Abstract
NF-YCs are important transcription factors with diverse functions in the plant kingdoms including seed development. NF-YC8, 9, 10, 11 and 12 are close homologs with similar seed-specific expression patterns. Despite the fact that some of the NF-YCs are functionally known; their biological roles have not been systematically explored yet, given the potential functional redundancy. In this study, we generated pentuple mutant pnfyc of NF-YC8-12 and revealed their functions in the regulation of grain quality and seed germination. pnfyc grains displayed significantly more chalkiness with abnormal starch granule packaging. pnfyc seed germination and post-germination growth are much slower than the wild-type NIP, largely owing to the GA-deficiency as exogenous GA was able to fully recover the germination phenotype. The RNA-seq experiment identified a total of 469 differentially expressed genes, and several GA-, ABA- and grain quality control-related genes might be transcriptionally regulated by the five NF-YCs, as revealed by qRT-PCR analysis. The results demonstrated the redundant functions of NF-YC8-12 in regulating GA pathways that underpin rice grain quality and seed germination, and shed a novel light on the functions of the seed-specific NF-YCs.
Keywords: rice (Oryza sativa L.), gibberellins, abscisic acid, NF-YCs
1. Introduction
Rice (Oryza sativa L.) is a major cereal crop in the world, as it is consumed as a staple food by more than half of the world’s population [1]. Rice seed is a complex organ that is comprised of a maternal caryopsis coat, a diploid embryo and a triploid endosperm. The nutrients such as starch, protein and lipids are accumulated in the endosperm underpinning seed germination or grain yield and quality for human consumption. It has been known that phytohormones are extensively involved in the regulation of plant seed development [2,3,4,5]. The action of GAs and ABA on seed development is strictly correlated and antagonistic [2]. Through the investigation of the rice seed hormonal dynamics during the grain filling stage, Yang et al. (2001) revealed that GAs play key roles in embryogenesis, while the ABA content reached the peak at a much later stage, thus it seemed to be more relevant to the seed maturation [6]. So far, numerous pieces of literature about genes controlling rice seed development have been published, and these genes are involved in transcriptional regulation, the ubiquitin–proteasome pathway, plant hormone response, and so on [7,8,9]. Specifically, Yao et al. (2017) found that NF-YC8 to NF-YC12 are five important genes involved in starch synthesis, seed storage protein and the stress response [7]. The study shows that NF-YC12 is a key transcription factor in regulating endosperm development [8] and storage material accumulation in rice seeds [10]. A novel transcription factor subunit NF-YC13 was identified in indica rice, which can respond to salt stress signals by interacting with the B-subunit [11]. It is studied that NF-YC2 and NF-YC4 proteins can interact with three flowering-time genes to regulate the photoperiodic flowering response under long sunlight conditions [12].
Nuclear Factor Y (NF-Y) is a family of transcription factors that are found in vast quantities in higher eukaryotes. The NF-Y protein complex consists of three subunits: NF-YA (CBF-B/HAP2), NF-YB (CBF-A/HAP3), and NF-YC (CBF-C/HAP5) which usually forms a heterotrimer to regulate the transcription of the target genes [13,14]. For yeast and animals, each NF-Y subunit is encoded by a single gene. However, the situation in plants is more complicated with multiple members of each subunit, which dramatically expanded the diversity of NF-Ys’ gene function in the plant kingdom [13,15]. In rice, each NF-Y subunit covers more than 10 gene members as reported, and many of them have been identified to participate in extensive developmental processes like nutrient accumulation in the endosperm, flowering regulation and ABA signal response [16,17,18,19]. NF-YBs are well-documented among those three subunits and have been implicated in plant height regulation, grain yield, carbon assimilation, photoperiodic flowering and other processes [20,21,22,23,24]. For example, NF-YB2, NF-YB3 and NF-YB4 are close homologs that are functionally redundant in regulating chloroplast biogenesis in rice [25]. It is noteworthy that several NF-YBs and NF-YCs were found to be specifically expressed in rice endosperm. Some seed-specific NF-YBs control rice seed development by affecting the nutrition accumulation and the loading of sucrose into developing seeds [26,27,28]. In addition, endosperm-specific NF-YBs and NF-YCs may also form heterotrimer complexes with other non-NF-Y transcription factors, hence regulating grain filling and quality via the ubiquitin–proteasome pathway [8,29]. For example, AtNF-YB9-YC12-bZIP67 can activate the expression of SUS2 and promote seed development [30]; OsNF-YB1 interacts with OsNF-YC12, and OsNF-YC12 can bind to the promoter of FLO6 and OsGS1;3 to regulate grain weight and chalky endosperm [10].
Several previous works have revealed that OsNF-YC8 (LOC_Os01g01290), OsNF-YC9 (LOC_Os01g24460), OsNF-YC10 (LOC_Os01g39850), OsNF-YC11 (LOC_Os10g23910) and OsNF-YC12 (LOC_Os05g11580) are close homologs with similar seed-specific expression pattern, implying that they play a role in rice seed development [7,8,29,31]. So far, it is known that the NF-YC9 controls cell proliferation to influence grain width, and NF-YC11 regulates the accumulation of storage substances in rice seeds [10,32]. In 2019, our lab reported that NF-YC12 forms a heterotrimer complex with NF-YB1 and bHLH144 to regulate rice grain quality [8]. Nevertheless, knowledge about the function of the five genes is rather fragmented, given that the high similarity of the genes may give rise to functional redundancy. Here, we report the systematic functional analysis of NF-YC8, 9, 10, 11 and 12 using single gene or pentuple gene mutants. The five genes may work redundantly to regulate ABA and GA response, thus determining grain quality and seed germination. This work sheds new insight into the functional roles of the seed-specific OsNF-YCs.
