Abstract
Fibroblast growth factor receptor (FGFR) genomic alterations (GAs) represent an actionable target, key to the pathogenesis of some urothelial cancers (UCs). Though FGFR GAs are common in noninvasive UC, little is known about their role in the metastatic(m) setting and response to therapy. This study aimed to assess the impact of FGFR alterations on sensitivity to systemic treatments and survival and to validate Bajorin’s and Bellmunt’s prognostic scores in mUC patients according to their FGFR status. We retrospectively analyzed data from 98 patients with tumor-sequenced UC who received treatment between January 2010 and December 2020. Up to 77 developed metastatic disease and were deemed the study population. Twenty-six showed FGFR GAs. A trend toward a better response to cisplatin and checkpoint inhibitors was suggested favoring FGFR GA tumors. FGFR GA patients who received an FGFR inhibitor as first-line had poorer responses compared with other options (20% vs. 68.4%, p = 0.0065). Median PFS was 6 vs. 5 months in the FGFR GA vs. FGFR WT cohort (p = 0.71). Median OS was significantly worse in the FGFR GA vs. FGFR WT cohort (16.2 vs. 31.9 months, p = 0.045). Multivariate analyses deemed FGFR GAs as a factor independently associated with the outcome (HR 2.59 (95% CI 1.21–5.55)). Bajorin’s model correctly predicted clinical outcomes in the whole study population but not in FGFR GA cases. FGFR GAs are a relevant biomarker in mUC that could condition the response to systemic therapy. New prognostic models, including this molecular determination, should be designed and validated.
Keywords: FGFR, independent factor, metastatic urothelial cancer
1. Introduction
Bladder cancer is the eleventh most common cancer worldwide [1]. Genetic and environmental factors, such as smoking, exposure to occupational carcinogens, and infections, can lead to this disease [2,3]. The prognosis for patients with mUC remains poor. Around 25% of cases will present advanced disease at diagnosis or will develop metastases [1].
Bajorin’s prognostic model has been widely adopted in this disease and classifies mUC in three risk categories based on two prognostic factors: a Karnofsky performance status (KPS) of <80% and visceral metastases. Median survival varies between 9.3 and 33 months for poor and favorable risk patients, respectively [4].
Fortunately, significant improvements have been made in the therapeutic landscape of mUC. New options include checkpoint inhibitors (CPIs), FGFR inhibitors (FGFRis), and antigen-directed cytotoxic therapies (enfortumab vedotin or sacituzumab govitecan), which have revolutionized the management of this disease [5,6,7]. Thus, understanding the clinical implications of the molecular background of individual patients will help to define the optimal sequence of treatment options.
Fibroblast growth factor receptor gene alterations are common molecular findings in UC. Activating somatic mutations of FGFR3 have been detected in 50–70% of nonmuscle-invasive carcinomas and in 20% of mUC [8,9].
FGFR alterations have been identified as an early event in UC development.
Most of the missense mutations in the FGFR3 gene are clustered in three hotspots in exons 7, 10, and 15. The substitution of a serine for a cysteine at position 249 (S249C) is the most frequent pathogenic mutation. [10]. These alterations can lead to ligand-independent dimerization, autophosphorylation, and activation of the receptor [8].
Another frequent alteration is the fusion of FGFR3 to transforming acid coiled-coil containing protein 3 (TACC3), which leads to constitutive activation of the tyrosine kinase dominium [11]. FGFR3 amplification and alternative splicing are less frequent, and their functional implications are not yet fully understood [12].
Erdafitinib is the first FGFR antagonist approved by the FDA (2019) for the treatment of mUC with FGFR3 or FGFR2 mutations, but many others, such as rogaratinib, pemigatinib, and infigratinib, are being explored in different clinical settings [5,8,13].
Since FGFR GAs have become a cornerstone in mUC, we aimed to assess the value of such alterations and validate the current prognostic models in this particular population.
2. Materials (Patients) and Methods
2.1. Study Design and Patients
We designed a multicenter observational retrospective study. Eligible patients were adults diagnosed with UC from four hospitals in Madrid (Spain) between January 2010 and December 2020. The study protocol was approved by the institutional ethics committee, and patients provided written consent. Clinical records of patients who were deceased or lost to follow-up were reviewed following the instructions of the ethics committee. Only patients with tumor-sequenced metastatic disease who had received treatment were included in the analysis.
2.2. Genomic Analysis
Detection of FGFR mutations and fusions was performed on DNA isolated from formalin-fixed and paraffinized tumor tissue samples. The next-generation sequencing panel Foundation One® or qualitative real-time polymerase chain reaction-based assays (n = 9) with TFGFR or QIAGEN therascreen® were performed as routine practice.
2.3. Outcomes
The radiological response was based on the investigators’ judgment. PFS was calculated from the treatment start date to progression or death; OS was defined as the time from the treatment start date to the date of death from any cause. Patients without an event were censored at the date of last follow-up.
2.4. Statistical Analysis
Descriptive analysis was used to summarize the baseline characteristics of the study population and every cohort (FGFR GA or FGFR WT). Categorical variables were summarized as absolute frequency (%), while numerical variables were summarized as mean ± SD for normally distributed variables (Shapiro test, p > 0.05) or median (IQR) otherwise. Associations between FGFR alterations and clinical factors were analyzed using X2 or Fisher’s exact tests when needed for categorical variables and t-test or Mann–Whitney U test for numerical variables. Univariate Cox proportional hazard regression was performed to assess for correlations between FGFR altered genes, relevant baseline clinical and treatment characteristics, and survival. Cox proportional hazard models were applied for multivariate analysis. The hazard ratio (HR) and 95% confidence interval (CI) were calculated for every prognostic factor. Results were considered statistically significant at two-sided p-values of <0.05. PFS and OS were estimated using the Kaplan–Meier method, and statistical significance was assessed using the log-rank test. All analyses were performed using R (version 4.1.1).
3. Results
3.1. Overall Study Population
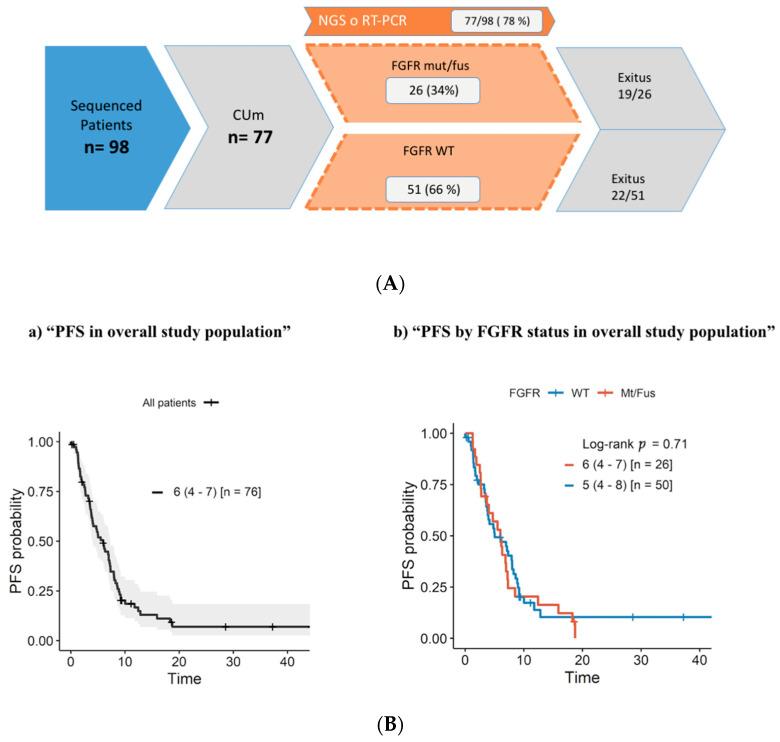
A total of 98 UC patients that underwent any form of FGFR testing were identified (Supplementary Table S1). Up to 77 developed metastatic disease and were deemed eligible for the study (Table 1; Figure 1A). The FGFR genomic alterations are summarized in Table 2.
