Abstract
Background: Immune-checkpoint inhibitors (ICIs) have increased and improved the treatment options for patients with non-oncogene-addicted advanced stage non-small cell lung cancer (NSCLC). However, the role of ICIs in oncogene-addicted advanced stage NSCLC patients is still debated. In this study, in an attempt to fill in the informational gap on the effect of ICIs on other driver mutations, we set out to provide a molecular landscape of clinically relevant oncogenic drivers in programmed death-ligand 1 (PD-L1) positive NSCLC patients. Methods: We retrospectively reviewed data on 167 advanced stage NSCLC PD-L1 positive patients (≥1%) who were referred to our clinic for molecular evaluation of five driver oncogenes, namely, EGFR, KRAS, BRAF, ALK and ROS1. Results: Interestingly, n = 93 (55.7%) patients showed at least one genomic alteration within the tested genes. Furthermore, analyzing a subset of patients with PD-L1 tumor proportion score (TPS) ≥ 50% and concomitant gene alterations (n = 8), we found that n = 3 (37.5%) of these patients feature clinical benefit with ICIs administration, despite the presence of a concomitant KRAS gene alteration. Conclusions: In this study, we provide a molecular landscape of clinically relevant biomarkers in NSCLC PD-L1 positive patients, along with data evidencing the clinical benefit of ICIs in patient NSCLC PD-L1 positive alterations.
Keywords: molecular oncology, molecular pathology, PD-L1, immune-checkpoint inhibitors, biomarkers
1. Introduction
Lung cancer represents the leading cause of cancer deaths worldwide [1]. About 85% of lung cancers are non-small cell lung cancer (NSCLC) [2,3]. In recent years, several efforts have been made to improve clinical outcomes of advanced stage NSCLC patients. Central to these efforts has been the advent of precision medicine. This approach, which involves the identification of actionable oncogenic driver alterations, has spurred the development of specific therapeutic agents capable of thwarting the molecular pathways involved in cancer progression. Among these agents are tyrosine kinase inhibitors (TKIs). Remarkably, these agents are able to target a long series of recently discovered oncogenetic mutations involved in several driver genes, namely, Epidermal Growth Factor Receptor (EGFR) [4,5,6,7], V-Raf Murine Sarcoma Viral Oncogene Homolog B1 (BRAF) [8,9], Kirsten Rat Sarcoma Viral Oncogene Homolog (KRAS) exon 2 p.G12C [10,11] and gene fusions in Anaplastic Lymphoma Receptor Tyrosine Kinase (ALK) [12,13,14,15,16] and ROS Proto-Oncogene 1, Receptor Tyrosine Kinase (ROS1) [17,18,19]. Another milestone in the clinical management of advanced stage NSCLC patients has been the development of immune-checkpoint inhibitors (ICIs) [20]. Currently, the evaluation of programmed death-ligand 1 (PD-L1) expression levels is the most widely adopted and standardized tool for ICI administration [21,22]. ICIs have indeed increased and improved the treatment options for non-oncogene-addicted advanced stage NSCLC patients [23,24,25,26]. However, the role of ICIs in oncogene-addicted advanced stage NSCLC patients is still debated [27]. For example, a recent review has highlighted the lack of efficacy of pembrolizumab in naïve EGFR-mutated advanced stage NSCLC patients expressing low levels of PD-L1 (1%) [28]. However, even less is known about the effect of ICIs on other clinically relevant biomarkers. Undoubtedly, paucity of data in this specific field is a major setback for lung cancer treatment. Indeed, evaluating PD-L1 expression levels and the genomic assessment of clinically relevant oncogenic targetable drivers would be crucial to broaden the treatment options for NSCLC patients. In our referral laboratory experience at the Molecular Predictive Pathology Laboratory at the Department of Public Health of the University of Naples Federico II, we routinely perform immunohistochemistry/immunocytochemistry (IHC/ICC) to evaluate PD-L1 expression [29,30]. In addition, we perform both DNA-based next generation sequencing (NGS) and fully automated real-time polymerase chain reaction (RT-qPCR), namely, Idylla™ (Biocartis, Mechelen, Belgium) to evaluate point mutations, deletions and insertions [31,32,33] and IHC/ICC and RNA-based NGS analysis to identify gene fusions [34].
In this study, in an attempt to fill in the informational gap on the effect of ICIs on other driver mutations, we set out to provide a molecular landscape of clinically relevant oncogenic drivers in PD-L1 positive NSCLC patients. To this aim, we retrospectively evaluated data collected from our archives of advanced stage NSCLC patients with positive PD-L1 expression (≥1%) who were referred to our clinic for evaluation of at least five of the most common driver mutations, namely, EGFR, KRAS, BRAF, ALK and ROS1. In addition, in a subset of patients, we were also able to retrieve information about patients’ medical treatments and performance status.
2. Results
2.1. Patient and Sample Characteristics
We retrospectively analyzed data on a total of 167 samples from advanced stage NSCLC PD-L1 positive patients (≥1%) who were referred to our clinic for molecular evaluation of at least five proto-oncogenes, namely, EGFR, KRAS, BRAF, ALK and ROS1. Overall, our study population was composed of n = 103 (61.7%) males and n = 64 (38.3%) females with a median age of 67.3 years (ranging from 43 to 93 years). The vast majority was diagnosed with adenocarcinoma (ADC) (n = 62, 37.1%), NSCLC favor ADC (n = 58, 34.7% and NSCLC not otherwise specified (NOS) (n = 32, 19.2%), followed by squamous cell carcinoma (SqCC) (n = 8, 4.8%), NSCLC favor SqCC (n = 4, 2.4%) and adeno-squamous carcinoma (n = 3, 1.8%). The number of histological samples (n = 110, 65.9%) was almost double that of cytological samples (n = 57, 34.1%). Histological samples comprised small biopsies (n = 86, 78.2%), and surgical resections (n = 24, 21.8%). As for the cytological samples, they were mostly made up of cell blocks (n = 52, 91.2%); of these, some were used for PD-L1 TPS assessment. Direct smears (n = 5, 8.8%), instead, were used for the assessment of other clinically relevant biomarkers.
Results are summarized in Table 1, Figure 1, Figure 2 and Figure 3 and Supplementary Table S1.
Table 1.
Clinical and molecular findings of the study population.
