Abstract
The aim of this study was to examine the level of physical activity during pregnancy in different populations worldwide. An intensive search was carried out from February until May 2021. The inclusion criteria were original studies of healthy pregnant women, and the main study variable was the assessment of physical activity. A total of 110 out of 1451 studies were assessed for inclusion, using the Newcastle–Ottawa Scale for quality, and for the risk of bias. The 44 analyzed articles were divided into 5 tables according to the characteristics of the intervention and the validated instrument used to measure physical activity (PA). A total of 59.09% of the studies indicated that participants had a low level of physical activity during pregnancy. In addition, the median quality score of the studies was 7.12, and 77.27% of the studies were cataloged as having a high-quality score. Although international guidelines recommend that women without a contraindication engage in prenatal physical activity, the results of the present study show that the level of PA is too low for women to achieve scientifically proven maternal-fetal benefits. Failure to achieve the recommended levels of weekly physical activity could pose significant risks to maternal well-being.
Keywords: pregnancy, physical activity, health promotion, active, prevalence
1. Introduction
Today’s society shows concerning and increasing levels of physical inactivity, which are associated with an alarming number of complications and associated pathologies, such as gestational diabetes or preeclampsia [1,2]. In parallel, people are experiencing higher levels of stress in the body and more health problems year after year, which support the appearance of diseases that reduce the quality of life [3,4] as well as high globalized economic costs; these diseases are directly responsible for the high cost of medicines, hospital stays and clinical consultations [5].
The gestational period is not excluded from this problem [6], which supports the appearance of diseases. A relevant consequence is the saturation of the health system with complications and pathologies that could be addressed with healthy lifestyle habits, good nutrition, and the regular practice of physical activity [7].
It should be added that pregnancy is considered a period with great influence on the establishment of certain healthy habits that the woman will continue (in some cases throughout her life) [8]; therefore, comprehensive wellness interventions are necessary. Balance in pregnant women’s systems is important to avoid maternal, fetal and newborn risks and ensure a healthy pregnancy. In this sense, physical inactivity can be a threat to this desired and complex situation [9], and therefore, it would be interesting to know the level of compliance to physical activity guidelines affecting the health of the mother and fetus [10]. Pregnant women spent more than 50% of their time in sedentary behaviors and it impacted on pregnancy outcomes for both mother (higher levels of C Reactive Protein or LDL Cholesterol) and child (larger newborn abdominal circumference or macrosomic infants) [11].
Increasingly, in recent years, the World Health Organization has been working on the creation of guidelines and recommendations for the practice of physical activity in the fight against high levels of sedentarism [12], and specifically, there are global organizations that, for more than 30 years, have been dealing with the complex relationship between physical activity and pregnancy for healthy pregnant women, publishing international clinical guidelines for physical exercise and pregnancy [13,14]. Specifically, recent universal guidelines (American, Canadian, Spanish), in consensus with others throughout the world, have established the recommendation for a healthy pregnant woman to stay physically active, performing 150 min of moderate physical activity at least 3 days a week and, whenever possible, under the supervision of a professional [13,14,15]. At this point, the current scientific consensus emerging from early evidence [16,17] suggests that an accumulation of 30 min or more of moderate exercise per day should occur on most, if not all, days of the week in the absence of either medical or obstetric complications, similar to the recommendation for the non-pregnant population.
In a global analysis, the scientific literature has confirmed a positive association between the regular practice of moderate physical activity by healthy pregnant women and maternal, fetal and newborn well-being [18]. Similarly, and in direct relation to the health of the mother and her future child, scientific evidence speculates that an active pregnancy produces an epigenetic effect of physical activity as a promoter of a healthy and balanced intrauterine environment as the basis of health, both during the gestational period and after it [19,20]. Accordingly, the objective of the present study was to examine the level of physical activity of the pregnant population in different regions of the world through the analysis of the scientific literature on this issue to be aware of the basis for future interventions.
2. Materials and Methods
This was a systematic review designed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (P.R.I.S.M.A.) [21]. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42021262193).
2.1. Data Sources
An intensive search was carried out in the following databases: PubMed, Scopus, Sport Discus and Web of Science. The search began in February 2021 and ended in April 2021. To guarantee equality in the selection process, the same article selection criteria were used for all databases considering the differences in controlled vocabulary and syntax rules. The detailed search strategy is presented in Supplementary Material.
The reference lists of the selected studies were reviewed to identify other studies that may have been missed in the electronic keyword search. The registries’ observational studies were reviewed to identify unpublished research. The search terms used were:
(“physical activity” OR “exercise” OR “training”)
(“barriers” OR “enablers” OR “access”)
(“pregnancy” OR “maternal” OR “antenatal” OR “pregnant”)
(“health” OR “wellbeing”)
(“maternal outcome” OR “pregnancy outcome”)
(“observational” OR “cohort” OR “qualitative” NOT “randomized clinical trial” NOT “RCT”)
2.2. Study Inclusion and Exclusion Criteria
To address the main objective, study inclusion and exclusion criteria were structured using the PICOS framework (population, intervention, comparison, outcome, study design) as a worksheet [22] (Table 1).
Table 1.
PICOS framework.
| PICOS | DEFINITIONS |
|---|---|
| Population | Healthy pregnant women. |
| Interventions | PA assessment carried out during pregnancy. |
| Comparison | Baseline data based on not practicing physical activity. |
| Outcome | The main variable of the study should record the PA developed during pregnancy. |
| Study design | Observational studies. |
Population:
The population of interest was healthy pregnant women without contraindications to physical exercise, regardless of their gestational age at the time of entry to the study. The characteristics of the population were reviewed in the methodology of each study. Women with contraindications were excluded from the analyses [13,14,15].
Intervention:
The investigated intervention was related to the different forms of assessment, analysis and recording of the prevalence of physical activity during pregnancy in different regions of the world. Due to the different nature of the studies and, therefore, their different forms of interventions, studies whose measurement of physical activity was assessed through validated questionnaires, including the Pregnancy Physical Activity Questionnaire (PPAQ) [23], the International Physical Activity Questionnaire (IPAQ) [24], and the Kaiser Modified Questionnaire (KPAS) [25], or by direct and mixed measurements (accelerometer and pedometer) [26,27] were included (Table 2).
Table 2.
NOS quality score.
| Studies | Selection | Comparability | Exposure | Total Quality Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author, Year | Representativeness of the Exposed Cohort | Selection of the Non-Exposed Cohort | Ascertainment of Exposure | Demonstration That Outcome of Interest was not Present at Start of Study | Comparability of Cohorts on the Basis of the Design or Analysis | Ascertainment of Outcome | Was Follow-Up Long Enough for Outcomes to Occur | Adequacy of Follow Up of Cohorts | ||
| Record of Physical Activity using PPAQ | Aburezq 2020 [32] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Antosiak-Cyrak 2019 [33] | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 1 | 7 | |
| Chasan-Taber 2015 [34] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | |
| Chasan-Taber 2014 [35] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | |
| Davoud 2020 [36] | 1 | 0 | 1 | 1 | 2 | 0 | 1 | 1 | 7 | |
| Gebregziabher 2019 [37] | 0 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 7 | |
| Hailemariam 2020 [38] | 1 | 1 | 0 | 1 | 2 | 1 | 0 | 1 | 7 | |
| Harrold 2014 [39] | 1 | 0 | 1 | 1 | 2 | 0 | 1 | 1 | 7 | |
| Ko 2016 [40] | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 5 | |
| Lee 2016 [41] | 1 | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 6 | |
| Lynch 2012 [42] | 0 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 6 | |
| Mourady 2013 [43] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | |
| Okafor 2020 [44] | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 8 | |
| Santos 2016 [45] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | |
| Schmidt 2017 [46] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Todorovic 2020 [47] | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | |
| Van der Waerden 2019 [48] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 | |
| Xiang 2019 [49] | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 7 | |
| Yin 2019 [50] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Zhang 2014 [51] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Record of Physical Activity using IPAQ | Bertolotto 2010 [52] | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 5 |
| Harizopoulou 2010 [53] | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 7 | |
| Padmapriya 2015 [54] | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | |
| Rêgo 2017 [55] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | |
| Román-Gálvez 2021 [56] | 1 | 1 | 0 | 1 | 2 | 0 | 1 | 1 | 7 | |
| Record of Physical Activity using KPAS | Bacchi 2016 [57] | 0 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 7 |
| Chasan-Taber 2007 [58] | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 0 | 6 | |
| Chasan-Taber 2008 [59] | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | |
| Currie 2014 [60] | 0 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 7 | |
| Fell 2009 [61] | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 | |
| Fortner 2011 [62] | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 6 | |
| Direct measurements of physical activity | Di Fabio 2015 [63] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Downs 2009 [64] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 6 | |
| Everson 2011 [65] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | |
| Hawkins 2014 [66] | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 8 | |
| Jiang 2012 [67] | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 7 | |
| Morgan 2014 [68] | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | |
| Morkrid 2014 [69] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 | |
| Rousham 2006 [70] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | |
| Sinclair 2019 [71] | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 8 | |
| Direct and indirect measurements with mixed studies of physical activity | Chadonnet 2012 [72] | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 6 |
| Cohen 2013 [73] | 0 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 7 | |
| Kominiarek 2018 [74] | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 5 | |
| Medek 2016 [75] | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 6 | |
Comparison:
In this case, the comparison was based on not practicing physical activity. For this comparison, women who engaged in physical activity were compared to those who did not.
Outcome:
The primary outcome was the level of physical activity performed during pregnancy, and the secondary outcomes were variables corresponding to the mother and the newborn (Table 3, Table 4, Table 5, Table 6 and Table 7). The studies must have contained at least one primary study variable that somehow recorded the quantity of physical activity performed during pregnancy using the aforementioned validated questionnaires or direct measurement methods.
Table 3.
