Abstract
Mid-regional proadrenomedullin (MR-proADM) is a new biomarker of endothelial damage and its clinical use is increasing in sepsis and respiratory infections and recently in SARS-CoV-2 infection. We conducted a systematic review and meta-analysis to clarify the use of MR-proADM in severe COVID-19 disease. After Pubmed, Embase, and Scopus search, registries, and gray literature, deduplication, and selection of full-texts, we found 21 studies addressing the use of proadrenomedullin in COVID-19. All the studies were published between 2020 and 2022 from European countries. A total of 9 studies enrolled Intensive Care Unit (ICU) patients, 4 were conducted in the Emergency Department, and 8 had mixed populations. Regarding the ICU critically ill patients, 4 studies evaluating survival as primary outcome were available, of which 3 reported completed data. Combining the selected studies in a meta-analysis, a total of 252 patients were enrolled; of these, 182 were survivors and 70 were non-survivors. At the admission to the ICU, the average MR-proADM level in survivor patients was 1.01 versus 1.64 in non-survivor patients. The mean differences of MR-proADM values in survivors vs. non-survivors was −0.96 (95% CI from −1.26, to −0.65). Test for overall effect: Z = 6.19 (p < 0.00001) and heterogeneity was I2 = 0%. MR-proADM ICU admission levels seem to predict mortality among the critical COVID-19 population. Further, prospective studies, focused on critically ill patients and investigating a reliable MR-proADM cut-off, are needed to provide adequate guidance to its use in severe COVID-19.
Keywords: proadrenomedullin, MR-proADM, SARS-CoV-2, COVID-19, biomarkers, intensive care, endothelitis
1. Introduction
Proadrenomedullin (pro-ADM) is a 52 multipotent regulatory amino acid peptide expressed in various tissues and organs, upregulated by hypoxia, inflammatory cytokines, bacterial products, and shear stress. Its precursor, mid-regional pro-ADM (MR-proADM), is currently considered an effective biomarker of endothelial damage as its increase in plasma seems to correlate with disease severity [1].
The mechanisms underlying this correlation are poorly defined even if associations with cardiovascular and thromboembolic complications, immunosuppression, and sepsis-like multiorgan dysfunction have been reported [2]. Regarding SARS-CoV-2, an association between MR-proADM levels and virus-induced endothelial damage is assumed. As endothelitis emerges as a prominent feature of the severe COVID-19 disease [3,4], an association between MR-proADM levels and virus-induced endothelial damage has been hypothesized as the pathophysiological mechanisms in COVID-19-induced critical illness seem related to an increased incidence of cardiovascular and thromboembolic complications, immune cell deactivation, and sepsis-like multiple organ failure. A rising number of studies has proposed that virus-induced endothelitis, resulting in impaired vascular blood flow, coagulation, and leakage, may partially explain the development of organ dysfunction and edema [5]. In this sense, since ADM has been shown to play a key role in reducing vascular hyper permeability and promoting endothelial stability and integrity following severe infections [3], MR-proADM might be a potential biomarker of COVID-19 severity and may be able to mimic disease progression, allowing the identification of patients most at risk of developing a severe form of SARS-CoV-2-related illness or multi-organ failure.
If the prognostic role of MR-proADM was demonstrated in the context of pneumonia, sepsis, and septic shock—currently the most studied areas evaluating the predictive capacity of this biomarker [3,6,7]—the pathological mechanism has not been fully clarified; nor is it in the case of severe COVID-19, where most of the studies have a limited size and were designed in the context of a pandemic emergency, with heterogeneity of objectives and study contexts.
To find an answer to uncertainties regarding the role of MR-proADM as a predictive marker of the severity of COVID-19 disease, we systematically present a review of the current literature. The possibility of constructing a meta-analysis capable of establishing the MR-proADM clinical severity cut-off in COVID-19 patients admitted to the Intensive Care Unit (ICU) was subsequently investigated.
2. Materials and Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [7]. The protocol was registered prospectively in OSF (DOI 10.17605/OSF.IO/V93EW, link https://osf.io/v93ew/ accessed on 1 May 2022). Since not all studies express values of pro-ADM levels by the same assessment technique, we refer to proADM when including results by all methods and to MR-proADM when levels were determined with the B.R.A.H.M.S. KRYPTOR compact PLUS (Thermo Fisher Scientific, Hennigsdorf, Germany) technique.
2.1. Eligibility Criteria
The research was conducted on 25 April 2022; randomized controlled trials (RCTs), non-randomized controlled trials (NRCTs), commentaries, letters, systematic reviews, and meta-analyses published in English and Italian were eligible for inclusion. The meta-analysis was then performed evaluating studies conducted only in the ICU setting to assess if MR-proADM levels may vary in survivors versus non survivors in critically ill patients with severe COVID-19 disease.
2.2. Information Sources
This systematic review was performed using Pubmed, Embase, and Scopus databases, and was implemented with the use of registries (clinicaltrials.gov, accessed on 25 April 2022) and gray literature searches.
