Abstract
Dendritic cells (DC) and leukaemia derived DC (DCleu) are potent stimulators of anti-leukaemic activity in acute myeloid leukaemia (AML) and can be generated from mononuclear cells in vitro following standard DC/DCleu-generating protocols. With respect to future clinical applications though, DC/DCleu-generating protocols specifically designed for application in a whole-blood-(WB)-environment must be established. Therefore, we developed ten new DC/DCleu-generating protocols (kits; Kit-A/-C/-D/-E/-F/-G/-H/-I/-K/-M) for the generation of DC/DCleu from leukaemic WB, containing calcium-ionophore, granulocyte-macrophage-colony-stimulating-factor (GM-CSF), tumour-necrosis-factor-alpha, prostaglandin-E1 (PGE1), prostaglandin-E2 (PGE2) and/or picibanil (OK-432). All protocols were evaluated regarding their performance in generating DC/DCleu using refined classification and/or ranking systems; DC/DCleu were evaluated regarding their performance in stimulating anti-leukaemic activity using a cytotoxicity fluorolysis assay. Overall, we found the new kits capable to generate (mature) DC/DCleu from leukaemic WB. Through refined classification and ranking systems, we were able to select Kit-I (GM-CSF + OK-432), -K (GM-CSF + PGE2) and -M (GM-CSF + PGE1) as the most efficient kits in generating (mature) DC/DCleu, which are further competent to stimulate immunoreactive cells to show an improved anti-leukaemic cytotoxicity as well. This great performance of Kit-I, -K and -M in mediating DC/DCleu-based anti-leukaemic immunity in a WB-environment in vitro constitutes an important and directive step for translating DC/DCleu-based immunotherapy of AML into clinical application.
Keywords: leukaemia derived DC, acute myeloid leukaemia, anti-leukaemic functionality
1. Introduction
Acute myeloid leukaemia is a malignant disorder of the hematopoietic system, characterised by an uncontrolled proliferation of abnormally differentiated myeloid blasts [1,2]. It is the predominant subtype of leukaemia in adults with a median age of 72 years at diagnosis [3]. Standard treatment options include chemotherapy with or without allogeneic stem cell transplantation (SCT) [4,5], which have an overall 5-year-survival-rate of 29.5% that decreases to only 8.9% for patients over the age of 65 years [6]; the outcome remains unsatisfactory.
Alternative treatment options are needed. Above all, immunomodulatory approaches aiming to (re-)activate the immune system against leukaemia have shown auspicious results [7]. Specifically, dendritic cells (DC) have been put into the limelight of research: DC are one of the most potent mediators of the immune system. As antigen-presenting cells, they have the competence to link the innate and adaptive immune system and mediate a comprehensive immune response to a particular antigen [8,9]. Based on these powerful properties, manipulating DC to present leukaemia-specific antigens proposes to be an auspicious way to induce leukaemia-specific immunity.
There are diverse approaches to manipulating DC: DC can be generated from monocytes in vitro (known as moDC), pulsed with leukaemic peptides [10], apoptotic leukaemic cells or leukaemic cell lysates [11,12], fused with leukaemic blasts [13] or electroporated with messenger ribonucleic acid (mRNA) encoding leukaemia-associated-antigens (LAA) [14] and transferred to the patient as a vaccine. Moreover, DC can be generated from leukaemic blasts in vitro and potentially in vivo (known as DCleu), which are characterised by the simultaneous expression of dendritic- and leukaemia-specific antigens [15,16,17,18,19]. The latter not only has the advantage of being unrestricted to a certain LAA, thereby being able to stimulate the immune system to the whole leukaemic antigen repertoire, but also of being less complex as in vivo generation of DC/DCleu circumvents cumbersome manufacturing of LAA-specific moDC under good manufacturing practice (GMP) conditions and administration to the patient.
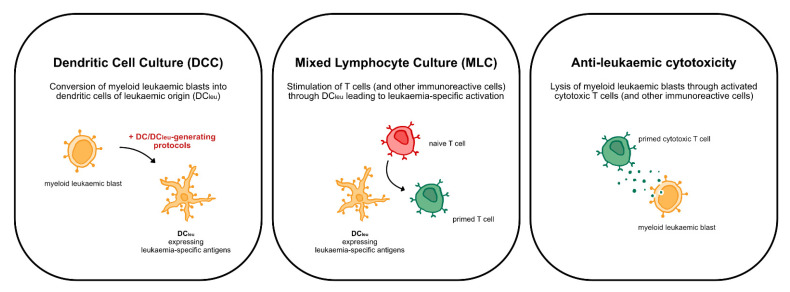
It is well known that myeloid leukaemic blasts can be converted into dendritic cells (DCleu) that have the competence to (re-)activate the innate and adaptive immune system against leukaemia [17,18,19,20,21,22,23,24] (Figure 1). This conversion of leukaemic blasts into DCleu can be induced using different sets of response modifiers (DC/DCleu-generating protocols) that incorporate the induction of hematopoietic differentiation, dendritic activation and maturation [16]. DC/DCleu-generating protocols like Ca, Mcm and Pici have already demonstrated their capacity to generate (mature) DC and DCleu (DC/DCleu) from peripheral blood mononuclear cells (MNC) and therewith stimulate an anti-leukaemic immune response in vitro [20,21,25].
Figure 1.
Overview of the concept of DC/DCleu-based immunotherapy depicting the steps of generating DCleu through DC/DCleu-generating protocols in the dendritic cell culture (DCC) and stimulating T cell enriched immunoreactive cells with generated DCleu in the mixed lymphocyte culture (MLC), overall leading to anti-leukaemic cytotoxicity of T cell enriched immunoreactive cells.
With regard to future clinical applications though, more physiological conditions have to be endeavoured, and thus, progression from DC/DCleu-generation in an MNC- to a whole-blood-(WB)-environment is inevitable. WB, in contrary to MNC, not only contains the full spectrum of soluble (e.g., cytokines, chemokines) and cellular (e.g., granulocytes, erythrocytes) components that take part in an immune response but also that determine the patient- and tumour-specific environment, consequently influencing the generation of DC/DCleu and the mediation of DC/DCleu-based anti-leukaemic immunity.
To ensure efficient DC/DCleu-generation in a novel WB-environment, we developed ten new DC/DCleu-generating protocols (kits; Kit-A, -C, -D, -E, -F, -G, -H, -I, -K, -M; deduced from Ca, Mcm, Pici), specifically designed for the generation of DC/DCleu from leukaemic WB. Kits were composed of up to three response modifiers, including granulocyte-macrophage-colony-stimulating-factor (GM-CSF), tumour-necrosis-factor-alpha (TNFa), interferon-alpha (IFNa), prostaglandin-E1 and -E2 (PGE1, PGE2), picibanil (OK-432) and Calcium-Ionophore A23187 (Ca-Iono). The success of kits in generating DC/DCleu though is strongly dependent on the response modifiers’ (synergistic) potential to induce hematopoietic differentiation, dendritic activation and maturation [16]. Setting up our kits, we thus decided upon GM-CSF, IFNa and Ca-Iono for their potential to induce differentiation of myeloid leukaemic blasts to dendritic cells [26,27,28,29,30], OK-432 for its potential to activate dendritic cells through danger signalling [31,32] and GM-CSF, TNFa, IFNa, OK-432, PGE1, PGE2 and Ca-Iono for their potential to induce maturation of dendritic cells through CCR7 expression [26,28,33,34,35,36,37]. Ultimately, to determine the best performing kits, in addition to the capacity of kits to generate DC/DCleu, the capacity of kit-generated DC/DCleu to stimulate anti-leukaemic immunity must be taken into consideration.
In this study, we developed DC/DCleu-generating protocols specifically designed for the generation of DC/DCleu in a WB-environment and evaluated their potential to generate DC/DCleu and mediate DC/DCleu-based anti-leukaemic immunity. This constitutes an important and directive step for translating DC/DCleu-based immunotherapy into clinical application.
2. Results
2.1. DC/DCleu-Generation Using Standard Protocols Is Comparable from MNC and WB
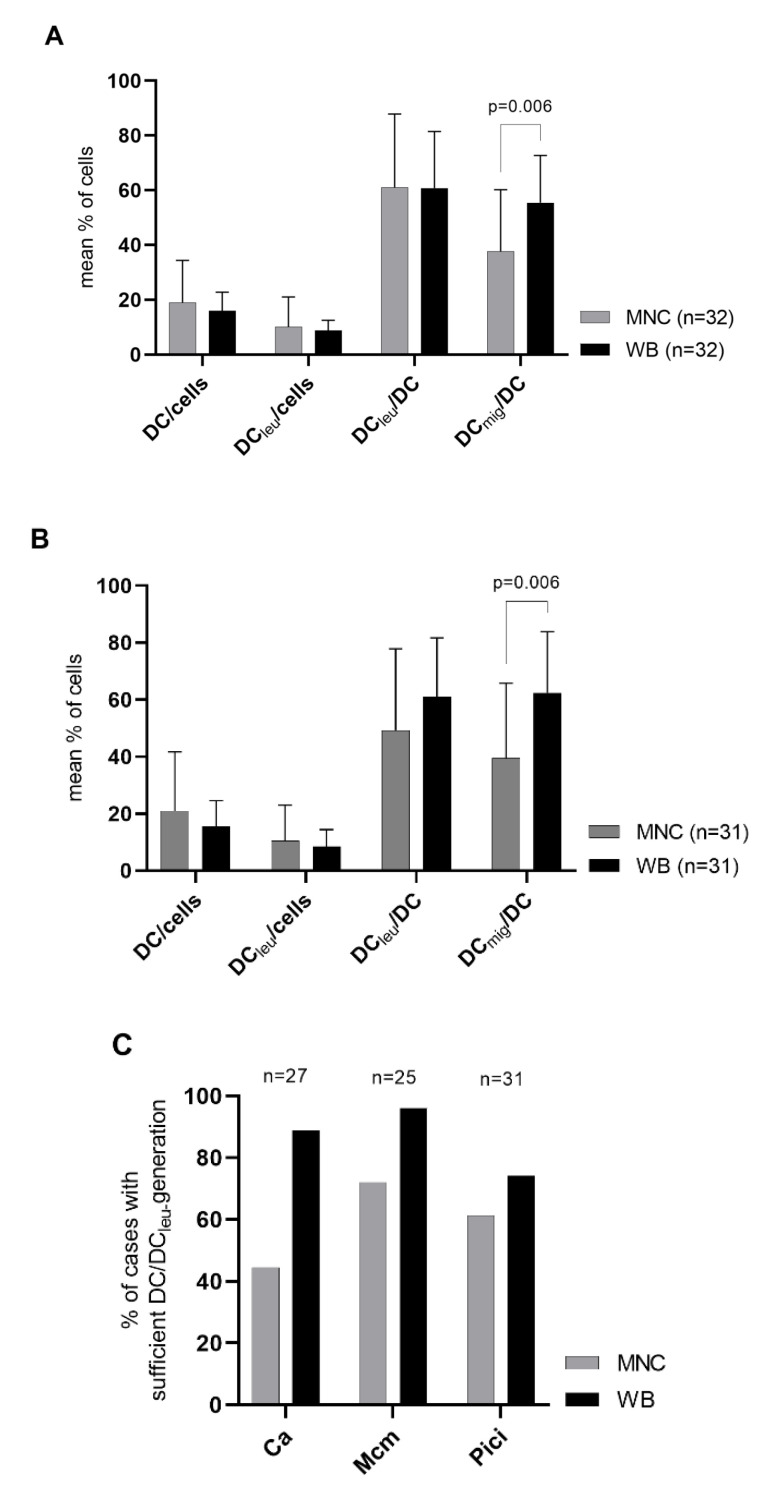
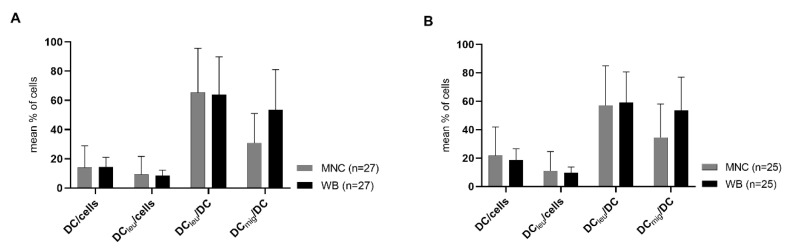
We were able to generate significant frequencies of (mature) DC and DCleu from both MNC and WB with standard methods (Ca, Mcm, Pici). Pooling all standard methods, we found no significant differences in frequencies of DC/cells, DCleu/cells and DCleu/DC comparing MNC- and WB-cultures, but significantly higher frequencies of DCmig/DC in WB-cultures compared to MNC-cultures (Figure 2A). Considering all standard methods separately, we found no significant differences in frequencies of DC/cells, DCleu/cells and DCleu/DC comparing MNC- and WB-cultures but (significantly) higher frequencies of DCmig/DC in WB-cultures compared to MNC-cultures (Figure 2B, exemplary shown Pici; Figure A1).