2. Results and Discussion
2.1. Five Seed-Specific NY-YCs Works Redundantly to Regulate Grain Quality
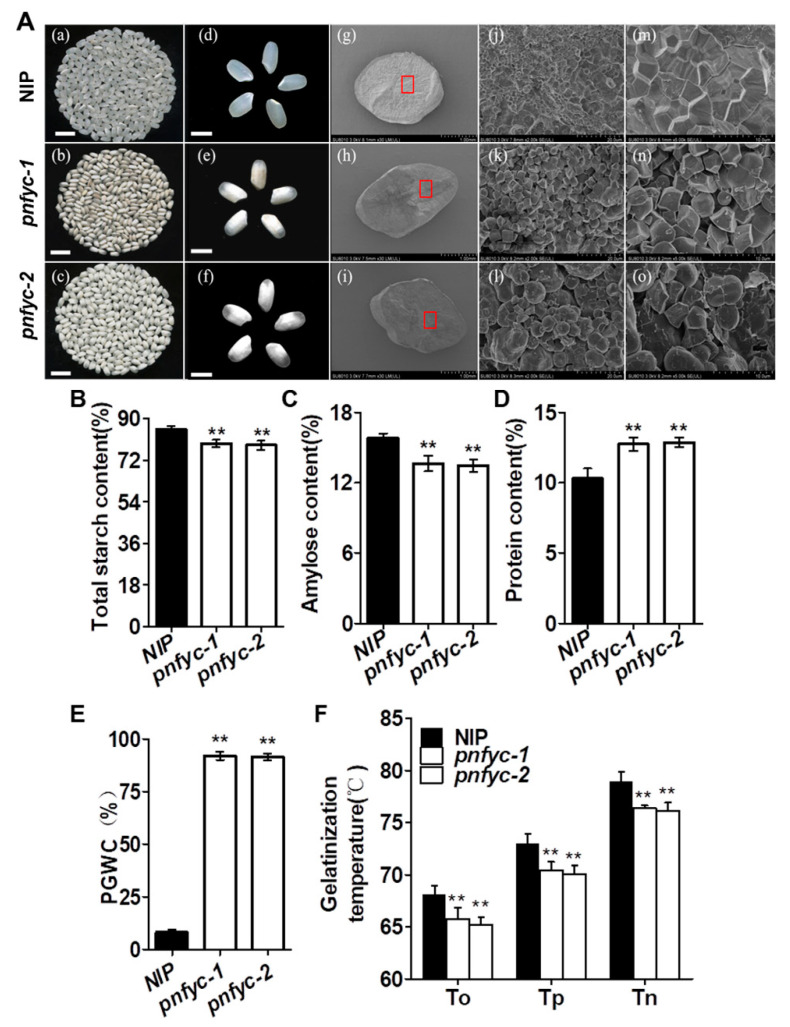
To specify the biological functions of OsNF-YC8, OsNF-YC9, OsNF-YC10, OsNF-YC11 and OsNF-YC12, we generated a single mutant of each gene in the background of Kitaake (Oryza sativa ssp. Japonica), respectively. Sanger sequencing further confirmed the mutations of the corresponding genes with insertion or deletions, which should have shifted the open reading frame and disrupted the resulting protein functions (Figure S1). Compared with Kitaake, nfyc8 exhibited increased percentage of grains with chalkiness (PGWC), nfyc12 had higher degree of chalkiness (DEC), while nfyc9 and nfyc10 exhibited increased PGWC and higher DEC (Figure S2A–C). We used the CRISPR/Cas9 technology to simultaneously knock out all the five NF-YC genes in Nipponbare (NIP, Oryza sativa ssp. japonica) to generate the pentuple nf-yc mutants (hereafter referred to as pnfyc) to assess the potential functional redundancy among the genes. Two representative lines pnfyc-1 and pnfyc-2 were selected for the followed genotyping and genetic analysis. As shown in Figure S3, Sanger sequencing detected various types of homozygous insertion or deletion mutations in each of the NF-YC8-12, suggesting all the five genes were successfully knocked-out. During the vegetative growth stage, no visible differences were observed in major agronomic traits such as plant height, flowering date, seed setting and spikelets per panicle in the pnfyc lines (Table S1). However, the milled grains of pnfyc lines showed obvious chalkiness. As revealed by the cross-sections of the pnfyc seeds, the starchy endosperm of pnfyc was floury-white when compared with NIP. Scanning electron microscopy (SEM) images of transverse sections indicated that the starch granule of NIP and pnfyc grains had different morphologies, shape and packaging densities. Unlike the regular shape of the starch granule of NIP, pnfyc had irregular, loosely packed starch granules, which might be responsible for the observed chalkiness (Figure 1A). Furthermore, we examined the contents of storage substances in the brown seeds, and found that the total starch and amylose contents of pnfyc were significantly lower than that of the NIP. Conversely, pnfyc had relatively higher crude protein contents than NIP (Figure 1B–D). The PGWC in pnfyc reached over 90%, while that of NIP was less than 10% (Figure 1E). Following the change in the starch contents, differential scanning calorimetry (DSC) analysis demonstrated that the gelatinization characteristics including the onset, peak as well as end gelatinization temperatures of pnfyc were also significantly altered (Figure 1F and Table S2). The results above indicated that OsNF-YC8, 9, 10, 11 and 12 work redundantly to positive regulate rice grain quality, particularly the grain chalkiness.
Figure 1.
(A) (a–o) Grain chalkiness phenotypical characterization (a–f), bar = 2 mm. Scanning electron microscopy (SEM) analysis (g–o). The central areas shown are indicated as red squares. The magnification is 30 times in (g,h,i); 2000 times in (j,k,l), and 5000 times in (m,n,o). (B–F) Quality trait parameters of mature seeds from pnfyc lines and NIP. Data are shown as means ± SD of at least three biological replicates. (** p < 0.01 by two-tailed Student’s t-test).
