Abstract
Globally, migraine is a leading cause of disability with a huge impact on both the work and private life of affected persons. To overcome the societal migraine burden, better treatment options are needed. Increasing evidence suggests that ATP-sensitive potassium (KATP) channels are involved in migraine pathophysiology. These channels are essential both in blood glucose regulation and cardiovascular homeostasis. Experimental infusion of the KATP channel opener levcromakalim to healthy volunteers and migraine patients induced headache and migraine attacks in 82-100% of participants. Thus, this is the most potent trigger of headache and migraine identified to date. Levcromakalim likely induces migraine via dilation of cranial arteries. However, other neuronal mechanisms are also proposed. Here, basic KATP channel distribution, physiology, and pharmacology are reviewed followed by thorough review of clinical and preclinical research on KATP channel involvement in migraine. KATP channel opening and blocking have been studied in a range of preclinical migraine models and, within recent years, strong evidence on the importance of their opening in migraine has been provided from human studies. Despite major advances, translational difficulties exist regarding the possible anti-migraine efficacy of KATP channel blockage. These are due to significant species differences in the potency and specificity of pharmacological tools targeting the various KATP channel subtypes.
Keywords: KATP channels, provoked migraine, SUR, Kir6.x, levcromakalim, glibenclamide, human migraine model, in vivo models, migraine
1. Introduction
According to the World Health Organization (WHO), more than a billion people are living with migraine, and among the 15–49 year-old population, headache disorders is the most burdensome of all disorders [1,2]. Migraine attacks are characterized by pulsating head pain of moderate to severe intensity, photo- and/or phonophobia, nausea, vomiting, and aggravation by routine physical activity [3]. Migraine has a tremendous impact on quality of life for sufferers and may affect sleep [4], cognitive function [5], and private and professional life [6]. Despite huge individual suffering and socioeconomic impact, the pathophysiological mechanisms of migraine remain incompletely understood and highly debated [7]. The brain is generally thought of as non-nociceptive, but plexuses of nociceptive nerve fibers from the trigeminal ganglion innervate the blood vessels of the meninges (dura, pia, and arachnoid mater), linking pain perception and the brain vascular system in what is described as the trigeminovascular system [8]. Nowadays, many think of migraine as a neurovascular [7] sensory threshold disease [9]. The identification of calcitonin gene-related peptide (CGRP) involvement in migraine is a translational success story culminating in the marketing of monoclonal antibodies targeting CGRP or its receptor as well as small molecule receptor antagonists [10]. However, these CGRP-targeting migraine preventatives are only effective in approximately 60% of patients [11,12,13,14,15], stressing the importance of continued research and drug development.
A range of migraine-provoking substances have been identified in human experiments. Common for all, is the dilation of cephalic arteries [16,17,18,19,20,21] via downstream opening of vascular smooth muscle ATP-sensitive potassium (KATP) channels [7]. The finding that the KATP channel opener levcromakalim is the most potent trigger of experimental migraine tested to date [22,23] has fueled interest in KATP channel involvement in migraine pain generation and, within recent years, a significant number of studies have addressed this topic.
The aim of this review is to collectively present the evidence on KATP channel involvement in migraine pain and review the underlying hypotheses of where and how the KATP channels are involved in migraine pathophysiology. Logically, the possibility of targeting antimigraine therapeutics against these channels will also be discussed.
2. Molecular Basis and Physiological Function of KATP Channels
2.1. Molecular Structure and Regulation of Channel Activity
The KATP channels were first identified in cardiac muscle cells by A. Noma in the early 1980s [24]. These channels were later shown in other tissues, such as pancreas, smooth muscle cells, and the nervous system [25,26,27]. They belong to the family of transmembrane potassium inward-rectifying (Kir) channels, which are predominantly found on the plasma membranes but are also present on the mitochondrial inner membrane [28]. Seven subfamilies within the Kir family have been identified with different molecular and physiological functions (Kir1.x through to Kir7.x), where ATP-sensitive K+ channels belong to the Kir6.x subfamily and are strongly associated with cellular metabolism and membrane electrophysiology [27]. Kir6.x have two subtypes, namely Kir6.1 and Kir6.2, which are expressed in various tissues [29].
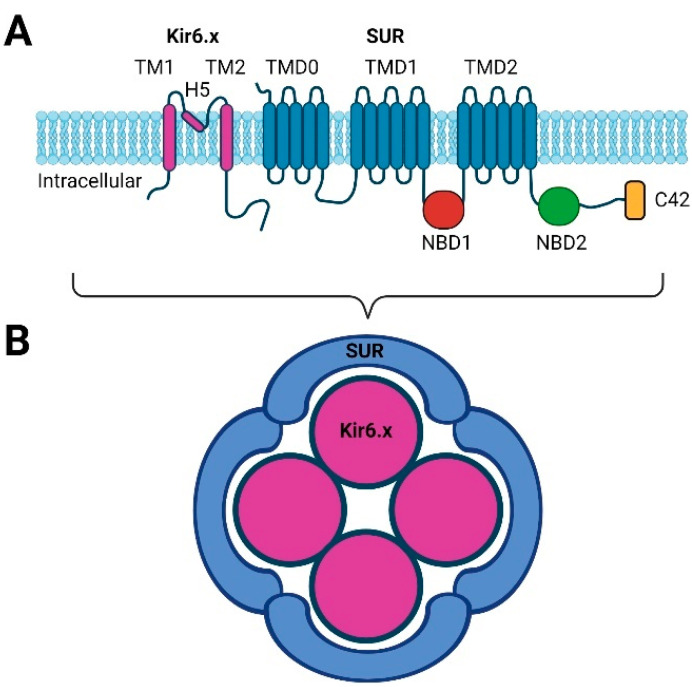
Kir channels have two transmembrane spanning regions (TM1 and TM2) with an extracellular pore-forming region (H5) and both the amino and carboxyl terminal are cytosolic (Figure 1A) [29,30]. However, to obtain a functional channel, four Kir subunits are necessary, and the activity of the channel is regulated by four sulfonylurea receptors (SUR), thus creating a hetero-octameric structure (Figure 1B) [29,31]. These SUR receptors are ATP-binding cassettes (ABCs) or transport ATPases and have 17 transmembrane regions arranged into three domains (TMD0, TMD1 and TMD2) together with two intracellular nucleotide binding domains (NBD1 and NBD2); see Figure 1A. SUR subunits SUR2A and SUR2B only differ at the carboxyl terminal 42 amino acids (C42), while the SUR1 subunit is more unique [27,29,30].
Figure 1.
Simple structure of the KATP channel. (A) The Kir6.x subunit is composed of a two transmembrane region (TM1 and TM2) connected by a pore-forming region (H5). The SURx subunit is composed of three domains of either five transmembrane regions (TMD0) or six transmembrane regions (TMD1 and TMD2). The nucleotide binding domains are found intracellularly (NBD1 and NBD2). SUR2A and SUR2B only differ in their C-terminal end (C42). (B) The functional KATP channel is formed by four Kir6.x subunits and four SURx subunits (created using BioRender.com).
The inward-rectifying function is a result of an intracellular blockage of the pore by Mg2+ or polyamines, which blocks the efflux of K+. During channel activation the blockage is removed and K+ efflux can occur [27]. High concentrations of ATP will inhibit the channel, while reduced ATP levels will activate and open the channel [32]. The activity of the channel is controlled by the SUR subunits due to their NBDs, where MgATP binds to NBD2 and MgADP binds to NBD1 [29,33]. Phosphatidylinositol 4,5-bisphosphate (PIP2) is suggested to play a role in channel activity regulation, as PIP2 activates the channel and reduces its sensitivity to ATP, thus counteracting the inhibitory effect at ATP [34,35,36]. Lastly, Kir6.x channels can be activated via phosphorylation by protein kinase A (PKA) or protein kinase G (PKG) [37,38,39,40]. These kinases are downstream targets of cAMP and cGMP, respectively, and have been suggested as molecular pathways in migraine pathophysiology (Figure 2) [41,42].
Figure 2.
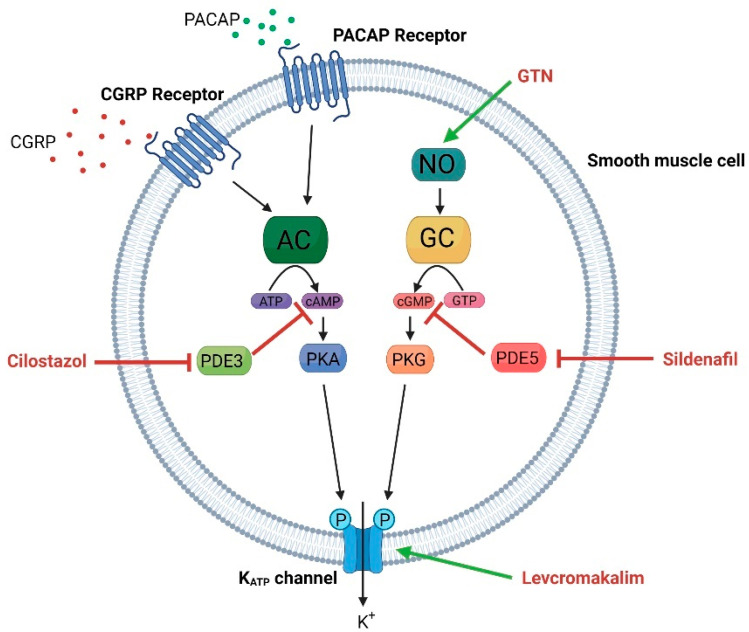
Molecular pathways and pharmacological agents leading to the opening of the KATP channel in vascular smooth muscle. The neuropeptides PACAP and CGRP activate KATP channels via the adenylyl cyclase pathway, while the NO donor GTN (glyceryl trinitrate) activates the channel via the guanylyl cyclase pathway. Cilostazol and Sildenafil are blockers of the phosphodiesterase 3 and 5 (PDE3 and PDE5), respectively, causing accumulation of cAMP and cGMP, which promote the opening of KATP channels. Levcromakalim causes vasodilation by direct action on the KATP channels (created using BioRender.com).
2.2. Tissue Distribution
The KATP channels are expressed throughout the body but the combination of the different subunits of Kir6.x and SURx vary in different tissues, such as the vascular system, neuronal system, and pancreas (see Table 1). The pancreatic β-cells express Kir6.2/SUR1, which control the glucose-stimulated insulin secretion (and represents the most studied channel), while the Kir6.2/SUR2A channels are the predominant form found in myocardia [25,29,33]. The vascular smooth muscle cells express Kir6.1/SUR2B and these have distinct structural features from the pancreatic Kir6.2/SUR1 isoforms, as the Kir6.1 cytoplasmic regions is placed too far from the membrane to interfere with the membrane-bound PIP2, which is known to activate or open the Kir6.2/SUR1 channels in pancreatic β-cells [33]. Furthermore, Kir6.1 channels do not show spontaneous channel activity, while pancreatic and myocardial Kir6.2 channels open spontaneously when ATP levels are low or absent [33,38,43]. In most tissue, channels are composed of two homogenous Kir subunits and four homogenous SUR subunits; however, examples of more heterogenous compositions have been reported [44]. The different compositions of KATP channel subunits in different tissues potentially allow for more specific therapeutic targets in the development of novel drug candidates for specific pathologies.
Table 1.
Subunits composition and tissue expression of KATP channels. For a more detailed overview of subunit composition, tissue distribution and physiological function, please see [48].
| Channel Subunit Composition | Tissue | References |
|---|---|---|
| Kir6.1/SUR1 | Retina | [49] |
| Nervous system | [46,48] | |
| Kir6.1/SUR2B | Vascular smooth muscle | [43,45,50,51,52] |
| Non-vascular smooth muscle | [48,53] | |
| Conduction system of the heart | [48,54] | |
| Kir6.2/SUR1 | Pancreatic β-cells | [52,55] |
| Arterial cardiac myocytes | [52,56] | |
| Nervous system | [48,52,57,58] | |
| Skeletal muscle | [48,59] | |
| Kir6.2/SUR2A | Ventricular myocytes | [54,60] |
| Skeletal muscle | [48,59] | |
| Kir6.2/SUR2B | Non-vascular smooth muscle | [53] |
| Nervous system | [48,57,61] | |
| Conduction system of the heart | [54,62] | |
| Skeletal muscle | [59] |
Kir6.1/SUR2B is found in the smooth muscle cells of the vascular system, and are the dominant form in brain arteries and dura mater [45], where they are involved in vasodilation and constriction. For this reason, Kir6.1/SUR2B have been suggested as a target for migraine pain intervention [46,47].
2.3. Physiological Functions of KATP Channels
In a physiological resting state, KATP channels are blocked but allow a small inward current of K+, while the active or open state of the channel results in the efflux of K+, resulting in hyperpolarization of the membrane. Below, the physiological consequence of this in different cell and tissue types is presented.
2.3.1. Vascular System
The tone of the vascular system is controlled by a sophisticated relationship of molecular functions causing vasoconstriction or vasodilation. KATP channels have long been known as the target of vasodilatory drugs like diazoxide and pinacidil [63] and their role in vasodilation have likewise been studied for decades [33,38,63,64,65].
In vascular smooth muscle cells, hyperpolarization caused by K+ efflux upon KATP channel opening will cause the inhibition of voltage-operated Ca2+ channels (VOCC), reducing Ca2+ influx and consequently causing smooth muscle relaxation and vasodilation (Figure 2) [33,66,67,68]. Many vasodilating substances target receptors or second messengers upstream from KATP channels. Nitric oxide (NO) binds to guanylyl cyclase (GC), which in turn converts GTP to cGMP, and cGMP can subsequently phosphorylate and open the KATP channels [68,69,70]. Additionally, KATP channels, located in the endothelium, mediate vasodilation to some extent [71]. The potent vasodilators CGRP and pituitary adenylate cyclase-activating peptide (PACAP) bind to their respective G-protein coupled receptors on vascular smooth muscle to activate the adenylyl cyclase (AC) enzyme, causing cAMP to be converted from ATP [40,72,73]. In addition, inhibitors of phosphodiesterase (PDE) type 3 and 5 (cilostazol and sildenafil) are marketed for their vasodilating effect for different indications [74,75]. PDEs degrade cAMP and cGMP; thus, the inhibition of PDEs causes the accumulation of cAMP and/or cGMP and downstream phosphorylation of the KATP channel [7]. Interestingly, all these drugs or substances have headache as a primary side effect [16,17,74,75,76]. Thus, the above mentioned mechanisms have been speculated to be important in migraine pathophysiology.
