Abstract
The presence of certain microorganisms in dairy products or silage is highly desirable. Among them are probiotic strains of lactic acid bacteria (LAB), which show many beneficial features, including antimicrobial properties that support the development of beneficial microflora; in addition, owing to their biochemical activity, they influence the nutritional, dietary, and organoleptic properties of food products. Before being placed on the market, each strain requires separate testing to determine its probiotic properties and effectiveness. The aim of this study was to isolate LAB strains from a pickled beetroot sample that could be used in the dairy industry and with the potential to be considered as a probiotic in the future. Two strains identified using the MALDI technique were selected—Lactococcus lactis and Weissella cibaria. The optimal growth conditions of the strains were determined, and their proteolytic properties were assessed with the use of the o-PA reagent and spectrophotometry. The lipid profile was analyzed using the SALDI (surface-assisted laser desorption/ionization) technique and silver nanoparticles. High-performance liquid chromatography was used to assess the ability of the strains to synthesize beneficial metabolites, such as B vitamins (B2, B3, and B9) or lactic acid, and gas chromatography was used to analyze the substances responsible for organoleptic properties. Moreover, the ability to inhibit the growth of pathogenic strains was also tested in the selected strains. Both tested strains demonstrated the desired properties of starter cultures for future use in functional food production, showing that fermented plant products can serve as valuable potential probiotic sources.
Keywords: lactic acid bacteria, laser desorption/ionization, B vitamins, metabolites
1. Introduction
Probiotics have been around humankind since people started consuming fermented milk and food. Nevertheless, their beneficial effects remained unexplored until the last century. During this time, the meaning of the word has been changed several times; however, it has always been equated with health benefits. Currently the FAO/WHO recommends using the word probiotic in the context of “live microorganisms which when administered in adequate amounts confer a health benefit on the host” [1]. The effectiveness of the action of probiotic microorganisms depends on the size of their populations, which the definition does not specify. It is assumed that probiotic food products should contain more than 106–107 colony forming units (CFU) per gram so that the consumer can easily supply 109 CFU per day [2,3]. During food production, in order to accelerate or start the fermentation process, starter cultures are added to raw products in addition to probiotics. Due to the fact that they exhibit enzymatic activity, both probiotics and starter cultures enable the desired properties to be obtained in the final product (e.g., flavor and texture) [4].
Many microorganism species can be used as a probiotic or starter culture, including bacteria, yeasts, and molds. These microorganisms are added or can be found naturally in many different foods, such as fermented dairy products (yoghurt, cheese, and kefir), pickled vegetables and fruits, cereal, fermented sausages, bread, beer, or wine [2,5]. In the food industry, lactic acid bacteria (LAB) predominate. They mainly include the following genera: Lactobacillus, Lactococcus, Enterococcus, Streptococcus, Pediococcus, Leuconostoc, and Weisella [6]. A common feature of all LAB is the ability to anaerobically metabolize a number of sugars via fermentation (depending on the species/strains). The main product of this process is lactic acid, which has preservative properties and contributes to the inhibition of the growth of pathogenic microorganisms. Homofermentative bacteria, such as those belonging to the Lactoccocus or Streptococcus genera, produce this acid via the Embden–Meyerhof (EM) pathway [7]. In contrast, heterofermentative LAB strains (Weissella, Leuconostoc, and certain Lactobacillus), in addition to lactic acid, can also produce acetic acid, ethanol, carbon dioxide, or acetate through the phosphoketolase pathways. Depending on inter alia temperature and the strain used, the amount of lactic acid produced may vary from a few to over 200 g per liter of medium [8]. Lactic acid neutralizes the electrochemical potential of cell membranes and denaturates the intracellular proteins of microorganisms by lowering the pH of the environment, which limits the growth of pathogenic and spoilage bacteria. In addition, the beneficial changes that take place in the host’s body under the influence of probiotics include an increase in the number of positive intestinal microflora. The antimicrobial activity of probiotic strains also results from their ability to form biofilms on the gut wall, compete for nutrients, and produce or release various metabolites (lactic acid, antimicrobial peptides, short-chain fatty acids, and hydrogen peroxide) [2,6]. The important properties of probiotics also include their ability to biosynthesize water-soluble B-group vitamins (folic acid, riboflavin, niacin, and cobalamin), which are not produced by the human body [7]. Each B-group vitamin is chemically different and acts in synergy to maintain the body’s homeostasis by playing major roles in metabolic processes such as energy production and red blood cell formation. These vitamins are easily removed or destroyed during cooking and food processing; thus, the use of bacteria makes it possible to enrich foods with these compounds in situ, which is an additional benefit for consumers. Moreover, LAB strains produce enzymes that cause the degradation of fats, proteins, and carbohydrates present in food products. As a result, volatile compounds, such as aldehydes, ketones, esters, or alcohols, are formed, which give the final food products specific organoleptic properties [9]. LABs are also a rich source of lipid compounds that form cell membranes, participate in metabolic pathways (in protein transport, DNA replication, etc.), and are a reserve energy material. Hence, it is important to analyze the qualitative and quantitative composition of bacterial lipid compounds in order to determine and characterize the unique properties of the LAB [10].
Many studies show that consuming products that contain probiotics contributes to the improvement of intestinal health by regulating the microflora and stimulating and developing the immune system. Their consumption has a beneficial health effect in the case of food and urogenital infections, colon cancerogenesis, coronary heart disease, blood pressure, diabetes and obesity, allergies [11,12], psychiatric disorders [13], and even vaccine responses [14]. The presence of L(+) lactic acid in probiotic products also contributes to the reduced absorption of toxic substances and cholesterol into the blood [15]. Increased consumer awareness of the positive properties of products containing probiotics has led to an increase in their consumption. This is related to the great interest of food producers (especially in dairy sectors) in searching for new strains or isolates that show the best probiotic properties.
The aim of this study was to isolate LAB strains from a pickled beetroot sample that could be used in the dairy industry as starter cultures for future use in the production of functional foods. For the selected and identified LAB strains, tests were carried out to assess their physiological characteristics, interactions with pathogenic bacteria, ability to synthesize B vitamins and lactic acid, and production of volatile compounds.
2. Materials and Methods
2.1. Isolation of Microorganisms
The LAB strains were isolated from homemade pickled beetroots. Pickled foods are widely regarded in Poland as having health-promoting properties and a being a good source of natural probiotics. A series of 10-fold dilutions ranging from 10−1 to 10−4 in peptone water (Peptone Water, Sigma-Aldrich, Germany) was prepared from the tested samples. The samples and all dilutions were then applied to 7 different culture media. A total of 100 μL of the appropriate dilution was applied to the medium and evenly distributed with a spreader. The prepared plates were incubated for 24–72 h in three conditions: aerobic conditions at 37 °C, aerobic conditions at 25 °C, and anaerobic conditions at 30 °C. Then, based on morphological differences, single colonies of bacteria were selected, from which reduction cultures were prepared on the same media to obtain pure cultures. The following culture media were used: M-17 Agar (Sigma Aldrich, Steinheim, Germany), MRS Agar (Sigma Aldrich, Steinheim, Germany), China Blue Lactose Agar (Sigma Aldrich, Steinheim, Germany), APT Agar (Merc, Darmstadt, Germany), and TOS (Merck, Darmstadt, Germany).
2.2. Identification Using the MALDI Technique
The identification of the isolated strains was carried out using the MALDI (matrix-assisted laser desorption/ionization) spectrometric technique (microflex LT MALDI-TOF MS) by the direct application of the sample to the plate (according to the procedure recommended by the manufacturer [16]). For this purpose, the isolated colony was spread as a thin layer directly onto the sample position. Then, 1 μL of 70% formic acid was applied to the sample; after it dried, 1 μL of HCCA matrix solution (10 mg/mL of a solution containing 50% acetonitrile, 47.5% water, and 2.5% trifluoroacetic acid) was applied. The collected spectra were analyzed with the help of the Biotyper platform. From the isolated strains, 2 with the expected properties were selected for further analysis.
2.3. Microscopic Observation
For the selected strains, Gram staining was performed followed by a microscopic examination of the cells’ morphology according to the following procedure: (1) suspend bacterial cells in a drop of distilled water applied to a microscopic slide using a microbial loop (1 μL); (2) fix the bacterial cells after water evaporation by transferring the slide over a burner (3 times); (3) apply the basic dye (crystal violet; 3 min); (4) rinse with distilled water; (5) apply the iodine solution (2 min); (6) rinse with distilled water; (7) apply the ethanol (30 s); (8) rinse with distilled water; (9) stain with an additive colorant (safranin; 30 s); (10) rinse with distilled water.
2.4. Confirmation of Identification by 16S rRNA Gene Sequencing
For DNA extraction, single colonies of the 2 selected strains were subcultured in liquid media (Tryptic Soy Agar, Sigma Aldrich, Steinheim, Germany) and incubated for 24 h at 30 °C. A total of 2 mL of liquid culture of each bacteria strain was centrifuged, and DNA was extracted using a combination of bead-beating and an E.Z.N.A. Bacterial DNA Kit (Omega BIO-TEK, Norcross, USA). The universal 16S rRNA bacterial primers 1492R (5′-GGTTACCTTGTTACGACTT-3′) and 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) were applied to amplify this gene [17]. The PCR products were visualized on a 1% agarose gel stained with ethidium bromide under UV light to confirm the presence of an approximately 1350 bp band. The PCR products were purified and sequenced by Genomed (Warsaw, Poland). The obtained results were analyzed using the BioEdit program and compared with the NCBI online database.
2.5. Growth Assessment of Selected Isolates
The aim of this step was to find the best culture conditions for the cultivation of the selected bacteria in view of biomass preparation for future starter cultures. To determine the optimal growth conditions, bacterial suspensions with optical densities of 0.5 McF were prepared in liquid culture media. The following media were used: MRS broth (Sigma Aldrich, Steinheim, Germany), tryptic soy broth (Sigma Aldrich, Steinheim, Germany), M-17 broth (Sigma Aldrich, Steinheim, Germany), and LAPTg. The LAPTg medium was prepared by dissolving the following ingredients in 1 L of water: 10 g of yeast extract, 15 g of peptone, 1 mL of Tween 80, 10 g of tryptone, and 18 g of lactose (pH approx. 6.5). The bacterial suspensions were incubated at 20 and 30 °C. The optical density of each sample after 6, 24, and 48 h was measured in triplicate. A DEN-1 densitometer (Biosan) was used for the measurements.
2.6. Determination of Proteolytic Properties
The proteolytic activity of the selected two strains was determined by means of the spectrophotometric test using the o-phthaldialdehyde (o-PA) reagent [18]. The o-PA solution was prepared by mixing the following reagents: 25 mL of 100 mM sodium tetraborate, 2.5 mL of 20% (m/V) sodium dodecyl sulfate, 1 mL of 40 mg/mL o-PA dissolved in methanol, and 0.1 mL of β-mercaptoethanol.
Before starting the tests, the bacteria were plated on solid MRS agar and incubated for about 24 h at 30 °C. The appropriate amount of biomass was then transferred to 0.9% saline via a loop to determine the optical density of 1 McF. Subsequently, 1 mL of the obtained suspension was transferred into 9 mL of 10% (m/V) RSM (reconstituted skimmed milk) and incubated at 37 °C for 24 h. After the incubation, 0.75 M trichloroacetic acid was added to the obtained cultures for deproteinization at a volume ratio of 1:2. The samples were centrifuged, and the obtained supernatants were incubated in o-Pa solution for 10 min at room temperature. Then, the absorbance (λ = 340 nm) was measured against the control sample using a UV-Vis spectrophotometer (NanoDrop 2000c, Thermo Scientific). Each sample was measured in triplicate. The results were expressed in millimoles (mM) of free amino acids (FAA) per liter of milk by referring to a prepared standard curve of glycine.
2.7. Volatile Organic Compounds Analysis
MRS agar was poured into sterile headspace tubes. The selected bacterial strains were plated on the medium and incubated at 37 °C for 48 h. Subsequently, solid phase microextraction (SPME) was performed using Carboxen/polydimethylsiloxane fiber. The extraction was performed for 15 min, and desorption was carried out in the dispenser for 2 min at 230 °C. A chromatographic analysis was performed using GC-MS (Agilent) with a splitless dispenser and a temperature gradient program. The temperature program for the chromatographic furnace was as follows: 40 °C (2 min), accretion at 10 °C/min to 140 °C (10 min), and accretion at 5 °C/min to 270 °C (5 min). The flow rate of the helium carrier gas through the column was 1.2 mL/min. Electron beam ionization (EI) at 70 eV was used. The temperature of the ion source was 250 °C and the temperature of the transfer line was 300 °C. MS spectra were recorded in the range of 35–550 m/z. The obtained data were deconvolved and equalized. Integrated peaks were identified using the NIST 17 library. The following quality control criteria were used: matching factor min. 70% and presence in min. 2 out of 3 analyzed repetitions.
