Abstract
Sequences of the 16S ribosomal DNA (rDNA) from psychrotolerant and mesophilic strains of the Bacillus cereus group revealed signatures which were specific for these two thermal groups of bacteria. Further analysis of the genomic DNA from a wide range of food and soil isolates showed that B. cereus group strains have between 6 and 10 copies of 16S rDNA. Moreover, a number of these environmental strains have both rDNA operons with psychrotolerant signatures and rDNA operons with mesophilic signatures. The ability of these isolates to grow at low temperatures correlates with the prevalence of rDNA operons with psychrotolerant signatures, indicating specific nucleotides within the 16S rRNA to play a role in psychrotolerance.
The cold shock response of Escherichia coli and Bacillus subtilis has been studied in some detail (for reviews see references 11, 18, and 39). It includes the induction of a number of cold shock proteins (13), among which are small acidic RNA- and DNA-binding proteins (12, 22, 24). Far less is known about the adaptation of bacteria to growth at low temperatures. To understand the physiological and molecular bases of psychrotolerance, a large number of strains of the Bacillus cereus group which differ mainly in their ability to grow at low temperatures (25, 26) have been investigated. The comparison of more than 20 mesophilic B. cereus and Bacillus thuringiensis strains, as well as psychrotolerant B. cereus and Bacillus mycoides strains revealed sequence differences in the 16S ribosomal DNA (rDNA), 23S rDNA, the 16S-23S rDNA spacer region, and the major cold shock protein CspA. A new psychrotolerant species of the B. cereus group, Bacillus weihenstephanensis, was therefore proposed (21). This new species can be distinguished from the mesophilic B. cereus by PCR assays directed against the major cold shock protein CspA (9) or the 16S rDNA (36).
Among the physiological differences found between mesophilic B. cereus and psychrotolerant B. weihenstephanensis was an improved protein synthesis of the psychrotolerant isolate at low temperature. The incorporation of l-[35S]methionine into protein was higher in B. weihenstephanensis when cold shocked to 7 or 12°C. The response occurred quickly after the cold shock. It was concluded that the improved protein synthesis was not a cold shock response but rather a constitutive ability of the psychrotolerant strain (20).
This ability may be due to a different structure of the ribosome. Therefore, the 16S rDNA sequences of 18 mesophilic B. cereus and B. thuringiensis strains and 9 psychrotolerant B. weihenstephanensis and B. mycoides strains were compared (for accession numbers for EMBL, see reference 21). The sequences revealed a very high degree of identity. Single base pair substitutions were randomly distributed over the gene. The most obvious difference (Table 1) was one signature that was characteristic for mesophilic B. cereus and B. thuringiensis (AACATTTTGAACCGCATGGTTC) and for psychrotolerant B. weihenstephanensis and B. mycoides (AATATTTTGAACTGCATAGTTC). This signature was located at bp 180 to 192 according to the E. coli nomenclature (2, 3, 8) and at bp 180 to 201 according to the B. cereus nomenclature (1). The difference between the two nomenclatures is due to the gapped alignment. All of the substitutions are transitions from C and G (mesophilic) to T and A (psychrotolerant). Three of the examined B. thuringiensis strains possessed a C-to-T transition at position 2 of the signature.
TABLE 1.
Bacterial strains and 16S rDNA sequences used in this study
| Species and/or straina | Origina | Phenotypeb | Signature (180–192)c | Source or reference(s)d |
|---|---|---|---|---|
| E. coli | Mesophilic | AACGT-------CGCAAGA--C | 2, 3, 8 | |
| B. subtilis | Mesophilic | GTTGTTT-GAACCGCATGGTTC | 14 | |
| B. cereus | ||||
| WSBC10027 | Weihenstephan, Germany; pasteurized milk | Mesophilic | AACATTTTGAACCGCATGGTTC | 21 |
| WSBC10028 | Weihenstephan, Germany; pasteurized milk | Mesophilic | ********************** | 21 |
| WSBC10030 | Weihenstephan, Germany; pasteurized milk | Mesophilic | ********************** | 21 |
| WSBC10032 | Weihenstephan, Germany; pasteurized milk | Mesophilic | ********************** | 21 |
| WSBC10033 | Weihenstephan, Germany; pasteurized milk | Mesophilic | ********************** | 21 |
| WSBC10034 | Weihenstephan, Germany; pasteurized milk | Mesophilic | ********************** | 21 |
| WSBC10035 | Weihenstephan, Germany; pasteurized milk | Mesophilic | ********************** | 21 |
| WSBC10037 | Weihenstephan, Germany; pasteurized milk | Mesophilic | ********************** | 21 |
| ATCC27877 | ATCC | Mesophilic | ********************** | 21 |
| IAM12605 | N.D. | ********************** | 32 | |
| NCTC11143 | N.D. | ********************** | 1 | |
| WSBC10312 | Thailand; kurkuma root | Mesophilic | N.D. | 36 |
