Abstract
Ustekinumab has shown efficacy in Crohn’s Disease (CD) patients. To identify patient profiles of those who benefit the most from this treatment would help to position this drug in the therapeutic paradigm of CD and generate hypotheses for future trials. The objective of this analysis was to determine whether baseline patient characteristics are predictive of remission and the drug durability of ustekinumab, and whether its positioning with respect to prior use of biologics has a significant effect after correcting for disease severity and phenotype at baseline using interpretable machine learning. Patients’ data from SUSTAIN, a retrospective multicenter single-arm cohort study, were used. Disease phenotype, baseline laboratory data, and prior treatment characteristics were documented. Clinical remission was defined as the Harvey Bradshaw Index ≤ 4 and was tracked longitudinally. Drug durability was defined as the time until a patient discontinued treatment. A total of 439 participants from 60 centers were included and a total of 20 baseline covariates considered. Less exposure to previous biologics had a positive effect on remission, even after controlling for baseline disease severity using a non-linear, additive, multivariable model. Additionally, age, body mass index, and fecal calprotectin at baseline were found to be statistically significant as independent negative risk factors for both remission and drug survival, with further risk factors identified for remission.
Keywords: Crohn’s Disease, ustekinumab, predictive factors
1. Introduction
Crohn’s Disease (CD) is a chronic inflammatory bowel disease that can result in ulceration, thickening of the intestinal wall, and a broad array of symptoms and complications. The immune mechanisms underlying CD are becoming better understood, and therefore more therapeutic options have appeared in recent years, including use of immunomodulators and biologic agents. Ustekinumab is a drug treatment in the latter category, targeting interleukin (IL)-12 and IL-23, that is indicated for the treatment of adult patients with moderately to severely active CD who have had an inadequate response with, lost response to, or were intolerant to either conventional therapy or a TNFα antagonist or have medical contraindications to such therapies [1]. The positioning of UST in particular versus anti-integrin medications is a topic of active discussion in the clinical literature [2,3].
The use of biomarkers, clinical data, and prediction models holds promise of finding the best drug for the right patient at the right time. Although clinical trials offer the gold standard by way of direct comparison between alternative treatments, they can also have certain limitations based on their generalizability (restrictive eligibility criteria, strict designs, and commonly binary endpoints). On the contrary, real-world studies and pragmatic observational trials can better reflect clinical practice and represent a greater diversity of patients, including ethnic background, biologic constituency, geographic representation, with sometimes longer follow ups and bigger cohort sizes, and hence are an important supplementary source of information to clinical trials. Real-world data from electronic medical records and claims data often come with very large sample sizes but are also constrained by data quality issues. In contrast, observational studies such as SUSTAIN [4] benefit from high-quality data capture and a pragmatic view of clinical practice. The objective of the current study was to determine whether baseline patient characteristics, including prior treatment history, are predictive of remission and drug durability after controlling for disease severity at baseline.
2. Materials and Methods
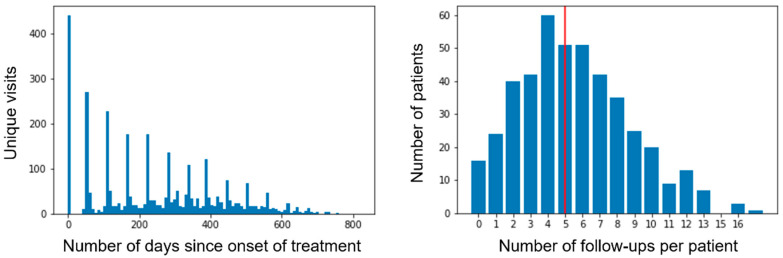
The SUSTAIN study [4] is a retrospective multicenter study comprising 463 patients on ustekinumab (UST) with active CD (Harvey-Bradshaw Index (HBI) > 4). The HBI index was recorded by gastroenterologists in the follow-up of patients. HBI measurement was not available for 24 patients, who were excluded from the analysis, leaving a cohort size of 439 from 61 centers. Patients were evaluated at baseline and then during the follow-up for an average of 5.5 visits (median 5), and an average frequency of every 8 weeks, generating a total of 2876 visits in total where HBI was recorded (Figure 1). This creates an opportunity for longitudinal analysis as well as drug durability, which are the focus of this study.
Figure 1.
Number of unique visits over time (left) and number of follow-up visits per patient; red line indicates the mean number of visits (right).
The patient cohort exhibited a median HBI of 8.0 at baseline, with just under 50% with mild disease (5 ≤ HBI < 7), 45% with moderate disease (7 ≤ HBI ≤ 16), and 5% with severe disease (HBI > 16). The cohort was refractory, defined as having failed to respond to or remain on at least one other treatment line in the past, with 96.1% of the cohort being anti-TNF experienced and 23.7% also anti-integrin experienced prior to the onset of treatment with UST. A total of 60% had had at least one surgery; at baseline, 28% were treated with concomitant steroids and 30% with immunosuppressants (65% azathioprine, 28.8% methotrexate, and 8% Mercaptopurine).
The analysis was primarily focused on remission as a dichotomous endpoint, achieving or not remission, defined as a HBI value of ≤ 4. Importantly, the analysis was longitudinal and considered all available follow-ups per patient, rather than focusing on a specific cross-section, with temporal dependence handled by adding “days since onset of treatment” as an independent variable. Drop-out bias was handled using the last-value-carried-forward (LVCF) imputation as detailed in Appendix A.
As a secondary endpoint, drug durability was considered, defined as the time to drop-out, which is right-censored by the last follow-up. In addition to its clinical significance, drug durability served to reinforce findings that replicate across both analyses, given its lack of sensitivity to drop-out bias.
A complete table of all features used can be found in Table 1. These included 20 baseline features, time as a longitudinal covariate, and the number of concomitant steroids courses administered during the treatment window. Both models (remission and durability) were able to handle both discrete and numeric variables. For interpretability reasons, all numeric variables, except days since the start of UST treatment, were discretized using a model-driven technique that automatically determines the cut-offs that are optimal for the given multivariable analysis (see Appendix A). For example, the model-driven lower cut-off for the Body-Mass-Index (BMI) is 27, rather than the clinical threshold of 17. The groups employed for discretization are shown in the fourth column of Table 1. Following discretization, a separate model of remission was built on discretized variables only. In particular, the time elapsed between diagnosis and start of treatment, the age at the time of consent, BMI, and laboratory tests (baseline albumin, baseline fecal calprotectin, baseline haemoglobin, and baseline CRP), the number of surgeries prior to onset of treatment, the number of comorbidities, and the number of anti-TNF episodes, and similarly with the number of anti-integrin episodes were used as raw numeric values initially and discretized for interpretability. Treatment episodes refer to the number of times a patient has been treated with the respective drug class. So, for example, a patient with 2 treatment episodes on anti-integrin would have been started and stopped anti-integrin treatment twice, but in both cases, using vedolizumab, which is the only anti-integrin used in this cohort. Alternative codifications of exposure to anti-TNF or anti-integrin were tried out, including total duration of treatment (number of days on drug) and exposure (yes/no). All had a similar effect directionally on the model, but number of treatment episodes offered the best fit overall.
