Abstract
It is well established that access to preventative care, such as breast or cervical cancer screening, can reduce morbidity and mortality. Certain groups may be missed out of these healthcare services, such as women with disabilities, as they face many access barriers due to underlying inequalities and negative attitudes. However, the data have not been reviewed on whether women with disabilities face inequalities in the uptake of these services. A systematic review and meta-analysis were conducted to compare the uptake of breast and cervical cancer screening in women with and without disabilities. A search was conducted in July 2021 across four databases: PubMed, MEDLINE, Global Health, and CINAHL. Quantitative studies comparing the uptake of breast or cervical cancer screening between women with and without disabilities were eligible. Twenty-nine studies were included, all from high-income settings. One third of the 29 studies (34.5%, n = 10) were deemed to have a high risk of bias, and the remainder a low risk of bias. The pooled estimates showed that women with disabilities have 0.78 (95% CI: 0.72–0.84) lower odds of attending breast cancer screening and have 0.63 (95% CI: 0.45–0.88) lower odds of attending cervical cancer screening, compared to women without disabilities. In conclusion, women with disabilities face disparities in receipt of preventative cancer care. There is consequently an urgent need to evaluate and improve the inclusivity of cancer screening programs and thereby prevent avoidable morbidity and mortality.
Keywords: disability, cancer, screening, mammography, pap smear
1. Introduction
Breast and cervical cancer are leading causes of cancer death in women, accounting for 15.5% and 7.7% of all cancer deaths, respectively [1]. The early detection of breast or cervical cancer significantly improves the prognosis. Participation in a cancer screening programme is consequently associated with an 89% reduction in cervical cancer mortality [2] and a 21–25% reduction in breast cancer mortality [3]. However, there is strong evidence that disparities exist in cancer screening uptakes, even in settings where cancer screening programmes are well-established [4]. As a result, countries are failing to reach their cancer screening targets and people are dying unnecessarily [5].
Disability is potentially an important predictor of cancer screening uptake. People with disabilities face a range of barriers to accessing screening, including a lack of accessibility (information, transport, equipment, and facilities), a lack of affordability, communication difficulties, and negative attitudes of healthcare professionals [6,7,8]. They are also on average poorer and with less education, two known predictors of low screening uptake [4]. These barriers are likely to translate into lower service coverage and there is growing evidence suggesting that cancer screening uptake is lower among people with disabilities [6]. For instance, a study from the UK showed that women with disabilities are 36% less likely to attend breast screening and 25% less likely to attend bowel cancer screening, when compared to women without disabilities [9]. Women with multiple difficulties, or difficulties with vision or self-care were least likely to attend screening. Similarly, a national study in South Korea showed that having any type of disability is associated with 29% lower odds of cervical cancer screening [10].
The lower uptake of screening among people with disabilities is an important issue, as globally there are at least one billion persons with disabilities [11]. In the UK alone, there are at least 11 million people with disabilities [12]. Disability is particularly common in older people, who are also the focus of cancer screening programmes. However, there are no systematic reviews on the association between disability with breast and cervical cancer screening uptake, except one from 2013 that focused on women in the USA only [13]. The aim of this paper is to systematically review the global data on disparities in uptake of breast and cervical cancer screening among women in relation to their disability status.
2. Materials and Methods
This systematic review of peer-reviewed articles that presents research findings on the uptakes or receipt of breast or cervical cancer screening in women with and without disabilities was conducted in July 2021. This review used both the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Guidelines for Meta-Analyses and Systematic Reviews of Observational Studies (MOOSE Guideline) for conducting and reporting [14,15].
2.1. Defining Disability
This review used the World Health Organization International Classification Functioning, Disability, and Health (WHO-ICF) framework in defining disability [16]. Therefore, disability was classified as any form of physical, sensory, cognitive, or psychosocial impairment associated with activity limitations or participation restrictions. Moreover, this review also included diagnostic codes for specific illnesses (e.g., psychosis) or impairment (e.g., visual impairment, functional hearing loss) considered likely to be disabling.
2.2. Outcome
The primary outcome of interest was uptake/receipt of either cervical or breast cancer screening, comparing women with and without disabilities.
2.3. Data Sources and Search Terms
The literature search was conducted up to July 2021 across four databases (MEDLINE, PubMed, CINAHL, and Global Health). A combination of subject headings and key terms were used to assess: (1) disability, (2) breast or cervical cancer, and (3) screening uptake or utilization. A string of search terminologies was constructed, to ensure a comprehensive and holistic search strategy, such as the term “cancer screening”; where this can include early diagnostics or detection, also the type of diagnostic methods (Pap smear, mammography), where applicable truncation was also utilised. Boolean operators (‘AND’, ‘OR’, and ‘NOT’) were used to string search terminologies together. This review used both persons with disabilities and women with disabilities in the search term, even though breast and cervical cancer already implies women as the target population. The search strategy for MEDLINE is included as a supplementary file (Supplementary File S1).
2.4. Inclusion Criteria
Studies were included if they quantitatively assessed the uptake and/or receipt of breast cancer screening (mammography, other radiological or laboratory examinations) or cervical cancer screening (Pap smear or visual inspection) in women with and without disability, aged 18–70. There were no limitations on study design (except the exclusion of qualitative studies); therefore, both observational and interventional studies were included. Eligible publication date was restricted to be between 2011 and 2021, to ensure relevance of the findings.
We excluded studies if they were not written in English; not peer reviewed, or did not compare disparities of uptakes in women with disabilities to women without disabilities. Moreover, review studies and studies lacking clarity in reporting of measure of effect (i.e., no information on lower or upper limits, or ability to calculate these values), were also excluded from this review.
2.5. Study Selection
After the search strategy developed for MEDLINE was deemed to provide sufficient results, it was transferred to other search databases. Results from the database searches were transferred to Mendeley, which automatically removed duplicates. Subsequently, articles were transferred to Rayyan for title, abstract, and keyword screening. The initial screening was conducted by a single reviewer, and the results were checked by a second reviewer. The full texts were then retrieved and were screened by two authors (FRA and AN) according to the eligibility criteria for this review.
2.6. Data Extraction
There were three main components extracted from the selected articles: (1) article information (author information, country, and study design); (2) participant information (disability assessment, number of participants screened and did not receive screening, and contextual setting); (3) outcome (measurement and measure of association). Odds Ratios were extracted, rather than calculated from data presented, for this review.
2.7. Risk of Bias Assessment
Appraisal of risk of bias of included studies was undertaken using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for observational studies [17]. The checklist was used to examine methodological components of each study, and the studies were scored as to whether they had a low, medium, or high risk of bias.
2.8. Data Synthesis and Meta-Analysis
Estimates were pooled based on type of screening, resulting in the estimate of odds ratio of breast or cervical cancer screening comparing women with and without disabilities. The pooled estimates were calculated using a random effects model, as variations between included studies (e.g., country setting, sampling method, types of disability, and outcome measurement) can result in between-study heterogeneity. Heterogeneity across analyses was assessed using the I2 statistic.
