Abstract
Metabolism and circadian rhythms are intimately linked, with circadian glucagon-like peptide-1 (GLP-1) secretion by the intestinal L-cell entraining rhythmic insulin release. GLP-1 secretion has been explored in the context of obesogenic diets, but never in a rodent model of type 2 diabetes (T2D). There is also considerable disagreement regarding GLP-1 levels in human T2D. Furthermore, recent evidence has demonstrated decreased expression of the β-cell exocytotic protein secretagogin (SCGN) in T2D. To extend these findings to the L-cell, we administered oral glucose tolerance tests at 6 time points in 4-hour intervals to the high-fat diet/streptozotocin (HFD-STZ) mouse model of T2D. This revealed a 10-fold increase in peak GLP-1 secretion with a phase shift of the peak from the normal feeding period into the fasting-phase. This was accompanied by impairments in the rhythms of glucose, glucagon, mucosal clock genes (Arntl and Cry2), and Scgn. Immunostaining revealed that L-cell GLP-1 intensity was increased in the HFD-STZ model, as was the proportion of L-cells that expressed SCGN; however, this was not found in L-cells from humans with T2D, which exhibited decreased GLP-1 staining but maintained their SCGN expression. Gcg expression in isolated L-cells was increased along with pathways relating to GLP-1 secretion and electron transport chain activity in the HFD-STZ condition. Further investigation into the mechanisms responsible for this increase in GLP-1 secretion may give insights into therapies directed toward upregulating endogenous GLP-1 secretion.
Keywords: diurnal, enteroendocrine, GIP, GLP-1, glucose, insulin
The incretin hormone glucagon-like peptide-1 (GLP-1) is secreted from the intestinal L-cell in response to food intake (1, 2). Due to its profound role in stimulating glucose-dependent insulin secretion, along with its beneficial effects to induce satiety and weight loss, long-acting GLP-1 receptor (GLP-1R) agonists are now widely used in the treatment of type 2 diabetes (T2D) and obesity (3, 4). However, at the physiological level, GLP-1 secretion is not continuously elevated, but exhibits a circadian pattern that plays a role in entraining the daily rhythms in insulin secretion and, therefore, glucose homeostasis (5-7). At the cellular level, circadian rhythms are orchestrated by the core clock genes, which are expressed in all nucleated mammalian cells, both centrally and peripherally (8, 9). Within the molecular clock, the transcriptional oscillators, BMAL1 (brain and muscle aryl hydrocarbon receptor nuclear translocator-like [ARNTL] protein 1) and CLOCK, bind E-box promoter elements to stimulate the expression of PERIOD (PER1,2,3) and CRYPTOCHROME (CRY1,2), which feedback to repress the BMAL1-CLOCK heterodimer (10). These rhythms are coordinated centrally in the suprachiasmatic nuclei of the hypothalamus, synchronized by light as the primary zeitgeber (ZT), and propogate rhythms in downstream tissues through behavioral, autonomic, and hormonal cues (11). While the suprachiasmatic nuclei are the master regulators of central rhythms, metabolic tissues such as the intestine, pancreatic α- and β- cells, hepatocytes, skeletal muscle, and adipose tissue exhibit peripheral secondary rhythms that are entrainable by food intake (12-21). Although cell-autonomous, these rhythms are strongly influenced by the sleep-wake cycle, which, in turn, coordinates the fasting-feeding schedule.
Disruptions in the circadian clock, such as in shift-workers, have been strongly implicated in the development of metabolic diseases, including T2D (22-25). Conversely, high-fat diets (HFD) have been shown to lengthen circadian periodicity and disrupt locomotor activity and feeding patterns, in some cases within 1 week of diet initiation (26). However, metabolic disruption has also been shown to affect peripheral circadian clocks, with abnormal patterns in diurnal insulin secretion demonstrated both in vivo and in pancreatic islets from patients with T2D (26-32). Further evidence from pancreatic islets shows that both insulin content and plasma glycated hemoglobin (HbA1c) concentrations are closely related to Per and Cry expression (30, 33), and sleep duration is inversely correlated with HbA1c and postprandial glycemia (34, 35). Finally, polymorphisms in ARNTL have been associated with both hypertension and T2D (36).
The bidirectional link between the clock and metabolism is also true at the level of the L-cell. Hence, both whole-body and L-cell (via the Gcg promoter) Arntl knockout mice demonstrate abnormal patterns in GLP-1 secretion, along with altered rhythms in insulinemia and glycemia (6, 37). Conversely, both palmitate exposure in vitro and diets that are high in fat in vivo cause decreased L-cell Arntl expression and disrupt the physiological patterns in GLP-1 release (7, 38, 39).
A number of exocytotic genes demonstrate rhythmic expression in the pancreatic β- and α-cells, including those for the core SNARE proteins, vesicle-associated membrane protein and syntaxin, as well as the accessory SNARE proteins, syntaxin-binding protein-1, and secretagogin (SCGN) (6, 16, 40, 41). Interestingly, loss of SCGN results in decreased insulin secretion, in vitro and in vivo (42-44). However, Scgn is also expressed by a wide variety of enteroendocrine cells in rodents and humans (45-47), and recent studies have demonstrated that Scgn plays a role in regulating peak circadian GLP-1 secretion in vitro (6). With rhythmic expression in the L-cell at both the mRNA and protein levels, SCGN also exerts time-dependent functional effects on the actin cytoskeleton which is critical for secretory granule movement through the cell (6, 48). This calcium-sensitive secretory protein is further involved in granule fusion at the cell membrane through time-independent interactions with the core SNARE protein, SNAP25 (6, 43, 49, 50).
Evidence from human studies has shown that pancreatic islet SCGN expression is reduced in T2D (42). However, nothing is known about the impact of this metabolic disease on L-cell expression of Scgn. We have therefore utilized a murine model of T2D to determine the relationship(s) between the circadian clock, L-cell Scgn, and rhythmic GLP-1 secretion. The results indicate that both clock gene expression and SCGN levels are altered in the L-cells of obese, diabetic mice, in association with profound alterations in the circadian secretion of GLP-1.
Methods
In Vivo Studies
Male 5- to 6-week-old C57Bl/6J (Jackson Laboratories) were allowed to acclimate for 1 week before being randomly assigned to treatment groups. Male and female Gcg-Venus (51) mice were bred at the University of Toronto, with littermates randomized into treatment groups at 6 to 7 weeks of age. HFD-STZ mice were put on a 60% high-fat diet (HFD; D12492, Research Diets) for 5 weeks and then injected intraperitoneally with 35 mg/kg streptozotocin (STZ) daily for 5 days (52) followed by a 2-week washout period to stabilize body weight (Fig. 1A). Rodent chow-fed (RC), control mice were given a matched diet containing 10% fat (D12450J, Research Diets) and received vehicle (citrate buffer) injections. Mice were weighed weekly. Overnight fasting glycemia (One Touch glucose meter; Lifescan), food intake (by weight, every 4 hours over 24 hours), and/or body composition (In Vivo Xtreme; Bruker) analyses were performed 2 weeks after STZ administration in a subgroup of mice, followed by euthanasia for tissue collection.
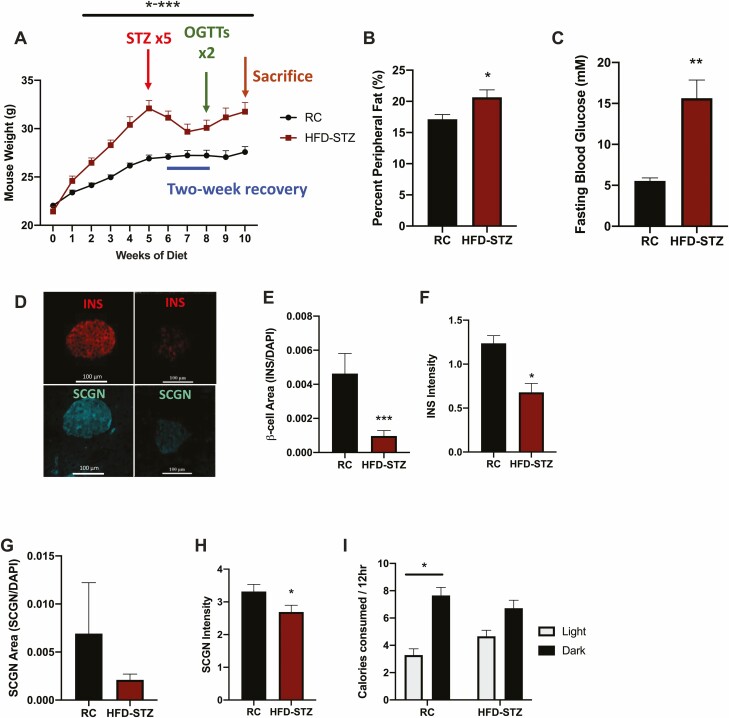
Figure 1.
HFD-STZ mice exhibit decreased pancreatic insulin and secretagogin. C57Bl/6J mice fed a high-fat diet (HFD) were treated with streptozotocin (STZ) as indicated or were fed regular chow (RC, control) followed by 2 oral glucose tolerance tests (OGTTs) separated by 1 week and then sacrificed (A, n = 15-20). Percent peripheral fat (B, n = 8) and overnight fasting blood glucose (C, n = 8) at 8 weeks. Representative images of INS and SCGN in pancreatic sections (D), β-cell area (E, n = 4-6), INS staining intensity (F, n = 4-6), SCGN area (G, n = 4-6) and SCGN staining intensity (H, n = 4-6). Light/dark calorie consumption at 8 weeks (I, n = 8). *P < 0.05, **P < 0.01, *** P < 0.001.