2.3. Search Strategy
To perform the systematic review, the following search strategies were selected:
PubMed: “proADM” [All Fields] AND (“COVID-19” [All Fields] OR “COVID-19” [MeSH Terms] OR “COVID-19 vaccines” [All Fields] OR “COVID-19 vaccines” [MeSH Terms] OR “COVID-19 serotherapy” [All Fields] OR “COVID-19 serotherapy” [Supplementary Concept] OR “COVID-19 nucleic acid testing” [All Fields] OR “COVID-19 nucleic acid testing” [MeSH Terms] OR “COVID-19 serological testing” [All Fields] OR “COVID-19 serological testing” [MeSH Terms] OR “COVID-19 testing” [All Fields] OR “COVID-19 testing” [MeSH Terms] OR “SARS-CoV-2” [All Fields] OR “SARS-CoV-2” [MeSH Terms] OR “severe acute respiratory syndrome coronavirus 2” [All Fields] OR “ncov” [All Fields] OR “2019 ncov” [All Fields] OR ((“coronavirus” [MeSH Terms] OR “coronavirus” [All Fields] OR “cov” [All Fields]) AND 1 November 2019:3000/12/31 [Date—Publication])); Embase, Scopus, clinicaltrials.gov, and greylit.org: (‘proadrenomedullin’/exp OR proadrenomedullin) AND (‘coronavirus disease 2019’/exp OR ‘coronavirus disease 2019’).
2.4. Selection and Data Collection Process
Search results were exported to EndNote V.X9 (Clarivate Analytics, Philadelphia, PA, USA). Duplicates were automatically removed. The review process was carried out in three steps consisting of title and abstract review process, full-text review process, and risk of bias assessment. For each level, four authors (G.M., E.B., D.L., and A.G.) independently screened the articles with conflicts resolved by a third author (L.B.).
2.5. Study Risk of Bias Assessment
To assess the risk of bias, the Risk Of Bias In Non-randomized Studies—of Interventions (ROBINS-i) tool [8] and the Rob 2.0 tool [9] were used for NRCTs and RCTs, respectively. Risk of bias assessment was carried out by four authors (G.M., E.B., D.L., and A.G.) independently; where discrepancies were noticed, a third author (L.B.) was involved to resolve them.
2.6. Synthesis Methods
The main outcome was the use of pro-ADM as a prognostic marker in patients with COVID-19. A planned Excel spreadsheet was used to extract data (patient’s characteristics, type of surgery, follow-up periods, outcome measures, and main results). The results of the systematic review were reported in a summary table with the main features described for each study. All eligible studies were evaluated to collect data regarding MR-proADM levels among survivors and non-survivors in ICU population with severe COVID-19 disease. Given that the primary outcome was MR-proADM levels, data presented as median and interquartile range were converted into mean and standard deviation using validated online converters [10]. Estimates of effect were derived from quantitative analysis utilizing Review Manager 5.4 [11]. MR-proADM mean levels and standard deviations were used to evaluate mean differences (MD) with a 95% confidence interval (95% CI). Inverse variance method and random effects were used to assess overall MD. Statistical significance was set at p < 0.05. To evaluate the size of the effect of the MD, we considered levels of 0.2, 0.5, and 0.8 as small, medium, and large effects. Heterogeneity was assessed using the I2 index, with values of 25%, 50%, and 75% taken to indicate low, moderate, and high levels of heterogeneity, respectively [12].
3. Results
3.1. Study Selection
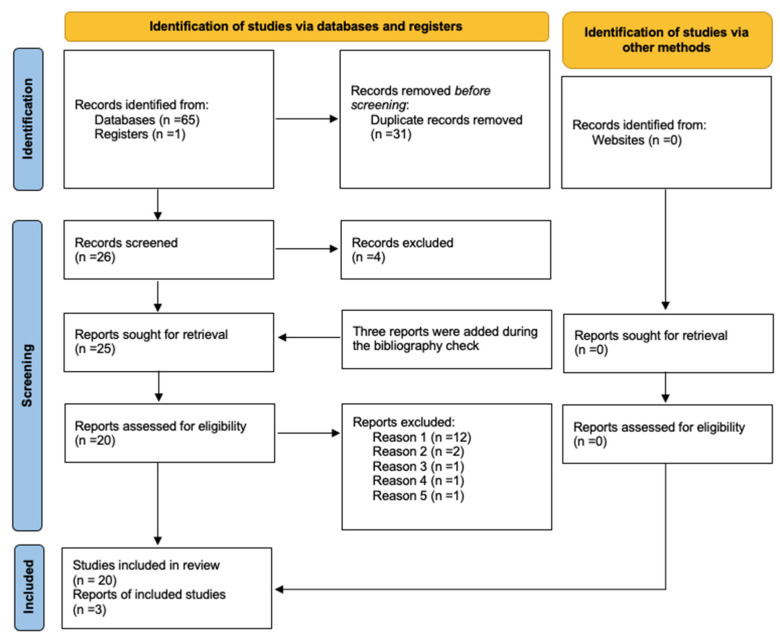
The systematic literature search retrieved 65 results in databases and one in registers. A flow chart describing the complete literature search process is reported in Figure 1.