Record of Physical Activity using the PPAQ questionnaire during pregnancy.
| Author, Year, Country | Type of Study | N | Sample | GEST. AGE | INST. | PA REG. | Main Conclusion |
Other Variables | |
|---|---|---|---|---|---|---|---|---|---|
| [32] | Aburezq 2020 Kuwait |
Cross-sectional study | 653 | Pregnant women from Kuwait | 3T: >32 wk | PPAQ | MET h /wk | PA helps control weight, gestational blood pressure, and birth weight. Vigorous PA is more common in women without GDM | Sociodemographic, anthropometric, and pregnancy variables |
| [33] | Antosiak-Cyrak 2019 Poland |
Observational study | 267 | Polish pregnant women | 1T: 9 wk 2T: 21 wk 3T: 33 wk |
PPAQ | MET h/wk | Women prefer low to moderate intensity exercises. Women with previous children perform more PA. |
Sociodemographic and pregnancy variables |
| [34] | Chasan-Taber 2014 USA |
Prospective cohort study | 1276 | Hispanic pregnant women | 1T: 12 wk 2T: 21 wk 3T: 30 wk |
PPAQ | MET h/wk | Women who meet PA guidelines have a lower and controlled GWG | Sociodemographic, behavioral, psychosocial and pregnancy variables |
| [35] | Chasan-Taber 2015 USA |
Prospective cohort study | 1240 | Hispanic pregnant women | 12.4 wk | PPAQ | MET h/wk | PA performed before and early in pregnancy does not significantly reduce the risk of GH | Sociodemographic, behavioral, psychosocial and pregnancy variables |
| [36] | Davoud 2020 Iran |
Cross-sectional study | 256 | Iranian pregnant women | 1T: <13 wk 2T:14–27 wk 3T: >28 wk |
PPAQ | MET ·min/day | PA decreases during 2T and 3T. PA is related to quality of life |
Sociodemographic, psychosocial and pregnancy variables |
| [37] | Gebregziabher 2019 Ethiopia |
Cross-sectional study | 458 | Ethiopian pregnant women | ND | PPAQ | MET h/sem | Non-primiparous women with a higher level of education perform more PA | Sociodemographic and pregnancy variables |
| [38] | Hailemariam 2020 Ethiopia |
Cross-sectional study | 299 | Ethiopian pregnant women | 1T: <13 wk 2T:14–27 wk 3T: >28 wk |
PPAQ | MET h/wk | Women with a lower academic level and who work at home are at greater risk of being sedentary | Sociodemographic and pregnancy variables. |
| [39] | Harrod 2014 USA |
Longitudinal cohort study | 826 | Pregnant women | 17 wk 27 wk 1 day postpartum |
PPAQ | MET h/wk | Higher PA and total energy expenditure in the last stage of pregnancy are related to lower neonatal adiposity. | Sociodemographic, pregnancy and anthropometric variables of mother and newborn. |
| [40] | Ko 2016 China |
Prospective descriptive study | 150 | Chinese pregnant women | 29 wk 40 wk |
PPAQ | MET h/wk | PA is higher in 2T and 3T. Women with low levels of PA have a greater chance of caesarean delivery |
Sociodemographic, behavioral and anthropometric variables |
| [41] | Lee 2016 Taiwan |
Cross-sectional study | 581 | Taiwanese pregnant women | 1T: 14–16 wk 2T: 24–28 wk 3T: 30–32 wk |
PPAQ | MET h/day | Higher energy expenditure in sports activities during 3T. | Sociodemographic and anthropometric variables |
| [42] | Lynch 2012 USA |
Prospective cohort study | 1355 | Hispanic pregnant women | 1T: 14–16 wk 2T: 24–28 wk 3T: 30–32 wk |
PPAQ | MET h/wk | The predominant energy expenditure is that of domestic and care activities. Multiparous women who underwent pre-pregnancy PA are less likely to be inactive. | Sociodemographic, psychosocial and behavioral variables |
| [43] | Mourady 2017 Lebanon |
Cross-sectional study | 141 | Lebanese pregnant women | 1T: 14–16 wk 2T: 24–28 wk 3T: 30–32 wk |
PPAQ | MET h/wk | Light PA is positively correlated with psychological health and social relationships. | Sociodemographic, psychosocial, anthropometric behavioral and pregnancy variables |
| [44] | Okafor 2020 South Africa |
Cross-sectional study | 1082 | South African pregnant women | ND | PPAQ | MET h/day | Younger women perform less PA. Single and unemployed women are less active. | Sociodemographic, behavioral anthropometric variables |
| [45] | Santos 2016 Portugal |
Longitudinal prospective study | 118 | Portuguese pregnant women | 1T: <12 wk 2T: 12–28 wk 3T: >28 wk |
PPAQ | MET h/wk | AF decreases significantly to a greater extent in 1T. | Sociodemographic and anthropometric variables |
| [46] | Schmidt 2017 Germany |
Observational study | 83 | German pregnant women | 1T: 14–16 wk 2T: 24–28 wk 3T: 30–32 wk |
PPAQ | MET h/wk | PA decreases during pregnancy despite showing the availability of the necessary information for it. | Sociodemographic, behavioral anthropometric and pregnancy variables |
| [47] | Todorovic 2020 Serbia |
Cross-sectional study | 162 | Serbian pregnant women | 12 wk | PPAQ | MET min/wk | One third of women have insufficient PA in 3T. Lower PA is associated with a lower educational level. | Sociodemographic, behavioral and anthropometric variables |
| [48] | Van der Waerden 2019 France |
2 cohort studies ELFE EDEN |
575 1745 |
French pregnant women | 3T <24–28 wk |
PPAQ | MET h/wk | Less PA and sedentary lifestyle seems to be associated with postpartum depression. | Sociodemographic, psychosocial and pregnancy variables. |
| [49] | Xiang 2019 China |
Cross-sectional study | 1077 | Chinese pregnant women | 1T: <13 wk 2T: 13–28 wk 3T: >28 wk |
PPAQ | MET h/wk | A high level of PA predominates, but not PE. Unemployed women without PA habits are more likely to fail to comply with PA guidelines, especially in 3T. | Sociodemographic, behavioral, anthropometric and pregnancy variables. |
| [50] | Yin 2019 China |
Cross-sectional study | 1179 | Chinese pregnant women | 1T: <12 wk 2T: 24–28 k 3T: >32 wk |
PPAQ | MET h/wk | During pregnancy, an inactive lifestyle predominates, with low intensity unit exercises. | Sociodemographic, behavioral and anthropometric variables |
| [51] | Zhang 2014 China |
Cross-sectional study | 1056 | Chinese pregnant women | 1T: <13 wk 2T: 14–27 wk 3T: >28 wk |
PPAQ | MET h/day | Women with a lower pre-pregnancy BMI, higher educational level and active husbands are more likely to perform PA and PE during pregnancy. | Sociodemographic, anthropometric and pregnancy variables |
N.: sample size. GEST. AGE.: gestational age. INST.: instrument for measuring physical activity. PA REG.: record of physical activity. T.: trimester. WK.: week. H.: hour. MET.: metabolic equivalent of task. MIN.: minutes. PA.: physical activity. GDM.: gestational diabetes mellitus. GWG.: gestational weigh gain. GH.: gestational hypertension. PE.: physical exercise. BMI.: body mass index.
Table 4.
Record of Physical Activity using the IPAQ questionnaire during pregnancy.
| Author, Year, Country | Type of Study | N | Sample | GEST. AGE | INST. | PA REG. | Main Conclusion |
Other Variables | |
|---|---|---|---|---|---|---|---|---|---|
| [52] | Bertolotto 2010 Italy |
Observational study | 268 | Italian pregnant women | 27 + 6 wk | IPAQ | MET min/wk | PA before pregnancy can lower the risk of GDM. | Sociodemographic, behavioral, anthropometric and pregnancy variables |
| [53] | Harizopoulou 2010 Greece |
Cross-sectional study | 160 | Pregnant women | 12 wk | IPAQ | MET min/wk | Physical inactivity before and during early pregnancy is associated with an increased risk of developing GDM | Sociodemographic, anthropometric, pregnancy, childbirth and newborn variables. |
| [54] | Padmapriya 2015 Singapore |
Cohort study | 1247 | Chinese, Malarian and Indian pregnant women | 26–28 wk | IPAQ | MET min/wk | The time spent on light, moderate and vigorous PA was reduced during pregnancy. | Sociodemographic, psychosocial, anthropometric and pregnancy variables |
| [55] | Rêgo 2017 Brazil |
Cohort study | 1447 | Nulliparous pregnant women | 25–26 wk | IPAQ | MET min/wk | No association was found between the level of PA in the 2T and 3T with adverse perinatal outcomes. | Sociodemographic, pregnancy and delivery variables |
| [56] | Román-Gálvez 2021 Spain |
Prospective cohort study | 463 | Healthy pregnant women | 1T: <14 wk 2T: 24 wk 3T: >32 wk |
IPAQ | MET min/wk | Two-thirds of women achieve enough PA. Energy expenditure decreases throughout pregnancy. | Sociodemographic, behavioral anthropometric and pregnancy variables. |
N.: sample size. GEST. AGE.: gestational age. INST.: instrument for measuring physical activity. PA REG.: record of physical activity. T.: trimester. WK.: week. H.: hour. MET.: metabolic equivalent of task. MIN.: minutes. PA.: physical activity. GDM.: gestational diabetes mellitus. GWG.: gestational weigh gain. GH.: gestational hypertension. PE.: physical exercise. BMI.: body mass index.
Table 5.
