Abstract
Nitrostyrene derivatives are widely used in organic syntheses as a substrate for Michael addition, photoisomerization and cycloaddition. In contrast, ortho-hydroxy derivatives exhibit unusual behaviors in these reactions. Conjugate addition proceeded upon treatment of the ortho-hydroxy-β-nitrostyrene with an amine; however, subsequent C–C bond cleavage readily occurred to afford the corresponding imine. Moreover, conversion of the trans-isomer to a cis-isomer did not occur efficiently, even when UV light was irradiated. We studied these unusual behaviors of β-nitrostyrene, focusing on the role of the ortho-hydroxy group.
Keywords: nitrostyrene; conjugate addition; C–C bond cleavage; photoisomerization; 1,3-dipolar cycloaddition
1. Introduction
A strongly electron-withdrawing nitro group activates the scaffold to undergo versatile reactions, which are used as building blocks for diverse purposes [1]. In the case of a nitroalkene, the resonance effect besides the induced effect generates biased electron-density of the scaffold and facilitates reactions such as nucleophilic addition and cycloaddition. Indeed, β-nitrostyrene serves as a good electrophile for the conjugate addition to afford α-substituted β-(nitro)ethylbenzenes [2], and serves as a good dienophile for the Diels–Alder reaction [3,4,5] or dipolarophile for cycloaddition with 1,3-dipole [6,7,8] to construct versatile cyclic systems. Nitrostyrenes also undergo denitrative cross-coupling reactions, affording disubstituted alkenes [9]. In addition, chemical conversion of the nitro group, such as reduction followed by diazotization and Sandmeyer reactions, facilitates the approach to versatile compounds from a nitro compound [10,11]. Furthermore, the leaving ability of a nitro group also plays an important role in organic synthesis [12,13,14,15]. Despite numerous reports, the chemistry of β-nitrostyrene is still a hot topic and is energetically studied.
In our previous work, we demonstrated aziridination of β-nitrostyrene 1a using a primary amine and N-chlorosuccinimide (NCS) [16], which proceeds via conjugate addition, N-chlorination and intramolecular nucleophilic substitution on the nitrogen atom (Scheme 1) [17]. The high electrophilicity and ring strain of the nitroaziridine facilitates further chemical conversions [16,18]. We considered that functionalized benzofurans could be synthesized by intramolecular nucleophilic substitution when a hydroxy group is introduced at the ortho-position (Scheme 2). According to this strategy, ortho-hydroxy-β-nitrostyrene 1b was subjected to reactions under the same conditions used for 1a; however, 1b exhibited different chemical behaviors from other β-nitrostyrenes. These unusual reactivities prompted us to study the influence of the ortho-hydroxy group. Since compounds derived from salicylaldehyde are widely used in organic synthesis [19,20,21], information on specific properties caused by the ortho-hydroxy group will be useful for many researchers.
Scheme 1.
A mechanism for the formation of nitroaziridine from β-nitrostyrene 1a.
Scheme 2.
A synthetic plan for functionalized benzofuran using an ortho-hydroxy group.
2. Results and Discussion
2.1. C–C Bond Cleavage
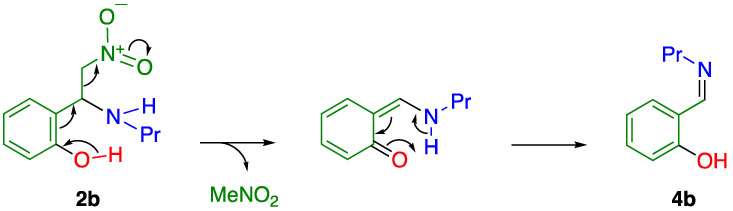
When nitrostyrene 1a was subjected to reaction with 3 equiv. propylamine at room temperature in THF, conjugate addition occurred and was completed within 5 min to afford the corresponding adduct 2a in an 86% yield (Table 1, Entry 1). In this reaction, further C–C bond cleavage leading to imine 4a was not observed. On the contrary, ortho-hydroxynitrostyrene 1b afforded adduct 2b in a 35% yield together with the formation of imine 4b in a 59% yield upon treatment with propylamine under the same conditions (Entry 2).
Table 1.
Effect of the ortho-substituent for the conjugate addition.

| Entry | X | Yield of 2/% | Yield of 4/% | |
|---|---|---|---|---|
| 1 | H | a | 86 | 1 |
| 2 | OH | b | 35 | 59 |
| 3 | OAc | c | 91 | 9 |
According to a report by Mpourmpakis and Lykakis, such imine formation predominantly proceeded in a protic solvent such as methanol [22]. In this process, the hydrogen bond between the substrate and solvent seems to be important. On the other hand, imine was easily formed even when the reaction was conducted in an aprotic solvent in the case of nitrostyrene 1b. Using dry solvent did not effectively suppress C–C bond cleavage, which rules out the possibility that water contained in the solvent is involved. Hence, an ortho-hydroxy group might facilitate C–C bond cleavage by intramolecular hydrogen bonds (Figure 1a). It is considered that hydrogen bonds increase the acidity of an amino group and the leaving ability of the methanenitronic acid moiety. Hence, the C–C bond cleavage readily occurs to furnish the corresponding imine 4b. As another possibility, denitromethylation via a quinoid intermediate is also considered, as illustrated in Scheme 3; that is, deprotonation of the acidic hydroxy proton occurred to form a quinoid intermediate accompanied by elimination of nitromethane, and subsequent tautomerism to afford imine 4b. To avoid imine formation, the ortho-hydroxy group was protected by an acetyl group. ortho-Acetoxy-β-nitrostyrene 1c efficiently underwent conjugate addition with propylamine to furnish adduct 2c in a 91% yield (Table 1, Entry 3). This result indicates that the ortho-hydroxy group facilitates C–C bond cleavage after conjugate addition.