2.2. pnfyc Are GA-Deficient with Retarded Seed Germination
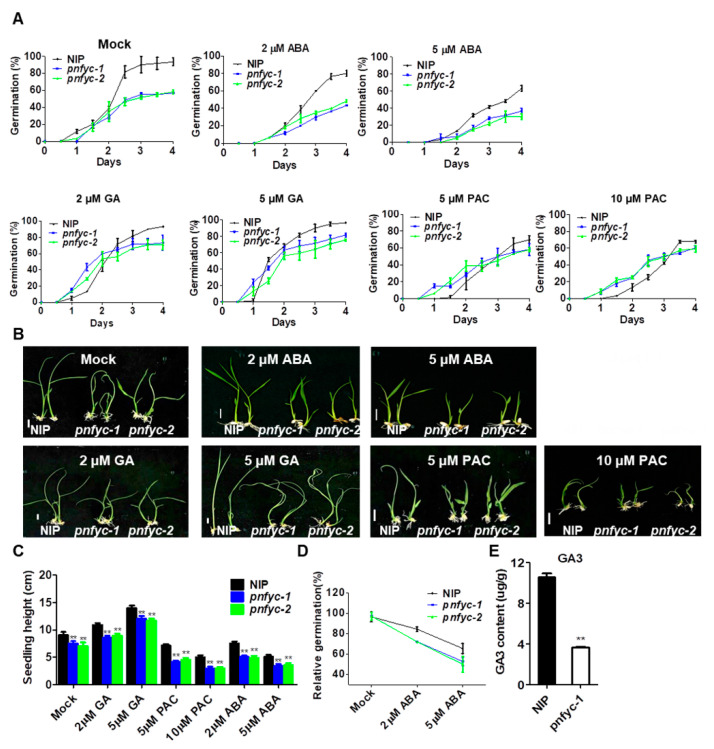
The altered storage substance proportion in pnfyc lines provoked us to test the roles of the five NF-YCs in seed germination. We carried out germination assays on the nfyc single mutants, pnfyc and NIP lines on ½ MS medium for 4 days. All the five nfyc single mutants showed slightly lower germination rates compared with Kitaake, which was followed by retarded post-germination growth (Figure S4A–C). However, the pnfyc seeds showed much retarded germination (56.7–60.0%) than the NIP (90.0–96.7%) at day 4, which further confirmed the functional redundancy among the five NF-YCs. Given the key roles of ABA and GA in seed germination regulation, we subsequently investigated the seed germination under exogenous ABA, GA and PAC (GA biosynthesis inhibitor) treatments (Figure 2A–C). The application of 2 μM exogenous ABA significantly restrained the seed germination of both pnfyc and NIP seeds. To evaluate the relative ABA sensitivity of the seeds, we calculated the relative germination rate of the seeds under mock and ABA treatments. The results showed that the WT relative germination rates of 2 μM ABA/mock and 5 μM ABA/mock were 72.2% and 65.5%, respectively. However, for the pnfyc seeds, 84.5% and 51.4% were obtained. Therefore, pnfyc is hypersensitive to exogenous ABA treatment in seed germination (p < 0.05) (Figure 2D). Similar hypersensitivity to ABA inhibition effects was also observed in the post-germination growth of pnfyc seedlings. In contrast to the ABA treatments, the application of 2 μM exogenous GA significantly recovered the seed germination of pnfyc, and a more intense recovering effect was observed when 5 μM exogenous GA was applied, suggesting the GA deficiency might be the major reason for the retarded seed germination in pnfyc. Exogenous PAC displayed similar inhibitory effects as ABA, as 10 μM exogenous PAC decreased the NIP germination rate and post-germination growth to the pnfyc level. In addition, we quantified the GA3 contents in the germinative seeds of NIP and pnfyc-1. The result showed that the GA3 content in pnfyc-1 was reduced to only 30% of the NIP (p < 0.01), which is in agreement with the recovered germination of pnfyc by GA (Figure 2E).
Figure 2.
(A) The germination rate of NIP and pnfyc lines under different concentrations of exogenous hormones. (B) The plant morphology of NIP and pnfyc lines treated with different exogenous hormones at 7 days after germination. (C) The seedling height of NIP and pnfyc lines under different exogenous hormones at 7 days after germination. Bar = 1 cm. (D) The relative germination of the NIP and pnfyc seeds under ABA treatments were determined after 4 days and expressed as a percentage of those grown under ‘mock’ conditions. (E) Quantification of GA3 derivatives in NIP and pnfyc seeds germinated for 6 h was analyzed with liquid chromatography-tandem mass spectrometry. Data are shown as means ± SD of at least three biological replicates. (** p < 0.01 by two-tailed Student’s t-test).
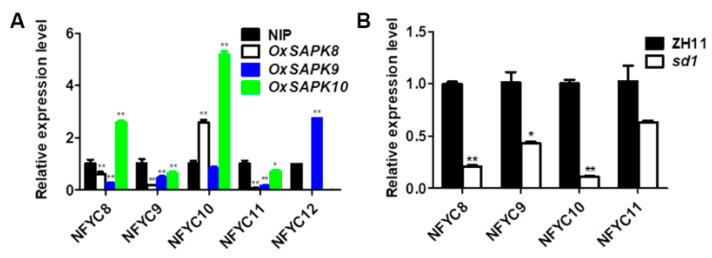
To test the involvement of the five NF-YCs in ABA and GA biosynthesis and signaling, we examined the transcription of the genes in the germinative seeds of various ABA or GA-related genetic lines by qRT-PCR. SAPK8, 9 and 10 are core elements of ABA signaling, and over-expression of the genes conferred plants ABA hypersensitivity [33]. We found that NF-YC10 was significantly up-regulated by SAPK8 and 10, while NF-YC8 and NF-YC12 were up-regulated by SAPK10 and SAPK9, respectively. However, NF-YC9 and NF-YC11 were down-regulated in all the three OxSAPK lines (Figure 3A). In the GA-deficient mutant sd1 [34], the transcription of NF-YC8, 9, 10 and 11 were all severely repressed, indicating the five NF-YCs are highly responsive to endogenous GA level (Figure 3B). Taken together, we proposed that NF-YC8, 9, 10, 11 and 12 may serve as key regulators mediating the balance of GA and ABA.
Figure 3.
(A) The expression level of NFYC8 to NFYC12 in the seeds of wild-type-NIP, OxSAPK8, OxSAPK9, and OxSAPK10 mutants that germinated for 6 h. (B) The expression level of NFYC8 to NFYC11 in the seeds of wild-type-ZH11 and sd1 mutant that germinated for 6 h (* p < 0.05, ** p < 0.01 by two-tailed Student’s t-test).
Although NF-YC10 and NF-YC12 have been reported as key regulators of rice seed development [8,10,32], the roles of the five seed-specific NY-YCs are still not very clear so far, given the potential functional redundancy among them. By simultaneously knocking out the five genes, we revealed their functions in grain quality and seed germination, and the GA-deficiency in pnfyc might be the major reason for the observed phenotype. Aside from the well-known function as a seed germination promoter, GA has been recently found to regulate endosperm development as well. Cui et al. (2020) reported that application of exogenous GA4+7 significantly altered the content of other phytohormones such as auxin, zeatin and ABA, increased the activities of superoxide dismutase, catalases, and peroxidases and reduced the malondialdehyde content, which finally improved grain filling and yield in maize [35]. In Arabidopsis, NF-YC3, NF-YC4 and NF-YC9 have redundant roles in the regulation of GA-ABA-mediated seed germination [36]. NF-YCs bind RGL2, a repressor during GA signaling, and then in the form of NF-YC–RGL2 module targets ABI5, a key factor in ABA signaling [37,38]. NF-YC can bind to the CCAAT-box on the ABI5 promoter to regulate ABI5 gene expression. The NF-YC–RGL2–ABI5 module integrates ABA and GA signaling to regulate seed germination [36].