Record of Physical Activity using the KPAS questionnaire during pregnancy.
| Author, Year, Country | Type of Study | N | Sample | GEST. AGE | INST. | PA REG. | Main Conclusion |
Other Variables | |
|---|---|---|---|---|---|---|---|---|---|
| [57] | Bacchi 2016 Italy |
Longitudinal descriptive study | 236 | Caucasian pregnant women | 1T: 14–16 wk 2T: 24–28 wk 3T: 30–32 wk |
Modified Kaiser Questionnaire KPAS |
Activity frequency | PA is generally low. In women with normal weight it increases in the 2T and 3T but in overweight women it remains stable. | Sociodemographic, behavioral anthropometric and pregnancy variables. |
| [58] | Chasan-Taber 2007 USA |
Prospective study | 1231 | Latina pregnant women | 15 wk 28 wk |
Modified Kaiser Questionnaire KPAS |
Activity frequency | Occupational PA is higher in women with a high academic level and higher income. Domestic PA is higher in multiparous and older women. | Sociodemographic, behavioral, anthropometric variables |
| [59] | Chasan-Taber 2008 USA |
Prospective cohort study | 1006 | Hispanic pregnant women | >24 wk | Modified Kaiser Questionnaire KPAS |
Activity frequency | A significant reduction in the risk of GDM is found in women who undergo some type of PA. | Sociodemographic, behavioral, anthropometric and pregnancy variables |
| [60] | Currie 2014 Canada |
Prospective cohort study | 1749 | Canadian pregnant women | 20 wk | Modified Kaiser Questionnaire KPAS |
Activity frequency | PA together with an active lifestyle is associated with a lower appearance of fetal macrosomia. | Sociodemographic, behavioral anthropometric variables, pregnancy and newborn. |
| [61] | Fell 2009 Canada |
Prospective cohort study | 1737 | Canadian pregnant women | 21+4 wk | Modified Kaiser Questionnaire KPAS |
Activity frequency | PA during the first 20 weeks of gestation is lower than pre-pregnancy PA. | Sociodemographic, behavioral, anthropometric variables. |
| [62] | Fortner 2011 USA |
Prospective cohort study | 1043 | Puerto Rican pregnant women | 15+5 wk | Modified Kaiser Questionnaire KPAS |
Activity frequency | Recreational PA in early pregnancy reduces the risk of GH. | Sociodemographic, anthropometric, psychosocial and pregnancy variables. |
N.: sample size. GEST. AGE.: gestational age. INST.: instrument for measuring physical activity. PA REG.: record of physical activity. T.: trimester. WK.: week. H.: hour. MET.: metabolic equivalent of task. MIN.: minutes. PA.: physical activity. GDM.: gestational diabetes mellitus. GWG.: gestational weigh gain. GH.: gestational hypertension. PE.: physical exercise. BMI.: body mass index.
Table 6.
Direct measurements of physical activity during pregnancy.
| Author, Year, Country | Type of Study | N | Sample | GEST. AGE | INST. | PA REG. | Main Conclusion |
Other Variables | |
|---|---|---|---|---|---|---|---|---|---|
| [63] | Di Fabio 2015 USA |
Longitudinal prospective study | 46 | American pregnant women | 18 wk 35 wk |
Accelerometer | Min/day in activity intensity | Total PA is higher in women who met recommendations for pre-pregnancy PA. | Sociodemographic and anthropometric variables |
| [64] | Downs 2009 Canada |
Cohort study | 80 | Pregnant women | 20 wk 32 wk |
Pedometer | Steps/day | Women’s PA behaviors decreased from the second to third trimesters of pregnancy | Sociodemographic and anthropometric variables |
| [65] | Everson 2011 USA |
Cross-sectional study | 359 | American pregnant women | 20–22 wk | Accelerometer | Min/day in activity intensity | Moderate/vigorous PA is higher in 1T and 2T compared to 3T. | Sociodemographic, psychosocial and pregnancy variables. |
| [66] | Hawkins 2014 USA |
Cross-sectional study | 294 | Healthy pregnant women | 1T: <14 wk 2T: 24 wk 3T: >32 wk |
Accelerometer | Min/day in activity intensity | Light PA has a protective effect on CRP in 2T. | Anthropometric and pregnancy sociodemographic variables. |
| [67] | Jiang 2012 China |
Cohort study | 919 | Healthy pregnant women | 18–28 wk 29–35 wk |
Pedometer | Steps/day | 50% of women in 2T and 60% in 3T had low levels of PA. | Sociodemographic and anthropometric variables |
| [68] |
Morgan 2014 United Kingdom |
Prospective cohort study | 466 | Healthy pregnant women | ND | Accelerometer | Min/day in activity intensity | A reduced PA is associated with instrumental deliveries. Being overweight and obese is related to pregnancy and childbirth problems. | Sociodemographic, anthropometric, pregnancy and delivery variables. |
| [69] | Morkrid 2014 Norway | Cohort study | 759 | Healthy pregnant women | 15 wk | Accelerometer | Min/day in activity intensity | Higher level of PA in early pregnancy reduces the risk of GDM development. | Sociodemographic, anthropometric and pregnancy variables. |
| [70] | Rousham 2006 United Kingdom |
Prospective cohort study | 57 | Pregnant women | wk: 12 16 25 34 38 |
Accelerometer | Min/day in activity intensity | PA levels are reduced during pregnancy. | Sociodemographic variables |
| [71] | Sinclair 2019 Canada |
Cohort study | 70 | Canadian pregnant women | 1T: 16–18 wk 2T: 24–26 wk 3T: 32–34 k |
Accelerometer | Min/day in activity intensity | A higher level of sedentary lifestyle is associated with a higher level of perceived stress. | Sociodemographic variables |
N.: sample size. GEST. AGE.: gestational age. INST.: instrument for measuring physical activity. PA REG.: record of physical activity. T.: trimester. WK.: week. H.: hour. MET.: metabolic equivalent of task. MIN.: minutes. PA.: physical activity. GDM.: gestational diabetes mellitus. GWG.: gestational weigh gain. GH.: gestational hypertension. PE.: physical exercise. BMI.: body mass index.
Table 7.
Direct and indirect measurements with mixed studies of physical activity during pregnancy.
| Author, Year, Country | Type of Study | N | Sample | GEST. AGE | INST. | PA REG. | Main Conclusion |
Other Variables | |
|---|---|---|---|---|---|---|---|---|---|
| [72] | Chandonnet 2012 Canada |
Cross-sectional study | 49 | Canadian pregnant women with obesity |
1T: 13 wk 2T: 25 wk 3T: 35 wk |
PPAQ Accelerometer |
MET h/wk Min/day in activity intensity |
PA is reduced during pregnancy. The highest energy expenditure occurs in housework and sedentary activities. | Sociodemographic, behavioral and anthropometric variables. |
| [73] | Cohen 2013 Canada |
Observational study | 54 | Canadian pregnant women | 26 wk | PPAQ Pedometer |
MET h/day Steps/day |
Women with a goal to perform PA are more likely to meet the guidelines. | Sociodemographic, anthropometric and pregnancy variables. |
| [74] | Kominiarek 2018 USA |
Observational study | 49 | Hispanic and american pregnant women | 28 wk 36 wk |
PPAQ Accelerometer |
MET h/wk MET min/day Steps/day |
PA is reduced and sedentary activity increases as the pregnancy progresses. | Sociodemographic, behavioral and anthropometric variables. |
| [75] | Medek 2016 Iceland | Observational study | 217 | Icelandic pregnant women | 24- 28 wk | IPAQ Pedodometer |
MET min/wk Steps/day |
Vigorous PA appears to be beneficial to maternal glucose tolerance, both in BMI and overweight and obese women. | Sociodemographic, behavioral and pregnancy variables. |
N.: sample size. GEST. AGE.: gestational age. INST.: instrument for measuring physical activity. PA REG.: record of physical activity. T.: trimester. WK.: week. H.: hour. MET.: metabolic equivalent of task. MIN.: minutes. PA.: physical activity. GDM.: gestational diabetes mellitus. GWG.: gestational weigh gain. GH.: gestational hypertension. PE.: physical exercise. BMI.: body mass index.
Study selection:
In all cases, observational studies were selected, and studies related to interventions (randomized clinical trials or quasi-randomized clinical trials), some type of review (narrative, systematic or systematic review with meta-analysis) and qualitative research were excluded. In addition, articles published between 2006 and 2021 and written in English, Spanish and Portuguese were considered for the search.
2.3. Data Extraction
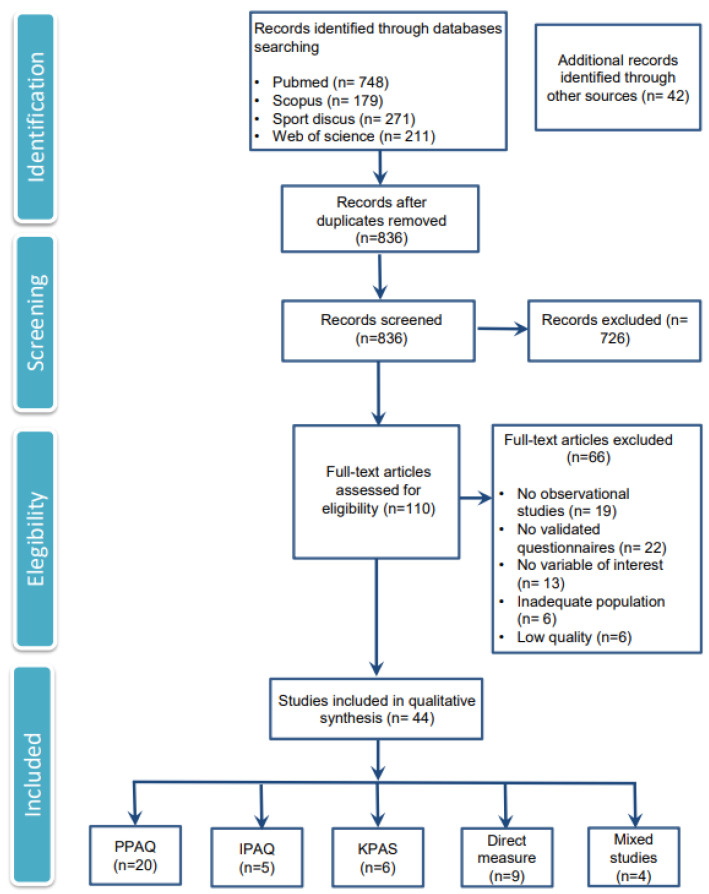
The selection process that was followed for the reviewed articles is captured in Figure 1 [28]. Titles and abstracts identified in the electronic searches were independently screened by two researchers to select potentially relevant studies. The abstracts were identified and passed an initial analysis, and posteriori searches of the full text were performed. The full texts were analyzed separately to search for priority outcomes for data extraction. Reference lists of selected articles were checked to identify possible additional studies not identified by electronic searches.