Figure 1.
PRISMA flow-diagram. The reasons for exclusion: reason 1: papers which did not consider ICU population; reason 2: papers which evaluated different outcome (i.e., renal replacement therapy, superinfections); reason 3: analyzed MR-proADM levels among children versus adult patients; reason 4: considered pro-ADM levels with a different technique (bioactive ADM); reason 5: presented a population already included in a previously published study.
After de-duplication, 26 studies were selected for full-text review. Four papers were then excluded because they did not match the inclusion criteria. After an additional literature check, three papers were included in the systematic review [13,14,15]. A total of 20 articles were submitted to the systematic review.
In order to determine ICU-admitted patients’ pro-ADM cut offs, a new revision of selected studies was made, with the aim to organize data in a meta-analysis. Only 4 studied satisfied meta-analysis inclusion criteria. The reasons for exclusion of the 17 papers were: 12 papers did not consider ICU population, 2 papers evaluated different outcomes (i.e., renal replacement therapy, superinfections) [15,16], 1 analyzed MR-proADM levels among children versus adults patients [17], 1 considered pro-ADM levels with a different technique (bioactive ADM) [18], and 1 was excluded because it presented a population already included in a previously published study [19].
One of the four remaining studies included in the meta-analysis process did not report the standard deviation, and for this reason, was not included in calculation [20]. The meta-analysis was then performed with the three remaining studies.
3.2. Systematic Review
Study Characteristics
Characteristics of the individual studies are provided in Table 1.
Table 1.
Descriptive table of systematic review results, including the 20 full texts analyzed.
| Author | Year | Type of Study | Country | Period | Number of Patient | Clinical Setting | Timing | Outcome | Findings | AUC | Cut Off |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Benedetti et al. [21] | 2021 | prospective observational | Italy | March–April 2020 | 21 | IMCU | admission (T0), 24 h (T1), T3 e 5 | severe disease |
|
0.81 | 1.07 nmol/L |
| García de Guadiana-Romualdo et al. [22] | 2021 | prospective observational | Spain | March–April 2021 | 99 | ED | T0 | mortality/severe disease progression |
|
0.871 | 0.80 nmol/L |
| Girona-Alarcon et al. [17] | 2021 | prospective observational cohort | Spain | March–June 2020 | 20 | ICU | hospitalization | pediatric vs. adult population |
|
||
| Gregoriano et al. [23] | 2021 | prospective observational | Switzerland | February–April 2020 | 89 | mixed population | T0, T1, T2, T3 | in-hospital mortality |
|
0.78 | 0.93 nmol/L |
| Indirli et al. [24] | 2022 | retrospective | Italy | March–June 2020 | 116 | IMCU | At admission | in-hospital mortality |
|
0.79 | >1 |
| Lhote et al. * | 2021 | prospective multicentric | France | July 2020 to February 2021 | 170 | ICU | T0 | SOFA at day 3 |
|
NA | NA |
| Lo Sasso et al. [25] | 2021 | retrospective observational | Italy | September–October 2020 | 110 | mixed population | hospitalization | Inhospital mortality |
|
0.95 | 1.73 nmol/L |
| Malinina et al. ** [15] | 2020 | retrospective observational | Russia | 37 | ICU | Bacterial superinfection |
|
||||
| Mendez et al. [26] | 2021 | longitudinal | Spain | March–June 2020 | 210 | ED | T0 | in-hospital mortality |
|
NA | 1.16 |
| Minieri et al. [27] | 2021 | not specified | Italy | not specified | 321 | ED | ED-triage | overall in-hospital mortality |
|
0.85 | 1.105 |
| Montrucchio et al. [28] | 2021 | prospective observational | Italy | March–June 2020 | 57 | ICU | T0–1, T3, T7, T14 | ICU mortality—trend |
|
0.85 | >1.8 nmol/L * |
| Moore et al. [29] | 2022 | prospective | UK | April–June 2020 | 135 | ED | at the admission | 30-days mortality |
|
0.8441 | 1.54 |
| Oblitas et al. [19] | 2021 | prospective | Spain | August–November 2020 | 95 | ICU | once within 72 h of ICU admission | 30-day mortality and 30-day combined event |
|
0.73 and 0.72 | ≥1 |
| Popov et al. [30] | 2021 | prospective observational | Russia | 97 | mixed population | mortality |
|
0.75 | 0.895 nmol/L | ||
| Roedl et al. [16] | 2021 | observational | Germany | March–September 2020 | 64 | ICU | ICU admission | RRT versus no-RRT |
|
0.69 | |
| Simon et al. [18] | 2021 | prospective observational | Germany | March–April 2020 | 53 | ICU | Daily, T1–7 | ARDS, ECMO, MV, RRT |
|
bio-ADM: 70 pg/mL * | |
| Sozio et al. [31] | 2021 | retrospective | Italy | March–May 2020 | 111 | mixed population | ED admission | severe disease |
|
0.85 | Mortality 0.895 nmol/L |
| Spoto et al. [32] | 2020 | prospective observational | Italy | April–June 2020 | 69 | mixed population | hospitalization | endothelial damage, MOF, severe disease |
|
0.78 | ARDS 3.04; mortality 2 nmol/L |
| Van Oers et al. [33] | 2021 | prospective | the Netherlands | March–May 2020 | 105 | ICU | on a daily basis, during the first 7 days | 28-day mortalit |
|
0.84 | 1.57 |
| Zaninotto et al. [34] | 2021 | retrospective | Italy | November | 135 | mixed population | 7 days | clinical outcomes |
|
0.900 | 1.50 |
List of abbreviations: Area Under the Curve, AUC; Emergency Department, ED; Intensive Care Unit, ICU; Intermediate Care Unit, IMCU; T: time express in days; Multiorgan Failure, MOF; Acute Respiratory Distress Syndrome, ARDS; Extracorporeal Membrane Oxygenation, ECMO; Diffusing capacity for carbon monoxide, DLCO; Mechanical Ventilation, MV; Renal Replacement Therapy, RRT; C-terminal proendothelin-1, CT-proET-1; MR-proadrenomedullin, MR-proADM; Sequential Organ Failure Assessment, SOFA. * only abstract available. ** full-text article provided by the corresponding author.
