Abstract
This study investigated the long-term functional changes in patients with moderate-to-severe ischemic stroke. In addition, we investigated whether there was a difference between the modified Barthel Index (MBI) and Functional Independence Measure (FIM) according to severity. To evaluate the changes in the long-term functional independence of the subjects, six evaluations were conducted over 2 years, and the evaluation was performed using MBI and FIM. A total of 798 participants participated in this study, of which 673 were classified as moderate and 125 as severe. During the first 3 months, the moderate group showed greater recovery than the severe group. The period of significant change in the National Institutes of Health Stroke Scale (NIHSS) score was up to 6 months after onset in the moderate group, and up to 3 months after onset in the severe group. In the severe group, MBI evaluation showed significant changes up to 6 months after onset, whereas FIM showed significant changes up to 18–24 months. Our results showed that functional recovery of patients with ischemic stroke in the 3 months after onset was greater in the moderate group than in the severe group. FIM is more appropriate than MBI for evaluating the functional status of patients with severe stroke.
Keywords: functional independence measure, independence, modified Barthel Index, severity, stroke
1. Introduction
Stroke is a major cause of mortality and morbidity in the United States. It is the second leading cause of death worldwide and a major cause of disability [1,2,3]. Ischemic stroke is the most common type of stroke and occurs when a blood vessel in the neck or brain is blocked [4]. It is estimated that 25% to 75% of the 50 million stroke survivors need help in their daily lives [5]. Such functional dependence of stroke patients can be a burden to their family members and caregivers [6]. Functional independence is a significant goal for stroke patients and a major focus in studies of stroke recovery [7,8].
Despite the neurological damage and impairment caused by stroke, most stroke patients achieve functional recovery over time [9,10]. Such functional recovery in stroke patients shows notable changes during the first 3 months after stroke onset and reaches a plateau between 3 and 6 months [11,12,13,14]. Several studies have shown that the functional status is significantly improved even after 6 months from stroke onset [15,16,17,18]. In another study, functional recovery was achieved by 12 months in 30% of stroke patients [19].
Recently, there have been major advances in the care and rehabilitation of stroke. Therefore, updated prognostic data are needed for the functional recovery of stroke patients [20]. The results of long-term functional recovery after stroke and the knowledge of such recovery periods are critical for determining proper treatment and the rehabilitation period [21,22]. Examining changes in the long-term functional recovery after 6 months from the onset of stroke may elucidate the full extent of recovery and the point of plateau where there is a much less dynamic rate.
The functional recovery of stroke patients is also associated with the initial severity of impairment [23,24]. The severity of initial neurological damage can be quantitatively measured and classified by the National Institutes of Health Stroke Scale (NIHSS) [25]. Moderate and severe stroke types exhibit a low degree and slow rate of functional recovery compared with mild stroke [26,27]. Although there have been several studies on the long-term functional recovery of stroke, studies comparing the changes in recovery according to severity are still lacking. Severe neurological damage at the initial stage of stroke has negative effects on functional recovery [28,29,30]. Therefore, changes in functional recovery should be closely monitored in patients with moderate and severe stroke.
The Barthel Index (BI) and Functional Independence Measure (FIM) are the most highly recommended tools for assessing functional recovery in patients with stroke. These tools are credible and have been proven to have high validity for measuring the functional outcome of stroke patients [31,32,33]. Glowinski and Blazejewski (2020) proposed a model for the evaluation of patient conditions and the rehabilitation progress [34]. The degree of functional changes depends on the severity of stroke, and a tool that enables a more sensitive assessment of such functional changes needs to be explored.
In this study, we aimed to examine long-term functional changes in patients with moderate and severe ischemic stroke for 2 years from onset and to determine the differences among the results according to stroke severity by comparing the modified Barthel Index (MBI) and FIM scores.
2. Materials and Methods
2.1. The Korean Stroke Cohort for Functioning and Rehabilitation (KOSCO)
KOSCO is a large multicenter prospective cohort study of patients with primary stroke who are admitted to university hospitals in nine regions of South Korea. Patients with primary stroke who were admitted to the hospital between August 2012 and June 2014 were recruited in the current study, although the KOSCO study is still ongoing. KOSCO is a 10-year longitudinal follow-up study investigating the factors that affect residual disabilities, activity limitation, and long-term quality of life in patients with first-time strokes.
The subjects were patients aged ≥19 years (the standard age for adults in Korea) who were diagnosed with primary stroke, admitted to the hospital within 7 days of onset, and met the selection criteria. Patients with a history of stroke, those diagnosed with transient ischemic attack, and those with traumatic intracerebral hemorrhage were excluded.
