In the 1960s bacterial bioluminescence attracted increasing interest among microbiologists and biochemists. Plating seawater samples taken from virtually any place on the globe revealed the presence of bioluminescent bacteria, but at a rather low level of abundance, not more than a few cells per milliliter. A question frequently raised was what function the light emission might have in such “free-living” planktonic bioluminescent bacteria. The question was even more compelling because the maximum light emission of these bacteria under ideal conditions in the laboratory is about 103 to 104 photons s−1 cell−1. Even at this level of luminescence, populations found free in seawater would produce nowhere near enough light to have physiological or ecological significance.
The answer, it turns out, is that there is not a function for bioluminescence when the bacteria are planktonic in seawater. Such cells do not produce luciferase and do not emit light. This answer, as well as new insights about cell-to-cell communication and gene regulation in many different bacterial species, came from experiments designed to explain what seemed a curious physiological phenomenon observed in the laboratory. The initial research in this area was published in the Journal of Bacteriology in the early 1970s. The editors of this journal showed considerable wisdom and foresight in accepting these research papers. It was not obvious that they would form the cornerstone of a very active research area some 30 years later, and the concepts in these papers were not readily accepted by the scientific community.
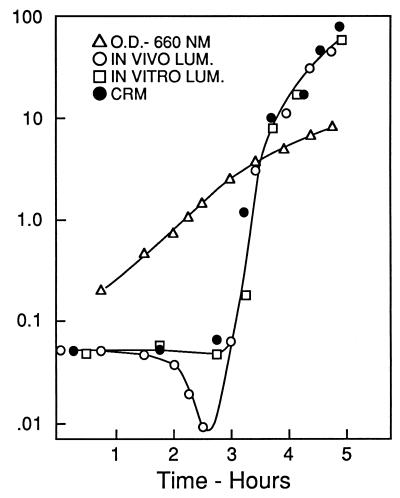
The basic observation was that in newly inoculated cultures of a luminescent marine bacterium like Vibrio fischeri, the onset of exponential growth occurs without a lag but bioluminescence does not increase until mid-logarithmic phase, when it literally shoots up. This is shown in Fig. 1, taken from the original report of the phenomenon (14), in which the lag and subsequent sharp rise in luminescence were attributed to transcriptional regulation and referred to as autoinduction. The figure also shows that extractable luciferase activity and the amount of luciferase protein rise in parallel with in vivo bioluminescence. Experiments carried out with inhibitors of RNA and protein synthesis indicated that in a freshly inoculated culture the luciferase genes are not transcribed; neither luciferase mRNA nor the protein was being synthesized during the eclipse period, as it was called. The autoinduction was attributed to a substance produced by the bacteria themselves, which was therefore dubbed the autoinducer. Autoinduction showed characteristics of a developmental process and differential gene expression. This analogy to development did not catch on; at the time bacteria were generally viewed as undifferentiated cells with a single-minded program focused on growth and division. This view was held in spite of the fact (hindsight shows) that there was also some evidence suggesting a role for cell-to-cell signals in the control of competence gene expression in gram-positive bacteria (16).
FIG. 1.
Time course for development of luminescence and luciferase as compared with growth, measured by optical density. Luciferase was measured with antibody as cross-reacting material (CRM); luminescence in vivo and in vitro was measured photometrically. Reprinted from reference 14.
Albert Szent-Gyorgyi has been quoted as saying, “Research is seeing what everyone else has seen and thinking what no one else has thought.” Indeed, autoinduction of bacterial luminescence was evident earlier in the work of Farghaly (5), who found that luminescence lagged well behind growth. He did not comment other than to say that it always occurred, but little could have been said anyway, since this was prior to knowledge of the structure of DNA, transcription and its activation, mRNA, and protein synthesis. The framework for understanding the molecular basis of autoinduction did not really exist.
Observations of this phenomenon were made in the Hastings laboratory in the 1960s, reported first in a presentation at the annual American Society for Microbiology meeting in 1968 (13). Learning of this, Kempner and Hanson were intrigued and undertook some experiments of their own (12). They concluded that the failure to emit light during the first stages of growth was due to an inhibitor in the complex medium and that its action was transient because it was removed by metabolic action of the bacteria themselves. They also concluded that the putative inhibitor, which they characterized only with regard to its dialyzability, acted by binding directly to the luciferase. This explanation fit better with the information available at the time on gene expression in other bacteria. Why the decrease in luminescence during the first hours took so long to occur and why the rise of luminescence after the eclipse was so rapid could not be explained. Also unexplained was the observation by the Hastings laboratory that the phenomenon occurred in a minimal medium (which Farghaly had also used), containing only salts and glycerol, in which the putative inhibitor(s) would presumably not be present.
In his Journal of Bacteriology study, Eberhard reanalyzed autoinduction in both minimal and complex media and with two strains (species, but not so recognized at the time) of luminous bacteria (2) but avoided the use of the terms repressor and inducer because the structures and mechanisms of action were not known. It was he, with coworkers, however, who was later responsible for the first determination of the structure of an autoinducer, an acylhomoserine lactone in V. fischeri (4). In his 1972 paper he had already shown that the activators from the different species were non-cross-reacting and differed in stability towards heating. He confirmed that an inhibitor(s) was present in complex medium but absent in minimal medium and that the phenomenon of autoinduction occurred in minimal medium, attributable to the production of an activator by the cells themselves.