2.3. NF-YCs Regulates the Transcription of ABA and GA Pathway Genes
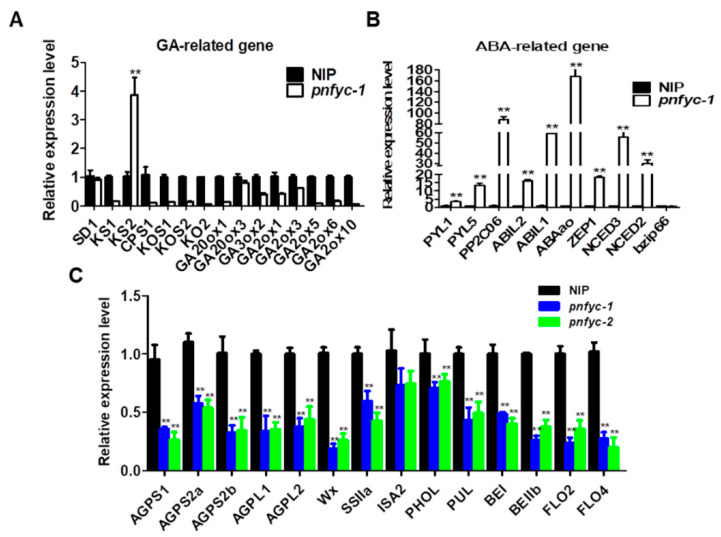
RNA sequencing experiments on the 6 HAI (6 h after imbibition) germinating seeds of pnfyc and WT were carried out to identify the potential target genes of the five NF-YCs. As a result, there were a total of 469 differentially expressed genes (DEGs) as shown in (Supplementary Table S1). KEGG analysis revealed predominant enrichment of the DEGs on the pathways of ‘phenylpropanoid biosynthesis’, ‘protein processing in endoplasmic reticulum’ and ‘plant hormone signal transduction’ (Figure S5A). We further carried out a qRT-PCR analysis on eight randomly selected DEGs to verify the RNA-seq results, and the results showed that seven of those genes had a similar transcriptional level inclination to that detected by the RNA-seq, indicating the high reliability of our RNA-seq results (Figure S5B). Notably, a series of genes reported as critical regulators of the ABA signal pathway were found to be down-regulated in pnfyc (Figure 4B), including positive ABA signaling factors like OsbZIP46 [39,40], OsbZIP12 [41,42] and OsNAC52 [43] as well as the OsHSP24.1 encoding an ABA-responsive heat shock protein [44]. Furthermore, we conducted the qRT-PCR and results showed that the expression of most GA biosynthesis genes was down-regulated in pnfyc plants, while the expression of ABA biosynthesis and negative signal pathway-related genes was up-regulated (Figure 4A,B).
Figure 4.
(A) The expression level of GA synthesis and metabolism-related genes in the seeds of wild-type-NIP and pnfyc mutants that germinated for 6 h. (B) The expression level of ABA biosynthesis and negative signal pathway-related genes in the seeds of wild-type-NIP and pnfyc mutants that germinated for 6 h. (C) The expression level of starch synthesis-related genes in wild-type-NIP and pnfyc mutant seeds 7 days after pollination. Data are shown as means ± SD of at least three biological replicates. (** p < 0.01 by two-tailed Student’s t-test).
Given the severely affected starch qualities in pnfyc seeds, we also examined the transcriptional levels of several starch biosynthesis enzyme or regulator genes in the developing seeds of pnfyc and NIP. It was found that, except for ISA2, all of these ADP-glucose pyrophosphorylase, granule-bound starch synthase, starch synthase and starch branching enzyme were mostly down-regulated in 7 DAP endosperm of pnfyc lines (Figure 4C) [45,46,47,48].
2.4. Potential Interactive Proteins of the Five NF-YCs
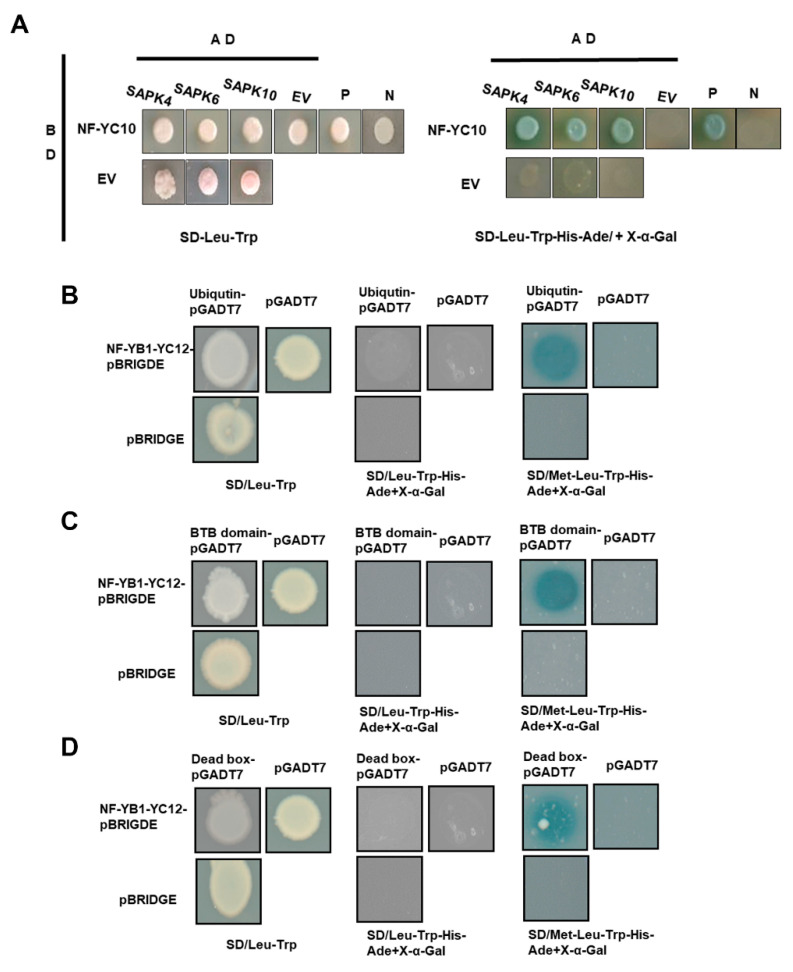
We tested the protein–protein interaction between the 5 NF-YCs and 10 SAPKs which are ABA signaling components. A total of 50 NF-YC-SAPK combinations were tested by yeast-two-hybrid, and results showed that NF-YC10 binds to SAPK4, 6 and 10, while all the other combinations were negative. Hence, the suggestion is that NF-YC10 perceives the ABA signal from SAPK4, 6 and 10 (Figure 5A).
