Abstract
Alcohol consumption is a global healthcare problem. Chronic alcohol consumption generates a wide spectrum of hepatic lesions, the most characteristic of which are steatosis, hepatitis, fibrosis, and cirrhosis. Alcoholic liver diseases (ALD) refer to liver damage and metabolomic changes caused by excessive alcohol intake. ALD present several clinical stages of severity found in liver metabolisms. With increased alcohol consumption, the gut microbiome promotes a leaky gut, metabolic dysfunction, oxidative stress, liver inflammation, and hepatocellular injury. Much attention has focused on ALD, such as alcoholic fatty liver (AFL), alcoholic steatohepatitis (ASH), alcoholic cirrhosis (AC), hepatocellular carcinoma (HCC), a partnership that reflects the metabolomic significance. Here, we report on the global function of inflammation, inhibition, oxidative stress, and reactive oxygen species (ROS) mechanisms in the liver biology framework. In this tutorial review, we hypothetically revisit therapeutic gut microbiota-derived alcoholic oxidative stress, liver inflammation, inflammatory cytokines, and metabolic regulation. We summarize the perspective of microbial therapy of genes, gut microbes, and metabolic role in ALD. The end stage is liver transplantation or death. This review may inspire a summary of the gut microbial genes, critical inflammatory molecules, oxidative stress, and metabolic routes, which will offer future promising therapeutic compounds in ALD.
Keywords: alcohol consumption, gut microbiome, metabolomics, liver injury, fibrosis, steatohepatitis, cirrhosis, hepatocellular carcinoma, liver transplantation
1. Introduction
The past decade has witnessed that the human gut microbiota is a key player in alcoholic liver disease (ALD). The genetic capacity of the gut microbiome evolves the human lifespan. Gut microbes are considered a potential source for new therapeutic biomarker discoveries in ALD. Gut intestine (GI) has a huge collection of various microorganisms (i.e., bacteria, viruses, archaea, and eukaryotes), which are found in human body systems. The majority of microorganisms reside throughout the skin, saliva, oral mucosa, oral cavity, respiratory tract, reproductive systems, and GI [1,2]. GI contains 95 % of the human microbiome (i.e., 100 trillion bacteria and 1000 isolated bacterial species) [3]. Curiously, it has been found that 99% of the human gut microbiota in the GI tract are anaerobic bacteria. The remaining 1% include fungi, protozoa, archaea, and many microorganisms [4,5].
The ALD refers to liver disease caused by excessive alcohol consumption, a direct cause of liver disease with a high mortality rate [6]. ALD takes many years to develop. It can start from acute fatty liver to chronic alcoholic cirrhosis (AC), which can then lead to liver cancer (i.e., hepatic carcinoma, HCC) and liver failure. An acute to chronic stage of liver condition, alcohol, or ethanol can contribute to liver cirrhosis and hepatocyte cellular death through an increasing reactive oxygen species (ROS) level [7,8].
Gut microbiota contribute to host metabolism via metabolic signaling and microbial pathways. The communication of microbiota with the host-interacting organisms is essential for ALD prevention [9]. The good and bad bacterial flora (e.g., good Escherichia coli, Lactobacilli, Bifidobacteria, and bad Clostridium difficile, Enterococcus faecalis, Lampyllobacter) are related to liver disease metabolism, obesity, and ALD. Good bacteria are involved in metabolic health maintenance, anti-aging effects, stimulating immunity role in ALD, and act as probiotic bacterium. Bad bacteria are related to pathogens, which could yield metabolites that might impact the metabolic ALD [10]. Strangely, decreased Bacteroides and increased Firmicutes have a wide tendency toward fatty liver formation (i.e., obesity). The phyla proteobacteria, actinobacteria, fusobacteria, and verrucomicrobia are relatively low in abundance in the gut. Gut microbiome colonization starts at birth and is established in the first three years [11,12].
The goal of this review article is to understand only the gut microbiota-derived alcoholic oxidative stress, liver inflammation, inflammatory cytokines, and metabolic regulation in the ALD profile. The molecular mechanisms behind the alcohol effects are not extensively studied in the literature, and therefore, we delineated and limited the current study only to the unexplored area (alterations in microbial gene communities and metabolite profile). The bulk of this review focuses on the microbial community and bioactive microbial products, such as small molecule profiling and disruption in various ALD. ALD exhibits greater healthcare problems and is a microbial disruptor affecting microbiome architecture, metabolic inhibition, and cellular partners. Metabolic functions in the GI tract can allow pathogenic bacteria to colonize, resulting in host infection [8]. Here, we chose to focus on several ALD-based clinical stages, such as AFL, alcoholic liver fibrosis, ASH, AC, and HCC. To address these gaps, the five clinical stages of ALD are discussed in this review manuscript. We hypothetically revisit therapeutic gut microbiota-derived alcoholic oxidative stress, liver inflammation, inflammatory cytokines, and metabolic regulation. The novelty of our manuscript is that acute to chronic ALD is proposed with every stage of metabolic damage.
2. Oxidative Stress Formation in ALD
Oxidative stress is formed by excessive ROS production, which leads to apoptosis and necrosis. Alcohol can enter the liver through the portal vein. Oxidative stress and oxidant stress are pathogenic factors, which result in the production of free radicals, acetaldehyde, and fatty acid ethyl esters that can damage the liver metabolisms [13].
ROS is highly involved in ALD. Alcohol-associated hepatocarcinogenesis is oxidative stress, which is secondary to ROS due to alcohol metabolism, inflammation, and expanded iron storage. ROS (i.e., hydrogen peroxide, H2O2; hydroxyl radical, ºOH; hydroxide, OH−, etc.,) can play a significant role in ALD development. Alcohol oxidation via alcohol dehydrogenase (ADH) and the microsomal ethanol-oxidizing system (MEOS) play a significant role in alcoholic metabolism [5].
In fact, ROS harms mobile macromolecules that may induce the development of liver carcinogenesis through the formation of lipid peroxides, including 4-hydroxynonenal [14]. The accumulation of ROS causes structural adjustments after damaging the DNA, mainly cell-cycle arrest or apoptosis. The DNA critically impacts genetic functions, including replication and transcription, and cancer proliferation [15]. The accumulation of ROS additionally induces the production of numerous cytokines, the activation of immune cells, angiogenesis, and the metastatic process [16]. Oxidative metabolites, such as acetaldehyde and ROS, produced by alcohol metabolism, can induce epigenetic changes through changes in the metabolism of folate, an essential component of DNA synthesis, and methylation [17,18].
Epigenetic regulation involves several chemical modifications, including DNA methylation [19,20]. The genetic polymorphisms in the methylene tetrahydrofolate reductase (MTHFR) gene can cause the changes in folate metabolism that have been reported to be associated with the development of AC, HCC [21], and ALD [22]. The oxidation of alcohol through the ADH paths delivers acetaldehyde, which is converted to acetate. Both the oxidation and reduction reaction reduce nicotinamide adenine dinucleotide (NAD) to its reduced form, NAD hydrogen (NADH).
Alcohol is metabolized into acetaldehyde in the liver, which causes toxicity. Liver cirrhosis is a process in which the normal liver structure is converted into abnormal nodules and eventually into fibrosis [23]. Liver cirrhosis is a chronic form of liver damage, and the causes of cirrhosis include excessive consumption of alcohol, chronic hepatitis C virus infection, and chronic hepatitis B virus infection [24]. Additionally, the symptoms of liver cirrhosis can lead to portal hypertension, enlarged spleen, poor nutrition, ascites, kidney failure, and liver cancer [25].
Fibrogenic mechanisms are initiated and maintained by alcohol metabolism [26]. The metabolism of alcohol in the liver is a highly oxidative event that results in the generation of acetaldehyde [27]. Acetaldehyde is a major toxic metabolite and is one of the major causes mediating the fibrogenic and mutagenic effects of alcohol in the liver [28]. Hepatocytes principally produce acetaldehyde and act on activated hepatic stellate cells (HSC) in a paracrine way, and they directly increase the expression of collagen-I in hepatic stellate cells by activating multiple signaling pathways and transcription factors. Acetaldehyde responds quickly to cellular components, manufacturing adducts, such as malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), and malondialdehyde-acetaldehyde (MAA), which help maintain HSC activation [29].
Drinking excessive amounts of alcohol might upset the equilibrium of intestinal microbes. Verrucomibrobia and Bacteroidetes grew in alcohol-fed animals. Firmicutes largely reduced. Firmicutes played a starring role [30]. The particular gut pathogen can cause the GI epithelial cells to produce ROS. Strains of the Lactobacillus genus of the GI symbiotic bacteria can cause intestinal phagocytes to produce ROS dependent on NADPH oxidase 1 (NOX1) [31]. Enhanced damage-related molecular patterns (DAMPs) were also produced by increased ROS/RNS, which worsened inflammation. The redox equilibrium of the gut is significantly influenced by gut microorganisms. Alcohol-induced gut microbiota imbalance results in deleterious metabolic expression that affects the liver.
3. Alcohol-Induced Metabolic Inflammation and Cellular Alterations
An inflammasome is a multiprotein oligomer comprising caspase-1, PYCARD, and NALP that promotes the activation and recruitment of inflammatory cells in response to cellular danger signals. The inflammasome initiates procaspase-1, which converts pro-interleukin (IL)-1 to functional IL-1 [32]. Oxidative stress also stimulates the conversion of pro-IL-18 to IL-18, which leads to IFN secretion and natural killer cell activation, as well as the degradation and inactivation of IL-33 [33,34]. Cytosolic caspase activation and recruitment domain (CARD), which recruits caspase-1 to the inflammasome, is also activated by inflammatory stimuli. Inflammasome and IL-1 are activated in ALD patients and rodent models [35]. Recently, multiple inflammasomes had mRNA expression in the liver, implying that inflammasome activation is part of the liver pathophysiology.
Alcohol metabolites, such as both acetaldehyde and acetate, directly induce an inflammatory response. However, lipopolysaccharide (LPS)-related initiation of proinflammatory cytokines in the Kupper cells/macrophages has activated. Acetaldehyde and acetate exposure of rodent macrophage cells caused the activation of NF-κB signaling and production of TNF-α [36]. This NF-κB activation is, in part, mediated via the downregulation of SIRT1, an NF-κB antagonist; such effect appears to be limited to alcohol metabolites, while alcohol itself has no effect [36].
Alcohol-induced intestinal permeability was substantially and negatively linked with Bifidobacterium and Faecalibacterium prausnitzii, supporting the notion that some bacteria actually strengthen the gut barrier. The anti-inflammatory effects of Bifidobacterium and Faecalibacterium prausnitzii are well recognized and may be lost due to persistent alcohol use.
4. Alcoholic Liver Diseases
4.1. Alcoholic Fatty Liver and Molecular Networking
Alcoholic fatty liver (AFL) is a build-up of fat inside the liver cells, which helps expand the liver size. Human AFL, liver mortality, and unintended injuries can be elevated among those consuming alcohol over several years [37]. A scar tissue forms when the liver is damaged. This is called liver fibrosis. At the same time, scar tissue replaces healthy tissue. AFL is associated with genetic disorders, lifestyle factors, and social factors. The liver fat concentration can be reduced by heavy alcohol drinking. Worldwide, 90% of chronic liver disease (CLD) and liver failures occur due to alcohol consumption. CLD is a significant public health concern [38].
The World Health Organization (WHO) globally estimates that the seventh major cause of death and physical disability is alcoholic disorders [39]. More than 1.32 million people are globally affected by ALD, which is the most common cause of AC in Europe, North America, South America, and central Asia [40]. Over the past two decades, the prevalence of AC has doubled [41]. Alcohol-related cirrhosis and liver cancer account for 1% of all deaths worldwide, and this is expected to increase and create a multilayered burden [42].
Human microbiome-derived translational medicinal research has clarified that alcohol consumption could be a global health problem. Alcohol is quickly absorbed from the GI tract and increases the concentration of alcohol in the blood. Alcohol-associated chemicals or small molecules play a significant role in liver diseases. The small molecules are mainly caused by excessive alcohol consumption [43]. Excessive drinking of alcohol causes the liver to become inflamed (swollen), which damages its tissues. ALD patients are affected by hepatitis and cirrhosis. The relationship between ALD and the gut microbiome is often neglected [44].
microRNAs (miRNAs) have a central role in protein synthesis. The miRNAs are involved in post-transcriptional modification, miRNAs degradation, and inhibition of miRNAs interactions [45]. In mice, excessive alcohol consumption affected the miRNAs, which led to fatty liver and CLD [46]. CLD has been studied in monozygotic twins, which could explain the miRNAs modification [47]. The genes involved in alcohol metabolism include ADH, acetaldehyde dehydrogenase, and cytochrome P450 2E1 genes that normalize the innate immune response (i.e., Interleukin-1, IL-1; tumor necrosis factor-alpha; TNF-α; and patatin link phospholipase domain containing 3, PNPLA3 genes). Those genes show molecular variances that have been widely considered. The genes of IL-1 and TNF-α related to alcoholic metabolism and AC have been summarized [48,49]. Gut environmental genes and metabolic chemicals may promote the accumulation of fat in the liver. The above genes play an important role in liver metabolic transformation and metabolic dysbiosis from the healthy liver to AFL.
