Abstract
Dehalogenases are key enzymes in the metabolism of halo-organic compounds. This paper describes a systematic approach to the isolation and molecular analysis of two families of bacterial α-halocarboxylic acid (αHA) dehalogenase genes, called group I and group II deh genes. The two families are evolutionarily unrelated and together represent almost all of the αHA deh genes described to date. We report the design and evaluation of degenerate PCR primer pairs for the separate amplification and isolation of group I and II deh genes. Amino acid sequences derived from 10 of 11 group I deh partial gene products of new and previously reported bacterial isolates showed conservation of five residues previously identified as essential for activity. The exception, DehD from a Rhizobium sp., had only two of these five residues. Group II deh gene sequences were amplified from 54 newly isolated strains, and seven of these sequences were cloned and fully characterized. Group II dehalogenases were stereoselective, dechlorinating l- but not d-2-chloropropionic acid, and derived amino acid sequences for all of the genes except dehII°P11 showed conservation of previously identified essential residues. Molecular analysis of the two deh families highlighted four subdivisions in each, which were supported by high bootstrap values in phylogenetic trees and by enzyme structure-function considerations. Group I deh genes included two putative cryptic or silent genes, dehI°PP3 and dehI°17a, produced by different organisms. Group II deh genes included two cryptic genes and an active gene, dehIIPP3, that can be switched off and on. All αHA-degrading bacteria so far described were Proteobacteria, a result that may be explained by limitations either in the host range for deh genes or in isolation methods.
The biosphere contains a multitude of halogenated organic compounds, more than 2,400 of which have been identified as occurring naturally; however, those constituting the bulk quantities are synthesized industrially (13). Many halo-organic compounds have been categorized as priority pollutants (8), even though a wide range of bacterial species that can degrade such substances and, in many cases, utilize them as sole sources of carbon and energy have been isolated in laboratory culture (11, 21). Notwithstanding the recalcitrance of halo-organic compounds in the biosphere, microbial catabolism is clearly a major latent route by which these compounds may be detoxified and recycled. Therefore, we need to understand much more about the process of microbial adaptation involved in order to harness this potential.
Dehalogenation is a key reaction in such recycling, and a variety of microbial enzymes which catalyze carbon-halogen bond cleavage have been described (11, 21, 56). α-Halocarboxylic acids (αHAs) were originally listed on the United Kingdom Department of the Environment’s “Red List” and have also been identified as intermediates in the biodegradation of halogenated solvents such as 1,2-dichloroethane (20). The pioneering studies of Jensen (23) and Goldman and colleagues (12) resulted in the identification and characterization of hydrolytic dehalogenases associated with the catabolism of αHAs. In the last decade, some 18 dehalogenase (deh) genes have been cloned and sequenced (24, 28, 30, 40, 41, 52, 64). However, despite the availability of molecular data, even recent attempts to classify dehalogenases have tended to focus on arbitrary characteristics such as substrate specificity, especially with optically active substrates such as 2-monochloropropionic acid (2MCPA) (16, 29, 55). Koonin and Tatusov (32) suggested that at least some αHA dehalogenases were evolutionarily related to a group of hydrolases, which they called the HAD superfamily. All of the HAD superfamily dehalogenases were active with the l- but not the d-isomer of 2MCPA, and Kurihara et al. (33) referred to them as the l-Dex family.
The evolution of αHA dehalogenases is of interest in terms of understanding the origin of novel enzyme activities and the adaptation of bacteria to degrade xenobiotic compounds. In this respect, it is important to establish the true evolutionary relationships between dehalogenase genes and to develop methods by which adaptive processes involving deh genes can be studied in the natural environment. This paper describes a genetic approach to investigate the diversity and molecular ecology of deh genes. The aim was to establish a molecular phylogenetic classification that would provide a solid framework for studies of the adaptation of bacteria to degrade halogenated aliphatic compounds. Degenerate oligonucleotide primers for PCR amplification of two distinct families of deh genes were designed and extensively evaluated. The use of these primers for isolation and characterization of active and silent (cryptic) deh genes, and their wider utility, is described.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and culture conditions.
Over 50 bacterial strains were isolated independently by enrichment culture with one of several carbon sources, enrichment source materials, and isolation temperatures. Enrichments were done with either SBS (53) or Brunner’s (6) minimal enrichment medium. Burkholderia (β subclass of Proteobacteria) sp. strains G02, I11, and K13 were isolated from separate enrichment cultures on monochloroacetic acid (MCA) (0.5 g of C liter−1) at 30°C by using three different bulk soil sources (natural woodland, rosebed, and cultivated woodland soils). The following strains were isolated from grass rhizosphere soil: Burkholderia sp. strain P11 (β subclass of Proteobacteria), isolated on 2,3-dichloropropionic acid (23DCPA) (0.5 g of C liter−1) at 30°C, and strain DA1 (β subclass of Proteobacteria) and Bradyrhizobium sp. strains DA2 and DA3 (α subclass of Proteobacteria), isolated on 2,2-dichloropropionic acid (22DCPA) (0.5 g of C liter−1) at 20°C. Pseudomonas sp. strains K55 and 18a (γ subclass of Proteobacteria) were isolated from river epilithon on 14 mM 2MCPA at 15 and 4°C, respectively, and Pseudomonas sp. strain 17a was isolated from river epilithon on MCA, also at 4°C. Once purified, all bacterial isolates were grown aerobically at 20°C and 150 rpm either in minimal medium with the appropriate carbon source (0.5 g of C liter−1) or in LBNS broth (10 g of peptone liter−1 and 5 g of yeast extract liter−1) supplemented with αHA (0.5 g of C liter−1).
Plasmid pYW2 contained a 12-kb HindIII fragment from Pseudomonas putida PP3 genomic DNA ligated into the pBluescript II KS(+/−) vector (Stratagene, Cambridge, United Kingdom). This fragment contains an active dehalogenase gene, dehII; a cryptic dehalogenase gene, dehI0; a putative permease gene, dehP; and a regulatory gene, dehRII (17). Plasmid pYW10 contains the 3.1-kb BamHI restriction fragment of pYW2 carrying dehII ligated into plasmid vector pUC18 (39).
Dehalogenase assays.
αHA dehalogenation in liquid cultures and enzyme assays was estimated by coulometric titration of free halide in samples by using a Sherwood Chloride Analyzer 926, as previously described (54). Growth and harvesting of bacterial cultures and cell breakage for preparation of crude cell extracts were carried out as described by Thomas et al. (59). Dehalogenase assays and zymography by native polyacrylamide gel electrophoresis were carried out as previously described (59).
DNA extraction and manipulation.
Genomic DNA was extracted from overnight cultures by the method of Ausubel et al. (2). Plasmid DNA from pYW2 and pYW10 was isolated by using Qiagen (Crawley, United Kingdom) Qiaquick miniprep kits according to the manufacturer’s instructions. All PCR products were purified by using the Qiagen Qiaquick PCR purification kit and cloned by ligation with plasmid vector pGEM-T Easy (Promega, Southampton, United Kingdom). Blue-white screening of Escherichia coli XL-1 Blue (Stratagene) transformants was done on Luria-Bertani agar (39) containing 50 μg of ampicillin ml−1 and top spread with 40 μl of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (20 mg ml−1) and 40 μl of IPTG (isopropyl-β-d-thiogalactopyranoside) (2%, wt/vol).
PCR primer design and amplification of group I deh genes.
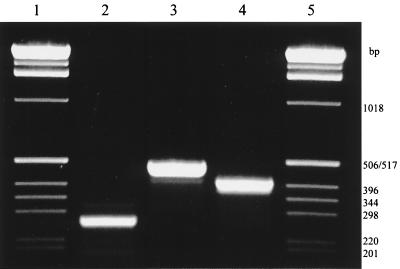
Degenerate group I deh PCR primers (Table 1) were designed (Oligo version 3.4; National Biosciences Inc., Plymouth, Minn.) on the basis of a consensus dehalogenase gene sequence derived from an alignment of the following genes: dehI from P. putida PP3, dhlIV from Alcaligenes xylosoxidans subsp. denitrificans ABIV (4), the dl-DEX gene from Pseudomonas sp. strain YL (42), and hadD from P. putida AJ1 (24). The primer pair dehIfor1 and dehIrev1 amplified a 230-bp product, while dehIfor1 and dehIrev2 amplified a 504-bp product (Fig. 1).
TABLE 1.
Group I deh PCR primer sequences, showing comparisons with binding sites in dehI-type genes from various sources
| dehI-type gene | Host strain | GenBank/EMBL accession no.a | Binding site sequence in comparison with primer sequenceb:
|
||
|---|---|---|---|---|---|
| dehIfor1ACGYTNSGSGTGCCNTGGGT | dehIrev1AWCARRTAYTTYGGATTRCCRTA | dehIrev2SGCMAKSRCNYKGWARTCACT | |||
| dehId | P. putida PP3 | AJ133460 (this study) | ...T.AC.C.....T..... | .A..GA..T..T.....A..A.. | G..C.GCG.TTG.A.A..... |
| dhlIVc | A. xylosoxidans ABIV | X77610 | ...T.AC.C.....T..... | .A..GA..T..T.....A..A.. | G..C.GCG.TTG.A.A..... |
| dl-DEX genec | Pseudomonas sp. strain YL | U97030 | ...C.GC.G.....A..... | .T..AG..C..T.....G..A.. | C..A.GGA.GCG.T.A..... |
| hadDc,d | P. putida AJ1 | M81841 | ...T.CG.C.....C..... | .T..GA..T..C.....G..G.. | C..C.TGG.CTT.T.G..... |
5′→3′. The predicted PCR product size is between 460 and 469 bp. IUPAC ambiguity code used: B = C, G, or T; D = A, G, or T; K = G or T; M = A or C; N = A, C, G, or T; R = A or G; S = C or G; W = A or T; Y = C or T. Nucleotides identical to those of the primer are indicated by dots.