Figure 5.
(A) Y2H assay of interaction between NF-YC10 and SAPKs. BD: pDEST32; AD: pDEST22; EV: empty vector, pDEST32 or pDEST22; P: positive control, pGBKT7-53/pGADT7-T; N: positive control, pGBKT7-Lam/pGADT7-T. (B) Y3H analysis of NF-YB1, NF-YC12, and Ubiquitin protein domain. (C) Y3H analysis of NF-YB1, NF-YC12, and BTB/POZ protein domain. (D) Y3H analysis of NF-YB1, NF-YC12, and Dead-box protein domain.
Our previous study has demonstrated that NF-YB1-YC12 dimer binds to bHLH144 to form a heterotrimer complex that regulates rice grain quality [8]. To identify other components that may interact with NF-YB1-YC12 dimer, we performed yeast-three hybrid (Y3H) experiments to screen a seed-derived prey library using NF-YB1-YC12-pBRIDGE as bait. We finally obtained three interactive proteins LOC_Os01g68950, LOC_Os07g46160 and LOC_Os11g38670 which are annotated as ubiquitin domain-containing protein, BTB/POZ domain-containing protein and dead-box ATP-dependent RNA helicase, respectively. Interestingly, the interactions are valid only on the SD/-Met/-Leu/-Trp/-Ade/-His/+X-α-Gal medium, in which the drop-out of methionine drove the expression of NF-YC12 under Met25 promoter. Meanwhile, the interactions were compromised on SD/-Leu/-Trp/-Ade/-His/+X-α-Gal medium, in which NF-YC12 was suppressed by the supplemented methionine in the medium. Hence, the binding of NF-YB1-YC12 is necessary for the formation of heterotrimer complexes with the three proteins (Figure 5B–D).
In conclusion, we report a rice pentuple gene mutant pnfyc, which knocked out five homologous genes OsNF-YC8, OsNF-YC9, OsNF-YC10, OsNF-YC11 and OsNF-YC12 simultaneously. The expression of starch synthesis genes decreased in pnfyc, resulting in the decrease of starch content and the increase of protein content, the change of grain quality and the significant increase of chalkiness trait. The results showed that NF-YC8 to NF-YC12 could regulate grain quality traits by regulating starch synthesis. In addition, NF-YC8-12 also inhibited seed germination by affecting the expression of GA-related genes, and the phenotype is significantly restored by applying exogenous GA. Finally, the expression of ABA-related genes in pnfyc increased, and pnfyc seeds were hypersensitive to exogenous ABA. NFYC10 could interact with SAPK to regulate ABA expression.
3. Materials and Methods
3.1. Plant Growth Conditions and Phenotype Measurement
To generate the CRISPR/Cas9-derived knock-out mutants, the specific target small-guide RNA (sgRNA) of each gene was designed and assembled in a pYLCRISPR/CAS9-MH vector system according to a previous report [49], and subsequently transformed into Nipponbare and Kitaake (Oryza sativa, ssp. japonica) backgrounds. All the plants were grown in the experimental greenhouse and field of the China National Rice Research Institute (CNRRI). Agronomic traits were analyzed with 10 replicates. Panicle length, number of primary branch panicles, number of effective panicles per plant, seed setting rate (%), and plant height were measured manually. Rice seed grains were harvested and air-dried at room temperature for at least 2 weeks. The thousand-grain-weight, seed length, width and chalkiness were examined by a seed phenotyping system (Wan Sheng, Hangzhou, China). Grain thickness was determined at the same time for each grain using an electronic digital calliper.
3.2. Physicochemical Properties of Seed Grain
Total starch content of the dried brown seeds was measured using a starch assay kits Megazyme K-TSTA and KAMYL (Megazyme, Ireland, UK, http://www.megazyme.com/ accessed on 6 May 2020). The total amylose and protein contents in the grains were measured by following a previous report [50]. The content is expressed as the percentage of total sample weight on an oven-dry basis. To analyze the gelatinization temperature, DSC assay was conducted on a differential scanning calorimeter DSC1 STARe system (METTLER-TOLEDO, Zurich, Switzerland). Briefly, 5 mg rice powder was sealed and placed in an aluminum sample cup, mixed with 10 μL distilled water, and then the samples were analyzed by the differential scanning calorimeter (METTLER-TOLEDO, Zurich, Switzerland). The heating rate was 10 °C min−1 over a temperature range of 40 °C to 100 °C [51].
3.3. Scanning Electron Microscopy (SEM) Assay
Prepare two types of milled rice, one was wild-type NIP and the other was the pnfyc mutant. The whole grains were cut transversely with a sharp blade and then sputtered with gold in order to increase electrical conductivity. Fractured rice grains were mounted on the copper stage and then viewed with a scanning electron microscope at 30, 2000 and 5000 times magnification. The analysis was performed based on three biological replicates at least. The experiment was conducted in institute of Agriculture and Biotechnology, ZheJiang University as described previously using a HITACHI S-3400N scanning electron microscope (HITACHI, Tokyo, Japan).
3.4. Seed Germination and Phenotypic Assay
Briefly, 100 dehusked seeds were surface-sterilized in 75% ethanol for 2 min, then in 50% bleach for 30 min, and then cleaned with sterilized ddH2O 5–8 times for 3 min each time. The sterilized seeds were air dried and sown on a ½ strength MS medium containing different concentrations of ABA (0, 2, 5 µM), GA (0, 2, 5 µM) and PAC (0, 2, 5, 10 µM). Germination rates were recorded every 12 h. The seedlings’ height above ground was measured and the growth status of seedlings was photographed after 7 days. Germination is established with the appearance of the emergence of 2 mm embryos through the seed coat. The data are the mean of 3 biological triplicates.