Table 1.
Study population demographics (n = 77) and comparison between FGFR GA and WT patients.
| Variable | Modality | Metastatic | Mt/Fus (n = 26) | WT (n = 51) | p-Value |
|---|---|---|---|---|---|
| Age | Median (IQR) | 69 (62–76) | 69 (63–77) | 69 (61–75) | 0.45 |
| Sex | Male | 55 (71.4%) | 17 (65.4%) | 38 (74.5%) | 0.57 |
| Female | 22 (28.6%) | 9 (34.6%) | 13 (25.5%) | ||
| ECOG PS | 0 | 26 (33.8%) | 9 (34.6%) | 17 (33.3%) | 0.15 |
| 1 | 25 (32.4%) | 14 (53.8%) | 11 (21.6%) | ||
| 2 | 2 (2.6%) | 0 (0%) | 2 (3.9%) | ||
| Not available | 24 (31.2%) | 3 (11.5%) | 21 (41.2%) | ||
| Smoking | Never smoked | 16 (25.4%) | 7 (26.9%) | 9 (17.6%) | 0.55 |
| Current smoker | 9 (14.3%) | 3 (11.5%) | 6 (11.8%) | ||
| Former smoker | 38 (60.3%) | 11 (42.3%) | 27 (52.9%) | ||
| Not available | 14 (18.2%) | 5 (19.2%) | 9 (17.6%) | ||
| Tumor location | Bladder | 62 (80.5%) | 17 (65.4%) | 45 (88.2%) | 0.037 |
| Nonbladder | 15 (19.5%) | 9 (34.6%) | 6 (11.8%) | ||
| Surgery | No | 4 (5.2%) | 1 (3.8%) | 3 (5.9%) | 1 |
| Yes | 73 (94.8%) | 25 (96.2%) | 48 (94.1%) | ||
| Surgery extension | Cystectomy (RC) | 38 (49.3%) | 7 (28.0%) | 31 (64.6%) | 0.0076 |
| Nephroureterectomy/nephrectomy(NU) | 12 (15.6%) | 8 (32.0%) | 4 (8.3%) | ||
| NU + RC | 3 (3.9%) | 1 (4.0%) | 2 (4.2%) | ||
| TURBT | 20 (26%) | 9 (36.0%) | 11 (22.9%) | ||
| Lymphadenectomy | No | 36 (46.8%) | 15 (57.7%) | 21 (41.2%) | 0.37 |
| Yes | 38 (49.3%) | 11 (42.3%) | 27 (52.9%) | ||
| Not available | 3 (3.9%) | 0 (0.0%) | 3 (5.9%) | ||
| Bladder preservation | Radiotherapy | 4 (5.2%) | 2 (7.7%) | 2 (3.9%) | 0.86 |
| Chemo-radiotherapy | 6 (7.8%) | 2 (7.7%) | 4 (7.8%) | ||
| No | 67 (87.0%) | 22 (84.6%) | 45 (88.2%) | ||
| pT | 1 | 7 (9.1%) | 3 (11.5%) | 4 (7.8%) | 0.022 |
| 2 | 28 (36.4%) | 8 (30.8%) | 20 (39.2%) | ||
| 3 | 28 (36.4%) | 6 (23.1%) | 22 (43.1%) | ||
| 4 | 13 (16.8%) | 9 (34.6%) | 4 (7.8%) | ||
| Not available | 1 (1.3%) | 0 (0.0%) | 1 (2.0%) | ||
| pN | 0 | 14 (36.8%) | 3 (27.3%) | 11 (40.7%) | 0.81 |
| 1 | 11 (28.9%) | 4 (36.4%) | 7 (25.9%) | ||
| 2 | 12 (31.6%) | 4 (36.4%) | 8 (29.6%) | ||
| 3 | 1 (2.6%) | 0 (0%) | 1 (3.7%) | ||
| Grade | 2 | 2 (2.6%) | 1 (3.8%) | 1 (2%) | 1 |
| 3 | 73 (94.8%) | 25 (96.2%) | 48 (94.1%) | ||
| NA, n (%) | 2 (2.6%) | 0 (0.0%) | 2 (3.9%) | ||
| Variant histologies | Transitional cells | 70 (90.9%) | 23 (88.5%) | 47 (92.2%) | 0.71 |
| Squamous | 5 (6.5%) | 2 (7.7%) | 3 (5.9%) | ||
| Anaplastic | 1 (1.3%) | 1 (3.8%) | 0 (0%) | ||
| Neuroendocrine | 1 (1.3%) | 0 (0%) | 1 (2%) | ||
| Perioperative chemotherapy | No | 52 (67.5%) | 16 (61.5%) | 36 (70.6%) | 0.67 |
| Neoadjuvant | 12 (15.6%) | 5 (19.2%) | 7 (13.7%) | ||
| Adjuvant | 13 (16.9%) | 5 (19.2%) | 8 (15.7%) | ||
| Liver metastases | No | 61 (79.2%) | 22 (84.6%) | 39 (76.5%) | 0.7 |
| Yes | 15 (19.5%) | 4 (15.4%) | 11 (21.6%) | ||
| Not available | 1 (1.3%) | 0 (0.0%) | 1 (2.0%) | ||
| Bone metastases | No | 46 (59.7%) | 15 (57.7%) | 31 (60.8%) | 0.91 |
| Yes | 30 (39.0%) | 11 (42.3%) | 19 (37.3%) | ||
| Not available | 1 (1.3%) | 0 (0.0%) | 1 (2.0%) | ||