Figure 1.
Flow chart of the results in each of the study development phases.
For studies where one of the investigators recommended their exclusion, both investigators agreed by consensus to make a final decision on whether they were included. In situations of absolute discrepancy, a third researcher provided his assessment on the inclusion of the study.
Finally, 110 studies from a total of 1451 studies were assessed for inclusion by two independent reviewers. Quality assessment and risk of bias were assessed following the Newcastle–Ottawa Scale (NOS), and studies classified as high or moderate quality were included [29,30]. This scale establishes an overall quality score that ranges from 0 to 9 stars using three factors in its measurement: selection, comparability, and results. A “high” quality score required 3 or 4 stars for selection, 1 or 2 stars for comparability, and 2 or 3 stars for results, and a “moderate” quality score required 2 stars for selection, 1 or 2 stars for comparability, and 2 or 3 stars for results [31]. Studies with lower scores were discarded.
2.4. Data Synthesis
Given our goal of knowing the levels of physical activity of the pregnant population in the existing literature, the heterogeneity in the study designs and in the techniques assessed and the measurement tools for the main variable, a narrative synthesis was carried out precluding the conduct of a formal metanalysis. The 44 analyzed articles were divided into 5 tables according to the characteristics of the intervention and the instrument used to measure physical activity.
As shown in Table 3, Table 4, Table 5, Table 6 and Table 7, the data extracted from each study were divided into three groups. First, the data on the author(s), type of study, sample size, year of publication and country where the study was carried out were used to determine the methodological design. Then, data from the study group, such as gestational period, and maternal outcomes were used to define the characteristics of the sample. Finally, the physical activity measuring instrument, definition of physical activity and main conclusions were used to determine the method of measuring the primary outcome variable of the study.
3. Results
The flow chart shows the results obtained in relation to each of the study development phases: identification, exploration, eligibility, and inclusion. Of a total of 1451 studies that were identified, 836 were screened, and 110 of them were full text assessed for eligibility. A total of 44 studies [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75] were finally analyzed and included for synthesis.
3.1. Quality Assessments
The quality assessments of the included studies are summarized in Table 2. The median quality score of the studies was 7.12 (range 5 to 9) after removing low-quality studies. With this assessment, 22.73% of the studies were rated as moderate quality (n = 10) [40,41,42,52,58,62,64,72,74,75], and 77.27% were rated as high quality (n = 34) [32,33,34,35,36,37,38,39,43,44,45,46,47,48,49,50,51,53,54,55,56,57,59,60,61,63,65,66,67,68,69,70,71,73].
In detail, when analyzing the selection quality factors, 52.27% of the studies [33,37,40,42,47,49,50,51,52,53,54,57,58,59,60,62,63,64,68,72,73,74,75] had flaws in the representativeness of the exposed cohort due to the calculation of the sample size being unusual. In terms of comparability, all studies displayed at least one control factor for the comparability of the cohorts based on the design or analysis. Finally, in relation to the evaluation of the quality of the results, 40.90% of the studies [36,39,40,41,42,44,52,56,57,60,62,66,67,69,71,72,73,74,75] lacked evidence in the control of the factor or ascertainment of the outcome.
3.2. Characteristics of the Included Studies
In a global perspective, 44.44% of the studies (n = 12) [33,37,38,42,44,47,49,51,56,57,58,64] reported, as a primary result, that PA levels were directly and positively related to maternal educational level, and the remaining 55.56% [32,34,35,36,39,40,41,43,45,46,48,50,52,53,54,55,59,60,61,62,63,65,66,67,68,69,70,71,72,73,74,75] showed, on one hand, the relationship of PA levels with other maternal factors; on the other hand, they focused on describing the practice of PA during pregnancy. PA carried out during this period is recorded as mostly in domestic activities [41,42,58,72].
In general, the analyzed studies covered two types of studies with different themes: on one hand, 61.36% (n = 27) [33,34,36,37,38,41,42,44,45,46,47,49,50,51,54,56,57,58,61,63,64,65,67,70,72,73,74] showed the descriptors of patterns and habits of physical activity during pregnancy, and on the other hand, 38.64% (n = 17) [32,35,39,40,43,48,52,53,55,59,60,62,66,68,69,71,75] showed the effect of a physically active lifestyle during the nine months of gestation on different maternal-fetal parameters. Over half of the articles (59.09%) (n = 26) [33,34,36,37,38,41,42,44,45,46,47,49,50,51,54,57,58,61,63,64,65,67,70,72,73,74] found levels below what is indicated in the international recommendations, namely 150 min per week of moderate PA.
Only one article found that two-thirds of the sample met the recommendations [56]. The remaining 17 articles also found inadequate PA levels associated with pregnancy problems (gestational hypertension, diabetes, depression, anxiety, or fetal macrosomia). Low to moderate intensity of gestational PA was registered more frequently than the vigorous one [33,43,50,66], without the weekly exercise being sufficient to meet the international standards.
The gestational period in which the information was collected in the studies is diverse, as is the methodology for describing it. Articles that compared physical activity during the three trimesters of pregnancy stand out [33,36,38,39,41,42,43,45,46,49,50,51,56,57,66,70,71,72], indicating the gestational weeks in which the data were collected. The other group of articles [32,33,34,35,40,43,44,45,58,59,60,61,62,63,64,65,66,67,69,73,74,75] collected data on PA performed in the first weeks of pregnancy (>15 weeks) middle of pregnancy (20–24 weeks) or at the end of pregnancy (>26 weeks) in a specific way. Physical activity decreases throughout pregnancy compared to pre-gestational PA [46,47,50,54]. Specifically, low and inadequate PA was found during the first trimester [60,61,70] and there was a progressive decrease from the second to the third trimester [36,49,56,64,65,67,72,74]. In contrast, 2 articles found a higher PA, without being enough, during the last trimester of pregnancy [40,45].
The included studies were found in a total of 22 countries and relate to a large sample of pregnant women (n = 28,728). Specifically, the largest number of studies have been carried out in Asia (10) [32,36,40,41,43,49,50,51,54,67], North Europe (n = 7) [33,46,48,68,69,70,75], South Europe (n = 6) [45,47,52,53,56,57], North America (n = 17) [34,35,39,42,58,59,60,61,62,63,64,65,66,71,72,73,74], Africa (n = 3) [37,38,44] and South America (n = 1) [55].
Asian countries find lower levels of PA during pregnancy and a progressive decrease in it, with the exception of the study by Ko (2016) [40]. The physical measurement study stands out, finding that 50% during the 2nd trimester and 60% during the third trimester do not reach the suggested recommendations.
As for the European continent, a decrease in the PA level during pregnancy is reflected both for the countries of North and South Europe. A lower PA is associated with mental [48] and physical [68] problems, as well as evidence for both the northern region [69] and the southern region [52,53] that PA from early pregnancy prevents complications, such as gestational diabetes. Specifically, it is recorded in South Europe that two thirds of the pregnant population do not reach the optimal energy expenditure during pregnancy [56].
In studies conducted in the North American area, PA generally decreases and sedentary lifestyle increases during pregnancy; however, a difference is visible between behavioral self-report studies that find lower PA in the first trimester [61], and studies with direct physical measurements [64,65], which record lower levels as gestation progresses. Similarly, it is found that performing PA only at the beginning of pregnancy lacks benefits [35]. Nevertheless, performing PA from the beginning and continuing throughout the pregnancy prevents complications such as gestational diabetes [34,59] or gestational hypertension. [62], as in European countries.
Studies conducted in Africa mainly associate the practice of PA with educational level and multiparity, with educated women and those with previous children being the most active [37,38,44]. Finally, the only study found in South America [55] shows no association between PA and perineal outcomes.
The study designs that are presented varied in their nomenclature, with 43.18% of the studies (n = 19) defined as cohort studies [34,35,39,42,48,54,55,56,58,59,60,61,62,64,67,68,69,70,71] and 34.09% defined as cross-sectional studies (n = 15) [32,36,37,38,40,43,44,47,49,50,51,53,65,66,72]. The remaining studies have been classified by their authors as observational [33,46,52,73,74,75] and prospective longitudinal [45,57,62]. Cohort studies base the selection of the cohort on the specific population of the corresponding regions.
Related to pregnancy outcomes, maternal variables that were collected were divided into sociodemographic (age, academic level, purchasing power, job occupation, marital status, parity or previous miscarriages) anthropometric (prepregnancy BMI, prenatal weight gain, height, waist, hip circumference, maternal BMI at delivery), psychosocial and behavioral (sleep habits, food intake, smoking or alcoholism), pregnancy (parity, ethnicity, age, education level, marriage, smoking previously and during pregnancy, employment status, household income, weight gain, skinfolds, body circumferences, chronic disease history, gestational diabetes, blood pressure, gestational hypertension, preeclampsia), perinatal (gestational age at delivery, birth <37 weeks, type of delivery, duration or injuries) and newborn (perinatal mortality, birth weight (<2500g, 2500–4000g, >4000g), body fat mass, Apgar 1, Apgar 5, length or head circumference) data. Studies found that performing PA helps control gestational weight gain [32], reduced risk of gestational diabetes [34,52,53,59,69] and gestational hypertension [62], lessened c-sections [40] and instrumental delivery [68], lower neonatal adiposity [39] and macrosomia [60] and stress [71] during pregnancy and lessened postpartum depression [48].