3.5. RNA-Seq Analysis and RT-PCR Analysis
For RNA-seq, total RNA of germinative seeds at 6 h-after-imbibition was extracted using Trizol as instructed (Yeasen, Shanghai, China). The high-throughput sequencing was performed using the Illumina HiSeq™ 2500 platform and the KEGG pathway analysis of the DEGs was ultimately done by Personalgene Technology Co (Personal, Shanghai, China). DEGs were defined as genes with |log2Fold change| ≥ 1 and FDR < 0.01 using EBSeq [52]. The endosperm of 7 days after fertilization and seeds of 6 h after germination were collected as samples for the extraction of RNA to detect the expression level of genes in starch biosynthesis and hormone synthesis, and the RNA was extracted by Trizol according to the kit manufacturer’s instructions (Yeasen, Shanghai, China).
For the RT-PCR analysis, the first-strand cDNA was synthesized using M-MLV reverse transcriptase according to the manufacturer’s instructions (Takara, Dalian, China). The expression levels of different samples were determined using CFX96 touch real-time PCR detection system (Bio-Rad, Hercules, CA, USA). Expression was assessed by evaluating threshold cycle (CT) values. The relative expression level of tested genes was normalized to ubiquitin gene and calculated by the 2−∆∆CT method. The experiment was performed in two biological replicates with three technical triplicates of each. Primer sequences are listed in Supplementary Table S3.
3.6. Yeast-Two-Hybrid Assay
The yeast two-hybrid assay was conducted based on the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). The coding sequence of NF-YC8 to NF-YC12 and SAPK1 to SAPK10 were amplified and cloned into bait vector pdest32 and prey vector pdest22, respectively. Two vectors, pDEST32-NFYCs and pDEST-SAPKs, were co-transfected into the Y2H Gold strain, and then yeast cells were grown on SD/-Trp/-Leu and SD/-Trp/-Leu/-His/-Ade medium for screening. pGBKT7-53 and pGADT7-T were used as positive controls, pGBKT7-Lam and pGADT7-T were used as negative controls. The primers used are listed in Supplementary Table S3.
3.7. Yeast-Three-Hybrid Assay
NF-YB1 CDS was cloned to fuse with GAL4 BD domain, and NF-YC12 was driven by a methionine-responsive promoter Met25 in pBRIDGE (Clontech, Dalian, China). NF-YB1-NF-YC12-pBRIDGE in strain Y2H Gold was mated with an AD domain-fused seed cDNA library in Y187 strain. The mated transformants were first selected on SD/-Leu/-Trp. Positive colonies were then transferred to SD/-Leu/-Trp/-His/-Ade/-Met/+X-a-Gal and SD/-Leu/-Trp/-His/-Ade/+X-a-Gal, respectively. The interaction was confirmed by the visualization of blue colonies on the medium.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23158382/s1.
Author Contributions
Conceptualization, J.Z.; Formal analysis, S.L.; Investigation, J.L.; Methodology, L.C., X.L., M.Y. and X.T.; Project administration, J.Z.; Resources, B.B.K., J.Y. and J.Z.; Software, C.C., B.N., A.A.A. and X.L.; Supervision, Y.W. and X.Z.; Writing—original draft, H.X.; Writing—review & editing, J.Z. All the authors read and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and within Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Natural Science Foundation of Zhejiang province (Grant No. 343 LZ21C130001), National Natural Science Foundation of China (Grant No. 32071986 and U20A2030), CNRRI key research and development project (CNRRI-2020-01), and ASTIP program of CAAS.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang N.L., Long T., Yao W., Xiong L.Z., Zhang Q.F., Wu C.Y. Mutant resources for the functional analysis of the rice genome. Mol. Plant. 2013;6:596–604. doi: 10.1093/mp/sss142. [DOI] [PubMed] [Google Scholar]
- 2.Locascio A., Roig-Villanova I., Bernardi J., Varotto S. Current perspectives on the hormonal control of seed development in Arabidopsis and maize: A focus on auxin. Front Plant Sci. 2014;5:412. doi: 10.3389/fpls.2014.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rijavec T., Dermastia M. Cytokinins and their function in developing seeds. Acta Chim. Slov. 2010;57:617–629. [PubMed] [Google Scholar]
- 4.Abbas M., Alabadi D., Blazquez M.A. Differential growth at the apical hook: All roads lead to auxin. Front. Plant Sci. 2013;4:441. doi: 10.3389/fpls.2013.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hedden P., Thomas S.G. Plant Hormone Signaling. Annu. Plant Rev. 2006;24:229–255. [Google Scholar]
- 6.Yang J., Zhang J., Wang Z., Zhu Q., Wang W. Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiol. 2001;127:315–323. doi: 10.1104/pp.127.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang W., Lu Z., Xiong Y., Yao J. Genome-wide identification and co-expression network analysis of the OsNF-Y gene family in rice. Crop J. 2017;5:21–31. doi: 10.1016/j.cj.2016.06.014. [DOI] [Google Scholar]
- 8.Bello B.K., Hou Y.X., Zhao J., Jiao G.A., Wu Y.W., Li Z.Y., Wang Y.F., Tong X.H., Wang W., Yuan W.Y., et al. NF-YB1-YC12-bHLH144 complex directly activates Wx to regulate grain quality in rice (Oryza sativa L.) Plant Biotechnol. J. 2019;17:1222–1235. doi: 10.1111/pbi.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamakawa H., Hirose T., Kuroda M., Yamaguchi T. Comprehensive expression profiling of rice grain filling-related genes under high temperature using DNA microarray. Plant Physiol. 2007;144:258–277. doi: 10.1104/pp.107.098665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong Y.F., Ren Y., Li W., Wu F.S., Yang W.J., Huang X.L., Yao J.L. NF-YC12 is a key multi-functional regulator of accumulation of seed storage substances in rice. J. Exp. Bot. 2019;70:3765–3780. doi: 10.1093/jxb/erz168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manimaran P., Venkata Reddy S., Moin M., Raghurami Reddy M., Yugandhar P., Mohanraj S.S., Balachandran S.M., Kirti P.B. Activation-tagging in indica rice identifies a novel transcription factor subunit, NF-YC13 associated with salt tolerance. Sci. Rep. 2017;7:9341. doi: 10.1038/s41598-017-10022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S.K., Park H.Y., Jang Y.H., Lee K.C., Chung Y.S., Lee J.H., Kim J.K. OsNF-YC2 and OsNF-YC4 proteins inhibit flowering under long-day conditions in rice. Planta. 2016;243:563–576. doi: 10.1007/s00425-015-2426-x. [DOI] [PubMed] [Google Scholar]
