Abstract
We localized five rpoC (β′) mutations affecting Escherichia coli RNA polymerase assembly. The Ts4, XH56, and R120 mutations changed β′ residues conserved throughout eubacteria; the JE10092 mutation occurred in the hypervariable region; rpoC1 (TsX) changed a universally conserved residue and corresponds to yeast rpb1-1. Thus, distinct, predominantly conserved β′ residues participate in interactions holding RNA polymerase together.
Cellular RNA polymerases (RNAP) are multifunctional, multisubunit enzymes. Isolated subunits do not possess RNAP partial functions (e.g., ability to melt DNA or bind nucleotide triphosphates [26]). Thus, functional sites are formed by allosteric changes and/or at subunit interfaces upon assembly of RNAP. Understanding of intra- and intersubunit interactions during enzyme assembly will be necessary to elucidate the mechanism of RNAP action. In Escherichia coli, the dimeric α subunit orchestrates RNAP assembly by interacting with the β subunit (8). The α2β subassembly then binds the β′ subunit to form the catalytic core, α2ββ′.
Conditional mutations had been used to define sites in α that interact with other RNAP subunits. The rpoA112 (R45A) mutation weakens the interaction with β; rpoA101 (C191R) interferes with β′ entry into the complex (7). Biochemical and structural work indicates that Arg45 and Cys191 directly contact β and β′, respectively (6, 25). Mutations in α and in the yeast counterpart, RPB3, that affect α2β formation in their respective systems occur in homologous positions (2), suggesting that the RNAP assembly pathway has been evolutionarily conserved.
The tripartite interaction of β′ with α2β does not easily lend itself to analysis. Consequently, we lack information about regions of β′ involved in RNAP assembly. Eighteen temperature-sensitive strains with mutations in E. coli rpoC, which codes for β′, were isolated by Miller and coworkers in 1976 (12). RNAP purified from some of the mutant strains was temperature sensitive and/or failed to reassemble after denaturation and renaturation (5, 6). Localization of these rpoC mutations could pinpoint β′ regions important for assembly. Unfortunately, many original mutations were lost (14a). We obtained six rpoC assembly mutants—rpoC110, Ts4, XH56, R120, JE10092, and RpoC1—from colleagues and localized the mutations.
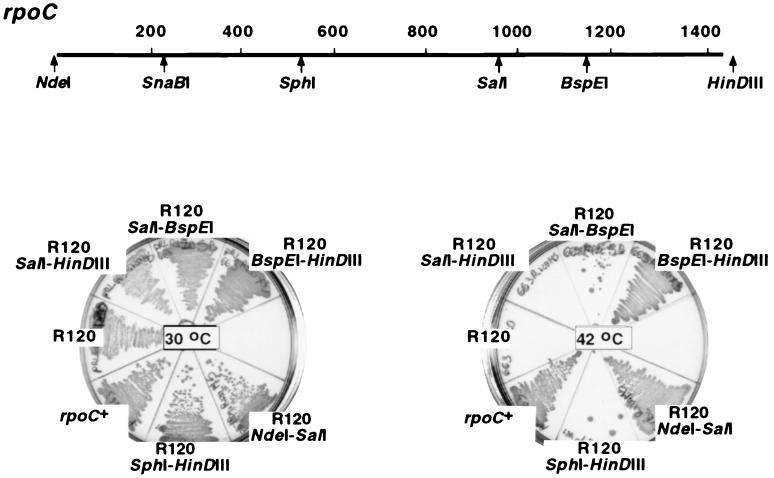