Kupffer cells, stellate cells activation, and macrophages are associated as being against metastatic cancer via CLD. Inflammatory cells, such as monocytes, macrophages, and neutrophils, are derived from the bone marrow, which can migrate to the liver and produce proinflammatory cytokines (i.e., TNF-α, IL-1β, IL-6, monocyte chemoattractant protein-1 (MCP1)) [50,51]. Inflammatory cells in the hepatic sinusoids lead to the activation of stellate cells and the initiation of fibrosis genes (that is, the production of alpha-1 (α-1) smooth muscle actin and collagen-1) [52].
As per the publications, studies of fatty liver have shown that alcohol can damage liver cells and activate the apoptotic pathway. The stress on the alcohol-treated endoplasmic reticulum (ER) activates the interferon gene (SIG), which leads to phosphorylation of the interferon regulatory factor 3 (IRF3). Fatty liver cells are recognized by liver immune cells as “danger” signals, leading to proinflammatory reactions [53]. In hepatocytes, alcohol abuse is characterized by an accumulation of fats (especially triglycerides, phospholipids, and cholesterol esters) [54].
In AFL, the gut microbiota is essential. Bacteroidales, Clostridiales, Enterobacteriales are gaining more attention in AFL. The alteration of gut microbes is brought about by excessive consumption of foods high in calories and alcohol. Due to unbalanced microorganisms, gut dysbiosis develops from chronic alcohol consumption [55]. In particular, alcohol-treated mice exhibited Bacteroidales and a few Prevotella OTUs, suggesting the effects of certain strains. Lachnospiraceae and Roseburia hominis were linked with resistant and FMT-protected mice. Precisely, the downregulated Lachnospiraceae and Roseburia in AC patients were described [56,57]. The different mechanisms and signaling molecules in mice are represented in Table 1.
Table 1.
Robust predictions of the recent genomics and metabolomics in alcoholic animal models.
| Animals | Exposures | Main Results | Ref. | |
|---|---|---|---|---|
| Mice | C57BL/6J male (6–8 weeks old) |
chronic 5% ethanol diet for 11, 22, and 33 days |
(↑) AST, ALT, amount of G-MDSC. | [58] |
| C57BL/6 mice | alcohol diet for 8 weeks | (↑) proportions of CA, total MCA, DCA in ileum unconjugated and total bile acid concentrations in the plasma hepatic Cyp7a1 protein expression, hepatic IL-1B, TNF protein. (↓) proportions of TCA and TDCA in ileum. |
[59] | |
| C57BL/6J female (7–8 weeks old) |
5% ethanol for 6 weeks in Lieber–DeCarli liquid diet |
(↑) liver mRNA expression level of TNF-α, IL-6, Cc2, Ccr5 liver protein level of TNF-α, IL-1β, IL-6, CD14 serum protein level. |
[60] | |
| Rats | Male Wistar rats | Non-stop ethanol supply for 3 weeks. Gut sterilization with polymyxin B and neomycin |
(↓) Plasma endotoxin levels (80–90 pg/mL → <25 pg/mL), average hepatic pathological score in ethanol-fed and antibiotic-treated rats. Antibiotics prevented elevated aspartate aminotransferase levels and hepatic surface hypoxia. |
[61] |
| Mice | Alcohol-fed NS5ATg mice |
Lieber–DeCarli diet containing 3.5% ethanol or isocaloric dextrin for long-term alcohol feeding, repetitive LPS injection |
(↑) Ethanol-induced endotoxemia, liver injury, and tumorigenesis after Toll-like receptor (TLR)-4 induction through hepatocyte-specific transgenic expression of the HCV non-structural protein NS5A. |
[62] |
| Mice | 60 male Kunming mice (18–22 g) (6–8 weeks old) |
alcohol gavage for 2–13 days |
(↑) AST, ALT, TG, Hepatic MDA, ADH, mRNA, and protein expression of Cyp2e1, CAT. (↓)Major endogenous antioxidant enzymes (SOD and GSH-Px) mRNA expression of Nrf2, NQO-1, ADH. |
[63] |
| Rats | Male Wistar rats (170–180 g) |
chronic ethanol feeding | (↑) ROS production by LPS in Kupffer cells isolated from ethanol-fed mice. ROS production in Kupffer cells by LPS stimulation were increased NADPH oxidase dependently. ERK1/2 contributed to the increase in TNF-α production in Kupffer cells by LPS stimulation. |
[64] |
| Mice | C57BL/6 male | EtOH-containing diets (35% of total calories, AF) ad libitum for 4 weeks |
(↑) Saturated fatty acid levels. PLS-DA performed for liver and fecal samples. Mouse liver damage can be improved. (↑) intestinal; (↓) hepatic fatty acids; (↑) amino acid concentration. |
[65] |
| Mice | C57BL/6 male (5–6 weeks old) |
EtOH-containing Lieber– DeCarli liquid diet or an isocaloric control diet |
(↑) ALT and AST. PCA, OPLS-DA, volcano maps, and correlation coefficient analyzed. |
[66] |
| Mice | C57BL/6 male (8 weeks old) |
Intermittent hypoxia exposure |
PCA, OPLS-DA, and volcano maps, heatmaps analyzed. (↑) N1-(5-Phospho-D-ribosyl)-AMP, stearidonic acid, adenine, arachidonic acid (peroxide-free), ergothioneine, betaine, cyclohexylamine, GSH, GSH disulfide. |
[67] |
| Mice | Kunming mice (7 weeks old) |
10% lard, 20% sucrose, 2.5% cholesterol, and 0.5% sodium cholate |
(↑) Taurochenodeoxycholic acid, taurine, chenodeoxycholic acid, (4Z,7Z,10Z,13Z,16Z,19Z)-4,7,10,13,16, 19-docosahexaenoic acid, oleic acid, alpha-linolenic acid. Enrichment analysis. |
[68] |
| Rats | Male Sprague Dawley rats (1 year old, 180–200g) |
CCl4 (1mL/kg 40% CCl4, diluted in olive oil); twice a week for eight weeks |
H&E and Masson’s trichrome staining, PLS-DA, heatmap, ROC curve analysis. (↑) L-tryptophan, L-valine, cholesterol, glycocholate, methylmalonic acid. |
[69] |
| Mice | BALB/c mice (8 weeks old) |
E. granulosus infection | 25mg of hepatic and fecal samples were analyzed. PCA, OPLS-DA, and volcano maps, heatmaps analyzed. (↑) 2-ethyl-2-hydroxybutyric acid, 2-hydroxyvaleric acid, cytidine 2’,3’-cyclic phosphate, sodium citrate, carboxytolbutamide, methylselenopyruvate. (↓) Pyronaridine, Bis(4-nitrophenyl) phosphate, Inosinic acid, 5-Phosphoribosyl-4-carboxy-5- aminoimidazole, tolclofos-methy, maduropeptin chromophore. |
[70] |
| Mice | Fat-1 transgenic mice (10–12 weeks old) |
EtOH diet | (↑) neutrophil accumulation, Pai-1 expression in wild-type mice. (↓) neutrophil accumulation, pai-1 expression, KC M1 abundance in fat-1 mice. Flow cytometry analysis of hepatic immune cells. |
[71] |
Notes and abbreviations: ↑, and ↓ show an increase and decrease in the condition. EtOH, ethanol; AST, aspartate transaminase; ALT, alanine transaminase; G-MDSC, granulocyte-like myeloid-derived suppressor cells; ADH, alcohol dehydrogenase; ALD, Alcoholic liver disease; IL, interleukin; LBP, Lipopolysaccharide binding protein; LPS, Lipopolysaccharide; NADPH, Nicotinamide adenine dinucleotide phosphate; ROS, reactive oxygen species; TLR-4, Toll-like receptor-4; TNF-α, Tumor necrosis factor-α; AHB, asymptomatic hepatitis B virus infection; CHB, chronic hepatitis B; CHC, chronic hepatitis C; CIR, cirrhosis type C; HCV, Hepatitis C virus; HCC, hepatocellular carcinoma; GSH, Glutathione; NAFLD, non-alcoholic fatty liver disease; PLS-DA, Partial least-squares discriminant analysis; OPLS-DA, Orthogonal PLS-DA; E. granulosus, Echinococcus granulosus.
4.2. Alcoholic Liver Fibrosis in Humans with Alcoholism
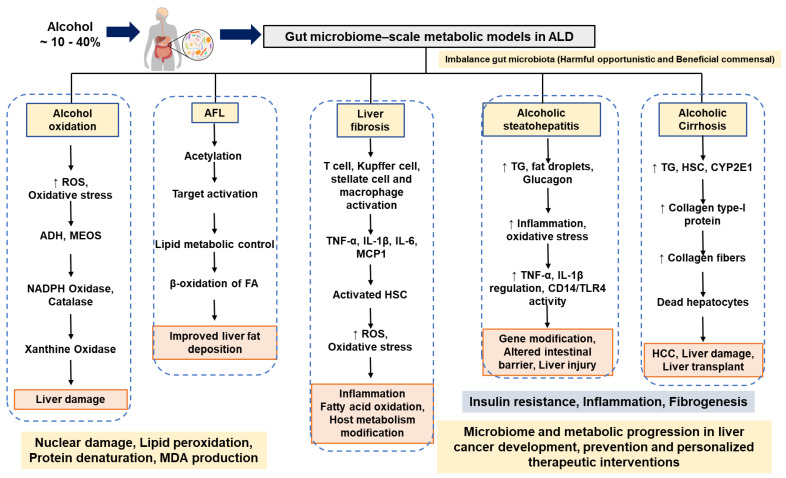
The fibrotic tissues are found in presinusoidal and pericentral areas in ALD. Alcoholic steatohepatitis (ASH) is mainly involved in the development of progressive fibrosis [72]. Collagen bands are noticeable and connected to fibrosis expansion. The ASH-based metabolic dysfunction leads to the development of proliferating nodules and cirrhosis of the liver. The cell and molecular metabolic mechanisms of advanced fibrosis, hepatic microcirculatory dysfunction, and vascular occlusion in ALD have not been fully studied [73]. Acetaldehyde (an alcohol metabolite) can directly activate HSC, which are involved in collagen-producing cells in the injured liver. HSC are activated by activated Kupffer cells, damaged hepatocytes, and infiltrating polymorphonuclear leukocytes (PMN) cells. These kinds of cells yield fibrogenic mediators (i.e., growth factors: TGF-β1, PDGF), cytokines (IL-8, TNF-α, leptin, and angiotensin-II), soluble mediators (nitric oxide), and ROS [74]. HSC activate and proliferate under the effect of acute and chronic liver damage [27]. Figure 1 presents the possible mechanisms of gut microbiome-derived metabolic oxidation, inflammation, and inhibition, which could be involved in the development of ALD.
Figure 1.
Summary of possible mechanisms and discussions of gut microbiome-derived metabolic oxidation, inflammation, and inhibition, which could be involved in the development of ALD. The possible metabolic influence of liver dysbiosis on ALD. Abbreviations: ALD, alcoholic liver disease; AFL, alcoholic fatty liver; TG, triglyceride; TNF-α, Tumor necrosis factor-α; IL, interleukin; ROS, reactive oxygen species; HS, hepatic stellate cell; NADPH, Nicotinamide adenine dinucleotide phosphate; ADH, alcohol dehydrogenase; TLR, Toll-like receptor.
ROS accumulation, mitochondrial damage, ER stress, DNA damage, and protein adducts broadly stimulate the promotion of fibrillation of intracellular signaling pathways in HSCs, including ERK, PI3K/AKT, and c-Jun N-terminal kinases (JNK) [75]. An increase in TIMP-1 and a decrease in the action of metal proteinase promote collagen accumulation. Cells synthesize unprocessed collagen from HSC. These include portal fibroblasts and bone-marrow-derived cells. A novel molecular mechanism in the epithelium-to-leaf transition of hepatocytes may play a key role in alcoholic liver fibrosis [76].
The mucosal microbiomes differ in that Enterococcus, Veillonella, Megasphaera, and Burkholderia are more prominent, and Roseburia is far less prevalent [77]. Alcoholics had much lower levels of intestinal bacteria, such as Enterococci, Bifidobacteria, Eubacterium g23, Oscillibacter, and Clostridiales [78].