- 13.Laloum T., De Mita S., Gamas P., Baudin M., Niebel A. CCAAT-box binding transcription factors in plants: Y so many? Trends Plant Sci. 2013;18:157–166. doi: 10.1016/j.tplants.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Zhao H., Wu D., Kong F., Lin K., Zhang H., Li G. The Arabidopsis thaliana nuclear factor Y transcription factors. Front. Plant Sci. 2017;7:2045. doi: 10.3389/fpls.2016.02045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swain S., Myers Z.A., Siriwardana C.L., Holt B.F., 3rd The multifaceted roles of NUCLEAR FACTOR-Y in Arabidopsis thaliana development and stress responses. Biochim. Biophys. Acta Gene Regul. Mech. 2017;1860:636–644. doi: 10.1016/j.bbagrm.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Petroni K., Kumimoto R.W., Gnesutta N., Calvenzani V., Fornari M., Tonelli C., Holt B.F., Mantovani R. The promiscuous life of plant NUCLEAR FACTOR Y transcription factors. Plant Cell. 2012;24:4777–4792. doi: 10.1105/tpc.112.105734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thirumurugan T., Ito Y., Kubo T., Serizawa A., Kurata N. Identification, characterization and interaction of HAP family genes in rice. Mol. Genet. Genom. 2008;279:279–289. doi: 10.1007/s00438-007-0312-3. [DOI] [PubMed] [Google Scholar]
- 18.Lee D.K., Kim H.I., Jang G., Chung P.J., Jeong J.S., Kim Y.S., Bang S.W., Jung H., Do Choi Y., Kim J.K. The NF-YA transcription factor OsNF-YA7 confers drought stress tolerance of rice in an abscisic acid independent manner. Plant Sci. 2015;241:199–210. doi: 10.1016/j.plantsci.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Li L., Zheng W., Zhu Y., Ye H., Tang B., Arendsee Z.W., Jones D., Li R., Ortiz D., Zhao X. QQS orphan gene regulates carbon and nitrogen partitioning across species via NF-YC interactions. Proc. Natl. Acad. Sci. USA. 2015;112:14734–14739. doi: 10.1073/pnas.1514670112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adachi S., Yoshikawa K., Yamanouchi U., Tanabata T., Sun J., Ookawa T., Yamamoto T., Sage R.F., Hirasawa T., Yonemaru J. Fine mapping of carbon assimilation rate 8, a quantitative trait locus for flag leaf nitrogen content, stomatal conductance and photosynthesis in rice. Front. Plant Sci. 2017;8:60. doi: 10.3389/fpls.2017.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan W.H., Wang P., Chen H.X., Zhou H.J., Li Q.P., Wang C.R., Ding Z.H., Zhang Y.S., Yu S.B., Xing Y.Z. A major QTL, Ghd8, plays pleiotropic roles in regulating grain productivity, plant height, and heading date in rice. Mol. Plant. 2011;4:319–330. doi: 10.1093/mp/ssq070. [DOI] [PubMed] [Google Scholar]
- 22.Wei X., Xu J., Guo H., Jiang L., Chen S., Yu C., Zhou Z., Hu P., Zhai H., Wan J. DTH8 suppresses flowering in rice, influencing plant height and yield potential simultaneously. Plant Physiol. 2010;153:1747–1758. doi: 10.1104/pp.110.156943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J.J., Xue H.W. OsLEC1/OsHAP3E participates in the determination of meristem identity in both vegetative and reproductive developments of rice F. J. Integr. Plant Biol. 2013;55:232–249. doi: 10.1111/jipb.12025. [DOI] [PubMed] [Google Scholar]
- 24.Ito Y., Thirumurugan T., Serizawa A., Hiratsu K., Ohme-Takagi M., Kurata N. Aberrant vegetative and reproductive development by overexpression and lethality by silencing of OsHAP3E in rice. Plant Sci. 2011;181:105–110. doi: 10.1016/j.plantsci.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Miyoshi K., Ito Y., Serizawa A., Kurata N. OsHAP3 genes regulate chloroplast biogenesis in rice. Plant J. 2003;36:532–540. doi: 10.1046/j.1365-313X.2003.01897.x. [DOI] [PubMed] [Google Scholar]
- 26.Sun X.C., Ling S., Lu Z.H., Ouyang Y.D., Liu S.S., Yao J.L. OsNF-YB1, a rice endosperm-specific gene, is essential for cell proliferation in endosperm development. Gene. 2014;551:214–221. doi: 10.1016/j.gene.2014.08.059. [DOI] [PubMed] [Google Scholar]
- 27.Bai A.N., Lu X.D., Li D.Q., Liu J.X., Liu C.M. NF-YB1-regulated expression of sucrose transporters in aleurone facilitates sugar loading to rice endosperm. Cell Res. 2016;26:384. doi: 10.1038/cr.2015.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nie D.M., Ouyang Y.D., Wang X., Zhou W., Hu C.-G., Yao J. Genome-wide analysis of endosperm-specific genes in rice. Gene. 2013;530:236–247. doi: 10.1016/j.gene.2013.07.088. [DOI] [PubMed] [Google Scholar]
- 29.Xu J.J., Zhang X.F., Xue H.W. Rice aleurone layer specific OsNF-YB1 regulates grain filling and endosperm development by interacting with an ERF transcription factor. J. Exp. Bot. 2016;67:6399–6411. doi: 10.1093/jxb/erw409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto A., Kagaya Y., Toyoshima R., Kagaya M., Takeda S., Hattori T. Arabidopsis NF-YB subunits LEC1 and LEC1-LIKE activate transcription by interacting with seed-specific ABRE-binding factors. Plant J. 2009;58:843–856. doi: 10.1111/j.1365-313X.2009.03817.x. [DOI] [PubMed] [Google Scholar]
- 31.E Z.G., Li T.T., Zhang H.Y., Liu Z.H., Deng H., Sharma S., Wei X.F., Wang L., Niu B.X., Chen C. A group of nuclear factor Y transcription factors are sub-functionalized during endosperm development in monocots. J. Exp. Bot. 2018;69:2495–2510. doi: 10.1093/jxb/ery087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia S.Z., Xiong Y.F., Xiao P.P., Wang X., Yao J.L. OsNF-YC10, a seed preferentially expressed gene regulates grain width by affecting cell proliferation in rice. Plant Sci. 2019;280:219–227. doi: 10.1016/j.plantsci.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi Y., Murata M., Minami H., Yamamoto S., Kagaya Y., Hobo T., Yamamoto A., Hattori T. Abscisic acid-activated SNRK2 protein kinases function in the gene-regulation pathway of ABA signal transduction by phosphorylating ABA response element-binding factors. Plant J. 2005;44:939–949. doi: 10.1111/j.1365-313X.2005.02583.x. [DOI] [PubMed] [Google Scholar]