Table 1.
Variables employed in their original form and discretized.
| Variable | Original (Non-Discretized) |
Post-Processed (Discretized) |
Description of Discretized Version |
Descriptives |
|---|---|---|---|---|
| Days since the start of UST treatment (longitudinal, not baseline) | Numeric | Numeric | - | |
| HBI at baseline, categorized | Ordinal (3) | - | [5–7] (mild), [8–16] (moderate), >16 (severe) | Distribution: Mild 50%, Moderate 45%, Severe 5% % missing values: 0% |
| Years between diagnosis and start of UST | Numeric | Dichotomous | ≤18, >18 | Median: 10.0 % missing values: 0% |
| Number of surgeries before UST treatment onset | Numeric | Ordinal (3) | 0, 1–2, ≥3 | Distribution: 0 (47%), 1–2 (43%), ≥3 (10%) |
| Number of anti-TNF episodes | Numeric | Dichotomous | ≤1, >1 | Median: 2.0 96.1% of patients ≥1 anti-TNF |
| Number of anti-integrin episodes | Numeric | Dichotomous | 0, 1 | Median: 0.0 23.7% of patients ≥1 anti-integrin |
| Age at the time of signing consent | Numeric | Dichotomous | ≤40, >40 | Median: 46.0 % missing values: 0% |
| BMI at baseline | Numeric | Ordinal (3) | <27, 27–38, >38 | Median: 23.81 % missing values: 16% |
| Sex | Dichotomous | Dichotomous | Female, Male | Distribution: Female 49%, Male 51% % missing values: 0% |
| Number of comorbidities | Numeric | Ordinal (4) | 0–1, 2–3, ≥4 | Median: 1.0 |
| Perianal disease | Categorical | Dichotomous | Never/Previous, Current | Distribution: Never/Previous 86%, Current 14% % missing values: 0% |
| CD location | Categorical | Dichotomous | Ileocolic/Ileum (L1/L3), Colon (L2) | Distribution: Ileocolic/Ileum 88%, Colon 12% % missing values: 0% |
| Patient ever had EIMs | Dichotomous | Dichotomous | Yes/No | Distribution: No 59%, Yes 41% |
| Family history of CD | Dichotomous | Dichotomous | Yes/No | Distribution: No 13%, Yes 87% % missing values: 16% |
| Upper gastrointenstinal tract (L4) involved | Dichotomous | Dichotomous | Yes/No | Distribution: No 92%, Yes 8% % missing values: 0% |
| Baseline albumin (g/L) | Numeric | Dichotomous | ≤3.8, >3.8 | Median: 4.0 % missing values: 33% |
| Baseline faecal calprotectin (mcg/g) | Numeric | Ordinal (3) | ≤80, 81–650, >650 | Median: 667.33 % missing values: 52% |
| Baseline haemoglobin (g/L) | Numeric | Dichotomous | ≤10.9, >10.9 | Median: 13.2 % missing values: 10% |
| Baseline CRP (mg/L) | Numeric | Ordinal (3) | ≤0.5, 0.6–6.1, >6.1 | Median: 7.2 % missing values: 10% |
| Steroid use at first UST dose | Dichotomous | Dichotomous | Yes/No | Distribution: No 72%, Yes 28% |
| Under Immunosuppressants at first UST dose | Dichotomous | Dichotomous | Yes/No | Distribution: No 70%, Yes 30% |
| Number of concomitant steroid courses | Numeric | Dichotomous | Yes/No | Median: 0.0 |
BMI, Body Mass Index; CD, Crohn’s Disease; CRP, C-reactive protein; EIM, extraintestinal manifestation; HBI, Harvey Bradshaw Index; TNF, Tumor Necrosis Factor; UST, ustekinumab.
Baseline measurements were used for two reasons: to control for disease severity at the onset of treatment, but also as predictors of remission given information that is available at a clinically actionable point and could in the future inform a decision to treat by the clinician.
HBI at baseline was the only numeric variable that was discretized pre-modelling into mild, moderate, and severe disease using the clinical definition described earlier, to facilitate investigation of its interactions with other variables. In particular, a large percentage of patients were under either steroids or immunosuppressants (or both) at the study onset, which might have masked what would have otherwise been a higher HBI value. These variables were considered as having interaction effects with baseline HBI.
BMI at baseline was estimated using height and weight measurements where possible and inferred using regression imputation otherwise. Sex was also considered. Given the wide range of different comorbidities, these were not considered individually but rather a single variable was introduced counting the number of comorbidities present.
Disease type was described by the following variables: whether the patient had perianal disease, CD location according to the Montreal classification, ileal (L1), colonic (L2), or ileocolonic (L3), whether the upper gastrointestinal tract (L4) was involved, and whether there was ever an extraintestinal manifestation. CD location was collapsed into a dichotomous variable capturing whether the ileum was involved (Yes/No), as it was observed that there was no statistically significant difference between the effect of ileocolonic (L3) vs. ileal only (L1). Additionally, a family history of CD was also encoded as a dichotomous variable.
The patient journey was a semi-structured data source that can be summarized in a tabular form in a variety of ways. This analysis considered the number of surgeries before UST treatment onset, discretized into groups for 0, 1–2, and 3+, and treated like an ordinal factor. Previous biologic use was a key object of interest for the study. It was encoded as the number of anti-TNF episodes and, separately, the number anti-integrin episodes. Sensitivity analyses were conducted with alternative encodings including the cumulative length of treatment episodes measured in days, or measured as a proportion of time since diagnosis, and maintained a similar direction and significance levels.
The number of concomitant steroid courses that were given concomitantly with UST treatment was also considered in the model as a control, to improve the generalizability of the findings and attempt to extrapolate on the possibility of steroid-free remission.