Genetic manipulations of specific subunits of the functional KATP channel may result in vascular issues like knockout of the Kcnj8, the gene that encodes Kir6.1, which was shown to cause sudden early death associated with an atrioventricular blockage that could not be rescued by the KATP channel opener pinacidil but was worsened by the vasoconstrictive agent methylergometrine [77]. A later in vivo study showed that the selective deletion of Kir6.1 in vascular smooth muscle cells resulted in hypertension and a loss of response to pinacidil but did not cause sudden death [64]. Thus, sudden death was likely related to the global deletion of Kir6.1. It is important to keep in mind that global knockout of specific genes might cause unexpected phenotypic traits due to a lack of expression of the gene during embryonic development and consequences thereof, which might not be expected if the gene had been expressed in the embryonic stages and silenced later in life [78,79,80].
2.3.2. Neuronal Function
KATP channels (Kir6.2/SUR1, Kir6.2/SUR2A, and Kir6.2/SUR2B) are also expressed in the brain, especially in neurons where their activation causes hyperpolarization and reduced excitability [27,29,30], which is often related to a reduction in neurotransmitter release [81,82]. Hyperpolarization may lead to the activation of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels of which the consequence is dependent on the given situation [83]. The excitatory neurotransmitter glutamate will cause an influx of Ca2+, which can result in an elevation in mitochondrial Ca2+ levels, depolarization of the mitochondria, loss of oxidative phosphorylation, ATP depletion, swelling and rupture of mitochondrial membrane and, subsequently, release of pro-apoptotic species, ultimately leading to neuronal cell death [81,84]. This cascade can be blocked or regulated by activation of the mitochondrial KATP channels due to their ability to cause hyperpolarization and reduced glutamate release, thus playing a role in neuronal protection from excitatory toxicity [81]. Moreover, neuronal mitochondrial KATP channels regulate intracellular Ca2+ concentrations during hypoxia, thus protecting the neuron from hypoxia [84]. The KATP channel opener, iptakalim, was used to rescue stress-induced mitochondrial damage and alleviate a depressive-like phenotype in the rodent chronic mild stress model of depression [85]. Altogether, activating KATP channels in neurons most likely serve a neuroprotective function during stressful stimuli like ischemia and oxidative stress [48,57,86].
2.3.3. Analgesia, Antinociception, and Opioid Signaling
KATP channels are, furthermore, involved in opioid signaling, which appears to be selective to these types of channels as no other K+ channels have been illustrated to bear similar properties [87]. Nevertheless, other K+ channels are implicated in pain perception but are beyond the scope of this review. For an extensive review on K+ channels in the pathophysiology of pain, please see [88].
The KATP channels have been shown to be downstream targets of the opioid receptor via regulation of nitric oxide synthase and NO generation and subsequent efflux of K+ and hyperpolarization [89]. This NO/cGMP/KATP channel pathway has further been suggested to be an important factor in the inflammatory nociceptive response [70,90,91]. Additionally, loss of KATP channels or their function in peripheral neurons has been implicated in pain perception in multiple studies [57,58,92,93]. For instance, expression of Kir6.1, SUR1, and SUR2 in rat spinal cord were downregulated after nerve injury [94] and knockout of SUR1 subtype resulted in the loss of the antinociceptive effect of morphine in mice [95,96]. Furthermore, the expression of Kir6.1/SUR2B were shown to be regulated by the inflammatory toll-like receptor 4 and NF-κB-dependent signaling, which was suggested to be a factor in the poor vasoconstriction during sepsis [97]. Furthermore, the KATP channel opener, cromakalim administered centrally, reduced astrocyte activation and lowered expression of IL-1β and TNF-α, thereby reducing neuroinflammation [98], and relieved injury-induced neuropathic pain both acutely (lasting hours) and chronically (lasting days) [94]. Khanna et al., 2011 showed the analgesic effect of the systemic administration of cromakalim in the formalin test to the same degree as morphine but only high doses of cromakalim (1 mg/kg and 5 mg/kg) induced an analgesic effect in the tail flick test [87]. Combining morphine and cromakalim only showed additive analgesic effects in the formalin test (inflammatory pain) and not in the tail flick test (heat sensitivity) [87]. Likewise, the KATP channel blocker, glibenclamide, increased the pain response to the formalin test but not in the tail flick test [87]. Interestingly, central administration of cromakalim did not induce an analgesic effect in the tail flick test [98]. One could speculate that the reason for this is the lack of inflammatory agents in the tail flick test, which would suggest that the antinociceptive effect of KATP channel openers is related to anti-inflammatory properties, resulting in analgesia in inflammatory pain rather than neuropathic pain.
Overall, the involvement of KATP channels in analgesic or antinociceptive mechanisms appear to be closely related to their ability to hyperpolarize the membrane and reduce hyperexcitability induced by inflammatory events. However, even though KATP channels in pain research have been studied for decades, no therapeutic agent has reached the market, and we speculate that the reason for this lack of drug development may be due to the high complexity of these mechanisms, the abundant distribution of KATP channels throughout the body and lack of subtype specific pharmacological tools.
Interestingly, when levcromakalim (KATP channel opener) was systemically administered, humans developed headache or migraine [22,23] and mice became hypersensitive [45,47,99]. When levcromakalim was centrally administered in mice, analgesic effects became evident [45,100]; see Section 4. (KATP Channels and Headache).
2.3.4. Insulin Secretion and Glucose Metabolism
The pancreatic islet β-cells are responsible for the secretion of insulin, and Kir6.2/SUR1 KATP channels play an important role in this mechanism. When blood glucose levels rise, cell metabolism increases, and the higher ATP levels will trigger the closing of KATP channels, resulting in membrane depolarization and the activation of voltage-gated Ca2+ channels and Ca2+ influx, which ultimately leads to insulin secretion [101,102]. It is generally accepted that the loss of function of KATP channels in islet β-cells leads to poor or no response of KATP channels to changes in ATP/ADP ratio, leaving the channels closed and the membrane at a depolarized state. This leads to a rise in cytosolic Ca2+ and the secretion of insulin, causing the phenotype of hyperinsulinism [103,104]. In contrast, gain-of-function mutations can lead to reduced or absent secretion of insulin, hyperglycemia, and diabetes due to a high degree of K+ efflux and membrane hyperpolarization [102]. Congenital hyperinsulinism is linked to the loss of function of the Kir6.2/SUR1 KATP channel expression in the β-cells [101,104].
Type 2 diabetes mellitus is linked to chronic low-grade systemic inflammation and oxidative stress in β-cell, leading to low or no insulin secretion due to a loss of β-cells [105,106]. Several studies have reported that reactive oxygen species (ROS) and reactive nitrogen species (RNS) modulate KATP channel activity by inhibition of mitochondrial ATP production [107,108,109,110,111]. One study illustrated that oxidative stress-induced loss of β-cells caused high blood glucose levels in wild-type (WT) mice, while in SUR1−/− mice, glucose levels only rose slightly over control levels and these mice had a significantly better survival rate compared to WT mice [107]. As the SUR subunit of the KATP channel holds the nucleotide binding sites, this illustrates that the mitochondrial ATP production is involved in the negative effects of oxidative stress on the insulin secretion pathway. One could speculate that oxidative stress in other tissues also disturbs the KATP channel function, for instance, in the migraine-relevant trigeminovascular system [112,113].
3. Pharmacological Tools Targeting KATP Channels
Pharmacological investigation using the different and more or less selective openers and inhibitors of the KATP channel is crucial for the understanding of channel function and interpretation of results. Both categories include several chemical classes. In summary, there is some selectivity of the KATP channel openers and blockers, but one should keep in mind that selectivity is commonly not an all or nothing phenomena but a function of dose.
3.1. KATP Channel Openers
Levcromakalim or cromakalim belongs to the benzopyran class and primarily opens smooth muscle and cardiomyocyte KATP channels via affinity to TMD2 on SUR2 [48]. PKi values for levcromakalim is 6.37 ± 0.04 on SUR2A and 6.95 ± 0.03 on SUR2B [114]. In vasomotor experiments, levcromakalim has a reported pEC50 of 6.36 ± 0.09 on human pial arteries and 6.32 ± 0.3 on omental arteries [115]. In rats, similar studies found values of 6.32 ± 0.09 and 5.46 ± 0.17 for levcromakalim on basilar and middle cerebral arteries, respectively [116], and 7.14 ± 0.11 on middle meningeal arteries [117].
The cyanoguanidines (pinacidil, P-1075) also display selectivity to SUR2 and have potent hypotensive effects [118,119], but like levcromakalim, pinacidil did not reverse glibenclamide-induced SUR1-mediated hyperglycemia [120].
In contrast, diazoxide (benzothiadiazine) is more specific for SUR1, but also activates SUR2B [121]. Accordingly, it displayed both hypotensive and hyperglycemic effects [120,122,123].
3.2. KATP Channel Blockers
The sulfonylureas (glibenclamide, glicazide, and gliquidone) are compounds that target the SUR subunits to inhibit KATP channels via NBD2 (Figure 1A) [124]. Sulfonylureas are used clinically to promote insulin secretion in type 2 diabetes [125]. Glibenclamide has a 50-fold higher affinity towards SUR1 over SUR2A and SUR2B [126], and the inhibition of Kir6.2/SUR1 is poorly reversed, whereas the blocking of Kir6.2/SUR2A is rapidly reversed [127]. In addition, gliquidone and glicazide display lower IC50 values in pancreatic cells than in cardiomyocytes and vascular smooth muscle, suggesting a higher affinity for SUR1 [128]. Species differences are reported on the activity of glibenclamide on the human and mouse Kir6.1/SUR1 channels. pIC50 is 8.37 on the human and 5.7 on the mouse channel [126,129] suggest a much larger potency of glibenclamide on human compared to mouse pancreatic KATP channels.
The thiazolidinediones (rosiglitazone, pioglitazone, etc.) are another class of drugs used clinically for the treatment of type 2 diabetes via their effect on peroxisome proliferator-activated receptors (PPARs) [125]. However, patch clamp experiments on HEK cells expressing various subtypes of KATP channels have shown that these compounds also block vascular KATP channels to various degrees [130]. Rosiglitazone was found to inhibit all isoforms of KATP channels. The IC50 was 10 µM for the Kir6.1/SUR2B channel and ∼45 µM for KIR6.2/SURx channels. The inhibition was also present without the SUR subunit. Additionally, rosiglitazone had no effect on Kir1.1, Kir2.1, and Kir4.1 channels, suggesting that the channel inhibitory effect is selective for Kir6.x channels [131]. In a subsequent study, the same group compared a large number of PPAR agonists and found some to potently inhibit Kir6.1/SUR2B. The most potent agonists were AS-252424, englitazone, A6355, rosiglitazone, and cay10415 with IC50 values of 4 μM, 7 μM, 8 μM, 12 μM, and 15 μM, respectively. Lastly, the morpholinoguanidine PNU-37883A needs mentioning. Both in vitro and in vivo evidence suggest that this compound is selective for vascular KATP channels over pancreatic ones and that it acts on the Kir6.1 rather than SUR components [132].
4. KATP Channels and Headache
Clinical trials with KATP channel openers for the treatment of hypertension and asthma had headache as a primary side effect [63,133,134]. A range of migraine- and headache-triggering substances (NO, CGRP, PACAP, cilostazol, sildenafil, and more) activate KATP channels downstream from target binding (Figure 2). This agrees with the theory that the arteries of the trigeminovascular system, are involved in the generation of migraine pain [7].
4.1. Levcromakalim Is a Potent Trigger of Experimental Headache and Migraine
In an experimental setting, recognized as the human model of migraine [135], intravenous infusion of levcromakalim (1 mg over 20 min) was studied in healthy volunteers [136], including migraine without aura (MO) patients [22] and migraine with aura (MA) patients [23].
After levcromakalim infusion, 12 of 14 healthy volunteers reported a headache with a median time to onset of 30 min (range 10–60 min) compared to 1 of 6 participants after placebo [136]. In MO patients, migraine attacks without aura were induced in 16 out of 16 patients contra 1 out of 16 participants after placebo [22]. The median time to migraine onset was 3 h (range 1-9 h) after levcromakalim infusion. In MA patients, attacks were induced in 14 out of 17 participants, and 1 participant after placebo. Four attacks were MO whereas ten were MA. Median time of onset for MO was 2.8 h (range 1–4 h) and 44 min (range 20–120 min) for MA [23].
Apart from headache characteristics, hemodynamic parameters and circumference or blood flow velocity of selected arteries were reported in the above-mentioned clinical studies. In healthy participants, the middle meningeal artery (MMA) had a 7–22% larger circumference throughout the 5 h test period measured with 3.0 Tesla (magnetic resonance angiography (MRA)). The superficial temporal artery (STA) also dilated, but less robustly throughout the test period. The middle cerebral artery (MCA) dilated but it was not significantly different from placebo. Heart rate (HR) AUC0–290 was significantly increased but mean arterial blood pressure (MAP) AUC0–290 was not significantly lowered [136]. However, in a larger study, all arteries (STA, MMA, and MCA) dilated, and HR and MAP also changed significantly in response to levcromakalim [137]. In MO and MA patients both HR AUC0–120 and MAP AUC0–120 were also significantly altered. In the MO patient study [22], STA and radial artery diameters were measured by ultrasonography and blood flow velocity of the MCA was measured by transcranial doppler as a proxy for arterial circumference. Thus, this method is inferior to MRA. Only the STA was found to dilate in response to levcromakalim. Effects on arterial dilation was not published in the MA study [23].
4.2. KATP Channel Opening in Preclinical Migraine Models
Preclinical models in which the effect of KATP channel opening and inhibition have been studied in the context of migraine include models of vasoactivity, CGRP release, mast cells degranulation and behavior in rodents.
