Abstract
Ethylene biosynthesis and signal transduction play critical roles in plant sex differentiation. ACS (1-aminocyclopropane-1-carboxylic acid synthase) is a rate-limiting enzyme in ethylene biosynthesis. However, the understanding of the ACS gene family in Cucurbita maxima is limited. Here, we identified and characterized 13 ACS genes in the C. maxima genome. All ACS genes could be divided into three groups according to a conserved serine residue at the C-terminus. Thirteen CmaACS genes were found to be randomly distributed on 10 of the 20 chromosomes of C. maxima. The ACS gene exhibits different tissue-specific expression patterns in pumpkin, and four ACS genes (CmaACS1, CmaACS4, CmaACS7, and CmaACS9) were expressed specifically in both the female and male flowers of C. maxima. In addition, the expression levels of CmaACS4 and CmaACS7 were upregulated after ethephon and IAA treatments, which ultimately increased the number of female flowers, decreased the position of the first female flower and decreased the number of bisexual flowers per plant. These results provide relevant information for determining the function of the ACS genes in C. maxima, especially for regulating the function of ethylene in sex determination.
Keywords: ACS gene family, Cucurbita maxima, sex determination, flower development
1. Introduction
The plant hormone ethylene is present in most plant tissues and plays important roles in the vegetative growth stage, reproductive growth stage, and mature stage of plants [1,2]. Ethylene is considered the main hormone affecting sex differentiation in Cucurbitaceae plants. This hormone determines the sex of each floral meristem by preventing the development of stamens and pistil primordia. It has been suggested that stamens or carpels may develop independently under different concentrations of ethylene due to different levels of sensitivity to the hormone [3]. Transformation of the meristem to a female flower requires high ethylene levels to prevent the development of the stamen primordium. However, low levels of ethylene lead to the transformation of the meristem into a male flower by preventing the development of carpel primordia, thus ensuring the normal development of flowers [4,5,6]. The ethylene-releasing agent ethephon has been widely used as a female flower induction reagent in melon [7], cucumber [8,9], and pumpkin [10]. At the same time, the use of ethylene-inhibiting treatments in melon [7], cucumber [11], and pumpkin was beneficial to the development of male flowers [7,11,12]. However, the role of ethylene in watermelon is opposite to that in other Cucurbitaceae species: ethephon treatment induces male flowers in watermelon [13,14].
In plants, ethylene is biosynthesized from methionine, which is converted to S-Adenosyl Methionine (ADOMet) under the action of ADOMet synthetase. ADOMet is converted to methylthioadenosine by ACS (EC4.4.1.14), and 1-aminocyclopropane-1-carboxylic acid (ACC) is the direct precursor of ethylene [15,16,17,18]. The conversion of SAM to ACC catalyzed by ACC synthase (ACS) is the rate-limiting step in the ethylene synthesis pathway, and ACS is the rate-limiting enzyme in this pathway [19,20,21,22]. ACS is encoded by polygene family members regulated by different plant development, environment, and hormone signals [23,24,25]. In addition, the nonfunctional ACS subtypes that interact with heterodimers between ACS proteins may regulate the enzyme activity of ACS through post-translational regulation [26]. Previous studies have shown that the orthologous genes CmACS7, CsACS2, CitACS4, and CpACS27 control stamen stagnation and mutation in cucumber, melon, watermelon, and pumpkin, respectively, leading to transformation into andromonoecy from monoecy [27,28,29,30,31]. On the other hand, the loss of function of the orthologous genes CsACS11 and CmACS11 of cucumber and melon, respectively, completely block the path of female flower development, resulting in androecy [31,32]. Collectively, previous studies have shown that the ethylene synthase gene is directly related to sex determination in Cucurbitaceae.
Genome-wide analysis of the ACS gene family in most cucurbitaceous species has been reported. However, it has not been reported in C. maxima plants. There is still a lack of comprehensive understanding of CmaACS in pumpkin. Hence, a genome-wide comprehensive study was performed to identify putative pumpkin ACS family genes. Additionally, their phylogenetic relationships, chromosome distribution, gene structure, conserved motifs, homology, and cis-regulatory elements were characterized to obtain insights into CmaACS genes. Furthermore, the expression profiles in different tissues/organs and under the effects of numerous hormones have been extensively assessed. This study provides valuable information for further study of the cloning of key genes involved in ethylene synthesis and signal transduction pathways in pumpkin.
2. Results
2.1. Genome-Wide Identification of ACSs in Pumpkin
In this study, 13 ACS members were detected in the genome of pumpkin (Table 1). We named these ACS genes CmaACS1–13 according to their gene IDs. Detailed information on these ACS genes, including their length, molecular weight (Mw), isoelectric point (pI), and location, is shown in Table 1. The lengths of the amino acids of CmaACS proteins ranged from 442 aa to 549 aa, with the pIs varying from 5.61 to 8.96 and the Mws ranging from 49.81 kD to 60.27 kD. Furthermore, the predicted grand average of hydropathy (GRAVY) values of the CmaACS proteins ranged from −0.325 to −0.098, suggesting that they were hydrophilic. Moreover, subcellular location prediction revealed that five CmaACS proteins were positioned in the chloroplast, that four CmaACS proteins were positioned in the nucleus, and that four CmaACS proteins were positioned in the cytoplasm.
Table 1.
The characteristics of 13 ACSs in Cucurbita maxima.
| Gene | Gene ID | Chromosomal Localization | CDS Length (bp) | Protein Length (aa) | Mw (kDa) | pI | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| CmaACS1 | CmaCh02G015170.1 | Chr02: 8650601~8653154 | 1650 | 549 | 60.14 | 5.65 | Chloroplast |
| CmaACS2 | CmaCh03G008840.1 | Chr03: 6510606~6512326 | 1437 | 478 | 53.80 | 7.98 | Nucleus |
| CmaACS3 | CmaCh04G007260.1 | Chr04: 3694549~3696868 | 1455 | 484 | 54.31 | 6.61 | Cytoplasm |
| CmaACS4 | CmaCh04G008430.1 | Chr04: 4336601~4338975 | 1410 | 469 | 53.05 | 8.96 | Nucleus |
| CmaACS5 | CmaCh05G001180.1 | Chr05: 508990~511072 | 1329 | 442 | 49.88 | 9.42 | Cytoplasm |
| CmaACS6 | CmaCh07G006250.1 | Chr07: 2713056~2714884 | 1428 | 475 | 53.48 | 8.43 | Nucleus |
| CmaACS7 | CmaCh10G007020.1 | Chr10: 3155561~3157085 | 1338 | 445 | 49.92 | 5.86 | Cytoplasm |
| CmaACS8 | CmaCh11G006760.1 | Chr11: 3270641~3272363 | 1326 | 441 | 49.81 | 5.61 | Chloroplast |
| CmaACS9 | CmaCh15G011790.1 | Chr15: 7462460~7465739 | 1650 | 549 | 60.27 | 5.76 | Chloroplast |
| CmaACS10 | CmaCh16G005820.1 | Chr16: 3012078~3013989 | 1455 | 484 | 54.22 | 7.60 | Cytoplasm |
| CmaACS11 | CmaCh16G007300.1 | Chr16: 3845420~3847307 | 1491 | 496 | 56.18 | 8.57 | Nucleus |
| CmaACS12 | CmaCh17G004550.1 | Chr17: 2713968~2716904 | 1395 | 464 | 52.53 | 6.90 | Chloroplast |
| CmaACS13 | CmaCh17G004560.1 | Chr17: 2729331~2731675 | 1482 | 493 | 55.90 | 6.77 | Chloroplast |
CDS—coding DNA sequences, bp—base pair, Mw—molecular weight, pI—isoelectric points.
