Abstract
Space travelers are exposed to microgravity (µg), which induces enhanced bone loss compared to the age-related bone loss on Earth. Microgravity promotes an increased bone turnover, and this obstructs space exploration. This bone loss can be slowed down by exercise on treadmills or resistive apparatus. The objective of this systematic review is to provide a current overview of the state of the art of the field of bone loss in space and possible treatment options thereof. A total of 482 unique studies were searched through PubMed and Scopus, and 37 studies met the eligibility criteria. The studies showed that, despite increased bone formation during µg, the increase in bone resorption was greater. Different types of exercise and pharmacological treatments with bisphosphonates, RANKL antibody (receptor activator of nuclear factor κβ ligand antibody), proteasome inhibitor, pan-caspase inhibitor, and interleukin-6 monoclonal antibody decrease bone resorption and promote bone formation. Additionally, recombinant irisin, cell-free fat extract, cyclic mechanical stretch-treated bone mesenchymal stem cell-derived exosomes, and strontium-containing hydroxyapatite nanoparticles also show some positive effects on bone loss.
Keywords: spaceflight, microgravity, astronauts, cells, animals, bone loss, signaling pathways, countermeasures, pharmacology
1. Introduction
The length of a space mission and, thus, the amount of time space pilots are spending in orbit have been increased since humans have started conquering space. Space travelers are affected by numerous stressors in space, which include psychological factors such as isolation, psychosocial factors such as family disruption, human factors such as food restrictions, habitability factors such as lack of privacy, and physical factors such as radiation and microgravity (µg) [1,2].
Microgravity is known to induce changes and health problems in the human body, such as cardiovascular, immune, or musculoskeletal health concerns [3,4,5]. One of the reasons for the problems in the musculoskeletal system is mechanical unloading caused by µg. In addition, µg leads to skeletal muscle atrophy and bone loss (BL), among other effects. BL can be seen as a reduction in bone mineral density (BMD). The µg-induced reduction in BMD occurs at a fast rate of 0.5–1.5% per month in space [5,6,7].
Therefore, a systematic review was conducted to investigate to what extent this phenomenon resembles postmenopausal (PMP) osteoporosis (OP) on Earth, keeping in mind that the main cause/mechanism of postmenopausal osteoporosis is different from the reduction in BMD in µg. For this purpose, recent findings on the mechanisms of µg-related changes in bone density and possible treatment options are discussed. Furthermore, clinical trials are presented to complement in vitro with in vivo data. Overall, this review provides a current overview of the state of the art of the field of BL in space and also discusses possible implications for OP patients on Earth.
1.1. Postmenopausal Osteoporosis
The OP diagnostic criterion is a T-score ≤ −2.5 standard deviation below the mean for young healthy adults at the femoral neck, lumbar spine, or total hip by BMD testing [8,9,10]. PMP OP is known as primary OP type I and is caused most likely by reduced estrogen levels, which eventually lead to increased bone resorption (BRS) [8]. The bone mass reaches its maximum, named the peak bone mass, at the end of the bone maturation process around the age of 28–30 years. It is particularly dependent on a sufficient calcium intake, adequate vitamin D intake, genetics, and physical activity [11,12,13].
The increased BRS is closely related to hormonal changes, especially to the decline in the endogenous estrogen production at menopause. Estrogen has an inhibitory effect on osteoclasts (OCs); therefore, the deprivation of estrogen is associated with the loss of BMD. Deprivation of estrogen also contributes to increased urinary calcium loss and reduced intestinal calcium absorption, which is caused by calcium influx from the estrogen-deprived bones into the plasma and eventually results in reduced levels of parathyroid hormone (PTH) [14,15].
Following the European guidelines and the guidelines of the American Association of Clinical Endocrinologists (AACE), the treatment of OP can be divided into nonpharmacological and pharmacological strategies. The nonpharmacological strategy consists of lowering the risk of falling and optimizing the dietary intake. The risk of falling is lowered by physical exercise, reduction in consumption of alertness- and balance-altering medication, and home environment improvement, among others, while optimizing the dietary intake is achieved by ensuring that the patient consumes enough protein and calories, as well as calcium and vitamin D, which can be achieved with supplements [10,16]. A diet with high potential renal acid load might also increase urinary calcium excretion, and a diet high in alkaline precursors might reduce urinary calcium excretion (uCaV) and urinary N-telopeptide (uNTx), while increasing femoral neck, lumbar spine, and total hip BMD. In spaceflight, the effect depends very much on the exercise level/mechanical loading [17,18].
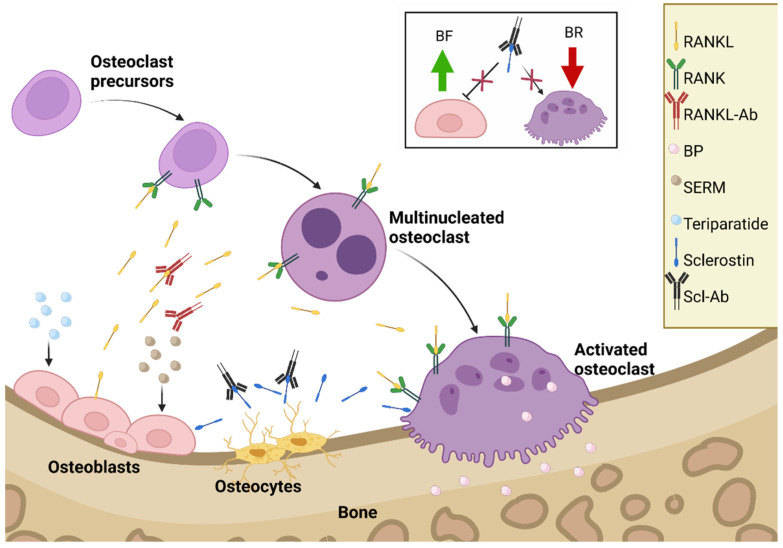
Pharmacological treatment can be divided into antiresorptive (bisphosphonate (BP)), receptor activator of nuclear factor κβ ligand (RANKL) antibody, selective estrogen receptor modulator (SERM), antisclerostin antibody, and anabolic medication (see Figure 1) [10,16,19].
Figure 1.
The treatment of menopausal osteoporosis. Abbreviations: RANKL, receptor activator of nuclear factor κβ ligand; SERMS, selective estrogen receptor modulator; Scl-Ab, antisclerostin antibody [10,16,19]. The insert shows the Scl-Ab’s dual mode of action in more detail. Created with BioRender.com (accessed on 27 July 2022).
SERMs, such as raloxifene, reduce BL by binding to the estrogen receptor, where they act as an estrogen agonist or antagonist, depending on the tissue. BPs, such as alendronate, reduce BL by reducing OC recruitment and activity and increasing the apoptosis of OCs. RANKL antibodies, such as denosumab, reduce BL by binding to RANKL, which prevents OC activation. Teriparatide is an anabolic medication. It is the 1–34 N-terminal fragment of PTH; it induces bone formation (BF) and improves the skeletal architecture by increasing the activity and number of osteoblasts (OBs) [10,16].
Sclerostin, synthesized in osteocytes, inhibits bone formation. It is responsible to keep the balance between new bone tissue formation and resorption of old bone tissue. It exerts antianabolic effects on bone. The protein sclerostin binds to LRP5/6 receptors and inhibits wingless-related integration site (Wnt) signaling, which results in a reduction in bone formation [20]. Targeting sclerostin is important because of the protein’s special antianabolic action on bone formation. The humanized monoclonal antibody romosozumab (brand name Evenity), indicated for osteoporosis to reduce the risk of fractures, was approved by the Food and Drug Administration for use in the USA in April 2019 and in the European Union in December 2019. Recent rodent data showed that romosozumab and abaloparatide have additive effects when used as a countermeasure against disuse osteopenia in female rats [19].
Romosozumab exhibits a dual mode of action. Firstly, it enhances bone formation; secondly, it inhibits bone resorption. This mechanism is shown in Figure 1. The development of romosozumab offers a safe and efficacious new drug to treat osteoporosis in postmenopausal women and men. In addition, the drug may be interesting to be tested in space travelers [21].
1.2. Microgravity-Induced Bone Loss
The decreased mechanical loading of weight-bearing bones caused by µg in space leads to BL in humans after more than 4 months of space travel [6,7]. This is a result of increased BRS and unchanged or decreased BF, which is seen in different human studies both in space and during bed rest [22,23,24] (see Figure 2). During the first 2 weeks of spaceflight, the BRS is especially exacerbated in humans [25,26,27]. Microgravity releases calcium from bone in humans, which suppresses PTH and lowers circulating 1,25-dihydroxyvitamin D, although 25-dihydroxyvitamin D concentrations are still adequate. This leads to decreased calcium absorption in humans [24,26,27,28]. The decreased BF is found to be a consequence of impaired OB function and increased osteocyte apoptosis which was detected in both in vivo and vitro studies, as well as in a study focusing on rats [5,29,30,31]. The latter used a total of 50 4-week-old male Wistar rats, which were not suspended (n = 25) or suspended by the tail for 2, 4, and 7 days (n = 25 total). The right tibia metaphyses were used for histomorphometric analysis, the right femurs were used for TUNEL assays, and the metaphyseal area in left femurs was used for Western blot and immunoprecipitation analyses. The authors could show that, in the samples, components of the antiapoptotic pathway were downregulated during unloading. These findings contribute to µg-induced BL of 0.5–1.5% every month in humans [6,7,22].
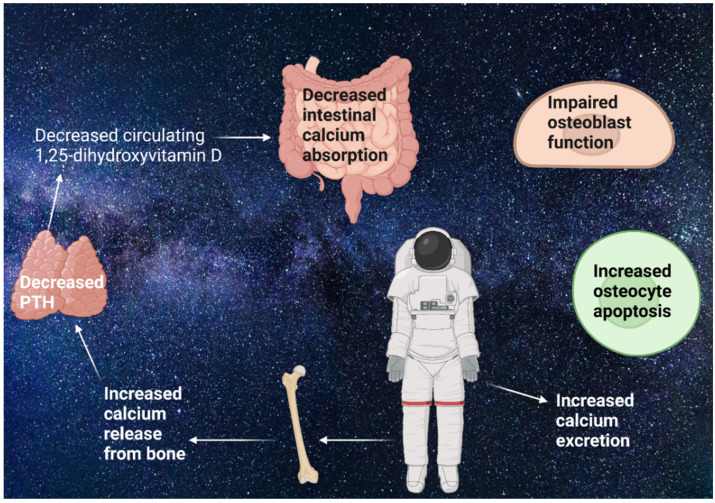
Figure 2.
Mechanisms of microgravity (µg)-induced bone loss: µg releases calcium from bone, which suppresses the parathyroid hormone (PTH). Afterward, the suppressed PTH then lowers the circulating 1,25-dihydroxyvitamin D. This leads to decreased calcium absorption [24,26,27,28]. Additionally, osteoblast function is impaired, and osteocyte apoptosis is increased [5,32]. This results in unchanged or decreased bone formation and increased bone resorption, which leads to bone loss [22,23,24]. Created with BioRender.com (accessed on 27 July 2022).
Because of the harmful effects induced on the human organ system by µg, it is necessary for a successful space mission to develop countermeasures (CMs) against the µg-induced changes [33]. The CMs can be divided into (1) preflight CMs, which include physical exercise and physiological adaptation training, (2) inflight CMs, which include physical exercise, sensory–motor training, pharmaceuticals, and nutritional health, and (3) postflight CMs, which include rehabilitation [34]. The Advanced resistive exercise device (ARED), a cycle ergometer, and treadmill exercise are current inflight CMs, routinely used directly against µg-induced BL [35]. The crewmembers on the International Space Station (ISS) are prescribed to do 2.5 h of exercise of personal preference per day for 6 days a week [36].
The ARED on board the ISS uses vacuum cylinders, making it effective in the µg environment. It can provide up to 272 kg of concentric resistance, with a constant load through the range of motion of the body; during the eccentric phase, the ARED provides ~90% of the concentric load. Additionally, the ARED replicates the inertial characteristics normally experienced during gravity on Earth [37].
Crewmembers of the ISS can use the treadmill by a subject-loading device to fix themselves to the device. The subject loading makes the crewmembers able to exercise partially loaded, where the typical load is ~70%. The cycle ergometers have clipless pedals to fix the feet to the device and can provide up to 350 W load [36].
Furthermore, different drugs promoting bone formation have been studied. These so-called exercise pills include urolithin A and kartogenin. Urolithin A increased the exercise capacity and counteracted the decline of muscle function caused by age in rodents. Kartogenin was effective in promoting the differentiation of chondrocytes and the repair of cartilage in an in vitro study [38,39].
2. Materials and Methods
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [40].
2.1. Eligibility Criteria
The inclusion criteria were defined by the PICO parameters, which include population, intervention, comparison, and outcome [41].