Genomic DNA extracted from mutant cells was used to amplify by PCR the whole rpoC gene. PCR products were cloned in the pCYB2 expression vector (New England Biolabs). A control plasmid, pCYB2β′WT, expressing wild-type rpoC was also constructed. Five strains, R120, Ts4, JE10092, XH56, and RpoC1, grew at a restrictive temperature (42°C) when transformed with pCYB2β′WT, confirming that rpoC mutations caused temperature sensitivity of mutant strains. In contrast, RpoC110 strains obtained from two different sources failed to grow at 42°C in the presence of pCYB2β′WT. Plasmids expressing rpoCR120, rpoCTs4, rpoCXH56, rpoCJE10092, and rpoC1 did not support growth of the 397c (rpoC397) temperature-sensitive strain (3) at 42°C; in contrast, 397c transformed with pCYB2β′WT or pCYB2β′110 grew at 42°C. Therefore, we (i) discontinued further analyses of rpoC110 and (ii) mapped the remaining mutations by swapping fragments between mutant and wild-type plasmids and assaying the ability of hybrid plasmids to support 397c growth at 42°C (Fig. 1). In this way, the determinants of temperature sensitivity were shown to reside in the 1,010-bp SalI-BspEI rpoC fragment (R120, XH56, and JE10092), between the engineered NdeI site at position 1 and the SnaBI site at codon 292 (Ts4), and between the BspEI site at codon 1213 and the HindIII site in the vector (RpoC1). These fragments and the corresponding pCYBβ′WT fragments were sequenced. Sequence comparisons revealed single-nucleotide differences between the wild type and each of the mutants: Ts4 has a GGG (Gly)-to-GAG (Glu) change at codon 181, XH56 has a CGT (Arg)-to-CAT (His) change at codon 883, R120 has a GGT (Gly)-to-GAT (Asp) change at codon 900, JE10092 has a GGT (Gly)-to-GAT (Asp) change at codon 1033, and RpoC1 has a GGT (Gly)-to-GAT (Asp) change at codon 1360. We conclude that G181E, R883H, G900D, G1033D, and G1360D cause the temperature-sensitive phenotypes of Ts4, R120, XH56, JE10092, and RpoC1, respectively. Genetic contexts of these mutations are presented in Fig. 2.
FIG. 1.
Localization of the R120 mutation to the SalI-BspEI fragment of rpoC. (Top) The E. coli rpoC gene and restriction sites used to construct hybrid plasmids are shown (numbers indicate codon positions). (Bottom) Plasmids pCYB2β′WT (labeled rpoC+) and pCYBβ′R120 (labeled R120) and the indicated hybrid plasmids were transformed into strain 397c. The transformants were streaked on Luria-Bertani–ampicillin plates, and the plates were incubated at 30 and 42°C overnight. The absence of growth of cells labeled R120 SalI-BspEI at 42°C indicates that the R120 mutation is within the 1,010-bp SalI-BspEI rpoC fragment.
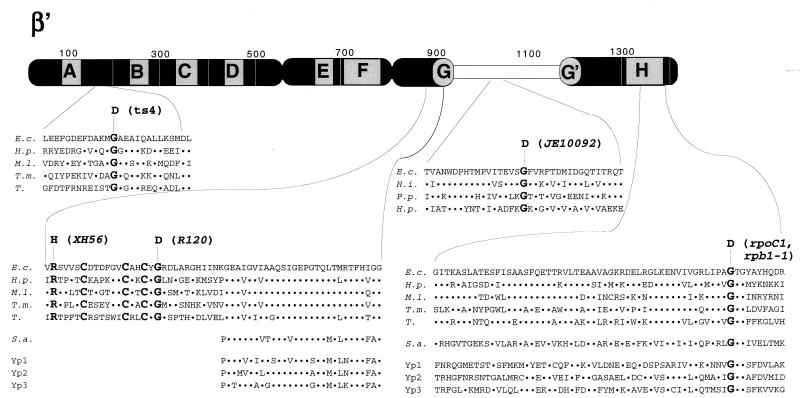
FIG. 2.