Three separate analyses were run, namely, longitudinal remission non-discretized, longitudinal remission discretized, and drug durability. The primary analysis was a longitudinal remission analysis that measured the effect of different covariates on the probability of remission at different times since onset of treatment. This model was fitted on continuous variables first and then on discretized versions for interpretability reasons. The secondary analysis was a drug durability analysis focused on the time each patient stayed on ustekinumab. In the longitudinal analysis, every patient follow-up constituted a single observation, and the set of covariates included the time since onset of treatment, allowing for follow-ups made at different times to become comparable by controlling for the effect of time, while at the same time extracting an explicit representation of a non-linear trajectory of the endpoint over the duration of the treatment. Such use of time as a predictor notably increases the effective sample size of the analysis. Baseline covariates featured identical values for all observations coming from the same patient. LVCF techniques were used to correct for possible drop-out bias (see Appendix A). Given a dichotomous dependent variable, we were able to assess goodness-of-fit by evaluating R2 (proportion of variance explained) as well as a standard metric of classification accuracy, Area Under the Curve (AUC), using 10-fold cross validation, treating the remission model as a classifier. Drug durability featured instead one observation per patient and hence had less power than the longitudinal analysis but benefitted from a lack of sensitivity to drop-out bias.
All three analyses relied on the Generalized Additive Model (GAM) framework, introduced [5] as an extension of linear and logistic regression that allows for data-driven discovery of non-linear effects between each predictor and the remission flag using splines, while preserving its interpretable properties such as p-values and confidence intervals on additive effects. Survival analysis is a recent use case for GAMs proposed in [6] and has certain advantages over classical techniques such as Cox regression and Kaplan–Meier estimates, such as the possibility to describe the hazard function while at the same time consider non-linear effects of additional covariates via the use of splines. A key benefit of using the GAM modeling framework for both longitudinal [7] and durability analyses is that it allows for direct comparison of the effects of different covariates across the models.
GAMs produce statistically accurate p-values if the number of spline knots (which controls the flexibility of each non-linear effect) is set in advance to a fixed value (in our case, this was 8 for main effects and 4 for interaction terms). Additionally, effect sizes can be read off a partial dependence plot which, for the longitudinal remission model, captures the effect on the log-odds of remission (on the y-axis) for changes in the underlying independent variable (x-axis). These plots and respective confidence bands were also used to guide variable discretization. Statistical analysis was performed in Python 3.0 using the PyGAM package (0.8.0) and Matplotlib 3.3.3 for plotting purposes.
3. Results
3.1. Performance of Models
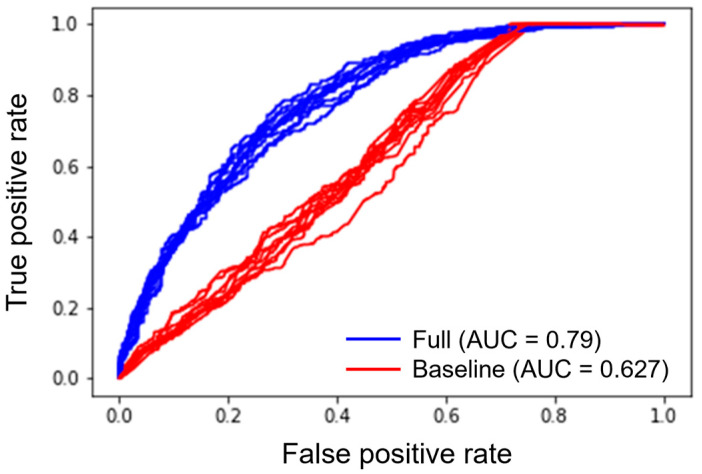
Performance of the longitudinal remission model in terms of cross-validated R2 was modest, around 0.25, though, as discussed, a more reliable metric of predictive performance in this case is AUC, where the model performed well with a value of 0.796 (0.78, 0.8) over 10 folds of cross-validation, compared to that of a baseline model which only considered time as a predictor variable, whose median AUC was 0.62 (0.61–0.64) (Figure 2). The performance is comparable with similar analyses such as [8], though the latter additionally used laboratory values shortly after onset of treatment, rather than only at baseline as conducted here.
Figure 2.
Ten iterations of a training-test split following which the longitudinal remission model is used to classify whether a patient will be in remission at a given week that was not observed in the training data. The full model makes use of all 22 variables (including non-significant ones), whereas the baseline model only uses time.
Given that information about the number of concomitant steroid courses is not available at the time of assessing a patient’s risk of not achieving remission, we reran the analysis without this variable in order to assess the sensitivity of the model to this variable, and the AUC dropped by less than 1%, from a median of 0.796 to one of 0.789.
3.2. Significant Variables
The longitudinal remission model considered 22 variables out of which 20 were baseline features and 2 were not (days since start of UST treatment and number of concomitant steroid courses). Out of the baseline patient characteristics, 16 proved significant in the non-discretized longitudinal remission model (Table 2). The discretized longitudinal remission model has by construction lower power and was fitted as an interpretability aid; it was performed only on those features that were already found significant from the non-discretized version of the primary analysis model. Most variables preserved their significance, but some lost it, reflecting the importance of discretizing numeric variables such as laboratory measurements. Reassuringly, the shapes, directions, and magnitudes of all reported effect sizes were preserved when discretizing, with the exception of baseline albumin and baseline hemoglobin which in the non-discretized version seem to have non-linearities not sufficiently captured by the discretized versions, which likely is the reason why these features ceased to be significant in the discretized model. Indeed, several variables lose significance because of discretization, which is expected as discretization loses both information and power.
Table 2.
Statistical significance (p-value) of the effect of each variable in longitudinal remission and durability.
| Longitudinal Remission | Durability | ||
|---|---|---|---|
| Feature | Non-Discretized | Discretized * | |
| Days since start of UST treatment | <0.05 | <0.05 | <0.05 |
| HBI at the first UST dose, categorized | <0.05 | <0.05 | 0.083 |
| Years between diagnosis and start of UST | <0.05 | 0.547 | 0.077 |
| Number of surgeries before UST treatment onset | <0.05 | <0.05 | 0.24 |
| Number of anti-TNF episodes | <0.05 | 0.09 | <0.05 |
| Number of anti-integrin episodes | <0.05 | <0.05 | <0.05 |
| Age at the time of signing consent | <0.05 | <0.05 | <0.05 |
| BMI at baseline | <0.05 | <0.05 | <0.05 |
| Sex | 0.888 | N/A | 0.842 |
| Number of comorbidities | <0.05 | <0.05 | 0.296 |
| Perianal disease | <0.05 | <0.05 | 0.541 |
| Location of CD | <0.05 | <0.05 | 0.137 |
| Patient ever had EIMs | 0.806 | N/A | 0.648 |
| Family history of CD | 0.958 | N/A | 0.998 |
| Upper gastrointestinal tract (L4) involved | 0.999 | N/A | 0.828 |
| Baseline albumin | <0.05 | 0.431 | 0.357 |
| Baseline fecal calprotectin | <0.05 | 0.26 | <0.05 |
| Baseline hemoglobin | <0.05 | 0.77 | <0.05 |
| Baseline CRP | <0.05 | <0.05 | <0.05 |
| Under Steroids at first UST dose | <0.05 | <0.05 | 0.237 |
| Under Immunosuppressants at first UST dose | <0.05 | <0.05 | 0.813 |
| Number of concomitant Steroid courses | <0.05 | <0.05 | <0.05 |
Variables that were significant in longitudinal remission (non-discretized) and durability models are indicated in bold. * All features discretized, except for “days since start of UST treatment”. BMI, Body Mass Index; CD, Crohn’s Disease; CRP, C-reactive protein; EIM, extraintestinal manifestation; HBI, Harvey Bradshaw Index; TNF, Tumor Necrosis Factor; UST, ustekinumab.