Figure 2.
DC/DCleu-generation from leukaemic WB and MNC with standard DC/DCleu-generating protocols (Ca, Mcm, Pici). Given are the mean ± SD of DC/cells, DCleu/cells, DCleu/DC and DCmig/DC generated with all standard protocols pooled (A) and with the standard protocol Pici (B) from MNC compared to WB. Further shown are proportions of cases with sufficient DC/DCleu-generation following the DC/DCleu-classification using standard protocols (Ca, Mcm, Pici) in MNC compared to WB (C). Statistically significant differences (p values < 0.05) (two-tailed t-test) are given.
Evaluating proportions of cases with sufficient and insufficient DC/DCleu-generation according to the DC/DCleu-classification (Table 1), we found higher proportions of cases with sufficient DC/DCleu-generation using WB compared to MNC after treatment with all three standard protocols (Figure 2C).
Table 1.
Classification of DC- and DCleu-generation.
| Subcategories | DC/Cells | DCleu/Cells | ||
|---|---|---|---|---|
| Sufficient | Excellent | ≥15% | and | ≥10% |
| Good | ≥15% | and | <10% and ≥5% | |
| or | ||||
| <15% and ≥10% | and | ≥ 10% | ||
| Satisfactory | <15% and ≥10% | and | <10% and ≥5% | |
| Insufficient | <10% | and/or | <5% |
DC/DCleu-generation is assessed with respect to frequencies of generated DC/cells and/or DCleu/cells. Results were divided in sufficient, further subdivided in excellent, good and satisfactory and insufficient.
In summary, we found the generation of (mature) DC/DCleu using standard protocols equivalent or even superior in WB compared to MNC. Subsequent experiments were thus performed in a WB-environment simulating more physiological conditions.
2.2. DC/DCleu-Generation from WB Is Comparable Using New Protocols (Kits) and Standard Protocols
We introduced nine new DC/DCleu-generating protocols (kits; Kit-A, -C, -D, -E, -F, -G, -H, -I, -K) and compared their capacity to generate DC and DCleu (subgroups) to the performance of the standard protocols they derived from in a WB-environment. We thus compared results of Kit-A, -C, -E, -G, -K to Mcm (sharing GM-CSF, TNFa and/or PGE2), Kit-E, -H to Mcm (all cytokine-based), Kit-D, -G, -I to Pici (sharing GM-CSF and/or OK-432) and Kit-F to Ca (sharing Ca-Iono).
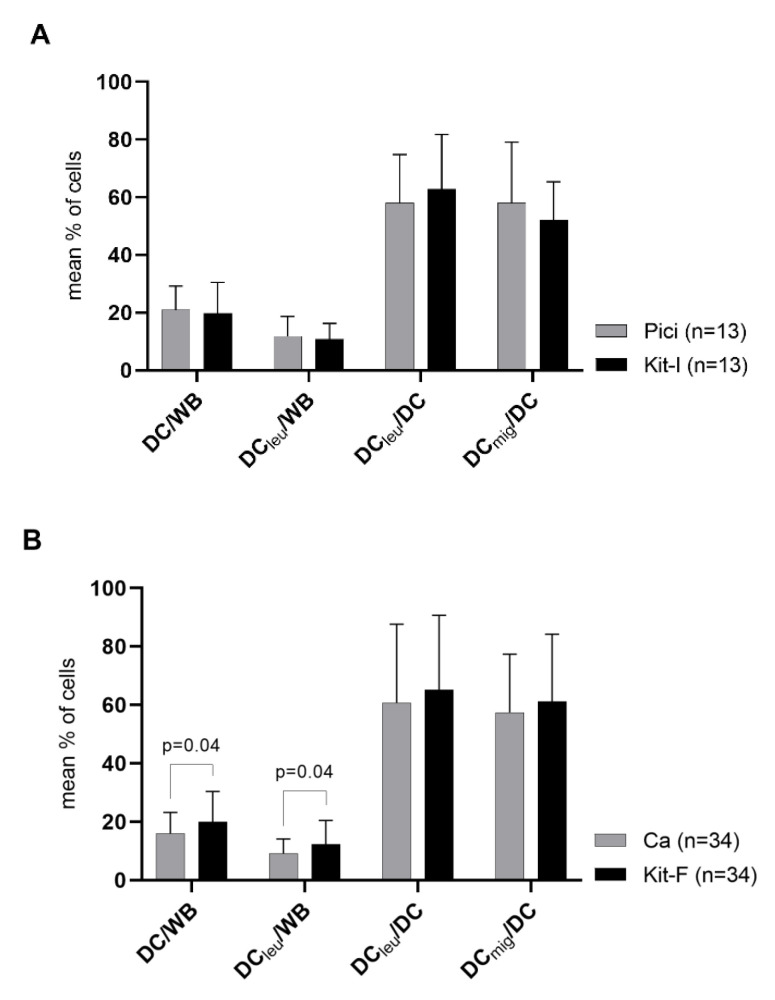
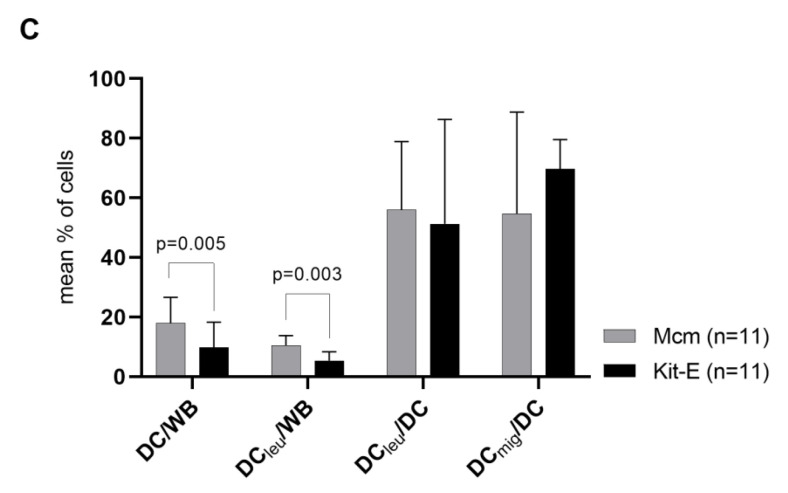
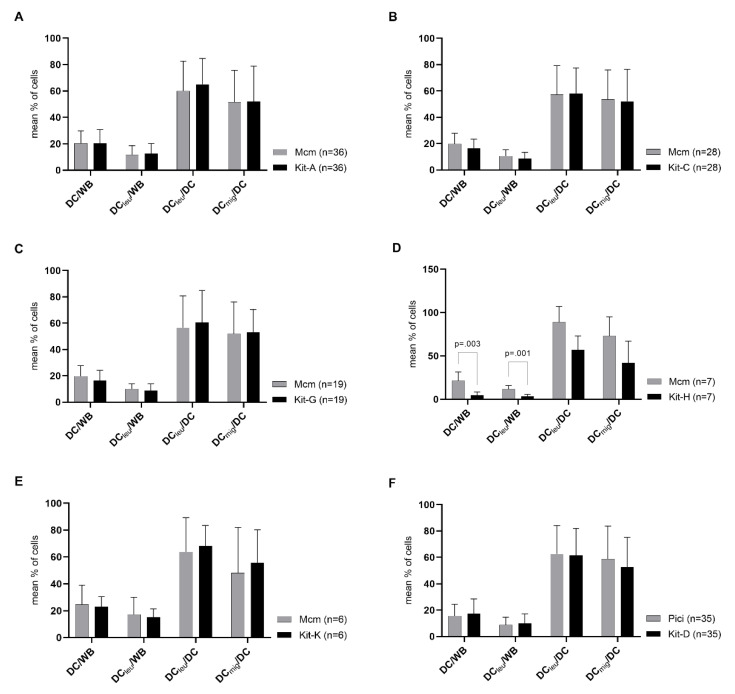
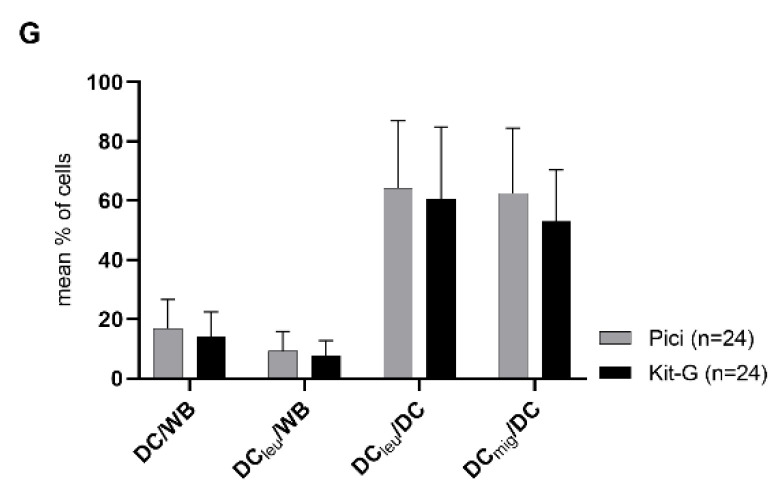
We found no significant differences in frequencies of DC and DCleu (subtypes) generated with Kit-A, -C, -D, -G, -I, -K compared to standard protocols (Figure 3A, exemplarily shown Kit-I vs. Pici; Figure A2). Kit-F even generated significantly higher frequencies of DC/WB and DCleu/WB compared to Ca (Figure 3B). However, Kit-E, -H generated significantly lower frequencies of DC/WB and DCleu/WB compared to Mcm (Figure 3C, exemplarily shown Kit-E vs. Mcm; Figure A2). Frequencies of DCleu/DC and DCmig/DC were comparable between kits and standard protocols.
Figure 3.
DC/DCleu-generation from leukaemic WB with standard (Ca, Mcm, Pici) and new DC/DCleu-generating protocols (kits). Given are the mean ± SD of DC/cells, DCleu/cells, DCleu/DC and DCmig/DC generated with Pici compared to Kit-I (A), Ca compared to Kit-F (B) and Mcm compared to Kit-E (C). Statistically significant differences (p values < 0.05) (two-tailed t-test) are given.
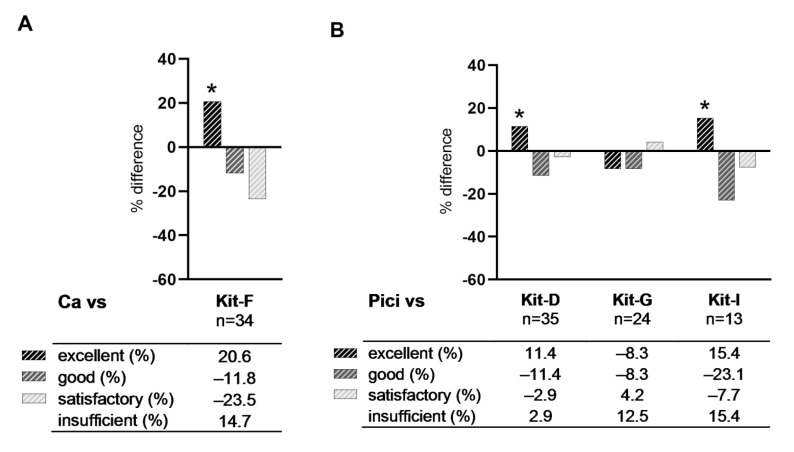
Evaluating proportions of cases with sufficient (excellent, good, satisfactory) and insufficient DC/DCleu-generation according to the DC/DCleu-classification, we found Kit-F (compared to Ca), Kit-D and -I (compared to Pici) and Kit-A, -G and -K (compared to Mcm) generating higher proportions of cases with excellent DC/DCleu-generation compared to their respective standard protocol. Proportions of cases with good DC/DCleu-generation were lower with all kits, proportions of cases with satisfactory DC/DCleu-generation were lower with Kit-A, -D, -F, -I and equal or higher with Kit-C, -E, -G, -H, -K compared to their respective standard protocol (Figure 4).
Figure 4.
Comparison of DC/DCleu-generation from leukaemic WB with standard (Ca, Mcm, Pici) and new DC/DCleu-generating protocols (kits). Given are the percentual differences of proportions of cases with sufficient (excellent, good, satisfactory) and insufficient DC/DCleu-generation following the DC/DCleu-classification when comparing the standard protocol Ca with its derivative protocol Kit-F (A), Pici with its derivative protocols Kit-D, -G, -I (B), and Mcm with its derivative protocols Kit-A, -C, -E, -G, -H, -K (C). An increase of proportions of cases with excellent DC/DCleu-generation is marked with *.