Gene used for design of group I deh primers.
Gene used as template in PCR evaluation of group I deh primers.
FIG. 1.
Agarose gel showing the PCR products obtained with universal group I and group II deh primer pairs. Lane 2, dehIfor1 and dehIrev1; lane 3, dehIfor1 and dehIrev2; lane 4, dehIIfor1 and dehIIrev1; lanes 1 and 5, 1-kb ladder size marker. The source DNA for the PCR was P. putida PP3.
The PCR mixtures (total volume, 25 μl) contained 50 pmol of each primer, 200 μM deoxynucleoside triphosphates, 0.5 U of Taq polymerase (Pharmacia, St. Albans, United Kingdom), 50 mM KCl, 1.5 mM MgCl2, and 10 mM Tris-HCl, pH 9.0. Approximately 200 to 300 ng of DNA from test strain cultures was added to the PCR mixtures. PCR was performed with either an MJ Research PTC-100 or MWG-Biotech (Milton-Keynes, United Kingdom) Primus 96 thermal cycler, which was programmed to perform a touchdown program of 94°C for 2 min; 20 cycles of 92°C for 20 s, 70°C for 30 s (minus 1°C per cycle), and 75°C for 30 s; and then 20 cycles of 92°C for 20 s, 51°C for 30 s, and 75°C for 30 s. PCR products were visualized by agarose gel electrophoresis (50).
PCR primer design and amplification of group II deh genes.
Two degenerate 23-mer PCR primers, designated dehIIfor1 and dehIIrev1, were designed by using Oligo version 3.4 (National Biosciences Inc.) from a consensus sequence alignment of the DehH2 gene, the DehH109 gene, and the deh genes dehCI, dehCII, and dhlB (Table 2). PCR was carried out with a programmable heating block (MJ Research PTC-100) and the same reaction mix as for amplification of group I deh genes. The PCR involved an initial denaturation step at 94°C for 10 min followed by 36 cycles of 94°C for 45 s, 55°C for 2 min, and 75°C for 45 s, with a final elongation step at 75°C for 5 min.
TABLE 2.
Group II deh PCR primer sequences, showing comparisons with binding sites in dehII-type genes from various sources
| dehII-type gene | Host strain | GenBank/EMBL accession no.a | Binding site sequence in comparison with primer sequenceb:
|
|
|---|---|---|---|---|
| dehIIfor1TGGCGVCARMRDCARCTBGARTA | dehIIrev1TCSMADSBRTTBGASGANACRAA | |||
| dehIId | P. putida PP3 | AJ133462 (this study) | .....G..AAAG..A..G..A.. | ..CC.CGCG..T..C..A..G.. |
| hadLd | P. putida AJ1 | M81841 | .....G..AAAG..AT.G..A.. | ..CC.CGCG..T..C..A..G.. |
| DehH109 genec | P. putida 109 | D17523 | .....A..GAAG..G..C..A.. | ..CC.ACTG..G..C..G..G.. |
| l-DEX gene | Pseudomonas sp. strain YL | S74078 | .....G..AAAG..G..C..G.. | ..CC.CGCG..C..C..C..G.. |
| DehH2 genece | Moraxella sp. strain B | D90423 | .....C..AAAG..A..C..A.. | ..CC.TGGA..C..C..T..A.. |
| dhlBc | Xanthobacter autotrophicus GJ10 | M81691 | .....G..GAAG..G..G..A.. | ..GA.GCCG..G..G..C..G.. |
| dehCIc,d | Pseudomonas sp. strain CBS3 | M62908 | .....A..ACGT..G..C..A.. | ..CC.GGCG..T..C..T..A.. |
| dehCIIc,d | Pseudomonas sp. strain CBS3 | M62909 | .....G..GAAA..G..T..G.. | ..CC.TGCA..C..C..A..A.. |
| hdlIVad | B. cepacia strain MBA4 | X66249 | .....T..ACGG..G..C..A.. | ..CC.CGCA..T..C..G..G.. |
References: hadL, 24; DehH109 gene, 28; l-DEX gene, 41; DehH2 gene, 27; dhlB, 64; dehCI and dehCII, 52; hdlIVa, 40.
5′→3′. The predicted PCR product size is 422 bp for all deh genes except dhlB (416 bp). IUPAC ambiguity code used: B = C, G, or T; D = A, G, or T; M = A or C; N = A, C, G, or T; R = A or G; S = C or G; V = A, C, or G. Nucleotides identical to those of the primer are indicated by dots.
Gene used for design of group II deh primers.
Gene used as template in PCR evaluation of group II deh primers.
A plasmid-encoded dehalogenase.
Sequencing of partial deh genes.
Unless otherwise stated, all PCR products obtained with primer pairs dehIfor1-dehIrev2 and dehIIfor1-dehIIrev1 were cloned, and DNA sequences were confirmed by analysis of at least two replicate products from separate PCRs. DNA sequencing was done with either a Prism 377 automated laser fluorescence sequencer (PE Applied Biosystems, Warrington, United Kingdom) or a Licor DNA4000L (MWG-Biotech), using fluorescent primer labelling with near-infrared IRD800 according to the manufacturers’ instructions. DNA for sequencing was prepared from clones by using either Wizard plus SV Minipreps (Promega) or Qiagen Qiaquick miniprep kits. Otherwise, protocols for DNA manipulation were as described by Sambrook et al. (50). Both strands were sequenced to ensure accuracy. Partial deh sequences were confirmed by comparison with complete gene sequences as far as possible.
Dehalogenase sequence and phylogenetic analysis.
Dehalogenase gene sequences were compared with those in the GenBank-EMBL database (release 55) (14) by using FASTA3 (46, 47) at the European Bioinformatics Institute (Cambridge, United Kingdom) (8a) to identify homologues. Dehalogenase sequences were aligned by using the CLUSTAL W program (61). Evolutionary distances were calculated by the method of Jukes and Cantor (25), and phylogenetic trees were constructed by the neighbor-joining method (49) with TREECON for Windows (63). Topologies were also compared between trees constructed by the method of maximum likelihood and maximum parsimony by using PHYLIP version 3.5 (10) and the neighbor-joining method. All reference sequences were obtained from EMBL (release 55). Bootstrap analysis (9) of up to 500 replicates was performed on the phylogeny.
Isolation of bacterial total RNA.
Strains DA1 and DA2 were grown at 20°C and 150 rpm in minimal medium (6) supplemented with 22DCPA at 0.5 g of C liter−1. Strains 17a, 18a, and K55 were grown at 20°C and 150 rpm in LBNS broth supplemented with 2MCPA at 0.5 g of C liter−1, and 5 ml was harvested when approximately 70% of substrate dechlorination was detected. Each culture (5 ml) was centrifuged (5 min at 14,000 × gav and 4°C) and resuspended in 1 ml of Tri-Reagent (Sigma, Poole, United Kingdom). Total RNA was isolated according to the manufacturer’s instructions and treated with DNase I (5 U in 10 mM Tris-HCl [pH 8.3]–1.5 mM MgCl2–25 mM KCl) at 37°C for 1 h, after which the enzyme was inactivated by heating at 75°C for 10 min. RNA was stored at −70°C until required.
RT-PCR of specific group I deh genes.
First-strand cDNA was synthesized by using Moloney murine leukemia virus reverse transcriptase according to the instructions of the manufacturer (Stratagene). The first-strand cDNA was used as the template for all of the PCRs. PCR primer pairs for amplification of specific group I deh sequences were designed to be nested within the DNA fragments amplified by the universal group I deh primers by using selected αHA-utilizing strains, as follows (annealing temperatures are given after the primer sequences; for and rev indicate forward and reverse primers, respectively): dehIDA1-for1, (5′-CGTGGCTGCGTTCGTTG) and dehIDA1-rev1 (5′-GCTTCACGCCGAGATTTG) (50°C), dehIDA2-for1 (5′-TGGGTGGCGTTCGGCATA) and dehIDA2-rev1 (5′-CGCCCCTTGAGCAGTTCC) (53°C), dehI17a-for1 (5′-CGGGGTTATTACGCAGG) and dehI17a-rev1 (5′-GCAGTAGCGAAAATCAGGT) (53°C), and dehI18a-for1 (5′-ATGGGTCGCCTTCGGTTG) and dehI18a-rev1 (5′-CCTCGGTGGATGCCTTGG) (53°C). Primer pair dehI17a-for1-dehI17a-rev1 was used to amplify group I deh gene sequences from strains K55 and PP3, as well as from 17a itself. PCR of cDNAs was carried out as follows: 94°C for 2 min, followed by 30 cycles of 92°C for 20 s, x°C for 30 s (where x is the specific annealing temperature for a given primer pair, as noted above), and 75°C for 30 s. For each reverse transcription-PCR (RT-PCR) the following controls were included: a non-DNase I-treated RNA sample, a DNase I-treated RNA sample, a DNase I-treated RNA sample spiked with genomic DNA from the strain, and a water control.