2.2. Multiple Sequence Alignment and Phylogenetic Analysis of CmaACS Gene Family
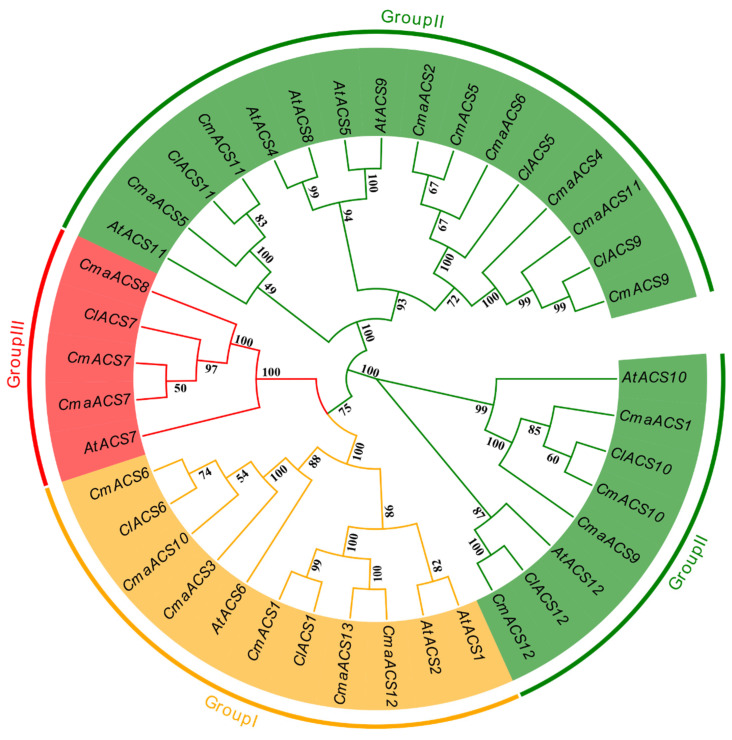
Studies have shown that amino acid residues play a key role in enzyme activity [33]. The 13 ACS proteins identified were subjected to sequence alignment (Figure S1). All ACSs contained seven conserved regions found in the ACS of Arabidopsis, tomato, and other plant species. In ACSs, the amino acid residues in two regions (II and VII) are particularly conserved; therefore, changes in these amino acids may lead to different functions. To further evaluate the evolution of the ACS gene family, MEGA-X software was used to construct a phylogenetic tree of the full-length protein sequences of 41 ACS genes from three cucurbit species (melon, watermelon, and pumpkin) and Arabidopsis (Figure 1). The ACS proteins of C. maxima can be classified into three groups. Strikingly, the members of group II formed two different branches on the phylogenetic tree instead of clustering on the same branch, which may be due to their different motifs at the N-terminus [34] (Figure S1). CmaACS1 and CmaACS9 were closely matched to AtACS10 and AtACS12, which are presumed to be amino acid transferases without ACS activity [25].
Figure 1.
Phylogenetic relationship of 13 ACSs in Cucurbita maxima and other plants. All ACSs genes were divided into three groups based on the high bootstrap values and the phylogenetic tree’s topology.
2.3. Gene Structure and Conserved Motif Composition of CmaACS Genes Family
The exon-intron configurations of CmaACS genes were examined to acquire further insights into the probable structural evolution of the CmaACS family of genes. Our results show that the number of exons of the CmaACS genes ranged from three to five (Figure 2). This number is similar to that of the ACS family genes of A. thaliana [25], melon and watermelon [34]. For genes that are relatively closely evolutionarily related, the length and distribution of exons were similar (Figure 2b). We also investigated the full-length protein sequences of the 13 CmaACSs to identify their conserved motifs (Figure 2c) and identified ten motifs distributed among the ACS members, including six (motifs 1, 2, 3, 4, 5, and 6) aminotransferase domains (Table S3). Interestingly, CmaACSs in the same group often had similar motif compositions (Figure 2c); for example, motif 4 is specific to group III. The similar arrangement of motifs in the subgroups indicates that the protein structure is conserved within a particular subfamily. Overall, the results show that the members of a group have the same genetic structure, and their phylogenetic relationships remain unchanged. By studying the conserved motif constitution, gene structure, and phylogenetic relationship, we found that the stability of the group classification was robust, indicating that the CmaACS proteins have very conserved amino acid residues, that members of the same group may have similar functions, and that members of different groups have unique structures and motifs that may play different roles during plant growth and development.
Figure 2.
Phylogenetic relationships, gene structure, and architecture of conserved protein motifs in CmaACSs. (a) A phylogenetic tree based on the CmaACSs sequences. According to phylogenetic relationships, 13 CmaACSs were clustered into three groups (I-III) and represented with different colors. (b) The exon-intron structure of CmaACSs. Green boxes represent UTR regions, yellow boxes represent exons, black lines represent introns, and pink boxes represent Aminotran_1_2 domain. (c) The motif composition of CmaACSs. Different colored boxes display different motifs.
2.4. Chromosomal Distribution and Synteny Analysis of CmaACS Genes
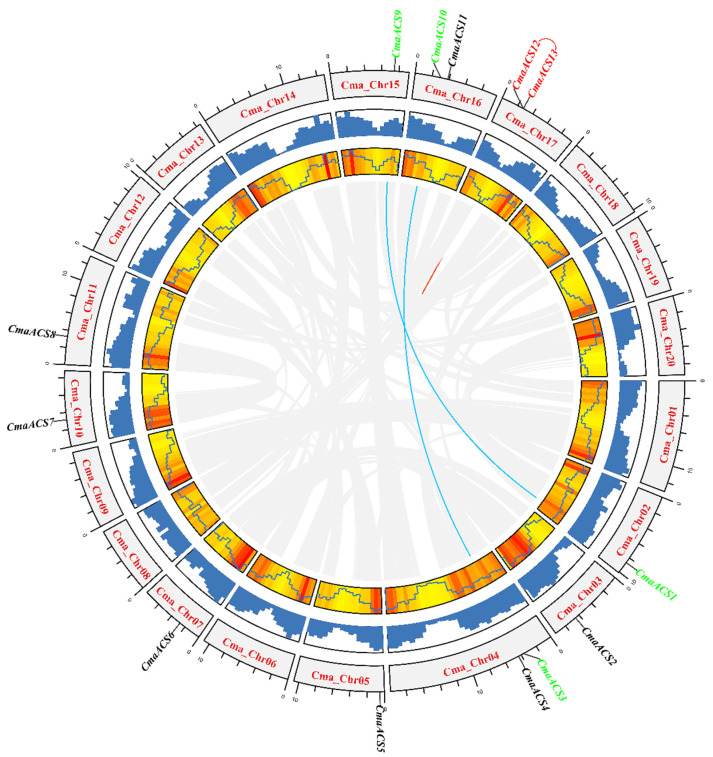
According to the chromosome annotation information of pumpkin, the identified pumpkin ACS gene was located on the chromosome (Figure 3). The results showed that there were two ACSs on chromosomes 4, 16, and 17 and one ACS each on chromosomes 2, 3, 5, 7, 10, 11, and 15, which were unevenly distributed. Gene duplication is ubiquitous and plays an important role in plant evolution. The built-in MCScanX software of TBtools (https://github.com/CJ-Chen/TBtools, accessed on 20 August 2021) was used to analyze the intraspecific collinearity of the ACS genes in pumpkin. Three pairs of collinear genes were detected, indicating that the six CmaACS genes were the result of duplication events (Figure 3). These results indicate that gene duplication promoted the increase in the number of ACS genes in the pumpkin genome to a large extent, and whole-genome duplication or fragment duplication may play a major role in driving this process.
