Abstract
The Saccharomyces cerevisiae maltose transporter is a 12-transmembrane segment protein that under certain physiological conditions is degraded in the vacuole after internalization by endocytosis. Previous studies showed that endocytosis of this protein is dependent on the actin network, is independent of microtubules, and requires the binding of ubiquitin. In this work, we attempted to determine which coat proteins are involved in this endocytosis. Using mutants defective in the heavy chain of clathrin and in several subunits of the COPI and the COPII complexes, we found that clathrin, as well as two cytosolic subunits of COPII, Sec23p and Sec24p, could be involved in internalization of the yeast maltose transporter. The results also indicate that endocytosis of the maltose transporter and of the α-factor receptor could have different requirements.
Vesicles are responsible for the traffic of proteins in the cells. Formation of vesicles requires the action of coat proteins which are recruited from the cytosol onto a particular membrane to drive budding and to select the vesicle cargo (for reviews, see references 31 and 49). In Saccharomyces cerevisiae cells, three types of vesicles which differ in their function and coat composition have been identified. Clathrin-coated vesicles, formed from the plasma membrane and trans-Golgi network, are involved in endocytosis as well as in the secretion of proteins (for a review, see reference 43). COPI (or coatomer) is a large cytosolic protein complex which forms a coat around vesicles budding from the Golgi apparatus and endoplasmic reticulum (ER) (3). Its role has been a subject of controversy, but accumulated data suggest that COPI is involved in both anterograde and retrograde transport in the ER-Golgi system (for reviews, see references 8 and 42). Some components of COPI might also play a role in early endocytosis in animal cells (1, 16, 49). COPII is another cytosolic complex which directs the budding of vesicles from the ER and is involved in the anterograde transport of proteins to Golgi (for a review, see reference 24). To our knowledge, evidence for a role of COPII in endocytosis has not been reported.
Solutes, receptors, and damaged or unneeded plasma membrane proteins are internalized by endocytic vesicles. Two markers are generally used to investigate endocytosis in S. cerevisiae: Lucifer yellow CH as a nonspecific marker of the fluid phase endocytosis by which bulk solutes are internalized; and a- and α-factors as specific markers of the receptor-mediated endocytosis by which specific solutes are internalized when bound to specific receptors on the cell surface (12, 47). a- and α-factors bind to their 7-transmembrane segment (7-TMS) receptors and are internalized and degraded in the vacuole (10). These 7-TMS receptors also show a constitutive endocytosis, which becomes apparent in the absence of the corresponding pheromone. In recent years, a number of plasma membrane proteins with 12-transmembrane segments (12-TMS) have been shown to follow endocytosis. Several transporters which are internalized without binding of external ligand to be degraded in the vacuole belong to this group of proteins (13, 18, 23, 26, 30, 35, 36, 48). Among these transporters, the maltose transporter seems well suited as an endocytic marker of the 12-TMS proteins since it is quite abundant in yeast cells, shows a biochemical activity that can be easily measured, and is well characterized both biochemically (for a review, see reference 25) and genetically (7). Previous studies showed that endocytosis of the maltose transporter is partially dependent on the actin network, is independent of microtubules (32), and requires binding of ubiquitin, probably as a signal for endocytosis (28). We attempt here to establish which of the coat proteins is involved in endocytosis of this transporter. We investigated two steps of endocytosis as follows. Internalization was investigated by measuring the decrease in transport activity with radioactive maltose as well as the disappearance of the transporter from the plasma membrane with antibodies. Degradation was investigated by following the cellular content of the transporter with antibodies. Using strains deficient in the clathrin heavy chain, in the α-, β′-, and γ-COPI subunits, or in the COPII components Sec23p, Sec24p, Sec13p, Sec31p, and Sec16p, we conclude that clathrin and two components of COPII, Sec23p and Sec24p, might play a role in the internalization of the maltose transporter.
MATERIALS AND METHODS
Reagents.
d-[U-14C]maltose and enhanced chemiluminescence reagents were from Amersham International (Little Chalfont, United Kingdom). Goat anti-rabbit antibody-peroxidase conjugate was from Biosource International (Camarillo, Calif.). All other reagents were of analytical grade.
Yeast strains and growth conditions.
The genotypes of the strains are described in Table 1. All these strains were unable to grow on maltose and were transformed with the multicopy plasmid pRM1-1, which carries the MAL1 locus (39). The transformed cells, which grew and transported maltose at rates the same as those of mal+ wild-type strains, were grown at 24°C in a rotary shaker (200 rpm) in a medium containing 2% peptone, 1% yeast extract, 2% maltose, and 3 ppm of antimycin A to force utilization of maltose. Cell growth was monitored by measuring optical densities at 640 nm.
TABLE 1.
Strains used in this work
| S. cerevisiae strain (reference) | Genotype |
|---|---|
| GPY1100 (47) | MATα leu2-3,112 ura3-52 his4-519 trp1 can1 |
| GPY418 (47) | MATα leu2-3,112 ura3-52 his4-519 trp1 can1 chc1-521 |
| RSY255 (21) | MATα ura3,52 leu2-3,112 |
| RSY1196 (21) | MATa ura3-52 leu2-3,112 sec24-1 |
| RSY617 (21) | MATα ura3-52 leu2-3,112 sec16-1 |
| RSY277 (21) | MATα ura3-52 sec21-1 |
| RSY281 (21) | MATα ura3-52 his4-619 sec23-1 |
| RSY265 (21) | MATα ura3-52 his4-619 sec13-1 |
| RSY952 (21) | MATα ura3-52 leu2-3 sec31-1 |
| RH402-6B (26a) | MATα ura3 leu2 his4 lys2 trp1 ret1-1 |
| RSY146 (11) | MATα ura3-52 leu2-3 trp1 sec27-1 |
| RH448 (19) | MATa ura3 leu2 his4 lys2 bar1-1 |
| RH1436 (19) | MATa ura3 leu2 his4 sec23-1 bar1-1 |
Conditions for endocytosis of the maltose transporter and of the α-factor receptor.