16S rRNA gene amplification and analysis.
The PCR primers and reaction conditions for amplification of 16S rRNA genes were those published by Marchesi et al. (37) and gave a product of about 1,320 bp. PCR products were purified by using a 100-kDa size exclusion column (Amicon, Watford, United Kingdom), and both strands were sequenced on an automated laser fluorescence sequencer (ABI 377; PE Applied Biosystems) by using primers 63f and 1387r (37), giving double coverage of >1 kbp of 16S rDNA for phylogenetic analysis. The 16S rRNA gene sequences of αHA-degrading bacteria were compared with those in the EMBL database (release 55) (14) by using FASTA3 (46, 47) at the European Bioinformatics Institute (8a) and with those from the Ribosomal Database Project by using SIMILARITY RANK (36) to identify closely related sequences. Identification of strains was based on alignments of their 16S RNA gene sequences to a database of known reference strains (data not shown) by using CLUSTAL W (61) followed by assignments to phylogenetic groups. Evolutionary distances were calculated with the Jukes-Cantor algorithm (25), and phylogenetic trees were determined by the neighbor-joining method (49) with TREECON for Windows (63).
Nucleotide sequence accession numbers.
The nucleotide sequences presented here have been submitted to the EMBL database under the following accession numbers: dehIDA1, AJ133455; dehIDA2, AJ133456; dehI°17a, AJ133457; dehI18a, AJ133458; dehI°PP3, AJ133461; dehIIPP3, AJ133462; dehIIDA3, AJ133463; dehIIG02, AJ133464; dehIII11, AJ133465; dehIIK13, AJ133466; dehIIK55, AJ133467; and dehII°P11, AJ133468.
RESULTS
16S rRNA gene analysis of αHA-degrading bacterial isolates.
Batch enrichment cultures were used to isolate αHA-utilizing bacteria from three soils. Eight different strains were isolated and purified on solid media with one of the following αHAs as the sole source of carbon and energy: MCA, 2MCPA, 22DCPA, and 23DCPA. Identification of the bacterial isolates, based on 16S rRNA gene analysis (43), showed that they were all Proteobacteria. Strains DA2 and DA3 were Bradyrhizobium species (α subclass of Proteobacteria); strains DA1, G02, I11, K13, and P11 were members of the β subclass of Proteobacteria (all assigned to the genus Burkholdaria except strain DA1, which remains unassigned); and strains 17a, 18a, and K55 were Pseudomonas sensu stricto (γ subclass of Proteobacteria).
PCR amplification of group I dehalogenase (deh) genes.
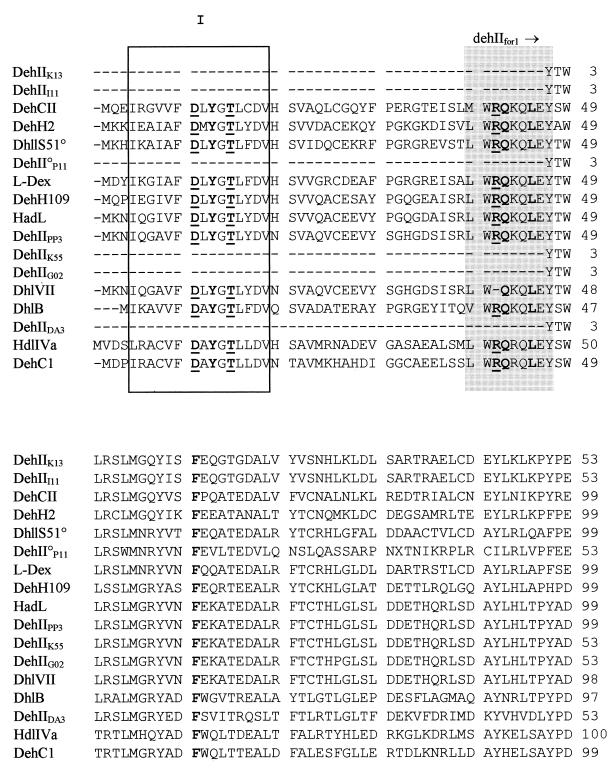
An alignment of four related deh genes (dehI, dhlIV, the dl-DEX gene, and hadD) was used to identify conserved regions as potential annealing sites for PCR primers that would enable amplification of these and other related αHA dehalogenase (αHA deh) genes. Three such regions were identified and used to design two sets of degenerate PCR primers pairs: dehIfor1 with either dehIrev1 or dehIrev2 (Table 1). It should be noted that primers dehIfor1 and dehIrev1 were both 128-fold degenerate, while dehIrev2 was 2,048-fold degenerate. The larger PCR product (504 bp, obtained with dehIfor1 and dehIrev2) was used in this study because of its higher information content (approximately 56% of the dehI gene). Testing of the primers was initially carried out with template DNAs from P. putida AJ1, which contains hadD (24), and P. putida PP3, containing dehI (59). In each case PCR products of the expected sizes (Fig. 1) and sequences (see database entries noted in Materials and Methods) were obtained. It should be noted that minor errors in the published sequence of hadD (3) were identified, and the published derived amino acid sequence had to be corrected to account for these (Fig. 2).
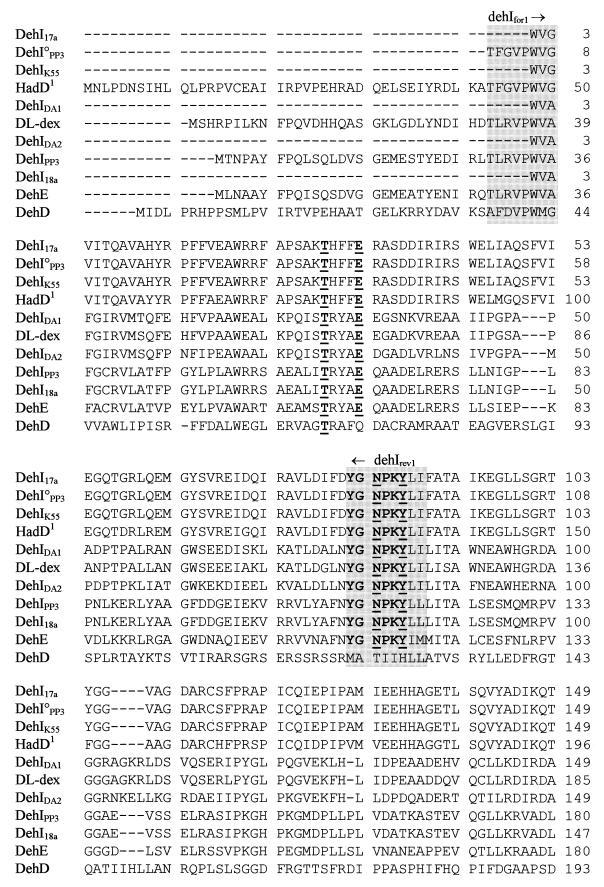
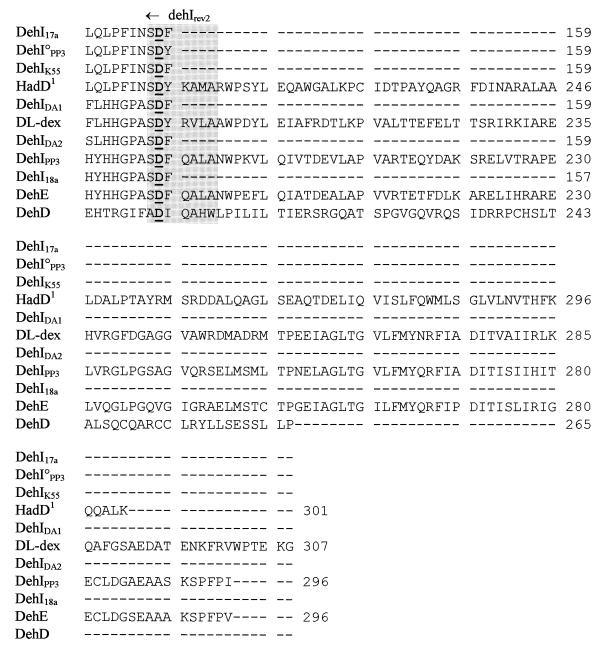
FIG. 2.
Alignment of deduced amino acid sequences of the group I Deh proteins. The conserved residues, described by Nardi-Dei et al. (42), are underlined and in boldface. The conserved amino acid motif, YGNPKY, is shown in boldface. The group I dehalogenase gene PCR priming sites dehIfor1, dehIrev1, and dehIrev2 are shown in the three shaded areas. The deduced amino acid sequence for HadD is based on the resequenced version (see text).
Primers dehIfor1 and dehIrev2 were further tested on a range of bacteria enriched and isolated on αHAs, all of which were shown to produce αHA dehalogenases by native polyacrylamide gel electrophoresis (gel zymography). All PCR products were cloned and sequenced, and a positive result was recorded only when products from at least two replicate PCRs were shown to be identical and homologous to deh genes in this group. A small number of PCR products of the expected size were found after sequencing to be obviously unrelated to dehI, even though they were amplified consistently from some bacterial isolates. These were excluded from further consideration in the present study. Five of 20 new bacterial isolates tested positive with the dehI PCR primers: strains 17a, K55, 18a, DA1, and DA2. Analysis of the dehalogenase genes thus obtained indicated that they were evolutionarily related in terms of both nucleotide and derived amino acid sequence alignments. Thus, the dehI PCR primers were successful in amplifying a family of related deh genes, designated group I deh genes, from a variety of bacteria.