Genetic context of rpoC assembly mutants. The thick bar represents the 1,407-amino-acid β′ subunit of E. coli RNAP. Stippled boxes labeled A to H represent segments of β′ highly conserved in evolution (1, 24). The E. coli hypervariable region (24), absent in homologues from most bacteria, archaea, and eukaryotes, is shown by an open box. The amino acid sequences of E. coli β′ (E.c.) harboring assembly mutations are expanded underneath. Homologous amino acid sequences from Haemophilus influenzae (H.i.), Pseudomonas putida (P.p.), Helicobacter pylori (H.p.), Mycobacterium leprae (M.l.), Thermotoga maritima (T.m.), chloroplasts from tobacco (T.), Sulfolobus acidocaldarium (S.a.), and yeast RNAP 1, 2, and 3 (Yp1, Yp2, and Yp3, respectively) are also shown. The dots symbolize identity to the E. coli sequence. Amino acids changed by assembly mutations, as well as three conserved cysteines close to segment G, are highlighted by bold typeface. Note that rpoC1 in E. coli and rpb1-1 in yeast cause the same substitution.
The Ts4 mutation occurred between β′ conserved segments A and B. Gly181 is the most conserved residue in the six-amino-acid motif, MGAEA, that is present only in eubacterial β′ subunits. RNAP purified from Ts4 cells is not temperature sensitive, but biochemical analysis suggested that β′Ts4 is defective in association with α2β (21).
The XH56 and R120 mutations occurred close to conserved segment G, involved in transcript elongation and cleavage (14). RNAPR120 is not temperature sensitive but fails to assemble into active RNAP after denaturation (4). β′XH56 can be assembled into active enzyme in vitro, but RNAPXH56 activity is temperature sensitive (5). Further biochemical analysis will be necessary to determine why the neighboring XH56 and R120 mutations result in different defects. Residues changed by the XH56 and R120 mutations are strictly conserved in eubacteria but are absent from archaeal and eukaryotic β′ homologues. Three additional neighboring β′ residues are conserved in eubacteria: Cys888, Cys895, and Cys898. Groups of cysteines can coordinate structural or catalytic zinc ions in proteins (22). It is tempting to speculate that Cys888, Cys895, and Cys898 coordinate RNAP zinc and that the temperature-sensitive mutations affect RNAP assembly by altering zinc binding.
The JE10092 mutation is a known mutation: E. coli rpoC907 also causes G1033D replacement and affects plasmid replication (17). Compared to the wild type, a fourfold increase in pBR322 copy number was observed in JE10092 grown at 39°C (data not shown). A similar value was reported for RpoC907 (18), confirming that the JE10092 and rpoC907 mutations are allelic. RNAPJE10092 is assembly defective in vivo (15) and is temperature sensitive (23). The JE10092 mutation occurred in the evolutionarily hypervariable region of β′, but in organisms where this region is present Gly1033 is conserved. Our recent analysis shows that the hypervariable region has only limited ability to tolerate mutations and may be indirectly involved in RNAP assembly (24).
The rpoC1 mutation was the first conditional mutation in RNAP to be isolated (11). The mutation changes the universally conserved Gly in segment H. Another temperature-sensitive mutation affecting RNAP assembly, rpoC397, occurred upstream of rpoC1 and resulted in substitution of 52 β′ amino acids distal to position 1355 with 23 unnatural residues (3). RNAPRpoC1 is extremely temperature sensitive, but normal activity of RNAPRpoC1 (and RNAP397C) can be restored by simple addition of purified β′WT under nondenaturing conditions (3, 16). Thus, mutant subunits bind α2β less tightly, suggesting that segment H forms the α2β binding site.
rpoC1 corresponds exactly to the yeast rpb1-1 mutation (19). Similar to rpoC1, rpb1-1 makes yeast temperature sensitive, and all transcription ceases upon temperature upshift. Suppressors of rpb1-1 map in the conserved segment I of the β homologue, RPB2 (14). In E. coli, opr mutations suppressing the temperature-sensitive phenotype of RpoC1 were isolated (10). Mapping of these suppressors is currently underway.
In summary, our data demonstrate that point mutations in β′ dramatically interfere with RNAP assembly. Four of five mutations changed evolutionarily conserved residues of β′ and may define sites of intersubunit contacts that hold the RNAP molecule together. Further in-depth analysis of mutant enzymes and site-specific mutagenesis of rpoC to generate new assembly-deficient RNAP should clarify the role of these β′ regions in RNAP assembly.
ACKNOWLEDGMENTS
This work was supported by NIH grant GM59295-01 and a Burroughs Wellcome Career Award to K.S.
