Abstract
Metal foams possess remarkable properties, such as lightweight, high compressive strength, lower specific weight, high stiffness, and high energy absorption. These properties make them highly desirable for many engineering applications, including lightweight materials, energy-absorption devices for aerospace and automotive industries, etc. For such potential applications, it is essential to understand the mechanical behaviour of these foams. Producing metal foams is a highly challenging task due to the coexistence of solid, liquid, and gaseous phases at different temperatures. Although numerous techniques are available for producing metal foams, fabricating foamed metal still suffers from imperfections and inconsistencies. Thus, a good understanding of various processing techniques and properties of the resulting foams is essential to improve the foam quality. This review discussed the types of metal foams available in the market and their properties, providing an overview of the production techniques involved and the contribution of metal foams to various applications. This review also discussed the challenges in foam fabrications and proposed several solutions to address these problems.
Keywords: compressive properties, Gibson and Ashby model, mechanical properties, metal foams, melt foaming, powder metallurgy
1. Introduction
Metal foams are cellular structures comprising solid materials with a large portion of gas-filled pores by volume. Due to their cellular structure, metal foams possess a set of unique mechanical and physical properties. These properties allow them to become highly efficient in several engineering applications, notably in components for blast resistance, fire resistance, thermal insulation, foam core sandwich panels, and sound and vibration damping [1,2]. In addition, they are recyclable, with no disposal issues [3]. Consequently, these materials have attracted immense attention in recent years. One of the exceptional features of foams is that their mechanical properties are flexible, and their pore size, geometry, density, and choice of foaming material can be controlled. When used as energy absorption materials, these foams could go through substantial deformations under nearly constant stress [4]. With the rapid advancements in defence, aerospace, and automotives, there is an increasing demand for lightweight materials with high specific strength, better fuel efficiency, and high energy absorption capacity to withstand impact forces [5,6]. Thus, their good mechanical, acoustic, electrical, thermal, and chemical properties make them ideal for structural and functional applications [6,7]. Metal foams generally consist of aluminium (Al), nickel (Ni), magnesium (Mg), copper (Cu), zinc (Zn), and steel. In particular, Al and their alloys are widely used as non-flammable materials for thermal and sound insulation, sandwich cores, mechanical damping, lightweight panels and impact resistance in transportation, strain insulators, and vibration control [8,9,10].
These foams are mostly developed by the addition of foaming agents or space holders in the matrix metal. Several methods have been used for foam development. These methods include adding foaming agent in liquid melt [11], compaction of metal powder and blowing agent [12], blowing gas [13], etc. This review discusses the microstructure of various metal foams, their mechanical properties, manufacturing methods, and industrial applications. Evaluating the properties of metal foams developed through different techniques is essential to find the optimum manufacturing strategies and the effects of various parameters on their microstructure and strength. After introducing different types of metal foams, we discuss the microstructure and properties of the metal foams. The fabrication techniques such as melt foaming and powder metallurgy techniques are discussed in the next section, Section 6 and Section 7 discusses the industrial applications of metal foams and the way forward respectively.
2. Types of Metal Foams
Foam structures are divisible into two types, i.e., the open- cell foams and closed-cell foams, as listed in Table 1. In open-cell foams, pores are connected to allow matters to pass through them. By contrast, pores are isolated in closed-cell foams. In general, open-cell foams are preferred for functional applications, such as in filters, catalyst supports, heat exchangers, etc., and closed-cell foams find applications in silencers, automobiles, bearings, sound and energy absorbers, etc. [14].
Table 1.
Microstructures of metal foams fabricated by melt foaming and powder metallurgy technique.
| Manufacturing Techniques | Material | Foaming Agent/Space Holders | Microstructure | Reference |
|---|---|---|---|---|
| Melt foaming | Al matrix, graphene |
NaCl, KCl and PMMA |
|
[49] |
| Al-Si13-MgX (X = 2.5–15 wt %) alloy | Mg |
|
[50] | |
| AlMg50, Ca | TiH2 |
|
[48] | |
| A356 foams | CaCO3 |
|
[51] | |
| Powder metallurgy | ||||
| Using foaming agent | AlSi10 alloy |
TiH2 |
|
[52] |
| Mg, Al, Cu, and Zn, yttrium | TiH2 |
|
[53] | |
| AlMg4Si8 alloy and multi-walled carbon nanotubes (MWCNT) | TiH2 |
|
[54] | |
| Space holder technique | Ti-based Cu alloy | Acrawax |
|
[55] |
| Steel (iron, graphite phosphorous) |
Urea granules |
|
[56] | |
| Aluminium, Graphene | NaCl, KCl, and PMMA |
|
[49] | |
| 316L austenitic stainless steel | Urea particles |
|
[57] | |
| Al matrix and MWCNT | Urea particles |
|
[58] | |
2.1. Open-Cell Foams
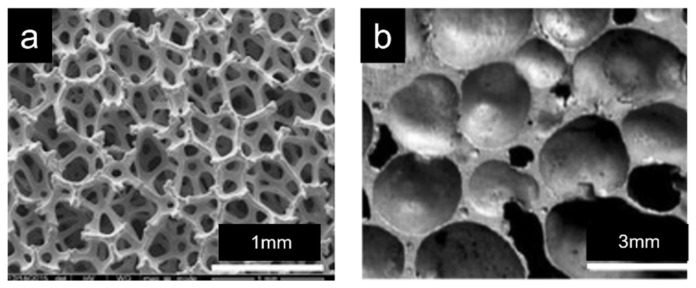
In open-cell foams, no film occurs between adjacent cells in the matrix material. A larger effective surface area (10–1000 times) is exposed to the surroundings compared to the dense material. They have sponge-like interconnected pores, as shown in Figure 1a [15]. Metal foams have a high specific surface area. These features make them suitable materials for heat exchangers, sound absorbers, catalysts, hydrogen storage, filter elements, etc. [16,17]. Meanwhile, high-porosity metal foams are commonly used in several real-world devices, including heat exchangers [18,19,20,21,22], fuel stacks [23], solar collectors [24,25], heat storage [26,27], etc. These foams possess excellent thermal properties, high permeability, high conductivity, and volume-to-area ratio.
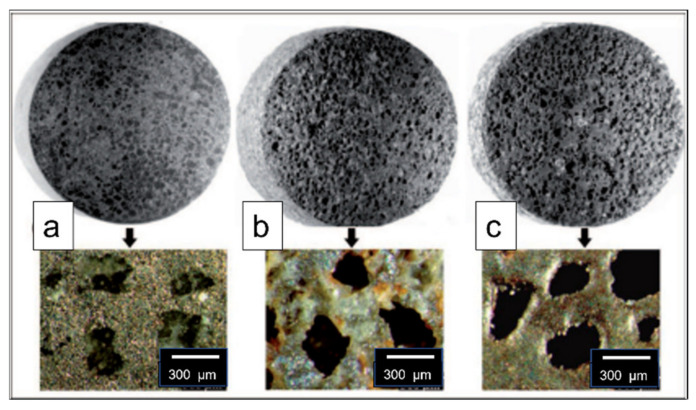
Figure 1.
The optical micrograph of microstructure of (a) open-cell metal foam [15] and (b) closed-cell metal foam [28].
2.2. Closed-Cell Foams
Cells in the closed-cell foams are separated by a thin film of matrix material (Figure 1b) [28,29]. Due to their remarkable energy absorption capabilities with high specific stiffness and high damping capacity [30,31,32], these foams are commonly used in foam-filled tubes, blast resistance, sound and noise insulation, foam core sandwich, shock absorbers, etc. [33]. Owing to their structure, closed-cell foams usually have a higher compressive strength and are denser, requiring more materials to manufacture. Since they are denser, they thus exhibit higher strength and need a special gas (inert gases) to fill the pores for better insulation and low thermal conductivity [34,35,36].
3. Microstructure of Metal Foams
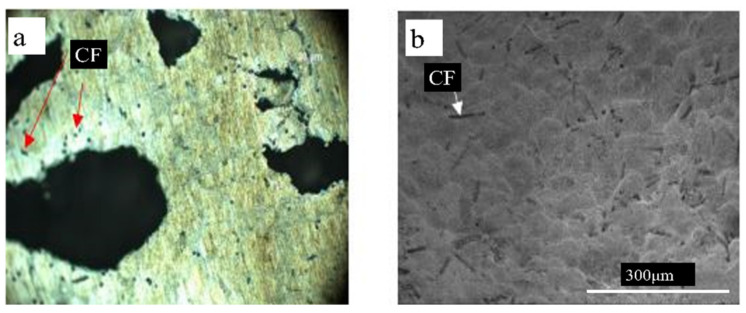
The microstructure of the cell-wall matrix influences mechanical properties and necessitates a controlled pore structure in metal foams. The microstructure analysis of Ni-coated carbon fibres reinforced AlSi7 foams was carried out. These foams were fabricated by a powder metallurgy technique using TiH2 as foaming agent. The resultant foams exhibited a uniform distribution of coated carbon fibre on the cell walls of aluminium foam, which means good wettability at the carbon fibre–Al foam interface and thus strong interfacial bonding of carbon fibres with the Al matrix was achieved. Figure 2 shows clear evidence of dispersed carbon fibres separately, stretched and aligned randomly in the aluminium matrix [37]. It was found that the stability and the maximum foam expansion for AlSi7 alloy foams depends on the size and volume fraction of SiC particles added. Increasing the particle size and/or decreasing the ceramic particle content results in a uniform cell structure formation. This was due to the cell wall thinning rate and viscosity of the Al melt affected by ceramic inclusion [38]. The A359 foams reinforced with Al2O3 particles were developed via melt foaming. These foams exhibited a closed cell, uniform cell size and roughly equiaxed polyhedral structure. In addition, the uniform distribution of the Al2O3 particles in the cell wall resulted in good interfacial interaction between the A359 matrix and Al2O3 particle [39].
Figure 2.
(a) Optical image; (b) SEM image of carbon fibres (CF) reinforced AlSi7 foams [37].
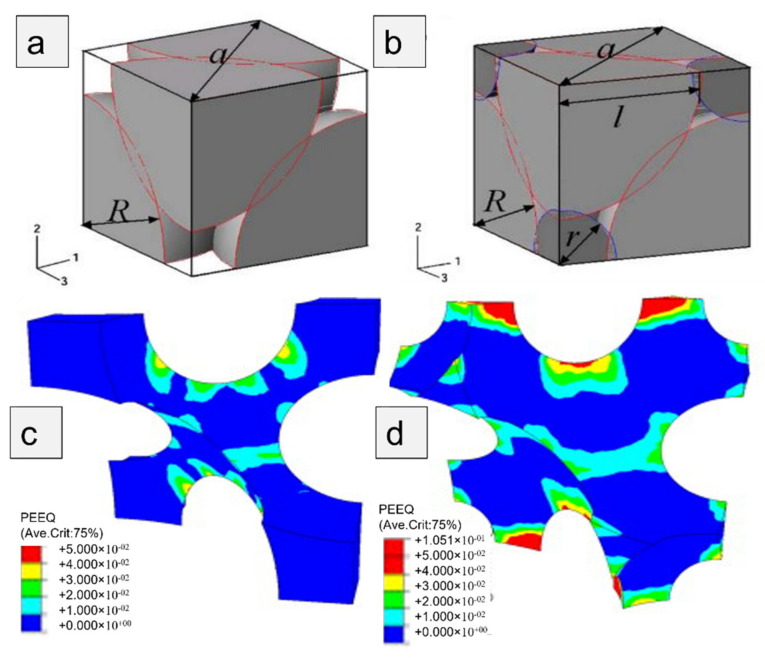
The uniform cellular structure and dual-size cellular structure (uniform-cell distribution embedded with secondary-size cells or/and bimodal cell size distribution) under compression as shown in Figure 3 were studied. The plastic deformation in the former case was concentrated in the primary inclining struts, while in the latter one, deformation was shared by both major as well as minor struts. Thus, secondary-size cells enhanced the strength and stiffness of the Al foams [40]. The pore structure of Mg alloy foams with varying cell shapes developed using melt foaming technique was analysed. The composites with porosities lower and higher than 70% exhibit spherical and polyhedron-shaped cells, respectively. In this case, due to 87% porosity, the cell shapes were polyhedron, as evident from Figure 4 [41].
Figure 3.
Compacted structures of fillers in (a) uniform-size cellular structure; (b) dual-size structure; Equivalent plastic strain distributions in: (c) uniform-size cellular structure; (d) a dual-size cellular structure (bimodal cell size distribution) (relative density = 0.1 and the compression strain = 2%) [40].
Figure 4.
Microstructure of Mg alloy foams with varying cell sizes: (a) D = 1.6 mm; (b) D = 1.2 mm; (c) D = 1 mm; (d) D = 0.9 mm [41].
Using potassium carbonate particles as space holders, copper foams were developed by the powder metallurgy technique. The two types of pore structure were formed: macro-pores and micro-pores. Macro-pores were interconnected spherical-shaped pores with the same size as potassium carbonate particles, while micro-pores were found in the pore struts and walls. The open-pore structure and connectivity of the macro-pores are formed by these micro-pores, resulting in foams of a high degree of pore connectivity [42]. The homogenous distribution of interconnected pores in Fe (Al) foams was obtained in a compacted specimen, as shown in Figure 5a. These pores after the leaching process replicate the size and shape of space holders (NaCl particles), as shown in Figure 5b. This demonstrates that the final morphology of pores can be tailored by selecting different types of space holders with varying sizes. Furthermore, the use of polyester resin as a binder provided adequate strength to prevent compact deformation and collapse and also reduces the corrosion and dissolution of the base metal during the leaching process. Figure 5c depicts the pore structure after sintering, when resin is removed [43].