Figure 1.
(a) A plausible intramolecular hydrogen bond of adduct 2b; (b) a plausible intramolecular hydrogen bond of adduct 2c.
Scheme 3.
Another plausible mechanism for the formation of imine 4b.
Different reactivity between 2a and 2b was also observed in the N-chlorination using NCS. While chlorination of half of 2a occurred just after the addition of equimolar NCS in CDCl3 (Table 2, Entry 1) [17], no reaction of the ortho-hydroxy derivative 2b proceeded upon treatment with NCS within 5 min (Entry 2). When the solution of the latter reaction mixture was left at room temperature for 2 h, the formation of N-chlorinated product 3b, imine 4b and nitrostyrene 1b was observed (Entry 3). The imine 4b was formed by C–C bond cleavage, and nitrostyrene 1b was a product of the E2 elimination of chloramine from 3b. Only small amounts of 3b were obtained, even though 2 equiv. of NCS was used and reaction time was prolonged (Entries 4 and 5). It is considered that the low reactivity of 2b is caused by the ortho-hydroxy group. The intramolecular hydrogen bond between the hydroxy and amino groups decreases the nucleophilicity of the amino group to suppress the N-chlorination, resulting in the low conversion of 2b. Even though 3b is produced, the hydroxy-supported formation of 4b might occur predominantly (Figure 1a). Furthermore, the leaving ability of the amino group was improved by N-chlorination, which undergoes E2 elimination to afford nitrostyrene 1b.
Table 2.
Effect of the ortho-substituent for the conjugate addition.

| Entry | X | Time/min | Yield/% | Recovery/% | |||
|---|---|---|---|---|---|---|---|
| 3 | 4 | 1 | 2 | ||||
| 1 | H | A | 5 | 50 | 0 | 0 | 50 |
| 2 | OH | B | 5 | 0 | 0 | 0 | 100 |
| 3 | OH | B | 120 | 18 | 21 | 10 | 51 |
| 4 a | OH | B | 120 | 35 | 39 | 7 | 19 |
| 5 a | OH | B | 1000 | 21 | 47 | 24 | 8 |
a As shown, 2 equiv. NCS was used.
To exclude the influence of the ortho-hydroxy group, the ortho-acetoxy derivative 2c was subjected to reaction with NCS at room temperature in THF, which is the best solvent for N-chlorination of 2a [16]. However, N-chlorination did not proceed at all, which might be due to the intramolecular hydrogen bond between the amino and acetoxy groups (Figure 1b). Although a base such as triethylamine and cesium carbonate was added to the reaction mixture to cleave the hydrogen bond, no positive effect was observed. Hence, the electron-withdrawing inductive effect and steric hindrance of the acetyl group might be additional factors used to prevent the N-chlorination of 2c.
Although a small amount of 3b was obtained through several attempts, its instability disturbed isolation and further chemical transformation. Thus, a synthetic study for benzofurans is under investigation.
2.2. Photoisomerization
When a solution of 1c (called E-1c hereafter) in CDCl3 was subjected to the measurement of 1H-NMR, new signals appeared in the lower field. A couple of doublet splits with a 9.0 Hz coupling constant indicated that isomerization to cis-form (called Z-1c hereafter) occurred, and half the amount of E-1c was consumed after 5 h under ambient conditions. While this isomerization was not influenced by the bulkiness of the O-acyl group and the addition of acids such as acetic acid, methanesulfonic acid and trifluoroborane–ether complex, the isomerization was accelerated under sunlight, which indicates that photoisomerization proceeded. On the contrary, E-1b revealed considerably lower reactivity for the photoisomerization in comparison with E-1a and E-1c.
To conduct the photoisomeric reaction of E-1a–c, UV-Vis. spectra were measured (Figure 2). The spectra of E-1a and E-1c were similar to each other, exhibiting an absorption band at around 320 nm. On the other hand, the spectrum of E-1b exhibited an absorption band with a vibronic band at 360 nm and 305 nm. TD-DFT calculations of E-1a and E-1c indicate that both absorption bands originated in the π→π* transition (Figure 3). The two maximum wavelengths of E-1b at 360 nm and 305 nm were assigned to the transitions HOMO to LUMO and HOMO−1 to LUMO, respectively.
Figure 2.
UV-Vis. spectra of 1a–c.
Figure 3.
TD-DFT calculations for 1b and 1c using CAM-B3LYP/6-31G(d,p), CPCM (CH2Cl2).
Based on the UV spectra, the photoisomeric reaction of nitrostyrenes E-1a–c was performed under irradiation using LED light (λ = 310 nm) for 2.5 h in CDCl3 (Scheme 4, upper). In cases of E-1a and E-1c, respectively, isomerization occurred efficiently to afford a Z-rich mixture without any other detectable by-product. On the contrary, almost all of the E-1b remained in the E-form after the irradiation, which might be due to the tautomerization. Among 1a–c, only 1b can isomerize between Z- and E-forms via a tautomeric structure, nitronic acid 5. Hence, it is considered that Z-1b was transformed into the more stable E-1b, even though photoisomerization occurred under irradiation of UV light (Scheme 4, lower).
Scheme 4.
Photoisomerization of E-form to Z-form (upper) and tautomerism of Z-1b via 5 (lower).