Figure 3.
Chromosomal distribution of CmaACS genes. From the outside to the inside, the first circle represents chromosome coordinates; the second and third circles represent gene density distribution; blue or red lines connect gene pairs.
To explore the evolution of the ACS gene family in Cucurbitaceae, we analyzed the homologous relationships between pumpkin, melon, and watermelon (Figure 4). Collinear analysis showed that there were a large number of linearly homologous ACS genes in pumpkin, melon, and watermelon. In addition, 16 pairs of genes in pumpkin and melon were collinear, 92.3% (12) of the pumpkin ACS genes had homologous genes in melon, 16 pairs of pumpkin and watermelon were collinear, and 92.3% (12) of the pumpkin ACS genes had homologous genes in watermelon. Taken together, these results showed that most of the ACS genes during the formation and evolution of pumpkin remained intact.
Figure 4.
Synteny analysis of ACSs in watermelon, melon, and C. maxima. The blue lines represent the syntenic ACS pairs between the two genomes. The chromosome number is shown at the top of each chromosome.
2.5. Cis-Elements in the Promoters of CmaACSs
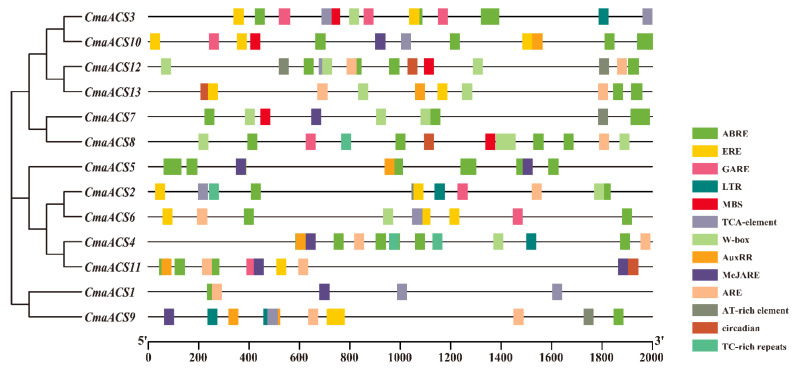
Predicting the cis-elements in promoters that regulate gene expression is essential for improving the understanding of gene regulation [35]. We studied the 2000 bp sequence upstream of the initiation codon of each CmaACS gene, analyzed its cis-elements online via PlantCARE tools (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 27 August 2021), and isolated the functional unknown elements and general transcriptional regulatory elements. As shown in Figure 5, various cis-elements related to stress and plant hormone responses were detected in these ACS genes, such as abscisic acid-responsive elements (ABREs), gibberellin-responsive elements (GARE motifs), salicylic acid-responsive elements (TCA elements), MeJARE-responsive elements (MeJAREs), auxin-responsive elements (AuxRRs), and ethylene-responsive elements (EREs and W-boxes). Stress-related elements, including AREs, LTRs, MBSs, AT-rich-elements, circadian-related elements, and TC-rich repeats, were present in a small number of ACS genes. Most hormone- and stress-responsive elements were present upstream of a specific small number of CmaACS genes, suggesting that these elements play key roles in the response to hormones and in stress response mechanisms.
Figure 5.
Cis-elements that are related to different stress and hormone responses in the putative promoters of CmaACSs. Cis-elements with similar functions are displayed in the same color. Different color boxes show different identified cis-elements.
2.6. Expression Analysis of CmaACSs in Pumpkin Tissue
To understand the potential function of specific ACS isozymes in pumpkin, we analyzed the tissue-specific expression patterns of the CmaACS genes in different tissues of pumpkin. The results showed that the 13 CmaACS genes were differentially expressed in the roots, stems, leaves, female flowers, and male flowers. CmaACS1, CmaACS4, CmaACS7, and CmaACS9 were specifically and highly expressed in the male and female flowers. Although CmaACS3 and CmaACS5 were highly expressed in the female flowers, the expression was not significantly different from that in the other organs. CmaACS8 and CmaACS11 were highly expressed in the leaves, CmaACS12 and CmaACS13 were specifically expressed in the roots, and the expression of the other genes was not significantly different across the various organs (Figure 6). Interestingly, a relatively strong expression of four CmaACSs (CmaACS1, CmaACS4, CmaACS7, and CmaACS9) was observed in the female and male flowers of subgynoecious pumpkin, suggesting that the different expression patterns were associated with flower development.
Figure 6.
Expression patterns of CmaACS genes in different tissues. (A–M) Relative expression levels of ACS gene family members in different tissues. Means followed by a different letter in each column are statistically different by SSR’s test at p > 0.05.
2.7. Expression Profiles of the CmaACSs of C. maxima at Different Flower Developmental Stages
To investigate whether CmaACS1, CmaACS4, CmaACS7, and CmaACS9 were related to pumpkin sex determination, qRT-PCR was used to measure their expression at different stages (S1–S7) in the stamens, ovaries, stigmas, and styles. As shown in Figure 7A, the expression in the ovaries, stigmas, and styles of CmaACS1 tended to increase first but then decrease during flower development; the expression level at S2 was the highest. The CmaACS1 expression in the stamens showed a bimodal trend, peaking at S1 and S5, and the highest expression level among the stamens was recorded at S5. These CmaACS1 expression trends indicate that CmaACS1 may play an important role in the early development of stamens, stigmas, and styles. The expression of CmaACS4 in the stigmas, styles, ovaries, and stamens showed a bimodal trend during flower development. The expression of CmaACS4 was the highest at S5 in the stigmas and styles and was the highest at S3 in the ovaries. The expression level at S7 was the highest in stamens (Figure 7B); it is speculated that CmaACS4 is related to pistil and stamen maturation. The expression of CmaACS7 in the ovaries, stigmas, and styles was significantly reduced during flower development, and the expression level was highest at S1. The expression of CmaACS7 in the stamens was very low at different stages of pumpkin stamens. Across S1–S7, the expression of CmaACS7 in the ovaries, stigmas, and styles was significantly higher than that in the stamens, and the expression in S1 was significantly higher than that in the stamens. The trend of CmaACS7 expression shown in Figure 7C indicates the important role of CmaACS7 in early sex differentiation. The expression of CmaACS9 in the stigmas, styles, ovaries, and stamens showed a bimodal trend. Moreover, CmaACS9 was highly expressed in the S2 phase of the ovaries, stigmas, and styles, and CmaACS9 was specifically and highly expressed in the S1 phase of the stamens (Figure 7D), which suggests that CmaACS9 may be involved in the early development of stamens.
Figure 7.
Comparison of expression patterns of CmaACS1 (A), CmaACS4 (B), CmaACS7 (C), and CmaACS9 (D) at different flower development stages.