4.2.1. Dilatory Effects on Cranial Arteries
The effect of KATP channel opening on cranial arteries have been studied both ex vivo using the wire myograph technique and in vivo using intravital microscopy through a closed cranial window in anesthetized rats. The latter allows simultaneous imaging of dural and pial arteries in an intact animal. It was found that levcromakalim infusion (0.1 mg/kg i.v.) increased dural artery diameter by 130 ± 24%, pial artery diameter by 18 ± 3%, and lowered MAP by 31% [138]. For pinacidil (0.38 mg/kg i.v.), the figures were 126 ± 8% and 17 ± 3%, respectively. The response was significantly lower in pial than in dural arteries for both KATP channel openers [138]. A lower dose of levcromakalim (0.025 mg/kg i.v.) sub-maximally dilated the MMA and decreased MAP by 29% [117]. In ex vivo artery preparations, the picture was similar. Levcromakalim (3 µM) induced relaxation of 74 ± 9% in rat dural arteries and 38 ± 8% in middle cerebral arteries, and pinacidil (3 µM) induced relaxation that amounted to 55 ± 11% and 26 ± 4%. Again, the dilatory responses were significantly different between the dural and cerebral arteries [138]. This is also reflected in the pEC50 values; see Section 3.1. (KATP Channel Openers), revealing an approximate 10-fold higher potency of levcromakalim on meningeal over cerebral arteries [117,139]. The in vivo findings could be explained by poor blood-brain barrier passage of levcromakalim and pinacidil, but this cannot explain the ex vivo difference as the blood-brain barrier is bypassed in the wire myograph technique. Therefore, it was suggested that KATP channels are heterogeneously distributed between cranial arteries [138].
4.2.2. Stimulation of CGRP Release
The CGRP release assay is an ex vivo technique in which CGRP release from isolated tissue can be investigated. In migraine research, CGRP release from relevant structures as the trigeminal ganglia, trigeminal nucleus caudalis and dura mater are commonly investigated [140]. Levcromakalim (1 µM) and diazoxide (10 µM) have been tested for their ability to stimulate CGRP release from all three tissues in rats [141]. Levcromakalim (0.1–100 µM) was tested in mouse trigeminal ganglia and brain stem [99]. In neither species nor tissue preparation did levcromakalim induce release of CGRP, supporting the hypothesis that KATP channel opening is a downstream event upon CGRP receptor activation within single cells [142]. For review of the effect of KATP channel blockage on CGRP release, see Section 4.3.1. (Effect of KATP Channel Blockers in Preclinical Models). From the CGRP release model, it is evident that the headache-inducing effect of levcromakalim is not caused by direct stimulation of neuronal CGRP release.
4.2.3. Mast Cell Degranulation
Dural mast cells may be involved in migraine attacks [143] and therefore mast cell degranulation assays and markers have been applied to study this possible aspect of headache mechanisms. Levcromakalim and diazoxide (10 µM) failed to degranulate rat dural mast cells in situ and, likewise, both drugs (0.01 µM–10 µM) failed to degranulate rat peritoneal mast cells in vitro [141]. Thus, degranulation of mast cells is not the primary mechanism in headache caused by levcromakalim.
4.2.4. In Vivo Mouse Model
Many migraine triggering substances defined in the human migraine model have also been used in mice where they induce a state of hypersensitivity to cutaneous stimulation with von Frey filaments [99,144,145,146]. This model is considered to be the mouse parallel to the human model of provoked migraine. Repeated (every 48 h) injections of levcromakalim (1 mg/kg i.p.) induce both cephalic and hind paw hypersensitivity to von Frey stimulation, peaking 2 h after the 3rd injection [45,47,99], whereas levcromakalim administered locally in the hind paw did not induce hypersensitivity, and intracerebroventricular administration provided analgesia on the hotplate [45]. The observed hypersensitivity is at odds with the study by Khanna et al., 2011 showing an antinociceptive effect of cromakalim and diazoxide delivered i.p. [87].
4.3. KATP Channel Blockage as Therapeutic Target in Migraine
The opening of KATP channels by systemic levcromakalim induces headache and migraine attacks with and without aura. Accordingly, blocking KATP channels may abort migraine attacks. A convincing amount of preclinical evidence suggests that KATP channel blockage is a promising drug target for migraine. However, translation to patients is pending better pharmacological tools. Table 2 summarizes the studies reviewed in the following sections and provides an overview of the doses of applied test substances expressed as µmol/kg and the ratio between blocker (glibenclamide) and headache trigger substance.
Table 2.
Details of human and rodent studies on KATP channel blockage in different migraine models. Rows in same color are compared. The ratio of blocker/migraine trigger are used for rough assessment of effectiveness across models. Effective Y/N/P: Y = yes, N = no, P = partially. Percentwise changes of arterial circumference and diameter are the same. Thus, 20% change in diameter = 20% change in circumference. Dose mol/kg = (dose g/kg)/(MW g/mol), dose umol/kg = (dose mol/kg) × 106. Glibenclamide 494 g/mol, levcromakalim 286 g/mol, PACAP 4534 g/mol, CGRP 3798 g/mol, PNU 382 g/mol. * Glibenclamide given after PACAP, # CGRP is accumulated dose in man/bolus in rat. Ratio will increase if the 1 min dose of CGRP is applied. $ Possible first pass metabolism of levcromakalim i.p will increase the mouse ratio, due to a smaller denominator. & PACAP s.c. may result in lower plasma concentrations than i.v. which will increase the mouse ratio.
| Species | Endpoint | Headache Trigger mg/kg | Headache Trigger, umol/kg | Blocker mg/kg |
Blocker, umol/kg | Ratio (Blocker/Trigger) | Effective Y/N/P |
|---|---|---|---|---|---|---|---|
| Rat | MMA diameter | Levcromakalim 0.025 mg/kg iv over 10 min | 0.087 | PNU-37883A 0.5 mg/kg i.v. over 10 min | 1.3 | 15 | P |
| Rat | MMA diameter | Levcromakalim 0.1 mg/kg iv over 20 min | 0.35 | Glibenclamide 20 mg/kg iv over 20 min | 40.5 | 116 | P |
| Rat | MMA diameter | Levcromakalim 0.1 mg/kg iv over 20 min | 0.35 | Glibenclamide 30 mg/kg iv over 20 min | 60.7 | 174 | Y |
| Human | MMA, STA, MCA circumference | Levcromakalim 0.014 mg/kg iv over 20 min | 0.049 | Glibenclamide 0.14 mg/kg p.o. | 0.3 | 5.8 | N |
| Rat | MMA diameter | CGRP 0.3 ug/kg iv bolus | 0.000079 | Glibenclamide 7 mg/kg iv over 20 min | 14.2 | 178,968 | P |
| Rat | MMA diameter | CGRP 0.3 ug/kg iv bolus | 0.000079 | Glibenclamide 30 mg/kg iv over 20 min | 60.7 | 767,004 | Y |
| Human | STA and RA diameter | CGRP 0.43 ug/kg iv over 20 min | 0.000011 | Glibenclamide 0.14 mg/kg p.o. | 0.3 | 24,972 # | N |
| Human | STA and RA diameter | CGRP 0.02 ug/kg/min i.v. | 0.0000053 | Glibenclamide 0.14 mg/kg p.o. | 0.3 | 53,690 | N |
| Human | MMA circumference | PACAP 200 picomol/kg over 20 min | 0.2 | Glibenclamide 0.14 mg/kg p.o. * | 0.3 | 1.4 | N |
| Human | Headache | Levcromakalim 0.014 mg/kg iv over 20 min | 0.049 | Glibenclamide 0.14 mg/kg p.o. | 0.3 | 5.8 | N/P |
| Mouse | Tactile hypersensitivity | Levcromakalim 1 mg/kg i.p $ | 3.5 | Glibenclamide 1 mg/kg i.p. | 2 | 0.6 | Y |
| Human | Headache | PACAP 200 picomol/kg over 20 min | 0.2 | Glibenclamide 0.14 mg/kg p.o. | 0.3 | 1.4 | N |
| Mouse | Tactile hypersensitivity | PACAP 0.2 ug/kg s.c. & | 0.000044 | Glibenclamide 1 mg/kg i.p. | 2 | 45,891 | P |
| Human | Headache | CGRP 0.43 ug/kg iv over 20 min | 0.000011 | Glibenclamide 0.14 mg/kg p.o. | 0.3 | 24,972 | N/P |
4.3.1. Effect of KATP Channel Blockers in Preclinical Models
The preclinical evidence suggesting the relevance of KATP channel blockage in migraine is based on evidence from studies of (a) cranial arteries, (b) CGRP release, (c) behavioral models, and (d) a genetically modified model:
(a) High dose glibenclamide (30 mg/kg i.v.) effectively blocked vasodilation in rats induced by levcromakalim and pinacidil in both dural and pial arteries in vivo [31,138]. Glibenclamide also inhibited dilation caused by migraine-triggering peptides CGRP [65] and PACAP [147] that support KATP channel activation by phosphorylation via cAMP and PKA [148]. PNU-37883A effectively inhibited dilatory responses to stimulation with KATP channel openers in various arteries of different species including the MMA in vitro and in vivo [117,132]. Interestingly, glibenclamide [65] and PNU-37883A [149] failed to inhibit arterial dilation caused by NO-donors in some reports whereas others did find a relationship between NO (cGMP) and KATP channel-mediated arterial dilation [45,150].
(b) Glibenclamide (3 µM) inhibited ex vivo capsaicin-induced CGRP release from trigeminal ganglia and dura mater from spontaneous trigeminal allodynic (STA) [151,152] rats via an unknown mechanism [47].
(c) Also, in the STA rat model of migraine, glibenclamide (1–10 mg/kg i.p.) and gliquidone (10-100 mg/kg i.p.) reversed spontaneous trigeminal allodynia [47]. In the mouse models of provoked migraine, glibenclamide (1 mg/kg i.p.) was highly effective against levcromakalim, cilostazol, and glyceryl-trinitrate (GTN)-induced tactile hypersensitivity [47,99], whereas it only partially blocked the effect of PACAP-38 [146].
(d) Mice lacking the Kir6.1 subunit in smooth muscle cells were less sensitive to CGRP [64], levcromakalim and GTN [45] induced vasodilation and hypersensitivity.
4.3.2. Clinical Effect of KATP Channel Inhibition in Human Migraine Models
Glibenclamide (10 mg p.o.) has been tested against levcromakalim [137,153], CGRP [154], and PACAP-38 [155] induced migraine or headache in healthy volunteers. Glibenclamide was given 2 h prior to levcromakalim and CGRP infusions, but after the PACAP infusion. Headache data and hemodynamic measures were obtained. In all studies, subjects continuously received glucose to counteract the pronounced drop in blood glucose caused by glibenclamide. Overall, glibenclamide was found ineffective against both hemodynamic changes and headache induction after infusion of all three migraine triggering compounds.
The three above-mentioned studies were all cross-over studies but with variations in experimental design. In the levcromakalim study, NCT03886922 [137,153,156], three study arms were included: placebo-placebo, glibenclamide-placebo, and glibenclamide-levcromakalim. The study did not have a placebo-levcromakalim group, making the conclusions a bit distorted. In total, 12/15 participants (80%) reported headache after glibenclamide-levcromakalim, 5/15 (33%) after glibenclamide-placebo, and 1/15 (7%) following placebo-placebo. Thus, glibenclamide itself did not induce headache at a rate significantly different from placebo. To test if glibenclamide protected against levcromakalim-induced headache, comparison was made to a previous study showing headache induction in 12/14 of participants (86%) after levcromakalim versus 1/6 (17%) after placebo [136]. Hence, glibenclamide pretreatment did not inhibit headache development but, noteworthily, the median time to headache onset was 30 min (range 10–60) after levcromakalim infusion without pretreatment and 180 min (range 20–600) with glibenclamide pretreatment (p = 0.007) [153]. Glibenclamide did not influence HR, MAP, nor the circumference of neither STA,, MMA, nor MCA, which, in this study, all significantly changed in response to levcromakalim [137].
The study on CGRP and glibenclamide included two experimental groups in a randomized cross-over design: placebo-CGRP and glibenclamide-CGRP. The incidence of headache on the placebo-CGRP day was 19/20 (95%) vs. 14/20 (70%) on the glibenclamide-CGRP day (p = 0.06). Biologically, this was a 25% reduction in headache inductions, but power was set to detect 50% reduction in the study; thus, we cannot with certainly say if this finding happened by chance. Glibenclamide clearly did not influence CGRP-mediated changes on the hemodynamic parameters arterial diameter, HR, MAP, and facial skin blood flow [154]. Similar findings were obtained with glibenclamide as posttreatment when headache was induced by PACAP-38 [155]. Here, 19/20 participants (95%) reported headache compared to 18/20 (90%) on the placebo-PACAP day (p = 0.698).
5. Discussion
Direct comparison between human and animal experiments is not straight forward [157]. Apart from the rule of thumb conversion factor for doses based on body surface area to account for a generally faster metabolism in smaller animals [158], several other factors may be of importance. For the studies reviewed here, the difficulties concern: (1) different routes of administration and lack of pharmacokinetic data to safely interpret their impact, (2) different measuring endpoints, (3) a lack of evidence on the exact KATP channel distribution and expression levels in different tissues and species, and (4) the potency of test compounds on different channel subtypes and receptors across species. In Table 2, these are mentioned with the possible effect it may have on the conclusions.
5.1. KATP Channel Opening Has Similar Effect in Preclinical and Clinical Studies
Across species, KATP channel openers dose-dependently dilate arteries and decrease blood pressure [48,137,138]. The headache- or migraine-inducing effect of levcromakalim infusion in humans was modeled in the mouse model of migraine, but with major differences that need mentioning. Humans received a single dose of 0.014 mg/kg i.v. to induce headache or migraine [22,23,136], whereas mice received one, two, or sometimes three i.p. injections of 1 mg/kg before hypersensitivity to tactile stimuli was evident [45,47,99]. Thus, translation is complicated by different routes of administration and different measuring endpoints, and no common readout to which the other measures can be related to. Some degree of first pass metabolism following i.p. administration of levcromakalim is likely due to portal absorption [159], thus somewhat lowering the actual mouse dose. The net conclusion is that, in addition to hemodynamic effects, migraine-relevant nociceptive pathways are also replicated in the mouse model but at a higher dosing regimen.
