Abstract
Glycoprotein (GP) VI is the major platelet collagen receptor and a promising anti-thrombotic target. This was first demonstrated in mice using the rat monoclonal antibody JAQ1, which completely blocks the Collagen-Related Peptide (CRP)-binding site on mouse GPVI and efficiently inhibits mouse platelet adhesion, activation and aggregation on collagen. Here, we show for the first time that JAQ1 cross-reacts with human GPVI (huGPVI), but not with GPVI in other tested species, including rat, rabbit, guinea pig, swine, and dog. We further demonstrate that JAQ1 differently modulates mouse and human GPVI function. Similar to its effects on mouse GPVI (mGPVI), JAQ1 inhibits CRP-induced activation in human platelets, whereas, in stark contrast to mouse GPVI, it does not inhibit the adhesion, activation or aggregate formation of human platelets on collagen, but causes instead an increased response. This effect was also seen with platelets from newly generated human GPVI knockin mice (hGP6tg/tg). These results indicate that the binding of JAQ1 to a structurally conserved epitope in GPVI differently affects its function in human and mouse platelets.
Keywords: glycoprotein VI, JAQ1, platelet receptors, platelet activation, platelet inhibition
1. Introduction
Platelets are small, anucleated blood cells produced by bone marrow-resident megakaryocytes (MK), that have key roles in hemostasis and thrombosis [1,2]. At the sites of vascular injury, the platelets recognize and bind to specific ligands on the exposed extracellular matrix, become activated and aggregate to form a hemostatic plug that seals the vessel and prevents excessive blood loss. However, in pathological conditions, the intravascular platelet activation can precipitate the formation of vessel-occluding thrombi, leading to ischemic disease states, such as stroke or myocardial infarction [3,4]. Therefore, anti-platelet drugs have become indispensable therapeutics to efficiently prevent or treat arterial thrombosis, but they carry an inherent risk of bleeding, most obviously in multimorbid patients requiring dual platelet inhibition or combined anticoagulation [5]. Among the major platelet receptors, glycoprotein (GP) VI has emerged as a promising therapeutic target, as its functional inhibition or genetic deletion provides protection from arterial thrombus formation in vivo, while only minimally affecting hemostasis [6]. GPVI is the main signaling receptor for collagen and is expressed exclusively on platelets and MK. GPVI is associated with the FcR γ (Fc receptor γ)-chain, which is responsible for the signaling via its immunoreceptor-tyrosine-based-activation-motif (ITAM). Besides collagen, several additional physiological agonists have been identified in recent years. These include fibrinogen, fibrillar fibrin [7,8,9,10] and fibronectin [11], the basement membrane protein nidogen-1 [12] and laminins [13]. These ligands are likely to—at least in part—contribute to the role of GPVI in pathophysiological processes beyond thrombosis, such as ischemia-reperfusion injury [14], sepsis [15], cancer and metastasis [16,17] and venous thrombosis [18]. Collectively, these studies highlight the potential benefits of efficient anti-GPVI agents. In fact, the first inhibitors of GPVI are now entering the clinic. The GPVI-blocking Fab (ACT017, glenzocimab) is assessed in the context of acute ischemic stroke [19,20,21]. In transgenic mice carrying the human GP6 gene, glenzocimab was found to be effective in thrombus suppression, without impacting GPVI-dependent inflammatory hemostasis [22]. The first studies on the (patho-)physiological function and in vivo relevance of GPVI were performed in mice and capitalized on the first reported anti-GPVI monoclonal antibody (mAb), JAQ1 (rat IgG2a) [23,24]. It was shown that JAQ1 completely blocks the collagen-related-peptide (CRP)-induced activation of mouse platelets and virtually abolishes mouse platelet adhesion, activation and aggregate formation on collagen [6,25]. Notably, a possible interaction between JAQ1 and human GPVI (huGPVI) has not been studied. Mouse and human GPVI share ~67.3% of their nucleotide sequence and ~64.4% of the amino-acid sequence, with huGPVI having an intracellular domain that is 24 amino acids longer than that of mouse GPVI (mGPVI) [26]. In addition, the extracellular domains of the receptor also differ between the two species, best documented by the ability of huGPVI, but not mGPVI, to support platelet spreading on fibrinogen [27]. As this raises the question as to whether a specific anti-mGPVI antibody could bind huGPVI and thereby modulate its function in a comparable manner [28], we assessed the effects of JAQ1 on huGPVI. Here, we show that JAQ1 recognizes a conformational epitope on huGPVI and efficiently activates the human platelets upon cross-linking by a secondary antibody. Similar to its effect on mGPVI, JAQ1 inhibited the human platelet activation by CRP, but, in stark contrast to mouse platelets, it did not inhibit, but rather promoted the adhesion, activation and aggregate formation of human platelets on collagen. These differential effects of JAQ1 on huGPVI were confirmed in platelets from a newly generated mouse line expressing huGPVI instead of mGPVI.