| B. weihenstephanensis | ||||
| WSBC10201 | Weihenstephan, Germany; pasteurized milk | Psychrotolerant | **T*********T****A**** | 21 |
| WSBC10204T | Weihenstephan, Germany; pasteurized milk | Psychrotolerant | **T*********T****A**** | 21 |
| WSBC10205 | Weihenstephan, Germany; pasteurized milk | Psychrotolerant | **T*********T****A**** | 21 |
| WSBC10206 | Weihenstephan, Germany; pasteurized milk | Psychrotolerant | **T*********T****A**** | 21 |
| WSBC10207 | Weihenstephan, Germany; pasteurized milk | Psychrotolerant | **T*********T****A**** | 21 |
| WSBC10210 | Weihenstephan, Germany; pasteurized milk | Psychrotolerant | **T*********T****A**** | 21 |
| WSBC10311 | Weihenstephan, Germany; soil | Psychrotolerant | N.D. | 36 |
| B. mycoides | ||||
| WSBC10276 | Weihenstephan, Germany; pasteurized milk | Psychrotolerant | **T*********T****A**** | 21 |
| WSBC10279 | Weihenstephan, Germany; pasteurized milk | Psychrotolerant | **T*********T****A**** | 21 |
| WS2641T | DSM2048 | Psychrotolerant | **T*********T****A**** | 21 |
| B. thuringiensis | ||||
| WS2614 | HER1211 | Mesophilic | AACATTTTGAACCGCATGGTTC | 21 |
| WS2617 | HER1232 | Mesophilic | ********************** | 21 |
| WS2618 | HER1233 | Mesophilic | ********************** | 21 |
| WS2625 | HER1387 | Mesophilic | ************T********* | 21 |
| WS2626 | HER1404 | Mesophilic | ********************** | 21 |
| WS2627 | HER1410 | Mesophilic | N.D. | Ackermann |
| WS2629 | HER1418 | Mesophilic | N.D. | Ackermann |
| IAM12077 | N.D. | ************T********* | 32 | |
| NCIMB9134 | N.D. | ************T********* | 1 | |
| B. anthracis “Sterne” | Mesophilic | ********************** | 1 | |
| Other strains | ||||
| WSBC10246 (S38/21) | Copenhagen, Denmark; soil | Mesophilic | N.D. | 6 |
| WSBC10250 (S38/25) | Copenhagen, Denmark; soil | Mesophilic | N.D. | 6 |
| WSBC10313 (3) | Kiel, Germany; milk powder | Mesophilic | N.D. | 38 |
| WSBC10314 (4) | Kiel, Germany; milk powder | Mesophilic | N.D. | 38 |
| WSBC10315 (6) | Kiel, Germany; milk powder | Psychrotolerant | N.D. | 38 |
| WSBC10316 (46) | Kiel, Germany; milk powder | Mesophilic | N.D. | 38 |
| WSBC10310 | Weihenstephan, Germany; soil | Mesophilic | N.D. | 36 |
| WSBC10297 | Weihenstephan, Germany; soil | Psychrotolerant | N.D. | 36 |
DSM, Deutsche Sammlung für Mikroorganismen und Zellkulturen, Braunschweig, Germany; ATCC, American Type Culture Collection, Rockville, Md.; HER, Centre de référence pour virus bactériens, Felix d’Herbelle Université Laval, H.-W. Ackermann, Quebec, Canada; WS, Weihenstephan Collection, Institute of Microbiology, FML Weihenstephan, Freising, Germany; WSBC, Weihenstephan Bacillus cereus Collection, Institute of Microbiology, FML Weihenstephan, Freising, Germany.
Psychrotolerant strains are able to grow at and below 7°C (27). N.D., not determined.
According to the E. coli nomenclature (2, 3, 8). According to the B. cereus nomenclature (1), the signature is located at bp 180 to 201. Letters in boldface are the signature bases. N.D., not determined.
References are given for the accession numbers of 16S rDNA sequences if sequences are available. In all other cases, the reference for the strain is given.
The signatures were used to develop a rapid PCR assay to discriminate between mesophilic and psychrotolerant strains (36). During the development of this assay, a primer combination that yielded exclusively a psychrotolerant signal for the psychrotolerant strains was found. However, some of the mesophilic strains showed both the mesophilic and the psychrotolerant signal. The occurrence of these two signals can be explained by the coexistence of unknown numbers of mesophilic and psychrotolerant 16S rDNA copies within a single organism. In this study, the proportion of mesophilic and psychrotolerant signatures of these intermediate strains was determined, and a correlation with growth at extreme temperatures was established.
Isolation of individual bacterial cells.
First, individual colonies were obtained by serial dilutions. However, due to hydrophobic interactions on the surfaces of the bacteria, cells sometimes stick together. A second approach was applied in order to exclude the possibility that the occurrence of the intermediate strains was a result of coisolated, mixed bacterial strains. Individual cells were isolated from four intermediate strains with a micromanipulator (10). These bacteria were grown to colonies, and the chromosomal DNA was isolated from five different colonies of each strain. The PCR assay (36) was repeated with these isolates. The PCR fragments from each of the isolates looked identical to the strain from which it was derived (data not shown). This indicates that the mixed patterns did not result from a mixed bacterial culture.
Psychrotolerance index as determined by restriction digest of rDNA.