The patients underwent a face-to-face baseline evaluation 7 days after onset. For the assessment, the tools that could determine the functional status of stroke patients, such as the NIHSS, MBI, FIM, Korean Mini-Mental State Examination (K-MMSE), Fugl-Meyer Assessment (FMA), Modified Ashworth Scale (MAS), 9-hole pegboard test, Functional Ambulatory Category (FAC), American Speech Language Hearing Association-National Outcome Measurement System Swallowing Scale (ASHA-NOMS), Korean Frenchay Aphasia Screening Test (K-FAST), and Modified Rankin Scale (MRS) were used. Additionally, the health conditions of patients and guardians, their mood, and changes in their quality of life were assessed. After the initial baseline assessment, the subjects were evaluated at hospital discharge and 3, 6, 12, 18, and 24 months after onset. Follow-up assessments were performed each year during the study period. During this time, the assessment was continued, except for those who withdrew their consent or died. Written consent was obtained from all participants prior to the study. The study protocol was approved by the ethics committee of each hospital.
2.2. Study Population
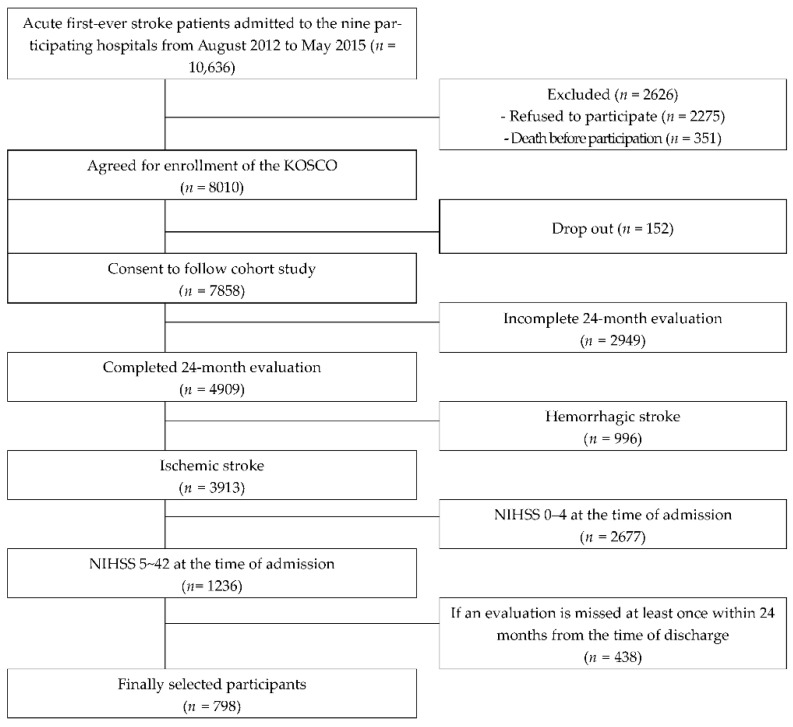
During the study period, 10,636 patients were admitted to each hospital. Among them, 7858 people agreed to long-term follow-up after discharge, and 4909 people completed the follow-up evaluation up to 24 months. Among them, patients diagnosed with ischemic stroke (n = 3913) were selected for the study, after excluding those with hemorrhagic stroke. In addition, those who had mild stroke at admission were excluded, and only patients with moderate and severe stroke were included. Finally, 798 patients were selected for the study (Figure 1).
Figure 1.
Flow diagram of the study participants.
2.3. Stroke Severity and Functional Assessments
The initial stroke severity was derived from a medical record review of the NIHSS scores obtained on patient arrival at the hospital. The assessment was performed at each time point, from hospital discharge to 24 months after onset. NIHSS is a tool that can quantitatively measure the level of neurological damage in stroke patients. The total NIHSS scores were classified according to severity as mild (0–4), moderate (5–15), or severe (16–42).
MBI and FIM, which can evaluate the level of functional independence, were used in this study. The assessment was performed at each time point, from hospital discharge to 24 months after onset. The Barthel Index was first published in 1965 and consists of 10 items. Shah et al. (1989) further improved the discriminative power of the instrument by standardizing the rating criteria and scale into a five-point Likert format. The MBI, as it is known, has increased the sensitivity of the instrument both at the item and scale levels, and yielded a higher content reliability and internal consistency [33,35,36]. MBI consists of 10 daily activity items and can be analyzed using a total score ranging from 0 to 100. Each item is scored on a 5-level scale, from 1 (completely dependent) to 5 (completely independent) [33]. FIM consists of 18 daily activity items with a total score ranging from 18 to 126. Each item is rated on a 7-point scale ranging from 1 (completely dependent) to 7 (independent), according to the level of independence. A total score of ≤108 indicates the need for help from others or restrictions in activities [37].
2.4. Statistical Analysis
Descriptive statistics were used to analyze the demographic and clinical characteristics of the patients and the characteristics of primary stroke. Changes in MBI, FIM, and NIHSS scores in the moderate and severe groups at each assessment time point were analyzed using one-way repeated-measures ANOVA. Statistical analysis was performed using SPSS for Windows 22.0. Statistical significance was set at p < 0.05.