Unexpectedly, two different group I deh genes were amplified from P. putida PP3. One was expected: the dehI that resides on a transposable element previously described by Thomas et al. (59) and Topping et al. (62). The other, designated dehI°PP3, was located immediately upstream of dehIIPP3, a group II deh, and was cloned separately from dehIPP3 on a 12-kbp HindIII restriction fragment of P. putida PP3 genomic DNA (see below).
Phylogenetic analysis of group I deh genes.
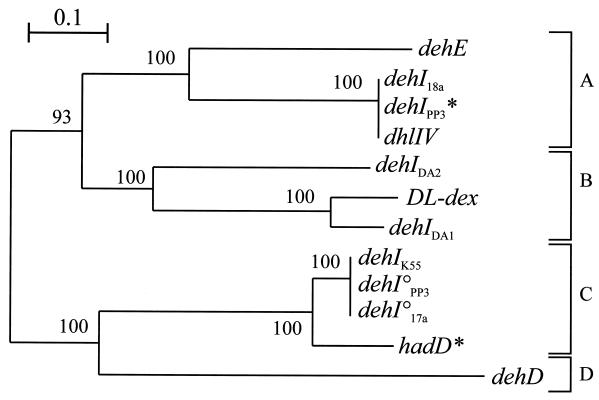
Figure 3 shows a phylogenetic tree illustrating the relationships between all known group I deh genes, based on a CLUSTAL W (61) nucleotide alignment over the whole region amplified with primers dehIfor1 and dehIrev2 (504 bp). Almost identical tree topologies were obtained whether they were constructed from alignments done by using maximum-parsimony, maximum-likelihood, or Jukes-Cantor algorithms (25). The four subdivisions identified from this tree were supported by high bootstrap values (Fig. 3), as well as by analysis of alignments with the deduced amino acid sequences for the dehalogenase gene products (data not shown). The gene products of the dl-DEX gene (subdivision B) and dhlIV (subdivision A) have basic structural similarities in that they are both homodimers made up of polypeptides of between 32 and 35 kDa, whereas HadD (subdivision C) is a tetrameric holoenzyme (57).
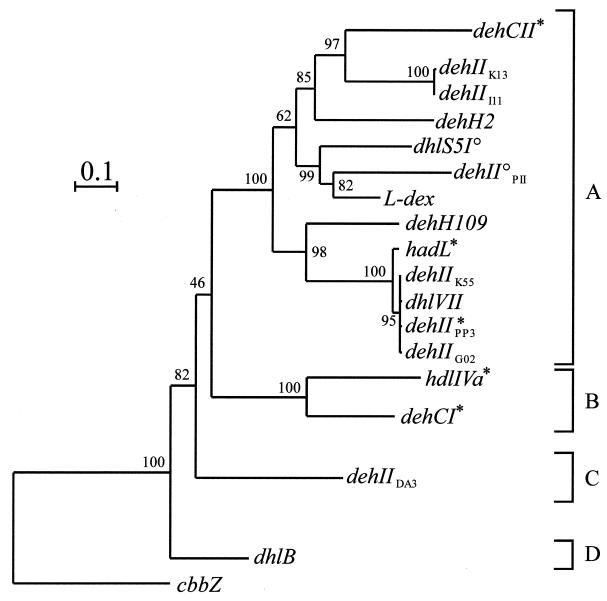
FIG. 3.
Dendrogram illustrating the relationships between the group I αHA dehalogenase genes (dehI type) from a variety of bacteria, including those isolated in this study (designated dehIX, where X is the bacterial strain containing the dehalogenase gene). DNA sequences (466 bp) were aligned by using CLUSTAL W, and the tree was constructed by the neighbor-joining program from a similarity matrix of pairwise comparisons made by using the Jukes-Cantor algorithm (25). Bootstrap values from 100 replicate trees are shown at the dendrogram nodes. °, cryptic deh gene. The scale bar represents sequence divergence (0.1 = 10%). The sequences of dehD (accession no. X93597) and dehE (accession no. Y15517) were reported by Cairns et al. (7) and Stringfellow et al. (58). Other reference sequences are listed in Table 1. ∗, dehalogenase genes from reference strains for which PCR products were cloned and sequenced in this study. A, B, C, and D are subdivisions based on >55% nucleotide sequence identity and supported by high bootstrap values as shown.
Nardi-Dei et al. (42) identified five amino acids in dl-DEX (T65, E69, N117, Y120, and D194) encoded within the region amplified by dehIfor1 and dehIrev2, all of which were essential for dehalogenase activity. Derived amino acid sequence alignments for all of the group I Deh proteins described in this study showed conservation of all of these residues, with the exception of DehD from a Rhizobium sp., which shared only T65. In addition, the deduced amino acid motif YGNPKY, positioned around the middle of the protein (including N117 and Y120 of dl-DEX) was a conserved feature of the group, again excepting DehD.
Design and evaluation of group II deh PCR amplification primers.
Alignments of five known deh gene sequences, related to each other but showing no obvious homology to the group I deh family (Table 2), were used to identify four conserved regions that were analyzed as potential binding sites for universal PCR primers for this group of genes. Primers dehIIfor1 and dehIIrev1 were designed to match the two most highly conserved potential primer-binding sites and were chosen for further evaluation. However, a potential concern was the degeneracy of these primers, since it was even greater than that of the group I deh primer pair and allowed 864 and 6,912 possible target sequence permutations with dehIIfor1 and dehIIrev1, respectively. In Table 2 these primers’ sequences are compared with corresponding target sites on nine deh genes. The size of the PCR products predicted from all of these templates except dhlB (416 bp [54.8% of the gene]) was 422 bp, representing 60.1 to 63.4% of the gene. Initial testing of PCR primer pair dehIIfor1-dehIIrev1 was carried out with genomic DNA templates from four bacteria, representing five deh genes: P. putida PP3 (dehII), P. putida AJ1 (hadL), Pseudomonas sp. strain CBS3 (dehCI and dehCII), and Burkholderia cepacia MBA4 (hdlIVa). In each case, products of the expected size were obtained (see, e.g., Fig. 1), and following their cloning and sequencing from replicate PCRs, they were confirmed as originating from the corresponding dehII-type templates. For example, two products of identical size, corresponding exactly to the DNA regions between the dehIIfor1 and dehIIrev1 binding sites shown in Table 2 for dehCI and dehCII, were amplified and cloned from Pseudomonas sp. strain CBS3 template DNA. It should be noted that single-base mismatches between the group II deh primers and target sites on three of the test dehII-type genes (dehII, hadL, and hdlIVa) did not prevent the amplification of the expected DNA fragments. The complete sequence of dehII from P. putida PP3 has not been reported before, and this gene was clearly shown to be a member of this group, as was the unpublished dhlVII (19), with which it had 98.8% nucleotide sequence identity. The dehIIPP3 gene was separately isolated on a 12-kbp HindIII restriction fragment, cloned to produce plasmid pYW2, and subsequently subcloned on a 3.0-kbp BamHI fragment to produce pYW10.
As might be expected from the use of such highly degenerate primers, some PCR artifacts were observed, usually as amplified DNA fragments outside the expected size range for group II deh genes. However, even when testing was extended to newly isolated αHA-degrading bacteria and mixed enrichment cultures (see below), such artifacts occurred only infrequently, were easily identified (confirmed by sequencing), and were eliminated from this study. Further comparisons between the predicted primer-binding sites in the five control dehII-type genes (Table 2) and the observed sequences of PCR product primer ends, obtained from replicate reactions, showed an unexpectedly high number of mismatches, i.e., up to 5 of 23 bp and 9 of 23 bp for dehIIfor1 and dehIIrev1, respectively. These data suggested that optimal primers from the degenerate mixture (7,776 different oligonucleotides, providing approximately 6 million possible PCR primer pair combinations) were not necessarily being selected in the annealing step of PCR. A likely explanation would be that optimal primers were used up in the early cycles of PCR, but as they were depleted, oligonucleotides with suboptimal matching to the primer-binding sites had sufficient homology to compete and prime elongation in the final cycles of the reaction. It should be noted that unusually high concentrations (2 μM) of the group II deh primers were used in PCRs so as to reduce this problem.
Identification of group II deh genes in newly isolated αHA-degrading bacteria.
Overall, 54 αHA-degrading bacteria were isolated from independent batch culture enrichments with combinations of eight soil or sediment samples and three different αHA substrates. PCR with the primer pair dehIIfor1-dehIIrev1 resulted in 43 isolates (i.e., 80%) testing positive for group II deh. Eight of these, including strains 17a, 18a, DA2, and K55 (Fig. 3), also gave a positive result with group I deh primers. Only two isolates, strains J14 and DA5, did not give rise to a PCR product with either group I or group II deh primers.