The signature of the psychrotolerant strain, AATATTTTGAACTGCATAGTTC, contains an SspI site. This restriction site includes the first signature base T. The mesophilic strains possess a C at this position and, therefore, do not contain this restriction site in their 16S rDNA signatures.
Chromosomal DNA was isolated from mesophilic, intermediate, and psychrotolerant strains. A PCR was performed (2 mM MgCl2, 50 pmol of each primer, 0.2 mM concentrations of each dNTP, 1 U of Taq polymerase) (30 cycles of 95°C for 15 s, 55°C for 30 s, and 72°C for 30 s) using 5′-GTC GAG CGA ATG GAT TAA G-3′ as the forward primer and 5′-GCT GCT GGC ACG TAG TTA-3′ as the reverse primer. This PCR yielded a 473-bp fragment extending from bp 61 to 534 (B. cereus nomenclature) and containing the signature. The PCR products were digested with SspI and separated on a 1.5% agarose gel. The agarose gel was scanned, and the intensity of the bands was measured with the Imagemaster 1D software package from Pharmacia Biotech (Braunschweig, Germany). This method allowed the estimation of the fraction of rDNA operons carrying mesophilic or psychrotolerant signatures (psychrotolerance index).
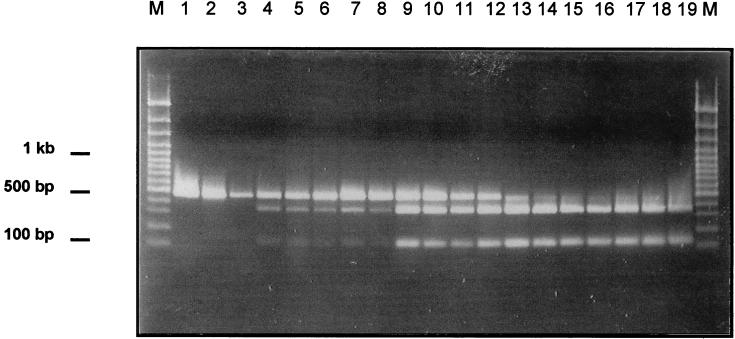
Figure 1 shows the restriction patterns of the SspI digests of the signature. The mesophilic strains (lanes 1 to 3) show the uncleaved 473-bp PCR product. The psychrotolerant strains (lanes 14 to 19) show the two cleavage products of the SspI restriction digest at 351 and 122 bp but not the uncleaved 473-bp PCR product. The remaining intermediate strains show all three bands. Strain WSBC10250 (lane 4) has a mesophilic band 6.6 times more intense than the psychrotolerant band, strain WSBC10316 (lane 6) has a mesophilic band 4 times more intense, and strains WSBC10246, HER1418, and HER1410 (lanes 5, 7, and 8) have a mesophilic band 3 times more intense. Strains WSBC10310, WSBC10313, WSBC10314, and WSBC10315 (lanes 9, 10, 11, and 12) show the mesophilic and the psychrotolerant bands at identical intensities, and strain WSBC10297 (lane 13) shows a psychrotolerant band 2.3 times more intense. We conclude from these data that intermediate strains have different percentages of rDNA operons with a psychrotolerant signature.
FIG. 1.
Restriction digests at the psychrotolerance signature. Chromosomal DNA was isolated. A PCR was performed using 5′-GTC GAG CGA ATG GAT TAA G-3′ as the forward primer and 5′-GCT GCT GGC ACG TAG TTA-3′ as the reverse primer. The PCR products were digested with SspI and separated on an agarose gel. The experiment was done twice, and one of the identical experiments is presented. Lanes: M, molecular weight standard, 100-bp ladder; 1, WSBC10028; 2, WSBC10030; 3, WSBC10312; 4, WSBC10250; 5, WSBC10246; 6, WSBC10316; 7, HER1418; 8, HER1410; 9, WSBC10310; 10, WSBC10313; 11, WSBC10314; 12, WSBC10315; 13, WSBC10297; 14, WSBC10311; 15, WSBC10201; 16, WSBC10204; 17, WSBC10206; 18, WSBC10276; 19, WSBC10279.
Psychrotolerance index as determined by inverse PCR and Southern hybridization.
Chromosomal DNA was digested with HhaI, whose restriction site is located 394 bp downstream of the last signature base, and self-ligated. An inverse PCR was performed (2 mM MgCl2, 50 pmol of each primer, 0.2 mM concentrations of each dNTP, 1 U of Taq polymerase) (35 cycles of 95°C for 15 s, 55°C for 60 s, and 72°C for 180 s) using the universal primers 5′-GGT GAG GTA ACG GCT CA-3′ and 5′-GGG TCC ATC CAT AAG TGA-3′. The binding site for these primers is located between the HhaI site and the signature (21). The PCR products were separated on an agarose gel. Due to the close proximity of the restriction site to the signature, every band on the gel corresponds to one individual operon, the smallest band possible being 591 bp. The fragments were blotted onto nitrocellulose. A Southern analysis (29) was performed using DIG-labelled oligonucleotide probes directed against the psychrotolerant (GAT AAT ATT TTG AAC TGC ATA G) and the mesophilic (GAT AAC ATT TTG AAC CGC ATG G) signature. Another probe from 16S rDNA was derived by PCR. This probe recognized a 211-bp region from 323 to 534 (B. cereus nomenclature) that was identical in all the 16S rDNA sequences. This probe was used to determine the total number of 16S rDNA operons.