Figure 5.
(a) Compacted; (b) Leached; (c) Sintered Fe(Al) foams [43].
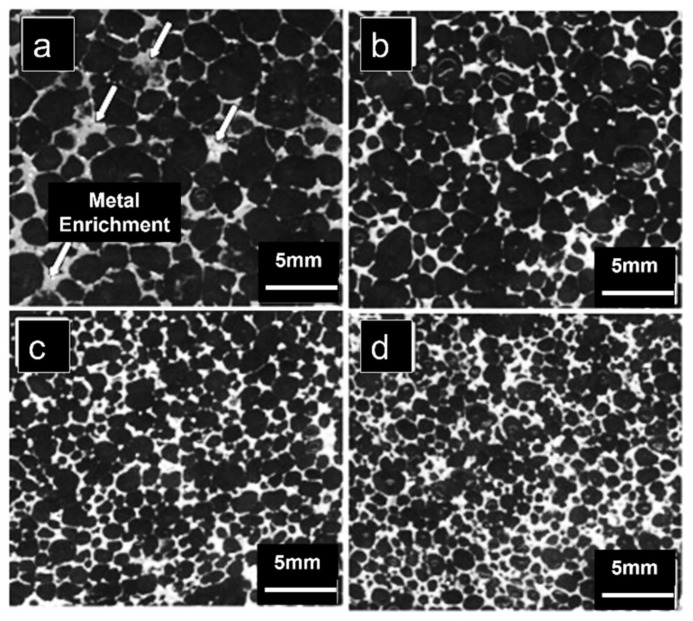
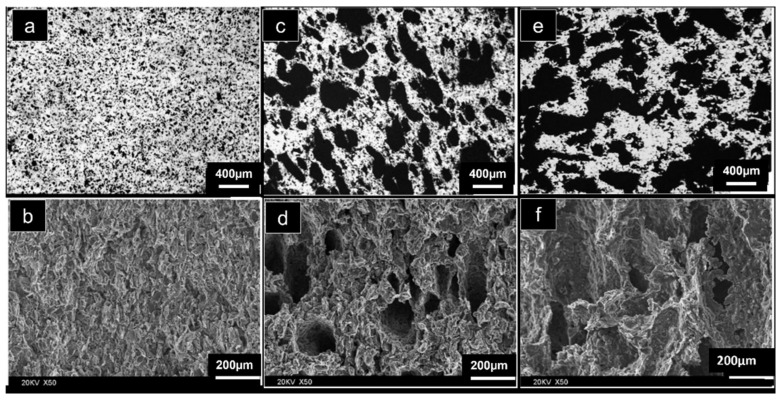
The selection of a proper compaction pressure is critical for producing high-strength foams because it affects their pore morphology. The influence of compaction pressure on pore morphology and densification was analysed using acrawax as space holders, which are lubricating and compressible. At lower compaction pressure (200 MPa), spherical pores were obtained, however, the cell walls were porous (micro-pores). Denser cell walls and mostly spherical pores were obtained when compacted at 300 MPa. At higher compaction pressure (400 MPa), the cell walls were more dense, but the high pressure deformed the pore morphology into an ellipsoidal shape [44]. The study of the pore microstructure of Mg/CNT composite foams developed via the powder metallurgy technique using carbamide particles as space holders revealed that the local porosity fluctuation increases as the overall porosity increases. The absolute fluctuation amplitudes are 1%, 2% and 3% for overall porosity of 29%, 39% and 49%, respectively. These variations are minor in comparison to the overall porosity and pores that are uniformly dispersed in each composite foam. Composite foams with higher overall porosity have large and many more connected pores than those with lower overall porosity. Thus, the maximum pore size as well as the total specific surface area increases significantly with the increase in the overall porosity [45]. Figure 6 depicts the optical and SEM images of these foams with varying porosities [46,47].
Figure 6.
Optical and SEM images of the Mg alloy foam with different porosities: (a,b) 7%; (c,d) 36%; (e,f) 55% [46].
The effect of anodization treatment on the morphology of primary α-Al grains, as well as the constitution and distribution of secondary phases induced by adding thickening and foaming agents to Al foams developed by melt foaming, were investigated. The results showed the formation of dendritic α-Al grains and CaAl4 phase. The cooling curve of Al foams showed that during solidification, primary α-Al formed first, followed by CaAl4; thus, CaAl4 was not present in the melt and does not contribute to thickening [48]. In addition, the microstructure of metal foams is also influenced by the fabrication technique used. Table 1 shows the various studies on the morphology and microstructures of metal foams developed by melt foaming and powder metallurgy The microstructures of metal foams formed using different foaming agents and space holders have been mentioned [48,49,50,51,52,53,54,55,56,57,58].
4. Mechanical Properties of Metal Foams
The mechanical properties of metal foams, particularly their compressive strength and energy absorption capacities, are dependent on their cell structure, porosities, and relative densities. Table 2 shows the mechanical properties of various metal foams with varying porosities and strain rates. The plateau stress has been found to have higher values at porosity varying from 60 to 70%, while energy absorption capacities are higher for higher porosity values [59,60,61,62]. The effect of pore morphology on the compressive strength of Al foam concluded that the pore shapes of foams had a greater influence on their mechanical properties than the pore size [63]. Furthermore, the addition of TiB2 particles to Al foam increases the maximum foam expansion significantly due to the solid phase lowering the minimum cell wall thickness, allowing larger expansions before cell rupture and collapse. This results into higher proof stress, yield strength and more energy absorption for a given strain as compared to pure Al foams [64,65].
Table 2.
Mechanical properties of metal foams with varying porosities and strain rates.
| Fabrication Technique (Strain Rate) |
Material | Porosity | Plateau Stress (MPa) |
Energy Absorption (MJ/m3) |
Reference |
|---|---|---|---|---|---|
| Space-holder technique (Strain rate = 0.01/s) |
Ti foam | 80 | 12.55 | - | [59] |
| Ti foam | 78 | 15.42 | - | ||
| Ti foam | 76 | 15.84 | - | ||
| Ti foam | 74 | 21.61 | - | ||
| Ti foam | 72.4 | 25.43 | - | ||
| Ti foam | 70 | 27.97 | - | ||
| Ti foam | 66.6 | 30.76 | - | ||
| Space-holder technique (Strain rate = 0.01/s) |
Al foam | 50 | 29.5 | 20.9 | [60] |
| Al foam | 60 | 18.8 | 13.5 | ||
| Al foam | 70 | 9.9 | 6.6 | ||
| Melt foaming (Strain rate = 3 × 10−3 s−1) |
Al/0.25 wt.% SiO2 | 86 | 0.8 | 13.7 | [61] |
| Al/0.5 wt.% SiO2 | 84 | 1.4 | 46.2 | ||
| Al/0.75 wt.% SiO2 | 91 | 0.4 | 23.0 | ||
| Al/1.0 wt.% SiO2 | 87 | 0.7 | 18.3 | ||
| Space-holder technique (Strain rate = 0.01/s) |
Ti foam | 68 | 100 | 120 | [62] |
| Ti foam | 57 | 180 | 160 | ||
| Ti foam | 46 | 260 | 220 |
The higher values of porosities can reduce energy absorption capacity of foams. Therefore, it is essential to develop foams with optimum porosities. As the porosity of AlCu5Mn foams increases from 45.8 to 91.2%, the corresponding energy absorption capacity decreased from 72.22 to 2.70 MJ m−3. The energy absorption capacity has the highest values of 72.22 MJ m−3 at 45.8% porosity. In addition, the compression properties of AlCu5Mn foams were found to be better than those of other Al-based foams [66]. The effect of porosity on the compressive strength of TiNi foams was investigated. The study revealed that increased porosity resulted in a continuous decline in the elastic modulus and compressive strength of foams [67]. In another study, the densification strain decreased with the increment in foam density and uniform deformation in compression and strain hardening, which were the same as the bulk material with no plateau stress [68]. The compressive properties and energy absorption behaviour of powder metallurgy fabricated Al foams reinforced with glass fibres was found to depend on the volume fraction of glass fibre and porosity fraction. In addition, the compressive strength of the composite foams was higher than that of the pure Al foam [69]. The compressive strength of Cu foams under quasi-static compressive conditions concluded that at high strain levels in the densification stage, the stress–strain curves slightly depend on the strain rate [70]. Open-celled Zn foams with porosities in the range of 74 to 92% were fabricated by the space-holder technique using spherical carbamide particles as space holders. The modulus of elasticity and compressive yield strength decreased with porosity, and they have good compatibility with the Gibson–Ashby model for cellular solids [71]. The angular carbamide particles as space holders in Al foams reduced their mechanical properties significantly; however, the desired properties were obtained by using spherical carbamide particles as space holders [72].
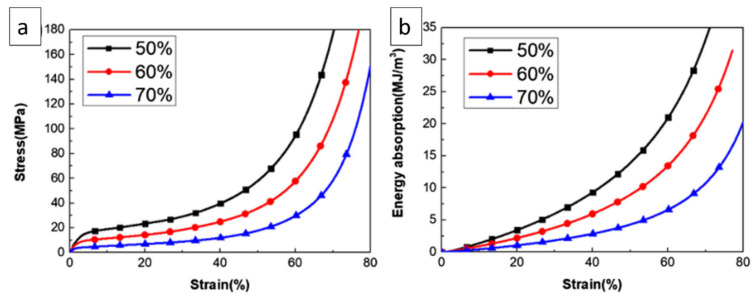
In an Al foam prepared by the space-holder technique, the plateau region as shown in Figure 7a exhibits a slowly progressive increase in stress, and no stress drop appears on the compression curves as a result of the hardening of foams. In addition, the uniform distribution and size of the pores also facilitates the smooth fluctuation of the stress–strain curves. The energy absorption of Al foams decreases with increasing porosity, as shown in Figure 7b. The values of Al foam parameters such as plateau stress and energy absorption at a strain rate of 60% under quasi-static compression are mentioned in Table 3 showing the same trend [60]. In addition, Table 3 shows the effect of fabrication techniques and the mechanical properties of various metallic foams developed by melt foaming and the powder metallurgy technique. The compressive strength of foams increased with the addition of reinforcements and the presence of a well-defined porous structure. This indicates the feasibility of these fabrication techniques for the development of metallic foams [65,73,74,75,76,77,78,79,80,81,82,83].
Figure 7.
(a) Stress–strain curves; (b) Energy absorption curves of Al foams with varying porosities [60].
Table 3.
Mechanical properties of various metallic foams developed by melt foaming and powder metallurgy technique.
| Foam Material | Foaming Agent | Fabrication Technique | Mechanical Properties | References |
|---|---|---|---|---|
| Al (ALPORAS) |
TiH2 | Melt foaming |
|
[73] |
| Al alloy | TiH2 | Melt foaming |
|
[74] |
| Mg–Al, Mg–Zn and Mg–Cu foams | CaCO3 | Powder metallurgy |
|
[76] |
| Al/ scandium |
TiH2 | Melt foaming |
|
[77] |
| Al/TiB2 | TiH2 | Powder metallurgy |
|
[65] |
| Zn foam | TiH2 | Powder metallurgy |
|
[78] |
| Al/3.7% Si/0.18% Mg | TiH2 | Melt foaming |
|
[79] |
| Al63Cu28Fe9 alloy | - | Melt foaming |
|
[80] |
| Al/Al2O3 | - | Powder metallurgy |
|
[81] |
| Zn–Mg alloy foam | CaCO3 | Powder metallurgy |
|
[82] |
| Al/Zn foams | CaCO3 | Melt foaming |
|
[83] |
| Mg/Al/Zn foams | CaCO3 | Powder metallurgy |
|
[75] |
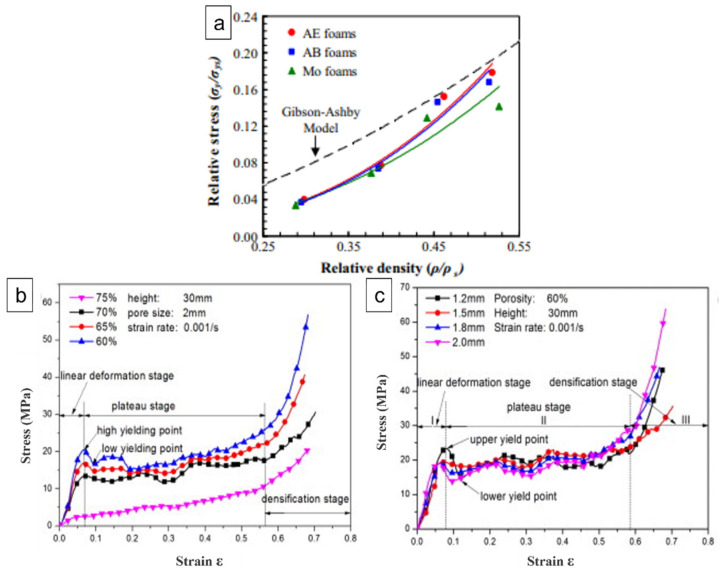
The mechanical properties of metal foam have been predicted using Gibson–Ashby mathematical models [84,85,86]. According to Gibson–Ashby, this model proposed the relationship between the relative stress and relative density and found the plateau stress or yield stress (ys) and elastic modulus Ef of metal foam.