The inclusion criteria were humans ≥18 years old, animal models, or cell cultures involved in BRS or BF. This population needed to be exposed to the intervention of either µg or µg analogs, or possible CMs against µg-induced BL. The population exposed to the interventions needed to be compared to healthy individuals, controls, or baseline. The controls included human, animals, and cells involved in BRS or BF.
The outcome needed to be either mechanism of µg-induced BL or the effect of possible CMs against µg-induced BL. The studies were not eligible if written in languages other than English or Danish, published before 1 January 2016, involving human individuals < 18 years old, or a systematic review, meta-analysis, or case report.
2.2. Information Sources
The literature search for this review was performed on the online repositories of PubMed (pubmed.ncbi.nlm.nih.gov) and Scopus (scopus.com), and the latest search was conducted on 28 March 2022.
2.3. Search
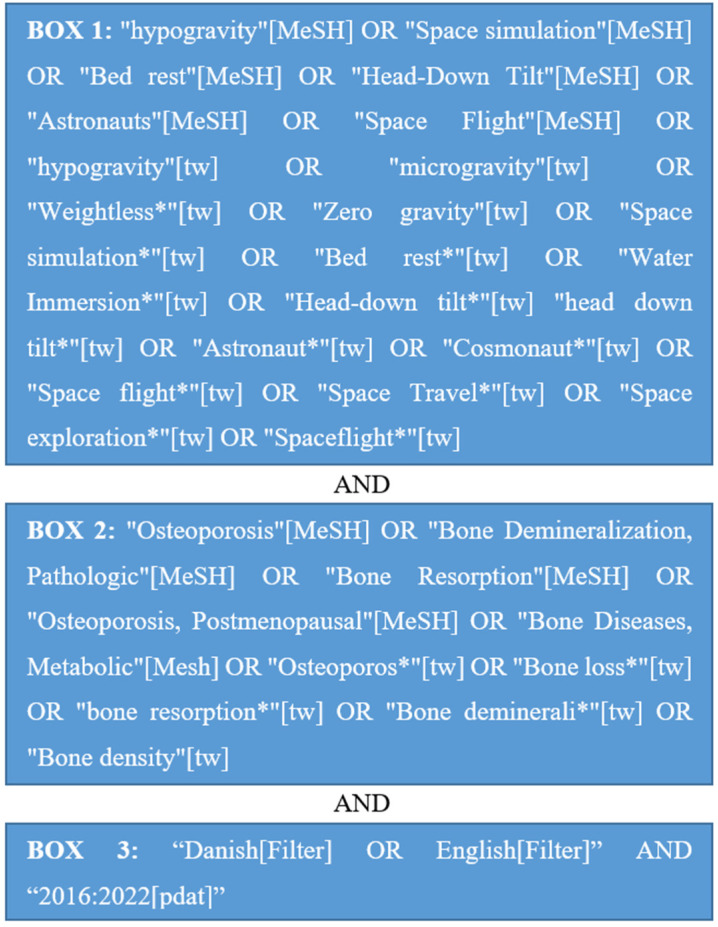
Through a preliminary search in PubMed, relevant search terms were found as illustrated in Figure 3. Each search term in box 1 was combined with each search term in box 2 and all the filters in box 3 in several searches on 28 March 2022.
Figure 3.
Search terms in PubMed.
2.4. Study Selection
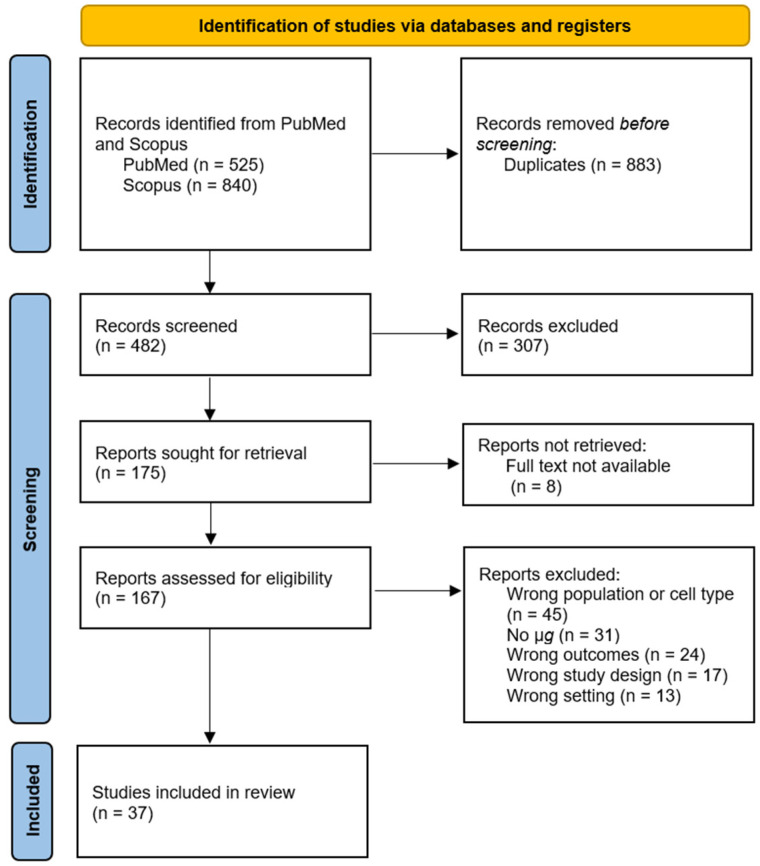
The literature search resulted in a total of 1365 studies of which 883 were duplicates, leaving 482 studies. The reason for the duplicates is that each search term in box 1 of Figure 3 was combined with each search term in box 2 in several searches. After title and abstract screening for eligibility criteria, there were 307 studies excluded, leaving 175 studies. It was not possible to retrieve eight studies; thus, 167 studies remained. The full articles were screened for the eligibility criteria and their relevance to the objectives of this systematic review, which yielded 37 studies (see individual reasons for exclusion in the flowchart in Figure 4).
Figure 4.
Flowchart of study selection.
2.5. Data Collection Process
The author of this review developed a data extraction sheet, which was pilot-tested on three of the studies in case of any missing or redundant data. This was applied singlehandedly by the author of this review.
2.6. Data Items
The data obtained from the studies were as follows: first author, year of publication, population characteristics (number of participants, age, and gender), intervention characteristics (µg/µg analog and possible CMs), and outcome of either mechanism of µg-related changes in bone density or the effect of possible CMs for µg-related changes in bone density.
2.7. Risk of Bias in Individual Studies
Studies in space are not only affected by µg, but also affected by radiation, vibration during the launch from and return to Earth, and other environmental stressors in space, which also have the potential of inducing BL. This point cannot be excluded and may have impact on the findings. Low numbers of participants in space studies are also a problem since it makes it difficult to generalize the findings. Because of the risk of bias, these items were kept in mind when searching for studies.
2.8. Summary Measures
The outcomes measured were changes in BMD and other bone characteristics, as well as markers of bone cell activity.
2.9. Risk of Bias across Studies
Since the literature search only included published studies in English and Danish, there was a risk of both language bias and publication bias. These types of bias were kept in mind when searching for studies.
3. Results
3.1. Study Selection
The selection of the studies resulted in 37 studies as described in detail in Section 2.4. and in Figure 4.
3.2. Study Characteristics and Results of Individual Studies
An overview of the results of the mechanisms of µg-related changes in bone density are provided in Table 1, while the results of the possible CMs are given in Table 2.
Table 1.
The mechanisms of µg-related changes in bone density.
| Author and Publication Year | Population | Intervention | Outcome |
|---|---|---|---|
| Real microgravity in humans | |||
| Bilancio et al., 2019 [42] | 1 male (52 years) and 1 female (37 years) | 6 months on ISS | Compared to BSL: Decrease of 0.9 to 1.4% in total BMD; Increase of 27 to 116% in urine calcium/creatinine ratio. |
| Burkhart et al., 2020 [43] | 1 female and 16 males (45 ± 4 years) | 4–7 months on ISS | Between 4.6% and 6.1% decline in vertebral volumetric BMD (p < 0.05) compared to preflight. Vertebral volumetric BMD decreased 1% and lumbar spine BMD decreased 0.5% per month. |
| Sibonga et al., 2020 [44] | 10 humans (gender N/A, 48 ± 5 years) | 157 ± 21 days on ISS | 5 of 10 astronauts had an incomplete recovery of BMD 1 year after return to Earth, which persisted in 4 of 5 astronauts after 2 years compared to BSL. |
| Real microgravity in animals | |||
| Berg-Johansen et al., 2016 [45] | 14 male C57BL/6 mice (19–20 weeks) | SG (n = 6): 30 days in low Earth orbit CG (n = 8): normal gravity |
SG showed 20% reduced BV/TV (p < 0.05), 18% reduced BMD (p < 0.05), and 14% reduced trabecular thickness (p = 0.001) compared to CG. No significant difference in the trabecular number or trabecular spacing. |
| Gerbaix et al., 2017 [46] | 22 C57/BL6 male mice (2 months) | SG (n = 10): 30 days in space CG (n = 12): normal gravity |
SG decreased femur BV 64% and vertebrae BV 35.7%. SG increased bone resorption 140% and empty lacunae 344%. No bone recovery in SG 8 days after landing despite normalized OC activity. |
| Chatani et al., 2016 [47] | Medaka fish larvae in stage 39 | SG (n = 3–9, depending on the anaylsis): 8 days on the ISS CG (n = 3–16, depending on the anaylsis): normal gravity |
SG had significantly enhanced osterix, osteocalcin, TRAP5, and matrix metallopeptidase-9. |
| Von Kroge et al., 2021 [48] | C57BL/6N male mice (8–9 weeks) | SG (n = 5): 4 weeks in space CG (n = 5): normal gravity |
After 4 weeks in space, BV/TV, cortical thickness, trabecular number, and thickness significantly decreased. This bone loss was only recovered in trabecular bone, and not in cortical thickness. |
| Simulated microgravity in humans | |||
| Bonnefoy et al., 2022 [49] | 20 male (34 ± 8 years) | SG: 60 days HDBR + antioxidant CG: 60 days HDBR |
Compared to BSL: Decreased bone density of 1.4% (p < 0.0001) and BV/TV of 1% (p < 0.05). |
| Bemben et al., 2021 [50] | 6 males and 5 females (25–50 years) | 30 d HDBR | Compared to BSL: Increase in sclerostin, TRAP5, P1NP, and calcium. Decrease in total hip BMD and PTH. Women had a greater decrease in total hip BMD and increase in TRAP5 than men. |
| Buehlmeier et al., 2017 [51] | 24 males (SG1 + SG2 ~60 years, SG3 ~23 years) | SG1 (n = 8): 14 days bed rest SG2 (n = 8): 14 days bed rest + CD SG3 (n = 7): 14 days bed rest |
Urinary N-telopeptide of type I collagen (p < 0.05), urinary C-telopeptide of type I collagen (p = N/A), and sclerostin (p < 0.05) increased during bed rest in all groups compared to BSL. |
| Simulated microgravity in animals | |||
| Cabahug-Zuckerman et al., 2016 [52] | 24 C57BL/6J male mice (4 months) | SG1 (n = 6): 14 days HLU SG2 (n = 6): 14 days HLU + PC-I SG3 (n = 6): PC-I CG (n = 6): normal gravity |
After 5 days: Increased RANKL-producing osteocytes and osteocyte apoptosis yjtrrfold in cortical bone and fourfold in trabecular SG1 compared to CG (p < 0.05). After 14 days: Only the increased osteocyte apoptosis in trabecular bone and RANKL-producing osteocytes remained significantly elevated threefold in SG1 compared to CG. |
| Chowdhury et al., 2016 [53] | 61 male rats (3–18 rats per SG and CG, age N/A) | SG1: 2 weeks HLU SG2: 2 weeks X-ray IR SG3: 2 weeks HLU + X-ray IR CG: normal gravity |
Significantly decreased BMD in distal femur and proximal tibia in SG1 and SG3 compared to CG (p < 0.05), but BMD was not significantly decreased in SG2. |
| Chen et al., 2021 [54] | 12 C57/BL6 mice (2 months) | SG (n = 6): 4 weeks HLU CG (n = 6): normal gravity |
MicroRNA-138-5p was upregulated during s-µg in SG compared to CG (p < 0.01). |
| Simulated microgravity in vitro | |||
| Chen et al., 2021 [54] | Murine pre-OB cells MC3T3-E1 | SG: 12 h RPM CG: normal gravity |
Compared to CG: MicroRNA-138-5p decreased ALP 93.1% and collagen type 1 α-1 64.9% in SG (p < 0.01). MicroRNA-138-5p decreased protein and mRNA expression of β-catenin in SG. |
| Cazzaniga et al., 2016 [55] | Human bMSCs | SG: 4 days RPM CG: normal gravity |
SG show a significant upregulation of heat shock protein 60, heat-shock protein 70, superoxide dismutase 2 and cylclooxygenase 2, and a significant increase in RUNX2 and osterix compared to CG. |
| Chen et al., 2016 [56] | Rat bMSCs | SG: 2 days clinostat CG: normal gravity |
Clinorotation significantly depolymerizes F-actin, and this hinders “transcriptional coactivator with PDZ-binding motif” nuclear. Furthermore, s-µg inhibited ALP and RUNX2. |
| Zhang et al., 2020 [57] | Murine pre-OB MC3T3-E1 | SG: 72 h clinorotation CG: normal gravity |
Expression of all but microRNA-30a in the microRNA-30 family is upregulated in s-µg. This is negatively correlated with expression of RUNX2, osteocalcin and ALP, which decreased during s-µg. |
Abbreviations: ISS: International Space Station, BSL: baseline, BMD; bone mineral density, N/A: not available, SG: study group, n: number of individuals, CG: control group, BV/TV: bone volume fraction, BV: bone volume OC: osteoclast, TRAP5: tartrate-resistant acid phosphatase 5b, HDBR: head-down tilt bed rest, P1NP: N-terminal propeptide of type 1 procollagen, PTH: parathyroid hormone, CD: cognitive training, and diet protein and alkaline, HLU: hindlimb-unloading, PC-I: pan-caspase inhibitor, RANKL: receptor activator of nuclear factor κβ ligand, IR: irradiation, s-µg: simulated microgravity, OB: osteoblast, RPM: random positioning machine, ALP: alkaline phosphatase, bMSC: bone mesenchymal stem cell, RUNX2: RUNX family transcription factor 2.