3. Results
3.1. Patients Characteristics
The final study included 798 patients according to the selection criteria. Among these 798 patients, 673 were categorized into the moderate group and 125 into the severe group. Regarding sex, there were more men (61%) in the moderate group, whereas there were more women (50.4%) in the severe group. In both groups, those who completed 12 years of education accounted for the highest percentage in the education category, and large-artery damage showed the highest percentage in the ischemia type category. The mean NIHSS scores at admission were 8.14 in the moderate group and 19.47 in the severe group. The mean K-MMSE score at admission was 20.19 in the moderate group and 14.09 in the severe group (Table 1).
Table 1.
Baseline characteristics of study population according to the severity.
| Moderate Group (n = 673) | Severe Group (n = 125) | ||
|---|---|---|---|
| Age (mean ± SD) | 65.98 ± 12.25 | 69.35 ± 10.81 | |
| Sex (%) | Male | 411 (61.1) | 62 (49.6) |
| Female | 262 (38.9) | 63 (50.4) | |
| Education (%) | Uneducated | 90 (13.4) | 20 (16.0) |
| 6 years | 140 (20.8) | 23 (18.4) | |
| 9 years | 128 (19.0) | 28 (22.4) | |
| 12 years | 196 (29.1) | 30 (24.0) | |
| Over 14 years | 90 (13.4) | 20 (16.0) | |
| N/A | 29 (4.3) | 4 (3.2) | |
| Ischemic Type (%) | Large artery atherosclerosis |
376 (55.9) | 65 (52.0) |
| Small vessel occlusion |
105 (15.6) | 12 (9.6) | |
| Cardio-embolism | 99 (14.7) | 32 (25.6) | |
| Other determined | 33 (4.9) | 7 (5.6) | |
| Undetermined ischemic stroke |
60 (8.9) | 9 (7.2) | |
| NIHSS at the time of hospitalization (mean ± SD) | 8.14 ± 2.99 | 19.47 ± 3.60 | |
| K-MMSE at the time of hospitalization (mean ± SD) | 20.19 ± 9.11 | 14.09 ± 11.33 | |
Data are presented as n (%) or as the mean ± standard deviation. SD—standard deviation, NIHSS—National Institute of Health Stroke Scale, K-MMSE—Korean Mini-Mental State Examination.
3.2. Changes in NIHSS Scores in the Moderate and Severe Groups
Changes in the NIHSS scores were examined in the moderate and severe groups at different time points. In the moderate group, there were significant changes between discharge and 3 months after onset (p < 0.000), and between discharge and 3–6 months (p < 0.006) after onset. In the severe group, there were significant changes between discharge and 3 months after onset (p < 0.001) (Table 2).
Table 2.
Changes in the mean NIHSS total scores by time period for moderate and severe groups.
| Time (I) | Time (J) | MD (I-J) | SE | p Value | 95% CI | |
|---|---|---|---|---|---|---|
| Moderate group (n = 673) |
Discharge | 3 months | 1.218 * | 0.117 | 0.000 * | 0.988–1.449 |
| 3 months | 6 months | 0.229 * | 0.083 | 0.006 * | 0.065–0.397 | |
| 6 months | 12 months | 0.080 | 0.096 | 0.405 | −0.109–0.269 | |
| 12 months | 18 months | 0.131 | 0.076 | 0.085 | −0.018–0.280 | |
| 18 months | 24 months | −0.083 | 0.072 | 0.250 | −0.225–0.059 | |
| Severe group (n = 125) |
Discharge | 3 months | 1.312 * | 0.383 | 0.001 * | 0.554–2.070 |
| 3 months | 6 months | 0.376 | 0.286 | 0.191 | −0.190–0.942 | |
| 6 months | 12 months | −0.096 | 0.357 | 0.789 | −0.804–0.612 | |
| 12 months | 18 months | −0.008 | 0.311 | 0.979 | −0.623–0.607 | |
| 18 months | 24 months | −0.448 | 0.276 | 0.107 | −0.994–0.098 |
* p < 0.05. MD—mean difference, SE—standard error, CI—confidence interval.
3.3. Long-Term Changes in Functional Independence in the Moderate and Severe Groups
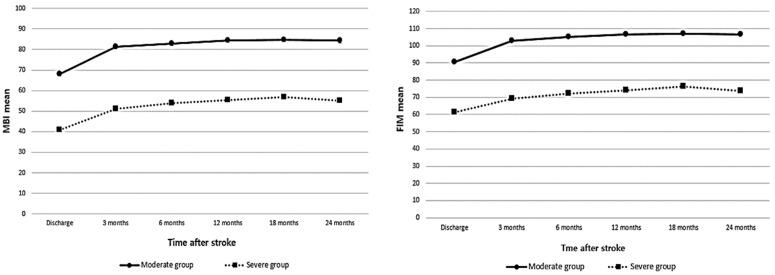
Changes in functional independence in patients with moderate and severe stroke over 24 months from hospital discharge were analyzed using the mean MBI and FIM scores. The results showed that the changes in the total scores of both measures were largest at 3 months after onset in both groups. In the moderate group, MBI showed improvement from 68.07 at discharge to 81.28 after onset. FIM also improved from 90.39 at discharge to 102.93 at 3 months after onset. In the severe group, MBI improved from 40.74 at discharge to 51.04 at 3 months after onset. FIM also improved from 61.33 at discharge to 69.12 at 3 months after onset (Table 3).