| Lymph node only metastases | Yes | 15 (19.5%) | 5 (19.2%) | 10 (19.6%) | 1 |
| Visceral metastases | Yes | 60 (77.9%) | 21 (80.8%) | 39 (76.5%) | 1 |
| Not available | 1 (1.3%) | 0 (0.0%) | 1 (2.0%) | ||
| First-line treatment for mUC | Cisplatin-based | 33 (42.8%) | 11 (42.3%) | 22 (44.0%) | |
| Checkpoint inhibitors | 23 (29.9%) | 5 (19.2%) | 18 (36.0%) | ||
| Carboplatin-based | 8 (10.4%) | 2 (7.7%) | 6 (12.0%) | ||
| FGFR inhibitor | 5 (6.5%) | 5 (19.2%) | 0 (0.0%) | ||
| Vinflunine | 3 (3.9%) | 1 (3.8%) | 2 (4.0%) | ||
| Best supportive care | 2 (2.6%) | 2 (7.7%) | 0 (0.0%) | ||
| Paclitaxel | 1 (1.3%) | 0 (0.0%) | 1 (2.0%) | ||
| Surgery | 1 (1.3%) | 0 (0.0%) | 1 (2.0%) | ||
| Not available | 1 (1.3%) | (0%) | 1 (2.0%) |
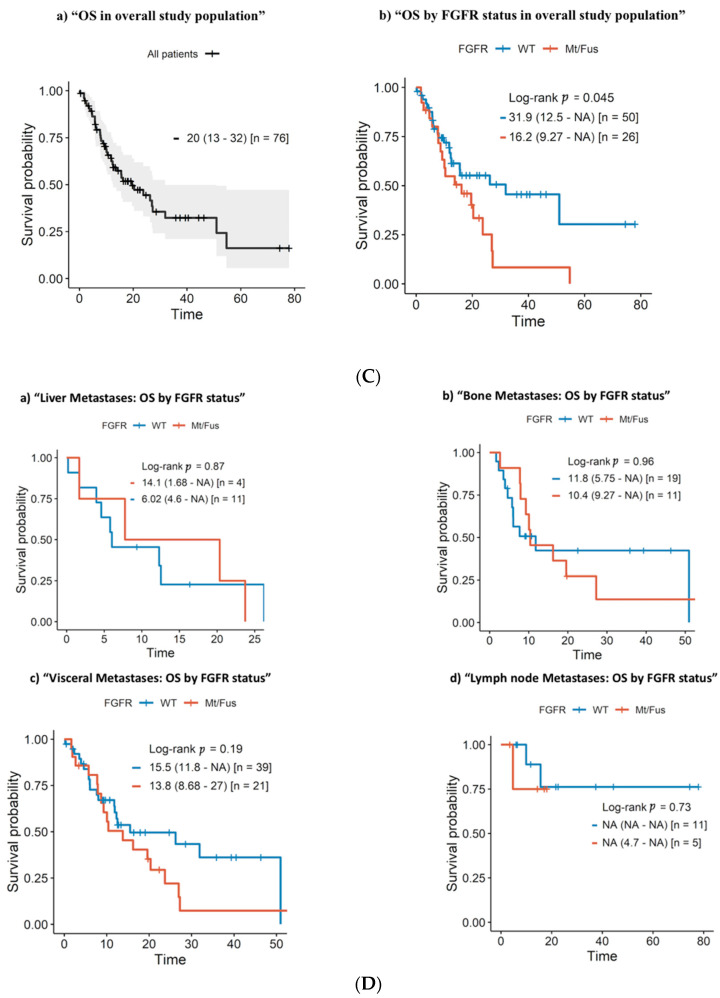
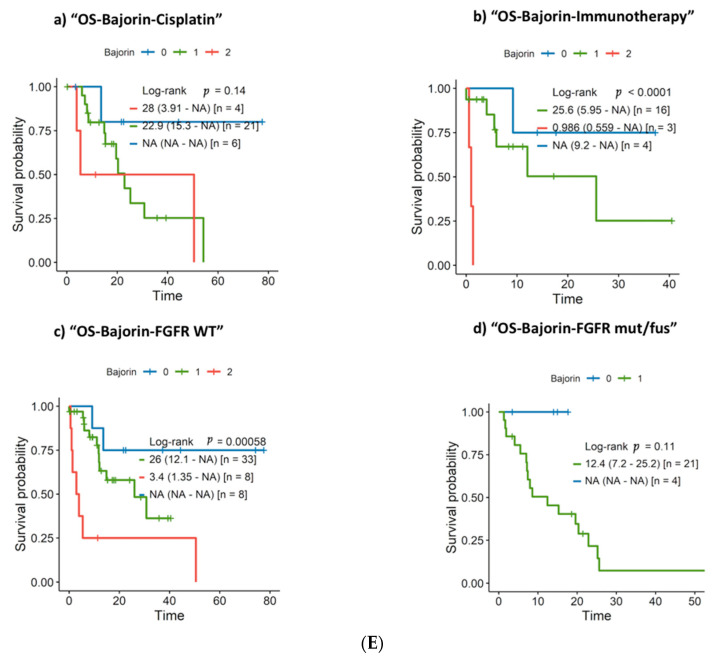
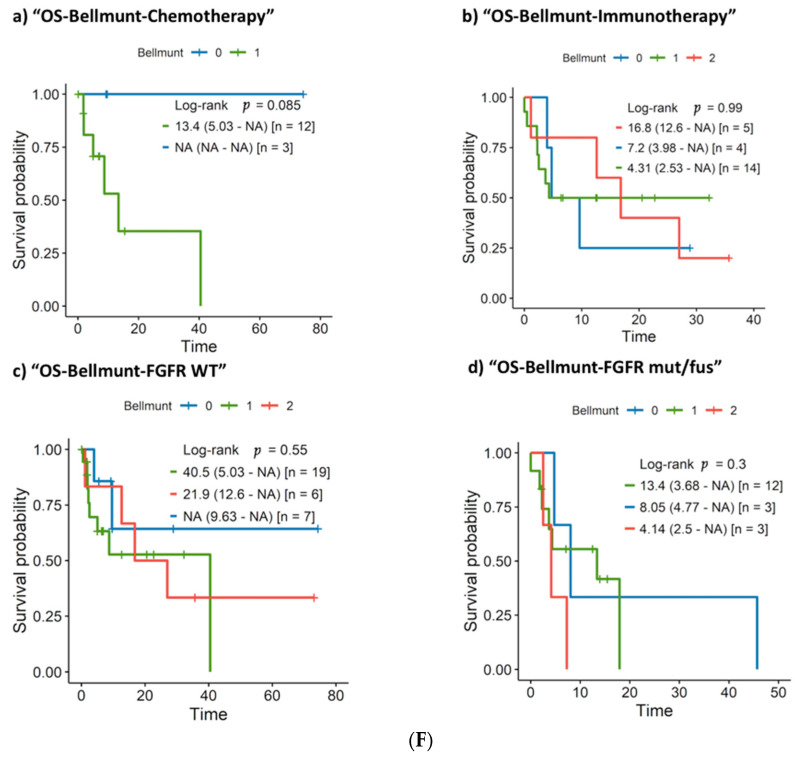
Figure 1.
(A) Study population flowchart. (B) Progression-free survival according to FGFR genomic alterations. (C) Overall survival according to FGFR status. (D) Overall survival according to FGFR status in patients with liver metastases, bone metastases, visceral metastases, or lymph node only metastases. (E) Bajorin’s criteria in patients treated with first-line cisplatin-based therapy (a) and immune therapy (b); Bajorin’s criteria in FGFR WT patients (c); Bajorin’s criteria in FGFR mut/fus patients (d). (F) Bellmunt’s criteria for overall survival in patients treated with second-line chemotherapy (a) and immune therapy (b); Bellmunt’s prognostic factors in FGFR WT patients (c) and FGFR GA patients (d).
Table 2.
FGFR genomic alterations.
| TYPE OF FGFR | N (26) | % |
|---|---|---|
| GENOMIC ALTERATION (Number of Cases) | ||
| MUTATION | 15 | 57.7% |
| -FGFR3 S249C (13) | ||
| -FGFR3 S249C // S783 frameshift mutation (1) | ||
| -FGFR3 S249C // H349D (1) | ||
| FUSION | 6 | 23.1% |
| -FGFR1-FGFR1 (1) | ||
| -FGFR3-TACC3 (3) | ||
| -FGFR2-OFD1 (1) -FGFR2-AFF3 (1) | ||
| MUTATION + AMPLIFICATION | 2 | 7.7% |
| -FGFR3 S249C + FGFR1 amplification (1) | ||
| -FGFR3 S249C + FGFR1 amplification (1) | ||
| MUTATION + FUSION | 1 | 3.8% |
| -FGFR3 R248C // S249C + FGFR3-TACC3 (1) | ||
| FUSION + AMPLIFICATION | 2 | 7.7% |
| -FGFR2-RTKN2 + FGFR2 amplification (1) -FGFR3-TACC3 + FGFR1 amplification (1) |
Next-generation sequencing with the Foundation One® test was performed in 68 cases, and qualitative real-time polymerase chain reaction-based assay TFGFR or QIAGEN therascreen® tests in 9. TFGFR or QIAGEN therascreen® tests evaluated somatic mutations within the FGFR3 gene: R248C, S249C, G370C, and Y373C, and fusions: FGFR3-TACC3v3, FGFR3-TACC3v1, FGFR3-BAIAP2L1, FGFR2-BICC1, and FGFR2-CASP7.
3.2. Patient Characteristics
We compared baseline characteristics between FGFR GA and WT patients. Age, sex, ECOG, and smoking history did not differ significantly between FGFR status groups. Upper tract tumor location was more common in the FGFR GA cohort (p = 0.037), and more nephroureterectomies were performed consequently (p = 0.0076). FGFR GAs were associated with more pT4 stages (p = 0.022). (Table 1).