A number of interaction terms were also included, as shown in Table A1. The drug durability model considered the same baseline variables and given its smaller effective sample size detected seven significant variables, all of which were reassuringly also significant in the remission model.
Variables that were statistically significant across both analyses (longitudinal remission and drug durability) are indicated in bold in Table 2, and comprise the number of concomitant steroid courses and the following baseline characteristics, number of anti-TNF episodes, number of anti-integrin episodes, BMI, age at the time of signing consent, baseline fecal calprotectin, baseline hemoglobin, and baseline CRP.
The estimated effect of each variable on longitudinal remission is summarized in Table 3. The direction of effect is given for statistically significant variables. The second column describes the directionality of the effect in the non-discretized model. In the third column, the exact effect is reported from the respective feature in the discretized version of the model, in a log-odds scale, as is typical in logistic regressions, including the non-linear version used here (see Appendix A). A positive estimated effect suggests that a higher value of this feature is a positive factor for remission, whereas a negative estimated effect suggests that a higher value correlates with less probability of remission. For factors with more than two values, we report the difference in log-odds of remission between patients in the group with the highest value for this particular feature versus those in the group with the lowest value (holding other things constant).
Table 3.
Estimated effects of each variable on longitudinal remission.
| Direction of Effect in Non-Discretized Longitudinal Remission Model | Exact Effect Size in Discretized Longitudinal Remission Model (Lowest vs. Highest) | Effect for Group with Lowest Value | Effect for Group with Highest Value | |
|---|---|---|---|---|
| HBI at the first UST dose, categorised | Negative | Negative: −2.32 | 1.09 (0.91, 1.27) | 0.14 (−0.04, 0.32) |
| Years between diagnosis and start of UST | Negative | Not significant, trending negative | 0.06 (−0.05, 0.17) | −0.06 (−0.17, 0.05) |
| Number of surgeries before UST treatment onset | Negative | −0.33 | 0.19 (0.06, 0.33) | −0.14 (−0.33, 0.05) |
| Number of anti-TNF episodes | Negative | Borderline insignificant, trending negative | 0.11 (−0.01, 0.24) | −0.11 (−0.24, 0.01) |
| Number of anti-integrin episodes | Negative | Negative: −1.96 | 0.7 (0.19, 1.22) | −1.26 (−2.27, −0.25) |
| Age at the time of signing consent | Negative | Negative: −0.31 | 0.16 (0.06, 0.26) | −0.16 (−0.26, −0.06) |
| BMI at baseline | Negative | Negative: −2 | 0.8 (0.52, 1.09) | −1.2 (−1.73, −0.68) |
| Sex | Insignificant | N/A | N/A | N/A |
| Number of comorbidities | Negative | Negative: −0.41 | 0.22 (0.06, 0.38) | −0.19 (−0.44, 0.07) |
| Perianal disease | Negative | Negative: −0.7 | 0.35 (0.21, 0.48) | −0.35 (−0.48, −0.21) |
| Location of CD (no ileal involvement) | Positive | Positive: 0.74 | −0.37 (−0.52, −0.23) | 0.37 (0.23, 0.52) |
| Patient ever had EIMs | Insignificant | N/A | N/A | N/A |
| Family history of CD | Insignificant | N/A | N/A | N/A |
| Upper gastrointenstinal tract (L4) involved | Insignificant | N/A | N/A | N/A |
| Baseline albumin | Negative | Negative: −0.13 | 0.07 (−0.03, 0.16) | −0.07 (−0.16, 0.03) |
| Baseline faecal calprotectin | Negative | Negative: −0.45 | 0.28 (0.02, 0.53) | −0.17 (−0.33, −0.0) |
| Baseline hemoglobin | Positive | Positive: 0.08 | −0.04 (−0.19, 0.11) | 0.04 (−0.11, 0.19) |
| Baseline CRP | Positive | Positive: 0.6 | −0.32 (−0.53, −0.11) | 0.28 (0.14, 0.43) |
| Under Steroids at first UST dose | Positive | Positive: 0.69 | −0.35 (−0.48, −0.22) | 0.35 (0.22, 0.48) |
| Under Immunosuppressants at first UST dose | Negative | Negative: −0.28 | 0.14 (0.04, 0.23) | −0.14 (−0.23, −0.04) |
| Number of concomitant Steroid courses | Negative | Negative: −0.83 | 0.42 (0.29, 0.54) | −0.42 (−0.54, −0.29) |
For factors with more than 2 values, the difference in log-odds of remission between patients in the group with the highest value vs. those with the lowest value are reported. BMI, Body Mass Index; CD, Crohn’s Disease; CRP, C-reactive protein; EIM, extraintestinal manifestation; HBI, Harvey Bradshaw Index; TNF, Tumor Necrosis Factor; UST, ustekinumab.