Table 3.
Changes in the mean of NIHSS total scores by time period for moderate and severe groups.
| Time | MBI | FIM | ||
|---|---|---|---|---|
| Moderate Group (n = 673) |
Severe Group (n = 125) |
Moderate Group (n = 673) |
Severe Group (n = 125) |
|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Discharge | 68.07 ± 28.04 | 40.74 ± 35.62 | 90.39 ± 28.02 | 61.33 ± 36.33 |
| 3 months | 81.28 ± 26.94 | 51.04 ± 38.09 | 102.93 ± 28.32 | 69.1 ± 38.88 |
| 6 months | 82.85 ± 26.90 | 53.94 ± 37.76 | 105.04 ± 28.05 | 72.20 ± 38.22 |
| 12 months | 84.48 ± 27.00 | 55.26 ± 38.94 | 106.57 ± 28.42 | 74.10 ± 39.33 |
| 18 months | 84.79 ± 27.52 | 56.95 ± 39.45 | 107.02 ± 28.77 | 76.27 ± 40.33 |
| 24 months | 84.21 ± 28.65 | 55.10 ± 40.22 | 106.80 ± 29.74 | 73.67 ± 41.24 |
MBI—modified Barthel Index, FIM—Functional Independence Measure, SD—standard deviation.
Functional recovery in the moderate and severe groups persisted up to 18 months after onset. The level of recovery was more significantly increased in the severe group than in the moderate group 3 months after onset. However, the severe group showed a more significant recovery from 3 to 18 months after onset. Functional recovery decreased between 18 and 24 months, with a more significant decrease in the severe group than in the moderate group (Figure 2).
Figure 2.
Changes in total MBI and FIM scores in moderate and severe patients.
3.4. Comparison of MBI and FIM Scores at Each Time Point in the Moderate and Severe Groups
The mean MBI and FMI scores were compared at each time point between the moderate and severe groups. The moderate group did not show any differences between the MBI and FIM scores. In both tools, significant changes were observed between discharge and 3, 3–6, and 6–12 months after onset. In the severe group, the MBI and FIM results significantly differed. MBI showed significant changes between discharge and 3 (p < 0.001) and 3–6 months after onset (p < 0.034), whereas FIM showed significant changes between discharge and 3 (p < 0.001), 3–6 (p < 0.017), 12–18 (p < 0.032), and 18–24 (p < 0.005) months after onset (Table 4).
Table 4.
Changes in the mean MBI and FIM total scores by time period for moderate and severe groups.
| Time (I) | Time (J) | MD (J-I) | SE | p Value | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Moderate group (n = 673) |
MBI | Discharge | 3 months | 13.214 * | 0.812 | 0.000 * | 11.6202–14.808 |
| 3 months | 6 months | 1.571 * | 0.405 | 0.000 * | 0.776–2.365 | ||
| 6 months | 12 months | 1.630 * | 0.440 | 0.000 * | 0.766–2.494 | ||
| 12 months | 18 months | 0.308 | 0.367 | 0.402 | 0.413–1.028 | ||
| 18 months | 24 months | −0.579 | 0.386 | 0.134 | −1.338–0.179 | ||
| FIM | Discharge | 3 months | 12.535 * | 0.817 | 0.000 * | 10.930–14.140 | |
| 3 months | 6 months | 2.108 * | 0.435 | 0.000 * | 1.255–2.962 | ||
| 6 months | 12 months | 1.533 * | 0.450 | 0.001 * | 0.649–2.418 | ||
| 12 months | 18 months | 0.447 | 0.381 | 0.241 | −0.300–1.195 | ||
| 18 months | 24 months | −0.214 | 0.374 | 0.568 | −0.949–0.521 | ||
| Severe group (n = 125) |
MBI | Discharge | 3 months | 10.296 * | 1.467 | 0.000 * | 7.393–13.199 |
| 3 months | 6 months | 2.896 * | 1.351 | 0.034 * | 0.221–5.571 | ||
| 6 months | 12 months | 1.328 | 1.106 | 0.232 | −0.861–3.517 | ||
| 12 months | 18 months | 1.688 | 1.067 | 0.116 | −0.423–3.799 | ||
| 18 months | 24 months | −1.856 | 1.135 | 0.105 | −4.103–0.391 | ||
| FIM | Discharge | 3 months | 7.792 * | 1.461 | 0.000 * | 4.900–10.684 | |
| 3 months | 6 months | 3.080 * | 1.276 | 0.017 * | 0.555–5.605 | ||
| 6 months | 12 months | 1.896 | 1.080 | 0.082 | −0.241–4.033 | ||
| 12 months | 18 months | 2.176 * | 1.006 | 0.032 * | 0.185–4.167 | ||
| 18 months | 24 months | −2.600 * | 0.900 | 0.005 * | −4.381–−0.819 | ||
* p < 0.05. MD—mean difference, SE—standard error, CI—confidence interval, MBI—modified Barthel Index, FIM—Functional Independence Measure.