5.2. Discrepant Results on KATP Channel Inhibition in Preclinical and Clinical Studies
Evidently, there has been a poor translation between preclinical and clinical studies looking at KATP channel blockage with glibenclamide in a migraine context, both on the hemodynamic parameters and headache readouts (hypersensitivity to tactile stimulation in rodents). These discrepancies may be explained by differences in dosing regimens, pharmacodynamic action of glibenclamide, subunit distributions, and trigger potency on various receptors.
5.2.1. Discrepant Effect of Glibenclamide on Cranial Arteries
In animal models using intravital microscopy of dural arteries, high doses of glibenclamide (7–30 mg/kg i.v.) were needed to prevent arterial dilation and a decrease in blood pressure followed by levcromakalim (0.1 mg/kg i.v.), pinacidil (0.38 mg/kg i.v.) [137], and CGRP (0.3 µg/kg i.v.) [65]. Looking at the glibenclamide/levcromakalim dose ratio (Table 2), it was 174 for full blockage and 116 for partial blockage of dural artery dilation in rats [138]. In the human equivalent study, the ratio was 5.8 (glibenclamide given to non-fasting participants has a high oral bioavailability, therefore this ratio is not adjusted). For CGRP, the ratio was 178,968 for the partially (but non-significant) effective dose of glibenclamide, and 767,004 for the fully effective dose in rats [65]. In the human experiment, the ratio was 24,972–53,690 depending on whether the total or 1 min CGRP dose was used. In animals, glibenclamide has only been tested against PACAP-induced arterial dilation in vitro. Here, the dose ratio calculation would not be meaningful to compare to the clinical data. In summary, the relative dose of glibenclamide contra trigger (levcromakalim, CGRP) was much higher in the rat studies. Given the SUR1 preference of glibenclamide, the relative low dose of glibenclamide given in humans may explain the lack of an effect of glibenclamide on human cranial arteries expressing SUR2B, while the higher dose applied to rats was sufficient to also inhibit SUR2B.
5.2.2. Discrepant Effect of Glibenclamide on Headache Measures
In terms of headache measures, the effectiveness of glibenclamide in mouse and rat models of migraine has not been seen in the human model of provoked migraine. In both rats and mice, glibenclamide (1 mg/kg i.p.) was sufficient to inhibit cutaneous tactile hypersensitivity, but in the human studies, 10 mg p.o. (0.14 mg/kg) was not convincingly effective. The rodent dose was 7 times higher than the human dose (assuming equal bioavailability), which is the typical conversion factor between rats and humans based on body surface area [158]. Looking at the glibenclamide/trigger ratio in the mouse model and the human model, we found that in mice, the ratio was 0.58 for levcromakalim and 45,891 for PACAP, the latter only partially preventing hypersensitivity. In the human studies, the ratios were 5.79 for levcromakalim and 1.42 for PACAP. Despite different routes of administration, we get a clear indication that the glibenclamide/PACAP ratio was smaller in the human study compared to the mouse study, which may explain the lack of translation between results. However, for levcromakalim, the ratio was 10-fold higher in the human experiment, which leaves no simple explanation for the lack of efficacy on the primary readout. Recall, however, that in this study [153], a comparison was made to a previous study [136]. A highly significant effect was found on a secondary output, median time to headache onset, which was 30 min after levcromakalim and 180 min after glibenclamide-levcromakalim, suggesting that glibenclamide was effective when plasma levels were high [153]. Glibenclamide has not been directly tested against CGRP in the mouse model of provoked migraine.
5.2.3. Target Engagement
The 10 mg dose of glibenclamide applied in the human studies was clearly insufficient in terms of blocking vascular KATP channels (Kir6.1/SUR2B), but the effect on blood glucose was shown to indicate the efficient blocking of pancreatic (Kir6.2/SUR1) channels in line with SUR1 selectivity of glibenclamide described in Section 3.2. (KATP channel blockers). In rats, the applied (high) dose was able to block the vascular KATP channels. In humans, larger doses cannot be applied due to the adverse effect on blood glucose [153]. The effect of blood glucose was not reported in the rat studies looking at hemodynamics after glibenclamide 7–30 mg/kg, i.v. [65,138]. In another rat study, both glibenclamide 1 mg/kg and 10 mg/kg i.p. decreased blood glucose from 7 mmol/L (vehicle treatment) to 3 mmol/L 2 h post-treatment. In mice, acute injection of glibenclamide (10 and 30 µg/mouse) caused a rapid, dose-dependent drop in blood glucose levels from approximately 170 to 120 mg/dL, peaking at 60 min. The two highest concentrations of glibenclamide caused a similar marked reduction of fed blood glucose after an extended period, consistent with a saturated effect of the drug in vivo [160]. Glibenclamide 5 mg/kg/day (delivered by a subcutaneous minipump) did not affect blood glucose in vivo [161]. The pronounced effect on human (but not mouse) glucose level is likely due to the reported > 100-fold higher potency of glibenclamide on human SUR1 over mouse SUR1 [126,162].
The higher selectivity of PNU-37883A on the vascular channels compared to glibenclamide is evident when the inhibition of levcromakalim is related across two studies from the same laboratory using the same in vivo model to study dilation of the MMA [117,138]. Here, 1.3 µmol/kg of PNU-37883A partly inhibited 0.087 µmol/kg of levcromakalim-induced dilation (ratio 15) and 40.5 µmol/kg of glibenclamide partly inhibited 0.35 µmol/kg of levcromakalim (ratio 116).
In both rats and mice, the lower dose of glibenclamide (1 mg/kg i.p. = 2 µmol/kg, ratio 0.58) was effective in different behavioral models of migraine [47,99]. The human studies on headache prevention by glibenclamide following provocation with levcromakalim, CGRP, and PACAP were negative on the primary outcome. Nevertheless, partial efficacy may have been present in the former two experiments; see Section 4.3.2. (Clinical effect of KATP channel inhibition in human migraine models). Partial inhibition of CGRP and PACAP induced alterations may be expected as the downstream effect from both neuropeptides likely also involve the opening of other ion channels [163].
5.3. Possible Mechanism of Headache Induction and Prevention
Different theories about where and how the opening of KATP channels causes headache exist. These are further fueled by the non-clarified effect of channel inhibition on headache readouts. Collectively, the interpretation regarding subunit contribution to headache is difficult as rodent and, to some extent, human data suggest that glibenclamide may inhibit headache (hypersensitivity in rodents) to some degree, without the relevant effect on the vascular Kir6.1/SUR2B channel, which is the proposed mediator of levcromakalim-induced migraine [22]. An effect on neuronal Kir6.2/SUR2 channels is also a possibility [164], albeit smooth muscle Kir6.1 subunits were identified as important [45].
5.3.1. Dilation of Meningeal Arteries
Dilation of intracranial arteries within the trigeminovascular system is the leading hypothesis of levcromakalim-induced headache and migraine [7,45,165]. Two proposed mechanisms are currently at play: (a) mechanical activation of trigeminal nociceptors by arterial dilation or (b) chemical activation of trigeminal nociceptors by high [K+] in the microenvironment between arteries and nerve endings [23]. These hypotheses need testing using a selective blocker of Kir6.1/SUR2B suitable for use in humans.
5.3.2. Effect on CGRP Signaling
A few preclinical studies suggest that KATP channels may affect CGRP signaling in different manners. In ex vivo organ preparations, glibenclamide inhibited the release of CGRP from trigeminal ganglia and dura mater [47]. In contrast, KATP channel openers did not directly stimulate the release of CGRP [99,141]. However, in vivo, the hypersensitivity induced by levcromakalim was abolished both by treatment with a CGRP-neutralizing antibody, and in genetically modified mice, by not expressing the CGRP receptor component RAMP1 [99], suggesting that CGRP is released by inter-tissue communication (not found ex vivo) following levcromakalim treatment and that this drives hypersensitivity. As an alternative to the vascular theory, this specific release of CGRP may in fact be what is inhibited by glibenclamide in vivo via its affinity to SUR1. This may also explain the speculative effect of glibenclamide on headache, in spite of the clear lack of a vascular effect on hemodynamics in human experiments.
5.3.3. Hyperpolarization-Activated Cyclic Nucleotide-Gated (HCN) Channels
Another alternative to the vascular theory of migraine induction by KATP channel opening is the involvement of HCN channels in the trigeminal nervous system [164]. Sustained hyperpolarization of neurons may engage HCN channels, and blockage of these have been suggested as therapeutic targets in diabetic neuropathy [166] and neuropathic [167] and inflammatory pain [168]. The proposed mechanism is that KATP channel openers lead to the long-lasting hyperpolarization of trigeminal nerves, in turn activating HCN channels that leads to augmented neuronal excitability and firing of the neurons [169,170]. In contrast, this hypothesis consists of the fact that, in CNS, the opening of KATP channels is involved in analgesia. Moreover, HCN channels are expressed in trigeminal and dorsal root ganglia, and systemic exposure to levcromakalim induces cephalic but not peripheral pain [22,23,136] and local administration of levcromakalim was unable to induce pain [171]. The HCN theory is currently being evaluated in a clinical trial (NCT04853797), testing HCN channel blocker ivabradine against levcromakalim-induced headache [156].
5.4. Clinical Therapeutic Perspectives
Ion channels are regarded as an important class of drug targets for modulating pain and is localized in primary sensory neurons and other key structures in pain processing [172]. KATP channels are probably the most diverse ion channel type, and each subtype has a specific physiological role. Developing drugs targeting all KATP channels may therefore be impossible since they are widespread and undesirable severe side effects would be expected. Thus, subtype selectivity is key and may be a very attractive target for the development of novel therapeutics for the acute and preventive treatment of migraine.
Accordingly, Kir6.1/SUR2B subunits are dominantly expressed in the vascular smooth muscle. In contrast, Kir6.2/SUR1 are expressed in the CNS and pancreas, and Kir6.2/SUR2A are expressed in cardiac and skeletal muscle [50,117]. However, the lack of detailed structural and functional insight of these channels poses a challenge for the development of selective drug candidates. The Kir6.1 selective KATP channel blocker, PNU-37883A, was developed as an orally effective non-kaliuretic diuretic in rats [149,173]. Because of its cardiac depressant activity, possibly related to its blockade of coronary artery Kir6.1 channels in animal experiments, the drug never advanced to human studies [174]. Thus, KATP channel blockers selective for Kir6.1 alone should be carefully considered. In addition, SUR2 null mice exhibited elevated resting blood pressure and sudden death from ST segment elevation and coronary artery vasospasm [175]. In SUR2, in null mice with a transgenic restoration of SUR2B, the above-mentioned side effects persisted [176]. Thus, these side effects seem to be caused by SUR2A knockout and are likely not related to knockout of the SUR2B subunit. A KATP channel blocker for the treatment of migraine should therefore preferably have an exclusive selectivity for Kir6.1/SUR2B KATP channels.
To date, most ion channel drug development has focused on identifying and developing small molecule and peptide modulators, mainly through serendipitous discovery [177]. Despite vastly improved screening tools for small molecule or compound libraries, only two novel ion channel drugs have been approved by the FDA since the 1990s [178]. The well-known disadvantage of small molecules is that they can bind to off-molecular targets, leading to more side effects and toxicity. Alternative modalities for targeting ion channels have recently included monoclonal antibodies (mAbs), which offer many additional advantages to selectivity and bioavailability. Yet, despite considerable interest, there are currently no marketed mAbs therapies that target an ion channel. This lack of success is mainly attributable to two important technical challenges. First, the ion channels have short extracellular loops displaying small epitope target areas over the plasma membrane, causing them to be challenging binding targets for large protein antibodies. Additionally, these extracellular loops tend to be highly conserved at the primary amino acid sequence level, and thus lack sufficient immunogenicity to generate robust antibody responses in mammalian hosts [178].
A major challenge and concern in developing KATP channel blockers is cardiac side effects. KATP channels are abundant in the myocardium and KATP channel openers have proven useful in ischemic heart disease through direct actions on the myocardium [179] and may prevent arrhythmias [180]. To overcome this problem, it is important to test with several heart assays, such as the ex vivo Langendorff heart model (perfused isolated heart model), to evaluate the direct effects of compounds on cardiac function and to ensure cardiovascular safety of new drug candidates [181].
In conclusion, KATP channels are recognized as promising therapeutic targets for migraine treatment but remain a major challenge for drug discovery. To move forward, we need further studies on the specific subtypes of the KATP channel to enable a deeper understanding of their structures, functions and distribution for more selective and successful drug development. Furthermore, knowledge on the consequences of activation or blockage of the KATP channels on a molecular- or pathway-specific level in the pathophysiology of migraine, is necessary to fully comprehend and predict the potential of this novel therapeutic target.
Acknowledgments
Thanks to Jes Olesen for proofreading the manuscript.
Author Contributions
Conceptualization, S.L.C. and A.C.; writing—original draft preparation, S.L.C., A.C. and S.G.; writing—review and editing, S.L.C., A.C., S.G. and I.J.-O.; supervision, I.J.-O. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the drafting, proofreading, and finalizing of the manuscript.