4.3. Alcoholic Steatohepatitis in Humans with Alcoholism
Patients with severe ASH fail to recover despite abstinence and medical therapy. There was no apparent improvement for the clinical treatment of ASH and AC over three months [79,80]. ASH is a condition caused by regular alcohol use, which results in long-term (chronic) inflammation and altered liver metabolism. Chronic ASH-B, C, and D virus infections are the most common causes of CLD. There are various genotypes of hepatitis-C virus. Heavy alcohol consumption is believed to act synergistically with hepatitis-C in the progression of advanced liver disease [81,82]. Moreover, alcoholic hepatitis, hepatitis-C, and hepatic iron are independent risk factors of higher mortality at 6 months [83]. Hepatitis-C virus is carcinogenic and is associated with the development of different types of malignancies.
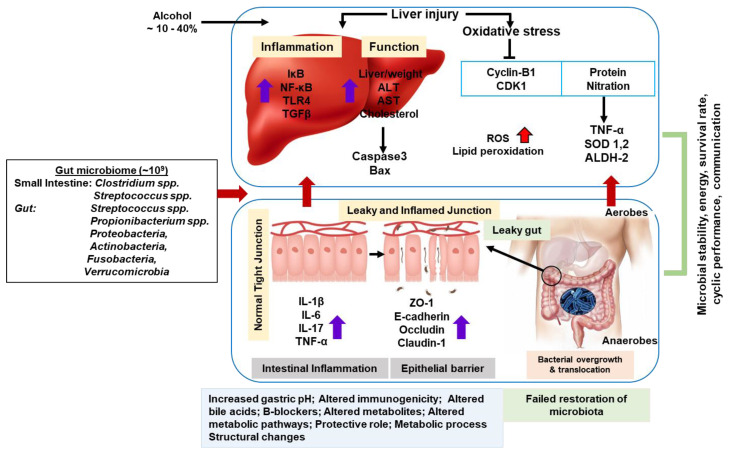
Adult human microbiome and microbiota mapping provides a basic outline of metabolic qualities (Figure 2). In ASH, microbial dysbiosis is characterized. Increased Bifidobacteria, Streptococci, and Enterobacteria in certain species (i.e., Clostridium leptum or F. prausnitzii) are well-established anti-inflammatory strains. They are found at reduced levels [84]. Another factor in ALD risk is hepatic iron, which could be identified as a predictor of mortality in AC. The molecular mechanism and significance of iron overload in the development of iron-burdened ALD and ASH have not been shown. Now, researchers are focusing on these areas of interest [85].
Figure 2.
Proposed models for understanding the alcohol-induced liver metabolic damages through gut–liver axis.
Acute ASH correlates with disease severity, where it is linked with inflammatory cytokine TNF-α and IL-1β upregulation [50]. The phosphodiesterase inhibitor, pentoxifylline, decreased the TNF-α transcription factor and associated promoter activity. The efficacy of pentoxifylline in ASH has been considered more systematically [86,87]. In particular, in severe ASH, pentoxifylline showed an auxiliary mortality improvement with a low incidence of the contraceptive syndrome [88]. Oxidative stress occurs in ASH. Recent studies have failed to prove the effectiveness of N-acetylcysteine (NAC) treatment in ASH [89,90,91,92]. Interestingly, a combination therapy with corticosteroids plus NAC increased the 1-month survival rate among patients with severe acute ASH, but the 6-month survival rate did not improve [92]. The ineffectiveness of anabolic steroids in ASH has been formerly revised [93]. Propylthiouracil (PTU) counters alcohol-induced hypermetabolism and suppresses oxidative stress metabolism [94,95,96]; however, a recent review of PTU exhibited that it is ineffective in ALD [97,98].
Ascites and jaundice are the main symptoms, and a significant number of patients have hepatic encephalopathy [99]. Generally, as per the current guidelines, liver transplantation is not advised for patients with ASH [100]. Liver biopsy shows progressive degeneration, focal hepatocyte necrosis, and neutrophilic infiltration [101]. Despite the good outcomes in various publications, liver transplantation as a treatment for ASH remains controversial, and there is currently an organ shortage. Hence, in most cases, liver transplantation is not recommended as the treatment option for ASH.
ASH is formed by hepatocellular damage and parenchymal inflammation and is a prerequisite for the development of fibrosis and cirrhosis. An episode of ASH may cause severe liver damage, increase resistance during blood circulation, and may be associated with a poor prognosis [102]. Many molecular mechanisms can contribute to the development of ASH. Acetaldehyde has a toxic effect. It binds to proteins and DNA to form self-antigens, resulting in fundamental changes and protein adducts that activate the immune system [103,104]. Mitochondrial damage and impaired glutathione functions develop, leading to oxidative stress and apoptosis [105]. The generation of ROS accompanying the formation of DNA damage and lipid peroxidation is initiated [106]. Major sources of ROS include the CYP2E1-dependent MEOS respiratory chain mitochondrial transport system, NADH-based cytochrome reductase, and xanthine oxidase, which are significantly correlated with ASH metabolism [107,108].
Chronic alcohol consumption has remarkably increased the CYP2E1 gene, which could metabolize ethanol to acetaldehyde and increased ROS and hydroxyl-ethyl radicals [109]. Alcohol metabolites and ROS interfere with signaling pathways, such as natural killer (NK) cells kβ, STAT-JAK, and JNK, in hepatocytes to induce local stimulation of inflammatory processes, such as TNF-α, CXC chemokines (e.g., IL-8), and osteopontin [110]. Alcohol intake may cause elevations of the gut microbial flora of the large intestine and serum lipopolysaccharides, which cause changes to intestinal permeability [111,112]. This induces inflammatory action by CD14/TLR4 activity in Kupffer cells [113]. Consequences of the inflammatory environment in alcoholic liver include PMN infiltration, ROS formation, and hepatic impairment. The reduction in the metabolic pathway to the ubiquitin proteasome leads to hepatocyte damage and the presence of aggregated sites of cytokeratin (i.e., Mallory–Denk bodies) in the liver [114].
Patients with ASH had higher concentrations of Acidaminococcus, Escherichia spp., and Bacteroides spp., which are linked to insulin resistance, and lower levels of Lachnospiraceae and Ruminococcaceae, which are responsible for butyrate production. These findings are consistent with those of a prior study, which found that Bacteroides vulgatus was one of the major species contributing to insulin resistance and circulating BCAA levels in humans [115].
The host gene Muc2 expression is primarily limited to the intestine and does not occur in the liver or in inflammatory cells. The Muc2 gene may cause ASH death. Probiotic Lactobacillus helped mice whose ability to restore ASH was impeded [116,117]. Bacillus and Veillonella were increased in the feces of patients with severe alcoholic hepatitis relative to healthy subjects [118].
Patients with ASH frequently have bacterial infections found. A crucial mechanistic role in ASH is played by the movement of bacteria and the bacterial proteins they produce throughout the GI tract. P. gingivalis is a significant periodontal pathogen that causes chronic periodontitis and can potentially have an impact on distant organs, such as the liver [119]. P. gingivalis might represent a brand-new ASH risk factor.
4.4. Alcoholic Cirrhosis in Humans with Alcoholism
AC is a result of severe damage to the liver cells. In this advanced stage of cirrhosis, the liver becomes stiff, swollen, and barely able to function. Hepatitis-B virus (HBV) and hepatitis-C virus (HCV) are major risk factors for AC development. HCV infection is one of the leading end-stage liver cirrhosis conditions that requires liver transplantation.
Alcohol increases the activity of cytochrome P450 2E1 (CYP2E1), which can metabolize alcohol and produce ROS [120]. ROS forms through CYP2E1-dependent oxidative ethanol metabolism that can involve collagen production in HSC cocultured with hepatocytes [26]. Ethanol metabolism induces oxidative stress and the secretion of inflammatory cytokines that can activate HSC. The triggered HSC move to the area of liver injury and secrete many ECMs, which act as the main events triggering the process of liver fibrogenesis [121]. In this study, HepG2 cells that do (E47 cells) or do not (C34 cells) have CYP2E1 with HSC were used to evaluate the potential fibrogenic effects of CYP2E1-dependent creation of ROS. Both intracellular and extracellular H2O2 and lipid peroxidation were increased in HSC-incubated E47 cells. This suggests that ROS is triggered by CYP2E1 metabolism. The HepG2 cells may spread and enter HSC after modulation of the collagen type-I protein. However, collagen type-I protein increased in AC [122]. We summarize the understanding that alcohol induces metabolic liver damage through the gut–liver axis (Figure 2).
Acetaldehyde binds to proteins to produce byproducts [123]. Malondialdehyde (MAA) and acetaldehyde react with proteins in vivo during liver ethanol metabolism to produce an MAA adduct, a hybrid protein. A study found that the absorption of chronic ethanol produces high levels of MAA-added proteins, which are associated with liver damage in humans with ALD [124]. MDA is also produced during lipid peroxidation in hepatocytes by aldehyde dehydrogenase. Both MDA and acetaldehyde can produce stable adducts, and while ethanol is oxidized in the liver, MDA and acetaldehyde can coexist at similar concentrations. In addition, the concentration of bio proteins increases, dependent on the coexistence of malonaldehyde and acetaldehyde, and MDA increases when the protein forms a structurally stable bond with acetaldehyde [125]. The adduct of ethanol metabolism and the MDA product of lipid peroxidation appear to progress during liver necrosis and liver fibrosis [126]. This includes oxidative tissue damage to the liver during the process of metabolizing excessive absorption of ethanol by the liver. After 12 months of ethanol consumption, osteoblastic fibrosis is widely developed. MDA additives caused by lipid peroxidation of chronic alcohol in the liver lead to liver necrosis and fibrosis [127].
AC is a major public health problem and is caused by excessive alcohol intake [128]. The molecular and analytical link in AC needs to be understood [129]. Liver cirrhosis, an irreversible liver disease, is accompanied by ascites and jaundice, and it has poor prognosis [130]. Studies have shown that one-third of patients die from liver cirrhosis during hospitalization, and the remaining 60 % only live for a short time [131]. As the most serious consequence of alcohol abuse, we revisited several metabolic chemical reactions that are necessary to reduce the negative consequences of AC [132].
In AC patients, there was an increase in Prevotellaceae. On the other hand, patients with ALD had lower levels of Firmicutes and Bacteroidaceae [57]. The Actinobacteria and Proteobacteria of the Gram-negative bacteria were raised as a result of heavy alcohol use. The Gammaproteobacteria class, which includes the Enterobacteriaceae and Pasteurellaceae, was primarily responsible for the enrichment of Proteobacteria. Patients with AC had 27 times more Enterobacteriaceae than healthy controls in their feces. In individuals with cirrhosis, Enterobacteriaceae were the most prevalent liver-translocating bacteria [133].
4.5. Hepatocellular Carcinoma in Humans with Alcoholism
Hepatocellular carcinoma (HCC) is the fifth and seventh most frequent cancer in men and women, respectively [134,135]. The incidence of HCC has been frequently amplified worldwide. Among patients with AC, 1–2 % develop HCC [136]. The CLD significantly increases the risk of HCC. HCC is the most common hepatic malignancy. The use of metabolomics for HCC prevention is still at an early stage, although it has many promising findings. There are few reports or studies about mortality prediction using HCC biomarkers.
In the liver cellular milieu, Kupffer cells and bone-marrow-derived macrophages recognize small sequences of molecules, formally called pathogen-associated molecular patterns (PAMPs), from endotoxins from the main circulation via TLR-4 [137]. The upregulation of TLR-4 promotes the binding of its ligand of myeloid differentiation primary response protein 88 (MyD88). This can induce the mitogen-activated protein kinase (MAPKs), p38, and (JNK). The inhibition of nuclear factor kappa-B (NK-κB), mitogen-activated protein kinase (MAPK), p38, and the NK-κB signaling is promoted by TLR-4 modification. These effects favor the release of TNF-α, IFN-γ, prostaglandin-2, chemokine C-C motif ligands, IL-1α, IL-1β, IL-6, ROS, and nitrates that can tolerate liver inflammation [138,139].
From the various carcinogenic effects, NF-κβ can induce the expression of antiapoptotic genes (TRAF-1 and TRAF-2) [8,140]. TNF-α deregulates the tight junctions (TJ) and induces an intestinal barrier disorder. Surprisingly, higher levels of TNF-α and IL-6 were found in alcohol-dependent focused duodenal biopsies, which could be established in in vivo studies [141]. According to publications, alcohol-dependent DSM-IV criteria, TNF-α, IL-6, and IL-10 showed important alterations that were considered candidate therapeutic biomarkers [142].