The studies were published between 2020 and 2022. Of the 21 selected articles, 9 enrolled an ICU population, 4 were conducted in an Emergency Department (ED), and 8 had mixed populations. All studies were conducted in European countries except for 2, conducted in Russia: 8 studies were from Italy, 4 from Spain, 2 from the Netherlands, 2 from Germany, 1 from France, 1 from the UK, and 1 from Switzerland. The outcome most frequently considered was mortality. Of the 21 selected articles, 16 agree that the value of proADM predicts mortality or poor outcomes.
The enrollment period, as shown in Table 1, was similar for almost all the studies considered. Other data such as Area Under the Curve (AUC) and proADM considered cut-off are shown in Table 1. All studies considered used as MR-proADM determining levels the B.R.A.H.M.S. KRYPTOR compact PLUS (Thermo Fisher Scientific, Hennigsdorf, Germany) technique, except for one paper [18].
3.3. Meta-Analysis
Considering the four studies that were candidates for inclusion in the meta-analysis, one [20] could not be included due to lack of total population number. The other three studies reported MR-proADM admission values in ICU patient populations with critical COVID-19 disease divided by survivors and non-survivors. All studies considered were conducted in 2021. Regarding the country, one was conducted in Spain, one in Italy, and one in the Netherlands.
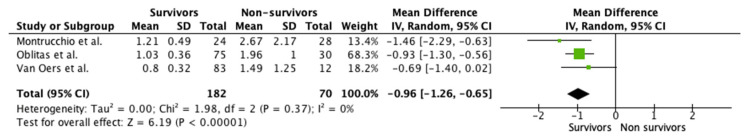
Among the selected studies, 252 patients were enrolled; of these, 182 were survivors and 70 non-survivors (Figure 2). At the admission to the ICU, the average MR-proADM level in survivor patients was 1.01 versus 1.64 in non-survivor patients. The MD of MR-proADM values in survivors vs. non-survivors was −0.96 (95% CI from −1.26, to −0.65). Test for overall effect: Z = 6.19 (p < 0.00001) and heterogeneity was I2 = 0% (Figure 2).
Figure 2.
Forest plot of the hypothetical meta-analyzed results [14,28,33]. One of the four studies selected could not be included as it did not report the standard deviation. Analysis conducted with Review manager 5.4 [11].
All studies were prospective non-randomized clinical trials; therefore, the ROBINS-i tool was applied to assess the risk of bias. The overall risk of bias was low (Supplementary Table S1). Publication bias was not tested because of the small number of studies.
4. Discussion
This systematic review of the literature highlights the potential role of MR-proADM as a clinical prognostic biomarker in critically ill patients with COVID-19, although a lack of an unequivocal explanation regarding its mechanism of action remains. The growing interest in this promising biomarker and its potential role in the context of COVID-19 pandemic should be underlined. The meta-analysis evaluating only studies conducted in ICU seems to confirm the efficacy of the use of this biomarker, although it deserves further studies to increase the sample size and better define a reliable cut-off. The COVID-19 pandemic has renewed attention to the well-known need for a biomarker capable of differentiating the most critical patients to whom interventions and resources should be targeted. In addition, the characteristics of the new infection—especially at the beginning—highlighted the “weaknesses” of traditional biomarkers, such as procalcitonin and C-reactive protein, but also, d-dimer and cardiac enzymes, which were progressively used as “surrogates” for possible damage mechanisms.