Table 2.
Possible CMs for µg-induced bone loss and their effects.
| Author and Publication Year | Population | Intervention | Outcome |
|---|---|---|---|
| Real microgravity in vitro | |||
| Colucci et al., 2020 [58] | System 1: OC/OB System 2: OC/EC System 3: OC/OB/EC |
SG1: 14 days ISS + R-irisin SG2: 14 days ISS CG1: normal gravity CG2: normal gravity + R-irisin |
R-irisin in SG1 prevented the downregulation of RUNX2, osterix, activating transcription factor 4, osteoprotegerin, and Collα1 caused by microgravity. |
| Cristofaro et al., 2019 [59] | Human bMSCs | SG: 88 h ISS + SCHN CG: normal gravity |
SCHN exhibited a protective effect in SG on the reduced ALP activity caused by microgravity compared to CG (p < 0.05). |
| Simulated microgravity in humans | |||
| Cavanagh et al., 2016 [60] | 6 male and 6 female (30.2 ± 6.8 years) | SG (n = 6): 84 days HDBR + LE CG (n = 6): 84 days HDBR |
BMD in the intertrochanteric and total hip regions was decreased in CG, but not in SG compared to baseline. The BMD loss was higher in CG than in SG. |
| Belavý et al., 2016 [61] | 24 males (32 ± 10.6 years) | SG1 (n = 7): 60 days BR + vibration RE SG2 (n = 8): 60 days BR + RE CG (n = 9): 60 days BR |
In SG1 and SG2 bone-specific ALP increased significantly more than in CG. SG1 also showed a greater proximal femur bone mineral content 6–24 months after BR compared to CG (p = 0.01). There was no significant difference on sclerostin and dickkopf-1 proteins. |
| Gao et al., 2019 [62] | 2 males and 4 females (30 ± 12 years) | Cross-over study: 4 days of BR with either PBD or hospital diet separated by 30 days | The PBD attenuated the increase of urinary N-telopeptide of type I collagen to 33% ± 20% from 89% ± 75% during hospital diet. PBD had no effect on BMD compared to hospital diet. |
| Buehlmeier et al., 2017 [51] | 24 males (SG1 + SG2 ~60 years, SG3 ~23 years) | SG1 (n = 8): 14 days BR SG2 (n = 8): 14 days BR + CD SG3 (n = 8): 14 days BR |
No systematic difference between the SGs. |
| Austermann et al., 2021 [63] | 20 males (3 ± 8 years) | SG (n = 10): 60 days HDBR + antioxidant CG (n = 10): 60 days HDBR |
Antioxidant supplement had no effect on bone resorption or formation. |
| Simulated microgravity in animals | |||
| Cabahug-Zuckerman et al., 2016 [52] | 24 C57BL/6J male mice (4 months) | SG1 (n = 6): 14 days HLU SG2 (n = 6): 14 days HLU + PC-I SG3 (n = 6): PC-I CG (n = 6): no intervention |
The PC-I used in SG2 prevented the HLU-induced increase in osteocyte apoptosis, osteocyte RANKL expression, and endocortical resorption in both cortical and trabecular bone compared to SG1. |
| Colaianni et al., 2017 [64] | 32 C57BL6 male mice (2 months) | SG1 (n = 8): 4 weeks HLU + R-irisin SG2 (n = 8): 4 weeks HLU SG3 (n = 8): R-irisin CG (n = 8): vehicle injection |
Compared to CG: R-irisin prevented loss of cortical or trabecular BMD in SG1. R-irisin induced the recovery of bone mass through attenuation of osteoprotegerin; thus, the RANKL/osteoprotegerin ratio was the same in SG1 as CG. R-irisin also inhibited the decrease in ALP and Collα1 mRNA expression caused by simulated microgravity in SG1. |
| Ding et al., 2021 [65] | 42 C57BL/6J male mice (12 weeks) | SG1 (n = 6): 28 days HLU + alendronate SG2 (n = 6): 28 days HLU + raloxifene SG3 (n = 6): 28 days HLU + teriparatide SG4 (n = 6): 28 days HLU + Anti-RANKL SG5 (n = 6): 28 days HLU + bortezomib CG1 (n = 6): normal gravity CG2 (n = 6): 28 days HLU |
SG1 and SG4 reduced urinary C-telopeptide of type I collagen compared to CG2 (p < 0.05) and restored BMD close to CG1. SG5 reduced urinary C-telopeptide of type I collagen (p < 0.05) and enhanced P1NP compared to CG2 (p < 0.05), which increased BMD and strength compared to CG2 (p < 0.05). SG2 had no effect on bone loss. SG3 only stimulated cortical bone formation. |
| Han et al., 2018 [66] | 48 WTC57BL/6J mice (2–3 months) 32 KOC mice (2–3 months) |
SG1 (n = 16): 4 weeks HLU + CDL SG2 (n = 16): 4 weeks HLU + KOC SG3 (n = 16): 4 weeks HLU + CDL + KOC CG1 (n = 16): 4 weeks HLU CG2 (n = 16): normal gravity |
Increased BV/TV, trabecular number and thickness, ALP activity, osteocalcin content, and mRNA level of bone morphogenetic protein-2, Collα1, ALP, and osteocalcin in SG1 compared to CG1, but this effect was even bigger in SG3 with combined CDL and KOC. Osteoprotegerin increased, while RANKL level decreased in SG3 compared to CG1; however, in SG1, only osteoprotegerin increased compared to CG1. |
| DeLong et al., 2020 [67] | 35 C57BL/6J male mice (16 weeks) | SG (n = 15): 3 weeks HLU + TC CG1 (n = 10): 3 weeks HLU CG2 (n = 10): 3 weeks TC |
In SG, a 2% loss in cortical thickness and 15% loss in trabecular BV/TV was observed compared to 6% and 50% corresponding losses in CG1. This protective effect did not influence the cortical bone at lower strained distal shaft. |
| Yang et al., 2021 [68] | 36 C57BL/6J male mice (8 weeks) | SG (n = 6): 4 weeks HLU + SMF CG1 (n = 6): 4 weeks GMF CG2 (n = 6): 4 weeks SMF CG3 (n = 6): 4 weeks HLU CG4 (n = 6): 4 weeks HLU + GMF |
SG had an increased BV/TV, trabecular number, connectivity density, cortical area, and femoral bone mineral content compared to CG4 (p < 0.05). Additionally, TRAP5 decreased during SMF compared to CG4 (p < 0.05). |
| Xu et al., 2022 [69] | 18 C57BL/6 male mice (8 weeks) | SG (n = 6): 4 weeks HLU + CEFFE CG1 (n = 6): normal gravity CG2 (n = 6): 4 weeks HLU + PBS |
Compared to CG2: CEFFE in SG increased BV/TV and trabecular number and cortical thickness. Additionally, the number of empty lacunae was reduced by CEFFE. |
| Xiao et al., 2021 [70] | 40 C57BL/6J male mice (6 months) | SG1 (n = 10): 28 dats HLU + CMS SG2 (n = 10): 28 dats HLU + STE CG1 (n = 10): normal gravity + PBS CG2 (n = 10): 28 days HLU + PBS |
Compared to CG2: CMS increased the BMD, BV/TV, cortical thickness, and trabecular thickness and number. CMS decreased trabecular spacing, number of OCs per field, and percentage of OC surface per bone surface. STE only increased BV/TV, as well as trabecular thickness and number, and decreased trabecular spacing. |
| Wakabayashi et al., 2020 [71] | 32 ddY male mice (8 weeks) | SG1 (n = 8): 4 weeks HLU + IL-6 mAb SG2 (n = 8): 4 weeks HLU + alendronate CG1 (n = 8): normal gravity + vehicle CG2 (n = 8): 4 weeks HLU + vehicle |
Compared to CG2: IL-6 mAb in SG1 reduced number of OCs per bone perimeter compared to CG2 (p < 0.05). Alendronate in SG2 increased BV/TV and trabecular number, while decreasing number of OCs per bone perimeter in both femur and tibia (p < 0.05). |
| Diao et al., 2018 [72] | 50 SD male rats (6 weeks) | SG1 (n = 30): 30 days HLU + polyphenol SG2 (n = 10): 30 days HLU CG (n = 10): normal gravity |
SG1 compared to SG2: SG1 alleviated the rise of bone surface/bone volume ratio and decreased ME, BMD, and BV/TV, but it differed from CG. SG1 increased ALP, P1NP, and expression of RUNX2, Collα1, ALP, osteonectin, osterix, osteocalcin, and β-catenin. |
| He et al., 2020 [73] | 32 C57BL/6J male mice (10 weeks) | SG1 (n = 8): 4 weeks HLU SG2 (n = 8): 4 weeks HLU + IL-6 mAb CG1 (n = 8): normal gravity CG2 (n = 8): normal gravity + IL-6 mAb |
Increased BMD, BV/TV, trabecular thickness, trabecular number, stiffness, and ultimate load in femur in SG2 compared to SG1 (p < 0.05). Serum osteocalcin and mRNA expression of ALP, osteocalcin and TRAP5 increased, while RANKL/osteoprotegerin ratio decreased in SG2 compared to SG1 (p < 0.05). All these factors were normalized compared to CG1. |
| Khajuria et al., 2015 [74] | 30 male Wistar rats (12 weeks) | SG1 (n = 6): 20 weeks RHLI SG2 (n = 6): 10 weeks RHLI+ 10 weeks RHLI/ZOL SG3 (n = 6): 10 weeks RHLI+ 10 weeks RHLI/ALF SG4 (n = 6): 10 weeks RHLI+ 10 weeks RHLI/ALF/ZOL CG (n = 6): nonimmobilized control |
The combination of ZOL + ALF was more effective in decreasing bone porosity, in improving the mechanical strength of the femoral midshaft, and in improving dry bone and ash weights than the respective monotherapies. |
| Simulated microgravity in vitro | |||
| Cristofaro et al., 2019 [59] | Human bMSCs | SG: 88 h RPM + SCHN CG: normal gravity |
SCHN had a promoting effect in SG on the deposition of hydroxyapatite crystals compared to CG (p < 0.05). |
| Braveboy-Wagner et al., 2022 [75] | 7F2 murine OBs | SG: 6 days RPM + nutraceuticals (curcumin, carnosic acid, and zinc) CG: 6 days RPM |
Compared to CG: In SG, ALP activity was elevated 160% by 50 µm zinc, 140% by 7.5 µm curcumin, and 113% by 10 µm carsonic acid. SG had an induced expression of ALP, RUNX2, and osteonectin in nonosteogenic maintenance medium. |
| Chen et al., 2020 [76] | Murine primary OBs | SG1: 48 h RPM + R-irisin SG2: 48 h RPM CG: normal gravity |
Lower doses of R-irisin promote both expression of Collα1 and ALP (p < 0.05), activity of ALP, and calcium deposition in OBs. R-irisin also recovered the microgravity-induced reduction of ALP and Collα1 (p < 0.001), ALP activity, and β-catenin expression. |
| Ethiraj et al., 2020 [77] | RAW264.7 pre-OCs | SG1: 24 h RCCS + MG-132 SG2: 24 h RCCS CG1: normal gravity CG2: normal gravity + MG-132 |
The proteasome inhibitor MG-132 in SG1 suppressed receptor activator of nuclear factor κβ receptor expression, compared to CG. MG-132 treatment in SG1 also showed a significant decrease in resorbed bone area compared to SG2. |
| Diao et al., 2018 [72] | OBs from newborn rat | SG1: 72 h RPM + polyphenols SG2: 72 h RPM CG: normal gravity |
Polyphenols promoted ALP activity in SG1 compared to SG2 (p < 0.01). Polyphenols had a dose–effect response, but were still decreased compared to CG (p < 0.05). |
| He et al., 2020 [73] | Murine pre-OB cell line MC3T3-E1 | SG1: 96 h RB SG2: 96 h RB + IL-6 mAb CG: normal gravity |
IL-6 mAb increased ALP activity, osteoprotegerin level, and mRNA expression of ALP, osteopontin and RUNX2, while RANKL decreased in SG2 compared to SG1 (p < 0.05). All these factors were normalized compared to CG. |
| He et al., 2020 [73] | Macrophage cell line RAW264.7 | SG1: 96 h RB SG2: 96 h RB + IL-6 mAb CG: normal gravity |
IL-6 mAb decreased mRNA expression of cathepsin K and TRAP5 in SG2 compared to SG1 (p < 0.05). All these factors were normalized compared to CG. |
Abbreviations: OC: osteoclast, OB: osteoblast, EC: endothelial cell, SG: study group, ISS: International Space Station, R-irisin: recombinant irisin, CG: control group, RUNX2: RUNX family transcription factor 2, Collα1: collagen type 1 α-1, bMSC: bone mesenchymal stem cell, SCHN: strontium-containing hydroxypatitie nanoparticles, ALP: alkaline phosphatase, n: number of individuals, HDBR: head-down tilt bed rest, LE: locomotor exercise, BMD; bone mineral density, BR: bed rest, RE: resistive exercise, PBD: pulse-based diet, CD: cognitive training, and diet protein and alkaline, HLU: hindlimb-unloading, PC-I: pan-caspase inhibitor, RANKL: receptor activator of nuclear factor κβ ligand, P1NP: N-terminal propeptide of type 1 procollagen, KOC: knockout casein kinase 2-interacting protein-1, CDL: constrained dynamic loading, BV/TV: bone volume fraction, TC: tibial compression, SMF: static magnetic field, GMF: geomagnetic field, TRAP5: tartrate-resistant acid phosphatase 5b, CEFFE: cell-free fat extract, PBS: phosphate-buffered saline, CMS: cyclic mechanical stretch treated bMSCs-derived exosomes, STE: static cultured bMSCs-derived exosomes, IL-6 mAb: interleukin-6 monoclonal antibody, ME: mechanical properties, RPM: random positioning machine, RCCS: rotary cell culture system, RB: rotary wall vessel bioreactor, RHLI: right hindlimb immobilization, ALF: alfacalcidol, ZOL: zoledronic acid.