3.3. Position of Ustekinumab in the Treatment Journey
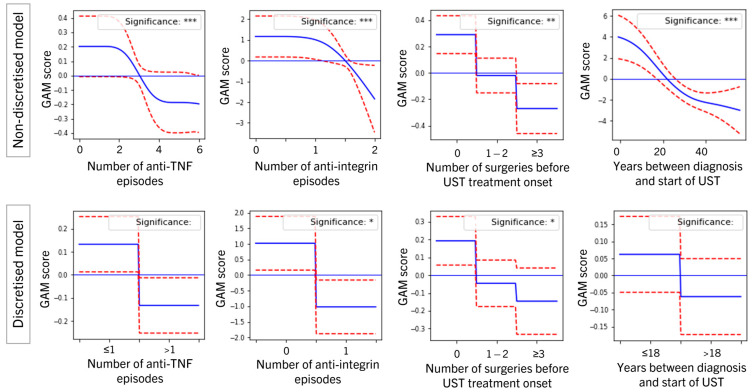
Previous exposure to biologics correlated with lower chances of remission and drug durability. The number of anti-TNF episodes and number of anti-integrin episodes were both independent risk factors for lower chances of remission and drug durability, with prior exposure to anti-integrin having a larger effect (Table 3 and Table A1). Although the number of anti-TNF episodes was a significant predictor in their continuous form for both remission and durability, the remission discretized version loses significance but maintains a downward trend (Figure 3). Exposure to anti-integrin remains significant in all models, with exposure having a negative effect on the log-odds of remission of up to −1.26 (−2.27, −0.25) compared with a positive effect of no exposure of 0.7 (0.19, 1.22). This ranks it among the variables with the biggest absolute effect in the remission model, with only BMI at baseline and baseline HBI ranking higher (see second column in Table 3). These effects persisted in significance and a trend under different ways of capturing exposure, such as replacing the number of episodes with total cumulative length of episodes and exposure (yes/no).
Figure 3.
Generalized additive model (GAM) plots showing estimated effects of selected significant variables in the longitudinal remission model, in the non-discretized model (top row) and discretized model (bottom row). Plots may be read by comparing one value on the x-axis to another and can be read off as the vertical distance between the respective y-values. Horizontal blue line denotates 0 (no effect), solid blue line represents the estimated effect of different values of the covariates and discontinuous red lines represent 95% confidence intervals. * p < 0.05, ** p < 0.01, *** p < 0.001.
Additionally, all other variables capturing the position of ustekinumab in the treatment journey (years between diagnosis and start of UST and number of surgeries before UST treatment onset) showed a similar correlation with increased odds of remission with earlier positioning (Figure 3 and Table 3).
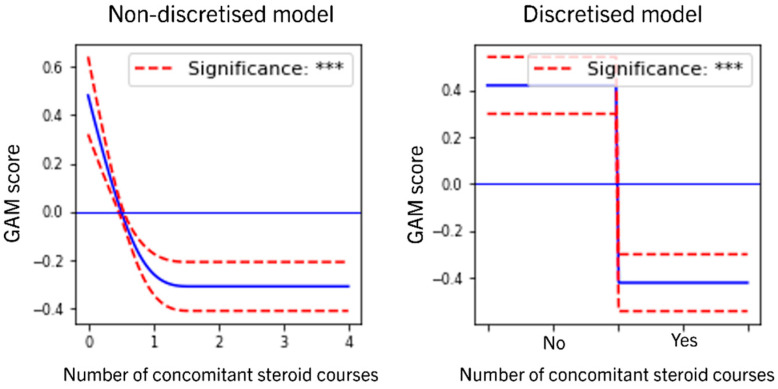
3.4. Role of Concomitant Steroids
The number of concomitant steroid courses was found to have a significantly negative effect on both remission and durability (Table 2 and Table 3); see the graph in Figure 4 for both longitudinal remission models. A positive association would have suggested that perhaps the observed real-world effect of remission under UST is at least in part due to concomitant steroids. An absent or negative association is consistent with the hypothesis that concomitant steroids are not improving the chances of remission but are instead used in a small subset of most refractory, severe patients who are not responding to treatment to temporarily/modestly alleviating symptoms. This, together with the fact that 57% of the cohort did not receive any concomitant steroids, could be seen as consistent with the hypothesis that a majority of patients experiences steroid-free remission.
Figure 4.
Visual summary of the effect of the number of concomitant steroid courses in the longitudinal remission model, non-discretized model (left) and discretized model (right). Horizontal blue line denotates 0 (no effect), solid blue line represents the estimated effect of different values of the covariates and discontinuous red lines represent 95% confidence intervals. *** p < 0.001.
4. Discussion
This analysis identified 12 baseline factors as negative predictors for achieving clinical remission over time and 4 positive predictors, even after controlling for possible confounders such as disease activity at onset and frailty as proxied by the HBI at baseline and the number of comorbidities, respectively. Baseline variables with a negative effect were HBI at the first UST dose, years between diagnosis and start of UST, number of surgeries before UST treatment onset number of anti-TNF episodes, number of anti-integrin episodes, age at the time of signing consent, BMI at baseline, number of comorbidities, perianal disease, baseline albumin, baseline fecal calprotectin, and being under Immunosuppressants at first UST dose. On the other hand, no ileal involvement, baseline hemoglobin, baseline CRP, and not being under steroids at first UST dose were associated with higher chances of achieving remission in the non-discretized model of longitudinal remission.
Seven variables were also found to be significant independent risk factors across both remission and durability, namely years between diagnosis and start of UST, BMI at baseline, baseline fecal calprotectin, baseline hemoglobin, and baseline CRP, as well as the number of anti-TNF episodes and number of anti-integrin episodes the patient was exposed to before the start of treatment, even after controlling for the HBI at baseline and number of comorbidities. Other patient journey characteristics such as the number of prior surgeries and the therapeutic latency, as well as disease characteristics such as the location of CD, were statistically significant in the remission model, but not in the durability model (it was expected that the latter would discover fewer findings due to it having less power).
In retrospective non-randomized data such as this cohort [4,9,10,11,12], prior treatment history correlates with disease severity and/or frailty which can therefore act as an unobserved confounder that would explain this finding. Nevertheless, disease severity at onset was controlled for using multivariable analysis involving disease characteristics, baseline HBI values, and laboratory tests, so the fact that this effect persists is conducive to the generation of a hypothesis that earlier positioning of UST in the treatment pathway might be beneficial for patients. It should be noted that this is a single-arm study, and therefore no direct comparison to other treatments is possible.
With regards to baseline laboratory measurements, the study demonstrated that baseline albumin, CRP, hemoglobin, and fecal calprotectin were simultaneously statistically significant predictors of remission, and all except albumin were also statistically significant predictors of durability—while still controlling for disease activity as described above. Higher values of baseline albumin and calprotectin correlated with lower odds of achieving remission, in accordance with others, whereas higher values of baseline CRP and hemoglobin correlated with higher chances of remission and durability. The results for CRP are surprising and in contrast with other reports [12]; given that the model is extensively controlling for disease severity at onset, a hypothesis that would be consistent with this data is that, among severe patients, those with high CRP can be most helped by UST. In particular, the effect size of fecal calprotectin was moderately sized, which confirms clinical practice. This is one of the first analyses also to consider BMI which is often excluded due to missing data considerations and showed it to be a significant independent risk factor for not achieving remission, with one of the largest effect sizes across all baseline covariates for remission. Finally, the location of CD (ileal involvement) and presence of perianal disease were found to be significant as independent risk factors of failing to achieve remission, but insignificant as a predictor of drug durability.