2. Results
2.1. Anti-Mouse GPVI Monoclonal Antibody JAQ1 Binds Human GPVI and Modulates Receptor Function
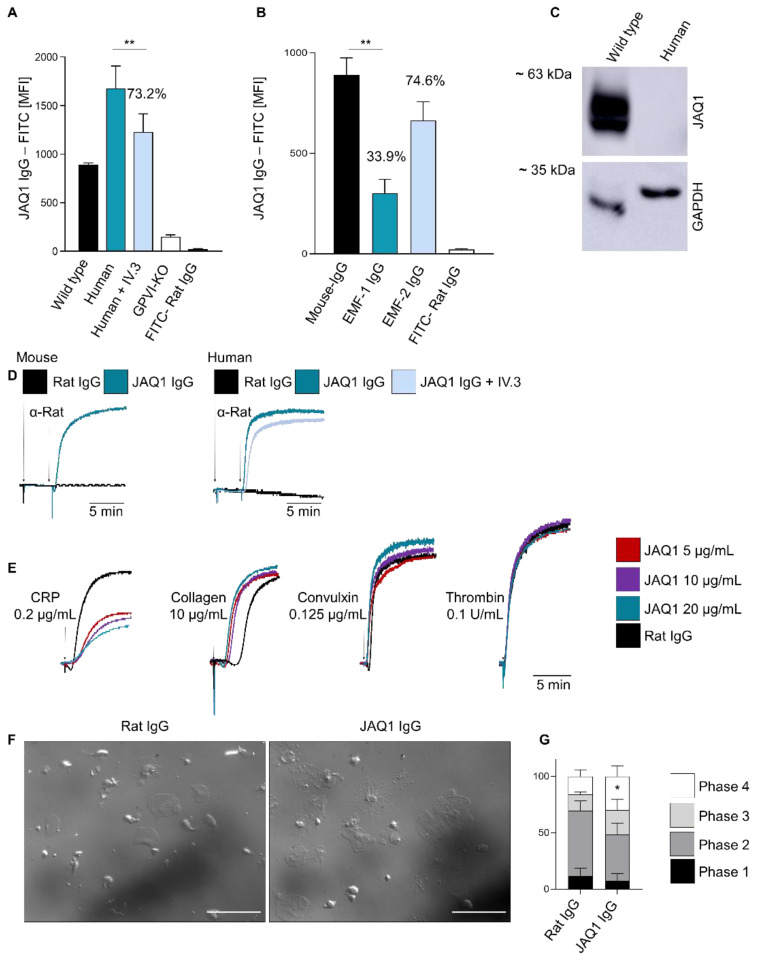
JAQ1 (rat IgG2a) is the first anti-GPVI mAb reported in the literature and was initially raised against mouse GPVI [24]. JAQ1 completely blocks the major collagen binding site/CRP-binding site in mGPVI [6], resulting in profound inhibition of platelet adhesion, activation and aggregate formation on collagen in vitro [6,24,29]. To assess potential cross-reactivity of JAQ1 with GPVI in other species, we assessed the binding of JAQ1-FITC to platelets in freshly prepared diluted heparinized blood by flow cytometry. In agreement with previous descriptions, JAQ1-FITC robustly bound to the wild type (WT), but not Gp6−/− mouse platelets (Figure 1A). In addition, we observed no binding to the platelets from closely related species, such as rat, rabbit, guinea pig, swine or dog (Table 1). Remarkably, however, JAQ1-FITC robustly bound to human platelets (Figure 1A, Table 1). Next, we assessed whether JAQ1 binding would be affected by the pre-incubation of human platelets with different, established anti-huGPVI monoclonal antibodies (mAbs). Indeed, JAQ1-FITC binding was reduced by ~66% after pre-incubation with the function-blocking anti-huGPVI mAb EMF-1 [30], but only partially by the non-function-blocking EMF-2 (~26%) (Figure 1B). Subsequently, we tested whether JAQ1 recognizes huGPVI in a Western blot analysis of human platelet lysate. However, while JAQ1 efficiently detected mGPVI no band in the human platelet lysates was observed, indicating that the epitope on huGPVI is conformation sensitive (Figure 1C).
Figure 1.
Anti-mouse GPVI monoclonal antibody JAQ1 binds to human GPVI and modulates its function. (A) Washed human or mouse blood was pre-incubated with JAQ1 IgG-FITC and Mean Fluorescence Intensity (MFI) was measured by flow cytometry; where indicated, human blood was pre-incubated with 20 µg/mL IV.3. Irrelevant rat-IgG-FITC was used as control; (B) Human blood was pre-incubated with 20 µg/mL EMF1, EMF-2 or control IgG for 10 min and subsequently incubated with JAQ1-FITC for 10 min, irrelevant IgG-FITC was used as control; (C) Mouse (WT) and human platelet lysates were separated by 10% SDS-PAGE under non-reducing condition and blotted onto PVDF membrane. JAQ1-HRP was used to detect GPVI on the membrane. GAPDH served as loading control; (D) Murine or human washed platelets were pre-incubated with either JAQ1 or a control IgG and aggregometry was performed; crosslinking of bound antibody was induced by adding an anti-Rat IgG antibody and light transmission was recorded for 15 min. When indicated, human platelets were incubated with IV.3 prior to JAQ1 addition; (E) Human washed platelets were pre-incubated with either JAQ1 or a control IgG and aggregometry was performed; aggregation was induced using the indicated agonists and for 10 min; (F,G) Human-washed platelets were pre-incubated with IV.3 plus JAQ1 IgG or a control IgG and let spread on a 100 µg/mL fibrinogen-coated surface for 45 min at 37 °C. Experiments shown are representative of n = 4. Flow cytometry and spreading data are expressed as mean ± SD, significance is expressed as * p < 0.05, ** p < 0.01, vs. indicated group (t-test).
Table 1.
Flow cytometric analysis of JAQ1 binding to the platelet surface of different species. The data are reported as MFI of JAQ1-FITC (JAQ1 MFI) and as percentages with respect to mouse MFI (JAQ1%). Mean Platelet Volume (MPV) of the different species are reported in femtoliter (MPV). “Negative” indicates MFI values comparable to isotype IgG-FITC control values. Data are reported as mean ± SD.
Next, we assessed the effects of JAQ1 on the human platelet aggregation. The cross-linking of JAQ1 with a secondary anti-rat IgG antibody triggered the rapid aggregation of human platelets, similar to mouse platelets (Figure 1D). Of note, this platelet response was not dependent on FcγRIIa, as blocking this with IV.3 antibody did not prevent JAQ1-cross-linking-induced platelet aggregation, but only minimally delayed it (Figure 1D; Figure S1A, Supplementary Materials). The pre-incubation of human-washed platelets with 5, 10 or 20 µg/mL JAQ1 reduced and delayed aggregation in response to CRP, but in contrast to mouse platelets (as shown in paragraph 2.3) this was not abrogated. Notably, the traces of the JAQ1-treated samples showed a reduced platelet-shape change, pointing to a JAQ1-dependent platelet pre-activation and indicating that the residual observable aggregation is partly due to this effect (Figure 1E; Figure S1B,C, Supplementary Materials). Intriguingly, the presence of JAQ1 rather promoted collagen- and convulxin- dependent aggregate formation, and this occurred independently of FcγRIIa (Figure 1E; Figure S1B,C, Supplementary Materials), indicating that JAQ1 modulates huGPVI towards a pre-active state. The treatment with higher concentrations of JAQ1 slightly exacerbated the CRP-inhibitory effect and convulxin-dependent increased aggregation, but not with collagen (Figure 1E; Figure S1B,C, Supplementary Materials). Finally, we tested the JAQ1 effect on the spreading of human platelets on a fibrinogen-coated surface in the absence of additional agonists. Interestingly, pre-incubation with JAQ1 but not the control IgG increased the percentage of the fully spread platelets (phase 4) on the surface (Figure 1F,G). Collectively, these data indicate that the binding epitope of JAQ1 may functionally not be fully conserved between mouse and human GPVI.