2.8. Analysis of Fats by Mass Spectrometry
The two selected strains were analyzed and cultured for 48 h at 37 °C in liquid medium (MRS broth). After centrifugation, the biomass was washed in 1 mL of 0.9% NaCl solution, then lipid extraction was performed using the Folch method. A total of 1.5 mL of a 2:1 (vol/vol) chloroform/methanol mixture was added to the biomass. The Eppendorf tubes were transferred to an ultrasonic bath and left for 15 min at room temperature. Then, 0.5 mL of 0.05 M NaCl was added to the suspension and vortexed for 10 min. In the next step, the suspensions were centrifuged for 15 min at 2415× g, the upper layer was collected in separate test tubes, 0.5 mL of 0.05 M NaCl solution was added, and the tubes were vortexed again. The two bottom layers were combined, and the solvent was evaporated using a vacuum centrifuge. The lipid samples were dissolved in chloroform and a volume of 0.5 µL was applied to the plates immediately before measurement.
A plate covered with a layer of silver nanostructures applied to the surface of H17 steel by means of electrodeposition was selected for the measurements [19]. Prior to the synthesis of silver nanostructures, the plates were cleaned in an ultrasonic cleaner with acetone, methanol, and acetonitrile. The two plates were then placed facing each other in a 100 mL beaker and connected to a Consort EV202 bench power supply. Ninety milliliters of silver trifluoroacetate solution (98%; Trimen Chemicals, Łódź, Poland) was poured into a beaker. The solution was prepared by dissolving 9 × 10−5 moles of AgTFA in a mixture of 80% isopropanol and 20% acetonitrile. Electrodeposition was carried out for 15 min at a voltage of 10V. The negative electrode was then cleaned with cotton wool and washed three times in boiling acetonitrile and isopropanol. LDI TOF MS measurements were performed in the m/z 80–2000 range with an UltrafleXtreme mass spectrometer (Bruker Daltonics, Bremen, Germany) equipped with a 355 nm laser with a frequency of 2 kHz. The number of laser shots was 2000 (4 × 500) per sample. The electrode voltages were 26.64 and 13.54 kV. At the first acceleration, the voltage was 25.08 kV, and for the second ion source it was 22.43 kV. The detector gain value for the reflectron was 30×. Cubic calibration was performed using silver cluster signals. An analysis of the data obtained was performed using the FlexAnalysis 3.3 software (Bruker Daltonics, Bremen, Germany) and mMass 5.5.0.
2.9. Analysis of B Vitamins and Lactic Acid
B vitamins: Bacterial biomass was added via a sterile loop into sterile tubes containing niacin test medium and MRS broth to obtain a 0.5 and 1 McF suspension. The cultures prepared in this way were incubated at 20 and 30 °C for 24 h. After this, the contents of the tubes were vortexed and then centrifuged (4000 rpm, 10 min at 4 °C). The obtained supernatant was used for HPLC analysis to determine B vitamin content (riboflavin—B2; niacin—B3; and folic acid—B9). A chromatographic analysis was performed using LC-MS 8050 and a Kinetex C8 column (100 × 2.1 mm, 1.7 µm). The mobile phase was a mixture of MeOH and 0.1% HCOOH in H2O in a gradient elution (0.01–5 min, 20–35% B; 5–6 min, 35–20% B; 6–7 min, 20% B), the flow was 0.2 mL/min, the oven temperature was 30 °C, and the injection was 3 µL.
Lactose (L) and lactic acid (LA): Sufficient bacterial biomass was added to sterile tubes containing LAPTg medium to obtain a suspension concentration of 1 McF. The cultures prepared in this way were incubated at 20/30 °C for 24 h. After this, the contents of the tubes were vortexed and then centrifuged (4000 rpm, 10 min at 4 °C). The obtained supernatant was used for the HPLC analysis of lactose and lactic acid. Chromatographic analysis was performed using LC-MS 8050 and a Kinetex C8 column (100 × 2.1 mm, 1.7 µm). The mobile phase was 0.1% HCOOH in H2O with a flow rate of 0.2 mL/min, oven temperature of 30 °C, and injection of 2 µL.
In order to perform the quantitative analysis, the MRM MS (multiple reaction monitoring) scanning mode was used (Table 1). The method was validated via the use of a series of standard solutions for each of the determined compounds. For the determination of vitamins on an analytical balance, 1 mg of vitamins B2, B3, and B9 were weighed and dissolved in 1 mL of distilled water. In turn, 2.5 mg of lactic acid and 1 mg of lactose were dissolved in 1 mL of 0.1% HCOOH in H2O. Dilutions were prepared from the stock solutions and used for method validation (Table 2).
Table 1.
Conditions for the detection of the analyzed compounds. B2—riboflavin; B3—niacin; B9—folic acid; L—lactose; LA—lactic acid.
| Retention Time (Min) | Ionization | Molecular Ion | Fragment Ion | Q1 (V) | CE | Q3 (V) | |
|---|---|---|---|---|---|---|---|
| B2 | 3.811 | Positive | 337 | 243 | −20 | −25 | −20 |
| B3 | 1.247 | Positive | 124 | 79 | −10 | −24 | −15 |
| B9 | 2.971 | Positive | 442 | 176 | −16 | −39 | −19 |
| L | 1.060 | Negative | 341 | 161.15 | 13 | 8 | 15 |
| LA | 1.594 | Negative | 89.25 | 43.05 | 19 | 13 | 14 |
Table 2.
Validation of the analytical method. LOD—limit of detection; LOQ—limit of quantification.
| Linearity (µg/mL) | R2 | Standard Curve Equation | LOD (µg/mL) | LOQ (µg/mL) | |
|---|---|---|---|---|---|
| B2 | 0.005–10 | 0.9941 | y = 1,137,260x − 89,427 | 0.001 | 0.0033 |
| B3 | 0.25–10 | 0.9985 | y = 8819.9x − 1000.03 | 0.050 | 0.1650 |
| B9 | 0.025–10 | 0.9980 | y = 104,830x + 11,539 | 0.005 | 0.0165 |
| L | 0.01–400 | 0.9963 | y = 19,947x + 122,904 | 0.005 | 0.0165 |
| LA | 0.01–2000 | 0.9972 | y = 18,770x + 651,622 | 0.005 | 0.0165 |
2.10. Inhibition of Pathogens
The antimicrobial activity of the selected LAB strains (Lactococcus lactis and Weissella cibaria) was tested against 3 strains of pathogenic bacteria: Escherichia coli, Enterobacter cloacae, Pseudomonas aeruginosa, and one food spoilage microorganism—Listeria innocua. All strains came from our own collection and were isolated from clinical specimens (diabetic foot swabs) or environmental specimens. In this study, we used the supernatant of LAB strains in liquid MRS broth medium (Sigma Aldrich, Germany). For this purpose, LAB cell biomass was added to the medium such that the optical density of the suspension was about 4 McF. The samples were then incubated for 24 h at 37 °C (aerobic conditions). In order to obtain a fluid containing no bacterial cells, after incubation, the samples were centrifuged (30 min, 4000 rpm), and the obtained supernatant was used for further studies. Mueller–Hinton agar (MH; Sigma Aldrich, Steinheim, Germany) was used in this assay. MH agar is a non-selective medium that is recommended for testing the sensitivity of bacteria to various substances, e.g., antibiotics, bacteriocins, etc. The assay consisted of spreading 100 μL of the pathogen suspension on the surface of the plate. Then, after 15 min, 0.3 mL of lactic acid bacteria supernatant was applied to the surface of the medium and incubated for 24 h at 25 °C (aerobic conditions). All the tests were repeated three times for each sample.
3. Results and Discussion
3.1. Isolation of Lactic Acid Bacteria—Identification and Phenotypic Characterization
Fermented vegetables are traditionally obtained through the spontaneous fermentation of lactic acid, in which a variety of microorganisms are involved. The microflora of vegetables is varied and depends on the type of plant and the part of it being processed. Its composition significantly affects the quality and safety of final products [20]. With the use of the MALDI technique, five Gram-positive bacteria (Lactococcus garvieae, Lactococcus lactis, Lactobacillus plantarum, Leuconostoc citreum, and Weissella cibaria) and two fungi (Geotrichum candidum and Pichia kluyveri) were identified in a pickled red beet sample. All of the isolated bacteria were lactic acid bacteria (LAB). Two isolated strains (W. cibaria and L. citereum) were heterofermentative bacteria, which play a role in the initial steps of vegetable fermentation. In turn, the remaining bacterial strains were homofermentative bacteria, which have a higher tolerance to high salt concentrations and acidification, meaning they play a role in the final stages of the fermentation process [21]. Two LAB strains—Lactococcus lactis and Weissella cibaria—were selected for further research. L. lactis is a well-known LAB strain that shows high potential as a probiotic; in addition, its use in food production has a long history. Nevertheless, L. lactis is mainly isolated from dairy products. In our work, we focused on another type of potential probiotic source, namely silage. The second strain, W. cibaria, has been found to have probiotic potential and various beneficial properties, including the functional production of exopolysaccharides and attenuation of pathogen-induced inflammatory responses. In addition, this strain was found to be the dominant species in the microflora of silage products, such as kimchi [22]. One of the requirements set for starter cultures strains is their precise characterization. For this reason, additional identification was performed for these strains by sequencing the 16S rRNA gene. This gene has been the basis of sequence-based bacterial analysis for decades because it is highly conservative and present in all bacteria [23]. A comparison of the identification results obtained using the MALDI and 16S rRNA techniques is presented in Table 3.
Table 3.
Comparison of identification results between the MALDI and 16S rRNA techniques.
| Identification by MALDI | 16S rRNA Identification | Number in PCM ** | |||
|---|---|---|---|---|---|
| Identification Result | MSP Log Score * | Identification Result | % Similarity | Strand Length (bp) | |
| Lactococcus lactis ssp lactis 3C1_QSA IBS | 2.02 |
Lactococcus lactis strain NBRC 100933 [NR_113960.1] Lactococcus lactis strain NCDO 604 [NR_040955.1] |
99.86 99.86 |
1412 | L. lactis Beet 1 [OM957496] |
| Weissella cibaria DSM 15878T DSM | 2.13 |
Weissella cibaria strain II-I-59 [NR_036924.1] Weissella confusa strain JCM 1093 [NR_040816.1] |
99.58 99.17 |
1442 | W. cibaria Beet 2 [OM957500] |
* log score >2.00—secure genus identification, probable species identification. ** PCM—Polish Collection of Microorganisms.
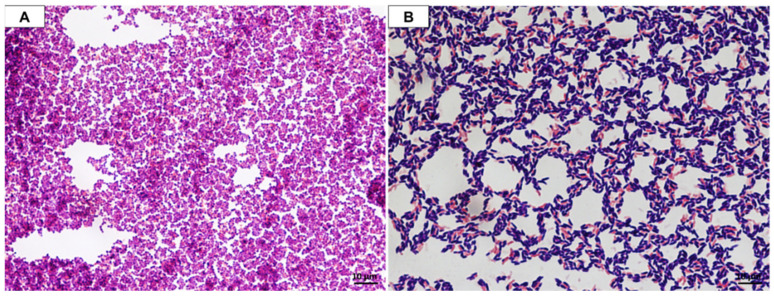
The microscopic observation of the cell morphology of the selected bacterial strains showed that L. lactis and W. cibaria are Gram-positive bacteria (Figure 1). The L. lactis cells demonstrated a spherical shape (Ø 1 µm) and occurred in chains (see Figure 1A), while W. cibaria had rod-shaped cells growing in pairs (2.0–2.5 µm long and 0.9–1.0 µm wide) (see Figure 1B), which is consistent with their taxonomic affiliation. The selected LAB strains were tested for their ability to: (1) produce catalase; (2) produce organic acids by metabolizing glucose; (3) metabolize citrate; and (4) produce the nitrate reductase enzyme. For both strains, the results of the tests mentioned above were negative, i.e., no activity in the examined aspect.
Figure 1.
Optical microscope photos of the Gram staining of the tested strains: (A)—Lactococcus lactis; (B)—Weissella cibaria.
3.2. Growth Assessment of Selected Isolates
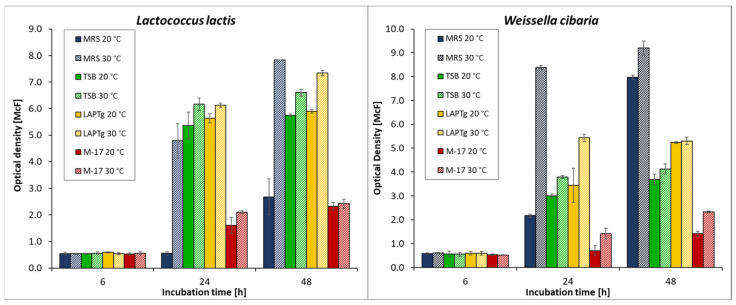
The ability of the selected LAB to grow under various culture conditions was assessed by measuring optical density (OD). Measuring the OD at 565 nm is the most common method for estimating the concentration of cells in a suspension liquid [24]. For the growth assessment of L. lactis and W. cibaria, four different culture media (MRSB, TSB, LAPTg and M17), two temperatures (20 °C and 30 °C), and three measurement points (6, 24, and 48 h) were selected. During the measurements, the effect of the background absorbance by the pure medium was taken into account (MRS—1.1; TSB—0.28; M-17—6.63; LAPTg—0.68). For all strains, an increase in optical density was observed with increasing cultivation time. The degree of growth depended on the medium used and the incubation temperature.