BMD was decreased in humans by 0.9% after 6 months [42] and 1% per month in space [43]. The decrease in BMD in animals was 18–35.7% after 1 month [45,46].
Both markers of BRS [46,47,50,51,52] and BF [47,50] were elevated during µg in humans and animals; meanwhile, several in vitro studies found inhibited OB differentiation markers (DMs) [54,56,57].
Additionally, sclerostin was increased in humans [51], while mRNA expression of β-catenin was decreased in OBs [54].
Resistive and locomotor exercise had an increasing effect on alkaline phosphatase (ALP) and attenuated the decrease in BMD during bed rest, respectively [60,61]. Resistive vibration exercise revealed an additional positive effect because it led to a greater proximal bone mineral content 6–24 months after bed rest compared to no exercise, which resistive exercise alone did not [61]. The positive effect of exercise on BL is supported by several animal studies [66,67].
BP, anti-RANKL, proteasome inhibitor (PI), pan-caspase inhibitor (PC-I), and interleukin-6 monoclonal antibody (IL-6 mAb) showed positive effects on BL [52,65,71,77], while SERMs and teriparatide did not reveal any effects on BL [65].
Additionally, recombinant irisin (R-irisin), cell-free fat extract, and injection of cyclic mechanical stretch-treated bone mesenchymal stem cell-derived exosomes (CMS) had protective effects on the bones [58,59,64,69,76].
Nanoparticles of hydroxyapatite (HA) loaded with risedronate were generated for bone-targeted drug delivery in ovariectomized rats (model for OP) [78]. This nanoparticle-based formulation was superior to risedronate sodium monotherapy in this model of postmenopausal osteoporosis. In addition, the zoledronic acid (ZOL)/HA nanoparticle-based drug formulation was tested in ovariectomized rats [79]. This formulation was highly effective in promoting BF. Moreover, the risedronate/zinc/HA-based nanomedicine revealed similar results. The findings showed that this nanomedicine had a therapeutic advantage over risedronate or risedronate/HA therapy for the treatment of osteoporosis in rats [80]. Furthermore, strontium hydroxyapatite (SrHA) and zoledronic acid (ZOL) nanoparticle-based drug formulation exerted therapeutic advantages over ZOL or SrHA monotherapy in experimental OP in rats [81].
Antioxidants (AO) only showed a positive effect on BL in animal and cell studies [72,75], but not in human studies [63].
3.3. Latest Clinical Trials
An overview of a selection of the latest clinical trials of CMs against and mechanisms of µg-induced BL listed in clinicaltrials.gov as assessed on 19 April 2022 is given in Table 3. These studies primarily examined the effect of CMs on BMD and markers of bone cell activity in µg analogs.
Table 3.
An overview of a selection of the latest clinical trials of µg-induced BL and countermeasures.
| Title and Identification Number | Subjects | Design | Outcome | Status/Conclusion |
|---|---|---|---|---|
| The Effects of Whole Body Unloading on Physiological Function (NCT03195348) | 12 | IV SG | Investigate the effects of hyper-buoyancy flotation in 7 days on skeletal muscles and bone mineral density. | Completed. No results posted yet. |
| A New Nutritional Countermeasure to Prevent the Deconditioning Induced by 60 Days of Antiorthostatic Bed Rest (NCT03594799) | 20 | IV RP Investigator masking |
Investigate if XXS-2A-BR2 prevents or reduces the harmful effects caused by physical inactivity through 60 days of bed rest. Secondary outcome is the change in urinary C-telopeptide of type I collagen. | Completed. No effect on urinary C-telopeptide of type I collagen, serum β-C-telopeptide of type I collagen, NTX, alkaline phosphatase, P1NP, or osteocalcin. |
| Thigh Cuffs to Prevent the Deconditioning Induced by 5 Days of Dry Immersion (NCT03915457) | 20 | IV RP No masking |
Investigate if thigh cuffs prevent or reduce the deconditioning caused by dry immersion. The outcome is the change in the balance of bone remodeling markers. | Completed. No results posted yet. |
| Planetary Habitat Simulation: Bone Metabolism Studies (NCT02637921) | 14 | IV RC Open-label masking |
Investigate the effect of hypoxia and bed rest on bone metabolism. Outcomes include the change in markers of bone cell activity. | Completed. Serum calcium and NTX increased, while P1NP decreased. No difference between normoxia or hypoxia. |
| Understanding the Negative Effects of Bed Rest and Using Exercise as Countermeasure (NCT04964999) | 24 | IV RP Open-label masking |
Investigate if exercise counteracts the negative effects caused by 2 week head-down tilt bed rest on bone markers among others. | Recruiting. |
| Integrative Study of Physiological Changes Induced by a 5-Day Dry Immersion on 20 Healthy Female Volunteers (NCT05043974) | 20 | IV SG Open-label masking |
Investigate the changes caused by dry immersion for 5 days in female physiology. The outcome is the change in bone metabolism and bone mineral density. | Recruiting. |
Abbreviations: NCT: national clinical trial, IV: interventional, SG: single-group assignment, RP: randomized parallel assignment, NTX: urinary N-telopeptide of type I collagen, P1NP: N-terminal propeptide of type 1 procollagen, RC: randomized crossover assignment.
4. Discussion
4.1. Summary of Evidence
4.1.1. Mechanisms of Microgravity-Related Changes in Bone Density
Earlier studies demonstrated a reduction in BMD of 0.5–1.5% per month in space [6,7]. This finding was supported by Burkhart et al., who reported a monthly reduction of 0.5–1% in BMD on the ISS in humans [43]. However, Bilancio et al. reported only a 0.9–1.4% decrease in total BMD after 6 months on the ISS in humans [42]. The rate of BMD reduction in mice seems to be higher because other studies found a reduction of 18–35.7% in BMD after 30 days in µg [45,46]. This difference could be caused by biological differences, because the skeleton of mice keeps growing after puberty and lacks Haversian systems among other elements compared to humans [82]. The highest rate of age-related BL is found during the late perimenopause and beginning of the menopause, where the decrease of BL is up to 2.5% in the lumbar spine annually [83,84]. This indicates that the age-related rate of BL is substantially lower than the µg-induced BMD reduction. However, the morphology of µg-induced BL resembles the MP BL. BL caused by µg reduced trabecular thickness and volume, and increased osteocyte apoptosis in the trabecular bone [45,46,52]. This resembles the MP BL, because the MP BL increases trabecular remodeling [14,85]. Sibonga et al. reported that, after a 6 month-stay in space, four of 10 astronauts had an incomplete recovery from µg-induced loss of BMD 2 years after returning to Earth [44]. Interestingly, it was observed that only the trabecular BL, but not the cortical BL was recovered in mice 1 week after 4 weeks of spaceflight [48].
The µg-induced decrease in BMD is caused by an elevated bone resorption. Several studies found elevated markers of bone matrix degradation and OC markers and activation in both humans and animals [47,50,51,52], which is supported by the 140% increase in BRS found by Gerbaix et al. [46]. However, markers of OBs were also elevated in several studies [47,50]. These findings suggest that both OC and OB activities are increased during µg, but that the process of BRS must be greater than the BF due to the decreased BMD. In untreated PMP osteoporotic women, there was an increase in BRS markers reported, whereas the BF markers differed more between different studies [86,87,88,89].
The increased sclerostin in humans and the decreased mRNA expression of β-catenin in OBs during µg suggest that the heightened bone turnover (BT) is caused by inhibition of the Wnt/β-catenin signaling pathway, leading to increased BRS and decreased BF [50,51,54,90]. However, numerous studies demonstrated that serum levels of sclerostin were significantly lower in PMP women with OP than in women without OP [91,92], which is different from the higher levels of sclerostin found during µg [50,51]. The discrepancy in sclerostin levels could be a consequence of biological differences because the majority of the subjects in the µg studies were male.
Several in vitro studies on cultured cells showed that the differentiation of OBs to osteocytes was inhibited, because OB DMs were decreased [54,56,57]. However, Cazzaniga et al. measured an increase in OB DMs after 4 days of µg in human bone mesenchymal stem cells (bMSCs) [55]. This inconsistency could be attributed to differences in the length of µg exposure and different cell types. To compare these data with findings in PMP women, we must focus on estrogen deficiency in cells, with studies showing that several OB DMs were decreased in estrogen-depleted bMSCs and pre-OBs [93,94]. These data suggest that the estrogen-depleted MP OP induces a decrease in OB differentiation, which is similar to several studies on µg-induced BL.
4.1.2. Possible Treatment Options for Microgravity-Induced Bone Loss
Both resistive exercise and locomotor exercise exhibited an increasing effect on the OB DM ALP and attenuated the decrease in BMD during bed rest, respectively [60,61]. Animal studies support the effectiveness of exercise on µg-induced BL. Tibial compression during hindlimb unloading (HLU) had a protective effect on BL, while constrained dynamic loading increased bone volume fraction (BV/TV) [67], trabecular number and thickness, and OB DM [66].
Exercise is an important part of treatment of PMP OP [10,16]. Several studies on PMP women showed an increase in BMD after resistive and aerobic exercise [95,96,97,98].