Two and a half years after the onset of SARS-CoV2 pandemic, the importance of the mechanism of endothelial damage at the microvascular level has been widely demonstrated. In this regard, the application of the pro-ADM biomarker in this specific context seemed to be of great interest right from the start, to identify—as early as possible—those patients at greatest risk of poor prognosis. The lack of a univocal explanation for its mechanism of action has not discouraged various authors from considering it in the clinical setting, even if its applications remain varied. Overall, the studies included in our review agree in defining the validity of MR-proADM in the early stages of hospitalization as a prognostic biomarker. Elevated values were found in patients with more severe disease and correlated with statistical significance with patient mortality [35]. This aspect emerged both in the ICU setting and in the ED, opening important perspectives not only in terms of patient allocation but also in terms of possible discharge.
However, although the total number of patients involved in the studies is increasing, there is a huge variation in terms of population, outcome, and methods of assessing MR-proADM (Table 1). The prominent discrepancies that had already emerged in studies on proADM in patients with sepsis and septic shock [36] were further enhanced in the pandemic setting.
The reviewed studies focused on two different populations, represented by ED and ICU patients. Among them, different outcomes were considered, sometimes compromising inter-study comparability (i.e., the use of RRT [16], superinfections [15], children versus adult population [17]).
Another source of dissimilarity is the timing of biomarker testing. Most of the studies evaluated the baseline value of MR-proADM at the patient admission, with a single determination (Table 1). Among the articles considered, only six considered more than a single measurement, but with different intervals (i.e., 3 repeated measurements, daily measurements, etc.) [18,21,23,28,33,34]. However, the role of trend analysis of biomarker values over time is recently emerging in the COVID-19 [28] population, but also in sepsis and septic shock [37].
A clear heterogeneity is also reported on cut-off adopted by different authors, as it was in the more studied context of pneumonia, sepsis, and septic shock [3,6,7], where it seems reasonable to consider a difference within settings (ICU, ED, general wards) and the relative expected severity of patients. As the literature is not consistent in establishing a precise cut-off for increase mortality/severity risk, some authors refer to a value derived from their internal cohort, while others relate to literature-reported previous values.
Considering that establishing a cut-off is one of the most important clinical goals, particularly in the context of a pandemic where a reduction in available resources has been experienced, we propose the use of a meta-analytic approach to determine a clinical severity cut-off derived from available studies on MR-proADM in ICU admitted critically ill COVID-19 patients, excluding all studies involving a mixed population. Our aim was to achieve a possible threshold value for evaluation and access to the critical care area, based on defined endothelial damage and related likely organ failure. Considering cut-off values identified from the available scientific literature (Table 1), MR-proADM cut-off values proposed by Elke et al. among patients with severe sepsis or septic shock (namely 2.75 for low-severity patients and 10.9 nmol/for high-severity patients at baseline) [3] might not represent a useful reference for studies still in progress and/or about to be published. However, those values appear quite in line with the previous cut-offs proposed for respiratory infections, while it appears lower than those identified in sepsis or septic shock [22].
We suggest a cut-off evaluating the values expressed in Table 1 for the ICU population and considering the mean difference of the mean MR-proADM values in the two high- and low-risk populations. It might be emphasized that this meta-analysis cannot be used to propose a MR-pro-ADM cut-off value for disease severity, as this would require an individual-patient meta-analysis followed by ROC curve construction and identification of the pro-ADM value corresponding to the best combination of sensitivity and specificity.
Furthermore, it is essential to note the significant difference between the values proposed in the meta-analysis concerning the ones expressed by Elke et al. (namely 0.96 in our meta-analysis vs. 2.5 in patients with sepsis and 10.9 in patients with septic shock in the manuscript by Elke et al. [3]). The reason for this discrepancy is currently not fully known. Although previous experience on the MR-proADM biomarker is related to sepsis and septic shock, the difference in the cut-offs underlines different physiopathological mechanisms. In septic shock, very high values refer to situations in which significant tissue hypoperfusion is present, with consequent organ failure. Otherwise, in the respiratory failure related to severe COVID-19 disease, the endothelial damage is likely to have a different origin, reflecting the need for specific cut-off values.
As discussed above, the overall number of articles on the subject is still limited. Furthermore, the studies considered show clinical heterogeneity concerning the type of population (ED versus ICU) and its severity, the outcomes, the timing of MR-proADM value(s), the cut-off considered, and the possible role of different confounders.
5. Conclusions
Despite the lack of randomized clinical trials and the clinical and methodological reported issues, an increased interest in the use of MRpro-ADM and its physiopathology implications in COVID-19 critically ill patients is emerging. In Europe, the current experience on the use of pro-ADM seems to highlight its validity in the early stages of hospitalization as a prognostic biomarker. High values have been found consistently in patients with more severe disease, both in ICUs and EDs, and correlated with statistical significance with patient mortality. Our meta-analysis confirms a significant difference in MR-proADM values at ICU admission between surviving and non-surviving patients.
