Abstract
This study aimed to identify the role of crosslinking agents in the resin–dentin bond strength (BS) when used as modifiers in adhesives or pretreatments to the dentin surface through a systematic review and meta-analysis. This paper was conducted according to the directions of the PRISMA 2020 statement. The research question of this review was: “Would the use of crosslinkers agents improve the BS of resin-based materials to dentin?” The literature search was conducted in the following databases: Embase, PubMed, Scielo, Scopus, and Web of Science. Manuscripts that reported the effect on the BS after the use of crosslinking agents were included. The meta-analyses were performed using Review Manager v5.4.1. The comparisons were performed by comparing the standardized mean difference between the BS values obtained using the crosslinker agent or the control group. The subgroup comparisons were performed based on the adhesive strategy used (total-etch or self-etch). The immediate and long-term data were analyzed separately. A total of 50 articles were included in the qualitative analysis, while 45 articles were considered for the quantitative analysis. The meta-analysis suggested that pretreatment with epigallocatechin-3-gallate (EGCG), carbodiimide, ethylenediaminetetraacetic acid (EDTA), glutaraldehyde, and riboflavin crosslinking agents improved the long-term BS of resin composites to dentin (p ≤ 0.02). On the other hand, the use of proanthocyanidins as a pretreatment improved both the immediate and long-term BS values (p ≤ 0.02). When incorporated within the adhesive formulation, only glutaraldehyde, riboflavin, and EGCG improved the long-term BS to dentin. It could be concluded that the application of different crosslinking agents such as carbodiimide, EDTA, glutaraldehyde, riboflavin, and EGCG improved the long-term BS of adhesive systems to dentin. This effect was observed when these crosslinkers were used as a separate step and when incorporated within the formulation of the adhesive system.
Keywords: aging, collagen, dentin-bonding agents, proanthocyanidins
1. Introduction
For years, there have been many efforts to accomplish the suitable and predictable adhesion of composite resins to the dental structure, since reliable bonds offer high retention strength, less microleakage, and restoration stability [1]. Collagen fibrils aid in anchoring resin composite restorations to the dentin substrate [2]. Therefore, the long-term adhesion is a direct result of the stability and integrity of the collagen fibrils within the resin–dentin hybrid layer [3]. Dentin is acellular, collagen-rich, and avascular and contains type I collagen, non-collagenous proteins, and carbonated apatite. The collagen structure is flanked by C-terminal and globular N-terminal propeptides along with undeviating telopeptides, thereby accommodating the hydroxyapatite crystals and supporting the tissue [4,5,6].
It is necessary to distinguish that the mechanism of hybridization relies on the creation of a suitable hybrid layer to achieve the micromechanical retention of the dental restoration [7]. With that said, in restorative dentistry, the high mechanical properties of collagen are desirable. The resin–tooth interface should also have a lower rate of biodegradation to ensure the longevity of the restoration [8]. The improvement of the mechanical stability and physicomechanical properties of the collagen can be achieved by protecting them with several biomodifications using different collagen crosslinking agents [9]. The pretreatment of the dentin surface with these agents before bonding procedures may help increase the bond strength (BS) values and improve the stiffness of the resin–dentin bond [9,10,11,12,13,14,15]. The dentinal mineral phase might be depleted, causing denudation of the collagen scaffold, which can become simply confronted by the bacterially derived enzymes or endogenous proteases. This can lead to irreversible destruction of the dentinal structure. This led researchers to consider it critical to identify solutions for the long-term bond durability of adhesive systems [12].
Collagen fibers are fashioned from bundles of crosslinked microfibrils with endogenous crosslinks and covalent bonds. The covalent crosslinks produced with external crosslinking agents can be very stable over time, thereby inactivating the active sites of the dentin proteases by reducing the molecular mobility and inducing conformational changes in their structure or changing the negatively charged ionized carboxyl groups to positively charged amides. This approach aims to strengthen the collagen fibrils in the hybrid layer via intermolecular crosslinking [10,11]. Intriguingly, it is well acknowledged that the stiffness of the collagen matrix decreases from 18,000 MPa in the mineralized tissue to 1–3 MPa in the demineralized tissue. This low elasticity modulus permits further movements, such as lateral and rotational movements of the adjacent collagen peptides, taking them within the extent of the active site of the matrix metalloproteinases (MMPs), which is an expected consequence of the dentin collagen degradation [16]. Correspondingly, many natural or synthetic crosslinking agents have been claimed to offer significant advantages in developing mechanically stable collagen scaffolds [12]. It should be noted that crosslinking agents yield crosslinks in collagen fibers that reinforce the scaffold sufficiently. Consequently, unwinding its structure becomes impossible. Further, crosslinking agents can crosslink proteases, which truthfully interferes with their molecular mobility; they can disable the C-terminal telopeptides, thereby maintaining the telopeptides’ aptitude to sterically block collagenase binding to the critical peptide bond. Thus, a comparison of each agent may be helpful to select the most effective one for everyday clinical practice [17].
However, this issue is still controversial, and it is unclear in the literature which crosslinking agents could be effective for application to the dentin substrate while performing contemporary clinical bonding procedures. Suitably, systematic reviews are important tools that can provide a map of the range of available evidence. Hence, this study aimed to identify whether the BS to dentin could be affected by modifying or pretreating the dentin with crosslinking agents through a systematic review and meta-analysis. Hence, the null hypothesis to be tested was that there is no effect on the immediate and long-term dentinal BS levels when a collagen crosslinker is used for the bonding procedures.
2. Materials and Methods
This systematic review and meta-analysis was conducted according to the directions of the PRISMA 2020 statement [18]. The protocol was registered in the Open Science Framework with the registration number 0000-0002-2759-8984. The following PICOS strategy was used: population, dentin substrate; intervention, use of collagen crosslinkers; control, adhesive application without the use of a collagen crosslinker; outcome, BS; study design, in vitro studies. The research question of this review was: “Does the use of crosslinker agents improve the BS of resin-based materials to dentin?”
2.1. Literature Search
The literature search was conducted until 24 May 2021. The following databases were screened: Embase, PubMed, Scielo, Scopus, and Web of Science. The search strategy and keywords used in PubMed are listed in Table 1, as this search strategy was adapted to be used in the other screened databases. After completing the search, all articles were imported into EndNote X7 software (Thomson Reuters) to eliminate duplicates. After this, the articles were imported into the Rayyan QCRI mobile app [19].
Table 1.
Search strategy used in PubMed.
| Search Strategy | |
|---|---|
| # 1 | Bond OR Bonding OR Dental bonding OR Bonding efficacy OR bond strength OR Bonding performance OR bonding effectiveness OR Bond performance OR adhesive properties OR microtensile strength OR Micro-tensile strength OR bonding properties OR Microtensile bond strength OR shear bond strength OR micro shear bond strength OR performance OR Dental Bonding* OR Dentin-Bonding Agents OR Dental Bonding OR Dentin-Bonding Agents* OR Dental Bonding* |
| # 2 | Dentine OR Dentin OR Dentin* |
| # 3 | Cross-linking OR Cross-linkers OR Crosslinking OR Cross-links OR Cross-Linking OR cross-linking agents OR Dentin collagen OR Cross-linking agent OR Collagen Matrix OR Crosslink OR Collagen degradation OR Crosslinking and biomimicry OR Cross linker |
| # 4 | #1 AND #2 AND #3 |
2.2. Study Selection
Two reviewers (C.E.C.-S. and R.B.) independently assessed the titles and abstracts of all studies. Manuscripts for full-text review were chosen in line with the inclusion and exclusion criteria depicted in Table 2. A full text was considered if there were insufficient data to make clear decisions. A structured and logical approach to the literature search was used to identify the relevant papers reporting the effect of crosslinking agents. Reference lists of the original studies were hand-searched to identify any articles that could have been missed during the initial search, keeping the inclusion criteria in mind. Those articles that seemed to meet the inclusion criteria or had inadequate data in the title and abstract to make a clear decision were selected for a full evaluation. Any disagreement or discrepancy regarding the eligibility of the included manuscripts was resolved and decided through consensus and agreement by a third reviewer (L.H.). The reviewers also hand-searched the list of references for the included articles to identify additional articles not found in the initial search.
Table 2.
Inclusion and exclusion criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
2.3. Data Extraction
Data of relevance from the studies involved were extracted using Microsoft Office Excel 2019 sheets (Microsoft Corporation, Redmond, WA, USA). These data comprised the study and year of publication, the type of tooth, the collagen crosslinker used, the mode of application of the crosslinker, the BS test used, the aging conditions for the BS test, the adhesive system used, the predominant failure mode evaluated, and the main results. If any data were missing or unclear, the corresponding author of the study was contacted via e-mail to retrieve the missing information. If the investigators did not respond within 2 weeks, the missing information was not included.
2.4. Quality Assessment
The methodological quality of each included manuscript was assessed independently by two authors (L.H. and R.B.). The risk of bias in each article was assessed via the description of the following parameters: specimen randomization, single-operator protocol implementation, operator-blinded, presence of a control group, standardized samples, failure mode, manufacturer’s instructions, and sample size calculation [1]. If the article included the parameter, the study received a “YES” for that specific parameter. In the case of missing data, the parameter received a “NO”. The risk of bias was classified regarding to the sum of “YES” answers received: 1 to 3 indicated a high bias, 4 to 6 medium, and 7 to 8 indicated a low risk of bias.
2.5. Statistical Analysis
Meta-analyses were performed using Review Manager v5.4.1 (The Cochrane Collaboration) software program. The comparisons were performed using the random-effects model, and the standardized mean difference between the BS values obtained using the crosslinker agent or the control group. Subgroup comparisons were made depending on the adhesive strategy used (total-etch or self-etch). Immediate and long-term BS data were analyzed separately. Additionally, a different analysis was performed when the crosslinking agent was used as a primer or was incorporated within the adhesive formulation. If the study compared several experimental groups against the same control group, data for the experimental groups (mean, standard deviation (SD), and sample size) were combined for the meta-analysis. The heterogeneity among studies was assessed using the Cochran Q test and the I2 test.
3. Results
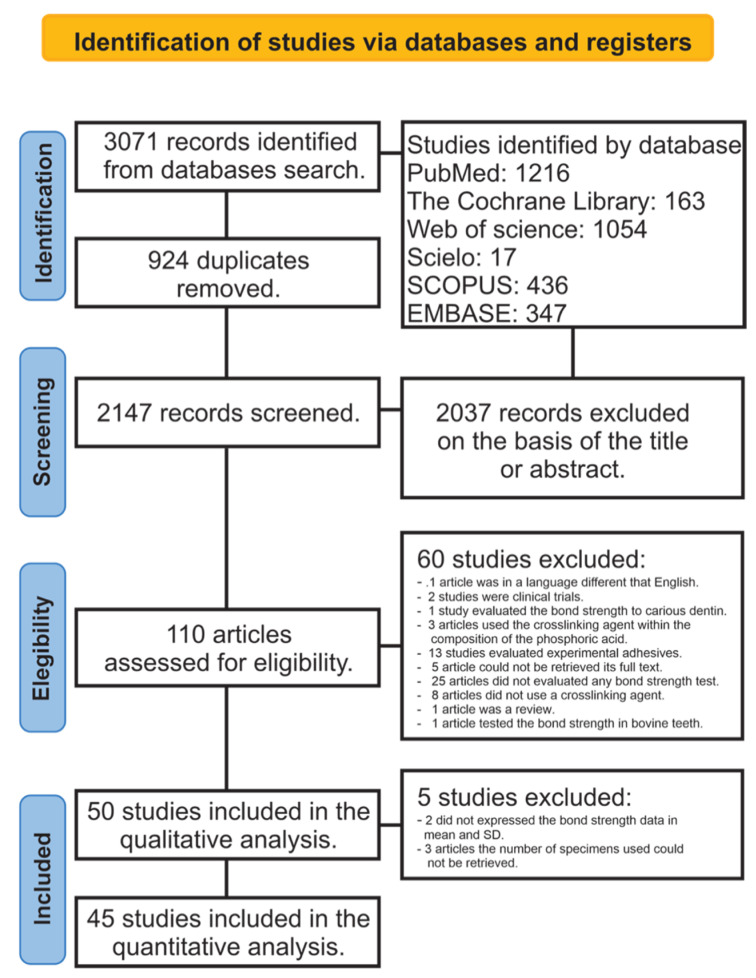
A total of 3071 papers were retrieved from all databases. After eliminating the duplicates, 2147 documents were examined by reading the title and abstract. After this, 2037 articles were excluded because they did not meet the inclusion criteria, leaving a total of 110 articles to be evaluated by full-text reading. Of these articles, 60 were not considered in the qualitative analysis: 1 article was in a language other than English [20], 2 studies were clinical trials [21,22], 1 study evaluated the BS only to carious dentin [23], 3 articles used the crosslinker agent within the composition of the phosphoric acid [24,25,26], 13 studies evaluated experimental adhesives [12,14,16,27,28,29,30,31,32,33,34,35,36], for 5 articles we could not retrieve the full text [37,38,39,40,41], 25 articles did not evaluate any BS test [42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66], 8 articles did not use a crosslinker agent [25,67,68,69,70,71,72,73], 1 article was a review [74], and 1 article tested the BS in bovine teeth as a substrate [75]. A total of 50 articles were included in the qualitative analysis. Of these, 5 articles were not considered for the meta-analysis because two of them did not express the BS data as means and SDs [76,77], and in 3 articles the number of specimens used could not be retrieved [78,79,80], totalling 45 articles considered for the quantitative analysis [17,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125]. A flowchart designating the study selection process according to the PRISMA statement is presented in Figure 1.
Figure 1.
Flowchart according to PRISMA guidelines.
The characteristics of the studies included in the qualitative analysis are shown in Table 3.
Table 3.