3.3. First-Line Treatment Outcome
First-line systemic treatment consisted of cisplatinum-based chemotherapy (n = 32), checkpoint inhibitors (CPIs) (n = 21), FGFRis (n = 5), and others (n = 19) in 26 FGFR GA and 51 FGFR WT patients.
In the whole study population, the ORR was 50%, including 7.1% (n = 5) CRs and 43% (n = 30) PRs. Eight additional patients (11.4%) achieved stable disease (SD) as the best response, with a disease control rate (DCR) of 61.5%.
The ORR to first-line cisplatin-based therapy was 59.4% and 38.1% with CPI. FGFR GA patients showed a trend toward a better response to cisplatin and checkpoint inhibitors with regards to the WT cohort. No statistically significant differences in PFS and OS according to FGFR status were found in the first-line therapy subgroups (Supplementary Figure S4).
Five FGFR GA patients who received a first-line FGFRi had poorer responses compared with other first-line options (20% vs. 68.4%, p = 0.0065). (Table 3).
Table 3.
Treatment response to first-line therapy according to FGFR status and specific therapy according to the RECIST criteria v1.1.
| Population | Treatment (n) | Type of Response | ||||||
|---|---|---|---|---|---|---|---|---|
| CR | PR | SD | PD | ORR | p-Value * | p-Value ORR | ||
| Overall | Any (70) | 5 (7.1%) | 30 (42.9%) | 8 (11.4%) | 27 (38.6%) | 35 (50.0%) | ||
| FGFR WT | 3 (6.5%) | 18 (39.1%) | 5 (10.9%) | 20 (43.5%) | 21 (45.7%) | 0.71 | 0.57 | |
| FGFR mut/fus | 2 (8.3%) | 12 (50.0%) | 3 (12.5%) | 7 (29.2%) | 14 (58.3%) | |||
| Overall | Cisplatinum-based (32) | 2 (6.3%) | 17 (53,1%) | 5 (15.6%) | 8 (25.0%) | 19 (59.4%) | ||
| FGFR WT | 1 (4.8%) | 10 (47.6%) | 3 (14.3%) | 7 (33.3%) | 11 (52.4%) | 0.43 | 0.45 | |
| FGFR mut/fus | 1 (9.1%) | 7 (63.6%) | 2 (18.2%) | 1 (9.1%) | 8 (72.7%) | |||
| Overall | Immunotherapy (21) | 2 (9.5%) | 6 (28.6%) | 2 (9.5%) | 11 (52.4%) | 8 (38.1%) | ||
| FGFR WT | 1 (6.3%) | 4 (25.0%) | 2 (12.5%) | 9 (56.3%) | 5 (31.3%) | 0.59 | 0.33 | |
| FGFR mut/fus | 1 (20.0%) | 2 (40.0%) | 0 (0.0%) | 2 (40.0%) | 3 (60.0%) | |||
| FGFR mut/fus |
FGFR inhibitors (5)
Other (19) |
0 (0.0%) | 1 (20.0%) | 0 (0.0%) | 4 (80.0%) | 1 (20.0%) | 0.065 | 0.12 |
| 2 (10.5%) | 11 (57.9%) | 3 (15.8%) | 3 (15.8%) | 13 (68.4%) | ||||
* (ref) p-values for the four types of response and for the proportion of ORR were obtained using Chi-squared tests or Fisher exact test when necessary.
3.4. Progression-Free Survival and Overall Survival
PFS and OS information was available in 76 of the 77 patients.
Median PFS after first-line treatment for the whole study population was 6 months in the FGFR mut/fus cohort and 5 months in the FGFR WT patient group (p = 0.71) (Figure 1B).
Median OS for the whole study population was 20 months and was significantly worse in FGFR GA (mutation/fusion) vs. FGFR WT tumors (16.2 vs. 31.9 months, p = 0.045; Figure 1C). When stratified by metastases location (liver, bone, visceral, and lymph node), there were no significant differences between FGFR GA and WT patients (Figure 1D).
No significant differences were found in OS in patients with variant histologies (n = 7) (p = 0.67) or when comparing urothelial with squamous (n = 5, p = 0.94). There were also no differences in clinical outcomes among patients with bladder and UTUC (p = 0.88) or when stratifying by FGFR status (Supplementary Figures S2 and S3).
3.5. Clinical and Molecular Prognostic Factors: Univariate and Multivariate Survival Analysis
The log-rank test revealed that FGFR GA tumors were associated with a shorter OS, and the univariate Cox regression model confirmed this result (HR 1.87 (95%CI 1.01 to 3.48)). Univariate analyses revealed other variables associated with survival: the presence of visceral metastases (HR 4.87 (95% CI 1.48 to 16.0)) and ECOG > 1 (HR 2.79 (95% CI 1.29 to 6.00)). A multivariable model, including the abovementioned variables as well as age, tumor location, and treatment, showed that FGFR GA was independently associated with survival (HR 2.59 (95% CI 1.21 to 5.55)). In addition, age (HR 1.03 (95% CI 1.00 to 1.07)), visceral metastases (HR 11.4 (95% CI 2.56 to 50.9)), and ECOG >1 (HR 6.4 (95% CI 2.43 to 16.9)) were independently associated with survival (Table 4).
Table 4.
Univariate and multivariate analysis for prognostic factors and overall survival.
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Variable | Modality | HR | 95% CI | HR | 95% CI |
| Age | (continuous) | 1.02 | 0.992–1.05 | 1.03 | 1.00–1.07 |
| Location | Nonbladder | 1 (ref) * | - | 1 (ref) | - |
| Bladder | 1.07 | 0.47–2.42 | 1.39 | 0.56–3.48 | |
| Treatment | Cisplatin | 1 (ref) | - | 1 (ref) | - |
| Immunotherapy | 1.32 | 0.60–2.90 | 2.40 | 0.97–5.90 | |
| Other | 1.71 | 0.84–3.48 | 3.17 | 1.38–7.24 | |
| Visceral metastases | No | 1 (ref) | - | 1 (ref) | - |
| Yes | 4.87 | 1.48–16.0 | 11.4 | 2.56–50.9 | |
| ECOG > 1 | No | 1 (ref) | - | 1 (ref) | - |
| Yes | 2.79 | 1.29–6.00 | 6.40 | 2.43–16.9 | |
| FGFR | Wild-type | 1 (ref) | - | 1 (ref) | - |
| Mutated | 1.87 | 1.01–3.48 | 2.59 | 1.21–5.55 | |
* (ref) denotes the category used as reference.
We aimed to analyze the validity of classical prognostic models in the study population, subdivided according to FGFR genomic alterations. First, we explored Bajorin’s model that considers visceral metastases and KPS < 80% as poor risk factors. Median OS in patients treated with first-line platinum-based chemotherapy with zero, one, or two risk factors were: not reached, 22.9, and 28 months, respectively (p = 0.14). The same analysis was performed in patients treated with first-line CPI with mOS: not reached, 25.6, and <1 month, respectively (p = 0.0001). When we analyzed Bajorin’s criteria according to FGFR status, they were fulfilled in FGFR WT patients (p = 0.00058) but did not reach statistical significance in FGFR GA patients (p = 0.11) (Figure 1E).
Subsequently, we tested Bellmunt’s model in the second line, which also includes anemia. We observed a trend toward better survival in chemotherapy-treated patients with no risk factors (p = 0.085). No trend was found in patients who received CPI (p = 0.99) or in FGFR GA patients (p = 0.3) (Figure 1F).
3.6. Interaction of FGFR GAs with Additional Biomarkers
In order to further characterize the associations among FGFR GAs with the outcome and other molecular biomarkers, we added four FGFR amplified cases to the FGFR mut/fus cohort (n = 30).