4. Discussion
In this study, long-term functional changes were examined in patients with moderate and severe ischemic stroke. The moderate and severe groups were classified according to the NIHSS scores obtained at admission, and changes in functional independence were evaluated from discharge to 24 months after onset. Functional independence was assessed using MBI and FIM, and we aimed to determine the differences between the two groups.
Our results showed that functional recovery was greatest 3 months after onset in both the moderate and severe groups. In addition, the level of functional recovery at 3 months after onset was higher in the moderate group than in the severe group. One previous study also aimed to compare the neurological and functional recovery of stroke at 3 months after stroke onset after classifying stroke patients into moderate and severe groups [38]. It showed stronger recovery in the moderate group than in the severe group, which is in line with our study results. Stroke severity at onset is a valuable predictor of long-term functional recovery [39,40]. In our study, the moderate group also showed a higher level of recovery compared with the severe group because neurological recovery that occurs at the early stage after the onset of stroke is possibly affected by the size of brain lesions [41]. Neuroplasticity is the capability of stimulation by a variety of stimuli for the modulation of brain activity [42]. Brains compensate damages through reorganization and the creation of new connections among undamaged neurons [43]. After the ischemia of cells, oxygen deprivation in neurons cascades destruction in the focus of infarction being formed which lasts for many hours, usually leading to the progression of damage.
To understand the level of neurological recovery in the moderate and severe groups, the NIHSS scores at each time point were examined in this study. The results showed significant changes up to 6 months after onset in the moderate group and up to 3 months after onset in the severe group. In addition, changes in MBI scores at each time point were analyzed to understand the level of functional recovery in both groups. The moderate group showed significant changes up to 12 months after onset, whereas the severe group showed significant changes up to 6 months after onset. In both NIHSS and MBI, the moderate group showed significant changes over a longer period than the severe group. The severity of initial stroke affects the rate of recovery [44,45]. The long-term recovery observed in the moderate group compared with the severe group could be due to the relatively slower recovery rate of severe stroke; thus, it can be speculated that the level of recovery at each assessment time point was not sufficient to be statistically significant. Between 12 and 18 months after stroke onset, the level of functional recovery was higher in the severe group than in the moderate group, although the change was not statistically significant. This suggests a steady but slower and lower level of recovery in the severe group 18 months after the onset of stroke, and may be a useful guide to the most appropriate time and length of rehabilitation.
In this study, the MBI and FIM scores were compared, and the differences between the results according to the severity of stroke were examined. The results showed that the period of significant change was the same between the two assessment tools in the moderate group, whereas it was different in the severe group. A comparison of the two assessment tools showed significant changes between discharge and 3 and 3–6 months after the onset of stroke for MBI, and between discharge and 3, 3–6, 12–18, and 18–24 months after the onset of FIM. MBI is a 5-level scale and is more convenient, and clinical researchers are more familiar with it; therefore, it is more widely used in the clinical field [46,47]. However, another study reported the limited applicability of MBI to moderate and severe stroke [48]. FIM, on the other hand, does not have limited application according to the severity of disease and has more specific assessment items as a 7-level Likert scale. Therefore, it can evaluate small changes in recovery with higher sensitivity [49]. Both MBI and FIM are highly valid tools for assessing the level of functional independence in stroke patients. However, because more severe stroke shows lower and slower levels of functional recovery, our study highlights the need for an assessment tool that is more suitable for severe stroke.
Among the participants in our study, the mean score of cognitive function at admission was higher in the moderate group than in the severe group. Hence, age and cognitive function might have affected changes in functional recovery. Furthermore, the difference in the number of participants between the moderate and severe groups made it difficult to statistically compare functional recovery between the groups. In addition, the type or amount of rehabilitation patients received during hospitalization, and the location after discharge, may also affect long-term functional recovery, although this study did not consider such points. Finally, our study determined the period of long-term functional changes in patients with moderate and severe stroke; however, the activities that led to such functional recovery were not determined. Despite these limitations, our study is significant because it assessed the functional status of ischemic stroke patients through face-to-face interviews instead of phone interviews and provided data on long-term functional status during the 2 years from the onset of stroke. In addition, our study suggests a tool that allows for a more sensitive assessment of the functional status of patients with severe stroke. These data can be used for additional functional assessments of patients with ischemic stroke.
5. Conclusions
Our study supports previous results on the neurological recovery period of ischemic stroke patients and presents additional information on the functional recovery period. In addition, we showed that MBI and FIM, which are commonly used tools for evaluating functional recovery, enable the sensitive assessment of patients with moderate stroke, whereas FIM offers a more sensitive assessment of the functional state in patients with severe stroke. Our study results provide useful data for healthcare specialists who evaluate the functional status of stroke patients.