Sub-group analyses were conducted for studies that comprised similar characteristics: type of disability and study methodology or design. Additionally, studies that were deemed with high risk of bias were excluded from the subgroup analyses. All statistical analyses were conducted using R version 1.4.1717 and package Meta [18].
3. Results
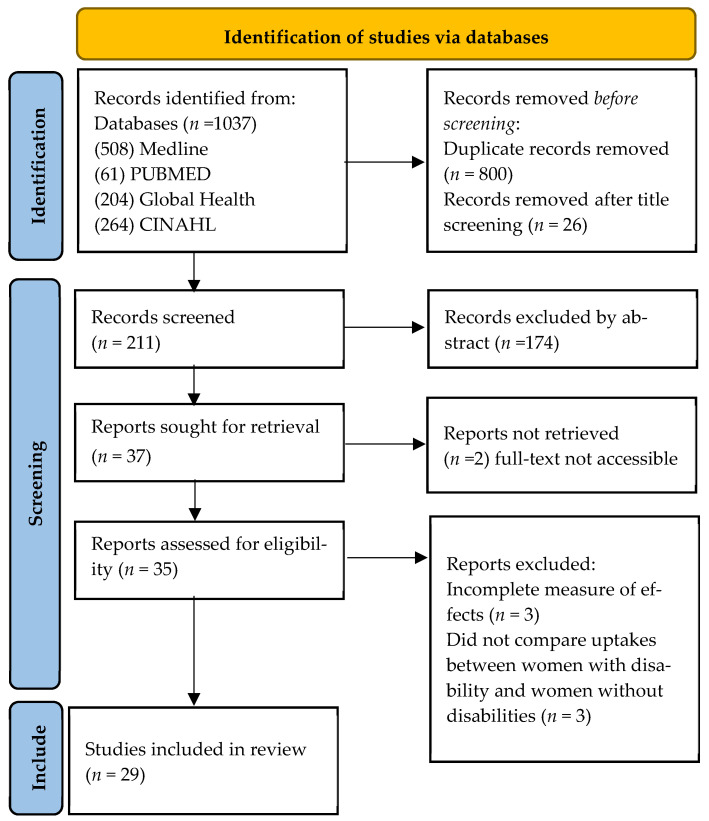
The database search was conducted on 21 July 2021 and resulted in 1037 papers (Figure 1). After duplicates were removed, 1037 titles were screened and ineligible studies were excluded. Consequently, 211 abstracts were screened, of which 35 full texts were selected for assessment. However, the full texts of two articles could not be retrieved. A further of six studies were excluded, due to incomplete information on the measure of effects or lack of reporting of differences between women with and without disabilities. Overall, 29 eligible studies were identified. No studies were added after conducting a further hand search and a reverse citation search.
Figure 1.
PRISMA flow chart of search results.
3.1. Study Characteristics
Table 1 summarizes the characteristics of the 29 studies included in the review. Thirteen studies (45%) focused on breast cancer screening alone, six (21%) on cervical screening alone, and ten (34%) addressed both breast and cervical screening uptake. All included studies were conducted in high-income countries. The greatest number were conducted in the US (37.7% of all the studies), followed by the UK (13.8%), Canada, South Korea, and Northern Ireland (10.3% each). Australia, Denmark, and Sweden contributed one study each.
Table 1.
Information and characteristics of included studies.
| Author | Study Location | Study Design | Type of Disability | Definition of Disability | Type of Screening | Participants | Age Range (Years) | Follow up Time | |
|---|---|---|---|---|---|---|---|---|---|
| With Disability | Without Disability | ||||||||
| Cobigo et al. (2013) [36] | Canada | Cohort | Learning | Intellectual developmental disabilities based on the ICD-10. | Both | 17,777 | 1,440,962 | 20–69 | Breast: 2 years, Cervix: 3 years |
| Horner-Johnson et al. (2015) [37] | USA | Cohort | Functional | Presence of limitations in basic actions involving physical functions, vision, hearing, or cognition. | Both | 10,985 (urban), 3108 (rural) | 42,834 (urban), 8579 (rural) | Breast: 40–64, Cervix: 18–64 |
6 years |
| Ko et al. (2011) [22] | South Korea | Cross-sectional | Physical and psychosocial. | ICF: Physical, internal organ, and mental. | Both | 23,511 | 11,660 | 42–69 | 2 years |
| Kushalnagar. (2019) [38] | USA | Cross-sectional | Hearing | Functional hearing impairment. | Both | Breast: 324 Cervix: 529 |
Breast: 1086 Cervix: 1119 |
Breast: 40–74 Cervix: 21–65 |
n/a |
| Murphy et al. (2021) [34] | USA | Mixed methods, retrospective for quantitative; and qualitative. | Psychosocial | Serious mental illness (SMI): schizophrenia, bipolar depression, major depression. | Both | Breast 94,921 Cervix 274,643 |
Breast: 11,955,674 Cervix: 31,949,537 |
21–64 | 7 years |
| Osborn et al. (2012) [25] | UK | Cohort | Learning | General terms and related terms (e.g., autism, down syndrome, and Fragile X syndrome). | Both | Breast: 2956 Cervix: 6254 |
50,779 | Breast: 50–64 Cervix: 20–65 |
10 years |
| Steele et al. (2017) [19] | USA | Cross-sectional | Physical and functional. | Self-report of disability. | Both | 2580 | 12,499 | 21–75 | n/a |
| Woodhead et al. (2016) [26] | UK | Cross-sectional | Psychosocial | Serious mental illness based on ICD-10 diagnosis. | Both | Breast: 625, Cervix: 1393 |
Breast: 25,385, Cervix: 106,554 |
Breast: 50–70, Cervix: 25–64 |
n/a |
| Xu et al. (2017) [39] | USA | Cohort | Visual | Clinical diagnosis of visual impairment. | Both | Breast: 1308, Cervix: 1247 | Breast: 2635, Cervix: 2483 |
Breast: 40–74, Cervix: 20–74 |
11 years |
| Assi et al. (2020) [23] | USA | Cross-sectional | Visual | Self-reported visual impairments. | Breast | 1915 | 10,205 | 50–74 | n/a |
| Caban et al. (2011) [40] | USA | Cohort | Functional and psychosocial. | Reported functional limitations of activity of daily living (ADL) and instrumental activities of daily living (IADL). | Breast | 2281 | 2329 | >65 | 2 years |
| Courtney-Long et al. (2011) [41] | USA | Cross-sectional | Physical and functional. | Self-report of disability. | Breast | 64,905 | 130,394 | 40–74 | 2 years |
| Fioravante et al. (2021) [21] | USA | Cross-sectional | Hearing | Functional hearing loss. | Breast | 2123 | 10,067 | 50–74 | n/a |
| Floud et al. (2017) [9] | UK | Cohort | Functional (including psychological) and physical. | Self-report of disability. | Breast | 109,869 | 363,316 | 50–70 | 5 years |