Characteristics of the studies included in the review.
| Study and Year | Type of Tooth | Collagen Crosslinkers | Mode of Application | Bond Strength Test Used | Adhesive Used | Storing Conditions | Predominant Failure Mode | Main Results |
|---|---|---|---|---|---|---|---|---|
| Munksgaard, 2002 [96] | Human teeth | HEMA and GA | Incorporated into the adhesive system | TBS | Concise Enamel Bond®) (3M Company, St. Paul, USA) | Distilled water at 37 °C for 24 h | NO | Bond strength was highly dependent on the HEMA and GA concentration used. |
| Osorio, 2005 [94] | Human molars | EDTA | Pretreatment | µTBS | Adper Scotchbond 1 (3M ESPE, St. Paul, MN, USA), and Clearfil SE Bond (Kuraray Co. Ltd., Osaka, Japan) | Distilled water at 37 °C for 24 h | Mixed | Collagen network is better-preserved after EDTA application. |
| Al-Ammar, 2009 [105] | Human molars | GA, GSE, and Genipin | Pretreatment | µTBS | Adper Single bond (3M ESPE, St. Paul, MN, USA) and One Step Plus | Distilled water at 37 °C for 24 h | Adhesive | Application of GA and GSE increased bond strength. |
| Bedran-Russo, 2010 [109] | Human molars | EDC, MES, and bacterial collagenase from Clostridium histolyticum—type I | Pretreatment | µTBS | Adper Single bond (3M ESPE, St. Paul, MN, USA) and One Step Plus | Distilled water at 37 °C for 24 h or 12 months | Not measured | EDC preserved the bond strength after aging. |
| Cova, 2011 [116] | Human molars | UVA-activated RF | Pretreatment | µTBS | XP Bond Adhesive (Dentsply) | Artificial saliva at 37 °C 24 h, 6 months, and 1 year | Adhesive | RF/UVA increased immediate and long-term bond strength. |
| Kim, 2011 [100] | Human molars | EDTA | Pretreatment | µTBS | Adper Single Bond 2 (3M ESPE, St. Paul, MN, USA) | Distilled water at 37 °C for 24 h | NO | Dentin treatment with EDTA improved the Bond strength durability. |
| Osorio, 2011 [93] | Human molars | Zinc | Incorporated into the adhesive system | µTBS | Single Bond Plus (3M ESPE, St Paul, MN, USA), and Clearfil SE Bond (Kuraray, Tokyo, Japan) | Distilled water at 37 °C for 24 h, 1 week, and 4 weeks. | Mixed | Zinc-doped resin did not affect bond strength. |
| Islam, 2012 [104] | Human molars | HP and GSE | Incorporated into the adhesive system | µTBS | Clearfil SE bond (Kuraray Noritake Dental Inc. Tokyo, Japan) | Distilled water at 37 °C for 24 h | Cohesive/adhesive | Incorporation of HP had a positive influence on the immediate bond strength. |
| Fang, 2017 [79] | Human molars | PRA | Pretreatment | µTBS | Adper Single Bond 2 (3M) and Prime&Bond NT (Dentsply) | Distilled water at 37 °C for 24 h | Mixed | PRA preconditioning improved resin–dentin bond strength. |
| Fawzy, 2012 [118] | Human molars | RF | Pretreatment | µTBS | Adper Single Bond 2 (3M) | Distilled water at 37 °C for 24 h or 4 months | Non measured | RF maintained the bond strength after short-term water storage. |
| Castellan, 2013 [112] | Human molars | GSE and cocoa seed | Pretreatment | µTBS | Single Bond Plus and One Step Plus | Simulated body fluid at 37 °C for 24 h, 3 months, 6 months, and 12 months. | Non measured | GSE enhanced immediate bond strength. |
| Chiang, 2013 [114] | Human molars | RF/UVA, and GA | Pretreatment | µTBS | Scotch Bond Multipurpose (3M) | Distilled water to normal water at 37 °C for 24 h and thermocycling. | Adhesive / Mixed | RF/UVA treatment enhanced bond strength. |
| Fawzy, 2013 [117] | Human molars and premolars | CH/RF | Pretreatment | µTBS | Adper Single Bond 2 (3M) | Distilled water at 37 °C for 24 h or 6 months. | Non measured | Significant improvement in bond strength was found. |
| Islam, 2014 [103] | Human molars | HP | Incorporated into the adhesive system | µTBS | Clearfil SE bond (Kuraray Noritake Dental Inc. Tokyo, Japan | Distilled water at 37 °C for 24 h | Mixed/Cohesive | The incorporation HPN into the self-etching had a positive effect on the immediate bond strength. |
| Liu, 2014 [78] | Human molars | PRA | Pretreatment | µTBS | Adper Single Bond 2 (3M ESPE, St. Paul, MN, USA) | Distilled water at 37 °C for 24 h | Mixed | PRA was effective stabilizing the adhesive–dentin bonding. |
| Sabatini, 2014 [91] | Human molars | BAC | Incorporated into the adhesive system | µTBS | Adper Single Bond Plus (3M ESPE, St. Paul, MN, USA) | Artificial saliva at 37 °C for 24 h and 6 months | Adhesive + Mixed | BAC preserved the resin–dentin bond. |
| Scheffel, 2015 [90] | Human molars | EDC | Pretreatment | µTBS | Single Bond 2 (3M ESPE, St. Paul, MN, USA) | Distilled water at 37 °C for 6 and 12 months | Adhesive + Mixed | EDC prevented resin–dentin bond degradation after aging. |
| Singh, 2015 [85] | Human molars | EDTA and EDC | Pretreatment | SBS | G-Bond (GC Corp., Tokyo, Japan) | Distilled water at 37 °C for 24 h and 6 months | Mixed | EDC pretreatment resulted in preservation of resin–dentin bond strength. |
| Yang, 2015 [87] | Human molars | EGCG | Pretreatment | µTBS | Adper Single Bond 2 (3M ESPE, St. Paul, MN, USA) | Thermocycling | Adhesive failure | Pretreatment with EGCG improve immediate bond strength and bond stability. |
| Gerhardt, 2016 [119] | Human molars | EGCG and GTE | Pretreatment | µTBS | Clearfil SE Bond (Kuraray) | Distilled water at 37 °C for 24 h and 6 months | Cohesive in resin | Dentin pretreatment with EGCG andGTE increased bond strength. |
| Hass, 2016 [17] | Human molars | PRA, RF, and GA | Pretreatment | µTBS | Single Bond Plus (SB)(3M ESPE, St. Paul, MN, USA), and Tetric N-Bond (TN) (Ivoclar Vivadent AG, Schaan, Liechtenstein) | Distilled water at 37 °C for 24 h and 18 months | Mixed | The PRA and GA treatments produced stable interfaces after aging. |
| Joseph, 2016 [102] | Human molars | RF | Pretreatment | SBS | Adper Single Bond 2 (3M ESPE, St. Paul, MN, USA) | Distilled water at 37 °C for 24 h | NO | The pretreatment with RF improved the immediate bond strength. |
| Leme-Kraus, 2017 [76] | Human molars | PRA | Pretreatment | µTBS | Experimental adhesives were applied | Simulated body fluid at 37 °C for 24 h and 1 year | NO | PRA produced a robust and stable adhesion. |
| Loguercio, 2016 [98] | Human molars | MC | Pretreatment | µTBS | Adper Single Bond 2 [3M ESPE, St Paul, MN, USA), and Prime&Bond NT (Dentsply De Trey, Konstanz, Germany) | Distilled water at 37 °C for 24 h and 2 years | Adhesive/mixed fractures | MC retarded the degradation of resin dentin interfaces over a 24-month period. |
| Neri, 2016 [95] | Human molars | EGCG | Pretreatment | µTBS | Adper Easy One (3M ESPE, St. Paul, MN, USA) | Distilled water at 37 °C for 24 h, 6 months, and 12 months | Mixed | Pretreatment with EGCG preserved the bond strength after aging. |
| Venigalla, 2016 [83] | Human molars | RF, EDC, and PRA | Pretreatment | µTBS | Adper Single Bond Adhesive (3M ESPE) | Artificial saliva for 24 h at 37 °C and 6 months | Adhesive failure | Modification of dentin using collagen crosslinking improved the bond strength durability. |
| Zhang, 2016 [60] | Human molars | EDC | Pretreatment | µTBS | Single Bond 2 (3M ESPE) | 0.9% NaCl solution at 37 °C for 24 h and 90 days | Adhesive failure | The EDC-treated groups exhibited significantly greater bond strength. |
| Zhang, 2016 (b) [82] | Human molars | EDC | Pretreatment | µTBS | Single Bond 2, 3MESPE | 0.9% NaCL at 37 °C for 24 h and 90 days | No | Treatment with EDC improved bond strength. |
| Zhou, 2016 [81] | Human molars | GSE | Pretreatment | µTBS | Single Bond Plus (3M ESPE, St. Paul, MN, USA) | Distilled water at 37 °C for 24 h | No | GSE pretreatment allowed to use the dry bond technique without lowering the bond strength values. |
| Abunawareg, 2017 [106] | Human molars | EDC and RF | Pretreatment | µTBS | Adper Single bond 2(3M ESPE, St. Paul, MN, USA) | Distilled water at 37 °C for 24 h, 6 months, and 12 months | Mixed | Collagen crosslinking induced by EDC and RF improved the durability of the resin–dentin bond. |
| Feiz, 2017 [80] | Human molars | GSE | Pretreatment | SBS | Adper Single Bond 2 (3M) | Distilled water at 37 °C for 24 h | Non measured | GSE material did not improve the bond strength. |
| Lee, 2017 [99] | Human molars | GA | Incorporated into the adhesive system | µTBS | Gluma Comfort Bond and Desensitizer and iBond Total Etch (both from Heraeus Kulzer)] | Distilled water at 37 °C for 24 h | Mixed | Collagen crosslinking with GA stabilized the bond strength after aging. |
| Paulose, 2017 [92] | Human molars | GSE | Pretreatment | µTBS | AdperTM Single Bond 2 (3M ESPE, St. Paul, MN, USA), and Single Bond Universal Adhesive (3M ESPE, St. Paul, MN, USA) | Distilled water at 37 °C for 24 h and 1 year | No | GSE was effective only by using it in the dry bond technique. |
| Yu, 2017 [84] | Human molars | EGCG | Incorporated into the adhesive system | µTBS | Single Bond 2 (3M ESPE) | Deionized water at 37 °C for 24 h and thermocycling. | No | EGCG-containing adhesive improved the long-term bond strength. |
| Bharti, 2018 [110] | Human molars | SA and PRA | Pretreatment | SBS | One Coat Bond SL (Coltene) | Distilled water to normal water at 37 °C for 24 h and thermocycling. | Non measured | Treatment with collagen crosslinking agent increased the bond strength- |
| Mazzoni, 2018 [97] | Human molars | EDC | Pretreatment | µTBS | Clearfil SE Bond (Kuraray Dental, Osaka, Japan), and XP Bond (Dentsply DeTrey GmbH, Konstanz, Deustche; abbreviation: XPB) | Artificial saliva at 37 °C for 24 h and 1 year. | Mixed | The use of EDC improved the bond strength over the time. |
| Sun, 2018 [86] | Human molars | EGCG | Pretreatment | µTBS | Adper Single Bond 2 (3M ESPE, St. Paul, MN, USA) | Thermocycling | No | EGCG preconditioning enhanced resin–dentin bond durability. |
| Chaharom, 2019 [113] | Human molars | GR | Non specified | µTBS | All-Bond 3, One-Step Plus, Clearfil SE Bond (Kuraray) and Clearfil S3 (Kuraray) | Thermocycling | Adhesive / Mixed | GR had no effect on the immediate bond strength. |
| Kasraei, 2019 [101] | Human premolars | RF | Pretreatment | µTBS | Clearfil SE Bond adhesive (Kuraray, Tokyo, Japan) | Distilled water at 37 °C for 24 h and thermocycling. | Mixed | Treatment of dentin with RF had a negative impact on the bond strength. |
| Silva, 2019 [89] | Human molars and premolars | GSE | Pretreatment | SBS | ClearfilTM SE Bond (Kuraray, Medical Inc., Tokyo, Japan) | Thermocycling | No | The application of GSE did not improve the bond strength. |
| Tang, 2016 [88] | Human molars | EDC | Pretreatment | SBS | Single Bond 2 (3M ESPE, St. Paul, MN, USA) | Distilled water at 37 °C for 24 h | Adhesive failure | EDC favored the bond strength. |
| Venkatachalam, 2019 [121] | Human premolars | RF | Pretreatment | SBS | Adper Single Bond 2 (3M ESPE, St. Paul, MN, USA) | Distilled water at 37 °C and thermocycling. | No | Pretreatment by application of RF improved the shear bond strength. |
| Baena, 2020 [107] | Human molars | CH | Pretreatment | µTBS | Optibond FL (Kerr) | Artificial saliva at 37 °C for 24 h or thermocycling. | Adhesive | Application of CH did not improve the long-term bond strength. |
| Comba, 2019 [115] | Human molars | EDC | Pretreatment | µTBS | Single Bond Universal (3M) | Artificial saliva at 37 °C 24 h and 1 year | Adhesive | The use of EDC preserved the stability of the adhesive interface. |
| Fu, 2020 [124] | Human molars | Modified RF | Incorporated into the adhesive system | µTBS | Single Bond Universal (3M) and Zipbond | Distilled water at 37 °C for 1 week and 6 months | Mixed | RF modified adhesives improved the long-term bond strength. |
| Li, 2020 [125] | Human molars | DMA | Pretreatment | µTBS | Adper Single Bond 2 (3M ESPE, St. Paul, MN, USA) | Distilled water at 37 °C for 24 h and thermocycling | Mixed | Pretreatment with DMA preserved the bond strength. |
| Nivedita, 2019 [123] | Human premolars | CH and PRA | Pretreatment | SBS | Adper Single Bond-2 (3M ESPE, St. Paul, MN, USA) | Distilled water at 37 °C for 24 h and thermocycling. | NO | Application of Chitosan and PRA improved the bond strength. |
| Baldion, 2021 [108] | Human molars | EDTA, GA, PRA, and MY. | Pretreatment | µTBS | Adper Single bond 2 (3M ESPE, St. Paul, MN, USA) | Artificial saliva at 37 °C for 24 h and 18 months. | Adhesive | Use of GA and PRA preserved the bond strength after aging. |
| Bourgi, 2021 [111] | Human molars | RB | Incorporated into the adhesive system | µTBS | Prime&Bond Active (Dentsply) | Distilled water at 37 °C for 24 h | Non measured | RB helped in maintaining bond strength. |
| Freitas, 2021 [77] | Human molars | DX | Incorporated into the adhesive system | µTBS | Adper Single Bond 2 (3M) | Distilled water at 37 °C for 24 h and 1 year. | DX adhesives maintained the bond strength after aging. |
HEMA: hydroxyethyl methacrylate; GA: glutaraldehyde; EDTA: ethylenediaminetetraacetic acid; GSE: grape seed extract; EDC: 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride; MES: N-hydroxysuccinimide-NHS, 2-(N-morpholino)ethanesulfonic acid; RF: riboflavin; HP: hesperidin; PRA: proanthocyanidin; RF/UVA: riboflavin-ultraviolet; CH/RF: chitosan/riboflavin; BAC: benzalkonium chloride; EDC: carbodiimide; EGCG: epigallocatechin gallate; GTE: green tea extract; MC: minocycline; SA: sodium ascorbate; DMA: dopamine methacrylamide; MY: myricetin; RB: ribose; DX: doxycline; GR: galardin; TBS: tensile bond strength; µTBS: micro-tensile bond strength; SBS: shear bond strength.
This review identified several substances proposed as crosslinking agents, including carbodiimide, epigallocatechin-3-gallate (EGCG), glutaraldehyde, riboflavin, proanthocyanidins, ethylenediaminetetraacetic acid (EDTA), benzalkonium chloride, zinc, chitosan, minocycline, hesperidin, myricetin, sodium ascorbate, and galardin. The crosslinking agents were used as primers or incorporated into the adhesive formulation.
