Abstract
We report Western blot data showing that the 42.8-kDa product of the previously characterized cotH locus (8) is a structural component of the Bacillus subtilis spore coat. We show that the assembly of CotH requires both CotE and GerE. In agreement with these observations, the ultrastructural analysis of purified spores suggests that CotH is needed for proper formation of both inner and outer layers of the coat.
Endospores of Bacillus subtilis are encased in a thick protein shell known as the coat (3, 12). The coat is composed of 20 or more polypeptides arranged in an electron-dense outer layer and a lamellar inner layer. Proper formation of the coat is essential for spore resistance to bactericidal enzymes and chemicals and for efficient spore response to germinants (3, 12).
Although some coat components are synthesized at stage II of sporulation, under the transcriptional control of early mother cell factor ςE (9, 15), coat material can be visualized only when the developing spore is present as a free protoplast within the mother cell compartment of the sporangium (12). Sporulation protein SpoIVA (2, 9, 11) has been proposed to control the assembly of a ring of CotE proteins around the forespore (2), and a scaffold-like structure is thought to separate the CotE ring from the outer surface of the forespore membrane (2). Outer and inner coat components are then assembled on the outer and inner sides of the CotE ring. Assembly of the two coat layers is controlled by different mechanisms, with the outer and inner layers requiring cotE (13) and gerE (7) expression, respectively. However, GerE action is not exclusively required for the assembly of the inner coat components but is also needed for the transcriptional regulation of several genes coding for outer coat components (10, 15).
We have previously reported on the characterization of the cotH locus, encoding a predicted polypeptide of 42.8 kDa. cotH is under the transcriptional control of ςK-containing RNA polymerase, and the transcriptional activator GerE is not required for its expression (8). Deletion of cotH has a pleiotropic effect on the assembly of several outer coat components, including the products of the previously characterized GerE-dependent genes cotB, cotC, and cotG (8). Based on the analysis of a cotE-cotH double mutant, we suggested that CotH is localized either in the inner coat or at the interface between the two layers (8).
Here we present Western blot data indicating that CotH is a structural component of the spore coat and that CotH assembly is under the dual control of CotE and GerE. Electron microscopy (EM) results suggest that CotH function is required for the correct formation of both inner and outer coat structures. These results suggest that CotH is either (i) a component of the two coat layers whose assembly is under complex control or (ii) in close proximity to components of both coat layers.
CotH is a structural component of the coat.
To show whether CotH is a structural component of the spore coat, a 0.7-kb PvuII-EcoRI DNA fragment internal to the cotH coding region (8) was cloned into plasmid pRSETB (Invitrogen) in frame with six histidine codons (polyhistidine tag). By using the QIAexpress System, the hybrid protein was overexpressed, purified, and used to generate CotH antisera. The anti-CotH polyclonal antibodies obtained were used in Western blot experiments. B. subtilis cells were grown in Difco sporulation (DS) medium for 48 h at 37°C, and spores were harvested by centrifugation and purified as described previously (1a, 6, 8).
Coat proteins were solubilized by treatment of the spores with 1% (wt/vol) sodium dodecyl sulfate–50 mM dithiothreitol (pH 9.5) at 65°C for 30 min (8). After centrifugation, the average amount of released proteins, measured by colorimetric assay, was 2 μg/ml of sporulation medium (corresponding to about 107 purified spores). Identical total protein concentrations were fractionated by sodium dodecyl sulfate–12.5% polyacrylamide gel electrophoresis and electrotransferred to a nitrocellulose membrane. Membranes were then probed with anti-CotH sera and developed by using the ECL detection system (Amersham) in accordance with the manufacturer’s instructions.
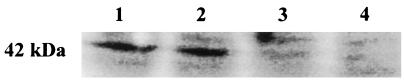
As shown in Fig. 1, a polypeptide of about 42 kDa, corresponding to the predicted size of CotH, was recognized by anti-CotH antibodies in coat material purified from wild-type spores (lane 1) but not in spores of the congenic cotH deletion mutant strain (lane 2). The same antibody preparation used for the experiment of Fig. 1 failed to specifically recognize CotH in crude extracts of wild-type sporulating cells collected 6 and 8 h after the onset of sporulation (1; see also below).
FIG. 1.
Western blot analysis. Spore coat proteins were extracted from congenic strains PY17 (wild type, lane 1), ER223 (cotH mutant, lane 2), BZ213 (cotE mutant, lane 3), and KS450 (gerE mutant, lane 4). One hundred micrograms of total protein was loaded in each lane. The arrow on the left points to the CotH band. The positions of molecular size (Mr) markers are indicated on the right.
The presence of CotH in purified Cot proteins, together with its absence in crude extracts of identical protein concentrations, suggests that CotH is enriched in purified coat material and therefore is a structural component of the spore coat.