| Guilcher et al. (2014) [42] | Canada | Cohort | Physical and functional. | Morbidity: presence of limiting disease, e.g., arthritis, hypertension. | Breast | 4660 | 5703 | 50–69 | 2 years |
| Jensen et al. (2016) [33] | Denmark | Cohort | Psychosocial | Schizophrenia, affective disorders, eating disorder. | Breast | 47,648 | 96,616 | 50–69 | Up to 10 years |
| Ross et al. (2020) [6] | Northern Ireland | Cohort | Physical and psychosocial. | Self-report of disability. | Breast | 20,541 | 36,787 | 48–70 | 1 year |
| Ross et al. (2020) [28] | Northern Ireland | Cohort | Psychosocial | Chronic poor mental health. | Breast | 6162 | 51,166 | 50–70 | 4 years |
| Ross et al. (2021) [27] | Northern Ireland | Cohort | Psychosocial | Record of psychotropic prescription. | Breast | 17,521 | 39,807 | 50–70 | 3 years |
| Sakellariou and Rotarou. (2019) [20] | UK | Cross-sectional | Physical | Lower limb impairment. | Breast | 2697 | 6794 | 20–70+ | n/a |
| Shin et al. (2020) [43] | South Korea | Cohort | Physical and psychosocial. | Diagnosis of disability by healthcare professional. | Breast | 419,376 | 5,864,247 | >40 | 10 years |
| Koroukian et al. (2012) [35] | USA | Cohort | Psychosocial | Morbidity: presence of limiting disease, e.g., arthritis, hypertension. | Breast | 61,661 | 68,427 | 50–64 | n/a |
| Wu et al. (2021) [44] | USA | Cohort | Visual | Partial vision loss (PVL) and severe vision loss (SVL). | Breast | PVL: 348, SVL: 348 |
348 | 65–72 | 5 years |
| Abrams et al. (2012) [24] | USA | Cohort | Psychosocial | Psychosis (schizophrenia), substance use disorder, bipolar disorder, or mania. | Cervical | 20,306 | 85,375 | 19–64 | 1 year |
| Brown et al. (2016) [30] | Canada | Cohort | Intellectual and developmental. | Clinical diagnosis of intellectual and developmental disabilities. | Cervical | 5033 | 527,437 | 20–64 | n/a |
| Eriksson et al. (2019) [29] | Sweden | Cohort | Psychosocial | Psychiatric diagnosis. | Cervical | 65,292 | 341,171 | 23–60 | 5 years |
| Shin et al. (2018) [10] | South Korea | Cohort | Physical and functional. | Diagnosis of disability by healthcare professional. | Cervical | 426,189 | 7,376,529 | >50 | 10 years |
| Tuesley et al. (2019) [32] | Australia | Cohort | Psychosocial | Classified as serious mental illnesses, based on prescriptions in the last 12 months. | Cervical | 18,363 | 899,777 | 18–69 | 10 years |
| Weitlauf et al. (2013) [31] | USA | Cohort | Psychosocial | PTSD and depression based on clinical diagnosis ICD 9. | Cervical | 17,295 | 16,828 | 18–65 | 1 year |
Breast: breast cancer screening. Cervix: cervical cancer screening.
For studies that included cervical cancer screening, participants’ starting age was generally younger (starting from 18) compared to studies on breast cancer screening (minimum age of 40 and above).
3.1.1. Study Design
Most of the included studies used a cohort study design (65.5%), utilizing data retrieved from national databases such as the National Health Insurance or Disability databases. Five included studies used a cross-sectional design (31%) [19,20,21,22,23] and one study used a mixed-method design [24].
3.1.2. Types of Disabilities
The most common type of disability included in this review was psychosocial disability, accounting for 47% of all studies (n = 15), assessed as a psychiatric or mental health diagnosis, a history of psychiatric prescription, or self-reported mental status [24,25,26,27,28,29,30,31,32,33,34,35,36]. One third (31.3%, n = 10) of the studies included used disability in general or combining different types of disability into a category of having a disability or no disability. Few studies (9.7%, n = 3) focused on vision impairment, intellectual, or learning disabilities (9.7% n = 3), physical impairment (6.9%, n = 2), and functional hearing loss (6.9%, n = 2). Four studies also considered the number of disabilities and the severity of disability in relation with breast or cervical cancer screening.
3.1.3. Outcome Measurement
The outcome of interest in this review is the uptake of breast or cervical cancer screening services. Of all the studies included in this review, 72% (n = 23) of the studies retrieved data on breast and cervical cancer screening utilization from central or national databases, where billing codes and examination or diagnostic history were linked to data on disability status. The other method of measurement was self-reporting through a questionnaire or interview (Table 2 and Table 3).
Table 2.
Details on outcome measurement and estimate of association between presence of any disability in women and breast cancer screening.