The results of the growth assessment showed that the six-hour incubation did not cause any significant differences between the set cultivation conditions, and the OD of both strains was comparable (0.53–0.60 McF); however, after 24 h, the differences began to become noticeable (Figure 2). Considering the lower temperature (20 °C), the growth of L. lactis was significantly improved when LAPTg or TSB medium was applied, and only a slight increase in OD between 24 and 48 h was observed. Similar observations were noted for W. cibaria after 1 day of incubation; however, the differences between these two media and the other two media were less than in the case of the former strain. Moreover, after 48 h, the best growth of W. cibaria was observed in MRSB, as shown by a considerable increase in the bacterial OD between 1 and 2 days of incubation—from 2.17 to 7.97 McF. Regarding the higher incubation temperature (30 °C), W. cibaria demonstrated a considerably higher growth in biomass after 24 and 48 hrs of incubation when MRSB was applied −1.5–5.9 times higher compared to the other culture media. Contrary to this, the growth of L. lactis was similar on all tested culture media except for the M17 medium, where the increase in biomass was significantly lower—by 2.2–3.2 times. In general, the M17 medium appeared to be the worst given the growth of both tested LAB strains.
Figure 2.
Graphs showing the growth of the tested LAB strains in various culture media and temperatures.
3.3. Proteolytic Properties
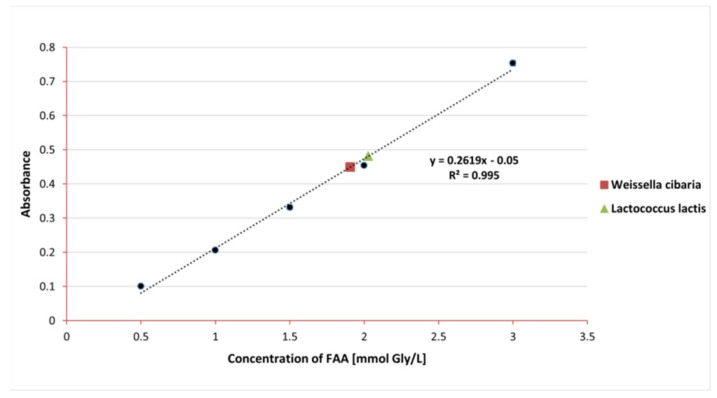
From an industrial point of view, proteolytic activity is an advantageous feature of LAB strains. In the dairy industry, proteolytic properties play a key role in ensuring successful fermentation. Proteolytic enzymes secreted by bacteria release peptides and amino acids from casein, which are involved in creating the aroma, texture, and taste of the final product [25]. The proteolytic properties of the tested strains were determined by means of a spectrophotometric test with the o-PA reagent. The concentration of FAA was calculated based on the equation of the glycine standard curve and the obtained absorbance results. The result for L. lactis was 2.01 ± 0.002 mmol Gly/L FFA, and for W. cibaria 1.91 ± 0.01 mmol Gly/L FFA (see Figure 3).
Figure 3.
Calibration curve showing the dependence of absorbance on the concentration of glycine.
According to data in the literature, proteolytic activity is classified as low (0–1 mmol/L), medium (1–2 mmol/L), and high (2–3 mmol/L) [25]. The proteolytic activity of the tested strains was between 1 and 2 mmol/L, which proves their intermediate activity. Joković et al. also examined the proteolytic activity of many different LAB strains isolated from dairy products using the same technique [26]. The strains of W. cibaria isolated by the authors were characterized with the lowest values of proteolytic activity (about 0.5 mmol Gly/L). In our work, we obtained activity at a level four times higher in the case of W. ciabria, which indicates the possibility of using this strain as a potential starter culture in the dairy industry. Joković et al. also tested fourteen L. lactis strains. As in our study, they observed that the proteolytic activity of L. lactis was higher than that of W. cibaria. In turn, the proteolytic activity of various L. lactis strains isolated by Gonzalez et al. during the cheese-making process ranged from 0.68 to 5.98 mMGly/L [27]. In the work of Herreros et al., the range of proteolytic activity of L. lactis isolated from cheese was 0.22 to 2.85 mMGly/L [28]. Our results for L. lactis are consistent with the above values from the literature. The differences in our results may have many causes, of which the choice of environmental matrix for the isolation of the strain has the greatest influence.
3.4. Volatile Compounds
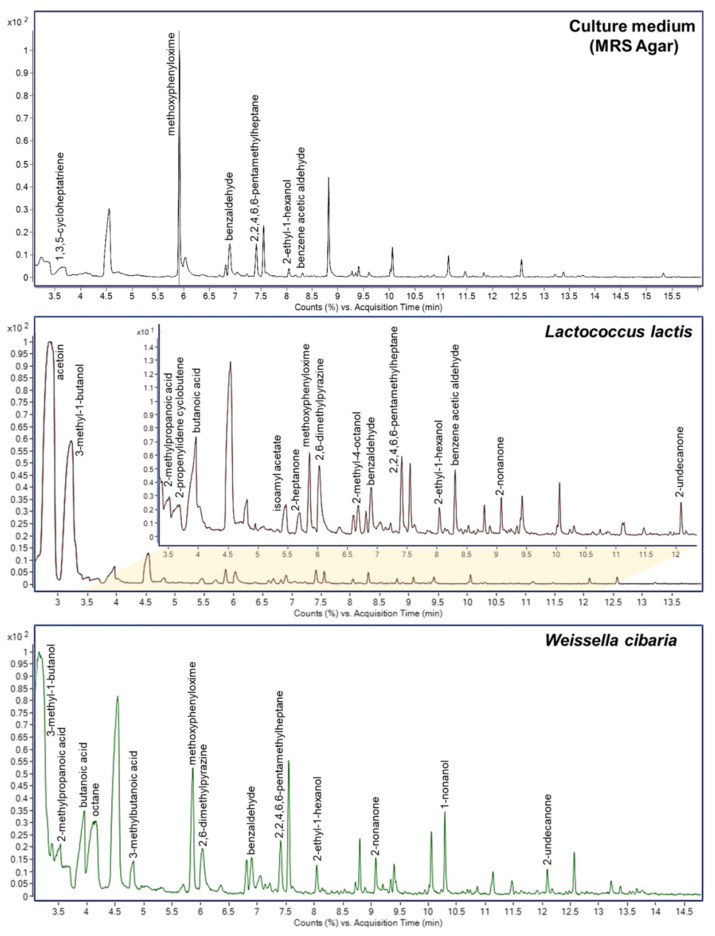
Lactic acid bacteria produce volatile organic compounds (VOCs) by fermenting various products. Their composition is responsible for the taste, smell, and texture of fermented products, e.g., dairy products. VOCs produced by L. lactis and W. cibaria strains were analyzed using gas chromatography (GC). A control analysis of an applied culture medium was also performed. The peaks in the chromatograms were matched with compounds in the NIST 17 library (see Figure 4). Sixteen compounds were identified in the L. lactis chromatogram and thirteen in W. cibaria. They were mainly alcohols, ketones, acids, and aldehydes. Ten of the identified compounds were present in both LAB strain samples. Six compounds were identified in the control sample. Four of them (methoxyphenyloxime, benzaldehyde, 2,2,4,6,6-pentamethylheptane, and 2-ethyl-1-hexanol) were also identified in samples of both LAB strains. In the case of the L. lactis strain, benzene acetic aldehyde was additionally identified, which was also present in the control sample.
Figure 4.
GC/MS chromatograms of control sample (MRS agar) and analyzed LAB strain samples.
LAB strains take part in three basic biochemical processes that are responsible for the basic taste of fermented milk products. They are glycolysis (the metabolism of lactose, lactate, and citrate), lipolysis, and proteolysis [29]. As a result of the metabolism of citrate or lactose, the main fragrance compound produced is acetoin, which gives a buttery (acidic, sour, cheesy, dairy, and creamy with a fruity nuance) odor [30,31]. In our research, this compound was identified only in the L. lactis strain; however, its amount was much higher compared to the other compounds. Interestingly, although L. lactis is one of the most commonly used strains in the production of fermented foods, it usually requires a chemical or genetic mutation to increase acetoin production [30]. In turn, as a result of the oxidative degradation of fatty acids and amino acids, methyl ketones and aldehydes are formed, which can be modified by dehydrogenases to form alcohols and organic acids [29]. For example, 2-nonanone, 1-nonanone, and 2-heptanone—identified in the samples—are compounds that are very common in dairy products, giving them a hot milk and dairy-like aroma and delicate taste [29,32]. 3-methyl-1-butanol, identified in both samples, and 3-methylbutanoic acid, identified in W. cibaria, give dairy products a characteristic cheese aroma. Butanoic acid is present in almost all dairy products (also in both of our strains) and is considered as the most potent odorant, giving a buttery, sweaty, cheesy, and rancid odor [32].
3.5. Analysis of Fats
During the surface-assisted laser desorption/ionization analysis, the spectra of the tested strains were recorded. The assignment of the probable metabolites, peptides, and lipids to the m/z values was carried out by searching the HMDB [33], LIPID MAPS [34], and MetaCyc [35] databases. The compounds identified in the spectra were present in the form of adducts with a proton, sodium, and potassium, and by using a plate covered with silver nanoparticles, we also found adducts with two Ag isotopes, i.e., [M + 107Ag]+ and [M + 109Ag]+. The possibility of the silver cationization of the analyzed compounds is not surprising and has been described by many authors who have used SALDI methods based on plates with silver nanoparticles [36,37,38,39]. SALDI methods based on silver nanoparticles have an established position in the analysis of compounds with low molecular weights, such as metabolites or lipids [40]; however, it is worth noting that none of these techniques have thus far been applied to the analysis of lipids from microorganisms.
For the analysis of the spectra, rigorous criteria were used when searching for compounds in databases: the signal-to-noise ratio was a minimum of 5, and the match error was no more than |10| ppm. In the SALDI MS spectrum of the L. lactis extract, 67 signals were identified that were assigned to 64 compounds (Table 4), while for W. cibaria, there were 84 signals matched with 77 compounds (Table 5). Despite the use of the Folch extraction method, the spectra showed many signals not only from lipids, but also from peptides. In some cases, extracts from bacterial cells may be contaminated with peptides, sugars, free amino acids, and other endogenous compounds. Peptides trapped in lipid micelles can enter the organic phase during the extraction process [41]. Lactic acid bacteria are known for their production of bioactive peptides produced from proteins by microbial proteolysis [42,43,44]; thus, it is not surprising that they were present in cell extracts. The remaining signals on the spectra were mainly assigned to lipids belonging to all classes, i.e., fatty acids, glycerolipids, glycerophospholipids, sphingolipids, and steroids. Fatty acids (FA) are one of the most dynamic components of bacterial cells. They are components of phospholipids and play a key role in maintaining the structure and function of the cell membrane [10]. The glycerolipids identified in the spectra were triacylglycerols (TGs) and diacylglycerols, which are a reserve and energy material in bacterial cells. Glycerolipids also regulate the level of lipids in cell membranes and are precursors in the synthesis of phospholipids [45,46,47]. Among the glycerophospholipids identified were phosphatidylglycerols (PGs), which are the main phospholipids among lactic bacteria; phosphatidylethanolamines (PEs), responsible for membrane architecture and bacterial mobility; phosphatidylinositols (PIs), playing a role in the dynamics of the bacterial membrane [48,49]; phosphatidylcholine (PC), which plays a key role in symbiotic interactions and resistance to bacteria and heavy metals; and phosphatidylserine (PS), which is a precursor in the synthesis of PEs [10,50]. Another group of lipids detected in the extracts were ceramides, which belong to sphingolipids. They act as a protective barrier by trapping water in cells [51]. Sterols are another class of lipids that were identified in the spectra. These compounds have been detected in bacteria; however, there is insufficient research to confirm their properties and importance [52]. Among the other compounds identified in the spectrum of the L. lactis extract, we distinguished celotriose (m/z 613.075), which is glucotriose, a compound that acts as a bacterial xenobiotic metabolite. Interestingly, in their work, Adsul et al. showed the possibility of using cellotriosis by Lactobacillus spp. bacteria as a carbon source and for the microbial production of lactic acid [53].
Table 4.
SALDI mass spectrum of Lactococcus lactis lipid extract made on an AgNLET plate with electroplated silver nanoparticles (S/N ≥ 5, Δ max. ± 10 ppm).