Group II deh PCR products from seven independently isolated αHA-utilizing bacteria were cloned and fully sequenced. Figure 4 shows a phylogenetic tree illustrating the relationships between the deh gene sequences isolated from these bacteria and the other group II deh genes previously reported in the literature (Table 2). The phylogenetic trees constructed by maximum parsimony, maximum likelihood, and neighbor joining all showed similar topologies. The range of nucleotide sequence similarities in the group II deh genes was 43.2 to 99.8%, and four subdivisions or clusters, supported by high bootstrap values, that grouped together genes showing >55% nucleotide sequence identity are shown in Fig. 4. Extensive sequence analysis, involving comparisons between the group II deh genes and representatives of HAD superfamily genes (32) suggested that the former constituted a monophyletic group of the latter. Hence, the tree was rooted with cbbZp, a gene from Alcaligenes eutrophus encoding 2-phosphoglycolate phosphatase, which was found to be most closely related to the group II deh genes.
FIG. 4.
Dendrogram illustrating the relationships between the group II αHA dehalogenases (dehII type) from a variety of bacteria, including those isolated in this study (designated dehIIX, where X is the bacterial strain containing the dehalogenase gene). Sequences (403 bp) were aligned by using CLUSTAL W, and the tree was constructed by the neighbor-joining program from a similarity matrix of pairwise comparisons made by using the Jukes-Cantor algorithm (25). Bootstrap values from 100 replicate trees are shown at the dendrogram nodes. °, cryptic deh gene. The scale bar represents sequence divergence (0.1 = 10%). Accession numbers and/or references for published αHA genes not already cited in Table 2: dhlS5I0, reference 30 (not deposited in EMBL); dhlVII, X94147 (19); cbbZp (2-phosphoglycolate phosphatase), M68905 (51). ∗, dehalogenase genes from reference strains for which PCR products were cloned and sequenced in this study. A, B, C, and D are subdivisions based on >55% nucleotide sequence identity and supported by high bootstrap values as shown.
Conservation of structure and function in group II dehalogenases.
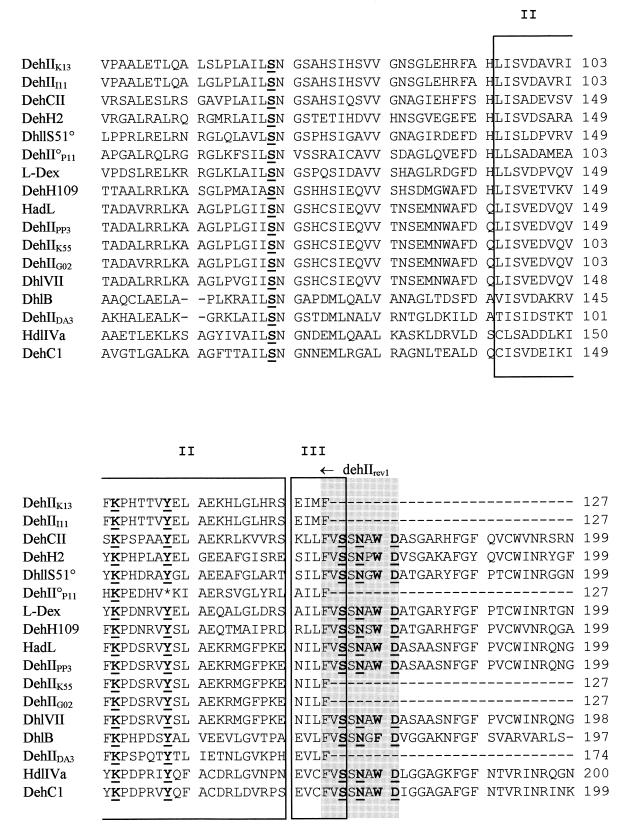
Figure 5 shows an alignment of amino acid sequences derived from all of the group II deh genes. The alignment shows the three conserved secondary-structure motifs defining the HAD superfamily (32). The group II deh PCR primers were designed to amplify a region of the deh gene containing only motif II and part of motif III; however, these are clearly conserved in all but one case, that of DhlVII. The crystal structures of l-DEX and DhlB have both been solved, and active-site amino acids have been identified (18, 33, 48). Figure 5 shows that all of these residues are conserved, except in the cases of DhlVII, which lacks R41 (cf. l-DEX [18]), and DehIIP11, which has a stop codon instead of Y157 (cf. l-DEX). It should be noted that Burkholdaria sp. strain P11 was isolated on 23DCPA, and no αHA dehalogenase activity was detected in this strain (Fig. 6, lane 9), suggesting that its group II deh gene may be cryptic. F60, which was suggested by Li et al. (35) to be associated with the substrate-stabilizing hydrophobic pocket of l-DEX, is also conserved in all of the group II deh-derived sequences.
FIG. 5.
Alignment of derived amino acid sequences of bacterial group II deh genes from the GenBank-EMBL database or from a sequenced cloned gene (dehIIPP3) or partial dehalogenase sequences derived from PCR products (dehIIK13, dehIII11, dehII°P11, dehIIK55, dehIIG02, and dehIIDA3) by using the CLUSTAL W program (where the X in DehIIX indicates the strain from which the sequence is derived). The regions corresponding to the group II dehalogenase gene PCR priming sites dehIIfor1 and dehIIrev1 are shown as shaded areas, and the three conserved secondary structure motifs I, II, and III (32) of the HAD superfamily are boxed. Nine residues essential for catalysis for the group II dehalogenases are shown underlined in boldface, based on l-DEX, as follows (18, 33): D10, T14, R41, S118, K151, Y157, S175, N177, and D180. Conserved amino acids forming the hydrophobic pocket (35) are shown in boldface: Y12, Q42, L45, F60, K151, N177, and W179.
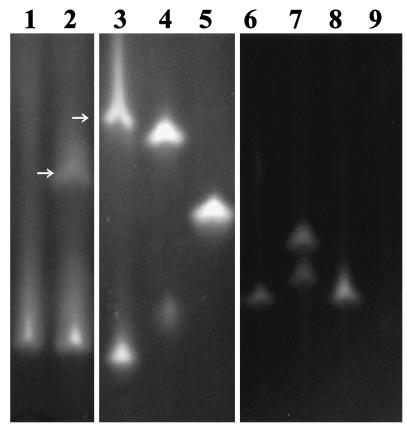
FIG. 6.
Native polyacrylamide gel electrophoresis gel showing dehalogenases in crude cell extracts from the following αHA-utilizing bacteria: lane 1, E. coli(pYW10); lane 2, P. putida PP3; lane 3, P. putida AJ1; lane 4, Pseudomonas sp. strain CBS3; lane 5, B. cepacia MBA4; lane 6, Burkholderia sp. strain K13; lane 7, Burkholderia sp. strain G02; lane 8, Burkholderia sp. strain I11; lane 9, Burkholderia sp. strain P11 (no αHA dehalogenase activity detected). Enzymes were visualized by activity straining with dichloroacetic acid (lanes 1, 2, and 6 to 9) or MCA (lanes 3 to 5) according to the method of Thomas et al. (59). Unless otherwise indicated, staining bands represent group II dehalogenases identified in separate zymography experiments by their activity with l-2MCPA but not d-2MCPA. Pseudomonas sp. strain CBS3 and Burkholderia sp. strain G02 each produced two separate group II dehalogenases. P. putida PP3 and AJ1 produced both group I (arrows) and group II dehalogenases.
Dehalogenases from the newly isolated αHA-degrading bacteria were characterized by assays and gel zymography. Figure 6 shows, not unexpectedly, that some of the strains produced more than one dehalogenase, but group II Deh proteins could be distinguished from group I Deh proteins by their stereoselective dechlorination of l-2MCPA but not d-2MCPA. Burkholdaria sp. strain G02 apparently produced two l-2MCPA-specific dehalogenases, but only one group II deh gene was cloned from this organism. The stereospecificity of DehIIPP3 for l-2MCPA observed in this study contradicts the results of Weightman et al. (66), who suggested that the enzyme dechlorinated both isomers of 2MCPA. The results from the earlier study are under further investigation.
Detection of group I deh gene expression in selected bacterial isolates.
Cryptic or silent deh genes have been reported in the literature (30, 55, 59), and two, dehII°P11 and dhlS51°, were assigned to the group II deh family (see above). Slater et al. (54) showed that another group II gene, dehIIPP3, could be silenced and subsequently switched on by use of appropriate selection media. Therefore, it was of interest to determine whether group I deh genes were cryptic or active. Initially, expression of dehalogenase genes in αHA-degrading bacteria was investigated by gel zymography, using the approach described above and in the legend to Fig. 6 to distinguish between group I and group II dehalogenases.
As indicated in Table 3, all except one of the bacteria containing group I deh genes produced at least one dehalogenase with activity against d-2MCPA. The exception, strain 17a, thus contained a dehI-type gene, assigned to subdivision C, that may be cryptic, although it should be noted that enzyme instability may have accounted for the absence of a group I dehalogenase with activity against d-2MCPA. Two other genes, dehI°PP3 and dehIK55, almost identical to dehI°17a (>99% nucleotide sequence identity) but from different bacterial isolates, clustered with hadD in subdivision C (Fig. 3). Whereas HadD, produced by Pseudomonas sp. strain AJ1 (Fig. 6, lane 3), has activity with d- but not l-2MCPA (3, 57), the only dehalogenase produced by Pseudomonas sp. strain K55 showing activity with d-2MCPA also had activity with l-2MCPA. The organism from which dehI°PP3 was isolated, P. putida PP3, has been studied extensively and found to produce only two dehalogenases, DehIPP3 and DehIIPP3 (54, 66), as indicated in Fig. 6 (lane 2). The dehI°PP3 gene was localized in a cluster alongside dehIIPP3 (17), and following cloning of the two genes together in E. coli DH5α(pYW2), the recombinant strain produced only one dehalogenase, corresponding to DehIIPP3 (Fig. 6, lane 1). Therefore, we propose that dehI°PP3 is a cryptic gene that does not produce an active dehalogenase.