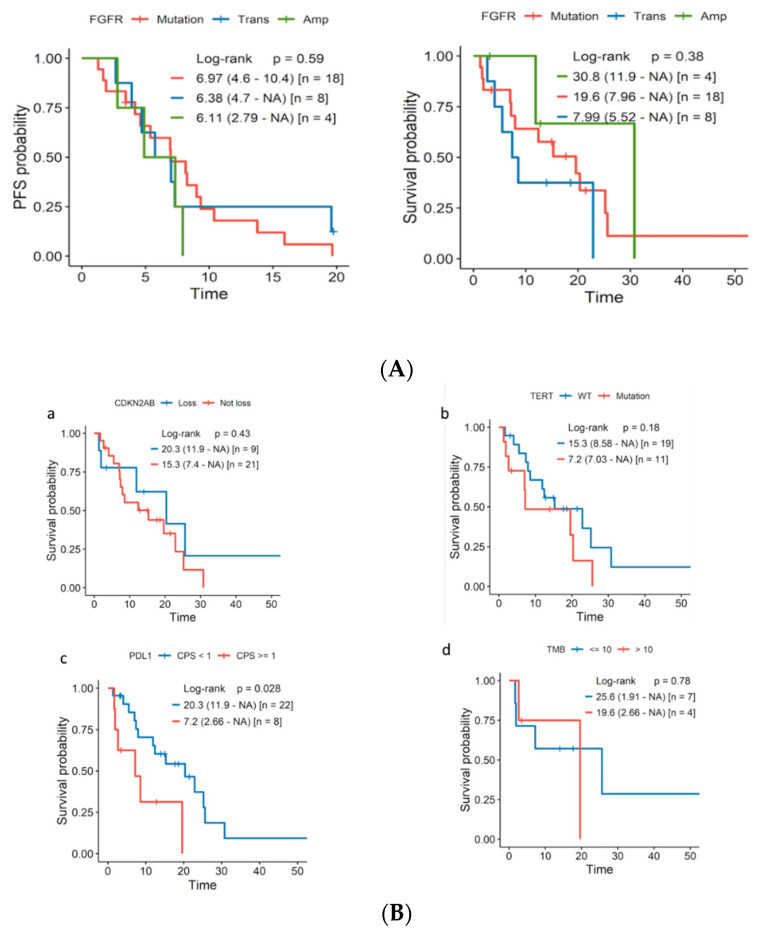
We explored the relationship between the FGFR genomic alteration type and treatment response (cisplatin, CPI, FGFRi). No significant differences in response rates were observed according to the aberration subtype (Supplementary Table S2 and Figure S1). Regarding PFS and OS, the outcomes were equally poor irrespective of the genomic alteration (Figure 2A).
Figure 2.
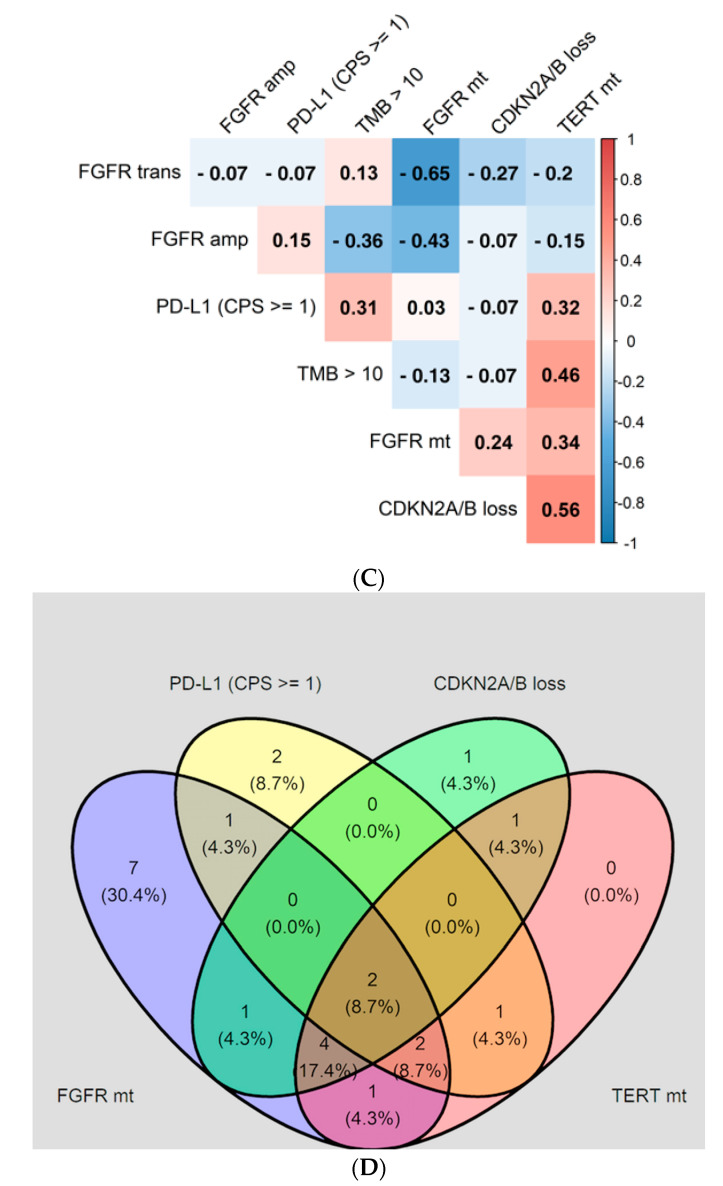
(A) PFS or OS according to the type of FGFR genomic alteration: translocation/fusion ( Trans) and amplification (Amp) cases were added. (B) Overall survival according to additional biomarkers: (a) CDKN2A/B loss; (b) TERT promoter mutations; (c) PD-L1 (CPS < 1 vs. CPS ≥ 1); (d) TMB (≤10 vs. >10). (C) Phi coefficient assessing the correlation between different biomarkers in the FGFR GA population. Phi correlation. Heat map: the phi index ranges from −1 to +1; red indicates a positive correlation (darker red indicates a stronger correlation between two biomarkers); blue indicates a negative correlation; white (phi = 0) represents no correlation. (D) Venn diagram representing the superposition of the expression of different biomarkers: expression of PD-L1 ≥ 1, FRGR genomic alterations, loss of cyclin-dependent kinase inhibitor (CDKN2A/B), and TERT promoter mutation.
Subsequently, we analyzed the interactions between FGFR GAs, the potential molecular biomarkers of the response to CPI (PD-L1, TMB ), and other mutations of interest, including CDKN2A/B loss and TERT promoter mutations. Phi correlation matrixes were calculated, and a Venn diagram was constructed for 30 FGFR GA tumors to determine the association with clinical outcomes (Figure 2C,D).
The prevalence, by NGS, of TERT promoter mutations was 11/30 (36%), and 9/30 (30%) had a loss of CDKN2A/B. PD-L1 positive immunohistochemistry expression was present in 8/30 (26%), and TMB results were available in 11/30 (36%) of the samples.
FGFR GA PD-L1 CPS ≥ 1 tumors were associated with worse clinical outcomes (p = 0.028). We also observed a trend toward a worse OS in FGFR GA patients with associated TERT promoter mutations or a TMB >10 (Figure 2B).
CDKN2A/B loss and TERT promoter mutations were positively correlated (Phi 0.56), as was PD-L1 (CPS ≥ 1) and a TMB > 10 (Phi 0.31). Another significant association was found between FGFR mutations and TERT promoter mutations with a medium–strong value (Phi 0.34); the correlation was weaker for FGFR mutations and CDKN2A/B loss (Phi 0.24). Interestingly, these relationships were not observed in the FGFR-translocated or amplified cases. A weak association between PD-L1 CPS ≥ 1 and FGFR mutated (Phi 0.03) or amplified (Phi 0.15) genes was also observed (Figure 2C,D).