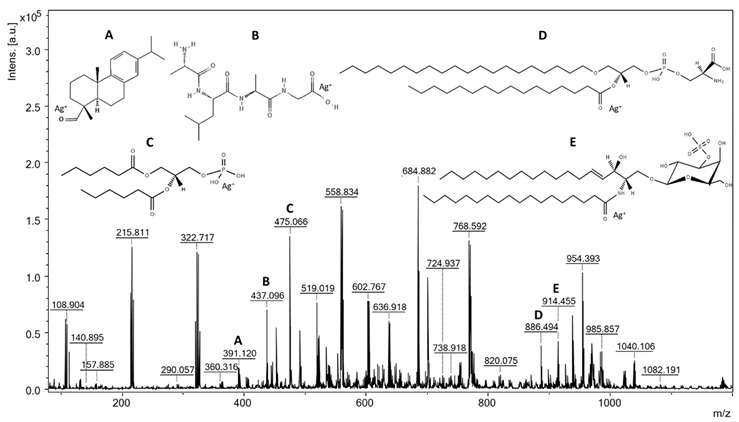
| |||||
|---|---|---|---|---|---|
| Compound * | Adduct Formula | m/z Exp. | m/z Calc. | Δ (ppm) | |
| 1. | [FA oxo(15:0)] 4-oxo-pentadecanoic acid | [C15H28O3 + 107Ag]+ | 363.108 | 363.108 | 0.0 |
| 2. | [FA hydroxy(17:2)]12-hydroxy-8E,10Eheptadecadienoic acid | [C17H30O3 + 107Ag]+ | 389.123 | 389.124 | −2.6 |
| 3. | Dehydroabietadienal | [C20H28O + 107Ag]+ | 391.120 | 391.119 | 2.6 |
| [C20H28O + 109Ag]+ | 393.119 | 393.118 | 2.5 | ||
| 4. | [GP (6:0/6:0)] 1,2-dihexanoyl-sn-glycero-3-phosphate | [C15H29O8P + K]+ | 407.123 | 407.123 | 0.0 |
| 5. | Ala-Leu-Ala-Gly | [C14H26N4O5 + 107Ag]+ | 437.096 | 437.095 | 2.3 |
| 6. | Met-Phe-Cys | [C17H25N3O4S2 + K]+ | 438.093 | 438.092 | 2.3 |
| 7. | Cys-Trp-Gly-Gly | [C18H23N5O5S + Na]+ | 444.130 | 444.131 | −2.3 |
| 8. | Asn-Thr-Cys-Ser | [C14H25N5O8S + Na]+ | 446.132 | 446.132 | 0.0 |
| 9. | Ala-Ala-Ala-Asp | [C13H22N4O7 + 107Ag]+ | 453.052 | 453.053 | −2.2 |
| 10. | Ala-Asn-Gly-Ser | [C12H21N5O7 + 107Ag]+ | 454.050 | 454.049 | 2.2 |
| 11. | [GP (6:0/6:0)] 1,2-dihexanoyl-sn-glycero-3-phosphate | [C15H29O8P + 107Ag]+ | 475.066 | 475.065 | 2.1 |
| 12. | Val-Asp-His | [C15H23N5O6 + 107Ag]+ | 476.069 | 476.069 | 0.0 |
| 13. | [GP (6:0/6:0)] 1,2-dihexanoyl-sn-glycero-3-phosphate | [C15H29O8P + 109Ag]+ | 477.065 | 477.064 | 2.1 |
| 14. | PA(17:2(9Z,12Z)/0:0) | [C20H37O7P + 109Ag]+ | 529.130 | 529.132 | −3.8 |
| 15. | Ala-Trp-Val-Tyr | [C28H35N5O6 + H]+ | 538.267 | 538.266 | 1.9 |
| 16. | C12-ACP (Dodecanoyl-ACP) | [C23H44N2O8PS + H]+ | 540.263 | 540.263 | 0.0 |
| 17. | LysoPE(20:3(8Z,11Z,14Z)/0:0) | [C25H46NO7P + K]+ | 542.259 | 542.264 | −9.2 |
| 18. | Asp-Leu-Met-His | [C21H34N6O7S + K]+ | 553.184 | 553.184 | 0.0 |
| 19. | Alpha-Trisaccharide | [C20H37NO14 + K]+ | 554.187 | 554.185 | 3.6 |
| 20. | Ala-Ala-Tyr-His | [C21H28N6O6 + 109Ag]+ | 569.110 | 569.111 | −1.8 |
| 21. | Glu-Phe-Ala-Pro | [C22H30N4O7 + 109Ag]+ | 571.116 | 571.116 | 0.0 |
| 22. | Cys-Pro-Pro-Tyr | [C22H30N4O6S + 107Ag]+ | 585.094 | 585.093 | 1.7 |
| 23. | Ala-Cys-Tyr-His | [C21H28N6O6S + 107Ag]+ | 599.084 | 599.084 | 0.0 |
| [C21H28N6O6S + 109Ag]+ | 601.085 | 601.083 | 3.3 | ||
| 24. | Cellotriose | [C18H32O16 + 109Ag]+ | 613.075 | 613.073 | 3.3 |
| 25. | Octacosanyl hexadecanoate | [C44H88O2 + H]+ | 649.684 | 649.686 | −3.1 |
| 26. | FAD stem group | [C15H26N6O13P2 + 109Ag]+ | 669.009 | 669.008 | 1.5 |
| 27. | GalCer(d18:2/20:0) | [C44H83NO8 + H]+ | 754.621 | 754.619 | 2.7 |
| 28. | TG(13:0/13:0/17:2(9Z,12Z)) | [C46H84O6 + Na]+ | 755.614 | 755.616 | −2.6 |
| 29. | PC(18:2(9Z,12Z)/P-18:1(11Z)) | [C44H82NO7P + H]+ | 768.592 | 768.590 | 2.6 |
| 30. | [PC (16:2/18:1)] 1-hexadecyl-2-(9Z-octadecenyl)sn- glycero-3-phosphocholine | [C42H86NO6P + K]+ | 770.587 | 770.582 | 6.5 |
| 31. | PA(19:0/22:2(13Z,16Z)) | [C44H83O8P + H]+ | 771.584 | 771.590 | −7.8 |
| 32. | PC(15:0/20:2(11Z,14Z)) | [C43H82NO8P + H]+ | 772.582 | 772.585 | −3.9 |
| 33. | PG(P-20:0/17:2(9Z,12Z)) | [C43H81O9P + H]+ | 773.572 | 773.569 | 3.9 |
| 34. | PC(14:1(9Z)/18:3(6Z,9Z,12Z)) | [C40H72NO8P + 109Ag]+ | 834.407 | 834.404 | 3.6 |
| 35. | [PE (6:0/8:0)] 1-(6-[5]-ladderane-hexanyl)-2(8-[3]- ladderane-octanyl)-sn-glycerophosphoethanolamine | [C43H72NO6P + 107Ag]+ | 836.407 | 836.414 | −8.4 |
| 36. | [PC (18:1/22:6)] 1-(11Z-octadecenoyl)-2-(4Z,7Z,10Z,13Z,16Z,19Z-docosahexaenoyl)-sn-glycero-3-phosphocholine | [C48H82NO8P + K]+ | 870.543 | 870.541 | 2.3 |
| 37. | PI(16:1(9Z)/19:1(9Z)) | [C44H81O13P + Na]+ | 871.535 | 871.531 | 4.6 |
| 38. | PS(O-20:0/16:0) | [C42H84NO9P + 109Ag]+ | 886.494 | 886.493 | 1.1 |
| 39. | PI(15:0/20:2(11Z,14Z)) | [C44H81O13P + K]+ | 887.497 | 887.505 | −9.0 |
| 40. | PE(P-18:0/22:4(7Z,10Z,13Z,16Z)) | [C45H82NO7P + 109Ag]+ | 888.495 | 888.487 | 9.0 |
| 41. | PS(O-16:0/21:0) | [C43H86NO9P + 107Ag]+ | 898.505 | 898.509 | −4.5 |
| 42. | PI(16:0/20:3(8Z,11Z,14Z)) | [C45H81O13P + K]+ | 899.504 | 899.505 | −1.1 |
| 43. | (3′-sulfo)Galbeta-Cer(d18:1/2-OH-16:0) | [C40H77NO12S + 107Ag]+ | 902.422 | 902.421 | 1.1 |
| 44. | PG(16:1(9Z)/22:4(7Z,10Z,13Z,16Z)) | [C44H77O10P + 107Ag]+ | 903.428 | 903.430 | −2.2 |
| 45. | PE(22:1(13Z)/24:1(15Z)) | [C51H98NO8P + Na]+ | 906.688 | 906.692 | −4.4 |
| 46. | PS(15:0/22:1(11Z)) | [C43H82NO10P + 107Ag]+ | 910.468 | 910.472 | −4.4 |
| 47. | PC(18:3(6Z,9Z,12Z)/20:4(5Z,8Z,11Z,14Z)) | [C46H78NO8P + 109Ag]+ | 912.451 | 912.451 | 0.0 |
| 48. | PG(P-18:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | [C46H79O9P + 107Ag]+ | 913.450 | 913.451 | −1.1 |
| 49. | (3′-sulfo)GalBeta-Cer(d18:1/18:0) | [C42H81NO11S + 107Ag]+ | 914.455 | 914.458 | −3.3 |
| 50. | PI(P-16:0/17:0) | [C42H81O12P + 107Ag]+ | 915.455 | 915.451 | 4.4 |
| 51. | (3′-sulfo)GalBeta-Cer(d18:1/18:0) | [C42H81NO11S + 109Ag]+ | 916.463 | 916.457 | 6.5 |
| 52. | PI(O-16:0/18:3(9Z,12Z,15Z)) | [C43H79O12P + 109Ag]+ | 927.437 | 927.435 | 2.2 |
| 53. | PS(17:2(9Z,12Z)/22:4(7Z,10Z,13Z,16Z)) | [C45H76NO10P + 107Ag]+ | 928.421 | 928.425 | −4.3 |
| 54. | PS(18:1(9Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | [C46H76NO10P + 107Ag]+ | 940.430 | 940.425 | 5.3 |
| 55. | PI(16:1(9Z)/18:1(9Z)) | [C43H79O13P + 107Ag]+ | 941.432 | 941.430 | 2.1 |
| 56. | [PE (22:6/22:6)] 1,2-di-(4Z,7Z,10Z,13Z,16Z,19Z-docosahexaenoyl)-sn-glycero-3-phosphoethanolamine | [C49H74NO8P + 107Ag]+ | 942.413 | 942.420 | −7.4 |
| 57. | GalCer(d18:0/26:1) | [C50H97NO8 + 109Ag]+ | 948.626 | 948.626 | 0.0 |
| 58. | [GL (18:0/20:0/20:0)] 1-octadecanoyl-2,3-dieicosanoyl-sn-glycerol | [C61H118O6 + K]+ | 985.857 | 985.856 | 1.01 |
| 59. | [PC (24:0/26:0)] 1-tetracosanoyl-2-hexacosanoyl-sn- glycero-3-phosphocholine | [C58H116NO8P + H]+ | 986.859 | 986.851 | 8.1 |
| 60. | PE(22:2(13Z,16Z)/24:1(15Z)) | [C51H96NO8P + 109Ag]+ | 990.593 | 990.592 | 1.0 |
| 61. | [GP (18:0/18:0/2:0/2:0)] 1,2-di-(9Z-octadecenoyl)-sn- glycero-3-cytidine-5′-diphosphate | [C48H85N3O15P2 + H]+ | 1006.555 | 1006.552 | 3.0 |
| 62. | Siroheme amide | [C42H47FeN5O15 + 107Ag]+ | 1024.151 | 1024.146 | 4.9 |
| 63. | 2-Oxo-delta3-4,5,5-trimethylcyclopentenylacetyl-CoA | [C31H48N7O18P3S + 109Ag]+ | 1040.106 | 1040.103 | 2.9 |
| 64. | CoA(22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | [C43H66N7O17P3S + 107Ag]+ | 1184.255 | 1184.249 | 5.1 |
| [C43H66N7O17P3S + 109Ag]+ | 1186.246 | 1186.249 | −2.5 | ||
* Putative metabolite.
Table 5.
SALDI mass spectrum of Weissella cibaria lipid extract made on an AgNLET plate with electroplated silver nanoparticles (S/N ≥ 5, Δ max. ± 10 ppm).