| Author | Outcome Definition | Assessment Method | Uptake (%) | Unadjusted OR | aOR (95% CI) |
Risk of Bias Rating | |
|---|---|---|---|---|---|---|---|
| Women with Disabilities |
Women without Disabilities |
||||||
| Assi et al. [23] | Receipt of mammography in the past 2 years. | Self-report | (−5.02%) difference in proportions | BRFSS: 0.63 (0.56–0.70) |
0.67 (0.59–0.75) |
High | |
| NHIS: 0.78 (0.68–0.89) |
0.82 (0.71–0.89) |
||||||
| Caban et al. [40] | Receipt of mammography in the past 1 year of the study period. | Self-report | n/a | n/a | Moderate disability: 0.76 (0.64–0.91) |
0.98 (0.81–1.18) |
Low |
| Severe disability: 0.46 (0.40–0.54) |
0.67 (0.54–0.83) |
||||||
| Cobigo et al. [36] | Receipt of mammography in the past 2 years. | Clinical record (Insurance code) | 42% | 60% | 0.47 (0.45–0.50) |
0.95 (0.84–1.08) |
Low |
| Courtney-Long et al. [41] | Receipt of mammogram within the past 2 years. | Self-report | Total group: 72% | 78% | n/a | 0.92 (0.87–0.98) |
High |
| Aged 50–74: 78% | 83% | n/a | 0.92 (0.85–0.99) |
||||
| Fioravante et al. [21] | Receipt of mammogram within past two years. | Self-report | n/a | n/a | 0.84 (0.73–0.96) |
0.83 (0.72–0.96) |
High |
| Floud et al. [9] | Clinical registration of breast cancer screening in the past 3 years. | Clinical record |
83% | 89% | n/a | 0.64 (0.62–0.65) |
Low |
| Guilcher, et al. [42] | Receipt of mammography within two years. | Clinical record |
Moderate disability: 67% | 68% | n/a | 1.22 (1.09–1.38) |
Low |
| Severe disability: 67% | 68% | n/a | 0.88 (0.78–0.99) |
||||
| Horner-Johnson et al. [37] | Receipt of mammography within two years. | Clinical record |
Rural: 67% | 70% | 0.63 (0.56–0.72) |
0.79 (0.68–0.91) |
Low |
| Urban: 73% | 76% | 0.85 (0.77–0.93) |
0.94 (0.84–1.04) |
||||
| Jensen et al. [33] | Rates of participation in the first 18 months of the screening round. | Clinical record |
74.5% | 81% | 0.65 (0.63–0.68) |
0.79 (0.77–0.82) |
Low |
| Ko et al. [22] | Utilisation of breast cancer screening services during the study period. | Self-report | 26% | 32% | n/a | 0.78 (0.43–1.4) |
|
| Koroukian et al. [35] | Receipt of screening mammography in the study period and adherence to national guideline. | Clinical record | 38% | 32% | n/a | 0.68 (0.66–0.7) | Low |
| Kushalnagar [38] | Adherence to mammography guidelines. | Self-report | 76% | 82% | n/a | 0.94 (0.77–0.94) |
Low |
| Murphy et al. [34] | Receipt of breast cancer screening, during the 6 year study period. | Clinical record |
51% | 62% | 0.88 (0.87–0.89) |
0.79 (0.78–0.8) |
Low |
| Osborn et al. [25] | Clinical record of attending for mammography or mammography results during the study period. | Clinical record |
44% | 52% | IRR = 0.78 (0.74–0.83) * |
IRR = 0.76 (0.72–0.81) * |
Low |
| Ross et al. (2021) [27] | Records of women attending the screening programme from 1 April 2011 to 31 March 2014. | Clinical record |
74% | 81% | 0.71 (0.68–0.74) |
0.67 (0.64–0.7) |
Low |
| Ross et al. (2020) [28] | Clinical attendance of screening invitation. | Clinical record |
75% | 81% | 0.53 (0.50–0.57) |
0.93 (0.89–0.98) |
Low |
| Ross et al. (2019) [6] | Breast cancer screening attendance. | Self-report | 68% | 80% | 0.67 (0.64–0.70) |
0.77 (0.73–0.82) |
Low |
| Sakellariou, Rotarou [20] | Receipt of mammogram within the past three years. | Secondary data analysis | 48% | 46% | n/a | 0.80 (0.70–0.92) |
|
| Shin et al. [43] | Clinical attendance or use of mammography for breast cancer screening during 2014–2015. | Clinical record |
41% | 54% | n/a | 0.82 (0.82–0.83) |
Low |
| Steele et al. [19] | Receipt of mammogram within the past 2 years. | Self-report | 67% | 73% | n/a | 0.79 (0.77–0.82) |
High |
| Woodhead et al. [26] | Receipt of mammography in the past three years, and for aged 50–64 in five years. | Clinical record |
58% | 66% | 0.72 (0.61–0.86) |
0.60 (0.49–0.73) | Low |
| Wu et al. [44] | Receipt of mammogram within past two years. | Insurance record | PVL: 77% | 81% | n/a | 0.56 (0.36–0.87) |
Low |
| SVL: 72% | 81% | n/a | 0.58 (0.37–0.9) | ||||
| Xu et al. [39] | Full adherence or partial adherence to screening guidelines, during the study period. | Insurance record | 65% | 75% | n/a | 0.49 (0.40–0.6) | Low |
aOR: adjusted Odds Ratio. OR: Odds ratio. n/a: Not available. *: reported as IRR.
Table 3.
Details on outcome measurement and estimate of association between presence of any type of disability in women and cervical cancer screening.
| Author | Outcome Definition |
Assessment Methods |
Uptake (%) | Risk of Bias Rating | |||
|---|---|---|---|---|---|---|---|
| Women with Disabilities |
Women without Disabilities |
Unadjusted OR (95% CI) |
aOR (95%CI) |
||||
| Abrams et al. [24] | Clinical attendance to cervical screening over the study period (July 2004–June 2004). | Clinical record | 25% | 18% | n/a | 1.46 (1.36–1.57) | Low |
| Brown et al. [30] | Clinical attendance to cervical screening between 1 April 2007 and 31 March 2010. | Clinical Record (Insurance code) |
68% | 77% | n/a | 0.61 (0.58–0.65) |
Low |
| Cobigo et al. [36] | Receipt of at least one Pap test over a 3 year period. | Clinical record |
34% | 67% | 0.26 (0.25–0.27) |
0.21 (0.2–0.21) |
Low |
| Eriksson et al. [29] | Clinical participation in cervical cancer screening over the 5 year study cohort period. | Clinical record |
86% | 89% | n/a | 0.98 (0.97–0.98) |
Low |
| Horner-Johnson et al. [37] | Receipt of Pap smear with three years. | Clinical record |
Rural: 77% | 84% | 0.50 (0.44–0.58) |
0.69 (0.59–0.81) |
Low |
| Urban: 82% | 87% | 0.67 (0.62–0.72) |
0.78 (0.87–0.96) |
||||
| Ko et al. [22] | Utilisation of cervical cancer screening services during the study period. | Self-report | >30 years: 29% | 45% | n/a | 0.71 (0.41–1.22) |
High |
| >40 years: 23% | 43% | n/a | 0.52 (0.27–0.98) |
||||
| Kushalnagar [38] | Adherence to pap smear guidelines. | Self-report | 78% | 85% | n/a | 0.71 (0.59–0.86) |
High |
| Murphy et al. [34] | Receipt of pap smear during the 6 year study period. | Clinical record |
52% | 61% | 0.92 (0.92–0.93) |
0.80 (0.80–0.81) |
Low |
| Osborn et al. [25] | Clinical record of attending for Pap smear during the study period. | Clinical record |
68% | 85% | IRR = 0.55 (0.53–0.57) * |
IRR = 0.54 (0.52–0.56) * |
Low |
| Shin et al. [43] | Use of the cervical cancer screening programme in the past ten years (2006–2015). | Administrative data (clinical record) | 54% | 60% | n/a | 0.71 (0.71–0.72) |
Low |
| Steele et al. [19] | Receipt of a Pap smear within the past 2 years. | Self-report | 72% | 82% | n/a | 0.77 (0.60–0.99) |
High |
| Weitlauf et al. [31] | Use of Pap smear test in outpatient setting during the study period. | Insurance record | n/a | Depression: 1.04 (0.98–1.09) |
1.05 (0.99–1.12) |
Low | |
| PTSD: 1.17 (1.09–1.26) |
1.14 (1.06–1.22) |
||||||
| Woodhead et al. [26] | Receipt of cervical cancer screening any time in the last three years for those aged up to 49 years, or any time in the last five years for those aged 50–64. | Clinical record |
80% | 78% | 1.16 (0.99–1.35) |
0.35 (0.29–0.42) |
Low |
| Xu et al. [39] | Full adherence or partial adherence to screening guidelines during the study period. | Insurance record | 64% | 81% | n/a | 0.32 (0.27–0.39) |
Low |
aOR: adjusted Odds Ratio. OR: Odds ratio. n/a: not available. *: reported as IRR.