Cells were harvested during exponential growth (about 0.7 mg [dry weight] per ml), washed, and suspended in 3 volumes of an ammonium-free medium as described previously (6) in the presence of 2% glucose and 250 μg of tetracycline chlorohydrate per ml to avoid bacterial contamination. The suspension was incubated at 24, 32, or 35°C in a rotary shaker (200 rpm). Samples of the suspensions were taken at various times, and endocytosis of the maltose transporter and of the α-factor receptor was measured.
Endocytosis of the maltose transporter.
To measure endocytosis of the maltose transporter, two steps were monitored, internalization and degradation. Internalization was measured by monitoring two parameters, the decrease in the rate of transport activity with radioactive maltose as previously described (35) and the disappearance of the transporter from the plasma membrane by immunoblotting purified plasma membrane preparations as previously described (29). In the latter case, the H+-ATPase was used as a marker protein of plasma membrane (45). Degradation of the maltose transporter was determined by immunoblotting crude extract preparations as previously described (29).
Endocytosis of the α-factor receptor.
Constitutive endocytosis of the α-factor receptor was monitored by measuring its rate of internalization in the absence of the pheromone. To this end, the disappearance of the receptor from the plasma membrane was monitored by measuring the binding of labeled α-factor by the procedure described in reference 40 with some modifications. Ten-milliliter aliquots of the yeast suspension in the ammonium-free medium (see above), containing about 5 × 107 cells, were incubated for various time periods at 24 or 32°C. Next, 0.5 ml of 200 mM NaN3 and 200 mM NaF, two inhibitors of the energy metabolism, was added to stop endocytosis. The cells were then harvested by centrifugation and suspended in 0.25 ml of the rich medium YPUAD previously described (51), in the presence of 10 mM NaN3 and 10 mM NaF. To 0.1 ml of this suspension, a 10−6 M final concentration of 35S-labeled α-factor, obtained and purified as described previously (12), was added. After incubation for 30 min at 30°C, 2 ml of the YPUAD medium in the presence of the inhibitors was added, and the cells were harvested by filtration through GF/C glass fiber filters (2.5 cm diameter), washed with 2 ml of the same medium, and counted for their radioactivity. To subtract the radioactivity due to unspecific binding of the labeled α-factor, controls were run in parallel. In this case, the cells were added to a 10−6 M final concentration of 35S-labeled α-factor plus 4 × 10−5 M unlabeled α-factor.
Crude extract preparation, plasma membrane purification, and immunoblotting.
Crude extract and crude membrane fraction were obtained as previously described (45). Plasma membrane purification was achieved by application of the crude membrane fraction to a discontinuous sucrose gradient as previously described (29). Samples of crude extract and purified plasma membrane preparations were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the maltose transporter and the H+-ATPase were detected by enhanced chemiluminescence by using the polyclonal antibodies anti-maltose transporter and anti-ATPase as previously described (29).
Protein measurements.
Protein was determined after precipitation with trichloroacetic acid by the method described previously (27).
RESULTS
Internalization and degradation of the maltose transporter in a clathrin-deficient mutant.
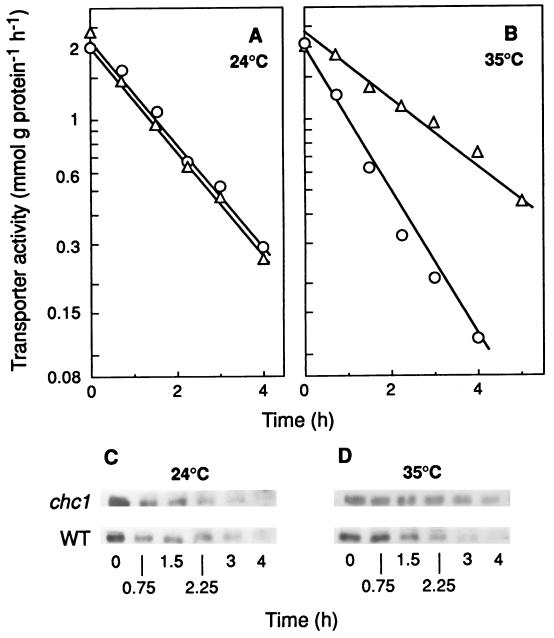
The clathrin coat consists of a basic building block, the triskelion, formed by three heavy chains and three light chains with different sizes (42), and of complexes of associated proteins, adaptors, which select cargo proteins by interacting with specific signals (43). A single gene, CHC1, codes for the clathrin heavy chain in S. cerevisiae, and a conditional mutant of this gene, chc1-525, which at the nonpermissive temperature of 35°C is immediately perturbed in clathrin secretory (44) and endocytic functions (47), has been isolated. We used this mutant, as well as its isogenic wild-type strain, to investigate if clathrin plays a role in endocytosis of the maltose transporter. Since the maltose transporter is internalized for proteolysis in the vacuole during nitrogen starvation in the presence of glucose (30, 33, 35), we triggered endocytosis of this protein by starving the cells of ammonium in the presence of this sugar. To monitor its internalization, we measured the decrease in transport activity (36) and found that, at 24°C, this activity decreased at a similar rate in mutant and wild-type cells (Fig. 1A) while, at 35°C, a reduction in this rate of about 50% was observed in the mutant compared with the wild-type cells (Fig. 1B). These results indicated that clathrin is required for a normal rate of internalization of the transporter. If this were the case, at 35°C, disappearance of the transporter from the plasma membrane and, consequently, degradation of this protein would occur at a lower rate in the mutant than in the wild-type cells, whereas at 24°C, differences between the two strains would not be observed.
FIG. 1.