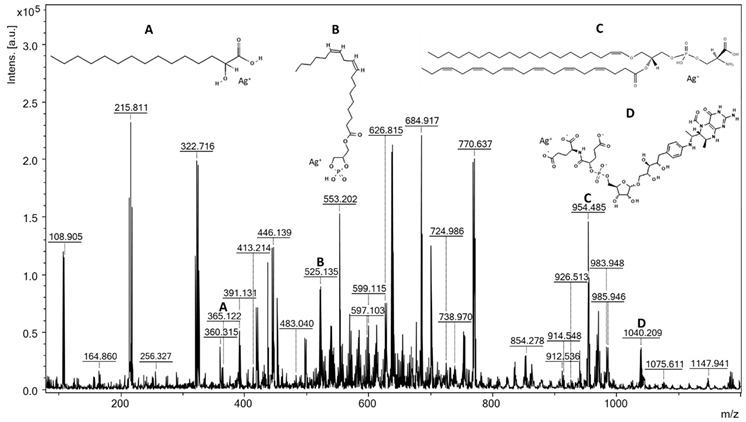
| |||||
|---|---|---|---|---|---|
| Compound * | Adduct Formula | m/z Exp. | m/z Calc. | Δ (ppm) | |
| 1. | [FA hydroxy(15:0)] 2-hydroxy-pentadecanoic acid | [C15H30O3 + 107Ag]+ | 365.12 | 365.12 | 0.0 |
| 2. | N-palmitoyl proline | [C21H39NO3 + Na]+ | 376.28 | 376.28 | 0.0 |
| 3. | Galactan | [C14H26O11 + Na]+ | 393.14 | 393.14 | 0.0 |
| 4. | [PC (6:0)] 1-hexanoyl-sn-glycero3-phosphocholine | [C14H30NO7P + K]+ | 394.14 | 394.14 | 0.0 |
| 5. | 2-hydroxy-9Z,12Z,15Z-octadecatrienoic acid | [C18H30O3 + 109Ag]+ | 403.12 | 403.12 | 0.0 |
| 6. | Met-Tyr | [C14H20N2O4S + 107Ag]+ | 419.02 | 419.02 | 0.0 |
| 7. | [PC (3:0)] 1-(2E-propionyl)sn-glycero-3- phosphocholine | [C11H22NO7P + 109Ag]+ | 420.02 | 420.02 | 0.0 |
| 8. | S-(2-Chloroacetyl)glutathione | [C12H18N3O7ClS + K]+ | 422.02 | 422.02 | 0.0 |
| 9. | Val-Gly-Arg | [C13H26N6O4 + 107Ag]+ | 437.11 | 437.11 | 0.0 |
| 10. | S-Adenosylmethionine | [C15H23N6O5S + K]+ | 438.11 | 438.11 | 0.0 |
| 11. | Ala-Cys-Gly-Arg | [C14H27N7O5S + K]+ | 444.14 | 444.14 | 0.0 |
| 12. | Phe-Trp-Gly | [C22H24N4O4 + K]+ | 447.14 | 447.14 | 0.0 |
| 13. | Asp-Gly-Arg | [C12H22N6O6 + 107Ag]+ | 453.07 | 453.06 | 22.1 |
| 14. | Ala-Ala-Ala-Asn | [C13H23N5O6 + 109Ag]+ | 454.07 | 454.07 | 0.0 |
| 15. | Dopaxanthin | [C18H18N2O8 + 107Ag]+ | 497.01 | 497.01 | 0.0 |
| [C18H18N2O8 + 109Ag]+ | 499.01 | 499.01 | 0.0 | ||
| 16. | CPA(18:2(9Z,12Z)/0:0) | [C21H37O6P + 109Ag]+ | 525.14 | 525.14 | 0.0 |
| 17. | CPA(18:1(11Z)/0:0) | [C21H39O6P + 109Ag]+ | 527.15 | 527.15 | 0.0 |
| 18. | Ile-Lys-Tyr | [C21H34N4O5 + 107Ag]+ | 529.16 | 529.16 | 0.0 |
| 19 | PS(17:2(9Z,12Z)/0:0) | [C23H42NO9P + Na]+ | 530.25 | 530.25 | 0.0 |
| 20. | PA(17:0/0:0) | [C20H41O7P + 107Ag]+ | 531.16 | 531.16 | 0.0 |
| 21. | Arg-Ile-Ile-Pro | [C23H43N7O5 + K]+ | 536.29 | 536.30 | −18.6 |
| 22. | PG(12:0/0:0) | [C18H37O9P + 109Ag]+ | 537.12 | 537.12 | 0.0 |
| 23. | LysoPC(18:4(6Z,9Z,12Z,15Z)) | [C26H46NO7P + Na]+ | 538.29 | 538.29 | 0.0 |
| 24. | Ala-Glu-Ile-Thr | [C18H32N4O8 + 107Ag]+ | 539.13 | 539.13 | 0.0 |
| 25. | Asn-Leu-Phe-Phe | [C28H37N5O6 + H]+ | 540.28 | 540.28 | 0.0 |
| 26. | Ala-Phe-Val-Pro | [C22H32N4O5 + 109Ag]+ | 541.14 | 541.14 | 0.0 |
| 27. | His-Lys-Val-His | [C23H37N9O5 + Na]+ | 542.28 | 542.28 | 0.0 |
| 28. | Glu-Glu-Met-Pro | [C20H32N4O9S + K]+ | 543.15 | 543.15 | 0.0 |
| 29. | PC(O-10:1(9E)/2:0) | [C20H40NO7P + 107Ag]+ | 544.16 | 544.16 | 0.0 |
| 30. | LysoPA(0:0/18:1(9Z)) | [C21H41O7P + 109Ag]+ | 545.16 | 545.16 | 0.0 |
| 31. | Asn-Trp-Asp-Pro | [C24H30N6O8 + Na]+ | 553.20 | 553.20 | 0.0 |
| 32. | Lys-Trp-Gly-Gly | [C21H30N6O5 + 109Ag]+ | 555.13 | 555.13 | 0.0 |
| 33. | Ala-Cys-Tyr-Tyr | [C24H30N4O7S + K]+ | 557.14 | 557.15 | −17.9 |
| 34. | Arg-Asn-Asp-Gly | [C16H28N8O8 + 107Ag]+ | 567.11 | 567.11 | 0.0 |
| 35. | Phe-Thr-Pro-Pro | [C23H32N4O6 + 109Ag]+ | 569.14 | 569.14 | 0.0 |
| 36. | Thr-Trp-Arg | [C21H31N7O5 + 109Ag]+ | 570.14 | 570.14 | 0.0 |
| 37. | Ile-Met-Thr-Thr | [C19H36N4O7S + 107Ag]+ | 571.13 | 571.14 | −17.5 |
| 38. | Cys-Ser-Tyr-Tyr | [C24H30N4O8S + K]+ | 573.14 | 573.14 | 0.0 |
| 39. | Phe-Gly-Ser-Tyr | [C23H28N4O7 + 109Ag]+ | 581.10 | 581.10 | 0.0 |
| 40. | Asn-Leu-Asn-Asp | [C18H30N6O9 + 109Ag]+ | 583.11 | 583.11 | 0.0 |
| 41. | Ala-Phe-Asn-Gln | [C21H30N6O7 + 107Ag]+ | 585.12 | 585.12 | 0.0 |
| [C21H30N6O7 + 109Ag]+ | 587.12 | 587.12 | 0.0 | ||
| 42. | His-Met-Phe-Gly | [C22H30N6O5S + 107Ag]+ | 597.10 | 597.10 | 0.0 |
| 43. | Arg-Cys-Gln-Ser | [C17H32N8O7S + 107Ag]+ | 599.12 | 599.12 | 0.0 |
| [C17H32N8O7S + 109Ag]+ | 601.12 | 601.12 | 0.0 | ||
| 44. | Cys-Met-Phe-Thr | [C21H32N4O6S2 + 109Ag]+ | 609.08 | 609.08 | 0.0 |
| 45. | Asp-Met-Thr-His | [C19H30N6O8S + 109Ag]+ | 611.09 | 611.09 | 0.0 |
| 46. | Ala-Trp-Cys-Gln | [C22H30N6O6S + 107Ag]+ | 613.10 | 613.10 | 0.0 |
| [C22H30N6O6S + 109Ag]+ | 615.10 | 615.10 | 0.0 | ||
| 47. | Asp-Met-Asp-His | [C19H28N6O9S + 107Ag]+ | 623.07 | 623.07 | 0.0 |
| 48. | Cys-Met-Thr-Tyr | [C21H32N4O7S2 + 109Ag]+ | 625.08 | 625.08 | 0.0 |
| 49. | Poly-g-D-glutamate | [C20H30N4O12 + 109Ag]+ | 627.09 | 627.09 | 0.0 |
| 50. | Asn-Met-Met-Gln | [C19H34N6O7S2 + 107Ag]+ | 629.10 | 629.10 | 0.0 |
| 51. | P-(bromoacetamido)phenyl uridylpyrophosphate | [C17H20BrN3O13P2 + Na]+ | 637.96 | 637.95 | 15.7 |
| 52. | Asn-Trp-Asp-Cys | [C22H28N6O8S + 107Ag]+ | 643.08 | 643.07 | 15.6 |
| 53. | TG(12:0/12:0/20:0) | [C47H90O6 + H]+ | 751.68 | 751.68 | 0.0 |
| 54. | Stigmast-5,22E-dien-3beta-yl (13Z-docosenoate) | [C51H88O2 + Na]+ | 755.67 | 755.67 | 0.0 |
| [C51H88O2 + K]+ | 771.64 | 771.64 | 0.0 | ||
| 55. | PE(15:0/22:0) | [C42H84NO8P + H]+ | 762.60 | 762.60 | 0.0 |
| 56. | GlcCer(d18:2/21:0) | [C45H85NO8 + H]+ | 768.64 | 768.63 | 13.0 |
| 57. | PS(17:1(9Z)/20:4(5Z,8Z,11Z,14Z)) | [C43H74NO10P + K]+ | 834.47 | 834.47 | 0.0 |
| 58. | PG(18:1(9Z)/20:4(5Z,8Z,11Z,14Z)) | [C44H77O10P + K]+ | 835.48 | 835.49 | −12.0 |
| 59. | N-(2-hydroxy-eicosanoyl)-1-beta-glucosyl-4E,6E-pentadecasphingadienine | [C41H77NO9 + 109Ag]+ | 836.47 | 836.46 | 12.0 |
| 60. | PG(20:5(5Z,8Z,11Z,14Z,17Z)/20:5(5Z,8Z,11Z,14Z,17Z)) | [C46H71O10P + Na]+ | 837.47 | 837.47 | 0.0 |
| 61. | TG(16:1(9Z)/18:3(6Z,9Z,12Z)/18:3(6Z,9Z,12Z)) | [C55H92O6 + H]+ | 849.70 | 849.70 | 0.0 |
| 62. | [PC (3:0/3:0/3:0)] 1-(2E,6E,10E-phytatrienyl)-2-(2E,6E10E-phytatrienyl)-sn-glycero-3-phosphocholine | [C48H88NO6P + 107Ag]+ | 912.54 | 912.54 | 0.0 |
| 63. | PG(O-20:0/19:1(9Z)) | [C45H89O9P + 109Ag]+ | 913.53 | 913.53 | 0.0 |
| 64. | PG(22:1(11Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | [C50H85O10P + K]+ | 915.56 | 915.55 | 10.9 |
| 65. | PC(22:4(7Z,10Z,13Z,16Z)/P-18:1(11Z)) | [C48H86NO7P + 107Ag]+ | 926.51 | 926.52 | −10.8 |
| 66. | PS(17:0/22:1(11Z)) | [C45H86NO10P + 109Ag]+ | 940.51 | 940.50 | 10.6 |
| 67. | PIP(16:0/18:0) | [C43H84O16P2 + Na]+ | 941.51 | 941.51 | 0.0 |
| 68. | LacCer(d18:0/14:0) | [C44H85NO13 + 107Ag]+ | 942.51 | 942.51 | 0.0 |
| 69. | PI(17:2(9Z,12Z)/18:4(6Z,9Z,12Z,15Z)) | [C44H73O13P + 107Ag]+ | 947.39 | 947.38 | 10.6 |
| [C44H73O13P + 109Ag]+ | 949.39 | 949.38 | 10.5 | ||
| 70. | PS(P-20:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | [C48H82NO9P + 107Ag]+ | 954.49 | 954.48 | 10.5 |
| [C48H82NO9P + 109Ag]+ | 956.48 | 956.48 | 0.0 | ||
| 71. | PI(O-16:0/20:2(11Z,14Z)) | [C45H85O12P + 107Ag]+ | 955.49 | 955.48 | 10.5 |
| 72. | PI(15:0/20:1(11Z)) | [C44H83O13P + 107Ag]+ | 957.47 | 957.46 | 10.4 |
| 73. | PI(17:1(9Z)/20:3(8Z,11Z,14Z)) | [C46H81O13P + 109Ag]+ | 981.44 | 981.45 | −10.2 |
| 74. | TG(19:1(9Z)/21:0/21:0) | [C64H122O6 + H]+ | 987.94 | 987.93 | 10.1 |
| 75. | 5-formyl-tetrahydrosarcinapterin | [C36H52N7O20P + 107Ag]+ | 1040.21 | 1040.21 | 0.0 |
| 76. | Lipid IVA | [C68H126N2O23P2 + H]+ | 1401.84 | 1401.83 | 7.1 |
| 77. | Tetrahexosylceramide (d18:1/26:1(17Z)) | [C70H128N2O23 + K]+ | 1403.85 | 1403.85 | 0.0 |
* Putative metabolite.