As mentioned above, Z-1b was found to be hardly isolable, which prompted us to fix the stereochemistry via concerted cycloaddition. For this purpose, 1,3-dipolar cycloaddition with nitrone 6 was employed because two bonds can be formed in a single reaction and the electron density of the double bond in the nitrostyrenes is polarized. Our strategy consists of three steps: (i) photoisomerization of E-1c to Z-1c, (ii) cycloaddition of Z-1c with nitrone 6 and (iii) hydrolysis of the acetate moiety of the cycloadduct, which afforded the formal adduct of Z-1b with nitrone 6 (Supplementary Materials).
Initially, 1,3-dipolar cycloaddition was performed with 1a to determine how much stereochemistry was retained during the reaction. When E-1a was used as a substrate, cycloaddition efficiently proceeded in a concerted manner to afford only trans-isomer 8 and 8′ (Table 3, Entry 1). In the cycloaddition using a diastereomeric mixture of 1a (Z/E = 76/24), four kinds of cycloadducts resulted in an 86% total yield. The stereochemistry for each isomer was determined by 1H-NssMR by comparing coupling constants with those of the literature [23] and was finally confirmed by X-ray crystallography of 8a. The ratio of cis-isomers (7a + 7′a) and trans-isomers (8a + 8′a) was 81/19, by which it was confirmed that the diastereomeric ratio of 1a was not changed significantly (Entry 2). The ortho-Acetoxy derivative 1c was less reactive than 1a under the same conditions, in which cis-cycloadducts (7c + 7′c) were predominantly formed, although isomerization competitively occurred to some extent (Entry 3). Although increasing the amount of nitrone 6 is effective to increase the cis/trans ratio of products up to 83/17, the yield did not increase satisfactorily (Entry 4). Heating at a higher temperature was more effective to increase the yield without observing a considerable decrease in the cis/trans ratio (Entry 5).
Table 3.
1,3-Dipolar cycloaddition of 1 and nitrone 6.

| Entry | X | Ratio | Yield of cis-Isomer/% | Yield of trans-Isomer/% | Ratio | |||
|---|---|---|---|---|---|---|---|---|
| Z-1/E-1 | 7 | 7′ | 8 | 8′ | cis/trans | |||
| 1 a | H | a | 0/100 | 0 | 0 | 80 | 11 | 0/100 |
| 2 a | H | a | 96/4 | 45 | 30 | 15 | 3 | 81/19 |
| 3 a | OAc | c | 97/3 | 18 | 12 | 8 | 2 | 75/25 |
| 4 b | OAc | c | 97/3 | 23 | 15 | 7 | 1 | 83/17 |
| 5 c | OAc | c | 99/1 | 43 | 10 | 18 | 3 | 72/28 |
a Heated with 1.5 equiv. of 6 at 110 °C. b Heated with 3 equiv. of 6 at 110 °C. c Heated with 2 equiv. of 6 at 150 °C.
Although the four cycloadducts could not be separated completely by column chromatography on silica gel, 7c was obtained as a pure form or as a mixture with 8′c. Next, hydrolysis of the ester moiety of 7c was attempted to obtain cis-cycloadduct 7b (X = OH), which is not directly synthesized from nitrostyrene 1b (Scheme 5). When a mixture of 7c and 8′c (cis/trans = 83/17) was treated with a base in methanol, 7b and 8′b were obtained in an 81% total yield; however, the isomeric ratio was inverted to 36/64. Since the α-proton of the nitro group is highly acidic, deprotonation easily occurs under basic conditions, which caused isomerization from 7 to 8 via nitronate ion. Then, hydrolysis under acidic conditions was conducted using pure 7c. In this reaction, the desired cycloadduct 7b was furnished in pure form without any detectable 8’b, which indicates that acid hydrolysis is better for this purpose; however, some side reactions, including the elimination of nitrone, also occurred, and conversion of 7b was still low (Scheme 6). Although further optimization of reaction conditions is necessary, there was shown to be a possibility that cycloadduct 7b formally derived from Z-1b could be synthesized.
Scheme 5.
Hydrolysis of acetoxy-substituted cycloadducts 7c and 8′c under basic conditions.
Scheme 6.
Hydrolysis of acetoxy-substituted cycloadduct 7c under acidic conditions.
3. Conclusions
Unusual reactivities of ortho-hydroxy-β-nitrostyrene 1b were studied. While nitrostyrenes usually undergo conjugate addition and subsequent aziridination, 1b underwent C–C bond fission after conjugate addition to afford imine 4b under the same conditions. Unusual behavior of 1b was also observed in the photoisomerization from E-form to Z-form; 1b was intact under irradiation of UV light, while other nitrostyrenes 1a and 1c efficiently isomerized under the same conditions. It is considered that these behaviors are caused by an intramolecular hydrogen bond and tautomerization. Salicylaldehyde condenses with carbonyl or nitro compounds to afford conjugate systems possessing a hydroxy group at the ortho-position. Since these compounds are often used as a substrate for various purposes in organic syntheses [19,20,21], the insights obtained here will provide useful information to researchers using such compounds.
4. Materials and Methods
4.1. General
All the reagents and solvents were commercially available and used as received. UV light (310 nm) was irradiated using Techno Sigma LED PER-AMP (Okayama, Japan). The 1H-NMR spectra were measured on a JEOL JMN-ECZ400S (Tokyo, Japan) at 400 MHz with tetramethylsilane as an internal standard. The 13C-NMR spectra were measured on a JEOL JMN-ECZ400S (Tokyo, Japan) at 100 MHz, and assignments of 13C-NMR spectra were performed via DEPT experiments. Absorption spectra were recorded on a JASCO V-650 spectrophotometer (Tokyo, Japan). The IR spectra were recorded on a JASCO FT/IR-4200 spectrometer (Tokyo, Japan). The high-resolution mass spectra were measured on an AB SCIEX Triple TOF 4600 (Tokyo, Japan). Diffraction data were collected at 93 K under a cold N2-gas stream on a Rigaku XtaLAB Synergy-S/Mo system (λ=0.71073 Å (Mo-Kα), Tokyo, Japan). The integrated data were analyzed by using a Yadokari-XG software package [24]. The structures were solved with the ShelXT structure solution program [25] using Intrinsic Phasing and refined with the ShelXL refinement package [26] using least-squares minimization. Anisotropic refinement was performed for all non-hydrogen atoms, and all the hydrogen atoms were put in calculated positions. The geometrical optimization was carried out at the CAM-B3LYP/6-31G(d,p) level of theory implemented on the Gaussian 09 package [27].