| Global | 1–49% | ≥50% | |
|---|---|---|---|
| Total (%) | 167 (100.0) | 84 (100.0) | 83 (100.0) |
| Sex (%) | M: 103 (61.7) F: 64 (38.3) |
M: 53 (63.1) F: 31 (36.9) |
M: 50 (60.2) F: 33 (39.8) |
| Median Age (range) | 67.3 y (43–93 y) | 66.9 y (43–92 y) | 67.8 y (44–93 y) |
| Sample type (n; %) - subtype (n; %) |
Histological (110, 65.9) - Biopsy (86, 78.2) - Resection (24, 21.8) Cytological (57, 34.1) - Cell block (52, 91.2) - Smear (5, 8.8) |
Histological (53, 63.1) - Biopsy (40, 75.5) - Resection (13, 24.5) Cytological (31, 36.9) - Cell block (29, 93.5) - Smear (2, 6.5) |
Histological (57, 68.7) - Biopsy (46, 80.7) - Resection (11, 19.3) Cytological (26, 31.3) - Cell block (23, 88.5) - Smear (3, 11.5) |
| Diagnosis (n, %) | ADC (62, 37.1) NSCLC favor ADC (58, 34.7) NSCLC NOS (32, 19.2) SqCC (8, 4.8) NSCLC favor SqCC (4, 2.4) ADC + SqCC (3, 1.8) |
ADC (41, 48.8) NSCLC favor ADC (24, 28.6) NSCLC NOS (11, 13.1) SqCC (6, 7.1) NSCLC favor SqCC (1, 1.2) ADC + SqCC (1, 1.2) |
NSCLC favor ADC (34, 41.0) ADC (21, 25.3) NSCLC NOS (21, 25.3) NSCLC favor SqCC (3, 3.6) SqCC (2, 2.4) ADC + SqCC (2, 2.4) |
| PD-L1 (n, %) | 1–49 (84, 50.3) ≥50 (83, 49.7) |
- | - |
| Clone (n, %) | SP263 (134, 80.2) 22C3 (33, 19.8) |
SP263 (67, 79.8) 22C3 (17, 20.2) |
SP263 (67, 80.7) 22C3 (16, 19.3) |
| DNA based-biomarker molecular platform (n, %) | NGS (164, 98.2) RT-qPCR (3, 1.8) |
NGS (83, 98.8) RT-qPCR (1, 1.2) |
NGS (81, 97.6) RT-qPCR (2, 2.4) |
| Molecular results (n, %) | WT (74, 44.3) Mutated (93, 55.7) |
WT (41, 48.8) Mutated (43, 51.2) |
WT (33, 39.8) Mutated (50, 60.2) |
| DNA-based biomarkers (n, %) |
EGFR (167, 100.0) - WT (146, 87.4) - mutated (21, 12.6) - p.L858R (9, 42.8) - p.E746_A750del (6, 28.4) - p.E709_T710insD (1, 4.8) - p.G719A + p.T790M (1, 4.8) - p.I744_K745insKIPVAI (1, 4.8) - p.E746_S752del (1, 4.8) - p.S768_D760dup (1, 4.8) - p.S768I (1, 4.8) KRAS (167, 100.0) - WT (111, 66.5) - mutated (56, 33.5) - p.G12C (27, 48.1) - p.G12V (13, 23.2) - p.G12A (5, 8.9) - p.G12D (3, 5.4) - p.Q61H (3, 5.4) - p.G13C (2, 3.6) - p.G12R (1, 1.8) - p.G13D (1, 1.8) - p.G13R (1, 1.8) BRAF (167, 100.0) - WT (163, 97.6) - mutated (4, 2.4) - p.V600E (2, 50.0) - p.G466V (1, 25.0) - p.G469A (1, 25.0) NRAS (164, 98.2) - WT (163, 99.4) - mutated (1, 0.6) - p.G12D (1, 100.0) KIT (164, 98.2) - WT (164, 100.0) PDGFRα (164, 98.2) - WT (164, 100.0) PIK3CA (164, 98.2) - WT (161, 98.2) - mutated (3, 1.8) - p.E545K (2, 66.7) - p.E542K (1, 33.3) |
EGFR (84, 100.0) - WT (72, 85.7) - mutated (12, 14.3) - p.E746_A750del (4, 33.4) - p.L858R (4, 33.4) - p.E709_T710indD (1, 8.3) - p.G719 + p.T790M (1, 8.3) - p.S768_D760dup (1, 8.3) - p.S768I (1, 8.3) KRAS (84, 100.0) - WT (59, 70.2) - mutated (25, 29.8) - p.G12C (10, 40.0) - p.G12V (8, 32.0) - p.G13C (2, 8.0) - p.G12A (1, 4.0) - p.G12D (1, 4.0) - p.G12R (1, 4.0) - p.G13R (1, 4.0) - p.Q61H (1, 4.0) BRAF (84, 100.0) - WT (83, 98.8) - mutated (1, 1.2) - p.G469A (1, 100.0) NRAS (83, 98.8) - WT (82, 98.8) - mutated (1, 1.2) - p.G12D (1, 100.0) KIT (83, 98.8) - WT (83, 100.0) PDGFRα (83, 98.8) - WT (83, 100.0) PIK3CA (83, 98.8) - WT (82, 98.8) - mutated (1, 1.2) - p.E542K (1, 100.0) |
EGFR (83, 100.0) - WT (74, 89.2) - mutated (9, 10.8) - p.L858R (5, 55.6) - p.E746_A750del (2, 22.2) - p.I744_K745insKIPVAI (1, 11.1) - p.E746_S752del (1, 11.1) KRAS (83, 100.0) - WT (52, 62.7) - mutated (31, 37.3) - p.G12C (17, 54.8) - p.G12V (5, 16.1) - p.G12A (4, 12.9) - p.G12D (2, 6.5) - p.Q61H (2, 6.5) - p.G13D (1, 3.2) BRAF (83, 100.0) - WT (80, 96.4) - mutated (3, 3.6) - p.V600E (2, 66.7) - p.G466V (1, 33.3) NRAS (81, 97.6) - WT (81, 100.0) KIT (81, 97.6) - WT (81, 100.0) PDGFRα (81, 97.6) - WT (81, 100.0) PIK3CA (81, 97.6) - WT (79, 97.5) - mutated (2, 2.5) - p.E545K (2, 100.0) |
| RNA-based biomarker assays (n, %) | IHC/ICC (152, 91.0) NGS (15, 9.0) |
IHC/ICC (78, 92.9) NGS (6, 7.1) |
IHC/ICC (74, 89.2) NGS (9, 10.8) |
| RNA-based biomarkers (n, %) |
ALK (167, 100.0) - Negative/WT (160, 95.8) - Positive/rearranged (7, 4.2) ROS1 (167, 100.0) - Negative/WT (166, 99.4) - Positive/rearranged (1, 0.6) RET (15, 9.0) - WT (15, 100.0) NTRK (15, 9.0) - WT (15, 100.0) MET (15, 9.0) - WT (15, 100.0) |
ALK (84, 100.0) - Negative/WT (81, 96.4) - Positive/rearranged (3, 3.6) ROS1 (84, 100.0) - Negative/WT (84, 100.0) RET (6, 7.1) - WT (6, 100.0) NTRK (6, 7.1) - WT (6, 100.0) MET (6, 7.1) - WT (6, 100.0) |
ALK (83, 100.0) - Negative/WT (79, 95.2) - Positive/rearranged (4, 4.8) ROS1 (83, 100.0) - Negative/WT (82, 98.8) - Positive/rearranged (1, 1.2) RET (9, 10.8) - WT (9, 100.0) NTRK (9, 10.8) - WT (9, 100.0) MET (9, 10.8) - WT (9, 100.0) |
Abbreviations: ADC: adenocarcinoma; ALK: Anaplastic Lymphoma Receptor Tyrosine Kinase; BRAF: V-Raf Murine Sarcoma Viral Oncogene Homolog B1; EGFR: Epidermal Growth Factor Receptor; F: female; ICC: immunocytochemistry; IHC: immunohistochemistry; KIT: KIT Proto-Oncogene, Receptor Tyrosine Kinase; KRAS: Kirsten Rat Sarcoma Viral Oncogene Homolog; M: male; MET: MET Proto-Oncogene, Receptor Tyrosine Kinase; n: number; NGS: next generation sequencing; NOS: not otherwise specified; NRAS: Neuroblastoma RAS Viral Oncogene Homolog; NSCLC: non-small cell lung cancer; NTRK: Neurotrophic Receptor Tyrosine Kinase; PD-L1: programmed death-ligand 1; PDGFRα: Platelet Derived Growth Factor Receptor Alpha; PIK3CA: Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha; RET: Rearranged During Transfection; RT-qPCR: real-time polymerase chain reaction; ROS1: ROS Proto-Oncogene 1, Receptor Tyrosine Kinase; SqCC: squamous cell carcinoma; WT: wild type; y: years.
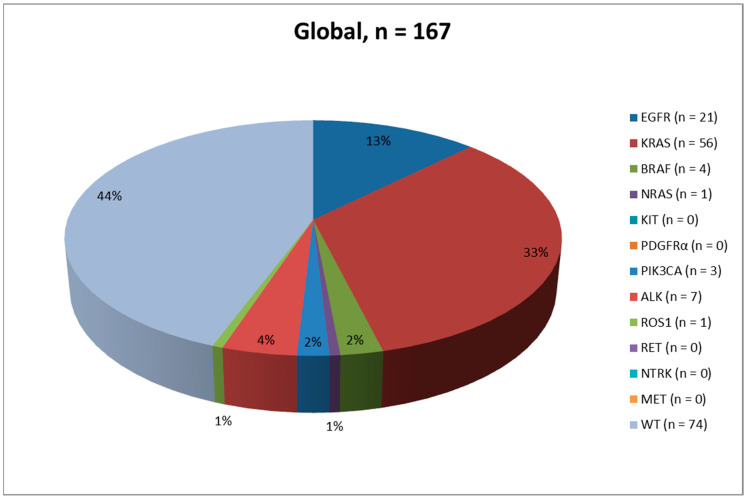
Figure 1.
Pie chart describing the mutational landscape in the global advanced stage NSCLC PD-L1 positive patients (≥1%) population.
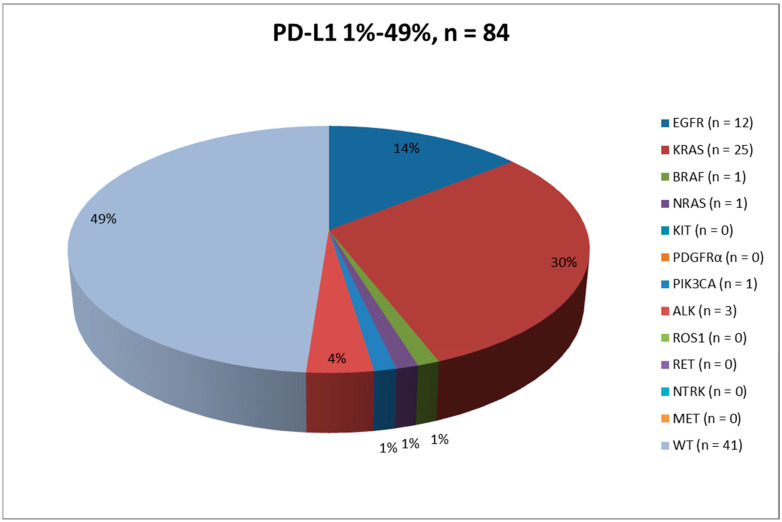
Figure 2.