In addition, it is relevant to show that 13 studies [34,35,36,42,43,44,48,51,56,62,67,69,71] administered other questionnaires on nutrition, sleep or depression to address and fully explore the complexity of the multifactorial course of pregnancy.
Research studies were divided into three different groups: studies with indirect methods which use validated questionnaires for data recording, studies with direct methods using on-site technological material to take measurements and mixed studies with direct and indirect methods of physical activity quantification. The data collection has been similar in all the studies analysed, since it is given by the scientifically validated protocol of each instrument used.
3.2.1. Studies with Indirect Methods of Physical Activity Quantification
Regarding the data collection instruments, within the indirect measurements, the use of the “Pregnancy Physical Activity Questionnaire” (PPAQ) was predominant, as a validated and reference questionnaire among the pregnant population (Table 3), followed by the “International Physical Activity Questionnaire” IPAQ (Table 4) and the “Modified Kaiser Questionnaire” KPAS (Table 5). These questionnaires are reliable and validated in pregnant women and provide information on the time spent on activities in the life of pregnant women, including items on family, work, or sports/exercise.
The main difference found in the collection of information resides in the fact that the PPAQ records of PA use METS hours/week, the IPAQ uses METS minutes/week and the KPAS has its own measurement on a Likert-type scale, making it difficult to compare the quantitative results among them. Furthermore, among the studies that used the PPAQ as a measurement instrument, 5 collected PA in METS hours/day, making extrapolation of the results even more difficult.
3.2.2. Studies with Direct Methods of Physical Activity Quantification
The main instruments for the objective measurement of PA during pregnancy that are reflected in the scientific literature are the use of accelerometers and pedometers, with 7 and 2 articles in each case, respectively (Table 6). The measurement system for the accelerometer is the min/day that are invested for each activity and for the pedometers, the amount of steps/day. In addition, a wide heterogeneity was observed in the duration of data collection, ranging between 3 and 7 consecutive days of recording the activity with these devices in the studies.
3.2.3. Mixed Studies with Direct and Indirect Methods for Quantifying Physical Activity
Publications that used both (4 studies) both direct and indirect measures focused on a more complete view of PA patterns and combined the two reference questionnaires (PPAQ and IPAQ) with objective data from accelerometers and pedometers (Table 7). The data collection system was identical to what was stated in the previous sections.
4. Discussion
This study aimed to examine the level of PA in pregnant populations from different regions of the world. When attempting a global analysis of the different studies, one of the main questions from a scientific point of view is the diversity found in the way of measuring the gestational PA as seen in the included tables. It is interesting to note that many of these studies, in addition to the assessment of the PA, combined the analysis of certain maternal-fetal parameters, which provides us with an additional analysis to consider. Regarding the division by geographical regions, despite finding differences in the study modalities and associations between registered variables, possibly due to the type of research that has been performed and the economic resources to carry it out, low patterns of PA are widely found.
In this sense, it can be observed that the studies that use direct measures of physical activity using technological elements are carried out in high-income countries, mainly in North America [63,64,65,66,71] and North Europe [68,69,70]. However, the compatibility of the results can become complex due to variations in the methodology, registration, and data processing [76] so the conclusions should be interpreted with caution. In contrast, studies conducted in the African [37,38,44] or South American [55] regions using self-report questionnaires associate this physical activity with maternal educational levels and parity, showing a great intrinsic social problem in these geographical areas. However, the quantity and quality of studies in low-income countries is scarce, so the use of a specific methodology to study the prevalence of PA and the increase in socioeconomic resources to obtain data that can be extrapolated worldwide is vital.
Similarly, previous studies with general populations, show a deep variation in trends in inactivity across regions, income groups, and countries [77]. Higher rates of insufficient physical activity have been recorded in high-income countries while East and Southeast Asia maintain better physical levels [77]. In contrast, women and populations with limited economic resources tend to be less physically active than those with greater resources [78] so this trend could be similar with the pregnant population.
In addition to the cultural paradigm associated with each geographical region, it is worrying that approximately 40% of the female population meets the minimum recommendations for weekly PA [79], evidencing as a latent problem among the population of reproductive age. Added to this is that, currently during the COVID-19 pandemic, the lowest PA levels are more frequent in women, being a potential risk group for physical inactivity [80] during all stages of life, especially during pregnancy.
It is clearly essential to educate women that physical activity promotes improved cardiovascular function, decreased risk of gestational diabetes mellitus, hypertension, and a lower percentage of body fat in the mother, increased gestational age, and improved neurodevelopment in the child [81]. Thus, a recommendation for PA with early onset and throughout pregnancy may be key to improving maternal-fetal parameters [34,35,59,62].
From a methodological point of view, the studies reviewed have great variation as to when they evaluated PA during the pregnancy which makes it difficult to compare across studies and represents an important future research topic. This problem was exposed in the study by Chan (2019) on regulated PA programs where inconsistencies in findings hamper the drawing of firm conclusions [82]. Therefore, an important scientific challenge exists in this area, needing future research.
As stated, the analysis of each of the aforementioned tables offers us different ways of studying and recording gestational PA, which at the same time causes significant confusion and makes data generalization impossible. However, in each of the cases, it can be verified that pregnancy is a period with a certain decrease, in some cases, the total absence, of a regular practice of physical activity. Although the recommendations were not followed, the realization of the minimum level of physical activity during pregnancy is supported by most of the articles reviewed and is in agreement with other previous studies [83,84].
At a general level, the results obtained show that, in most populations studied, PA levels do not reach universal basic recommendations to achieve the already proven benefits of physical practice in maternal, fetal, and newborn outcomes. This has been previously exposed by Borondulin and colleagues (2008) who showed that only a small proportion of pregnant women reached the recommended level of PA [85], and by Lindqvist and colleagues (2016) who found that less than half of the sample met the guidelines [86]. Likewise, scientific studies with the adult population find that less than 10% of adults perform 150 min per week of moderate PA [87]. Specifically in pregnant women, it has been observed that, maintaining levels of light physical activity can promote wellbeing [88] as well as controlling the presence of gestational diabetes, even when starting from a lower intensity physical activity measured with accelerometry [89]. However, previous studies with direct measurements of quality accelerometry in this population are limited and the studies analyzed do not provide generalized conclusions.
In the same way, it was observed that higher levels of physical practice are associated with higher socioeconomic and cultural conditions, and many of the studies that were analyzed remind us of the positive effects of moderate physical practice on maternal parameters, not only of a physical or physiological nature but also psychological and emotional. This has been demonstrated by studies on this subject [90,91,92]. Together, these results show the need to establish a minimum guideline of moderate PA during pregnancy as a basic and fundamental element for the health of future populations.
Even when a more detailed analysis was carried out and attention was focused on the European population, studies developed in Germany [46], Spain [56], France [48], Greece [53], Italy [52,57], Iceland [75], Norway [69], Portugal [45], the United Kingdom [68,69,70], Serbia [47] and Poland [33] clearly and specifically show this decrease in PA in the gestational period. Some of these studies also reaffirmed the beneficial effects mentioned previously that are associated with a more active pregnancy.
Beyond the European analysis, in our opinion, the excellent study carried out by Evenson and Wen in 2011 [65] with 359 pregnant American women aged ≥16 years, who wore an accelerometer for 1 week, is remarkable. This study described the prevalence and correlations between physical activity and sedentary behaviors measured among these pregnant women. For this, in addition to the accelerometer, cross-sectional data collected from the United States National Health and Nutrition Survey (NHANES) from 2003 to 2006 were used. The authors found that most monitored pregnant women spent more than half of the day performing sedentary behaviors and did not comply with the established physical activity recommendations. This sedentary behavior was directly associated with lower levels of economic income and was less significant at high levels of income.
The central idea of a physically active pregnancy as a health promoter continues to be a necessary objective and directly related to universal strategies such as the Sustainable Development Goals proposed by the United Nations (SDG) [93], specifically with objectives 3 (to ensure healthy lives and promote well-being for all at all ages) and 5 (to achieve gender equality and empower all women and girls); but this undoubtedly still needs greater promotion, especially from institutions in charge of the health of pregnant women. The lack of progress in the different regions could explained by the fact that, although more than 70% of countries have an operational physical activity policy, the scale and scope of its implementation has not yet had a national impact [94,95].
Future studies in this scientific field should focus on the prevalence of physical activity during pregnancy in different populations and its relationship with different maternal, fetal and newborn parameters during and after the gestational period. In addition, it is necessary to pay attention to determining the barriers that pregnant women face in their limited access (currently) to the regular and safe practice of physical activity and even more if this low level of practice is in some way affecting the health of the mother and child. The main limitation observed in the present work is the diversity of intervention designs presented by the analyzed studies, which logically prevents generalization in the analysis and discussion of the results, affecting, to a certain extent, the validity of the conclusions reached. Another limitation to consider is related to the different gestational periods studied by the different authors, which naturally causes significant heterogeneity in the targeting of the periods of pregnancy in which the results of each investigation were obtained.
This article provides an updated view of worldwide data on the level of physical exercise performed by different pregnant populations around the world. Health practitioners should continue to promote moderate physical activity during pregnancy; in fact, not reaching 150 min of weekly moderate physical activity could mean significant risks in all areas of maternal health (physiological, mental, emotional) and child health. From the research point of view, new clinical guidelines, and recommendations for exercise during pregnancy, should be developed in different settings.
5. Conclusions
The results of the present study allow us to conclude that even though in the last 15 years there has been a significant increase in physical practice in the pregnant population, the current levels observed in the present review are still very far from the universal recommendations proposed by international organizations in their related publications.