The meta-analysis was conducted considering 45 articles. Separate analyses were performed for the crosslinking agents used as pretreatments or incorporated within the adhesive formulation. Additionally, separate analyses were performed for the immediate and long-term bond strengths. Separate subgroups were analyzed for each condition considering the type of adhesive used (self-etch or total-etch).
Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8 show the analyses of the crosslinkers agents when applied as a separate step as a primer.
Figure 2.
Forest plot of the immediate (A) and long-term (B) bond strength comparison between the chitosan crosslinking agent and the control according to the adhesive used [107,123].
Figure 3.
Forest plot of the immediate (A) and long-term (B) bond strength comparison between the EGCG crosslinking agent and the control according to the adhesive used [67,86,95,119].
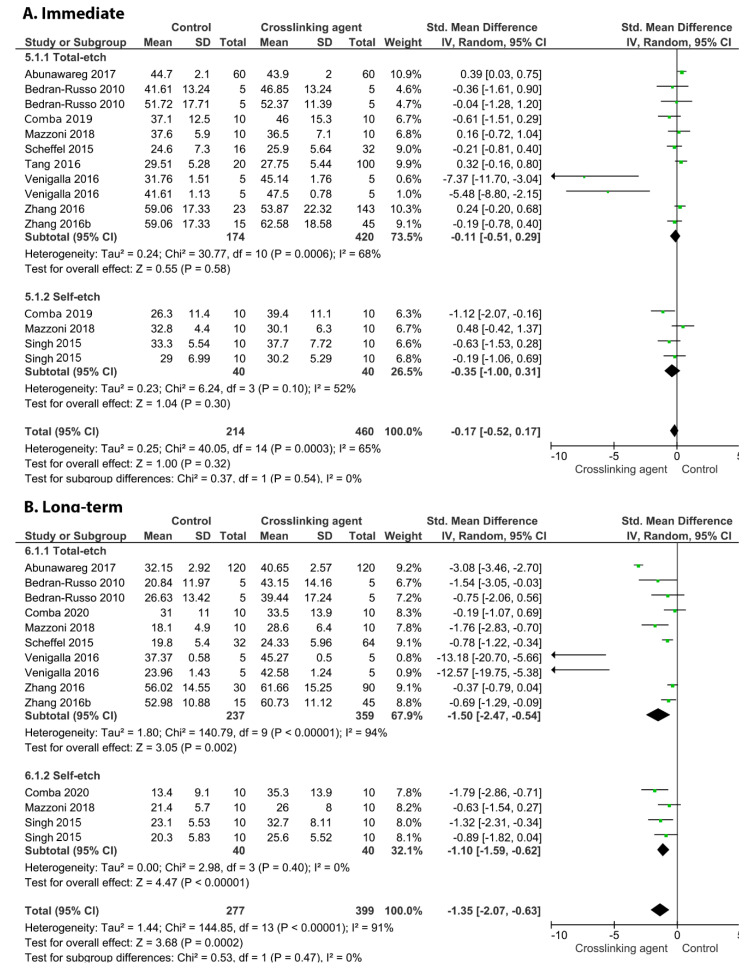
Figure 4.
Forest plot of the immediate (A) and long-term (B) bond strength comparison between the carbodiimide crosslinking agent and the control according to the adhesive used [60,82,83,85,90,97,106,109,115].
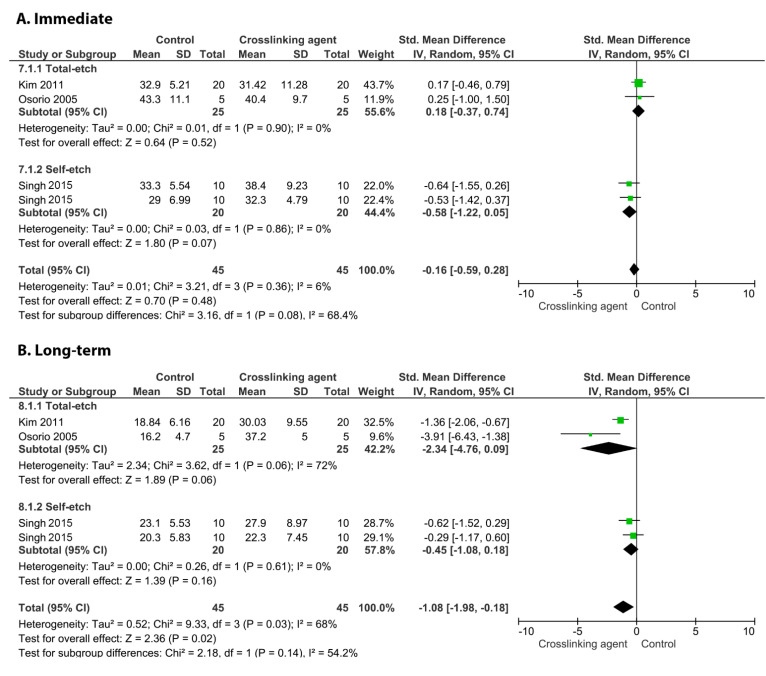
Figure 5.
Forest plot of the immediate (A) and long-term (B) bond strength comparison between the EDTA crosslinking agent and the control according to the adhesive used [85,94,111].
Figure 6.
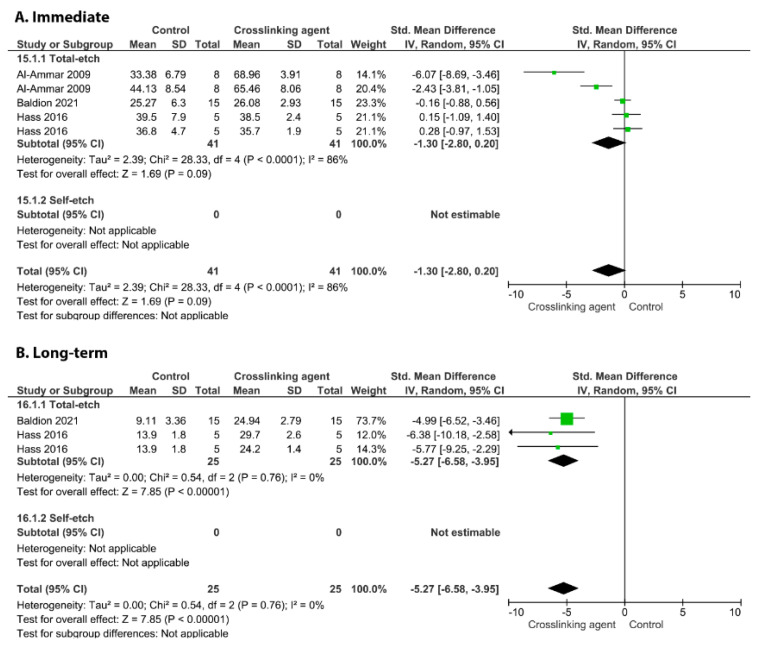
Forest plot of the immediate (A) and long-term (B) bond strength comparison between the glutaraldehyde crosslinking agent and the control according to the adhesive used [17,105,108].
Figure 7.
Forest plot of the immediate (A) and long-term (B) bond strength comparison between the riboflavin crosslinking agent and the control according to the adhesive used [17,83,102,106,114,116,117,118].
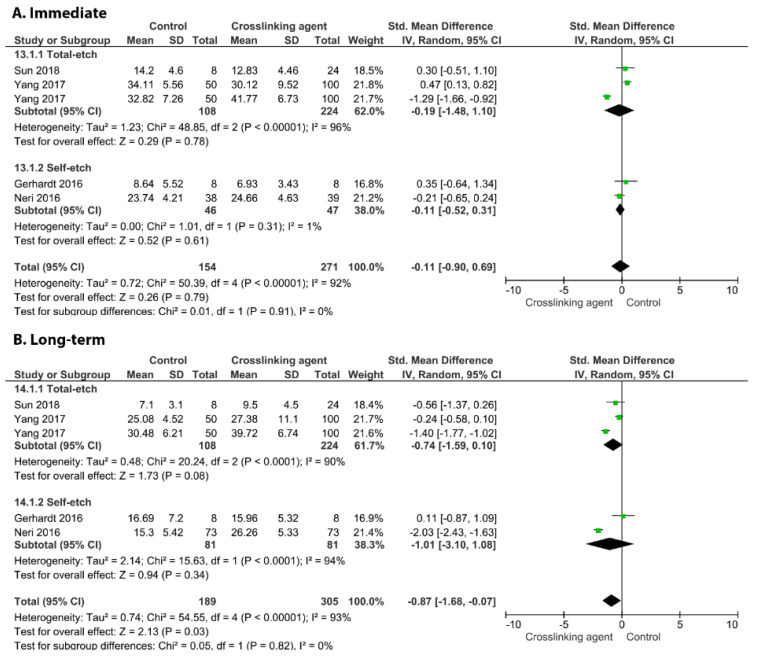
Figure 8.
Forest plot of the immediate (A) and long-term (B) bond strength comparison between the proanthocyanidins crosslinking agent and the control according to the adhesive used [17,81,83,89,92,105,108,110,112,123].
When chitosan was used prior to the application of an adhesive system, neither the immediate nor long-term BS values were improved (Figure 2; p = 0.3, and p = 0.97).
Figure 3 shows the effects of the application of the EGCG crosslinker agent. The meta-analysis favoured the use of this crosslinking agent only for the long-term BS (p = 0.03) while an improvement in the immediate BS was not observed (p = 0.79). This behaviour was found for the carbodiimide (Figure 4; long-term p = 0.002, immediate, p = 0.32), EDTA (Figure 5; long-term p = 0.02, immediate, p = 0.48), glutaraldehyde (Figure 6; immediate, p = 0.09; long-term, p < 0.00001), and riboflavin (Figure 7; immediate, p = 0.31; long-term, p < 0.00001).
Figure 8 shows a forest plot of the comparison of the proanthocyanidins used as a crosslinking agent. According to the analysis, both the immediate and long-term BS values are improved when this crosslinking agent is used (p ≤ 0.02).
Figure 9, Figure 10, Figure 11 and Figure 12 show the analyses of the crosslinkers agents when incorporated within the formulation of the adhesive system. According to the analyses, the incorporation of proanthocyanidins did not improve the immediate BS (Figure 9; p = 0.21).
Figure 9.
Forest plot of the immediate bond strength comparison between the proanthocyanidins crosslinking agent and the control according to the adhesive used [103].
Figure 10.
Forest plot of the immediate (A) and long-term (B) bond strength comparison between the glutaraldehyde crosslinking agent and the control according to the adhesive used [99].
Figure 11.
Forest plot of the immediate (A) and long-term (B) bond strength comparison between the riboflavin crosslinking agent and the control according to the adhesive used [124].
Figure 12.
Forest plot of the immediate (A) and long-term (B) bond strength comparison between the EGCG crosslinking agent and the control according to the adhesive used [84].
On the other hand, the incorporation of glutaraldehyde (Figure 10, p = 0.009), riboflavin (Figure 11, p < 0.00001), and EGCG (Figure 12, p < 0.00001) into an adhesive system improved the long-term BS to dentin.
Conferring to the parameters contemplated in the risk of bias assessment, the majority of manuscripts were categorized with a medium risk of bias (Table 4). Numerous studies failed to report the single-operator, operator-blinded, and sample size calculation parameters.
Table 4.
Risk of bias of the included studies.
| Study | Specimen Randomization | Single Operator | Operator Blinded | Control Group | Standardized Specimens | Failure Mode | Manufacturer’s Instructions | Sample size Calculation | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|
| Abunawareg, 2017 [106] | YES | NO | NO | YES | YES | YES | YES | NO | Medium |
| Al-Ammar, 2009 [105] | YES | NO | NO | YES | YES | YES | YES | NO | Medium |
| Baena, 2020 [107] | NO | NO | NO | YES | YES | YES | YES | NO | Medium |
| Baldion, 2021 [108] | YES | NO | NO | YES | YES | YES | YES | NO | Medium |
| Bedran-Russo 2010 [109] | YES | NO | NO | YES | YES | NO | YES | NO | Medium |
| Bharti, 2018 [110] | YES | NO | NO | YES | YES | NO | YES | NO | Medium |
| Bourgi, 2021 [111] | YES | NO | NO | YES | YES | NO | YES | YES | Medium |
| Castellan, 2013 [112] | NO | NO | NO | YES | YES | NO | YES | NO | High |
| Chaharom, 2019 [113] | YES | NO | NO | YES | YES | YES | YES | NO | Medium |
| Chiang, 2013 [114] | YES | NO | NO | YES | YES | YES | YES | NO | Medium |
| Comba, 2019 [115] | YES | YES | NO | YES | YES | YES | YES | NO | Medium |
| Cova 2011 [116] | YES | NO | NO | YES | YES | YES | YES | NO | Medium |
| Fu, 2020 [124] | YES | NO | YES | YES | YES | YES | YES | NO | Medium |
| Fang, 2017 [79] | YES | NO | NO | YES | YES | YES | YES | NO | Medium |
| Fawzy, 2012 [118] | NO | NO | NO | YES | YES | YES | YES | NO | Medium |
| Fawzy, 2013 [117] | YES | NO | NO | YES | YES | NO | YES | NO | Medium |
| Feiz, 2017 [80] | NO | NO | NO | YES | YES | NO | YES | NO | High |
| Freitas, 2021 [77] | YES | NO | NO | YES | YES | NO | YES | NO | Medium |
| Gerhardt 2016 [119] | YES | NO | NO | YES | YES | YES | YES | NO | Medium |
| Hass, 2016 [17] | YES | NO | YES | YES | YES | YES | YES | NO | Medium |
| Islam, 2012 [104] | NO | NO | NO | YES | YES | YES | YES | NO | Medium |
| Islam, 2014 [103] | NO | NO | NO | YES | YES | YES | YES | NO | Medium |
| Joseph, 2016 [102] | YES | NO | NO | YES | YES | NO | YES | NO | Medium |
| Kasraei, 2019 [101] | YES | YES | NO | YES | YES | YES | YES | NO | Medium |
| Kim, 2011 [100] | YES | NO | NO | YES | YES | NO | YES | NO | Medium |
| Lee, 2017 [99] | YES | YES | NO | YES | YES | YES | YES | NO | Medium |
| Leme-Kraus, 2017 [76] | NO | NO | NO | YES | YES | NO | YES | NO | High |
| Li, 2020 [125] | YES | NO | NO | YES | YES | YES | YES | NO | Medium |
| Liu, 2014 [78] | YES | NO | NO | YES | YES | YES | YES | NO | Medium |
| Loguercio, 2016 [98] | YES | YES | NO | YES | YES | YES | YES | NO | Medium |
| Mazzoni, 2018 [97] | YES | YES | NO | YES | YES | YES | YES | NO | Medium |
| Munksgaard, 2002 [96] | NO | NO | NO | YES | YES | NO | YES | NO | High |
| Neri, 2016 [95] | YES | NO | NO | YES | YES | YES | YES | NO | Medium |
| Nivedita, 2019 [123] | NO | NO | NO | YES | YES | NO | YES | NO | High |
| Osorio, 2005 [94] | NO | NO | NO | YES | YES | YES | YES | NO | Medium |
| Osorio, 2011 [93] | NO | NO | NO | YES | YES | YES | YES | NO | Medium |
| Paulose, 2017 [92] | YES | NO | NO | YES | YES | NO | YES | NO | Medium |
| Sabatini, 2014 [91] | YES | NO | NO | YES | YES | YES | YES | NO | Medium |
| Scheffel, 2015 [90] | YES | NO | NO | YES | YES | YES | YES | NO | Medium |
| Silva, 2019 [89] | YES | NO | NO | YES | YES | NO | YES | NO | Medium |
| Singh, 2015 [85] | YES | NO | NO | YES | YES | YES | YES | NO | Medium |
| Sun, 2018 [86] | NO | NO | NO | YES | YES | NO | YES | NO | High |
| Tang, 2016 [88] | YES | NO | NO | YES | YES | YES | YES | NO | Medium |
| Venigalla, 2016 [83] | YES | NO | NO | YES | YES | YES | YES | NO | Medium |
| Venkatachalam, 2019 [121] | YES | NO | NO | YES | YES | NO | YES | NO | Medium |
| Yang, 2015 [87] | YES | NO | NO | YES | YES | YES | YES | NO | Medium |
| Yu, 2017 [84] | YES | NO | NO | YES | YES | NO | YES | YES | Medium |
| Zhang, 2016 [60] | NO | NO | NO | YES | YES | YES | YES | NO | Medium |
| Zhang, 2016 (b) [82] | NO | NO | NO | YES | YES | NO | YES | NO | High |
| Zhou, 2016 [81] | NO | NO | NO | YES | YES | NO | YES | NO | High |
4. Discussion
A systematic review and meta-analysis were performed regarding the immediate and long-term BS levels of adhesive systems to dentin depending on the crosslinking agents used as pretreatments or incorporated within the adhesive formulation. Some of the crosslinkers increased the BS, while others lacked any influence. Hence, the hypothesis examined in this review was partially accepted.