| σpl = σysCρrel3/2 (open cell) | (1) |
| Ef/Es = ρrel2 (open cell) | (2) |
| σpl = σysCρrel2 (closed cell) | (3) |
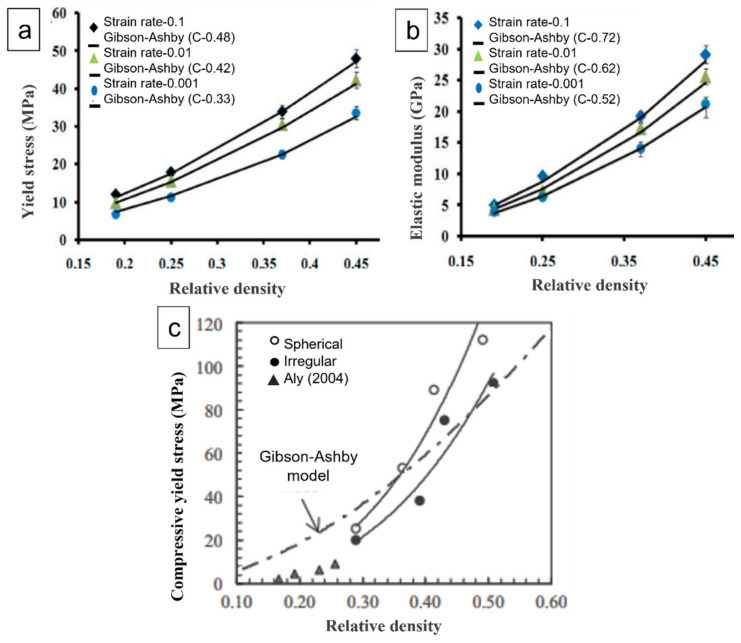
where σpl = plateau stress of the metal foam, σys = yield stress of the cell metal foam; ρrel is the relative density; C = shape factor = 0.3 Ef = elastic modulus of the metal foam, and Es = elastic modulus of the solid metal. As shown in Figure 8a,b, Jain et al. [57] investigated the compressive behaviour of austenitic stainless steel foam (ASSF) at three different compressive strain rates at varying relative densities. The stress–strain curves revealed three regions: (a) elastic, (b) plateau, and (c) densified region. The compressive yield stress and Ef values when compared to the predictions of Gibson–Ashby model agreed with the model, as evident from Figure 8a,b. For yield stress, at strain rates of 0.001, 0.01, and 0.1 s−1, the shape factor values were found to be C = 0.33, 0.42, and 0.48, respectively. In addition, for Ef shape factor, values were 0.52, 0.62, and 0.72 at the strain rates of 0.001, 0.01, 0.1 s−1, respectively. The value of ‘C’ was found to increase with strain rate. Furthermore, Jiang et al. [87] developed Al foams with different shapes of carbamide particles. The compressive strength of specimens with spherical pores was found to be greater than that of specimens with strip-shaped pores. At low relative densities, the strength values of steel foams are lower than predicted by the Gibson–Ashby model. In this model, pore walls are assumed to be solid metal. However, large pores, broken walls, anisotropic pore structure, micropores in cell walls, and non-uniform foam density significantly affect the mechanical properties of foams.
Figure 8.
(a) Yield stress (σys), and (b) Elastic modulus (Ef), as a function of relative density at varying stain rates [57] and (c) Relative density vs. compressive yield stress of Fe–1.5% Mo steel foams [88].
Similarly, Bekoz and Oktay [88] developed steel foam using different shapes of carbamide particles. The compressive yield strengths of the steel foams obtained during the investigation were compared with the predictions of the Gibson and Ashby model in Figure 8c. The compressive yield stress at lower relative densities was lower than the values predicted by the Gibson–Ashby model. The contribution of cell face stretching to the overall strength and stiffness of foam was discovered to vary linearly with relative density. In contrast, the contribution of cell edge bending was nonlinear. The findings of Aly’s study [89] are also included in Figure 8c for comparison. It is clear from Figure 8c that the compressive yield stress is affected by relative density, and better results were obtained for foams with spherical pores. This is due to the fact that these foams exhibited comparatively smoother cell wall surfaces. However, stress concentration occurs easily at the sharp edges of irregular pores, resulting in reduced strength. According to Gibson and Ashby, the plateau stress (collapse stress) and post-collapse behaviour also depend on the type of foam, whether it is open or closed cell. Bekoz and Oktay [90] also obtained lower values of compressive yield strengths for Cu–Ni–Mo steel foams as compared to values predicted from Gibson–Ashby mathematical models, as evident from Figure 9a. It is clear that the relative stress is influenced by the relative density. In addition, the compressive yield stresses for all foams were lower than the values predicted by model, especially at low relative densities. Gibson and Ashby [86] used a simple model to analyse the yield strength of a porous metal and found that the collapse stress is not influenced by pore size [91]. The quasi-static compressive stress–strain curves of AZ31 magnesium alloy foams with different pore sizes revealed that the yield strength of specimens with pore sizes of 1.5, 1.8, and 2.0 mm were almost the same as shown in Figure 9b,c, showing consistency with Gibson and Ashby’s results [86,91], while with porosities, it varied.
Figure 9.
(a) Relationship between relative stress and relative density of the steel foams; Stress–strain curves with varying (b) pore sizes; (c) porosities [91].
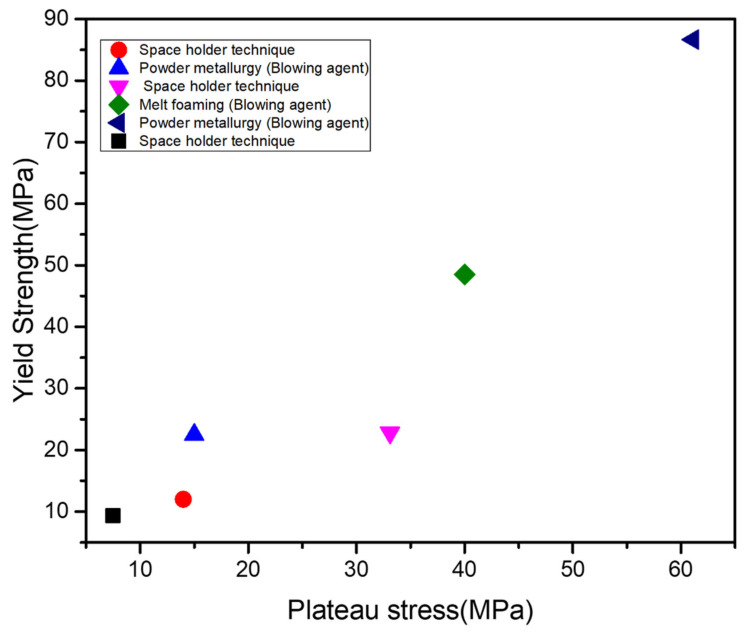
As it is clear that the plateau stress and yield strength are a function of relative density [86], Figure 10 shows their values by employing different fabrication techniques at same relative density of 0.4. In addition, Table 4 mentions the foam type, space holder or blowing agent used in the fabrication of Al foams. From Figure 10, it can be seen that the powder metallurgy results in increased values as compared to melt foaming.
Figure 10.
Plateau stress versus yield strength of the metal foam (Al) at a relative density of 0.4.
Table 4.
Information regarding Figure 10.
| Processing Technique | Foam Type | Material | Space Holder/Blowing Agent | Reference |
|---|---|---|---|---|
| Space-holder technique | Closed cell | Al | Space holder (Carbamide) | [92] |
| Space-holder technique | Open cell | Al-Al2O3 | Space holder (Carbamide) | [93] |
| Powder metallurgy (blowing agent) | Closed cell | Al | Blowing agent (Dolomite) |
[11] |
| Space-holder technique | Closed cell | Al-CNT | Space holder (Carbamide) |
[94] |
| Melt foaming | Closed cell | AlMnCu | Blowing agent (TiH2) |
[66] |
| Powder metallurgy (blowing agent) |
Closed cell | AA7075/SiC | Foaming agent (CaCO3) |
[95] |
5. Fabrication Techniques of Metal Foams
Metal foams are primarily manufactured using either liquid-phase (or melt foaming) or solid-phase (powder metallurgy) techniques.
5.1. Melt Foaming
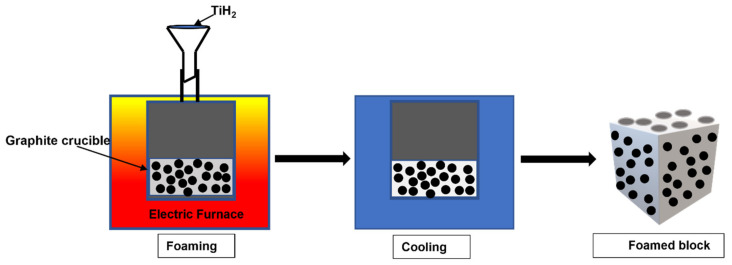
Melt foaming is particularly popular because it produces relatively inexpensive foams with desirable properties [96,97]. The foam quality depends on various parameters, such as composition, the temperature of the forming process, holding time and cooling conditions, size, distribution and the volume fraction of reinforced foam-stabilizing particles (e.g., SiC, Al2O3, etc.) [98,99]. In melt foaming, the composite powder mix is placed in a graphite crucible and is melted using an electric resistance furnace, as demonstrated in Figure 11. The melt is maintained at a relatively low temperature to keep it sufficiently viscous when adding foaming agents or injecting gas [100].
Figure 11.
The schematic illustration of melt foaming [113].
Some of the common foaming agents and injection gases include TiH2 [101,102,103,104,105], CaCO3 [106,107], zirconium hydride [108,109], dolomite (CaMg(CO3)2) [110,111], etc. The stabilising particles such as Ca, ZrB2, CaO, Al2O3, etc., are also added into the melt [83,112]. The melt is then stirred continuously for the foaming agent and stabilizing particles to distribute evenly. The crucible is kept in the furnace to sustain the required temperature for decomposing TiH2 and releasing gas and bubbles. The foamed melt is taken out of the furnace and cooled in the air. This technique was used to fabricate A356/20SiC composite foams with varying porosities and cell sizes with TiH2 as a blowing agent [113].
In another study, AlSi9Mg/SiC composite foams were successfully developed using direct melt foaming with CaCO3 as a blowing agent and SiC as a foam stabiliser. The yield stress and collapse plateau stress of composite foams increased along with the SiC volume fraction [114]. Similarly, SiC-reinforced AlSi9Mg composite foams fabricated by this technique resulted in an increased elastic limit of composite foams along with the strain hardening [115]. In addition, Mg alloy foam was fabricated with this method to examine the microstructure of the foams [116]. Additionally, the AlSiCu cellular foams with TiH2 were developed to investigate the effect of the thermal decomposition of TiH2 on the foaming behaviour of the Al alloy. The effectiveness of the melt foaming of the Al alloy is highly dependent on the decomposition properties of TiH2 [117]. Mg alloys foams were fabricated by the melt processing method using CaCO3, TiH2, or MgH2 powders as blowing agents, which release gas during decomposition and gradually foam the magnesium alloy melt [118,119,120]. Various additives, such as SiC, carbon, and calcium particles were added to the melt to increase its viscosity [121]. Several metallic foams have been developed by using the melt foaming technique, as mentioned in Table 5, where TiH2 and CaCO3 were mostly used as foaming agents for the development of a foam structure.
Table 5.
Metal foams developed by melt foaming.
| Foam Material | Foam Type | Foaming Agents | Reference |
|---|---|---|---|
| Al | Closed cell | TiH2 | [122] |
| Al/SiC | Closed cell | TiH2 | [123] |
| Al/Ca | Closed cell | TiH2 | [124] |
| Al 6061/Cu | Closed cell | TiH2 | [125] |
| ZA22/SiC | Closed cell | CaCO3 | [126] |
| Zn/22Al/SiC | Closed cell | CaCO3 | [127] |
| Al alloy (ALPORAS) | Closed cell | TiH2 | [128] |
| Al (ALPORAS) | Closed cell | TiH2 | [129] |
| Al/Si/Mg | Closed cell | CaCO3 | [130] |
5.2. Powder Metallurgy Foaming Techniques/Methods
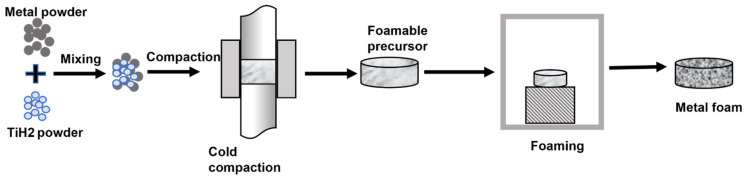
In this technique, the metallic powder was mixed with some mass fraction of foaming agent (TiH2 powder) or space holders in a powder mixer. The mixed powders are then compacted cold, using a uniaxial compaction, in a lubricated tool-steel die at required pressures to achieve precursor green densities. The precursor specimens are later led to a furnace, so that the foaming procedure take place under high temperatures in case of green compacts with foaming agents, while for compacts with space holders, porosities are acquired by a leaching process followed by sintering.