Moreover, IL-37 requires anti-inflammatory receptors through IL-18Rα and IL-1R8 for transportation from the extracellular space to the cytoplasm. IL-37 expression is significantly lower than that in non-AFL patients [143]. According to in vivo analysis, IL-37 expression was downregulated in wild-type mice after ethanol administration [143]. This change in IL-37 expression plays important roles in HCC. IL-37 is correlated with tumor size and is linked to disease-free survival and quantity by inducing tumor-infiltrating CD571 natural killer cells [144]. Molecular variations in HCC and their biological interpretation are fully documented through genomics. Clinical therapeutic targets (i.e., MyD88, IRAK4, IRAK1, TRAF6, IKKβ) and the discovery of anti-inflammation pathways (i.e., AMPK, STAT3, STAT6, MER, PTEN) have been summarized in liver cancer [145,146].
In liver cell metabolism, TLR-4 expression has been found in hepatic stellate cells (HSC), hepatocytes, and endothelial cells [147]. In HSC, an upregulation of hepatocyte epiregulin has been found [148], which stimulates epidermal growth factor protein, which has mitogenic properties in hepatocytes [149]. The antiapoptotic properties of NF-κβ promote hepatocarcinogenesis development. TLR-4 deficiency and intestinal stabilization analysis in knockout mice have been studied in steatosis, oxidative stress, and inflammation, with a resulting decrease in HCC risk. The risk of liver injury was increased by the lack of innate immunity triggered by TLR-4 suppression [150]. Chronic alcohol consumption is linked to immunosuppression with reduced CD8 T cells, which play an important role in antitumor effects [151]. In Table 2, a summary of gut microbiota-derived mechanistic candidate publications is presented with various targets for human liver treatment responses in ALD. Finally, abstinence is most important to prevent liver injury and is beneficial at all stages of liver diseases. We suggest that multidisciplinary approaches are required to treat alcohol disorders.
Table 2.
Robust predictions of the recent genomic and metabolic properties in humans with ALD.
| Animals | Exposures | Main results | Ref. | |
|---|---|---|---|---|
| Human | 14 alcoholic patients | chronic alcohol intake | (↑) Plasma endotoxin levels and serum IL-6 and IL-8 levels of patients compared to healthy subjects. Serum LBP was positively correlated with white blood cell and neutrophil counts as an indicator of an inflammatory response. |
[152] |
| recombinant HepG2 ADH1/CYP2E1 cells | 100 mM ethanol for 6, 24, 48, 72, 96, and 110 h | (↓) CYP1A2, CYP2B6, CYP2C9, CYP2E1, and CYP3A4 expression. (↓) AGO1 knockdown, HNF4A RNA levels. |
[153] | |
| severe AH (n = 161), and HC (n = 28) |
chronic alcohol intake | (↑) level of sST2 was increased in SAH, higher levels of 3-HM in patients compared with controls, expression at baseline of GRK2 in circulating PMNs. (↓) expression of the chemokine receptor CXCR2 on the surface of circulating PMNs. |
[154] | |
| Human |
51 alcoholic patients | consumed excessive alcohol, tobacco smoking |
(↑) CYP2E1 activity, oxidative stress. (↑) chlorzoxazone oxidation. |
[109] |
| 10 liver samples of AC | chronic alcohol intake | (↑) increased CCL2, CCL3, CCL4, CCL5, CCL8, CCL5 mRNA expression in AC liver, increased MØ infiltration. |
[60] | |
| healthy control (n = 33), alcoholic liver cirrhosis (n = 23) |
chronic alcohol intake | (↑) tumor volume and tumor maximum diameter expression of BCL-xl, CCL2, IL-4, IL-10, TIMP1, col1a1, and PCNA the frequency and number of macrophages in the liver hepatic CD206 expression. M2-associated protumor genes in the liver. |
[151] | |
| Human | 53 cirrhosis cohort patients |
alcohol intake, 1 yr follow-up, underwent liver transplantation |
Small intestinal bacterial overgrowth was seen in 59% of patients with cirrhosis and was significantly related to systemic endotoxemia. |
[155] |
| AH patients (n = 6); HC persons (n = 6) |
Ethanol consumption of at least 80 g/day | (↑) NF-kB activity in the monocytes of six patients with AH as compared with normal subjects. (↑) NF-kB activity, TNF-α RNA expression, and TNF-α release by endotoxin in AH patients. |
[156] | |
| Human | HCC, Late intrahepatic recurrence (n = 18); Early intrahepatic recurrence (n = 22) |
HCC patients | (↓) Plasma specimens, tryptophan, cholesterol glucuronide, LysoPC (20:5), LysoPC (22:6). ROC curves based on methionine, GCDCA, and cholesterol sulfate was selected. AUC equal to 0.95. |
[157] |
| Human | 46 patients | HCV-related HCC patients |
PCA and PLS-DA score-plot has found. ROC curve analyzed for N-acetyl-lysine, L-glutamine, L-aspartate, and L-proline. Heatmap presenting the hierarchical clustering analysis. |
[158] |
| Human | 248 serum samples | AHB, CHB, CHC with many types of liver disease |
Heatmap analysis, γ-glutamyl peptides mechanism, GSH oxidation and reduction. (↓) GSH level. |
[159] |
| Human | 52 serum samples | HCV, HCC patients | Serum sample analysis, 73 metabolites detected, Sensitivity of 97%, specificity of 95%, and an AUROC of 0.98 found. Sixteen metabolites were significantly altered. |
[159] |
| Human | 559 patients | NAFLD patients | AUROC of 0.92, sensitivity of 73%, and specificity of 94%. | [160] |
| Human | 117 patients | HCV (n = 67), HBV (n = 50 patients) | OPLS-DA analysis, metabolites and their pathway analysis, Fold-change analysis. |
[161] |
Notes and abbreviations: ↑ and ↓ show an increase and decrease in the condition. ALD, Alcoholic liver disease; IL, interleukin; LBP, Lipopolysaccharide binding protein; LPS, Lipopolysaccharide; NADPH, Nicotinamide adenine dinucleotide phosphate; ROS, reactive oxygen species; TLR-4, Toll-like receptor-4; TNF-α, Tumor necrosis factor-α; AHB, asymptomatic hepatitis B virus infection; CHB, chronic hepatitis B; CHC, chronic hepatitis C; CIR, cirrhosis type C; HCV, Hepatitis C virus; HCC, hepatocellular carcinoma; GSH, Glutathione; NAFLD, non-alcoholic fatty liver disease; OPLS-DA, Orthogonal Projections to Latent Structures Discriminant Analysis.
All patients with cirrhosis also had lower levels of Akkermansia and higher levels of Enterobacteriaceae, whereas the HCC subset had higher levels of Bacteroides and Ruminococcus with lower levels of Bifidobacterium. When a correlation network was built, these patterns were discovered to be constant within the increased inflammation. In contrast to Bacteroides, which were linked to higher levels of the proinflammatory cytokines IL8 and IL13, this network revealed an inverse association between Akkermansia and fecal calprotectin [162,163]. ALD is a major indication of liver transplantation worldwide.
5. Probiotics and Antioxidant Activity in ALD
As per a new study, pre-/probiotics are living microorganisms that play a beneficial role. Probiotics can be obtained in a variety of ways, including diet and human gut microbiota. The TJ proteins occludin and claudin-3 were frequently expressed by the probiotic Akkermansia muciniphila, which improved the liver damage caused by alcohol. Akkermansia muciniphila plays a more promising probiotic role [164,165]. Alcohol consumption quantitively decreased Bacteroides spp. and Firmicutes. Similarly, Proteobacteria and Actinobacteria were increased by alcohol [166]. Lactobacillus spp. has been shown to improve various liver diseases. Lactobacillus and Bifidobacterium lactis were used together to treat functional bowel disorders and ALD. In liver and intestine, probiotics were involved in modulating the gut microbiota and immune response, decreasing inflammatory cytokine and ROS expression. Probiotics play a significant role in reducing the fat accumulation in liver while increasing the fatty acid β-oxidation [167].
Nutritional care based on prebiotics and probiotics is an important part of ALD treatment. Probiotics were shown to be effective in reducing or preventing some ALD. Probiotics are based on quantitative changes in bacterial overgrowth in the intestine, which is common in ALD patients. These probiotics of Akkermansia muciniphila and Lactobacillus spp. could help improve the ALD survival rate. Recent studies investigating the use of pre-, pro-, and symbiotics in ALD and cirrhosis have found that they improve clinical and biochemical markers of liver disease [168].
6. Microbiome-Wide Dynamic Microbial Proof-of-Concept Clinical Validation
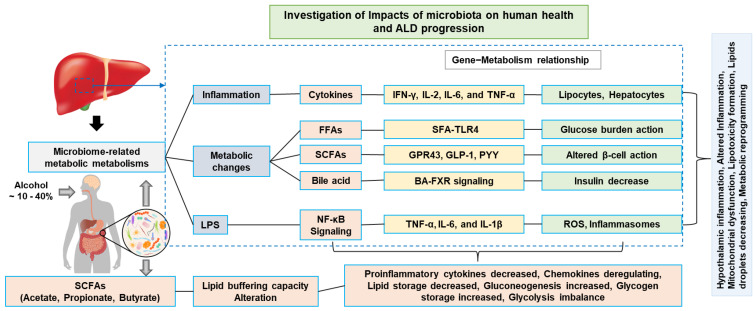
The relationship between the gut microbiome and liver diseases (i.e., dysbiosis, fibrosis, AFL, ASH, AC, and HCC) is more complex than the involvement of the microbiota in other diseases. Norfloxacin and rifaximin are used to increase the survival rate of patients with cirrhosis and HCC [169,170,171,172,173,174]. These drug treatments had better efficacy. These drugs are associated with severe liver disease (i.e., hepatic encephalopathy, portal hypertension, and spontaneous bacterial peritonitis) [175,176]. Probiotic drug-based gut microbes with modulating effects are being developed in order to restore bacterial diversity. Prebiotics have the effect of alleviating alcoholic liver injury [177]. At present, many pharmaceutical companies have made profits with strong microbial mixtures sold in both pharmacies and superstores. This kind of bacterial microbial compound has not been proven effective in cancer environments. However, bacterial microbial compounds have shown good efficacy in GI conditions, such as Clostridium difficile infection (CDI), where they can distribute fecal microbiota by transplantation and improve the anticancer immune response by preventing cancer progression [178]. CDI is notable for its increased virulence in ALD. However, these kinds of treatments are not included in the protocol of HCC treatment due to a lack of standardization. New clinical analysis is focusing on gut microbiota-based liver dysbiosis to increase therapeutic options. It was also seen in various studies that HCC served as an advanced stage of liver diseases. Nowadays, a variety of anti-inflammatory and anti-cytokine therapies are being used to treat ALD (Figure 3). However, these treatments were not very effective, and 15 % of patients continued to experience a worsening of liver function. Liver transplantation is the only option for people with end-stage or advanced ALD [179]. Accurate non-invasive metabolomic techniques to replace liver biopsy are a gold standard diagnostic tool for ALD. Death could occur due to hepatic failure induced by ALD. Finally, an emerging technology, such as miRNA analysis and artificial-intelligence-based methods to examine the metabolomic, genomic, and overall functional profile, are particularly promising.
Figure 3.
Proposed pathogenic mechanisms of ALD. An alcohol-induced liver metabolic intracellular imbalance through gut–liver axis. LPS, lipopolysaccharide; FFAs, free fatty acids; SCFAs, short-chain fatty acids.
7. Summary and Future Outlook
According to the present study, we live with a huge number of microbes in our gut, ranging from metabolic inhibition, inflammation, and various metabolic stresses. There are no questions about the fact that microbiota-based metabolomic signatures in ALD have been reported. ALD has rapidly become a global health problem. It is urgent to provide new therapeutic microbiota-associated genetic factors, proteins, and metabolites, especially for late-stage ALD. The therapeutic biomarkers of IL-1α, IL-1β, IL-6, IL-37, TNF-α, NK-kB, TGF-β1, CD14, CD571, and metabolites were summarized in various ALDs. Metagenomic comparisons predict genomic functions. Metabolomics provides an expansive biochemical profile of individual microbial strains. The clinical application of gut microbiota-based metabolomic signature flows, small molecule databases, and novel techniques specific to metabolomics profiling are useful and attractive platforms.
The probiotics of Akkermansia muciniphila and Lactobacillus spp. play a significant role in ALD survival rate. Probiotics provide a promising new approach for improving ALD. In food science research, probiotics explore new paths of ALD survival rate extension. Probiotics provide promising new approaches to improve ALD.