Acknowledgments
The authors would like to thank the help of the Own Program of Universidad Politécnica de Madrid for the financial support of the predoctoral contract of the first author.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11154638/s1, Table S1: PICOS framework.
Author Contributions
Conceptualization, R.B., I.R. and J.G.-A.; Data curation, C.S.-J., R.B., I.R. and M.S.-P.; Formal analysis, C.S.-J. and M.S.-P.; Investigation, C.S.-J., M.S.-P., J.G.-A., R.B. and I.R.; Methodology, C.S.-J., M.S.-P., R.B., J.G.-A. and I.R.; Project administration, R.B. and I.R.; Resources, C.S.-J., M.S.-P., and J.G.-A.; Software, C.S.-J., M.S.-P. and J.G.-A.; Supervision, R.B., I.R. and J.G.-A.; Validation, C.S.-J., M.S.-P., and R.B.; Visualization, C.S.-J., M.S.-P., R.B. and I.R.; Writing–original draft, C.S.-J., R.B. and M.S.-P.; Writing–review and editing, C.S.-J., M.S.-P. and R.B.; Funding acquisition, I.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding, and it is associated with Research Project 47/1ACT/21. Universidad Politécnica de Madrid.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arocha Rodulfo J.I. Sedentary lifestyle a disease from xxi century. Sedentarismo, la enfermedad del siglo xxi. Clin. Investig. Arterioscler. 2019;31:233–240. doi: 10.1016/j.arteri.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Lee I.M., Shiroma E.J., Lobelo F., Puska P., Blair S.N., Katzmarzyk P.T. Lancet Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet. 2012;380:219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teel Z., Marnane C., Iranpour C., Chey T., Jackson J.W., Patel V., Silove D. The global prevalence of common mental disorders: A systematic review and meta-analysis 1980–2013. Int. J. Epidemiol. 2014;43:476–493. doi: 10.1093/ije/dyu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grandes G., Montoya I., Arietaleanizbeaskoa M.S., Arce V., Sanchez A., MAS Group The burden of mental disorders in primary care. Eur. Psychiatry. 2011;26:428–435. doi: 10.1016/j.eurpsy.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Bueno D.R., Marucci Mde F., Codogno J.S., Roediger Mde A. Os custos da inatividade física no mundo: Estudo de revisão [The costs of physical inactivity in the world: A general review] Cien Saude Colet. 2016;21:1001–1010. doi: 10.1590/1413-81232015214.09082015. [DOI] [PubMed] [Google Scholar]
- 6.Meander L., Lindqvist M., Mogren I., Sandlund J., West C.E., Domellöf M. Physical activity and sedentary time during pregnancy and associations with maternal and fetal health outcomes: An epidemiological study. BMC Pregnancy Childbirth. 2021;21:166. doi: 10.1186/s12884-021-03627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poudevigne M.S., O’Connor P.J. A review of physical activity patterns in pregnant women and their relationship to psychological health. Sports Med. 2006;36:19–38. doi: 10.2165/00007256-200636010-00003. [DOI] [PubMed] [Google Scholar]
- 8.Atkinson L., Shaw R.L., French D.P. Is pregnancy a teachable moment for diet and physical activity behaviour change? An interpretative phenomenological analysis of the experiences of women during their first pregnancy. Br. J. Health Psychol. 2016;21:842–858. doi: 10.1111/bjhp.12200. [DOI] [PubMed] [Google Scholar]
- 9.Mate A., Reyes-Goya C., Santana-Garrido Á., Vázquez C.M. Lifestyle, Maternal Nutrition and Healthy Pregnancy. Curr. Vasc. Pharmacol. 2021;19:132–140. doi: 10.2174/1570161118666200401112955. [DOI] [PubMed] [Google Scholar]
- 10.Barakat R., Perales M., Garatachea N., Ruiz J.R., Lucia A. Exercise during pregnancy. A narrative review asking: What do we know? Br. J. Sports Med. 2015;49:1377–1381. doi: 10.1136/bjsports-2015-094756. [DOI] [PubMed] [Google Scholar]
- 11.Fazzi C., Saunders D.H., Linton K., Norman J.E., Reynolds R.M. Sedentary behaviours during pregnancy: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2017;14:32. doi: 10.1186/s12966-017-0485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bull F.C., Al-Ansari S.S., Biddle S., Borodulin K., Buman M.P., Cardon G., Carty C., Chaput J.P., Chastin S., Chou R. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020;54:1451–1462. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mottola M.F., Davenport M.H., Ruchat S.M., Davies G.A., Poitras V.J., Gray C.E., Jaramillo Garcia A., Barrowman N., Adamo K.B., Duggan M. 2019 Canadian guideline for physical activity throughout pregnancy. Br. J. Sports Med. 2018;52:1339–1346. doi: 10.1136/bjsports-2018-100056. [DOI] [PubMed] [Google Scholar]
- 14.Committee on Obstetric Practice Physical Activity and Exercise During Pregnancy and the Postpartum Period: ACOG Committee Opinion, Number 804. Obstet Gynecol. 2020;135:e178–e188. doi: 10.1097/AOG.0000000000003772. [DOI] [PubMed] [Google Scholar]
- 15.Barakat R., Díaz-Blanco A., Franco E., Rollán-Malmierca A., Brik M., Vargas M., Silva C., Sánchez-Polán M., Gil J., Perales M., et al. Guías clínicas para el ejercicio físico durante el embarazo. Prog. Obs. Ginecol. 2019;62:464–471. doi: 10.20960/j.pog.00231. [DOI] [Google Scholar]
- 16.ACOG Committee Obstetric Practice ACOG Committee opinion. Number 267, January 2002: Exercise during pregnancy and the postpartum period. Obs. Gynecol. 2002;99:171–173. doi: 10.1097/00006250-200201000-00030. [DOI] [PubMed] [Google Scholar]
- 17.American College of Sports Medicine . ACSM’s Guidelines for Exercise Testing and Prescription. 6th ed. Lippincott Williams and Wilkins; Philadelphia, PA, USA: 2000. [Google Scholar]
- 18.Dipietro L., Evenson K.R., Bloodgood B., Sprow K., Troiano R.P., Piercy K.L., Vaux-Bjerke A., Powell K.E., 2018 Physical Activity Guidelines Advisory Committee Benefits of Physical Activity during Pregnancy and Postpartum: An Umbrella Review. Med. Sci. Sports Exerc. 2019;51:1292–1302. doi: 10.1249/MSS.0000000000001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers J.M. Smoking and pregnancy: Epigenetics and developmental origins of the metabolic syndrome. Birth Defects Res. 2019;111:1259–1269. doi: 10.1002/bdr2.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badon S.E., Littman A.J., Chan K.C.G., Tadesse M.G., Stapleton P.L., Bammler T.K., Sorensen T.K., Williams M.A., Enquobahrie D.A. Physical activity and epigenetic biomarkers in maternal blood during pregnancy. Epigenomics. 2018;10:1383–1395. doi: 10.2217/epi-2017-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A., PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amir-Behghadami M., Janati A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg. Med. J. 2020;37:387. doi: 10.1136/emermed-2020-209567. [DOI] [PubMed] [Google Scholar]
- 23.Chasan-Taber L., Schmidt M.D., Roberts D.E., Hosmer D., Markenson G., Freedson P.S. Development and validation of a Pregnancy Physical Activity Questionnaire. Med. Sci. Sports Exerc. 2004;36:1750–1760. doi: 10.1249/01.MSS.0000142303.49306.0D. [DOI] [PubMed] [Google Scholar]
- 24.Sanda B., Vistad I., Haakstad L.A.H. Reliability and concurrent validity of the International Physical Activity Questionnaire short form among pregnant women. BMC Sports Sci. Med. Rehabil. 2017;9:7. doi: 10.1186/s13102-017-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt M.D., Freedson P.S., Pekow P., Roberts D., Sternfeld B., Chasan-Taber L. Validation of the Kaiser Physical Activity Survey in pregnant women. Med. Sci. Sports Exerc. 2006;38:42–50. doi: 10.1249/01.mss.0000181301.07516.d6. [DOI] [PubMed] [Google Scholar]
- 26.Aguilar Cordero M.J., Sánchez López A.M., Guisado Barrilao R., Rodríguez Blanque R., Noack Segovia J., Pozo Cano M.D. Descripción del acelerómetro como método para valorar la actividad física en los diferentes periodos de la vida; revisión sistemática [Accelerometer description as a method to assess physical activity in different periods of life; systematic review] Nutr. Hosp. 2014;29:1250–1261. doi: 10.3305/nh.2014.29.6.7410. [DOI] [PubMed] [Google Scholar]
- 27.Evenson K.R., Chasan-Taber L., Symons Downs D., Pearce E.E. Review of self-reported physical activity assessments for pregnancy: Summary of the evidence for validity and reliability. Paediatr. Perinat. Epidemiol. 2012;26:479–494. doi: 10.1111/j.1365-3016.2012.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Page M.J., Moher D., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. The Ottawa Hospital Research Institute. 2014. [(accessed on 14 December 2015)]. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 30.Zeng X., Zhang Y., Kwong J.S., Zhang C., Li S., Sun F., Niu Y., Du L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J. Evid. Based Med. 2015;8:2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 31.McPheeters M.L., Kripalani S., Peterson N.B., Idowu R.T., Jerome R.N., Potter S.A., Andrews J.C. Closing the quality gap: Revisiting the state of the science (vol. 3: Quality improvement interventions to address health disparities) [(accessed on 15 March 2021)];Evid. Rep. Technol. Assess. (Full Rep). 2012 208:1–475. (Evidence Reports/Technology Assessments, No. 208.3.) Appendix G, Thresholds for Quality Assessment. Available online: https://www.ncbi.nlm.nih.gov/books/NBK107322/ [PMC free article] [PubMed] [Google Scholar]