Funding Statement
This research was funded by the Candys Foundation. Song Guo is supported by the BRIDGE – Translational Excellence Programme (bridge.ku.dk) at the Faculty of Health and Medical Sciences, University of Copenhagen, funded by the Novo Nordisk Foundation. Grant agreement no. NNF20SA0064340 (2021 fellows).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Steiner T.J., Stovner L.J., Jensen R., Uluduz D., Katsarava Z. Migraine Remains Second among the World’s Causes of Disability, and First among Young Women: Findings from GBD2019. J. Headache Pain. 2020;21:4–7. doi: 10.1186/s10194-020-01208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James S.L., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A., et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olesen J. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd Edition. Cephalalgia. 2018;38:1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 4.Vgontzas A., Pavlović J.M. Sleep Disorders and Migraine: Review of Literature and Potential Pathophysiology Mechanisms. Headache. 2018;58:1030–1039. doi: 10.1111/head.13358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vuralli D., Ayata C., Bolay H. Cognitive Dysfunction and Migraine 17 Psychology and Cognitive Sciences 1701 Psychology 11 Medical and Health Sciences 1103 Clinical Sciences 11 Medical and Health Sciences 1109 Neurosciences. J. Headache Pain. 2018;19:109. doi: 10.1186/s10194-018-0933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashina M., Katsarava Z., Do T.P., Buse D.C., Pozo-Rosich P., Özge A., Krymchantowski A.V., Lebedeva E.R., Ravishankar K., Yu S., et al. Migraine: Epidemiology and Systems of Care. Lancet. 2021;397:1485–1495. doi: 10.1016/S0140-6736(20)32160-7. [DOI] [PubMed] [Google Scholar]
- 7.Ashina M. Migraine. N. Engl. J. Med. 2020;383:1866–1876. doi: 10.1056/NEJMra1915327. [DOI] [PubMed] [Google Scholar]
- 8.Ashina M., Hansen J.M., Do T.P., Melo-Carrillo A., Burstein R., Moskowitz M.A. Migraine and the Trigeminovascular System-40 Years and Counting. Lancet. Neurol. 2019;18:795–804. doi: 10.1016/S1474-4422(19)30185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng K.P., May A. Migraine Understood as a Sensory Threshold Disease. Pain. 2019;160:1494–1501. doi: 10.1097/j.pain.0000000000001531. [DOI] [PubMed] [Google Scholar]
- 10.Edvinsson L., Haanes K.A., Warfvinge K., Krause D.N. CGRP as the Target of New Migraine Therapies—Successful Translation from Bench to Clinic. Nat. Rev. Neurol. 2018;14:338–350. doi: 10.1038/s41582-018-0003-1. [DOI] [PubMed] [Google Scholar]
- 11.Stauffer V.L., Dodick D.W., Zhang Q., Carter J.N., Ailani J., Conley R.R. Evaluation of Galcanezumab for the Prevention of Episodic Migraine: The EVOLVE-1 Randomized Clinical Trial. JAMA Neurol. 2018;75:1080–1088. doi: 10.1001/jamaneurol.2018.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Förderreuther S., Zhang Q., Stauffer V.L., Aurora S.K., Láinez M.J.A. Preventive Effects of Galcanezumab in Adult Patients with Episodic or Chronic Migraine Are Persistent: Data from the Phase 3, Randomized, Double-Blind, Placebo-Controlled EVOLVE-1, EVOLVE-2, and REGAIN Studies. J. Headache Pain. 2018;19:121. doi: 10.1186/s10194-018-0951-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashina M., Goadsby P.J., Reuter U., Silberstein S., Dodick D.W., Xue F., Zhang F., Paiva da Silva Lima G., Cheng S., Mikol D.D. Long-Term Efficacy and Safety of Erenumab in Migraine Prevention: Results from a 5-Year, Open-Label Treatment Phase of a Randomized Clinical Trial. Eur. J. Neurol. 2021;28:1716–1725. doi: 10.1111/ene.14715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silberstein S.D., Dodick D.W., Bigal M.E., Yeung P.P., Goadsby P.J., Blankenbiller T., Grozinski-Wolff M., Yang R., Ma Y., Aycardi E. Fremanezumab for the Preventive Treatment of Chronic Migraine. N. Engl. J. Med. 2017;377:2113–2122. doi: 10.1056/NEJMoa1709038. [DOI] [PubMed] [Google Scholar]
- 15.Ashina M., Saper J., Cady R., Schaeffler B.A., Biondi D.M., Hirman J., Pederson S., Allan B., Smith J. Eptinezumab in Episodic Migraine: A Randomized, Double-Blind, Placebo-Controlled Study (PROMISE-1) Cephalalgia. 2020;40:241–254. doi: 10.1177/0333102420905132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lassen L.H., Haderslev P.A., Jacobsen V.B., Iversen H.K., Sperling B., Olesen J. CGRP May Play a Causative Role in Migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 17.Schytz H.W., Birk S., Wienecke T., Kruuse C., Olesen J., Ashina M. PACAP38 Induces Migraine-like Attacks in Patients with Migraine without Aura. Brain. 2009;132:16–25. doi: 10.1093/brain/awn307. [DOI] [PubMed] [Google Scholar]
- 18.Thomsen L.L. Investigations into the Role of Nitric Oxide and the Large Intracranial Arteries in Migraine Headache. Cephalalgia. 1997;17:873–895. doi: 10.1046/j.1468-2982.1997.1708873.x. [DOI] [PubMed] [Google Scholar]
- 19.Pellesi L., Al-Karagholi M.A.M., Chaudhry B.A., Lopez C.L., Snellman J., Hannibal J., Amin F.M., Ashina M. Two-Hour Infusion of Vasoactive Intestinal Polypeptide Induces Delayed Headache and Extracranial Vasodilation in Healthy Volunteers. Cephalalgia. 2020;40:1212–1223. doi: 10.1177/0333102420937655. [DOI] [PubMed] [Google Scholar]
- 20.Kruuse C., Thomsen L.L., Birk S., Olesen J. Migraine Can Be Induced by Sildenafil without Changes in Middle Cerebral Artery Diameter. Brain. 2003;126:241–247. doi: 10.1093/brain/awg009. [DOI] [PubMed] [Google Scholar]
- 21.Guo S., Olesen J., Ashina M. Phosphodiesterase 3 Inhibitor Cilostazol Induces Migraine-like Attacks via Cyclic AMP Increase. Brain. 2014;137:2951–2959. doi: 10.1093/brain/awu244. [DOI] [PubMed] [Google Scholar]
- 22.Al-Karagholi M.A.M., Hansen J.M., Guo S., Olesen J., Ashina M. Opening of ATP-Sensitive Potassium Channels Causes Migraine Attacks: A New Target for the Treatment of Migraine. Brain. 2019;142:2644–2654. doi: 10.1093/brain/awz199. [DOI] [PubMed] [Google Scholar]
- 23.Al-Karagholi M.A.M., Ghanizada H., Nielsen C.A.W., Hougaard A., Ashina M. Opening of ATP Sensitive Potassium Channels Causes Migraine Attacks with Aura. Brain. 2021;144:2322–2332. doi: 10.1093/brain/awab136. [DOI] [PubMed] [Google Scholar]
- 24.Noma A. ATP-Regulated K+ Channels in Cardiac Muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 25.Miki T., Nagashima K., Seino S. The Structure and Function of the ATP-Sensitive K+ Channel in Insulin-Secreting Pancreatic Beta-Cells. J. Mol. Endocrinol. 1999;22:113–123. doi: 10.1677/jme.0.0220113. [DOI] [PubMed] [Google Scholar]
- 26.Proks P., Ashcroft F.M. Modeling KATP Channel Gating and Its Regulation. Prog. Biophys. Mol. Biol. 2009;99:7–19. doi: 10.1016/j.pbiomolbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Hibino H., Inanobe A., Furutani K., Murakami S., Findlay I., Kurachi Y. Inwardly Rectifying Potassium Channels: Their Structure, Function, and Physiological Roles. Physiol. Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 28.Choma K., Bednarczyk P., Koszela-Piotrowska I., Kulawiak B., Kudin A., Kunz W.S., Dołowy K., Szewczyk A. Single Channel Studies of the ATP-Regulated Potassium Channel in Brain Mitochondria. J. Bioenerg. Biomembr. 2009;41:323–334. doi: 10.1007/s10863-009-9233-7. [DOI] [PubMed] [Google Scholar]
- 29.Babenko A.P., Aguilar-Bryan L., Bryan J. A View of Sur/Kir6.X, KATP Channels. Annu. Rev. Physiol. 1998;60:667–687. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- 30.Seino S., Miki T. Physiological and Pathophysiological Roles of ATP-Sensitive K+ Channels. Prog. Biophys. Mol. Biol. 2003;81:133–176. doi: 10.1016/S0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- 31.Syed A.U., Koide M., Brayden J.E., Wellman G.C. Tonic Regulation of Middle Meningeal Artery Diameter by ATP-Sensitive Potassium Channels. J. Cereb. Blood Flow Metab. 2019;39:670–679. doi: 10.1177/0271678X17749392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Standen N.B., Quayle J.M., Davies N.W., Brayden J.E., Huang Y., Nelson M.T. Hyperpolarizing Vasodilators Activate ATP-Sensitive K+ Channels in Arterial Smooth Muscle. Science. 1989;245:177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- 33.Sung M.W., Yang Z., Driggers C.M., Patton B.L., Mostofian B., Russo J.D., Zuckerman D.M., Shyng S.L. Vascular KATP Channel Structural Dynamics Reveal Regulatory Mechanism by Mg-Nucleotides. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2109441118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ribalet B., John S.A., Xie L.H., Weiss J.N. Regulation of the ATP-Sensitive K Channel Kir6.2 by ATP and PIP(2) J. Mol. Cell. Cardiol. 2005;39:71–77. doi: 10.1016/j.yjmcc.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 35.Baukrowitz T., Schulte U., Oliver D., Herlitze S., Krauter T., Tucker S.J., Ruppersberg J.P., Fakler B. PIP2 and PIP as Determinants for ATP Inhibition of KATP Channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- 36.Larsson O., Barker C.J., Berggren P.O. Phosphatidylinositol 4,5-Bisphosphate and ATP-Sensitive Potassium Channel Regulation: A Word of Caution. Diabetes. 2000;49:1409–1412. doi: 10.2337/diabetes.49.9.1409. [DOI] [PubMed] [Google Scholar]
- 37.Shi Y., Cui N., Shi W., Jiang C. A Short Motif in Kir6.1 Consisting of Four Phosphorylation Repeats Underlies the Vascular KATP Channel Inhibition by Protein Kinase C. J. Biol. Chem. 2008;283:2488–2494. doi: 10.1074/jbc.M708769200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi W.-W., Yang Y., Shi Y., Chun J. KATP Channel Action in Vascular Tone Regulation: From Genetics to Diseases. Sheng Li Xue Bao. 2012;64:1–13. [PMC free article] [PubMed] [Google Scholar]
- 39.Quinn K.V., Giblin J.P., Tinker A. Multisite Phosphorylation Mechanism for Protein Kinase A Activation of the Smooth Muscle ATP-Sensitive K+ Channel. Circ. Res. 2004;94:1359–1366. doi: 10.1161/01.RES.0000128513.34817.c4. [DOI] [PubMed] [Google Scholar]
- 40.Shi Y., Chen X., Wu Z., Shi W., Yang Y., Cui N., Jiang C., Harrison R.W. CAMP-Dependent Protein Kinase Phosphorylation Produces Interdomain Movement in SUR2B Leading to Activation of the Vascular KATP Channel. J. Biol. Chem. 2008;283:7523–7530. doi: 10.1074/jbc.M709941200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kokoti L., Al-Karagholi M.A.M., Ashina M. Latest Insights into the Pathophysiology of Migraine: The ATP-Sensitive Potassium Channels. Curr. Pain Headache Rep. 2020;24:77. doi: 10.1007/s11916-020-00911-6. [DOI] [PubMed] [Google Scholar]
- 42.Schytz H.W., Schoonman G.G., Ashina M. What Have We Learnt from Triggering Migraine? Curr. Opin. Neurol. 2010;23:259–265. doi: 10.1097/WCO.0b013e328337b884. [DOI] [PubMed] [Google Scholar]
- 43.Yamada M., Isomoto S., Matsumoto S., Kondo C., Shindo T., Horio Y., Kurachi Y. Sulphonylurea Receptor 2B and Kir6.1 Form a Sulphonylurea-Sensitive but ATP-Insensitive K+ Channel. J. Physiol. 1997;499:715–720. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teramoto N., Zhu H.-L., Shibata A., Aishima M., Walsh E.J., Nagao M., Cole W.C. ATP-Sensitive K+ Channels in Pig Urethral Smooth Muscle Cells Are Heteromultimers of Kir6.1 and Kir6.2. Am. J. Physiol Ren. Physiol. 2009;296:107–117. doi: 10.1152/ajprenal.90440.2008. [DOI] [PubMed] [Google Scholar]
- 45.Christensen S.L., Rasmussen R.H., Cour S.L., Ernstsen C., Hansen T.F., Kogelman L.J., Lauritzen S.P., Guzaite G., Styrishave B., Janfelt C., et al. Smooth Muscle ATP-Sensitive Potassium Channels Mediate Migraine-Relevant Hypersensitivity in Mouse Models. Cephalalgia. 2022;42:93–107. doi: 10.1177/03331024211053570. [DOI] [PubMed] [Google Scholar]