In summary, most kits were able to generate comparable or, in the case of Kit-F, even higher frequencies of DC/DCleu (subgroups) and higher proportions of cases with excellent DC/DCleu-generation compared to their respective standard protocol. Of note, Kit-E and -H were not able to generate comparable frequencies of DC/DCleu (subgroups) but rather generated significantly lower frequencies compared to their standard protocol.
2.3. Ranking of Kits
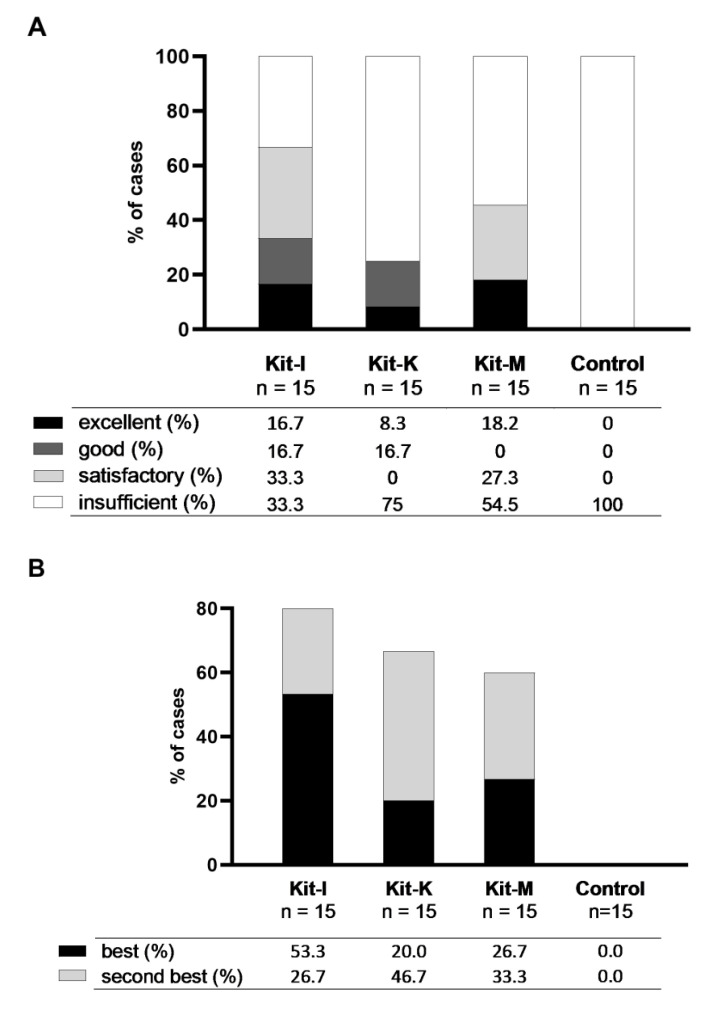
We assessed the potential of kits based on two different ranking methods: (1) the class-ranking based on the proportions of excellent, good, satisfactory and insufficient results of DC/DCleu-generation using quantities of generated DC/WB and DCleu/WB, and (2) the best-ranking based on the proportions of best and second-best results of DC/DCleu-generation using quantities of generated DC/WB.
2.3.1. Class-Ranking of Kits
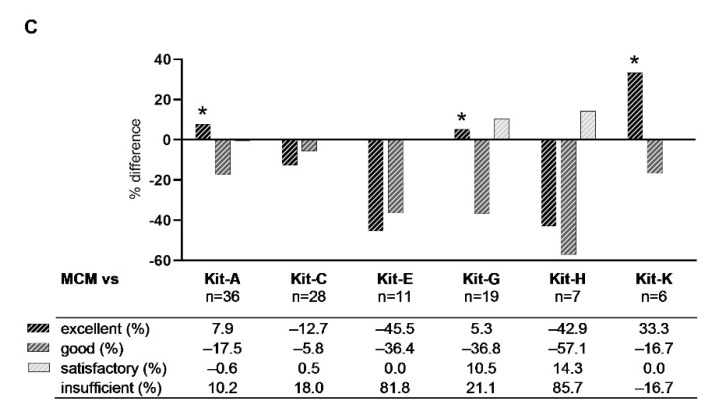
We ranked kits based on quantities of generated DC/WB and DCleu/WB. We therefore developed three subordinate rankings (ranking 1–3) and one superordinate ranking (ranking 4) (Figure 5A, Table 2). Ranking 1 considers kits based on their proportion of cases with excellent DC/DCleu-generation. In doing so, Kit-I and -K performed the best (68% and 58% of cases, respectively), followed by Kit-F, -A, -D, -G, -C, -E and -H. Ranking 2 considers kits based on their proportion of cases with excellent and good DC/DCleu-generation. In doing so, Kit-K and -I performed the best (83% and 72% of cases, respectively), followed by Kit-A, -F, -D, -C, -G, -E and -H. Ranking 3 considers kits based on their proportion of cases with sufficient (excellent, good, satisfactory) DC/DCleu-generation. In doing so, Kit-K and -I performed the best (83% and 79% of cases, respectively), followed by Kit-A, -F, -D, -C, -G, -E and -H. Ranking 4 considers results of rankings 1–3, creating a superordinate ranking, nominating Kit-K and -I as the best performing kits, followed by Kit-A, -F, -D, -C, -G, -E and -H.
Figure 5.
Class- and best-ranking of Kit-A, -C, -D, -E, -F, -G, -H, -I and -K. Given are (A) the class-ranking with the proportions of cases with sufficient (excellent, good, satisfactory) and insufficient DC/DCleu-generation using kits and (B) the best-ranking with proportions of cases with best- and second-best DC/DCleu-generation using kits.
Table 2.
Class-ranking of all kits.
| Rank | Ranking 1 Excellent |
Ranking 2 Excellent, Good |
Ranking 3 Excellent, Good, Satisfactory |
Ranking 4 Combined |
|
|---|---|---|---|---|---|
| 1 | I | K | K | → | K |
| 2 | K | I | I | → | I |
| 3 | F | A | A | → | A |
| 4 | A | F | F | → | F |
| 5 | D | D | D | → | D |
| 6 | G | C | C | → | C |
| 7 | C | G | G | → | G |
| 8 | E | E | E | → | E |
| 9 | H | H | H | → | H |
Kits (A, C, D, E, F, G, H, I, K) were ranked based on the proportions of cases with excellent, good, and/or satisfactory generation of DC/WB and DCleu/WB.
2.3.2. Best-Ranking of Kits
We further ranked kits based on the best and second-best results of quantities of generated DC/WB. We therefore developed two subordinate rankings (ranking 1–2) and one superordinate ranking (ranking 3) (Figure 5B, Table 3). Ranking 1 considers kits based on their proportion of cases with the best DC/DCleu-generation. In doing so, Kit-F and -A performed the best (33% and 30% of cases, respectively), followed by Kit-D, -I, -E, -C, -K, -G and -H. Ranking 2 considers kits based on their proportion of cases with the best and second-best DC/DCleu-generation. In doing so, Kit-F and -I performed the best (52% and 47% of cases, respectively), followed by Kit-D, -A, -E, -K, -C, -G and -H. Ranking 3 considers results of rankings 1–2, creating a superordinate ranking, nominating Kit-F and -A/-D/-I (the latter equally) as the best performing kits, followed by Kit-E, -K, -C, -G and -H.
Table 3.
Best-ranking of all kits.
| Rank | Ranking 1 Best |
Ranking 2 Best, Second-Best |
Ranking 3 Combined |
|
|---|---|---|---|---|
| 1 | F | F | → | F |
| 2 | A | I | → | A = D = I |
| 3 | D | D | → | |
| 4 | I | A | → | |
| 5 | E | E | → | E |
| 6 | C | K | → | K = C |
| 7 | K | C | → | |
| 8 | G | G | → | G |
| 9 | H | H | → | H |
Kits (A, C, D, E, F, G, H, I, K) were ranked based on the proportions of cases with best and/or second-best generation of DC/WB.
In summary, the class-ranking of kits found Kit-K and -I to be the best performing kits, the best-ranking of kits found Kit-F, -A, -D and -I to be the best performing kits. Overall, Kit-I appears to be the most proficient kit in generating DC/DCleu.
2.4. Selection of Best Kits and Evaluation of Their DC/DCleu-Generating and Anti-Leukaemic Performance
2.4.1. Selection of Best Kits
We selected the best performing kits for further evaluation. Ranking of kits found Kit-K, -I, -A, -D and -F to be the most proficient kits. As TNFa and Ca-Iono were no longer approved for human systemic treatment though, we had to exclude Kit-A and -F from further considerations. As Kit-D, composed of three response modifiers (GM-CSF, OK-432, PGE2), showed no advantage in generating DC/DCleu compared to Kit-I and -K, composed of two response modifiers (GM-CSF with OK-432 or PGE2), we moreover excluded Kit-D from further considerations. Lastly, as parallel studies with PGE1 showed PGE2 and PGE1 to have comparable effects on the generation of DC/DCleu, we introduced a new Kit-M, derived from Kit-K, consisting of GM-CSF and PGE1, for further evaluation [37].
Overall, we selected Kit-I, -K and -M as the most promising kits and evaluated their capacity to generate (mature) DC/DCleu as well as their potential to initiate anti-leukaemic cytotoxicity.
2.4.2. Kit-I, -K and -M Generate Significantly Higher Frequencies of DC/DCleu Compared to Control
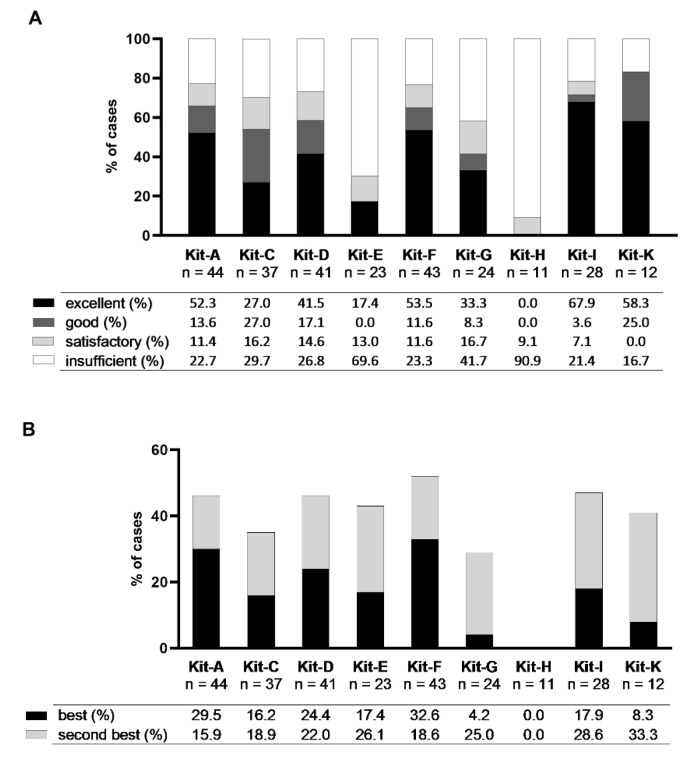
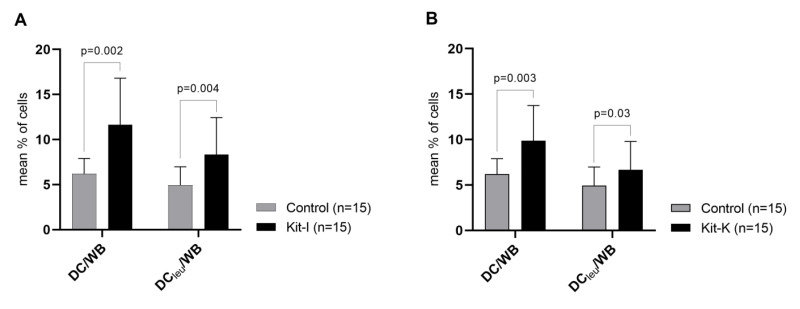
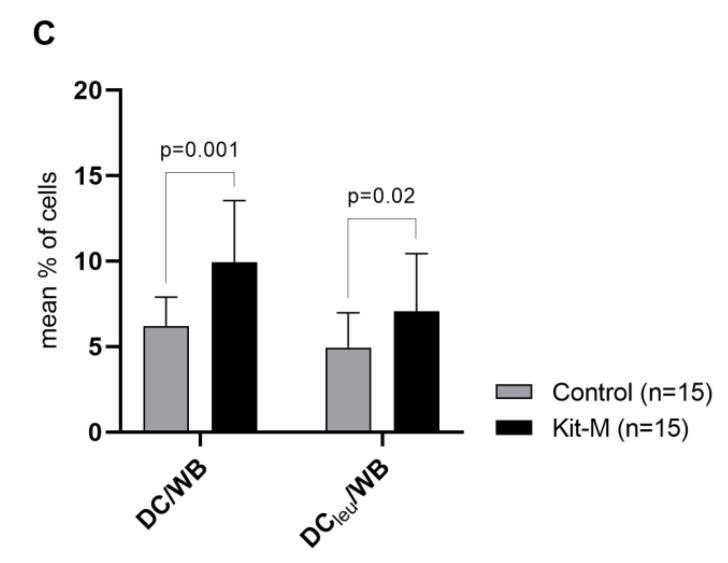
All three kits (Kit-I, -K, -M) were able to generate significantly higher frequencies of DC/WB and DCleu/WB compared to control (Figure 6). Kit-generated DC/DCleu hereby consisted of significant frequencies of mature DC. Comparing kits among themselves, we found no significant differences in frequencies of generated DC/WB, DCleu/WB, DCleu/DC and DCmig/DC (data not shown). Ranking kits according to the class-ranking and best-ranking, we found Kit-I to be the most efficient kit in generating DC/DCleu, followed by Kit-M and -K (Figure 7A,B and Table 4 and Table 5).