4. Discussion
Growing evidence suggests that FGFR GAs are associated with worse outcomes in different tumor types [14,15,16]. In spite of the general correlation of FGFR GA with lower grades and stages of nonmuscle-invasive UC, there is no evidence to support that FGFR GAs are associated with a favorable phenotype once urothelial carcinoma advances [17].
Thus, we aimed to assess the value of FGFR GAs in mUC, as it remains unknown [18]. Among the 77 mUC patients analyzed, 26 presented FGFR GAs, and 51 were FGFR WT. Patients showing FGFR GAs had a significantly worse overall survival compared with the WT cohort.
In the multivariate analysis, an ECOG PS > 1, visceral metastasis, and treatment regimens other than cisplatin-based were confirmed as independent prognostic factors. Moreover, FGFR GAs were significantly associated with worse OS (HR 2.59 (95% CI 1.21–5.55)).
Unfortunately, since all patients had received several types of treatment, this study could not differentiate between a prognostic or predictive role of FGFR GAs. Thus, the outcome could be influenced by the response to treatments rather than by the biological action of these alterations.
The impact of FGFR GAs was irrespective of the type of molecular alteration (mutation, fusion, amplification), supporting the driver role of this gene.
Studies are controversial regarding FGFR GA and CPI responsiveness [19,20,21]. Though our results did not show a statistically significant difference between FGFR GA and WT tumors, a trend toward a better response to both treatments was suggested favoring GA tumors. A statistically significant increase in PFS after first-line treatment in the FGFR GA cohort was also observed. This is in contrast with the worse OS for this population and with the worse OS for this population. Therefore, FGFR GA UC might represent a distinct biological entity that correlates with an aggressive phenotype once the UC becomes advanced.
A rapid progression after perioperative treatment could explain these differences, which reinforces the aggressiveness of its nature. These results are in line with prior communications, including muscle-invasive bladder cancer, in which alterations in the FGFR gene have been associated with inferior responses to neoadjuvant platinum-based chemotherapy and a higher recurrence rate [22]. Intriguingly, this difference did not lead to a worse OS as in our study, which included only metastatic UC.
Hence, an adequate sequenced approach must be considered in this population, and a phase III clinical trial is underway (NCT03390504).
Interestingly, first-line treatment with an FGFRi showed worse response rates compared with other therapeutic approaches in FGFR GA patients. However, only five cases presented this condition.
Surprisingly, we did not find significant differences in the ORR to any of the treatments according to the FGFR genomic alteration type, as different susceptibilities to erdafitinib have been described in FGFR3 mutations (ORR: 49%), fusions (ORR: 16%), and for FGFR3:TACC3v1 (ORR: 36%) [5].
Since the sample size of our study was small, larger studies should elucidate the specific role of the different genomics alterations described in FGFR in mUC.
Clinical prognostic scores are commonly used in mUC to stratify patients and predict outcomes. Bajorin showed that a poor KPS and visceral metastases were independently associated with worse outcomes [4]. Bellmunt reported that an ECOG PS ≥ 1, anemia, and liver metastasis predict OS in platinum-refractory patients [23].
Both models have been widely adopted. However, the treatment landscape of mUC has significantly changed, and, currently, FGFR molecular alterations determine the choice of the treatment strategy.
In this study, Bajorin’s model correctly predicted clinical outcomes in the whole study population, and its accuracy even improved when restricted to the FGFR WT cases.
These results lead to the notion that classical algorithms could work for FGFR WT cases but not for FGFR GAs tumors. However, such findings should be confirmed in larger studies since none of the FGFG GA patients had ≥2 poor prognostic factors in this study, likely due to the low numbers.
Although UC has been considered a single entity, the evidence points toward distinct biological differences between UTUC and UBC. [24,25]. Nevertheless, the outcomes were equivalent irrespective of the tumor location, and the multivariate analysis was not significant. Outcomes in UTUC tumors harboring FGFR GA vs. FGFR WT were also similar.
Finally, we explored the incidence and potential role of additional biomarkers and genomic alterations in the FGFR GA population.
FGFR mutations have been associated with a lower PD-L1 expression, decreased T-cell infiltration, and a predominantly luminal-papillary subtype [26,27,28,29]. In total, 26% of the FGFR GA patients had a PD-L1 CPS ≥ 1 tumoral expression and were associated with a worse outcome. This finding supports the importance of several ongoing clinical trials combining FGFRi and CPI that could specifically benefit these poor-prognosis patients [30].
A high TMB is commonly used as a predictive biomarker for CPI [31]. Unfortunately, prior authors have communicated a low TMB in the FGFR GA tumors [32]. In our study, 4/10 FGFR GA patients had >10 mutations/Mb. However, they did not present a different clinical course, and conclusions must be taken with caution due to the limited sample size.
TERT promoter mutations are frequent GAs in UC and indicate poor prognostic and increased recurrence rates [33,34]. Interestingly, we identified a correlation between FGFR GAs and TERT promoter mutations, although no impact on response to therapy was observed. TERT mutations also seem to correlate with a higher TMB and PD-L1 expression, which may predict immunotherapy response in the FGFR GA population. [35].
CDKN2A is a tumor suppressor that renders the retinoblastoma inactive [36]. We observed a positive association between FGFR mutations and CDKN2A/B loss, as described with the TCGA [36,37]. Such alterations showed a trend toward increased survival. CDKN2A loss may cause resistance to CPI in UC [38], as CDKN2A and CD274 (encoding PD-L1) are both encoded in p9 chromosome 9 [39]. However, further validation is required.
Finally, it must be highlighted that some pathological and molecular factors previously associated with the outcome are missing in our study. As an example, FGFR GAs are overrepresented in the luminal subtype, which seems to have a better prognosis [28,29]. In addition, lymphovascular invasion and fibronectin expression could play a role when determining the best therapeutic option for every patient [40,41].
5. Conclusions
Our hypothesis-generating real-world data analysis suggests that the aberrations in FGFR may be an independent biomarker in mUC that should be included in new prognostic models.
Acknowledgments
We would like to thank the HM Hospitals for assisting with data collection and management. We also wish to thank Kyra Kennedy for her review of the manuscript.
Abbreviations
Checkpoint inhibitor (CPI); Eastern Cooperative Oncology Group Performance Status (ECOG PS); fibroblast growth factor receptor (FGFR); genomic alteration (GA); hazard ratio (HR); Karnofsky performance status (KPS); next-generation sequencing (NGS); overall response rate (ORR); overall survival (OS); progression-free survival (PFS); The Cancer Genome Atlas (TCGA); tumor mutational burden (TMB); urothelial bladder cancer (UBC); upper tract urothelial cancer (UTUC); wild-type (WT).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11154483/s1, Table S1: Clinical and anatomopathological characteristics of 98 UC patients sequenced for FGFR alterations, including 77 cases with advanced disease (study population). Table S2: ORR according to the type of FGFR genomic alteration (mutation, fusion, amplification). Figure S1: ORR according to the type of FGFR genomic alteration (mutation, fusion, amplification). Figure S2: OS according to the histology type: (a) urothelial vs non urothelial (b) squamous vs urothelial. Figure S3: OS in UTUC in general population and stratified by FGFR status: (a) UTUC vs bladder (b) UTUC by FGFR status; Figure S4: (a) PFS for tumors with FGFR GAs assessed from the exposition to platinum-based chemotherapy; (b) OS for tumors with FGFR GAs assessed from the exposition to platinum-based chemotherapy; (c) PFS for tumors with FGFR GAs assessed from the exposition to checkpoint in-hibitors; (d) OS for tumors with FGFR GAs assessed from the exposition to checkpoint inhibitors.