Knowing that the luciferase gene is not transcribed at low cell densities because the autoinducer cannot accumulate to the level needed, the more interesting question then became, what purpose does autoinduction serve? With the isolation and characterization of bacteria from light organs of fish (10), the answer became evident and compelling. The bacteria in these light organs are packed in like sardines, about 1010 per ml, so an autoinducer can accumulate and the bacteria can emit a very bright light, which the fish uses for its own purpose (11). But the bacteria that overflow from such organs to the open sea will not produce more luciferase in their new environment. They can survive long periods in seawater, indeed for many years in laboratory experiments (3), with little or no growth.
The autoinduction of bacterial luminescence has now been worked out in considerable mechanistic detail. It is evident that it falls into the category of cell-cell communication and that the luminescence genes are activated under conditions of high cell density where the aggregate light emission is bright enough to be seen and have functional importance. What is not evident is why it was not perceived 30 years ago that bacteria other than the bioluminescent bacteria would be found with other genes having a similar developmental differentiation. This was certainly foreshadowed and suggested in a report on alloinducer signals (signals produced by heterologous species) of bacterial luminescence in the late 1970s (9).
Indeed, it was not until the 1990s that mounting evidence, including sequence similarities with the autoinduction genes for the bioluminescent system and identification of acylhomoserine lactones in other bacteria, led to our current view that bacterial cell-to-cell signaling is a common phenomenon. Again, a Journal of Bacteriology article, a minireview published in 1994, was seminal. Here the term quorum sensing was coined (8) and used in the title to encourage people to read on as much as anything else. The rapidly developing field of bacterial cell-to-cell signaling has somehow crystallized around this term.
It is now common knowledge among microbiologists that quorum-sensing systems analogous to those described for the luminescent marine bacteria regulate gene expression in a great variety of gram-negative bacteria (for example see references 1, 6–8, and 17). In fact, quorum sensing controls expression of important virulence factors in pathogenic bacteria like Pseudomonas aeruginosa (for reviews see references 6 and 17). It has now been clearly established that cell-to-cell signals control expression of competence genes in streptococci and bacilli, and the identities of the molecules have been determined. Furthermore, chemical signals are involved in control of other genes in other gram-positive bacteria, for example in control of virulence genes in staphylococci (for a recent review on signaling in gram-positive bacteria see reference 15). The gram-positive bacteria use peptides rather than the acylhomoserine lactone signals that are common in gram-negative bacteria. One wonders if other secondary metabolites may also have roles as signals in bacterial communication. We may have just scratched the surface of the language of bacterial communication.
Footnotes
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1.Dunny G M, Winans S C. Cell-cell signaling in bacteria. Washington, D.C: ASM Press; 1999. [Google Scholar]
- 2.Eberhard A. Inhibition and activation of bacterial luciferase synthesis. J Bacteriol. 1972;109:1101–1105. doi: 10.1128/jb.109.3.1101-1105.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eberhard A. Long term survival of bioluminescent bacteria in sea water. Biol Bull. 1972;143:459. [Google Scholar]
- 4.Eberhard A, Burlingame A L, Eberhard C, Kenyon G L, Nealson K H, Oppenheimer N J. Stuctural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981;20:2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- 5.Farghaly A H. Factors influencing the growth and light production of luminous bacteria. J Cell Comp Physiol. 1950;36:165–184. doi: 10.1002/jcp.1030360205. [DOI] [PubMed] [Google Scholar]
- 6.Fuqua C, Greenberg E P. Self perception in bacteria. Curr Opin Microbiol. 1998;1:183–189. doi: 10.1016/s1369-5274(98)80009-x. [DOI] [PubMed] [Google Scholar]
- 7.Fuqua C, Winans S C, Greenberg E P. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing gene regulators. Annu Rev Microbiol. 1996;50:591–564. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 8.Fuqua W C, Winans S C, Greenberg E P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberg E P, Hastings J W, Ulitzur S. Induction of luciferase synthesis in Beneckea harveyi by other marine bacteria. Arch Microbiol. 1979;120:87–91. [Google Scholar]
- 10.Hastings J W, Mitchell G. Endosymbiotic bioluminescent bacteria from the light organ of pony fish. Biol Bull. 1971;141:261–268. [Google Scholar]
- 11.Hastings J W. Light to hide by: ventral luminescence to camouflage the silhouette. Science. 1971;173:1016–1017. doi: 10.1126/science.173.4001.1016. [DOI] [PubMed] [Google Scholar]
- 12.Kempner E, Hanson F. Aspects of light production by Photobacterium fischeri. J Bacteriol. 1968;95:975–979. doi: 10.1128/jb.95.3.975-979.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nealson K H, Platt T, Hastings J W. Bacterial luciferase as an inducible enzyme. Bacteriol. Proc. GP2; 1968. [Google Scholar]
- 14.Nealson K H, Platt T, Hastings J W. Cellular control of the synthesis and activity of the bacterial luminescence system. J Bacteriol. 1970;104:313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novick R P, Muir T W. Virulence gene regulation by peptides in staphylococci and other Gram-positive bacteria. Curr Opin Microbiol. 1999;2:40–45. doi: 10.1016/s1369-5274(99)80007-1. [DOI] [PubMed] [Google Scholar]
- 16.Tomaz A, Hotchkiss R D. Regulation of the transformability of pneumococcal cultures by macromolecular cell products. Proc Natl Acad Sci USA. 1964;51:480–487. doi: 10.1073/pnas.51.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Delden C, Iglewski B H. Cell-to-cell signaling in Pseudmonas aeruginosa. Emerg Infect Dis. 1998;4:551–560. doi: 10.3201/eid0404.980405. [DOI] [PMC free article] [PubMed] [Google Scholar]