4.2. Preparation of β-Nitrostyrenes 1
Nitrostyrene 1a is commercially available, and 1b and 1c were obtained as follows.
4.2.1. Synthesis of Nitrostyrene 1b
To a solution of ammonium acetate (524 mg, 6.8 mmol) in acetic acid (4 mL) were added nitromethane (1.18 mL, 19.6 mmol) and salicylaldehyde (0.34 mL, 3.24 mmol), and the resultant mixture was heated under reflux for 6 h. After cooling down to room temperature, water (40 mL) was added and the mixture was extracted with dichloromethane (30 mL × 3). The organic layer was dried over magnesium sulfate and concentrated under reduced pressure to afford E-2-(2-hydroxyphenyl)-1-nitroethene (1b) [28,29] (466 mg, 2.8 mmol, 64% yield) as a brown solid. 1H-NMR (400 MHz, CDCl3) δ 5.7 (br s, 1H), 6.85 (dd, J = 8.2, 1.0 Hz, 1H), 7.02 (ddd, J = 7.6, 7.6, 1.0 Hz, 1H), 7.35 (ddd, J = 8.2, 7.6, 1.4 Hz, 1H), 7.44 (dd, J = 7.6, 1.4 Hz, 1H), 7.95 (d, J = 14.0 Hz, 1H), 8.14 (d, J = 14.0 Hz, 1H); 13C-NMR (100 MHz, CDCl3) δ 116.6 (CH), 117.8 (C), 121.7 (CH), 132.8 (CH), 133.4 (CH), 135.7 (CH), 138.7 (CH), 156.1 (C); HR-MS (ESI/TOF) Calcd. for C8H7NO3 [(M + Na)+]: 188.0318, found: 188.0319.
4.2.2. Acetylation of 1b
To a solution of 1b (317 mg, 1.9 mmol) in dichloromethane (8 mL) were added triethylamine (0.27 mL, 1.9 mmol) and acetyl chloride (0.21 mL, 2.9 mmol) at 0 °C, then the mixture was warmed to room temperature. After the mixture was stirred at room temperature for 14 h, water (50 mL) was added and was extracted with dichloromethane (30 mL × 3). The organic layer was dried over magnesium sulfate and concentrated under reduced pressure to afford E-2-(2-acetoxyphenyl)-1-nitroethene (1c) [30] (376 mg, 1.3 mmol, 70% yield) as a yellow solid. 1H-NMR (400 MHz, CDCl3) δ 2.42 (s, 3H), 7.23 (dd, J = 8.0, 1.2 Hz, 1H), 7.32 (ddd, J = 8.0, 8.0, 1.2 Hz, 1H), 7.52 (ddd, J = 8.0, 8.0, 1.6 Hz, 1H), 7.59 (dd, J = 8.0, 1.6 Hz, 1H), 7.60 (d, J = 13.6 Hz, 1H), 8.06 (d, J = 13.6 Hz, 1H); 13C-NMR (100 MHz, CDCl3) δ 21.2 (CH3), 123.0 (C), 123.8 (CH), 126.8 (CH), 129.1 (CH), 133.2 (CH), 138.7 (CH), 150.2 (C), 168.9 (C). A signal of tertiary carbon was not observed, presumably due to overlapping; HR-MS (ESI/TOF) calcd. for C10H9NO4 [(M + Na)+]: 230.0424, found: 230.0423.
4.3. Conjugate Addition of Amine to β-Nitrostyrene
To a solution of nitrostyrene 1a (45 mg, 0.3 mmol) in THF (3 mL), propylamine (74 μL, 0.9 mmol) was added and the resultant mixture was stirred at room temperature for 5 min. The solvent was removed under reduced pressure to afford adduct 2a (62.5 mg, 0.26 mmol, 86% yield) as a yellow oil. When 1b and 1c were used, the reaction was conducted in the same way; however, adduct 2b could not be isolated upon treatment with column chromatography on silica gel, presumably due to the decomposition of the product. So, assignment of 1H-NMR was performed using a reaction mixture.
1-Nitro-2-phenyl-2-(propylamino)ethane (2a): Yellow oil. 1H-NMR (400 MHz, CDCl3) δ 0.87 (t, J = 7.2 Hz, 3H), 1.41–1.51 (m, 2H), 2.45 (t, J = 7.2 Hz, 2H), 4.40 (dd, J = 8.8, 4.8 Hz, 1H), 4.49 (dd, J = 12.0, 4.8 Hz, 1H), 4.57 (dd, J = 12.0, 8.8 Hz, 1H), 7.30–7.40 (m, 5H); 13C-NMR (100 MHz, CDCl3) δ 11.7 (CH3), 23.2 (CH2), 49.3 (CH2), 61.1 (CH), 81.1 (CH2), 127.2 (CH), 128.5 (CH), 129.1 (CH), 138.8 (C); IR (KBr/cm−1) 3400–3280 (br), 1634, 1602, 1553, 1521, 1380, 1343; HR-MS (ESI/TOF) calcd for C11H16N2O2 [(M + H)+]: 209.1277, found: 209.1285.