Figure 6.
DC/DCleu-generation from leukaemic WB with Kit-I, -K and -M. Given are the mean ± SD of DC/cells and DCleu/cells generated with Kit-I (A), Kit-K (B), and Kit-M (C) compared to control. Statistically significant differences (p values < 0.05) (two-tailed t-test) are given.
Figure 7.
Class- and best-ranking of Kit-I, -K and -M. Given are (A) the class-ranking with the proportions of cases with sufficient (excellent, good, satisfactory) and insufficient DC/DCleu-generation using kits and (B) the best-ranking with proportions of cases with best- and second-best DC/DCleu-generation using kits.
Table 4.
Class-ranking of selected kits.
| Rank | Ranking 1 Excellent |
Ranking 2 Excellent, Good |
Ranking 3 Excellent, Good, Satisfactory |
Ranking 4 Combined |
|
|---|---|---|---|---|---|
| 1 | I | I | → | I | |
| 2 | I, M | K | M | → | M |
| 3 | K | M | K | → | K |
Selected kits (I, K, M) were ranked based on the proportions of cases with excellent, good and/or satisfactory generation of DC/WB and DCleu/WB.
Table 5.
Best-ranking of selected kits.
| Rank | Ranking 1 Best |
Ranking 2 Best, Second-Best |
Ranking 3 Combined |
|
|---|---|---|---|---|
| 1 | I | I | → | I |
| 2 | M | K | → | M = K |
| 3 | K | M | → |
Selected kits (I, K, M) were ranked based on the proportions of cases with best and/or second-best generation of DC/WB.
2.4.3. DC/DCleu Generated with Kit-I, -K and -M Stimulate Anti-Leukaemic Activity
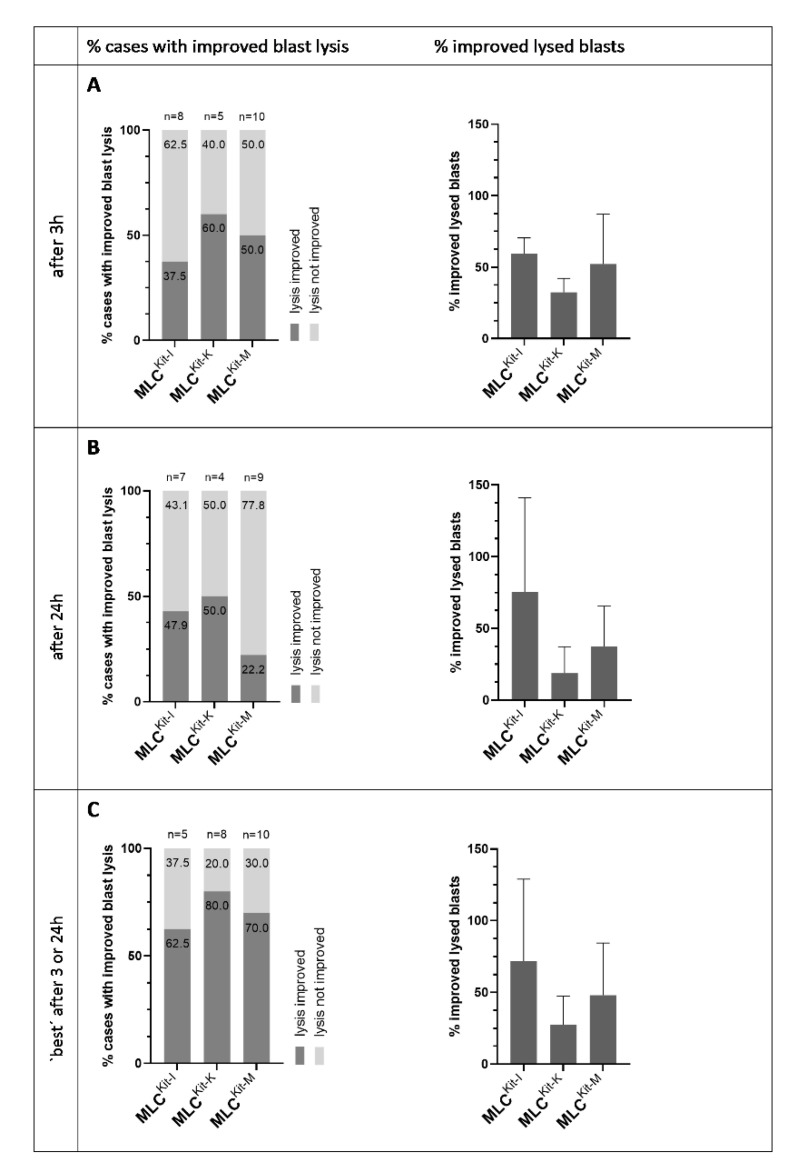
We analysed the improvement in blast lytic activity of MLCKit-I, MLCKit-K and MLCKit-M compared to MLCControl through CTX after 3 h and 24 h of incubation of effector and target cells to assess the anti-leukaemic activity of DC/DCleu-stimulated immunoreactive cells.
After 3 h, we could observe an improvement in blast lysis in about 40% of cases in MLCKit-I, 60% of cases in MLCKit-K and 50% of cases in MLCKit-M compared to control. The average improvement in blast lysis was about 60% in MLCKit-I, about 30% in MLCKit-K and 50% in MLCKit-M (Figure 8A). After 24 h, we could observe an improvement in blast lysis in about 40% of cases in MLCKit-I, 50% of cases in MLCKit-K and 20% of cases in MLCKit-M compared to control. The average improvement in blast lysis was about 75% in MLCKit-I, about 20% in MLCKit-K and 40% in MLCKit-M (Figure 8B). Selecting the best-achieved improvement in blast lysis after 3 h or 24 h, we could observe an improvement in blast lysis in about 60% of cases in MLCKit-I, 80% of cases in MLCKit-K and 70% of cases in MLCKit-M compared to control. The average improvement in blast lysis was about 70% in MLCKit-I, about 30% in MLCKit-K and 50% in MLCKit-M (Figure 8C).
Figure 8.
Stimulatory impact of DC/DCleu generated with Kit-I, -K and -M on the anti-leukaemic cytotoxicity of T cell enriched immunoreactive cells. Given are the proportion of cases with an improvement in blast lysis (“% cases with improved blast lysis”) and the mean ± SD of improved lysed blasts (“% improved lysed blasts”) in MLCKit-I, MLCKit-K and MLCKit-M in relation to MLCControl after 3 h (A) and 24 h (B), and the “best” achieved results after 3 h and 24 h (C).
In summary, we found Kit-I, -K and -M gave rise to comparable frequencies of DC/DCleu. All three kits were able to improve the anti-leukaemic activity of immunoreactive cells compared to control. Overall, Kit-I, followed by Kit-M and -K, performed the best regarding the class-ranking and best-ranking, with the highest proportions of excellent and best DC/DCleu-generation, as well as the best-achieved improvement in blast lysis after 3 h or 24 h. We thus perceive Kit-I and -M to have the highest potential in generating DC/DCleu and stimulating anti-leukaemic activity.
3. Discussion
3.1. DCleu-Based Immunotherapy
DC are one of the most potent mediators of the immune system. Manipulated to express leukaemia-specific antigens, they impose an auspicious way to (re-)activate the immune system to a leukaemia-specific immunity. Particularly, DC generated from myeloid leukaemic blasts (known as DCleu), which are characterised by the simultaneous expression of dendritic- and leukaemia-specific antigens, hold the great potential of stimulating the immune system to the whole leukaemic antigen repertoire [15,16,17,18,19]. The process of converting myeloid leukaemic blasts into DC/DCleu in vitro using DC/DCleu-generating protocols consisting of various response modifiers has been demonstrated multiple times before [20,21,25]. Most protocols are based on an MNC-setting, though. With respect to future clinical applications, progression to DC/DCleu-generation in a WB-setting simulating a more physiological patient- and tumour-specific environment is inevitable. We thus developed new DC/DCleu-generating protocols specifically designed for the generation of DC/DCleu from leukaemic WB and evaluated their potential to generate DC/DCleu and mediate DC/DCleu-based anti-leukaemic activity.
3.2. Standard Protocols—DC/DCleu Generation from Leukaemic MNC and WB
Standard DC/DCleu-generating protocols like Ca, Mcm and Pici have been shown to be competent in generating significant frequencies of (mature) DC/DCleu from leukaemic MNC [20,21]. Although their compositions and operations were specifically designed for MNC-application, we nevertheless wanted to examine their performance in a novel WB-environment, simulating more physiological conditions.
We found all standard protocols competent to generate DC/DCleu from MNC and WB to a comparable extent, with higher frequencies of mature DC generated from WB (Figure 2). This is an interesting finding as the stimulation of maturation and CCR7-dependent (lymph node) migration is essential for DC/DCleu to activate T-cells as well as other immunoreactive cells [38,39,40]. DC maturation and migration is a tightly regulated process, controlled by a large variety of chemotactic and non-chemotactic factors like danger-associated-molecular-patterns (DAMPs), acute-phase-proteins (APPs), proinflammatory cytokines or eicosanoids produced by different cell types [41]. A superior maturation of DC/DCleu in a WB- compared to an MNC-environment may be attributed to the fact that WB comprises the full spectrum of soluble (including chemotactic and non-chemotactic) and cellular factors necessary for the homeostasis of the blood and immune system and thus can readily support DC/DCleu maturation and migration. An inferior maturation of DC/DCleu in a (serum-free) MNC-environment in contrary suggests a restriction of this process due to the absence of (readily) available maturation and migration factors. This emphasises the importance of incorporating the full spectrum of soluble and cellular factors present in WB when developing immunomodulatory agents for in vivo use.
Assessing the overall DC/DCleu-generating potential of standard protocols using the DC/DCleu-classification, we found higher proportions of cases with sufficient DC/DCleu-generation using WB than MNC, letting DC/DCleu-generation with standard protocols appear more reliable from WB than MNC.
Overall, we found the standard protocols perform better in a WB-environment than in the MNC-environment they were originally designed for.
3.3. New Kits Specifically Designed for WB
We developed nine new DC/DCleu-generating protocols (kits) specifically designed for the generation of DC/DCleu from leukaemic WB. The new kits were derived from the standard protocols and composed of one to three response modifiers, including GM-CSF, IFNa, TNFa, PGE1, OK-432 and Ca-Iono.
We compared the performance of the new kits to their respective standard protocols and found Kit-F generated significantly higher, Kit-A, -C, -D, -G, -I and -K generated equal and Kit-E and -H generated significantly lower frequencies of DC/DCleu from WB compared to their respective standard protocol. Frequencies of mature DC were comparable between kits and standard protocols (Figure 3). Assessing kits using the DC/DCleu-classification showed a similar picture: we found Kit-F, -G (when compared to Mcm), -D, -I, -A and -K achieved higher proportions of cases with excellent DC/DCleu-generation and Kit-C, -E, -G (when compared to Pici) and -H achieved lower proportions of cases with excellent DC/DCleu-generation compared to their respective standard protocol (Figure 4).
Overall, most kits performed comparable or even better compared to their respective standard protocol. Only Kit-E and -H performed worse in comparison.
3.4. Ranking of Kits
We further ranked kits according to their performance in generating DC/DCleu. Ranking kits with the class-ranking (based on proportions of cases with excellent, good and satisfactory generation of DC/DCleu), we found Kit-K and -I achieved the best results (Figure 5A, Table 2). Ranking kits with the best-ranking (based on proportions of cases with the best and second-best generation of DC), we found Kit-F and -A = -D = -I achieved the best results (Figure 5B, Table 3).