Pie chart describing the mutational landscape in the 1–49% PD-L1 positive advanced stage NSCLC patients population.
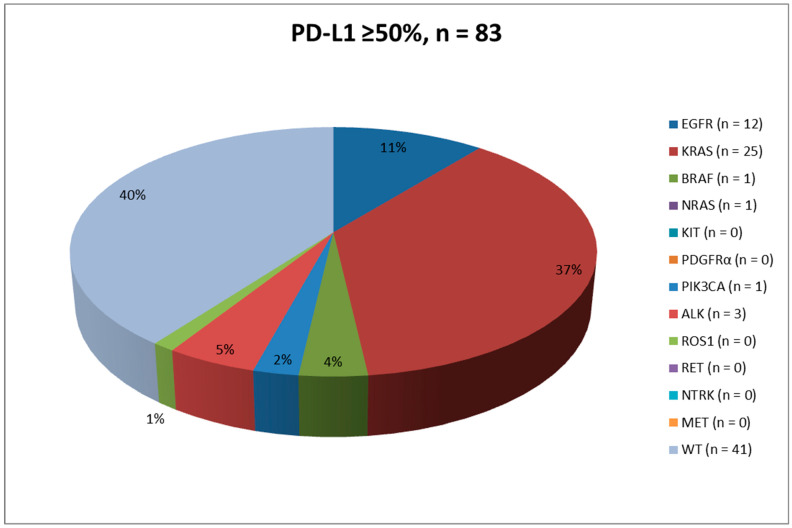
Figure 3.
Pie chart describing the mutational landscape in the ≥50% PD-L1 positive advanced stage NSCLC patients population.
2.2. PD-L1 Status and Molecular Evaluation
For the evaluation of the expression level of PD-L1, SP263 (n = 134, 80.2%) was more commonly used than 22C3 (n = 33, 19.8%). Overall, n = 84 (50.3%) samples expressed PD-L1 levels between 1% and 49%, and n = 83 (49.7%) samples expressed PD-L1 levels ≥50% (Figure 4). For the evaluation of DNA-based biomarkers, NGS was used to analyze 164/167 (98.2%) cases, whereas RT-qPCR analysis was used for the remaining 3 cases (1.8%). Remarkably, KRAS was the most commonly mutated gene (n = 56, 33.5%), followed by EGFR (n = 21, 12.6%), BRAF (n = 4, 2.4%), Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha (PIK3CA, n = 3, 1.8%), and Neuroblastoma RAS Viral Oncogene Homolog (NRAS, n = 1, 0.6%). No alterations were reported in KIT Proto-Oncogene, Receptor Tyrosine Kinase (KIT) and Platelet Derived Growth Factor Receptor Alpha (PDGFRα). For the evaluation of ALK and ROS1 gene rearrangements, IHC/ICC was employed in the vast majority of cases (n = 152, 91.0%), whereas RNA-based NGS analysis was adopted in only n = 15 (9.0%) instances. Interestingly, ALK fusions emerged in n = 7 (4.2%) cases and ROS1 in only n = 1 (0.6%) case. No additional Rearranged During Transfection (RET) and Neurotrophic Receptor Tyrosine Kinase (NTRK) gene fusions or MET Proto-Oncogene and Receptor Tyrosine Kinase (MET) exon 14 skipping alterations were reported. As for the biomarker analyses, n = 93 (55.7%) cases showed at least one genomic alteration within the tested genes, whereas no concomitant clinically relevant biomarker alterations were detected in the remaining n = 74 (44.3%) cases.
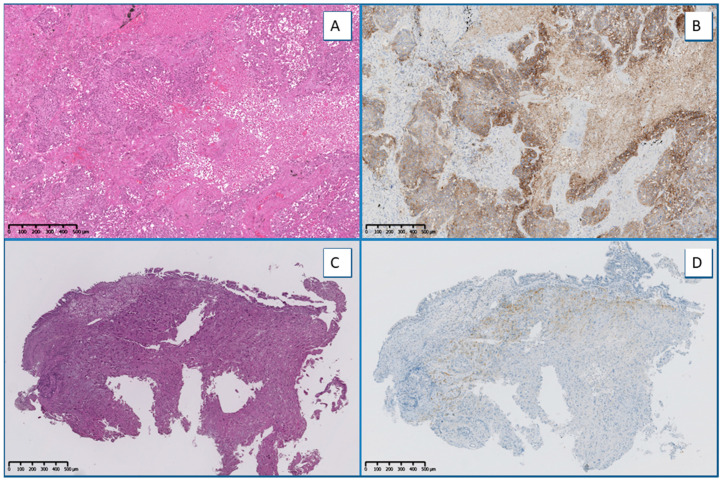
Figure 4.
Exemplificative cases of PD-L1 SP263 clone IHC evaluation. Original magnification 5×: (A) H and E stained slide with the corresponding PD-L1 evaluation (1–49%, (B)); (C) H and E stained slide with the corresponding PD-L1 evaluation (≥50%, (D)).
Results are summarized in Table 1, Figure 1, Figure 2, Figure 3 and Figure 4 and Supplementary Table S1.
2.3. PD-L1 Expression: 1–49%
For DNA-based biomarker analyses, NGS was applied to almost all cases (n = 83, 98.8%), whereas RT-qPCR was used for only n = 1 (1.2%) case. Remarkably, KRAS was the most commonly mutated gene (n = 25, 29.8%), followed by EGFR (n = 12, 14.3%), BRAF (n = 1, 1.2%), PIK3CA (n = 1, 1.2%) and NRAS (n = 1, 1.2%). Regarding the evaluation of ALK and ROS1 gene rearrangements, IHC/ICC was employed in the vast majority of cases (n = 78, 92.9%), whereas RNA-based NGS analysis was adopted in only n = 6 (7.1%) instances. Interestingly, n = 3 (3.6%) cases showed ALK gene fusion, whereas no ROS1 gene rearrangements were reported. Concerning the biomarker analyses, n = 43 (51.2%) cases showed at least one genomic alteration within the tested genes, whereas no concomitant clinically relevant biomarker alterations were detected in the remaining n = 41 (48.8%) cases.
Results are summarized in Table 1, Figure 1, Figure 2 and Figure 3 and Supplementary Table S1.
2.4. PD-L1 Expression: ≥50%
For DNA-based biomarker analyses, NGS was applied to almost all cases (n = 81, 97.6%), whereas RT-qPCR was employed in only n = 2 (2.4%) instances. Remarkably, KRAS was the most frequently mutated gene (n = 31, 37.3%), followed by EGFR (n = 9, 10.8%), BRAF (n = 3, 3.6%) and PIK3CA (n = 2, 2.5%), No alterations were reported in NRAS. Regarding the evaluation of ALK and ROS1 gene rearrangements, IHC/ICC was employed in the vast majority of cases (n = 74, 89.2%), whereas RNA-based NGS analysis was adopted in only n = 9 (10.8%) instances. Interestingly, whereas ALK fusions were identified in n = 4 (4.8%) cases, ROS1 fusions were detected in only n = 1 (1.2%) case. As for the biomarker analyses, at least one genomic alteration was detected in n = 50 (60.2%) cases, whereas no concomitant clinically relevant biomarker alterations were detected in the remaining n = 33 (39.8%) cases.
Results are summarized in Table 1, Figure 1, Figure 2 and Figure 3 and Supplementary Table S1.
2.5. Clinical Management
Overall, data on the clinical management of n = 41 patients were retrieved. Among these, n = 16 (39.0%) showed a PD-L1 expression ≥50%. Half of these patients did not show concomitant gene alterations. In this subset, n = 6 (75.0%) patients received immunotherapy alone, n = 1 patient chemotherapy alone (1/8, 12.5%), and n = 1 patient supportive care (1/8, 12.5%). Interestingly, five out of six patients (83.3%) are still receiving frontline treatments comprising ICIs alone or combination therapies. In the abovementioned subset of patients with concomitant gene alterations, almost all patients (7/8, 87.5%) presented with KRAS gene mutations (n = 4 KRAS exon 2 p.G12C, n = 1 KRAS exon 2 p.G12A, n = 1 KRAS exon 2 p.G12V and n = 1 KRAS exon 3 p.Q61H), whereas one patient harbored one type of EML4(6)-ALK(20) gene rearrangement. Seven cases harboring KRAS gene mutations were treated with pembrolizumab. Overall, n = 3 (37.5%) of these patients (n = 2 with a KRAS exon 2 p.G12C and n = 1 with KRAS exon 2 p.G12A) are still being treated with the same therapeutic regimen.