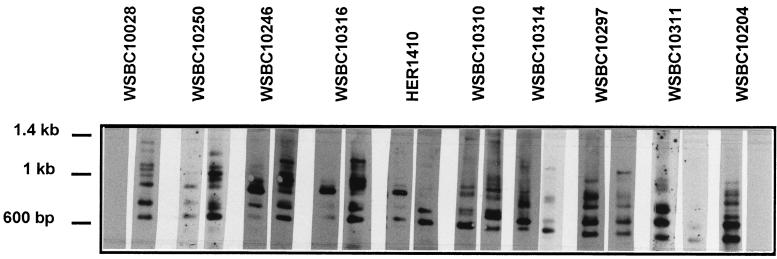
Figure 2 shows the Southern blots of the inverse PCR of selected strains. Among the members of the B. cereus group we detected between 6 and 10 copies of 16S rDNA. The PCR products of the mesophilic strain WSBC10028 (lane 1) reacted only with the mesophilic probe. The PCR products of the psychrotolerant strains WSBC10311 (lane 9) and WSBC10204 (lane 10) reacted only with the psychrotolerant probe. The same was true for the mesophilic B. cereus (WSBC10030 and WSBC10312) and the psychrotolerant B. weihenstephanensis (WSBC10201 and WSBC10206) and B. mycoides (WSBC10276 and WSBC10279) strains not shown in Fig. 2. The control experiment with the nonspecific 16S rDNA probe showed that all the bands that hybridized with the nonspecific rDNA probe also reacted with either the mesophilic or the psychrotolerant probe (data not shown).
FIG. 2.
Southern blot of inverse PCR fragments. Chromosomal DNA was digested with HhaI and self-ligated. An inverse PCR was performed using 5′-GGT GAG GTA ACG GCT CA-3′ as the forward primer and 5′-GGG TCC ATC CAT AAG TGA-3′ as the reverse primer. The PCR products were separated on an agarose gel and blotted onto nitrocellulase. A Southern analysis using DIG-labelled oligonucleotide probes directed against the psychrotolerant (GAT AAT ATT TTG AAC TGC ATA G) and the mesophilic (GAT AAC ATT TTG AAC CGC ATG G) signature 1 was performed. The experiment was done two or three times, and one of the identical experiments is presented. Lanes: 1, WSBC10028; 2, WSBC10250; 3, WSBC10246; 4, WSBC10316; 5, HER1410; 6, WSBC10310; 7, WSBC10314; 8, WSBC10297; 9, WSBC10311; 10, WSBC10204. Left lane of each pair of blots, psychrotolerant probe; right lane, mesophilic probe.
The remaining strains yielded mixed patterns. WSBC10250 (lane 2) reacted predominantly with the mesophilic probe but also slightly with the psychrotolerant probe. WSBC10246 (lane 3) and WSBC10316 (lane 4) showed one strong psychrotolerant band, several weak psychrotolerant bands, and numerous mesophilic bands. B. thuringiensis HER1410 (lane 5) and WSBC10310 (lane 6) also had only one strong psychrotolerant band. Two bands reacted predominantly with the mesophilic probe. Several bands showed up slightly with both probes. Three more bands were detected for B. thuringiensis HER1410 with the nonspecific 16S rDNA probe (data not shown) which were not detectable with either the mesophilic or psychrotolerant probe. WSBC10314 (lane 7) possessed six 16S rDNA copies. One of them hybridized with the psychrotolerant probe, the other with the mesophilic probe. Two more bands hybridized weakly with the mesophilic probe and two with the psychrotolerant probe. WSBC10297 (lane 8) showed mostly psychrotolerant bands. The majority of these also showed up slightly with the mesophilic probe. Only one band at high molecular weight hybridized exclusively with the mesophilic probe.
Comparison of psychrotolerance indices.
The intensity of the bands resulting from the SspI restriction (Fig. 1) was measured with the Imagemaster 1D software package. The proportion of the psychrotolerant (351-bp) band was determined as a percentage of the total PCR product (the 351-bp band plus the 473-bp band). The proportion of psychrotolerant signatures from the inverse PCR (Fig. 2) was determined as a percentage of those bands that appeared predominantly with the psychrotolerant probe. The psychrotolerance index (expressed as a percentage) as determined by restriction digest was plotted against the psychrotolerance index as determined by inverse PCR. Figure 3 shows that the correlation between the two different approaches to determine the prevalence of psychrotolerant signatures of the strains is reasonably good.
FIG. 3.
Correlation of the psychrotolerance indices obtained by restriction digest (Fig. 1) and inverse PCR (Fig. 2). The proportion of psychrotolerant signatures from the inverse PCR is determined as a percentage of those bands that appear predominantly with the psychrotolerant probe. These data are plotted on the abscissa. The intensity of the bands resulting from the SspI restriction was measured with the Imagemaster 1D software package. The proportion of the psychrotolerant (351-bp) band is expressed as a percentage of the total PCR product (the 351-bp band plus the 473-bp band). These data are plotted on the ordinate. The experiment was done twice, and the means of the populations were determined. Standard errors were less than 10%.