TABLE 3.
Comparison of group I deh genes and their expression in selected αHA-utilizing bacteria
| Organism (dehalogenase gene) | Group I deh PCR product (bp)a | RT-PCR product (bp)b | Presence of dehalogenase showing activity with d-2MCPAc |
|---|---|---|---|
| P. putida AJ1 (hadD) | 462 | NDd | + |
| Pseudomonas sp. strain 17a (dehI°17a) | 466 | 270 | − |
| Pseudomonas sp. strain 18a (dehI18a) | 460 | 400 | + |
| Pseudomonas sp. strain K55 (dehIK55) | 466 | 270 | + |
| Bradyrhizobium sp. strain DA2 (dehIDA2) | 466 | 330 | + |
| Strain DA1, β subclass of Proteobacteria (dehIDA1) | 466 | 400 | + |
| P. putida PP3 (dehIPP3) | 460 | ND | + |
| E. coli(pYW10) (dehI°PP3) | 469 | 290 | − |
Size determined by sequencing but not including the primer sequences.
RT-PCR was performed with specific primers as described in Materials and Methods.
As determined by gel zymography.
ND, not determined.
RT-PCR experiments using oligonucleotide primers specific for each of the group I deh genes in subdivision C (see Materials and Methods) showed that they were all transcribed (Table 3). RT-PCR was also carried out with strains DA1, DA2, and 18a, each of which produced a single dehalogenase showing activity against d- and l-2MCPA (38), confirming that transcription of group I deh genes could be detected (Table 3).
DISCUSSION
In describing dehCI and dehCII, Schneider et al. (52) were first to report the full sequence of an αHA deh gene. Since then, the sequences of at least 18 bacterial αHA deh genes have been reported, and the structures of two of the proteins, l-DEX and DhlB (18, 48), have been solved. The possibility of using molecular systematic methods to classify αHA dehalogenases has been considered by several groups (18, 34, 48), but until now has been applied to only a limited extent. The problem of classification of isofunctional dehalogenases is apparent in recent reviews (29, 56), where these enzymes have been grouped arbitrarily on the basis of characters such as electrophoretic mobility and substrate specificity. The work described in this paper provides a comprehensive molecular systematic classification of almost all of the αHA dehalogenases described to date and identifies two distinct evolutionary families: group I and group II deh genes. The results provide a rational framework for studying dehalogenase diversity and evolution and a basis for understanding the adaptation of bacteria to degrade xenobiotic haloaliphatic compounds.
Despite the fact that they are highly degenerate, the deh PCR primers described in this paper are useful molecular tools, facilitating rapid and efficient isolation and identification of deh genes in bacteria isolated from the natural environment. We have used the group I and group II deh primers to amplify deh genes directly from soil and enrichment culture samples (38), thus identifying dehalogenase genes in environmental samples without the need for bacterial cultivation. To maximize their specificity, the group II deh PCR primers were designed on the basis of conserved regions that did not overlap extensively with regions corresponding to the HAD superfamily motifs (32), thus limiting the number of potential universal primer-binding sites. Indeed, on the basis of theoretical considerations alone, these highly degenerate primers (Table 2) might easily have been discounted. However, the utility of the group II deh primers for isolation of a range of dehII-type genes is indicated by the observation from our wider screening program that 42 of 50 newly isolated αHA-utilizing bacteria tested positive. This result also suggested that group II deh genes may predominate over other αHA deh genes in the natural environment.
Group I now comprises 11 deh genes and is split into four subdivisions. Prior to this study, all of the deh genes described in the literature that were assigned to group I were produced by Pseudomonas species. Although subdivision C group I deh genes are also contained within host species from the genus Pseudomonas (sensu stricto), subdivisions A and B contain genes from various species of the α, β, and γ subclasses of Proteobacteria. Group I deh genes do not share any obvious feature with group II deh genes in terms of DNA or deduced amino acid sequences, suggesting that they are not evolutionarily related. They also seem to be functionally distinct in that all of the noncryptic group I deh genes tested encoded dehalogenases that dechlorinated d-2MCPA, whereas all group II dehalogenases tested lacked this activity. However, consideration of substrate specificities in isolation can be misleading in terms of investigating the evolutionary relationships between the dehalogenases. For example, of all the group I deh genes, hadD is most distantly related to dehD, even though these two genes uniquely encode dehalogenases that can dechlorinate d- but not l-2MCPA (3, 7). Also, dehL appears to be similar to the group II deh genes, in that it encodes a dehalogenase with activity against l- but not d-2MCPA; however, it seems to be phylogenetically unrelated to any other deh gene.
The group II deh family seems to constitute a branch of the HAD superfamily (32), which was recently proposed to be linked with the evolution of P-type ATPases (1), and contains at least four major subdivisions defining closer deh gene relationships (Fig. 4). The conservation of amino acid motifs and active-site residues derived from PCR-amplified group II deh gene sequences (Fig. 5) supports the results of previous studies and helped to validate the approach used here.
All but one of the αHA-utilizing bacteria isolated in this study were shown to produce αHA dehalogenases. The exception, Burkholdaria sp. strain P11, utilized only 23DCPA (an αβHA), and no αHA dehalogenase activity was detected in this strain, suggesting that dehII°P11 is a cryptic or silent gene. This would be consistent with the amino acid sequence derived from dehII°P11, which gave a stop codon in the place corresponding to the conserved Y157, which was identified as essential for the activity of l-DEX by Soda and colleagues (18, 33). Ridder et al. (48) reported that the same conserved residue (Y153) bordered the active site of DhlB. Bacterial degradation of βHAs has been reported to involve a pathway quite different from that for the αHAs (31, 65). Therefore, the cryptic group II deh gene in strain P11 may not be directly associated with the organism’s ability to utilize 23DCPA.
The approach described here allowed us to identify cryptic or silent, as well as active, deh genes. The association between cryptic genes and adaptive evolution has been widely discussed (15, 44, 45, 60), and various mechanisms have been proposed to explain their cryptic state and potential for being activated. Dehalogenase genes seem to show some of this variety. The cryptic group I gene dehI°PP3 was found to be transcribed (Table 3) but apparently not translated into an active dehalogenases. This gene was cloned and was localized upstream of dehIIPP3 and downstream of dehP, encoding a putative permease, and dehRII, encoding a putative regulator (17). The cloned dehI°PP3 was not expressed, even under conditions where the downstream gene, dehIIPP3, gave high levels of dehalogenases activity (Fig. 6). However, analysis of the complete open reading frame for this gene did not provide any obvious explanation as to why dehI°PP3 was cryptic. Interestingly, our analysis of unpublished sequence data from Honnens et al. (19) showed a partial gene sequence with high homology (95% identity over 474 nucleotides) to group I deh subdivision C, upstream from dhlVII (a group II deh) in Pseudomonas fluorescens ABVII. Furthermore, the remarkable similarity between the regions flanking the dehII gene in P. putida PP3 and the sequence reported previously (19) upstream and downstream of dhlVII (98% identity over 1,663 nucleotides, including dehIIPP3 and dehI°PP3 homologues) suggested that adaptation of the host organisms, one isolated in the United Kingdom and the other isolated in Germany, to utilize αHAs may have involved horizontal transfer of linked group I and group II deh genes.
Group II was found to contain two cryptic genes, dhlS51° and dehII°P11, and an active gene that can be switched off, dehIIPP3. The first was recently reported by Köhler et al. (30), but this gene’s cryptic state seemed to be associated with the lack of an active promoter rather than with a nonfunctional gene product. As noted above, the open reading frame of the partial sequence of dehII°P11 showed a missing essential amino acid and at least one stop codon. The dehIIPP3 gene can be switched on and off by appropriate environmental selection, and although the mechanism for this has not been elucidated, it is likely that it involves interaction with a transposable element, DEH, containing dehIPP3, a group I deh gene (59, 60). Clearly, further investigation is needed to uncover how genes that are silenced in these various ways contribute to the potential of bacteria to degrade xenobiotic compounds.
There was no apparent correlation between the assignments of deh genes to subdivisions of group I and group II families and the 16S rRNA based phylogenetic assignments of their bacterial hosts. In itself this was not surprising, since horizontal transfer of deh genes carried on plasmids has previously been reported (27, 28). Also, Brokamp et al. (5) have described five plasmid-encoded dehalogenases, and at least three other dehalogenase genes, dehIPP3 (59), the DehH2 gene (29), and dhlIV (4), are known to be carried on transposon-like mobile genetic elements. As far as we know, no dehalogenase-producing organism outside the phylum Proteobacteria has so far been reported, and this may reflect a limited phylogenetic range of organisms producing αHA dehalogenases. However, given their potential for horizontal transfer and the catabolic versatility of phyla such as the low- and the high-G+C gram-positive bacteria and the Cytophagales, each of which contains many xenobiotic-degrading species, confinement of αHA deh genes within Proteobacteria appears to be anomalous. Haloalkane dehalogenases are produced by species outside the Proteobacteria, so that the restricted range of species containing group I and group II deh genes may simply reflect limitations in currently used enrichment and isolation procedures related to the growth requirements of other bacterial groups.