Current evidence encourages further prospective and adequate studies on this promising predictive biomarker in the COVID-19 population, providing more specific guidance on its use and specific cut-offs. Other areas to be investigated in the next future are possibly confounding factors and the role of the biomarker trend during the time.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11154543/s1, Table S1: Adrenomedullin’s definition and characteristics [38,39].
Author Contributions
Conceptualization, G.M. (Giorgia Montrucchioand), E.B. and L.B.; methodology, G.M. (Giorgia Montrucchioand) and E.B.; software, E.B.; validation, G.M. (Giorgia Montrucchioand) and L.B.; formal analysis, G.M. (Giorgia Montrucchioand), E.B., D.L., A.G., A.V. and G.D.; investigation, G.M. (Giorgia Montrucchioand), E.B., D.L., A.G., A.V. and G.D.; data curation, G.M. (Giorgia Montrucchioand), E.B., D.L., A.G., A.V. and G.D.; writing—original draft preparation, G.M. (Giorgia Montrucchioand) and E.B.; writing—review and editing, F.R., G.M. (Giulio Mengozzi) and L.B.; supervision, F.R. and G.M. (Giulio Mengozzi). All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Patient consent was waived as this is a systematic review and meta-analysis, which therefore uses data already extracted from other studies. All studies considered collected informed consent from their patients.
Data Availability Statement
The datasets used and analyzed during the current meta-analysis are available from the corresponding author upon reasonable request.
Conflicts of Interest
All the authors declare that they have no conflict of interest in the field of the present article.
Funding Statement
Funds required are provided by the Department of Surgical Sciences, University of Turin, 10126 Turin, Italy.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saeed K., Legramante J.M., Angeletti S., Curcio F., Miguens I., Poole S., Tascini C., Sozio E., Del Castillo J.G. Mid-regional pro-adrenomedullin as a supplementary tool to clinical parameters in cases of suspicion of infection in the emergency department. Expert Rev. Mol. Diagn. 2021;21:397–404. doi: 10.1080/14737159.2021.1902312. [DOI] [PubMed] [Google Scholar]
- 2.Saeed K., Wilson D.C., Bloos F., Schuetz P., van der Does Y., Melander O., Hausfater P., Legramante J.M., Claessens Y.-E., Amin D., et al. The early identification of disease progression in patients with suspected infection presenting to the emergency department: A multi-centre derivation and validation study. Crit. Care. 2019;23:40. doi: 10.1186/s13054-019-2329-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elke G., Bloos F., Wilson D.C., Brunkhorst F.M., Briegel J., Reinhart K., Loeffler M., Kluge S., Nierhaus A., Jaschinski U., et al. The use of mid-regional proadrenomedullin to identify disease severity and treatment response to sepsis-a secondary analysis of a large randomised controlled trial. Crit. Care. 2018;22:79. doi: 10.1186/s13054-018-2001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson D.C., Schefold J.C., Baldirà J., Spinetti T., Saeed K., Elke G. Adrenomedullin in COVID-19 induced endotheliitis. Crit. Care. 2020;24:411. doi: 10.1186/s13054-020-03151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renaud B., Schuetz P., Claessens Y.E., Labarère J., Albrich W., Mueller B. Proadrenomedullin improves Risk of Early Admission to ICU score for predicting early severe community-acquired pneumonia. Chest. 2012;142:1447–1454. doi: 10.1378/chest.11-2574. [DOI] [PubMed] [Google Scholar]
- 7.Van Paassen J., Van Dissel J.T., Hiemstra P.S., Zwaginga J.J., Cobbaert C.M., Juffermans N.P., De Wilde R.B., Stijnen T., De Jonge E., Klautz R.J., et al. Perioperative proADM-change is associated with the development of acute respiratory distress syndrome in critically ill cardiac surgery patients: A prospective cohort study. Biomark. Med. 2019;13:1081–1091. doi: 10.2217/bmm-2019-0028. [DOI] [PubMed] [Google Scholar]
- 8.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 10.Luo D., Wan X., Liu J., Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018;27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 11.Revman Cp. Nordic Cochrane Centre, The Cochrane Collaboration . Review Manager 5 (RevMan 5) Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen, Denmark: 2020. Version 5.4. [Google Scholar]
- 12.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; Hoboken, NJ, USA: 2019. [Google Scholar]