We are grateful to R. Hayward (University of Edinburgh) for the R120, XH56, and Ts4 strains; to D. J. Jin (NIH) for JE10092; to E. Kalayeva (Moscow Institute of Molecular Genetics) for the T16 (rpoC1) strain; and to Y. Ohnishi (University of Tokushima) and the Yale Genetic Center for RpoC110 strains.
D.M. and E.C.N. contributed equally to this work.
REFERENCES
- 1.Allison L A, Moyle M, Shales M, Ingles C J. Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell. 1985;42:599–610. doi: 10.1016/0092-8674(85)90117-5. [DOI] [PubMed] [Google Scholar]
- 2.Azuma Y, Yasui K, Yamagishi M, Ishihama A. Isolation of thermolabile mutant RNA polymerase II from fission yeast Schizosaccharomyces pombe with mutations in the subunit 3 gene. J Biochem. 1995;118:216–220. doi: 10.1093/oxfordjournals.jbchem.a124881. [DOI] [PubMed] [Google Scholar]
- 3.Christie G E, Cale S B, Isaksson L A, Jin D J, Xu M, Sauer B, Calendar R. Escherichia coli rpoC397 encodes a temperature-sensitive C-terminal frameshift in the β′ subunit of RNA polymerase that blocks growth of bacteriophage P2. J Bacteriol. 1996;178:6991–6993. doi: 10.1128/jb.178.23.6991-6993.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross G C, Fields D A, Bautz E K F. Characterization of ts beta′ mutant RNA polymerase of Escherichia coli. Mol Gen Genet. 1976;147:337–341. doi: 10.1007/BF00582886. [DOI] [PubMed] [Google Scholar]
- 5.Gross G C, Fields D A, Bautz E K F. Temperature-sensitive mutants of Escherichia coli with defects in the assembly of RNA polymerase in vitro. Eur J Biochem. 1977;81:333–338. doi: 10.1111/j.1432-1033.1977.tb11956.x. [DOI] [PubMed] [Google Scholar]
- 6.Heyduk T, Heyduk E, Severinov K, Tang H, Ebright R H. Rapid epitope mapping by hydroxyl-radical protein footprinting: determinants of RNA polymerase alpha subunit for interaction with beta, beta′ and sigma subunits. Proc Natl Acad Sci USA. 1996;93:10162–10166. doi: 10.1073/pnas.93.19.10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Igarashi K, Fujita N, Ishihama A. Sequence analysis of two temperature-sensitive mutations in the alpha subunit gene (rpoA) of Escherichia coli RNA polymerase. Nucleic Acids Res. 1990;18:5945–5948. doi: 10.1093/nar/18.20.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishihama A. Molecular assembly and functional modulation of Escherichia coli RNA polymerase. Adv Biophys. 1990;26:19–31. doi: 10.1016/0065-227x(90)90005-e. [DOI] [PubMed] [Google Scholar]
- 9.Ito R, Akimoto S, Ohnishi Y. Expression of srnB gene of F plasmid by altered RNA polymerase in Escherichia coli. Biochim Biophys Acta. 1985;825:1–11. doi: 10.1016/0167-4781(85)90073-9. [DOI] [PubMed] [Google Scholar]
- 10.Kalyaeva E S, Sever I S, Nikiforov V G, Danilevskaya O N. A mutation suppressing the overproduction of RNA polymerase ββ′ subunits in the RpoC1 strain of Escherichia coli. Mol Gen Genet. 1980;178:669–674. doi: 10.1007/BF00337877. [DOI] [PubMed] [Google Scholar]
- 11.Khesin R B, Mindlin S Z, Gorlenko Z M, Ilyina T S. Temperature sensitive mutations affecting RNA synthesis in Escherichia coli. Mol Gen Genet. 1968;103:194–208. doi: 10.1007/BF00427146. [DOI] [PubMed] [Google Scholar]
- 12.Kirschbaum J B, Claeys I V, Nasi S, Molholt B, Miller J H. Temperature-sensitive RNA polymerase mutants with altered subunit synthesis and degradation. Proc Natl Acad Sci USA. 1975;72:2375–2379. doi: 10.1073/pnas.72.6.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markovtsov V, Mustaev A, Goldfarb A. Protein-RNA interactions in the active center of transcription elongation complex. Proc Natl Acad Sci USA. 1996;93:3221–3226. doi: 10.1073/pnas.93.8.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin C, Okamura S, Young R. Genetic exploration of interactive domains in RNA polymerase II subunits. Mol Cell Biol. 1990;10:1908–1914. doi: 10.1128/mcb.10.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Miller, J., and E. Bautz. Personal communication.