The pharmacological treatment of MP OP is an important part of the therapy according to the European and AACE guidelines [10,16]. In addition, these treatments were tested in simulated µg (s-µg). BP and anti-RANKL inhibited the BRS and restored BMD in mice exposed to HLU close to mice in normal gravity in several studies [65,71]. Furthermore, 3-month-old male Wistar rats subjected to right hindlimb immobilization for 10 weeks to induce osteopenia were treated with zoledronic acid and alfacalcidol. This combination therapy was more effective than each drug administered as a monotherapy for the treatment of disuse osteoporosis [74]. However, SERM and teriparatide did not reveal any effect on BL [65], while BP, SERM, anti-RANKL, and teriparatide demonstrated a significant increase in BMD in MP women [99,100,101,102,103,104]. It should not be forgotten, however, that administration of BP and anti-RANKL can also cause some more or less severe side-effects. Overall, BP reduces fracture risk, but also induces changes in the bone material, which can reduce bone toughness [105]. More severely, BP and anti-RANKL are also linked to the development of osteonecrosis of the jaw, a condition which can lead to life-threatening complications [106,107,108]. The missing effect of raloxifene and teriparatide in µg could be attributed to the biological difference between humans and mice. Additionally, the PI inhibited BRS, promoted BF, and induced an increase in BMD in mice exposed to HLU [65], while it decreased the area of resorbed bone in pre-OCs exposed to s-µg [77]. Ovariectomy in animals is used as a model for PMP OP [109]. The PI increased the bone volume in ovariectomized animals, while it inhibited the OC formation and BRS in vitro, as well as stimulated OB differentiation [110,111]. PC-Is prevented the HLU-induced increase in osteocyte apoptosis, osteocyte RANKL expression, and endocortical resorption in mice [52]. PC-Is might have a positive effect on PMP OP. The OC differentiation was blocked by PC-Is in OCs [112], and the decreases in BV/TV, as well as trabecular number and thickness, caused by ovariectomy in rats were alleviated [113].
Furthermore, IL-6 mAb alleviates the µg-induced BL by normalizing the BF and BRS, which leads to an increased BMD [73]. Meanwhile, another study only measured a decrease in the number of OCs [71]. This difference could be credited to different experimental protocols and strains of mice. Antibodies are already used in the treatment of MP OP, but not IL-6 mAb, which shows varying results. Some in vitro studies showed a suppressed development of OCs caused by IL-6 mAb [114,115]. Moreover, other studies, both in vitro and in vivo, showed no effect of IL-6 mAb on BRS, BL, or OC development [114,116,117]. The use of IL-6 mAb in the treatment of MP OP is still quite unknown, and further studies are necessary.
Pharmacological treatments are vital for future space exploration, especially because the pharmacodynamics and pharmacokinetics might be different in space [118,119]. Absorption of paracetamol showed both slower and faster peak concentration during µg in different studies [118,119,120], while metabolism through P450 enzymes in rats was reduced by 0–50% after 7–14 days of spaceflight [118,121,122,123].
Furthermore, the effect of different supplements on µg-induced BL was evaluated. R-irisin injection on mice during HLU prevented BL. There was no measurable loss of BMD, and R-irisin recovered the bone mass through attenuation of the OC inhibitor osteoprotegerin and increased activity and differentiation of OBs [58,64,76]. In ovariectomized mice, R-irisin prevented trabecular BL and induced greater BMD, while the number of OBs was increased, and the number of OCs was decreased in parallel [124]. Additionally, R-irisin returns the serum levels of osteocalcin, ALP, and tartrate-resistant acid phosphatase 5b in ovariectomized rats back to the same serum levels as non-ovariectomized rats [125]. Addition of SCHN to bMSCs had a protective effect on µg-induced reduction of ALP activity and a promoting effect on the deposition of hydroxyapatite crystals [59]. Strontium ranelate is already used in OP treatment, where it showed a significant increase in BMD on PMP OP [126,127]. The injection of cell-free fat extract also has a positive effect on BL, because it alleviates the HLU-induced decrease in BV/TV, trabecular number, and cortical thickness [69]. Furthermore, the injection of CMS in mice exposed to HLU increased BMD, BV/TV, cortical thickness, and trabecular thickness and number, while reducing the number of OCs [70]. However, pulse-based diet and dietary protein and alkaline supplement did not show any effect on BL in humans [51,62].
Application of AO had an effect in studies on cells and animals. Both nutraceuticals and polyphenols have an elevating effect on ALP activity in OBs exposed to s-µg [72,75]. The reduced BL in rats could be due to alleviation of the µg-induced inhibition of Wnt/β-catenin pathway, which enhances BF, because ALP, N-terminal propeptide of type 1 procollagen, and β-catenin are increased [72]. However, no significant effect in humans on BL, who already consumed sufficient amounts of polyphenols, was found [63], which questions the use of AO against µg-induced BL. AO for MP women also showed variable results. A study found no effect of vitamin C in PMP women [128]. However, other studies showed that the polyphenol resveratrol alone and curcumin combined with alendronate increased BMD in PMP women [129,130]. Here, the question remains whether the respective test subjects received adequate amounts of antioxidants before starting the study. These findings suggest that AO might have, under certain circumstances, an effect as a supplement to the treatment as advised in the European and AACE guidelines [10,16].
4.2. Strengths and Limitations
There were several strengths of this review. The data were individually searched and collected from PubMed and Scopus, and then systemically and manually examined on the basis of PICO parameters. Another strength is that the results were based on the latest data from 2016 onward, and we did not use systematic reviews or meta-analyses, where data are interpreted by additional authors, or case reports in the results.
There were also limitations to this work. This systematic review focused on the mechanisms behind µg-induced BL and the possible treatment thereof on humans, but this review used studies examining animals and cell cultures, as well as studies examining s-µg, which cannot replicate human studies in space. Nevertheless, the use of animals, cell cultures, and s-µg have shown to be great alternatives to human studies in space [131]; therefore, this review argues that studies of animals and cell cultures and studies using s-µg are justified in this case. Furthermore, the studies on CM in real µg were performed on cells and not any on animals or humans. Since pharmacological treatments can be different in space [118,119,120,121,122,123], this may also be a limitation.
5. Conclusions
Space travelers experience µg, which enhances BL by 0.5–1.5% and reduces BMD per month in space. Many, but not all studies indicated an elevated BF and a greater increase in BRS, which led to increased BT and enhanced BL. One reason for this finding is, among others, the inhibition of the Wnt/β-catenin signaling pathway. Space travels will be more common in the future. Therefore, the enhanced BL needs to be alleviated so that it does not limit the possibility of space travel. To counteract the µg-induced BL, numerous CMs have been proposed. Firstly, adequate nutrient intake, particularly sufficient amounts of energy, protein, calcium, and vitamin D and a diet high in alkaline precursors, might reduce bone loss. Secondly, different types of exercise showed a positive effect on BF and BMD. Thirdly, pharmacological treatment with BP, anti-RANKL, PI, PC-I, and IL-6 mAb decreased BRS and promoted BF, which in turn induced an increase in BMD. However, SERM and teriparatide did not reveal a significant effect on BL. Additionally, injection of r-irisin, cell-free fat extract, and CMS, as well as the addition of SCHN, showed positive effects on BL. However, AO and other supplements delivered various results. Although, several CMs revealed promising results, the results are still too variable and the number of replications for any human study are still too small to propose definitive guidelines for space travels and long-term space exploration.
Taken together, more research is needed to expand the current knowledge of the mechanisms of µg-induced BL. Studies on BL in space can be favorable and support the treatment of osteoporosis on Earth.
Abbreviations
| AACE | American Association of Clinical Endocrinologists |
| ALF | Alfacalcidol |
| ALP | Alkaline phosphatase |
| AO | Antioxidants |
| ARED | Advanced resistive exercise device |
| BP | Bisphosphonate |
| BF | Bone formation |
| BL | Bone loss |
| BR/BRS | Bone resorption |
| BMD | Bone mineral density |
| BT | Bone turnover |
| bMSC | Bone mesenchymal stem cell |
| BV/TV | Bone volume fraction |
| CM | Countermeasure |
| CMS | Cyclic mechanical stretch treated bone mesenchymal stem cell-derived exosomes |
| DM | Differentiation marker |
| HLU | Hindlimb unloading |
| IL-6 mAb | Interleukin-6 monoclonal antibody |
| ISS | International Space Station |
| MP | Menopausal |
| OB | Osteoblast |
| OC | Osteoclast |
| OP | Osteoporosis |
| PC-I | Pan-caspase inhibitor |
| PI | Proteasome inhibitor |
| PMP | Postmenopausal |
| PTH | Parathyroid hormone |
| RANKL | Receptor activator of nuclear factor κβ ligand |
| RHLI | Right hindlimb immobilization |
| R-irisin | Recombinant irisin |
| SCHN | Strontium-containing hydroxyapatite nanoparticles |
| SERM | Selective estrogen receptor modulator |
| S-µg | Simulated microgravity |
| uCaV | Urinary calcium excretion |
| uNTx | Urinary N-telopeptide |
| Wnt | Wingless-related integration site |
| ZOL | Zoledronic acid |
| µg | Microgravity |
Author Contributions
Conceptualization, R.B.; methodology, R.B. and M.W.; validation, D.G., H.S., M.H., M.W. and M.I.; investigation, R.B.; resources, M.I.; writing—original draft preparation, R.B.; writing—review and editing, R.B., M.W., H.S., D.G. and M.H.; visualization, R.B.; supervision, D.G.; project administration, D.G. and M.I.; funding acquisition, D.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Deutsches Zentrum für Luft und Raumfahrt (DLR), BMWi projects 50WB1924 (DG) and 50WB2219 (DG).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morphew M.E. Psychological and human factors in long duration spaceflight. McGill J. Med. 2001;6:74–80. doi: 10.26443/mjm.v6i1.555. [DOI] [Google Scholar]
- 2.Furukawa S., Nagamatsu A., Nenoi M., Fujimori A., Kakinuma S., Katsube T., Wang B., Tsuruoka C., Shirai T., Nakamura A.J., et al. Space Radiation Biology for “Living in Space”. Biomed. Res. Int. 2020;2020:4703286. doi: 10.1155/2020/4703286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen M., Frishman W.H. Effects of Spaceflight on Cardiovascular Physiology and Health. Cardiol Rev. 2019;27:122–126. doi: 10.1097/CRD.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 4.Hughes-Fulford M. To infinity … and beyond! Human spaceflight and life science. FASEB J. 2011;25:2858–2864. doi: 10.1096/fj.11-0902ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juhl O.J.T., Buettmann E.G., Friedman M.A., DeNapoli R.C., Hoppock G.A., Donahue H.J. Update on the effects of microgravity on the musculoskeletal system. NPJ Microgravity. 2021;7:28. doi: 10.1038/s41526-021-00158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeBlanc A., Schneider V., Shackelford L., West S., Oganov V., Bakulin A., Voronin L. Bone mineral and lean tissue loss after long duration space flight. J. Musculoskelet Neuronal Interact. 2000;1:157–160. [PubMed] [Google Scholar]