2.2. A humanized GP6 Mouse Line to Study the Effect of JAQ1 on huGPVI
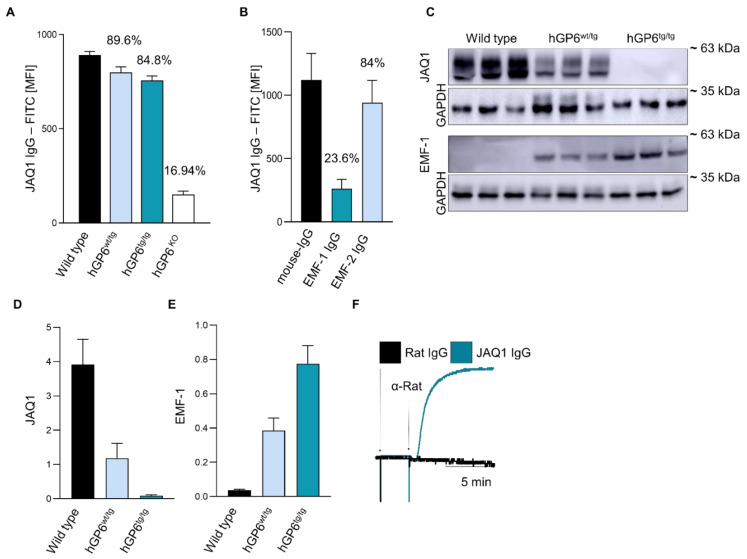
In order to study the JAQ1 effects on huGPVI in the absence of possible FcγRIIa interferences, we capitalized on a newly generated mouse line humanized for the GP6 gene (hGP6tg/tg). The mouse line generation is thoroughly described in the Materials and Methods Section 4.3; (Figure S4, Supplementary Materials). To ensure the suitability of the newly generated mouse line for further experiments, we analyzed the platelet count, size and expression levels of prominent membrane glycoproteins and found no alteration compared to the wild-type controls, except for the selective expression of human or mouse GPVI (Tables S1 and S2, Supplementary Materials). As expected, we also did not find any difference in the overall platelet activation (Figure S4A,B, Supplementary Materials) and GPVI-dependent platelet aggregation and thrombus formation under flow (Figure S4C–F, Supplementary Materials). Next, we confirmed by flow cytometry that JAQ1 binds to the platelets of hGP6tg/tg, hGP6wt/tg and wild-type mice in a comparable manner (Figure 2A). Furthermore, we tested whether EMF-1 or EMF-2 compete with JAQ1 for binding to the hGP6tg/tg platelets. In line with the results obtained with the human platelets, the JAQ1-FITC binding was profoundly reduced upon pre-incubating the platelets with EMF-1 (~76.4% reduction), but very marginally by EMF-2 (~16%) (Figure 2B). To exclude that the inability of JAQ1 to recognize human GPVI in a Western blot analysis was due to species-specific glycosylation, we probed the platelet lysates from the WT, hGP6tg/tg and hGP6wt/tg animals with JAQ1. As expected, the signals were only obtained in the samples from the WT or hGP6wt/tg mice, but not from the hGP6tg/tg platelet lysates. Of note, less mGPVI was detected in the hGP6wt/tg platelet lysates as compared to the WT platelets (70% reduction). Likewise, EMF-1 recognized huGPVI in the lysates of hGP6tg/tg as well as the hGP6wt/tg platelets (Figure 2C). As expected, the signal obtained from the hGP6wt/tg lysates was reduced (−49.3%) as compared to that of the hGP6tg/tg platelet lysates (Figure 2D,E). Finally, pre-incubation with JAQ1 IgG did not induce an evident platelet aggregation, whereas the cross-linking of JAQ1 with a secondary anti-rat IgG antibody induced a rapid aggregate formation of hGP6tg/tg-derived platelets (Figure 2F; Figure S2, Supplementary Materials). Overall, these data confirm the specific JAQ1 binding to huGPVI and illustrate that the newly generated mouse line is a suitable model for testing the huGPVI-targeting molecules.
Figure 2.
hGP6tg/tg mice confirm that JAQ1 binds to native human GPVI on the platelet surface, but not in Western blot analysis. (A) WT, hGP6wt/tg, hGP6tg/tg and Gp6−/−-washed blood was incubated JAQ1-FITC and MFI was measured by flow cytometry; (B) hGP6tg/tg-washed blood was pre-incubated with either EMF-1, EMF-2 or control IgG and subsequently incubated with JAQ1-FITC; (C) WT, hGP6wt/tg and hGP6tg/tg platelet lysates were separated by SDS-PAGE under non-reducing conditions and blotted onto a PVDF membrane. JAQ1 or EMF-1 were used to detect GPVI on the membrane. GAPDH served as loading control; (D,E) Western blot quantitative analysis relative to loading control; (F) hGP6tg/tg washed platelets were pre-incubated with JAQ1 or control IgG and aggregate formation was induced using anti-rat IgG antibodies (20 µg/mL). Experiments shown are representative of n = 4, Western blot of n = 3. Flow cytometry and Western blot data are expressed as means ± SD.