Author Contributions
Conceptualization, E.Y.L. and M.C.J.; methodology, E.Y.L., M.K.S. (Min Kyun Sohn), J.M.L., D.Y.K., Y.I.S., G.J.O., Y.S.L., S.Y.L., M.K.S. (Min Keun Song), J.H.H., J.H.A., Y.H.L., W.H.C., S.M.C., S.K.L., M.C.J. and Y.H.K.; formal analysis, E.Y.L.; writing—original draft preparation, E.Y.L.; writing—review and editing, E.Y.L. and M.C.J.; visualization, E.Y.L.; supervision, M.C.J.; project administration, Y.H.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was approved by the Research Ethics Committee of the Wonkwang University Hospital (WKUH 1515).
Informed Consent Statement
Written informed consent was obtained from all patients or from their legally authorized representative prior to inclusion in the study, if the patient was unable to provide informed consent.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by a grant from the Korea Disease Control and Prevention Agency (2022-11-006) and by a National Research Foundation (NRF) grant, provided by the Korean government (MSIP, NRF-2020R1A2C3010304).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Naghavi M., Abajobir A.A., Abbafati C., Abbas K.M., Abd-Allah F., Abera S.F., Fischer F. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brott T., Bogousslavsky J. Treatment of acute ischemic stroke. N. Engl. J. Med. 2000;343:710–722. doi: 10.1056/NEJM200009073431007. [DOI] [PubMed] [Google Scholar]
- 3.Thorvaldsen P., Kuulasmaa K., Rajakangas A.M., Rastenyte D., Sari C., Wilhelmsen L. Stroke trends in the WHO MONICA project. Stroke. 1997;28:500–506. doi: 10.1161/01.STR.28.3.500. [DOI] [PubMed] [Google Scholar]
- 4.Wallace J.B. Researchers document troubling rise in strokes in young adults, starting at age 25. The Washington Post. May 11, 2016.
- 5.Miller E.L., Murray L., Richards L., Zorowitz R.D., Bakas T., Clark P., Billinger S.A. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: A scientific statement from the American Heart Association. Stroke. 2010;41:2402–2448. doi: 10.1161/STR.0b013e3181e7512b. [DOI] [PubMed] [Google Scholar]
- 6.Ostwald S.K., Swank P.R., Khan M.M. Predictors of functional independence and stress level of stroke survivors at discharge from inpatient rehabilitation. J. Cardiovasc. Nurs. 2008;23:371–377. doi: 10.1097/01.JCN.0000317435.29339.5d. [DOI] [PubMed] [Google Scholar]
- 7.Jaracz K., Grabowska-Fudala B., Gorna K., Kozubski W. Consequences of stroke in the light of objective and subjective indices: A review of recent literature. Neurol Neurochir. Pol. 2014;48:280–286. doi: 10.1016/j.pjnns.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Wood J.P., Connelly D.M., Maly M.R. Getting back to real living’: A qualitative study of the process of community reintegration after stroke. Clin. Rehabil. 2010;24:1045–1056. doi: 10.1177/0269215510375901. [DOI] [PubMed] [Google Scholar]
- 9.Wade D.T., Hewer R.L. Functional abilities after stroke: Measurement, natural history and prognosis. J. Neurol. Neurosurg. Psychiatry. 1987;50:177–182. doi: 10.1136/jnnp.50.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan P.W., Lai S.M., Keighley J. Defining post-stroke recovery: Implications for design and interpretation of drug trials. Neuropharmacology. 2000;39:835–841. doi: 10.1016/S0028-3908(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 11.Au-Yeung S.S., Hui-Chan C.W. Predicting recovery of dextrous hand function in acute stroke. Disabil. Rehabil. 2009;31:394–401. doi: 10.1080/09638280802061878. [DOI] [PubMed] [Google Scholar]
- 12.Kwakkel G., Kollen B., Lindeman E. Understanding the pattern of functional recovery after stroke: Facts and theories. Restor. Neurol. Neu-Rosci. 2004;22:281–299. [PubMed] [Google Scholar]
- 13.Lee K.B., Lim S.H., Kim K.H., Kim K.J., Kim Y.R., Chang W.N., Hwang B.Y. Six-month functional recovery of stroke patients: A multi-time-point study. International journal of rehabilitation research. Internationale Zeitschrift fur Rehabilitationsforschung. Rev. Int. De Rech. De Readapt. 2015;38:173. doi: 10.1097/MRR.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verheyden G., Nieuwboer A., De Wit L., Thijs V., Dobbelaere J., Devos H. Time course of trunk, arm, leg, and functional recovery after ischemic stroke. Neurorehabil. Neural Repair. 2008;22:173–179. doi: 10.1177/1545968307305456. [DOI] [PubMed] [Google Scholar]