- 7.Vico L., van Rietbergen B., Vilayphiou N., Linossier M.T., Locrelle H., Normand M., Zouch M., Gerbaix M., Bonnet N., Novikov V., et al. Cortical and Trabecular Bone Microstructure Did Not Recover at Weight-Bearing Skeletal Sites and Progressively Deteriorated at Non-Weight-Bearing Sites During the Year Following International Space Station Missions. J. Bone Mineral. Res. 2017;32:2010–2021. doi: 10.1002/jbmr.3188. [DOI] [PubMed] [Google Scholar]
- 8.Glaser D.L., Kaplan F.S. Osteoporosis. Definition and clinical presentation. Spine. 1997;22:12S–16S. doi: 10.1097/00007632-199712151-00003. [DOI] [PubMed] [Google Scholar]
- 9.Siris E.S., Adler R., Bilezikian J., Bolognese M., Dawson-Hughes B., Favus M.J., Harris S.T., Jan de Beur S.M., Khosla S., Lane N.E., et al. The clinical diagnosis of osteoporosis: A position statement from the National Bone Health Alliance Working Group. Osteoporos. Int. 2014;25:1439–1443. doi: 10.1007/s00198-014-2655-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanis J.A., Cooper C., Rizzoli R., Reginster J.Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019;30:3–44. doi: 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Recker R.R., Davies K.M., Hinders S.M., Heaney R.P., Stegman M.R., Kimmel D.B. Bone gain in young adult women. Jama. 1992;268:2403–2408. doi: 10.1001/jama.1992.03490170075028. [DOI] [PubMed] [Google Scholar]
- 12.Rizzoli R., Bianchi M.L., Garabédian M., McKay H.A., Moreno L.A. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305. doi: 10.1016/j.bone.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Hopper J.L., Green R.M., Nowson C.A., Young D., Sherwin A.J., Kaymakci B., Larkins R.G., Wark J.D. Genetic, common environment, and individual specific components of variance for bone mineral density in 10- to 26-year-old females: A twin study. Am. J. Epidemiol. 1998;147:17–29. doi: 10.1093/oxfordjournals.aje.a009361. [DOI] [PubMed] [Google Scholar]
- 14.Armas L.A., Recker R.R. Pathophysiology of osteoporosis: New mechanistic insights. Endocrinol. Metab. Clin. N. Am. 2012;41:475–486. doi: 10.1016/j.ecl.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Heaney R.P., Recker R.R., Saville P.D. Menopausal changes in calcium balance performance. J. Lab. Clin. Med. 1978;92:953–963. doi: 10.1111/j.1753-4887.1983.tb07709.x. [DOI] [PubMed] [Google Scholar]
- 16.Camacho P.M., Petak S.M., Binkley N., Diab D.L., Eldeiry L.S., Farooki A., Harris S.T., Hurley D.L., Kelly J., Lewiecki E.M., et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis 2020 Update. Endocr. Pract. 2020;26:1–46. doi: 10.4158/GL-2020-0524SUPPL. [DOI] [PubMed] [Google Scholar]
- 17.Zwart S.R., Rice B.L., Dlouhy H., Shackelford L.C., Heer M., Koslovsky M.D., Smith S.M. Dietary acid load and bone turnover during long-duration spaceflight and bed rest. Am. J. Clin. Nutr. 2018;107:834–844. doi: 10.1093/ajcn/nqy029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han Y., An M., Yang L., Li L., Rao S., Cheng Y. Effect of Acid or Base Interventions on Bone Health: A Systematic Review, Meta-Analysis, and Meta-Regression. Adv. Nutr. 2021;12:1540–1557. doi: 10.1093/advances/nmab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brent M.B., Brüel A., Thomsen J.S. Anti-sclerostin antibodies and abaloparatide have additive effects when used as a countermeasure against disuse osteopenia in female rats. Bone. 2022;160:116417. doi: 10.1016/j.bone.2022.116417. [DOI] [PubMed] [Google Scholar]
- 20.Li X., Zhang Y., Kang H., Liu W., Liu P., Zhang J., Harris S.E., Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 21.Rauner M., Taipaleenmäki H., Tsourdi E., Winter E.M. Osteoporosis Treatment with Anti-Sclerostin Antibodies-Mechanisms of Action and Clinical Application. J. Clin. Med. 2021;10:787. doi: 10.3390/jcm10040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Man J., Graham T., Squires-Donelly G., Laslett A.L. The effects of microgravity on bone structure and function. NPJ Microgravity. 2022;8:9. doi: 10.1038/s41526-022-00194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith S.M., McCoy T., Gazda D., Morgan J.L., Heer M., Zwart S.R. Space flight calcium: Implications for astronaut health, spacecraft operations, and Earth. Nutrients. 2012;4:2047–2068. doi: 10.3390/nu4122047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith S.M., Wastney M.E., Morukov B.V., Larina I.M., Nyquist L.E., Abrams S.A., Taran E.N., Shih C.Y., Nillen J.L., Davis-Street J.E., et al. Calcium metabolism before, during, and after a 3-mo spaceflight: Kinetic and biochemical changes. Am. J. Physiol. 1999;277:R1–R10. doi: 10.1152/ajpregu.1999.277.1.R1. [DOI] [PubMed] [Google Scholar]
- 25.Caillot-Augusseau A., Vico L., Heer M., Voroviev D., Souberbielle J.C., Zitterman A., Alexandre C., Lafage-Proust M.H. Space flight is associated with rapid decreases of undercarboxylated osteocalcin and increases of markers of bone resorption without changes in their circadian variation: Observations in two cosmonauts. Clin. Chem. 2000;46:1136–1143. doi: 10.1093/clinchem/46.8.1136. [DOI] [PubMed] [Google Scholar]
- 26.Caillot-Augusseau A., Lafage-Proust M.H., Soler C., Pernod J., Dubois F., Alexandre C. Bone formation and resorption biological markers in cosmonauts during and after a 180-day space flight (Euromir 95) Clin. Chem. 1998;44:578–585. doi: 10.1093/clinchem/44.3.578. [DOI] [PubMed] [Google Scholar]
- 27.Smith S.M., Wastney M.E., O’Brien K.O., Morukov B.V., Larina I.M., Abrams S.A., Davis-Street J.E., Oganov V., Shackelford L.C. Bone markers, calcium metabolism, and calcium kinetics during extended-duration space flight on the mir space station. J. Bone Miner. Res. 2005;20:208–218. doi: 10.1359/JBMR.041105. [DOI] [PubMed] [Google Scholar]
- 28.Grimm D., Grosse J., Wehland M., Mann V., Reseland J.E., Sundaresan A., Corydon T.J. The impact of microgravity on bone in humans. Bone. 2016;87:44–56. doi: 10.1016/j.bone.2015.12.057. [DOI] [PubMed] [Google Scholar]
- 29.Bucaro M.A., Fertala J., Adams C.S., Steinbeck M., Ayyaswamy P., Mukundakrishnan K., Shapiro I.M., Risbud M.V. Bone cell survival in microgravity: Evidence that modeled microgravity increases osteoblast sensitivity to apoptogens. Ann. N. Y. Acad. Sci. 2004;1027:64–73. doi: 10.1196/annals.1324.007. [DOI] [PubMed] [Google Scholar]
- 30.Bucaro M.A., Zahm A.M., Risbud M.V., Ayyaswamy P.S., Mukundakrishnan K., Steinbeck M.J., Shapiro I.M., Adams C.S. The effect of simulated microgravity on osteoblasts is independent of the induction of apoptosis. J. Cell. Biochem. 2007;102:483–495. doi: 10.1002/jcb.21310. [DOI] [PubMed] [Google Scholar]
- 31.Dufour C., Holy X., Marie P.J. Skeletal unloading induces osteoblast apoptosis and targets α5β1-PI3K-Bcl-2 signaling in rat bone. Exp. Cell Res. 2007;313:394–403. doi: 10.1016/j.yexcr.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 32.Carmeliet G., Vico L., Bouillon R. Space flight: A challenge for normal bone homeostasis. Crit. Rev. Eukaryot. Gene Expr. 2001;11:131–144. doi: 10.1615/CritRevEukarGeneExpr.v11.i1-3.70. [DOI] [PubMed] [Google Scholar]
- 33.DiFrancesco J.M., Olson J.M. The economics of microgravity research. NPJ Microgravity. 2015;1:15001. doi: 10.1038/npjmgrav.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mars K., Bollweg L., Norsk P. About Human Health Countermeasures (HHC) [(accessed on 5 April 2022)]; Available online: http://www.nasa.gov/hrp/elements/hhc/about.
- 35.Orwoll E.S., Adler R.A., Amin S., Binkley N., Lewiecki E.M., Petak S.M., Shapses S.A., Sinaki M., Watts N.B., Sibonga J.D. Skeletal health in long-duration astronauts: Nature, assessment, and management recommendations from the NASA Bone Summit. J. Bone Miner. Res. 2013;28:1243–1255. doi: 10.1002/jbmr.1948. [DOI] [PubMed] [Google Scholar]
- 36.Trappe S., Costill D., Gallagher P., Creer A., Peters J.R., Evans H., Riley D.A., Fitts R.H. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. J. Appl. Physiol. 2009;106:1159–1168. doi: 10.1152/japplphysiol.91578.2008. [DOI] [PubMed] [Google Scholar]
- 37.Loehr J.A., Lee S.M., English K.L., Sibonga J., Smith S.M., Spiering B.A., Hagan R.D. Musculoskeletal adaptations to training with the advanced resistive exercise device. Med. Sci. Sports Exerc. 2011;43:146–156. doi: 10.1249/MSS.0b013e3181e4f161. [DOI] [PubMed] [Google Scholar]
- 38.Furukawa S., Chatani M., Higashitani A., Higashibata A., Kawano F., Nikawa T., Numaga-Tomita T., Ogura T., Sato F., Sehara-Fujisawa A., et al. Findings from recent studies by the Japan Aerospace Exploration Agency examining musculoskeletal atrophy in space and on Earth. NPJ Microgravity. 2021;7:18. doi: 10.1038/s41526-021-00145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson K., Zhu S., Tremblay M.S., Payette J.N., Wang J., Bouchez L.C., Meeusen S., Althage A., Cho C.Y., Wu X., et al. A stem cell-based approach to cartilage repair. Science. 2012;336:717–721. doi: 10.1126/science.1215157. [DOI] [PubMed] [Google Scholar]
- 40.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009;151:W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 41.Aslam S., Emmanuel P. Formulating a researchable question: A critical step for facilitating good clinical research. Indian J. Sex. Transm. Dis. AIDS. 2010;31:47–50. doi: 10.4103/0253-7184.69003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bilancio G., Cavallo P., Lombardi C., Guarino E., Cozza V., Giordano F., Cirillo M. Urea and minerals monitoring in space missions by spot samples of saliva and urine. Aerosp. Med. Hum. Perform. 2019;90:43–47. doi: 10.3357/AMHP.5200.2019. [DOI] [PubMed] [Google Scholar]
- 43.Burkhart K., Allaire B., Anderson D.E., Lee D., Keaveny T.M., Bouxsein M.L. Effects of Long-Duration Spaceflight on Vertebral Strength and Risk of Spine Fracture. J. Bone Mineral. Res. 2020;35:269–276. doi: 10.1002/jbmr.3881. [DOI] [PubMed] [Google Scholar]
- 44.Sibonga J.D., Spector E.R., Keyak J.H., Zwart S.R., Smith S.M., Lang T.F. Use of Quantitative Computed Tomography to Assess for Clinically-relevant Skeletal Effects of Prolonged Spaceflight on Astronaut Hips. J. Clin. Densitom. 2020;23:155–164. doi: 10.1016/j.jocd.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Berg-Johansen B., Liebenberg E.C., Li A., Macias B.R., Hargens A.R., Lotz J.C. Spaceflight-induced bone loss alters failure mode and reduces bending strength in murine spinal segments. J. Orthop. Res. 2016;34:48–57. doi: 10.1002/jor.23029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerbaix M., Gnyubkin V., Farlay D., Olivier C., Ammann P., Courbon G., Laroche N., Genthial R., Follet H., Peyrin F., et al. One-month spaceflight compromises the bone microstructure, tissue-level mechanical properties, osteocyte survival and lacunae volume in mature mice skeletons. Sci. Rep. 2017;7:2659. doi: 10.1038/s41598-017-03014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chatani M., Morimoto H., Takeyama K., Mantoku A., Tanigawa N., Kubota K., Suzuki H., Uchida S., Tanigaki F., Shirakawa M., et al. Acute transcriptional up-regulation specific to osteoblasts/osteoclasts in medaka fish immediately after exposure to microgravity. Sci. Rep. 2016;6:39545. doi: 10.1038/srep39545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Kroge S., Wölfel E.M., Buravkova L.B., Atiakshin D.A., Markina E.A., Schinke T., Rolvien T., Busse B., Jähn-Rickert K. Bone loss recovery in mice following microgravity with concurrent bone-compartment-specific osteocyte characteristics. Eur. Cell Mater. 2021;42:220–231. doi: 10.22203/eCM.v042a16. [DOI] [PubMed] [Google Scholar]