2.8. Expression Profiles of CmaACSs in Pumpkin under Phytohormones and Ethylene Inhibitors Treatment
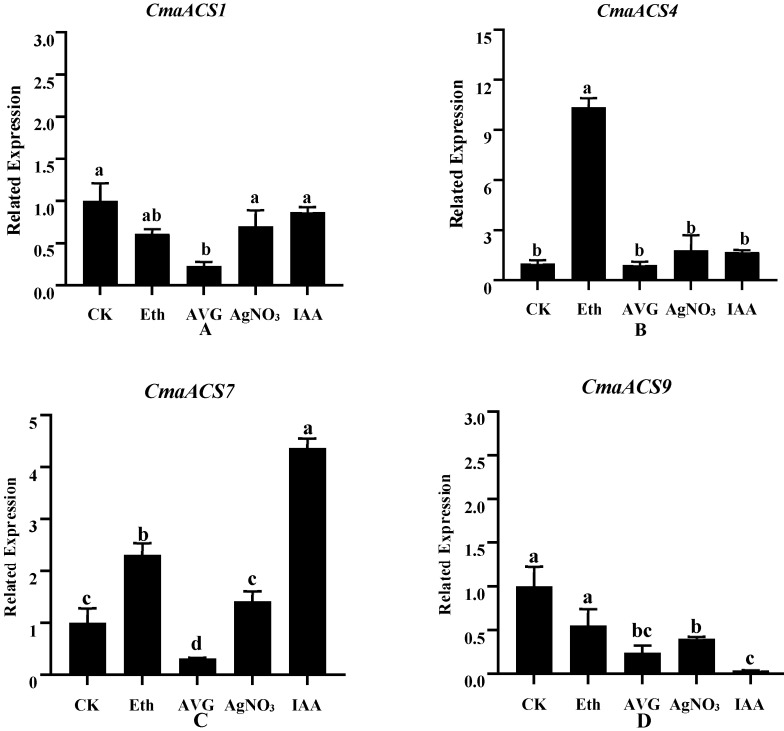
To reveal the possible role of CmaACSs after Ethephon (an ethylene-releasing agent), AVG (an inhibitor of ethylene biosynthesis), AgNO3 (an inhibitor of ethylene biological response), and IAA treatment, gene expression analysis of four selected ACSs were performed using quantitative RT-PCR. In the treatment with exogenous hormones, ethephon and IAA increased the number of female flowers per plant in ‘2013-12’, decreased the position of the first female flower, and reduced the number of bisexual flowers per plant (Table 2). Compared with that of the ‘2013-12’ control, the expression level of CmaACS1 after AVG treatment was significantly reduced, while ethephon, IAA and AgNO3 had no significant effect on the expression of CmaACS1 (Figure 8A). As shown in Figure 8B, compared to the ‘2013–12’ control, the expression level of CmaACS4 significantly increased after ethephon treatment. Compared with that of the ‘2013-12′ control, the expression level of CmaACS7 after ethephon and IAA treatment significantly increased, while the mRNA expression level of CmaACS7 significantly decreased after AVG treatment. AgNO3, an ethylene biosynthesis inhibitor, had no significant effect on the expression of CmaACS7 (Figure 8C). As shown in Figure 8D, compared with that of the ‘2013-12′ control, the expression level of CmaACS9 after treatment with ethephon, AVG, AgNO3 and IAA was significantly reduced. Taken together, these results indicate that CmaACSs may play crucial roles in ethylene synthesis and signal transduction pathways as well as in the sex determination of pumpkin.
Table 2.
Effects of phytohormones and ethylene inhibitors treatments on sex differentiation of ‘2013-12′.
| Treatments | Number of Female Flowers Per Plant |
First Female Flower Position |
Number of Bisexual Flowers Per Plant |
|---|---|---|---|
| Ethephon | 14.8 ± 0.6a | 5.7 ± 0.5c | 0.5 ± 0.2b |
| IAA | 14.3 ± 1.2a | 6.8 ± 1.4c | 1.3 ± 0.4b |
| AVG | 9.8 ± 0.8b | 11.3 ± 0.9b | 5.4 ± 0.9a |
| AgNO3 | 6.5 ± 0.2c | 12.9 ± 1.0a | 6.4 ± 0.9a |
| CK | 10.2 ± 0.8b | 10.1 ± 1.1b | 5.2 ± 1.1a |
Note: Values are the mean (± standard error) of 45 plants. Means followed by a different letter in each column are statistically different by SSR’s test at p > 0.05.
Figure 8.
Comparison of expression patterns of CmaACS1 (A), CmaACS4 (B), CmaACS7 (C), and CmaACS9 (D) Under Different Hormone Treatments. Means followed by a different letter in each column are statistically different by SSR’s test at p > 0.05.
3. Discussion
ACS is one of the rate-limiting enzymes in the process of endogenous ethylene biosynthesis and is very important for the regulation of endogenous ethylene biosynthesis. Members of the ACS polygene family have been cloned and identified from various plant species. The results of genome screening showed that there are 12 ACS genes in A. thaliana [25], 14 ACS genes in bananas [36], 9 ACS genes in tomatoes [21], and 10 ACS genes in grapes [37] suggesting that gene expansion, diversification, and functional redundancy contributed to the evolution of the gene family [33,38]. The number of ACS genes is similar among Cucurbitaceae species. The number of ACS genes were increased in pumpkin compared to cucumber [33], melon, and watermelon [34]. The intraspecific collinearity of the ACS genes in a pumpkin was analyzed by TBtools built-in MCScanX software (https://github.com/CJ-Chen/TBtools, accessed on 20 August 2021), and three pairs of collinear genes were detected (Figure 3). Among them, the amino acid sequence identity of CmaACS2 and CmaACS6 reached 90.79%, the amino acid sequence identity of CmaACS10 and CmaACS3 reached 89.69%, and the amino acid sequence identity of CmaACS13 and CmaACS12 reached 85.94% (Table S2). This shows that certain isozymes encoded by the ACS genes in pumpkin are the results of gene duplication or diversification. Previous studies have shown that the chromosome distribution of the ACS gene is uneven. In this study, we identified and characterized 13 ACS genes in the C. maxima genome; these genes were distributed on 10 chromosomes, and there were two ACS genes on chromosomes 4, 16 and 17. Consistent with the results of previous studies, we found that the ACS gene is unevenly distributed on multiple chromosomes. The ACS gene structure analysis, nucleotide sequence and motif arrangement of pumpkin are consistent with those in previous studies on AtACS and CsACS genes in Arabidopsis, melon, and watermelon [25,34]. ACS proteins can be divided into three categories based on the sequence characteristics of the C-terminal [25,39]. According to this principle, all the identified CmaACS proteins were clustered into three categories, showing high similarity with the proteins in A. thaliana, melon, and watermelon [25,34]. In addition, the phylogenetic results are also supported by the gene structure and motif distribution. ACS genes clustered into distinct clades are associated with distinct motifs that are regulatory sites for post-transcriptional regulatory mechanisms [40]. CmaACS1 and CmaACS9 clustered together with AtACS10 and AtACS12. AtACS10, and AtACS12 are ACS-like, which are considered to be amino acid transferases without ACS activity [27]. CsACS10 and CsACS12 clustered with AtACS10 and AtACS12 in cucumber and were abundantly expressed in cucumber leaves, cotyledons, and tendrils. Enzyme activity assays showed that CsACS1-2 and CsACS6 were active, CsACS9, CsACS10, and CsACS12 were inactive, and denatured CsACS1-2, CsACS2, and CsACS6 were inactive. Combined with a comparison of amino acid sequences, their studies suggest that amino acid conservation in invariant residues and conserved motifs of CsACS proteins is important for their in vitro ACS activity [33]. In the present study, we found that CmaACS1 and CmaACS9 were highly expressed in male flowers, and there were significant changes in the conserved residues and domain amino acids of ACS proteins and transaminases (Figure S1). Therefore, further experiments are needed to determine whether CmaACS1 and CmaACS9 have ACC-producing activity.