Demineralized collagen fibrils are susceptible to hydrolytic or enzymatic degradation over time, leaving voids or demineralized nanochannels within the resin dentin hybrid layer [11]. With time, the degree of degradation increases and is mostly concerted at the bottom part of the hybrid layer, where the collagen appears to be less penetrated by resin monomers [126] and displays elevated enzymatic activity [127]. Collagen fibrils are deemed essential for helping in anchoring the composite resin to the dentinal surface. Consequently, they might be recognized as an imperative feature for the durability of dentin adhesion [2,128]. In an attempt to improve the resin–dentin interface, numerous researchers have focused on lessening the enzymatic biodegradation through the use of MMP inhibitors [129,130,131] separately from other recommended clinically relevant approaches [132]. Biomodifications and increases in the chemical and mechanical properties of collagen are deemed essential to improve the stiffness of collagen fibers [111]. This could be possible with the use of collagen crosslinking agents.
Appropriately, several substances have been argued to offer noteworthy benefits in protecting collagen fibers, and they were proposed as crosslinking agents, including carbodiimide, EGCG, glutaraldehyde, riboflavin, proanthocyanidin, EDTA, benzalkonium chloride, zinc, chitosan, minocycline, hesperidin, myricetin, sodium ascorbate, and galardin. The crosslinking agents were used as primers or incorporated into the adhesive formulation.
The analyses of the crosslinkers agents when applied (as a separate step) as primers showed that when chitosan was used prior to the application of an adhesive system, neither the immediate nor long-term BS values were improved (p = 0.77, and p = 0.97).
One should bear in mind that chitosan is known for being biocompatible and antibacterial, and has a broad spectrum of dental applications [49,133]. It possesses an affinity to amino acids and can generate microfibrillar arrangements in the collagen structure [134]. This combination provides benefits for tissue engineering purposes [135], as type I collagen has an aptitude to form ionic complexes with chitosan, increasing the collagen strength [136]. Previous studies [137,138] have reported that the incorporation of chitosan into dentin adhesives seemingly did not have any influence on the BS of the adhesive to the dentin structure. This could be similar to the results of this study, which showed that using chitosan as a separate primer solution could not enhance the BS. This might be explained by the fact that chitosan may increase the hydrophilicity of some adhesive systems, thereby permitting the pathway of nanometer-sized defects within the hybrid layer and making it more porous. In addition, chitosan used alone was considered a weak crosslinker, since it was demonstrated to lessen the activity of collagenolytic enzymes when applied to dentin without adhesive. Further, a detrimental interaction between the chitosan and adhesive could be the reason for this BS reduction [107].
The effect of the application of the EGCG favoured only the long-term BS (p = 0.03), while an improvement in the immediate BS was not observed (p = 0.79). This behaviour was observed for the carbodiimide (long-term p = 0.002, immediate, p = 0.32), EDTA (long-term p = 0.02, immediate, p = 0.48), glutaraldehyde (immediate, p = 0.09; long-term, p < 0.00001), and riboflavin (immediate, p = 0.31; long-term, p < 0.00001).
The use of an EGCG crosslinker had been advocated to impair the immediate BS. EGCG, a major green tea polyphenol, provides numerous functions, such as anti-inflammatory, antimicrobial, anticollagenolytic, antioxidant, and anticancerogenic effects [139,140]. This crosslinker was able to make the collagen chain stable [141,142]. In addition, it can reduce the biodegradation of collagen and increase the number of collagen crosslinks through the interaction of hydrogen molecules of galloyl groups [141]. It was demonstrated that EGCG applied for 60 s was efficient in maintaining the resin–dentin bond durability. Although this application period is clinically possible, it includes adding a step to the adhesive technique, thereby increasing the clinical time of the process, which contradicts the trend of simplifying materials [95]. Previous research revealed that this crosslinker enhanced the BS of some adhesive systems [143]. However, EGCG at higher concentrations could interrupt the adhesive polymerization, thereby affecting the BS [144]. Even at a concentration of 0.1%, this crosslinker did not show promising results [95]. The EGCG is probably entrapped within the linear chains after curing, thereby interfering with the monomer conversion and BS. These data corroborate the findings of this study.
Likewise, carbodiimides are a substitute agent for crosslinking to glutaraldehyde that do not contain toxic components and show greater biocompatibility because of their urea derivative, which can be easily rinsed from collagen without leaving any residual chemicals. They enhance the mechanical properties of collagen, making it more difficult to unwind and strengthening the hybrid layer [55]. A higher carbodiimide concentration can inactivate the dentin proteases, especially MMP-9 with a one minute application period after the use of acid-etched dentin [145]. They crosslink proteins (collagen) via the activation of the carboxylic acid groups of glutamic and aspartic acid residues in peptides by donating O-acylisourea groups within a treatment time of 1 h, which is clinically intolerable [146]. Perdigão et al. reported that 1 to 4 h are needed for an effective carbodiimide application for a stronger hybrid layer. Hence, their effect is concentration-dependent [145]. Carbodiimides enhanced the resistance to fatigue cracking after 6 months of water storage in dentin-bonded specimens according to Zhang et al. in 2016 [82]. This could correlate with the result of this study.
Equally, EDTA with 4 carboxylic groups showed promising results after aging. This agent is used for irrigation during the mechanical instrumentation of the root canal system and is known to decalcify smear-layer-covered dentin. EDTA might inhibit MMP-2 and MMP-9 activity when applied for 1 to 5 min because it is an effective Zn2+ and Ca2+ chelator. This helps in improving the durability of the resin–dentin bond [147]. This conclusion seems to support the results of this meta-analysis. It also increases the BS to dentin when compared to phosphoric-acid-treated dentin [148], but it is important to mention that in 2009, Sauro et al. suggested that EDTA can be removed easily by simple water rinsing; hence, this may result in no residual EDTA remaining, consequently leading to an incapacity to inhibit the activity of MMPs [149]. It has been suggested that the efficacy of EDTA relies on numerous features, such as the hardness of the dentin structure; the duration of application depth of the material; the pH, the form (gel or liquid), and the concentration of the material [149]. Additionally, according to the meta-analysis, dentin pretreatment with EDTA improved the long-term BS. This result may have been due to an improved resin infiltration into the collagen matrix [94]. It has been previously demonstrated that EDTA improves the removal of the smear layer, and as a consequence a better interaction of the functional monomers with the dentin may occur [94,150].
With regards to glutaraldehyde, it acts as a true crosslinking agent, containing five carbon aliphatic molecules. It contains two aldehyde groups at each end of the chain able to react with the amino groups of the collagen fibrils, reinforcing the hybrid layer and making the whole adhesive interface stronger via the chemical covalent bonds formed (Schiff base crosslinks) [65]. In addition, when 5% glutaraldehyde is applied for 1 min after acid-etched dentin according to Frassetto et al. 2016 [2], this consequently leads to an increase in the dentin collagen properties. Formerly, a commercial glutaraldehyde-based desensitizer, Gluma (Heraeus Kulzer GmgH, Germany), was tested and shown to contain 5.0% glutaraldehyde, 35% hydroxy ethyl methacrylate (HEMA), and 60% water. The dentin-treated interface with Gluma revealed bond stability and positive effects; however, despite the influence of HEMA on the stability of resin–dentin bonds, previous studies confirmed that the 25% glutaraldehyde-treated dentin increased the elastic modulus and improved the resistance of collagen to degradation [45,59]. Glutaraldehyde was also successful in caries-affected dentin according to Macedo et al. [151], and promoted the remineralization of the dentin via the reaction of the carbonyl group in the glutaraldehyde and hydroxyapatite in the dentin according to Chen et al. in 2016 [152]. Despite its efficacy, glutaraldehyde is strongly toxic, thereby limiting its clinical application [153]. The shortcoming of this agent is that it causing quick surface crosslinking of the tissue, producing a barrier that hinders its supplementary dispersion into the tissue bulk, risking the fixation of the tissue since the depth of the tissue increases [154]. This was elucidated by the fact that this agent was not able to totally inhibit the activity of collagenolytic enzymes in the deeper area of the hybrid layer, meaning an improvement in the immediate BS could not be observed in some adhesive systems [17]. This conclusion seems to support the results of this meta-analysis.
Recently, physical methods, specifically ultraviolet radiation, have been tested in dentistry and ophthalmology. Riboflavin, known as vitamin B2, is a crosslinking agent that produces free radicals via photooxidation (UVA), which improves the rigidity and mechanical stability of the collagen matrix, as well as the penetration of the adhesive resin (collagen became more resistant to enzymatic degradation) [2]. Riboflavin plays an important role in adhesive dentistry due to its biocompatibility, easy application, and activation by blue light. Note that riboflavin possesses optimum absorption peaks for UVA at 270, 366, and 445 nm wavelengths. The high energy of UVA breaks down the weak intrinsic crosslinks in the collagen and generates free oxygen radicals. The latter creates a new stronger covalent bond between the collagen fibers and leads to successful results. Indeed, studies have shown that 0.1% riboflavin-treated dentin is sufficient to obtain good adhesion in bonding protocols [16,65]. Notwithstanding an immediately restricted influence in terms of the collagenolytic enzyme inhibition, the binding effect of this crosslinker can be maintained for a long time [17], as suggested also by this systematic review and meta-analysis.
According to the analysis, both the immediate and long-term BS levels were improved when the proanthocyanidins crosslinker was used (immediate, p = 0.02; long-term, p = 0.001). Proanthocyanidins are natural crosslinkers derived from polyphenols (polyphenolic compounds) that are known as tannins and could improve the mechanical properties of dentin, thereby enhancing the quality of the hybrid layer. They are prevalent in elm trees, vegetables, fruits, nuts, flowers, pine bark, and grape seeds. They are known as crosslinkers and antioxidant agents with very low toxicity [2]. In 2017, Münchow et al. suggested that the incorporation of proanthocyanidins in an experimental adhesive did not show any negative effects on the immediate resistance of the resin–dentin bond when its concentration was ≤2% [11]. However, Perdigão et al. reported that 2% proanthocyanidins had no adverse effects on the dentin BS, but 3% decreased the BS [145]. In 2016, Frassetto et al. indicated that the application of 3.75 mass% proanthocyanidins for 5, 15, or 30 s in 10-μm-deep demineralized dentin offered adequate crosslinking to protect the dentin matrix from the catalytic activity of MMP-1 and MMP-9 [2]. Another study pointed out that the interaction between collagen and proanthocyanidins is not time-dependent, even within a short time exposure of 10 s [53]. Suitably, higher concentrations of proanthocyanidins can form a denser collagen matrix, which can hinder the water leaching and decrease the vapor permeability of the proanthocyanidin–collagen film [155]. Hence, proanthocyanidins can displace the water between collagen microfibrils by creating in this way a new hydrogen bond between the fibrils, aggregating them and protecting the collagen triple helix [156]. Additionally, proanthocyanidins are extraordinarily effective in stabilizing demineralized dentinal collagen against enzymatic activity in a clinically relevant context, presumably due to the non-covalent nature and covalent, electrostatic, and hydrophobic interactions with proline-rich proteins such as collagen molecules [145]. In 2018, Gré et al. confirmed that strong bonds can be formed between the amide carbonyl group of collagens and the phenolic hydroxyl group of proanthocyanidins, leading to the formation of the proline–proanthocyanidin complex [65]. Next, in 2021, a report suggested that the biomodification of deep dentin with proanthocyanidins showed the highest shear BS, followed by riboflavin and the control group (no collagen crosslinker modification) [157]. Proanthocyanidins can be incorporated into the etching solution and experimental primer to enhance the stability of bonding, while proanthocyanidins incorporated into an experimental adhesive showed no negative effect but stabilized the interfacial resin–dentin bonds, confirming the outcome obtained in this analysis. Other studies showed a drawback of proanthocyanidins in terms of discoloration (a brownish color) of the substrate treated [65,157].
The analyses of the crosslinker agents when incorporated within the formulation of the adhesive system revealed that the incorporation of proanthocyanidins did not improve either the immediate or long-term BS (p = 0.21). As described before, proanthocyanidins are considered well-known crosslinkers that can enhance both the immediate and aged BS levels of pretreated dentin [105]; nevertheless, their incorporation into the self-etch primer seemed to be unsuccessful to display such an influence in this present review. As elucidated in a previous manuscript, the large molecular size of the proanthocyanidins may have impeded the etching effect of the self-etch primer, hindering the ideal hybridization between the resin monomers and dentinal structure [103]. Another study indicated a comparable effect of this crosslinker when incorporated into an adhesive system on the dentin BS [36].