2.3. Differential Effect of JAQ1 on huGPVI and mGPVI
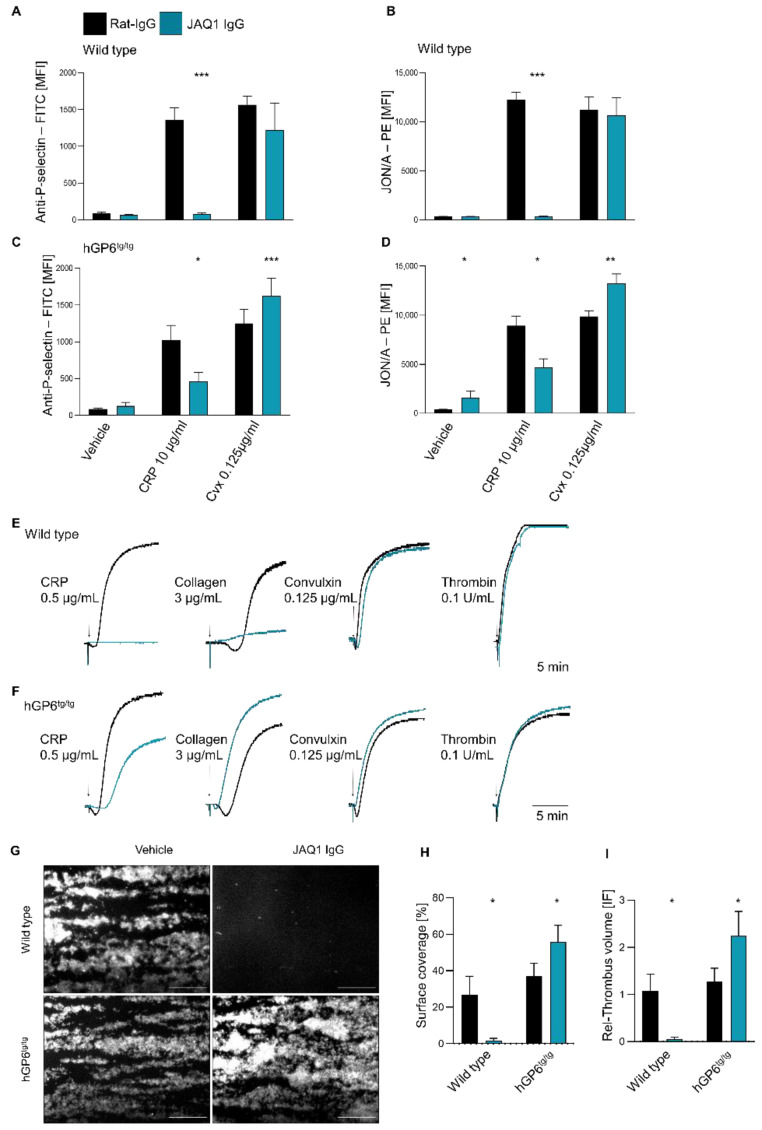
The effect of JAQ1 on the GPVI-mediated platelet activation was assessed by flow cytometry, using WT and hGP6tg/tg platelets. In line with previous reports [6], JAQ1 abrogated the CRP-induced platelet activation of the WT platelets, while convulxin (CVX)-induced activation remained intact (Figure 3A,B). In contrast, the hGP6tg/tg platelets displayed only moderately inhibited CRP-dependent platelet activation, while convulxin-induced integrin activation was even enhanced (Figure 3C,D). Next, the effect of JAQ1 on the platelet aggregation was assessed. On the WT platelets, JAQ1 abrogated the CRP-induced and dramatically reduced the collagen-induced aggregation, while aggregation to the convulxin or non-GPVI agonist was unaltered (Figure 3E; Figure S3A,C, Supplementary Materials; and data not shown). Interestingly, the aggregation of the hGP6tg/tg platelets in response to CRP was delayed and reduced following the JAQ1-pretreatment. In stark contrast, JAQ1 accelerated and fostered the collagen- and convulxin-induced hGP6tg/tg platelet aggregation (Figure 3F; Figure S3B,D, Supplementary Materials), confirming our results with the human platelets (Figure 1). To assess whether the enhancement of platelet activation and aggregation would result in an accelerated thrombus formation, we tested the effect of JAQ1 on the WT and hGP6tg/tg platelets in a whole mouse blood-flow adhesion assay on a collagen-coated surface. As expected, JAQ1 completely abolished the thrombus formation in the WT blood. Strikingly, however, the formation of the thrombi was potently enhanced after incubation of the hGP6tg/tg-derived blood, thus illustrating the differential effects of JAQ1 on mouse versus human GPVI (Figure 3G–I).
Figure 3.
Differential effect of JAQ1 on huGPVI and mGPVI. (A–D) WT (A,B) or hGP6tg/tg (C,D) diluted heparinized blood was pre-incubated with 20 µg/mL JAQ1 or control-IgG. Treated platelets were incubated with WUG 1.9-FITC (A,C), JON/A-PE (B,D) and stimulated with CRP (10 µg/mL), convulxin (1.25 µg/mL) or vehicle; (E,F) Washed platelets from WT (E) or hGP6tg/tg (F) were pre-incubated with 20 µg/mL JAQ1 or control IgG and aggregation was induced with the indicated agonists; aggregation was measured for 10 min; (G–I) Heparinized WT or hGP6tg/tg blood was pre-incubated with 20 µg/mL JAQ1 or control IgG and tested in flow adhesion assay on a collagen-coated surface. Percentage of the covered surface (H) and relative volume of thrombi (I) were analyzed based on fluorescence intensity of anti-GPIX-AF488 derivative. Experiments shown are representative of n = 4. Flow cytometry data are expressed as means ± SD, significance is expressed as * p < 0.05, ** p < 0.01, *** p < 0.001 vs. indicated group (t-test).
3. Discussion
The monoclonal antibody JAQ1 has become a widely used tool for platelet research and its inhibitory effect on mouse GPVI has been thoroughly characterized. Here, we report, for the first time, that this antibody also cross-reacts with huGPVI. Interestingly, however, our data suggest that the binding epitope is functionally different between mGPVI and huGPVI. While JAQ1 inhibits the CRP-dependent platelet activation in both species (albeit at different levels), it differs in its effects on convulxin- and collagen-induced platelet activation (Figure 1, Figure 2 and Figure 3). On the murine platelets, JAQ1 blocks activation in response to low and intermediate collagen concentrations (Figure 3, [38]), while it enhances collagen-induced activation of huGPVI expressing platelets (Figure 1 and Figure 3; Figures S1 and S3, Supplementary Materials). One explanation could be that JAQ1 stabilizes the GPVI dimers (or clusters), which ‘prime’ GPVI for subsequent platelet activation, thereby accelerating and enhancing the platelet activation. Of note, JAQ1 even triggers a pre-activation of the human but not the mouse GPVI, resulting in subtle integrin activation (already in the absence of further agonists) as revealed by flow cytometry (Figure 3A–D).
Very unexpectedly, our data show that JAQ1 binds to a conserved epitope in mouse and human GPVI that somehow evolved to differ in its functional significance. Mouse, rat and guinea pig diverged from human ~90 million years ago [39], and are more closely related within each other with respect to other species, such as dog and swine [40]. These data are in agreement with our flow cytometry results, showing no binding to GPVI on the platelet surface in these animals. The previous studies revealed discrepancies between the mouse and human GPVI affinity to specific ligands and discussed possible differences with regard to the relevance of this receptor in thrombosis [41], thus, underscoring the need to utilize humanized animal models. Indeed, our newly generated mouse line allowed us to faithfully reproduce the results obtained with human platelets, thereby excluding the possible overlapping effects of FcγRIIa, which is present in human but not mouse platelets and signals through a similar pathway as GPVI. These data clearly show that the hGP6tg/tg mice reported here are a suitable model system for testing GPVI modulators in vitro and in vivo.
