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009;139:1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Candela M., Perna F., Carnevali P., Vitali B., Ciati R., Gionchetti P., Rizzello F., Campieri M., Brigidi P. Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: Adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int. J. Food Microbiol. 2008;125:286–292. doi: 10.1016/j.ijfoodmicro.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 159.Chen L., Li H., Li J., Chen Y., Yang Y. Lactobacillus rhamnosus GG treatment improves intestinal permeability and modulates microbiota dysbiosis in an experimental model of sepsis. Int. J. Mol. Med. 2019;43:1139–1148. doi: 10.3892/ijmm.2019.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gu Z., Wu Y., Wang Y., Sun H., You Y., Piao C., Liu J., Wang Y. Lactobacillus rhamnosus Granules Dose-Dependently Balance Intestinal Microbiome Disorders and Ameliorate Chronic Alcohol-Induced Liver Injury. J. Med. Food. 2020;23:114–124. doi: 10.1089/jmf.2018.4357. [DOI] [PubMed] [Google Scholar]
- 161.Panpetch W., Chancharoenthana W., Bootdee K., Nilgate S., Finkelman M., Tumwasorn S., Leelahavanichkul A. Lactobacillus rhamnosus L34 Attenuates Gut Translocation-Induced Bacterial Sepsis in Murine Models of Leaky Gut. Infect. Immun. 2018;86:e00700-17. doi: 10.1128/IAI.00700-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mardani F., Mahmoudi M., Esmaeili S.A., Khorasani S., Tabasi N., Rastin M. In vivo study: Th1-Th17 reduction in pristane-induced systemic lupus erythematosus mice after treatment with tolerogenic Lactobacillus probiotics. J. Cell Physiol. 2018;234:642–649. doi: 10.1002/jcp.26819. [DOI] [PubMed] [Google Scholar]
- 163.Khorasani S., Mahmoudi M., Kalantari M.R., Lavi Arab F., Esmaeili S.A., Mardani F., Tabasi N., Rastin M. Amelioration of regulatory T cells by Lactobacillus delbrueckii and Lactobacillus rhamnosus in pristane-induced lupus mice model. J. Cell Physiol. 2019;234:9778–9786. doi: 10.1002/jcp.27663. [DOI] [PubMed] [Google Scholar]
- 164.Chen F., Wang H., Chen J., Liu Y., Wen W., Li Y., Huang X. Lactobacillus delbrueckii Ameliorates Intestinal Integrity and Antioxidant Ability in Weaned Piglets after a Lipopolysaccharide Challenge. Oxid. Med. Cell Longev. 2020;2020:6028606. doi: 10.1155/2020/6028606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schultz M., Veltkamp C., Dieleman L.A., Grenther W.B., Wyrick P.B., Tonkonogy S.L., Sartor R.B. Lactobacillus plantarum 299V in the treatment and prevention of spontaneous colitis in interleukin-10-deficient mice. Inflamm. Bowel Dis. 2002;8:71–80. doi: 10.1097/00054725-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 166.Mao Y., Nobaek S., Kasravi B., Adawi D., Stenram U., Molin G., Jeppsson B. The effects of Lactobacillus strains and oat fiber on methotrexate-induced enterocolitis in rats. Gastroenterology. 1996;111:334–344. doi: 10.1053/gast.1996.v111.pm8690198. [DOI] [PubMed] [Google Scholar]
- 167.Karczewski J., Troost F.J., Konings I., Dekker J., Kleerebezem M., Brummer R.J., Wells J.M. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G851-9. doi: 10.1152/ajpgi.00327.2009. [DOI] [PubMed] [Google Scholar]
- 168.Morshedi M., Saghafi-Asl M., Hosseinifard E.S. The potential therapeutic effects of the gut microbiome manipulation by synbiotic containing-Lactobacillus plantarum on neuropsychological performance of diabetic rats. J. Transl. Med. 2020;18:18. doi: 10.1186/s12967-019-02169-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Manirarora J.N., Kosiewicz M.M., Alard P. Feeding lactobacilli impacts lupus progression in (NZBxNZW)F1 lupus-prone mice by enhancing immunoregulation. Autoimmunity. 2020;53:323–332. doi: 10.1080/08916934.2020.1777282. [DOI] [PubMed] [Google Scholar]
- 170.Schepper J.D., Collins F.L., Rios-Arce N.D., Raehtz S., Schaefer L., Gardinier J.D., Britton R.A., Parameswaran N., McCabe L.R. Probiotic Lactobacillus reuteri Prevents Postantibiotic Bone Loss by Reducing Intestinal Dysbiosis and Preventing Barrier Disruption. J. Bone Miner. Res. 2019;34:681–698. doi: 10.1002/jbmr.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zelante T., Iannitti R.G., Cunha C., De Luca A., Giovannini G., Pieraccini G., Zecchi R., D’Angelo C., Massi-Benedetti C., Fallarino F., et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 172.Tzang B.S., Liu C.H., Hsu K.C., Chen Y.H., Huang C.Y., Hsu T.C. Effects of oral Lactobacillus administration on antioxidant activities and CD4+CD25+forkhead box P3 (FoxP3)+ T cells in NZB/W F1 mice. Br. J. Nutr. 2017;118:333–342. doi: 10.1017/S0007114517002112. [DOI] [PubMed] [Google Scholar]
- 173.Eun C.S., Kim Y.S., Han D.S., Choi J.H., Lee A.R., Park Y.K. Lactobacillus casei prevents impaired barrier function in intestinal epithelial cells. APMIS. 2011;119:49–56. doi: 10.1111/j.1600-0463.2010.02691.x. [DOI] [PubMed] [Google Scholar]
- 174.Zakostelska Z., Kverka M., Klimesova K., Rossmann P., Mrazek J., Kopecny J., Hornova M., Srutkova D., Hudcovic T., Ridl J., et al. Lysate of probiotic Lactobacillus casei DN-114 001 ameliorates colitis by strengthening the gut barrier function and changing the gut microenvironment. PLoS ONE. 2011;6:e27961. doi: 10.1371/journal.pone.0027961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mike A., Nagaoka N., Tagami Y., Miyashita M., Shimada S., Uchida K., Nanno M., Ohwaki M. Prevention of B220+ T cell expansion and prolongation of lifespan induced by Lactobacillus casei in MRL/lpr mice. Clin. Exp. Immunol. 1999;117:368–375. doi: 10.1046/j.1365-2249.1999.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang S., Ahmadi S., Nagpal R., Jain S., Mishra S.P., Kavanagh K., Zhu X., Wang Z., McClain D.A., Kritchevsky S.B., et al. Lipoteichoic acid from the cell wall of a heat killed Lactobacillus paracasei D3-5 ameliorates aging-related leaky gut, inflammation and improves physical and cognitive functions: From C. elegans to mice. Geroscience. 2020;42:333–352. doi: 10.1007/s11357-019-00137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hu W.S., Rajendran P., Tzang B.S., Yeh Y.L., Shen C.Y., Chen R.J., Ho T.J., Vijaya Padma V., Chen Y.H., Huang C.Y. Lactobacillus paracasei GMNL-32 exerts a therapeutic effect on cardiac abnormalities in NZB/W F1 mice. PLoS ONE. 2017;12:e0185098. doi: 10.1371/journal.pone.0185098. [DOI] [PMC free article] [PubMed] [Google Scholar]