- 32.Aburezq M., AlAlban F., Alabdulrazzaq M., Badr H. Risk factors associated with gestational diabetes mellitus: The role of pregnancy-induced hypertension and physical inactivity. Pregnancy Hypertens. 2020;22:64–70. doi: 10.1016/j.preghy.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Antosiak-Cyrak K.Z., Demuth A. A study of physical activity levels of pregnant women using the Polish version of Pregnancy Physical Activity Questionnaire (PPAQ-Pl) Ginekol. Pol. 2019;90:250–255. doi: 10.5603/GP.2019.0047. [DOI] [PubMed] [Google Scholar]
- 34.Chasan-Taber L., Silveira M., Lynch K.E., Pekow P., Solomon C.G., Markenson G. Physical activity and gestational weight gain in Hispanic women. Obesity. 2014;22:909–918. doi: 10.1002/oby.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chasan-Taber L., Silveira M., Pekow P., Braun B., Manson J.E., Solomon C.G., Markenson G. Physical activity, sedentary behavior and risk of hypertensive disorders of pregnancy in Hispanic women. Hypertens Pregnancy. 2015;34:1–16. doi: 10.3109/10641955.2014.946616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davoud A., Abazari M. The Relationship between Quality of Life and Physical Activity, Worry, Depression, and Insomnia in Pregnant Women. Iran J. Psychiatry. 2020;15:159–168. doi: 10.18502/ijps.v15i2.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gebregziabher D., Berhe H., Kassa M., Berhanie E. Level of physical activity and associated factors during pregnancy among women who gave birth in Public Zonal Hospitals of Tigray. BMC Res Notes. 2019;12:454. doi: 10.1186/s13104-019-4496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hailemariam T.T., Gebregiorgis Y.S., Gebremeskel B.F., Haile T.G., Spitznagle T.M. Physical activity and associated factors among pregnant women in Ethiopia: Facility-based cross-sectional study. BMC Pregnancy Childbirth. 2020;20:92. doi: 10.1186/s12884-020-2777-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrod C.S., Chasan-Taber L., Reynolds R.M., Fingerlin T.E., Glueck D.H., Brinton J.T., Dabelea D. Physical activity in pregnancy and neonatal body composition: The Healthy Start study. Obs. Gynecol. 2014;124:257–264. doi: 10.1097/AOG.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ko Y.L., Chen C.P., Lin P.C. Physical activities during pregnancy and type of delivery in nulliparae. Eur. J. Sport Sci. 2016;16:374–380. doi: 10.1080/17461391.2015.1028468. [DOI] [PubMed] [Google Scholar]
- 41.Lee C.F., Hwang F.M., Lin H.M., Chi L.K., Chien L.Y. The Physical Activity Patterns of Pregnant Taiwanese Women. J. Nurs. Res. 2016;24:291–299. doi: 10.1097/JNR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 42.Lynch K.E., Landsbaugh J.R., Whitcomb B.W., Pekow P., Markenson G., Chasan-Taber L. Physical activity of pregnant Hispanic women. Am. J. Prev. Med. 2012;43:434–439. doi: 10.1016/j.amepre.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mourady D., Richa S., Karam R., Papazian T., Hajj Moussa F., El Osta N., Kesrouani A., Azouri J., Jabbour H., Hajj A. Associations between quality of life, physical activity, worry, depression and insomnia: A cross-sectional designed study in healthy pregnant women. PLoS ONE. 2017;12:e0178181. doi: 10.1371/journal.pone.0178181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okafor U.B., Goon D.T. Physical Activity Level during Pregnancy in South Africa: A Facility-Based Cross-Sectional Study. Int. J. Environ. Res. Public Health. 2020;17:7928. doi: 10.3390/ijerph17217928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos P.C., Abreu S., Moreira C., Santos R., Ferreira M., Alves O., Moreira P., Mota J. Physical Activity Patterns During Pregnancy in a Sample of Portuguese Women: A Longitudinal Prospective Study. Iran Red Crescent Med. J. 2016;18:e22455. doi: 10.5812/ircmj.22455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt T., Heilmann T., Savelsberg L., Maass N., Weisser B., Eckmann-Scholz C. Physical Exercise During Pregnancy—How Active Are Pregnant Women in Germany and How Well Informed? Geburtshilfe Frauenheilkd. 2017;77:508–515. doi: 10.1055/s-0043-107785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Todorovic J., Terzic-Supic Z., Bjegovic-Mikanovic V., Piperac P., Dugalic S., Gojnic-Dugalic M. Factors Associated with the Leisure-Time Physical Activity (LTPA) during the First Trimester of the Pregnancy: The Cross-Sectional Study among Pregnant Women in Serbia. Int. J. Environ. Res. Public Health. 2020;17:1366. doi: 10.3390/ijerph17041366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Waerden J., Nakamura A., Pryor L., Charles M.A., El-Khoury F., Dargent-Molina P., EDEN Mother–Child Cohort Study Group Domain-specific physical activity and sedentary behavior during pregnancy and postpartum depression risk in the French EDEN and ELFE cohorts. Prev. Med. 2019;121:33–39. doi: 10.1016/j.ypmed.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 49.Xiang M., Zhang J., Liang H., Zhang Z., Konishi M., Hu H., Nishimaki M., Kim H.K., Tabata H., Shimizu H. Physical activity and dietary intake among Chinese pregnant women: An observational study. BMC Pregnancy Childbirth. 2019;19:295. doi: 10.1186/s12884-019-2452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin Y.-N., Huang Y., Liu X.-H., Luo B.-R. Assessment of physical activity status among pregnant women in southwestern China. Front. Nurs. 2019;6:135–141. doi: 10.2478/FON-2019-0020. [DOI] [Google Scholar]
- 51.Zhang Y., Dong S., Zuo J., Hu X., Zhang H., Zhao Y. Physical activity level of urban pregnant women in Tianjin, China: A cross-sectional study. PLoS ONE. 2014;9:e109624. doi: 10.1371/journal.pone.0109624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertolotto A., Volpe L., Calianno A., Pugliese M.C., Lencioni C., Resi V., Di Cianni G. Physical activity and dietary habits during pregnancy: Effects on glucose tolerance. J. Matern Fetal Neonatal. Med. 2010;23:1310–1314. doi: 10.3109/14767051003678150. [DOI] [PubMed] [Google Scholar]
- 53.Harizopoulou V.C., Kritikos A., Papanikolaou Z., Saranti E., Vavilis D., Klonos E., Papadimas I., Goulis D.G. Maternal physical activity before and during early pregnancy as a risk factor for gestational diabetes mellitus. Acta Diabetol. 2010;47:83–89. doi: 10.1007/s00592-009-0136-1. [DOI] [PubMed] [Google Scholar]
- 54.Frioux S., Wood J.N., Fakeye O., Luan X., Localio R., Rubin D.M. Physical Activity and Sedentary Behavior Patterns Before and During Pregnancy in a Multi-ethnic Sample of Asian Women in Singapore. Matern Child Health J. 2015;19:2523–2535. doi: 10.1007/s10995-015-1773-3. [DOI] [PubMed] [Google Scholar]
- 55.Rêgo A.S., Alves M.T., Batista R.F., Ribeiro C.C., Bettiol H., Cardoso V.C., Barbieri M.A., Loureiro F.H., Silva A.A. Physical activity in pregnancy and adverse birth outcomes. Cad. Saude Publica. 2016;32:e00086915. doi: 10.1590/0102-311x00086915. [DOI] [PubMed] [Google Scholar]
- 56.Román-Gálvez M.R., Amezcua-Prieto C., Salcedo-Bellido I., Olmedo-Requena R., Martínez-Galiano J.M., Khan K.S., Bueno-Cavanillas A. Physical activity before and during pregnancy: A cohort study. Int. J. Gynaecol. Obstet. 2021;152:374–381. doi: 10.1002/ijgo.13387. [DOI] [PubMed] [Google Scholar]
- 57.Bacchi E., Bonin C., Zanolin M.E., Zambotti F., Livornese D., Donà S., Tosi F., Baldisser G., Ihnatava T., Di Sarra D. Physical Activity Patterns in Normal-Weight and Overweight/Obese Pregnant Women. PLoS ONE. 2016;11:e0166254. doi: 10.1371/journal.pone.0166254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chasan-Taber L., Schmidt M.D., Pekow P., Sternfeld B., Manson J., Markenson G. Correlates of physical activity in pregnancy among Latina women. Matern Child Health J. 2007;11:353–363. doi: 10.1007/s10995-007-0201-8. [DOI] [PubMed] [Google Scholar]
- 59.Chasan-Taber L., Schmidt M.D., Pekow P., Sternfeld B., Manson J.E., Solomon C.G., Braun B., Markenson G. Physical activity and gestational diabetes mellitus among Hispanic women. J. Womens Health. 2008;17:999–1008. doi: 10.1089/jwh.2007.0560. [DOI] [PubMed] [Google Scholar]
- 60.Currie L.M., Woolcott C.G., Fell D.B., Armson B.A., Dodds L. The association between physical activity and maternal and neonatal outcomes: A prospective cohort. Matern Child Health J. 2014;18:1823–1830. doi: 10.1007/s10995-013-1426-3. [DOI] [PubMed] [Google Scholar]
- 61.Fell D.B., Joseph K.S., Armson B.A., Dodds L. The impact of pregnancy on physical activity level. Matern Child Health J. 2009;13:597–603. doi: 10.1007/s10995-008-0404-7. [DOI] [PubMed] [Google Scholar]
- 62.Fortner R.T., Pekow P.S., Whitcomb B.W., Sievert L.L., Markenson G., Chasan-Taber L. Physical activity and hypertensive disorders of pregnancy among Hispanic women. Med. Sci. Sports Exerc. 2011;43:639–646. doi: 10.1249/MSS.0b013e3181f58d3e. [DOI] [PubMed] [Google Scholar]