- 46.Al-Karagholi M.A.-M., Hansen J.M., Severinsen J., Jansen-Olesen I., Ashina M. The KATP Channel in Migraine Pathophysiology: A Novel Therapeutic Target for Migraine. J. Headache Pain. 2017;18:90. doi: 10.1186/s10194-017-0800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christensen S.L., Munro G., Petersen S., Shabir A., Jansen-Olesen I., Kristensen D.M., Olesen J. ATP Sensitive Potassium (KATP) Channel Inhibition: A Promising New Drug Target for Migraine. Cephalalgia. 2020;40:650–664. doi: 10.1177/0333102420925513. [DOI] [PubMed] [Google Scholar]
- 48.Li Y., Aziz Q., Tinker A. The Pharmacology of ATP-Sensitive K + Channels (K ATP) Handb. Exp. Pharmacol. 2021;267:357–378. doi: 10.1007/164_2021_466. [DOI] [PubMed] [Google Scholar]
- 49.Skatchkov S.N., Rojas L., Eaton M.J., Orkand R.K., Biedermann B., Bringmann A., Pannicke T., Veh R.W., Reichenbach A. Functional Expression of Kir 6.1/SUR1-KATP Channels in Frog Retinal Müller Glial Cells. Glia. 2002;38:256–267. doi: 10.1002/glia.10073. [DOI] [PubMed] [Google Scholar]
- 50.Ploug K.B., Sørensen M.A., Strøbech L., Klaerke D.A., Hay-Schmidt A., Sheykhzade M., Olesen J., Jansen-Olesen I. KATP Channels in Pig and Human Intracranial Arteries. Eur. J. Pharmacol. 2008;601:43–49. doi: 10.1016/j.ejphar.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 51.Aziz Q., Li Y., Tinker A. ATP-Sensitive Potassium Channels and Vascular Function. Channels. 2015;9:3–4. doi: 10.1080/19336950.2015.1004289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ploug K.B., Baun M., Hay-Schmidt A., Olesen J., Jansen-Olesen I. Presence and Vascular Pharmacology of KATP Channel Subtypes in Rat Central and Peripheral Tissues. Eur. J. Pharmacol. 2010;637:109–117. doi: 10.1016/j.ejphar.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 53.Rodrigo G., Standen N. ATP-Sensitive Potassium Channels. Curr. Pharm. Des. 2005;11:1915–1940. doi: 10.2174/1381612054021015. [DOI] [PubMed] [Google Scholar]
- 54.Bao L., Kefaloyianni E., Lader J., Hong M., Morley G., Fishman G.I., Sobie E.A., Coetzee W.A. Unique Properties of the ATP-Sensitive K+ Channel in the Mouse Ventricular Cardiac Conduction System. Circ. Arrhythm. Electrophysiol. 2011;4:926–935. doi: 10.1161/CIRCEP.111.964643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aguilar-Bryan L., Nichols C.G., Wechsler S.W., Clement IV J.P., Boyd A.E., González G., Herrera-Sosa H., Nguy K., Bryan J., Nelson D.A. Cloning of the Beta Cell High-Affinity Sulfonylurea Receptor: A Regulator of Insulin Secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- 56.Flagg T.P., Kurata H.T., Masia R., Caputa G., Magnuson M.A., Lefer D.J., Coetzee W.A., Nichols C.G. Differential Structure of Atrial and Ventricular KATP: Atrial KATP Channels Require SUR1. Circ. Res. 2008;103:1458–1465. doi: 10.1161/CIRCRESAHA.108.178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zoga V., Kawano T., Liang M.Y., Bienengraeber M., Weihrauch D., McCallum B., Gemes G., Hogan Q., Sarantopoulos C. KATPchannel Subunits in Rat Dorsal Root Ganglia: Alterations by Painful Axotomy. Mol. Pain. 2010;6 doi: 10.1186/1744-8069-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawano T., Zoga V., McCallum J.B., Wu H.E., Gemes G., Liang M.Y., Abram S., Kwok W.M., Hogan Q.H., Sarantopoulos C.D. ATP-Sensitive Potassium Currents in Rat Primary Afferent Neurons: Biophysical, Pharmacological Properties, and Alterations by Painful Nerve Injury. Neuroscience. 2009;162:431–443. doi: 10.1016/j.neuroscience.2009.04.076. [DOI] [PubMed] [Google Scholar]
- 59.Tricarico D., Mele A., Lundquist A.L., Desai R.R., George A.L., Conte Camerino D. Hybrid Assemblies of ATP-Sensitive K+ Channels Determine Their Muscle-Type-Dependent Biophysical and Pharmacological Properties. Proc. Natl. Acad. Sci. USA. 2006;103:1118–1123. doi: 10.1073/pnas.0505974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inagaki N., Gonoi T., Clement IV J.P., Wang C.Z., Aguilar-Bryan L., Bryan J., Seino S. A Family of Sulfonylurea Receptors Determines the Pharmacological Properties of ATP-Sensitive K+ Channels. Neuron. 1996;16:1011–1017. doi: 10.1016/S0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 61.Sun H.S., Feng Z.P., Barber P.A., Buchan A.M., French R.J. Kir6.2-Containing ATP-Sensitive Potassium Channels Protect Cortical Neurons from Ischemic/Anoxic Injury in Vitro and in Vivo. Neuroscience. 2007;144:1509–1515. doi: 10.1016/j.neuroscience.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 62.Jovanović S., Ballantyne T., Du Q., Blagojević M., Jovanović A. Phenylephrine Preconditioning in Embryonic Heart H9c2 Cells Is Mediated by Up-Regulation of SUR2B/Kir6.2: A First Evidence for Functional Role of SUR2B in Sarcolemmal KATP Channels and Cardioprotection. Int. J. Biochem. Cell Biol. 2016;70:23–28. doi: 10.1016/j.biocel.2015.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jahangir A., Terzic A. K(ATP) Channel Therapeutics at the Bedside. J. Mol. Cell. Cardiol. 2005;39:99–112. doi: 10.1016/j.yjmcc.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aziz Q., Thomas A.M., Gomes J., Ang R., Sones W.R., Li Y., Ng K.E., Gee L., Tinker A. The ATP-Sensitive Potassium Channel Subunit, Kir6.1, in Vascular Smooth Muscle Plays a Major Role in Blood Pressure Control. Hypertension. 2014;64:523–529. doi: 10.1161/HYPERTENSIONAHA.114.03116. [DOI] [PubMed] [Google Scholar]
- 65.Gozalov A., Jansen-Olesen I., Klaerke D., Olesen J. Role of KATP Channels in Cephalic Vasodilatation Induced by Calcitonin Gene-Related Peptide, Nitric Oxide, and Transcranial Electrical Stimulation in the Rat. Headache. 2008;48:1202–1213. doi: 10.1111/j.1526-4610.2008.01205.x. [DOI] [PubMed] [Google Scholar]
- 66.Nelson M.T., Quayle J.M. Physiological Roles and Properties of Potassium Channels in Arterial Smooth Muscle. Am. J. Physiol. Cell Physiol. 1995;268:799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 67.Tinker A., Aziz Q., Thomas A. The Role of ATP-Sensitive Potassium Channels in Cellular Function and Protection in the Cardiovascular System. Br. J. Pharmacol. 2014;171:12–23. doi: 10.1111/bph.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murphy M.E., Brayden J.E. Nitric Oxide Hyperpolarizes Rabbit Mesenteric Arteries via ATP-Sensitive Potassium Channels. J. Physiol. 1995;486:58. doi: 10.1113/jphysiol.1995.sp020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olesen J. The Role of Nitric Oxide (NO) in Migraine, Tension-Type Headache and Cluster Headache. Pharmacol. Ther. 2008;120:157–171. doi: 10.1016/j.pharmthera.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 70.Duarte I.D.G., dos Santos I.R., Lorenzetti B.B., Ferreira S.H. Analgesia by Direct Antagonism of Nociceptor Sensitization Involves the Arginine-Nitric Oxide-CGMP Pathway. Eur. J. Pharmacol. 1992;217:225–227. doi: 10.1016/0014-2999(92)90881-4. [DOI] [PubMed] [Google Scholar]
- 71.Aziz Q., Li Y., Anderson N., Ojake L., Tsisanova E., Tinker A. Molecular and Functional Characterization of the Endothelial ATP-Sensitive Potassium Channel. J. Biol. Chem. 2017;292:17587–17597. doi: 10.1074/jbc.M117.810325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Logu F., Landini L., Janal M.N., Li Puma S., De Cesaris F., Geppetti P., Nassini R. Migraine-Provoking Substances Evoke Periorbital Allodynia in Mice. J. Headache Pain. 2019;20:18. doi: 10.1186/s10194-019-0968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schytz H.W., Olesen J., Ashina M. The PACAP Receptor: A Novel Target for Migraine Treatment. Neurotherapeutics. 2010;7:191–196. doi: 10.1016/j.nurt.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown T., Forster R.B., Cleanthis M., Mikhailidis D.P., Stansby G., Stewart M. Cilostazol for Intermittent Claudication. Cochrane Database Syst. Rev. 2021;2021 doi: 10.1016/j.jvs.2021.07.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moore R.A., Edwards J.E., McQuay H.J. Sildenafil (Viagra) for Male Erectile Dysfunction: A Meta-Analysis of Clinical Trial Reports. BMC Urol. 2002;2:1–12. doi: 10.1186/1471-2490-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trainor D.C., Jones R.C. Headaches in Explosive Magazine Workers. Arch. Environ. Health. 1966;12:231–234. doi: 10.1080/00039896.1966.10664362. [DOI] [PubMed] [Google Scholar]
- 77.Miki T., Suzuki M., Shibasaki T., Uemura H., Sato T., Yamaguchi K., Koseki H., Iwanaga T., Nakaya H., Seino S. Mouse Model of Prinzmetal Angina by Disruption of the Inward Rectifier Kir6.1. Nat. Med. 2002;8:466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- 78.Porter A. Controlling Your Losses: Conditional Gene Silencing in Mammals. Trends Genet. 1998;14:73–79. doi: 10.1016/S0168-9525(97)01326-7. [DOI] [PubMed] [Google Scholar]
- 79.Sauer B. Inducible Gene Targeting in Mice Using the Cre/Lox System. Methods. 1998;14:381–392. doi: 10.1006/meth.1998.0593. [DOI] [PubMed] [Google Scholar]
- 80.Utomo A.R.H., Nikitin A.Y., Lee W.H. Temporal, Spatial, and Cell Type-Specified Control of Cre-Mediated DNA Recombination in Transgenic Mice. Nat. Biotechnol. 1999;17:1091–1096. doi: 10.1038/15073. [DOI] [PubMed] [Google Scholar]
- 81.Soundarapandian M.M., Zhong X., Peng L., Wu D., Lu Y. Role of K ATP Channels in Protection against Neuronal Excitatory Insults. J. Neurochem. 2007;103:1721–1729. doi: 10.1111/j.1471-4159.2007.04963.x. [DOI] [PubMed] [Google Scholar]
- 82.Yildirim C., Özkaya B., Bal R. KATP and TRPM2-like Channels Couple Metabolic Status to Resting Membrane Potential of Octopus Neurons in the Mouse Ventral Cochlear Nucleus. Brain Res. Bull. 2021;170:115–128. doi: 10.1016/j.brainresbull.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 83.Kase D., Imoto K. The Role of HCN Channels on Membrane Excitability in the Nervous System. J. Signal. Transduct. 2012;2012:1–11. doi: 10.1155/2012/619747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Country M.W., Jonz M.G. Mitochondrial KATP Channels Stabilize Intracellular Ca2+ during Hypoxia in Retinal Horizontal Cells of Goldfish (Carassius Auratus) J. Exp. Biol. 2021;224 doi: 10.1242/jeb.242634. [DOI] [PubMed] [Google Scholar]
- 85.Guo W., Tang Z.Y., Cai Z.Y., Zhao W.E., Yang J., Wang X.P., Ji J., Huang X.X., Sun X.L. Iptakalim Alleviates Synaptic Damages via Targeting Mitochondrial ATP-Sensitive Potassium Channel in Depression. FASEB J. 2021;35 doi: 10.1096/fj.202100124RR. [DOI] [PubMed] [Google Scholar]
- 86.Toulorge D., Guerreiro S., Hirsch E.C., Michel P.P. KATP Channel Blockade Protects Midbrain Dopamine Neurons by Repressing a Glia-to-Neuron Signaling Cascade That Ultimately Disrupts Mitochondrial Calcium Homeostasis. J. Neurochem. 2010;114:553–564. doi: 10.1111/j.1471-4159.2010.06785.x. [DOI] [PubMed] [Google Scholar]
- 87.Khanna N., Malhotra R.S., Mehta A.K., Garg G.R., Halder S., Sharma K.K. Interaction of Morphine and Potassium Channel Openers on Experimental Models of Pain in Mice. Fundam. Clin. Pharmacol. 2011;25:479–484. doi: 10.1111/j.1472-8206.2010.00880.x. [DOI] [PubMed] [Google Scholar]