2-(Hydroxyphenyl)-1-nitro-2-(propylamino)ethane (2b): 1H-NMR (400 MHz, CDCl3) δ 0.93 (t, J = 7.2 Hz, 3H), 1.57 (tq, J = 7.2, 7.2 Hz, 2H), 2.61 (t, J = 7.2 Hz, 2H), 4.50 (dd, J = 10.6, 3.2 Hz, 1H), 4.53 (dd, J = 13.4, 3.2 Hz, 1H), 4.77 (dd, J = 13.4, 10.6 Hz, 1H), 6.83 (dd, J = 8.0, 8.0 Hz, 1H), 6.84 (d, J = 8.0 Hz, 1H), 7.02 (d, J = 8.0 Hz, 1H), 7.22 (dd, J = 8.0, 8.0 Hz, 1H). N-H and O-H signals were not observed.
2-(Acetoxyphenyl)-1-nitro-2-(propylamino)ethane (2c): 1H-NMR (400 MHz, CDCl3) δ 0.86 (t, J = 7.2 Hz, 3H), 1.36–1.63 (m, 2H), 2.12 (s, 3H), 3.14–3.33 (m, 2H) 4.90 (dd, J = 13.6, 4.8 Hz, 1H), 5.43 (dd, J = 13.6, 9.6 Hz, 1H), 5.90 (dd, J = 9.6, 4.8 Hz, 1H), 6.85 (dd, J = 7.6, 7.2 Hz, 1H), 6.95 (d, J = 7.6 Hz, 1H), 7.06 (dd, J = 7.2, 1.2 Hz, 1H), 7.26 (ddd, J = 7.6, 7.6, 1.2 Hz, 1H).
4.4. Photoisomerization of β-Nitrostyrene
A solution of nitrostyrene 1a (45 mg, 0.3 mmol) in acetonitrile (3 mL) was irradiated by UV light (310 nm) in the dark at room temperature for 17 h. After removal of the solvent under reduced pressure, the isomeric ratio was determined by 1H-NMR of the residue. The cis-isomer could be isolated by column chromatography on silica gel (eluted with hexane/ethyl acetate = 90/10) and the structure was confirmed by comparison with NMR data of authentic sample [31]. Yellow oil, 1H-NMR (400 MHz, CDCl3) δ 6.78 (d, J = 9.6 Hz, 1H), 6.97 (d, J = 9.6 Hz, 1H), 7.37-7.44 (m, 3H), 7.48-7.53 (m, 2H).
(Z)-2-(2-Acetoxyphenyl)-1-nitroethene (Z-1c): Yellow solid (eluted with hexane/ethyl acetate = 90/10), 1H-NMR (400 MHz, CDCl3) δ 2.27 (s, 3H), 6.80 (d, J = 9.2 Hz, 1H), 7.03 (d, J = 9.2 Hz, 1H), 7.17 (dd, J = 7.6, 1.2 Hz, 1H), 7.28 (ddd, J = 7.6, 7.6, 1.2 Hz, 1H), 7.42 (dd, J = 7.6, 1.6 Hz, 1H), 7.43 (ddd, J = 7.6, 7.6, 1.6 Hz, 1H); 13C-NMR (100 MHz, CDCl3) δ 21.0 (CH3), 122.7 (CH), 124.5 (C), 126.1 (CH), 129.2 (CH), 129.9 (CH), 131.2 (CH), 138.1 (CH), 148.3 (C), 168.9 (C).
4.5. 1,3-Dipolar Cycloaddition of β-Nitrostyrene of 1 with Nitrone 7
4.5.1. 2-(4-Chlorophenyl)-3,5-diphenyl-4-nitroisooxazolidine 8a and 9a
In a sealed tube, a solution of nitrostyrene 1a (30 mg, 0.2 mmol) and nitrone 7 (70 mg, 0.3 mmol) in toluene (2 mL) was heated at 110 °C in the dark for 17 h. After removal of the solvent, the isomeric ratio was determined by 1H-NMR of the residue. Cis-isomers 8a and 8a’ could be separated by column chromatography on silica gel (eluted with hexane/ethyl acetate = 95/5), but trans-isomers 9a and 9a’ were obtained as a mixture. In the case of 8a, a single crystal was obtained through recrystallization from dichloromethane−methanol, and it was subjected to X-ray crystallography.
3,4-trans-4,5-cis-2-(4-Chlorophenyl)-3,5-diphenyl-4-nitroisoxazolidine (8a): Yellow plates, mp 125.2–125.7 °C 1H-NMR (400 MHz, CDCl3) δ 5.23 (d, J = 4.0 Hz, 1H), 5.43 (dd, J = 6.0, 4.0 Hz, 1H), 5.74 (d, J =6.0 Hz, 1H), 7.02 (d, J = 9.0 Hz, 2H), 7.23 (d, J = 9.0 Hz, 2H), 7.36–7.56 (m, 10H); 13C-NMR (100 MHz, CDCl3) δ 74.7 (CH), 81.4 (CH), 99.8 (CH), 118.8 (CH), 126.6 (CH), 127.4 (CH), 128.9 (CH), 129.0 (CH), 129.3 (C), 129.4 (CH), 129.8 (CH × 2), 131.4 (C), 137.4 (C), 147.4 (C); HR-MS (ESI/TOF) calcd. for C21H17ClN2O3 [(M + H)+]: 381.1001, found: 381.1000. Crystallographic data: empirical formula C21H17ClN2O3; formula weight 380.82; temperature (K) 93(2); crystal system orthorhombic; space group Pbca; unit cell dimensions a = 14.3835(8) Å, b = 10.4902(4) Å, c = 23.8298(12) Å; volume (Å) 3595.6(3); Z 8; ρcalc (g·cm–3) 1.407; absorption coefficient (mm–1) 0.237; θ range (°) 2.220–25.496; reflections collected 3345; independent reflections 2579; completeness to θ 99.9; goodness of fit 1.082; final R indices [I > 2σ(I)] R1 = 0.0499, wR2 = 0.1319; R indices (all data) R1 = 0.0642, wR2 = 0.1426; largest diff. peak (e Å) 0.391; largest diff. hole (e Å) -0.357; CCDC 2,178,609.