Oral glucose tolerance tests (OGTT; 5 g dextrose/kg body weight) were conducted on 4-hour fasted mice at ZT (hours after lights-on) 2, 6, 10, 14, 18, and 22 in C57Bl/6J mice, and at ZT2 and 14 (established peak and trough of GLP-1 secretion) (5-7, 53) in Gcg-Venus mice. Blood samples were collected at t = 0, 10, and 60 minutes and plasma was stored at −80 °C. Mice were tested twice, at 2 different time points in random sequence, with a 1-week recovery period between tests. Plasma glucose was determined using a GM9 Glucose Analyzer (Analox), plasma total GLP-1, insulin, and glucagon by U-PLEX immunoassay (MesoScale Discovery Assay; RRIDs: AB_2801383, AB_2784505, and AB_2801382, respectively), and plasma glucose-dependent insulinotrophic polypeptide (GIP) by enzyme-linked immunosorbent assay (ELISA, Millipore; RRID: AB_2801384). Mice were then euthanized for tissue collection. All animal studies were approved by the Animal Care Committee at the University of Toronto and were conducted in accordance with the Canadian Council on Animal Care guidelines.
RNA Expression Analyses
Quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) was conducted on RNA extracts (RNeasy kit, Qiagen) from ileal mucosal scrapes using the Taqman Gene Expression Assay (ThermoFisher) with primers (ThermoFisher) as listed in (54). Data were analyzed using the ΔΔCT method (55), using H3f3a as the internal control, as it has previously been validated for use in circadian studies of the gut mucosa (7).
Immunostaining
Murine ileum and pancreas were formalin-fixed and paraffin-embedded for sectioning (UHN Pathology Services, Toronto, ON, Canada). Formalin-fixed, paraffin-embedded ileal sections from normal humans and subjects with T2D were obtained from BioChain. Sections were dewaxed and rehydrated as previously described (6). Ileal and pancreatic sections were stained for GLP-1 and SCGN or insulin and SCGN, respectively, using the antisera listed in (54); 4′,6-diamidino-2-phenylindole (DAPI) was used to detect nuclei. The specificity of the SCGN antiserum was established by the complete lack of staining in intestinal sections from whole-body Scgn knockout mice, and negative controls omitting the primary antisera were run for all tissues (data not shown). Immunostained intestinal cells were counted in a blinded fashion for the presence or absence of GLP-1 and SCGN co-staining, with the average percent distribution calculated as the number of cells in each population divided by the total number of cells counted (20-40 cells per mouse and 15-20 per human, to make n = 1). The surface area of pancreatic insulin and SCGN staining was determined relative to the surface area of the entire section in a blinded fashion, using NIS-Elements Imaging software (Nikon Corporation).
RNAscope for duplex detection of GCG and SCGN was conducted using the Multiplex Fluorescent Reagent Kit v2 from ACD Biotechnie (RRID: SCR_012481) as per the manufacturer’s instructions. Co-expression of GCG and SCGN was counted in 10 cells per section to make n = 1; L-cell numbers could not be reliably determined in these sections due to lack of villi.
All images were collected using an inverted Nikon eclipse Ti microscope connected to a Prairie swept field confocal module. The excitation lasers (405, 561, 640 nm) were powered by an Agilent Technologies laser launch (Model MLC400B), and the emission filter was multiband pass at 400-450 nm, 600-650 nm, and 680-700 nm. Samples were imaged through a 40× water immersion objective (Nikon Apo 1.25 WI, lS) with an ultrafast Andor iXon3 detector. Intensities for both immunostaining and RNAscope were quantified by establishing regions-of-interest with background subtractions, and then determining the mean fluorescence intensity as the average intensity per pixel, using NIS-Elements Imaging software.
Gcg-Venus L-Cell Isolation and RNA-Sequencing Analyses
Primary ileal epithelial cells were isolated from Gcg-Venus mice (51) at ZT14 (established physiological peak of GLP-1 secretion) (5-7, 53). Fluorescence-activated cell sorting was conducted using the FACS Melody cell sorter (BD Bioscience) at the University of Toronto Temerty Faculty of Medicine Flow Cytometry Facility. DAPI-staining as well as side- and forward-scatter were used to remove dead and non–Venus-positive cells. Venus-positive cells were sorted into RLT lysis buffer (Qiagen) in LoBind tubes (Eppendorf). RNA was extracted using the RNeasyPlus Micro Kit (Qiagen), with gDNA removal. Purified RNA was then sent for RNA-sequencing (RNA-seq) at the Donnelly Sequencing Centre and sequenced on the NovaSeq SP 300c using 150bp paired-end reads. FASTQ files were aligned using kallisto (56), and count tables were generated using tximport (57). Genes of low expression were removed and data were normalized using EdgeR (58). Differential expression analyses were conducted using limma (59) (Bioconductor), with an adjusted P value set to 0.01. Gene set enrichment analyses were then run using the camera function in limma, using the Bader Lab gene set resource (http://download.baderlab.org/EM_Genesets). Network analyses were generated using the EnrichmentMap (60, 61) software in Cytoscape, where nodes represent up- or downregulated pathways. Edges connecting nodes were calculated by the number of shared genes between pathways. Clusters of functionally related pathways were grouped and labeled using AutoAnnotate (62). The datasets generated and/or analyzed during the current study are available in the ArrayExpress repository at https://www.ebi.ac.uk/biostudies/arrayexpress/studies/E-MTAB-11636.
Statistical Analyses
All data are expressed as mean ± SEM. All in vivo time-dependent analyses were conducted using MetaCycle (63) and CircaCompare (64) in a parametric fashion to identify 24-hour rhythms and peak values and to compare rhythms between conditions, respectively. Delta area under the curve (ΔAUC) was calculated using the trapezoidal rule for the changes in hormonal and glycemic responses from fasting over the entire 60-minute study. Statistical analyses were conducted using GraphPad Prism with Student’s t test used to compare 2 groups, and ANOVA (2- or 3-way in combination with Tukey’s post hoc analysis, when appropriate) for 3 or more groups. Some data were log-transformed prior to statistical analyses to normalize variance.
Results
Metabolic and Circadian Disruptions in HFD-STZ Mice
To develop a model of T2D, mice were fed a high-fat diet (HFD) to induce obesity and then treated with low-dose STZ to create a relative insulin deficiency. Accordingly, HFD-fed mice exhibited rapid weight gain, with significant differences from control animals observed within 2 weeks of diet initiation. STZ administration resulted in weight loss; however, the HFD-STZ animals remained heavier than their matched controls (P < 0.05-0.001, Fig. 1A) and exhibited an increased percentage of peripheral fat (P < 0.05, Fig. 1B). HFD-STZ mice also demonstrated an increase in overnight fasting glycemia (P < 0.01, Fig. 1C), accompanied by a significant reduction in β-cell area (P < 0.01) as well as in insulin staining intensity (P < 0.01, Fig. 1D-1F); total pancreatic weight was not different between the groups (data not shown). SCGN area was not significantly reduced in the T2D model, likely as this staining included α-cells which are known to expand under conditions of T2D as well as with loss of Scgn (65) (Fig. 1G). This finding was concomitant to a reduction in the overall intensity of the islet SCGN staining in the HFD-STZ mice (P < 0.01), consistent with a previous report on islets from patients with T2D (42) (Fig. 1H). Analysis of caloric intake during the light (inactive; ZT0-12) and dark (active; ZT12-24) periods showed that the normal rhythm in calorie consumption in the RC animals (P < 0.05) was ablated in the HFD-STZ condition, largely due to an increase in food intake during the light period (Fig. 1I). Additionally, small intestinal weight and length were increased; however, this difference did not persist when normalized to body weight (P < 0.05, Fig. S1A-S1D) (54). All of these parameters were significantly increased for the colons of the HFD-STZ mice (P < 0.05, Fig. S1E-S1H) (54).