Internalization and degradation of the maltose transporter in a mutant defective in clathrin. Strains GPY1100 (CHC1, wild type [WT]; ○) and GPY 418 (chc1-1; ▵), transformed with the plasmid pRM1-1 carrying the MAL1 locus, were harvested during exponential growth at 24°C, washed, and suspended in 3 volumes of the endocytosis medium. After incubation at 24°C (A and C) or 35°C (B and D) for the indicated times, the cells were harvested and assayed for maltose transporter activity (A and B). The maltose transporter band was detected by immunoblotting aliquots, containing 40 μg of protein of cellular extracts obtained at the indicated times (C and D).
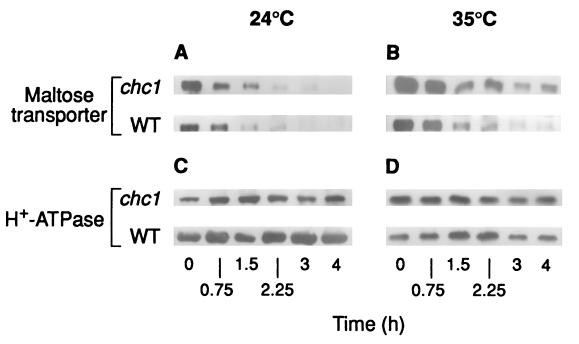
To check these predictions, degradation (Fig. 1) and disappearance of the transporter from the plasma membrane (Fig. 2) were monitored by immunoblotting crude extracts and plasma membrane preparations, respectively. The results showed that, in both cases, the intensity of the band corresponding to the transporter decreased at 35°C, at a lower rate in mutant cells than in wild-type cells (Fig. 1D and 2B) while at 24°C, no differences were observed (Fig. 1C and 2A). In the experiments with plasma membrane, the H+-ATPase was used as a marker protein (45). It has been shown that under the conditions used in this work, the H+-ATPase remains stable (4, 29) and, in accordance with this, we found that the intensity of the band corresponding to this protein remained constant (Fig. 2C and D).
FIG. 2.
Disappearance of the maltose transporter from the plasma membrane in a mutant defective in clathrin. Strains GPY1100 (CHC1, wild type [WT]) and GPY418 (chc1-1), transformed with the plasmid pRM1-1 carrying the MAL1 locus, were grown and treated as described in the legend for Fig. 1. After incubation at 24°C (A and C) or 35°C (B and D) maltose transporter (A and B) and H+-ATPase (C and D) were detected by immunoblotting aliquots containing 7 and 3 μg of protein, respectively, of purified plasma membrane preparations obtained at the indicated times.
These results indicate that clathrin-coated vesicles could play a role in internalization of the maltose transporter, accounting for about 50% of endocytosis of the transporter. This partial contribution of clathrin to endocytosis suggests that clathrin is not the sole mediator of plasma membrane vesiculation and that another protein(s) can perform the complementing function. The components of the two other known coat complexes, COPI and COPII, seem to be good candidates to provide this function.
Internalization and degradation of the transporter in mutants deficient in COPI components.
COPI is a protein complex consisting of seven subunits, α, β, β′, γ, δ, ɛ, and ζ, which are found in the cytosol and on the cytoplasmic side of the Golgi compartment and which are assembled to form coated vesicles by the action of the small GTP-binding protein ARF. Although the biochemical description of COPI and its association with membranes in vitro is very detailed, its precise role in living cells is not well defined. COPI-coated vesicles seem responsible for steps in both anterograde and retrograde transport in the ER-Golgi system (42) and certain subunits of COPI might play a role in endocytosis in animal cells (1, 16, 49).
In S. cerevisiae, α-, β′-, and γ-COP are the products of the RET1, SEC27, and SEC21 genes, respectively. Temperature-sensitive mutants in these genes have been isolated which, at the nonpermissive temperature of 35°C, show a severe defect in protein transport from the ER and accumulation of ER membranes (11, 15, 20, 46). We used these mutants, as well as their isogenic wild-type strain, to investigate whether α-, β′-, and γ-COPI are involved in endocytosis of the maltose transporter. Cells growing exponentially at 24°C were suspended in the ammonium-free medium to trigger endocytosis and were separated into two aliquots that were incubated at 24 and 35°C. We found that internalization and degradation of the transporter occurred at similar rates at the two temperatures independent of the presence of a mutation in COPI genes (results not shown), thus indicating that α-, β′-, and γ-COP do not play a role in endocytosis of the 12-TMS maltose transporter.
Internalization and degradation of the transporter in mutants deficient in COPII components.
The COPII coat consists of three elements: one small GTP-binding protein, Sar1p, and two coat complexes, Sec23/24p and Sec13/31p, each one consisting of two subunits. Formation of COPII-coated vesicles (for reviews, see references 24 and 41) begins with recruitment from the cytosol of Sar1p to the ER membrane where Sar1p exchanges GDP for GTP by the action of an integral membrane glycoprotein, Sec12p (2). Then, the Sec23/24p complex binds to Sarp1-GTP. Finally, Sec23p stimulates the GTPase activity of Sarp1 and the Sec13/31p complex binds to initiate budding (50). An additional protein, Sec16p, is also essential for budding of COPII-coated vesicles, although it may not contribute directly to vesicle morphogenesis. This protein interacts with Sec23p and Sec24p and appears to act on the GTPase cycle (14). In contrast to the other COPII components mentioned above, Sec16p cannot be readily removed but remains firmly associated with ER membranes (14). COPII-coated vesicles are involved in sorting of proteins from ER and intra-Golgi apparatus (for a review, see reference 24).