On the other hand, the incorporation of glutaraldehyde (p = 0.009), riboflavin (p < 0.00001), and EGCG (p < 0.00001) into an adhesive system improved the long-term BS to dentin. Concerning glutaraldehyde, the mechanism appears to rely on covalent chemical bond formation between the aldehyde group of glutaraldehyde and the amino groups of hydroxylysine and lysine in collagen fibrils [158,159], which increases the ability of the collagen to resist enzymatic degradation [160]. Additionality, this agent has been able to infiltrate more deeply into the etched dentinal matrix than might the other agents, possibly enhancing the collagen crosslinking at the bottom part of the hybrid layer and diminishing the bond degradation over time, indicating that this technique has been proven as a substitute to increase the long-term BS of adhesive systems to dentin. Similar findings were observed for riboflavin incorporated into adhesive systems. This could be clarified by the fact that no competition was found between the adhesive and riboflavin in absorbing light. Furthermore, this could be explained by the crosslinking effect of riboflavin and the dual effect of not just activating the adhesive monomers, but also the riboflavin. Universal adhesives seem to benefit from the long-term 0.1% riboflavin modification, as previously reported [124]. Additionally, Daood et al. [16] presented that the 3% riboflavin incorporated into the adhesive system seems to increase the immediate BS and preserve the durability of the resin–dentinal bond, without negatively influencing the degree of conversion. Moreover, this study revealed that EGCG enhanced the bonding stability of the adhesive systems after a long aging period. A preceding manuscript also established that 200 μg/mL of an EGCG-modified adhesive system might maintain the stability of the interface between the resin monomers and dentin, and perhaps this was observed after six months of distilled water aging [144]. Further, another study evidenced that the BS of an EGCG-modified adhesive system did not diminish within six months of water storage, while a dentin pretreatment with EGCG combined with a water solution or ethanol solution may enhance the bonding performance [87]. It was recently demonstrated that the EDC crosslinking effect stayed within the hybrid layer for 5 years in terms of the BS, collagen structure maintenance, and dentinal enzyme inhibition [161]. The researchers have recognized the impact of EGCG on improving the bonding stability to the matrix-bound MMPs’ inhibition effect [84]. This could be in agreement with the findings of this study and could explain the reason behind the bond stability after aging.
The present analysis noted the effect of modifying or pretreating dentin with crosslinking agents on the bonding ability of the adhesive systems. Novel dental material types might, however, be fashioned using nanotechnologies and other new methods within the fields of biomaterials and material sciences. Such progress may include antimicrobial properties, MMP and cathepsin inhibition, collagen-strengthening properties, and dental hard tissue regeneration. In conclusion, while there are still some unsettled difficulties regarding the durability of the adhesive interface, it is truly remarkable to see how far adhesive systems have come in the past 50–60 years. Methods that are capable of creating stable resin–dentin bonds and resisting the collagenolytic hydrolysis will probably be available in the next few years, improving the quality of the dental therapies. The results of this review should be taken with caution, since some future techniques should be tested, including nanoleakage, wetting ability, scanning electron microscopy, and micro-raman spectroscopy techniques. Additionally, most of the manuscripts included were categorized as having a high or medium risk of bias, and consequently superior experimental designs should be used to acquire a higher degree of evidence. Several crosslinking agents were used to show the importance of collagen strengthening; however, many other crosslinkers were not studied and evaluated in this study. In addition, there is an opportunity for slight changes in the chemistry of these materials, causing significantly different reactions and binding with the amino acid groups of collagen fibrils. Further, one should bear in mind that the pretreatment methods might be uncontrolled, and it could be problematic to define the crosslinking effect. Supplementary studies should be carried out to investigate the intrinsic protective effects of crosslinking agents on collagen, besides their mechanism of inhibition on MMP enzymes. A previous systematic review exhibited that there is no clinical effectiveness to defending the use of collagen crosslinking agents in combination with the adhesive technique in restorative procedures [162]. This did not match the results of this systematic review; thus, randomized controlled clinical trials should be considered in future investigations. Moreover, commercial products based on crosslinkers are somewhat absent in the dental market, meaning this could be a difficulty for a dentist in their daily clinical practice. The future research directions should be focused on inventing new adhesive systems containing crosslinkers as commercial products.
The aim of introducing an additional crosslinker is to stop collagen molecules from sliding past each other under stress inside the dentinal substrate [13], which will decrease the extensibility and enhance the biomechanical strength of the collagen fibers [15]. Collagen crosslinkers were used as a substitute dentin pretreatment to improve the durability of the dentinal bonding and to strengthen the dentinal collagen fibrils [111]. This conclusion seems to match the finding of this paper, since the influence of the crosslinkers appears to develop with time to favour collagen in terms of preserving an expanded position, whereby monomers and solvents can be received inside the adhesive formulation [14].
5. Conclusions
Within the limitations of this systematic review, it can be concluded that the application of different crosslinking agents such as carbodiimide, EDTA, glutaraldehyde, riboflavin, and EGCG improved the long-term BS of adhesive systems to dentin. This effect was observed when these crosslinkers were used as a separate step and when incorporated within the formulation of the adhesive system.
Acknowledgments
Authors Louis Hardan and Rim Bourgi would like to thank the Saint-Joseph University of Beirut, Lebanon, and the International Medical University of Kuala Lumpur for this research. Furthermore, the authors would also like to recognize the University of Hidalgo State, Mexico, the Polytechnic University of Marche, and the Medical University of Lodz for supporting this research.
Author Contributions
Conceptualization, L.H., R.B., U.D. and C.E.C.-S.; methodology, L.H., R.B., U.D. and C.E.C.-S.; software L.H., R.B., M.L.-S., U.D. and C.E.C.-S.; validation, L.H., R.B., U.D. and C.E.C.-S.; formal analysis, L.H., R.B., U.D., G.O., M.L.-S. and C.E.C.-S.; investigation, L.H., R.B., G.O., W.D., M.Z., N.J., M.L.-S., U.D. and C.E.C.-S.; resources, L.H., R.B., W.D., M.L.-S., J.E.Z.-C., M.R., U.D. and C.E.C.-S.; data curation, L.H., R.B., U.D. and C.E.C.-S.; writing—original draft preparation, L.H., R.B., U.D. and C.E.C.-S.; writing—review and editing, L.H., R.B., U.D., M.L.-S. and C.E.C.-S.; visualization, L.H., R.B., U.D., M.R., M.Z., N.J., W.D., G.O., J.E.Z.-C., M.L.-S. and C.E.C.-S.; supervision, L.H.; project administration, L.H.; funding acquisition, M.L.-S. and L.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the first author (L.H.) upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bourgi R., Hardan L., Rivera-Gonzaga A., Cuevas-Suárez C.E. Effect of Warm-Air Stream for Solvent Evaporation on Bond Strength of Adhesive Systems: A Systematic Review and Meta-Analysis of in Vitro Studies. Int. J. Adhes. Adhes. 2021;105:102794. doi: 10.1016/j.ijadhadh.2020.102794. [DOI] [Google Scholar]
- 2.Frassetto A., Breschi L., Turco G., Marchesi G., Di Lenarda R., Tay F.R., Pashley D.H., Cadenaro M. Mechanisms of Degradation of the Hybrid Layer in Adhesive Dentistry and Therapeutic Agents to Improve Bond Durability—A Literature Review. Dent. Mater. 2016;32:e41–e53. doi: 10.1016/j.dental.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Breschi L., Maravic T., Cunha S.R., Comba A., Cadenaro M., Tjäderhane L., Pashley D.H., Tay F.R., Mazzoni A. Dentin Bonding Systems: From Dentin Collagen Structure to Bond Preservation and Clinical Applications. Dent. Mater. 2018;34:78–96. doi: 10.1016/j.dental.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Tjäderhane L., Nascimento F.D., Breschi L., Mazzoni A., Tersariol I.L., Geraldeli S., Tezvergil-Mutluay A., Carrilho M.R., Carvalho R.M., Tay F.R. Optimizing Dentin Bond Durability: Control of Collagen Degradation by Matrix Metalloproteinases and Cysteine Cathepsins. Dent. Mater. 2013;29:116–135. doi: 10.1016/j.dental.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tjäderhane L., Carrilho M.R., Breschi L., Tay F.R., Pashley D.H. Dentin Basic Structure and Composition—An overview. Endod. Top. 2009;20:3–29. doi: 10.1111/j.1601-1546.2012.00269.x. [DOI] [Google Scholar]
- 6.Mazzoni A., Breschi L., Carrilho M., Nascimento F.D., Orsini G., Ruggeri Jr A., Gobbi P., Manzoli L., Tay F.R., Pashley D.H. A Review of the Nature, Role, and Function of Dentin Non-collagenous Proteins. Part II: Enzymes, Serum Proteins, and Growth Factors. Endod. Top. 2009;21:19–40. doi: 10.1111/j.1601-1546.2012.00268.x. [DOI] [Google Scholar]
- 7.Takamizawa T., Imai A., Hirokane E., Tsujimoto A., Barkmeier W.W., Erickson R.L., Latta M.A., Miyazaki M. SEM Observation of Novel Characteristic of the Dentin Bond Interfaces of Universal Adhesives. Dent. Mater. 2019;35:1791–1804. doi: 10.1016/j.dental.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Vidal C.M., Aguiar T.R., Phansalkar R., McAlpine J.B., Napolitano J.G., Chen S.-N., Araújo L.S., Pauli G.F., Bedran-Russo A. Galloyl Moieties Enhance the Dentin Biomodification Potential of Plant-Derived Catechins. Acta Biomater. 2014;10:3288–3294. doi: 10.1016/j.actbio.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou W., Liu S., Zhou X., Hannig M., Rupf S., Feng J., Peng X., Cheng L. Modifying Adhesive Materials to Improve the Longevity of Resinous Restorations. Int. J. Mol. Sci. 2019;20:723. doi: 10.3390/ijms20030723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daood U., Akram Z., Matinlinna J., Fawzy A. Dentine Collagen Cross-Linking Using Tiopronin-Protected Au/EDC Nanoparticles Formulations. Dent. Mater. 2019;35:1017–1030. doi: 10.1016/j.dental.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Münchow E.A., Bottino M.C. Recent Advances in Adhesive Bonding: The Role of Biomolecules, Nanocompounds, and Bonding Strategies in Enhancing Resin Bonding to Dental Substrates. Curr. Oral Health Rep. 2017;4:215–227. doi: 10.1007/s40496-017-0146-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daood U., Tsoi J., Neelakantan P., Matinlinna J., Omar H., Al-Nabulsi M., Fawzy A. In Vitro Assessment of Ribose Modified Two-Step Etch-and-Rinse Dentine Adhesive. Dent. Mater. 2018;34:1175–1187. doi: 10.1016/j.dental.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Bailey A., Light N., Atkins E. Chemical Cross-Linking Restrictions on Models for the Molecular Organization of the Collagen Fibre. Nature. 1980;288:408–410. doi: 10.1038/288408a0. [DOI] [PubMed] [Google Scholar]
- 14.Daood U., Sauro S., Pichika M.R., Omar H., Lin S.L., Fawzy A. Novel Riboflavin/VE-TPGS Modified Universal Dentine Adhesive with Superior Dentine Bond Strength and Self-Crosslinking Potential. Dent. Mater. 2020;36:145–156. doi: 10.1016/j.dental.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Gu L., Shan T., Ma Y., Tay F.R., Niu L. Novel Biomedical Applications of Crosslinked Collagen. Trends Biotechnol. 2019;37:464–491. doi: 10.1016/j.tibtech.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Daood U., Swee Heng C., Neo Chiew Lian J., Fawzy A.S. In Vitro Analysis of Riboflavin-Modified, Experimental, Two-Step Etch-and-Rinse Dentin Adhesive: Fourier Transform Infrared Spectroscopy and Micro-Raman Studies. Int. J. Oral Sci. 2015;7:110–124. doi: 10.1038/ijos.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hass V., Luque-Martinez I.V., Gutierrez M.F., Moreira C.G., Gotti V.B., Feitosa V.P., Koller G., Otuki M.F., Loguercio A.D., Reis A. Collagen Cross-Linkers on Dentin Bonding: Stability of the Adhesive Interfaces, Degree of Conversion of the Adhesive, Cytotoxicity and in Situ MMP Inhibition. Dent. Mater. 2016;32:732–741. doi: 10.1016/j.dental.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 19.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu N., Li F., Chen Y., Dou Q., Shen L., Chen J. Effect of Quaternary Ammonium Methacrylates Incorporation into a Dental Adhesive on the Resistance of Enzymatic Degradation of Resin-Dentine Interfaces. Zhonghua Kou Qiang Yi Xue Za Zhi Zhonghua Kouqiang Yixue Zazhi. 2013;48:414–418. [PubMed] [Google Scholar]