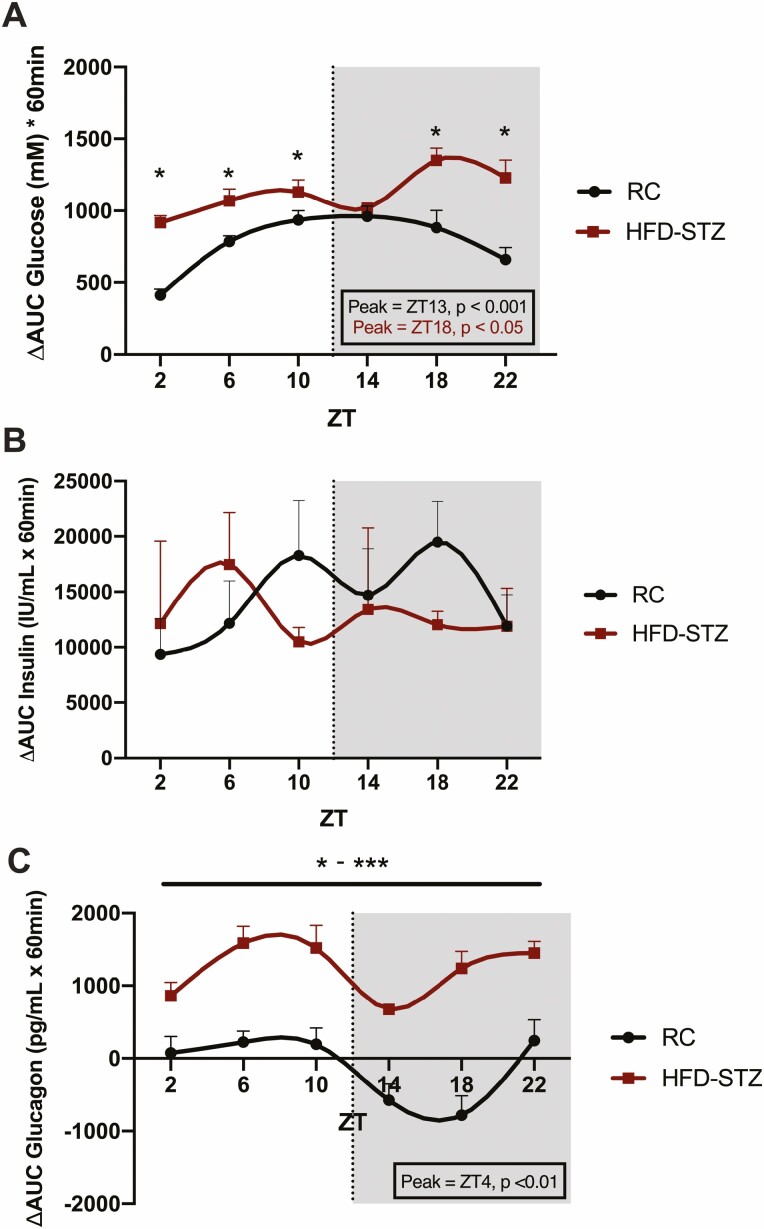
As compared with control, RC animals, HFD-STZ mice demonstrated 4-hour fasting hyperglycemia at all time points assessed over the 24-hour day (P < 0.05 at ZT2, 6, 10, 14, 18, and 22; Fig. S2A) (54). Furthermore, an impaired glucose response to an OGTT was also found in these animals, with increased concentrations at 5 of the 6 time points (P < 0.05 at ZT2, 6, 10, 18, and 22) and a shift in the peak of the normal, physiological rhythm in ∆AUC glucose, from ZT13 in the RC animals to ZT18 (Fig. 2A, S2A) (54). As previously observed (7), basal insulin levels and responses to the OGTT were found to be quite variable, likely reflecting individual differences in insulin sensitivity. However, 4-hour fasting insulin levels in HFD-STZ animals were not different from those of control mice at any time point, nor were there any differences in the plasma insulin responses to the timed OGTTs, which were arrhythmic in both groups of mice (Fig. 2B, S2B) (54). However, when taken with the increase in glycemia, these findings indicate a state of relative insulin deficiency in the HFD-STZ mice. Furthermore, despite a slight reduction in 4-hour fasting glucagonemia, the dysglycemia in the HFD-STZ mice given oral glucose was compounded by loss of the normal decrease in glucagon responses observed during the feeding/active period, as well as ablation of the circadian pattern, which reached a nadir at ZT16 in control animals (P < 0.01; Fig. 2C, S2C) (54).
Figure 2.
HFD-STZ mice exhibit hyperglycemia and hyperglucagonemia as well as abnormal circadian rhythms in response to timed OGTTs. OGTTs were administered at 6 time points (ZT2, 6, 10, 14, 18, and 22; shaded area from ZT12 to ZT24 indicates lights-out) over the 24-hour clock to 4-hour fasted C57Bl/6J RC and HFD-STZ mice (n = 10), expressed as ∆AUC responses in plasma glucose (A), insulin (B) and glucagon (C). Peak time points of significant rhythms are shown in the boxed insets and are color-coded accordingly.*P < 0.05, **P < 0.01, ***P < 0.001.
Profoundly Increased GLP-1 Responses and Altered Rhythmicity in HFD-STZ Mice
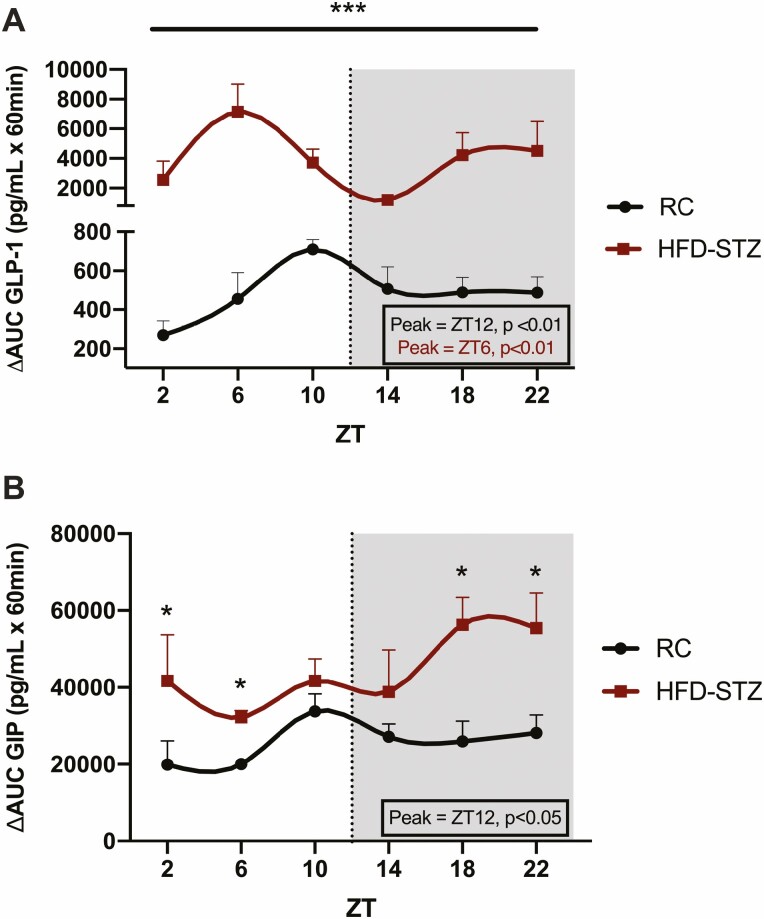
Despite no change in basal levels of GLP-1, HFD-STZ mice exhibited a drastic increase in the ∆AUC GLP-1 responses to oral glucose loads administered at 6 different time points over the 24 hour day (by ~7×, P < 0.01-0.001; Fig. 3A, S2D) (54), accompanied by a phase shift in the normal physiological rhythm of GLP-1 secretion, peaking in the light, fasting period (i.e., ZT6, P < 0.01) rather than at the onset of the dark, feeding period as found for the control, RC mice (i.e., ZT12, P < 0.01). Although the response of the K-cells, which secrete the other incretin hormone, GIP, was not as dramatically affected by the diabetic state as GLP-1, HFD-STZ mice demonstrated an increase in basal levels (P < 0.05 at ZT 2, 10, 18, and 22) as well as an increase in the response to oral glucose (i.e., by ~2×; P < 0.05 at ZT2, 6, 18 and 22) and loss of the normal rhythm exhibited by the control animals (peak = ZT12, P < 0.05; Fig. 3B, S2E) (54).
Figure 3.
HFD-STZ mice exhibit increased GLP-1 and GIP levels and altered rhythms in response to timed OGTTs. OGTTs were administered at 6 time points (ZT2, 6, 10, 14, 18, and 22; shaded area from ZT12 to ZT24 indicates lights-out) over the 24-hour clock to 4-hour fasted C57Bl/6J RC and HFD-STZ mice (n = 10), expressed as ∆AUC responses in GLP-1 (A) and GIP (B). Peak time points of significant rhythms are shown in the boxed insets and are color-coded accordingly. *P < 0.05, ***P < 0.001.
Disrupted Rhythms in Intestinal Clock Genes and Secretagogin in HFD-STZ Mice
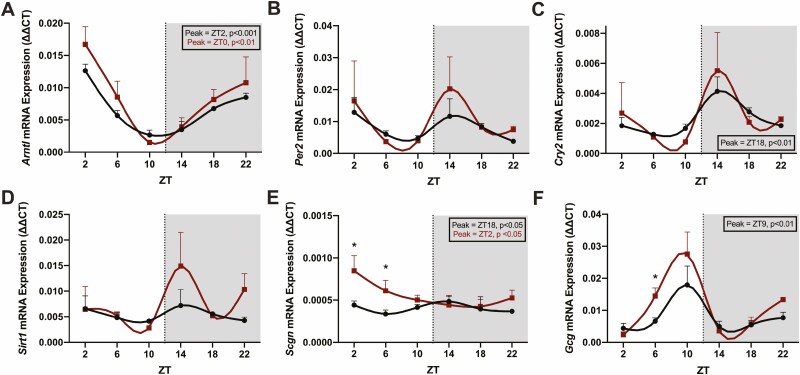
Ileal mucosal scrapes were collected at 4-hour intervals over the 24-hour day for qRT-PCR. Transcript levels in control, RC-fed animals showed an anti-phasic rhythm in Arntl (peak at ZT0, P < 0.001) as compared to Cry2 (peak at ZT18, P < 0.01) and Scgn (peak at ZT18, P < 0.05), with trends to peaks in the dark phase also observed for both Per2 and Sirt1 (Fig. 4A-4E). Interestingly, the HFD-STZ mice exhibited shifted rhythms in both Arntl (peak at ZT2, P < 0.01) and Scgn (peak at ZT16, P < 0.05) as well as an ablated rhythm in Cry2. Scgn expression was also significantly increased at ZT2 and ZT6 (by ~2×, P < 0.05; Fig. 4E). Finally, the rhythm in Gcg was significant, peaking immediately prior to the peak in GLP-1 secretion in the RC animals (at ZT9, P < 0.01; Fig. 4F). Although this pattern was lost in the HFD-STZ condition, a significant elevation in Gcg was detected in these animals during the light period (P < 0.05 at ZT6).