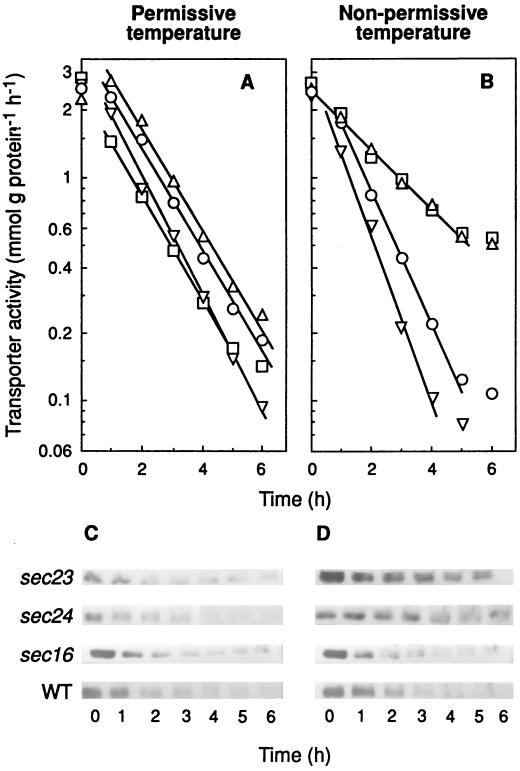
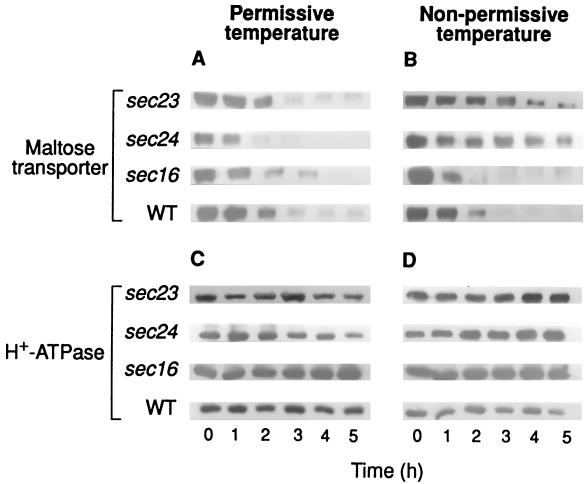
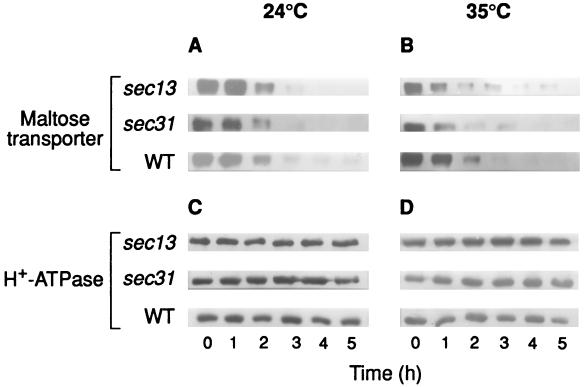
To investigate if some element(s) of COPII could play a role in endocytosis of the maltose transporter, we used temperature-sensitive mutants deficient in Sec23p, Sec24p, Sec13p, and Sec31p which, when exposed to the nonpermissive temperature, exhibit defective secretory functions and an excess of ER-like membrane structures (21). The results showed that sec23 and sec24 mutant cells exhibited, at 32 and 35°C, respectively, a reduced rate of internalization when this parameter was followed by either measuring the decrease in transport activity (Fig. 3B) or the disappearance of the transporter from the plasma membrane (Fig. 4B). In addition, and as expected from this reduction of internalization, a reduction in the rate of degradation of the transporter at the nonpermissive temperature was also observed (Fig. 3D). As in previous experiments, the H+-ATPase was used as a marker protein of plasma membrane and, in accordance with its known stability, a constant amount of this protein was detected (Fig. 4C and D). Data shown in Fig. 3A and B indicated half-life values for the transporter at 24°C of about 1 h in all strains, whereas at the nonpermissive temperatures tested, values of about 1 h in wild-type and 2.5 h in sec23 and sec24 strains were calculated.
FIG. 3.
Internalization and degradation of the maltose transporter in mutants defective in Sec23p, Sec24p, and Sec16p. Strains RSY255 (wild type [WT]; ○), RSY617 (sec16-1; ▿), RSY1196 (sec24-1; □), and RSY218 (sec23-1; ▵), transformed with the plasmid pRM1-1 carrying the MAL1 locus, were grown and treated as described in the legend for Fig. 1. After incubation at 24°C (A and C) or 35°C (except for RSY617, which was incubated at 32°C) (B and D) for the indicated times, the cells were harvested and assayed for maltose transporter activity (A and B). The maltose transporter band was detected by immunoblotting aliquots containing 40 μg of protein of cellular extracts obtained at the indicated times (C and D).
FIG. 4.
Disappearance of the maltose transporter from the plasma membrane in mutants defective in Sec23p, Sec24p, and Sec16p. Strains RSY255 (wild type [WT]), RSY617 (sec16-1), RSY1196 (sec24-1), and RSY218 (sec23-1), transformed with the plasmid pRM1-1 carrying the MAL1 locus, were grown and treated as described in the legend for Fig. 1. After incubation at 24°C (A and C) or 35°C (except for RSY617, which was incubated at 32°C) (B and D), maltose transporter (A and B) and H+-ATPase (C and D) were detected by immunoblotting aliquots containing 7 and 3 μg of protein, respectively, of purified plasma membrane preparations obtained at the indicated times.
These results suggest that a normal internalization of the maltose transporter requires the action of the two cytosolic proteins Sec23 and Sec24. But there is another possible explanation for the results: some sec mutants show inhibition of the secretory pathway even during growth at the permissive temperature (31). If sec23 and sec24 mutants show this inhibition, the transporter could accumulate into the cells during growth at 24°C and, in this case, the accumulated transporter could be secreted to the plasma membrane during the endocytosis experiments. As a consequence, two processes could take place during these experiments: arrival of the transporter at the plasma membrane by secretion of the accumulated transporter and internalization of the transporter by endocytosis. The simultaneous occurrence of these two opposite processes would result in an apparent decrease in the rate of endocytosis. However, this possibility seems very unlikely, since the cells used to investigate endocytosis at both the permissive and the nonpermissive temperature came from aliquots of the same culture (see legends to Fig. 3 and 4) and should have a similar accumulation of the transporter, if any. Therefore, if the decrease in endocytosis was due to this accumulation, it would appear at both temperatures and not only at the nonpermissive one as observed.