Three studies measured the adherence of women with disability to national cancer screening guidelines, thus, to be categorized as “screened” or “have utilized the service” if they had met criteria for the number of visits [26,35,45]. Table 2 and Table 3 describes how screening uptakes were measured in each study and its measure of association between disability status and breast or cervical cancer screening.
Generally, the included studies all presented ORs with a 95% confidence interval to estimate the measure of association between disability status and the utilization of breast or cervical screening services. Only two studies used the incidence rate ratio (IRR) to estimate the use of the cancer screening services comparing women with and without disabilities [25,32]. Some studies provided two estimates, depending on how disability status was coded, participants settings, and the severity of disability or impairment.
3.1.4. Risk of Bias
One third of the 29 studies (34.5%, n = 10) were deemed to have a high risk of bias, and the remainder a low risk of bias (Table 2 and Table 3). Generally, studies that have a high risk of bias was due to not providing sufficient information on how the population or participants were categorized as exposed, and how the outcome was measured. Many studies were also marked down for using a self-reporting questionnaire as the sole measure of uptake or receipt of cancer screening.
3.1.5. Breast Cancer Screening Uptake in Women with Disability
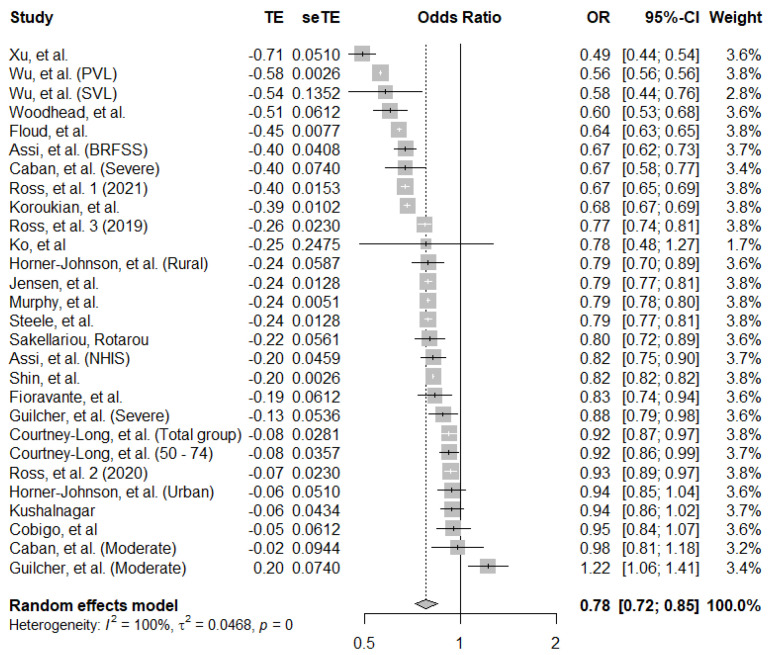
There were 28 data points included in the pooled analysis for breast cancer screening uptake, taken from 21 studies. The pooled estimate showed that women with disability have 0.78 (95% CI: 0.72–0.85) lower odds of breast cancer screening compared to women without disability. Individual estimates ranged from 0.49 to 1.22, and there was strong evidence for heterogeneity (I2 = 100%, p < 0.001) (Figure 2).
Figure 2.
Pooled adjusted odds ratio estimates of breast cancer screening uptake by disability status [6,9,19,20,21,22,23,26,27,28,33,34,35,36,37,38,39,40,41,42,43,44].
3.1.6. Cervical Cancer Screening
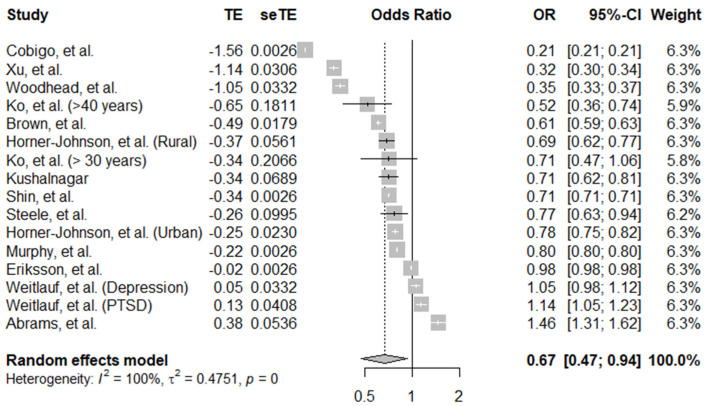
There were 16 data points included in the pooled analysis for cervical cancer screening uptake, taken from 13 different studies (Figure 3). The overall pooled estimate of aORs is 0.67 (95% CI, 0.47–0.94), showing that women with disabilities have 0.67 lower odds of receiving cervical cancer screening compared to women without disability. From all the data points, only two data points showed significantly lower screening odds in women with disabilities. Moreover, one data point showed significantly higher screening in women with disabilities. There was evidence of high between-study heterogeneity (I2 = 100%, p ≤ 0.001).
Figure 3.
Pooled adjusted odds ratio estimates of cervical cancer screening uptake by disability status [19,22,24,26,29,30,31,34,36,37,38,39,43].
3.2. Subgroup Analyses
Limited subgroup analyses were possible (Table 4).
Table 4.
Summary of subgroup analyses.
| Screening Type | Sub-Group | Studies Included (References) |
Pooled Estimate (95% CI) |
Heterogeneity (I2) |
|---|---|---|---|---|
| Breast cancer | Visual impairment | N = 3 studies [5,20,33] | 0.63 (0.51–0.77) | 95% |
| Breast cancer | Psychosocial | N = 7 studies [22,23,24,25,26,31,32] | 0.69 (0.60–0.80) | 100% |
| Cervical cancer | Psychosocial | N = 6 studies [22,24,27,28,29,32] | 0.64 (0.34–1.18) | 100% |
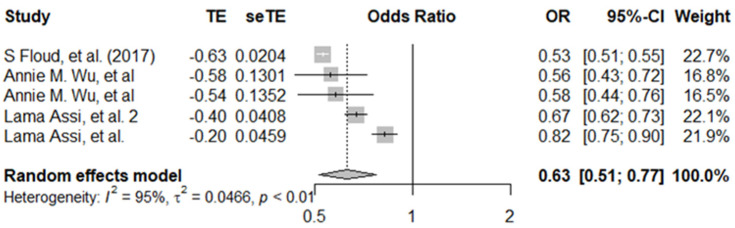
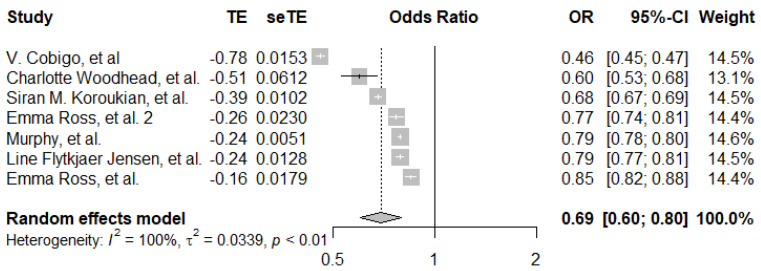
For breast cancer screening, the pooled estimate of five data points from three studies showed that women with visual impairment had a 0.63 (95% CI: 0.51–0.77) lower odds of breast cancer screening compared to women without visual impairment. There was evidence of high between-study heterogeneity (I2 = 95%, p ≤ 0.001) (Figure 4). For psychosocial disabilities, the pooled estimate was an OR of 0.69 (95%CI, 0.60–80) (Figure 5), across seven data points from seven different studies.