As an additional approach, the 16S rDNA PCR products obtained from strains WSBC10246, WSBC10314, and WSBC10297 (Fig. 2) were purified and directly sequenced. The sequences of these three strains were 100% identical, with the following exceptions: strain S38/21 possessed 30% T and 70% C in the positions of the first (182) and second (192) signature bases (A[C70T30]ATTTTGAAC[C70T30]GCATGGT), with the third (197) signature base being a mesophilic G. This strain was found to be 24.5% psychrotolerant according to the SspI digest and 12% psychrotolerant according to the inverse PCR. Strain S74/3 possessed 70% T and 30% C in the position of the first signature base (A[C30T70]ATTTTGAACTGCATGGT), with the second signature base being a psychrotolerant T and the third signature base being a mesophilic G. This strain was found to be 39% psychrotolerant according to the SspI digest and 50% psychrotolerant according to the inverse PCR. Strain V1R/14 possessed 80 to 90% T and 10 to 20% C in a position between the first and the second signature bases (ATATTT[T85C15]GAACTGCATGGT), with the first and second signature bases being the psychrotolerant T and the last signature base being the mesophilic G. This strain was found to be 70% according to the SspI digest and 87% psychrotolerant according to the inverse PCR. Therefore, the three different methods to determine a psychrotolerance index of the strains yielded comparable results.
Growth rates at different temperatures.
It was unclear whether the presence of these signatures has any functional consequence for growth at low or high temperature. To test this possibility, bacteria were grown from an overnight culture in plate count broth (5 g of casein peptone/liter, 2.5 g of yeast extract/liter, 1 g of glucose/liter [pH 6.8]) at 10, 28, and 42°C under continuous shaking at 150 rpm.
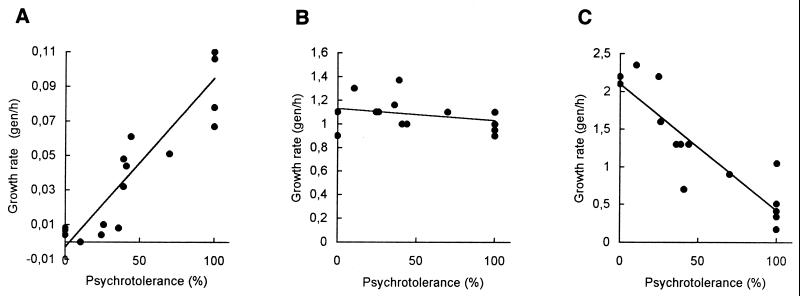
Figure 4 shows the growth rates as generations per hour (gen/h) plotted versus the psychrotolerance index as estimated by restriction digest with SspI. At 10°C, a clear correlation between the growth rate and the psychrotolerance index was observed. The growth rates increased with an increasing psychrotolerance index of from 0.0063 ± 0.0009 gen/h for the mesophiles to 0.097 ± 0.0079 for the psychrotolerant strains. The experiment was also done at 7°C (data not shown). All psychrotolerant B. weihenstephanensis and B. mycoides strains, with the exception of WSBC10311, were capable of growing at a slow rate, as were the strains WSBC10297 (83% psychrotolerant) and WSBC1315 (50% psychrotolerant). The other strains did not grow and are considered mesophilic according to the definition of psychrotolerance (27). At 28°C, all strains grew at approximately the same rate of 1.08 ± 0.033 gen/h. A correlation between the psychrotolerance index and the growth rate at 42°C was also seen. The growth rates decreased with increasing psychrotolerance index from rates around 2.1 gen/h for the mesophilic B. cereus to rates between 0.17 and 0.51 gen/h for the psychrotolerant B. weihenstephanensis and B. mycoides. An exception was strain WSBC10311, which grew faster (1.05 gen/h) than the other psychrotolerant strains.
FIG. 4.
Comparison of growth rates and psychrotolerance indices. Cultures were grown in plate count broth at 10°C (A), 28°C (B), and 42°C (C). The optical densities were measured at 600 nm, and the growth rates were determined during exponential growth as generations per hour (gen/h). The growth rates are plotted against the percentage of psychrotolerant signatures as determined by restriction digest with SspI (Fig. 1). The experiment was done two to six times, and the means of the populations were determined. Standard errors were less than 20%. The exact psychrotolerance indices were as follows: WSBC10028, 0%; WSBC10030, 0%; WSBC10312, 0%; WSBC10250, 10%; WSBC10246, 12.5%; WSBC10316, 14.3%; HER1418, 25%; HER1410, 33%; WSBC10310, 33%; WSBC10313, 43%; WSBC10314, 50%; WSBC10315, 50%; WSBC10297, 87%; WSBC10311, 100%; WSBC10201, 100%; WSBC10204, 100%; WSBC10206, 100%; WSBC10276, 100%; WSBC10279, 100%.
Discussion and concluding remarks.