In conclusion, we present a previously undescribed and unexpected pre-activating effect of JAQ1 on huGPVI. This study also illustrated how the same epitope in human and mouse GPVI has genetically diverged during evolution, leading to a different functional significance.
4. Materials and Methods
4.1. Antibodies and Reagents
The collagen-related peptide (CRP) was a generous gift from Paul Bray (Baylor College, USA); Horm collagen was purchased from Takeda (Linz, Austria); convulxin was purchased from Enzo Life Sciences (New York, NY, USA); thrombin was purchased from Roche Diagnostic (Mannheim, Germany); rabbit anti-GAPDH and rat anti-mouse IgG-HRP antibodies were purchased from Sigma-Aldrich (Steinheim, Germany); goat anti-rat IgG-HRP was purchased from Dianova (Hamburg, Germany); anti-rabbit IgG-HRP was purchased from Jackson Immuno (Suffolk, UK). For the human blood collection, the S-monovettes 3.2% citrate and Safety-Fly-Needle 21G were purchased from Sarstedt (Nümbrecht, Germany). The micro-cuvettes for aggregometry were purchased from LABITec (Ahrensburg, Germany). The 5 mL Polystyrene Round-Bottom Tubes for flow cytometry were purchased from Corning Inc. (New York, NY, USA). The heparin was purchased from Ratiopharm (Ulm, Germany). The JAQ1 [24], EMF-1 [30], EMF-2, JON/A [42,43] and WUG 1.9 were generated in house. The IV.3 antibody was purchased from Biozol (Eching, Germany).
4.2. Blood Donors and Blood Collection
The blood was obtained from healthy volunteers not undergoing anticoagulant or antiplatelet drug therapy for at least 4 weeks before recruitment. For the present study, the volunteers signed a written informed consent after approval by the Institutional Review Boards of the University of Würzburg and in accordance with the Declaration of Helsinki. The relevant guidelines and regulations were followed during the performance of all of the described methods. Butterfly needles were used for the collection of blood by venipuncture; the samples were collected into 9 mL tubes containing 3.2% trisodium citrate. For all of the studies, the blood was used within 4 h from withdrawal and kept at room temperature.
4.3. Animals
The animals used in this study were matched based on genetic background, sex and age. The experiments with animals shown in this article had been previously approved by the district government of Lower Franconia (Regierung von Unterfranken) and performed following the current Animal Research: Reporting of In Vivo Experiments guidelines (https://www.nc3rs.org.uk/arrive-guidelines, accessed on 15 June 2022).In order to generate the humanized GP6 (hGP6tg/tg) mouse line, the cDNA-expressing human GPVI (huGPVI) was inserted at the level of the murine ATG of the mouse Gp6 gene via CRISPR-Cas9 technology. The mutagenesis was carried out on the base of previous publications [44] via inserting the cDNA-expressing huGPVI within the exon 1 of the WT gene, thus allowing for the selective expression of the human but not mouse GPVI (Figure S5, Supplementary Materials). The mice were genotyped by PCR with the forward primer 5′-TGGCAAGAAGAGATAAGTGGTGGCT-3′, the reverse primers 5′-CAGGTCACCTTCAGGACTCACCAAT-3′ for the wild-type amplification and 5′-CAGACTTCTCTTCATGGCCGGGAT-3′ for the transgenic mouse amplification. For the experiments, venous blood was collected in 300 µL of 20 U/mL heparin via retro-orbital bleeding.
4.4. Measurement of Platelet Count and Size
For the assessment of platelet size and count, the mice were bled into tubes coated with EDTA; the parameters were measured using an automated cell counter (ScilVet, scil animal care company GmbH, Viernheim, Germany).
4.5. Washed Human and Murine Platelets
The human-washed platelets were obtained as follows: 2 mL of ACD pH4.5 was added to the citrated blood and centrifuged for 20′ at 300× g at room temperature. The Platelet-Rich-Plasma was collected in new 15 mL tubes and supplemented with 1/10 ACD, 2 μL of apyrase/mL (0.02 U mL−1; A6410, Sigma-Aldrich) and 5 μL PGI2/mL (0.1 μg mL−1; P6188, Sigma-Aldrich). The platelets were pelleted by centrifugation for 10 min at 500× g, washed twice with Tyrode’s buffer containing 2 μL apyrase/mL and 5 μL PGI2/mL. The murine platelets were washed as previously described [45].
4.6. Flow Cytometry Assays
The human blood diluted 1:10 and the murine blood diluted 1:20 in Tyrode’s buffer—Ca2+ was used for the flow cytometry analysis. For the platelet activation analysis, the murine blood was diluted in Tyrode’s buffer + Ca2+. JON/A-PE (Emfret Analytics, Eibelstadt, Germany) was used to detect the activated integrin αIIbβ3 [42] whilst P-selectin exposure was detected with a specific anti-mPselectin FITC-conjugated antibody WUG 1.9 [42]. The diluted murine blood was incubated with either CRP (10 µg/mL), convulxin (1.25 µg/mL), thrombin (0.1 U/mL) or vehicle control, together with JON/A-PE and anti-Psel-FITC for 12 min and finally diluted in 500 µL PBS. The measurement of the MFI was performed on a FACSCelesta (BD Biosciences, Gurugram, India).
4.7. Western Blot
The human and murine platelets were lysed at a concentration of 5 × 108/mL in an IP-buffer containing a protease inhibitor cocktail. The platelet lysates were mixed with a loading buffer containing SDS and pre-heated to 95 °C for 5 min before loading in a 10% polyacrylamide gel, and the immunoblotting was performed as previously described [46,47].
4.8. Aggregometry Assay
Human- and murine-washed platelets were resuspended at a concentration of 2 × 108/mL in Tyrode’s buffer—Ca2+. The aggregometry was performed as previously described [48]. The human or murine platelets were activated using 0.5 µg/mL Collagen-Related-Peptide (CRP), convulxin (0.125 µg/mL) or collagen 3 µg/mL (mouse) and 10 µg/mL (human). The measurements were performed using an APACT 4 aggregometer from LABITec (Ahrensburg, Germany).