5.2.1. Using Foaming Agent
Metallic powders and a foaming agent such as titanium hydride powder are mixed and then cold compacted, as illustrated in Figure 12. This compacted sample is then placed inside the furnace for sintering where titanium hydride decomposes, resulting in high-pressure voids. These expand by semi-solid flow, and the metal swells, forming foam that fills the mould before cooling and stabilising it. The process produces components that have a similar shape as the mould but have a lower relative density. Metals such as tin, brass, zinc, lead and bronze can also be foamed using the correct process parameters and foaming agents. The foaming agents (TiH2 or MgH2) were used to fabricate ZnAl4Cu1-alloy and AlSi12 alloy foams [131]. It was revealed that in order to foam zinc, the foam must be significantly overheated above the melting temperature of the metal, or a greater amount of foaming agent must be used. The deformation behaviour of zinc foams was comparable to that of Al foams. The compression strength of zinc foams was significantly lower at the same density but similar at equal porosity [131].
Figure 12.
Schematic diagram of powder metallurgy technique for metallic foams.
5.2.2. Space-Holder Technique
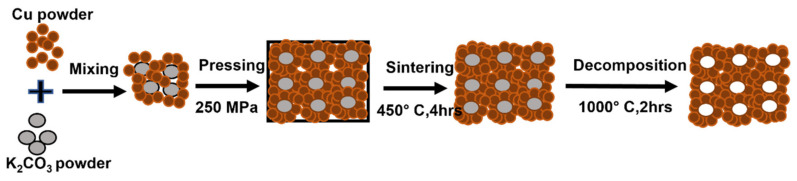
The space-holder technique enables producing metal foams with controlled pore morphology [132]. Figure 13 shows the processing of metal foams via the space-holder technique. The space holders are firstly mixed with the metal powders. These space holders can be polymeric materials, or salts, such as NaCl particles [133,134,135,136,137,138], K2CO3 [69], carbamide particles [60,139], carbohydrate particles [140], polymethylmethacrylate (PMMA), etc. [141]. Approximately 1 to 2% binders are added to increase the strength of the final part. The mixture is compressed, or injection moulded, which is followed by sintering where space holders are removed due to heating, solvent debinding (before sintering), or by carrying out a dissolution process after sintering [142,143]. The removal of the space-holder material produces connected pores that may be open or closed. Finally, the green sample is sintered to enhance its structural strength. Overall, the space-holder method is a comparatively simple method for developing metal foams [144].
Figure 13.
Schematic representation of the Cu foam fabricated by the space-holder technique [69].
Many authors used this technique to produce metal foams focusing on processing parameters, feasibility, and efficiency [145,146,147]. Iron-based foams are quite often manufactured using this technique, as it allows the production of pure materials almost free from inclusions and impurities. It also enables the preparation of materials in a near-final shape with an interconnected pore structure and the required properties [148,149]. Metal foams fabricated by a pherical space holder generally show a higher compressive strength [87,150]. The effects of shape and space holder particle content were investigated; the cell wall of spherical pores was more uniform due to the increased contact between the metal particles during sintering [88]. The compressive strength of Al foams fabricated by this technique decreased with the increase in particle size of the space holder (using two particle sizes: 5–10 mm and 10–15 mm) due to the formation of thin walls. However, their energy-absorption capacity increased with the increase in the particle size of space holders, except for compacted samples [151]. Carbamide particles as space holders were employed to develop Al foams and porosities were efficiently controlled, resulting in a higher compressive strength that further increased along with sintering time and temperature [72]. Mg alloy foams were also developed using carbamide particles as space holders. The water-soluble polymers such as PMMA particles can be considered as promising space holders for developing foams. Al and Mg foams were developed through the powder metallurgy technique, using (PMMA) particles as space holders, resulting in efficient control over porosities and densities by varying the PMMA particle content. PMMA particles leave almost negligible residue on decomposition during sintering [152,153,154]. This technique has been employed to fabricate various metal foams, as mentioned in Table 6. Different foaming agents or space holders have been used to fabricate these metallic foams.
Table 6.
Metal foams fabricated by powder metallurgy technique.
| Foam Material | Foaming Agent/Space Holders | Fabrication Technique | Reference |
|---|---|---|---|
| Cu | Potassium carbonate | Space-holder method | [155] |
| Cu | Potassium carbonate | Space-holder method | [156] |
| Cu/CuO | Oxide | Powder metallurgy | [157] |
| AlSi10Mg | TiH2 | Powder metallurgy | [158] |
| Al/Mg | NaCl | Space-holder method | [159] |
| Al/Y2O3 | NaCl | Space-holder method | [133] |
| Al/SiC | TiH2 | Powder metallurgy | [28] |
| Al–Sn foams | TiH2 | Powder metallurgy | [160] |
| Al 6061-Al2O3 | TiH2 | Powder metallurgy | [161] |
| Al-Sn (Co, Mg, Mn, Ni, and Ti) | TiH2 | Powder metallurgy | [162] |
| Al6061 and AlSi7 alloys | TiH2 | Powder metallurgy | [163] |
| Fe/Titanium (Ti) | CO2 | Powder metallurgy | [164] |
| AA2014-SiC | Calcium hydride | Liquid metallurgy | [165] |
| Al/MWCNTs | TiH2 | Powder metallurgy | [166] |
In addition, the advantages and disadvantages of these techniques are mentioned in Table 7.
Table 7.
Advantages and disadvantages of melt foaming and powder metallurgy technique.
| Fabrication Techniques | Advantages | Disadvantages |
|---|---|---|
| Melt foaming |
|
|
| Powder metallurgy |
|
|
6. Applications of Metal Foams
Metal foams encompass a wide range of applications as mentioned in Table 8, and new uses emerge all the time. A European distributor of Alporas foams (Gleich) from Shinko developed a vacuum lifting tool for a large-scale producer (Pilkington) of flat glass products using the float glass process. Replacing the entire Al part of the tool with Alporas foam reduced the weight from 82 to 32 kg. Thus, this tool becomes handy when they are changed manually. Although the foam tools are manufactured on a small scale, i.e., five to six pieces per year, these Al foams could withstand temperatures as high as 400 °C due to their higher heat resistance. Their outstanding machinability further encourages the usage of this material [167].
Table 8.
Various potential applications of metal foams.
| Foams | Applications | Reference |
|---|---|---|
| Mg foams | Bone implants | [46] |
| Metallic foam | Heat exchanger | [168] |
| Ni-Cu | Electrodes for super capacitors | [169] |
| Al7075 and 6061 alloy | Crash boxes | [170] |
| Drug delivery | [171] | |
| Al alloy (AlSi12 or 6061) | Foaming around fastening elements | [172] |
| A356/steel | Radiation shielding | [173] |
| Al-foam (Duocel®) | Military-medium tactical vehicles | [174] |
| Fe/Mg/CNT foam | Bone implant | [175] |
| Al foam | Crash box for Valeo’s front-end module systems | [176] |
| Fe/P foam | Bone replacement | [177] |
| Al foam | Ship structure | [178] |
| Cu foam | Heat exchangers | [179] |
| Alulight | Tail lifts, Alimex panel | [180] |
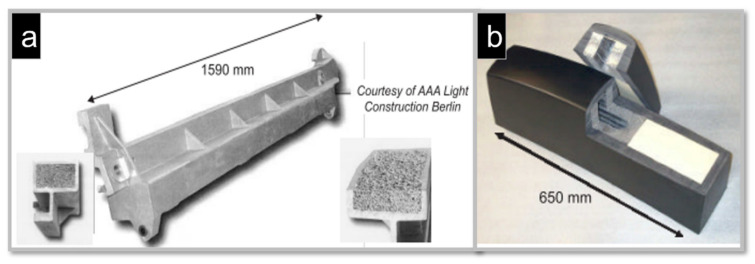
Another closed-cell Alporas (Al foam core) was fabricated into a composite beam (Figure 14a) by embedding the AlZn10Si8Mg alloy foam completely in the denser skin of AlZn10Si8Mg alloy using the sand-casting technique. The transverse beam dampened vibration frequencies up to 370 Hz by internal friction or interface slipping between the core and the skin. This part was equipped in 700 machines approximately so far. In this frequency range, sound attenuation of up to 60% was achieved [181]. Since 2004, the company (Gleich) has been producing 2500 parts, each weighing 21 kg. Figure 14b shows the system of the modular tram concept (COMBINO) produced by three German manufacturing companies: Siemens (tram), Huebner (impact absorber), and Schunk Sintermetalltechnik (metal foam). This system meets customer needs basing on the same framework. In addition, the Al foam core for the impact energy absorber was developed by the extrusion of powder mixtures, which was followed by the embedment of a rubber shell with the foam. These absorbers are manufactured in hundreds of units for other tram operators and manufacturers [182].
Figure 14.
Applications of closed-cell Alporas: (a) a transverse beam of a machine and two insets showing its cross-sections in different directions (courtesy of AAA Light Construction Berlin); (b) an energy absorber for a tram built for the COMBINO vehicle system (courtesy of Hubner, Schunk, Siemens).
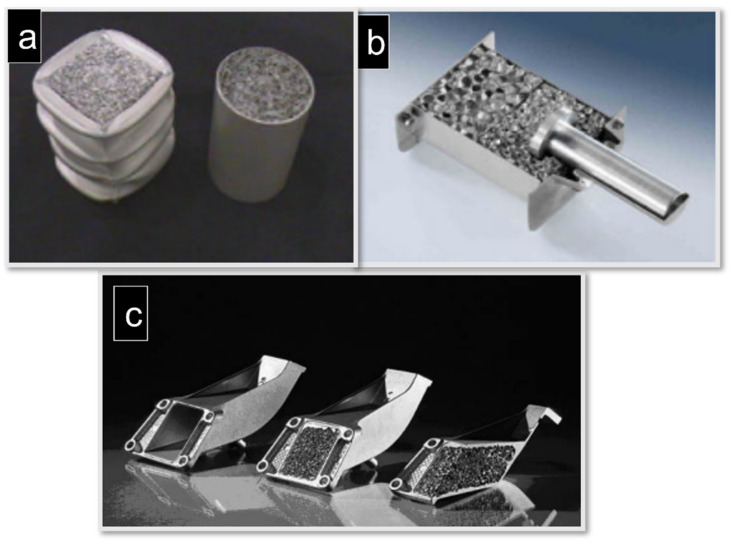
Figure 15a shows a crash box for the front-end module systems of Valeo, which was designed by Cymat and Valeo in a joint development programme. Figure 15b shows a slightly different design concept, consisting of foams with varying densities for adjusting the absorber’s deformation curve [181]. The Austrian manufacturer Alulight manufactures 100,000 pieces of closed-cell Al foams annually as a crash absorber element for Audi cars, making it the only large-scale production of closed cell-Al foams through automated production technology [183]. The automobile manufacturer BMW (München, Germany) and LKR (Ranshofen, Austria) jointly developed an engine mounting bracket using Alulight foams as a lightweight inner core (Figure 15c). This bracket could bear the heavier weight of the car engine and mechanical vibrations by dissipating thermal energy. The high fracture toughness and stiffness of these composites were improved, resulting in enhanced safety in crash situations [181].
Figure 15.
Applications of metal foams: (a) prototypes of crash absorbers made of extruded Al filled with Cymat foam core (courtesy of Cymat); (b) design based on Al foams (Metcomb) with two different densities (courtesy of Hutte Kleinreichenbech); (c) prototype of a BMW engine mounting bracket produced by LKR Ranshofen. From left: empty casting, composite part comprising foam core and cast shell, and section through composite part (courtesy of LKR).
Alcoa (the USA) developed a new method based on the continuous casting technique to reduce the cost of Al foams for inexpensive products [184]. The technology has matured to mass produce low-cost foams. AlSi alloy were used as a filling foam in the crash element, with a 30% improvement in absorbing the impact energy, while the vehicle weight increased by 3% only [185]. NiMH and NiCd battery electrodes are nowadays perhaps the primary industrial application for metal foams. Vale Inco manufactures 4 million m2 of Ni foam annually for these applications [186]. Ti-based foam with ammonium bicarbonate (NH4HCO3) as a space holder was developed by powder metallurgy for bone implant application [187].
7. Challenges and Way Forward
The technology of metal foaming is developing at an accelerated pace with considerable progress. This research area involves interdisciplinary collaborations among physics, chemistry, and materials engineering to produce the required quality economically with reproducible foamed materials that possessed a unique range of properties. These diverse applications allow the construction of functionally new parts or devices. Since foams have many competitors that are mostly less expensive, it becomes crucial to improve fabrication technologies for large-scale foam production, generating a greater variety of low-cost metal foam products. Despite the successful development, more basic research remains essential to understanding the correlation between the composition of metal alloy and foamability. Producing foams of consistent quality, morphology, and control of structure is challenging. However, the foam quality and properties can be controlled by optimizing the factors and parameters. In addition, the foam’s porosities can be controlled by heat treatment or coating the blowing agent to stop the decomposition of the gas-blowing agent in the melt. Overall, to produce uniformly structured metal foams, it is necessary to optimize the processing parameters in the existing fabrication techniques. In addition, further studies are required to compare the available processes to produce metal foams from raw material (powders) or Al scrap. The development of alloy foams for the core with improved properties may contribute to their successful usage in various applications.
The academic and industrial research plays a crucial role in eradicating problems that otherwise limit the wider applications of metal foams. Improvement is required to ensure the reproducibility and uniformity of the foam’s cell structures for attaining even densities and distribution of pore sizes in the entire part or component, resulting in uniform foam structures. Additionally, new processing routes, cheaper raw materials, and less wastage may reduce the processing and material costs of metal foams. Case studies based on simulation, innovative design, and testing could show end-users that despite the higher costs of some foams or parts, there are more benefits associated with these new structures and materials, such as savings in weight and energy.