The organization of the rrn operons has been extensively studied in E. coli and B. subtilis. E. coli contains 7 rrn operons (16), and B. subtilis possesses 10 rrn operons (28, 31). In both organisms, it is possible to delete one of these operons without any major influence on cell growth or physiology under normal conditions (5, 37). Yet, the existence of multiple rrn operons suggests some selective advantage to the organism. Condon and coworkers (4) found that seven rrn operons are required for E. coli to rapidly adapt to nutrient and temperature changes. The time taken to adapt to a temperature shift from 28 to 42°C increased with decreasing numbers of intact operons. It was suggested that the ability to adapt to changing environmental conditions has provided the selective pressure to retain several operons. As observed previously (17, 21), strains of the B. cereus group possess between 6 and 10 rDNA operons. The overall number of operons varies among members of the group but does not correlate with the growth rate at any temperature (data not shown).
It has been suggested before that the ribosome acts as a procaryotic sensor for the heat and cold shock response (33, 35). It was postulated that the physiological signal for the induction of the cold shock response may be the inhibition of initiation of translation caused by the temperature shift. Three ribosome-associated proteins, IF2, CsdA, and RbfA, are induced during the transient block in initiation of translation in E. coli (7, 15, 34) to convert the cold-sensitive nontranslatable ribosome into a cold-resistant translatable state. At least one of these three proteins, CsdA, was also involved in optimal growth at low temperature, possibly by unwinding stable secondary structures in mRNA (19). The inhibition of protein synthesis upon cold shock was significantly more dramatic for mesophilic B. cereus than for psychrotolerant B. weihenstephanensis (20). Since protein synthesis of the psychrotolerant strain occurred instantaneously after cold shock, it was postulated that the ability of this strain to synthesize protein at low temperature was a constitutive ability, its ribosomes being in a cold-resistant, translatable state.
This study is in agreement with the idea of the ribosome being a critical factor for the adaptation to growth at low temperature. The observed differences in the 16S rDNA sequence correlated significantly with the growth behavior of the strains at extreme temperatures. At low (10°C) and high (42°C) temperature, we obtained a correlation of the growth rate with the psychrotolerance index as estimated by restriction digest with SspI (Fig. 3). The same data plotted versus the percentage of operons that hybridized with a probe directed against the psychrotolerant signature in the 16S rDNA looked similar (not shown). A high percentage of psychrotolerant signatures may help the strain to grow at low temperatures, and a high percentage of mesophilic signatures may help the cells to grow at high temperatures. Sequence analysis of selected strains and the occurrence of weak hybridizations of the inverse PCR products with both the mesophilic and the psychrotolerant probes (Fig. 2) show that intermediate forms of the signature are as well possible.
The 16S rDNA is part of the small (30S) ribosomal subunit which also contains 21 ribosomal proteins. This subunit is involved in the early steps of translation initiation and is the site of codon-anticodon interaction. In E. coli, the structure around the region of the signature (bp 100 to 190) is involved in binding of the primary binding protein S20 (30). Our data imply that the 16S rDNA molecule of the B. cereus group species may exist in two different states. This may lead to a different structure of the 30S subunit since binding of primary binding proteins affects binding of the secondary and tertiary binding proteins (30). One of these structures may allow translation initiation at low temperature, and the other one may allow it at high temperature. The ability of a strain to grow at low or high temperature may be influenced by the ratio of these two structures, considering that intermediate forms are possible.
Particularly striking is the fact that the base pair substitutions are all from G and C in the mesophilic strains to A and T in the psychrotolerant strains. With respect to the lower melting temperature of A-T bonds, it is possible that the 16S rDNA forms hydrogen bonds at the position of the signature which cannot be dissolved at low temperature in case of G-C bonding but can be dissolved in case of A-T bonding. This transition may yield a state of the ribosome which is capable of translation at low temperature. On the other hand, the A-T bonding may be too weak to guarantee stable structures at higher temperatures. Lodmell and Dahlberg (23) have observed that even single point mutations in the 912 region of the E. coli 16S rDNA enhanced the stability of one or the other of two proposed 16S rDNA conformations. In order to finally prove that the different 16S rDNA operons are responsible for efficient translation at different temperatures, one would have to construct knockout mutants of the various different copies of 16S rDNA. However, a genetic system for B. cereus which would allow these mutants to be constructed is not yet available.
Acknowledgments
We thank H.-W. Ackermann (Felix d’Herbelle Université Laval, Quebec, Canada), C. Wiebe (Bundesanstalt für Milchforschung, Kiel, Germany), P. Damgaard (Denmark), and R. Mayr (our laboratory) for providing strains. We thank J. Fröhlich and H. König (Universität Mainz, Mainz, Germany) for isolating bacteria with the micromanipulator, and we thank H. Hermann (our laboratory) for technical assistance. W. Metzger (Sequiserve, Vaterstetten, Germany) performed the sequence analysis.
This work was supported in part by the Deutsche Forschungsgemeinschaft (Sche 316/3-1).