Members of the ACS gene family exhibit different tissue expression patterns in different plant species, suggesting that these genes have potentially different functions in plant development. In this study, 13 CmaACS genes were differentially expressed in the roots, stems, leaves, female flowers, and male flowers. CmaACS7 was highly expressed specifically in the female flowers (Figure 6); CmaACS7 was expressed specifically in S1 female flowers; and its expression in the ovaries, stigmas, and styles was higher than that in the stamens (Figure 7C). The homologous genes CmACS7 and ClACS7 in the third group are directly associated with andromonoecy [31,32,41,42], which suggests that CmaACS7 is also involved in female development. CmaACS4 is highly expressed in females. CmaACS4 is homologous to CmACS11 and ClACS11, and both CmACS11 and ClACS11 are involved in female flower development [31,32,34]. The expression of CmaACS4 was the highest at S5 in the stigmas and styles, the highest at S3 in the ovaries, and the highest at S7 in the stamens (Figure 7B). It is thus speculated that CmaACS4 is also involved in female flower development. CmaACS1 and CmaACS9 are homologous to CmACS11 and ClACS11, but CmaACS1 and CmaACS9 are highly expressed in male flowers (Figure 6). It was found that the double peaks of CmaACS1 expression in the stamens appeared at S1 and S5, and the expression at S5 was higher than that at S1. The expression level of CmaACS9 in the stamens at S1 was significantly higher than that in other tissues (Figure 7A,D), indicating that these genes are involved in male flower development. It is speculated that homologous genes may play different functions in different plant species.
The use of ethylene and ethylene inhibitors may directly affect the expression of sex-determining genes [43,44]. The expression of CitACS1 and CitACS4 was upregulated after watermelon was treated with ethephon, while the expression of CitACS2 and CitACS3 was downregulated; in A. thaliana, the expression of AtACS2~7 and AtACS9~12 was upregulated in response to IAA, and that of AtACS4, AtACS6, and AtACS7 was upregulated in response to ethephon [25,45,46]. After applying AgNO3, we found that the expression of CsACS2 in gynoecious tissues was higher than that in subgynoecious tissues. After AgNO3 treatment, the expression level of gynoecious and subgynoecious proteins increased [47]. In our study, both ethylene and IAA treatments could increase the number of female flowers and reduce the node position of female flowers. After the application of ethylene, the expression of CmaACS4 and CmaACS7 in pumpkin increased. Analysis of cis-elements showed that all CmaACS genes except CmaACS1, CmaACS5, and CmaACS8 contained a large number of EREs (Figure 5). This difference in sequence structure suggests that the inducible expression of CmaACSs by ethylene is associated with the occurrence of ethylene-inducible regulatory elements in their promoter sequences (Figure 5). After the application of IAA, the expression of CmaACS7 increased, the number of female flowers increased, the number of bisexual flowers decreased, and the first female flower appeared earlier. IAA treatment can promote ethylene production and ethylene-related gene expression [10,48,49,50]. Therefore, we speculated that IAA may affect the sex determination of pumpkin by inducing the expression of CmaACS7 and indirectly affecting the production of ethylene. Moreover, it was observed that the expression of CmaACS9 was downregulated after ethylene treatment, and the increase in exogenous ethylene content inhibited the production of male flowers. It is thus presumed that CmaACS9 is closely related to the production of male flowers. The application of the ethylene biosynthesis inhibitor AVG and the ethylene sensing inhibitor silver nitrate has been shown to delay the transition to female flowers and reduce the number of female flowers per plant [7,51]. Our study also confirmed this result. AVG is a non-specific competitive inhibitor of ACS and has been shown to block ethylene biosynthesis [52,53,54]. ACS genes are regulated by ethylene through negative feedback or feedforward mechanisms. In this study, the expression levels of CmaACS1, CmaACS4, CmaACS7, and CmaACS9 were decreased after AVG treatment (Figure 8), so it is speculated that the ACS genes may be regulated by feedback of the ethylene mechanism. Silver ions were found to block ethylene sensing, and their effects were associated with inhibition of ethylene metabolism [55]. Furthermore, silver nitrate has nonspecific and undesirable off-target effects [54]. We verified the expression changes of ACS genes after silver nitrate treatment. The expressions of CmaACS1, CmaACS4, and CmaACS7 were not significantly different compared with the control group, and only the expression of CmaACS9 was decreased (Figure 8). The results showed that silver nitrate treatment only affected the expression of some ACS genes. It was speculated that the ethylene sensor may play a more important role in the sex determination of pumpkins. The direct relationship between the expression of CmaACSs and sex determination is currently unclear. Therefore, in situ hybridization should be used to further study the expression kinetics of CmaACSs at specific developmental stages.
4. Materials and Methods
4.1. Prediction and Identification of CmaACSs in C. maxima
To identify potential CmaACSs in pumpkin, a hidden Markov model (HMM) of the transaminase I and II domain (PF00155) was obtained from the Pfam protein family database and used to identify the putative ACS sequence with default parameters by the BlastP method. Searches for ACSs were performed using HMMER 3.0 (Howard Hughes Medical Institute: Cambridge, MA) (E-value ≤ 10−5). According to a recently published study, the amino acid sequence of the protein encoded by the AtACS, CmACS, and ClACS genes was retrieved from the TAIR (http://www.arabidopsis.org/ accessed on 5 June 2020), melon and watermelon (http://cucurbitgenomics.org/ accessed on 7 June 2020) genome database [25,56,57]. Afterwards, all the putative ACS amino acid sequences of pumpkin were obtained from the cucurbit genomic database (http://cucurbitgenomics.org/ accessed on 24 May 2020). Furthermore, candidate proteins were confirmed with SMART (http://smart.embl.de/ accessed on 10 June 2020) [58] and Pfam databases (http://pfam.xfam.org/ accessed on 15 June 2020) [59]. The information on molecular weights, isoelectric points, and predicted subcellular location was obtained from the ExPasy website (http://web.e-xpasy.org/protparam/ accessed on 15 August 2020) [60]. The WoLF PSORT II (https://www.genscript.c-om/wolf-psort.html accessed on 15 August 2020) online server was used to predict subcellular locations [61].
4.2. Sequence Analysis and Structural Characterization
Multiple Expectation Maximization for Motif Elicitation (http://meme-suite.org accessed on 17 August 2020) was used to analyze the conserved C. maxima protein sequences. For conserved motifs discovery, the maximum number of motifs was set to 10. The classic mode and motif width of 6–200 amino acids were selected. TBtools (V 1.098) software (https://github.com/CJ-Chen/TBtools, accessed on 20 August 2021) was used to display the CmaACSs gene structures [62]. The amino acid sequence encoded by the ACS gene of pumpkin in the database was analyzed by ClustalW with default parameters [63].
4.3. Phylogenetic Analysis
To investigate the phylogenetic relationships of CmaACS genes, 13 CmaACSs and ACS sequences of other species were used for phylogenetic analysis. The phylogenetic tree was constructed through the Neighbor–Joining (NJ) method under the parameters of the Poisson model, complete deletion, and 1000 bootstrap replicates using MEGAX software v.10.1.8 (Mega Limited, Auckland, New Zealand) (https://www.megasoftwar-e.net/ accessed on 2 April 2021). The results were formatted for display using the Evolview V3 (https://www.evolgenius.info/evolview/ accessed on 2 April 2021) [64].