Author Contributions
Conceptualization, E.S.F. and J.G.-D.J.; methodology, E.S.F. and R.M.d.L.; software, R.M.d.L.; validation, E.S.F., J.G.-D.J. and R.M.d.L.; formal analysis, E.S.F., J.G.-D.J. and R.M.d.L.; investigation, E.S.F. and J.G.-D.J.; resources, E.S.F., M.Y.F., M.B.L., P.N.A. and M.Q.P.; data curation, E.S.F., J.G.-D.J. and R.M.d.L.; writing—original draft preparation, E.S.F.; writing—review and editing, E.S.F., J.G.-D.J., J.F.R.M., A.B.G. and R.M.d.L.; visualization, E.S.F.; supervision, J.G.-D.J. and J.F.R.M.; project administration, E.S.F. and J.G.-D.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board and Ethics Committee of HM HOSPITALES (IRB No. 19.07.1433-GHM on 18 September 2019).
Informed Consent Statement
Written informed consent for clinical data records and genetic testing was obtained from all patients.
Data Availability Statement
The data presented in this study is available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Teoh J.Y.C., Huang J., Ko W.Y.K., Lok V., Choi P., Ng C.F., Sengupta S., Mostafid H., Kamat A.M., Black P.C., et al. Global Trends of Bladder Cancer Incidence and Mortality, and Their Associations with Tobacco Use and Gross Domestic Product per Capita. Eur. Urol. 2020;78:893–906. doi: 10.1016/j.eururo.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Lin J., Spitz M.R., Dinney C.P., Etzel C.J., Grossman H.B., Wu X. Bladder cancer risk as modified by family history and smoking. Cancer. 2006;107:705–711. doi: 10.1002/cncr.22071. [DOI] [PubMed] [Google Scholar]
- 4.Bajorin D.F., Dodd P.M., Mazumdar M., Fazzari M., McCaffrey J.A., Scher H.I., Herr H., Higgins G., Boyle M.G. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1999;17:3173–3181. doi: 10.1200/JCO.1999.17.10.3173. [DOI] [PubMed] [Google Scholar]
- 5.Loriot Y., Necchi A., Park S.H., Garcia-Donas J., Huddart R., Burgess E., Fleming M., Rezazadeh A., Mellado B., Varlamov S., et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2019;381:338–348. doi: 10.1056/NEJMoa1817323. [DOI] [PubMed] [Google Scholar]
- 6.Powles T., Rosenberg J.E., Sonpavde G.P., Loriot Y., Durán I., Lee J.L., Matsubara N., Vulsteke C., Castellano D., Wu C., et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021;384:1125–1135. doi: 10.1056/NEJMoa2035807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tagawa S.T., Balar A.V., Petrylak D.P., Kalebasty A.R., Loriot Y., Fléchon A., Jain R.K., Agarwal N., Bupathi M., Barthelemy P., et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients with Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J. Clin. Oncol. 2021;39:2474–2485. doi: 10.1200/JCO.20.03489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garje R., An J., Obeidat M., Kumar K., Yasin H.A., Zakharia Y. Fibroblast Growth Factor Receptor (FGFR) Inhibitors in Urothelial Cancer: Fibroblast Growth Factor Receptor (FGFR) Inhibitors in Urothelial Cancer. Oncologist. 2020;25:e1711–e1719. doi: 10.1634/theoncologist.2020-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helsten T., Elkin S., Arthur E., Tomson B.N., Carter J., Kurzrock R. The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clin. Cancer Res. 2016;22:259–267. doi: 10.1158/1078-0432.CCR-14-3212. [DOI] [PubMed] [Google Scholar]
- 10.Al-Obaidy K.I., Cheng L. Fibroblast growth factor receptor (FGFR) gene: Pathogenesis and treatment implications in urothelial carcinoma of the bladder. J. Clin. Pathol. 2021;74:491–495. doi: 10.1136/jclinpath-2020-207115. [DOI] [PubMed] [Google Scholar]
- 11.Costa R., Carneiro B.A., Taxter T., Tavora F.A., Kalyan A., Pai S.A., Chae Y.K., Giles F.J. FGFR3-TACC3 fusion in solid tumors: Mini review. Oncotarget. 2016;7:55924–55938. doi: 10.18632/oncotarget.10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomlinson D.C., L’Hôte C.G., Kennedy W., Pitt E., Knowles M.A. Alternative splicing of fibroblast growth factor receptor 3 produces a secreted isoform that inhibits fibroblast growth factor-induced proliferation and is repressed in urothelial carcinoma cell lines. Cancer Res. 2005;65:10441–10449. doi: 10.1158/0008-5472.CAN-05-1718. [DOI] [PubMed] [Google Scholar]
- 13.Pal S.K., Bajorin D., Dizman N., Hoffman-Censits J., Quinn D.I., Petrylak D.P., Galsky M.D., Vaishampayan U., de Giorgi U., Gupta S., et al. Infigratinib in upper tract urothelial carcinoma versus urothelial carcinoma of the bladder and its association with comprehensive genomic profiling and/or cell-free DNA results. Cancer. 2020;126:2597–2606. doi: 10.1002/cncr.32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J.J., Yan S., Pan Y., Liu Z., Liu Y., Deng Q., Tan Q., Woodward E.R. FGFR genes mutation is an independent prognostic factor and associated with lymph node metastasis in squamous non-small cell lung cancer. Cancer Biol. Ther. 2018;19:1108–1116. doi: 10.1080/15384047.2018.1480294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ipenburg N.A., Koole K., Liem K.S., van Kempen P.M.W., Koole R., van Diest P.J., van Es R.J.J., Willems S.M. Fibroblast Growth Factor Receptor Family Members as Prognostic Biomarkers in Head and Neck Squamous Cell Carcinoma: A Systematic Review. Target Oncol. 2016;11:17–27. doi: 10.1007/s11523-015-0374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amina B., Lynda A.K., Sonia S., Adel B., Jelloul B.H., Miloud M., Tewfik S. Fibroblast growth factor receptor 1 protein (FGFR1) as potential prognostic and predictive marker in patients with luminal B breast cancers overexpressing human epidermal receptor 2 protein (HER2) Indian J. Pathol. Microbiol. 2021;64:254–260. doi: 10.4103/IJPM.IJPM_87_20. [DOI] [PubMed] [Google Scholar]
- 17.Lee H.W., Seo H.K. Fibroblast Growth Factor Inhibitors for Treating Locally Advanced/Metastatic Bladder Urothelial Carcinomas via Dual Targeting of Tumor-Specific Oncogenic Signaling and the Tumor Immune Microenvironment. Int. J. Mol. Sci. 2021;22:9526. doi: 10.3390/ijms22179526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kardoust Parizi M., Margulis V., Lotan Y., Mori K., Shariat S.F. Fibroblast growth factor receptor: A systematic review and meta-analysis of prognostic value and therapeutic options in patients with urothelial bladder carcinoma. Urol. Oncol. Semin. Orig. Investig. 2021;39:409–421. doi: 10.1016/j.urolonc.2021.01.025. [DOI] [PubMed] [Google Scholar]
- 19.Santiago-Walker A.E., Chen F., Loriot Y., Siefker-Radtke A.O., Sun L., Sundaram R., de Porre P., Patel K., Wan Y. Predictive value of fibroblast growth factor receptor (FGFR) mutations and gene fusions on anti-PD-(L)1 treatment outcomes in patients (pts) with advanced urothelial cancer (UC) J. Clin. Oncol. 2019;37((Suppl. S7)):419. [Google Scholar]