3,4-cis-4,5-cis-2-(4-Chlorophenyl)-3,5-diphenyl-4-nitroisoxazolidine (8′a): 1H-NMR (400 MHz, CDCl3) δ 5.31 (d, J = 6.2 Hz, 1H), 5.59 (d, J = 4.2 Hz, 1H), 5.95 (dd, J = 6.2, 4.2 Hz, 1H), 6.94 (d, J = 9.2 Hz, 2H), 7.23 (d, J = 9.2 Hz, 2H), 7.34–7.58 (m, 10H).
3,4-trans-4,5-trans-2-(4-Chlorophenyl)-3,5-diphenyl-4-nitroisoxazolidine (9a): 1H-NMR (400 MHz, CDCl3) δ 5.32 (dd, J = 6.0, 4.0 Hz, 1H), 5.52 (d, J = 4.0 Hz, 1H), 5.75 (d, J = 6.0Hz, 1H), 7.03 (d, J = 8.6 Hz, 2H), 7.26 (d, J = 8.6 Hz, 2H), 7.34–7.57 (m, 10H).
3,4-cis-4,5-trans-2-(4-Chlorophenyl)-3,5-diphenyl-4-nitroisoxazolidine (9′a): 1H-NMR (400 MHz, CDCl3) δ 4.93 (d, J = 9.6 Hz, 1H), 5.46 (dd, J =9.6, 7.2 Hz, 1H), 6.05 (d, J = 7.2 Hz, 1H), 6.96 (d, J = 9.2 Hz, 2H), 7.16 (d, J = 9.2 Hz, 2H), 7.35–7.56 (m, 10H).
4.5.2. 5-(2-Acetoxyphenyl)-2-(4-chlorophenyl)-4-nitro-3-phenylisoxazolidine 8c and 9c
The reaction of 1c and nitrone 7 was conducted in the same way as 1a. Only cis-isomer 8c could be separated by column chromatography on silica gel (eluted with hexane/ethyl acetate = 95/5), but other isomers were obtained as a mixture.
3,4-trans-4,5-cis-5-(2-Acetoxyphenyl)-2-(4-chlorophenyl)-4-nitro-3-phenylisoxazolidine (8c): 1H-NMR (400 MHz, CDCl3) δ 2.25 (s, 3H), 5.21 (d, J = 3.6 Hz, 1H), 5.44 (dd, J = 6.0, 3.6 Hz, 1H), 5.77 (d, J = 6.0 Hz, 1H), 7.01 (d, J = 8.4 Hz, 2H), 7.21 (dd, J = 7.6, 0.8 Hz, 1H), 7.24 (d, J = 8.4 Hz, 2H), 7.28 (ddd, J = 7.6, 7.6, 0.8 Hz, 1H), 7.42 (ddd, J = 7.6, 7.6, 1.2 Hz, 1H), 7.59 (dd, J = 7.6, 1.2 Hz, 1H), 7.42–7.54 (m, 5H).
3,4-cis-4,5-cis-5-(2-Acetoxyphenyl)-2-(4-chlorophenyl)-4-nitro-3-phenylisoxazolidine (8′c): 1H-NMR (400 MHz, CDCl3) δ 2.35 (s, 3H), 5.34 (d, J = 6.8 Hz, 1H), 5.53 (d, J = 4.0 Hz, 1H), 5.98 (dd, J = 6.8, 4.0 Hz, 1H), 6.93 (d, J = 8.6 Hz, 2H), 7.18 (dd, J = 8.0, 0.8 Hz, 1H)7.24 (d, J = 8.6 Hz, 2H), 7.31 (ddd, J = 8.0, 8.0, 0.8 Hz, 1H), 7.35–7.41 (m, 3H), 7.40 (ddd, J = 8.0, 8.0, 1.2 Hz, 1H), 7.56 (d, J = 7.2 Hz, 2H), 7.78 (dd, J = 8.0, 1.2 Hz, 1H).
3,4-trans-4,5-trans-5-(2-Acetoxyphenyl)-2-(4-chlorophenyl)-4-nitro-3-phenylisoxazolidine (9c): Yellow oil, 1H-NMR (400 MHz, CDCl3) δ 2.21 (s, 3H), 5.20 (d, J = 4.8 Hz, 1H), 5.33 (dd, J = 4.8, 4.0 Hz, 1H), 6.02 (d, J = 4.0 Hz, 1H), 7.02 (d, J = 9.2 Hz, 2H), 7.15 (dd, J = 7.6, 1.2 Hz, 1H), 7.25 (d, J = 9.2 Hz, 2H), 7.33 (m, 7H), 7.67 (dd, J = 7.6, 1.2 Hz, 1H).