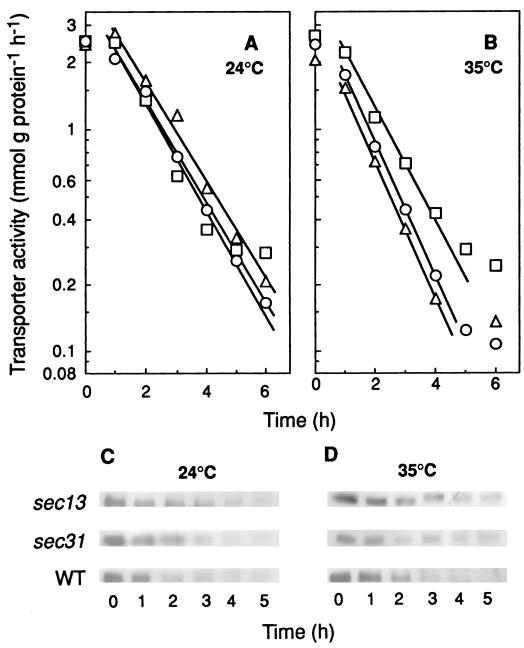
Experiments carried out with the temperature-sensitive mutants sec13 and sec31 (Fig. 5 and 6) indicated that neither of the two subunits of the Sec13/31p COPII complex is required for the internalization. In addition, the results shown in Fig. 3 and 4 obtained by using a temperature-sensitive sec16 mutant strain (21) demonstrated that Sec16p is not required for the process.
FIG. 5.
Internalization and degradation of the maltose transporter in mutants defective in Sec31p and Sec13p. Strains RSY255 (wild type [WT]; ○), RSY952 (sec31-1; ▵), and RSY265 (sec13-1; □), transformed with the plasmid pRM1-1 carrying the MAL1 locus, were grown and treated as described in the legend for Fig. 1. After incubation at 24°C (A and C) or 35°C (B and D) for the indicated times, the cells were harvested and assayed for maltose transporter activity (A and B). The maltose transporter band was detected by immunoblotting aliquots of cellular extracts containing 40 μg of protein that were obtained at the indicated times (C and D).
FIG. 6.
Disappearance of the maltose transporter from the plasma membrane in mutants defective in Sec31p and Sec13p. Strains RSY255 (wild type [WT]), RSY952 (sec31-1), and RSY265 (sec13-1), transformed with the plasmid pRM1-1 carrying the MAL1 locus, were grown and treated as described in the legend for Fig. 1. After incubation at 24°C (A and C) or 35°C (B and D) maltose transporter (A and B) and H+-ATPase (C and D) were detected by immunoblotting aliquots containing 7 and 3 μg of protein, respectively, of purified plasma membrane preparations obtained at the indicated times.
Internalization of the α-factor receptor in a mutant deficient in Sec23p.
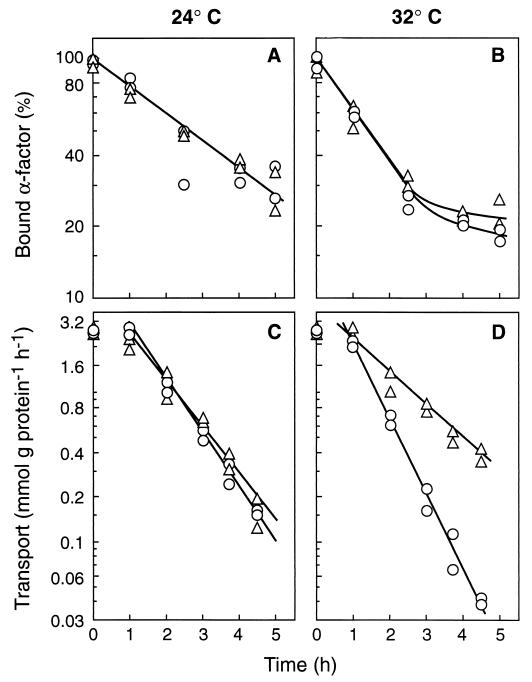
Previous results reported by Hicke et al. (19) suggested that a normal internalization of the α-factor receptor does not require the presence of a functional Sec23p. These results contrast with ours regarding the maltose transporter and suggest that endocytosis of these two proteins could show different requirements. However, this apparent difference might not be real but rather might be due to differences in the experimental conditions: whereas in the measurements of the receptor endocytosis, cells were preincubated in complete medium for 5 min at the nonpermissive temperature (19), in the measurements of the transporter endocytosis, the preincubation was performed in ammonium-free medium and lasted for several hours (Fig. 3 and 4). Therefore, to be able to compare endocytosis of the two proteins, we investigated the internalization of the α-factor receptor by using experimental conditions the same as those used with the transporter and by using wild-type and sec23 mutant strains with the appropriate genotype, MATa bar1-1 (19). We found that the response of the receptor internalization to a shift from 24 to 32°C was similar in the wild-type and the mutant strains (Fig. 7A and B) while in parallel experiments, and in agreement with data of Fig. 3 and 4, a different response was observed in the two strains in the case of the transporter (Fig. 7C and D). These results confirmed previous findings indicating that a normal internalization of the α-factor receptor does not require a functional Sec23p (19), and they suggest that, in fact, endocytosis of this 7-TMS receptor and of the 12-TMS maltose transporter could show different requirements.
FIG. 7.
Internalization of the α-factor receptor and of the maltose transporter in a mutant deficient in Sec23p. Strains RH448 (wild type; ○) and RH1416 (sec23-1; ▵), transformed with the plasmid pRM1-1 carrying the MAL1 locus, were grown and treated as described in the legend for Fig. 1. After incubation at 24 or 32°C for the indicated times, the cells were harvested and assayed for internalization of the α-factor receptor with 35S-labeled α-factor (A and B). Internalization of the maltose transporter was monitored in parallel experiments by measuring the decrease in transport activity (C and D). Data of two experiments are shown.