- 13.Lippi G., Henry B.M. Pooled analysis of mid-regional pro-adrenomedullin values in COVID-19 patients with critical illness. Intern. Emerg. Med. 2021;16:1723–1725. doi: 10.1007/s11739-021-02756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oblitas C.-M., Galeano-Valle F., Ramírez-Navarro J., López-Cano J., Monterrubio-Manrique Á., García-Gámiz M., Sancho-González M., Arenal-López S., Walther L.-A.-S., Demelo-Rodríguez P. Mid-Regional Pro-Adrenomedullin, Methemoglobin and Carboxyhemoglobin as Prognosis Biomarkers in Critically Ill Patients with COVID-19: An Observational Prospective Study. Viruses. 2021;13:2445. doi: 10.3390/v13122445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malinina D.A., Shlyk I.V., Polushin Y.S., Afanasiev A.A., Stanevich O.V., Bakin E.A. The informative value of proadrenomedullin in patients with severe COVID-19. Messenger Anesthesiol. Resusc. 2020;17:31–38. doi: 10.21292/2078-5658-2020-17-6-31-38. [DOI] [Google Scholar]
- 16.Roedl K., Jarczak D., Fischer M., Haddad M., Boenisch O., de Heer G., Burdelski C., Frings D., Sensen B., Karakas M., et al. MR-proAdrenomedullin as a predictor of renal replacement therapy in a cohort of critically ill patients with COVID-19. Biomarkers. 2021;26:417–424. doi: 10.1080/1354750X.2021.1905067. [DOI] [PubMed] [Google Scholar]
- 17.Girona-Alarcon M., Bobillo-Perez S., Sole-Ribalta A., Hernandez L., Guitart C., Suarez R., Balaguer M., Cambra F.J., Jordan I. The different manifestations of COVID-19 in adults and children: A cohort study in an intensive care unit. BMC Infect. Dis. 2021;21:87. doi: 10.1186/s12879-021-05786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon T.P., Stoppe C., Breuer T., Stiehler L., Dreher M., Kersten A., Kluge S., Karakas M., Zechendorf E., Marx G., et al. Prognostic Value of Bioactive Adrenomedullin in Critically Ill Patients with COVID-19 in Germany: An Observational Cohort Study. J. Clin. Med. 2021;10:1667. doi: 10.3390/jcm10081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oblitas C.M., Galeano-Valle F., Lopez-Cano J., Monterrubio-Manrique A., Garcia-Gamiz M., Ramirez-Navarro J., Sancho-Gonzalez M., Arenal-Lopez S., Alvarez-Sala Walther L., Demelo-Rodriguez P. Potential prognostic biomarkers in COVID19: Role of mid-regional pro-adrenomedullin, methemoglobin and carboxyhemoglobin. Intensive Care Med. Exp. 2021;9:50. doi: 10.1186/s40635-021-00415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lhote S., Van Grunderbeeck N., Colling D., Verchain S., Varillon C., Floch P., Vinsonneau C., Caulier T., Granier M., Mallat J. Proadrenomedullin assessment of multi-organ failure in COVID-19 sepsis (PAMOCOS): A prospective, multicentric observational study. Crit. Care. 2021;25:383. doi: 10.1186/s13054-021-03769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benedetti I., Spinelli D., Callegari T., Bonometti R., Molinaro E., Novara E., Cassinari M., Frino C., Guaschino R., Boverio R., et al. High levels of mid-regional proadrenomedullin in ARDS COVID-19 patients: The experience of a single, italian center. Eur. Rev. Med. Pharmacol. Sci. 2021;25:1743–1751. doi: 10.26355/eurrev_202102_24885. [DOI] [PubMed] [Google Scholar]
- 22.García de Guadiana-Romualdo L., Martínez Martínez M., Rodríguez Mulero M.D., Esteban-Torrella P., Hernández Olivo M., Alcaraz García M.J., Campos-Rodríguez V., Sancho-Rodríguez N., Galindo Martínez M., Alcaraz A., et al. Circulating MR-proADM levels, as an indicator of endothelial dysfunction, for early risk stratification of mid-term mortality in COVID-19 patients. Int. J. Infect. Dis. 2021;111:211–218. doi: 10.1016/j.ijid.2021.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregoriano C., Koch D., Kutz A., Haubitz S., Conen A., Bernasconi L., Hammerer-Lercher A., Saeed K., Mueller B., Schuetz P. The vasoactive peptide MR-pro-adrenomedullin in COVID-19 patients: An observational study. Clin. Chem. Lab. Med. 2021;59:995–1004. doi: 10.1515/cclm-2020-1295. [DOI] [PubMed] [Google Scholar]
- 24.Indirli R., Bandera A., Valenti L., Ceriotti F., Di Modugno A., Tettamanti M., Gualtierotti R., Peyvandi F., Montano N., Blasi F., et al. Prognostic value of copeptin and mid-regional proadrenomedullin in COVID-19-hospitalized patients. Eur. J. Clin. Investig. 2022;52:e13753. doi: 10.1111/eci.13753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo Sasso B., Gambino C.M., Scichilone N., Giglio R.V., Bivona G., Scazzone C., Muratore R., Milano S., Barbagallo M., Agnello L., et al. Clinical Utility of Midregional Proadrenomedullin in Patients with COVID-19. Lab. Med. 2021;52:493–498. doi: 10.1093/labmed/lmab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Méndez R., González-Jiménez P., Latorre A., Piqueras M., Bouzas L., Yépez K., Ferrando A., Zaldívar-Olmeda E., Moscardó A., Alonso R., et al. Acute and sustained increase in endothelial biomarkers in COVID-19. Thorax. 2022;77:400–403. doi: 10.1136/thoraxjnl-2020-216797. [DOI] [PubMed] [Google Scholar]