Figure 4.
HFD-STZ animals exhibit altered patterns in clock gene and Scgn expression in the ileal mucosa. mRNA was extracted from ileal mucosal scrapes at 6 time points (ZT2, 6, 10, 14, 18, and 22; shaded area from ZT12 to ZT24 indicates lights-out) over the 24-hour clock in 4-hour fasted C57Bl/6J RC (black) and HFD-STZ (red) mice (n = 4-5) and analyzed by qRT-PCR for Arntl (A), Per2 (B), Cry2 (C), Sirt1 (D), Scgn (E), and Gcg (F). Peak time points of significant rhythms are shown in the boxed insets and are color-coded accordingly. *P < 0.05.
Altered L-Cell Expression Profiles
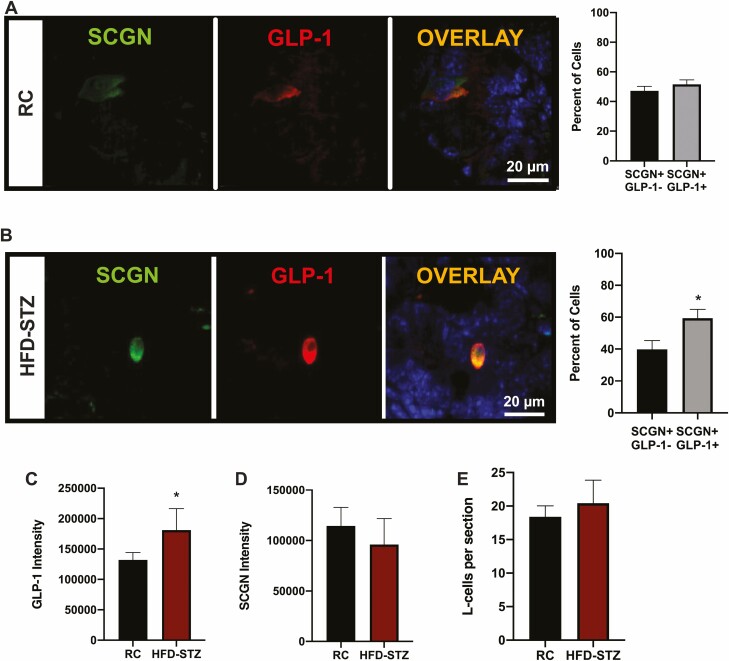
To examine the L-cells more specifically, immunostaining for GLP-1 and SCGN was conducted on ileal sections collected from both RC and HFD-STZ animals. All L-cells in both groups of mice were found to express SCGN (SCGN−/GLP-1+ = 0%), with localization in both the cytoplasm and nucleus, as previously reported (6, 66). RC animals exhibited similar percentages of enteroendocrine cells that expressed SCGN only (SCGN+/GLP-1−) as compared to SCGN cells that also expressed GLP-1 (i.e., L-cells: SCGN+/GLP-1+) (Fig. 5A). In contrast, HFD-STZ diabetes resulted in an increased percentage of SCGN + L-cells as compared to the other enteroendocrine cells that expressed SCGN but not GLP-1 (P < 0.05; Fig. 5B). L-cells in the HFD-STZ animals also exhibited increased GLP-1 staining intensity (P < 0.05, Fig. 5C), although the SCGN intensity was unchanged (Fig. 5D). The total number of L-cells in the villi and crypts was also unaffected by the HFD-STZ treatment (Fig. 5E). However, small intestinal and colon weights and lengths were increased in HFD-STZ condition, in agreement with previous reports of STZ exposure causing an increase in L-cell secretion of the intestinal growth factor, GLP-2, that is co-synthesized with GLP-1 (67). Together, these findings suggest that the increase in SCGN staining was related to a shift in the expression profile of the L-cell in HFD-STZ animals.
Figure 5.
HFD-STZ mice exhibit an increase in L-cell GLP-1 and SCGN expression. Murine ileal sections were immunostained for GLP-1 and SCGN and the percentage of SCGN+/GLP-1- and SCGN+/GLP-1 + cells was determined in C57Bl/6J RC (A, n = 4-6) and HFD-STZ mice (B, n = 4-6); representative images are shown (DAPI: blue). (C-E) L-cell GLP-1 (C, n = 4-6) and SCGN (D, n = 4-6) intensity, and the number of L-cells per section (E, n = 6). *P < 0.05.
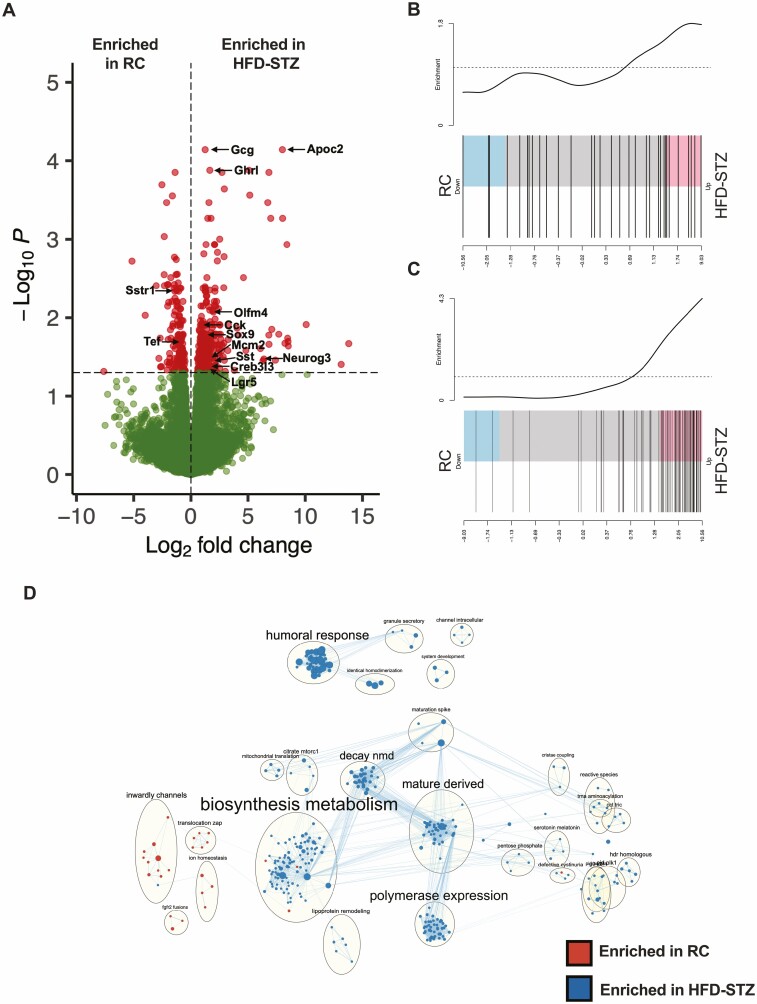
Gcg-Venus mice were subjected to the HFD-STZ protocol to enable direct examination of the L-cell transcriptome. In comparison to RC-fed Gcg-Venus mice, these animals exhibited similar metabolic changes in fasting glycemia, as well as in the glucose, insulin, glucagon, and GLP-1 responses to an OGTT (at ZT2 and ZT14) as observed for the C57Bl/6J HFD-STZ mice (Fig. S3) (54). RNA-seq of the Gcg-Venus L-cells collected at ZT14 (at which time GLP-1 secretion was ~4-fold elevated in the HFD-STZ animals, (P < 0.001; Fig. 3A) demonstrated that Gcg was the most significantly upregulated gene in the HFD-STZ condition (P < 0.001, Fig. 6A). Other genes that were significantly increased in the HFD-STZ condition included the intestinal hormones Sst, Cck, and Grhl, the enteroendocrine lineage markers, Neurog3 and Sox9, and the stem cell/proliferative markers, Lgr5, Olfm4, and Mcm2, in line with the finding of a shift in the L-cell expression of SCGN. Furthermore, the GLP-1 Secretion (Fig. 6B) and Electron Transport Chain (Fig. 6C) pathways were both increased in the HFD-STZ animals. In contrast, expression of the clock regulator Tef, as well as of Sstr1 were both decreased in the L-cells from the diabetic mice. An EnrichmentMap pathway grouping analysis further revealed HFD-STZ-induced upregulation of pathways relating to Biosynthesis Metabolism and Granule Secretion (Fig. 6D).
Figure 6.
Altered gene expression in L-cells from Gcg-Venus HFD-STZ mice. Volcano plot of the transcriptomes of L-cells isolated from Gcg-Venus RC and HFD-STZ mice at ZT14 (A). (B-C) Barcode plots of L-cell GLP-1 Secretion (B) and Electron Transport Chain (C) pathways. EnrichmentMap analysis comparing L-cells from Gcg-Venus RC and HFD-STZ mice (D). n = 8 (4 males + 4 females per condition).