The idea is to examine whether physical exercise changes the microbiota, so that the accumulation of fat in the liver decreases. The gut and the liver are in constant interaction with one another (for example, through metabolic compounds produced by gut bacteria). Some chemicals found in the gut–liver microenvironment are known as metabolic disruptors that affect glucose and fat metabolism. We hope that the microbiota-based genetic and metabolic profile role of ALD will provide input to the scientific community discussion. As a first step, we plan to outline various gut microbiota, phenotypic variations, and metabolite information, extending to biomarker discovery, which may be helpful in underlining phenotypic mechanisms. This review can be used as a direct reference and to suggest new molecular targets for therapeutic interventions in ALD.
Acknowledgments
Raja Ganesan would like to thank Ki Tae Suk for providing their precious support and granting this wonderful research opportunity. We would like to thank and acknowledge the National Research Foundation of Korea and Ministry of Education, Science and Technology for funding support.
Author Contributions
All authors contributed to conceptualizing, drafting, and revising the manuscript. All authors have read and agreed to the final version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Hallym University Research Fund and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (NRF-2018M3A9F3020956, NRF-2019R1I1A3A01060447, NRF-2020R1I1A3073530 and NRF-2020R1A6A1A03043026).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sender R., Fuchs S., Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Tierney B.T., Yang Z., Luber J.M., Beaudin M., Wibowo M.C., Baek C., Mehlenbacher E., Patel C.J., Kostic A.D. The landscape of genetic content in the gut and oral human microbiome. Cell Host Microbe. 2019;26:283–295.e8. doi: 10.1016/j.chom.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharjee S., Lukiw W. Alzheimer’s disease and the microbiome. Front. Cell. Neurosci. 2013;7:153. doi: 10.3389/fncel.2013.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schnabl B., Brenner D.A. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513–1524. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganesan R., Jeong J.-J., Kim D.J., Suk K.T. Recent trends of microbiota-based microbial metabolites metabolism in liver disease. Front. Med. 2022;9:1346. doi: 10.3389/fmed.2022.841281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30–36. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veith A., Moorthy B. Role of cytochrome p450s in the generation and metabolism of reactive oxygen species. Curr. Opin. Toxicol. 2018;7:44–51. doi: 10.1016/j.cotox.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raja G., Gupta H., Gebru Y.A., Youn G.S., Choi Y.R., Kim H.S., Yoon S.J., Kim D.J., Kim T.-J., Suk K.T. Recent advances of microbiome-associated metabolomics profiling in liver disease: Principles, mechanisms, and applications. Int. J. Mol. Sci. 2021;22:1160. doi: 10.3390/ijms22031160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan V., Putluri N., Sreekumar A., Mindikoglu A.L. Current applications of metabolomics in cirrhosis. Metabolites. 2018;8:67. doi: 10.3390/metabo8040067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 11.Palmer C., Bik E.M., DiGiulio D.B., Relman D.A., Brown P.O. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi Y.R., Kim H.S., Yoon S.J., Lee N.Y., Gupta H., Raja G., Gebru Y.A., Youn G.S., Kim D.J., Ham Y.L., et al. Nutritional status and diet style affect cognitive function in alcoholic liver disease. Nutrients. 2021;13:185. doi: 10.3390/nu13010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuma D.J., Casey C.A. Dangerous byproducts of alcohol breakdown—Focus on adducts. Alcohol Res. Health J. Natl. Inst. Alcohol Abus. Alcohol. 2003;27:285–290. [PMC free article] [PubMed] [Google Scholar]
- 14.Sarsour E.H., Kumar M.G., Chaudhuri L., Kalen A.L., Goswami P.C. Redox control of the cell cycle in health and disease. Antioxid Redox Signal. 2009;11:2985–3011. doi: 10.1089/ars.2009.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldar S., Khaniani M.S., Derakhshan S.M., Baradaran B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac. J. Cancer Prev. APJCP. 2015;16:2129–2144. doi: 10.7314/APJCP.2015.16.6.2129. [DOI] [PubMed] [Google Scholar]
- 16.Hagymási K., Blázovics A., Lengyel G., Kocsis I., Fehér J. Oxidative damage in alcoholic liver disease. Eur. J. Gastroenterol. Hepatol. 2001;13:49–53. doi: 10.1097/00042737-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Varela-Rey M., Woodhoo A., Martinez-Chantar M.-L., Mato J.M., Lu S.C. Alcohol, DNA methylation, and cancer. Alcohol. Res. 2013;35:25–35. [PMC free article] [PubMed] [Google Scholar]
- 18.Raja G., Jang Y.-K., Suh J.-S., Prabhakaran V.-S., Kim T.-J. Advanced understanding of genetic risk and metabolite signatures in construction workers via cytogenetics and metabolomics analysis. Process Biochem. 2019;86:117–126. doi: 10.1016/j.procbio.2019.07.016. [DOI] [Google Scholar]
- 19.Baylin S.B., Herman J.G., Graff J.R., Vertino P.M., Issa J.P. Alterations in DNA methylation: A fundamental aspect of neoplasia. Adv. Cancer Res. 1998;72:141–196. [PubMed] [Google Scholar]
- 20.Karthi S., Vasantha-Srinivasan P., Ganesan R., Ramasamy V., Senthil-Nathan S., Khater H.F., Radhakrishnan N., Amala K., Kim T.-J., El-Sheikh M.A., et al. Target activity of isaria tenuipes (hypocreales: Clavicipitaceae) fungal strains against dengue vector Aedes aegypti (linn.) and its non-target activity against aquatic predators. J. Fungi. 2020;6:196. doi: 10.3390/jof6040196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan J.M., Lu S.C., Van Den Berg D., Govindarajan S., Zhang Z.Q., Mato J.M., Yu M.C. Genetic polymorphisms in the methylenetetrahydrofolate reductase and thymidylate synthase genes and risk of hepatocellular carcinoma. Hepatology. 2007;46:749–758. doi: 10.1002/hep.21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabris C., Toniutto P., Falleti E., Fontanini E., Cussigh A., Bitetto D., Fornasiere E., Fumolo E., Avellini C., Minisini R., et al. Mthfr c677t polymorphism and risk of hcc in patients with liver cirrhosis: Role of male gender and alcohol consumption. Alcohol. Clin. Exp. Res. 2009;33:102–107. doi: 10.1111/j.1530-0277.2008.00816.x. [DOI] [PubMed] [Google Scholar]
- 23.Pinzani M., Rosselli M., Zuckermann M. Liver cirrhosis. Best Pract. Res. Clin. Gastroenterol. 2011;25:281–290. doi: 10.1016/j.bpg.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Zhou W.-C., Zhang Q.-B., Qiao L. Pathogenesis of liver cirrhosis. World J. Gastroenterol. WJG. 2014;20:7312. doi: 10.3748/wjg.v20.i23.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruha R., Dvorak K., Petrtyl J. Alcoholic liver disease. World J. Hepatol. 2012;4:81. doi: 10.4254/wjh.v4.i3.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyun J., Han J., Lee C., Yoon M., Jung Y. Pathophysiological aspects of alcohol metabolism in the liver. Int. J. Mol. Sci. 2021;22:5717. doi: 10.3390/ijms22115717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaffert C.S., Duryee M.J., Hunter C.D., Hamilton B.C., 3rd, DeVeney A.L., Huerter M.M., Klassen L.W., Thiele G.M. Alcohol metabolites and lipopolysaccharide: Roles in the development and/or progression of alcoholic liver disease. World J. Gastroenterol. WJG. 2009;15:1209. doi: 10.3748/wjg.15.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Setshedi M., Wands J.R., de la Monte S.M. Acetaldehyde adducts in alcoholic liver disease. Oxidative Med. Cell. Longev. 2010;3:178–185. doi: 10.4161/oxim.3.3.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao B., Bataller R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan A.W., Fouts D.E., Brandl J., Stärkel P., Torralba M., Schott E., Tsukamoto H., Nelson K.E., Brenner D.A., Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology (Baltimore, Md.) 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones R.M., Luo L., Ardita C.S., Richardson A.N., Kwon Y.M., Mercante J.W., Alam A., Gates C.L., Wu H., Swanson P.A., et al. Symbiotic lactobacilli stimulate gut epithelial proliferation via nox-mediated generation of reactive oxygen species. EMBO J. 2013;32:3017–3028. doi: 10.1038/emboj.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 33.Cayrol C., Girard J.-P. The il-1-like cytokine il-33 is inactivated after maturation by caspase-1. Proc. Natl. Acad. Sci. USA. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinon F., Mayor A., Tschopp J. The inflammasomes: Guardians of the body. Annu. Rev. Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 35.Valles S.L., Blanco A.M., Azorin I., Guasch R., Pascual M., Gomez-Lechon M.J., Renau-Piqueras J., Guerri C. Chronic ethanol consumption enhances interleukin-1-mediated signal transduction in rat liver and in cultured hepatocytes. Alcohol. Clin. Exp. Res. 2003;27:1979–1986. doi: 10.1097/01.ALC.0000099261.87880.21. [DOI] [PubMed] [Google Scholar]
- 36.Shen Z., Ajmo J.M., Rogers C.Q., Liang X., Le L., Murr M.M., Peng Y., You M. Role of sirt1 in regulation of lps- or two ethanol metabolites-induced tnf-alpha production in cultured macrophage cell lines. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G1047–G1053. doi: 10.1152/ajpgi.00016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rehm J., Room R., Monteiro M., Gmel G., Graham K., Rehn N., Sempos C.T., Jernigan D. Alcohol as a risk factor for global burden of disease. Eur. Addict. Res. 2003;9:157–164. doi: 10.1159/000072222. [DOI] [PubMed] [Google Scholar]
- 38.Buchanan R., Sinclair J.M. Alcohol use disorder and the liver. Addiction. 2021;116:1270–1278. doi: 10.1111/add.15204. [DOI] [PubMed] [Google Scholar]
- 39.Griswold M.G., Fullman N., Hawley C., Arian N., Zimsen S.R., Tymeson H.D., Venkateswaran V., Tapp A.D., Forouzanfar M.H., Salama J.S. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet. 2018;392:1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou M., Wang H., Zeng X., Yin P., Zhu J., Chen W., Li X., Wang L., Wang L., Liu Y. Mortality, morbidity, and risk factors in china and its provinces, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet. 2019;394:1145–1158. doi: 10.1016/S0140-6736(19)30427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flemming J.A., Djerboua M., Groome P.A., Booth C.M., Terrault N.A. Nafld and alcohol-related liver disease will be responsible for almost all new diagnoses of cirrhosis in canada by 2040. Hepatology. 2021;74:3330–3344. doi: 10.1002/hep.32032. [DOI] [PubMed] [Google Scholar]
- 42.Asrani S.K., Mellinger J., Arab J.P., Shah V.H. Reducing the global burden of alcohol-associated liver disease: A blueprint for action. Hepatology. 2021;73:2039–2050. doi: 10.1002/hep.31583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Won S.-M., Park E., Jung J.-J., Ganesan R., Gupta H., Gebru Y.A., Sharma S., Kim D.-J., Suk K.-T. The gut microbiota-derived immune response in chronic liver disease. Int. J. Mol. Sci. 2021;22:8309. doi: 10.3390/ijms22158309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma P., Arora A. Clinical presentation of alcoholic liver disease and non-alcoholic fatty liver disease: Spectrum and diagnosis. Transl. Gastroenterol. Hepatol. 2020;5:19. doi: 10.21037/tgh.2019.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lieber C.S. Hepatic and metabolic effects of ethanol: Pathogenesis and prevention. Ann. Med. 1994;26:325–330. doi: 10.3109/07853899409148346. [DOI] [PubMed] [Google Scholar]
- 46.Xu T., Li L., Hu H.Q., Meng X.M., Huang C., Zhang L., Qin J., Li J. Micrornas in alcoholic liver disease: Recent advances and future applications. J. Cell. Physiol. 2018;234:382–394. doi: 10.1002/jcp.26938. [DOI] [PubMed] [Google Scholar]