- 21.Costa C., Albuquerque N., Mendonça J., Loguercio A., Saboia V., Santiago S. Catechin-Based Dentin Pretreatment and the Clinical Performance of a Universal Adhesive: A Two-Year Randomized Clinical Trial. Oper. Dent. 2020;45:473–483. doi: 10.2341/19-088-C. [DOI] [PubMed] [Google Scholar]
- 22.de Souza L.C., Rodrigues N.S., Cunha D.A., Feitosa V.P., Santiago S.L., Reis A., Loguercio A.D., Perdigão J., de Paulo Aragao Saboia V. Two-Year Clinical Evaluation of a Proanthocyanidins-Based Primer in Non-Carious Cervical Lesions: A Double-Blind Randomized Clinical Trial. J. Dent. 2020;96:103325. doi: 10.1016/j.jdent.2020.103325. [DOI] [PubMed] [Google Scholar]
- 23.Hass V., De Paula A.M., Parreiras S., Gutiérrez M.F., Luque-Martinez I., de Paris Matos T., Bandeca M.C., Loguercio A.D., Yao X., Wang Y. Degradation of Dentin-Bonded Interfaces Treated with Collagen Cross-Linking Agents in a Cariogenic Oral Environment: An in Situ Study. J. Dent. 2016;49:60–67. doi: 10.1016/j.jdent.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Hass V., Luque-Martinez I., Muñoz M.A., Reyes M.F.G., Abuna G., Sinhoreti M.A.C., Liu A.Y., Loguercio A.D., Wang Y., Reis A. The Effect of Proanthocyanidin-Containing 10% Phosphoric Acid on Bonding Properties and MMP Inhibition. Dent. Mater. 2016;32:468–475. doi: 10.1016/j.dental.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Li J., Chen B., Hong N., Wu S., Li Y. Effect of Baicalein on Matrix Metalloproteinases and Durability of Resin-Dentin Bonding. Oper. Dent. 2018;43:426–436. doi: 10.2341/17-097-L. [DOI] [PubMed] [Google Scholar]
- 26.Paludo T., Marcondes M.L., Souto A.A., Lopes G.C., Loguércio A.D., Spohr A.M. Effect of Grape Seed Extract-Containing Phosphoric Acid Formulations on Bonding to Enamel and Dentin. Braz. Oral. Res. 2019;33:e098. doi: 10.1590/1807-3107bor-2019.vol33.0098. [DOI] [PubMed] [Google Scholar]
- 27.Hechler B., Yao X., Wang Y. Proanthocyanidins Alter Adhesive/Dentin Bonding Strengths When Included in a Bonding System. Am. J. Dent. 2012;25:276. [PMC free article] [PubMed] [Google Scholar]
- 28.Fonseca B.M., Barcellos D.C., da Silva T.M., Borges A.L.S., das Neves Cavalcanti B., Prakki A., de Oliveira H.P.M., de Paiva Gonçalves S.E. Mechanical-Physicochemical Properties and Biocompatibility of Catechin-Incorporated Adhesive Resins. J. Appl. Oral. Sci. 2019;27 doi: 10.1590/1678-7757-2018-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu L., Cai X., Guo J., Pashley D., Breschi L., Xu H., Wang X., Tay F., Niu L. Chitosan-Based Extrafibrillar Demineralization for Dentin Bonding. J. Dent. Res. 2019;98:186–193. doi: 10.1177/0022034518805419. [DOI] [PubMed] [Google Scholar]
- 30.Daood U., Omar H., Tsoi J., Fawzy A. Long-Term Bond Strength to Dentine of a Chitosan-Riboflavin Modified Two-Step Etch-and-Rinse Adhesives. Int. J. Adhes. Adhes. 2018;85:263–273. doi: 10.1016/j.ijadhadh.2018.06.015. [DOI] [Google Scholar]
- 31.Daood U., Omar H., Qasim S., Nogueira L.P., Pichika M.R., Mak K.-K., Steier L., Yiu C., Lin S.L., Fawzy A.S. New Antimicrobial and Collagen Crosslinking Formulated Dentin Adhesive with Improved Bond Durability. J. Mech. Behav. Biomed. Mater. 2020;110:103927. doi: 10.1016/j.jmbbm.2020.103927. [DOI] [PubMed] [Google Scholar]
- 32.Daood U., Balasankar M.P., Ibrahim M.A., Pichika M.R., Mak K.-K., Fawzy A.S. PLGA Nanoparticles Loaded with Quaternary Ammonium Silane and Riboflavin for Potential Applications in Adhesive Dentistry. Int. J. Adhes. Adhes. 2021;105:102797. doi: 10.1016/j.ijadhadh.2020.102797. [DOI] [Google Scholar]
- 33.Dávila-Sánchez A., Gutierrez M.F., Bermudez J.P., Méndez-Bauer L., Pulido C., Kiratzc F., Alegria-Acevedo L.F., Farago P.V., Loguercio A.D., Sauro S. Effects of Dentine Pretreatment Solutions Containing Flavonoids on the Resin Polymer-Dentine Interface Created Using a Modern Universal Adhesive. Polymers. 2021;13:1145. doi: 10.3390/polym13071145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Macedo F.A.A., Souza N.O., Lemos M.V.S., De-Paula D.M., Santiago S.L., Feitosa V.P. Dentin Bonding and Physicochemical Properties of Adhesives Incorporated with Epigallocatechin-3-Gallate. Odontology. 2019;107:23–28. doi: 10.1007/s10266-018-0367-0. [DOI] [PubMed] [Google Scholar]
- 35.Dias P.G., Da Silva E.M., Carvalho C., Miranda M., Portela M.B., Amaral C.M. Characterization and Antibacterial Effect of an Experimental Adhesive Containing Different Concentrations of Proanthocyanidin. J. Adhes. Dent. 2020;22:139–147. doi: 10.3290/j.jad.a44280. [DOI] [PubMed] [Google Scholar]
- 36.Epasinghe D., Yiu C., Burrow M., Tay F., King N. Effect of Proanthocyanidin Incorporation into Dental Adhesive Resin on Resin–Dentine Bond Strength. J. Dent. 2012;40:173–180. doi: 10.1016/j.jdent.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Osorio R., Cabello I., Medina-Castillo A.L., Osorio E., Toledano M. Zinc-Modified Nanopolymers Improve the Quality of Resin–Dentin Bonded Interfaces. Clin. Oral Investig. 2016;20:2411–2420. doi: 10.1007/s00784-016-1738-y. [DOI] [PubMed] [Google Scholar]
- 38.Maravic T., Breschi L., Comba A., Cunha S.R., Angeloni V., Nucci C., Hebling J., Pashley D., Tay F., Mazzoni A. Experimental Use of an Acrolein-Based Primer as Collagen Cross-Linker for Dentine Bonding. J. Dent. 2018;68:85–90. doi: 10.1016/j.jdent.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Khalid S., Rafique M.A., Khan A.S. Application of Natural Crosslinkers on Tooth Surface: An in-Vitro Comparative Evaluation of Resin-Dentin Bond Strength. JPMA. 2020;70:1363. doi: 10.5455/JPMA.17870. [DOI] [PubMed] [Google Scholar]
- 40.Jain K., Beri L., Kunjir K., Borse N., Neekhara N., Kadam A. Comparative Evaluation of the Effect of 10% Sodium Ascorbate, 10% Hesperidin, 1% Riboflavin 5 Phosphate, Collagen Cross-Linkers, on the Pushout Bond Strength of Fiber Postluted to Radicular Dentin: In Vitro Study. J. Conserv. Dent. JCD. 2018;21:95. doi: 10.4103/JCD.JCD_116_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Autieri G., Mussano F., Petruzzi M., Carossa M., Genova T., Corsalini M., Carossa S. Proanthocyanidin May Improve the Shear Bond Strength at the Composites/Dentine Interface. J. Biol. Regul. Homeost. Agents. 2018;32:1021–1025. [PubMed] [Google Scholar]
- 42.Abuelenain D.A., Abou Neel E.A., Abu-Haimed T. Effects of Dentin Modifiers on Surface and Mechanical Properties of Acid-Etched Dentin. Int. J. Adhes. Adhes. 2018;81:43–47. doi: 10.1016/j.ijadhadh.2017.11.006. [DOI] [Google Scholar]
- 43.Aydin B., Hassan L.S., Viana G., Bedran-Russo A.K. Assessing Collagen and Micro-Permeability at the Proanthocyanidin-Treated Resin-Dentin Interface. J. Adhes. Dent. 2016;18:529–534. doi: 10.3290/j.jad.a37359. [DOI] [PubMed] [Google Scholar]
- 44.Bedran-Russo A.K.B., Pereira P.N., Duarte W.R., Drummond J.L., Yamauchi M. Application of Crosslinkers to Dentin Collagen Enhances the Ultimate Tensile Strength. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2007;80:268–272. doi: 10.1002/jbm.b.30593. [DOI] [PubMed] [Google Scholar]
- 45.Bedran-Russo A.K.B., Pashley D.H., Agee K., Drummond J.L., Miescke K.J. Changes in Stiffness of Demineralized Dentin Following Application of Collagen Crosslinkers. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2008;86:330–334. doi: 10.1002/jbm.b.31022. [DOI] [PubMed] [Google Scholar]
- 46.Bedran-Russo A., Yoo K., Ema K., Pashley D.H. Mechanical Properties of Tannic-Acid-Treated Dentin Matrix. J. Dent. Res. 2009;88:807–811. doi: 10.1177/0022034509342556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bortolotto T., Ryabova A., Nerushay I., Kling S., Hafezi F., Garcia-Godoy F., Krejci I. Effects of Riboflavin, Calcium-Phosphate Layer and Adhesive System on Stress-Strain Behavior of Demineralized Dentin. Am. J. Dent. 2017;30:179–184. [PubMed] [Google Scholar]
- 48.Castellan C.S., Bedran-Russo A.K., Karol S., Pereira P.N.R. Long-Term Stability of Dentin Matrix Following Treatment with Various Natural Collagen Cross-Linkers. J. Mech. Behav. Biomed. Mater. 2011;4:1343–1350. doi: 10.1016/j.jmbbm.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daood U., Iqbal K., Nitisusanta L.I., Fawzy A.S. Effect of Chitosan/Riboflavin Modification on Resin/Dentin Interface: Spectroscopic and Microscopic Investigations. J. Biomed. Mater. Res. A. 2013;101:1846–1856. doi: 10.1002/jbm.a.34482. [DOI] [PubMed] [Google Scholar]
- 50.Dennis D., Maitari F., Abidin T., Farahanny W. Evaluation of Grape Seed Extract (Vitis Vinifera) as a Crosslinker on the Stability of Dentine Collagen in Total-Etch Adhesive Systems: An in-Vitro Study. Asian J. Pharm. Clin. Res. 2019;12:164–166. doi: 10.22159/ajpcr.2019.v12i5.32506. [DOI] [Google Scholar]
- 51.Zhang Z., Mutluay M., Tezvergil-Mutluay A., Tay F.R., Pashley D.H., Arola D. Effects of EDC Crosslinking on the Stiffness of Dentin Hybrid Layers Evaluated by NanoDMA over Time. Dent. Mater. 2017;33:904–914. doi: 10.1016/j.dental.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Green B., Yao X., Ganguly A., Xu C., Dusevich V., Walker M.P., Wang Y. Grape Seed Proanthocyanidins Increase Collagen Biodegradation Resistance in the Dentin/Adhesive Interface When Included in an Adhesive. J. Dent. 2010;38:908–915. doi: 10.1016/j.jdent.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Y., Chen M., Yao X., Xu C., Zhang Y., Wang Y. Enhancement in Dentin Collagen’s Biological Stability after Proanthocyanidins Treatment in Clinically Relevant Time Periods. Dent. Mater. 2013;29:485–492. doi: 10.1016/j.dental.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X., Zhou J., Chen L., Yang Y., Tan J. UVA-Activated Riboflavin Improves the Strength of Human Dentin. J. Oral Sci. 2015;57:229–234. doi: 10.2334/josnusd.57.229. [DOI] [PubMed] [Google Scholar]
- 55.Mazzoni A., Angeloni V., Apolonio F.M., Scotti N., Tjäderhane L., Tezvergil-Mutluay A., Di Lenarda R., Tay F.R., Pashley D.H., Breschi L. Effect of Carbodiimide (EDC) on the Bond Stability of Etch-and-Rinse Adhesive Systems. Dent. Mater. 2013;29:1040–1047. doi: 10.1016/j.dental.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 56.Mazzoni A., Apolonio F., Saboia V., Santi S., Angeloni V., Checchi V., Curci R., Di Lenarda R., Tay F., Pashley D.H. Carbodiimide Inactivation of MMPs and Effect on Dentin Bonding. J. Dent. Res. 2014;93:263–268. doi: 10.1177/0022034513516465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreira M., Souza N., Sousa R., Freitas D., Lemos M., De Paula D., Maia F., Lomonaco D., Mazzetto S., Feitosa V. Efficacy of New Natural Biomodification Agents from Anacardiaceae Extracts on Dentin Collagen Cross-Linking. Dent. Mater. 2017;33:1103–1109. doi: 10.1016/j.dental.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 58.dos Santos P.H., Karol S., Bedran-Russo A.K.B. Nanomechanical Properties of Biochemically Modified Dentin Bonded Interfaces. J. Oral. Rehabil. 2011;38:541–546. doi: 10.1111/j.1365-2842.2010.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu C., Wang Y. Cross-linked Demineralized Dentin Maintains Its Mechanical Stability When Challenged by Bacterial Collagenase. J. Biomed. Mater. Res. B Appl. Biomater. 2011;96:242–248. doi: 10.1002/jbm.b.31759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y., Tang L., Liu Y., Zhou Y., Chung K., Chan D.C. Effects of Carbodiimide Dentin Surface Treatment on Resin-Dentin Bonding. Am. J. Dent. 2016;29:208–212. [PubMed] [Google Scholar]
- 61.Xu C., Wang Y. Collagen Cross-Linking Increases Its Biodegradation Resistance in Wet Dentin Bonding. J. Adhes. Dent. 2012;14:11. doi: 10.3290/j.jad.a21494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma P., Nagpal R., Tyagi S.P., Manuja N. Bonding Efficacy of Etch-and-Rinse Adhesives after Dentin Biomodification Using Ethanol Saturation and Collagen Cross-Linker Pretreatment. J. Conserv. Dent. JCD. 2015;18:331. doi: 10.4103/0972-0707.159751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seseogullari-Dirihan R., Tjäderhane L., Pashley D.H., Tezvergil-Mutluay A. Effect of Ultraviolet A-Induced Crosslinking on Dentin Collagen Matrix. Dent. Mater. 2015;31:1225–1231. doi: 10.1016/j.dental.2015.08.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ekambaram M., Epasinghe D.J., Yiu C.K.Y., Matinlinna J.P. Effect of Solvents on Inhibition of Collagen-Bound Proteases by Chlorhexidine. Int. J. Adhes. Adhes. 2015;60:83–87. doi: 10.1016/j.ijadhadh.2015.03.010. [DOI] [Google Scholar]
- 65.Gre C.P., Lise D.P., Ayres A.P., De Munck J., Tezvergil-Mutluay A., Seseogullari-Dirihan R., Lopes G.C., Van Landuyt K., Van Meerbeek B. Do Collagen Cross-Linkers Improve Dentin’s Bonding Receptiveness? Dent. Mater. 2018;34:1679–1689. doi: 10.1016/j.dental.2018.08.303. [DOI] [PubMed] [Google Scholar]