Results are summarized in Table 2.
Table 2.
Clinical management.
| Sex | Age | Sample Type | Sample Subtype | Site | Diagnosis | PD-L1 | Clone | Alteration | First Oncological Observation Date | Performance Status | First Line Treatment | First-Line Treatment Starting Date | First-Line Treatment End Date |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 70 | Histological | Biopsy | Brain | NSCLC favor ADC | ≥50% | SP263 | KRAS exon 2 p.G12C | March 2019 | 1 | Pembrolizumab | May 2019 | Ongoing |
| F | 66 | Histological | Resection | Brain | ADC | ≥50% | SP263 | WT | February 2020 | 1 | Pembrolizumab | March 2021 | December 2021 |
| M | 77 | Histological | Biopsy | Lymphnode | NSCLC favor ADC | 1–49% | SP263 | KRAS exon 2 p.G12V | May 2020 | 1 | Carboplatino + Pemetrexed + Pembrolizumab | June 2020 | April 2021 |
| M | 75 | Histological | Biopsy | Lymphnode | ADC | ≥50% | SP263 | WT | April 2020 | 1 | Durvalumab | February 2021 | Ongoing |
| F | 75 | Histological | Biopsy | Lung | ADC | 1–49% | SP263 | KRAS exon 2 p.G12C | April 2020 | 0 | Carboplatino-Pemetrexed | July 2020 | Ongoing |
| M | 57 | Histological | Biopsy | Lung | ADC-SqCC | ≥50% | SP263 | WT | June 2020 | 0 | Pembrolizumab | July 2020 | Ongoing |
| M | 77 | Histological | Resection | Lung | ADC | ≥50% | SP263 | KRAS exon 2 p.G12C | February 2020 | 0 | Pembrolizumab | September 2021 | March 2022 |
| F | 69 | Histological | Biopsy | Lung | NSCLC favor ADC | 1–49% | SP263 | BRAF exon 11 p.G469A | June 2020 | 2 | Carboplatino + Pemetrexed + Pembrolizumab | September 2020 | February 2021 |
| F | 69 | Histological | Biopsy | Lung | NSCLC favor ADC | ≥50% | SP263 | EML4(6)-ALK(20) | September 2020 | 2 | Brigatinib | February 2021 | June 2021 |
| F | 68 | Histological | Resection | Lymphnode | ADC | 1–49% | SP263 | WT | April 2019 | 0 | Carboplatino + Pemetrexed | April 2019 | Ongoing with only pemetrexed |
| F | 69 | Cytological | Cell block | Soft tissue | ADC | 1–49% | SP263 | EGFR exon 20 p.S768_D760dup | December 2019 | 1 | Carboplatino + Pemetrexed + Pembrolizumab | February 2020 | September 2020 |
| M | 57 | Histological | Biopsy | Lung | NSCLC-NOS | ≥50% | SP263 | KRAS exon 2 p.G12C | February 2020 | 2 | Pembrolizumab | March 2020 | March 2020 |
| F | 55 | Cytological | Cell block | Lung | NSCLC-NOS | ≥50% | SP263 | WT | December 2020 | 1 | Pembrolizumab | January 2021 | Ongoing |
| M | 62 | Histological | Biopsy | Pleura | ADC | 1–49% | SP263 | ALK positive | April 2021 | 1 | Alectinib | April 2021 | Ongoing |
| M | 72 | Cytological | Cell block | Lung | NSCLC favor ADC | 1–49% | 22C3 | WT | June 2018 | 2 | Carboplatin + Pemetrexed | July 2018 | September 2018 |
| M | 78 | Cytological | Smear | Lung | ADC | ≥50% | SP263 | WT | May 2019 | 2 | Carboplatin | June 2019 | September 2019 |
| M | 72 | Cytological | Cell block | Lung | NSCLC favor ADC | 1–49% | SP263 | KRAS exon 2 p.G12C | March 2019 | 1 | Carboplatin + Pemetrexed | March 2019 | January 2020 |
| F | 73 | Histological | Biopsy | Lung | NSCLC favor ADC | ≥50% | SP263 | KRAS exon 2 p.G12V | June 2019 | 0 | Pembrolizumab | June 2019 | January 2021 |
| M | 79 | Histological | Biopsy | Lung | ADC | 1–49% | SP263 | WT | September 2019 | 0 | Carboplatin + Pemetrexed | October 2019 | January 2020 |
| M | 49 | Cytological | Cell block | Lung | NSCLC favor ADC | ≥50% | SP263 | WT | December 2019 | 0 | Pembrolizumab | December 2019 | Ongoing |
| M | 60 | Histological | Biopsy | Lung | NSCLC favor ADC | ≥50% | SP263 | KRAS exon 3 p.Q61H | December 2019 | 0 | Pembrolizumab | January 2020 | April 2020 |
| F | 62 | Histological | Biopsy | Brain | NSCLC-NOS | 1–49% | SP263 | EGFR exon 19 p.E746_A750del | December 2020 | 0 | Osimertinib | January 2021 | Ongoing |
| F | 58 | Histological | Resection | Brain | ADC | 1–49% | SP263 | WT | March 2021 | 0 | Carboplatin + Pemetrexed + Pembrolizumab | April 2021 | Ongoing |
| M | 61 | Cytological | Cell block | Lung | ADC | 1–49% | 22C3 | WT | July 2018 | 0 | Cisplatino + Pemetrexed | July 2018 | September 2018 |
| F | 56 | Histological | Biopsy | Lung | NSCLC favor ADC | 1–49% | 22C3 | KRAS exon 2 p.G13C | December 2018 | 2 | Carboplatin + Pemetrexed | January 2019 | August 2019 |
| M | 71 | Histological | Biopsy | Lung | NSCLC favor ADC | 1–49% | SP263 | KRAS exon 2 p.G12V | May 2019 | 1 | Cisplatin + Pemetrexed | May 2019 | July 2019 |
| M | 48 | Histological | Biopsy | Pleura | NSCLC favor ADC | 1–49% | SP263 | WT | June 2019 | 1 | Cisplatin + Pemetrexed | June 2019 | October 2019 |
| F | 67 | Cytological | Cell block | Lymphnode | NSCLC favor ADC | 1–49% | SP263 | WT | December 2019 | 2 | Carboplatin + Pemetrexed | January 2020 | January 2020 |
| M | 70 | Cytological | Cell block | Lymphnode | NSCLC favor ADC | 1–49% | SP263 | KRAS exon 2 p.G12V | June 2021 | 1 | Carboplatin + Gemcitabina | July 2021 | September 2021 |
| F | 74 | Cytological | Cell block | Lung | ADC | 1–49% | SP263 | KRAS exon 2 p.G12C | September 2020 | 1 | Pemetrexed + Pembrolizumab | October 2020 | October 2020 |
| M | 70 | Histological | Biopsy | Lung | NSCLC favor ADC | ≥50% | SP263 | WT | June 2021 | 3 | Supportive care | - | - |
| M | 77 | Cytological | Cell block | Lung | NSCLC favor SqCC | 1–49% | SP263 | WT | April 2021 | 2 | Atezolizumab | May 2021 | August 2021 |
| F | 71 | Cytological | Cell block | Lymphnode | NSCLC favor ADC | ≥50% | SP263 | KRAS exon 2 p.G12A | February 2021 | 1 | Pembrolizumab | March 2021 | Ongoing |
| F | 76 | Histological | Biopsy | Lung | SqCC | 1–49% | 22C3 | WT | August 2018 | 1 | Nivolumab | January 2019 | January 2019 |
| M | 72 | Cytological | Cell block | Lymphnode | NSCLC favor ADC | 1–49% | 22C3 | WT | October 2017 | 1 | Cisplatin + Pemetrexed | October 2017 | November 2017 |
| M | 46 | Histological | Biopsy | Brain | NSCLC favor ADC | 1–49% | SP263 | WT | May 2019 | 0 | Cisplatin + Pemetrexed | June 2019 | January 2020 |
| M | 73 | Histological | Resection | Lung | ADC | 1–49% | SP263 | WT | June 2020 | 0 | Carboplatin + Pemetrexed | August 2020 | October 2020 |
| M | 59 | Cytological | Cell block | Lung | NSCLC favor ADC | ≥50% | SP263 | WT | July 2020 | 0 | Pembrolizumab | August 2020 | Ongoing |
| F | 64 | Histological | Biopsy | Lung | ADC | 1–49% | SP263 | WT | September 2020 | 1 | Carboplatin + Pemetrexed + Pembrolizumab | October 2020 | November 2020 |
| M | 75 | Histological | Biopsy | Liver | ADC | 1–49% | SP263 | EGFR exon 19 p.E746_A750del | January 2021 | 1 | Osimertinib | January 2021 | Ongoing |
| M | 68 | Histological | Biopsy | Lung | ADC | ≥50% | SP263 | KRAS exon 2 p.G12C | December 2020 | 1 | Pembrolizumab | December 2020 | Ongoing |
Abbreviations: ADC: adenocarcinoma; ALK: Anaplastic Lymphoma Receptor Tyrosine Kinase; BRAF: V-Raf Murine Sarcoma Viral Oncogene Homolog B1; EGFR: Epidermal Growth Factor Receptor; F: female; KRAS: Kirsten Rat Sarcoma Viral Oncogene Homolog; M: male; NOS: not otherwise specified; NSCLC: non-small cell lung cancer; WT: wild type; y: years.