- 63.Di Fabio D.R., Blomme C.K., Smith K.M., Welk G.J., Campbell C.G. Adherence to physical activity guidelines in mid-pregnancy does not reduce sedentary time: An observational study. Int. J. Behav. Nutr. Phys. Act. 2015;12:27. doi: 10.1186/s12966-015-0191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Downs D.S., LeMasurier G.C., DiNallo J.M. Baby steps: Pedometer-determined and self-reported leisure-time exercise behaviors of pregnant women. J. Phys. Act. Health. 2009;6:63–72. doi: 10.1123/jpah.6.1.63. [DOI] [PubMed] [Google Scholar]
- 65.Evenson K.R., Wen F. Prevalence and correlates of objectively measured physical activity and sedentary behavior among US pregnant women. Prev. Med. 2011;53:39–43. doi: 10.1016/j.ypmed.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 66.Hawkins M., Pekow P., Chasan-Taber L. Physical activity, sedentary behavior, and C-reactive protein in pregnancy. Med. Sci. Sports Exerc. 2014;46:284–292. doi: 10.1249/MSS.0b013e3182a44767. [DOI] [PubMed] [Google Scholar]
- 67.Jiang H., Qian X., Li M. Can physical activity reduce excessive gestational weight gain? Findings from a Chinese urban pregnant women cohort study. Int. J. Behav. Nutr. Phys. Act. 2012;9:12. doi: 10.1186/1479-5868-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morgan K.L., Rahman M.A., Hill R.A. Physical activity and excess weight in pregnancy have independent and unique effects on delivery and perinatal outcomes. PLoS ONE. 2014;9:e94532. doi: 10.1371/journal.pone.0094532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mørkrid K., Jenum A.K., Berntsen S., Sletner L., Richardsen K.R., Vangen S., Holme I., Birkeland K.I. Objectively recorded physical activity and the association with gestational diabetes. Scand. J. Med. Sci. Sports. 2014;24:e389–e397. doi: 10.1111/sms.12183. [DOI] [PubMed] [Google Scholar]
- 70.Rousham E.K., Clarke P.E., Gross H. Significant changes in physical activity among pregnant women in the UK as assessed by accelerometry and self-reported activity. Eur. J. Clin. Nutr. 2006;60:393–400. doi: 10.1038/sj.ejcn.1602329. [DOI] [PubMed] [Google Scholar]
- 71.Sinclair I., St-Pierre M., Elgbeili G., Bernard P., Vaillancourt C., Gagnon S., Dancause K.N. Psychosocial Stress, Sedentary Behavior, and Physical Activity during Pregnancy among Canadian Women: Relationships in a Diverse Cohort and a Nationwide Sample. Int. J. Environ. Res. Public Health. 2019;16:5150. doi: 10.3390/ijerph16245150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chandonnet N., Saey D., Alméras N., Marc I. French Pregnancy Physical Activity Questionnaire compared with an accelerometer cut point to classify physical activity among pregnant obese women. PLoS ONE. 2012;7:e38818. doi: 10.1371/journal.pone.0038818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cohen T.R., Plourde H., Koski K.G. Use of the Pregnancy Physical Activity Questionnaire (PPAQ) to identify behaviours associated with appropriate gestational weight gain during pregnancy. J. Phys. Act. Health. 2013;10:1000–1007. doi: 10.1123/jpah.10.7.1000. [DOI] [PubMed] [Google Scholar]
- 74.Kominiarek M.A., Vyhmeister H., Balmert L.C., Fairchild P., Tolo H., Grobman W., Simon M. Activity Tracking Devices in Group Prenatal Care: A Feasibility Study. Biores. Open Access. 2018;7:165–176. doi: 10.1089/biores.2018.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Medek H., Halldorsson T., Gunnarsdottir I., Geirsson R.T. Physical activity of relatively high intensity in mid-pregnancy predicts lower glucose tolerance levels. Acta Obs. Gynecol. Scand. 2016;95:1055–1062. doi: 10.1111/aogs.12931. [DOI] [PubMed] [Google Scholar]
- 76.Wijndaele K., Westgate K., Stephens S.K., Blair S.N., Bull F.C., Chastin S.F., Dunstan D.W., Ekelund U., Esliger D.W. Utilization and Harmonization of Adult Accelerometry Data: Review and Expert Consensus. Med. Sci. Sports Exerc. 2015;47:2129–2139. doi: 10.1249/MSS.0000000000000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morales-Suárez-Varela M., Clemente-Bosch E., Peraita-Costa I., Llopis-Morales A., Martínez I., Llopis-González A. Maternal Physical Activity During Pregnancy and the Effect on the Mother and Newborn: A Systematic Review. J. Phys. Act Health. 2021;18:130–147. doi: 10.1123/jpah.2019-0348. [DOI] [PubMed] [Google Scholar]
- 78.Guthold R., Stevens G.A., Riley L.M., Bull F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 19 million participants. Lancet Glob. Health. 2018;6:e1077–e1086. doi: 10.1016/S2214-109X(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 79.Organización Panamericana de la Salud . La Inactividad Física: Un Factor Principal de Riesgo Para la Salud en las Américas. OPS; Washington, DC, USA: 2002. [Google Scholar]
- 80.Belza B., Warms C. Physical activity and exercise in women’s health. Nurs. Clin. N. Am. 2004;39:181–193. doi: 10.1016/j.cnur.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 81.Gonzalo-Encabo P., Cereijo L., Remón Á.L.C., Jiménez-Beatty J.E., Díaz-Benito V.J., Santacruz Lozano J.A. Associations between individual and environmental determinants and physical activity levels of an active population during the Spanish lockdown. Prev. Med. 2021;153:106719. doi: 10.1016/j.ypmed.2021.106719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan C.W.H., Au Yeung E., Law B.M.H. Effectiveness of Physical Activity Interventions on Pregnancy-Related Outcomes among Pregnant Women: A Systematic Review. Int. J. Environ. Res. Public Health. 2019;16:1840. doi: 10.3390/ijerph16101840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oliveira C., Imakawa T.D.S., Moisés E.C.D. Physical Activity during Pregnancy: Recommendations and Assessment Tools. Rev. Bras. Ginecol. Obstet. 2017;39:424–432. doi: 10.1055/s-0037-1604180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nascimento S.L., Surita F.G., Godoy A.C., Kasawara K.T., Morais S.S. Physical Activity Patterns and Factors Related to Exercise during Pregnancy: A Cross Sectional Study. PLoS ONE. 2015;10:e0128953. doi: 10.1371/journal.pone.0128953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Borodulin K., Evenson K.R., Wen F., Herring A.H., Benson A. Physical activity patterns during pregnancy. Med. Sci. Sports Exerc. 2008;40:1901. doi: 10.1249/MSS.0b013e31817f1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lindqvist M., Lindkvist M., Eurenius E., Persson M., Ivarsson A., Mogren I. Leisure time physical activity among pregnant women and its associations with maternal characteristics and pregnancy outcomes. Sex Reprod. Health. 2016;9:14–20. doi: 10.1016/j.srhc.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 87.Hagströmer M., Oja P., Sjöström M. Physical activity and inactivity in an adult population assessed by accelerometry. Med. Sci. Sports Exerc. 2007;39:1502–1508. doi: 10.1249/mss.0b013e3180a76de5. [DOI] [PubMed] [Google Scholar]
- 88.Yeo S., Kang J.H. Low-Intensity Exercise and Pregnancy Outcomes: An Examination in the Nurses’ Health Study II. Womens Health Rep. (New Rochelle) 2021;15:389–395. doi: 10.1089/whr.2021.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen H., Fang X., Wong T.H., Chan S.N., Akinwunmi B., Ming W.K., Zhang C.J.P., Wang Z. Physical Activity during Pregnancy: Comparisons between Objective Measures and Self-Reports in Relation to Blood Glucose Levels. Int. J. Environ. Res. Public Health. 2022;19:8064. doi: 10.3390/ijerph19138064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Evenson K.R., Savitz D.A., Huston S.L. Leisure-time physical activity among pregnant women in the US. Paediatr. Perinat. Epidemiol. 2004;18:400–407. doi: 10.1111/j.1365-3016.2004.00595.x. [DOI] [PubMed] [Google Scholar]
- 91.Catov J.M., Parker C.B., Gibbs B.B., Bann C.M., Carper B., Silver R.M., Simhan H.N., Parry S., Chung J.H., Haas D.M., et al. Patterns of leisure-time physical activity across pregnancy and adverse pregnancy outcomes. Int. J. Behav. Nutr. Phys. Act. 2018;15:68. doi: 10.1186/s12966-018-0701-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ahmadi K., Amiri-Farahani L., Haghani S., Hasanpoor-Azghady S.B., Pezaro S. Exploring the intensity, barriers and correlates of physical activity In Iranian pregnant women: A cross-sectional study. BMJ Open Sport Exerc. Med. 2021;7:e001020. doi: 10.1136/bmjsem-2020-001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Objetivos y Metas de Desarrollo Sostenible. Www.un.org. [(accessed on 29 May 2021)]. Available online: https://www.un.org/sustainabledevelopment/es/sustainable-development-goals/
- 94.Sallis J.F., Bull F., Guthold R., Heath G.W., Inoue S., Kelly P., Oyeyemi A.L., Perez L.G., Richards J., Hallal P.C. Lancet Physical Activity Series 2 Executive Committee. Progress in physical activity over the Olympic quadrennium. Lancet. 2016;388:1325–1336. doi: 10.1016/S0140-6736(16)30581-5. [DOI] [PubMed] [Google Scholar]
- 95.WHO . Global Action Plan on Physical Activity 2018–2030. More Active People for a Healthier World. World Health Organization; Geneva, Switzerland: 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.