- 66.Seseogullari-Dirihan R., Mutluay M.M., Pashley D.H., Tezvergil-Mutluay A. Is the Inactivation of Dentin Proteases by Crosslinkers Reversible? Dent. Mater. 2017;33:e62–e68. doi: 10.1016/j.dental.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 67.Yang H.Y., Li K., Yan H.Y., Liu S.Y., Wang Y.K., Huang C. High-Performance Therapeutic Quercetin-Doped Adhesive for Adhesive-Dentin Interfaces. Sci. Rep. 2017;7:8189. doi: 10.1038/s41598-017-08633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Porto I., Nascimento T.G., Oliveira J.M.S., Freitas P.H., Haimeur A., Franca R. Use of Polyphenols as a Strategy to Prevent Bond Degradation in the Dentin-Resin Interface. Eur. J. Oral Sci. 2018;126:146–158. doi: 10.1111/eos.12403. [DOI] [PubMed] [Google Scholar]
- 69.Peng W.A., Yi L.Y., Wang Z.M., Yang H.Y., Huang C. Effects of Resveratrol/Ethanol Pretreatment on Dentin Bonding Durability. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020;114:111000. doi: 10.1016/j.msec.2020.111000. [DOI] [PubMed] [Google Scholar]
- 70.Pedrosa V.O., Franca F.M.G., Turssi C.P., do Amaral F.L.B., Teixeira L.N., Martinez E.F., Basting R.T. Effects of Caffeic Acid Phenethyl Ester Application on Dentin MMP-2, Stability of Bond Strength and Failure Mode of Total-Etch and Self-Etch Adhesive Systems. Arch. Oral Biol. 2018;94:16–26. doi: 10.1016/j.archoralbio.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 71.Mapar M., Moalemnia M., Kalantari S. Comparison of the Effect of Chlorhexidine and Collagen Cross-Linking Agent (Quercus Extract) on the Tensile Bond Strength of Composite to Dentin. Med. Sci. 2020;24:1168–1175. [Google Scholar]
- 72.Chappelow C.C., Power M.D., Bowles C.Q., Miller R.G., Pinzino C.S., Eick J.D. Novel Priming and Crosslinking Systems for Use with Isocyanatomethacrylate Dental Adhesives. Dent. Mater. 2000;16:396–405. doi: 10.1016/S0109-5641(00)00034-8. [DOI] [PubMed] [Google Scholar]
- 73.Moussa H., Jones M.M., Huo N., Zhang R., Keskar M., Visser M.B., Swihart M.T., Cheng C., Sabatini C. Biocompatibility, Mechanical, and Bonding Properties of a Dental Adhesive Modified with Antibacterial Monomer and Cross-Linker. Clin. Oral Investig. 2021;25:2877–2889. doi: 10.1007/s00784-020-03605-w. [DOI] [PubMed] [Google Scholar]
- 74.Singh P., Nagpal R., Singh U.P., Manuja N. Effect of Carbodiimide on the Structural Stability of Resin/Dentin Interface. J. Conserv. Dent. 2016;19:501–509. doi: 10.4103/0972-0707.194020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pinto C.F., Berger S.B., Cavalli V., Bedran-Russo A.K., Giannini M. Influence of Chemical and Natural Cross-Linkers on Dentin Bond Strength of Self-Etching Adhesives. Int. J. Adhes. Adhes. 2015;60:117–122. doi: 10.1016/j.ijadhadh.2015.04.008. [DOI] [Google Scholar]
- 76.Leme-Kraus A.A., Aydin B., Vidal C.M., Phansalkar R.M., Nam J.W., McAlpine J., Pauli G.F., Chen S., Bedran-Russo A.K. Biostability of the Proanthocyanidins-Dentin Complex and Adhesion Studies. J. Dent. Res. 2017;96:406–412. doi: 10.1177/0022034516680586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Freitas P.H., Andre C.B., Fronza B.M., Giannini M., Rosalen P.L., Consani S., Franca R. Physicochemical Properties, Metalloproteinases Inhibition, and Antibiofilm Activity of Doxycycline-Doped Dental Adhesive. J. Dent. 2021;104:103550. doi: 10.1016/j.jdent.2020.103550. [DOI] [PubMed] [Google Scholar]
- 78.Liu R.R., Fang M., Zhang L., Tang C.F., Dou Q., Chen J.H. Anti-Proteolytic Capacity and Bonding Durability of Proanthocyanidin-Biomodified Demineralized Dentin Matrix. Int. J. Oral Sci. 2014;6:168–174. doi: 10.1038/ijos.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fang H., Li Q.L., Han M., Mei M.L., Chu C.H. Anti-Proteolytic Property and Bonding Durability of Mussel Adhesive Protein-Modified Dentin Adhesive Interface. Dent. Mater. 2017;33:1075–1083. doi: 10.1016/j.dental.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 80.Feiz A., Badrian H., Goroohi H., Mojtahedi N. The Effect of Synthetic Grape Seed Extract (GSE) on the Shear Bond Strength of Composite Resin to Dentin. J. Res. Med. Dent. Sci. 2017;5:65–70. [Google Scholar]
- 81.Zhou J.F., Chiba A., Scheffel D.L.S., Hebling J., Agee K., Tagami J., Tan J.Q., Abuelenain D., Abu Nawareg M., Hassan A.H., et al. Cross-Linked Dry Bonding: A New Etch-and-Rinse Technique. Dent. Mater. 2016;32:1124–1132. doi: 10.1016/j.dental.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Z., Beitzel D., Majd H., Mutluay M., Tezvergil-Mutluay A., Tay F.R., Pashley D.H., Arola D. Effect of Carbodiimide on the Fatigue Crack Growth Resistance of Resin-Dentin Bonds. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2016;32:211–222. doi: 10.1016/j.dental.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Venigalla B.S., Jyothi P., Kamishetty S., Reddy S., Cherukupalli R.C., Reddy D.A. Resin Bond Strength to Water versus Ethanol-Saturated Human Dentin Pretreated with Three Different Cross-Linking Agents. J. Conserv. Dent. 2016;19:555–559. doi: 10.4103/0972-0707.194019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu H.H., Zhang L., Yu F., Li F., Liu Z.Y., Chen J.H. Epigallocatechin-3-Gallate and Epigallocatechin-3-O-(3-O-Methyl)-Gallate Enhance the Bonding Stability of an Etch-and-Rinse Adhesive to Dentin. Materials. 2017;10:183. doi: 10.3390/ma10020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh S., Nagpal R., Tyagi S.P., Manuja N. Effect of EDTA Conditioning and Carbodiimide Pretreatment on the Bonding Performance of All-in-One Self-Etch Adhesives. Int. J. Dent. 2015;2015:141890. doi: 10.1155/2015/141890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun Q.R., Gu L.S., Quan J.J., Yu X.R., Huang Z.H., Wang R.X., Mai S. Epigallocatechin-3-Gallate Enhance Dentin Biomodification and Bond Stability of an Etch-and-Rinse Adhesive System. Int. J. Adhes. Adhes. 2018;80:115–121. doi: 10.1016/j.ijadhadh.2017.11.001. [DOI] [Google Scholar]
- 87.Yang H.Y., Guo J.M., Deng D.L., Chen Z.Y., Huang C. Effect of Adjunctive Application of Epigallocatechin-3-Gallate and Ethanol-Wet Bonding on Adhesive-Dentin Bonds. J. Dent. 2016;44:44–49. doi: 10.1016/j.jdent.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 88.Tang L., Zhang Y., Liu Y., Zhou Y. Influence of EDC on Dentin-Resin Shear Bond Strength and Demineralized Dentin Thermal Properties. Materials. 2016;9:920. doi: 10.3390/ma9110920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Silva A.C., Melo P., Ferreira J., Oliveira S., Gutknecht N. Influence of Grape Seed Extract in Adhesion on Dentin Surfaces Conditioned with Er,Cr:YSGG Laser. Lasers Med. Sci. 2019;34:1493–1501. doi: 10.1007/s10103-019-02749-w. [DOI] [PubMed] [Google Scholar]
- 90.Scheffel D.L., Delgado C.C., Soares D.G., Basso F.G., de Souza Costa C.A., Pashley D.H., Hebling J. Increased Durability of Resin-Dentin Bonds Following Cross-Linking Treatment. Oper. Dent. 2015;40:533–539. doi: 10.2341/13-211-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sabatini C., Kim J.H., Alias P.O. In Vitro Evaluation of Benzalkonium Chloride in the Preservation of Adhesive Interfaces. Oper. Dent. 2014;39:283–290. doi: 10.2341/13-131-LR. [DOI] [PubMed] [Google Scholar]
- 92.Paulose N.E., Fawzy A.S. Effect of Grape Seed Extract on the Bond Strength and Durability of Resin-Dentin Interface. J. Adhes. Sci. Technol. 2017;31:2525–2541. doi: 10.1080/01694243.2017.1308304. [DOI] [Google Scholar]
- 93.Osorio R., Yamauti M., Osorio E., Ruiz-Requena M.E., Pashley D., Tay F., Toledano M. Effect of Dentin Etching and Chlorhexidine Application on Metalloproteinase-Mediated Collagen Degradation. Eur. J. Oral Sci. 2011;119:79–85. doi: 10.1111/j.1600-0722.2010.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Osorio R., Erhardt M.C., Pimenta L.A., Osorio E., Toledano M. EDTA Treatment Improves Resin-Dentin Bonds’ Resistance to Degradation. J. Dent. Res. 2005;84:736–740. doi: 10.1177/154405910508400810. [DOI] [PubMed] [Google Scholar]
- 95.Neri J.R., Yamauti M., da Silveira F.D., Mendonca J.S., de Carvalho R.M., Santiago S.L. Influence of Dentin Biomodification with Epigallocatechin-3-Gallate on the Bond Strength of Self-Etch Adhesive: Twelve-Month Results. Int. J. Adhes. Adhes. 2016;71:81–86. doi: 10.1016/j.ijadhadh.2016.08.007. [DOI] [Google Scholar]
- 96.Munksgaard E.C. Wet or Dry, Normal or Deproteinized Dentin Surfaces as Substrate for Dentin Adhesives. Acta Odontol. Scand. 2002;60:60–64. doi: 10.1080/000163502753472023. [DOI] [PubMed] [Google Scholar]
- 97.Mazzoni A., Angeloni V., Comba A., Maravic T., Cadenaro M., Tezvergil-Mutluay A., Pashley D.H., Tay F.R., Breschi L. Cross-Linking Effect on Dentin Bond Strength and MMPs Activity. Dent. Mater. 2018;34:288–295. doi: 10.1016/j.dental.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 98.Loguercio A.D., Hass V., Gutierrez M.F., Luque-Martinez I.V., Szezs A., Stanislawczuk R., Bandeca M.C., Reis A. Five-Year Effects of Chlorhexidine on the In Vitro Durability of Resin/Dentin Interfaces. J. Adhes. Dent. 2016;18:35–42. doi: 10.3290/j.jad.a35514. [DOI] [PubMed] [Google Scholar]
- 99.Lee J., Sabatini C. Glutaraldehyde Collagen Cross-Linking Stabilizes Resin-Dentin Interfaces and Reduces Bond Degradation. Eur. J. Oral Sci. 2017;125:63–71. doi: 10.1111/eos.12317. [DOI] [PubMed] [Google Scholar]
- 100.Kim D.S., Park S.H., Choi G.W., Choi K.K., Kim S.Y. Effect of EDTA Treatment on the Hybrid Layer Durability in Total-Etch Dentin Adhesives. Dent. Mater. J. 2011;30:717–722. doi: 10.4012/dmj.2011-056. [DOI] [PubMed] [Google Scholar]
- 101.Kasraei S., Mojtahedi M., Goodarzi M.T., Azarsina M., Khamverdi Z. The Effect of Dentin PreTreatment with Activated Riboflavin on the Bond Strength of a Two-Step Self-Etch Adhesive System. Dent. Med. Probl. 2019;56:143–148. doi: 10.17219/dmp/105408. [DOI] [PubMed] [Google Scholar]
- 102.Joseph V.M., Thomas M.S., Ginjupalli K., Kundabala M. Effect Of 3% Riboflavin on the Adhesion of Dental Composite Resin to Etched Dentin. Res. J. Pharm. Biol. Chem. Sci. 2016;7:1829–1834. [Google Scholar]
- 103.Islam M.S., Hiraishi N., Nassar M., Yiu C., Otsuki M., Tagami J. Effect of Hesperidin Incorporation into a Self-Etching Primer on Durability of Dentin Bond. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2014;30:1205–1212. doi: 10.1016/j.dental.2014.08.371. [DOI] [PubMed] [Google Scholar]
- 104.Islam S., Hiraishi N., Nassar M., Yiu C., Otsuki M., Tagami J. Effect of Natural Cross-Linkers Incorporation in a Self-Etching Primer on Dentine Bond Strength. J. Dent. 2012;40:1052–1059. doi: 10.1016/j.jdent.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 105.Al-Ammar A., Drummond J.L., Bedran-Russo A.K. The Use of Collagen Cross-Linking Agents to Enhance Dentin Bond Strength. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2009;91B:419–424. doi: 10.1002/jbm.b.31417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abunawareg M., Abuelenain D.A., Elkassas D., Abu Haimed T., Al-Dharrab A., Zidan A., Hassan A.H., Pashley D. Role of Dentin Cross-Linking Agents in Optimizing Dentin Bond Durability. Int. J. Adhes. Adhes. 2017;78:83–88. doi: 10.1016/j.ijadhadh.2017.06.009. [DOI] [Google Scholar]
- 107.Baena E., Cunha S.R., Maravic T., Comba A., Paganelli F., Alessandri-Bonetti G., Ceballos L., Tay F.R., Breschi L., Mazzoni A. Effect of Chitosan as a Cross-Linker on Matrix Metalloproteinase Activity and Bond Stability with Different Adhesive Systems. Mar. Drugs. 2020;18:263. doi: 10.3390/md18050263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baldion P.A., Cortes C.C., Castellanos J.E., Betancourt D.E. Effect of Myricetin on Odontoblast-like Cells and Its Potential to Preserve Resin & Ndash;Dentin Bonds. J. Mech. Behav. Biomed. Mater. 2021;117:104392. doi: 10.1016/j.jmbbm.2021.104392. [DOI] [PubMed] [Google Scholar]
- 109.Bedran-Russo A.K.B., Vidal C.M.P., Dos Santos P.H., Castellan C.S. Long-Term Effect of Carbodiimide on Dentin Matrix and Resin-Dentin Bonds. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2010;94B:250–255. doi: 10.1002/jbm.b.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bharti N., Chandra A., Tikku A.P., Verma P., Bharti R., Shakya V.K., Bains R. An Ex Vivo Evaluation of Effect of Dentin Pretreatment with Various Agents for Varying Time Intervals on the Shear Bond Strength of Resin. J. Conserv. Dent. 2018;21:37–41. doi: 10.4103/jcd.jcd_227_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bourgi R., Daood U., Bijle M.N. Reinforced Universal Adhesive by Ribose Crosslinker: A Novel Strategy in Adhesive Dentistry. Polymers. 2021;13:704. doi: 10.3390/polym13050704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Castellan C.S., Bedran-Russo A.K., Antunes A., Pereira P.N. Effect of Dentin Biomodification Using Naturally Derived Collagen Cross-Linkers: One-Year Bond Strength Study. Int. J. Dent. 2013;2013:918010. doi: 10.1155/2013/918010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chaharom M.E.E., Bahari M., Rahbar M., Golbaz S. Effect of Galardin and Its Solvents on the Microtensile Bond Strength of Different Adhesive Systems to Dentin. Pesqui. Bras. Odontopediatria Clin. Integr. 2019;19:e4438. doi: 10.4034/PBOCI.2019.191.12. [DOI] [Google Scholar]