3. Discussion
The evaluation of PD-L1 expression is now one of the mandatory predictive tests to conduct in advanced stage NSCLC patients. In this study, we retrospectively analyzed a total of 167 advanced stage NSCLC PD-L1 positive patients (≥1%) who were referred to our referral clinic for the molecular evaluation of at least five driver genes, namely, EGFR, KRAS, BRAF, ALK and ROS1. In our experience, both histological (n = 110, 65.9%) and cytological (n = 57, 34.1%) samples were analyzed, strongly supporting the evidence that evaluation of PD-L1 expression levels and molecular profiling of advanced stage NSCLC patients is feasible by using both types of specimens [21,22,29,30]. In this context, studies have shown that NGS (both DNA- and RNA-based approaches) represents a valid solution to analyze clinically relevant biomarkers simultaneously in small tissue samples [32,33]. Overall, n = 84 (50.3%) and n = 83 (49.7%) patients showed a PD-L1 expression level of 1–49% and ≥50%, respectively. As in other experiences, most of the patients (n = 103, 61.7%; n = 53, 63.1%; n = 50, 60.2% were males [35]. Most cases were diagnosed as ADC (n = 62, 37.1%; n = 41, 48.8%; n = 21, 25.3%), NSCLC favor ADC (n = 58, 34.7%; n = 24, 28.6%; n = 34, 41.0%), and NSCLC NOS (n = 32, 19.2%; n = 11, 13.1%; n = 21, 25.3%), followed by SqCC (n = 8, 4.8%; n = 6, 7.1%; n = 2, 2.4%), NSCLC favor SqCC (n = 4, 2.4%; n = 1, 1.2%; n = 3, 3.6%) and adeno-squamous carcinomas (n = 3, 1.8%; n = 1, 1.2%; n = 2, 2.4%). Interestingly, n = 93 (55.7%) patients showed at least one genomic alteration within the tested genes. From an epidemiological point of view, the most common genomic alterations were reported within the KRAS gene (n = 56, 33.5%), followed by EGFR (n = 21, 12.6%), ALK (n = 7, 4.2%), BRAF (n = 4, 2.4%), PIK3CA (n = 3, 1.8%), ROS1 (n = 1, 0.6%), and NRAS (n = 1, 0.6%) (Table 1 and Supplementary Table S1). The strong correlation between PD-L1 expression and KRAS mutations has been previously demonstrated. Karatrasoglou et al. highlighted that PD-L1 positive (TPS ≥ 1%) NSCLC patients showed a concomitant KRAS mutation, and in particular KRAS exon 2 p,G12C point mutation, in a higher percentage of patients with respect to PD-L1 negative patients [36]. This phenomenon may be related to the induction of PD-L1 by KRAS mutations as it has been demonstrated in human NSCLC cell lines [37,38,39].
As for the data on treatment regimens, the seven cases harboring KRAS gene mutations received pembrolizumab alone (6/7, 85.7%) or pembrolizumab plus carboplatin and pemetrexed (1/7, 14.3%). Overall, in n = 3 (37.5%) of these patients (n = 2 with a KRAS exon 2 p.G12C and n = 1 with KRAS exon 2 p.G12A) the treatment is still ongoing. These data support the role of KRAS mutations (in particular KRAS exon 2 p.G12C point mutation) in increasing ICI sensitivity [40].
In this setting, despite the role of ICIs has been clearly demonstrated in the treatment of high PD-L1 expressers [23], little is known about the role of concomitant genomic alterations on this regimen. Lee et al. showed that ICI administration in KRAS mutated patients may determine an overall survival (OS) benefit respect to KRAS wild-type patients [41]. Similarly, Bodor et al. highlighted that KRAS-mutated NSCLC patients with PD-L1 TPS≥1% had a longer progression-free survival respect to PD-L1 negative patients (4.1 vs. 3.2 months, p = 0.001) [42]. A possible explanation may be the presence of a specific interaction between the tumor microenvironment and ICIs for this specific subset of patients as demonstrated by Falk et al. [43]. Similarly, the adoption of front-line pembrolizumab in PD-L1 positive advanced stage NSCLC patients harboring a KRAS exon 2 p.G12C point mutation seemed to be predictive of higher objective response rate (ORR, 57% versus 29%), median progression free survival (PFS, 12 versus 6 months) and OS (28 versus 15 months) [44]. Different from KRAS exon 2 p.G12C, the identification of other concomitant driver mutations is predictive of poor response to ICIs administration in the PD-L1 positive population [27]. The limited efficacy of ICIs in patients harboring EGFR mutations has been widely demonstrated [45]. In a phase II study, Lisberg et al. highlighted the absence of response to pembrolizumab as first line approach in advanced stage PD-L1 positive EGFR-mutant NSCLC patients naïve to TKI administration [28]. Similar data have been reported for other ICI drugs, such as atezolizumab and durvalumab [46,47]. The role of ICIs is controversial in BRAF-mutated patients [48]. In fact, in a multicentric retrospective cohort, Dudnik et al. showed promising data in terms of clinical efficacy of ICIs in BRAF-mutated advanced stage NSCLC [49]. Conversely, in a small retrospective study, Tan et al. highlighted an inferior OS in BRAF-mutated patients receiving ICI respect to those treated with front-line chemotherapy [50]. Regarding gene rearrangements, a very limited efficacy of ICIs in ALK- [47,51,52,53,54], ROS1- [55,56], RET- [57] and NTRK-rearranged [27] NSCLC patients has been highlighted. Considering MET exon 14 skipping, despite some evidence reporting response to ICIs [58], the overall efficacy of immunotherapy respect to target therapy is quite modest [59].
In conclusion, in this study we have provided a real-world practice experience on the molecular landscape of clinically relevant biomarkers in NSCLC PD-L1-positive patients. The most significant limitations of our study were the limited number of cases, the absence of molecular data on PD-L1 negative patients, the limited number of gene alterations analyzed and clinical data on progression-free survival and overall survival and the lack of clinical data on the vast majority of patients. Further studies are thus needed to better assess the role of the complex genomic landscape in advanced stage NSCLC patients.
4. Materials and Methods
4.1. Study Design
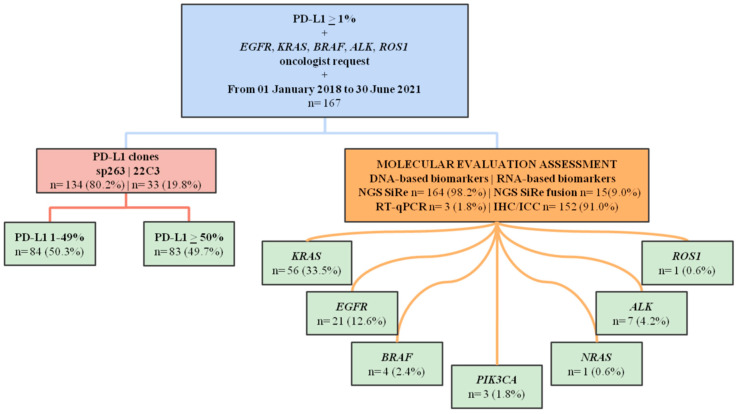
In this study, we retrospectively reviewed cases referred to our clinic from 1 January 2018 to 30 June 2021 for molecular evaluation of at least five driver druggable oncogenes, namely, EGFR, KRAS, BRAF, ALK, ROS1 and PD-L1 expression assessment; PD-L1 positive cases (expression in ≥1% tumor cells) were selected. Information regarding sex, median age, sample type and subtype and diagnosis was also retrieved. (Figure 5) Furthermore, for a subset of patients, data related to the duration of the first-line treatment, or until the loss of data for any causes, were also gathered.
Figure 5.