3.6. Synthesis of Vitamins and Lactic Acid
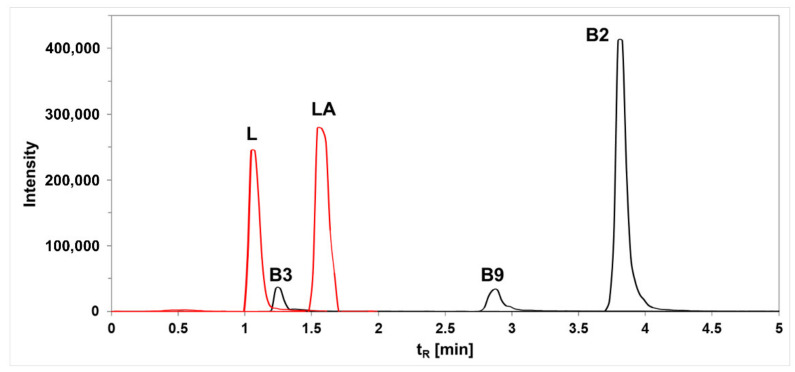
A growing consumer market demand for products containing functional ingredients that provide medical or health benefits has caused probiotic selection to be currently focused on finding certain strains of LAB that are able to produce or increase the content of specific beneficial compounds found in foods [54]. Such compounds could be micronutrients, for example, B-group vitamins, including riboflavin (B2), niacin (B3), and folate (B9), or substances with preservative properties, such as lactic acid. This study determined the ability of the L. lactis and W. cibaria strains to synthesize B vitamins and lactic acid. The concentration of these compounds was determined using HPLC in the selected LAB supernatants obtained after cultivation in two different culture media (niacin assay medium, and MRS broth) (Figure 5).
Figure 5.
Exemplary chromatograms obtained during the analysis of selected compounds by HPLC. L—lactose; LA—lactic acid; B2—riboflavin; B3—niacin; B9—folic acid; tR—retention time.
The results from the analysis of vitamin B (B2, B3, and B9) production capacity among the tested LABs revealed that L. lactis secreted niacin and riboflavin regardless of the type of culture medium used, and the amounts were highly temperature dependent (Table 6). The concentrations of secreted niacin were higher compared to that of riboflavin by 1.7 (30 °C) and 2.5 (20 °C) times and reached values of 1.273 ± 0.177 and 3.582 ± 0.132 µg/mL, respectively. The difference between niacin and riboflavin secretion levels in L. lactis increased up to 4.9 times when the MRS broth medium was applied; however, the measured amounts were five (niacin) and ten (riboflavin) times lower compared to those obtained using the niacin assay medium. Although W. cibaria did not demonstrate the ability to produce any type of the investigated B vitamins on the niacin assay medium, this changed when MRSB was applied and the secreted niacin and riboflavin amounts reached 1.465 ± 0.188 and 0.109 ± 0.002 µg/mL, respectively. Both strains revealed the high effect of the incubation temperature on the levels of secreted B vitamins. In each case, the use of a lower temperature (20 °C) stimulated vitamin production. This was particularly evident in the MRS broth medium, where lowering the temperature from 30 °C to 20 °C caused the strains to shift from consumers to vitamin B producers. Regarding folates, the analysis revealed that none of the investigated LAB were able to secrete vitamin B9 regardless of the temperature and type of culture medium used.
Table 6.
Concentration of the analyzed vitamins in the niacin assay medium and the post-culture fluid of LAB strains obtained using HPLC analysis. During the analysis, the concentrations of the analyzed vitamins in the clean medium were taken into account (values for LAB strains shown in the table are after background subtraction). nd—not detected; LOD—limit of detection; LOQ—limit of quantification.
| Concentration (µg/mL) | |||
|---|---|---|---|
| Sample | Niacin—B3 | Folic Acid—B9 | Riboflavin—B2 |
| TEMPERATURE 20 °C | |||
| Niacin Assay Medium | 0.178 ± 0.009 | nd | 4.104 ± 0.126 |
| L. lactis | 3.582 ± 0.132 | nd | 1.409 ± 0.045 |
| W. cibaria | nd | LOD | −2.276 ± 0.118 |
| MRS Broth Medium | nd | nd | nd |
| L. lactis | 0.718 ± 0.000 | nd | 0.147 ± 0.006 |
| W. cibaria | 1.465 ± 0.188 | nd | 0.109 ± 0.002 |
| TEMPERATURE 30 °C | |||
| Niacin Assay Medium | 0.174 ± 0.001 | nd | 4.592 ± 0.095 |
| L. lactis | 1.273 ± 0.177 | LOD | 0.754 ± 0.065 |
| W. cibaria | 0.081 ± 0.005 LOQ | LOD | −0.307 ± 0.495 |
| MRS Broth Medium | 1.350 ± 0.073 | nd | 4.011 ± 0.067 |
| L. lactis | −0.874 ± 0.072 | nd | −3.871 ± 0.001 |
| W. cibaria | −0.872 ± 0.058 | nd | −1.314 ± 0.115 |
Our studies revealed that one of the two investigated LAB strains derived from the pickled beetroots, L. lactis, was able to secrete niacin and riboflavin in the CDM medium. Thus far, riboflavin-producing LAB have been isolated from different ecological niches and demonstrated great variation in their ability to increase the concentrations of this vitamin in food matrices, including milk, soymilk, whey, and pseudocereals, from 0.50 to 6.57 mg/L [55,56,57]. However, the vast majority of work on riboflavin production relates to lactobacilli species, such as L. fermentum, L. acidophilus, or L. plantarum [58,59]. Natural (wild types) of Lactococcus lactis are considered as riboflavin consumers who can be easily converted into riboflavin producers through the overexpression of riboflavin biosynthesis genes via genetic engineering and exposure to purines or a toxic riboflavin analogue (roseoflavin) [60,61]. The niacin assay medium used in our studies contains sources of purines (adenine sulfate and guanine hydrochloride) and was likely responsible for observed significant increase in riboflavin production in L. lactis compared to the MRS broth medium. The recorded level was as high as that within the lactobacilli group—1.409 mg/L—and considering that the recommended daily intake for riboflavin is 1.3 mg/day for men and 1.1 mg/day for women [62], isolated L. lactis demonstrates high potential for the fortification of foods with vitamin B2 providing that suitable culture conditions (20 °C, purine addition) are established.
Interestingly, although Lactococcus members are not known to produce nicotinate metabolites, to which niacin belongs, the investigated L. lactis strain was characterized by the highest vitamin B3 secretion among all tested B-group vitamins. In their work, Jung et al. detected niacin production for the first time in a Lactococcus member, namely in L. raffinolactis WiKim0068, isolated from fermented cabbage (kimchi) [63]. The detected niacin concentration (0.932 mg/L) was similar to that obtained for Leuconostoc spp. (0.837–1.05 mg/L) and was far higher when Lactobacillus species were applied −0.05–0.1 mg/L. The niacin production capacity of L. raffinolactis WiKim0068 was slightly higher compared to that of L. lactis cultured on the same medium (MRSB) in our study; however, such differences may have resulted from varied incubation times, i.e., 48 h compared to 24 h, respectively. Moreover, as we showed, the niacin secretion level of L. lactis was significantly increased when vitamin-free medium combined with a lower temperature (20 °C) was applied. Another strain investigated in our studies, Weissella cibaria, is believed to be an interesting probiotic candidate that is already being used as a commercially available oral care probiotic in Korea (W. cibaria CMU) [64] and has been shown to produce B-group vitamins (e.g., folic acid [65]). Although the W. cibaria strain did not secrete any of the investigated B vitamins under most of the culture conditions used, the application of MRS broth medium combined with lower temperature brought about a switch from consumption to vitamin B production. Nevertheless, in most cases, W. cibaria appeared to be auxotrophic for riboflavin, since its initial concentration after incubation significantly decreased. As both strains investigated in our studies originated from the same source (pickled beetroot), it is possible that the sharing of the same ecological niche by L. lactis and W. cibaria has conditioned riboflavin production by the former strain that further can be consumed by the latter.
Similar to vitamin B production, the investigated LABs demonstrated great differences in their ability to utilize lactose and produce lactic acid (Table 7). At the lower temperature, L. lactis addition caused a 93% decrease in the lactose concentration of the medium, while in the presence of W. cibaria it was decreased only by 40%. The opposite phenomenon was observed at a higher temperature, wherein W. cibaria metabolized almost all lactose present in the culture medium (98%); in the case of L. lactis, this value was 67%. Regardless of the temperature and lactose metabolism rate, the highest lactic acid production was noted for variants with L. lactis inoculation, in which it was 2.3–3.3 times higher than that of W. cibaria. The lactic acid concentration produced by L. lactis was 1014.99 ± 47.59 µg/mL at 20 °C and 638.21 ± 8.03 µg/mL at 30 °C. Despite the great variation in lactose utilization by W. cibaria depending on the applied temperature, the differences in the level of lactic acid production under different incubation conditions were only slight—310.99 ± 7.48 and 277.36 ± 5.82 µg/mL at 20 °C and 30 °C, respectively.
Table 7.
Concentration of the analyzed compounds in the LAPTg medium and the post-culture fluid of LAB strains obtained using HPLC analysis. During the analysis, the concentrations of the analyzed lactose and lactic acid in the clean medium were taken into account (values for LAB strains shown in the table are after background subtraction).
| Sample | Lactose (µg/mL) | Lactic Acid (µg/mL) |
|---|---|---|
| TEMPERATURE 20 °C | ||
| LAPTg Medium | 38.61 ± 0.58 | 34.01 ± 1.88 |
| L. lactis | 2.61 ± 0.81 | 1014.99 ± 47.59 |
| W. cibaria | 14.49 ± 1.14 | 310.99 ± 7.48 |
| TEMPERATURE 30 °C | ||
| LAPTg Medium | 49.91 ± 1.00 | 16.43 ± 0.22 |
| L. lactis | 12.38 ± 0.95 | 638.21 ± 8.03 |
| W. cibaria | 0.91 ± 0.50 | 277.36 ± 5.82 |
The main product of the fermentation process carried out by LAB is lactic acid (LA), which is authorized by the U.S. Food and Drug Administration as GRAS (generally regarded as safe). Differences between the main types of LAB may explain the observed variation in lactose metabolism and lactic acid production between the investigated strains, since L. lactis is a homofermentative LAB while W. cibaria is a heterofermentative LAB. Indeed, the greater consumption of lactose by the L. lactis strain was accompanied by a greater production of lactic acid, while in the case of W. cibaria, despite the large differences in the percentage of metabolized lactose depending on the temperature used, the amount of lactic acid secreted was very similar. This suggests that in the case of W. cibaria, which demonstrated high lactose consumption under 30 °C, raising the temperature primarily causes an increase in the secretion of fermentation products other than LA, e.g., acetate or ethanol, which were already detected in the W. cibaria strain 92 previously [66]. L. lactis was found to be far more effective than W. cibaria at the decreasing the lactose concentration and LA production when the lower temperature was applied; a 93% decrease in lactose was accompanied by LA production at a level of 1 g/L, while for the second strain it was only a 40% decrease and the LA level was 0.310 g/L. These findings agree with the common sentiment that only homofermentative LAB is available for commercial LA production due to the high yield, productivity, and a high optical purity of lactic acid [8]. Although lactic acid is especially in demand on the market due to its many applications in the food industry as a food preservative, fermentation agent, acidulant, flavour enhancer, etc., other products of fermentation carried out by LAB can also be beneficial, e.g., acetate can serve as a nutrition source for other microorganisms in the colon, including propionate and butyrate-producing microorganisms [67]. In light of this, both tested strains demonstrated the desired probiotic properties, showing that fermented plant products can serve as valuable potential probiotic sources. Furthermore, during the evaluation of potential probiotic sources, it is crucially important to check their activity under different culture conditions, including different temperatures and culture media compositions, as this will help in planning their future application.
3.7. Study of Antagonistic Abilities
One of the criteria for selecting a good probiotic or starter culture strain is its ability to enhance the host’s natural defense against food pathogens. The reason for the inhibition of the growth of pathogenic bacteria is most likely the presence of organic acids, hydrogen peroxide, and bacteriocins in the culture fluid. Bacteriocins are proteins or peptides produced by bacteria that exhibit a bacteriostatic or bactericidal activity that is specific to some organisms [68]. In order to investigate the antibacterial properties of the isolated LAB strains, we used three pathogenic strains and one food spoilage strain. The general effect of the LAB supernatant on the growth of the selected bacteria on the medium was checked. Table 8 shows the average colony numbers of the pathogenic bacteria without and with the addition of the LAB supernatant. In order to better visualize the reduction in the number of pathogenic colonies, the numerical values were converted into percentage values. Similar antimicrobial activity was observed for both strains, W. cibaria and L. lactis. Both strains completely inhibited the growth of P. aeruginosa and L. innocua. They inhibited the growth of E. coli and B. cereus to a similar extent. In the case of E. cloacae, the W. cibaria strain showed greater antimicrobial properties.
Table 8.