3,4-cis-4,5-trans-5-(2-Acetoxyphenyl)-2-(4-chlorophenyl)-4-nitro-3-phenylisoxazolidine (9′c): Signals of 9′c were too small to be analyzed, except for signals of the acetyl group and ring protons of isoxazolidine. 1H-NMR (400 MHz, CDCl3) δ 2.40 (s, 3H), 4.87 (d, J = 8.8 Hz, 1H), 5.50 (dd, J = 8.8, 6.0 Hz, 1H), 6.21 (d, J = 6.0 Hz, 1H).
4.6. Hydrolysis of 8c under Acidic Conditions
To a solution of isoxazolidine 8c (19 mg, 0.043 mmol) in a mixed solvent (ethanol/water = 1/1, 2 mL), 1 M hydrochloric acid (43 μL, 0.043 mmol) was added, and the resultant mixture was heated in a sealed tube at 100 °C for 3 h. After cooling to room temperature, water (10 mL) was added, and the mixture was extracted with dichloromethane (10 mL × 3). The organic layer was dried over magnesium sulfate and concentrated under reduced pressure to afford a brown oil as a residue. The formation of the hydrolyzed product was confirmed via observation of a newly formed isoxazolidine ring; however, other aromatic protons could not be analyzed because of overlap with those by-products.
3,4-trans-4,5-cis-2-(4-Chlorophenyl)-5-(2-hydroxyphenyl)-4-nitro-3-phenylisoxazolidine (8b): 1H-NMR (400 MHz, CDCl3) δ 5.19 (d, J = 3.4 Hz, 1H), 5.61 (dd, J = 5.4, 3.4 Hz, 2H), 5.96 (d, J = 5.4 Hz, 1H).
Supplementary Materials
The following are available online: https://www.mdpi.com/article/10.3390/molecules27154804/s1. 1H- and 13C-NMR NMR spectra of 1b, 1c and 8a. 1H NMR spectra of reaction mixture for conjugate addition, photoisomerization and 1,3-dipolar cycloaddition.
Author Contributions
K.I. wrote a draft; K.W. did experiments; K.I. and N.N. discussed with K.W.; all authors contributed to the revision. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 1b, 1c and 8a are available from the authors.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nishiwaki N. A Walk through Recent Nitro Chemistry Advances. Molecules. 2020;25:3680. doi: 10.3390/molecules25163680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki Y. Asymmetric Michael Addition Mediated by Chiral Ionic Liquids. Mini Rev. Org. Chem. 2018;15:236–245. doi: 10.2174/1570193X15666171211165344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elkin P.K., Durfee N.D., Rawal V.H. Diels-Alder Reactions of 1-Alkoxy-1-amino-1,3-butadienes: Direct Synthesis of 6-Substituted and 6,6-Disubstituted 2-Cyclohexenones and 6-Substituted 5,6-Dihydropyran-2-ones. Org. Lett. 2021;23:5288–5293. doi: 10.1021/acs.orglett.1c01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcantonio E., Curti C., Battistini L., Sartori A., Cardinale L., Pelosi G., Zanardi F. Direct, Asymmetric Synthesis of Carbocycle-Fused Uracils via [4+2] Cycloadditions: A Noncovalent Organocatalysis Approach. Adv. Synth. Catal. 2021;363:2625–2633. doi: 10.1002/adsc.202100082. [DOI] [Google Scholar]
- 5.Asahara H., Hiraishi M., Nishiwaki N. One-Pot and Metal-Free Synthesis of 3-Arylated 4-Nitrophenols via Polyfunctionalized Cyclohexanones from β-Nitrostyrenes. Beistein. J. Org. Chem. 2020;16:1830–1836. doi: 10.3762/bjoc.16.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu M.-Y., He W.-W., Liu X.-Y., Tan B. Asymmetric Construction of Spirooxindoles by Organocatalytic Multicomponent Reactions Using Diazooxindoles. Angew. Chem. Int. Ed. 2015;54:9409–9413. doi: 10.1002/anie.201504640. [DOI] [PubMed] [Google Scholar]
- 7.Banerji A., Gupta M., Biswas P.K., Prange T., Neuman A. 1,3-Dipolar Cycloadditions. Part XII—Selective Cycloaddition Route to 4-Nitroisoxazolidine Ring Systems. J. Heterocycl. Chem. 2007;44:1045–1049. doi: 10.1002/jhet.5570440511. [DOI] [Google Scholar]
- 8.Sridharan V., Muthusubramanian S., Sivasubramanian S., Polborn K. Diastereoselective Synthesis of 2,3,4,5-Tetrasubstituted Isoxazolidines via 1,3-Dipolar Cycloaddition. Tetrahedron. 2004;60:8881–8892. doi: 10.1016/j.tet.2004.07.021. [DOI] [Google Scholar]
- 9.Marčeková M., Ferko B., Detková K.R., Jakubec P. Denitrative Cross-Couplings of Nitrostyrenes. Molecules. 2020;25:3390. doi: 10.3390/molecules25153390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akhtar R., Zahoor A.F., Rasool N., Ahmad M., Ali K.G. Recent Trends in the Chemistry of Sandmeyer Reaction: A Review. Mol. Divers. 2022;26:1837–1873. doi: 10.1007/s11030-021-10295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukhopadhyay S., Batra S. Applications of Sodium Nitrite in Organic Synthesis. Eur. J. Org. Chem. 2019:6424–6451. doi: 10.1002/ejoc.201900951. [DOI] [Google Scholar]