8. Summary
This review presents an overview of the metal foams developed by various techniques to enhance their properties and performance, summarising the efforts to improve the foaming behaviour and characteristics. Depending on their applications, foam structures could be tailored to generate open- or closed-cell foams. Microstructural studies evaluated the effect of reinforcements and fabrication techniques on the foams’ microstructure, and it defines their mechanical behaviour. The foams’ properties, particularly the compressive strengths and energy absorption, could be improved by adding different reinforcements, such as metal or ceramic particles, to stabilise the foam structure. The porosities in these foams are dependent on foaming agents and space-holder particles. The processing techniques that fabricate metal foams play a vital role in deciding their properties and foaming behaviour. Their applications as structural components in automotive parts, such as ships and aerospace transportation, and as sound dampers, filters, electrodes, etc. can be effectively attained. However, improvement would be possible by identifying the downsides and feasible solutions for better performances under various working conditions and production techniques. Although metal foams find applications in many sectors due to their lightweight and high strength, the high cost of fabrication techniques hinders large-scale production. The challenge of future process development should therefore focus on reducing the production cost as well.
Acknowledgments
This research is supported by the Structures and Materials (S&M) Research Lab of Prince Sultan University, and the authors acknowledge the Prince Sultan university for paying the article processing charges (APC).
Author Contributions
Writing—original draft preparation, B.P.; conceptualization, N.A.J.; validation, H.A.; resources, Y.A.; review and editing, A.A.; evaluation, M.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by the Ministry of Higher Education (MOHE) of Malaysia and International Islamic University Malaysia (IIUM) (FRGS/1/2019/TK08/UIAM/02/5).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fleck N. Metal Foams: A Design Guide. Volume 3069. Elsevier; Amsterdam, The Netherlands: 2016. p. 264. [DOI] [Google Scholar]
- 2.Goel M.D., Matsagar V.A., Marburg S., Gupta A.K. Comparative Performance of Stiffened Sandwich Foam Panels under Impulsive Loading. J. Perform. Constr. Facil. 2013;27:540–549. doi: 10.1061/(ASCE)CF.1943-5509.0000340. [DOI] [Google Scholar]
- 3.Raj R.E., Daniel B.S.S. Aluminum melt foam processing for light-weight structures. Mater. Manuf. Process. 2007;22:525–530. doi: 10.1080/10426910701236072. [DOI] [Google Scholar]
- 4.Banhart J., Baumeister J. Deformation characteristics of metal foams. J. Mater. Sci. 1998;33:1431–1440. doi: 10.1023/A:1004383222228. [DOI] [Google Scholar]
- 5.Jeenager V.K., Pancholi V., Daniel B.S.S. The effect of aging on energy absorption capability of closed cell aluminum foam. Adv. Mater. Res. 2012;585:327–331. doi: 10.4028/www.scientific.net/AMR.585.327. [DOI] [Google Scholar]
- 6.Ozan S., Taskin M., Kolukisa S., Ozerdem M.S. Application of ANN in the prediction of the pore concentration of aluminum metal foams manufactured by powder metallurgy methods. Int. J. Adv. Manuf. Technol. 2008;39:251–256. doi: 10.1007/s00170-007-1218-2. [DOI] [Google Scholar]
- 7.Jiang W., Fan Z., Liao D., Dong X., Zhao Z. A new shell casting process based on expendable pattern with vacuum and low-pressure casting for aluminum and magnesium alloys. Int. J. Adv. Manuf. Technol. 2010;51:25–34. doi: 10.1007/s00170-010-2596-4. [DOI] [Google Scholar]
- 8.Rajak D.K., Kumaraswamidhas L.A., Das S. An Energy Absorption Behaviour of Foam Filled Structures. Procedia Mater. Sci. 2014;5:164–172. doi: 10.1016/j.mspro.2014.07.254. [DOI] [Google Scholar]
- 9.Wang Y.C., Li D.Y., Peng Y.H., Zeng X.Q. Numerical simulation of low pressure die casting of magnesium wheel. Int. J. Adv. Manuf. Technol. 2007;32:257–264. doi: 10.1007/s00170-005-0325-1. [DOI] [Google Scholar]
- 10.Lara-Rodriguez G.A., Figueroa I.A., Suarez M.A., Novelo-Peralta O., Alfonso I., Goodall R. A replication-casting device for manufacturing open-cell Mg foams. J. Mater. Process. Technol. 2017;243:16–22. doi: 10.1016/j.jmatprotec.2016.11.041. [DOI] [Google Scholar]
- 11.Papadopoulos D.P., Omar H., Stergioudi F., Tsipas S.A., Lefakis H., Michailidis N. A novel method for producing Al-foams and evaluation of their compression behavior. J. Porous Mater. 2010;17:773–777. doi: 10.1007/s10934-009-9349-5. [DOI] [Google Scholar]
- 12.Paulin I., Šuštaršič B., Kevorkijan V., Škapin S.D., Jenko M. Synthesis of aluminium foams by the powder-metallurgy process: Compacting of precursors. Mater. Tehnol. 2011;45:13–19. [Google Scholar]
- 13.Bisht A., Gangil B., Patel V.K. Selection of blowing agent for metal foam production: A review. J. Met. Mater. Miner. 2020;30:1–10. doi: 10.14456/jmmm.2020.1. [DOI] [Google Scholar]
- 14.Banhart J. Manufacture, characterisation and application of cellular metals and metal foams. Prog. Mater. Sci. 2001;46:559–632. doi: 10.1016/S0079-6425(00)00002-5. [DOI] [Google Scholar]
- 15.Alavi R., Trenggono A., Champagne S., Hermawan H. Investigation on mechanical behavior of biodegradable iron foams under different compression test conditions. Metals. 2017;7:202. doi: 10.3390/met7060202. [DOI] [Google Scholar]
- 16.Davies G.J., Zhen S. Metallic foams: Their production, properties and applications. J. Mater. Sci. 1983;18:1899–1911. doi: 10.1007/BF00554981. [DOI] [Google Scholar]
- 17.Rajendran R., Prem Sai K., Chandrasekar B., Gokhale A., Basu S. Preliminary investigation of aluminium foam as an energy absorber for nuclear transportation cask. Mater. Des. 2008;29:1732–1739. doi: 10.1016/j.matdes.2008.03.028. [DOI] [Google Scholar]
- 18.Ghafarian M., Mohebbi-Kalhori D., Sadeghi J. Analysis of heat transfer in oscillating flow through a channel filled with metal foam using computational fluid dynamics. Int. J. Therm. Sci. 2013;66:42–50. doi: 10.1016/j.ijthermalsci.2012.11.008. [DOI] [Google Scholar]
- 19.Kamath P.M., Balaji C., Venkateshan S.P. Convection heat transfer from aluminium and copper foams in a vertical channel-An experimental study. Int. J. Therm. Sci. 2013;64:1–10. doi: 10.1016/j.ijthermalsci.2012.08.015. [DOI] [Google Scholar]
- 20.Dukhan N., Ali M. Strong wall and transverse size effects on pressure drop of flow through open-cell metal foam. Int. J. Therm. Sci. 2012;57:85–91. doi: 10.1016/j.ijthermalsci.2012.02.017. [DOI] [Google Scholar]
- 21.Han X.H., Wang Q., Park Y.G., T’Joen C., Sommers A., Jacobi A. A review of metal foam and metal matrix composites for heat exchangers and heat sinks. Heat Transf. Eng. 2012;33:991–1009. doi: 10.1080/01457632.2012.659613. [DOI] [Google Scholar]
- 22.Yang C., Nakayama A., Liu W. Heat transfer performance assessment for forced convection in a tube partially filled with a porous medium. Int. J. Therm. Sci. 2012;54:98–108. doi: 10.1016/j.ijthermalsci.2011.10.023. [DOI] [Google Scholar]
- 23.Yuan W., Tang Y., Yang X., Wan Z. Porous metal materials for polymer electrolyte membrane fuel cells-A review. Appl. Energy. 2012;94:309–329. doi: 10.1016/j.apenergy.2012.01.073. [DOI] [Google Scholar]
- 24.Chen Z., Gu M., Peng D. Heat transfer performance analysis of a solar flat-plate collector with an integrated metal foam porous structure filled with paraffin. Appl. Therm. Eng. 2010;30:1967–1973. doi: 10.1016/j.applthermaleng.2010.04.031. [DOI] [Google Scholar]
- 25.Wang P., Liu D.Y., Xu C. Numerical study of heat transfer enhancement in the receiver tube of direct steam generation with parabolic trough by inserting metal foams. Appl. Energy. 2013;102:449–460. doi: 10.1016/j.apenergy.2012.07.026. [DOI] [Google Scholar]
- 26.Liu Z., Yao Y., Wu H. Numerical modeling for solid-liquid phase change phenomena in porous media: Shell-and-tube type latent heat thermal energy storage. Appl. Energy. 2013;112:1222–1232. doi: 10.1016/j.apenergy.2013.02.022. [DOI] [Google Scholar]
- 27.Lafdi K., Mesalhy O., Shaikh S. Experimental study on the influence of foam porosity and pore size on the melting of phase change materials. J. Appl. Phys. 2007;102:083549. doi: 10.1063/1.2802183. [DOI] [Google Scholar]
- 28.Guden M., Yüksel S. SiC-particulate aluminum composite foams produced from powder compacts: Foaming and compression behavior. J. Mater. Sci. 2006;41:4075–4084. doi: 10.1007/s10853-006-7645-x. [DOI] [Google Scholar]
- 29.Kennedy A. Porous Metals and Metal Foams Made from Powders. Powder Metall. 2012;2:31–46. [Google Scholar]
- 30.Mondal D.P., Goel M.D., Das S. Compressive deformation and energy absorption characteristics of closed cell aluminum-fly ash particle composite foam. Mater. Sci. Eng. A. 2009;507:102–109. doi: 10.1016/j.msea.2009.01.019. [DOI] [Google Scholar]
- 31.Wang Z., Shen J., Lu G., Zhao L. Compressive behavior of closed-cell aluminum alloy foams at medium strain rates. Mater. Sci. Eng. A. 2011;528:2326–2330. doi: 10.1016/j.msea.2010.12.059. [DOI] [Google Scholar]
- 32.McCullough K.Y.G., Fleck N.A., Ashby M.F. Uniaxial stress-strain behaviour of aluminum alloy foams. Acta Mater. 1999;47:2323–2330. doi: 10.1016/S1359-6454(99)00128-7. [DOI] [Google Scholar]
- 33.Liu X., Zhang J., Fang Q., Wu H., Zhang Y. Response of closed-cell aluminum foams under static and impact loading: Experimental and mesoscopic numerical analysis. Int. J. Impact Eng. 2017;110:382–394. doi: 10.1016/j.ijimpeng.2016.11.004. [DOI] [Google Scholar]
- 34.Moran J.L., Cottrill A.L., Benck J.D., Liu P., Yuan Z., Strano M.S., Buongiorno J. Noble-gas-infused neoprene closed-cell foams achieving ultra-low thermal conductivity fabrics. RSC Adv. 2018;8:21389–21398. doi: 10.1039/C8RA04037K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banhart J., Ashby M.F., Fleck N.A. Metal foams and porous metal structures. Met. Innov. Technol. 1999;83:255–262. [Google Scholar]
- 36.Rajak D.K., Kumaraswamidhas L.A., Das S., Senthil Kumaran S. Characterization and analysis of compression load behaviour of aluminium alloy foam under the diverse strain rate. J. Alloys Compd. 2016;656:218–225. doi: 10.1016/j.jallcom.2015.09.255. [DOI] [Google Scholar]
- 37.Damanik F.S., Damanik M.S.F., Lange P.G. Effect of Nickel Coated of Carbon Fiber on Distribution of Carbon Fiber Reinforced Aluminium (AlSi7) Foam Composite by Powder Metallurgy. Int. Conf. Innov. Technol. 2019;1381:9–13. [Google Scholar]
- 38.Esmaeelzadeh S., Simchi A., Lehmhus D. Effect of ceramic particle addition on the foaming behavior, cell structure and mechanical properties of P/M AlSi7 foam. Mater. Sci. Eng. A. 2006;424:290–299. doi: 10.1016/j.msea.2006.03.013. [DOI] [Google Scholar]
- 39.Daoud A. Compressive response and energy absorption of foamed A359–Al2O3 particle composites. J. Alloys Compd. 2009;486:597–605. doi: 10.1016/j.jallcom.2009.07.013. [DOI] [Google Scholar]
- 40.Kou D.P., Li J.R., Yu J.L., Cheng H.F. Mechanical behavior of open-cell metallic foams with dual-size cellular structure. Scr. Mater. 2008;59:483–486. doi: 10.1016/j.scriptamat.2008.04.022. [DOI] [Google Scholar]