The conventional analysis of this same cohort [4] found three factors associated with ustekinumab drug durability; previous abdominal surgery and concomitant steroid use correlated with drug discontinuation, whereas a maintenance schedule with ustekinumab every 8 or 12 week correlated with drug durability. The differences between the two analyses, conventional multivariate analysis vs. interpretable machine learning, highlight how this novel tool can aid in data-driven discovery of non-linear effects between each predictor and the analyzed outcome.
This report introduces the use of GAMs for flexible interpretable modelling of multiple risk factors in IBD. The additive nature of the model retains an interpretation which is very similar to a logistic regression, and therefore can be couched in language, which is familiar and well understood to the clinician, such as log-odds, but the non-linearity improves the predictive ability of these models for variables that take values over large intervals, such as some laboratory measurements and patient demographics. Traditionally, this is resolved by discretizing the values using clinical intuition. Any form of discretization significantly decreases the statistical power of the model. Expert-based discretization, though helpful for explicability, can suffer in multivariable models where multiple baseline risk factors are controlled for simultaneously, rendering standard thresholds less relevant: for example, after controlling for the BMI and HBI at baseline, the threshold above which a third variable adds extra risk of poor outcomes might differ, as opposed to when it is being considered in isolation. In this work, we used the GAM’s estimated non-linear effects to ascertain the exact threshold at which a variable’s contribution crosses from subtracting risk or being neutral to adding risk, holding everything else constant. The findings between the original form of the model and its discretized version stayed directionally the same, which is reassuring, though the number of statistically significant findings for the latter model fell somewhat, as was also expected.
5. Conclusions
Predictors of ustekinumab effectiveness and durability were identified using a cutting-edge approach based on interpretable machine learning and relying on a non-linear multivariable model to control for confounders and identify independent risk factors, which increases the robustness of individual insights. The non-linearity of the model allows for capturing complex effects of time on remission over time, and hence makes use of all follow-up observations rather than selecting a single cross-section, increasing the power of the analysis. It also offers more flexible confounder control, which can increase statistical stability and robustness. Baseline risk for poor outcomes with UST is shown in this analysis to be multifactorial, involving multiple additive risk factors, with no single factor sufficiently explaining poor response by itself, but a combination of several achieving good performance both in terms of statistical goodness-of-fit as well as classification accuracy. In particular, after controlling carefully for disease severity, it was shown that prior exposure to anti-integrins, or higher prior exposure to anti-TNFs, is correlated with worse outcomes, suggesting that earlier positioning of UST might produce better outcomes, though this finding would have to be validated in a randomized trial.
Acknowledgments
We thank Beatriz Garcillán for revision and critical reading of the manuscript. Cristina Rubin is supported by a grant from the Ministerio de Economía y Competitividad (Instituto de Salud Carlos III, Río Hortega CM21/00025) and co-funded by Fondo Europeo de Desarrollo Regional (FEDER).
Appendix A. Details of Statistical Methodology
Appendix A.1. Missing Values
Missing values in either height or weight preclude the calculation of BMI, which is the clinically relevant combination of these two features. Without sophisticated missing data imputation [13], the analysis would either focus on weight alone, which is less clinically relevant, or impute the missing values for height and weight without taking into consideration their correlation and their relationship with other factors. To preserve as much structure as possible in this set of features, an advanced imputation technique known as “iterative Bayesian ridge regression” was used, which is a variant of the well-established Expectation-Maximization algorithm for missing data imputation [7] and relies on a small standalone regression model to predict the missing values using a selection of significant other features as input. Sex, age, and phenotype at the start of UST treatment were found to be significant. Laboratory biomarkers (fecal calprotectin, hemoglobin, albumin, and C-reactive protein) were first carefully calibrated to ensure they all used the same units of measurement and were then imputed using the hot-deck K-nearest neighbors imputation as per [14].
Appendix A.2. Drop-Out in Longitudinal Analysis
Drop-out bias is a well-known challenge in longitudinal analysis. Analyses that have a non-linear estimation shape are more robust to such bias in the sense that it is unlikely to impact the shape of the remission trajectory earlier on in the treatment window but can still be affected at later parts of the trajectory where the prevalence of drop-out increases. In a longitudinal setting, additional timestamps were generated for drop-out patients using the LVCF imputation, so that each of these patients would not have a number of visits smaller than a value randomly drawn from the distribution of the number of doses received by non-drop-out patients.
Appendix A.3. Shape Constraints
The user can either manually pre-specify the number of splines (which control the smoothness of the function ) or use cross-validation to determine the optimal number instead, in which case, however, the p-values and confidence intervals are no longer valid. For this reason, splines were pre-set to fixed values. All continuous variables were modeled using splines, whereas factors were modeled as in logistic regression as constant effects.
Modern implementations of GAM additionally allow the introduction of shape constraints into the smoothing functions, including monotonically decreasing, monotonically increasing, convex and concave shapes. Monotonicity is a natural generalization of linearity. For each variable, a monotonic decreasing and a monotonic increasing constraint were tried out, and the one resulting in the best fitting model was used, presented in Table 3.
Appendix A.4. Interaction Effects
In all models, a number of interaction effects were introduced in the model only where the main effects were first found to be significant: these were an interaction between hemoglobin and sex, interactions between each baseline lab and the HBI at the first UST dose, and, for longitudinal remission only, an interaction term between the days since the start of treatment and years since diagnosis, as well as an interaction between days since the start of UST treatment and BMI. These improved the fit of the model without modifying the shape or direction of the main effects.
Table A1.