Results of the experiment aimed at examining the antagonistic properties of LAB strains against pathogenic bacteria.
| Pathogenic Strain | Number of Grown Pathogen Colonies (Percentage of Inhibition) | ||
|---|---|---|---|
| Control | L. lactis | W. cibaria | |
| Escherichia coli | 340 ± 63 | 174 | 156 |
| 49% | 54% | ||
| Enterobacter cloacae | 62 ± 13 | 22 | 5 |
| 65% | 92% | ||
| Pseudomonas aeruginosa | 204 ± 12 | 0 | 0 |
| 100% | 100% | ||
| Listeria innocua | 956 ± 35 | 0 | 0 |
| 100% | 100% | ||
Two types of bacteriocins have been identified in lactic acid bactera, lanthibiotics (characterized by the presence of dehydrated and/or thioether amino acids) and non-lanthibiotics (those containing unmodified amino acids) [69]. Lactococcus lactis is a very popular component of commercial starter cultures applied by the dairy industry; thus, its bacteriocins have been widely characterised. The first bacteriocin isolated from L. lactis was nisin, which can kill a wide range of Gram-positive bacteria. For this reason, it is widely used as a food preservative in processed cheeses and dairy products [70]. L. lactis can also produce other lanthibiotics, such as the single peptide lacticin 481 and the two-component system lacticin 3147, and non-lanthibiotics, including lactococcin MMFII and lactococcin B or G [69]. By reviewing the literature on the antagonistic properties of the Weissella cibaria strain, one can find confirmation of the results obtained during our research. Srionnual et al. focused on the characterization of the bacteriocins responsible for the antagonistic properties of this strain [71]. They showed that this strain produced a bacteriocin, which they named Weissellicin 110. It was stable after high temperature treatment but had a narrow inhibitory spectrum against Listeria monocytogenes. Their research also indicated that the optimal temperature for the production of bacteriocins by W. cibaria was around 30 °C. In our research, the W. cibaria isolated from beetroot showed the strongest properties against bacteria of the same genus (Listeria innocua in our research). Kant Lakra et al. also showed in their research that the Weissella cibaria MD2 strain had potential probiotic properties [72]. The strain showed high survival in the artificial environment of the gastrointestinal tract, adhesion to intestinal epithelial cells, and antimicrobial activity against food-borne pathogens. They indicated that the antimicrobial properties of the strain could be used for packaging (as edible films) in the food industry in order to extend shelf-life. The strain they isolated also showed pro-health effects, such as high cholesterol removal and antioxidant activity, as well as a lack of haemolytic activity and sensitivity to conventional antibiotics, which makes it safe to use as a starter culture in the food industry or for the preparation of new functional foods.
4. Conclusions
Although a number of probiotic strains have been isolated and characterized, the search for more effective strains is still ongoing. The most attractive source of new bacteria with potential probiotic properties is fermented food, including popular dairy products, as well as silage, which is becoming more and more popular. In this study, two LAB strains—Lactococcus lactis and Weissella cibaria—isolated from a pickled beetroot sample were identified and characterized. During the analyses, two aspects were taken into account: their potential probiotic properties and their applicability in the food industry. Both lactic acid bacteria showed antimicrobial activity against four pathogenic bacteria (Escherichia coli, Enterobacter cloacae, Pseudomonas aeruginosa, and Listeria innocua). However, L. lactis showed much more interesting properties from the point of view of the food industry. The proteolytic activity of L. lactis was higher than that of W. cibaria. This strain produced 2.3–3.3 times more lactic acid than W. cibaria and secreted niacin and riboflavin regardless of the type of culture medium used, and the amounts were highly temperature dependent. Moreover, the analysis of volatile compounds showed that L. lactis produces large amounts of acetoin, which gives dairy products a desirable buttery odor. The lipid profile of the tested strains was also characterized using the modern SALDI technique, which allowed for the detection of lipids belonging to all classes, i.e., fatty acids, glycerolipids, glycerophospholipids, sphingolipids, and steroids.
Both tested strains demonstrated desirable probiotic properties, showing that fermented plant products can serve as valuable potential probiotic sources. Furthermore, during the evaluation of potential probiotic sources, it is crucially important to check their activity under different culture conditions, including temperatures and culture media compositions, to help plan their future applications.
Acknowledgments
This research was supported by the Toruń Center of Excellence “Towards Personalized Medicine” operating under the Excellence Initiative-Research University (B.B., E.M., M.Z., K.R., P.P.).
Author Contributions
Conceptualization, P.P.; methodology, E.M., M.Z., A.A., M.S., I.J., J.R., J.W.-S., M.B.-F., K.R., and P.P.; analysis and interpretation of data, E.M., M.Z., A.A., M.S., I.J., J.R., J.W.-S., M.B.-F., and K.R.; writing—original draft preparation, E.M., M.Z., and A.A.; writing—review and editing, P.P.; supervision, P.P. and B.B.; funding acquisition, D.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All sequence data that support the findings of this study have been deposited in GenBank and the public URL for the sequence collection is https://www.ncbi.nlm.nih.gov/sites/myncbi/1F1i8raBplj5d/collections/61883288/public/ (accessed on 1 June 2022) Lactococcus lactis strain Beet1 (OM957496) and Weissella cibaria strain Beet2 (OM957500).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported under the Intelligent Development Operational Program 2014–2020 subsidy 1.1/1.1.1–competition 7/1.1.1/2020 in framework Fast track Agrotech project no. POIR.01.01.01.-00-2294120 financed by National Centre for Research and Development.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.FAO/WHO . Guidelines for the Evaluation of Probiotics in Food. FAO; Rome, Italy: 2002. pp. 1–11. [Google Scholar]
- 2.Santacroce L., Charitos I.A., Bottalico L. A successful history: Probiotics and their potential as antimicrobials. Expert Rev. Anti-Infect. Ther. 2019;17:635–645. doi: 10.1080/14787210.2019.1645597. [DOI] [PubMed] [Google Scholar]
- 3.Fenster K., Freeburg B., Hollard C., Wong C., Laursen R.R., Ouwehand A.C. The production and delivery of probiotics: A review of a practical approach. Microorganisms. 2019;7:83. doi: 10.3390/microorganisms7030083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Worku H., Amenu K., Fesseha H. The Role of Starter Culture in Producing Probiotic Yoghurt: Significance for Human Health—A Review. Int. J. Recent Biotechnol. 2020;8:20–34. [Google Scholar]
- 5.Kumar B.V., Vijayendra S.V.N., Reddy O.V.S. Trends in dairy and non-dairy probiotic products—A review. J. Food Sci. Technol. 2015;52:6112–6124. doi: 10.1007/s13197-015-1795-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khaneghah A.M., Abhari K., Eş I., Soares M.B., Oliveira R.B.A., Hosseini H., Rezaei M., Balthazar C.F., Silva R., Cruz A.G., et al. Interactions between probiotics and pathogenic microorganisms in hosts and foods: A review. Trends Food Sci. Technol. 2020;95:205–218. doi: 10.1016/j.tifs.2019.11.022. [DOI] [Google Scholar]
- 7.Hatti-Kaul R., Chen L., Dishisha T., el Enshasy H. Lactic acid bacteria: From starter cultures to producers of chemicals. FEMS Microbiol. Lett. 2018;365:fny213. doi: 10.1093/femsle/fny213. [DOI] [PubMed] [Google Scholar]
- 8.Abedi E., Hashemi S.M.B. Lactic acid production—Producing microorganisms and substrates sources-state of art. Heliyon. 2020;6:e04974. doi: 10.1016/j.heliyon.2020.e04974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu Y., Zhang L., Wen R., Chen Q., Kong B. Role of lactic acid bacteria in flavor development in traditional Chinese fermented foods: A review. Crit. Rev. Food Sci. Nutr. 2022;62:2741–2755. doi: 10.1080/10408398.2020.1858269. [DOI] [PubMed] [Google Scholar]
- 10.Walczak-Skierska J., Złoch M., Pauter K., Pomastowski P., Buszewski B. Lipidomic analysis of lactic acid bacteria strains by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J. Dairy Sci. 2020;103:11062–11078. doi: 10.3168/jds.2020-18753. [DOI] [PubMed] [Google Scholar]
- 11.Nagpal R., Kumar A., Kumar M., Behare P.V., Jain S., Yadav H. Probiotics, their health benefits and applications for developing healthier foods: A review. FEMS Microbiol. Lett. 2012;334:1–15. doi: 10.1111/j.1574-6968.2012.02593.x. [DOI] [PubMed] [Google Scholar]
- 12.Kerry R.G., Patra J.K., Gouda S., Park Y., Shin H.S., Das G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018;26:927–939. doi: 10.1016/j.jfda.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbosa R.S.D., Vieira-Coelho M.A. Probiotics and prebiotics: Focus on psychiatric disorders- A systematic review. Nutr. Rev. 2020;78:437–450. doi: 10.1093/nutrit/nuz080. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann P., Curtis N. The influence of the intestinal microbiome on vaccine responses. Vaccine. 2018;36:4433–4439. doi: 10.1016/j.vaccine.2018.04.066. [DOI] [PubMed] [Google Scholar]
- 15.Shori A.B. The potential applications of probiotics on dairy and non-dairy foods focusing on viability during storage. Biocatal. Agric. Biotechnol. 2015;4:423–431. doi: 10.1016/j.bcab.2015.09.010. [DOI] [Google Scholar]
- 16.Bruker Daltonics . MALDI Biotyper 3.1 User Manual. Volume 1. Bruker Daltonics; Billerica, MA, USA: 2012. pp. 1–212. [Google Scholar]
- 17.Pomastowski P., Złoch M., Rodzik A., Ligor M., Kostrzewa M., Buszewski B. Analysis of bacteria associated with honeys of different geographical and botanical origin using two different identification approaches: MALDI-TOF MS and 16S rDNA PCR technique. PLoS ONE. 2019;14:e0217078. doi: 10.1371/journal.pone.0217078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Church F.C., Swaisgood H.E., Porter D.H., Catignani G.L. Spectrophotometric Assay Using o-Phthaldialdehyde for Determination of Proteolysis in Milk and Isolated Milk Proteins. J. Dairy Sci. 1983;66:1219–1227. doi: 10.3168/jds.S0022-0302(83)81926-2. [DOI] [Google Scholar]
- 19.Arendowski A., Sagandykova G., Mametov R., Rafińska K., Pryshchepa O., Pomastowski P. Nanostructured Layer of Silver for Detection of Small Biomolecules in Surface-Assisted Laser Desorption Ionization Mass Spectrometry. Materials. 2022;15:4076. doi: 10.3390/ma15124076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.These H. Food Microbiology: Principles into Practice. John Wiley & Sons; Hoboken, NJ, USA: 2016. Spoilage of Vegetables and Fruits; pp. 337–363. [DOI] [Google Scholar]
- 21.Bautista-Gallego J., Medina E., Sánchez B., Benítez-Cabello A., Arroyo-López F.N. Role of lactic acid bacteria in fermented vegetables. Grasas Aceites. 2020;71:e358. doi: 10.3989/gya.0344191. [DOI] [Google Scholar]
- 22.Yu H.S., Lee N.K., Choi A.J., Choe J.S., Bae C.H., Paik H.D. Anti-inflammatory potential of probiotic strain Weissella cibaria JW15 isolated from kimchi through regulation of NF-κB and MAPKs pathways in LPS-induced RAW 264.7 cells. J. Microbiol. Biotechnol. 2019;29:1022–1032. doi: 10.4014/jmb.1903.03014. [DOI] [PubMed] [Google Scholar]
- 23.Buszewski B., Rogowska A., Pomastowski P., Złoch M., Railean-Plugaru V. Identification of microorganisms by modern analytical techniques. J. AOAC Int. 2017;100:1607–1623. doi: 10.5740/jaoacint.17-0207. [DOI] [PubMed] [Google Scholar]
- 24.Model M.A. Methods for cell volume measurement. Cytom. Part A. 2018;93:281–296. doi: 10.1002/cyto.a.23152. [DOI] [PubMed] [Google Scholar]
- 25.Sáez G.D., Flomenbaum L., Zárate G. Lactic acid bacteria from argentinean femented foods: Isolation and characterization for their potential use as starters for fermentation of vegetables. Food Technol. Biotechnol. 2018;56:398–410. doi: 10.17113/ftb.56.03.18.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joković N., Rajković J., Veljović K., Tolina M. Screening of lactic acid bacteria isolated from Serbian kajmak for use in starter cultures. Biol. Nyssana. 2014;5:37–46. [Google Scholar]