- 41.Xu Z.G., Fu J.W., Luo T.J., Yang Y.S. Effects of cell size on quasi-static compressive properties of Mg alloy foams. Mater. Des. 2012;34:40–44. doi: 10.1016/j.matdes.2011.07.066. [DOI] [Google Scholar]
- 42.Shahzeydi M.H., Parvanian A.M., Panjepour M. The distribution and mechanism of pore formation in copper foams fabricated by Lost Carbonate Sintering method. Mater. Charact. 2016;111:21–30. doi: 10.1016/j.matchar.2015.11.010. [DOI] [Google Scholar]
- 43.Golabgir M.H., Ebrahimi-Kahrizsangi R., Torabi O., Saatchi A. Fabrication of open cell Fe-10%Al foam by space-holder technique. Arch. Metall. Mater. 2014;59:41–45. doi: 10.2478/amm-2014-0007. [DOI] [Google Scholar]
- 44.Sharma M., Modi O.P., Kumar P. Synthesis and characterization of copper foams through a powder metallurgy route using a compressible and lubricant space-holder material. Int. J. Miner. Metall. Mater. 2018;25:902–912. doi: 10.1007/s12613-018-1639-y. [DOI] [Google Scholar]
- 45.Li Q. Effect of porosity and carbon composition on pore microstructure of magnesium/carbon nanotube composite foams. Mater. Des. 2016;89:978–987. doi: 10.1016/j.matdes.2015.09.134. [DOI] [Google Scholar]
- 46.Zhuang H., Han Y., Feng A. Preparation, mechanical properties and in vitro biodegradation of porous magnesium scaffolds. Mater. Sci. Eng. C. 2008;28:1462–1466. doi: 10.1016/j.msec.2008.04.001. [DOI] [Google Scholar]
- 47.Wen C.E., Yamada Y., Shimojima K., Chino Y., Hosokawa H., Mabuchi M. Compressibility of porous magnesium foam: Dependency on porosity and pore size. Mater. Lett. 2004;58:357–360. doi: 10.1016/S0167-577X(03)00500-7. [DOI] [Google Scholar]
- 48.Hu L., Li Y., Zhou X., Yuan G. Characterization of as-cast microstructure of aluminum foams by melt foaming method. Mater. Lett. 2022;308:131112. doi: 10.1016/j.matlet.2021.131112. [DOI] [Google Scholar]
- 49.Awad M., Hassan N.M., Kannan S. Mechanical properties of melt infiltration and powder metallurgy fabricated aluminum metal matrix composite. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2021;235:2093–2107. doi: 10.1177/09544054211015956. [DOI] [Google Scholar]
- 50.Georgy K., Kumar K.C.H., Mukherjee M. Optimization of Mg Blowing Agent Content for Foaming Aluminum. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2022;53:1089–1102. doi: 10.1007/s11663-021-02403-3. [DOI] [Google Scholar]
- 51.Ghaleh M.H., Ehsani N., Baharvandi H.R. High-Porosity Closed-Cell Aluminum Foams Produced by Melting Method Without Stabilizer Particles. Int. J. Met. 2020;15:899–905. doi: 10.1007/s40962-020-00528-w. [DOI] [Google Scholar]
- 52.Linul E., Pietras D., Sadowski T., Marşavina L., Rajak D.K., Kovacik J. Crashworthiness performance of lightweight Composite Metallic Foams at high temperatures. Compos. Part A Appl. Sci. Manuf. 2021;149:106516. doi: 10.1016/j.compositesa.2021.106516. [DOI] [Google Scholar]
- 53.Palaniswamy S., Arunagiri K., Prakash S. Corrosion behaviour of closed cell magnesium foam with rare earth elements by powder metallurgy process. AIP Conf. Proc. 2020;2311:040010. doi: 10.1063/5.0034354. [DOI] [Google Scholar]
- 54.Damanik F., Lange G. Uniformly Dispersion of Multi-Walled Carbon Nanotubes in AlMg4Si8 Foam by Powder Metallurgy; Proceedings of the 3rd Asia Pacific Conference on Research in Industrial and Systems Engineering; Depok, Indonesia. 16–17 June 2020; pp. 230–235. [DOI] [Google Scholar]
- 55.Sharma A., Mishra P. Microstructure and mechanical behaviour of Ti-Cu foams synthesized via powder metallurgy technique. Mater. Res. Express. 2021;8:035402. doi: 10.1088/2053-1591/abed69. [DOI] [Google Scholar]
- 56.Sazegaran H. Investigation on Production Parameters of Steel Foam Manufactured Through Powder Metallurgical Space Holder Technique. Met. Mater. Int. 2020;27:3371–3384. doi: 10.1007/s12540-020-00659-z. [DOI] [Google Scholar]
- 57.Jain H., Mondal D.P., Gupta G., Kumar R. Effect of compressive strain rate on the deformation behaviour of austenitic stainless steel foam produced by space holder technique. Mater. Chem. Phys. 2021;259:124010. doi: 10.1016/j.matchemphys.2020.124010. [DOI] [Google Scholar]
- 58.Abo Sbia A.E.S., Uzun A. Production of MWCNT-Reinforced Aluminum Foams Via Powder Space-Holder Technique and Investigation of their Mechanical Properties. Trans. Indian Inst. Met. 2022;75:1–13. doi: 10.1007/s12666-022-02589-3. [DOI] [Google Scholar]
- 59.Jha N., Mondal D.P., Dutta Majumdar J., Badkul A., Jha A.K., Khare A.K. Highly porous open cell Ti-foam using NaCl as temporary space holder through powder metallurgy route. Mater. Des. 2013;47:810–819. doi: 10.1016/j.matdes.2013.01.005. [DOI] [Google Scholar]
- 60.Yang X., Hu Q., Du J., Song H., Zou T., Sha J., He C., Zhao N. Compression fatigue properties of open-cell aluminum foams fabricated by space-holder method. Int. J. Fatigue. 2019;121:272–280. doi: 10.1016/j.ijfatigue.2018.11.008. [DOI] [Google Scholar]
- 61.Salehi A., Babakhani A., Zebarjad S.M. Microstructural and mechanical properties of Al-SiO2 nanocomposite foams produced by an ultrasonic technique. Mater. Sci. Eng. A. 2015;638:54–59. doi: 10.1016/j.msea.2015.04.024. [DOI] [Google Scholar]
- 62.Jain H., Mondal D.P., Gupta G., Kothari A., Kumar R., Pandey A., Shiva S. Microstructure and high temperature compressive deformation in lightweight open cell titanium foam. Manuf. Lett. 2021;27:67–71. doi: 10.1016/j.mfglet.2020.12.007. [DOI] [Google Scholar]
- 63.Torres-Sanchez C., McLaughlin J., Bonallo R. Effect of Pore Size, Morphology and Orientation on the Bulk Stiffness of a Porous Ti35Nb4Sn Alloy. J. Mater. Eng. Perform. 2018;27:2899–2909. doi: 10.1007/s11665-018-3380-0. [DOI] [Google Scholar]
- 64.Nieh T.G., Higashi K., Wadsworth J. Effect of cell morphology on the compressive properties of open-cell aluminum foams. Mater. Sci. Eng. A. 2000;283:105–110. doi: 10.1016/S0921-5093(00)00623-7. [DOI] [Google Scholar]
- 65.Kennedy A.R., Asavavisitchai S. Effects of TiB2 particle addition on the expansion, structure and mechanical properties of PM Al foams. Scr. Mater. 2004;50:115–119. doi: 10.1016/j.scriptamat.2003.09.026. [DOI] [Google Scholar]
- 66.Yang D.H., Yang S.R., Ma A.B., Jiang J.H. Compression properties of cellular AlCu5Mn alloy foams with wide range of porosity. J. Mater. Sci. 2009;44:5552–5556. doi: 10.1007/s10853-009-3777-0. [DOI] [Google Scholar]
- 67.Ipek Nakaş G., Dericioglu A.F., Bor Ş. Fatigue behavior of TiNi foams processed by the magnesium space holder technique. J. Mech. Behav. Biomed. Mater. 2011;4:2017–2023. doi: 10.1016/j.jmbbm.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 68.San Marchi C., Mortensen A. Deformation of open-cell aluminum foam. Acta Mater. 2001;49:3959–3969. doi: 10.1016/S1359-6454(01)00294-4. [DOI] [Google Scholar]
- 69.Parvanian A.M., Panjepour M. Mechanical behavior improvement of open-pore copper foams synthesized through space holder technique. Mater. Des. 2013;49:834–841. doi: 10.1016/j.matdes.2013.01.077. [DOI] [Google Scholar]
- 70.Wang Q.Z., Lu D.M., Cui C.X., Liang L.M. Compressive behaviors and energy-absorption properties of an open-celled porous Cu fabricated by replication of NaCl space-holders. J. Mater. Process. Technol. 2011;211:363–367. doi: 10.1016/j.jmatprotec.2010.10.008. [DOI] [Google Scholar]
- 71.Sadighikia S., Abdolhosseinzadeh S., Asgharzadeh H. Production of high porosity Zn foams by powder metallurgy method. Powder Metall. 2015;58:61–66. doi: 10.1179/1743290114Y.0000000109. [DOI] [Google Scholar]
- 72.Bafti H., Habibolahzadeh A. Production of aluminum foam by spherical carbamide space holder technique-processing parameters. Mater. Des. 2010;31:4122–4129. doi: 10.1016/j.matdes.2010.04.038. [DOI] [Google Scholar]
- 73.Kenesei P., Kádár C., Rajkovits Z., Lendvai J. The influence of cell-size distribution on the plastic deformation in metal foams. Scr. Mater. 2004;50:295–300. doi: 10.1016/j.scriptamat.2003.09.046. [DOI] [Google Scholar]
- 74.Miyoshi T., Hara S., Mukai T., Higashi K. Development of a Closed Cell Aluminum Alloy Foam with Enhancement of the Compressive Strength. Mater. Trans. 2001;42:2118–2123. doi: 10.2320/matertrans.42.2118. [DOI] [Google Scholar]
- 75.Yang D., Guo S., Chen J., Qiu C., Agbedor S.O., Ma A., Jiang J., Wang L. Preparation principle and compression properties of cellular Mg–Al–Zn alloy foams fabricated by the gas release reaction powder metallurgy approach. J. Alloys Compd. 2021;857:158112. doi: 10.1016/j.jallcom.2020.158112. [DOI] [Google Scholar]
- 76.Wang H., Zhu D.F., Wu Y., Liu X.J., Jiang S.H., Nieh T.G., Lu Z.P. New insight into fabrication of shaped Mg–X alloy foams with cellular structure via a gas release reaction powder metallurgy route. J. Iron Steel Res. Int. 2021;28:125–132. doi: 10.1007/s42243-020-00543-5. [DOI] [Google Scholar]
- 77.Huang L., Wang H., Yang D., Ye F., Lu Z.P. Effects of scandium additions on mechanical properties of cellular Al-based foams. Intermetallics. 2012;28:71–76. doi: 10.1016/j.intermet.2012.03.050. [DOI] [Google Scholar]
- 78.Chethan A., Garcia-Moreno F., Wanderka N., Murty B.S., Banhart J. Influence of oxides on the stability of zinc foam. J. Mater. Sci. 2011;46:7806–7814. doi: 10.1007/s10853-011-5761-8. [DOI] [Google Scholar]
- 79.Song Y.H., Tane M., Ide T., Seimiya Y., Hur B.Y., Nakajima H. Fabrication of Al-3.7 PCT Si-0.18 PCT MG foam strengthened by aln particle dispersion and its compressive properties. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2010;41:2104–2111. doi: 10.1007/s11661-010-0247-x. [DOI] [Google Scholar]
- 80.Suárez M.A., Delgado-Pamanes M.F., Chávez-Alcalá J.F., Cruz-Ramírez A., Guadarrama I., Figueroa I.A. Microstructural and mechanical characterization of quasicrystalline Al-Cu-Fe foams. Mater. Today Commun. 2022;30:103043. doi: 10.1016/j.mtcomm.2021.103043. [DOI] [Google Scholar]
- 81.Tripathi O., Dwivedi V.K., Agarwal M. Microstructural-mechanical co-relation for Al2O3 reinforced aluminum metallic foam processed by compaction and sintering. J. Aust. Ceram. Soc. 2021;58:367–377. doi: 10.1007/s41779-021-00698-8. [DOI] [Google Scholar]
- 82.Yang D., Chen J., Chen W., Wang L., Wang H., Jiang J., Ma A. Fabrication of cellular Zn–Mg alloy foam by gas release reaction via powder metallurgical approach. J. Mater. Sci. Technol. 2017;33:1141–1146. doi: 10.1016/j.jmst.2017.03.019. [DOI] [Google Scholar]
- 83.Farahani M.R., Rezaei Ashtiani H.R., Elahi S.H. Effect of Zinc Content on the Mechanical Properties of Closed-Cell Aluminum Foams. Int. J. Met. 2021;16:713–722. doi: 10.1007/s40962-021-00635-2. [DOI] [Google Scholar]
- 84.Gibson L.J., Ashby M.F. The Mechanics of Three-Dimensional Cellular Materials. Proc. R. Soc. London A Math. Phys. Sci. 1982;382:43–59. [Google Scholar]
- 85.Gibson L.J., Editor G. Cellular Solids. Cambridge University Press; Cambridge, UK: 2021. pp. 270–274. [Google Scholar]
- 86.Ashby M.F., Gibson L.J. Cellular Solids: Structure and Properties. Cambridge University Press; Cambridge, UK: 1997. [Google Scholar]
- 87.Jiang B., Zhao N.Q., Shi C.S., Li J.J. Processing of open cell aluminum foams with tailored porous morphology. Scr. Mater. 2005;53:781–785. doi: 10.1016/j.scriptamat.2005.04.055. [DOI] [Google Scholar]