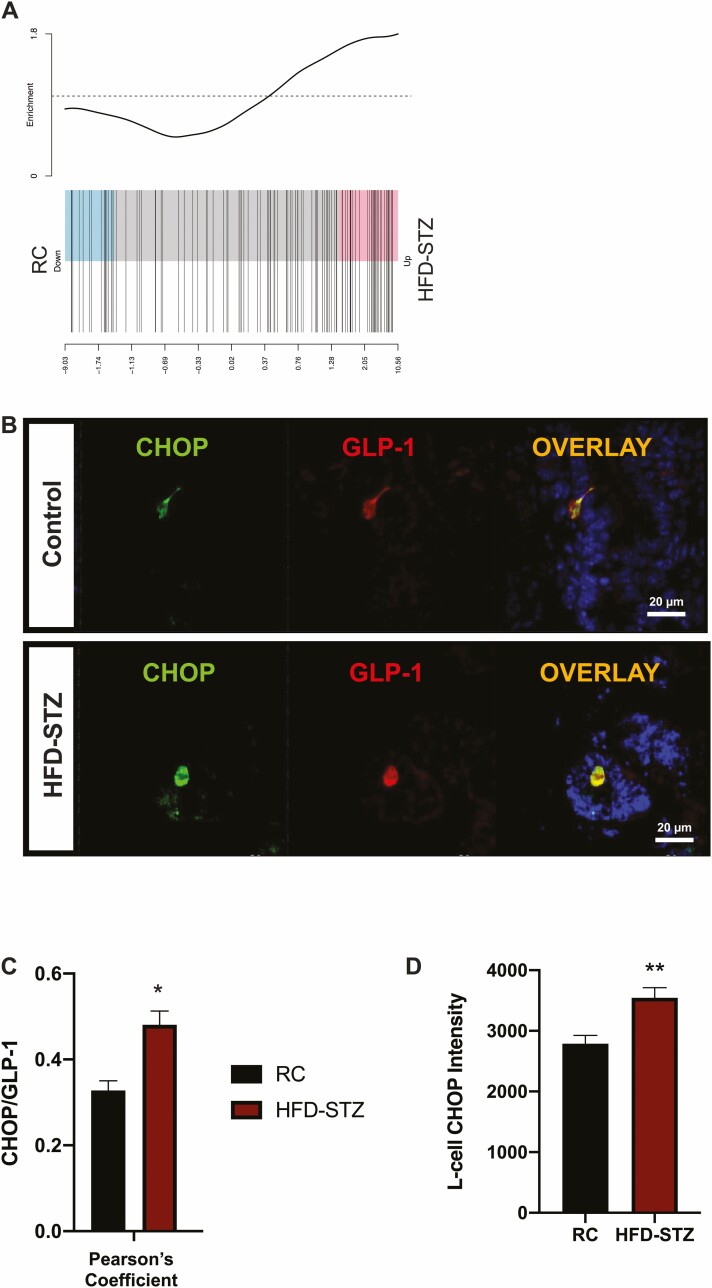
RNA-seq analysis also revealed that L-cells isolated from HFD-STZ Gcg-Venus mice exhibit increased expression of genes related to the Unfolded Protein Response (Fig. 7A). This finding was confirmed by immunostaining of L-cells from the C57Bl/6J mice for GLP-1 and the ER stress marker CHOP, which showed increased co-localization as well as CHOP intensity (Fig. 7B-7D).
Figure 7.
L-cells from HFD-STZ mice exhibit increased expression of unfolded protein response genes and CHOP intensity. Barcode plots (n = 8) of the Unfolded Protein Response pathway in L-cells isolated from Gcg-Venus RC and HFD-STZ mice at ZT14 (A). Representative ileal sections from C57Bl/6J mice co-stained for CHOP and GLP-1 SCGN (DAPI: blue) (B), Pearson’s Coefficient analysis of CHOP co-localization with GLP-1 (C, n = 4-6), and L-cell CHOP intensity (D, n = 4-6). *P < 0.05, **P < 0.01.
Ileal Sections from Humans With Type 2 Diabetes Exhibit Decreased GLP-1, but Unchanged GCG
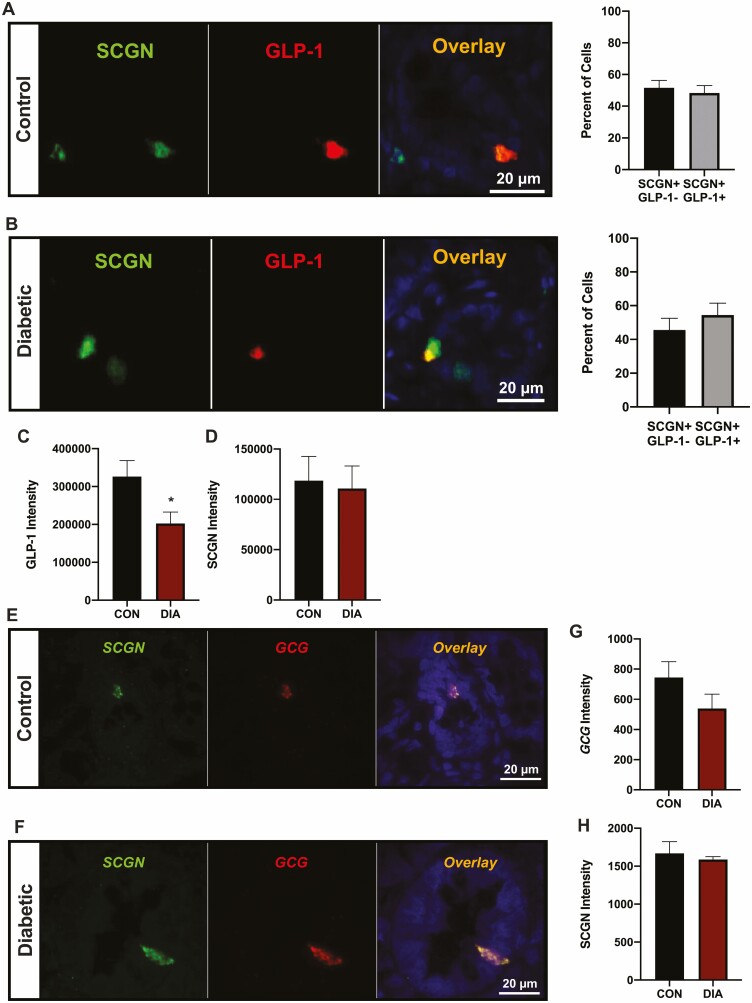
Finally, to determine whether the findings made in the HFD-STZ model of T2D might translate to humans, ileal sections from healthy controls and subjects with T2D were evaluated for GLP-1/SCGN as well as GCG/SCGN expression. As found in the mice, all of the human L-cells expressed SCGN, with equal proportions of SCGN + enteroendocrine (SCGN+/GLP-1−) and L-cells (SGCN+/GLP-1+) detected in healthy controls (Fig. 8A). However, no shift in the distribution of these cell populations was found in the samples from subjects with T2D (Fig. 8B) and, despite unchanged SCGN intensity, a decrease in GLP-1 staining intensity was observed (P < 0.05, Fig. 8C and 8D). RNAscope analysis showed a trend toward decreased L-cell GCG expression (P = 0.16) but no differences in L-cell SCGN expression between control and diabetic samples (Fig. 8E-8H).
Figure 8.
Ileal sections from subjects with type 2 diabetes exhibit decreased GLP-1 expression. (A-D) Human ileal sections from healthy controls (CON) and subjects with T2D (DIA) were co-stained for GLP-1 and SCGN (n = 3-5). (A-B) Percentage of SCGN+/GLP-1- and SCGN+/GLP-1 + cells in control (A) and diabetic (B) subjects; representative images are shown (DAPI: blue). (C-D) L-cell GLP-1 (C) and SCGN (D) staining intensity. (E-H) Human ileal sections from healthy controls and subjects with T2D were analyzed by RNAscope for GCG and SCGN expression (n = 3-5). (E-F) Representative images are shown for control (E) and diabetic (F) subjects. (G-H) GCG (G) and SCGN (H) intensity. *P < 0.05.
Discussion
It has been well-established that there is a reciprocal relationship between the circadian clock and metabolism (8) and that circadian GLP-1 secretion plays a role in entraining rhythmic insulin release (5, 7). However, it is currently unknown whether metabolic disruption, such as in patients with T2D, can alter the normal circadian pattern of GLP-1 release. The results of the present study demonstrate that, in the murine HFD-STZ model of T2D, the circadian secretion of GLP-1 is highly abnormal, with profound increases in the L-cell response to oral glucose as well as a phase shift in the circadian pattern to peak during the normal fasting period. Although similar effects were also noted in the diurnal rhythm of GIP release, these changes were much less marked than observed for GLP-1, as also reported for mice chronically fed a Western diet (7). When taken together with previous findings that GLP-1 exerts a greater ability than GIP to entrain insulin secretion by the β-cell (5, 7), these findings identify GLP-1 and the intestinal L-cell as key players in the complex interplay between the circadian clock and whole-body metabolism.
The results of the present study demonstrated increased stimulated GLP-1 secretion, but no differences in basal levels, in mice fed a HFD for 10 weeks in association with 3 to 4 weeks of STZ-induced diabetes. These changes were concomitant to an upregulation of genes related to metabolic and exocytotic pathways in the L-cell. Although not examining the effect of insulin-resistant diabetes, the intestinal L-cell has previously been interrogated after feeding of a high-fat (60%) and a Western (41% fat, 29% sucrose) diet for up to 16 weeks (7, 68). The findings indicate that ingestion of the high-fat diet for 2 weeks increases basal GLP-1 secretion and decreases small intestinal L-cell secretory responses to a variety of nutrient and chemical secretagogues in vitro. Consistent with this altered function, L-cell expression of a number of nutrient-sensing genes was decreased after 16 weeks of high-fat feeding (68). Conversely, feeding of a HFD for 6 weeks had no effect on basal but markedly enhanced the GLP-1 secretory response to oral glucose, in association with decreased mitochondrial gene expression but increased expression of genes related to cellular stimulation (39). Finally, the consumption of a Western diet for 16 weeks also had no effect on basal L-cell secretion but markedly increased stimulated GLP-1 release in vivo (7). Furthermore, transcriptomic pathways related to synaptic transmission and SNARE complex were upregulated in the L-cells from Western diet–fed mice (7). Of note, in this latter study, the microbiome and/or microbial metabolites were found to be a key modulator of L-cell secretory dynamics in vivo, as L-cell secretion was normal in vitro, in the absence of the gut microbiota (7). As all of these studies were carried out in Gcg-Venus animals, albeit in 2 different facilities (Cambridge and Toronto), it is therefore clear that diet composition, the duration of feeding, the intestinal microbiome, and the metabolic status of the animal all play critical roles in the regulation of L-cell GLP-1 release.