- 47.Stickel F., Hampe J. Genetic determinants of alcoholic liver disease. Gut. 2012;61:150–159. doi: 10.1136/gutjnl-2011-301239. [DOI] [PubMed] [Google Scholar]
- 48.Khoruts A., Stahnke L., McClain C.J., Logan G., Allen J.I. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology. 1991;13:267–276. doi: 10.1002/hep.1840130211. [DOI] [PubMed] [Google Scholar]
- 49.Menon K.V., Gores G.J., Shah V.H. Pathogenesis, diagnosis, and treatment of alcoholic liver disease. Mayo Clin. Proc. 2001;76:1021–1029. doi: 10.4065/76.10.1021. [DOI] [PubMed] [Google Scholar]
- 50.Szabo G., Petrasek J., Bala S. Innate immunity and alcoholic liver disease. Dig. Dis. 2012;30((Suppl. S1)):55–60. doi: 10.1159/000341126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petrasek J., Mandrekar P., Szabo G. Toll-like receptors in the pathogenesis of alcoholic liver disease. Gastroenterol. Res. Pract. 2010;2010:710381. doi: 10.1155/2010/710381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Louvet A., Mathurin P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 2015;12:231–242. doi: 10.1038/nrgastro.2015.35. [DOI] [PubMed] [Google Scholar]
- 53.Missiroli S., Patergnani S., Caroccia N., Pedriali G., Perrone M., Previati M., Wieckowski M.R., Giorgi C. Mitochondria-associated membranes (mams) and inflammation. Cell Death Dis. 2018;9:329. doi: 10.1038/s41419-017-0027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He S., McPhaul C., Li J.Z., Garuti R., Kinch L., Grishin N.V., Cohen J.C., Hobbs H.H. A sequence variation (i148m) in pnpla3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J. Biol. Chem. 2010;285:6706–6715. doi: 10.1074/jbc.M109.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hartmann P., Chen P., Wang H.J., Wang L., McCole D.F., Brandl K., Stärkel P., Belzer C., Hellerbrand C., Tsukamoto H., et al. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology (Baltimore, Md.) 2013;58:108–119. doi: 10.1002/hep.26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bajaj J.S., Heuman D.M., Hylemon P.B., Sanyal A.J., White M.B., Monteith P., Noble N.A., Unser A.B., Daita K., Fisher A.R., et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J. Hepatol. 2014;60:940–947. doi: 10.1016/j.jhep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y., Yang F., Lu H., Wang B., Chen Y., Lei D., Wang Y., Zhu B., Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology (Baltimore, Md.) 2011;54:562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 58.Li S., Wang N., Tan H.Y., Chueng F., Zhang Z.J., Yuen M.F., Feng Y. Modulation of gut microbiota mediates berberine-induced expansion of immuno-suppressive cells to against alcoholic liver disease. Clin. Transl. Med. 2020;10:e112. doi: 10.1002/ctm2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hartmann P., Hochrath K., Horvath A., Chen P., Seebauer C.T., Llorente C., Wang L., Alnouti Y., Fouts D.E., Stärkel P. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology. 2018;67:2150–2166. doi: 10.1002/hep.29676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ambade A., Lowe P., Kodys K., Catalano D., Gyongyosi B., Cho Y., Iracheta-Vellve A., Adejumo A., Saha B., Calenda C. Pharmacological inhibition of ccr2/5 signaling prevents and reverses alcohol-induced liver damage, steatosis, and inflammation in mice. Hepatology. 2019;69:1105–1121. doi: 10.1002/hep.30249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adachi Y., Moore L.E., Bradford B.U., Gao W., Thurman R.G. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 62.Machida K., Tsukamoto H., Mkrtchyan H., Duan L., Dynnyk A., Liu H.M., Asahina K., Govindarajan S., Ray R., Ou J.H., et al. Toll-like receptor 4 mediates synergism between alcohol and hcv in hepatic oncogenesis involving stem cell marker nanog. Proc. Natl. Acad. Sci. USA. 2009;106:1548–1553. doi: 10.1073/pnas.0807390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang C., Zheng L., Liu S., Guo X., Qu Y., Gao M., Cui X., Yang Y. A novel acidic polysaccharide from the residue of panax notoginseng and its hepatoprotective effect on alcoholic liver damage in mice. Int. J. Biol. Macromol. 2020;149:1084–1097. doi: 10.1016/j.ijbiomac.2020.02.034. [DOI] [PubMed] [Google Scholar]
- 64.Thakur V., Pritchard M.T., McMullen M.R., Wang Q., Nagy L.E. Chronic ethanol feeding increases activation of nadph oxidase by lipopolysaccharide in rat kupffer cells: Role of increased reactive oxygen in lps-stimulated erk1/2 activation and tnf-alpha production. J. Leukoc. Biol. 2006;79:1348–1356. doi: 10.1189/jlb.1005613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi X., Wei X., Yin X., Wang Y., Zhang M., Zhao C., Zhao H., McClain C.J., Feng W., Zhang X. Hepatic and fecal metabolomic analysis of the effects of lactobacillus rhamnosus gg on alcoholic fatty liver disease in mice. J. Proteome Res. 2015;14:1174–1182. doi: 10.1021/pr501121c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma T., Li Y., Zhu Y., Jiang S., Cheng C., Peng Z., Xu L. Differential metabolic pathways and metabolites in a c57bl/6j mouse model of alcoholic liver disease. Med. Sci. Monit. 2020;26:e924602. doi: 10.12659/MSM.924602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen L.-D., Huang Z.-W., Huang Y.-Z., Huang J.-F., Zhang Z.-P., Lin X.-J. Untargeted metabolomic profiling of liver in a chronic intermittent hypoxia mouse model. Front. Physiol. 2021;12:701035. doi: 10.3389/fphys.2021.701035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cai H., Wen Z., Meng K., Yang P. Metabolomic signatures for liver tissue and cecum contents in high-fat diet-induced obese mice based on uhplc-q-tof/ms. Nutr. Metab. 2021;18:69. doi: 10.1186/s12986-021-00595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chang H., Meng H.-Y., Liu S.-M., Wang Y., Yang X.-X., Lu F., Wang H.-Y. Identification of key metabolic changes during liver fibrosis progression in rats using a urine and serum metabolomics approach. Sci. Rep. 2017;7:11433. doi: 10.1038/s41598-017-11759-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu M., Du X., Xu H., Yang S., Wang C., Zhu Y., Zhang T., Zhao W. Metabolic profiling of liver and faeces in mice infected with echinococcosis. Parasites Vectors. 2021;14:324. doi: 10.1186/s13071-021-04807-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Warner J., Hardesty J., Song Y., Sun R., Deng Z., Xu R., Yin X., Zhang X., McClain C., Warner D., et al. Fat-1 transgenic mice with augmented n3-polyunsaturated fatty acids are protected from liver injury caused by acute-on-chronic ethanol administration. Front. Pharmacol. 2021;12:711590. doi: 10.3389/fphar.2021.711590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bataller R., Brenner D.A. Liver fibrosis. J. Clin. Investig. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cubero F.J., Urtasun R., Nieto N. Alcohol and liver fibrosis. Semin. Liver Dis. 2009;29:211–221. doi: 10.1055/s-0029-1214376. [DOI] [PubMed] [Google Scholar]
- 74.Park E., Jeong J.-J., Won S.-M., Sharma S.P., Gebru Y.A., Ganesan R., Gupta H., Suk K.T., Kim D.J. Gut microbiota-related cellular and molecular mechanisms in the progression of nonalcoholic fatty liver disease. Cells. 2021;10:2634. doi: 10.3390/cells10102634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moreno M., Bataller R. Cytokines and renin-angiotensin system signaling in hepatic fibrosis. Clin. Liver Dis. 2008;12:825–852. doi: 10.1016/j.cld.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 76.Firrincieli D., Boissan M., Chignard N. Epithelial-mesenchymal transition in the liver. Gastroenterol. Clin. Biol. 2010;34:523–528. doi: 10.1016/j.gcb.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 77.Bajaj J.S., Hylemon P.B., Ridlon J.M., Heuman D.M., Daita K., White M.B., Monteith P., Noble N.A., Sikaroodi M., Gillevet P.M. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012;303:G675–G685. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kirpich I.A., Solovieva N.V., Leikhter S.N., Shidakova N.A., Lebedeva O.V., Sidorov P.I., Bazhukova T.A., Soloviev A.G., Barve S.S., McClain C.J., et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: A pilot study. Alcohol. 2008;42:675–682. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carithers R.L., Jr., Herlong H.F., Diehl A.M., Shaw E.W., Combes B., Fallon H.J., Maddrey W.C. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Ann. Intern. Med. 1989;110:685–690. doi: 10.7326/0003-4819-110-9-685. [DOI] [PubMed] [Google Scholar]
- 80.Veldt B.J., Lainé F., Guillygomarc’h A., Lauvin L., Boudjema K., Messner M., Brissot P., Deugnier Y., Moirand R. Indication of liver transplantation in severe alcoholic liver cirrhosis: Quantitative evaluation and optimal timing. J. Hepatol. 2002;36:93–98. doi: 10.1016/S0168-8278(01)00228-8. [DOI] [PubMed] [Google Scholar]
- 81.Corrao G., Aricò S. Independent and combined action of hepatitis c virus infection and alcohol consumption on the risk of symptomatic liver cirrhosis. Hepatology. 1998;27:914–919. doi: 10.1002/hep.510270404. [DOI] [PubMed] [Google Scholar]
- 82.Hutchinson S.J., Bird S.M., Goldberg D.J. Influence of alcohol on the progression of hepatitis c virus infection: A meta-analysis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2005;3:1150–1159. doi: 10.1016/S1542-3565(05)00407-6. [DOI] [PubMed] [Google Scholar]
- 83.Punzalan C.S., Bukong T.N., Szabo G. Alcoholic hepatitis and hcv interactions in the modulation of liver disease. J. Viral Hepat. 2015;22:769–776. doi: 10.1111/jvh.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sokol H., Pigneur B., Watterlot L., Lakhdari O., Bermúdez-Humarán L.G., Gratadoux J.-J., Blugeon S., Bridonneau C., Furet J.-P., Corthier G., et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of crohn disease patients. Proc. Natl. Acad. Sci. USA. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gleeson D., Evans S., Bradley M., Jones J., Peck R.J., Dube A., Rigby E., Dalton A. Hfe genotypes in decompensated alcoholic liver disease: Phenotypic expression and comparison with heavy drinking and with normal controls. Am. J. Gastroenterol. 2006;101:304–310. doi: 10.1111/j.1572-0241.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- 86.Strieter R.M., Remick D.G., Ward P.A., Spengler R.N., Lynch J.P., 3rd, Larrick J., Kunkel S.L. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem. Biophys. Res. Commun. 1988;155:1230–1236. doi: 10.1016/S0006-291X(88)81271-3. [DOI] [PubMed] [Google Scholar]
- 87.Morgan T.R., McClain C.J. Pentoxifylline and alcoholic hepatitis. Gastroenterology. 2000;119:1787–1791. doi: 10.1053/gast.2000.20826. [DOI] [PubMed] [Google Scholar]
- 88.Akriviadis E., Botla R., Briggs W., Han S., Reynolds T., Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: A double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–1648. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 89.Moreno C., Langlet P., Hittelet A., Lasser L., Degré D., Evrard S., Colle I., Lemmers A., Devière J., Le Moine O. Enteral nutrition with or without n-acetylcysteine in the treatment of severe acute alcoholic hepatitis: A randomized multicenter controlled trial. J. Hepatol. 2010;53:1117–1122. doi: 10.1016/j.jhep.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 90.Stewart S., Prince M., Bassendine M., Hudson M., James O., Jones D., Record C., Day C.P. A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. J. Hepatol. 2007;47:277–283. doi: 10.1016/j.jhep.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 91.Phillips M., Curtis H., Portmann B., Donaldson N., Bomford A., O’Grady J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis—A randomised clinical trial. J. Hepatol. 2006;44:784–790. doi: 10.1016/j.jhep.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 92.Nguyen-Khac E., Thevenot T., Piquet M.A., Benferhat S., Goria O., Chatelain D., Tramier B., Dewaele F., Ghrib S., Rudler M., et al. Glucocorticoids plus n-acetylcysteine in severe alcoholic hepatitis. N. Engl. J. Med. 2011;365:1781–1789. doi: 10.1056/NEJMoa1101214. [DOI] [PubMed] [Google Scholar]