- 88.Bekoz N., Oktay E. Effects of carbamide shape and content on processing and properties of steel foams. J. Mater. Process. Technol. 2012;212:2109–2116. doi: 10.1016/j.jmatprotec.2012.05.015. [DOI] [Google Scholar]
- 89.Aly M.S.M.A., Bleck W. High Temperature Mechanical Properties of Cast as Well as Powder Metallurgical Manufactured Metallic Foams. RWTH Aachen University; Aachen, Germany: 2004. [Google Scholar]
- 90.Bekoz N., Oktay E. The role of pore wall microstructure and micropores on the mechanical properties of Cu-Ni-Mo based steel foams. Mater. Sci. Eng. A. 2014;612:387–397. doi: 10.1016/j.msea.2014.06.064. [DOI] [Google Scholar]
- 91.Xia X.C., Chen X.W., Zhang Z., Chen X., Zhao W.M., Liao B., Hur B. Effects of porosity and pore size on the compressive properties of closed-cell Mg alloy foam. J. Magnes. Alloys. 2013;1:330–335. doi: 10.1016/j.jma.2013.11.006. [DOI] [Google Scholar]
- 92.Hassanli F., Paydar M.H. Improvement in energy absorption properties of aluminum foams by designing pore-density distribution. J. Mater. Res. Technol. 2021;14:609–619. doi: 10.1016/j.jmrt.2021.06.073. [DOI] [Google Scholar]
- 93.Alizadeh M., Mirzaei-Aliabadi M. Compressive properties and energy absorption behavior of Al-Al2O3 composite foam synthesized by space-holder technique. Mater. Des. 2012;35:419–424. doi: 10.1016/j.matdes.2011.09.059. [DOI] [Google Scholar]
- 94.Yang K., Yang X., Liu E., Shi C., Ma L., He C., Li Q., Li J., Zhao N. Elevated temperature compressive properties and energy absorption response of in-situ grown CNT- reinforced Al composite foams. Mater. Sci. Eng. A. 2017;690:294–302. doi: 10.1016/j.msea.2017.03.004. [DOI] [Google Scholar]
- 95.Rathore R.K., Singh N.K., Xavier J. Processing and Characterization of Materials. Vol. 13. Springer; Berlin/Heidelberg, Germany: 2021. Epilogue; pp. 369–370. [DOI] [Google Scholar]
- 96.Banhart J. Manufacturing routes for metallic foams. Jom. 2000;52:22–27. doi: 10.1007/s11837-000-0062-8. [DOI] [Google Scholar]
- 97.Jiejun W., Chenggong L., Dianbin W., Manchang G. Damping and sound absorption properties of particle reinforced Al matrix composite foams. Compos. Sci. Technol. 2003;63:569–574. doi: 10.1016/S0266-3538(02)00215-4. [DOI] [Google Scholar]
- 98.Mondal D.P., Goel M.D., Das S. Effect of strain rate and relative density on compressive deformation behaviour of closed cell aluminum-fly ash composite foam. Mater. Des. 2009;30:1268–1274. doi: 10.1016/j.matdes.2008.06.059. [DOI] [Google Scholar]
- 99.Deqing W., Ziyuan S. Effect of ceramic particles on cell size and wall thickness of aluminum foam. Mater. Sci. Eng. A. 2003;361:45–49. doi: 10.1016/S0921-5093(03)00557-4. [DOI] [Google Scholar]
- 100.Malekjafarian M., Sadrnezhaad S.K., Abravi M.S., Golestanipour M., Mashhadi H.A. Manufacturing aluminum foams by melt gas injection process; Proceedings of the 7th International Conference on Porous Metals and Metallic Foams; Busan, Korea. 18–21 September 2011; pp. 195–202. [Google Scholar]
- 101.Surace R., De Filippis L., Ludovico A., Boghetich G. Experimental analysis of the effect of control factors on aluminium foam produced by powder metallurgy. Est. J. Eng. 2007;13:156–167. [Google Scholar]
- 102.Jeenager V.K., Pancholi V. Influence of cell wall microstructure on the energy absorption capability of aluminium foam. Mater. Des. 2014;56:454–459. doi: 10.1016/j.matdes.2013.08.109. [DOI] [Google Scholar]
- 103.Uzun A., Turker M. The effect of production parameters on the foaming behavior of spherical-shaped aluminum foam. Mater. Res. 2014;17:311–315. doi: 10.1590/S1516-14392014005000006. [DOI] [Google Scholar]
- 104.Mukherjee M., García-Moreno F., Jiménez C., Rack A., Banhart J. Microporosity in aluminium foams. Acta Mater. 2017;131:156–168. doi: 10.1016/j.actamat.2017.03.039. [DOI] [Google Scholar]
- 105.Asavavisithchai S., Kennedy A.R. The effect of compaction method on the expansion and stability of aluminium foams. Adv. Eng. Mater. 2006;8:810–815. doi: 10.1002/adem.200600067. [DOI] [Google Scholar]
- 106.Nakamura T., Gnyloskurenko S.V., Sakamoto K., Byakova A.V., Ishikawa R. Development of new foaming agent for metal foam. Mater. Trans. 2002;43:1191–1196. doi: 10.2320/matertrans.43.1191. [DOI] [Google Scholar]
- 107.Amirjan M., Bozorg M. Properties and corrosion behavior of Al based nanocomposite foams produced by the sintering-dissolution process. Int. J. Miner. Metall. Mater. 2018;25:94–101. doi: 10.1007/s12613-018-1551-5. [DOI] [Google Scholar]
- 108.Li D.W., Jie L.I., Tao L.I., Ting SU N., Zhang X.M., Yao G.C. Preparation and characterization of aluminum foams with ZrH2 as foaming agent. Trans. Nonferrous Met. Soc. China. 2011;21:346–352. doi: 10.1016/S1003-6326(11)60720-6. [DOI] [Google Scholar]
- 109.Matijasevic B., Görke O., Schubert H., Banhart J. Zirconium Hydride-A Possible Blowing Agent for Making Aluminium Alloy Foams. Porous Met. Met. Foam. Technol. 2006:107–110. [Google Scholar]
- 110.Haesche M., Lehmhus D., Weise J., Wichmann M., Mocellin I.C.M. Carbonates as foaming agent in chip-based aluminium foam precursor. J. Mater. Sci. Technol. 2010;26:845–850. doi: 10.1016/S1005-0302(10)60135-1. [DOI] [Google Scholar]
- 111.Kevorkijan V., Škapin S.D., Paulin I., Šuštaršič B., Jenko M. Synthesis and characterisation of closed cells aluminium foams containing dolomite powder as foaming agent. Mater. Tehnol. 2010;44:363–371. [Google Scholar]
- 112.Sasikumar S., Georgy K., Mukherjee M., Kumar G.S.V. Production, stability, and properties of in-situ Al–5ZrB2 composite foams. Mater. Sci. Eng. A. 2022;849:143501. doi: 10.1016/j.msea.2022.143501. [DOI] [Google Scholar]
- 113.Gui M.C., Wang D.B., Wu J.J., Yuan G.J., Li C.G. Deformation and damping behaviors of foamed Al-Si-SiCp composite. Mater. Sci. Eng. A. 2000;286:282–288. doi: 10.1016/S0921-5093(00)00789-9. [DOI] [Google Scholar]
- 114.Luo Y., Yu S., Li W., Liu J., Wei M. Compressive behavior of SiCp/AlSi9Mg composite foams. J. Alloys Compd. 2008;460:294–298. doi: 10.1016/j.jallcom.2007.06.041. [DOI] [Google Scholar]
- 115.Yu S., Luo Y., Liu J. Effects of strain rate and SiC particle on the compressive property of SiC p/AlSi 9 Mg composite foams. Mater. Sci. Eng. A. 2008;487:394–399. doi: 10.1016/j.msea.2007.11.025. [DOI] [Google Scholar]
- 116.Orbulov I.N., Ginsztler J. Compressive characteristics of metal matrix syntactic foams. Compos. Part A Appl. Sci. Manuf. 2012;43:553–561. doi: 10.1016/j.compositesa.2012.01.008. [DOI] [Google Scholar]
- 117.Yang D.H., Hur B.Y., He D.P., Yang S.R. Effect of decomposition properties of titanium hydride on the foaming process and pore structures of Al alloy melt foam. Mater. Sci. Eng. A. 2007;445:415–426. doi: 10.1016/j.msea.2006.09.064. [DOI] [Google Scholar]
- 118.Yang D.H., Hur B.Y., Yang S.R. Study on fabrication and foaming mechanism of Mg foam using CaCO3 as blowing agent. J. Alloys Compd. 2008;461:221–227. doi: 10.1016/j.jallcom.2007.07.098. [DOI] [Google Scholar]
- 119.Erryani A., Pramuji F., Annur D., Amal M.I., Kartika I. IOP Conference Series: Materials Science and Engineering. Volume 202. IOP Publishing; East Java, Indonesia: 2017. Microstructures and Mechanical Study of Mg Alloy Foam Based on Mg-Zn-Ca-CaCO3 System. [DOI] [Google Scholar]
- 120.Tane M., Nakajima H. Fabrication of porous magnesium with directional pores through use of hydrogen thermally decomposed from MgH2 powders during unidirectional solidification. J. Mater. Res. 2008;23:849–855. doi: 10.1557/JMR.2008.0105. [DOI] [Google Scholar]
- 121.Yang D., Seo C., Hur B.Y. Mg alloy foam fabrication via melt foaming method. J. Mater. Sci. Technol. 2008;24:302–304. [Google Scholar]
- 122.Papadopoulos D.P., Konstantinidis I.C., Papanastasiou N., Skolianos S., Lefakis H., Tsipas D.N. Mechanical properties of Al metal foams. Mater. Lett. 2004;58:2574–2578. doi: 10.1016/j.matlet.2004.03.004. [DOI] [Google Scholar]
- 123.Kadoi K., Nakae H. Relationship between foam stabilization and physical properties of particles on aluminum foam production. Mater. Trans. 2011;52:1912–1919. doi: 10.2320/matertrans.F-M2011817. [DOI] [Google Scholar]
- 124.Mu Y., Yao G., Liang L., Luo H., Zu G. Deformation mechanisms of closed-cell aluminum foam in compression. Scr. Mater. 2010;63:629–632. doi: 10.1016/j.scriptamat.2010.05.041. [DOI] [Google Scholar]
- 125.Raj R.E., Daniel B.S.S. Structural and compressive property correlation of closed-cell aluminum foam. J. Alloys Compd. 2009;467:550–556. doi: 10.1016/j.jallcom.2007.12.040. [DOI] [Google Scholar]
- 126.Yu S., Liu J., Luo Y., Liu Y. Compressive behavior and damping property of ZA22/SiCp composite foams. Mater. Sci. Eng. A. 2007;457:325–328. doi: 10.1016/j.msea.2006.12.089. [DOI] [Google Scholar]
- 127.Liu J., Yu S., Zhu X., Wei M., Luo Y., Liu Y. Correlation between ceramic additions and compressive properties of Zn-22Al matrix composite foams. J. Alloys Compd. 2009;476:220–225. doi: 10.1016/j.jallcom.2008.09.069. [DOI] [Google Scholar]
- 128.Amsterdam E., De Hosson J.T.M., Onck P.R. Failure mechanisms of closed-cell aluminum foam under monotonic and cyclic loading. Acta Mater. 2006;54:4465–4472. doi: 10.1016/j.actamat.2006.05.033. [DOI] [Google Scholar]
- 129.Markaki A.E., Clyne T.W. The effect of cell wall microstructure on the deformation and fracture of aluminium-based foams. Acta Mater. 2001;49:1677–1686. doi: 10.1016/S1359-6454(01)00072-6. [DOI] [Google Scholar]
- 130.Heidari Ghaleh M., Ehsani N., Baharvandi H.R. Compressive Properties of A356 Closed-Cell Aluminum Foamed with a CaCO3 Foaming Agent Without Stabilizer Particles. Met. Mater. Int. 2021;27:3856–3861. doi: 10.1007/s12540-020-00807-5. [DOI] [Google Scholar]
- 131.Kováčik J., Simančík F. Comparison of zinc and aluminium foam behaviour. Kov. Mater. 2004;42:79–90. [Google Scholar]
- 132.Mutlu I., Oktay E. Production and characterisation of Cr-Si-Ni-Mo steel foams. Indian J. Eng. Mater. Sci. 2011;18:227–232. [Google Scholar]
- 133.Zhao N.Q., Jiang B., Du X.W., Li J.J., Shi C.S., Zhao W.X. Effect of Y2O3 on the mechanical properties of open cell aluminum foams. Mater. Lett. 2006;60:1665–1668. doi: 10.1016/j.matlet.2005.11.088. [DOI] [Google Scholar]
- 134.Hussain Z., Suffin N.S.A. Microstructure and Mechanical Behaviour of Aluminium Foam Produced by Sintering Dissolution Process Using NaCl Space Holder. J. Eng. Sci. 2011;7:37–49. [Google Scholar]
- 135.Mohammed S.H. Manufacturing of Aluminum Foam as a Light Weight Structural Material. Eng. Tech. J. 2016;34:697–702. [Google Scholar]
- 136.Despois J.F., Marmottant A., Salvo L., Mortensen A. Influence of the infiltration pressure on the structure and properties of replicated aluminium foams. Mater. Sci. Eng. A. 2007;462:68–75. doi: 10.1016/j.msea.2006.03.157. [DOI] [Google Scholar]
- 137.Gaillard C., Despois J.F., Mortensen A. Processing of NaCI powders of controlled size and shape for the microstructural tailoring of aluminium foams. Mater. Sci. Eng. A. 2004;374:250–262. doi: 10.1016/j.msea.2004.03.015. [DOI] [Google Scholar]
- 138.Sun D.X., Zhao Y.Y. Static and dynamic energy absorption of Al foams produced by the sintering and dissolution process. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2003;34:69–74. doi: 10.1007/s11663-003-0056-3. [DOI] [Google Scholar]