- 49.Bonnefoy J., Baselet B., Moser D., Ghislin S., Miranda S., Riant E., Vermeesen R., Keiler A.M., Baatout S., Choukér A., et al. B-Cell Homeostasis Is Maintained During Two Months of Head-Down Tilt Bed Rest With or Without Antioxidant Supplementation. Front. Immunol. 2022;13:830662. doi: 10.3389/fimmu.2022.830662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bemben D.A., Baker B.S., Buchanan S.R., Ade C.J. Circulating MiR-21 expression is upregulated after 30° days of head-down tilt bed rest. Osteoporos. Int. 2021;32:1369–1378. doi: 10.1007/s00198-020-05805-2. [DOI] [PubMed] [Google Scholar]
- 51.Buehlmeier J., Frings-Meuthen P., Mohorko N., Lau P., Mazzucco S., Ferretti J.L., Biolo G., Pisot R., Simunic B., Rittweger J. Markers of bone metabolism during 14 days of bed rest in young and older men. J. Musculoskelet Neuronal Interact. 2017;17:399–408. [PMC free article] [PubMed] [Google Scholar]
- 52.Cabahug-Zuckerman P., Frikha-Benayed D., Majeska R.J., Tuthill A., Yakar S., Judex S., Schaffler M.B. Osteocyte Apoptosis Caused by Hindlimb Unloading is Required to Trigger Osteocyte RANKL Production and Subsequent Resorption of Cortical and Trabecular Bone in Mice Femurs. J. Bone Miner. Res. 2016;31:1356–1365. doi: 10.1002/jbmr.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chowdhury P., Akel N., Jamshidi-Parsian A., Gaddy D., Griffin R.J., Yadlapalli J.S.K., Dobretsov M. Degenerative tissue responses to space-like radiation doses in a rodent model of simulated microgravity. Ann. Clin. Lab. Sci. 2016;46:190–197. [PubMed] [Google Scholar]
- 54.Chen Z., Zhang Y., Zhao F., Yin C., Yang C., Huai Y., Liang S., Liu S., Xu X., Wu Z., et al. miR-138–5p negatively regulates osteoblast differentiation through inhibiting β-catenin under simulated microgravity in MC3T3-E1 cells. Acta Astronaut. 2021;182:240–250. doi: 10.1016/j.actaastro.2021.01.052. [DOI] [Google Scholar]
- 55.Cazzaniga A., Maier J.A.M., Castiglioni S. Impact of simulated microgravity on human bone stem cells: New hints for space medicine. Biochem. Biophys. Res. Commun. 2016;473:181–186. doi: 10.1016/j.bbrc.2016.03.075. [DOI] [PubMed] [Google Scholar]
- 56.Chen Z., Luo Q., Lin C., Kuang D., Song G. Simulated microgravity inhibits osteogenic differentiation of mesenchymal stem cells via depolymerizing F-actin to impede TAZ nuclear translocation. Sci. Rep. 2016;6:30322. doi: 10.1038/srep30322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang L., Li G., Wang K., Wang Y., Dong J., Wang H., Xu L., Shi F., Cao X., Hu Z., et al. MiR-30 family members inhibit osteoblast differentiation by suppressing Runx2 under unloading conditions in MC3T3-E1 cells. Biochem. Biophys. Res. Commun. 2020;522:164–170. doi: 10.1016/j.bbrc.2019.11.057. [DOI] [PubMed] [Google Scholar]
- 58.Colucci S., Colaianni G., Brunetti G., Ferranti F., Mascetti G., Mori G., Grano M. Irisin prevents microgravity-induced impairment of osteoblast differentiation in vitro during the space flight CRS-14 mission. FASEB J. 2020;34:10096–10106. doi: 10.1096/fj.202000216R. [DOI] [PubMed] [Google Scholar]
- 59.Cristofaro F., Pani G., Pascucci B., Mariani A., Balsamo M., Donati A., Mascetti G., Rea G., Rizzo A.M., Visai L. The NATO project: Nanoparticle-based countermeasures for microgravity-induced osteoporosis. Sci. Rep. 2019;9:17141. doi: 10.1038/s41598-019-53481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cavanagh P.R., Rice A.J., Novotny S.C., Genc K.O., Englehaupt R.K., Owings T.M., Comstock B., Cardoso T., Ilaslan H., Smith S.M., et al. Replacement of daily load attenuates but does not prevent changes to the musculoskeletal system during bed rest. Bone Rep. 2016;5:299–307. doi: 10.1016/j.bonr.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belavý D.L., Baecker N., Armbrecht G., Beller G., Buehlmeier J., Frings-Meuthen P., Rittweger J., Roth H.J., Heer M., Felsenberg D. Serum sclerostin and DKK1 in relation to exercise against bone loss in experimental bed rest. J. Bone Mineral. Metab. 2016;34:354–365. doi: 10.1007/s00774-015-0681-3. [DOI] [PubMed] [Google Scholar]
- 62.Gao R., Duff W., Chizen D., Zello G.A., Chilibeck P.D. The Effect of a Low Glycemic Index Pulse-Based Diet on Insulin Sensitivity, Insulin Resistance, Bone Resorption and Cardiovascular Risk Factors during Bed Rest. Nutrients. 2019;11:2012. doi: 10.3390/nu11092012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Austermann K., Baecker N., Zwart S.R., Fimmers R., Frippiat J.P., Stehle P., Smith S.M., Heer M. Antioxidant Supplementation Does Not Affect Bone Turnover Markers During 60 Days of 6° Head-Down Tilt Bed Rest: Results from an Exploratory Randomized Controlled Trial. J. Nutr. 2021;151:1527–1538. doi: 10.1093/jn/nxab036. [DOI] [PubMed] [Google Scholar]
- 64.Colaianni G., Mongelli T., Cuscito C., Pignataro P., Lippo L., Spiro G., Notarnicola A., Severi I., Passeri G., Mori G., et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci. Rep. 2017;7:2811. doi: 10.1038/s41598-017-02557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ding Y., Cui Y., Yang X., Wang X., Tian G., Peng J., Wu B., Tang L., Cui C.P., Zhang L. Anti-RANKL monoclonal antibody and bortezomib prevent mechanical unloading-induced bone loss. J. Bone Mineral. Metab. 2021;39:974–983. doi: 10.1007/s00774-021-01246-x. [DOI] [PubMed] [Google Scholar]
- 66.Han B., Wei S.P., Zhang X.C., Li H., Li Y., Li R.X., Li K., Zhang X.Z. Effects of constrained dynamic loading, CKIP-1 gene knockout and combination stimulations on bone loss caused by mechanical unloading. Mol. Med. Rep. 2018;18:2506–2514. doi: 10.3892/mmr.2018.9222. [DOI] [PubMed] [Google Scholar]
- 67.DeLong A., Friedman M.A., Tucker S.M., Krause A.R., Kunselman A., Donahue H.J., Lewis G.S. Protective Effects of Controlled Mechanical Loading of Bone in C57BL6/J Mice Subject to Disuse. JBMR Plus. 2020;4:e10322. doi: 10.1002/jbm4.10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang J., Zhou S., Lv H., Wei M., Fang Y., Shang P. Static magnetic field of 0.2–0.4 T promotes the recovery of hindlimb unloading-induced bone loss in mice. Int. J. Radiat. Biol. 2021;97:746–754. doi: 10.1080/09553002.2021.1900944. [DOI] [PubMed] [Google Scholar]
- 69.Xu M., Du J., Cui J., Zhang S., Zhang S., Deng M., Zhang W., Li H., Yu Z. Cell-Free Fat Extract Prevents Tail Suspension–Induced Bone Loss by Inhibiting Osteocyte Apoptosis. Front. Bioeng. Biotechnol. 2022;10:818572. doi: 10.3389/fbioe.2022.818572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiao F., Zuo B., Tao B., Wang C., Li Y., Peng J., Shen C., Cui Y., Zhu J., Chen X. Exosomes derived from cyclic mechanical stretch-exposed bone marrow mesenchymal stem cells inhibit RANKL-induced osteoclastogenesis through the NF-κB signaling pathway. Ann. Transl. Med. 2021;9:798. doi: 10.21037/atm-21-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wakabayashi H., Miyamura G., Nagao N., Kato S., Naito Y., Sudo A. Functional Block of Interleukin-6 Reduces a Bone Pain Marker but Not Bone Loss in Hindlimb-Unloaded Mice. Int. J. Mol. Sci. 2020;21:3521. doi: 10.3390/ijms21103521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diao Y., Chen B., Wei L., Wang Z. Polyphenols (S3) Isolated from Cone Scales of Pinus koraiensis Alleviate Decreased Bone Formation in Rat under Simulated Microgravity. Sci. Rep. 2018;8:12719. doi: 10.1038/s41598-018-30992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He B., Yin X., Hao D., Zhang X., Zhang Z., Zhang K., Yang X. Blockade of IL-6 alleviates bone loss induced by modeled microgravity in mice. Can. J. Physiol. Pharmacol. 2020;98:678–683. doi: 10.1139/cjpp-2019-0632. [DOI] [PubMed] [Google Scholar]
- 74.Khajuria D.K., Disha C., Razdan R., Mahapatra D.R. Additive effect of zoledronic acid and alfacalcidol in the treatment of disuse osteoporosis in rats. Rev. Bras. Reumatol. 2015;55:240–250. doi: 10.1016/j.rbr.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 75.Braveboy-Wagner J., Sharoni Y., Lelkes P.I. Nutraceuticals synergistically promote osteogenesis in cultured 7F2 osteoblasts and mitigate inhibition of differentiation and maturation in simulated microgravity. Int. J. Mol. Sci. 2022;23:136. doi: 10.3390/ijms23010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Z., Zhang Y., Zhao F., Yin C., Yang C., Wang X., Wu Z., Liang S., Li D., Lin X., et al. Recombinant irisin prevents the reduction of osteoblast differentiation induced by stimulated microgravity through increasing β-catenin expression. Int. J. Mol. Sci. 2020;21:1259. doi: 10.3390/ijms21041259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ethiraj P., Ottinger A.M., Singh T., Singh A., Haire K.M., Reddy S.V. Proteasome inhibition suppress microgravity elevated RANK signaling during osteoclast differentiation. Cytokine. 2020;125:154821. doi: 10.1016/j.cyto.2019.154821. [DOI] [PubMed] [Google Scholar]
- 78.Sahana H., Khajuria D.K., Razdan R., Mahapatra D.R., Bhat M.R., Suresh S., Rao R.R., Mariappan L. Improvement in bone properties by using risedronate adsorbed hydroxyapatite novel nanoparticle based formulation in a rat model of osteoporosis. J. Biomed. Nanotechnol. 2013;9:193–201. doi: 10.1166/jbn.2013.1482. [DOI] [PubMed] [Google Scholar]
- 79.Khajuria D.K., Razdan R., Mahapatra D.R. Development, in vitro and in vivo characterization of zoledronic acid functionalized hydroxyapatite nanoparticle based formulation for treatment of osteoporosis in animal model. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2015;66:173–183. doi: 10.1016/j.ejps.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 80.Khajuria D.K., Disha C., Vasireddi R., Razdan R., Mahapatra D.R. Risedronate/zinc-hydroxyapatite based nanomedicine for osteoporosis. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;63:78–87. doi: 10.1016/j.msec.2016.02.062. [DOI] [PubMed] [Google Scholar]
- 81.Khajuria D.K., Vasireddi R., Trebbin M., Karasik D., Razdan R. Novel therapeutic intervention for osteoporosis prepared with strontium hydroxyapatite and zoledronic acid: In vitro and pharmacodynamic evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;71:698–708. doi: 10.1016/j.msec.2016.10.066. [DOI] [PubMed] [Google Scholar]
- 82.Jilka R.L. The relevance of mouse models for investigating age-related bone loss in humans. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:1209–1217. doi: 10.1093/gerona/glt046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Finkelstein J.S., Brockwell S.E., Mehta V., Greendale G.A., Sowers M.R., Ettinger B., Lo J.C., Johnston J.M., Cauley J.A., Danielson M.E., et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J. Clin. Endocrinol. Metab. 2008;93:861–868. doi: 10.1210/jc.2007-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guthrie J.R., Ebeling P.R., Hopper J.L., Barrett-Connor E., Dennerstein L., Dudley E.C., Burger H.G., Wark J.D. A prospective study of bone loss in menopausal Australian-born women. Osteoporos. Int. 1998;8:282–290. doi: 10.1007/s001980050066. [DOI] [PubMed] [Google Scholar]
- 85.Keshawarz N.M., Recker R.R. Expansion of the medullary cavity at the expense of cortex in postmenopausal osteoporosis. Metab. Bone Dis. Relat. Res. 1984;5:223–228. doi: 10.1016/0221-8747(84)90063-8. [DOI] [PubMed] [Google Scholar]
- 86.Akesson K., Ljunghall S., Jonsson B., Sernbo I., Johnell O., Gärdsell P., Obrant K.J. Assessment of biochemical markers of bone metabolism in relation to the occurrence of fracture: A retrospective and prospective population-based study of women. J. Bone Miner. Res. 1995;10:1823–1829. doi: 10.1002/jbmr.5650101127. [DOI] [PubMed] [Google Scholar]
- 87.Garnero P., Hausherr E., Chapuy M.C., Marcelli C., Grandjean H., Muller C., Cormier C., Bréart G., Meunier P.J., Delmas P.D. Markers of bone resorption predict hip fracture in elderly women: The EPIDOS Prospective Study. J. Bone Miner. Res. 1996;11:1531–1538. doi: 10.1002/jbmr.5650111021. [DOI] [PubMed] [Google Scholar]
- 88.Garnero P., Shih W.J., Gineyts E., Karpf D.B., Delmas P.D. Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J. Clin. Endocrinol. Metab. 1994;79:1693–1700. doi: 10.1210/jcem.79.6.7989477. [DOI] [PubMed] [Google Scholar]