4.4. Promoter Sequence Analysis
The sequences of 2 kb upstream from the start codon were downloaded from Genoscope and defined as the promoter of each CmaACSs. Then, PlantCARE [65] was used to predict cis-elements and display them using Tbtools [62].
4.5. Analysis of the Collinearity and Selection Pressure of the ACS Gene Family
One Step MCScanX of TBtools was used to detect gene duplication events. The gene density of the genomes, the position of the CmaACSs on the chromosome, and the gene replication relationship are displayed by the Advanced Circos of Tbtools. TBtools built-in McScanX software were used to analyze the ACS genes of A. thaliana, watermelon, melon, and pumpkin with collinearity, and use Circos (http://circos.ca/software/download/cir-cos/ accessed on 2 June 2021) to draw the relationship diagram. Ka/Ks_Calculator of TBtools was used to calculate non-synonymous (ka) and synonymous (ks) substitution [62].
4.6. Expression Pattern of CmaACSs in C. maxima
The experiment was carried out with the subgynoecious inbred line ‘2013-12’. The seeds of ‘2013-12’ were germinated at 28 °C in the dark for 36 h after being treated with 55 °C water for 24 h and then transplanted to greenhouse breeding plots at Northeast Agricultural University. Three-leaf stage seedlings were transferred to a greenhouse, and the required irrigation and fertilizer were applied under natural photoperiodic conditions in the spring of 2017. Three biological replicates of plant tissues, including roots, stems, leaves, female flowers, male flowers, and flowers of different developmental stages, were sampled and frozen in liquid nitrogen to extract RNA.
In order to study the effects of phytohormones and ethylene inhibitor treatments on CmaACS gene expression, we selected CmaACS genes specifically expressed in flowers for further qRT-PCR analysis. The shoot apices of ‘2013-12’ at the four-true leaf stage were sprayed continuously with 100 mg/L 2-chloroethylphosphonic acid (Ethephon) (an ethylene-releasing agent), 100 mg/L Aminoethoxyvinyl glycine (AVG) (an inhibitor of ethylene biosynthesis), 200 mg/L AgNO3 (an inhibitor of ethylene biological response), and 200 mg/L indoleacetic acid (IAA) for 4 times (continuously) once per day, and each treatment was composed of 15 plants and three biological replicates. After treatment for one day, the shoot apices of ‘2013–12’ that had been subjected to the different chemical solutions were selected. All treated tissue samples were immediately frozen in liquid nitrogen and stored at −80 °C for subsequent analysis.
4.7. RNA Extraction and Gene Expression Analysis
Total RNA was extracted using the Trizol method as described by Ma et al. [66]. All RNA was analyzed by agrose gel electrophoresis and then quantified with a Nanodrop ND-1000 spectrophotometer. First-strand cDNA was synthesized from the total RNA by using a PrimeScript RT reagent kit (Takara Biomedical Technology, Dalian, China) according to the manufacturer’s instructions. The quantitative RT-PCR was carried out with the Roche Lightcyler® 480 instrument (Roche Diagnostics Nederland BV, Almere, Holland) using SYBR Green chemistry. The β-actin gene was used as an internal control. The reaction was carried out as follows: 95 °C for 30 s, followed by 40 cycles of 95 °C/10 s, 60 °C/30 s. Each reaction was performed in biological triplicates and the data from real-time PCR amplification was analyzed using 2−△△CT method [67]. The sequences of the primers used in this study are shown in detail in Table S1.
5. Conclusions
In this study, 13 CmaACS genes were characterized, their expression profiles were investigated, and their possible functions were discussed. The results showed that CmaACS1, CmaACS4, CmaACS7, and CmaACS9 were highly expressed in both female and male flowers of pumpkin, and their expression patterns were analyzed at different flower development stages and in response to different treatments. It is speculated that CmaACS1 has little effect on sex differentiation but that CmaACS4, CmaACS7, and CmaACS9 may be involved in sex differentiation. Our study provides comprehensive information on CmaACS genes and provides relevant information for understanding the function of ethylene in sex differentiation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23158476/s1.
Author Contributions
Conceptualization, data analysis, experimentation, and manuscript preparation, C.W. and B.Z.; Supervision, Y.W.; Plant growth management, W.X., W.L., F.C., Y.C. and X.H.; corresponding author, conceptualization, project implementation and manuscript development, S.Q. All authors reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This research was supported by grants from the National Natural Science Foundation of China (32072590).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang S.J., Chang C.L., Wang P.H., Tsai M.C., Hsu P.H., Chang I.F. A type III ACC synthase, ACS7, is involved in root gravitropism in Arabidopsis thaliana. J. Exp. Bot. 2013;64:4343–4360. doi: 10.1093/jxb/ert241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang F., Cui X., Sun Y., Dong C.-H. Ethylene signaling and regulation in plant growth and stress responses. Plant Cell Rep. 2013;32:1099–1109. doi: 10.1007/s00299-013-1421-6. [DOI] [PubMed] [Google Scholar]
- 3.Yin T., Quinn J.A. Tests of a mechanistic model of one hormone regulating both sexes in Cucumis sativus (Cucurbitaceae) Am. J. Bot. 1995;82:1537–1546. doi: 10.1002/j.1537-2197.1995.tb13856.x. [DOI] [Google Scholar]
- 4.Malepszy S., Niemirowicz-Szczytt K. Sex determination in cucumber (Cucumis sativus) as a model system for molecular biology. Plant Sci. 1991;80:39–47. doi: 10.1016/0168-9452(91)90271-9. [DOI] [Google Scholar]
- 5.Kater M.M., Franken J., Carney K.J., Colombo L., Angenent G.C. Sex determination in the monoecious species cucumber is confined to specific floral whorls. Plant Cell. 2001;13:481–493. doi: 10.1105/tpc.13.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.García A., Aguado E., Garrido D., Martínez C., Jamilena M. Two androecious mutations reveal the crucial role of ethylene receptors in the initiation of female flower development in Cucurbita pepo. Plant J. 2020;103:1548–1560. doi: 10.1111/tpj.14846. [DOI] [PubMed] [Google Scholar]
- 7.Byers R.E., Baker L.R., Sell H.M., Herner R.C., Dilley D.R. Ethylene: A Natural Regulator of Sex Expression of Cucumis melo L. Proc. Natl. Acad. Sci. USA. 1972;69:717–720. doi: 10.1073/pnas.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudich J., Halevy A., Kedar N. Increase in femaleness of three cucurbits by treatment with ethrel, an ethylene releasing compound. Planta. 1969;86:69–76. doi: 10.1007/BF00385305. [DOI] [PubMed] [Google Scholar]
- 9.Rudich J. Biochemical aspects of hormonal regulation of sex expression in Cucurbits. Biol. Util. Cucurbitaceae. 1990:269–280. doi: 10.7591/9781501745447-023. [DOI] [Google Scholar]