4.9. Spreading Assay
The human platelets were washed and resuspended at a concentration of 108/mL in Tyrode’s buffer—Ca2+. The washed platelets were incubated with either JAQ1 IgG or control rat IgG for 1 min and then pipetted onto a 100 µg/mL fibrinogen-coated surface. The platelets were allowed to spread for 45 min. Fixation, coating and visualization were performed, as previously described [49].
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23158610/s1.
Author Contributions
S.N. performed the experiments, analyzed the data, and wrote the manuscript. A.S. performed experiments. J.W.M.H. and M.J.E.K. provided supervision, analyzed data and did proofreading of the manuscript. B.N. and D.S. designed research, analyzed data, provided expert supervision and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
For this study, blood samples were obtained from healthy volunteers following written informed consent in accordance with the Declaration of Helsinki and after approval by the Institutional Review Boards (IRB) of University of Würzburg (295/20). The experiments with animals shown in this article had been previously approved by the district government of Lower Franconia (Regierung von Unterfranken) and performed following the current Animal Research: Reporting of In Vivo Experiments guidelines (https://www.nc3rs.org.uk/arrive-guidelines, accessed on 15 June 2022).
Informed Consent Statement
Informed consent was obtained from all of the subjects involved in the study.
Data Availability Statement
All data are included in the manuscript as figures, tables or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
S.N. is supported by the European Union’s Horizon 2020 Research and Innovation program under the Marie Sklodowska-Curie grant agreement No.766118; and is enrolled in a joint PhD program at the Universities of Maastricht (The Netherlands) and Würzburg (Germany).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Italiano J.E., Jr., Lecine P., Shivdasani R.A., Hartwig J.H. Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J. Cell Biol. 1999;147:1299–1312. doi: 10.1083/jcb.147.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michelson A.D., Cattaneo M., Frelinger A., Newman P. Platelets. 4th ed. Elsevier; Amsterdam, The Netherlands: 2019. [Google Scholar]
- 3.Vogtle T., Cherpokova D., Bender M., Nieswandt B. Targeting platelet receptors in thrombotic and thrombo-inflammatory disorders. Hamostaseologie. 2015;35:235–243. doi: 10.5482/HAMO-14-10-0049. [DOI] [PubMed] [Google Scholar]
- 4.Nieswandt B., Pleines I., Bender M. Platelet adhesion and activation mechanisms in arterial thrombosis and ischaemic stroke. J. Thromb. Haemost. 2011;9((Suppl. 1)):92–104. doi: 10.1111/j.1538-7836.2011.04361.x. [DOI] [PubMed] [Google Scholar]
- 5.McFadyen J.D., Schaff M., Peter K. Current and future antiplatelet therapies: Emphasis on preserving haemostasis. Nat. Rev. Cardiol. 2018;15:181–191. doi: 10.1038/nrcardio.2017.206. [DOI] [PubMed] [Google Scholar]
- 6.Nieswandt B., Schulte V., Bergmeier W., Mokhtari-Nejad R., Rackebrandt K., Cazenave J.P., Ohlmann P., Gachet C., Zirngibl H. Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in mice. J. Exp. Med. 2001;193:459–469. doi: 10.1084/jem.193.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mammadova-Bach E., Ollivier V., Loyau S., Schaff M., Dumont B., Favier R., Freyburger G., Latger-Cannard V., Nieswandt B., Gachet C., et al. Platelet glycoprotein VI binds to polymerized fibrin and promotes thrombin generation. Blood. 2015;126:683–691. doi: 10.1182/blood-2015-02-629717. [DOI] [PubMed] [Google Scholar]
- 8.Onselaer M.B., Hardy A.T., Wilson C., Sanchez X., Babar A.K., Miller J.L.C., Watson C.N., Watson S.K., Bonna A., Philippou H., et al. Fibrin and D-dimer bind to monomeric GPVI. Blood Adv. 2017;1:1495–1504. doi: 10.1182/bloodadvances.2017007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alshehri O.M., Hughes C.E., Montague S., Watson S.K., Frampton J., Bender M., Watson S.P. Fibrin activates GPVI in human and mouse platelets. Blood. 2015;126:1601–1608. doi: 10.1182/blood-2015-04-641654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrella G., Huang J., Provenzale I., Swieringa F., Heubel-Moenen F., Farndale R.W., Roest M., van der Meijden P.E.J., Thomas M., Ariens R.A.S., et al. Nonredundant Roles of Platelet Glycoprotein VI and Integrin alphaIIbbeta3 in Fibrin-Mediated Microthrombus Formation. Arterioscler. Thromb. Vasc. Biol. 2021;41:e97–e111. doi: 10.1161/ATVBAHA.120.314641. [DOI] [PubMed] [Google Scholar]
- 11.Maurer E., Schaff M., Receveur N., Bourdon C., Mercier L., Nieswandt B., Dubois C., Jandrot-Perrus M., Goetz J.G., Lanza F., et al. Fibrillar cellular fibronectin supports efficient platelet aggregation and procoagulant activity. Thromb. Haemost. 2015;114:1175–1188. doi: 10.1160/TH14-11-0958. [DOI] [PubMed] [Google Scholar]