- 27.Minieri M., Di Lecce V.N., Lia M.S., Maurici M., Bernardini S., Legramante J.M. Role of MR-proADM in the risk stratification of COVID-19 patients assessed at the triage of the Emergency Department. Crit. Care. 2021;25:407. doi: 10.1186/s13054-021-03834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montrucchio G., Sales G., Rumbolo F., Palmesino F., Fanelli V., Urbino R., Filippini C., Mengozzi G., Brazzi L. Effectiveness of mid-regional pro-adrenomedullin (MR-proADM) as prognostic marker in COVID-19 critically ill patients: An observational prospective study. PLoS ONE. 2021;16:e0246771. doi: 10.1371/journal.pone.0246771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore N., Williams R., Mori M., Bertolusso B., Vernet G., Lynch J., Philipson P., Ledgerwood T., Kidd S.P., Thomas C., et al. Mid-regional proadrenomedullin (MR-proADM), C-reactive protein (CRP) and other biomarkers in the early identification of disease progression in patients with COVID-19 in the acute NHS setting. J. Clin. Pathol. 2022. in press . [DOI] [PubMed]
- 30.Popov D.A., Borovkova U.L., Rybka M.M., Ramnenok T.V., Golukhova E.Z. Predictive value of proadrenomedullin in patients with COVID-19. Russ. J. Anesthesiol. Reanimatol. 2020;6:6–12. doi: 10.17116/anaesthesiology20200626. [DOI] [Google Scholar]
- 31.Sozio E., Tascini C., Fabris M., D’Aurizio F., De Carlo C., Graziano E., Bassi F., Sbrana F., Ripoli A., Pagotto A., et al. MR-proADM as prognostic factor of outcome in COVID-19 patients. Sci. Rep. 2021;11:5121. doi: 10.1038/s41598-021-84478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spoto S., Agrò F.E., Sambuco F., Travaglino F., Valeriani E., Fogolari M., Mangiacapra F., Costantino S., Ciccozzi M., Angeletti S. High value of mid-regional proadrenomedullin in COVID-19: A marker of widespread endothelial damage, disease severity, and mortality. J. Med. Virol. 2021;93:2820–2827. doi: 10.1002/jmv.26676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Oers J.A.H., Kluiters Y., Bons J.A.P., de Jongh M., Pouwels S., Ramnarain D., de Lange D.W., de Grooth H.J., Girbes A.R.J. Endothelium-associated biomarkers mid-regional proadrenomedullin and C-terminal proendothelin-1 have good ability to predict 28-day mortality in critically ill patients with SARS-CoV-2 pneumonia: A prospective cohort study. J. Crit. Care. 2021;66:173–180. doi: 10.1016/j.jcrc.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaninotto M., Maria Mion M., Marchioro L., Padoan A., Plebani M. Endothelial dysfunction and Mid-Regional proAdrenomedullin: What role in SARS-CoV-2 infected Patients? Clin. Chim. Acta. 2021;523:185–190. doi: 10.1016/j.cca.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agnello L., Bellia C., Iacolino G., Gambino C.M., Petrancosta R., Lo Sasso B., Ciaccio M. Mid-regional pro-adrenomedullin predicts poor outcome in non-selected patients admitted to intensive care unit. Biochim. Clin. 2018;42:S171. doi: 10.1515/cclm-2018-0645. [DOI] [PubMed] [Google Scholar]
- 36.Piccioni A., Saviano A., Cicchinelli S., Valletta F., Santoro M.C., de Cunzo T., Zanza C., Longhitano Y., Tullo G., Tilli P., et al. Proadrenomedullin in Sepsis and Septic Shock: A Role in the Emergency Department. Med. Kaunas. 2021;57:920. doi: 10.3390/medicina57090920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bima P., Montrucchio G., Caramello V., Rumbolo F., Dutto S., Boasso S., Ferraro A., Brazzi L., Lupia E., Boccuzzi A., et al. Prognostic Value of Mid-Regional Proadrenomedullin Sampled at Presentation and after 72 Hours in Septic Patients Presenting to the Emergency Department: An Observational Two-Center Study. Biomedicines. 2022;10:719. doi: 10.3390/biomedicines10030719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marino R., Struck J., Maisel A.S., Magrini L., Bergmann A., Di Somma S. Plasma adrenomedullin is associated with short-term mortality and vasopressor requirement in patients admitted with sepsis. Crit. Care. 2014;18:R34. doi: 10.1186/cc13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laterre P.F., Pickkers P., Marx G., Wittebole X., Meziani F., Dugernier T., Huberlant V., Schuerholz T., François B., Lascarrou J.B., et al. Safety and tolerability of non-neutralizing adrenomedullin antibody adrecizumab (HAM8101) in septic shock patients: The AdrenOSS-2 phase 2a biomarker-guided trial. Intensive Care Med. 2021;47:1284–1294. doi: 10.1007/s00134-021-06537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current meta-analysis are available from the corresponding author upon reasonable request.