Interaction effects.
| Feature Interactions | p-Value of Effect | ||
|---|---|---|---|
| Longitudinal Remission | Durability | ||
| Numeric Values Used for Several Features | All Features Discretized, Except for “Days since Start of UST Treatment” | ||
| (Baseline hemoglobin) × (Sex) | 0.626 | N/A | 0.821 |
| (HBI at the first UST dose, categorized) × (Baseline fecal calprotectin) | <0.05 | N/A | 0.307 |
| (HBI at the first UST dose, categorized) × (Baseline albumin) | <0.05 | N/A | 0.239 |
| (HBI at the first UST dose, categorized) × (Baseline hemoglobin) | <0.05 | N/A | 0.306 |
| (HBI at the first UST dose, categorized) × (Baseline CRP) | <0.05 | N/A | <0.05 |
| (Days since the start of UST treatment) × (Years between diagnosis and start of UST) | <0.05 | N/A | N/A |
| (Days since the start of UST treatment) × (BMI) | <0.05 | N/A | N/A |
Author Contributions
Conceptualization, M.C. (María Chaparro), C.N., M.B.-d.A. and J.P.G.; Formal analysis, S.K. and A.P.; Funding acquisition, C.N. and E.V.; Investigation, M.C. (María Chaparro), I.B.-R., E.F.S., J.G.G., L.R., M.T.D.-L.P., F.A.-A., E.I.F., M.C. (Mercedes Cabello), S.R.I., A.N.O., M.C. (Mara Charro), D.G., C.D.S., O.M.O., D.B., E.I., A.G.C., P.R.d.l.P., M.M.B.-W., M.A., M.J.G., E.H., J.G., P.M.M., B.V.J., C.Q.I., J.M.V.M., J.M.H., Y.G.-L., A.I.M.S., V.M.A., M.D.M.A., F.B., J.M.C., C.R.d.C., P.F.S., A.L.S.R., N.J., S.G.-L., A.F., I.J., F.J.M.C., C.T., P.V., R.d.F., D.M., G.M.A., A.H.-C., F.J.G.A., M.V.D., R.P.-V., A.N., F.R.M., I.M.-J., V.R.A., M.d.M.M.R., P.C.-M., I.G.T., M.N.-L., L.A.G., D.H.C., M.B.-d.A. and J.P.G.; Methodology, S.K. and A.P.; Resources, C.N.; Validation, S.K. and A.P.; Writing—original draft, S.K., A.P. and C.N.; Writing—review and editing, M.C. (María Chaparro), S.K., A.P., C.N., M.B.-d.A. and J.P.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki (revision of Seoul, October 2008) and current legislation in Spain regarding post-authorization observational studies for medicinal products for human use (Ministerial Order SAS/3470/2009) (protocol code JAN-CRD-2018-01//CNTO1275CRD4028, date of approval 23 May 2019)” for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data used for this study cannot be made available due to patient confidentiality, but the first publication of this data may be found at https://academic.oup.com/ibdjournal/advance-article/doi/10.1093/ibd/izab357/6528818 (accessed on 28 July 2022).
Conflicts of Interest
M.Ch. has served as a speaker, or has received research or education funding from MSD, AbbVie, Hospira, Pfizer, Takeda, Janssen, Ferring, Shire Pharmaceuticals, Falk Pharma, and Tillotts Pharma. I.B.-R. has served as a speaker for MSD, Janssen, Pfizer, and Takeda. E.F.S. has served as a speaker for Janssen, AbbVie, Tillotts Pharma, Ferring, MSD, Shire, Takeda, Falk, and Faes Farma. L.R. has served as a speaker or has received education funding from MSD, AbbVie, Takeda, Janssen, and Ferring. F.A.-A. has served as a speaker, consultant, and advisory member for or has received research funding from Janssen, MSD, AbbVie, Pfizer, Kern Pharma, Biogen, Sandoz, Takeda, Ferring, Faes Farma, Shire Pharmaceuticals, Falk Pharma, Tillotts Pharma, Gebro Pharma, Amgen, and Vifor Pharma. E.I.F. has served as a consultant or advisory member or received research and education funding from AbbVie, MSD, Pfizer, Takeda, Janssen, Ferring, and Falk Pharma. S.R.I. has served as a speaker or received research or education funding from MSD, AbbVie, Pfizer, Takeda, Janssen, Ferring, Faes Farma, Shire Pharmaceuticals, Chiesi, Gebro Pharma, Otsuka Pharmaceuticals, and Tillotts Pharma. D.B. has served as a speaker for AbbVie, Pfizer, Takeda, Janssen, Ferring, and Tillots Pharma. A.G.C. has served as a speaker, consultant, and advisory member for or received research funding from MSD, AbbVie, Pfizer, Kern Pharma, Takeda, Janssen, Ferring, Faes Farma, Shire Pharmaceuticals, Tillotts Pharma, Chiesi, and Otsuka Pharmaceutical. M.M.B.-W. has served as speaker or advisory board member or has received research and education funding from MSD, Ferring, AbbVie, Janssen, Biogen, and Takeda. M.A. has served as speaker for Takeda, Janssen, and AbbVie. M.J.G. has received financial support for traveling and educational activities from MSD, Janssen, AbbVie, Takeda, and Ferring. P.M.M. has served as a speaker, consultant, and advisory member for or received research funding from MSD, AbbVie, Pfizer, Takeda, Janssen, Roche, Ferring, Faes Farma, Shire Pharmaceuticals, Falk Pharma, Tillotts Pharma, and Otsuka Pharmaceutical. J.M.V.M. has served as a speaker, consultant, and advisory member for or received research funding from MSD, AbbVie, Takeda, Janssen, Kern, Ferring, Faes Farma, Shire Pharmaceuticals, Falk Pharma, Chiesi, and Gebro Pharma. J.M.H. has served as a speaker or received research or education funding from MSD, AbbVie, Pfizer, Takeda, Janssen, Sandoz, Ferring, and Faes Farma. Y.G.-L. has served as speaker, consultant, and advisory member for or has received unrestricted grants from MSD, AbbVie, Takeda, Janssen, Ferring, Shire Pharmaceuticals, and GebroPharma. A.I.M.S. has received fees from Jansen and Takeda. M.D.M.-A. has received fees as a speaker, consultant, and advisory member for or received research funding from MSD, AbbVie, Hospira, Pfizer, Takeda, Janssen, Shire Pharmaceuticals, Tillotts Pharma, and Faes Pharma. F.B. has served as a speaker, consultant, and advisory member for or received research funding from MSD, AbbVie, Takeda, Janssen, Pfizer, Biogen, Amgen, Ferring, Faes Farma, Tillotts Pharma, Falk Pharma, Chiesi, and Gebro Pharma. N.J. has received fees from Janssen, Takeda, AbbVie, and Tillots. C.T. has served as a speaker, consultant, and advisory board member for MSD, AbbVie, Pfizer, Takeda, Janssen, Ferring, Faes Farma, Shire Pharmaceuticals, Falk Pharma, and Tillots. A.H.-C. has served as speaker for or received research or education funding from AbbVie, Takeda, Kern Pharma, Pfizer, Janssen, Adacyte Therapeutics, and Ferring. F.R.M. has served as advisory member or received financial support for traveling and educational activities from Pfizer, Jansen, MSD, AbbVie, and Takeda. M.d.M.M.R. has served as speaker or received financial support for educational activities from MSD, Takeda, Janssen, AbbVie, Tillotts Pharma, and Ferring. I.G.T. has served as a speaker from MSD, AbbVie, Pfizer, Takeda, Kern, Shire Pharmaceuticals, and Janssen. M.N.-L. has served as a speaker or received financial support for traveling or received research funding from AbbVie, Janssen, Takeda, MSD, Pfizer, Ferring, Falk Pharma, Tillots Pharma, and Biogen. M.B.-d.A. has served as a speaker, consultant, and advisory member for or received research funding from MSD, AbbVie, Hospira, Takeda, Janssen, Kern, Ferring, Faes Farma, Shire Pharmaceuticals, Falk Pharma, Chiesi, Gebro Pharma, Otsuka Pharmaceuticals, and Tillotts Pharma. J.P.G. has served as a speaker, consultant, and advisory member for or received research funding from MSD, AbbVie, Hospira, Pfizer, Kern Pharma, Biogen, Takeda, Janssen, Roche, Ferring, Faes Farma, Shire Pharmaceuticals, Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, and Vifor Pharma. S.K., A.P., C.N., and E.V. are employees of Janssen. All other authors have nothing to declare.