Overall, we found Kit-K, -I, -A, -D and -F containing GM-CSF combined with OK-432, PGE2, TNFa and/or Ca-Iono the best performing kits, initiating sufficient DC/DCleu-generation in most cases. Interestingly, although GM-CSF has been shown to mediate differentiation, activation and maturation of dendritic cells [26,27], GM-CSF on its own, as in Kit-G, did not show sufficient DC/DCleu generation, which is in line with previous findings [42,43,44]. DC/DCleu-generation with GM-CSF seems to be dependent on the synergetic effects of additional response modifiers, like OK-432, PGE2 or Ca-Iono, that further enhance dendritic differentiation and proliferation and moreover stimulate activation and maturation, properties essential for mediating a comprehensive immune response [42,45,46]. Of note, a combination of GM-CSF with two other response modifiers, OK-432 and PGE2, as in Kit-D, showed no advantage over a combination of GM-CSF with either OK-432 or PGE2, as in Kit-I and -K. Saturation of the DC/DCleu-generation-pathway might be at play here. The Ca-Iono containing Kit-F showed good results in line with previous findings [47,48]. Mentionable, Ca-Iono containing protocols like Kit-F hold the significant advantage of reduced culture times of two days without constrained success of DC/DCleu-generation and T-cell stimulation through generated DC/DCleu; however, such protocols have constrained overall viability of DC/DCleu compared to other protocols [47,48]. When comparing the TNFa containing Kit-C to Kit-A, we found Kit-C performed worse than -A. This is dissenting with other findings that suggest PGE2 to further enhance the effect GM-CSF together with TNFa [46,49]. The IFNa containing Kit-E and -H showed poor performance. This might be surprising at first as IFNa is well-known for its anti-tumour effects and its role in the treatment of several solid and haematological malignancies [50,51,52,53]. In AML, IFNa has been shown to directly inhibit blast proliferation and moreover stimulate blast apoptosis [51,54]—properties that yet come amiss when aiming to convert myeloid leukaemic blasts into DCleu, hampering the overall DC/DCleu-generating process.
3.5. Selection of Kits for (Potential) Treatment of Patients
In regard to future clinical applications, the development of kits has to consider not only the potential of kits but also their biocompatibility and applicability. Of the nine new kits we developed, Kit-K, -I, -A, -D and -F showed the best performance, thus qualifying for further in-depth evaluation. As TNFa and Ca-Iono were no longer approved for human systemic treatment, we had to exclude Kit-A and -F from further considerations. As Kit-D constitutes a combination of Kit-I and -K with no advantage to the individual protocols but with a more intricate composition of three response modifiers in contrast to two response modifiers of the individual protocols, we also excluded Kit-D from further considerations. Lastly, as parallel studies with PGE1 showed PGE2 and PGE1 to have comparable effects on the generation of DC/DCleu, we introduced a new Kit-M, derived from Kit-K, consisting of GM-CSF and PGE1, for further evaluation [37].
Overall, we selected Kit-I, -K and -M as the most promising kits and further scrutinised their capacity to generate (mature) DC/DCleu as well as—and most importantly—their potential to initiate anti-leukaemic cytotoxicity through generated DC/DCleu. All three kits were able to generate significantly higher frequencies of DC/WB and DCleu/WB compared to the control (Figure 6). Notably, Kit-generated DC/DCleu hereby consisted of significant frequencies of mature DC. Both class- and order-ranking determined Kit-I to be superior to Kit-M and -K (Figure 7A,B and Table 4 and Table 5). All three kits were able to improve the anti-leukaemic cytotoxicity of immunoreactive cells through generated DC/DCleu in most of the cases. Interestingly, some cases achieved an improvement in lysis after 3 h and some cases only after 24 h, whereas the average improvement in lysis was the highest in MLCKit-K and MLCKit-M after 3 h and in MLCKit-I after 24 h. This can be explained by the different mechanisms by which immunoreactive cells exert cytotoxicity: the early and fast-acting perforin-granzyme pathway (as likely seen in MLCKit-K and MLCKit-M) and the late and slow-acting Fas/FasL pathway (as likely seen in MLCKit-I), which can run separately or synergistically [55,56]. Overall, pooling the best anti-leukaemic cytotoxicity after 3 or 24 h, DC/DCleu generated with Kit-I appear to be the most efficient in stimulating blast-lytic activity, followed by Kit-M and -K (Figure 8).
Taken together, all three kits were able to generate significant amounts of (mature) DC/DCleu, which have the competence to regularly mediate anti-leukaemic cytotoxicity. Kit-I hereby performed the best, followed by Kit-M and -K, regarding the class- and best-ranking as well as the overall best-achieved improvement in blast lysis. We thus perceive Kit-I and -M to have the highest potential in generating DC/DCleu and stimulating anti-leukaemic activity.
3.6. Ongoing Kit Studies
Kit-I, -K, -M have shown auspicious results. On the way to successful in vivo treatment of patients with AML, kits though have to undergo further investigations regarding their performance and toxicity.
In this study, we could show that kits are competent to generate (mature) DC/DCleu that regularly initiate anti-leukaemic activity. We could confirm these results in multiple studies [24,37]. Furthermore, we could enlighten the leukaemia-specific activity of innate and adaptive immunoreactive cells on a single-cell level by analysing their IFNy secretion profiles and correlating them with the overall achieved anti-leukaemic cytotoxicity, thereby being able to display (indirectly and directly) participating immunoreactive cells of DC/DCleu-mediated leukaemia-specific and anti-leukaemic immune responses: levels of IFNy secreting innate (NK cells, CIK cells, iNKT cells) and adaptive (T-cells) immune cells were significantly higher in MLCKits compared to MLCControl, with frequencies of IFNy secreting TCD3+, TCD4+, TCD8+ and NK56+ cells positively correlating with the overall achieved anti-leukaemic activity [24]. When assessing compositions of DC/DCleu stimulated T-enriched immunoreactive cells, we found lower frequencies of regulatory T-cells (Treg) in MLCKits compared to MLCControl [57]; an important finding as the success of immunomodulation is directly affected by the Tregs immunosuppressive disposition [58]. A milestone in drug development though not only is the verification of anti-leukaemic effects but also the exclusion of pro-leukaemic effects. We thus investigated the proliferation of blasts in relation to kit treatment but found no induction of blast proliferation through kit treatment in this regard [59]. Of note, DC/DCleu-generation and -functioning was comparable under normoxic (21% oxygen) and hypoxic (10% oxygen, resembling the in vivo situation) conditions [57].
Ultimately, DC/DCleu-based immunotherapy needs to be transferred from in vitro to in vivo settings to further assess its value in the treatment of AML. We thus extended our preclinical studies from in vitro to in vivo animal trials to gather more information about the kits’ efficiency and toxicity. Kit treatment (Kit-M) of leukaemically diseased rats resulted in the generation of DC/DCleu without induction of blast proliferation and consecutively in an improved anti-leukaemic (blast-lytic) activity [60], displaying in vivo functionality of kits. For future studies, we are now focusing on conducting kit studies in patients with refractory AML, and striving to replicate the promising in vitro and in vivo results of DC/DCleu-based immunotherapy in clinic.
4. Materials and Methods
4.1. Sample Collection and Preparation
Samples were collected in the form of heparinised whole blood from patients in acute stages of AML and MDS after obtaining written consent and in accordance with the World Medical Association Declaration of Helsinki and the ethics committee of the Ludwig-Maximilian-University Hospital (vote no 33905). Samples were provided by the university hospitals of Munich, Tuebingen, Oldenburg and Augsburg.
MNC were isolated from the patients’ blood samples using density gradient centrifugation (Biocoll-Separating-Solution, Biochrom, Berlin, Germany). T-cells were isolated from MNC by CD3+ positive MACS microbead and column-based immunomagnetic cell separation technology (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. MNC and T-cells, unless directly used, were frozen with 70% RPMI-1640 medium (Biochrom), 20% human serum (Health Care Europa GmbH, Vienna, Austria) and 10% dimethyl sulfoxide (Sigma Aldrich Chemie GmbH, Steinheim, Germany), stored at −80 °C and thawed when required.
4.2. Patients’ Characteristics
Blood samples were obtained from patients in acute phases of AML (n = 65) and MDS (n = 2) with an average age of 54.3 (range 21–88) years and a female-to-male ratio of 1:1.3. Patients were classified based on the French-American-British (FAB) classification (M0-M5), the aetiology (primary AML, secondary AML), the stage of disease (diagnosis, persistence, relapse, relapse after SCT), the blast phenotype and frequency. An overview is given in Table 6.
Table 6.
Patients’ characteristics.
| Patient No. | Age, Sex | Stage | FAB Type | Blast Phenotype (CD) | IC Blasts (%) | Source | Conducted Experiments |
|---|---|---|---|---|---|---|---|
| AML | |||||||
| 1172 | 24, m | dgn | p-M0 | 117,13,33,34 | 92 | MNC, WB | DCC |
| 993 | 71, m | dgn | p-M1 | 34,13,33,117 | 95 | MNC, WB | DCC |
| 1289 | 65, w | dgn | p-M1 | 34,33, 56,117 | 60 | WB | DCC |
| 1300 | 24, w | dgn | p-M1 | 34,13,33,65,117 | 75 | WB | DCC, CTX |
| 1190 | 72, m | dgn | p-M2 | 117,33,34 | 28 | MNC, WB | DCC |
| 1292 | 44, w | dgn | p-M2 | 117,13,33,34 | 78 | WB | DCC, CTX |
| 1295 | 40, m | dgn | p-M2 | 34,13,33,117 | 45 | WB | DCC |
| 1225 | 48, m | dgn | p-M3 | 117,13,33 | 77 | MNC, WB | DCC |
| 1327 | 61, m | dgn | p-M4 | 117,7,13,33,65 | 75 | WB | DCC |
| 1453 | 54, w | dgn | p-M4 | 15,14,33,64,56 | 52 | WB | DCC, CTX |
| 1459 | 54, m | dgn | p-M4 | 56,4,11c,11b,33,38,64 | 14 | WB | DCC, CTX |
| 1460 | 78, w | dgn | p-M4 | 15,34,117 | 68 | WB | DCC, CTX |
| 1430 | 79, m | dgn | p-M5 | 34,117,13,33 | 70 | WB | DCC, CTX |
| 1432 | 34, m | dgn | p-M5 | 34,13,33,64 | 81 | WB | DCC, CTX |
| 1447 | 21, m | dgn | p-M5 | 33,56,45 | 65 | WB | DCC, CTX |
| 1443 | 64, m | dgn | p-M? | 34,117,13,33 | 50 | WB | DCC |
| 1452 | 44, m | dgn | p-M? | 34,117,33,13,45 | 55 | WB | DCC, CTX |
| 1050 | 32, w | dgn | s-M1 | 117,14,13,33 | 90 | MNC, WB | DCC |
| 1144 | 32, m | dgn | s-M1 | 34,13,19,56,117 | 60 | MNC, WB | DCC |
| 1204 | 72, m | dgn | s-M2 | 117,13,56 | 43 | MNC, WB | DCC |
| 1131 | 53, w | dgn | s-M4 | 117,33 | 15 | WB | DCC |
| 1207 | 56, w | dgn | s-M4 | 117,4,13,15,33,34 | 10 | MNC, WB | DCC |
| 1442 | 73, w | dgn | s-M4 | 117,33,61,138 | 14 | WB | DCC, CTX |
| 1426 | 61, w | dgn | s-M5 | 34,117,13,33,64 | 93 | WB | DCC, CTX |
| 1439 | 61, w | dgn | s-M5 | 34,117,13,33 | 17 | WB | DCC |
| 999 | 51, w | dgn | s-M? | 34,13,33,65,117 | 14 | MNC, WB | DCC |
| 1056 | 27, m | dgn | s-M? | 117,13,15,33,34 | 42 | MNC, WB | DCC |
| 1165 | 59, m | dgn | s-M? | 34,13,33,117 | 46 | MNC, WB | DCC |
| 1194 | 72, m | dgn | s-M? | 34,33,117 | 18 | MNC, WB | DCC |
| 1196 | 39, w | dgn | s-M? | 117,33 | 11 | MNC, WB | DCC |
| 1226 | 69, m | dgn | s-M? | 117,33,34 | 65 | MNC, WB | DCC |
| 1385 | 82, m | dgn | s-M? | 34,13,56,117 | 23 | WB | DCC, CTX |
| 1434 | 61, w | dgn | s-M? | 34,117,7,13,33,56,64 | 61 | WB | DCC, CTX |
| 1449 | 78, m | dgn | s-M? | 15,65,4,45,64 | 62 | WB | DCC, CTX |
| 1454 | 60, w | dgn | s-M? | 34,117,20,61 | 33 | WB | DCC |
| 1024 | 39, m | pers | p-M2 | 34,33,117 | 88 | MNC, WB | DCC |
| 1218 | 54, m | pers | p-M2 | 33,56,13 | 30 | MNC, WB | DCC |
| 1201 | 60, w | pers | p-M5b | 34,4,13,14,15,33,56,6,5 | 63 | WB | DCC |
| 1320 | 66, m | pers | s-M6 | 34,65,117 | 15 | WB | DCC, CTX |
| 1310 | 77, w | pers | s-M? | 117,33,34,56 | 52 | WB | DCC |
| 1011 | 88, w | rel | p-M1 | 34,13,33,65,117 | 88 | MNC, WB | DCC |
| 1127 | 61, w | rel | p-M1 | 34,13,33,117 | 46 | MNC, WB | DCC |
| 1138 | 24, m | rel | p-M1 | 34,4,14,33,56,65,117 | 50 | MNC, WB | DCC |
| 1222 | 40, m | rel | p-M1 | 117,33,34,56 | 50 | MNC, WB | DCC |
| 1080 | 37, w | rel | p-M2 | 34,4,13,33,117 | 34 | MNC, WB | DCC |
| 1123 | 70, m | rel | p-M2 | 34,13,33,117 | 47 | MNC, WB | DCC |
| 1205 | 60, m | rel | p-M2 | 34,2,13,33,117 | 67 | MNC, WB | DCC |
| 1206 | 74, w | rel | p-M2 | 34,4,33,117 | 27 | MNC, WB | DCC |
| 1243 | 34, m | rel | p-M2 | 34,2,13,33,117 | 32 | MNC, WB | DCC |
| 1376 | 52, w | rel | p-M2 | 34,33,117 | 60 | WB | DCC, CTX |
| 1387 | 63, m | rel | p-M2 | 34,13,33,117 | 37 | WB | DCC, CTX |
| 1001 | 60, w | rel | p-M4 | 34,33,117 | 35 | MNC, WB | DCC |
| 1017 | 67, m | rel | p-M4 | 34,33,117 | 34 | MNC, WB | DCC |
| 1018 | 40, w | rel | p-M4 | 34,15,33,64,117 | 17 | MNC, WB | DCC |
| 1143 | 46, w | rel | p-M4 | 117,2,7,13,34,65 | 75 | MNC, WB | DCC |
| 998 | 67, m | rel | p-M5a | 34,4,64,117 | 92 | MNC, WB | DCC |
| 1263 | 40, m | rel | p-M5 | 34,19,33,117 | 18 | WB | DCC |
| 1375 | 44, w | rel | p-M5 | 34,15,33,117 | 35 | WB | DCC, CTX |
| 1303 | 64, w | rel | s-M4 | 34,4,13,33,14,56,65 | 65 | WB | DCC, CTX |
| 1286 | 21, m | rel | s-M5 | 117,33,34 | 35 | WB | DCC, CTX |
| 987 | 61, m | rel | s-M? | 34,13,33,117 | 21 | MNC, WB | DCC |
| 1183 | 36, w | rel | s-M? | 34,33,117 | 33 | MNC, WB | DCC |
| 1307 | 54, m | rel | s-M? | 117,13,15,19,33,34,65 | 30 | WB | DCC, CTX |
| 1386 | 57, m | rel | s-M? | 34,33,117 | 78 | WB | DCC, CTX |
| MDS | |||||||
| 1433 | 59, m | 34,117,14,33 | 14 | WB | DCC, CTX | ||
| 1306 | 42, m | 117,13,33,34 | 11 | WB | DCC, CTX | ||
AML acute myeloid leukaemia; MDS myelodysplastic syndrome; m male; f female; pAML primary AML; sAML secondary AML; FAB french-american-british classification; M? FAB type not classified; dgn diagnosis; pers persistance; rel relapse; IC blasts immunocytologically determined blasts; WB whole blood; MNC mononuclear cells; DCC dendritic cell culture; CTX cytotoxicity fluorolysis assay; bold markers were used to detect DCleu.