Study design and results. Abbreviations: ALK: Anaplastic Lymphoma Receptor Tyrosine Kinase; BRAF: V-Raf Murine Sarcoma Viral Oncogene Homolog B1; EGFR: Epidermal Growth Factor Receptor; ICC: immunocytochemistry; IHC: immunohistochemistry; KRAS: Kirsten Rat Sarcoma Viral Oncogene Homolog; NGS: next generation sequencing; NRAS: Neuroblastoma RAS Viral Oncogene Homolog; PD-L1: programmed death-ligand 1; PIK3CA: Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha; RT-qPCR: real-time polymerase chain reaction; ROS1: ROS Proto-Oncogene 1, Receptor Tyrosine Kinase.
All information regarding human material was managed using anonymous numerical codes, and all samples were handled in compliance with the Declaration of Helsinki (http://www.wma.net/en/30publications/10policies/b3/, last accessed 30 June 2022).
4.2. IHC/ICC Analysis
PD-L1 IHC/ICC evaluation was performed with a validated laboratory developed test (LDT), consisting of the use of Dako’s concentrate 22C3 anti-PD-L1 primary antibody with a Ventana’s detection systems on the BenchMark XT platform, or by using the companion diagnostic kit SP263 assay (Ventana Medical Systems, Tucson, AZ, USA) [29,30]. The level of PD-L1 expression was determined by using tumor proportion score (TPS). PD-L1 positive cases were classified either as low-positive PD-L1 expression (1–49%) or as high-positive PD-L1 expression (≥50%) [29,30].
ALK IHC/ICC evaluation was performed by using the Ventana ALK D5F3 companion diagnostic (CDx) assay (Ventana Medical Systems) together with the OptiView (Ventana) detection system. The latter system features a tyramide-based amplification phase in addition to the polymeric step. In particular, by increasing the signal difference between the specific immunoreaction of neoplastic cells and the background, the amplification phase significantly reduces equivocal results. Thus, only positive or negative ALK cases can be reported. Typically, only strong and granular cytoplasmic signals are scored as positive, regardless of the percentage of stained neoplastic cells [60,61,62].
ROS1 IHC/ICC evaluation was carried out with the D4D6 (Cell Signaling Technology, Inc., Danvers, MA, USA) clone. Generally, only tumors with a moderate- to strong staining intensity signal (2+ or 3+ scores) in more than half of the neoplastic cells are considered positive [60,63,64].
Finally, ALK and ROS1 IHC/ICC assays were adopted to confirm RNA-based NGS positive cases.
4.3. Molecular Testing
DNA- and RNA- based analyses of samples were carried out. DNA-based NGS analysis was performed with our narrow NGS panel, namely, SiRe® [65]; this panel was designed, developed and validated in the Molecular Predictive Pathology Laboratory of the Department of Public Health at the University of Naples Federico II [65]. SiRe® can simultaneously detect multiple hotspot gene alterations in seven genes (EGFR, KRAS, BRAF, NRAS, KIT, PDGFRα, and PIK3CA) [31,65]. In the present study, only variants with allele coverage >20X and a quality score >20, with an amplicon coverage of at least 500X alleles, were called.
RNA-based NGS analysis was performed with a narrow NGS panel, namely, SiRe fusion [34]. This panel was also designed, developed, and validated in the Molecular Predictive Pathology Laboratory of the Department of Public Health at University of Naples Federico II [34]. It simultaneously detects alterations in six oncogenic genes, namely, ALK, ROS1, RET, NTRK gene rearrangements, MET exon 14 skipping alterations [34]. In all the study cases, ALK and ROS1 status was further confirmed with IHC/ICC.
In a limited number of cases, the fully automated Idylla™ RT-qPCR platform was adopted to evaluate the molecular status of EGFR, KRAS and BRAF [32,33,66].
Acknowledgments
We thank Paola Merolla for editing the manuscript.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23158541/s1.
Author Contributions
Conceptualization, P.P., A.I., C.D.L., G.T., E.V. and U.M.; methodology, all authors; software, all authors; validation, all authors; formal analysis, all authors; investigation, all authors; resources, all authors; data curation, all authors; writing–original draft preparation, P.P.; writing–review and editing, all authors; visualization, all authors; supervision, G.T., E.V. and U.M.; project administration, G.T., E.V. and U.M.; funding acquisition, G.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to written informed consent was obtained from all patients and documented in accordance with the general authorisation to process personal data for scientific research purposes from ‘The Italian Data Protection Authority’ (http://www.garanteprivacy.it/web/guest/home/docweb/-/docwebdisplay/export/2485392). All information regarding human material was managed using anonymous numerical codes, and all samples were handled in compliance with the Helsinki Declaration (http://www.wma.net/en/30publications/10policies/b3/).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Pasquale Pisapia has received personal fees as speaker bureau from Novartis, unrelated to the current work. Giancarlo Troncone reports personal fees (as speaker bureau or advisor) from Roche, MSD, Pfizer, Boehringer Ingelheim, Eli Lilly, BMS, GSK, Menarini, AstraZeneca, Amgen and Bayer, unrelated to the current work. Elena Vigliar has received personal fees (as consultant and/or speaker bureau) from Diaceutics, AstraZeneca unrelated to the current work. Umberto Malapelle has received personal fees (as consultant and/or speaker bureau) from Boehringer Ingelheim, Roche, MSD, Amgen, Thermo Fisher Scientifics, Eli Lilly, Diaceutics, GSK, Merck and AstraZeneca, Janssen, Diatech, Novartis, Hedera unrelated to the current work. The other Authors have nothing to disclose.
Funding Statement
1. Monitoraggio ambientale, studio ed approfondimento della salute della popolazione residente in aree a rischio—In attuazione della D.G.R. Campania n.180/2019 to G.T. 2. POR Campania FESR 2014–2020 Progetto “Sviluppo di Approcci Terapeutici Innovativi per patologie Neoplastiche resistenti ai trattamenti—SATIN” to G.T. The funding agencies had no role in the design and performance of the study.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Travis W.D., Brambilla E., Nicholson A.G., Yatabe Y., Austin J.H.M., Beasley M.B., Chirieac L.R., Dacic S., Duhig E., Flieder D.B., et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson A.G., Tsao M.S., Beasley M.B., Borczuk A.C., Brambilla E., Cooper W.A., Dacic S., Jain D., Kerr K.M., Lantuejoul S., et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. 2022;17:362–387. doi: 10.1016/j.jtho.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Mok T.S., Wu Y.L., Thongprasert S., Yang C.H., Chu D.T., Saijo N., Sunpaweravong P., Han B., Margono B., Ichinose Y., et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 5.Rosell R., Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E., Palmero R., Garcia-Gomez R., Pallares C., Sanchez J.M., et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 6.Yang J.C.-H., Wu Y.L., Schuler M., Sebastian M., Popat S., Yamamoto N., Zhou C., Hu C.-P., O’Byrne K., Feng J., et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–151. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 7.Soria J.C., Ohe Y., Vansteenkiste J., Reungwetwattana T., Chewaskulyong B., Lee K.H., Dechaphunkul A., Imamura F., Nogami N., Kurata T., et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 8.Planchard D., Smit E.F., Groen H.J.M., Mazieres J., Besse B., Helland Å., Giannone V., D’Amelio A.M., Jr., Zhang P., Mookerjee B., et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: An open-label, phase 2 trial. Lancet Oncol. 2017;18:1307–1316. doi: 10.1016/S1470-2045(17)30679-4. [DOI] [PubMed] [Google Scholar]