Secretion of GLP-1 in normal rodents follows a circadian pattern (5-7), as confirmed in the present study. Similarly, GLP-1 release in normal humans demonstrates differences by time of day although, of note, these rhythms are disrupted by both obesity and diabetes (69). The markedly increased and shifted pattern of GLP-1 secretion in response to an OGTT observed in the obese, diabetic mice herein is consistent with these findings in humans. Furthermore, transcriptomic analysis revealed that the L-cells of the HFD-STZ mice had decreased expression of Tef, an accessory regulator of the molecular clock that negatively regulates peak GLP-1 release (5). Clock disruption via Arntl knockdown and knockout also impairs GLP-1 release at the peak secretory time point (53), consistent with the findings of altered patterns in both Arntl and Cry2 in the HFD-STZ mice. While these findings indicate key roles for the clock in determining the temporal pattern of GLP-1 secretion, other studies have shown essential roles for downstream exocytotic proteins, most notably the SNARE accessory proteins STXBP1 and SCGN, both of which demonstrate circadian rhythms in the intestinal L-cell and are essential for the peak time point of GLP-1 secretion (6, 40). Interestingly, Scgn expression was found to oscillate by time of day in the intestinal mucosa, which exhibited a shifted and enhanced rhythm in the HFD-STZ mice. Although the finding was not specific to the intestinal L-cell, as Scgn is a general marker of enteroendocrine cells (45-47), mucosal levels peaked immediately prior to the peak in GLP-1 secretion in the HFD-STZ mice. This correlation supports a role for increased L-cell Scgn expression in the abnormal circadian pattern of GLP-1 release observed in the HFD-STZ mice. Future studies utilizing L-cell Scgn knockout mice will be required to definitively establish the role of Scgn in circadian GLP-1 release in vivo.
In agreement with the immunostaining evidence, Gcg expression was increased in the HFD-STZ condition. Levels of Sst, Ghrl, and Cck were also increased in the L-cells from these animals, in line with evidence that diet-induced prediabetes can cause shifts in enteroendocrine cell lineages and populations (70). Indeed, expression of Neurog3, an enteroendocrine lineage marker, was significantly increased in L-cells from the HFD-STZ mice, as was that of the endocrine transcription factor Sox9, as seen in db/db mice (71), also supporting the finding of a shift toward increased SCGN-expressing L-cells (72). Additionally, the stem cell markers Lgr5, Oflm4, and Mcm2 were found to be upregulated in the HFD-STZ L-cells, suggesting an increased de-differentiation of L-cells in the HFD-STZ mice and in line with previous findings that Lgr5 cell numbers are increased with STZ exposure (73). Such plasticity of L-cell expression patterns has been reported previously (74). Finally, L-cell Sstr1 expression was decreased in the HFD-STZ condition, which may contribute to the upregulation of GLP-1 secretion, as SSTR antagonism elicits beneficial effects on glycemia through a GLP-1-dependent mechanism (75).
Pathway analysis showed that there was an increase in transcripts relating to both GLP-1 secretion and electron transport chain activity in L-cells from the HFD-STZ animals. Indeed, normal mitochondrial function has previously been demonstrated to be critical for rhythmic GLP-1 secretion (38). However, the increased mRNA, protein, and secretion of GLP-1 in these mice may result in ER stress, as the unfolded protein response pathway was also upregulated in these cells. L-cell ER stress has been previously reported in the context of lipo- and gluco-toxicity in vitro (76) and, consistent with these findings, the localization and staining intensity of the ER stress marker, CHOP, were both increased in L-cells. Similar findings have been reported for other endocrine lineages, with β-cells exhibiting ER stress in the context of the hyper- production and secretion of insulin in T2D (77).
The validity of the HFD-STZ mice as a model of T2D was established by the demonstration of increased fasting glycemia, insufficient insulin secretion to overcome the obesity-associated insulin resistance, and hyperglucagonemia (78, 79). Furthermore, as reported for humans with T2D (42), as well as for mice fed a HFD alone (80), islet SCGN expression was reduced in the HFD-STZ mice. It was therefore somewhat unexpected that differences were noted in the murine L-cells as compared to those from humans with T2D. Specifically, the GLP-1 staining intensity that was observed to be increased in the murine L-cells was actually decreased in the human intestinal L-cells. Unfortunately, no clinical data were available for the human samples, and it is therefore unknown as to whether these samples were collected from individuals with early- and/or late-stage disease, nor was there any information as to any medications that may have been utilized. Furthermore, previous reports have suggested that GLP-1 levels are reduced, unchanged, or even increased in T2D (81-85). It is therefore not currently possible to determine whether the present findings on rhythmic GLP-1 secretion in the HFD-STZ mice can be translated to the human, even though defective circadian GLP-1 responses have been reported in obese subjects with T2D following weight loss surgery (69, 86, 87).
Although there was a trend toward higher insulin responses in control animals during the normal feeding period, this did not reach statistical significance. However, the finding of a lack of rhythmicity in the insulin responses to oral glucose is consistent with previous reports on normal mice (7, 39, 53). Notably, these results differ from those obtained in similar rat studies, wherein significantly higher insulin responses were noted at the onset of the feeding period (5, 88). Although regulated by incretin responses to oral glucose administration, contributions from the cell-autonomous β-cell clock, as well as species-dependent variations in the timing of insulin resistance are also determinants of insulin release (7, 16, 41). Of similar note, the α-cell has also been demonstrated to exhibit a cell-autonomous clock which is phase-delayed as compared to that of the β-cell, at least in vitro (41). It was therefore of interest that suppression of glucagon secretion by oral glucose was only observed during the normal feeding period. This resulted in a circadian rhythm that is consistent with the general physiological principle of reducing glucagon-induced hepatic glucose production when not in the fasting state (89).
Finally, it is acknowledged that there are several limitations of the present study. The first of these is the correlational evidence linking enhanced GLP-1 secretion to the suggested molecular mechanisms. While the clock genes are clearly involved, the exact identity of the downstream mediators requires further elucidation. In addition, although female Gcg-Venus mice were included in the L-cell transcriptomic studies, the immunostaining analyses were conducted on samples from male animals only. Finally, the transcriptomic analysis of the primary L-cells was a priori limited to a single time point at the onset of the feeding period (ZT14), as similar increases in GLP-1 responses were also found at this time in Gcg-Venus mice fed a Western diet (7). Analysis of these cells at multiple time points throughout the 24-hour clock may have revealed additional targets for future studies.
In summary, although previous studies have shown that obesogenic feeding is a circadian disruptor, rhythmic GLP-1 secretion has never been studied in the context of T2D. Current GLP-1 based treatments for T2D rely on the administration of exogenous peptides or degradation inhibitors, raising GLP-1 bioactivity to high levels throughout the 24-hour day. However, therapeutic avenues upregulating endogenously secreted GLP-1 remain largely uninvestigated, despite the importance of the circadian rhythm in GLP-1 for regulation of the downstream patterns of insulin release and, thus, of nutrient deposition. The results of the present study demonstrate marked changes in the normal circadian pattern of GLP-1 secretion and in the transcriptome and proteome of the intestinal L-cell, as well as in downstream glucose homeostasis in a murine model of T2D. Further interrogation into this mechanism may provide insights into therapies directed toward increasing endogenous GLP-1 secretion in the context of T2D.
Acknowledgments
The authors are grateful to Drs. F. Reimann and F. Gribble for the gift of the Gcg-Venus mice, A. Srikrishnaraj for the RNAscope analyses, and N. Simard from the University of Toronto Faculty of Medicine Flow Cytometry Facility. A.D.B was supported by Ontario Graduate Scholarships and graduate studentships from the Banting and Best Diabetes Centre, University of Toronto; and P.L.B. by a Canada Research Chair.
Glossary
Abbreviations
- ARNTL
aryl hydrocarbon receptor nuclear translocator-like protein 1
- AUC
area under the curve
- BMAL1
brain and muscle ARNTL protein 1
- DAPI
4′,6-diamidino-2-phenylindole
- GCG
proglucagon
- GIP
glucose-dependent insulinotrophic polypeptide
- GLP-1
glucagon-like peptide-1
- GLP-1R
glucagon-like peptide-1 receptor
- HFD
high-fat diet
- OGTT
oral glucose tolerance test
- qRT-PCR
quantitative reverse-transcriptase polymerase chain reaction
- RC
rodent chow
- SCGN
secretagogin
- STZ
streptozotocin
- T2D
type 2 diabetes
- ZT
zeitgeber
Contributor Information
Andrew D Biancolin, Departments of Physiology, University of Toronto, Toronto ON M5S 1A8, Canada.
Hyerin Jeong, Departments of Physiology, University of Toronto, Toronto ON M5S 1A8, Canada.
Kimberly W Y Mak, Departments of Physiology, University of Toronto, Toronto ON M5S 1A8, Canada.
Zixuan Yuan, Departments of Physiology, University of Toronto, Toronto ON M5S 1A8, Canada.