DISCUSSION
The basic block of the clathrin coat is the triskelion, and the clathrin heavy chain is one of the two components of this basic block (43). The available evidence suggests that a deficiency in the clathrin heavy chain in yeast produces only a partial decrease in endocytosis of the 12-TMS maltose transporter (this work) and of the 7-TMS a- and α-factor receptors (47). Since in all instances examined in detail, a coat is used as a mechanical device to bud off vesicles (38), this partial contribution of clathrin to endocytosis suggests that, in addition to clathrin, another coat protein(s) could contribute to endocytosis in this organism. A similar conclusion has been reached with regard to animal cells in which β-COP was found in endosomes (1), microinjection of anti-β-COP antibodies inhibited the entry of viruses by endocytosis (49), and a defect of ɛ-COP was accompanied by a defect in endocytosis (16). In the case of yeast, the results indicate that α-, β′-, and γ-COPI are involved neither in endocytosis of the maltose transporter (this work) nor in endocytosis of the α-factor receptor (19), but the possibility that another COPI subunit(s) plays a role in endocytosis of these proteins cannot be ruled out.
The results obtained with mutants deficient in Sec23p and Sec24p suggest that a normal internalization of the maltose transporter could require the action of these two subunits of the COPII complex. The possibility that these two cytosolic coat proteins play a direct role in the formation of endocytic vesicles containing the transporter in yeast cells seems very atractive. These two proteins, independent of the other COPII components, might be recruited from the cytosol onto the plasma membrane to facilitate invagination. But an indirect effect of these proteins in internalization of the transporter cannot be excluded.
A variety of proteins involved in early and late secretory pathway have been investigated for their role in α-factor endocytosis (19). Apparently, some of these proteins, i.e., Sec12, Sec13, Sec16, Sec17, Sec20, Sec23, Sec7, and Sec14, were required for a normal degradation of the α-factor in the vacuole through their effect in traffic from early to late endosomes. But none of these proteins was found to be required for internalization of the α-factor receptor (19). This observation, which in the case of Sec23p was confirmed in our experimental conditions, contrasts with the results obtained with the maltose transporter, which suggests a role of Sec23p and Sec24p in the internalization of the transporter. In our experiments with the transporter (this work) and in those with the α-factor receptor (this work and reference 19), the same mutant allele of the SEC23 gene (sec23-1) was used. Therefore, whether Sec23p plays a direct or an indirect role in internalization, what seems clear is that a mutation in gene SEC23 differentially affects internalization of the transporter and of the α-factor receptor. Interestingly, a mutation in another gene has also been found to differentially affect the internalization of these two proteins: sec18-1 cells showed a normal internalization of the pheromone (37) whereas these cells were severely affected in internalization of the transporter (35). There is not a simple explanation for this different response to sec mutations of plasma membrane proteins. In this regard, one interesting question which has not yet received a clear answer is whether vesicles formed at a given cellular compartment are all the same or whether different types of vesicles exist (for a review, see reference 22). In yeast cells, evidence for at least two classes of secretory vesicles has been reported (17). These two classes of vesicles, although they use a common fusion machinery, show different cargo proteins and different coat composition, as suggested by their different densities (17). Regarding endocytosis, there is not evidence for the occurrence of distinct endocytic vesicles in yeast, but in animal cells four different classes of these vesicles which seem to differ in size, coat composition, cargo proteins, and sensitivity to a variety of inhibitors have been detected (5). The relative contribution of these vesicles to endocytosis may vary between cells and change in response to stimuli (5). Therefore, one possibility is that, in yeast, the different response of plasma membrane proteins to factors involved in protein traffic could be due to the occurrence of different classes of endocytic vesicles, the formation of which could respond to different stimuli. Consistent with this possibility is the observation that the carboxyl terminus of the a-factor receptor is required for constitutive but not for ligand-stimulated endocytosis (9), indicating that the two processes involve different parts of the receptor protein and, probably, different components of the endocytic machinery.
In conclusion, the results shown in this work indicate that clathrin and two cytosolic components of the COPII complex, Sec23p and Sec24p, could play a role in internalization of the yeast maltose transporter and that internalization of this 12-TMS transporter and of the 7-TMS α-factor receptor could have different requirements.
ACKNOWLEDGMENTS
The experiments with the α-factor receptor were performed in the laboratory of H. Riezman in Basel, Switzerland. We are indebted to H. Riezman for this, and also for his generous help and advice during the performance of these experiments. We are also very grateful to M. Herweijer and R. Serrano for the gift of the polyclonal antibodies against the maltose transporter and the plasma membrane ATPase, to M. J. LaFuente for her generous help and suggestions, to A. Fernández and J. Pérez for help in the preparation of the figures, and to J. M. Gancedo and M. J. Mazón for critical readings of the manuscript.
This work was supported by the Spanish Dirección General Científica y Técnica (PB97-1213-CO2-01) and by the European Commission (BIO4-CT95-01).