REFERENCES
- 1.Ash C A, Farrow J A E, Wallbanks S, Collins M D. Phylogenetic analysis of Bacillus anthracis and Bacillus cereus and related species based on reverse transcriptase sequencing of 16S ribosomal RNA. Lett Appl Microbiol. 1991;13:202–206. [Google Scholar]
- 2.Brosius J, Palmer M L, Kennedy P J, Noller H F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci USA. 1978;75:4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carbon P, Ehresmann C, Ehresmann B, Ebel J P. The complete nucleotide sequence of the ribosomal 16S RNA from Escherichia coli. Experimental details and cistron heterogeneities. Eur J Biochem. 1979;100:399–410. doi: 10.1111/j.1432-1033.1979.tb04183.x. [DOI] [PubMed] [Google Scholar]
- 4.Condon C, Liveris D, Squires C, Schwartz I, Squires C L. rRNA operon multiplicity in Escherichia coli and the physiological implications of rrn inactivation. J Bacteriol. 1995;177:4152–4156. doi: 10.1128/jb.177.14.4152-4156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Condon C, Philips J, Fu Z-Y, Squires C, Squires C L. Comparison of the expression of the seven ribosomal RNA operons in Escherichia coli. EMBO J. 1992;11:4175–4185. doi: 10.1002/j.1460-2075.1992.tb05511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damgaard P H, Jacobsen C S, Soerensen J. Development and application of a primer set for specific detection of Bacillus thuringiensis and Bacillus cereus in soil using magnetic capture hybridization and PCR amplification. Syst Appl Microbiol. 1996;19:436–441. [Google Scholar]
- 7.Dammel C S, Noller H F. Suppression of a cold-sensitive mutation in 16S rRNA by overexpression of a novel ribosome-binding factor, RbfA. Genes Dev. 1995;9:626–637. doi: 10.1101/gad.9.5.626. [DOI] [PubMed] [Google Scholar]
- 8.Ehresmann C, Stiegler P, Fellner P, Ebel J P. The determination of the primary structure of the 16S ribosomal RNA of Escherichia coli. 2. Nucleotide sequences of products from partial enzymatic hydrolosis. Biochimie. 1972;54:901–967. doi: 10.1016/s0300-9084(72)80007-5. [DOI] [PubMed] [Google Scholar]
- 9.Francis K P, Mayr R, von Stetten F, Stewart G S A B, Scherer S. Discrimination of psychrotolerant and mesophilic strains of the Bacillus cereus group by PCR targeting of major cold shock protein genes. Appl Environ Microbiol. 1998;64:3525–3529. doi: 10.1128/aem.64.9.3525-3529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fröhlich J, Kahle D, König H. Isolation of single bacteria from mixed populations with the aid of a micromanipulator. Biospectrum (Sonderausgabe) 1998. p. 110. [Google Scholar]
- 11.Graumann P, Marahiel M A. Some like it cold: response of microorganisms to cold shock. Arch Microbiol. 1996;166:293–300. doi: 10.1007/s002030050386. [DOI] [PubMed] [Google Scholar]
- 12.Graumann P, Wendrich T M, Weber M H W, Schröder K, Marahiel M A. A family of cold shock proteins in Bacillus subtilis is essential for cellular growth and for efficient protein synthesis at optimal and low temperatures. Mol Microbiol. 1997;25:741–756. doi: 10.1046/j.1365-2958.1997.5121878.x. [DOI] [PubMed] [Google Scholar]
- 13.Graumann P, Schröder K, Schmid R, Marahiel M A. Cold shock stress-induced proteins in Bacillus subtilis. J Bacteriol. 1996;178:4611–4619. doi: 10.1128/jb.178.15.4611-4619.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green C J, Stewart G C, Hollis M A, Vold B S, Bott K F. Nucleotide sequence of the Bacillus subtilis ribosomal RNA operon, rrnB. Gene. 1985;37:261–266. doi: 10.1016/0378-1119(85)90281-1. [DOI] [PubMed] [Google Scholar]
- 15.Gualerzi C O, Pon C L. Initiation of mRNA translation in procaryotes. Biochemistry. 1990;29:5881–5889. doi: 10.1021/bi00477a001. [DOI] [PubMed] [Google Scholar]
- 16.Jinks-Robertson S, Nomura M. Ribosomes and tRNA. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 1358–1385. [Google Scholar]
- 17.Johansen T, Carlson C R, Kolsto A-B. Variable numbers of rRNA gene operons in Bacillus cereus strains. FEMS Microbiol Lett. 1996;136:325–328. doi: 10.1111/j.1574-6968.1996.tb08068.x. [DOI] [PubMed] [Google Scholar]
- 18.Jones P G, Inouye M. The cold-shock response—a hot topic. Mol Microbiol. 1994;11:811–818. doi: 10.1111/j.1365-2958.1994.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 19.Jones P G, Mitta M, Kim Y, Jiang W, Inouye M. Cold shock induces a major ribosomal-associated protein that unwinds double-stranded RNA in Escherichia coli. Proc Natl Acad Sci USA. 1996;93:76–80. doi: 10.1073/pnas.93.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan, T., B. M. Prüß, S. Lechner, K. Neuhaus, and S. Scherer. Low temperature adaptation of a psychrotolerant strain of the Bacillus cereus group involves amino acid utilization, methionine uptake and protein synthesis. Submitted for publication.