- 93.Rambaldi A., Gluud C. Anabolic-androgenic steroids for alcoholic liver disease. Cochrane Database Syst. Rev. 2006;2006:Cd003045. doi: 10.1002/14651858.CD003045.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Israel Y., Kalant H., Orrego H., Khanna J.M., Videla L., Phillips J.M. Experimental alcohol-induced hepatic necrosis: Suppression by propylthiouracil. Proc. Natl. Acad. Sci. USA. 1975;72:1137–1141. doi: 10.1073/pnas.72.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iturriaga H., Ugarte G., Israel Y. Hepatic vein oxygenation, liver blood flow, and the rate of ethanol metabolism in recently abstinent alcoholic patients. Eur. J. Clin. Investig. 1980;10:211–218. doi: 10.1111/j.1365-2362.1980.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 96.Kettle A.J., Gedye C.A., Winterbourn C.C. Mechanism of inactivation of myeloperoxidase by 4-aminobenzoic acid hydrazide. Pt 2Biochem. J. 1997;321:503–508. doi: 10.1042/bj3210503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rambaldi A., Gluud C. Propylthiouracil for alcoholic liver disease. Cochrane Database Syst. Rev. 2005:Cd002800. doi: 10.1002/14651858.CD002800. [DOI] [PubMed] [Google Scholar]
- 98.Fede G., Germani G., Gluud C., Gurusamy K.S., Burroughs A.K. Propylthiouracil for alcoholic liver disease. Cochrane Database Syst. Rev. 2011 doi: 10.1002/14651858.CD002800.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mendenhall C.L., Moritz T.E., Roselle G.A., Morgan T.R., Nemchausky B.A., Tamburro C.H., Schiff E.R., McClain C.J., Marsano L.S., Allen J.I., et al. A study of oral nutritional support with oxandrolone in malnourished patients with alcoholic hepatitis: Results of a department of veterans affairs cooperative study. Hepatology. 1993;17:564–576. doi: 10.1002/hep.1840170407. [DOI] [PubMed] [Google Scholar]
- 100.Murray K.F., Carithers R.L., Jr. Aasld practice guidelines: Evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407–1432. doi: 10.1002/hep.20704. [DOI] [PubMed] [Google Scholar]
- 101.Ishak K.G., Zimmerman H.J., Ray M.B. Alcoholic liver disease: Pathologic, pathogenetic and clinical aspects. Alcohol. Clin. Exp. Res. 1991;15:45–66. doi: 10.1111/j.1530-0277.1991.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 102.Chedid A., Mendenhall C.L., Gartside P., French S.W., Chen T., Rabin L. Prognostic factors in alcoholic liver disease. Va cooperative study group. Am. J. Gastroenterol. 1991;86:210–216. [PubMed] [Google Scholar]
- 103.Niemelä O., Juvonen T., Parkkila S. Immunohistochemical demonstration of acetaldehyde-modified epitopes in human liver after alcohol consumption. J. Clin. Investig. 1991;87:1367–1374. doi: 10.1172/JCI115141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Theruvathu J.A., Jaruga P., Nath R.G., Dizdaroglu M., Brooks P.J. Polyamines stimulate the formation of mutagenic 1,n2-propanodeoxyguanosine adducts from acetaldehyde. Nucleic Acids Res. 2005;33:3513–3520. doi: 10.1093/nar/gki661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seitz H.K., Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol. Chem. 2006;387:349–360. doi: 10.1515/BC.2006.047. [DOI] [PubMed] [Google Scholar]
- 106.Wang Y., Millonig G., Nair J., Patsenker E., Stickel F., Mueller S., Bartsch H., Seitz H.K. Ethanol-induced cytochrome p4502e1 causes carcinogenic etheno-DNA lesions in alcoholic liver disease. Hepatology. 2009;50:453–461. doi: 10.1002/hep.22978. [DOI] [PubMed] [Google Scholar]
- 107.Albano E. Alcohol, oxidative stress and free radical damage. Proc. Nutr. Soc. 2006;65:278–290. doi: 10.1079/PNS2006496. [DOI] [PubMed] [Google Scholar]
- 108.Lieber C.S. Cytochrome p-4502e1: Its physiological and pathological role. Physiol. Rev. 1997;77:517–544. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- 109.Dupont I., Lucas D., Clot P., Ménez C., Albano E. Cytochrome p4502e1 inducibility and hydroxyethyl radical formation among alcoholics. J. Hepatol. 1998;28:564–571. doi: 10.1016/S0168-8278(98)80279-1. [DOI] [PubMed] [Google Scholar]
- 110.Seth D., Gorrell M.D., Cordoba S., McCaughan G.W., Haber P.S. Intrahepatic gene expression in human alcoholic hepatitis. J. Hepatol. 2006;45:306–320. doi: 10.1016/j.jhep.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 111.Urbaschek R., McCuskey R.S., Rudi V., Becker K.P., Stickel F., Urbaschek B., Seitz H.K. Endotoxin, endotoxin-neutralizing-capacity, scd14, sicam-1, and cytokines in patients with various degrees of alcoholic liver disease. Alcohol. Clin. Exp. Res. 2001;25:261–268. doi: 10.1111/j.1530-0277.2001.tb02207.x. [DOI] [PubMed] [Google Scholar]
- 112.Gebru Y.A., Choi M.R., Raja G., Gupta H., Sharma S.P., Choi Y.R., Kim H.S., Yoon S.J., Kim D.J., Suk K.T. Pathophysiological roles of mucosal-associated invariant t cells in the context of gut microbiota-liver axis. Microorganisms. 2021;9:296. doi: 10.3390/microorganisms9020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thurman R.G., II Alcoholic liver injury involves activation of kupffer cells by endotoxin. Am. J. Physiol. 1998;275:G605–G611. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- 114.Bardag-Gorce F., Li J., French B.A., French S.W. The effect of ethanol-induced cyp2e1 on proteasome activity: The role of 4-hydroxynonenal. Exp. Mol. Pathol. 2005;78:109–115. doi: 10.1016/j.yexmp.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 115.Pedersen H.K., Gudmundsdottir V., Nielsen H.B., Hyotylainen T., Nielsen T., Jensen B.A., Forslund K., Hildebrand F., Prifti E., Falony G., et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376–381. doi: 10.1038/nature18646. [DOI] [PubMed] [Google Scholar]
- 116.Nanji A.A., Khettry U., Sadrzadeh S.M. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease) Proc. Soc. Exp. Biol. Med. 1994;205:243–247. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- 117.Forsyth C.B., Farhadi A., Jakate S.M., Tang Y., Shaikh M., Keshavarzian A. Lactobacillus gg treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol. 2009;43:163–172. doi: 10.1016/j.alcohol.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim S.S., Eun J.W., Cho H.J., Song D.S., Kim C.W., Kim Y.S., Lee S.W., Kim Y.K., Yang J., Choi J., et al. Microbiome as a potential diagnostic and predictive biomarker in severe alcoholic hepatitis. Aliment. Pharmacol. Ther. 2021;53:540–551. doi: 10.1111/apt.16200. [DOI] [PubMed] [Google Scholar]
- 119.Zhou Y., Vatsalya V., Gobejishvili L., Lamont R.J., McClain C.J., Feng W. Porphyromonas gingivalis as a possible risk factor in the development/severity of acute alcoholic hepatitis. Hepatol. Commun. 2019;3:293–304. doi: 10.1002/hep4.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu D., Zhai Q., Shi X. Alcohol-induced oxidative stress and cell responses. J. Gastroenterol. Hepatol. 2006;21:S26–S29. doi: 10.1111/j.1440-1746.2006.04589.x. [DOI] [PubMed] [Google Scholar]
- 121.Nan Y.-M., Kong L.-B., Ren W.-G., Wang R.-Q., Du J.-H., Li W.-C., Zhao S.-X., Zhang Y.-G., Wu W.-J., Di H.-L. Activation of peroxisome proliferator activated receptor alpha ameliorates ethanol mediated liver fibrosis in mice. Lipids Health Dis. 2013;12:11. doi: 10.1186/1476-511X-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nieto N., Friedman S.L., Cederbaum A.I. Cytochrome p450 2e1-derived reactive oxygen species mediate paracrine stimulation of collagen i protein synthesis by hepatic stellate cells. J. Biol. Chem. 2002;277:9853–9864. doi: 10.1074/jbc.M110506200. [DOI] [PubMed] [Google Scholar]
- 123.Rasineni K., Donohue T.M., Jr., Thomes P.G., Yang L., Tuma D.J., McNiven M.A., Casey C.A. Ethanol-induced steatosis involves impairment of lipophagy, associated with reduced dynamin2 activity. Hepatol. Commun. 2017;1:501–512. doi: 10.1002/hep4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tuma D.J. Role of malondialdehyde-acetaldehyde adducts in liver injury. Free. Radic. Biol. Med. 2002;32:303–308. doi: 10.1016/S0891-5849(01)00742-0. [DOI] [PubMed] [Google Scholar]
- 125.Tuma D.J., Thiele G.M., Xu D., Klassen L.W., Sorrell M.F. Acetaldehyde and malondialdehyde react together to generate distinct protein adducts in the liver during long-term ethanol administration. Hepatology. 1996;23:872–880. doi: 10.1002/hep.510230431. [DOI] [PubMed] [Google Scholar]
- 126.Nlemelä O., Parkkila S., Ylä-herttuala S., Villanueva J., Ruebner B., Halsted C.H. Sequential acetaldehyde production, lipid peroxidation, and fibrogenesis in micropig model of alcohol-induced liver disease. Hepatology. 1995;22:1208–1214. doi: 10.1002/hep.1840220428. [DOI] [PubMed] [Google Scholar]
- 127.Kamimura S., Gaal K., Britton R.S., Bacon B.R., Triadafilopoulos G., Tsukamoto H. Increased 4-hydroxynonenal levels in experimental alcoholic liver disease: Association of lipid peroxidation with liver fibrogenesis. Hepatology. 1992;16:448–453. doi: 10.1002/hep.1840160225. [DOI] [PubMed] [Google Scholar]
- 128.Stasi C., Silvestri C., Voller F., Cipriani F. Epidemiology of liver cirrhosis. J. Clin. Exp. Hepatol. 2015;5:272. doi: 10.1016/j.jceh.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rehm J., Taylor B., Mohapatra S., Irving H., Baliunas D., Patra J., Roerecke M. Alcohol as a risk factor for liver cirrhosis: A systematic review and meta-analysis. Drug Alcohol Rev. 2010;29:437–445. doi: 10.1111/j.1465-3362.2009.00153.x. [DOI] [PubMed] [Google Scholar]
- 130.Yatsuhashi H., Sano H., Hirano T., Shibasaki Y. Real-world hospital mortality of liver cirrhosis inpatients in japan: A large-scale cohort study using a medical claims database: Prognosis of liver cirrhosis. Hepatol. Res. 2021;51:682–693. doi: 10.1111/hepr.13635. [DOI] [PubMed] [Google Scholar]
- 131.Jain P., Shasthry S.M., Choudhury A.K., Maiwall R., Kumar G., Bharadwaj A., Arora V., Vijayaraghavan R., Jindal A., Sharma M.K. Alcohol associated liver cirrhotics have higher mortality after index hospitalization: Long-term data of 5,138 patients. Clin. Mol. Hepatol. 2021;27:175. doi: 10.3350/cmh.2020.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Regier D.A., Kuhl E.A., Kupfer D.J. The dsm-5: Classification and criteria changes. World Psychiatry Off. J. World Psychiatr. Assoc. (WPA) 2013;12:92–98. doi: 10.1002/wps.20050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tuomisto S., Pessi T., Collin P., Vuento R., Aittoniemi J., Karhunen P.J. Changes in gut bacterial populations and their translocation into liver and ascites in alcoholic liver cirrhotics. BMC Gastroenterol. 2014;14:40. doi: 10.1186/1471-230X-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.El-Serag H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 135.Mocan T., Simão A.L., Castro R.E., Rodrigues C.M.P., Słomka A., Wang B., Strassburg C., Wöhler A., Willms A.G., Kornek M. Liquid biopsies in hepatocellular carcinoma: Are we winning? J. Clin. Med. 2020;9:1541. doi: 10.3390/jcm9051541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Seitz H.K., Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat. Rev. Cancer. 2007;7:599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 137.Gupta H., Youn G.S., Shin M.J., Suk K.T. Role of gut microbiota in hepatocarcinogenesis. Microorganisms. 2019;7:121. doi: 10.3390/microorganisms7050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Duque Correa M.A., Rojas López M. Activación alternativa del macrófago: La diversidad en las respuestas de una célula de la inmunidad innata ante la complejidad de los eventos de su ambiente. Inmunología. 2007;26:73–86. doi: 10.1016/S0213-9626(07)70077-X. [DOI] [Google Scholar]