Patricia L Brubaker, Departments of Physiology, University of Toronto, Toronto ON M5S 1A8, Canada; Department of Medicine, University of Toronto, Toronto ON M5S 1A8, Canada.
Funding
Operating support for this study was obtained from the Canadian Institutes of Health Research (PJT-14853). Some of the equipment was provided by the 3D (Diet, Digestive Tract and Disease) Centre funded by the Canadian Foundation for Innovation and Ontario Research Fund (project numbers 19442 and 30961).
Author Contributions
A.D.B. researched data, analyzed data, and wrote the manuscript; H.J, K.W.Y.M, and Z.Y. researched data and edited the manuscript; and P.L.B. provided funding, edited the manuscript, and is the guarantor of the work.
Disclosures
A.D.B., H.J., K.W.Y.M., Z.Y., and P.L.B. have nothing to declare.
Data Availability
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153-165. doi: 10.1016/j.cmet.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 2. Müller TD, Finan B, Bloom SR, et al. . Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019;30:72–130. [DOI] [PMC free article] [PubMed]
- 3. Campbell JEE, Drucker DJJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819-837. [DOI] [PubMed] [Google Scholar]
- 4. Drucker DJ, Habener JF, Holst JJ. Discovery, characterization, and clinical development of the glucagon-like peptides. J Clin Invest. 2017;127(12):4217-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gil-Lozano M, Mingomataj EL, Wu WK, Ridout SA, Brubaker PL. Circadian secretion of the intestinal hormone GLP-1 by the rodent L cell. Diabetes. 2014;63(11):3674-3685. [DOI] [PubMed] [Google Scholar]
- 6. Biancolin AD, Martchenko A, Mitova E, et al. . The core clock gene, Bmal1, and its downstream target, the SNARE regulatory protein secretagogin, are necessary for circadian secretion of glucagon-like peptide-1. Mol Metab. 2020;31:124-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martchenko SE, Martchenko A, Cox BJ, et al. . Circadian GLP-1 secretion in mice is dependent on the intestinal microbiome for maintenance of diurnal metabolic homeostasis. Diabetes. 2020;69(12):258. [DOI] [PubMed] [Google Scholar]
- 8. Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science. 2016;354(6315):994-999. [DOI] [PubMed] [Google Scholar]
- 9. Buhr ED, Takahashi JS. Molecular components of the mammalian circadian clock. Handb Exp Pharmacol. 2013;217(217):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feng D, Lazar MA. Clocks, metabolism, and the epigenome. Mol Cell. 2012;47(2):158-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35(1):445-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tahara Y, Shibata S. Circadian rhythms of liver physiology and disease: experimental and clinical evidence. Nat Rev Gastroenterol Hepatol. 2016;13(4):217-226. [DOI] [PubMed] [Google Scholar]
- 13. Johnston JD, Ordovás JM, Scheer FA, Turek FW. Circadian rhythms, metabolism, and chrononutrition in rodents and humans. Adv Nutr. 2016;7(2):399-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoogerwerf WA, Hellmich HL, Cornélissen G, et al. . Clock gene expression in the murine gastrointestinal tract: Endogenous rhythmicity and effects of a feeding regimen. Gastroenterology. 2007;133(4):1250-1260. [DOI] [PubMed] [Google Scholar]
- 15. Mayeuf-Louchart A, Staels B, Duez H. Skeletal muscle functions around the clock. Diabetes Obes Metab. 2015;17:39-46. [DOI] [PubMed] [Google Scholar]
- 16. Marcheva B, Ramsey KM, Buhr ED, et al. . Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466(7306):627-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rey G, Cesbron F, Rougemont J, Reinke H, Brunner M, Naef F. Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. Kramer A, ed. PLoS Biol. 2011;9(2):e1000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koike N, Yoo SHS-H, Huang H-CHC, et al. . Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338(6105):349-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCarthy JJ, Andrews JL, McDearmon EL, et al. . Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol Genomics. 2007;31(1):86-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zvonic S, Ptitsyn AA, Conrad SA, et al. . Characterization of peripheral circadian clocks in adipose tissues. Diabetes. 2006;55(4):962-970. [DOI] [PubMed] [Google Scholar]
- 21. Gachon F, Loizides-Mangold U, Petrenko V, Dibner C. Glucose homeostasis: regulation by peripheral circadian clocks in rodents and humans. Endocrinology. 2017;158(5):1074-1084. [DOI] [PubMed] [Google Scholar]
- 22. Shan Z, Li Y, Zong G, et al. . Rotating night shift work and adherence to unhealthy lifestyle in predicting risk of type 2 diabetes: results from two large US cohorts of female nurses. BMJ. 2018;363:k4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: Two prospective cohort studies in women. Groop L, ed. PLoS Med. 2011;8(12):e1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Q, Shi J, Duan P, et al. . Is shift work associated with a higher risk of overweight or obesity? A systematic review of observational studies with meta-analysis. Int J Epidemiol. 2018;47(6):1956-1971. [DOI] [PubMed] [Google Scholar]
- 25. Antunes LC, Levandovski R, Dantas G, Caumo W, Hidalgo MP. Obesity and shift work: chronobiological aspects. Nutr Res Rev. 2010;23(1):155-168. [DOI] [PubMed] [Google Scholar]
- 26. Kohsaka A, Laposky AD, Ramsey KM, et al. . High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6(5):414-421. [DOI] [PubMed] [Google Scholar]
- 27. Challet E, Van Reeth O, Turek FW. Altered circadian responses to light in streptozotocin-induced diabetic mice. Am J Physiol. 1999;277(2):E232-E237. doi: 10.1152/AJPENDO.1999.277.2.E232 [DOI] [PubMed] [Google Scholar]
- 28. Javeed N, Matveyenko AV. Circadian etiology of type 2 diabetes mellitus. Physiology. 2018;33(2):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grosbellet E, Dumont S, Schuster-Klein C, et al. . Circadian phenotyping of obese and diabetic db/db mice. Biochimie. 2016;124:198-206. [DOI] [PubMed] [Google Scholar]
- 30. Gabriel BM, Altintaş A, Smith JAB, et al. . Disrupted circadian oscillations in type 2 diabetes are linked to altered rhythmic mitochondrial metabolism in skeletal muscle. Sci Adv. 2021;7(43):eabi9654. doi: 10.1126/SCIADV.ABI9654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Petrenko V, Gandasi NR, Sage D, Tengholm A, Barg S, Dibner C. In pancreatic islets from type 2 diabetes patients, the dampened circadian oscillators lead to reduced insulin and glucagon exocytosis. Proc Natl Acad Sci USA. 2020;117(5):2484-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schade DS, Eaton RP, Mitchell W, Ortega T. Glucose and insulin response to high carbohydrate meals in normal and maturity-onset diabetic subjects. Diabetes Care. 1980;3(2):242-244. [DOI] [PubMed] [Google Scholar]
- 33. Stamenkovic JA, Olsson AH, Nagorny CL, et al. . Regulation of core clock genes in human islets. Metabolism. 2012;61(7):978-985. [DOI] [PubMed] [Google Scholar]
- 34. Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166(16):1768-1774. [DOI] [PubMed] [Google Scholar]
- 35. Buxton OM, Cain SW, O’Connor SP, et al. . Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4(129):129ra43. doi: 10.1126/SCITRANSLMED.3003200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woon PY, Kaisaki PJ, Braganca J, et al. . Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci. 2007;104(36):14412-14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martchenko A, Martchenko SE, Biancolin AD, Brubaker PL. Circadian rhythms and the gastrointestinal tract: Relationship to metabolism and gut hormones. Endocrinology. 2020;161(12):bqaa167. doi: 10.1210/endocr/bqaa167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martchenko A, Oh RH, Wheeler SE, Gurges P, Chalmers JA, Brubaker PL. Suppression of circadian secretion of glucagon-like peptide-1 by the saturated fatty acid, palmitate. Acta Physiol. 2018;222(4):e13007. [DOI] [PubMed] [Google Scholar]
- 39. Martchenko A, Biancolin AD, Martchenko SE, Brubaker PL. Nobiletin ameliorates high fat-induced disruptions in rhythmic glucagon-like peptide-1 secretion. Sci Rep. 2022;12(1):7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Campbell JR, Martchenko A, Sweeney ME, et al. . Essential role of syntaxin-binding protein-1 in the regulation of glucagon-like peptide-1 secretion. Endocrinology. 2020;161(5):bqaa039. doi: 10.1210/endocr/bqaa039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Petrenko V, Saini C, Giovannoni L, et al. . Pancreatic α- and β-cellular clocks have distinct molecular properties and impact on islet hormone secretion and gene expression. Genes Dev. 2017;31(4):383-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Malenczyk K, Girach F, Szodorai E, et al. . A TRPV1-to-secretagogin regulatory axis controls pancreatic β-cell survival by modulating protein turnover. EMBO J. 2017;36(14):2107-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ferdaoussi M, Fu J, Dai X, et al. . SUMOylation and calcium control syntaxin-1A and secretagogin sequestration by tomosyn to regulate insulin exocytosis in human ß cells. Sci Rep. 2017;7(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee J-J, Yang S-Y, Park J, Ferrell JE, Shin D-H, Lee K-J. Calcium ion induced structural changes promote dimerization of secretagogin, which is required for its insulin secretory function. Sci Rep. 2017;7(1):6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gehart H, van Es JH, Hamer K, et al. . Identification of enteroendocrine regulators by real-time single-cell differentiation mapping. Cell. 2019;176(5):1158-1173.e16. [DOI] [PubMed] [Google Scholar]