REFERENCES
- 1.Aniento F, Gu F, Parton R G, Gruenberg J. β-COP is present on endosomes and enables the formation of vesicles that ensure transport from early to late endosomes. J Cell Biol. 1996;133:29–41. doi: 10.1083/jcb.133.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barlowe C, Schekman R. SEC12 encodes a guanine nucleotide exchange factor essential for transport vesicle budding from the ER. Nature. 1993;365:347–349. doi: 10.1038/365347a0. [DOI] [PubMed] [Google Scholar]
- 3.Bednarek S Y, Ravazzola M, Hosobuchi M, Amherdt M, Perrelet A, Schekman R, Orci L. COPI- and COPII-coated vesicles bud directly from the endoplasmic reticulum in yeast. Cell. 1995;83:1183–1196. doi: 10.1016/0092-8674(95)90144-2. [DOI] [PubMed] [Google Scholar]
- 4.Benito B, Moreno E, Lagunas R. Half-life of the plasma membrane ATPase and its activating system in resting yeast cells. Biochim Biophys Acta. 1991;1063:265–268. doi: 10.1016/0005-2736(91)90381-h. [DOI] [PubMed] [Google Scholar]
- 5.Bishop N E. An update on non-clathrin-coated endocytosis. Rev Med Virol. 1997;7:199–209. doi: 10.1002/(sici)1099-1654(199712)7:4<199::aid-rmv203>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 6.Busturia A, Lagunas R. Catabolite inactivation of the glucose transport system in Saccharomyces cerevisiae. J Gen Microbiol. 1986;132:379–385. doi: 10.1099/00221287-132-2-379. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Q, Michels C A. The maltose permease encoded by MAL61 gene of Saccharomyces cerevisiae exhibits both sequence and structural homology to the sugar transporters. Genetics. 1989;123:477–484. doi: 10.1093/genetics/123.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosson P, Letourneur F. Coatomer (COPI)-coated vesicles: role in intracellular transport and protein sorting. Curr Opin Cell Biol. 1997;9:484–487. doi: 10.1016/s0955-0674(97)80023-3. [DOI] [PubMed] [Google Scholar]
- 9.Davis N G, Horecka J L, Sprague G F. cis- and trans-acting functions required for endocytosis of the yeast pheromone receptors. J Cell Biol. 1993;122:53–65. doi: 10.1083/jcb.122.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dohlman H G, Throner J, Caron M G, Lefkowitz R J. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- 11.Duden R, Hosobuchi M, Hamamoto S, Winey S, Byers B, Schekman R. Yeast β- and β′-coat proteins (COP). Two coatomer subunits essential for endoplasmic reticulum-to-Golgi protein traffic. J Biol Chem. 1994;269:24486–24495. [PubMed] [Google Scholar]
- 12.Dulic V, Egerton M, Elguindi I, Raths S, Singer B, Riezman H. Yeast endocytosis assays. Methods Enzymol. 1991;194:697–710. doi: 10.1016/0076-6879(91)94051-d. [DOI] [PubMed] [Google Scholar]
- 13.Egner R, Mahé Y, Pandjaitan R, Kuchler K. Endocytosis and vacuolar degradation of the plasma membrane-localized Pdr5 ATP-binding cassette multidrug transporter in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:5789–5887. doi: 10.1128/mcb.15.11.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espenshade P, Gimeno R E, Holzmacher E, Teung P, Kaiser C A. Yeast SEC16 gene encodes a multidomain vesicle coat protein that interacts with Sec 23p. J Cell Biol. 1995;131:311–324. doi: 10.1083/jcb.131.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerich B, Orci L, Tschochner H, Lottspeich F, Ravazzola M, Amherdt M, Wieland F, Harter C. Non-clathrin-coat protein α-COPI is a conserved subunit of coatomer and in Saccharomyces cerevisiae is essential for growth. Proc Natl Acad Sci USA. 1995;92:3229–3233. doi: 10.1073/pnas.92.8.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Q, Vasile E, Krieger M. Disruptions in Golgi structure and membrane traffic in a conditional lethal mammalian cell mutant are corrected by ɛ-COP. J Cell Biol. 1994;125:1213–1224. doi: 10.1083/jcb.125.6.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harsay E, Bretscher A. Parallel secretory pathways to the cell surface in yeast. J Cell Biol. 1995;131:297–305. doi: 10.1083/jcb.131.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hein C, Springael J Y, Volland C, Haguenauer-Tsapis R, André B. NPI1, an essential yeast gene involved in induced degradation of Gap1 and Fur4 permeases, encodes Rsp5 ubiquitin-protein ligase. Mol Microbiol. 1995;18:77–87. doi: 10.1111/j.1365-2958.1995.mmi_18010077.x. [DOI] [PubMed] [Google Scholar]
- 19.Hicke L, Zanolari B, Pypaert M, Rohrer J, Riezman H. Transport through the yeast endocytic pathway occurs through morphologically distinct compartments and requires an active secretory pathway and Sec18p/N-ethylmaleimide-sensitive fusion protein. Mol Biol Cell. 1997;8:13–31. doi: 10.1091/mbc.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosobuchi M, Kreis T, Schekman R. SEC21 is a gene required for ER to Golgi protein transport that encodes a subunit of a yeast coatomer. Nature. 1992;360:603–605. doi: 10.1038/360603a0. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser C, Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990;61:723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser C A, Gimeno R E, Shaywitz D A. Protein secretion, membrane biogenesis, and endocytosis. In: Pringel J R, Broach J R, Jones W, editors. Molecular and cellular biology of the yeast Saccharomyces. Vol. 3. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 91–227. [Google Scholar]
- 23.Kölling R, Hollenberg C P. The ABC-transporter Ste6 accumulates in the plasma membrane in a ubiquitinated form in endocytosis mutants. EMBO J. 1994;13:3261–3271. doi: 10.1002/j.1460-2075.1994.tb06627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuehn M J, Schekman R. COPII and secretory cargo capture into transport vesicles. Curr Opin Cell Biol. 1997;8:477–483. doi: 10.1016/s0955-0674(97)80022-1. [DOI] [PubMed] [Google Scholar]