- 114.Chiang Y.S., Chen Y.L., Chuang S.F., Wu C.M., Wei P.J., Han C.F., Lin J.C., Chang H.T. Riboflavin-Ultraviolet-A-Induced Collagen Cross-Linking Treatments in Improving Dentin Bonding. Dent. Mater. 2013;29:682–692. doi: 10.1016/j.dental.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 115.Comba A., Maravic T., Valente L., Girlando M., Cunha S.R., Checchi V., Salgarello S., Tay F.R., Scotti N., Breschi L., et al. Effect of Benzalkonium Chloride on Dentin Bond Strength and Endogenous Enzymatic Activity. J. Dent. 2019;85:25–32. doi: 10.1016/j.jdent.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 116.Cova A., Breschi L., Nato F., Ruggeri A., Carrilho M., Tjaderhane L., Prati C., Di Lenarda R., Tay F.R., Pashley D.H., et al. Effect of UVA-Activated Riboflavin on Dentin Bonding. J. Dent. Res. 2011;90:1439–1445. doi: 10.1177/0022034511423397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fawzy A.S., Nitisusanta L.I., Iqbal K., Daood U., Beng L.T., Neo J. Chitosan/Riboflavin-Modified Demineralized Dentin as a Potential Substrate for Bonding. J. Mech. Behav. Biomed. Mater. 2013;17:278–289. doi: 10.1016/j.jmbbm.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 118.Fawzy A.S., Nitisusanta L.I., Iqbal K., Daood U., Neo J. Riboflavin as a Dentin Crosslinking Agent: Ultraviolet A versus Blue Light. Dent. Mater. 2012;28:1284–1291. doi: 10.1016/j.dental.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 119.Gerhardt K.M.F., Oliveira C.A.R., Franca F.M.G., Basting R.T., Turssi C.P., Amaral F.L.B. Effect of Epigallocatechin Gallate, Green Tea Extract and Chlorhexidine Application on Long-Term Bond Strength of Self-Etch Adhesive to Dentin. Int. J. Adhes. Adhes. 2016;71:23–27. doi: 10.1016/j.ijadhadh.2016.08.005. [DOI] [Google Scholar]
- 120.Zhang Z., Beitzel D., Majd H., Mutluay M., Tezvergil-Mutluay A., Tay F.R., Pashley D.H., Arola D. Fatigue Resistance of Dentin Bonds Prepared with Two- vs. Three-Step Adhesives: Effect of Carbodiimide. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2017;33:1340–1350. doi: 10.1016/j.dental.2017.08.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Venkatachalam P., Varghese A.S., Suresh M., Mary N.S.G.P., Vivekanandan P., Subbiya A. Effect of Collagen Stabilizing Agents on the Shear Bond Strength to Dentin: An in Vitro Study. Int. Res. J. Pharm. 2019;10:127–130. doi: 10.7897/2230-8407.1005177. [DOI] [Google Scholar]
- 122.Sabatini C., Ortiz P.A., Pashley D.H. Preservation of Resin-Dentin Interfaces Treated with Benzalkonium Chloride Adhesive Blends. Eur. J. Oral Sci. 2015;123:108–115. doi: 10.1111/eos.12176. [DOI] [PubMed] [Google Scholar]
- 123.Nivedita L., Prakash V., Mitthra S., Mary N.S.G.P., Venkatesh A., Subbiya A. Evaluation of the Effect of Collagen Stabilizing Agents like Chitosan and Proanthocyanidin on the Shear Bond Strength to Dentin and Microleakage of Resin Composite at Enamel and Cemental Walls: An in Vitro Study. J. Conserv. Dent. JCD. 2019;22:483. doi: 10.4103/JCD.JCD_195_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fu C., Deng S., Koneski I., Awad M.M., Akram Z., Matinlinna J., Pichika M.R., Daood U., Fawzy A.S. Multiscale In-Vitro Analysis of Photo-Activated Riboflavin Incorporated in an Experimental Universal Adhesive. J. Mech. Behav. Biomed. Mater. 2020;112 doi: 10.1016/j.jmbbm.2020.104082. [DOI] [PubMed] [Google Scholar]
- 125.Li K., Sun Y., Tsoi J.K.H., Yiu C.K.Y. The Application of Mussel-Inspired Molecule in Dentin Bonding. J. Dent. 2020;99:103404. doi: 10.1016/j.jdent.2020.103404. [DOI] [PubMed] [Google Scholar]
- 126.Carrilho M., Carvalho R.M., De Goes M., Di Hipolito V., Geraldeli S., Tay F.R., Pashley D.H., Tjäderhane L. Chlorhexidine Preserves Dentin Bond in Vitro. J. Dent. Res. 2007;86:90–94. doi: 10.1177/154405910708600115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mazzoni A., Nascimento F.D., Carrilho M., Tersariol I., Papa V., Tjaderhane L., Di Lenarda R., Tay F.R., Pashley D.H., Breschi L. MMP Activity in the Hybrid Layer Detected with in Situ Zymography. J. Dent. Res. 2012;91:467–472. doi: 10.1177/0022034512439210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tjaderhane L., Nascimento F.D., Breschi L., Mazzoni A., Tersariol I.L.S., Geraldeli S., Tezvergil-Mutluay A., Carrilho M., Carvalho R.M., Tay F.R., et al. Strategies to Prevent Hydrolytic Degradation of the Hybrid Layer-A Review. Dent. Mater. 2013;29:999–1011. doi: 10.1016/j.dental.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Daood U., Yiu C., Burrow M.F., Niu L.-N., Tay F. Effect of a Novel Quaternary Ammonium Silane on Dentin Protease Activities. J. Dent. 2017;58:19–27. doi: 10.1016/j.jdent.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 130.Bafail A., Carrilho M.R., Kishen A., Prakki A. Effect of Protease Inhibitor Specificity on Dentin Matrix Properties. J. Mech. Behav. Biomed. Mater. 2020;109:103861. doi: 10.1016/j.jmbbm.2020.103861. [DOI] [PubMed] [Google Scholar]
- 131.de Moraes I.Q.S., do Nascimento T.G., da Silva A.T., de Lira L.M.S.S., Parolia A., de Moraes Porto I.C.C. Inhibition of Matrix Metalloproteinases: A Troubleshooting for Dentin Adhesion. Restor. Dent. Endod. 2020;45:e31. doi: 10.5395/rde.2020.45.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hardan L., Bourgi R., Kharouf N., Mancino D., Zarow M., Jakubowicz N., Haikel Y., Cuevas-Suárez C.E. Bond Strength of Universal Adhesives to Dentin: A Systematic Review and Meta-Analysis. Polymers. 2021;13:814. doi: 10.3390/polym13050814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Urbanczyk G., Lipp-Symonowicz B. The Influence of Processing Terms of Chitosan Membranes Made of Differently Deacetylated Chitin on the Crystalline Structure of Membranes. J. Appl. Polym. Sci. 1994;51:2191–2194. doi: 10.1002/app.1994.070511311. [DOI] [Google Scholar]
- 134.Kumar M.N.R. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000;46:1–27. doi: 10.1016/S1381-5148(00)00038-9. [DOI] [Google Scholar]
- 135.Gingras M., Paradis I., Berthod F. Nerve Regeneration in a Collagen–Chitosan Tissue-Engineered Skin Transplanted on Nude Mice. Biomaterials. 2003;24:1653–1661. doi: 10.1016/S0142-9612(02)00572-0. [DOI] [PubMed] [Google Scholar]
- 136.Taravel M., Domard A. Collagen and Its Interaction with Chitosan: II. Influence of the Physicochemical Characteristics of Collagen. Biomaterials. 1995;16:865–871. doi: 10.1016/0142-9612(95)94149-F. [DOI] [PubMed] [Google Scholar]
- 137.Diolosà M., Donati I., Turco G., Cadenaro M., Di Lenarda R., Breschi L., Paoletti S. Use of Methacrylate-Modified Chitosan to Increase the Durability of Dentine Bonding Systems. Biomacromolecules. 2014;15:4606–4613. doi: 10.1021/bm5014124. [DOI] [PubMed] [Google Scholar]
- 138.Elsaka S., Elnaghy A. Effect of Addition of Chitosan to Self-Etching Primer: Antibacterial Activity and Push-out Bond Strength to Radicular Dentin. J. Biomed. Res. 2012;26:288–294. doi: 10.7555/JBR.26.20120042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ferrazzano G.F., Amato I., Ingenito A., Zarrelli A., Pinto G., Pollio A. Plant Polyphenols and Their Anti-Cariogenic Properties: A Review. Molecules. 2011;16:1486–1507. doi: 10.3390/molecules16021486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Garbisa S., Biggin S., Cavallarin N., Sartor L., Benelli R., Albini A. Tumor Invasion: Molecular Shears Blunted by Green Tea. Nat. Med. 1999;5:1216–1216. doi: 10.1038/15145. [DOI] [PubMed] [Google Scholar]
- 141.Goo H.C., Hwang Y.-S., Choi Y.R., Cho H.N., Suh H. Development of Collagenase-Resistant Collagen and Its Interaction with Adult Human Dermal Fibroblasts. Biomaterials. 2003;24:5099–5113. doi: 10.1016/S0142-9612(03)00431-9. [DOI] [PubMed] [Google Scholar]
- 142.Tang H., Covington A.D., Hancock R. Structure–Activity Relationships in the Hydrophobic Interactions of Polyphenols with Cellulose and Collagen. Biopolym. Orig. Res. Biomol. 2003;70:403–413. doi: 10.1002/bip.10499. [DOI] [PubMed] [Google Scholar]
- 143.Santiago S.L., Osorio R., Neri J.R., Carvalho R.M., Toledano M. Effect of the Flavonoid Epigallocatechin-3-Gallate on Resin-Dentin Bond Strength. J. Adhes. Dent. 2013;15:535–540. doi: 10.3290/j.jad.a29532. [DOI] [PubMed] [Google Scholar]
- 144.Du X., Huang X., Huang C., Wang Y., Zhang Y. Epigallocatechin-3-Gallate (EGCG) Enhances the Therapeutic Activity of a Dental Adhesive. J. Dent. 2012;40:485–492. doi: 10.1016/j.jdent.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 145.Perdigao J., Reis A., Loguercio A.D. Dentin Adhesion and MMPs: A Comprehensive Review. J. Esthet. Restor. Dent. 2013;25:219–241. doi: 10.1111/jerd.12016. [DOI] [PubMed] [Google Scholar]
- 146.Cammarata C.R., Hughes M.E., Ofner C.M., III Carbodiimide Induced Cross-Linking, Ligand Addition, and Degradation in Gelatin. Mol. Pharm. 2015;12:783–793. doi: 10.1021/mp5006118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Thompson J.M., Agee K., Sidow S.J., McNally K., Lindsey K., Borke J., Elsalanty M., Tay F.R., Pashley D.H. Inhibition of Endogenous Dentin Matrix Metalloproteinases by Ethylenediaminetetraacetic Acid. J. Endod. 2012;38:62–65. doi: 10.1016/j.joen.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mohammadi Z., Shalavi S., Jafarzadeh H. Ethylenediaminetetraacetic Acid in Endodontics. Eur. J. Dent. 2013;7:S135–S142. doi: 10.4103/1305-7456.119091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sauro S., Mannocci F., Toledano M., Osorio R., Pashley D.H., Watson T.F. EDTA or H3PO4/NaOCl Dentine Treatments May Increase Hybrid Layers’ Resistance to Degradation: A Microtensile Bond Strength and Confocal-Micropermeability Study. J. Dent. 2009;37:279–288. doi: 10.1016/j.jdent.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 150.Luque-Martinez I.V., Muñoz M.A., Hass V., Sutil E., Reis A., Loguercio A.D. EDTA Conditioning Increases the Long-Term Microtensile Bond Strength to Sclerotic Dentin Mediated by Self-Etch Adhesives. J. Adhes. Dent. 2018;20:397–403. doi: 10.3290/j.jad.a41358. [DOI] [PubMed] [Google Scholar]
- 151.Macedo G., Yamauchi M., Bedran-Russo A. Effects of Chemical Cross-Linkers on Caries-Affected Dentin Bonding. J. Dent. Res. 2009;88:1096–1100. doi: 10.1177/0022034509351001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen C., Mao C., Sun J., Chen Y., Wang W., Pan H., Tang R., Gu X. Glutaraldehyde-Induced Remineralization Improves the Mechanical Properties and Biostability of Dentin Collagen. Mater. Sci. Eng. C. 2016;67:657–665. doi: 10.1016/j.msec.2016.05.076. [DOI] [PubMed] [Google Scholar]
- 153.Cai J., Palamara J.E.A., Burrow M.F. Effects of Collagen Crosslinkers on Dentine: A Literature Review. Calcif. Tissue Int. 2018;102:265–279. doi: 10.1007/s00223-017-0343-7. [DOI] [PubMed] [Google Scholar]
- 154.Sato K., Taguchi H., Maeda T., Minami H., Asada Y., Watanabe Y., Yoshikawa K. The Primary Cytotoxicity in Ultraviolet-a-Irradiated Riboflavin Solution Is Derived from Hydrogen Peroxide. J. Investig. Dermatol. 1995;105:608–612. doi: 10.1111/1523-1747.ep12323724. [DOI] [PubMed] [Google Scholar]
- 155.Balalaie A., Rezvani M.B., Basir M.M. Dual Function of Proanthocyanidins as Both MMP Inhibitor and Crosslinker in Dentin Biomodification: A Literature Review. Dent. Mater. J. 2018;37:173–182. doi: 10.4012/dmj.2017-062. [DOI] [PubMed] [Google Scholar]
- 156.Wang R., Li Y., Hass V., Peng Z., Wang Y. Methacrylate-Functionalized Proanthocyanidins as Novel Polymerizable Collagen Cross-Linkers—Part 2: Effects on Polymerization, Microhardness and Leaching of Adhesives. Dent. Mater. 2021 doi: 10.1016/j.dental.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Marya P., Handa M. Effect of proanthocyanidin and riboflavin on shear bond strength of a nanocomposite to deep dentin—An in vitro Study. J. Conserv. Dent. 2021;24:480–484. doi: 10.4103/jcd.jcd_126_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang Y., Liu Y., Liu H., Li S. Dual-Functionality Evaluation of a Novel Collagen Crosslinking Resin. J. Dent. Res. 2021;100:1251–1257. doi: 10.1177/00220345211007428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Scheffel D.L., Hebling J., Scheffel R.H., Agee K.A., Cadenaro M., Turco G., Breschi L., Mazzoni A., de Souza Costa C.A., Pashley D.H. Stabilization of Dentin Matrix after Cross-Linking Treatments, in Vitro. Dent. Mater. 2014;30:227–233. doi: 10.1016/j.dental.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ritter A.V., Swift E.J., Yamauchi M. Effects of Phosphoric Acid and Glutaraldehyde-HEMA on Dentin Collagen. Eur. J. Oral Sci. 2001;109:348–353. doi: 10.1034/j.1600-0722.2001.00088.x. [DOI] [PubMed] [Google Scholar]
- 161.Maravic T., Mancuso E., Comba A., Checchi V., Generali L., Mazzitelli C., Josic U., Hass V., Reis A., Loguercio A.D., et al. Dentin Cross-linking Effect of Carbodiimide After 5 Years. J. Dent. Res. 2021;100:1090–1098. doi: 10.1177/00220345211014799. [DOI] [PubMed] [Google Scholar]
- 162.Silva J.C., Cetira Filho E.L., Silva P.G.B., Costa F.W.G., Saboia V.P.A. Is dentin biomodification with collagen cross-linking agents effective for improving dentin adhesion? A systematic review and meta-analysis. Restor. Dent. Endod. 2022;47:23. doi: 10.5395/rde.2022.47.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the first author (L.H.) upon reasonable request.