Almost all of the αHA dehalogenases isolated in this laboratory and described in the literature are encoded by genes now assigned to either the group I or group II deh genes. Only two exceptions were identified: the DehH1 gene from a Moraxella sp. (27) and dehL from a Rhizobium sp. (7). On the basis of derived amino acid sequence alignments, Janssen et al. (22) proposed that the DehH1 gene is related to the haloalkane dehalogenase genes, dhlA and dhaA, and the α-hexachlorocyclohexadiene dehalogenase gene, linB. This indicates the existence of a third family of αHA deh genes encoding dehalogenases with broader substrate specificities, including activities against haloalkanes. It should be noted that DehHI is different from all of the group I and II dehalogenases in that it is a defluorinating enzyme, showing high activity with fluoroacetic acid but much lower activity with chloro- and bromo- analogues and virtually no activity with higher chemical homologues (26).
The dehL gene from a Rhizobium sp. (7) shows the same stereospecificity as group II deh genes (i.e., dechlorination of l- but not d-2MCPA) but is not a member of this family. No significant match in either nucleotide or derived amino acid alignments between dehL and group II deh genes was found, and the derived DehL amino acid sequence lacks almost all of the conserved residues of the group II dehalogenases. Since this gene shows no significant match with any other sequence currently deposited in the GenBank and EMBL databases (on the basis of FASTA searches), it might tentatively be suggested that dehL uniquely represents a fourth deh family.
ACKNOWLEDGMENTS
We gratefully acknowledge funding for the research described in this paper from the European Commission (project no. ENV4-CT95-0086; support for K.E.H.) and the Wellcome Trust (research fellowship to J.R.M.).
Many thanks are due to Lee Parry for providing expert technical assistance and important preliminary data. Thanks also go to Andrew Gane and Gareth Lewis for sequencing.
The first two authors contributed equally to this paper.
REFERENCES
- 1.Aravind L, Galperin M Y, Koonin E V. The catalytic domain of the P-type ATPase has the haloacid dehalogenase fold. Trends Biochem Sci. 1998;23:127–129. doi: 10.1016/s0968-0004(98)01189-x. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1989. [Google Scholar]
- 3.Barth P T, Bolton L, Thomson J C. Cloning and partial sequencing of an operon encoding two Pseudomonas putida haloalkanoate dehalogenases of opposite sterospecificity. J Bacteriol. 1992;174:2612–2619. doi: 10.1128/jb.174.8.2612-2619.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brokamp A, Happe B, Schmidt F R J. Cloning and nucleotide sequence of a d,l-haloalkanoic acid dehalogenase encoding gene from Alcaligenes xylosoxidans ssp. denitrificans ABIV. Biodegradation. 1997;7:383–396. doi: 10.1007/BF00056422. [DOI] [PubMed] [Google Scholar]
- 5.Brokamp A, Schwarze R, Schmidt F R J. Homologous plasmids from soil bacteria encoding d,l-halidohydrolases. Curr Microbiol. 1997;34:97–102. doi: 10.1007/s002849900151. [DOI] [PubMed] [Google Scholar]
- 6.Brunner W, Staub D, Leisinger T. Bacterial degradation of dichloromethane. Appl Environ Microbiol. 1980;40:950–958. doi: 10.1128/aem.40.5.950-958.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cairns S S, Cornish A, Cooper R A. Cloning, sequencing and expression in Escherichia coli of two Rhizobium sp. genes encoding haloalkanoate dehalogenases of opposite stereospecificity. Eur J Biochem. 1996;235:744–749. doi: 10.1111/j.1432-1033.1996.t01-1-00744.x. [DOI] [PubMed] [Google Scholar]
- 8.Environment Protection Act. c.43. London, United Kingdom: Her Majesty’s Stationery Office; 1990. [Google Scholar]
- 8a.European Bioinformatics Institute. 25 July 1997, revision date. [Online.] http://www.ebi.ac.uk. [8 March 1999, last date accessed.]
- 9.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 10.Felsenstein J. PHYLIP (Phylogeny Inference Package), version 3.5c. Distributed by the author. Seattle: Department of Genetics, University of Washington; 1993. [Google Scholar]
- 11.Fetzner S, Lingens F. Bacterial dehalogenases: biochemistry, genetics, and biotechnological applications. Microbiol Rev. 1994;58:641–685. doi: 10.1128/mr.58.4.641-685.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldman P. The enzymatic cleavage of the C-F bond in fluoroacetate. J Biol Chem. 1965;240:3434–3438. [PubMed] [Google Scholar]
- 13.Gribble G W. The diversity of natural organochlorines in living organisms. Pure Appl Chem. 1996;68:1699–1712. [Google Scholar]
- 14.Guenter S, Moseley M A, Sleep J, McGowran M, Garcia-Pastor M, Sterk P. The EMBL nucleotide sequence database. Nucleic Acids Res. 1998;26:8–15. doi: 10.1093/nar/26.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall B G. Activation of the bgl operon by adaptive mutation. Mol Biol Evol. 1998;15:1–5. doi: 10.1093/oxfordjournals.molbev.a025842. [DOI] [PubMed] [Google Scholar]
- 16.Hardman D J. Biotransformation of halogenated compounds. Crit Rev Biotechnol. 1991;11:1–40. doi: 10.3109/07388559109069182. [DOI] [PubMed] [Google Scholar]
- 17.Hill, K. E., L. O’Sullivan, J. R. Marchesi, and A. J. Weightman. Unpublished data.
- 18.Hisano T, Hata Y, Fujii T, Liu J-Q, Kurihara T, Esaki N, Soda K. Crystal structure of l-2-haloacid dehalogenase from Pseudomonas sp. YL J Biol Chem. 1996;271:20322–20330. doi: 10.1074/jbc.271.34.20322. [DOI] [PubMed] [Google Scholar]
- 19.Honnens, E., R. Reiting, A. Brokamp, and F. J. R. Schmidt. GenBank accession no. X94147, unpublished data.
- 20.Janssen D B, Pries F, van der Ploeg J R, Kazemier B, Terpstra P, Witholt B. Degradation of halogenated aliphatic compounds by Xanthobacter autotrophicus GJ10. Appl Environ Microbiol. 1985;49:673–677. doi: 10.1128/aem.49.3.673-677.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssen D B, Pries F, van der Ploeg J R. Genetics and biochemistry of dehalogenating enzymes. Annu Rev Microbiol. 1994;48:163–191. doi: 10.1146/annurev.mi.48.100194.001115. [DOI] [PubMed] [Google Scholar]
- 22.Janssen D B, Bosma T, Poelarends G J. Diversity and mechanisms of bacterial dehalogenation reactions. In: Janssen D B, Soda K, Wever R, editors. Proceedings of Colloquium on Mechanisms of Biodehalogenation and Dehalogenation, National Academy of Sciences, Amsterdam. Royal Netherlands Academy of Sciences, Amsterdam. Royal Netherlands Academy of Arts and Sciences, Amsterdam, The Netherlands. 1997. pp. 119–129. [Google Scholar]
- 23.Jensen H L. Decomposition of chloroacetates and chloropropionates by bacteria. Acta Agric Scand. 1960;10:83–103. [Google Scholar]
- 24.Jones D H, Barth P T, Byrom D, Thomas C M. Nucleotide sequence of the structural gene encoding a 2-haloalkanoic acid dehalogenase of Pseudomonas putida strain AJ1 and purification of the encoded protein. J Gen Microbiol. 1992;138:675–683. doi: 10.1099/00221287-138-4-675. [DOI] [PubMed] [Google Scholar]
- 25.Jukes T H, Cantor C R. Evolution of protein molecules. In: Munro H N, editor. Mammalian protein metabolism. New York, N.Y: Academic Press; 1969. pp. 21–132. [Google Scholar]
- 26.Kawasaki H, Yahara H, Tonomura K. Isolation and characterization of plasmid pUO1 mediating dehalogenation of haloacetate and mercury resistance in Moraxella sp. B Agric Biol Chem. 1981;45:1477–1481. [Google Scholar]
- 27.Kawasaki H, Tsuda K, Matsusita I, Tonomura K. Lack of homology between two haloacetate dehalogenase genes encoded on a plasmid from Moraxella sp. strain B. J Gen Microbiol. 1992;138:1317–1323. doi: 10.1099/00221287-138-7-1317. [DOI] [PubMed] [Google Scholar]
- 28.Kawasaki H, Toyama T, Maeda T, Nishino H, Tonomura K. Cloning and sequence analysis of a plasmid-encoded 2-haloacid dehalogenase gene from Pseudomonas putida no. 109. Biosci Biotechnol Biochem. 1994;58:160–163. doi: 10.1271/bbb.58.160. [DOI] [PubMed] [Google Scholar]
- 29.Kawasaki H. The haloacetate dehalogenase gene dehH2 carried on a transposon residing in a plasmid of Moraxella sp. B. In: Janssen D B, Soda K, Wever R, editors. Proceedings of Colloquium on Mechanisms of Biodehalogenation and Dehalogenation, National Academy of Sciences, Amsterdam. Royal Netherlands Academy of Arts and Sciences, Amsterdam, The Netherlands. 1997. pp. 175–184. [Google Scholar]