Figure 4.
Pooled adjusted odds ratio estimates of breast cancer screening uptake by visual impairment status [9,23,44].
Figure 5.
Pooled adjusted odds ratio estimates of breast cancer screening uptake by psychosocial disability status [26,27,28,33,34,35,36].
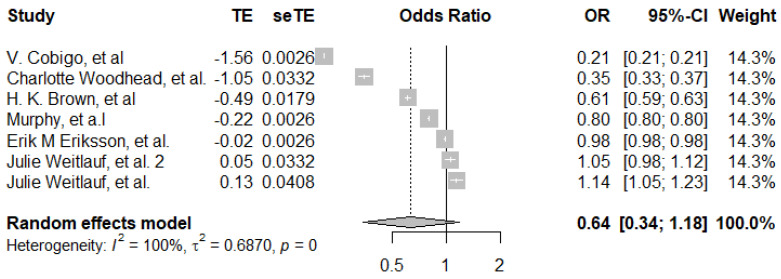
For cervical cancer screening, the pooled estimate for the association of psychosocial disability and screening uptake was 0.64 (95%CI, 0.34–1.18) (Figure 6), using seven data points. Individual estimates ranged from 0.21 to 1.14. Furthermore, all sub-group analysis showed evidence of heterogeneity among each study.
Figure 6.
Pooled adjusted odds ratio estimates of cervical cancer screening uptake by psychosocial disability status [26,29,30,31,34,36].
4. Discussion
This systematic review and meta-analysis identified 29 studies across 8 different countries, evaluating the uptake of breast and cervical cancer screening by disability status. All studies included in this review were conducted in high-income countries. Overall, women with disabilities were 22% less likely to undergo breast cancer screening and 33% less likely to attend for cervical cancer screening compared to women without disabilities. The individual study results followed this pattern and out of the 29 studies only 3 did not show lower screening among women with disabilities.
The results of this review are consistent with the broader literature on this topic. A 2013 systematic review on cervical and breast screening and disability in the USA only identified five studies [13]. It showed evidence for a lower uptake of mammography among women with disabilities, but the evidence for clinical breast examination or cervical cancer screening was less clear. Qualitative data shows that women with disabilities report multiple barriers to accessing breast and cervical cancer screening, including physical barriers, cost, a lack of knowledge, fear, and attitudes of healthcare workers [46,47,48]. Studies have also shown that colorectal cancer screening is less frequent among people with disabilities compared to those without [49,50]. More broadly, it is well established that people with disabilities face greater challenges in accessing health care services [51].
This review showed clear evidence of disparities in breast and cervical cancer screening services experienced by women with disabilities. These findings further emphasize the importance of an inclusive cancer screening program and accessible healthcare services. However, this review did not provide details on the quality and effectiveness of healthcare received. Additionally, the review only found studies from high-income countries, making generalizability an issue. More evidence is therefore needed from low- and middle-income countries. The results do indicate that it is appropriate to design and evaluate interventions to improve cancer screening uptake among women with disabilities. These interventions should address the common barriers encountered, for instance, through providing training on disability to screening care providers, encouraging carers to support screening uptake and ensuring facilities and information are accessible. It may also be important to review policies and targets related to cancer screening to ensure that they are inclusive of people with disabilities. If these changes are not made, then women with disabilities will continue to have lower screening rates and face avoidable cancer-related deaths.
There are strengths and limitations of this review that should be taken into account when interpreting the results. The level of heterogeneity was high, likely because of differences in the measurement of disability between studies. Most studies used clinical diagnosis, such as vision impairment, hearing, and other psychiatric or mental diagnoses, and only one study explicitly explored disability through the ICF framework [17]. Another limitation is that most screening and data extraction was conducted by a single reviewer, which can potentially lead to selection bias. Additionally, this review only included studies published in English and did not explore the grey literature. In terms of strengths, the search strategy implemented a holistic approach to the definition of disability, which was achieved by using search terms that were in-line with the ICF framework. Furthermore, it included clinical diagnosis and conditions that reflect disability. Adhering to the PRISMA and MOOSE guideline also provided this review with methodological rigor.
5. Conclusions
Women with disabilities face disparities in receipt of preventative cancer care. There is consequently an urgent need to evaluate and improve the inclusivity of cancer screening programs and thereby prevent avoidable morbidity and mortality.
Acknowledgments
The authors would like to acknowledge Ford Hickson as the Programme Director of the MSc Public Health at the London School of Hygiene and Tropical Medicine, for providing the administrative guidance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19159465/s1, Supplementary File S1: MEDLINE search strategy.