CotH assembly is under CotE and GerE control.
cotE mutant spores appear to lack the outer coat completely (13), while gerE mutant spores have been reported to lack the inner coat structure (7). Figure 1 shows that a polypeptide corresponding to CotH was not detected in either cotE or gerE mutant spores (lanes 3 and 4).
In agreement with the previously reported GerE-independent expression of cotH (8), we observed that anti-CotH antibodies recognized CotH in crude extracts of gerE mutant cells collected 6 and 7 h after the onset of sporulation (Fig. 2).
FIG. 2.
Western blot analysis. Crude extracts were prepared from congenic strains KS450 (gerE mutant; lanes 1 and 2) and ER228 (gerE cotH::cat mutant, lanes 3 and 4). Cells were collected 6 (lanes 1 and 3) and 7 (lanes 2 and 4) h after the onset of sporulation. One hundred micrograms of total protein was loaded in each lane.
Since CotE has a regulatory effect on coat components only at the assembly level (13) and GerE does not affect cotH expression (Fig. 2 and reference 8), we propose that in cotE and gerE mutant strains, CotH is normally produced but not deposited around the forming spore and that CotH assembly is therefore under the dual control of CotE and GerE.
CotH is required for proper spore coat assembly.
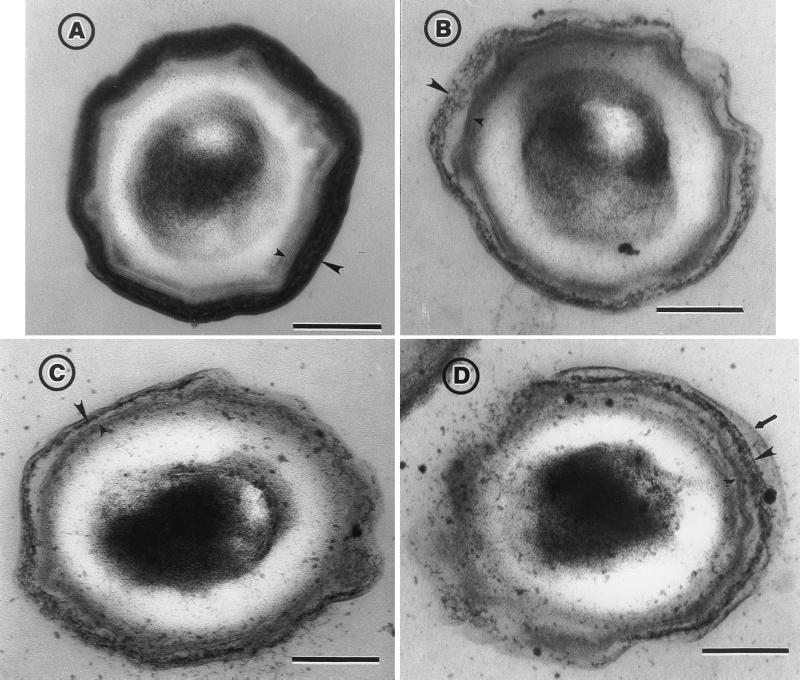
EM experiments were carried out to check whether the pleiotropic effect of CotH on the assembly of several coat components (8) causes any detectable alteration of the coat ultrastructure. Wild-type strain MB24 (identical to strain PY17 [used for the experiment of Fig. 1] in the pattern of extracted coat protein and by EM) and its congenic derivative AH1103 (constructed by transformation of MB24 with chromosomal DNA from strain ER209 [cotH::cat] [8]) were grown for 48 h in DS medium, and the spores were purified by Renografin gradients and processed for EM analysis as previously described (5). As shown in Fig. 3, the coat of cotH mutant spores (panels B to D) presented several deviations from what was observed with the wild-type strain (panel A; see also reference 4) at both the outer and inner coat levels. (i) The outer coat layers appeared diffuse, lacking the characteristic multilayered pattern, electron density, and a defined outer edge. (ii) The most external outer coat layers did not adhere closely to the rest of the outer coat structure and occasionally appeared to peel off (shown in panel D). (iii) The typical lamellar structure of the inner coat, in which three to five lamellae can usually be recognized, was reduced to one or two lamellae. (iv) The inner and outer coats did not seem tightly associated.
FIG. 3.
EM analysis of spores purified from wild-type strain MB24 (A) and congenic cotH deletion mutant AH1103 (B to D). The small and large arrowheads point to the inner and outer coat structures, respectively. The arrow in panel D indicates a coat structure that appears to peel off the spores (see text). Bars, 0.2 μm.
An important determinant of outer coat structural organization is the CotG protein (5). Since CotG assembly is dependent on cotH expression (8), the observed effects of CotH on the outer layer of the coat could be mediated in part by CotG. However, it is interesting that the cotH mutant is not morphologically equivalent to a cotG null mutant (5): while in cotG mutant spores, the outer coat region is surrounded by a well-defined outer layer (5), cotH spores lack a defined outer edge (Fig. 3). Thus, the role of CotH in outer coat assembly may not be restricted to the recruitment of CotG.