- 9.Planchard D., Besse B., Groen H.J.M., Souquet P.J., Quoix E., Baik C.S., Barlesi F., Kim T.M., Mazieres J., Novello S., et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: An open-label, multicentre phase 2 trial. Lancet Oncol. 2016;17:984–993. doi: 10.1016/S1470-2045(16)30146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skoulidis F., Li B.T., Dy G.K., Price T.J., Falchook G.S., Wolf J., Italiano A., Schuler M., Borghaei H., Barlesi F., et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021;384:2371–2381. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallin J., Engstrom L.D., Hargis L., Calinisan A., Aranda R., Briere D.M., Sudhakar N., Bowcut V., Baer B.R., Ballard J.A., et al. The KRASG12C Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer Discov. 2020;10:54–71. doi: 10.1158/2159-8290.CD-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw A.T., Kim D.-W., Nakagawa K., Seto T., Crinó L., Ahn M.-J., De Pas T., Besse B., Solomon B.J., Blackhall F., et al. Crizotinib versus Chemotherapy in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 13.Shaw A.T., Kim D.W., Mehra R., Tan D.S., Felip E., Chow L.Q., Camidge D.R., Vansteenkiste J., Sharma S., De Pas T., et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N. Engl. J. Med. 2014;370:1189–1197. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters S., Camidge D.R., Shaw A.T., Gadgeel S., Ahn J.S., Kim D.W., Ou S.H.I., Pérol M., Dziadziuszko R., Rosell R., et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 15.Camidge D.R., Kim H.R., Ahn M.-J., Yang J.C.-H., Han J.-Y., Lee J.-S., Hochmair M.J., Li J.Y.-C., Chang G.-C., Lee K.H., et al. Brigatinib versus Crizotinib in ALK-Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018;379:2027–2039. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 16.Shaw A.T., Bauer T.M., de Marinis F., Felip E., Goto Y., Liu G., Mazieres J., Kim D.-W., Mok T., Polli A., et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2020;383:2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 17.Shaw A.T., Ou S.H., Bang Y.J., Camidge D.R., Solomon B.J., Salgia R., Riely G.J., Varella-Garcia M., Shapiro G.I., Costa D.B., et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N. Engl. J. Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw A.T., Solomon B.J., Chiari R., Riely G.J., Besse B., A Soo R., Kao S., Lin C.-C., Bauer T.M., Clancy J.S., et al. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: A multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2019;20:1691–1701. doi: 10.1016/S1470-2045(19)30655-2. [DOI] [PubMed] [Google Scholar]
- 19.Drilon A., Siena S., Dziadziuszko R., Barlesi F., Krebs M.G., Shaw A.T., de Braud F., Rolfo C., Ahn M.-J., Wolf J., et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21:261–270. doi: 10.1016/S1470-2045(19)30690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Passiglia F., Reale M.L., Cetoretta V., Novello S. Immune-Checkpoint Inhibitors Combinations in Metastatic NSCLC: New Options on the Horizon? Immunotargets Ther. 2021;10:9–26. doi: 10.2147/ITT.S253581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirsch F.R., McElhinny A., Stanforth D., Ranger-Moore J., Jansson M., Kulangara K., Richardson W., Towne P., Hanks D., Vennapusa B., et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J. Thorac. Oncol. 2017;12:208–222. doi: 10.1016/j.jtho.2016.11.2228. [DOI] [PubMed] [Google Scholar]
- 22.Tsao M.S., Kerr K.M., Kockx M., Beasley M.B., Borczuk A.C., Botling J., Bubendorf L., Chirieac L., Chen G., Chou T.Y., et al. PD-L1 Immunohistochemistry Comparability Study in Real-Life Clinical Samples: Results of Blueprint Phase 2 Project. J. Thorac. Oncol. 2018;13:1302–1311. doi: 10.1016/j.jtho.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 24.Mok T.S.K., Wu Y.L., Kudaba I., Kowalski D.M., Cho B.C., Turna H.Z., Castro G., Jr., Srimuninnimit V., Laktionov K.K., Bondarenko I., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 25.Gandhi L., Rodríguez-Abreu D., Gadgeel S., Esteban E., Felip E., De Angelis F., Domine M., Clingan P., Hochmair M.J., Powell S.F., et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 26.Akinboro O., Larkins E., Pai-Scherf L.H., Mathieu L.N., Ren Y., Cheng J., Fiero M.H., Fu W., Bi Y., Kalavar S., et al. FDA Approval Summary: Pembrolizumab, Atezolizumab, and Cemiplimab-rwlc as single agents for first-line treatment of advanced/metastatic PD-L1 high NSCLC. Clin. Cancer Res. 2022;28:2221–2228. doi: 10.1158/1078-0432.CCR-21-3844. [DOI] [PubMed] [Google Scholar]
- 27.Addeo A., Passaro A., Malapelle U., Banna G.L., Subbiah V., Friedlaender A. Immunotherapy in non-small cell lung cancer harbouring driver mutations. Cancer Treat. Rev. 2021;96:102179. doi: 10.1016/j.ctrv.2021.102179. [DOI] [PubMed] [Google Scholar]
- 28.Lisberg A., Cummings A., Goldman J.W., Bornazyan K., Reese N., Wang T., Coluzzi P., Ledezma B., Mendenhall M., Hunt J., et al. A Phase II Study of Pembrolizumab in EGFR-Mutant, PD-L1+, Tyrosine Kinase Inhibitor Naïve Patients with Advanced NSCLC. J. Thorac. Oncol. 2018;13:1138–1145. doi: 10.1016/j.jtho.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vigliar E., Iaccarino A., Campione S., Campanino M.R., Clery E., Pisapia P., De Luca C., Bellevicine C., Malapelle U., De Dominicis G., et al. PD-L1 expression in cell-blocks of non-small cell lung cancer: The impact of prolonged fixation. Diagn. Cytopathol. 2020;48:595–603. doi: 10.1002/dc.24439. [DOI] [PubMed] [Google Scholar]
- 30.Vigliar E., Malapelle U., Iaccarino A., Acanfora G., Pisapia P., Clery E., De Luca C., Bellevicine C., Troncone G. PD-L1 expression on routine samples of non-small cell lung cancer: Results and critical issues from a 1-year experience of a centralised laboratory. J. Clin. Pathol. 2019;72:412–417. doi: 10.1136/jclinpath-2019-205732. [DOI] [PubMed] [Google Scholar]
- 31.Pepe F., De Luca C., Smeraglio R., Pisapia P., Sgariglia R., Nacchio M., Russo M., Serra N., Rocco D., Battiloro C., et al. Performance analysis of SiRe next-generation sequencing panel in diagnostic setting: Focus on NSCLC routine samples. J. Clin. Pathol. 2019;72:38–45. doi: 10.1136/jclinpath-2018-205386. [DOI] [PubMed] [Google Scholar]
- 32.De Luca C., Gragnano G., Pisapia P., Vigliar E., Malapelle U., Bellevicine C., Troncone G. EGFR mutation detection on lung cancer cytological specimens by the novel fully automated PCR-based Idylla EGFR Mutation Assay. J. Clin. Pathol. 2017;70:295–300. doi: 10.1136/jclinpath-2016-203989. [DOI] [PubMed] [Google Scholar]
- 33.Gragnano G., Nacchio M., Sgariglia R., Conticelli F., Iaccarino A., De Luca C., Troncone G., Malapelle U. Performance evaluation of a fully closed real-time PCR platform for the detection of KRAS p.G12C mutations in liquid biopsy of patients with non-small cell lung cancer. J. Clin. Pathol. 2021;75:350–353. doi: 10.1136/jclinpath-2021-207416. [DOI] [PubMed] [Google Scholar]
- 34.De Luca C., Pepe F., Iaccarino A., Pisapia P., Righi L., Listì A., Greco L., Gragnano G., Campione S., De Dominicis G., et al. RNA-Based Assay for Next-Generation Sequencing of Clinically Relevant Gene Fusions in Non-Small Cell Lung Cancer. Cancers. 2021;13:139. doi: 10.3390/cancers13010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans M., O’Sullivan B., Hughes F., Mullis T., Smith M., Trim N., Taniere P. The Clinicopathological and Molecular Associations of PD-L1 Expression in Non-small Cell Lung Cancer: Analysis of a Series of 10,005 Cases Tested with the 22C3 Assay. Pathol. Oncol. Res. 2020;26:79–89. doi: 10.1007/s12253-018-0469-6. [DOI] [PubMed] [Google Scholar]
- 36.Karatrasoglou E.A., Chatziandreou I., Sakellariou S., Stamopoulos K., Kavantzas N., Lazaris A.C., Korkolopoulou P., Saetta A.A. Association between PD-L1 expression and driver gene mutations in non-small cell lung cancer patients: Correlation with clinical data. Virchows Arch. 2020;477:207–217. doi: 10.1007/s00428-020-02756-1. [DOI] [PubMed] [Google Scholar]
- 37.Sumimoto H., Takano A., Teramoto K., Daigo Y. RAS–Mitogen-Activated Protein Kinase Signal Is Required for Enhanced PD-L1 Expression in Human Lung Cancers. PLoS ONE. 2016;11:e0166626. doi: 10.1371/journal.pone.0166626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen N., Fang W., Lin Z., Peng P., Wang J., Zhan J., Hong S., Huang J., Liu L., Sheng J., et al. KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol. Immunother. 2017;66:1175–1187. doi: 10.1007/s00262-017-2005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MMiura Y., Sunaga N. Role of Immunotherapy for Oncogene-Driven Non-Small Cell Lung Cancer. Cancers. 2018;10:245. doi: 10.3390/cancers10080245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong Z.-Y., Zhong W.-Z., Zhang X.-C., Su J., Xie Z., Liu S.-Y., Tu H.-Y., Chen H.-J., Sun Y.-L., Zhou Q., et al. Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin. Cancer Res. 2017;23:3012–3024. doi: 10.1158/1078-0432.CCR-16-2554. [DOI] [PubMed] [Google Scholar]