- 12.Lakshmanan H.H.S., Melrose A.R., Sepp A.I., Mitrugno A., Ngo A.T.P., Khader A., Thompson R., Sallee D., Pang J., Mangin P.H., et al. The basement membrane protein nidogen-1 supports platelet adhesion and activation. Platelets. 2021;32:424–428. doi: 10.1080/09537104.2020.1745170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue O., Suzuki-Inoue K., McCarty O.J., Moroi M., Ruggeri Z.M., Kunicki T.J., Ozaki Y., Watson S.P. Laminin stimulates spreading of platelets through integrin alpha6beta1-dependent activation of GPVI. Blood. 2006;107:1405–1412. doi: 10.1182/blood-2005-06-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pachel C., Mathes D., Arias-Loza A.P., Heitzmann W., Nordbeck P., Deppermann C., Lorenz V., Hofmann U., Nieswandt B., Frantz S. Inhibition of Platelet GPVI Protects Against Myocardial Ischemia-Reperfusion Injury. Arterioscler. Thromb. Vasc. Biol. 2016;36:629–635. doi: 10.1161/ATVBAHA.115.305873. [DOI] [PubMed] [Google Scholar]
- 15.Weiss L.J., Manukjan G., Pflug A., Winter N., Weigel M., Nagler N., Kredel M., Lam T.T., Nieswandt B., Weismann D., et al. Acquired platelet GPVI receptor dysfunction in critically ill patients with sepsis. Blood. 2021;137:3105–3115. doi: 10.1182/blood.2020009774. [DOI] [PubMed] [Google Scholar]
- 16.Volz J., Mammadova-Bach E., Gil-Pulido J., Nandigama R., Remer K., Sorokin L., Zernecke A., Abrams S.I., Ergun S., Henke E., et al. Inhibition of platelet GPVI induces intratumor hemorrhage and increases efficacy of chemotherapy in mice. Blood. 2019;133:2696–2706. doi: 10.1182/blood.2018877043. [DOI] [PubMed] [Google Scholar]
- 17.Mammadova-Bach E., Gil-Pulido J., Sarukhanyan E., Burkard P., Shityakov S., Schonhart C., Stegner D., Remer K., Nurden P., Nurden A.T., et al. Platelet glycoprotein VI promotes metastasis through interaction with cancer cell-derived galectin-3. Blood. 2020;135:1146–1160. doi: 10.1182/blood.2019002649. [DOI] [PubMed] [Google Scholar]
- 18.Perrella G., Nagy M., Watson S.P., Heemskerk J.W.M. Platelet GPVI (Glycoprotein VI) and Thrombotic Complications in the Venous System. Arterioscler. Thromb. Vasc. Biol. 2021;41:2681–2692. doi: 10.1161/ATVBAHA.121.316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleinschnitz C., Pozgajova M., Pham M., Bendszus M., Nieswandt B., Stoll G. Targeting platelets in acute experimental stroke: Impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation. 2007;115:2323–2330. doi: 10.1161/CIRCULATIONAHA.107.691279. [DOI] [PubMed] [Google Scholar]
- 20.Voors-Pette C., Lebozec K., Dogterom P., Jullien L., Billiald P., Ferlan P., Renaud L., Favre-Bulle O., Avenard G., Machacek M., et al. Safety and Tolerability, Pharmacokinetics, and Pharmacodynamics of ACT017, an Antiplatelet GPVI (Glycoprotein VI) Fab. Arterioscler. Thromb. Vasc. Biol. 2019;39:956–964. doi: 10.1161/ATVBAHA.118.312314. [DOI] [PubMed] [Google Scholar]
- 21.ACTICOR BIOTECH: Presentation of Positive Results from the ACTIMIS Phase 1b/2a Study in Stroke at ESOC 2022. 2022. [(accessed on 20 June 2022)]. Press release . Available online: https://www.businesswire.com/news/home/20220509005836/en/ACTICOR-BIOTECH-Presentation-of-Positive-Results-From-the-ACTIMIS-Phase-1b2a-Study-in-Stroke-at-ESOC-2022.
- 22.Jadoui S., Le Chapelain O., Ollivier V., Mostefa-Kara A., Di Meglio L., Dupont S., Gros A., Nomenjanahary M.S., Desilles J.P., Mazighi M., et al. Glenzocimab does not impact glycoprotein VI-dependent inflammatory haemostasis. Haematologica. 2021;106:2000–2003. doi: 10.3324/haematol.2020.270439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massberg S., Gawaz M., Gruner S., Schulte V., Konrad I., Zohlnhofer D., Heinzmann U., Nieswandt B. A crucial role of glycoprotein VI for platelet recruitment to the injured arterial wall in vivo. J. Exp. Med. 2003;197:41–49. doi: 10.1084/jem.20020945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieswandt B., Bergmeier W., Schulte V., Rackebrandt K., Gessner J.E., Zirngibl H. Expression and function of the mouse collagen receptor glycoprotein VI is strictly dependent on its association with the FcRgamma chain. J. Biol. Chem. 2000;275:23998–24002. doi: 10.1074/jbc.M003803200. [DOI] [PubMed] [Google Scholar]
- 25.Schulte V., Rabie T., Prostredna M., Aktas B., Gruner S., Nieswandt B. Targeting of the collagen-binding site on glycoprotein VI is not essential for in vivo depletion of the receptor. Blood. 2003;101:3948–3952. doi: 10.1182/blood-2002-10-3242. [DOI] [PubMed] [Google Scholar]
- 26.Jandrot-Perrus M., Busfield S., Lagrue A.H., Xiong X., Debili N., Chickering T., Le Couedic J.P., Goodearl A., Dussault B., Fraser C., et al. Cloning, characterization, and functional studies of human and mouse glycoprotein VI: A platelet-specific collagen receptor from the immunoglobulin superfamily. Blood. 2000;96:1798–1807. doi: 10.1182/blood.V96.5.1798. [DOI] [PubMed] [Google Scholar]
- 27.Slater A., Perrella G., Onselaer M.B., Martin E.M., Gauer J.S., Xu R.G., Heemskerk J.W., Ariens R.A.S., Watson S.P. Does fibrin(ogen) bind to monomeric or dimeric GPVI, or not at all? Platelets. 2019;30:281–289. doi: 10.1080/09537104.2018.1508649. [DOI] [PubMed] [Google Scholar]
- 28.Lecut C., Feeney L.A., Kingsbury G., Hopkins J., Lanza F., Gachet C., Villeval J.L., Jandrot-Perrus M. Human platelet glycoprotein VI function is antagonized by monoclonal antibody-derived Fab fragments. J. Thromb. Haemost. 2003;1:2653–2662. doi: 10.1111/j.1538-7836.2003.00495.x. [DOI] [PubMed] [Google Scholar]
- 29.EMFRET Analytics Home Page. 2022. [(accessed on 10 May 2022)]. Available online: https://www.emfret.com/index.php?id=2.