- 20.Rose T.L., Hayward M.C., Salazar A.H., Eulitt P., McGinty K., Drier A., Wobker S.E., Whang Y.E., Brower B.Y., Dunn M., et al. Fibroblast growth factor receptor status and response to immune checkpoint inhibition in metastatic urothelial cancer. J. Clin. Oncol. 2019;37((Suppl. S7)):458. [Google Scholar]
- 21.Wang L., Gong Y., Saci A., Szabo P.M., Martini A., Necchi A., Pal S., Plimack E.R., Sfakianos J.P., Bhardwaj N., et al. Fibroblast Growth Factor Receptor 3 Alterations and Response to PD-1/PD-L1 Blockade in Patients with Metastatic Urothelial Cancer. Eur. Urol. 2019;76:599–603. doi: 10.1016/j.eururo.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teo M.Y., Mota J.M., Whiting K.A., Li H.A., Funt S.A., Lee C.H., Solit D.B., Al-Ahmadie H., Milowsky M.I., Balar A.V., et al. Fibroblast Growth Factor Receptor 3 Alteration Status is Associated with Differential Sensitivity to Platinum-based Chemotherapy in Locally Advanced and Metastatic Urothelial Carcinoma. Eur. Urol. 2020;78:907–915. doi: 10.1016/j.eururo.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellmunt J., Choueiri T.K., Fougeray R., Schutz F.A.B., Salhi Y., Winquist E., Culine S., von der Maase H., Vaughn D.J., Rosenberg J.E. Prognostic Factors in Patients with Advanced Transitional Cell Carcinoma of the Urothelial Tract Experiencing Treatment Failure With Platinum-Containing Regimens. J. Clin. Oncol. 2010;28:1850–1855. doi: 10.1200/JCO.2009.25.4599. [DOI] [PubMed] [Google Scholar]
- 24.Sfakianos J.P., Cha E.K., Iyer G., Scott S.N., Zabor E.C., Shah R.H., Ren Q., Bagrodia A., Kim P.H., Hakimi A.A., et al. Genomic Characterization of Upper Tract Urothelial Carcinoma. Eur. Urol. 2015;68:970–977. doi: 10.1016/j.eururo.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Audenet F., Isharwal S., Cha E.K., Donoghue M.T.A., Drill E.N., Ostrovnaya I., Pietzak E.J., Sfakianos J.P., Bagrodia A., Murugan P., et al. Clonal Relatedness and Mutational Differences between Upper Tract and Bladder Urothelial Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019;25:967–976. doi: 10.1158/1078-0432.CCR-18-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galsky M.D., Arija J.Á.A., Bamias A., Davis I.D., De Santis M., Kikuchi E., Garcia-Del-Muro X., de Giorgi U., Mencinger M., Izumi K., et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1547–1557. doi: 10.1016/S0140-6736(20)30230-0. [DOI] [PubMed] [Google Scholar]
- 27.Jing W., Wang G., Cui Z., Xiong G., Jiang X., Li Y., Li W., Han B., Chen S., Shi B., et al. FGFR3 Destabilizes PD-L1 via NEDD4 to Control T-cell–Mediated Bladder Cancer Immune Surveillance. Cancer Res. 2022;82:114–129. doi: 10.1158/0008-5472.CAN-21-2362. [DOI] [PubMed] [Google Scholar]
- 28.Robinson B.D., Vlachostergios P.J., Bhinder B., Liu W., Li K., Moss T.J., Bareja R., Park K., Tavassoli P., Cyrta J., et al. Upper tract urothelial carcinoma has a luminal-papillary T-cell depleted contexture and activated FGFR3 signaling. Nat. Commun. 2019;10:2977. doi: 10.1038/s41467-019-10873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson A.G., Kim J., Al-Ahmadie H., Bellmunt J., Guo G., Cherniack A.D., Hinoue T., Laird P.W., Hoadley K.A., Akbani R., et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell. 2018;174:1033. doi: 10.1016/j.cell.2018.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palakurthi S., Kuraguchi M., Zacharek S.J., Zudaire E., Huang W., Bonal D.M., Liu J., Dhaneshwar A., DePeaux K., Gowaski M.R., et al. The Combined Effect of FGFR Inhibition and PD-1 Blockade Promotes Tumor-Intrinsic Induction of Antitumor Immunity. Cancer Immunol. Res. 2019;7:1457–1471. doi: 10.1158/2326-6066.CIR-18-0595. [DOI] [PubMed] [Google Scholar]
- 31.Halbert B., Einstein D.J. Hot or Not: Tumor Mutational Burden (TMB) as a Biomarker of Immunotherapy Response in Genitourinary Cancers. Urology. 2021;147:119–126. doi: 10.1016/j.urology.2020.10.030. [DOI] [PubMed] [Google Scholar]
- 32.Pal S.K., Agarwal N., Choueiri T.K., Stephens P.J., Ross J.S., Miller V.A., Ali S.M., Chung J., Grivas P. Comparison of tumor mutational burden (TMB) in relevant molecular subsets of metastatic urothelial cancer (MUC) Ann. Oncol. 2017;28:v297. [Google Scholar]
- 33.Hosen I., Rachakonda P.S., Heidenreich B., de Verdier P.J., Ryk C., Steineck G., Hemminki K., Kumar R. Mutations in TERT promoter and FGFR3 and telomere length in bladder cancer: TERT, FGFR3, Telomere Length in Bladder Cancer. Int. J. Cancer. 2015;137:1621–1629. doi: 10.1002/ijc.29526. [DOI] [PubMed] [Google Scholar]
- 34.Rachakonda P.S., Hosen I., de Verdier P.J., Fallah M., Heidenreich B., Ryk C., Wiklund N.P., Steineck G., Schadendorf D., Hemminki K., et al. TERT promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc. Natl. Acad. Sci. USA. 2013;110:17426–17431. doi: 10.1073/pnas.1310522110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H., Li J., Zhang C., Zhang C., Wang H. TERT mutations correlate with higher TMB value and unique tumor microenvironment and may be a potential biomarker for anti-CTLA4 treatment. Cancer Med. 2020;9:7151–7160. doi: 10.1002/cam4.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cancer Genome Atlas Research Network Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rebouissou S., Hérault A., Letouzé E., Neuzillet Y., Laplanche A., Ofualuka K., Maillé P., Leroy K., Riou A., Lepage M.-L., et al. CDKN2A homozygous deletion is associated with muscle invasion in FGFR3-mutated urothelial bladder carcinoma. J. Pathol. 2012;227:315–324. doi: 10.1002/path.4017. [DOI] [PubMed] [Google Scholar]
- 38.Nassar A., Adib E., Akl E.W., Abou Alaiwi S., Nuzzo P.V., Mouhieddine T.H., Sonpavde G., Haddad R.I., Giannakis M., Hodi F.S., et al. CDKN2A alterations as markers of immune checkpoint blockade (ICB) resistance in urothelial carcinoma (UC) J. Clin. Oncol. 2021;39((Suppl. S6)):475. [Google Scholar]
- 39.Banchereau R., Leng N., Zill O., Sokol E., Liu G., Pavlick D., Maund S., Liu L.-F., Kadel E., III, Balswin N., et al. Molecular determinants of response to PD-L1 blockade across tumor types. Nat. Commun. 2021;12:3969. doi: 10.1038/s41467-021-24112-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stangl-Kremser J., Muto G., Grosso A.A., Briganti A., Comperat E., Di Maida F. The impact of lymphovascular invasion in patients treated with radical nephroureterectomy for upper tract urothelial carcinoma: An extensive updated systematic review and meta-analysis. Urol. Oncol. 2022;40:243–261. doi: 10.1016/j.urolonc.2022.01.014. [DOI] [PubMed] [Google Scholar]
- 41.Di Maida F., Scalici Gesolfo C., Tellini R., Mari A., Sanfilippo C., Lambertini L. Fibronectin urothelial gene expression as a new reliable biomarker for early detection of local toxicity secondary to adjuvant intravesical therapy for non-muscle invasive bladder cancer. Ther. Adv. Urol. 2021;13:1756287221995683. doi: 10.1177/1756287221995683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study is available in this article.