- 88.Tsantoulas C., McMahon S.B. Opening Paths to Novel Analgesics: The Role of Potassium Channels in Chronic Pain. Trends Neurosci. 2014;37:146–158. doi: 10.1016/j.tins.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cunha T.M., Roman-Campos D., Lotufo C.M., Duarte H.L., Souza G.R., Verri W.A., Funez M.I., Dias Q.M., Schivo I.R., Domingues A.C., et al. Morphine Peripheral Analgesia Depends on Activation of the PI3Kγ/AKT/NNOS/NO/KATP Signaling Pathway. Proc. Natl. Acad. Sci. USA. 2010;107:4442–4447. doi: 10.1073/pnas.0914733107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kusuda R., Carreira E.U., Ulloa L., Cunha F.Q., Kanashiro A., Cunha T.M. Choline Attenuates Inflammatory Hyperalgesia Activating Nitric Oxide/CGMP/ATP-Sensitive Potassium Channels Pathway. Brain Res. 2020;1727:146567. doi: 10.1016/j.brainres.2019.146567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fakhri S., Ahmadpour Y., Rezaei H., Kooshki L., Moradi S.Z., Iranpanah A., Gravandi M.M., Abbaszadeh F., Ghanbarveisi F. The Antinociceptive Mechanisms of Melatonin: Role of L-Arginine/Nitric Oxide/Cyclic GMP/KATP Channel Signaling Pathway. Behav. Pharmacol. 2020;31:728–737. doi: 10.1097/FBP.0000000000000579. [DOI] [PubMed] [Google Scholar]
- 92.Gong L., Gao F., Li J., Li J., Yu X., Ma X., Zheng W., Cui S., Liu K., Zhang M., et al. Oxytocin-Induced Membrane Hyperpolarization in Pain-Sensitive Dorsal Root Ganglia Neurons Mediated by Ca(2+)/NNOS/NO/KATP Pathway. Neuroscience. 2015;289:417–428. doi: 10.1016/j.neuroscience.2014.12.058. [DOI] [PubMed] [Google Scholar]
- 93.Sarantopoulos C., McCallum B., Sapunar D., Kwok W.M., Hogan Q. ATP-Sensitive Potassium Channels in Rat Primary Afferent Neurons: The Effect of Neuropathic Injury and Gabapentin. Neurosci. Lett. 2003;343:185–189. doi: 10.1016/S0304-3940(03)00383-5. [DOI] [PubMed] [Google Scholar]
- 94.Wu X.F., Liu W.T., Liu Y.P., Huang Z.J., Zhang Y.K., Song X.J. Reopening of ATP-Sensitive Potassium Channels Reduces Neuropathic Pain and Regulates Astroglial Gap Junctions in the Rat Spinal Cord. Pain. 2011;152:2605–2615. doi: 10.1016/j.pain.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 95.Sakamaki G., Johnson K., Mensinger M., Hmu E., Klein A.H. Loss of SUR1 Subtype KATP Channels Alters Antinociception and Locomotor Activity after Opioid Administration. Behav. Brain Res. 2021;414 doi: 10.1016/j.bbr.2021.113467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fisher C., Johnson K., Okerman T., Jurgenson T., Nickell A., Salo E., Moore M., Doucette A., Bjork J., Klein A.H. Morphine Efficacy, Tolerance, and Hypersensitivity Are Altered After Modulation of SUR1 Subtype KATP Channel Activity in Mice. Front. Neurosci. 2019;13 doi: 10.3389/fnins.2019.01122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shi W., Cui N., Wu Z., Yang Y., Zhang S., Gai H., Zhu D., Jiang C. Lipopolysaccharides Up-Regulate Kir6.1/SUR2B Channel Expression and Enhance Vascular KATP Channel Activity via NF-KappaB-Dependent Signaling. J. Biol. Chem. 2010;285:3021–3029. doi: 10.1074/jbc.M109.058313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cao Z., Dai W., Zhang R., Chen L., Yang X., Hu L., Chiang L.Y., Liu W. Opening of the Adenosine Triphosphate-Sensitive Potassium Channel Attenuates Morphine Tolerance by Inhibiting JNK and Astrocyte Activation in the Spinal Cord. Clin. J. Pain. 2016;32:617–623. doi: 10.1097/AJP.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 99.Christensen S.L., Rasmussen R.H., Ernstsen C., La Cour S., David A., Chaker J., Haanes K.A., Christensen S.T., Olesen J., Kristensen D.M. CGRP-Dependent Signalling Pathways Involved in Mouse Models of GTN- Cilostazol- and Levcromakalim-Induced Migraine. Cephalalgia. 2021;41:1413–1426. doi: 10.1177/03331024211038884. [DOI] [PubMed] [Google Scholar]
- 100.Narita M., Suzuki T., Misawa M., Nagase H., Nabeshima A., Ashizawa T., Ozawa H., Saito T., Takahata N. Role of Central ATP-Sensitive Potassium Channels in the Analgesic Effect and Spinal Noradrenaline Turnover-Enhancing Effect of Intracerebroventricularly Injected Morphine in Mice. Brain Res. 1992;596:209–214. doi: 10.1016/0006-8993(92)91549-T. [DOI] [PubMed] [Google Scholar]
- 101.Saint-Martin C., Arnoux J.B., de Lonlay P., Bellanné-Chantelot C. KATP Channel Mutations in Congenital Hyperinsulinism. Semin. Pediatr. Surg. 2011;20:18–22. doi: 10.1053/j.sempedsurg.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 102.De Franco E., Saint-Martin C., Brusgaard K., Knight Johnson A.E., Aguilar-Bryan L., Bowman P., Arnoux J.B., Larsen A.R., May S., Greeley S.A.W., et al. Update of Variants Identified in the Pancreatic β-Cell KATP Channel Genes KCNJ11 and ABCC8 in Individuals with Congenital Hyperinsulinism and Diabetes. Hum. Mutat. 2020;41:884–905. doi: 10.1002/humu.23995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kane C., Shepherd R.M., Squires P.E., Johnson P.R.V., James R.F.L., Milla P.J., Aynsley-Green A., Lindley K.J., Dunne M.J. Loss of Functional KATP Channels in Pancreatic Beta-Cells Causes Persistent Hyperinsulinemic Hypoglycemia of Infancy. Nat. Med. 1996;2:1344–1347. doi: 10.1038/nm1296-1344. [DOI] [PubMed] [Google Scholar]
- 104.Li C., Ackermann A.M., Boodhansingh K.E., Bhatti T.R., Liu C., Schug J., Doliba N., Han B., Cosgrove K.E., Banerjee I., et al. Functional and Metabolomic Consequences of K ATP Channel Inactivation in Human Islets. Diabetes. 2017;66:1901–1913. doi: 10.2337/db17-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lontchi-Yimagou E., Sobngwi E., Matsha T.E., Kengne A.P. Diabetes Mellitus and Inflammation. Curr. Diab. Rep. 2013;13:435–444. doi: 10.1007/s11892-013-0375-y. [DOI] [PubMed] [Google Scholar]
- 106.Duncan B.B., Schmidt M.I. The Epidemiology of Low-Grade Chronic Systemic Inflammation and Type 2 Diabetes. Diabetes Technol. Ther. 2006;8:7–17. doi: 10.1089/dia.2006.8.7. [DOI] [PubMed] [Google Scholar]
- 107.Gier B., Krippeit-Drews P., Sheiko T., Aguilar-Bryan L., Bryan J., Düfer M., Drews G. Suppression of KATP Channel Activity Protects Murine Pancreatic Beta Cells against Oxidative Stress. J. Clin. Investig. 2009;119:3246–3256. doi: 10.1172/JCI38817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krippeit-Drews P., Lang F., Häussinger D., Drews G. H2O2 Induced Hyperpolarization of Pancreatic B-Cells. Pflug. Arch. 1994;426:552–554. doi: 10.1007/BF00378534. [DOI] [PubMed] [Google Scholar]
- 109.Maechler P., Jornot L., Wollheim C.B. Hydrogen Peroxide Alters Mitochondrial Activation and Insulin Secretion in Pancreatic Beta Cells. J. Biol. Chem. 1999;274:27905–27913. doi: 10.1074/jbc.274.39.27905. [DOI] [PubMed] [Google Scholar]
- 110.Nakazaki M., Kakei M., Koriyama N., Tanaka H. Involvement of ATP-Sensitive K+ Channels in Free Radical-Mediated Inhibition of Insulin Secretion in Rat Pancreatic Beta-Cells. Diabetes. 1995;44:878–883. doi: 10.2337/diab.44.8.878. [DOI] [PubMed] [Google Scholar]
- 111.Edalat A., Schulte-Mecklenbeck P., Bauer C., Undank S., Krippeit-Drews P., Drews G., Düfer M. Mitochondrial Succinate Dehydrogenase Is Involved in Stimulus-Secretion Coupling and Endogenous ROS Formation in Murine Beta Cells. Diabetologia. 2015;58:1532–1541. doi: 10.1007/s00125-015-3577-9. [DOI] [PubMed] [Google Scholar]
- 112.Benemei S., Fusi C., Trevisan G., Geppetti P. The TRPA1 Channel in Migraine Mechanism and Treatment. Br. J. Pharmacol. 2014;171:2552–2567. doi: 10.1111/bph.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Akerman S., Salvemini D., Romero-Reyes M. Targeting Reactive Nitroxidative Species in Preclinical Models of Migraine. Cephalalgia. 2021;41:1187–1200. doi: 10.1177/03331024211017884. [DOI] [PubMed] [Google Scholar]
- 114.Hambrock A., Löffler-Walz C., Kloor D., Delabar U., Horio Y., Kurachi Y., Quast U. ATP-Sensitive K+ Channel Modulator Binding to Sulfonylurea Receptors SUR2A and SUR2B: Opposite Effects of MgADP. Mol. Pharmacol. 1999;55:832–840. [PubMed] [Google Scholar]
- 115.Petersson J., Ryman T., Högestätt E.D. Vasodilator Effects of KRN2391, Levcromakalim and 3-Morpholino- Sydnonimin in Human Pial and Omental Arteries. Naunyn. Schmiedebergs. Arch. Pharmacol. 2000;362:68–73. doi: 10.1007/s002100000270. [DOI] [PubMed] [Google Scholar]
- 116.Jansen-Olesen I., Mortensen C.H., El-Bariaki N., Ploug K.B. Characterization of KATP-Channels in Rat Basilar and Middle Cerebral Arteries: Studies of Vasomotor Responses and MRNA Expression. Eur. J. Pharmacol. 2005;523:109–118. doi: 10.1016/j.ejphar.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 117.Ploug K.B., Boni L.J., Baun M., Hay-Schmidt A., Olesen J., Jansen-Olesen I. KATP Channel Expression and Pharmacological in Vivo and in Vitro Studies of the KATP Channel Blocker PNU-37883A in Rat Middle Meningeal Arteries. Br. J. Pharmacol. 2008;154:72–81. doi: 10.1038/bjp.2008.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carlsen J.E., Kardel T., Jensen H., Tangø M., Trap-Jensen J. Pinacidil, a New Vasodilator: Pharmacokinetics and Pharmacodynamics of a New Retarded Release Tablet in Essential Hypertension. Eur. J. Clin. Pharmacol. 1983;25:557–561. doi: 10.1007/BF00542128. [DOI] [PubMed] [Google Scholar]
- 119.Ward J.W., McBurney A., Farrow P.R., Sharp P. Pharmacokinetics and Hypotensive Effect in Healthy Volunteers of Pinacidil, a New Potent Vasodilator. Eur. J. Clin. Pharmacol. 1984;26:603–608. doi: 10.1007/BF00543493. [DOI] [PubMed] [Google Scholar]
- 120.Clapham J.C., Trail B.K., Hamilton T.C. K+ Channel Activators, Acute Glucose Tolerance and Glibenclamide-Induced Hypoglycaemia in the Hypertensive Rat. Eur. J. Pharmacol. 1994;257:79–85. doi: 10.1016/0014-2999(94)90697-1. [DOI] [PubMed] [Google Scholar]
- 121.Mannhold R. KATP Channel Openers: Structure-Activity Relationships and Therapeutic Potential. Med. Res. Rev. 2004;24:213–266. doi: 10.1002/med.10060. [DOI] [PubMed] [Google Scholar]
- 122.Wolff F. Diazoxide Hyperglycaemia and Its Continued Relief by Tolbutamide. Lancet. 1964;1:309–310. doi: 10.1016/S0140-6736(64)92414-6. [DOI] [PubMed] [Google Scholar]
- 123.Rubin A.A., Roth F.E., Taylor R.M., Rosenkilde H. Pharmacology of Diazoxide, an Antihypertensive, Nondiuretic Benzothiadiazine. J. Pharmacol. Exp. Ther. 1962;136:344–352. [PubMed] [Google Scholar]
- 124.Lodwick D., Rainbow R.D., Rubaiy H.N., Al Johi M., Vuister G.W., Norman R.I. Sulfonylurea Receptors Regulate the Channel Pore in ATP-Sensitive Potassium Channels via an Intersubunit Salt Bridge. Biochem. J. 2014;464:343–354. doi: 10.1042/BJ20140273. [DOI] [PubMed] [Google Scholar]
- 125.Maruthur N.M., Tseng E., Hutfless S., Wilson L.M., Suarez-Cuervo C., Berger Z., Chu Y., Iyoha E., Segal J.B., Bolen S. Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes: A Systematic Review and Meta-Analysis. Ann. Intern. Med. 2016;164:740–751. doi: 10.7326/M15-2650. [DOI] [PubMed] [Google Scholar]
- 126.Coghlan M.J., Carroll W.A., Gopalakrishnan M. Recent Developments in the Biology and Medicinal Chemistry of Potassium Channel Modulators: Update from a Decade of Progress. J. Med. Chem. 2001;44:1627–1653. doi: 10.1021/jm000484+. [DOI] [PubMed] [Google Scholar]
- 127.Gribble F.M., Tucker S.J., Seino S., Ashcroft F.M. Tissue Specificity of Sulfonylureas Studies on Cloned Cardiac and β- Cell K(ATP) Channels. Diabetes. 1998;47:1412–1418. doi: 10.2337/diabetes.47.9.1412. [DOI] [PubMed] [Google Scholar]
- 128.Liu S.Y., Tian H.M., Liao D.Q., Chen Y.F., Gou Z.P., Xie X.Y., Li X.J. The Effect of Gliquidone on KATP Channels in Pancreatic β-Cells, Cardiomyocytes, and Vascular Smooth Muscle Cells. Diabetes Res. Clin. Pract. 2015;109:334–339. doi: 10.1016/j.diabres.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 129.Glibenclamide|Ligand Activity Charts|IUPHAR/BPS Guide to PHARMACOLOGY. [(accessed on 26 June 2022)]. Available online: https://www.guidetopharmacology.org/GRAC/LigandActivityRangeVisForward?ligandId=2414.