- 30.Navarro S., Stegner D., Nieswandt B., Heemskerk J.W.M., Kuijpers M.J.E. Temporal Roles of Platelet and Coagulation Pathways in Collagen- and Tissue Factor-Induced Thrombus Formation. Int. J. Mol. Sci. 2021;23:358. doi: 10.3390/ijms23010358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balkenhol J., Kaltdorf K.V., Mammadova-Bach E., Braun A., Nieswandt B., Dittrich M., Dandekar T. Comparison of the central human and mouse platelet signaling cascade by systems biological analysis. BMC Genom. 2020;21:897. doi: 10.1186/s12864-020-07215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson C.W., Hutson N.K., Steward S.A., Ashmun R.A., Davis D.S., Edwards H.H., Rehg J.E., Dockter M.E. The Wistar Furth rat: An animal model of hereditary macrothrombocytopenia. Blood. 1988;71:1676–1686. doi: 10.1182/blood.V71.6.1676.1676. [DOI] [PubMed] [Google Scholar]
- 33.Massanyi M., Kohut L., Argente M.J., Halo M., Kovacik A., Kovacikova E., Ondruska L., Formicki G., Massanyi P. The effect of different sample collection methods on rabbit blood parameters. Saudi J. Biol. Sci. 2020;27:3157–3160. doi: 10.1016/j.sjbs.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Genzer S.C., Huynh T., Coleman-Mccray J.D., Harmon J.R., Welch S.R., Spengler J.R. Hematology and Clinical Chemistry Reference Intervals for Inbred Strain 13/n Guinea Pigs (Cavia Porcellus) J. Am. Assoc. Lab. Anim. Sci. 2019;58:293–303. doi: 10.30802/AALAS-JAALAS-18-000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pliszczak-Krol A., Rzasa A., Gemra M., Krol J., Luczak G., Zyzak A., Zalewski D., Iwaszko-Simonik A., Graczyk S. Age-related changes of platelet and plasma coagulation parameters in young pigs. J. Vet. Diagn. Investig. 2016;28:561–567. doi: 10.1177/1040638716658928. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz D., Sharkey L., Armstrong P.J., Knudson C., Kelley J. Platelet volume and plateletcrit in dogs with presumed primary immune-mediated thrombocytopenia. J. Vet. Intern. Med. 2014;28:1575–1579. doi: 10.1111/jvim.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korniluk A., Koper-Lenkiewicz O.M., Kaminska J., Kemona H., Dymicka-Piekarska V. Mean Platelet Volume (MPV): New Perspectives for an Old Marker in the Course and Prognosis of Inflammatory Conditions. Mediat. Inflamm. 2019;2019:9213074. doi: 10.1155/2019/9213074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulte V., Snell D., Bergmeier W., Zirngibl H., Watson S.P., Nieswandt B. Evidence for two distinct epitopes within collagen for activation of murine platelets. J. Biol. Chem. 2001;276:364–368. doi: 10.1074/jbc.M007536200. [DOI] [PubMed] [Google Scholar]
- 39.Ernst P.B., Carvunis A.R. Of mice, men and immunity: A case for evolutionary systems biology. Nat. Immunol. 2018;19:421–425. doi: 10.1038/s41590-018-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirkness E.F., Bafna V., Halpern A.L., Levy S., Remington K., Rusch D.B., Delcher A.L., Pop M., Wang W., Fraser C.M., et al. The dog genome: Survey sequencing and comparative analysis. Science. 2003;301:1898–1903. doi: 10.1126/science.1086432. [DOI] [PubMed] [Google Scholar]
- 41.Janus-Bell E., Ahmed M.U., Receveur N., Mouriaux C., Nieswandt B., Gardiner E.E., Gachet C., Jandrot-Perrus M., Mangin P.H. Differential Role of Glycoprotein VI in Mouse and Human Thrombus Progression and Stability. Thromb. Haemost. 2021;121:543–546. doi: 10.1055/s-0040-1718737. [DOI] [PubMed] [Google Scholar]
- 42.Bergmeier W., Schulte V., Brockhoff G., Bier U., Zirngibl H., Nieswandt B. Flow cytometric detection of activated mouse integrin alphaIIbbeta3 with a novel monoclonal antibody. Cytometry. 2002;48:80–86. doi: 10.1002/cyto.10114. [DOI] [PubMed] [Google Scholar]
- 43.Stegner D., Göb V., Krenzlin V., Beck S., Hemmen K., Schuhmann M.K., Schörg B.F., Hackenbroch C., May F., Burkard P., et al. Foudroyant cerebral venous (sinus) thrombosis triggered through CLEC-2 and GPIIb/IIIa dependent platelet activation. Nat. Cardiovasc. Res. 2022;1:132–141. doi: 10.1038/s44161-021-00017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mangin P.H., Tang C., Bourdon C., Loyau S., Freund M., Hechler B., Gachet C., Jandrot-Perrus M. A humanized glycoprotein VI (GPVI) mouse model to assess the antithrombotic efficacies of anti-GPVI agents. J. Pharmacol. Exp. Ther. 2012;341:156–163. doi: 10.1124/jpet.111.189050. [DOI] [PubMed] [Google Scholar]
- 45.Bender M., Stritt S., Nurden P., van Eeuwijk J.M., Zieger B., Kentouche K., Schulze H., Morbach H., Stegner D., Heinze K.G., et al. Megakaryocyte-specific Profilin1-deficiency alters microtubule stability and causes a Wiskott-Aldrich syndrome-like platelet defect. Nat. Commun. 2014;5:4746. doi: 10.1038/ncomms5746. [DOI] [PubMed] [Google Scholar]
- 46.Heib T., Hermanns H.M., Manukjan G., Englert M., Kusch C., Becker I.C., Gerber A., Wackerbarth L.M., Burkard P., Dandekar T., et al. RhoA/Cdc42 signaling drives cytoplasmic maturation but not endomitosis in megakaryocytes. Cell Rep. 2021;35:109102. doi: 10.1016/j.celrep.2021.109102. [DOI] [PubMed] [Google Scholar]
- 47.Neagoe R.A.I., Gardiner E.E., Stegner D., Nieswandt B., Watson S.P., Poulter N.S. Rac Inhibition Causes Impaired GPVI Signalling in Human Platelets through GPVI Shedding and Reduction in PLCgamma2 Phosphorylation. Int. J. Mol. Sci. 2022;23:3746. doi: 10.3390/ijms23073746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown H.C., Beck S., Navarro S., Di Y., Soriano Jerez E.M., Kaczmarzyk J., Thomas S.G., Mirakaj V., Watson S.P., Nieswandt B., et al. Antibody-mediated depletion of human CLEC-2 in a novel humanised mouse model. bioRxiv. 2021 doi: 10.1101/2021.10.03.462933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheller I., Beck S., Gob V., Gross C., Neagoe R.A.I., Aurbach K., Bender M., Stegner D., Nagy Z., Nieswandt B. Thymosin beta4 is essential for thrombus formation by controlling the G-actin/F-actin equilibrium in platelets. Haematologica. 2021 doi: 10.3324/haematol.2021.278537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript as figures, tables or Supplementary Materials.