- 139.El-Serag H.B., Rudolph K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 140.Teschke R. Alcoholic liver disease: Current mechanistic aspects with focus on their clinical relevance. Biomedicines. 2019;7:68. doi: 10.3390/biomedicines7030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Saffroy R., Pham P., Chiappini F., Gross-Goupil M., Castera L., Azoulay D., Barrier A., Samuel D., Debuire B., Lemoine A. The mthfr 677c > t polymorphism is associated with an increased risk of hepatocellular carcinoma in patients with alcoholic cirrhosis. Carcinogenesis. 2004;25:1443–1448. doi: 10.1093/carcin/bgh147. [DOI] [PubMed] [Google Scholar]
- 142.Leclercq S., Cani P.D., Neyrinck A.M., Stärkel P., Jamar F., Mikolajczak M., Delzenne N.M., de Timary P. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav. Immun. 2012;26:911–918. doi: 10.1016/j.bbi.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 143.Grabherr F., Grander C., Adolph T.E., Wieser V., Mayr L., Enrich B., Macheiner S., Sangineto M., Reiter A., Viveiros A., et al. Ethanol-mediated suppression of il-37 licenses alcoholic liver disease. Liver Int. Off. J. Int. Assoc. Study Liver. 2018;38:1095–1101. doi: 10.1111/liv.13642. [DOI] [PubMed] [Google Scholar]
- 144.Zhao J.-J., Pan Q.-Z., Pan K., Weng D.-S., Wang Q.-J., Li J.-J., Lv L., Wang D.-D., Zheng H.-X., Jiang S.-S., et al. Interleukin-37 mediates the antitumor activity in hepatocellular carcinoma: Role for cd57+ nk cells. Sci. Rep. 2014;4:5177. doi: 10.1038/srep05177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nezami Ranjbar M.R., Luo Y., Di Poto C., Varghese R.S., Ferrarini A., Zhang C., Sarhan N.I., Soliman H., Tadesse M.G., Ziada D.H., et al. Gc-ms based plasma metabolomics for identification of candidate biomarkers for hepatocellular carcinoma in egyptian cohort. PLoS ONE. 2015;10:e0127299. doi: 10.1371/journal.pone.0127299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Safaei A., Arefi Oskouie A., Mohebbi S.R., Rezaei-Tavirani M., Mahboubi M., Peyvandi M., Okhovatian F., Zamanian-Azodi M. Metabolomic analysis of human cirrhosis, hepatocellular carcinoma, non-alcoholic fatty liver disease and non-alcoholic steatohepatitis diseases. Gastroenterol. Hepatol. Bed Bench. 2016;9:158–173. [PMC free article] [PubMed] [Google Scholar]
- 147.Yu L.X., Schwabe R.F. The gut microbiome and liver cancer: Mechanisms and clinical translation. Nat. Rev. Gastroenterol. Hepatol. 2017;14:527–539. doi: 10.1038/nrgastro.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dapito D.H., Mencin A., Gwak G.Y., Pradere J.P., Jang M.K., Mederacke I., Caviglia J.M., Khiabanian H., Adeyemi A., Bataller R., et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and tlr4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Toyoda H., Komurasaki T., Uchida D., Takayama Y., Isobe T., Okuyama T., Hanada K. Epiregulin. A novel epidermal growth factor with mitogenic activity for rat primary hepatocytes. J. Biol. Chem. 1995;270:7495–7500. doi: 10.1074/jbc.270.13.7495. [DOI] [PubMed] [Google Scholar]
- 150.Uesugi T., Froh M., Arteel G.E., Bradford B.U., Thurman R.G. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 151.Yan G., Wang X., Sun C., Zheng X., Wei H., Tian Z., Sun R. Chronic alcohol consumption promotes diethylnitrosamine-induced hepatocarcinogenesis via immune disturbances. Sci. Rep. 2017;7:2567. doi: 10.1038/s41598-017-02887-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fujimoto M., Uemura M., Nakatani Y., Tsujita S., Hoppo K., Tamagawa T., Kitano H., Kikukawa M., Ann T., Ishii Y., et al. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: Relation to severity of liver disturbance. Alcohol. Clin. Exp. Res. 2000;24:48s–54s. doi: 10.1111/j.1530-0277.2000.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 153.Luo J., Xie M., Hou Y., Ma W., Jin Y., Chen J., Li C., Zhao K., Chen N., Xu L. A novel epigenetic mechanism unravels hsa-mir-148a-3p-mediated cyp2b6 downregulation in alcoholic hepatitis disease. Biochem. Pharmacol. 2021;188:114582. doi: 10.1016/j.bcp.2021.114582. [DOI] [PubMed] [Google Scholar]
- 154.Artru F., Saleh M.B., Maggiotto F., Lassailly G., Ningarhari M., Demaret J., Ntandja-Wandji L.-C., de Barros J.-P.P., Labreuche J., Drumez E. Il-33/st2 pathway regulates neutrophil migration and predicts outcome in patients with severe alcoholic hepatitis. J. Hepatol. 2020;72:1052–1061. doi: 10.1016/j.jhep.2019.12.017. [DOI] [PubMed] [Google Scholar]
- 155.Bauer T.M., Schwacha H., Steinbrückner B., Brinkmann F.E., Ditzen A.K., Aponte J.J., Pelz K., Berger D., Kist M., Blum H.E. Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. Am. J. Gastroenterol. 2002;97:2364–2370. doi: 10.1111/j.1572-0241.2002.05791.x. [DOI] [PubMed] [Google Scholar]
- 156.Hill D.B., Barve S., Joshi-Barve S., McClain C. Increased monocyte nuclear factor-kappab activation and tumor necrosis factor production in alcoholic hepatitis. J. Lab. Clin. Med. 2000;135:387–395. doi: 10.1067/mlc.2000.106451. [DOI] [PubMed] [Google Scholar]
- 157.Zhou L., Liao Y., Yin P., Zeng Z., Li J., Lu X., Zheng L., Xu G. Metabolic profiling study of early and late recurrence of hepatocellular carcinoma based on liquid chromatography-mass spectrometry. J. Chromatogr. B. 2014;966:163–170. doi: 10.1016/j.jchromb.2014.01.057. [DOI] [PubMed] [Google Scholar]
- 158.Liu Z., Nahon P., Li Z., Yin P., Li Y., Amathieu R., Ganne-Carrié N., Ziol M., Sellier N., Seror O., et al. Determination of candidate metabolite biomarkers associated with recurrence of hcv-related hepatocellular carcinoma. Oncotarget. 2017;9:6245–6258. doi: 10.18632/oncotarget.23500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Baniasadi H., Gowda G.A., Gu H., Zeng A., Zhuang S., Skill N., Maluccio M., Raftery D. Targeted metabolic profiling of hepatocellular carcinoma and hepatitis c using lc-ms/ms. Electrophoresis. 2013;34:2910–2917. doi: 10.1002/elps.201300029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Khusial R.D., Cioffi C.E., Caltharp S.A., Krasinskas A.M., Alazraki A., Knight-Scott J., Cleeton R., Castillo-Leon E., Jones D.P., Pierpont B., et al. Development of a plasma screening panel for pediatric nonalcoholic fatty liver disease using metabolomics. Hepatol. Commun. 2019;3:1311–1321. doi: 10.1002/hep4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Meoni G., Lorini S., Monti M., Madia F., Corti G., Luchinat C., Zignego A.L., Tenori L., Gragnani L. The metabolic fingerprints of hcv and hbv infections studied by nuclear magnetic resonance spectroscopy. Sci. Rep. 2019;9:4128. doi: 10.1038/s41598-019-40028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ponziani F.R., Bhoori S., Castelli C., Putignani L., Rivoltini L., Del Chierico F., Sanguinetti M., Morelli D., Paroni Sterbini F., Petito V., et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology (Baltimore, Md.) 2019;69:107–120. doi: 10.1002/hep.30036. [DOI] [PubMed] [Google Scholar]
- 163.Zheng R., Wang G., Pang Z., Ran N., Gu Y., Guan X., Yuan Y., Zuo X., Pan H., Zheng J., et al. Liver cirrhosis contributes to the disorder of gut microbiota in patients with hepatocellular carcinoma. Cancer Med. 2020;9:4232–4250. doi: 10.1002/cam4.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Grander C., Adolph T.E., Wieser V., Lowe P., Wrzosek L., Gyongyosi B., Ward D.V., Grabherr F., Gerner R.R., Pfister A., et al. Recovery of ethanol-induced akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018;67:891–901. doi: 10.1136/gutjnl-2016-313432. [DOI] [PubMed] [Google Scholar]
- 165.Corb Aron R.A., Abid A., Vesa C.M., Nechifor A.C., Behl T., Ghitea T.C., Munteanu M.A., Fratila O., Andronie-Cioara F.L., Toma M.M., et al. Recognizing the benefits of pre-/probiotics in metabolic syndrome and type 2 diabetes mellitus considering the influence of akkermansia muciniphila as a key gut bacterium. Microorganisms. 2021;9:618. doi: 10.3390/microorganisms9030618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bull-Otterson L., Feng W., Kirpich I., Wang Y., Qin X., Liu Y., Gobejishvili L., Joshi-Barve S., Ayvaz T., Petrosino J., et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of lactobacillus rhamnosus gg treatment. PLoS ONE. 2013;8:e53028. doi: 10.1371/journal.pone.0053028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jeong J.-J., Park H.J., Cha M.G., Park E., Won S.-M., Ganesan R., Gupta H., Gebru Y.A., Sharma S.P., Lee S.B., et al. The lactobacillus as a probiotic: Focusing on liver diseases. Microorganisms. 2022;10:288. doi: 10.3390/microorganisms10020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schwenger K.J.P., Clermont-Dejean N., Allard J.P. The role of the gut microbiome in chronic liver disease: The clinical evidence revised. JHEP Rep. 2019;1:214–226. doi: 10.1016/j.jhepr.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nishida S., Hamada K., Nishino N., Fukushima D., Koyanagi R., Horikawa Y., Shiwa Y., Saitoh S. Efficacy of long-term rifaximin treatment for hepatic encephalopathy in the japanese. World J. Hepatol. 2019;11:531–541. doi: 10.4254/wjh.v11.i6.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kang S.H., Lee Y.B., Lee J.H., Nam J.Y., Chang Y., Cho H., Yoo J.J., Cho Y.Y., Cho E.J., Yu S.J., et al. Rifaximin treatment is associated with reduced risk of cirrhotic complications and prolonged overall survival in patients experiencing hepatic encephalopathy. Aliment. Pharmacol. Ther. 2017;46:845–855. doi: 10.1111/apt.14275. [DOI] [PubMed] [Google Scholar]
- 171.Elfert A., Abo Ali L., Soliman S., Ibrahim S., Abd-Elsalam S. Randomized-controlled trial of rifaximin versus norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis. Eur. J. Gastroenterol. Hepatol. 2016;28:1450–1454. doi: 10.1097/MEG.0000000000000724. [DOI] [PubMed] [Google Scholar]
- 172.Kalambokis G.N., Mouzaki A., Rodi M., Pappas K., Fotopoulos A., Xourgia X., Tsianos E.V. Rifaximin improves systemic hemodynamics and renal function in patients with alcohol-related cirrhosis and ascites. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2012;10:815–818. doi: 10.1016/j.cgh.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 173.Vlachogiannakos J., Viazis N., Vasianopoulou P., Vafiadis I., Karamanolis D.G., Ladas S.D. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J. Gastroenterol. Hepatol. 2013;28:450–455. doi: 10.1111/jgh.12070. [DOI] [PubMed] [Google Scholar]
- 174.Shariff M.I.F., Tognarelli J.M., Lewis M.R., Want E.J., Mohamed F.E.Z., Ladep N.G., Crossey M.M.E., Khan S.A., Jalan R., Holmes E., et al. Plasma lipid profiling in a rat model of hepatocellular carcinoma: Potential modulation through quinolone administration. J. Clin. Exp. Hepatol. 2015;5:286–294. doi: 10.1016/j.jceh.2015.07.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ginés P., Rimola A., Planas R., Vargas V., Marco F., Almela M., Forné M., Miranda M.L., Llach J., Salmerón J.M., et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: Results of a double-blind, placebo-controlled trial. Hepatology. 1990;12:716–724. doi: 10.1002/hep.1840120416. [DOI] [PubMed] [Google Scholar]
- 176.Fernández J., Navasa M., Gómez J., Colmenero J., Vila J., Arroyo V., Rodés J. Bacterial infections in cirrhosis: Epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 177.Li J., Sung C.Y., Lee N., Ni Y., Pihlajamäki J., Panagiotou G., El-Nezami H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. USA. 2016;113:E1306–E1315. doi: 10.1073/pnas.1518189113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Delaune V., Orci L.A., Lacotte S., Peloso A., Schrenzel J., Lazarevic V., Toso C. Fecal microbiota transplantation: A promising strategy in preventing the progression of non-alcoholic steatohepatitis and improving the anti-cancer immune response. Expert Opin. Biol. Ther. 2018;18:1061–1071. doi: 10.1080/14712598.2018.1518424. [DOI] [PubMed] [Google Scholar]
- 179.Zakhari S. Overview: How is alcohol metabolized by the body? Alcohol Res. Health J. Natl. Inst. Alcohol Abus. Alcohol. 2006;29:245–254. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.