- 21.Lechner S, Mayr R, Francis K P, Prüß B M, Kaplan T, Wießner-Gunkel E, Stewart G S A B, Scherer S. Bacillus weihenstephanensis sp. nov. is a new psychrotolerant species of the Bacillus cereus group. Int J Syst Bacteriol. 1998;48:1373–1382. doi: 10.1099/00207713-48-4-1373. [DOI] [PubMed] [Google Scholar]
- 22.Lee S J, Xie A, Jiang W, Etchegaray J-P, Jones P G, Inouye M. Family of the major cold-shock protein, CspA CS7.4, of Escherichia coli, whose members show a high sequence similarity with the eukaryotic Y-box binding proteins. Mol Microbiol. 1994;11:833–839. doi: 10.1111/j.1365-2958.1994.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 23.Lodmell J S, Dahlberg A E. A conformational switch in Escherichia coli 16S ribosomal RNA during decoding messenger RNA. Science. 1997;277:1262–1267. doi: 10.1126/science.277.5330.1262. [DOI] [PubMed] [Google Scholar]
- 24.Mayr B, Kaplan T, Lechner S, Scherer S. Identification and purification of a family of dimeric major cold shock protein homologs from the psychrotrophic Bacillus cereus WSBC 10201. J Bacteriol. 1996;178:2916–2925. doi: 10.1128/jb.178.10.2916-2925.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayr R. Kältetolerante Bacillus-Arten in pasteurisierter Trinkmilch: Flora, Verderbsanalysen und Phagentypisierung von Bacillus cereus. Ph.D. dissertation. Freising-Weihenstephan, Germany: Technische Universität München; 1997. [Google Scholar]
- 26.Mayr R, Eppert I, Scherer S. Incidence and identification of psychrotrophic (7°C) Bacillus spp. in German HTST pasteurized milk. Milchwissenschaft, 1998;54:26–30. [Google Scholar]
- 27.Meer R R, Baker J, Bodyfelt F W, Griffiths M W. Psychrotrophic Bacillus ssp. in fluid milk products: a review. J Food Prot. 1991;54:969–979. doi: 10.4315/0362-028X-54.12.969. [DOI] [PubMed] [Google Scholar]
- 28.Moran C P, Jr, Bott K F. Organization of transfer and ribosomal ribonucleic acid genes in Bacillus subtilis. J Bacteriol. 1979;140:742–744. doi: 10.1128/jb.140.2.742-744.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 30.Stern S, Powers T, Changchien L-M, Noller H F. RNA-protein interactions in 30S ribosomal subunits: folding and function of 16S rRNA. Science. 1989;244:783–790. doi: 10.1126/science.2658053. [DOI] [PubMed] [Google Scholar]
- 31.Stewart G C, Wilson F E, Bott K F. Detailed physical mapping of the ribosomal RNA genes of Bacillus subtilis. Gene. 1982;19:153–162. doi: 10.1016/0378-1119(82)90001-4. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki T, Yamasato K. Phylogeny of spore-forming lactic acid bacteria based on 16S rRNA gene sequences. FEMS Microbiol Lett. 1994;115:13–17. doi: 10.1111/j.1574-6968.1994.tb06607.x. [DOI] [PubMed] [Google Scholar]
- 33.Thieringer H A, Jones P G, Inouye M. Cold shock and adaptation. Bioessays. 1998;20:49–57. doi: 10.1002/(SICI)1521-1878(199801)20:1<49::AID-BIES8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 34.Toone W M, Rudd K E, Friesen J D. deaD, a new Escherichia coli gene encoding a presumed ATP-dependent RNA helicase, can suppress a mutation in rpsB, the gene encoding ribosomal protein S2. J Bacteriol. 1991;173:3291–3302. doi: 10.1128/jb.173.11.3291-3302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Bogelen R A, Neidhardt F C. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc Natl Acad Sci USA. 1990;87:5589–5593. doi: 10.1073/pnas.87.15.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Stetten F, Francis K P, Lechner S, Neuhaus K, Scherer S. Rapid discrimination of psychrotolerant and mesophilic strains of the Bacillus cereus group by PCR targeting of 16S rDNA. J Microbiol Methods. 1998;34:99–106. [Google Scholar]
- 37.Widom R L, Jarvis E D, LaFauci G, Rudner R. Instability of rRNA operons in Bacillus subtilis. J Bacteriol. 1988;170:605–610. doi: 10.1128/jb.170.2.605-610.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiebe C, Hammer P. Zum Vorkommen von B. cereus in einem Milchtrocknungsbetrieb—Phänotypische Charakterisierung der isolierten Stämme. Presented at the Milchkonferenz, Deutsche Gesellschaft für Milchwissenschaft. 1997. [Google Scholar]
- 39.Yamanaka K, Fang L, Inouye M. The CspA family in Escherichia coli: multiple gene duplication for stress adaptation. Mol Microbiol. 1998;27:247–255. doi: 10.1046/j.1365-2958.1998.00683.x. [DOI] [PubMed] [Google Scholar]