- 12.Mukaijo Y., Yokoyama S., Nishiwaki N. Comparison of Substitutiong Ability of Nitronate versus Enolate for Direct Substitution of a nitro Group. Molecules. 2020;25:2048. doi: 10.3390/molecules25092048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gharui C., Pan S.C. Employment of α-Nitroketones in Organic Synthesis. Org. Biomol. Chem. 2019;17:5190–5211. doi: 10.1039/C9OB00828D. [DOI] [PubMed] [Google Scholar]
- 14.Asahara H., Sofue A., Kuroda Y., Nishiwaki N. Alkynylation and Cyanation of Alkenes Using Diverse Properties of a Nitro Group. J. Org. Chem. 2018;83:13691–13699. doi: 10.1021/acs.joc.8b01865. [DOI] [PubMed] [Google Scholar]
- 15.Ballini R., Petrini M. The Nitro to Carbonyl Conversion (Nef Reaction): New Perspectives for a Classical Transformation. Adv. Synth. Catal. 2015;357:2371–2402. doi: 10.1002/adsc.201500008. [DOI] [Google Scholar]
- 16.Hao F., Asahara H., Nishiwaki N. Direct Aziridination of Nitroalkenes Affording N-Alkyl-C-nitroaziridines and the Subsequent Lewis Acid Mediated Isomerization to β-Nitroenamines. Org. Lett. 2017;19:5442–5445. doi: 10.1021/acs.orglett.7b02724. [DOI] [PubMed] [Google Scholar]
- 17.Iwai K., Wada K., Hao F., Asahara H., Nishiwaki N. A Mechanistic Study for Aziridination of Nitroalkenes Mediated by N-Chlorosuccinimide. J. Oleo Sci. 2022;71:897–903. doi: 10.5650/jos.ess21406. [DOI] [PubMed] [Google Scholar]
- 18.Hao F., Nishiwaki N. Chemistry of Nitroaziridines. Heterocycles. 2019;99:54–72. doi: 10.3987/REV-18-SR(F)2. [DOI] [Google Scholar]
- 19.Sebastian A., Srinivasulu V., Abu-Yousef I.A., Gorka O., Al-Tel T.H. Domino Transformations of Ene/Yne Tethered Salicylaldehyde Derivatives: Pluripotent Platforms for the Construction of High sp3 Content and Privileged Architectures. Chem. Eur. J. 2019;25:15710–15735. doi: 10.1002/chem.201902596. [DOI] [PubMed] [Google Scholar]
- 20.Nayar C.R., Ravikumar R. Review: Second Order Nonlinearities of Schiff Bases Derived from Salicylaldehyde and Their Metal Complexes. J. Coord. Chem. 2014;67:1–16. doi: 10.1080/00958972.2013.864759. [DOI] [Google Scholar]
- 21.Masesane I.B., Desta Z.Y. Reactions of Salicylaldehyde and Enolates or Their Equivalents: Versatile Synthetic Routes to Chromane Derivatives. Beilstein. J. Org. Chem. 2012;8:2166–2175. doi: 10.3762/bjoc.8.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kallitsakis M.G., Tancini P.D., Dixit M., Mpourmpakis G., Lykakis I.N. Mechanistic Studies on the Michael Addition of Amines and Hydrazones to Nitrostyrenes: Nitroalkane Elimination via a Retro-aza-Henry-Type Process. J. Org. Chem. 2018;83:1176–1184. doi: 10.1021/acs.joc.7b02637. [DOI] [PubMed] [Google Scholar]
- 23.Yakura T., Nakazawa M., Takino T., Ikeda M. Stereochemistry of 1,3-Dipolar Cycloadditions of Nitrones with (E)-1-Alkyl-2-nitroethenes. Chem. Pharm. Bull. 1992;40:2014–2018. doi: 10.1248/cpb.40.2014. [DOI] [Google Scholar]
- 24.Wakita K., Kabuto C., Akine S., Nemoto T., Kwon E.J. 2001, Release of Software (Yadokari-XG 2009) for Crystal Structure Analyses. Cryst. Soc. Jpn. 2009;51:218–224. doi: 10.5940/jcrsj.51.218. [DOI] [Google Scholar]
- 25.Sheldrick G.M. SHELXT—Integrated Space-Group and Crystal Structure Determination. Acta Crystallogr. 2015;A71:3–8. doi: 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheldrick G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015;C71:3–8. doi: 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Revision C., Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., et al. Gaussian, 09. Gaussian, Inc.; Wallingford, CT, USA: 2010. [Google Scholar]
- 28.Pérez V.T., de Arriba F.Á.L., Monleón L.M., Simón L., Rubio O.H., Sanz F., Morán J.R. A High Yield Procedure for the Preparation of 2-Hydroxynitrostyrenes: Synthesis of Imines and Tetracyclic 1,3-Benzoxazines. Eur. J. Org. Chem. 2014;2014:3242–3248. doi: 10.1002/ejoc.201402016. [DOI] [Google Scholar]
- 29.Liu Y.F., Liu S.N., Zhao P.H., Li X.H., Liang W.J., Liu Y.Q. Synthesis, Characterization and Crystal Structure of trans-2-(2-Hydroxyphenyl)-1-nitroethylene. Asian J. Chem. 2014;26:2475–2478. doi: 10.14233/ajchem.2014.16446. [DOI] [Google Scholar]
- 30.Lewis K.G., Ghosh S.K., Bhuvanesh N., Gladysz J.A. Cobalt(III) Werner Complexes with 1,2-Diphenylethylenediamine Ligands: Readily Available, Inexpensive, and Modular Chiral Hydrogen Bond Donor Catalysts for Enantioselective Organic Synthesis. ACS Cent. Sci. 2015;1:50–56. doi: 10.1021/acscentsci.5b00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vuagnoux-d’Augustin M., Alexakis A. Influenece of the Double-Bond Geometry of the Michael Acceptor on Copper-Catalyzed Asymmetric Conjugate Addition. Eur. J. Org. Chem. 2007:5852–5860. doi: 10.1002/ejoc.200700424. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.