- 41.Lee C.K., Man J., Lord S., Cooper W., Links M., Gebski V., Herbst R.S., Gralla R.J., Mok T., Yang J.C. Clinical and Molecular Characteristics Associated with Survival among Patients Treated with Checkpoint Inhibitors for Advanced Non-Small Cell Lung Carcinoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4:210–216. doi: 10.1001/jamaoncol.2017.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bodor J.N., Bauman J.R., Handorf E.A., Ross E.A., Clapper M.L., Treat J. Real-world progression-free survival (rwPFS) and the impact of PD-L1 and smoking in driver-mutated non-small cell lung cancer (NSCLC) treated with immunotherapy. J. Cancer Res. Clin. Oncol. 2022. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 43.Falk A.T., Yazbeck N., Guibert N., Chamorey E., Paquet A., Ribeyre L., Bence C., Zahaf K., Leroy S., Marquette C.-H., et al. Effect of mutant variants of the KRAS gene on PD-L1 expression and on the immune microenvironment and association with clinical outcome in lung adenocarcinoma patients. Lung Cancer. 2018;121:70–75. doi: 10.1016/j.lungcan.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Herbst R.S., Lopes G., Kowalski D.M., Kasahara K., Wu Y.L., De Castro G., Jr., Cho B.C., Turna H.Z., Cristescu R., Aurora-Garg D., et al. LBA4 Association of KRAS mutational status with response to pembrolizumab monotherapy given as first-line therapy for PD-L1-positive advanced non-squamous NSCLC in Keynote-042. Ann. Oncol. 2019;30:xi63–xi64. doi: 10.1093/annonc/mdz453.001. [DOI] [Google Scholar]
- 45.Mazieres J., Drilon A., Lusque A.B., Mhanna L., Cortot A., Mezquita L., Thai A.A., Mascaux C., Couraud S., Veillon R., et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann. Oncol. 2019;30:1321–1328. doi: 10.1093/annonc/mdz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peters S., Gettinger S., Johnson M.L., Jänne P.A., Garassino M.C., Christoph D., Toh C.K., Rizvi N.A., Chaft J.E., Costa E.C., et al. Phase II Trial of Atezolizumab as First-Line or Subsequent Therapy for Patients with Programmed Death-Ligand 1–Selected Advanced Non–Small-Cell Lung Cancer (BIRCH) J. Clin. Oncol. 2017;35:2781–2789. doi: 10.1200/JCO.2016.71.9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garassino M.C., Cho B.-C., Kim J.-H., Mazières J., Vansteenkiste J., Lena H., Jaime J.C., Gray J.E., Powderly J., Chouaid C., et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): An open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19:521–536. doi: 10.1016/S1470-2045(18)30144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frisone D., Friedlaender A., Malapelle U., Banna G., Addeo A. A BRAF new world. Crit. Rev. Oncol. Hematol. 2020;152:103008. doi: 10.1016/j.critrevonc.2020.103008. [DOI] [PubMed] [Google Scholar]
- 49.Dudnik E., Peled N., Nechushtan H., Wollner M., Onn A., Agbarya A., Moskovitz M., Keren S., Popovits-Hadari N., Urban D., et al. BRAF Mutant Lung Cancer: Programmed Death Ligand 1 Expression, Tumor Mutational Burden, Microsatellite Instability Status, and Response to Immune Check-Point Inhibitors. J. Thorac. Oncol. 2018;13:1128–1137. doi: 10.1016/j.jtho.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 50.Tan I., Stinchcombe T.E., Ready N.E., Crawford J., Datto M.B., Nagy R.J., Lanman R.B., Gu L., Clarke J.M. Therapeutic outcomes in non-small cell lung cancer with BRAF mutations: A single institution, retrospective cohort study. Transl. Lung Cancer Res. 2019;8:258–267. doi: 10.21037/tlcr.2019.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gainor J.F., Shaw A.T., Sequist L.V., Fu X., Azzoli C.G., Piotrowska Z., Huynh T.G., Zhao L., Fulton L., Schultz K.R., et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non–Small Cell Lung Cancer: A Retrospective Analysis. Clin. Cancer Res. 2016;22:4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bylicki O., Guisier F., Monnet I., Doubre H., Gervais R., Janicot H., Perol M., Fournel P., Lamy R., Auliac J.B., et al. Efficacy and safety of programmed cell-death-protein-1 and its ligand inhibitors in pretreated patients with epidermal growth-factor receptor-mutated or anaplastic lymphoma kinase-translocated lung adenocarcinoma. Medicine. 2020;99:e18726. doi: 10.1097/MD.0000000000018726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.West H., McCleod M., Hussein M., Morabito A., Rittmeyer A., Conter H.J., Kopp H.-G., Daniel D., McCune S., Mekhail T., et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 54.Socinski M.A., Jotte R.M., Cappuzzo F., Orlandi F., Stroyakovskiy D., Nogami N., Rodríguez-Abreu D., Moro-Sibilot D., Thomas C.A., Barlesi F., et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 55.Chen D.S., Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 56.Jain A., Fujioka N., Patel M. Immune Checkpoint Inhibitors in ROS1-Rearranged Non–Small Cell Lung Cancer: A Report of Two Cases. J. Thorac. Oncol. 2019;14:e165–e167. doi: 10.1016/j.jtho.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Hegde A., Andreev-Drakhlin A.Y., Roszik J., Huang L., Liu S., Hess K., Cabanillas M., Hu M.I., Busaidy N.L., Sherman S.I., et al. Responsiveness to immune checkpoint inhibitors versus other systemic therapies in RET-aberrant malignancies. ESMO Open. 2020;5:e000799. doi: 10.1136/esmoopen-2020-000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guisier F., Dubos-Arvis C., Viñas F., Doubre H., Ricordel C., Ropert S., Janicot H., Bernardi M., Fournel P., Lamy R., et al. Efficacy and Safety of Anti-PD-1 Immunotherapy in Patients with Advanced NSCLC With BRAF, HER2, or MET Mutations or RET Translocation: GFPC 01-2018. J. Thorac. Oncol. 2020;15:628–636. doi: 10.1016/j.jtho.2019.12.129. [DOI] [PubMed] [Google Scholar]
- 59.Sabari J., Leonardi G., Shu C., Umeton R., Montecalvo J., Ni A., Chen R., Dienstag J., Mrad C., Bergagnini I., et al. PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann. Oncol. 2018;29:2085–2091. doi: 10.1093/annonc/mdy334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pisapia P., Lozano M.D., Vigliar E., Bellevicine C., Pepe F., Malapelle U., Troncone G. ALK and ROS1 testing on lung cancer cytologic samples: Perspectives. Cancer Cytopathol. 2017;125:817–830. doi: 10.1002/cncy.21899. [DOI] [PubMed] [Google Scholar]
- 61.Conde E., Hernandez S., Prieto M., Martinez R., Lopez-Rios F. Profile of Ventana ALK (D5F3) Companion Diagnostic Assay for Non-Small-Cell Lung Carcinomas. Expert Rev. Mol. Diagn. 2016;16:707–713. doi: 10.1586/14737159.2016.1172963. [DOI] [PubMed] [Google Scholar]
- 62.Kerr K.M., López-Ríos F. Precision medicine in NSCLC and pathology: How does ALK fit in the pathway? Ann. Oncol. 2016;27((Suppl. 3)):iii16–iii24. doi: 10.1093/annonc/mdw302. [DOI] [PubMed] [Google Scholar]
- 63.Rossi G., Ragazzi M., Tamagnini I., Mengoli M.C., Vincenzi G., Barbieri F., Piccioli S., Bisagni A., Vavala T., Righi L., et al. Does Immunohistochemistry Represent a Robust Alternative Technique in Determining Drugable Predictive Gene Alterations in Non-Small Cell Lung Cancer? Curr. Drug Targets. 2017;18:13–26. doi: 10.2174/1389450116666150330114441. [DOI] [PubMed] [Google Scholar]
- 64.Selinger C.I., Li B.T., Pavlakis N., Links M., Gill A.J., Lee A., Clarke S., Tran T.N., Lum T., Yip P.Y., et al. Screening for ROS1 gene rearrangements in non-small-cell lung cancers using immunohistochemistry with FISH confirmation is an effective method to identify this rare target. Histopathology. 2017;70:402–411. doi: 10.1111/his.13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malapelle U., Mayo de-Las-Casas C., Rocco D., Garzon M., Pisapia P., Jordana-Ariza N., Russo M., Sgariglia R., De Luca C., Pepe F., et al. Development of a gene panel for next-generation sequencing of clinically relevant mutations in cell-free DNA from cancer patients. Br. J. Cancer. 2017;116:802–810. doi: 10.1038/bjc.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Haele M., Vander Borght S., Ceulemans A., Wieërs M., Metsu S., Sagaert X., Weynand B. Rapid clinical mutational testing of KRAS, BRAF and EGFR: A prospective comparative analysis of the Idylla technique with high-throughput next-generation sequencing. J. Clin. Pathol. 2020;73:35–41. doi: 10.1136/jclinpath-2019-205970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.