- 15.Wabl R., Williamson C.A., Pandey A.S., Rajajee V. Long-term and delayed functional recovery in patients with severe cerebrovascular and traumatic brain injury requiring tracheostomy. J. Neurosurg. 2018;131:114–121. doi: 10.3171/2018.2.JNS173247. [DOI] [PubMed] [Google Scholar]
- 16.Dhamoon M.S., Moon Y.P., Paik M.C., Boden-Albala B., Rundek T., Sacco R.L. Long-term functional recovery after first ischemic stroke: The Northern Manhattan Study. Stroke. 2009;40:2805–2811. doi: 10.1161/STROKEAHA.109.549576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feigin V.L., Barker-Collo S., Parag V., Senior H., Lawes C.M., Ratnasabapathy Y. ASTRO study group. Auckland Stroke Outcomes Study. Part 1: Gender, stroke types, ethnicity, and functional outcomes 5 years post stroke. Neurology. 2010;75:1597–1607. doi: 10.1212/WNL.0b013e3181fb44b3. [DOI] [PubMed] [Google Scholar]
- 18.Kwakkel G., Kollen B.J. Predicting activities after stroke: What is clinically relevant? Int. J. Stroke. 2013;8:25–32. doi: 10.1111/j.1747-4949.2012.00967.x. [DOI] [PubMed] [Google Scholar]
- 19.Ullberg T., Zia E., Petersson J., Norrving B. Changes in functional outcome over the first year after stroke: An observational study from the Swedish stroke register. Stroke. 2015;46:389–394. doi: 10.1161/STROKEAHA.114.006538. [DOI] [PubMed] [Google Scholar]
- 20.Sennfält S., Norrving B., Petersson J., Ullberg T. Long-term survival and function after stroke: A longitudinal observational study from the Swedish Stroke Register. Stroke. 2019;50:53–61. doi: 10.1161/STROKEAHA.118.022913. [DOI] [PubMed] [Google Scholar]
- 21.Duncan P. Clinical and measurement issues in selecting stroke outcome measures in clinical trials. In: Goldstein L., editor. Restorative Neurology. Advances in the Pharmacotherapy of Recovery after Stroke. Wiley-Blackwell; Mt. Kisco, NY, USA: 1998. [Google Scholar]
- 22.Pedersen P.M., Jorgensen H.S., Nakayama H., Raaschou H.O., Olsen T.S. Comprehensive assessment of activities of daily living in stroke. The Copenhagen Stroke Study. Arch. Phys. Med. Rehabil. 1997;78:161–165. doi: 10.1016/S0003-9993(97)90258-6. [DOI] [PubMed] [Google Scholar]
- 23.Jørgensen H.S., Nakayama H., Raaschou H.O., Vive-Larsen J., Støier M., Olsen T.S. Outcome and time course of recovery in stroke. Part I: Outcome. The Copenhagen Stroke Study. Arch. Phys. Med. Rehabil. 1995;76:399–405. doi: 10.1016/S0003-9993(95)80567-2. [DOI] [PubMed] [Google Scholar]
- 24.Jørgensen H.S., Nakayama H., Reith J., Raaschou H.O., Olsen T.S. Acute stroke with atrial fibrillation. The Copenhagen stroke study. Stroke. 1996;10:1765–1769. doi: 10.1161/01.STR.27.10.1765. [DOI] [PubMed] [Google Scholar]
- 25.Jørgensen H.S., Nakayama H., Raaschou H.O., Olsen T.S. Intracerebral hemorrhage versus infarction: Stroke severity, risk factors and prognosis. Ann. Neurol. 1995;38:45–50. doi: 10.1002/ana.410380110. [DOI] [PubMed] [Google Scholar]
- 26.Ancheta J., Husband M., Law D., Reding M. Initial functional independence measure score and interval post stroke help assess outcome, length of hospitalization, and quality of care. Neurorehabil. Neural Repair. 2000;14:127–134. doi: 10.1177/154596830001400205. [DOI] [PubMed] [Google Scholar]
- 27.Ween J.E., Mernoff S.T., Alexander M.P. Recovery rates after stroke and their impact on outcome prediction. Neurorehabil. Neural Repair. 2000;14:229–235. doi: 10.1177/154596830001400309. [DOI] [PubMed] [Google Scholar]
- 28.Jiminez J., Morgan P.P. Predicting improvement in stroke patients referred for inpatient rehabilitation. Can. Med. Assoc. J. 1979;121:1481–1484. [PMC free article] [PubMed] [Google Scholar]
- 29.Feigenson J.S., McDowell F.H., Meese P., McCarthy M.L., Greenberg S.D. Factors influencing outcome and length and stay in a stroke rehabilitation unit. Stroke. 1977;8:651–656. doi: 10.1161/01.STR.8.6.651. [DOI] [PubMed] [Google Scholar]
- 30.Stern P.H., McDowell F., Miller S.M., Robinson M. Factors influencing stroke rehabilitation. Stroke. 1971;3:213–218. doi: 10.1161/01.STR.2.3.213. [DOI] [PubMed] [Google Scholar]