- 130.Wang Y., Yu L., Cui N., Jin X., Zhu D., Jiang C. Differential Sensitivities of the Vascular K(ATP) Channel to Various PPAR Activators. Biochem. Pharmacol. 2013;85:1495–1503. doi: 10.1016/j.bcp.2013.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yu L., Jin X., Cui N., Wu Y., Shi Z., Zhu D., Jiang C. Rosiglitazone Selectively Inhibits KATP Channels by Acting on the KIR6 Subunit. Br. J. Pharmacol. 2012;167:26. doi: 10.1111/j.1476-5381.2012.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Humphrey S.J. Pharmacology of the K-ATP Channel Blocking Morpholinoguanidine PNU- 37883A. Cardiovasc. Drug Rev. 1999;17:295–328. doi: 10.1111/j.1527-3466.1999.tb00022.x. [DOI] [Google Scholar]
- 133.D’Arcy V., Laher M., McCoy D., Sullivan P., Walsh C.H., Hickey M.P. Pinacidil, a New Vasodilator, in the Treatment of Mild to Moderate Essential Hypertension. Eur. J. Clin. Pharmacol. 1985;28:347–349. doi: 10.1007/BF00543335. [DOI] [PubMed] [Google Scholar]
- 134.Kidney J.C., Fuller R.W., Worsdell Y.M., Lavender E.A., Chung K.F., Barnes P.J. Effect of an Oral Potassium Channel Activator, BRL 38227, on Airway Function and Responsiveness in Asthmatic Patients: Comparison with Oral Salbutamol. Thorax. 1993;48:130–133. doi: 10.1136/thx.48.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ashina M., Hansen J.M., Olesen J. Pearls and Pitfalls in Human Pharmacological Models of Migraine: 30 Years’ Experience. Cephalalgia. 2013;33:540–553. doi: 10.1177/0333102412475234. [DOI] [PubMed] [Google Scholar]
- 136.Al-Karagholi M.A.-M., Ghanizada H., Hansen J.M., Skovgaard L.T., Olesen J., Larsson H.B.W., Amin F.M., Ashina M. Levcromakalim, an Adenosine Triphosphate-Sensitive Potassium Channel Opener, Dilates Extracerebral but Not Cerebral Arteries. Headache. 2019;59:1468–1480. doi: 10.1111/head.13634. [DOI] [PubMed] [Google Scholar]
- 137.Al-Karagholi M.A.M., Ghanizada H., Nielsen C.A.W., Ansari A., Gram C., Younis S., Vestergaard M.B., Larsson H.B.W., Skovgaard L.T., Amin F.M., et al. Cerebrovascular Effects of Glibenclamide Investigated Using High-Resolution Magnetic Resonance Imaging in Healthy Volunteers. J. Cereb. Blood Flow Metab. 2021;41:1328–1337. doi: 10.1177/0271678X20959294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gozalov A., Petersen K.A., Mortensen C., Jansen-Olesen I., Klaerke D., Olesen J. Role of KATP Channels in the Regulation of Rat Dura and Pia Artery Diameter. Cephalalgia. 2005;25:249–260. doi: 10.1111/j.1468-2982.2004.00848.x. [DOI] [PubMed] [Google Scholar]
- 139.Ploug K.B., Edvinsson L., Olesen J., Jansen-Olesen I. Pharmacological and Molecular Comparison of KATP Channels in Rat Basilar and Middle Cerebral Arteries. Eur. J. Pharmacol. 2006;553:254–262. doi: 10.1016/j.ejphar.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 140.Rasmussen R.H., Jansen-Olesen I., Kristensen D.M., Christensen S.L. Ex Vivo Release of Calcitonin Gene-Related Peptide from the Trigeminovascular System in Rodents|Protocol. J. Vis. Exp. 2022;183 doi: 10.3791/63723. [DOI] [PubMed] [Google Scholar]
- 141.Ploug K.B., Amrutkar D.V., Baun M., Ramachandran R., Iversen A., Lund T.M., Gupta S., Hay-Schmidt A., Olesen J., Jansen-Olesen I. K ATP Channel Openers in the Trigeminovascular System. Cephalalgia. 2012;32:55–65. doi: 10.1177/0333102411430266. [DOI] [PubMed] [Google Scholar]
- 142.Russell F.A., King R., Smillie S.-J., Kodji X., Brain S.D. Calcitonin Gene-Related Peptide: Physiology and Pathophysiology. Physiol. Rev. 2014;94:1099–1142. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sicuteri F. Mast cells and their active substances: Their role in the pathogenesis of migraine. Headache. 1963;3:86–92. doi: 10.1111/j.1526-4610.1963.hed0303086.x. [DOI] [PubMed] [Google Scholar]
- 144.Bates E.A., Nikai T., Brennan K.C., Fu Y.-H., Charles A.C., Basbaum A.I., Ptácek L.J., Ahn A.H. Sumatriptan Alleviates Nitroglycerin-Induced Mechanical and Thermal Allodynia in Mice. Cephalalgia. 2010;30:170–178. doi: 10.1111/j.1468-2982.2009.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pradhan A.A., Smith M.L., McGuire B., Tarash I., Evans C.J., Charles A. Characterization of a Novel Model of Chronic Migraine. PAIN®. 2014;155:269–274. doi: 10.1016/j.pain.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ernstsen C., Christensen S.L., Rasmussen R.H., Nielsen B.S., Jansen-Olesen I., Olesen J., Kristensen D.M. The PACAP Pathway Is Independent of CGRP in Mouse Models of Migraine: Possible New Drug Target? Brain. 2022;145:2450–2460. doi: 10.1093/brain/awac040. [DOI] [PubMed] [Google Scholar]
- 147.Bruch L., Bychkov R., Kästner A., Bülow T., Ried C., Gollasch M., Baumann G., Luft F.C., Haller H. Pituitary Adenylate-Cyclase-Activating Peptides Relax Human Coronary Arteries by Activating K(ATP) and K(Ca) Channels in Smooth Muscle Cells. J. Vasc. Res. 1997;34:11–18. doi: 10.1159/000159197. [DOI] [PubMed] [Google Scholar]
- 148.Hayabuchi Y., Davies N.W., Standen N.B. Angiotensin II Inhibits Rat Arterial KATP Channels by Inhibiting Steady-State Protein Kinase A Activity and Activating Protein Kinase Cε. J. Physiol. 2001;530:193. doi: 10.1111/j.1469-7793.2001.0193l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Meisheri K.D., Humphrey S.J., Khan S.A., Cipkus-Dubray L.A., Smith M.P., Jones A.W. 4-Morpholinecarboximidine-N-1-Adamantyl-N’-Cyclohexylhydrochloride (U- 37883A): Pharmacological Characterization of a Novel Antagonist of Vascular ATP-Sensitive K+ Channel Openers. J. Pharmacol. Exp. Ther. 1993;266:655–665. [PubMed] [Google Scholar]
- 150.Armstead W.M. Role of Nitric Oxide, Cyclic Nucleotides, and the Activation of ATP-Sensitive K+ Channels in the Contribution of Adenosine to Hypoxia-Induced Pial Artery Dilation. J. Cereb. Blood Flow Metab. 1997;17:100–108. doi: 10.1097/00004647-199701000-00013. [DOI] [PubMed] [Google Scholar]
- 151.Oshinsky M.L., Sanghvi M.M., Maxwell C.R., Gonzalez D., Spangenberg R.J., Cooper M., Silberstein S.D. Spontaneous Trigeminal Allodynia in Rats: A Model of Primary Headache. Headache. 2012;52:1336–1349. doi: 10.1111/j.1526-4610.2012.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Munro G., Petersen S., Jansen-Olesen I., Olesen J. A Unique Inbred Rat Strain with Sustained Cephalic Hypersensitivity as a Model of Chronic Migraine-like Pain. Sci. Rep. 2018;8:1836. doi: 10.1038/s41598-018-19901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Al-Karagholi M.A.M., Ghanizada H., Kokoti L., Paulsen J.S., Hansen J.M., Ashina M. Effect of KATP Channel Blocker Glibenclamide on Levcromakalim-Induced Headache. Cephalalgia. 2020;40:1045–1054. doi: 10.1177/0333102420949863. [DOI] [PubMed] [Google Scholar]
- 154.Coskun H., Elbahi F.A., Al-Karagholi M.A.M., Ghanizada H., Sheykhzade M., Ashina M. The Effect of KATP Channel Blocker Glibenclamide on CGRP-Induced Headache and Hemodynamic in Healthy Volunteers. Front. Physiol. 2021;12:652136. doi: 10.3389/fphys.2021.652136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kokoti L., Al-Mahdi Al-Karagholi M., Elbahi F.A., Coskun H., Ghanizada H., Amin F.M., Ashina M. Effect of KATP Channel Blocker Glibenclamide on PACAP38-Induced Headache and Hemodynamic. Cephalalgia. 2022;42:846–858. doi: 10.1177/03331024221080574. [DOI] [PubMed] [Google Scholar]
- 156.Home—ClinicalTrials.Gov. [(accessed on 27 June 2022)]; Available online: https://www.clinicaltrials.gov/
- 157.Nair A., Jacob S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016;7:27. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Reagan-Shaw S., Nihal M., Ahmad N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 159.Lukas G., Brindle S.D., Greengard P. The Route of Absorption of Intraperitoneally Administered Compounds. J. Pharmacol. Exp. Ther. 1971;178:562–564. [PubMed] [Google Scholar]
- 160.Remedi M.S., Nichols C.G. Chronic Antidiabetic Sulfonylureas In Vivo: Reversible Effects on Mouse Pancreatic β-Cells. PLoS Med. 2008;5:e206. doi: 10.1371/journal.pmed.0050206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lahmann C., Kramer H.B., Ashcroft F.M. Systemic Administration of Glibenclamide Fails to Achieve Therapeutic Levels in the Brain and Cerebrospinal Fluid of Rodents. PLoS ONE. 2015;10:1–18. doi: 10.1371/journal.pone.0134476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ashcroft F.M., Gribble F.M. ATP-Sensitive K+ Channels and Insulin Secretion: Their Role in Health and Disease. Diabetologia. 1999;42:903–919. doi: 10.1007/s001250051247. [DOI] [PubMed] [Google Scholar]
- 163.Sheykhzade M., Berg Nyborg N.C. Mechanism of CGRP-Induced Relaxation in Rat Intramural Coronary Arteries. Br. J. Pharmacol. 2001;132:1235–1246. doi: 10.1038/sj.bjp.0703936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Haanes K.A., Edvinsson L. Hyperpolarization through ATP-Sensitive Potassium Channels; Relevance to Migraine Pathology. Brain. 2020;143:e13. doi: 10.1093/brain/awaa003. [DOI] [PubMed] [Google Scholar]
- 165.Al-Mahdi Al-Karagholi M., Olesen J., Ashina M. Reply: Hyperpolarization through ATP-Sensitive Potassium Channels; Relevance to Migraine Pathology. Brain. 2020;143:E14. doi: 10.1093/brain/awaa004. [DOI] [PubMed] [Google Scholar]
- 166.Tsantoulas C., Lainez S., Wong S., Mehta I., Vilar B., McNaughton P.A. Hyperpolarization-Activated Cyclic Nucleotide-Gated 2 (HCN2) Ion Channels Drive Pain in Mouse Models of Diabetic Neuropathy. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aam6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bernard Healey S.A., Scholtes I., Abrahams M., McNaughton P.A., Menon D.K., Lee M.C. Role of Hyperpolarization-Activated Cyclic Nucleotide-Gated Ion Channels in Neuropathic Pain: A Proof-of-Concept Study of Ivabradine in Patients with Chronic Peripheral Neuropathic Pain. Pain Rep. 2021;6:e967. doi: 10.1097/PR9.0000000000000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Young G.T., Emery E.C., Mooney E.R., Tsantoulas C., McNaughton P.A. Inflammatory and Neuropathic Pain Are Rapidly Suppressed by Peripheral Block of Hyperpolarisation-Activated Cyclic Nucleotide-Gated Ion Channels. Pain. 2014;155:1708–1719. doi: 10.1016/j.pain.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 169.Tu H., Deng L., Sun Q., Yao L., Han J.S., Wan Y. Hyperpolarization-Activated, Cyclic Nucleotide-Gated Cation Channels: Roles in the Differential Electrophysiological Properties of Rat Primary Afferent Neurons. J. Neurosci. Res. 2004;76:713–722. doi: 10.1002/jnr.20109. [DOI] [PubMed] [Google Scholar]
- 170.Momin A., Cadiou H., Mason A., Mcnaughton P.A. Role of the Hyperpolarization-Activated Current Ih in Somatosensory Neurons. J. Physiol. 2008;586:5911–5929. doi: 10.1113/jphysiol.2008.163154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Al-Karagholi M.A.M., Ghanizada H., Hansen J.M., Aghazadeh S., Skovgaard L.T., Olesen J., Ashina M. Extracranial Activation of ATP-Sensitive Potassium Channels Induces Vasodilation without Nociceptive Effects. Cephalalgia. 2019;39:1789–1797. doi: 10.1177/0333102419888490. [DOI] [PubMed] [Google Scholar]
- 172.Zhang J., Yao J., Rong M. Editorial: Role of Ion Channels in Pain. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.884665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Perricone S.C., Humphrey S.J., Skaletzky L.L., Graham B.E., Zandt R.A., Zins G.R. Synthesis and Diuretic Activity of Alkyl- and Arylguanidine Analogs of N,N’-Dicyclohexyl-4-Morpholinecarboxamidine in Rats and Dogs. J. Med. Chem. 1994;37:3693–3700. doi: 10.1021/jm00048a005. [DOI] [PubMed] [Google Scholar]
- 174.Humphrey S.J., Smith M.P., Cimini M.G., Buchanan L.V., Gibson J.K., Khan S.A., Meisheri K.D. Cardiovascular Effects of the K-ATP Channel Blocker U-37883A and Structurally Related Morpholinoguanidines. Methods Find. Exp. Clin. Pharmacol. 1996;18:247–260. [PubMed] [Google Scholar]
- 175.Chutkow W.A., Pu J., Wheeler M.T., Wada T., Makielski J.C., Burant C.F., McNally E.M. Episodic Coronary Artery Vasospasm and Hypertension Develop in the Absence of Sur2 K(ATP) Channels. J. Clin. Investig. 2002;110:203–208. doi: 10.1172/JCI0215672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kakkar R., Ye B., Stoller D.A., Smelley M., Shi N.Q., Galles K., Hadhazy M., Makielski J.C., McNally E.M. Spontaneous Coronary Vasospasm in KATP Mutant Mice Arises from a Smooth Muscle-Extrinsic Process. Circ. Res. 2006;98:682–689. doi: 10.1161/01.RES.0000207498.40005.e7. [DOI] [PubMed] [Google Scholar]
- 177.Kalia J., Milescu M., Salvatierra J., Wagner J., Klint J.K., King G.F., Olivera B.M., Bosmans F. From Foe to Friend: Using Animal Toxins to Investigate Ion Channel Function. J. Mol. Biol. 2015;427:158–175. doi: 10.1016/j.jmb.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hutchings C.J., Colussi P., Clark T.G. Ion Channels as Therapeutic Antibody Targets. MAbs. 2019;11:265–296. doi: 10.1080/19420862.2018.1548232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gross G.J., Peart J.N. KATP Channels and Myocardial Preconditioning: An Update. Am. J. Physiol. Hear. Circ. Physiol. 2003;285:921–930. doi: 10.1152/ajpheart.00421.2003. [DOI] [PubMed] [Google Scholar]
- 180.Antzelevitch C., Di Diego J.M. Role of K+ Channel Activators in Cardiac Electrophysiology and Arrhythmias. Circulation. 1992;85:1627–1629. doi: 10.1161/01.CIR.85.4.1627. [DOI] [PubMed] [Google Scholar]
- 181.King D.R., Hardin K.M., Hoeker G.S., Poelzing S. Re-Evaluating Methods Reporting Practices to Improve Reproducibility: An Analysis of Methodological Rigor for the Langendorff Whole-Heart Technique. Am. J. Physiol. Circ. Physiol. 2022 doi: 10.1152/ajpheart.00164.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.