- 27.González L., Sacristán N., Arenas R., Fresno J.M., Tornadijo M.E. Enzymatic activity of lactic acid bacteria (with antimicrobial properties) isolated from a traditional Spanish cheese. Food Microbiol. 2010;27:592–597. doi: 10.1016/j.fm.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Herreros M.A., Fresno J.M., Prieto M.J.G., Tornadijo M.E. Technological characterization of lactic acid bacteria isolated from Armada cheese (a Spanish goats’ milk cheese) Int. Dairy J. 2003;13:469–479. doi: 10.1016/S0958-6946(03)00054-2. [DOI] [Google Scholar]
- 29.McSweeney P.L.H., Fox P.F. Advanced Dairy Chemistry. Springer; New York, NY, USA: 2009. [DOI] [Google Scholar]
- 30.Liu J.M., Chen L., Dorau R., Lillevang S.K., Jensen P.R., Solem C. From Waste to Taste—Efficient Production of the Butter Aroma Compound Acetoin from Low-Value Dairy Side Streams Using a Natural (Nonengineered) Lactococcus lactis Dairy Isolate. J. Agric. Food Chem. 2020;68:5891–5899. doi: 10.1021/acs.jafc.0c00882. [DOI] [PubMed] [Google Scholar]
- 31.Capozzi V., Lonzarich V., Khomenko I., Cappellin L., Navarini L., Biasioli F. Unveiling the molecular basis of mascarpone cheese aroma: VOCs analysis by SPME-GC/MS and PTR-ToF-MS. Molecules. 2020;25:1242. doi: 10.3390/molecules25051242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mallia S., Escher F., Schlichtherle-Cerny H. Aroma-active compounds of butter: A review. Eur. Food Res. Technol. 2008;226:315–325. doi: 10.1007/s00217-006-0555-y. [DOI] [Google Scholar]
- 33.Wishart D.S., Guo A., Oler E., Wang F., Anjum A., Peters H., Dizon R., Sayeeda Z., Tian S., Lee B.L., et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022;50:D622–D631. doi: 10.1093/nar/gkab1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fahy E., Sud M., Cotter D., Subramaniam S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 2007;35:W606–W612. doi: 10.1093/nar/gkm324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caspi R., Billington R., Keseler I.M., Kothari A., Krummenacker M., Midford P.E., Ong W.K., Paley S., Subhraveti P., Karp P.D. The MetaCyc database of metabolic pathways and enzymes-a 2019 update. Nucleic Acids Res. 2020;48:D445–D453. doi: 10.1093/nar/gkz862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arendowski A., Nizioł J., Ossoliński K., Ossolińska A., Ossoliński T., Dobrowolski Z., Ruman T. Laser desorption/ionization MS imaging of cancer kidney tissue on silver nanoparticle-enhanced target. Bioanalysis. 2018;10:83–94. doi: 10.4155/bio-2017-0195. [DOI] [PubMed] [Google Scholar]
- 37.Nizioł J., Rode W., Zieliński Z., Ruman T. Matrix-free laser desorption-ionization with silver nanoparticle-enhanced steel targets. Int. J. Mass Spectrom. 2013;335:22–32. doi: 10.1016/j.ijms.2012.10.009. [DOI] [Google Scholar]
- 38.Cioffi N., Colaianni L., Pilolli R., Calvano C.D., Palmisano F., Zambonin P.G. Silver nanofractals: Electrochemical synthesis, XPS characterization and application in LDI-MS. Anal. Bioanal. Chem. 2009;394:1375–1383. doi: 10.1007/s00216-009-2820-y. [DOI] [PubMed] [Google Scholar]
- 39.Silina Y.E., Meier F., Nebolsin V.A., Koch M., Volmer D.A. Novel galvanic nanostructures of Ag and Pd for efficient laser desorption/ionization of low molecular weight compounds. J. Am. Soc. Mass Spectrom. 2014;25:841–851. doi: 10.1007/s13361-014-0853-8. [DOI] [PubMed] [Google Scholar]
- 40.Sekuła J., Nizioł J., Rode W., Ruman T. Silver nanostructures in laser desorption/ionization mass spectrometry and mass spectrometry imaging. Analyst. 2015;140:6195–6209. doi: 10.1039/C5AN00943J. [DOI] [PubMed] [Google Scholar]
- 41.Ulmer C.Z., Jones C.M., Yost R.A., Garrett T.J., Bowden J.A. Optimization of Folch, Bligh-Dyer, and Matyash sample-to-extraction solvent ratios for human plasma-based lipidomics studies. Anal. Chim. Acta. 2018;1037:351–357. doi: 10.1016/j.aca.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mierau I., Venema G., Kok J., Kunji E.R.S. Casein and peptide degradation in lactic acid bacteria. Biotechnol. Genet. Eng. Rev. 1997;14:279–302. doi: 10.1080/02648725.1997.10647945. [DOI] [PubMed] [Google Scholar]
- 43.Raveschot C., Cudennec B., Coutte F., Flahaut C., Fremont M., Drider D., Dhulster P. Production of bioactive peptides by Lactobacillus species: From gene to application. Front. Microbiol. 2018;9:2354. doi: 10.3389/fmicb.2018.02354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tagliazucchi D., Martini S., Solieri L. Bioprospecting for bioactive peptide production by lactic acid bacteria isolated from fermented dairy food. Fermentation. 2019;5:96. doi: 10.3390/fermentation5040096. [DOI] [Google Scholar]
- 45.Doi Y. Glycerol metabolism and its regulation in lactic acid bacteria. Appl. Microbiol. Biotechnol. 2019;103:5079–5093. doi: 10.1007/s00253-019-09830-y. [DOI] [PubMed] [Google Scholar]
- 46.Gao Y., Li D. Screening of lactic acid bacteria with cholesterol-lowering and triglyceride-lowering activity in vitro and evaluation of probiotic function. Ann. Microbiol. 2018;68:537–545. doi: 10.1007/s13213-018-1360-0. [DOI] [Google Scholar]
- 47.Alvarez H.M., Steinbüchel A. Triacylglycerols in prokaryotic microorganisms. Appl. Microbiol. Biotechnol. 2002;60:367–376. doi: 10.1007/s00253-002-1135-0. [DOI] [PubMed] [Google Scholar]
- 48.López-Lara I.M., Geiger O. Bacterial lipid diversity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2017;1862:1287–1299. doi: 10.1016/j.bbalip.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Sohlenkamp C., Geiger O. Bacterial membrane lipids: Diversity in structures and pathways. FEMS Microbiol. Rev. 2015;40:133–159. doi: 10.1093/femsre/fuv008. [DOI] [PubMed] [Google Scholar]
- 50.Kondakova T., D’Heygère F., Feuilloley M.J., Orange N., Heipieper H.J., Poc C.D. Glycerophospholipid synthesis and functions in Pseudomonas. Chem. Phys. Lipids. 2015;190:27–42. doi: 10.1016/j.chemphyslip.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Lew L.-C., Liong M.-T. Bioactives from probiotics for dermal health: Functions and benefits. J. Appl. Microbiol. 2013;114:1241–1253. doi: 10.1111/jam.12137. [DOI] [PubMed] [Google Scholar]
- 52.Wei J.H., Yin X., Welander P.V. Sterol Synthesis in Diverse Bacteria. Front. Microbiol. 2016;7:990. doi: 10.3389/fmicb.2016.00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adsul M., Khire J., Bastawde K., Gokhale D. Production of Lactic Acid from Cellobiose and Cellotriose by Lactobacillus delbrueckii Mutant Uc-3. Appl. Environ. Microbiol. 2007;73:5055–5057. doi: 10.1128/AEM.00774-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LeBlanc J.G., Laiño J.E., del Valle M.J., Vannini V., van Sinderen D., Taranto M.P., de Valdez G.F., de Giori G.S., Sesma F. B-Group vitamin production by lactic acid bacteria—Current knowledge and potential applications. J. Appl. Microbiol. 2011;111:1297–1309. doi: 10.1111/j.1365-2672.2011.05157.x. [DOI] [PubMed] [Google Scholar]
- 55.Yépez A., Russo P., Spano G., Khomenko I., Biasioli F., Capozzi V., Aznar R. In situ riboflavin fortification of different kefir-like cereal-based beverages using selected Andean LAB strains. Food Microbiol. 2019;77:61–68. doi: 10.1016/j.fm.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 56.Ewe J., Liong M. Viability and growth characteristics of Lactobacillus in soymilk supplemented with B-vitamins. Int. J. Food Sci. Nutr. 2010;61:87–107. doi: 10.3109/09637480903334163. [DOI] [PubMed] [Google Scholar]
- 57.Thakur K., Lule V.K., Rajni C.S., Kumar N., Mandal S., Anand S., Kumari V., Tomar S.K. Riboflavin Producing Probiotic Lactobacilli as a Biotechnological Strategy to Obtain Riboflavin-enriched Fermented Foods. J. Pure Appl. Microbiol. 2016;10:161–166. [Google Scholar]
- 58.Jayashree S., Jayaraman K., Kalaichelvan G. Isolation, Screening and Characterization of Riboflavin. Recent Res. Sci. Technol. 2010;2:83–88. [Google Scholar]
- 59.Levit R., de Giori G.S., de LeBlanc A.M., LeBlanc J.G. Recent update on lactic acid bacteria producing riboflavin and folates: Application for food fortification and treatment of intestinal inflammation. J. Appl. Microbiol. 2021;130:1412–1424. doi: 10.1111/jam.14854. [DOI] [PubMed] [Google Scholar]
- 60.LeBlanc J.G., Burgess C., Sesma F., de Giori G.S., van Sinderen D. Lactococcus lactis is capable of improving the riboflavin status in deficient rats. Br. J. Nutr. 2005;94:262–267. doi: 10.1079/BJN20051473. [DOI] [PubMed] [Google Scholar]
- 61.Burgess C., O’Connell-Motherway M., Sybesma W., Hugenholtz J., van Sinderen D. Riboflavin Production in Lactococcus lactis: Potential for In Situ Production of Vitamin-Enriched Foods. Appl. Environ. Microbiol. 2004;70:5769–5777. doi: 10.1128/AEM.70.10.5769-5777.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zeisel H.S., Stover P. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline1. National Agricultural Library; Baltimore, MD, USA: 1998. pp. 35–50. [PubMed] [Google Scholar]
- 63.Jung M.Y., Lee C., Seo M.J., Roh S.W., Lee S.H. Characterization of a potential probiotic bacterium Lactococcus raffinolactis WiKim0068 isolated from fermented vegetable using genomic and in vitro analyses. BMC Microbiol. 2020;20:1–10. doi: 10.1186/s12866-020-01820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang M.-S., Yeu J.-E., Hong S.-P. Safety Evaluation of Oral Care Probiotics Weissella cibaria CMU and CMS1 by Phenotypic and Genotypic Analysis. Int. J. Mol. Sci. 2019;20:2693. doi: 10.3390/ijms20112693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Divya J.B., Varsha K.K., Nampoothiri K.M. Newly isolated lactic acid bacteria with probiotic features for potential application in food industry. Appl. Biochem. Biotechnol. 2012;167:1314–1324. doi: 10.1007/s12010-012-9561-7. [DOI] [PubMed] [Google Scholar]
- 66.Månberger A., Verbrugghe P., Guðmundsdóttir E.E., Santesson S., Nilsson A., Hreggviðsson G.Ó., Linares-Pastén J.A., Karlsson E.N. Taxogenomic assessment and genomic characterisation of Weissella cibaria strain 92 able to metabolise oligosaccharides derived from dietary fibres. Sci. Rep. 2020;10:5853. doi: 10.1038/s41598-020-62610-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thursby E., Juge N. Introduction to the human gut microbiota. Biochem. J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bungenstock L., Abdulmawjood A., Reich F. Evaluation of antibacterial properties of lactic acid bacteria from traditionally and industrially produced fermented sausages from Germany. PLoS ONE. 2020;15:e0230345. doi: 10.1371/journal.pone.0230345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alegría Á., Delgado S., Roces C., López B., Mayo B. Bacteriocins produced by wild Lactococcus lactis strains isolated from traditional, starter-free cheeses made of raw milk. Int. J. Food Microbiol. 2010;143:61–66. doi: 10.1016/j.ijfoodmicro.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 70.Suzuki A., Suzuki M. Antimicrobial activity of Lactococcus lactis subsp. lactis isolated from a stranded cuvier’s beaked whale (Ziphius cavirostris) against gram-positive and -negative bacteria. Microorganisms. 2021;9:243. doi: 10.3390/microorganisms9020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Srionnual S., Yanagida F., Lin L.H., Hsiao K.N., Chen Y.S. Weissellicin 110, a newly discovered bacteriocin from Weissella cibaria 110, isolated from plaa-som, a fermented fish product from Thailand. Appl. Environ. Microbiol. 2007;73:2247–2250. doi: 10.1128/AEM.02484-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lakra A.K., Domdi L., Hanjon G., Tilwani Y.M., Arul V. Some probiotic potential of Weissella confusa MD1 and Weissella cibaria MD2 isolated from fermented batter. LWT. 2020;125:109261. doi: 10.1016/j.lwt.2020.109261. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All sequence data that support the findings of this study have been deposited in GenBank and the public URL for the sequence collection is https://www.ncbi.nlm.nih.gov/sites/myncbi/1F1i8raBplj5d/collections/61883288/public/ (accessed on 1 June 2022) Lactococcus lactis strain Beet1 (OM957496) and Weissella cibaria strain Beet2 (OM957500).