- 46. Beumer J, Puschhof J, Bauzá-Martinez J, et al. . High-resolution mRNA and secretome atlas of human enteroendocrine cells. Cell. 2020;181(6):1291-1306.e19. [DOI] [PubMed] [Google Scholar]
- 47. Burclaff J, Bliton RJ, Breau KA, et al. . A proximal-to-distal survey of healthy adult human small intestine and colon epithelium by single-cell transcriptomics. Cell Mol Gastroenterol Hepatol. 2022;13(5):1554-1589. doi: 10.1016/J.JCMGH.2022.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sifuentes-Dominguez L, Li H, Llano E, et al. . SCGN deficiency results in colitis susceptibility. Elife. 2019;8:e49910. doi: 10.7554/eLife.49910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang SY, Lee JJ, Lee JH, et al. . Secretagogin affects insulin secretion in pancreatic β-cells by regulating actin dynamics and focal adhesion. Biochem J. 2016;473(12):1791-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rogstam A, Linse S, Lindqvist A, James P, Wagner L, Berggård T. Binding of calcium ions and SNAP-25 to the hexa EF-hand protein secretagogin. Biochem J. 2007;401(1):353-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: A primary cell study. Cell Metab. 2008;8(6):532-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Polotsky VY, Wilson JA, Haines AS, et al. . The impact of insulin-dependent diabetes on ventilatory control in the mouse. Am J Respir Crit Care Med. 2001;163(3 Pt 1):624-632. [DOI] [PubMed] [Google Scholar]
- 53. Martchenko SE, Martchenko A, Biancolin AD, Waller A, Brubaker PL. L-cell Arntl is required for rhythmic glucagon-like peptide-1 secretion and maintenance of intestinal homeostasis. Mol Metab. 2021;54:101340. doi: 10.1016/J.MOLMET.2021.101340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Biancolin AD, et al. . Disrupted and elevated circadian secretion of glucagon-like peptide-1 in a murine model of type 2 diabetes - University of Toronto Dataverse. Accessed July 8, 2022. 10.5683/SP3/MOKB9M [DOI] [PMC free article] [PubMed]
- 55. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402-408. [DOI] [PubMed] [Google Scholar]
- 56. Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34(5):525-527. [DOI] [PubMed] [Google Scholar]
- 57. Soneson C, Love MI, Robinson MD. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Research. 2015;4:1521. doi: 10.12688/F1000RESEARCH.7563.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Smyth GK. Limma: Linear models for microarray data. In: Gentleman R, Carey VJ, Huber W, Irizarry RA, Dudoit S (eds). Bioinformatics and Computational Biology Solutions Using R and Bioconductor; Springer, New York, NY, 2005:397–420. [Google Scholar]
- 60. Reimand J, Isserlin R, Voisin V, et al. . Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat Protoc. 2019;14(2):482-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Merico D, Isserlin R, Stueker O, Emili A, Bader GD. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS One. 2010;5(11):e13984. doi 10.1371/JOURNAL.PONE.0013984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kucera M, Isserlin R, Arkhangorodsky A, Bader GD. AutoAnnotate: a cytoscape app for summarizing networks with semantic annotations. F1000Research. 2016;5:1717. doi: 10.12688/F1000RESEARCH.9090.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wu G, Anafi RC, Hughes ME, Kornacker K, Hogenesch JB. MetaCycle: an integrated R package to evaluate periodicity in large scale data. Bioinformatics. 2016;32(21):3351-3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Parsons R, Parsons R, Garner N, Oster H, Rawashdeh O. CircaCompare: a method to estimate and statistically support differences in mesor, amplitude and phase, between circadian rhythms. Valencia A, ed. Bioinformatics. 2019;36(4):1208-1212. [DOI] [PubMed] [Google Scholar]
- 65. Malenczyk K, Szodorai E, Schnell R, et al. . Secretagogin protects pdx1 from proteasomal degradation to control a transcriptional program required for β cell specification. Mol Metab. 2018;14:108-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Khandelwal R, Sharma AK, Chadalawada S, Sharma Y. Secretagogin is a redox-responsive Ca 2+ sensor. Biochemistry. 2017;56(2):411-420. [DOI] [PubMed] [Google Scholar]
- 67. Fischer KD, Dhanvantari S, Drucker DJ, Brubaker PL. Intestinal growth is associated with elevated levels of glucagon-like peptide 2 in diabetic rats. Am J Physiol. 1997;273(4):E815-E820. doi: 10.1152/AJPENDO.1997.273.4.E815 [DOI] [PubMed] [Google Scholar]
- 68. Richards P, Pais R, Habib AM, et al. . High fat diet impairs the function of glucagon-like peptide-1 producing L-cells. Peptides. 2016;77:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mingrone G, Nolfe G, Castagneto Gissey G, et al. . Circadian rhythms of GIP and GLP1 in glucose-tolerant and in type 2 diabetic patients after biliopancreatic diversion. Diabetologia. 2009;52(5):873-881. [DOI] [PubMed] [Google Scholar]
- 70. Aliluev A, Tritschler S, Sterr M, et al. . Diet-induced alteration of intestinal stem cell function underlies obesity and prediabetes in mice. Nat Metab. 2021;3(9):1202-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Huang CZ, Xu JH, Zhong W, et al. . Sox9 transcriptionally regulates Wnt signaling in intestinal epithelial stem cells in hypomethylated crypts in the diabetic state. Stem Cell Res Ther. 2017;8(1):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li HJ, Ray SK, Kucukural A, Gradwohl G, Leiter AB. Reduced neurog3 gene dosage shifts enteroendocrine progenitor towards goblet cell lineage in the mouse intestine. Cell Mol Gastroenterol Hepatol. 2021;11(2):433-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhong XY, Yu T, Zhong W, et al. . Lgr5 positive stem cells sorted from small intestines of diabetic mice differentiate into higher proportion of absorptive cells and Paneth cells in vitro. Dev Growth Differ. 2015;57(6):453-465. [DOI] [PubMed] [Google Scholar]
- 74. Beumer J, Artegiani B, Post Y, et al. . Enteroendocrine cells switch hormone expression along the crypt-to-villus BMP signalling gradient. Nat Cell Biol. 2018;20(8):909-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jepsen SL, Wewer Albrechtsen NJ, Windeløv JA, et al. . Antagonizing somatostatin receptor subtype 2 and 5 reduces blood glucose in a gut- and GLP-1R-dependent manner. JCI Insight. 2021;6(4):e143228. doi: 10.1172/JCI.INSIGHT.143228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vasu S, Charlotte Moffett R, McClenaghan NH, Flatt PR. Differential molecular and cellular responses of GLP-1 secreting L-cells and pancreatic alpha cells to glucotoxicity and lipotoxicity. Exp Cell Res. 2015;336(1):100-108. [DOI] [PubMed] [Google Scholar]
- 77. Shrestha N, De Franco E, Arvan P, Cnop M. Pathological β-cell endoplasmic reticulum stress in type 2 diabetes: current evidence. Front Endocrinol (Lausanne). 2021;12:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mahler RJ, Adler ML. Type 2 diabetes mellitus: update on diagnosis, pathophysiology, and treatment. J Clin Endocrinol Metab. 1999;84(4):1165-1171. [DOI] [PubMed] [Google Scholar]
- 79. Galicia-Garcia U, Benito-Vicente A, Jebari S, et al. . Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21(17):1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hasegawa K, Wakino S, Kimoto M, et al. . The hydrolase DDAH2 enhances pancreatic insulin secretion by transcriptional regulation of secretagogin through a Sirt1-dependent mechanism in mice. FASEB J. 2013;27(6):2301-2315. [DOI] [PubMed] [Google Scholar]
- 81. Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: What is up, what is down? Diabetologia. 2011;54(1):10-18. [DOI] [PubMed] [Google Scholar]
- 82. Vollmer K, Hoist JJ, Bailer B, et al. . Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57(3):678-687. [DOI] [PubMed] [Google Scholar]
- 83. Smushkin G, Sathananthan A, Man CD, et al. . Defects in GLP-1 response to an oral challenge do not play a significant role in the pathogenesis of prediabetes. J Clin Endocrinol Metab. 2012;97(2):589-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Færch K, Vaag A, Holst JJ, Glümer C, Pedersen O, Borch-Johnsen K. Impaired fasting glycaemia vs impaired glucose tolerance: similar impairment of pancreatic alpha and beta cell function but differential roles of incretin hormones and insulin action. Diabetologia. 2008;51(5):853-861. [DOI] [PubMed] [Google Scholar]
- 85. Fritsche L, Heni M, Eckstein SS, et al. . Incretin hypersecretion in gestational diabetes mellitus. J Clin Endocrinol Metab. 2022;107(6):e2425-e2430. doi: 10.1210/CLINEM/DGAC095 [DOI] [PubMed] [Google Scholar]
- 86. Furman BL. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr Protoc. 2021;1(4):e78. [DOI] [PubMed] [Google Scholar]
- 87. Cefalu WT. Animal models of type 2 diabetes: clinical presentation and pathophysiological relevance to the human condition. ILAR J. 2006;47(3):186-198. [DOI] [PubMed] [Google Scholar]
- 88. Kalsbeek A, Strubbe JH. Circadian control of insulin secretion Is independent of the temporal distribution of feeding. Physiol Behav. 1998;63(4):553-560. [DOI] [PubMed] [Google Scholar]
- 89. Miller RA, Birnbaum MJ. Glucagon: acute actions on hepatic metabolism. Diabetologia. 2016;59(7):1376-1381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in References.