- 139.Ertürk A.T. Production of aluminum glass fiber reinforced foam synthesized by space-holder technique. Acta Phys. Pol. A. 2016;129:592–595. doi: 10.12693/APhysPolA.129.592. [DOI] [Google Scholar]
- 140.Michailidis N., Stergioudi F., Tsouknidas A., Pavlidou E. Compressive response of Al-foams produced via a powder sintering process based on a leachable space-holder material. Mater. Sci. Eng. A. 2011;528:1662–1667. doi: 10.1016/j.msea.2010.10.088. [DOI] [Google Scholar]
- 141.Jamal N.A., Maizatul O., Anuar H., Yusof F., Nor Y.A., Khalid K., Zakaria M.N. Preliminary development of porous aluminum via powder metallurgy technique. Mater. Werkst. 2018;49:460–466. doi: 10.1002/mawe.201700269. [DOI] [Google Scholar]
- 142.Tatt T.K., Muhamad N., Muchtar A., Sulong A.B., Shia K.Y. Production of porous stainless steel using the space holder method. Sains Malays. 2021;50:507–514. doi: 10.17576/jsm-2021-5002-21. [DOI] [Google Scholar]
- 143.Hong K., Kádár C., Knapek M., Drozdenko D., Jenei P., Kim M.Y., Choe H., Máthis K., Park H., Gubicza J. Comparison of morphology and compressive deformation behavior of copper foams manufactured via freeze-casting and space-holder methods. J. Mater. Res. Technol. 2021;15:6855–6865. doi: 10.1016/j.jmrt.2021.11.108. [DOI] [Google Scholar]
- 144.Stanev L., Kolev M., Drenchev B., Drenchev L. Open-cell metallic porous materials obtained through space holders-Part II: Structure and properties. A review. J. Manuf. Sci. Eng. Trans. ASME. 2017;139:050802. doi: 10.1115/1.4034440. [DOI] [Google Scholar]
- 145.Brothers A.H., Scheunemann R., DeFouw J.D., Dunand D.C. Processing and structure of open-celled amorphous metal foams. Scr. Mater. 2005;52:335–339. doi: 10.1016/j.scriptamat.2004.10.002. [DOI] [Google Scholar]
- 146.Abdullah Z., Ahmad S., Ramli M. The impact of composition and sintering temperature for stainless steel foams (SS316l) fabricated by space holder method with urea as space holder. Mater. Sci. Forum. 2017;888:413–417. doi: 10.4028/www.scientific.net/MSF.888.413. [DOI] [Google Scholar]
- 147.Ozan S., Bilhan S. Effect of fabrication parameters on the pore concentration of the aluminum metal foam, manufactured by powder metallurgy process. Int. J. Adv. Manuf. Technol. 2008;39:257–260. doi: 10.1007/s00170-007-1207-5. [DOI] [Google Scholar]
- 148.Čapek J., Vojtěch D. Microstructural and mechanical characteristics of porous iron prepared by powder metallurgy. Mater. Sci. Eng. C. 2014;43:494–501. doi: 10.1016/j.msec.2014.06.046. [DOI] [PubMed] [Google Scholar]
- 149.Čapek J., Vojtěch D., Oborná A. Microstructural and mechanical properties of biodegradable iron foam prepared by powder metallurgy. Mater. Des. 2015;83:468–482. doi: 10.1016/j.matdes.2015.06.022. [DOI] [Google Scholar]
- 150.Jiang B., Wang Z., Zhao N. Effect of pore size and relative density on the mechanical properties of open cell aluminum foams. Scr. Mater. 2007;56:169–172. doi: 10.1016/j.scriptamat.2006.08.070. [DOI] [Google Scholar]
- 151.Mohd Razali R.N., Abdullah B., Ismail M.H., Ahmad U.K., Idham M.F., Rasmli A. Mechanical properties of aluminium foam by conventional casting combined with NaCL space holder. Appl. Mech. Mater. 2013;393:156–160. doi: 10.4028/www.scientific.net/AMM.393.156. [DOI] [Google Scholar]
- 152.Jamal N.A., Tan A.W., Yusof F., Katsuyoshi K., Hisashi I., Singh S., Anuar H. Fabrication and compressive properties of low to medium porosity closed-cell porous Aluminum using PMMA space holder technique. Materials. 2016;9:254. doi: 10.3390/ma9040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tan P.P., Mohamad H., Anasyida A.S. Properties of Porous Magnesium Using Polymethyl Methacrylate (PMMA) as a Space Holder. J. Phys. Conf. Ser. 2018;1082:1–6. doi: 10.1088/1742-6596/1082/1/012063. [DOI] [Google Scholar]
- 154.Bi Y., Zheng Y., Li Y. Microstructure and mechanical properties of sintered porous magnesium using polymethyl methacrylate as the space holder. Mater. Lett. 2015;161:583–586. doi: 10.1016/j.matlet.2015.09.039. [DOI] [Google Scholar]
- 155.Zhao Y.Y., Fung T., Zhang L.P., Zhang F.L. Lost carbonate sintering process for manufacturing metal foams. Scr. Mater. 2005;52:295–298. doi: 10.1016/j.scriptamat.2004.10.012. [DOI] [Google Scholar]
- 156.Parvanian A.M., Saadatfar M., Panjepour M., Kingston A., Sheppard A.P. The effects of manufacturing parameters on geometrical and mechanical properties of copper foams produced by space holder technique. Mater. Des. 2014;53:681–690. doi: 10.1016/j.matdes.2013.07.047. [DOI] [Google Scholar]
- 157.Cuba Ramos A.I., Dunand D.C. Preparation and characterization of directionally freeze-cast copper foams. Metals. 2012;2:265–273. doi: 10.3390/met2030265. [DOI] [Google Scholar]
- 158.Körner C., Berger F., Arnold M., Stadelmann C., Singer R.F. Influence of processing conditions on morphology of metal foams produced from metal powder. Mater. Sci. Technol. 2000;16:781–784. doi: 10.1179/026708300101508432. [DOI] [Google Scholar]
- 159.Surace R., De Filippis L.A.C., Ludovico A.D., Boghetich G. Influence of processing parameters on aluminium foam produced by space holder technique. Mater. Des. 2009;30:1878–1885. doi: 10.1016/j.matdes.2008.09.027. [DOI] [Google Scholar]
- 160.Aguirre-Perales L.Y., Jung I.H., Drew R.A.L. Foaming behavior of powder metallurgical Al-Sn foams. Acta Mater. 2012;60:759–769. doi: 10.1016/j.actamat.2011.10.011. [DOI] [Google Scholar]
- 161.Mahmutyazicioglu N., Albayrak O., Ipekoglu M., Altintas S. Effects of alumina (Al2O3) addition on the cell structure and mechanical properties of 6061 foams. J. Mater. Res. 2013;28:2509–2519. doi: 10.1557/jmr.2013.187. [DOI] [Google Scholar]
- 162.Aguirre-perales L.Y., Drew R.A.L., Jung I. The Effect of In Situ Intermetallic Formation on Al-Sn Foaming Behavior. Metall. Mater. Trans. A. 2014;45:3714–3727. doi: 10.1007/s11661-014-2313-2. [DOI] [Google Scholar]
- 163.Duarte I., Banhart J. A study of aluminium foam formation—Kinetics and microstructure. Acta Mater. 2000;48:2349–2362. doi: 10.1016/S1359-6454(00)00020-3. [DOI] [Google Scholar]
- 164.Jee C.S.Y., Özgüven N., Guo Z.X., Evans J.R.G. Preparation of high porosity metal foams. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2000;31:1345–1352. doi: 10.1007/s11663-000-0021-3. [DOI] [Google Scholar]
- 165.Sahu S., Mondal D.P., Cho J.U., Goel M.D., Ansari M.Z. Low-velocity impact characteristics of closed cell AA2014-SiCp composite foam. Compos. Part B Eng. 2019;160:394–401. doi: 10.1016/j.compositesb.2018.12.054. [DOI] [Google Scholar]
- 166.Duarte I., Ventura E., Olhero S., Ferreira J.M.F. An effective approach to reinforced closed-cell Al-alloy foams with multiwalled carbon nanotubes. Carbon. 2015;95:589–600. doi: 10.1016/j.carbon.2015.08.065. [DOI] [Google Scholar]
- 167.Schäffler P., Hanko G., Mitterer H., Zach P. Alulight metal foam products; Proceedings of the 5th International Conference on Porous Metals and Metallic Foams; Lancaster, PA, USA. 5–7 September 2008; pp. 7–10. [Google Scholar]
- 168.Guo J., Du Z., Liu G., Yang X., Li M.-J. Compression effect of metal foam on melting phase change in a shell-and-tube unit. Appl. Therm. Eng. 2022;206:118124. doi: 10.1016/j.applthermaleng.2022.118124. [DOI] [Google Scholar]
- 169.Eugénio S., Silva T.M., Carmezim M.J., Duarte R.G., Montemor M.F. Electrodeposition and characterization of nickel-copper metallic foams for application as electrodes for supercapacitors. J. Appl. Electrochem. 2014;44:455–465. doi: 10.1007/s10800-013-0646-y. [DOI] [Google Scholar]
- 170.Campana F., Pilone D. Effect of heat treatments on the mechanical behaviour of aluminium alloy foams. Scr. Mater. 2009;60:679–682. doi: 10.1016/j.scriptamat.2008.12.045. [DOI] [Google Scholar]
- 171.Aghion E., Yered T., Perez Y., Gueta Y. The prospects of carrying and releasing drugs via biodegradable magnesium foam. Adv. Eng. Mater. 2010;12:374–379. doi: 10.1002/adem.200980044. [DOI] [Google Scholar]
- 172.Duarte I.M.A., Banhart J., Ferreira A.J.M., Santos M.J.G. Foaming around fastening elements. Mater. Sci. Forum. 2006;514:712–717. doi: 10.4028/www.scientific.net/MSF.514-516.712. [DOI] [Google Scholar]
- 173.Chen S., Bourham M., Rabiei A. Applications of Open-cell and Closed-cell Metal Foams for Radiation Shielding. Procedia Mater. Sci. 2014;4:293–298. doi: 10.1016/j.mspro.2014.07.560. [DOI] [Google Scholar]
- 174.Aerospace E. Duocel® Aluminum Foam–ERG Aerospace. [(accessed on 21 January 2021)]. Available online: http://ergaerospace.com/materials/duocel-aluminum-foam/
- 175.Oriňák A., Oriňáková R., Králová Z.O., Turoňová A.M., Kupková M., Hrubovčáková M., Radoňák J., Džunda R. Sintered metallic foams for biodegradable bone replacement materials. J. Porous Mater. 2014;21:131–140. doi: 10.1007/s10934-013-9757-4. [DOI] [Google Scholar]
- 176.Banhart From fundamental research to applications. Europhys. News. 2010;41:3. [Google Scholar]
- 177.Hrubovčáková M., Kupková M., Džupon M. Fe and Fe-P Foam for Biodegradable Bone Replacement Material: Morphology, Corrosion Behaviour, and Mechanical Properties. Adv. Mater. Sci. Eng. 2016;2016:6257368. doi: 10.1155/2016/6257368. [DOI] [Google Scholar]
- 178.Filetin T., Marić G., Kramer I. Metal foams in shipbuilding. Brodogradnja. 2005;56:228–237. [Google Scholar]
- 179.Huisseune H., De Schampheleire S., Ameel B., De Paepe M. Comparison of metal foam heat exchangers to a finned heat exchanger for low Reynolds number applications. Int. J. Heat Mass Transf. 2015;89:1–9. doi: 10.1016/j.ijheatmasstransfer.2015.05.013. [DOI] [Google Scholar]
- 180.Banhart J., Seeliger H.-W. Aluminium Foam Sandwich Panels: Manufacture, Metallurgy and Applications. Adv. Eng. Mater. 2008;10:793–802. doi: 10.1002/adem.200800091. [DOI] [Google Scholar]
- 181.Banhart J. Metal foams—from fundamental research to applications. Front. Des. Mater. 2007;279:280–289. [Google Scholar]
- 182.García-Moreno F. Commercial applications of metal foams: Their properties and production. Materials. 2016;9:85. doi: 10.3390/ma9020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Banhart J. Aluminium foams for lighter vehicles. Int. J. Veh. Des. 2005;37:114–125. doi: 10.1504/IJVD.2005.006640. [DOI] [Google Scholar]
- 184.Bryant J.D., Crowley M., Wang W., Wilhelmy D., Kallivayalil J. Development of Alcoa aluminum foam products. Porous Met. Met. Foam. 2008:19–22. [Google Scholar]
- 185.Hanssen A.G., Stöbener K., Rausch G., Langseth M., Keller H. Optimisation of energy absorption of an A-pillar by metal foam insert. Int. J. Crashworthiness. 2006;11:231–242. doi: 10.1533/ijcr.2005.0396. [DOI] [Google Scholar]
- 186.Walther G., Klöden B., Büttner T., Weissgärber T., Kieback B., Böhm A., Naumann D., Soberi S., Timberg L. A new class of high temperature and corrosion resistant nickel-based open-cell foams. Adv. Eng. Mater. 2008;10:803–811. doi: 10.1002/adem.200800088. [DOI] [Google Scholar]
- 187.Torres Y., Rodríguez J.A., Arias S., Echeverry M., Robledo S., Amigo V., Pavón J.J. Processing, characterization and biological testing of porous titanium obtained by space-holder technique. J. Mater. Sci. 2012;47:6565–6576. doi: 10.1007/s10853-012-6586-9. [DOI] [Google Scholar]