4.3. Flow Cytometry
Flow cytometric analyses were performed to assess and quantify frequencies, phenotypes and subsets of leukaemic blasts, DC and T-cells. In cases of aberrant expression of T, B, NK or monocytic antigens, proportions were not included in the analyses. Abbreviations of all cell types are given in Table 7.
Table 7.
Cell types.
| Cell Types | Cell Abbreviation | Surface Marker | Referred To | Abbreviation | Reference |
|---|---|---|---|---|---|
| Blasts | Bla | Bla+ (CD15+, CD34+, CD65+, CD117+) | MNC or WB WB |
Bla/cells Bla/WB |
[10] |
| Dendritic Cells | DC | DC+ (CD80+, CD83+, CD86+, CD206+, CD209+,) | MNC or WB WB |
DC/cells DC/WB |
[10] |
|
Leukaemia Derived
Dendritic Cells |
DCleu | DC+Bla+ | MNC or WB WB DC |
DCleu/cells DCleu/WB DCleu/DC |
[10] |
|
Mature Migratory
Dendritic Cells |
DCmig | DC+CCR7+ | DC | DCmig/DC | [12] |
Cell types and their surface marker combinations for flow cytometric staining and analysis. WB whole blood; MNC mononuclear cells.
Therefore, cells were stained with various monoclonal antibodies (moAbs) labelled with fluorescein isothiocyanate (FITC)a, phycoerythrin (PE)b, phycoerythrin-cyanine-7 tandem conjugate (PE-Cy7)c or allophycocyanin (APC)d. Antibodies were provided by Becton Dickinson (Heidelberg, Germany) (CD1ab, CD1ba, CD14c, CD15d, CD71c, CD206d, 7AADc, CCR7c), Beckmann Coulter (Krefeld, Germany) (CD1ab, CD3a, CD19c, CD33d, CD56b, CD80b, CD117b,d, CD206b, CD34a,c,CD65a, CD83a) and Thermo Fisher Scientific (Darmstadt, Germany) (CD1ab,CD13c,CD34d and CD86a).
Cell staining was performed by incubating cells with corresponding moAbs for 15 min in the dark using a staining medium containing 80% PBS (Biochrom) and 20% FCS (Biochrom). In the case of WB samples, erythrocytes were lysed prior to staining using a lysing buffer (Becton Dickinson) according to the manufacturers’ instructions. Cells were analysed with the fluorescence-activated cell sorting flow cytometer FACS Calibur (Becton Dickinson) and the acquisition and analysis software CellQuestPro (Becton Dickinson). Isotype controls were conducted according to the manufacturer’s instructions.
4.4. Dendritic Cell Culture (DCC)
DC/DCleu were generated from MNC and WB following refined DC/DCleu-generating protocols using specific response modifiers, including Ca-Ionophore A23187 (Ca-Iono) (Sigma Aldrich Chemie GmbH), FLT3-ligand (FL) (PromoCell, Heidelberg, Germany), granulocyte-macrophage-colony-stimulating-factor (GM-CSF) (Sanofi-Aventis, Frankfurt, Germany), interferon-alpha (IFNa) (Essex Pharma, Munich, Germany), interleukin-1-beta (IL-1b) (Cell Concepts, Umkirch, Germany), interleukin-4 (IL-4) (PeproTech, Berlin, Germany), interleukin-6 (IL-6) (Cell Concepts); picibanil (OK-432) (Chugai Pharmaceutical, Kajiwara, Japan); tumor-necrosis-factor-alpha (TNFa) (Cell Concepts), prostaglandin-E1 (PGE1) (PeproTech) and prostaglandin-E2 (PGE2) (PeproTech) (Table 8).
Table 8.
DC/DCleu-generating protocols.
| DC/DCleu-Protocol | DC/DCleu-Source | Composition (Total) and Time Added (d) | Culture Time (d) | References |
|---|---|---|---|---|
| Ca | MNC WB |
Ca-Iono 375 ng/mL (d1) IL-4 250 U/mL (d1) |
3–4 | [11,19] |
| Mcm | MNC WB |
GM-CSF 800 U/mL (d1, d4-5) TNFa 200 U/mL (d7-8) PGE2 1 μg/mL (d7-8) IL-1b 5 ng/mL (d7-8) IL-4 500 U/mL (d1, d4-5) IL-6 150 ng/mL (d7-8) FL 40 ng/mL (d, d4-5) |
10–14 | [11,19] |
| Pici | MNC WB |
GM-CSF 500 U/mL (d1) OK-432 10 μg/mL (d7-8) IL-4 250 U/mL (d1) |
9–11 | [11] |
| Kit-A | WB | GM-CSF 800 U/mL (d1, d2-3) TNFa 200 U/mL (d1, d2-3) |
7–10 | European Patent EP 3217975 B1 |
| Kit-C | WB | GM-CSF 800 U/mL (d1, d2-3) TNFa 200 U/mL (d, d2-3) PGE2 1 µg/mL (d1, d2-3) |
7–10 | |
| Kit-D | WB | GM-CSF 800 U/mL (d1, d2-3) OK-432 10 µg/mL (d1, d2-3) PGE2 1 µg/mL (d1, d2-3) |
7–10 | |
| Kit-E | WB | GM-CSF 800 U/mL (d1, d2-4, d7-8) IFNa 500 U/mL (d1, d2-4, d7-8) |
8–11 | [19] |
| Kit-F | WB | Ca-Iono 250 ng/mL (d1, d2-3) GM-CSF 800 U/mL (d1, d2-3) |
3–4 | European Patent EP 3217975 B1 |
| Kit-G | WB | GM-CSF 800 U/mL (d1, d2-3) | 7–10 | |
| Kit-H | WB | IFNa 500 U/mL (d1, d2-4, d7-8) | 7–10 | [19] |
| Kit-I | WB | GM-CSF 800 U/mL (d1, d2-3) OK-432 10 μg/mL (d1, d2-3) |
7–10 | European Patent EP 3217975 B1 US Patent US 10,912,820 |
| Kit-K | WB | GM-CSF 800 U/mL (d1, d2-3) PGE2 1 μg/mL (d1, d2-3) |
7–10 | European Patent EP 3217975 B1 |
| Kit-M | WB | GM-CSF 800 U/mL (d1, d2-3) PGE1 1 μg/mL (d1, d2-3) |
7–10 | European Patent EP 3217975 B1 US Patent US 10,912,820 |
Ca-Iono Ca-Ionophore A23187; FL FLT3-ligand; GM-CSF granulocyte-macrophage-colony-stimulating-factor; IFNa interferon-alpha; IL-1b interleukin-1-beta; IL-4 interleukin-4; IL-6 interleukin-6; OK-432 picibanil; TNFa tumor-necrosis-factor-alpha; PGE1 prostaglandin-E1; PGE2 prostaglandin-E2; d day.
For MNC-based cultures (as used for Ca, Mcm, Pici), 7 × 105 MNC/mL (Ca), 2.5 × 106 MNC/mL (Mcm) or 1 × 106 MNC/mL (Pici) were cultured in 12-multiwell-culture-plates (Thermo Fisher Scientific) and diluted with 2 mL x-vivo-15-medium (Lonza, Basel, Switzerland). For WB-based cultures (as used for Ca, Mcm, Pici, Kit-A, -C, -D, -E, -F, -G, -H, -I, -K and -M), 0.5–2 mL WB were cultured in 12-multiwell-culture-plates and diluted with 0.5–2 mL x-vivo-15-medium. Response modifiers were added to all cultures according to the protocols. One culture without added response modifiers severed as control. Cells were cultured under standard conditions at 21% O2, 5% CO2 and 37 °C, and harvested following the protocols. A half-medium-exchange was carried out after 7–8 days in Mcm-cultures.
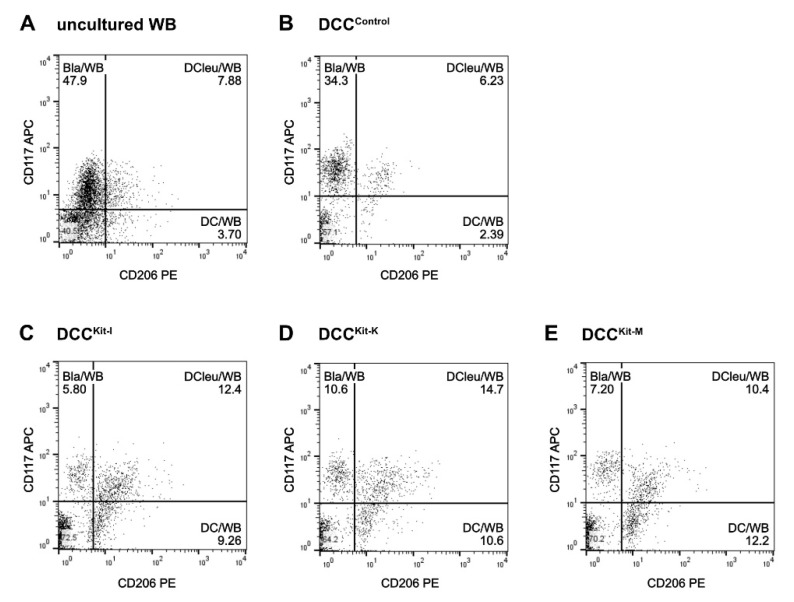
Flow cytometric analyses of (mature) DC and DCleu (subgroups) were performed following a refined gating strategy [10,11,17]. DCleu were analysed by the co-expression of at least one blast marker (CD15, CD34, CD65, CD117), including lineage-aberrant markers (CD56) and one dendritic marker (CD80, CD83, CD86, CD206, CD209) that had not been expressed on naive blasts (Figure 9). The premise for DC subgroup analyses was the presence of ≥10% DC/cells.