Author Contributions
F.R.A., conception of the idea, article screening, statistical analysis, wrote the manuscript. H.K., conception of the idea, wrote the manuscript. C.D., conception of the idea, wrote the manuscript. K.B., conception of the idea, wrote the manuscript. A.N., article screening, wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Jansen E.E.L., Zielonke N., Gini A., Anttila A., Segnan N., Vokó Z., Ivanuš U., McKee M., de Koning H.J., de Kok I.M.C.M., et al. Effect of organised cervical cancer screening on cervical cancer mortality in Europe: A systematic review. Eur. J. Cancer. 2020;127:207–223. doi: 10.1016/j.ejca.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Nelson H.D., Fu R., Cantor A., Pappas M., Daeges M., Humphrey L. Effectiveness of Breast Cancer Screening: Systematic Review and Meta-analysis to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann. Intern. Med. 2016;164:244–255. doi: 10.7326/M15-0969. [DOI] [PubMed] [Google Scholar]
- 4.Damiani G., Federico B., Basso D., Ronconi A., Bianchi C.B.N.A., Anzellotti G.M., Nasi G., Sassi F., Ricciardi W. Socioeconomic disparities in the uptake of breast and cervical cancer screening in Italy: A cross sectional study. BMC Public Health. 2012;12:99. doi: 10.1186/1471-2458-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Research UK Cancer Target Missed for 55,000 Patients over 6 Years 2021. [(accessed on 15 July 2021)]. Available online: https://news.cancerresearchuk.org/2021/09/22/cancer-target-missed-for-55000-patients-over-six-years/
- 6.Ross E., Maguire A., Donnelly M., Mairs A., Hall C., O’Reilly D. Disability as a predictor of breast cancer screening uptake: A population-based study of 57,328 women. J. Med. Screen. 2019;27:194–200. doi: 10.1177/0969141319888553. [DOI] [PubMed] [Google Scholar]
- 7.Casebolt M.T. Barriers to reproductive health services for women with disability in low- and middle-income countries: A review of the literature. Sex. Reprod. Healthc. 2020;24:100485. doi: 10.1016/j.srhc.2020.100485. [DOI] [PubMed] [Google Scholar]
- 8.Chan D.N.S., Law B.M.H., Au D.W.H., So W.K.W., Fan N. A systematic review of the barriers and facilitators influencing the cancer screening behaviour among people with intellectual disabilities. Cancer Epidemiol. 2022;76:102084. doi: 10.1016/j.canep.2021.102084. [DOI] [PubMed] [Google Scholar]
- 9.Floud S., Barnes I., Verfürden M., Kuper H., Gathani T., Blanks R.G., Alison R., Patnick J., Beral V., Green J., et al. Disability and participation in breast and bowel cancer screening in England: A large prospective study. Br. J. Cancer. 2017;117:1711–1714. doi: 10.1038/bjc.2017.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin D.W., Lee J.-W., Jung J.H., Han K., Kim S.Y., Choi K.S., Park J.H., Park J.H. Disparities in Cervical Cancer Screening Among Women With Disabilities: A National Database Study in South Korea. J. Clin. Oncol. 2018;36:2778–2786. doi: 10.1200/JCO.2018.77.7912. [DOI] [PubMed] [Google Scholar]
- 11.Kuper H., Heydt P. The Missing Billion Report. 2019. [(accessed on 16 July 2021)]. Available online: https://www.lshtm.ac.uk/media/38726.
- 12.Office for Disability Issues UK Disability Facts and Figures. [(accessed on 16 July 2021)];2021 Available online: https://www.gov.uk/government/statistics/disability-facts-and-figures/disability-facts-and-figures.
- 13.Andresen E.M., Peterson-Besse J.J., Krahn G.L., Walsh E.S., Horner-Johnson W., Iezzoni L.I. Pap, Mammography, and Clinical Breast Examination Screening Among Women with Disabilities: A Systematic Review. Women’s Health Issues. 2013;23:e205–e214. doi: 10.1016/j.whi.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooke B.S., Schwartz T.A., Pawlik T.M. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021;156:787–788. doi: 10.1001/jamasurg.2021.0522. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization . International Classification of Functioning, Disability and Health: ICF. World Health Organization; Geneva, Switzerland: 2001. [(accessed on 26 July 2021)]. Available online: https://apps.who.int/iris/handle/10665/42407. [Google Scholar]
- 17.Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., Currie M., Qureshi R., Mattis P., Lisy K., et al. Checklist for Cohort Studies. [(accessed on 26 July 2021)];Joanna Briggs Inst. Rev. Man. 2017 :1–7. Available online: https://joannabriggs.org/ebp/critical_appraisal_tools. [Google Scholar]
- 18.RStuio Team No Title 2020. [(accessed on 30 July 2021)]. Available online: http://www.rstudio.com/
- 19.Steele C.B., Townsend J.S., Courtney-Long E.A., Young M. Prevalence of Cancer Screening Among Adults With Disabilities, United States, 2013. Prev. Chronic Dis. 2017;14:E09. doi: 10.5888/pcd14.160312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakellariou D., Rotarou E.S. Utilisation of mammography by women with mobility impairment in the UK: Secondary analysis of cross-sectional data. BMJ Open. 2019;9:e024571. doi: 10.1136/bmjopen-2018-024571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fioravante N., Deal J.A., Willink A., Myers C., Assi L. Preventive Care Utilization among Adults with Hearing Loss in the United States. Semin. Hear. 2021;42:37–46. doi: 10.1055/s-0041-1725999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko K.D., Lee K.Y., Cho B., Park M.S., Son K.Y., Ha J.H., Park S.M. Disparities in health-risk behaviors, preventive health care utilizations, and chronic health conditions for people with disabilities: The Korean National Health and Nutrition Examination Survey. Arch. Phys. Med. Rehabil. 2011;92:1230–1237. doi: 10.1016/j.apmr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Assi L., Varadaraj V., Shakarchi A.F., Sheehan O.C., Reed N.S., Ehrlich J.R., Swenor B.K. Association of Vision Impairment With Preventive Care Use Among Older Adults in the United States. JAMA Ophthalmol. 2020;138:1298–1306. doi: 10.1001/jamaophthalmol.2020.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abrams M.T., Myers C.S., Feldman S.M., Boddie-Willis C., Park J., McMahon R.P., Kelly D.L. Cervical cancer screening and acute care visits among Medicaid enrollees with mental and substance use disorders. Psychiatr. Serv. 2012;63:815–822. doi: 10.1176/appi.ps.201100301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osborn D.P.J., Horsfall L., Hassiotis A., Petersen I., Walters K., Nazareth I. Access to Cancer Screening in People with Learning Disabilities in the UK: Cohort Study in the Health Improvement Network, a Primary Care Research Database. PLoS ONE. 2012;7:e43841. doi: 10.1371/journal.pone.0043841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodhead C., Cunningham R., Ashworth M., Barley E., Stewart R.J., Henderson M.J. Cervical and breast cancer screening uptake among women with serious mental illness: A data linkage study. BMC Cancer. 2016;16:819. doi: 10.1186/s12885-016-2842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross E., Maguire A., Mairs A., Hall C., Donnelly M.J.C., O’Reilly D.P.J. Disparities in Breast Cancer Screening Uptake for Women With Mental Illness in the United Kingdom. Am. J. Prev. Med. 2021;60:e123–e130. doi: 10.1016/j.amepre.2020.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Emma R., Aideen M., Michael D., Adrian M., Clare H., Dermot O. Does poor mental health explain socio-demographic gradients in breast cancer screening uptake? A population-based study. Eur. J. Public Health. 2020;30:538–543. doi: 10.1093/eurpub/ckz220. [DOI] [PubMed] [Google Scholar]