- 34.Ayele B.T., Magome H., Lee S., Shin K., Kamiya Y., Soh M.S., Yamaguchi S. GA-sensitive dwarf1-1D (gsd1-1D) Defines a New Mutation that Controls Endogenous GA Levels in Arabidopsis. J. Plant Growth Regul. 2014;33:340–354. doi: 10.1007/s00344-013-9385-x. [DOI] [Google Scholar]
- 35.Cui W., Song Q., Zuo B., Han Q., Jia Z. Effects of Gibberellin (GA4+7) in Grain Filling, Hormonal Behavior, and Antioxidants in High-Density Maize (Zea mays L.) Plants. 2020;9:978. doi: 10.3390/plants9080978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X., Hu P., Huang M., Tang Y., Li Y., Li L., Hou X. The NF-YC-RGL2 module integrates GA and ABA signalling to regulate seed germination in Arabidopsis. Nat. Commun. 2016;7:12768. doi: 10.1038/ncomms12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finkelstein R.R., Lynch T.J. The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell. 2000;12:599–609. doi: 10.1105/tpc.12.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carles C., Bies-Etheve N., Aspart L., Léon-Kloosterziel K.M., Koornneef M., Echeverria M., Delseny M. Regulation of Arabidopsis thaliana Em genes: Role of ABI5. Plant J. 2002;30:373–383. doi: 10.1046/j.1365-313X.2002.01295.x. [DOI] [PubMed] [Google Scholar]
- 39.Yang X., Yang Y.N., Xue L.J., Zou M.J., Liu J.Y., Chen F., Xue H.W. Rice ABI5-Like1 regulates abscisic acid and auxin responses by affecting the expression of ABRE-containing genes. Plant Physiol. 2011;156:1397–1409. doi: 10.1104/pp.111.173427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang N., Zhang H., Li X., Xiao J., Xiong L. Constitutive activation of transcription factor OsbZIP46 improves drought tolerance in rice. Plant Physiol. 2012;158:1755–1768. doi: 10.1104/pp.111.190389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joo J., Lee Y.H., Song S.I. Overexpression of the rice basic leucine zipper transcription factor OsbZIP12 confers drought tolerance to rice and makes seedlings hypersensitive to ABA. Plant Biotechnol. Rep. 2014;8:431–441. doi: 10.1007/s11816-014-0335-2. [DOI] [Google Scholar]
- 42.Hossain M.A., Lee Y., Cho J.-I., Ahn C.-H., Lee S.-K., Jeon J.-S., Kang H., Lee C.-H., An G., Park P.B. The bZIP transcription factor OsABF1 is an ABA responsive element binding factor that enhances abiotic stress signaling in rice. Plant Mol. Biol. 2010;72:557–566. doi: 10.1007/s11103-009-9592-9. [DOI] [PubMed] [Google Scholar]
- 43.Gao F., Xiong A., Peng R., Jin X., Xu J., Zhu B., Chen J., Yao Q. OsNAC52, a rice NAC transcription factor, potentially responds to ABA and confers drought tolerance in transgenic plants. Plant Cell Tissue Organ Cult. (PCTOC) 2010;100:255–262. doi: 10.1007/s11240-009-9640-9. [DOI] [Google Scholar]
- 44.Zou J., Liu A., Chen X., Zhou X., Gao G., Wang W., Zhang X. Expression analysis of nine rice heat shock protein genes under abiotic stresses and ABA treatment. J. Plant Physiol. 2009;166:851–861. doi: 10.1016/j.jplph.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Lee S.-K., Hwang S.-K., Han M., Eom J.-S., Kang H.-G., Han Y., Choi S.-B., Cho M.-H., Bhoo S.H., An G. Identification of the ADP-glucose pyrophosphorylase isoforms essential for starch synthesis in the leaf and seed endosperm of rice (Oryza sativa L.) Plant Mol. Biol. 2007;65:531–546. doi: 10.1007/s11103-007-9153-z. [DOI] [PubMed] [Google Scholar]
- 46.Tang X.J., Peng C., Zhang J., Cai Y., You X.-M., Kong F., Yan H.-G., Wang G.-X., Wang L., Jin J. ADP-glucose pyrophosphorylase large subunit 2 is essential for storage substance accumulation and subunit interactions in rice endosperm. Plant Sci. 2016;249:70–83. doi: 10.1016/j.plantsci.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Wei X., Jiao G., Lin H., Sheng Z., Shao G., Xie L., Tang S., Xu Q., Hu P. GRAIN INCOMPLETE FILLING 2 regulates grain filling and starch synthesis during rice caryopsis development. J. Integr. Plant Biol. 2017;59:134–153. doi: 10.1111/jipb.12510. [DOI] [PubMed] [Google Scholar]
- 48.Ryoo N., Yu C., Park C.-S., Baik M.-Y., Park I.M., Cho M.-H., Bhoo S.H., An G., Hahn T.-R., Jeon J.-S. Knockout of a starch synthase gene OsSSIIIa/Flo5 causes white-core floury endosperm in rice (Oryza sativa L.) Plant Cell Rep. 2007;26:1083–1095. doi: 10.1007/s00299-007-0309-8. [DOI] [PubMed] [Google Scholar]
- 49.Ma X., Zhang Q., Zhu Q., Liu W., Chen Y., Qiu R., Wang B., Yang Z., Li H., Lin Y. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant. 2015;8:1274–1284. doi: 10.1016/j.molp.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 50.Kang H.G., Park S., Matsuoka M., An G. White-core endosperm floury endosperm-4 in rice is generated by knockout mutations in the C-type pyruvate orthophosphate dikinase gene (OsPPDKB) Plant J. 2005;42:901–911. doi: 10.1111/j.1365-313X.2005.02423.x. [DOI] [PubMed] [Google Scholar]
- 51.Nishi A., Nakamura Y., Tanaka N., Satoh H. Biochemical and genetic analysis of the effects ofamylose-extender mutation in rice endosperm. Plant Physiol. 2001;127:459–472. doi: 10.1104/pp.010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leng N., Dawson J.A., Thomson J.A., Ruotti V., Rissman A.I., Smits B.M., Haag J.D., Gould M.N., Stewart R.M., Kendziorski C. EBSeq: An empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics. 2013;29:1035–1043. doi: 10.1093/bioinformatics/btt087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article and within Supplementary Material.