Figure 9.
Flow cytometric analyses of DCleu. Examplary plots show DCleu, characterised by the coexpression of the blast marker CD117 and the DC marker CD206, in uncultured WB (A) and after kit treatment with Kit-I (DCCKit-I) (C), Kit-K (DCCKit-K) (D), Kit-M (DCCKit-M) (E) as well as the Control (DCCControl) (B).
To analyse the competence of DC/DCleu-generating protocols to generate DC/DCleu, we developed a refined classification system, the DC/DCleu-classification, based on frequencies of DC/cells and DCleu/cells generated. DC/DCleu-generation was classified as ‘sufficient’, further subdivided into ‘excellent’, ‘good’ and ‘satisfactory’ or as ‘insufficient’, as given in Table 1.
4.5. Mixed Lymphocyte Culture (MLC)
DC/DCleu from WB-cultures were used to stimulate T-cell enriched immunoreactive cells. Therefore, a fraction of DCC containing 25 × 103 DC/DCleu and 1 × 106 T-cells were co-cultured in 24-multiwell-culture-plates (Thermo Fisher Scientific) and diluted in 1 mL RMPI-1640 containing 100 U/mL penicillin. 50 U/mL interleukin-2 (IL-2) (PeproTech) were added to all cultures on day 0 and days 2–3. Cells were cultured following the protocols under standard conditions at 21% O2, 5% CO2 and 37 °C. A half-medium exchange was carried out after 2–3 days.
4.6. Cytotoxicity Fluorolysis Assay (CTX)
The blast lytic activity of DC/DCleu stimulated T-cell enriched immunoreactive cells in MLC from WB-cultures was analysed using a cytotoxicity fluorolysis assay (CTX). Therefore, a fraction of MLC containing 1 × 106 T-cells (effector cells) was co-cultured with 1 × 106 thawed autologous leukaemic blasts (target cells) for 3 and 24 h at 37 °C, 21% O2 and 5% CO2. Target cells were stained with blast specific moAbs before culture and with 7AAD and a particular number of fluorosphere beads (Beckman Coulter) after culture when harvested. As a control, effector and target cells were cultured under the same conditions but separated and only combined prior to flow cytometric analyses.
Flow cytometric analyses were performed using a refined gating strategy [21], whereas the achieved blast lytic activity was defined as the percentual difference of viable target cells (blasts) between the effector-target-co-culture and the control.
4.7. Statistical Methods
Data are presented as mean ± standard deviation (SD). Statistical analyses were implemented using the two-tailed t-test. Significance was defined as ‘not significant’ (n.s.) with p values > 0.10, as ‘borderline significant’ with p values 0.10 to 0.05, as ‘significant’ with p values 0.05 to 0.005 and as ‘highly significant’ with p values < 0.005. All statistical analyses and figures were implemented using Excel 2013 (Microsoft, Redmond, WA, USA), SPSS (IBM, Armonk, NY, USA) and Prism 9 (GraphPad Software, San Diego, CA, USA).
5. Conclusions
In this study, we developed DC/DCleu-generating protocols (kits; comprising one to three response modifiers) specifically designed for the application in a WB-environment. We assessed kits based on their capacity to generate mature DC/DCleu from leukaemic myeloid blasts and to mediate DC/DCleu-based anti-leukaemic immunity. Ultimately, we identified Kit-I, -K and -M as the best performing kits in vitro, qualifying for further evaluation in vitro and vivo. In regard to future clinical applications, these findings are of particular importance as they are the basis for establishing clinical protocols for successful DC/DCleu-based immunotherapy of AML patients in vivo.
6. Patents
Modiblast Pharma GmbH (Oberhaching, Germany) holds the European Patent ‘EP 3,217,975 B1′ and the US Patent ‘US 10,912,820′, in which H.M.S. is involved.
Acknowledgments
The authors thank patients, nurses, and physicians for their support with sample materials and diagnostic reports.
Appendix A
Figure A1.
DC/DCleu-generation from leukaemic WB and MNC with standard DC/DCleu-generating protocols (Ca, Mcm, Pici). Given are the mean ± SD of DC/cells, DCleu/cells, DCleu/DC and DCmig/DC generated with the standard protocol Ca (A) and Pici (B) from MNC compared to WB. Statistically significant differences (p values < 0.05) (two-tailed t-test) are given.
Figure A2.
DC/DCleu-generation from leukaemic WB with standard (Ca, Mcm, Pici) and new DC/DCleu-generating protocols (kits). Given are the mean ± SD of DC/cells, DCleu/cells, DCleu/DC and DCmig/DC generated with Mcm compared to Kit-A (A), to Kit-C (B), to Kit-G (C), to Kit-H (D), and to Kit-K (E), and Pici compared to Kit-D (F) and Kit-G (G). Statistically significant differences (p values < 0.05) (two-tailed t-test) are given.
Author Contributions
C.S. (Christoph Schwepcke) conducted DCC, MLC and CTX experiments. L.K.K., D.D., D.C.A., Z.F., F.D.-G., C.G., A.H.-L., T.K., J.T. and M.W. performed additional DCC, MLC and CTX experiments. C.S. (Christoph Schwepcke) performed data analyses, statistical analyses and visualisation. L.K.K. performed additional data analyses, statistical analyses and visualisation. J.-O.W., A.R. and C.S. (Christoph Schmid) provided leukaemic whole blood samples and corresponding diagnostic reports. H.M.S. designed the study. L.K.K., C.S. (Christoph Schwepcke) and H.M.S. drafted the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Ludwig Maximilian University Hospital Munich (vote No. 33905).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Modiblast Pharma GmbH (Oberhaching, Germany) holds the European Patent ‘EP 3217975 B1′ and the US Patent ‘US 10,912,820′, in which H.M.S. is involved.
Funding Statement
The project was financially supported by the DAAD (ID 21520154) and intramural funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sawyers C.L., Denny C.T., Witte O.N. Leukemia and the disruption of normal hematopoiesis. Cell. 1991;64:337–350. doi: 10.1016/0092-8674(91)90643-D. [DOI] [PubMed] [Google Scholar]
- 2.Lowenberg B., Downing J.R., Burnett A. Acute myeloid leukemia. N. Engl. J. Med. 1999;341:1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 3.Shallis R.M., Wang R., Davidoff A., Ma X., Zeidan A.M. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019;36:70–87. doi: 10.1016/j.blre.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Döhner H., Estey E., Grimwade D., Amadori S., Appelbaum F.R., Büchner T., Dombret H., Ebert B.L., Fenaux P., Larson R.A., et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Short N.J., Rytting M.E., Cortes J.E. Acute myeloid leukaemia. Lancet. 2018;392:593–606. doi: 10.1016/S0140-6736(18)31041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Stat Facts: Leukaemia—Acute Myeloid Leukaemia (AML) [(accessed on 11 February 2021)];2020 Available online: https://seer.cancer.gov/statfacts/html/amyl.html.
- 7.Moeinafshar A., Hemmati S., Rezaei N. Immunotherapy in AML: A brief review on emerging strategies. Clin. Transl. Oncol. 2021;23:2431–2447. doi: 10.1007/s12094-021-02662-1. [DOI] [PubMed] [Google Scholar]
- 8.Chaplin D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010;125:S3–S23. doi: 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mellman I. Dendritic Cells: Master Regulators of the Immune Response. Cancer Immunol. Res. 2013;1:145–149. doi: 10.1158/2326-6066.cir-13-0102. [DOI] [PubMed] [Google Scholar]
- 10.Ogasawara M., Miyashita M., Yamagishi Y., Ota S. Wilms’ tumor 1 peptide-loaded dendritic cell vaccination in patients with relapsed or refractory acute leukemia. Ther. Apher. Dial. 2022;26:537–547. doi: 10.1111/1744-9987.13828. [DOI] [PubMed] [Google Scholar]
- 11.Lee J.-J., Kook H., Park M.-S., Nam J.-H., Choi B.-H., Song W.-H., Park K.-S., Lee I.-K., Chung I.-J., Hwang T.-J., et al. Immunotherapy using autologous monocyte-derived dendritic cells pulsed with leukemic cell lysates for acute myeloid leukemia relapse after autologous peripheral blood stem cell transplantation. J. Clin. Apher. 2004;19:66–70. doi: 10.1002/jca.10080. [DOI] [PubMed] [Google Scholar]
- 12.Kitawaki T., Kadowaki N., Fukunaga K., Kasai Y., Maekawa T., Ohmori K., Itoh T., Shimizu A., Kuzushima K., Kondo T., et al. Cross-priming of CD8(+) T cells in vivo by dendritic cells pulsed with autologous apoptotic leukemic cells in im-munotherapy for elderly patients with acute myeloid leukemia. Exp. Hematol. 2011;39:424–433.e2. doi: 10.1016/j.exphem.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Rosenblatt J., Stone R.M., Uhl L., Neuberg D., Joyce R., Levine J.D., Arnason J., McMasters M., Luptakova K., Jain S., et al. Individualized vaccination of AML patients in remission is associated with induction of antileukemia immunity and prolonged remissions. Sci. Transl. Med. 2016;8:368ra171. doi: 10.1126/scitranslmed.aag1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anguille S., Van de Velde A.L., Smits E.L., Van Tendeloo V.F., Juliusson G., Cools N., Nijs G., Stein B., Lion E., Van Driessche A., et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood. 2017;130:1713–1721. doi: 10.1182/blood-2017-04-780155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L., Giannopoulos K., Reinhardt P., Tabarkiewicz J., Schmitt A., Greiner J., Rolinski J.M., Hus I., Dmoszynska A., Wiesneth M., et al. Immunotherapy for patients with acute myeloid leukemia using autologous dendritic cells generated from leukemic blasts. Int. J. Oncol. 2006;28:855–861. doi: 10.3892/ijo.28.4.855. [DOI] [PubMed] [Google Scholar]
- 16.Amberger D.C., Schmetzer H.M. Dendritic Cells of Leukemic Origin: Specialized Antigen-Presenting Cells as Potential Treat-ment Tools for Patients with Myeloid Leukemia. Transfus. Med. Hemotherapy. 2020;47:432–443. doi: 10.1159/000512452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmetzer H.M., Kremser A., Loibl J., Kroell T., Kolb H.-J. Quantification of ex vivo generated dendritic cells (DC) and leukemia-derived DC contributes to estimate the quality of DC, to detect optimal DC-generating methods or to optimize DC-mediated T-cell-activation-procedures ex vivo or in vivo. Leukemia. 2007;21:1338–1341. doi: 10.1038/sj.leu.2404639. [DOI] [PubMed] [Google Scholar]
- 18.Narita M., Takahashi M., Liu A., Ayres F., Satoh N., Abe T., Nikkuni K., Furukawa T., Toba K., Aizawa Y. Generation of Dendritic Cells from Leukaemia Cells of a Patient with Acute Promyelocytic Leukaemia by Culture with GM-CSF, IL-4 and TNF-α. Acta Haematol. 2001;106:89–94. doi: 10.1159/000046595. [DOI] [PubMed] [Google Scholar]
- 19.Cignetti A., Vallario A., Roato I., Circosta P., Allione B., Casorzo L., Ghia P., Caligaris-Cappio F. Leukemia-derived immature dendritic cells differentiate into functionally competent mature dendritic cells that effi-ciently stimulate T cell responses. J. Immunol. 2004;173:2855–2865. doi: 10.4049/jimmunol.173.4.2855. [DOI] [PubMed] [Google Scholar]
- 20.Kremser A., Dreyig J., Grabrucker C., Liepert A., Kroell T., Scholl N., Schmid C., Tischer J., Kufner S., Salih H., et al. Dendritic cells (DCs) can be successfully generated from leukemic blasts in individual patients with AML or MDS: An evaluation of different methods. J. Immunother. 2010;33:185–199. doi: 10.1097/CJI.0b013e3181b8f4ce. [DOI] [PubMed] [Google Scholar]
- 21.Grabrucker C., Liepert A., Dreyig J., Kremser A., Kroell T., Freudenreich M., Schmid C., Schweiger C., Tischer J., Kolb H.-J., et al. The Quality and Quantity of Leukemia-derived Dendritic Cells from Patients with Acute Myeloid Leukemia and Myelodysplastic Syndrome Are a Predictive Factor for the Lytic Potential of Dendritic Cells-primed Leukemia-Specific T Cells. J. Immunother. 2010;33:523–537. doi: 10.1097/cji.0b013e3181d87ffd. [DOI] [PubMed] [Google Scholar]