- 29.Eriksson E.M., Lau M., Jonsson C., Zhang C., Riso Bergerlind L.-L., Jonasson J.M., Strander B. Participation in a Swedish cervical cancer screening program among women with psychiatric diagnoses: A population-based cohort study. BMC Public Health. 2019;19:313. doi: 10.1186/s12889-019-6626-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown H.K., Plourde N., Ouellette-Kuntz H., Vigod S., Cobigo V. Brief report: Cervical cancer screening in women with intellectual and developmental disabilities who have had a pregnancy. J. Intellect. Disabil. Res. 2016;60:22–27. doi: 10.1111/jir.12225. [DOI] [PubMed] [Google Scholar]
- 31.Weitlauf J.C., Jones S., Xu X., Finney J.W., Moos R.H., Sawaya G.F., Frayne S.M. Receipt of cervical cancer screening in female veterans: Impact of posttraumatic stress disorder and depression. Womens. Health Issues. 2013;23:e153–e159. doi: 10.1016/j.whi.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuesley K.M., Jordan S.J., Siskind D.J., Kendall B.J., Kisely S. Colorectal, cervical and prostate cancer screening in Australians with severe mental illness: Retrospective nation-wide cohort study. Aust. N. Z. J. Psychiatry. 2019;53:550–558. doi: 10.1177/0004867418814945. [DOI] [PubMed] [Google Scholar]
- 33.Jensen L.F., Pedersen A.F., Bech B.H., Andersen B., Vedsted P. Psychiatric morbidity and non-participation in breast cancer screening. Breast. 2016;25:38–44. doi: 10.1016/j.breast.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Murphy K.A., Stone E.M., Presskreischer R., McGinty E.E., Daumit G.L., Pollack C.E. Cancer Screening Among Adults With and Without Serious Mental Illness: A Mixed Methods Study. Med. Care. 2021;59:327–333. doi: 10.1097/MLR.0000000000001499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koroukian S.M., Bakaki P.M., Golchin N., Tyler C., Loue S. Mental illness and use of screening mammography among Medicaid beneficiaries. Am. J. Prev. Med. 2012;42:606–609. doi: 10.1016/j.amepre.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cobigo V., Ouellette-Kuntz H., Balogh R., Leung F., Lin E., Lunsky Y. Are cervical and breast cancer screening programmes equitable? The case of women with intellectual and developmental disabilities. J. Intellect. Disabil. Res. 2013;57:478–488. doi: 10.1111/jir.12035. [DOI] [PubMed] [Google Scholar]
- 37.Horner-Johnson W., Dobbertin K., Iezzoni L.I. Disparities in receipt of breast and cervical cancer screening for rural women age 18 to 64 with disabilities. Womens Health Issues. 2015;25:246–253. doi: 10.1016/j.whi.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Kushalnagar P., Engelman A., Simons A.N. Deaf Women’s Health: Adherence to Breast and Cervical Cancer Screening Recommendations. Am. J. Prev. Med. 2019;57:346–354. doi: 10.1016/j.amepre.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.XinLing X., McDermott S.W., Mann J.R., Hardin J.W., Deroche C.B., Carroll D.D., Courtney-Long E.A. A longitudinal assessment of adherence to breast and cervical cancer screening recommendations among women with and without intellectual disability. Prev. Med. 2017;100:167–172. doi: 10.1016/j.ypmed.2017.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caban M., Kuo Y.F., Raji M., Tan A., Freeman J. Predictors of mammography use in older women with disability: The patients’ perspectives. Med. Oncol. 2011;28((Suppl. S1)):S8–S14. doi: 10.1007/s12032-010-9656-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Courtney-Long E., Armour B., Frammartino B., Miller J. Factors associated with self-reported mammography use for women with and women without a disability. J. Womens Health. 2011;20:1279–1286. doi: 10.1089/jwh.2010.2609. [DOI] [PubMed] [Google Scholar]
- 42.Guilcher S.J.T., Lofters A., Glazier R.H., Jaglal S.B., Voth J., Bayoumi A.M. Level of disability, multi-morbidity and breast cancer screening: Does severity matter? Prev. Med. 2014;67:193–198. doi: 10.1016/j.ypmed.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 43.DongWook S., Jonghan Y., Juhee C., SeKyung L., JinHyung J., Kyungdo H., SoYoung K., Yoo J.E., KyoungEun Y., YeonYong K., et al. Breast cancer screening disparities between women with and without disabilities: A national database study in South Korea. Cancer. 2020;126:1522–1529. doi: 10.1002/cncr.32693. [DOI] [PubMed] [Google Scholar]
- 44.Wu A.M., Morse A.R., Seiple W.H., Talwar N., Hansen S.O., Lee P.P., Stein J.D. Reduced Mammography Screening for Breast Cancer among Women with Visual Impairment. Ophthalmology. 2021;128:317–323. doi: 10.1016/j.ophtha.2020.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.XinLing X., Mann J.R., McDermott S.W., Deroche C.B., Gustafson E., Hardin J.W. Women with visual impairment and insured by Medicaid or Medicare are less likely to receive recommended screening for breast and cervical cancers. Ophthalmic Epidemiol. 2017;24:168–173. doi: 10.1080/09286586.2016.1213302. [DOI] [PubMed] [Google Scholar]
- 46.Ramjan L., Cotton A., Algoso M., Peters K. Barriers to breast and cervical cancer screening for women with physical disability: A review. Women Health. 2016;56:141–156. doi: 10.1080/03630242.2015.1086463. [DOI] [PubMed] [Google Scholar]
- 47.Kilic A., Tastan S., Guvenc G., Akyuz A. Breast and cervical cancer screening for women with physical disabilities: A qualitative study of experiences and barriers...First International Congress of Nursing (Icon-2017), 16–18 March 2017, Grand Park Lara Convention Center, Lara-Antalya, Turkey. J. Adv. Nurs. 2019;75:1976–1986. doi: 10.1111/jan.14048. [DOI] [PubMed] [Google Scholar]
- 48.Merten J.W., Pomeranz J.L., King J.L., Moorhouse M., Wynn R.D. Barriers to cancer screening for people with disabilities: A literature review. Disabil. Health J. 2015;8:9–16. doi: 10.1016/j.dhjo.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Liao C.-M., Huang W.-H., Kung P.-T., Chiu L.-T., Tsai W.-C. Comparison of colorectal cancer screening between people with and without disability: A nationwide matched cohort study. BMC Public Health. 2021;21:1034. doi: 10.1186/s12889-021-11105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin D.W., Chang D., Jung J.H., Han K., Kim S.Y., Choi K.S., Lee W.C., Park J.H., Park J.H. Disparities in the Participation Rate of Colorectal Cancer Screening by Fecal Occult Blood Test among People with Disabilities: A National Database Study in South Korea. Cancer Res. Treat. 2020;52:60–73. doi: 10.4143/crt.2018.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.UN Realization of the sustainable development goals by, for and with persons with disabilities. [(accessed on 10 July 2022)];Dep. Econ. Soc. Aff. 2018 :390. Available online: https://www.un.org/development/desa/disabilities/wp-content/uploads/sites/15/2018/12/UN-Flagship-Report-Disability.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon reasonable request.