- 15.Nakada N, Yoshinaga K, Ishihama A, Nagasawa-Fujimori H. Non-coordinate synthesis of RNA polymerase ββ′ subunits in a temperature-sensitive β′-subunit mutant of Escherichia coli. Mol Gen Genet. 1982;188:173–178. doi: 10.1007/BF00332671. [DOI] [PubMed] [Google Scholar]
- 16.Panny S R, Heil A, Mazus B, Palm P, Zillig W, Mindlin S Z, Ilyina T S, Khesin R B. A temperature sensitive mutation of the β′-subunit of DNA-dependent RNA polymerase from E. coli T16. FEBS Lett. 1974;48:241–245. doi: 10.1016/0014-5793(74)80477-1. [DOI] [PubMed] [Google Scholar]
- 17.Petersen S K, Hansen F G. A missense mutation in the rpoC gene affects chromosomal replication control in Escherichia coli. J Bacteriol. 1991;173:5200–5206. doi: 10.1128/jb.173.16.5200-5206.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasmussen K V, Atlung T, Kerzman G, Hansen G E, Hansen F G. Conditional change of DNA replication control in an RNA polymerase mutant of Escherichia coli. J Bacteriol. 1983;154:443–451. doi: 10.1128/jb.154.1.443-451.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scafe C, Martin C, Nonet M, Podos S, Okamura S, Young R A. Conditional mutations occur predominantly in highly conserved residues of RNA polymerase II subunits. Mol Cell Biol. 1990;10:1270–1275. doi: 10.1128/mcb.10.3.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taketo M, Ishihama A. Biosynthesis of RNA polymerase in Escherichia coli. IV. Accumulation of intermediates in mutants defective in the subunit assembly. J Mol Biol. 1976;102:297–310. doi: 10.1016/s0022-2836(76)80055-1. [DOI] [PubMed] [Google Scholar]
- 21.Taketo M, Ishihama A, Kirschbaum J B. Altered synthesis and stability of RNA polymerase holoenzyme subunits in mutants of Escherichia coli with mutations in the β or β′ subunit genes. Mol Gen Genet. 1976;147:139–143. doi: 10.1007/BF00267565. [DOI] [PubMed] [Google Scholar]
- 22.Valee B L, Auld D S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990;29:5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- 23.Yoshinaga K, Suguira M. Physiological studies on a temperature-sensitive Escherichia coli mutant with an altered RNA polymerase β′-subunit. Biochim Biophys Acta. 1977;479:172–179. doi: 10.1016/0005-2787(77)90137-x. [DOI] [PubMed] [Google Scholar]
- 24.Zakharova N, Bass I A, Arsenieva E, Nikiforov V, Severinov K. Mutations in and monoclonal antibody binding to evolutionary hypervariable region of E. coli RNA polymerase β′ subunit inhibit transcript cleavage and transcript elongation. J Biol Chem. 1998;273:19371–19374. doi: 10.1074/jbc.273.38.24912. [DOI] [PubMed] [Google Scholar]
- 25.Zhang G, Darst S A. Structure of the Escherichia coli RNA polymerase alpha subunit amino-terminal domain. Science. 1998;281:262–266. doi: 10.1126/science.281.5374.262. [DOI] [PubMed] [Google Scholar]
- 26.Zillig W, Palm P, Heil A. Function and reassembly of subunits of DNA-dependent RNA polymerase. In: Losick R, Chamberlin M, editors. RNA polymerase. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1976. pp. 101–126. [Google Scholar]