- 22.Liepert A., Grabrucker C., Kremser A., Dreyßig J., Ansprenger C., Freudenreich M., Kroell T., Reibke R., Tischer J., Schweiger C., et al. Quality of T-cells after stimulation with leukemia-derived dendritic cells (DC) from patients with acute myeloid leu-kemia (AML) or myeloid dysplastic syndrome (MDS) is predictive for their leukemia cytotoxic potential. Cell. Immunol. 2010;265:23–30. doi: 10.1016/j.cellimm.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Boeck C.L., Amberger D.C., Doraneh-Gard F., Sutanto W., Guenther T., Schmohl J., Schuster F., Salih H., Babor F., Borkhardt A., et al. Significance of Frequencies, Compositions, and/or Antileukemic Activity of (DC-stimulated) Invariant NKT, NK and CIK Cells on the Outcome of Patients With AML, ALL and CLL. J. Immunother. 2017;40:224–248. doi: 10.1097/CJI.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 24.Klauer L.K., Schutti O., Ugur S., Doraneh-Gard F., Amberger D.C., Rogers N., Krämer D., Rank A., Schmid C., Eiz-Vesper B., et al. Interferon Gamma Secretion of Adaptive and Innate Immune Cells as a Parameter to Describe Leukaemia-Derived Dendritic-Cell-Mediated Immune Responses in Acute Myeloid Leukaemia in vitro. Transfus. Med. Hemotherapy. 2021;49:44–61. doi: 10.1159/000516886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirn-Lopez A., Deen D., Fischer Z., Rabe A., Ansprenger C., Stein K., Vogt V., Schick J., Kroell T., Kraemer D., et al. Role of Interferon (IFN)α in “Cocktails” for the Generation of (Leukemia-derived) Dendritic Cells (DCleu) From Blasts in Blood from Patients (pts) With Acute Myeloid Leukemia (AML) and the Induction of Antileukemic Reactions. J. Immunother. 2019;42:143–161. doi: 10.1097/CJI.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 26.Yan W.-L., Shen K.-Y., Tien C.-Y., Chen Y.-A., Liu S.-J. Recent progress in GM-CSF-based cancer immunotherapy. Immunotherapy. 2017;9:347–360. doi: 10.2217/imt-2016-0141. [DOI] [PubMed] [Google Scholar]
- 27.van de Laar L., Coffer P.J., Woltman A.M. Regulation of dendritic cell development by GM-CSF: Molecular control and impli-cations for immune homeostasis and therapy. Blood. 2012;119:3383–3393. doi: 10.1182/blood-2011-11-370130. [DOI] [PubMed] [Google Scholar]
- 28.Tough D.F. Type I Interferon as a Link Between Innate and Adaptive Immunity through Dendritic Cell Stimulation. Leuk. Lymphoma. 2004;45:257–264. doi: 10.1080/1042819031000149368. [DOI] [PubMed] [Google Scholar]
- 29.Koski G.K., Schwartz G.N., Weng D.E., Gress R.E., Engels F.H., Tsokos M., Czerniecki B.J., Cohen P.A. Calcium ionophore-treated myeloid cells acquire many dendritic cell characteristics independent of prior differentiation state, transformation status, or sensitivity to biologic agents. Blood. 1999;94:1359–1371. doi: 10.1182/blood.V94.4.1359. [DOI] [PubMed] [Google Scholar]
- 30.Czerniecki B.J., Carter C., Rivoltini L., Koski G.K., Kim H.I., Weng D.E., Roros J.G., Hijazi Y.M., Xu S., Rosenberg S.A., et al. Calcium ionophore-treated peripheral blood monocytes and dendritic cells rapidly display characteristics of acti-vated dendritic cells. J. Immunol. 1997;159:3823–3837. [PubMed] [Google Scholar]
- 31.Ryoma Y., Moriya Y., Okamoto M., Kanaya I., Saito M., Sato M. Biological effect of OK-432 (picibanil) and possible application to dendritic cell therapy. Anticancer Res. 2004;24:3295–3302. [PubMed] [Google Scholar]
- 32.Sato M., Takayama T., Tanaka H., Konishi J., Suzuki T., Kaiga T., Tahara H. Generation of mature dendritic cells fully capable of T helper type 1 polarization using OK-432 combined with prosta-glandin E2. Cancer Sci. 2003;94:1091–1098. doi: 10.1111/j.1349-7006.2003.tb01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalinski P. Regulation of Immune Responses by Prostaglandin E2. J. Immunol. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jonuleit H., Kühn U., Müller G., Steinbrink K., Paragnik L., Schmitt E., Knop J., Enk A.H. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur. J. Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 35.Kuroki H., Morisaki T., Matsumoto K., Onishi H., Baba E., Tanaka M., Katano M. Streptococcal preparation OK-432: A new maturation factor of monocyte-derived dendritic cells for clinical use. Cancer Immunol. Immunother. 2003;52:561–568. doi: 10.1007/s00262-003-0394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ritter U., Meissner A., Ott J., Korner H. Analysis of the maturation process of dendritic cells deficient for TNF and lymphotoxin-α reveals an essential role for TNF. J. Leukoc. Biol. 2003;74:216–222. doi: 10.1189/jlb.1202587. [DOI] [PubMed] [Google Scholar]
- 37.Amberger D.C., Doraneh-Gard F., Gunsilius C., Weinmann M., Möbius S., Kugler C., Rogers N., Böck C., Ködel U., Werner J.-O., et al. PGE1-Containing Protocols Generate Mature (Leukemia-Derived) Dendritic Cells Directly from Leukemic Whole Blood. Int. J. Mol. Sci. 2019;20:4590. doi: 10.3390/ijms20184590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogihara T., Iinuma H., Okinaga K. Usefulness of immunomodulators for maturation of dendritic cells. Int. J. Oncol. 2004;25:453–459. doi: 10.3892/ijo.25.2.453. [DOI] [PubMed] [Google Scholar]
- 39.Platt A.M., Randolph G.J. Dendritic Cell Migration Through the Lymphatic Vasculature to Lymph Nodes. Adv. Immunol. 2013;120:51–68. doi: 10.1016/b978-0-12-417028-5.00002-8. [DOI] [PubMed] [Google Scholar]
- 40.Förster R., Davalos-Misslitz A.C., Rot A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 41.Tiberio L., Del Prete A., Schioppa T., Sozio F., Bosisio D., Sozzani S. Chemokine and chemotactic signals in dendritic cell migration. Cell. Mol. Immunol. 2018;15:346–352. doi: 10.1038/s41423-018-0005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bedrosian I., Roros J.G., Xu S., Nguyen H.Q., Engels F., Faries M.B., Koski G.K., Cohen P.A., Czerniecki B.J. Granulocyte-macrophage colony-stimulating factor, interleukin-2, and interleukin-12 synergize with calcium ion-ophore to enhance dendritic cell function. J. Immunother. 2000;23:311–320. doi: 10.1097/00002371-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Basak S.K., Harui A., Stolina M., Sharma S., Mitani K., Dubinett S.M., Roth M.D. Increased dendritic cell number and function following continuous in vivo infusion of granulocyte macrophage–colony-stimulating factor and interleukin-4. Blood. 2002;99:2869–2879. doi: 10.1182/blood.V99.8.2869. [DOI] [PubMed] [Google Scholar]
- 44.Romani N., Gruner S., Brang D., Kämpgen E., Lenz A., Trockenbacher B., Konwalinka G., Fritsch P.O., Steinman R.M., Schuler G. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naito K., Ueda Y., Itoh T., Fuji N., Shimizu K., Yano Y., Yamamoto Y., Imura K., Kohara J., Iwamoto A., et al. Mature dendritic cells generated from patient-derived peripheral blood monocytes in one-step culture using streptococcal preparation OK-432 exert an enhanced antigen-presenting capacity. Int. J. Oncol. 2006;28:1481–1489. doi: 10.3892/ijo.28.6.1481. [DOI] [PubMed] [Google Scholar]
- 46.Rieser C., Böck G., Klocker H., Bartsch G., Thurnher M. Prostaglandin E2 and Tumor Necrosis Factor α Cooperate to Activate Human Dendritic Cells: Synergistic Activation of Interleukin 12 Production. J. Exp. Med. 1997;186:1603–1608. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waclavicek M., Berer A., Oehler L., Stöckl J., Schloegl E., Majdic O., Knapp W. Calcium ionophore: A single reagent for the differentiation of primary human acute myelogenous leukaemia cells towards dendritic cells. Br. J. Haematol. 2001;114:466–473. doi: 10.1046/j.1365-2141.2001.02970.x. [DOI] [PubMed] [Google Scholar]
- 48.Westers T.M., Houtenbos I., Snoijs N.C.L., A Van De Loosdrecht A., Ossenkoppele G.J. Leukemia-derived dendritic cells in acute myeloid leukemia exhibit potent migratory capacity. Leukemia. 2005;19:1270–1272. doi: 10.1038/sj.leu.2403794. [DOI] [PubMed] [Google Scholar]
- 49.Kaliński P., Hilkens C.M., Snijders A., Snijdewint F.G., Kapsenberg M.L. Dendritic Cells in Fundamental and Clinical Immunology. Springer; Berlin/Heidelberg, Germany: 1997. Dendritic cells, obtained from peripheral blood precursors in the presence of PGE 2, promote Th2 responses; pp. 363–367. [DOI] [PubMed] [Google Scholar]
- 50.Quesada J.R., Rios A., Swanson D., Trown P., Gutterman J.U. Antitumor activity of recombinant-derived interferon alpha in metastatic renal cell carcinoma. J. Clin. Oncol. 1985;3:1522–1528. doi: 10.1200/JCO.1985.3.11.1522. [DOI] [PubMed] [Google Scholar]
- 51.Smits E.L., Anguille S., Berneman Z.N. Interferon α may be back on track to treat acute myeloid leukemia. OncoImmunology. 2013;2:e23619. doi: 10.4161/onci.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang H., Liu X.H., Kong J., Wang J., Jia J.S., Lu S.Y., Gong L.Z., Zhao X.S., Jiang Q., Chang Y.J., et al. Interferon-α as maintenance therapy can significantly reduce relapse in patients with favorable-risk acute myeloid leukemia. Leuk Lymphoma. 2021;62:2949–2956. doi: 10.1080/10428194.2021.1948027. [DOI] [PubMed] [Google Scholar]
- 53.Ives N.J., Suciu S., Eggermont A.M., Kirkwood J., Lorigan P., Markovic S.N., Garbe C., Wheatley K., Bufalino R., Cameron D., et al. Adjuvant interferon-α for the treatment of high-risk melanoma: An individual patient data meta-analysis. Eur. J. Cancer. 2017;82:171–183. doi: 10.1016/j.ejca.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 54.Anguille S., Lion E., Willemen Y., Van Tendeloo V.F.I., Berneman Z.N., Smits E.L.J.M. Interferon-α in acute myeloid leukemia: An old drug revisited. Leukemia. 2011;25:739–748. doi: 10.1038/leu.2010.324. [DOI] [PubMed] [Google Scholar]
- 55.Lowin B., Hahne M., Mattmann C., Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 56.Hassin D., Garber O.G., Meiraz A., Schiffenbauer Y.S., Berke G. Cytotoxic T lymphocyte perforin and Fas ligand working in concert even when Fas ligand lytic action is still not detectable. Immunology. 2011;133:190–196. doi: 10.1111/j.1365-2567.2011.03426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gard F.D., Amberger D., Weinmann M., Boeck C., Gunsilius C., Kugler C., Werner J., Kraemer D., Rank A., Schmid C., et al. Standard normoxic versus physiological hypoxic culture of AML patients’ (pts) whole blood (WB) samples with immune modulatory kits yields comparable proportions of dendritic cells and functional results. Eur. J. Cancer. 2018;92:S10–S11. doi: 10.1016/j.ejca.2018.01.027. [DOI] [Google Scholar]
- 58.Pepeldjiyska E., Li L., Gao J., Seidel C.L., Blasi C., Özkaya E., Schmohl J., Kraemer D., Schmid C., Rank A., et al. Leukemia derived dendritic cell (DCleu) mediated immune response goes along with reduced (leukemia-specific) regulatory T-cells. Immunobiology. 2022;227:152237. doi: 10.1016/j.imbio.2022.152237. [DOI] [PubMed] [Google Scholar]
- 59.Plett C., Amberger D.C., Rabe A., Deen D., Stankova Z., Hirn Lopez A., Vokac Y., Werner J.O., Krämer D., Rank A. Kits do not induce AML-blasts’ proliferation ex vivo. IPO-38 is an appropriate and reliable marker to detect and quantify proliferating blasts. Eur. J. Cancer. 2017;5:3–4. [Google Scholar]
- 60.Schmid C., Atzler M., Rank A., Inngjerdingen M., Rabe A., Deen D., Wang R., Eiz-Vesper B., Schmetzer H. Immune modulation of AML-blasts in therapy refractory AML-patient in vivo with clinically approved response modifiers improves clinical status, blood cell regeneration and gives rise to leukaemia specific adaptive and innate immune reactive cells. Eur. J. Cancer. 2018;92:S15. doi: 10.1016/j.ejca.2018.01.038. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.