- 30.Köhler R, Brokamp A, Schwarze R, Reiting R H, Schmidt F R J. Characteristics and DNA-sequence of a cryptic haloalkanoic acid dehalogenase from Agrobacterium tumefaciens RS5. Curr Microbiol. 1998;36:96–101. doi: 10.1007/s002849900286. [DOI] [PubMed] [Google Scholar]
- 31.Kohler-Staub D, Kohler H-P E. Microbial degradation of β-chlorinated four-carbon aliphatic acids. J Bacteriol. 1989;171:1428–1434. doi: 10.1128/jb.171.3.1428-1434.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koonin E V, Tatusov R L. Computer analysis of bacterial haloacid dehalogenases defines a large superfamily of hydrolases with diverse specificity: application of an iterative approach to database search. J Mol Biol. 1994;244:125–132. doi: 10.1006/jmbi.1994.1711. [DOI] [PubMed] [Google Scholar]
- 33.Kurihara T, Liu J Q, Nardi-Dei V, Koshikawa H, Esaki N, Soda K. Comprehensive site-directed mutagenesis of l-2-halo acid dehalogenase to probe catalytic amino-acid-residues. J Biochem. 1995;117:1317–1322. doi: 10.1093/oxfordjournals.jbchem.a124861. [DOI] [PubMed] [Google Scholar]
- 34.Leisinger T, Bader R. Microbial dehalogenation of synthetic organohalogen compounds—hydrolytic dehalogenases. Chimia. 1993;47:116–121. [Google Scholar]
- 35.Li Y-F, Hata Y, Fujii T, Hisano T, Nishihara M, Kurihara T, Esaki N. Crystal structures of reaction intermediates of l-2-haloacid dehalogenase and implications for the reaction mechanism. J Biol Chem. 1998;273:15035–15044. doi: 10.1074/jbc.273.24.15035. [DOI] [PubMed] [Google Scholar]
- 36.Maidak B L, Olsen G J, Larsen N, Overbeek R, McCaughey M J, Woese C R. The RDP (Ribosomal Database Project) Nucleic Acids Res. 1997;25:109–110. doi: 10.1093/nar/25.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchesi J R, Sato T, Weightman A J, Martin T A, Fry J C, Hiom S J, Dymock D, Wade W G. Design and evaluation of useful bacterial specific PCR primers that amplify bacterial 16S rDNA genes. Appl Environ Microbiol. 1998;64:795–799. doi: 10.1128/aem.64.2.795-799.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchesi, J. R., K. E. Hill, and A. J. Weightman. Unpublished results.
- 39.Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- 40.Murdiyatmo U, Asmara W, Tsang J S, Baines A J, Bull A T, Hardman D J. Molecular biology of the 2-haloacid halidohydrolase IVa from Pseudomonas cepacia MBA4. Biochem J. 1992;284:87–93. doi: 10.1042/bj2840087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nardi-Dei V, Kurihara T, Okamura T, Liu J Q, Koshikawa H, Ozaki H, Terashima Y, Esaki N, Soda K. Comparative studies of genes encoding thermostable l-2-halo acid dehalogenase from Pseudomonas sp. strain YL, other dehalogenases, and two related hypothetical proteins from Escherichia coli. Appl Environ Microbiol. 1994;60:3375–3380. doi: 10.1128/aem.60.9.3375-3380.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nardi-Dei V, Kurihara T, Park C, Esaki N, Soda K. Bacterial dl-2-haloacid dehalogenase from Pseudomonas sp. strain 113: gene cloning and structural comparison with d- and l-2-haloacid dehalogenases. J Bacteriol. 1997;179:4232–4238. doi: 10.1128/jb.179.13.4232-4238.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olsen G J, Woese C R, Overbeek R. The winds of (evolutionary) change—breathing new life into microbiology. J Bacteriol. 1994;176:1–6. doi: 10.1128/jb.176.1.1-6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parker L L, Hall B G. Characterization and nucleotide sequence of the cryptic cel operon of Escherichia coli K12. Genetics. 1990;124:455–471. doi: 10.1093/genetics/124.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parker L L, Hall B G. Mechanisms of activation of the cryptic cel operon of Escherichia coli K12. Genetics. 1990;124:473–482. doi: 10.1093/genetics/124.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearson W R, Lipman D J. Improved tools for biological sequence analysis. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pearson W R. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 1990;183:63–98. doi: 10.1016/0076-6879(90)83007-v. [DOI] [PubMed] [Google Scholar]
- 48.Ridder I S, Rozeboom H J, Kalk K H, Janssen D B, Dijkstra B W. Three-dimensional structure of l-2-haloacid dehalogenase from Xanthobacter autotrophicus GJ10 with the substrate analogue formate. J Biol Chem. 1997;272:33015–33022. doi: 10.1074/jbc.272.52.33015. [DOI] [PubMed] [Google Scholar]
- 49.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 50.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 51.Schäferjohann J, Yoo J-G, Kusian B, Bowein B. The cbb operons of the facultative chemoautotroph Alcaligenes eutrophus encode phosphoglycolate phosphatases. J Bacteriol. 1993;175:7329–7340. doi: 10.1128/jb.175.22.7329-7340.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schneider B, Muller R, Frank R, Lingens F. Complete nucleotide sequences and comparison of the structural genes of two 2-haloalkanoic acid dehalogenases from Pseudomonas sp. strain CBS3. J Bacteriol. 1991;173:1530–1535. doi: 10.1128/jb.173.4.1530-1535.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slater J H, Lovatt D, Weightman A J, Senior E, Bull A T. The growth of Pseudomonas putida on chlorinated aliphatic acids and its dehalogenase activity. J Gen Microbiol. 1979;114:125–136. [Google Scholar]
- 54.Slater J H, Weightman A J, Hall B G. Dehalogenase genes of Pseudomonas putida PP3 on chromosomally located transposable elements. Mol Biol Evol. 1985;2:557–567. doi: 10.1093/oxfordjournals.molbev.a040366. [DOI] [PubMed] [Google Scholar]
- 55.Slater J H, Bull A T, Hardman D J. Microbial dehalogenation. Biodegradation. 1995;6:181–189. doi: 10.1007/BF00700463. [DOI] [PubMed] [Google Scholar]
- 56.Slater J H, Bull A T, Hardman D J. Microbial dehalogenation of halogenated alkanoic acids, alcohols and alkanes. Adv Microb Physiol. 1997;38:133–176. doi: 10.1016/s0065-2911(08)60157-5. [DOI] [PubMed] [Google Scholar]
- 57.Smith J M, Harrison K, Colby J. Purification and characterization of d-2-haloacid dehalogenase from Pseudomonas putida strain AJ1/23. J Gen Microbiol. 1990;136:881–886. doi: 10.1099/00221287-136-5-881. [DOI] [PubMed] [Google Scholar]
- 58.Stringfellow J M, Cairns S S, Cornish A, Cooper R A. Haloalkanoate dehalogenase II (dehE) of a Rhizobium sp: molecular analysis of the gene and formation of carbon monoxide from trihaloacetate by the enzyme. Eur J Biochem. 1997;250:789–793. doi: 10.1111/j.1432-1033.1997.00789.x. [DOI] [PubMed] [Google Scholar]
- 59.Thomas A W, Slater J H, Weightman A J. The dehalogenase gene dehI from Pseudomonas putida PP3 is carried on an unusual mobile genetic element designated deh. J Bacteriol. 1992;174:1932–1940. doi: 10.1128/jb.174.6.1932-1940.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas A W, Lewington J, Hope S, Topping A W, Weightman A J, Slater J H. Environmentally directed mutations in the dehalogenase system of Pseudomonas putida strain PP3. Arch Microbiol. 1992;158:176–182. doi: 10.1007/BF00290813. [DOI] [PubMed] [Google Scholar]
- 61.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Topping A W, Thomas A W, Slater J H, Weightman A J. The nucleotide-sequence of a transposable haloalkanoic acid dehalogenase regulatory gene (dehRI) from Pseudomonas putida strain PP3 and its relationship with ς(54)-dependent activators. Biodegradation. 1995;6:247–255. doi: 10.1007/BF00700464. [DOI] [PubMed] [Google Scholar]
- 63.van de Peer Y, De Wachter R. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment Comput. Applic Biosci. 1994;10:569–570. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- 64.van der Ploeg J, van Hall G, Janssen J B. Characterization of the haloacid dehalogenase from Xanthobacter autotrophicus GJ10 and sequencing of the dhlB gene. J Bacteriol. 1991;173:7925–7933. doi: 10.1128/jb.173.24.7925-7933.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Hylckama Vlieg, J. E. T., and D. B. Janssen. Personal communication.
- 66.Weightman A J, Weightman A L, Slater J H. Stereospecificity of 2-monochloropropionate dehalogenation by the two dehalogenases of Pseudomonas putida PP3: evidence for two different dehalogenation mechanisms. J Gen Microbiol. 1982;128:1755–1762. doi: 10.1099/00221287-128-8-1755. [DOI] [PubMed] [Google Scholar]