- 25.Lagunas R. Sugar transport in Saccharomyces cerevisiae. FEMS Microbiol Rev. 1993;104:229–242. doi: 10.1016/0378-1097(93)90598-v. [DOI] [PubMed] [Google Scholar]
- 26.Lai K, Bolognese C P, Swift S, McGraw P. Regulation of inositol transport in Saccharomyces cerevisiae involves inositol-induced changes in permease stability and endocytic degradation. J Biol Chem. 1995;270:2525–2534. doi: 10.1074/jbc.270.6.2525. [DOI] [PubMed] [Google Scholar]
- 26a.Letourneur F, Gaynor E C, Hennecke S, Demollière C, Duden R, Emr S D, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 27.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 28.Lucero P, Lagunas R. Catabolite inactivation of the yeast maltose transporter requires ubiquitin-ligase npi1/rsp5 and ubiquitin-hydrolase npi2/doa4. FEMS Microbiol Lett. 1997;147:273–277. doi: 10.1111/j.1574-6968.1997.tb10253.x. [DOI] [PubMed] [Google Scholar]
- 29.Lucero P, Peñalver E, Moreno E, Lagunas R. Moderate concentrations of ethanol inhibit endocytosis of the yeast maltose transporter. Appl Environ Microbiol. 1997;63:3831–3836. doi: 10.1128/aem.63.10.3831-3836.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medintz I, Jiang H, Han E K, Cui W, Michels C A. Characterization of the glucose-induced inactivation of maltose permease in Saccharomyces cerevisiae. J Bacteriol. 1996;178:2245–2254. doi: 10.1128/jb.178.8.2245-2254.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novick P, Ferro S, Schekman R. Order of events in the yeast secretory pathway. Cell. 1980;25:461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- 32.Peñalver E, Ojeda L, Moreno E, Lagunas R. Role of the cytoskeleton in endocytosis of the yeast maltose transporter. Yeast. 1997;13:541–549. doi: 10.1002/(SICI)1097-0061(199705)13:6<541::AID-YEA112>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 33.Peñalver E, Lucero P, Moreno E, Lagunas R. Catabolite inactivation of the maltose transporter in nitrogen starved cells could be due to the stimulation of the general protein turnover. FEMS Microbiol Lett. 1998;166:317–324. doi: 10.1111/j.1574-6968.1998.tb13907.x. [DOI] [PubMed] [Google Scholar]
- 34.Raths S, Rohrer J, Crausaz F, Riezman H. end3 and end4: two mutants defective in receptor-mediated endocytosis in Saccharomyces cerevisiae. J Cell Biol. 1993;120:55–65. doi: 10.1083/jcb.120.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riballo E, Herweijer M, Wolf D H, Lagunas R. Catabolite inactivation of the yeast maltose transporter occurs in the vacuole after internalization by endocytosis. J Bacteriol. 1995;177:5622–5627. doi: 10.1128/jb.177.19.5622-5627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riballo E, Lagunas R. Involvement of endocytosis in catabolite inactivation of the K+ and glucose transport systems in Saccharomyces cerevisiae. FEMS Microbiol Lett. 1994;121:77–80. doi: 10.1111/j.1574-6968.1994.tb07078.x. [DOI] [PubMed] [Google Scholar]
- 37.Riezman H. Yeast endocytosis. Trends Cell Biol. 1993;3:273–277. doi: 10.1016/0962-8924(93)90056-7. [DOI] [PubMed] [Google Scholar]
- 38.Robinson M S. Coats and vesicle budding. Trends Cell Biol. 1997;7:99–102. doi: 10.1016/S0962-8924(96)10048-9. [DOI] [PubMed] [Google Scholar]
- 39.Rodicio R. Insertion of non-homologous DNA sequences into a regulatory gene causes a constitutive maltase synthesis in yeast. Curr Genet. 1986;11:235–241. doi: 10.1007/BF00420612. [DOI] [PubMed] [Google Scholar]
- 40.Rohrer J, Bénédetti H, Zanolari B, Riezman H. Identification of a novel sequence mediating regulated endocytosis of the G protein-coupled α-pheromone receptor in yeast. Mol Biol Cell. 1993;4:511–521. doi: 10.1091/mbc.4.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rothman J E, Orci L. Molecular dissection of the secretory pathway. Nature. 1992;355:409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- 42.Rothman J E, Wieland F T. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- 43.Schmid S L. Clathrin-coated vesicle formation and protein sorting. Annu Rev Biochem. 1997;65:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 44.Seeger M, Payne G S. A role for clathrin in the sorting of vacuolar proteins in the Golgi complex of yeast. EMBO J. 1992;11:2811–2818. doi: 10.1002/j.1460-2075.1992.tb05348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serrano R. H+-ATPase from plasma membrane of Saccharomyces cerevisiae and Avena sativa roots. Purification and reconstitution. Methods Enzymol. 1988;157:533–544. doi: 10.1016/0076-6879(88)57102-1. [DOI] [PubMed] [Google Scholar]
- 46.Stenbeck G, Schreiner R, Herrmann D, Aubarch S, Lottspeich F, Rothman J E, Wieland F T. γ-COP, a coat subunit of non-clathrin-coated vesicles with homology to sec21p. FEBS Lett. 1992;314:195–198. doi: 10.1016/0014-5793(92)80973-k. [DOI] [PubMed] [Google Scholar]
- 47.Tan P K, Davis N G, Sprague G F, Payne G S. Clathrin facilitates the internalization of seven transmembrane segment receptors for mating pheromones in yeast. J Cell Biol. 1993;123:1707–1716. doi: 10.1083/jcb.123.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volland C, Urban-Grimal D, Géraud G, Haguenauer-Tsapis R. Endocytosis and degradation of the yeast uracil permease under adverse conditions. J Biol Chem. 1994;269:9833–9841. [PubMed] [Google Scholar]
- 49.Whitney J A, Gomez M, Sheff D, Kreis T E, Mellman I. Cytoplasmic coat proteins involved in endosome function. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 50.Yoshihisa T, Barlowe C, Schekman R. Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science. 1993;259:1466–1468. doi: 10.1126/science.8451644. [DOI] [PubMed] [Google Scholar]
- 51.Zanolari B, Raths S, Singer-Krüger B, Riezman H. Yeast pheromone receptor endocytosis and hyperphosphorylation are independent of G protein-mediated signal transduction. Cell. 1992;71:755–763. doi: 10.1016/0092-8674(92)90552-n. [DOI] [PubMed] [Google Scholar]