- 10.Martínez C., Manzano S., Megías Z., Garrido D., Picó B., Jamilena M. Involvement of ethylene biosynthesis and signalling in fruit set and early fruit development in zucchini squash (Cucurbita pepo L.) BMC Plant Biol. 2013;13:139. doi: 10.1186/1471-2229-13-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Den Nijs A., Visser D. Induction of male flowering in gynoecious cucumbers (Cucumis sativus L.) by silver ions. Euphytica. 1980;29:273–280. doi: 10.1007/BF00025124. [DOI] [Google Scholar]
- 12.Manzano S., Martínez C., Megías Z., Gómez P., Garrido D., Jamilena M. The role of ethylene and brassinosteroids in the control of sex expression and flower development in Cucurbita pepo. Plant Growth Regul. 2011;65:213–221. doi: 10.1007/s10725-011-9589-7. [DOI] [Google Scholar]
- 13.Manzano S., Martínez C., García J.M., Megías Z., Jamilena M. Involvement of ethylene in sex expression and female flower development in watermelon (Citrullus lanatus) Plant Physiol. Biochem. 2014;85:96–104. doi: 10.1016/j.plaphy.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J., Jianting S., Gaojie J., Zhang H., Guoyi G., Shaogui G., Yi R., Jianguang F., Shouwei T., Yong X. Modulation of sex expression in four forms of watermelon by gibberellin, ethephone and silver nitrate. Hortic. Plant J. 2017;3:91–100. doi: 10.1016/j.hpj.2017.07.010. [DOI] [Google Scholar]
- 15.Khan Z.I., Ahmad K., Ashraf M., Parveen R., Bibi Z., Mustafa I., Noorka I.R., Tahir H.M., Akram N.A., Ullah M.F., et al. Risk assessment of heavy metal and metalloid toxicity through a contaminated vegetable (Cucurbita maxima) from wastewater irrigated area: A case study for a site-specific risk assessment in Jhang, Pakistan. Hum. Ecol. Risk Assess. 2016;22:86–98. doi: 10.1080/10807039.2015.1055709. [DOI] [Google Scholar]
- 16.Bleecker A.B., Kende H. Ethylene: A gaseous signal molecule in plants. Annu. Rev. Cell Dev. Biol. 2000;16:1–18. doi: 10.1146/annurev.cellbio.16.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Wang K.L.-C., Li H., Ecker J.R. Ethylene biosynthesis and signaling networks. Plant Cell. 2002;14((Suppl. 1)):S131–S151. doi: 10.1105/tpc.001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarembinski T.I., Theologis A. Signals and Signal Transduction Pathways in Plants. Springer; Berlin/Heidelberg, Germany: 1994. Ethylene biosynthesis and action: A case of conservation; pp. 343–361. [DOI] [PubMed] [Google Scholar]
- 19.Adams D., Yang S. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc. Natl. Acad. Sci. USA. 1979;76:170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choudhury S.R., Roy S., Sengupta D.N. C-Terminal phosphorylation is essential for regulation of ethylene synthesizing ACC synthase enzyme. Plant Signal. Behav. 2013;8:491–511. doi: 10.4161/psb.23000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Z., Zhong S., Grierson D. Recent advances in ethylene research. J. Exp. Bot. 2009;60:3311–3336. doi: 10.1093/jxb/erp204. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y., Zhang S. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell. 2004;16:3386–3399. doi: 10.1105/tpc.104.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng H.-P., Lin T.-Y., Wang N.-N., Shih M.-C. Differential expression of genes encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis during hypoxia. Plant Mol. Biol. 2005;58:15–25. doi: 10.1007/s11103-005-3573-4. [DOI] [PubMed] [Google Scholar]
- 24.Wang N.N., Shih M.-C., Li N. The GUS reporter-aided analysis of the promoter activities of Arabidopsis ACC synthase genes AtACS4, AtACS5, and AtACS7 induced by hormones and stresses. J. Exp. Bot. 2005;56:909–920. doi: 10.1093/jxb/eri083. [DOI] [PubMed] [Google Scholar]
- 25.Yamagami T., Tsuchisaka A., Yamada K., Haddon W.F., Harden L.A., Theologis A. Biochemical Diversity among the 1-Amino-cyclopropane-1-Carboxylate Synthase Isozymes Encoded by the Arabidopsis Gene Family. J. Biol. Chem. 2003;278:49102–49112. doi: 10.1074/jbc.M308297200. [DOI] [PubMed] [Google Scholar]
- 26.Tsuchisaka A., Theologis A. Heterodimeric interactions among the 1-amino-cyclopropane-1-carboxylate synthase polypeptides encoded by the Arabidopsis gene family. Proc. Natl. Acad. Sci. USA. 2004;101:2275–2280. doi: 10.1073/pnas.0308515101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manzano S., Aguado E., Martinez C., Megias Z., Garcia A., Jamilena M. The ethylene biosynthesis gene CitACS4 regulates monoecy/andromonoecy in watermelon (Citrullus lanatus) PLoS ONE. 2016;11:e0154362. doi: 10.1371/journal.pone.0154362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji G., Zhang J., Zhang H., Sun H., Gong G., Shi J., Tian S., Guo S., Ren Y., Shen H. Mutation in the gene encoding 1-aminocyclopropane-1-carboxylate synthase 4 (CitACS4) led to andromonoecy in watermelon. J. Integr. Plant Biol. 2016;58:762–765. doi: 10.1111/jipb.12466. [DOI] [PubMed] [Google Scholar]
- 29.Martínez C., Manzano S., Megías Z., Barrera A., Boualem A., Garrido D., Bendahmane A., Jamilena M. Molecular and functional characterization of CpACS27A gene reveals its involvement in monoecy instability and other associated traits in squash (Cucurbita pepo L.) Planta. 2014;239:1201–1215. doi: 10.1007/s00425-014-2043-0. [DOI] [PubMed] [Google Scholar]
- 30.Boualem A., Troadec C., Kovalski I., Sari M.-A., Perl-Treves R., Bendahmane A. A conserved ethylene biosynthesis enzyme leads to andromonoecy in two Cucumis species. PLoS ONE. 2009;4:e6144. doi: 10.1371/journal.pone.0006144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boualem A., Fergany M., Fernandez R., Troadec C., Martin A., Morin H., Sari M.-A., Collin F., Flowers J.M., Pitrat M. A conserved mutation in an ethylene biosynthesis enzyme leads to andromonoecy in melons. Science. 2008;321:836–838. doi: 10.1126/science.1159023. [DOI] [PubMed] [Google Scholar]
- 32.Boualem A., Troadec C., Camps C., Lemhemdi A., Morin H., Sari M.A., Fraenkel-Zagouri R., Kovalski I., Dogimont C., Perl-Treves R. A cucurbit androecy gene reveals how unisexual flowers develop and dioecy emerges. Science. 2015;350:688–691. doi: 10.1126/science.aac8370. [DOI] [PubMed] [Google Scholar]
- 33.Lee J.H., Kim Y.C., Choi D., Han J.H., Jung Y., Lee S. RNA expression, protein activity, and interactions in the ACC synthase gene family in cucumber (Cucumis sativus L.) Hortic. Environ. Biotechnol. 2018;59:81–91. doi: 10.1007/s13580-018-0009-z. [DOI] [Google Scholar]
- 34.Wang Z., Yadav V., Yan X., Cheng D., Wei C., Zhang X. Systematic genome-wide analysis of the ethylene-responsive ACS gene family: Contributions to sex form differentiation and development in melon and watermelon. Gene. 2021;805:145910. doi: 10.1016/j.gene.2021.145910. [DOI] [PubMed] [Google Scholar]
- 35.Hernandez-Garcia C.M., Finer J.J. Identification and validation of promoters and cis-acting regulatory elements. Plant Sci. 2014;217–218:109–119. doi: 10.1016/j.plantsci.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Dong C., Li W., Zhang C., Wei Y., Wang Y., Hu H. Phylogenetic Analysis of ACS Gene Family in Musa acuminata. Mol. Plant Breed. 2017;15:425–432. [Google Scholar]
- 37.Zhai J. The Cloning and Expression Analysis of the ACS Family Gene of Vitis Tinifera; Liaoning Normal University, Liaoning, China, 2012