- 89.Delmas P.D., Eastell R., Garnero P., Seibel M.J., Stepan J. The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the International Osteoporosis Foundation. Osteoporos. Int. 2000;11((Suppl. S6)):S2–S17. doi: 10.1007/s001980070002. [DOI] [PubMed] [Google Scholar]
- 90.Fabre S., Funck-Brentano T., Cohen-Solal M. Anti-Sclerostin Antibodies in Osteoporosis and Other Bone Diseases. J. Clin. Med. 2020;9:3439. doi: 10.3390/jcm9113439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chan C.Y., Subramaniam S., Mohamed N., Muhammad N., Ramli F.F., Ima-Nirwana S., Chin K.Y. Circulating Biomarkers Related to Osteocyte and Calcium Homeostasis Between Postmenopausal Women with and without Osteoporosis. Endocr. Metab. Immune Disord. Drug Targets. 2021;21:2273–2280. doi: 10.2174/1871530321666210809154456. [DOI] [PubMed] [Google Scholar]
- 92.Xu X.J., Shen L., Yang Y.P., Lu F.R., Zhu R., Shuai B., Li C.G., Wu M.X. Serum sclerostin levels associated with lumbar spine bone mineral density and bone turnover markers in patients with postmenopausal osteoporosis. Chin. Med. J. 2013;126:2480–2484. [PubMed] [Google Scholar]
- 93.Wang Y., Song W., Jing S., Yu J. Effect of estrogen deficiency on the proliferation and osteogenic differentiation potential of mandibular bone marrow stromal cells. Zhonghua Kou Qiang Yi Xue Za Zhi. 2014;49:619–624. [PubMed] [Google Scholar]
- 94.Schiavi J., Fodera D.M., Brennan M.A., McNamara L.M. Estrogen depletion alters osteogenic differentiation and matrix production by osteoblasts in vitro. Exp. Cell Res. 2021;408:112814. doi: 10.1016/j.yexcr.2021.112814. [DOI] [PubMed] [Google Scholar]
- 95.Stolzenberg N., Belavý D.L., Beller G., Armbrecht G., Semler J., Felsenberg D. Bone strength and density via pQCT in post-menopausal osteopenic women after 9 months resistive exercise with whole body vibration or proprioceptive exercise. J. Musculoskelet Neuronal Interact. 2013;13:66–76. [PubMed] [Google Scholar]
- 96.Kerr D., Morton A., Dick I., Prince R. Exercise effects on bone mass in postmenopausal women are site-specific and load-dependent. J. Bone Miner. Res. 1996;11:218–225. doi: 10.1002/jbmr.5650110211. [DOI] [PubMed] [Google Scholar]
- 97.Nelson M.E., Fisher E.C., Dilmanian F.A., Dallal G.E., Evans W.J. A 1-y walking program and increased dietary calcium in postmenopausal women: Effects on bone. Am. J. Clin. Nutr. 1991;53:1304–1311. doi: 10.1093/ajcn/53.5.1304. [DOI] [PubMed] [Google Scholar]
- 98.Bloomfield S.A., Williams N.I., Lamb D.R., Jackson R.D. Non-weightbearing exercise may increase lumbar spine bone mineral density in healthy postmenopausal women. Am. J. Phys. Med. Rehabil. 1993;72:204–209. doi: 10.1097/00002060-199308000-00006. [DOI] [PubMed] [Google Scholar]
- 99.Sanad Z., Ellakwa H., Desouky B. Comparison of alendronate and raloxifene in postmenopausal women with osteoporosis. Climacteric. 2011;14:369–377. doi: 10.3109/13697137.2010.537408. [DOI] [PubMed] [Google Scholar]
- 100.Nakamura T., Matsumoto T., Sugimoto T., Hosoi T., Miki T., Gorai I., Yoshikawa H., Tanaka Y., Tanaka S., Sone T., et al. Clinical Trials Express: Fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: Denosumab fracture intervention randomized placebo controlled trial (DIRECT) J. Clin. Endocrinol. Metab. 2014;99:2599–2607. doi: 10.1210/jc.2013-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kendler D.L., Palacios S., Cox D.A., Stock J., Alam J., Dowsett S.A., Zanchetta J. Arzoxifene versus raloxifene: Effect on bone and safety parameters in postmenopausal women with osteoporosis. Osteoporos. Int. 2012;23:1091–1101. doi: 10.1007/s00198-011-1587-0. [DOI] [PubMed] [Google Scholar]
- 102.Bone H.G., Wagman R.B., Brandi M.L., Brown J.P., Chapurlat R., Cummings S.R., Czerwiński E., Fahrleitner-Pammer A., Kendler D.L., Lippuner K., et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: Results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513–523. doi: 10.1016/S2213-8587(17)30138-9. [DOI] [PubMed] [Google Scholar]
- 103.Tsai J.N., Uihlein A.V., Lee H., Kumbhani R., Siwila-Sackman E., McKay E.A., Burnett-Bowie S.A., Neer R.M., Leder B.Z. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: The DATA study randomised trial. Lancet. 2013;382:50–56. doi: 10.1016/S0140-6736(13)60856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gossiel F., Scott J.R., Paggiosi M.A., Naylor K.E., McCloskey E.V., Peel N.F.A., Walsh J.S., Eastell R. Effect of Teriparatide Treatment on Circulating Periostin and Its Relationship to Regulators of Bone Formation and BMD in Postmenopausal Women With Osteoporosis. J. Clin. Endocrinol. Metab. 2018;103:1302–1309. doi: 10.1210/jc.2017-00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Allen M.R., Burr D.B. Bisphosphonate effects on bone turnover, microdamage, and mechanical properties: What we think we know and what we know that we don’t know. Bone. 2011;49:56–65. doi: 10.1016/j.bone.2010.10.159. [DOI] [PubMed] [Google Scholar]
- 106.Qaisi M., Hargett J., Loeb M., Brown J., Caloss R. Denosumab Related Osteonecrosis of the Jaw with Spontaneous Necrosis of the Soft Palate: Report of a Life Threatening Case. Case Rep. Dent. 2016;2016:5070187. doi: 10.1155/2016/5070187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Diz P., López-Cedrún J.L., Arenaz J., Scully C. Denosumab-related osteonecrosis of the jaw. J. Am. Dent. Assoc. 2012;143:981–984. doi: 10.14219/jada.archive.2012.0323. [DOI] [PubMed] [Google Scholar]
- 108.Hamadeh I.S., Ngwa B.A., Gong Y. Drug induced osteonecrosis of the jaw. Cancer Treat. Rev. 2015;41:455–464. doi: 10.1016/j.ctrv.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 109.Komori T. Animal models for osteoporosis. Eur. J. Pharmacol. 2015;759:287–294. doi: 10.1016/j.ejphar.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 110.Wang H., Zhang H., Srinivasan V., Tao J., Sun W., Lin X., Wu T., Boyce B.F., Ebetino F.H., Boeckman R.K., Jr., et al. Targeting Bortezomib to Bone Increases Its Bone Anabolic Activity and Reduces Systemic Adverse Effects in Mice. J. Bone Miner. Res. 2020;35:343–356. doi: 10.1002/jbmr.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim S.H., Kim M.O., Kim H.J., Neupane S., Kim H.J., Lee J.H., Kim H.H., Kim J.Y., Lee Y. Bortezomib prevents ovariectomy-induced osteoporosis in mice by inhibiting osteoclast differentiation. J. Bone Miner. Metab. 2018;36:537–546. doi: 10.1007/s00774-017-0871-2. [DOI] [PubMed] [Google Scholar]
- 112.Kim H.Y., Kim K.S., Kim M.J., Kim H.S., Lee K.Y., Kang K.W. Auranofin Inhibits RANKL-Induced Osteoclastogenesis by Suppressing Inhibitors of κB Kinase and Inflammasome-Mediated Interleukin-1β Secretion. Oxid. Med. Cell Longev. 2019;2019:3503912. doi: 10.1155/2019/3503912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.He B., Zhu Y., Cui H., Sun B., Su T., Wen P. Comparison of Necroptosis With Apoptosis for OVX-Induced Osteoporosis. Front. Mol. Biosci. 2021;8:790613. doi: 10.3389/fmolb.2021.790613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kitazawa R., Kimble R.B., Vannice J.L., Kung V.T., Pacifici R. Interleukin-1 receptor antagonist and tumor necrosis factor binding protein decrease osteoclast formation and bone resorption in ovariectomized mice. J. Clin. Investig. 1994;94:2397–2406. doi: 10.1172/JCI117606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Girasole G., Jilka R.L., Passeri G., Boswell S., Boder G., Williams D.C., Manolagas S.C. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: A potential mechanism for the antiosteoporotic effect of estrogens. J. Clin. Investig. 1992;89:883–891. doi: 10.1172/JCI115668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Littlewood A.J., Aarden L.A., Evans D.B., Russell R.G., Gowen M. Human osteoblastlike cells do not respond to interleukin-6. J. Bone Miner. Res. 1991;6:141–148. doi: 10.1002/jbmr.5650060207. [DOI] [PubMed] [Google Scholar]
- 117.Kimble R.B., Bain S., Pacifici R. The functional block of TNF but not of IL-6 prevents bone loss in ovariectomized mice. J. Bone Miner. Res. 1997;12:935–941. doi: 10.1359/jbmr.1997.12.6.935. [DOI] [PubMed] [Google Scholar]
- 118.Wotring V. Evidence Report: Risk of Therapeutic Failure Due to Ineffectiveness of Medication. [(accessed on 5 April 2022)]; Available online: https://humanresearchroadmap.nasa.gov/Evidence/reports/Pharm.pdf.
- 119.Gandia P., Saivin S., Houin G. The influence of weightlessness on pharmacokinetics. Fundam. Clin. Pharmacol. 2005;19:625–636. doi: 10.1111/j.1472-8206.2005.00374.x. [DOI] [PubMed] [Google Scholar]
- 120.Gandia P., Bareille M.P., Saivin S., Le-Traon A.P., Lavit M., Guell A., Houin G. Influence of simulated weightlessness on the oral pharmacokinetics of acetaminophen as a gastric emptying probe in man: A plasma and a saliva study. J. Clin. Pharmacol. 2003;43:1235–1243. doi: 10.1177/0091270003257229. [DOI] [PubMed] [Google Scholar]
- 121.Merrill A.H., Jr., Wang E., LaRocque R., Mullins R.E., Morgan E.T., Hargrove J.L., Bonkovsky H.L., Popova I.A. Differences in glycogen, lipids, and enzymes in livers from rats flown on COSMOS 2044. J. Appl. Physiol. 1992;73:142S–147S. doi: 10.1152/jappl.1992.73.2.S142. [DOI] [PubMed] [Google Scholar]
- 122.Merrill A.H., Jr., Hoel M., Wang E., Mullins R.E., Hargrove J.L., Jones D.P., Popova I.A. Altered carbohydrate, lipid, and xenobiotic metabolism by liver from rats flown on Cosmos 1887. FASEB J. 1990;4:95–100. doi: 10.1096/fasebj.4.1.2295381. [DOI] [PubMed] [Google Scholar]
- 123.Merrill A.H., Jr., Wang E., Jones D.P., Hargrove J.L. Hepatic function in rats after spaceflight: Effects on lipids, glycogen, and enzymes. Am. J. Physiol. 1987;252:R222–R226. doi: 10.1152/ajpregu.1987.252.2.R222. [DOI] [PubMed] [Google Scholar]
- 124.Luo Y., Ma Y., Qiao X., Zeng R., Cheng R., Nie Y., Li S., A R., Shen X., Yang M., et al. Irisin ameliorates bone loss in ovariectomized mice. Climacteric. 2020;23:496–504. doi: 10.1080/13697137.2020.1745768. [DOI] [PubMed] [Google Scholar]
- 125.Morgan E.N., Alsharidah A.S., Mousa A.M., Edrees H.M. Irisin Has a Protective Role against Osteoporosis in Ovariectomized Rats. Biomed. Res. Int. 2021;2021:5570229. doi: 10.1155/2021/5570229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu J.M., Wai-Chee Kung A., Pheng C.S., Zhu H.M., Zhang Z.L., Wu Y.Y., Xu L., Meng X.W., Huang M.L., Chung L.P., et al. Efficacy and safety of 2 g/day of strontium ranelate in Asian women with postmenopausal osteoporosis. Bone. 2009;45:460–465. doi: 10.1016/j.bone.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 127.Reginster J.Y., Bruyère O., Sawicki A., Roces-Varela A., Fardellone P., Roberts A., Devogelaer J.P. Long-term treatment of postmenopausal osteoporosis with strontium ranelate: Results at 8 years. Bone. 2009;45:1059–1064. doi: 10.1016/j.bone.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 128.Talaulikar V.S., Chambers T., Manyonda I. Exploiting the antioxidant potential of a common vitamin: Could vitamin C prevent postmenopausal osteoporosis? J. Obstet. Gynaecol. Res. 2012;38:253–257. doi: 10.1111/j.1447-0756.2011.01629.x. [DOI] [PubMed] [Google Scholar]
- 129.Wong R.H., Thaung Zaw J.J., Xian C.J., Howe P.R. Regular Supplementation With Resveratrol Improves Bone Mineral Density in Postmenopausal Women: A Randomized, Placebo-Controlled Trial. J. Bone Miner. Res. 2020;35:2121–2131. doi: 10.1002/jbmr.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Khanizadeh F., Rahmani A., Asadollahi K., Ahmadi M.R.H. Combination therapy of curcumin and alendronate modulates bone turnover markers and enhances bone mineral density in postmenopausal women with osteoporosis. Arch. Endocrinol. Metab. 2018;62:438–445. doi: 10.20945/2359-3997000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fournier R., Harrison R.E. Strategies for studying bone loss in microgravity. Reach. 2020;17:100036. doi: 10.1016/j.reach.2020.100036. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.