In conclusion, our results establish that CotH is a structural component of the coat. CotH may be localized at the interface between the two coat layers, in which case changes in either coat layer could affect its assembly. Alternatively, CotH is a component of both the inner and outer coat layers, in which case its assembly would always require both CotE and GerE functions. In either case, formation of the inner coat and that of the outer coat appear to be not independent but rather interconnected processes, with the CotH protein influencing the course of events in both spore coat layers.
Acknowledgments
We are grateful to R. Losick and M. De Felice for critical reading of the manuscript and to M. Serrano for helping us in antibody preparation.
This work was supported by a bilateral Italy/Portugal (CNR/JNICT) travel grant to E.R. and R.Z., by PRAXIS XXI grant 129 to R.Z., and by a CNR grant (PF Biotecnologie) to E.R. Work in the C.P.M. laboratory was supported by PHS grant GM54395 from the National Institutes of Health. A.O.H. was the recipient of a postdoctoral fellowship from the Junta Nacional de Investigação Científica e Tecnológica (J.N.I.C.T.).
REFERENCES
- 1.Baccigalupi, L., and E. Ricca. Unpublished data.
- 1a.Donovan W, Zheng L, Sandman K, Losick R. Genes encoding spore coat polypeptides from Bacillus subtilis. J Mol Biol. 1987;196:1–10. doi: 10.1016/0022-2836(87)90506-7. [DOI] [PubMed] [Google Scholar]
- 2.Driks A, Roels S, Beall B, Moran C P, Jr, Losick R. Subcellular localization of proteins involved in the assembly of the spore coat of Bacillus subtilis. Genes Dev. 1994;8:234–244. doi: 10.1101/gad.8.2.234. [DOI] [PubMed] [Google Scholar]
- 3.Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993;57:1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henriques A O, Beall B W, Moran C P., Jr CotM of Bacillus subtilis, a member of the α-crystalin family of stress proteins, is induced during development and participates in spore outer coat formation. J Bacteriol. 1997;176:1887–1897. doi: 10.1128/jb.179.6.1887-1897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henriques A O, Melsen L R, Moran C P., Jr Involvement of superoxide dismutase in spore coat assembly in Bacillus subtilis. J Bacteriol. 1998;180:2285–2291. doi: 10.1128/jb.180.9.2285-2291.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenkinson H F, Sawyer W D, Mandelstam J. Synthesis and order of assembly of spore coat proteins in Bacillus subtilis. J Gen Microbiol. 1981;123:1–16. [Google Scholar]
- 7.Moir A. Germination properties of a spore coat-defective mutant of Bacillus subtilis. J Bacteriol. 1981;146:1106–1116. doi: 10.1128/jb.146.3.1106-1116.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naclerio G, Baccigalupi L, Zilhão R, de Felice M, Ricca E. Bacillus subtilis spore coat assembly requires cotH gene expression. J Bacteriol. 1996;178:4375–4380. doi: 10.1128/jb.178.15.4375-4380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roels S, Driks A, Losick R. Characterization of spoIVA, a sporulation gene involved in coat morphogenesis in Bacillus subtilis. J Bacteriol. 1992;174:575–585. doi: 10.1128/jb.174.2.575-585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacco M, Ricca E, Losick R, Cutting S. An additional GerE-controlled gene encoding an abundant spore coat protein from Bacillus subtilis. J Bacteriol. 1995;177:372–377. doi: 10.1128/jb.177.2.372-377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens C M, Daniel R, Illing N, Errington J. Characterization of a sporulation gene, spoIVA, involved in spore coat morphogenesis in Bacillus subtilis. J Bacteriol. 1992;174:586–594. doi: 10.1128/jb.174.2.586-594.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–341. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 13.Zheng L, Donovan W, Fitz-James P C, Losick R. Gene encoding a morphogenic protein required in the assembly of the outer coat of the Bacillus subtilis endospore. Genes Dev. 1988;2:1047–1054. doi: 10.1101/gad.2.8.1047. [DOI] [PubMed] [Google Scholar]
- 14.Zheng L, Halberg R, Roels S, Ichikawa H, Kroos L, Losick R. Sporulation regulatory protein GerE from Bacillus subtilis binds to and can activate or repress transcription from promoters for mother-cell-specific genes. J Mol Biol. 1992;226:1037–1050. doi: 10.1016/0022-2836(92)91051-p. [DOI] [PubMed] [Google Scholar]
- 15.Zheng L, Losick R. Cascade regulation of spore coat gene expression in Bacillus subtilis. J Mol Biol. 1990;212:645–660. doi: 10.1016/0022-2836(90)90227-d. [DOI] [PubMed] [Google Scholar]