Funding Statement
This work was supported by Janssen-Cilag Spain. This sponsor had a partial role in study design, analysis, and interpretation of data.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Feagan B.G., Sandborn W.J., Gasink C., Jacobstein D., Lang Y., Friedman J.R., Blank M.A., Johanns J., Gao L.L., Miao Y., et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 2016;375:1946–1960. doi: 10.1056/NEJMoa1602773. [DOI] [PubMed] [Google Scholar]
- 2.Danese S., Bonovan S., Peyrin-Biroulet L. Positioning ustekinumab in Crohn’s disease: From clinical evidence to clinical practice. J. Crohn’s Colitis. 2017;11:1258–1266. doi: 10.1093/ecco-jcc/jjx079. [DOI] [PubMed] [Google Scholar]
- 3.Pacou M., Mesana L., Gauthier A., Naessens D., Sloan S., Danese S., Bonovas S., Abrams K. Indirect treatment comparison of ustekinumab versus other biologics in moderate to severe Crohn’s disease: A 1-year treatment sequence analysis. Value Health. 2016;19:576. doi: 10.1016/j.jval.2016.09.1323. [DOI] [Google Scholar]
- 4.Chaparro M., Baston-Rey I., Fernández-Salgado E., González García J., Ramos L., Diz-Lois Palomares M.T., Argüelles-Arias F., Iglesias Flores E., Cabello M., Rubio Iturria S., et al. Long-Term Real-World Effectiveness and Safety of Ustekinumab in Crohn’s Disease Patients: The SUSTAIN Study. Inflamm. Bowel Dis. 2022. online ahead of print . [DOI] [PMC free article] [PubMed]
- 5.Hastie T.J., Tibshirani R.J. Generalized Additive Models. Volume 43 CRC Press; Boca Raton, FL, USA: 1990. [Google Scholar]
- 6.Argyropoulos C., Unruh M.L. Analysis of time to event outcomes in randomized controlled trials by generalized additive models. PLoS ONE. 2015;10:e0123784. doi: 10.1371/journal.pone.0123784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berhane K., Tibshirani R.J. Generalized Additive Models for Longitudinal Data. Can. J. Stat. 1998;26:517–535. doi: 10.2307/3315715. [DOI] [Google Scholar]
- 8.Waljee A.K., Wallace B.I., Cohen-Mekelburg S., Liu Y., Liu B., Sauder K., Stidham R.W., Zhu J., Higgins P.D. Development and validation of machine learning models in prediction of remission in patients with moderate to severe Crohn disease. JAMA Netw. Open. 2019;2:e193721. doi: 10.1001/jamanetworkopen.2019.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biemans V.B., van der Meulen-de Jong A.E., Van Der Woude C.J., Löwenberg M., Dijkstra G., Oldenburg B., De Boer N.K., Van Der Marel S., Bodelier A.G., Jansen J.M., et al. Ustekinumab for Crohn’s Disease: Results of the ICC Registry, a Nationwide Prospective Observational Cohort Study. J. Crohn’s Colitis. 2020;14:33–45. doi: 10.1093/ecco-jcc/jjz119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Straatmijer T., Biemans V.B., Hoentjen F., De Boer N.K., Bodelier A.G., Dijkstra G., Van Dop W.A., Haans J.J., Jansen J.M., Maljaars P.J., et al. Ustekinuma b for Crohn’s Disease: Two-Year Results of the Initiative on Crohn and Colitis (ICC) Registry, a Nationwide Prospective Observational Cohort Study. J. Crohn’s Colitis. 2021;15:1920–1930. doi: 10.1093/ecco-jcc/jjab081. [DOI] [PubMed] [Google Scholar]
- 11.Iborra M., Beltrán B., Fernández-Clotet A., Iglesias-Flores E., Navarro P., Rivero M., Gutiérrez A., Sierra-Ausin M., Mesonero F., Ferreiro-Iglesias R., et al. Real-world long-term effectiveness of ustekinumab in Crohn’s disease: Results from the ENEIDA registry. Aliment. Pharm. Ther. 2020;52:1017–1030. doi: 10.1111/apt.15958. [DOI] [PubMed] [Google Scholar]
- 12.Park J., Chun J., Yoon H., Cheon J.H. Feasibility of a Clinical Decision Support Tool for Ustekinumab to Predict Clinical Remission and Relapse in Patients with Crohn’s Disease: A Multicenter Observational Study. Inflamm. Bowel Dis. 2022:izac105. doi: 10.1093/ibd/izac105. [DOI] [PubMed] [Google Scholar]
- 13.Little R.J., Rubin D.B. Statistical Analysis with Missing Data. John Wiley and Sons; Hoboken, NJ, USA: 2019. [Google Scholar]
- 14.Garcia-Laencina P.J., Sancho-Gomez J.-L., Figueiras-Vidal A.R., Verleysen M. K Nearest Neighbours with Mutual Information for Simultaneous Classification and Missing Data Imputation. Neurocomputing. 2009;72:1483–1493. doi: 10.1016/j.neucom.2008.11.026. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used for this study cannot be made available due to patient confidentiality, but the first publication of this data may be found at https://academic.oup.com/ibdjournal/advance-article/doi/10.1093/ibd/izab357/6528818 (accessed on 28 July 2022).