- 38.Robert; Finn; Penelope; Coggill; Ruth; Eberhardt; Sean, The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016;44:D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chae H.S., Kieber J.J. Eto Brute? Role of ACS turnover in regulating ethylene biosynthesis. Trends Plant Sci. 2005;10:291–296. doi: 10.1016/j.tplants.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Booker M.A., DeLong A. Producing the ethylene signal: Regulation and diversification of ethylene biosynthetic enzymes. Plant Physiol. 2015;169:42–50. doi: 10.1104/pp.15.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin A., Troadec C., Boualem A., Rajab M., Fernandez R., Morin H., Pitrat M., Dogimont C., Bendahmane A. A transposon-induced epigenetic change leads to sex determination in melon. Nature. 2009;461:1135–1237. doi: 10.1038/nature08498. [DOI] [PubMed] [Google Scholar]
- 42.Tan J., Tao Q., Niu H., Zhang Z., Li D., Gong Z., Weng Y., Li Z. A novel allele of monoecious (m) locus is responsible for elongated fruit shape and perfect flowers in cucumber (Cucumis sativus L.) Theor. Appl. Genet. 2015;128:2483–2493. doi: 10.1007/s00122-015-2603-0. [DOI] [PubMed] [Google Scholar]
- 43.Salman-Minkov A., Levi A., Wolf S., Trebitsh T. ACC synthase genes are polymorphic in watermelon (Citrullus spp.) and differentially expressed in flowers and in response to auxin and gibberellin. Plant Cell Physiol. 2008;49:740–750. doi: 10.1093/pcp/pcn045. [DOI] [PubMed] [Google Scholar]
- 44.Li D., Sheng Y., Niu H., Li Z. Gene interactions regulating sex determination in cucurbits. Front. Plant Sci. 2019;10:1231. doi: 10.3389/fpls.2019.01231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong H., Zhen Z., Peng J., Chang L., Gong Q., Wang N.N. Loss of ACS7 confers abiotic stress tolerance by modulating ABA sensitivity and accumulation in Arabidopsis. J. Exp. Bot. 2011;62:4875–4887. doi: 10.1093/jxb/err143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Achard P., Cheng H., De Grauwe L., Decat J., Schoutteten H., Moritz T., Van Der Straeten D., Peng J., Harberd N.P. Integration of plant responses to environmentally activated phytohormonal signals. Science. 2006;311:91–94. doi: 10.1126/science.1118642. [DOI] [PubMed] [Google Scholar]
- 47.Li Z. Ph.D. Thesis. Shanghai Jiao Tong University; Shanghai, China: 2010. Cloning and Characterization of Unisexual Flower-Controlling M/m Gene in Cucumber. [Google Scholar]
- 48.Li Z., Wang S., Tao Q., Pan J., Si L., Gong Z., Cai R. A putative positive feedback regulation mechanism in CsACS2 expression suggests a modified model for sex determination in cucumber (Cucumis sativus L.) J. Exp. Bot. 2012;63:4475–4484. doi: 10.1093/jxb/ers123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tatsuki M., Nakajima N., Fujii H., Shimada T., Nakano M., Hayashi K.-I., Hayama H., Yoshioka H., Nakamura Y. Increased levels of IAA are required for system 2 ethylene synthesis causing fruit softening in peach (Prunus persica L. Batsch) J. Exp. Bot. 2013;64:1049–1059. doi: 10.1093/jxb/ers381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y., Yan C., Zou B., Wang C., Xu W., Cui C., Qu S. Morphological, Transcriptomic and Hormonal Characterization of Trimonoecious and Subandroecious Pumpkin (Cucurbita maxima) Suggests Important Roles of Ethylene in Sex Expression. Int. Int. J. Mol. Sci. 2019;20:3185. doi: 10.3390/ijms20133185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Owens K., Peterson C., Tolla G. Induction of perfect flowers on gynoecious muskmelon by silver nitrate and aminoethoxyvinylglycine. HortScience. 1980;15:654–655. [Google Scholar]
- 52.Baker J.E., Lieberman M., Anderson J.D. Inhibition of Ethylene Production in Fruit Slices by a Rhizobitoxine Analog and Free Radical Scavengers. Plant Physiol. 1978;61:886–888. doi: 10.1104/pp.61.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bangerth F. The effect of a substituted amino acid on ethylene biosynthesis, respiration, ripening and preharvest drop of apple fruits. J. Am. Soc. Hortic. Sci. 1978;103:401–404. [Google Scholar]
- 54.Schaller G.E., Binder B.M. Ethylene Signaling. Springer; Berlin/Heidelberg, Germany: 2017. Inhibitors of ethylene biosynthesis and signaling; pp. 223–235. [DOI] [PubMed] [Google Scholar]
- 55.Beyer Jr E.M. Effect of silver ion, carbon dioxide, and oxygen on ethylene action and metabolism. Plant Physiol. 1979;63:169–173. doi: 10.1104/pp.63.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garcia-Mas J., Benjak A., Sanseverino W., Bourgeois M., Mir G., González V.M., Hénaff E., Câmara F., Cozzuto L., Lowy E., et al. The genome of melon (Cucumis melo L.) Proc. Natl. Acad. Sci. USA. 2012;109:11872–11877. doi: 10.1073/pnas.1205415109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo S., Zhang J., Sun H., Salse J., Lucas W.J., Zhang H., Zheng Y., Mao L., Ren Y., Wang Z., et al. The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nat. Genet. 2013;45:51–58. doi: 10.1038/ng.2470. [DOI] [PubMed] [Google Scholar]
- 58.Ivica L., Peer B. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018;46:D493–D496. doi: 10.1093/nar/gkx922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sara E.G., Jaina M., Alex B., Eddy S.R., Aurélien L., Potter S.C., Matloob Q., Richardson L.J., Salazar G.A., Alfredo S. The Pfam protein families database in 2019. Nucleic Acids Res. 2018;47:D427–D432. doi: 10.1093/nar/gky995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Artimo P., Jonnalagedda M., Arnold K., Baratin D., Csardi G., de Castro E., Duvaud S., Flegel V., Fortier A., Gasteiger E., et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012;40:W597–W603. doi: 10.1093/nar/gks400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horton P., Park K.J., Obayashi T., Fujita N., Harada H., Adams-Collier C.J., Nakai K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007;35:W585–W587. doi: 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen C., Chen H., Zhang Y., Thomas H.R., Frank M.H., He Y., Xia R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 63.Li K.B. ClustalW-MPI: ClustalW analysis using distributed and parallel computing. Bioinformatics. 2003;19:1585–1586. doi: 10.1093/bioinformatics/btg192. [DOI] [PubMed] [Google Scholar]
- 64.Balakrishnan S., Gao S., Lercher M.J., Hu S., Chen W.H. Evolview v3: A webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019;47:W270–W275. doi: 10.1093/nar/gkz357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lescot M., Déhais P., Thijs G., Marchal K., Moreau Y., Van de Peer Y., Rouzé P., Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30:325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma J., He Y., Wu C., Liu H., Hu Z., Sun G. Cloning and Molecular Characterization of a SERK Gene Transcriptionally Induced During Somatic Embryogenesis in Ananas comosus cv. Shenwan. Plant Mol. Biol. Report. 2012;30:195–203. doi: 10.1007/s11105-011-0330-5. [DOI] [Google Scholar]
- 67.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2 (-delta delta c (t)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.