- 31.Duncan P.W., Lai S.M., van Culin V., Huang L., Clausen D., Wallace D. Development of a comprehensive assessment toolbox for stroke. Clin. Geriatr. Med. 1999;15:885–916. doi: 10.1016/S0749-0690(18)30037-5. [DOI] [PubMed] [Google Scholar]
- 32.Kidd D., Stewart G., Baldry J., Johnson J., Rossiter D., Petruckevitch A., Thompson A.J. The Functional Independence Measure: A comparative validity and reliability study. Disabil. Rehabil. 1995;17:10–14. doi: 10.3109/09638289509166622. [DOI] [PubMed] [Google Scholar]
- 33.Shah S., Vanclay F., Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 1989;42:703–709. doi: 10.1016/0895-4356(89)90065-6. [DOI] [PubMed] [Google Scholar]
- 34.Glowinski S., Blazejewski A. Spider as a rehabilitation tool for patients with neurological disabilities: The preliminary research. J. Pers. Med. 2020;10:33. doi: 10.3390/jpm10020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah S., Vanclay F., Cooper B. Predicting discharge status at commencement of stroke rehabilitation. Stroke. 1989;20:766–769. doi: 10.1161/01.STR.20.6.766. [DOI] [PubMed] [Google Scholar]
- 36.Keith R.A. Observations in the rehabilitation hospital: Twenty years of research. Arch. Phys. Med. Rehabil. 1988;69:625–631. [PubMed] [Google Scholar]
- 37.Sandhaug M., Andelic N., Vatne A., Seiler S., Mygland A. Functional level during sub-acute rehabilitation after traumatic brain injury: Course and predictors of outcome. Brain Inj. 2010;24:740–747. doi: 10.3109/02699051003652849. [DOI] [PubMed] [Google Scholar]
- 38.Murie-Fernández M., Marzo M.M. Predictors of Neurological and Functional Recovery in Patients with Moderate to Severe Ischemic Stroke: The EPICA Study. Stroke Res. Treat. 2020;2020:1–13. doi: 10.1155/2020/1419720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hankey G.J. Long-term outcome after ischaemic stroke/transient ischaemic attack. Cereb. Dis. 2003;16:14–19. doi: 10.1159/000069936. [DOI] [PubMed] [Google Scholar]
- 40.Wouters A., Nysten C., Thijs V., Lemmens R. Prediction of outcome in patients with acute ischemic stroke based on initial severity and improvement in the first 24 h. Front. Neurol. 2018;9:308. doi: 10.3389/fneur.2018.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teasell R.W., Foley N.C., Bhogal S.K., Speechley M.R. An evidence-based review of stroke rehabilitation. Top. Stroke Rehabil. 2003;10:29–58. doi: 10.1310/8YNA-1YHK-YMHB-XTE1. [DOI] [PubMed] [Google Scholar]
- 42.Voss P., Thomas M.E., Cisneros-Franco J.M., de Villers-Sidani É. Dynamic brains and the changing rules of neuroplasticity: Implications for learning and recovery. Front. Psychol. 2017;8:1657. doi: 10.3389/fpsyg.2017.01657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin M.P., Liebeskind D.S. Imaging of ischemic stroke. Contin. Lifelong Learn. Neurol. 2016;22:1399. doi: 10.1212/CON.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richards C.L., Malouin F., Nadeau S. Stroke rehabilitation: Clinical picture, assessment, and therapeutic challenge. Prog. Brain Res. 2015;218:253–280. doi: 10.1016/bs.pbr.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Teasell R., Foley N., Salter K., Bhogal S., Jutai J., Speechley M. Evidence-based review of stroke rehabilitation: Executive summary. Top. Stroke Rehabil. 2009;16:463–488. doi: 10.1310/tsr1606-463. [DOI] [PubMed] [Google Scholar]
- 46.Hinkle J.L. The use of the FIM™ instrument in the post stroke population. Top. Stroke Rehabil. 2000;7:47–66. doi: 10.1310/3LB6-CGVN-EAAU-0TEQ. [DOI] [Google Scholar]
- 47.Hsueh I.P., Lin J.H., Jeng J.S., Hsieh C.L. Comparison of the psychometric characteristics of the functional independence measure, 5 item Barthel index, and 10 item Barthel index in patients with stroke. J. Neurol. Neurosurg. Psychiatry. 2002;73:188–190. doi: 10.1136/jnnp.73.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hobart J.C., Thompson A.J. The five item Barthel index. J. Neurol. Neurosurg. Psychiatry. 2001;71:225–230. doi: 10.1136/jnnp.71.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z.C., Zhang S.P., Yuen P.C., Chan K.W., Chan Y.Y., Cheung C.H., Zhang X. Intra-rater and inter-rater reliability of tongue coating diagnosis in traditional chinese medicine using smartphones: Quasi-delphi study. JMIR Mhealth Uhealth. 2020;8:e16018. doi: 10.2196/16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.