Abstract
Background: Delay in dietetic service provision for upper gastrointestinal cancer exacerbates disease-related malnutrition and consequently increases morbidity and mortality. Dietetic services are usually referral-based and provided face-to-face in inpatient or outpatient settings, which can delay the commencement of nutrition care. The aim of this study was to provide intensive dietetic intervention close to the time of diagnosis for upper gastrointestinal cancer and assess the effect on quality-adjusted life years. Methods: A three-arm randomised controlled trial of adults newly diagnosed with upper gastrointestinal cancer was performed. A behavioural-based, individually tailored, symptom-directed nutrition intervention was provided in addition to usual care, delivered by a dietitian using a telephone (synchronously) or a mobile application (asynchronously) for 18 weeks, compared with a usual care control group. Data were collected at baseline, three, six, and twelve months post-randomisation. The primary outcome was quality-adjusted life years (EQ-5D-5L quality of life assessment tool). Data were analysed using linear mixed models. Results: One hundred and eleven participants were randomised. Quality-adjusted life years were not different in the intervention groups compared with control (telephone: mean (95% CI) 0.04 (0.43, 2.3), p = 0.998; App: −0.08 (−0.18, 0.02), p = 0.135) after adjustment for baseline, nutrition risk status, age, and gender. Survival was similar between groups over 12 months. The asynchronous mobile app group had a greater number of withdrawals compared with the telephone group. Conclusion: Early and intensive nutrition counselling, delivered at home, during anticancer treatment did not change quality-adjusted life years or survival over 12 months compared with usual care. Behavioural counselling alone was unable to achieve nutritional adequacy. Dietetic services delivered asynchronously using a mobile app had low acceptance for patients undergoing anticancer treatment. Trial Registration: 27 January 2017 Australian and New Zealand Clinical Trial Registry, ACTRN12617000152325.
Keywords: mHealth, malnutrition, upper gastrointestinal cancer, dietetic intervention, quality-adjusted life years, behaviour change
1. Introduction
Cancers of the stomach, oesophagus, and pancreas (upper gastrointestinal, UGI) are leading causes of cancer deaths worldwide [1]. These cancers and treatments cause declines in nutritional status and quality of life (QoL) [2,3]. Weight loss prior to chemotherapy has been associated with poorer overall survival in numerous cancer types, including gastric cancer [4]. Weight loss is often a presenting feature of UGI cancers, although the time to initiation of nutrition intervention varies considerably, resulting in the inconsistent provision of dietetic services to patients [5,6,7]. In practice, referral to dietetics services occurs very late in the care pathway, enabling nutritional decline and malnutrition to become established and nutrition impact symptoms to become barriers to effective food-based interventions leading to poor QoL [3,6,7,8,9].
A systematic review of the literature found that for people with cancer (including gastrointestinal cancers), a better nutritional state is associated with better QoL [10]. There is limited high-quality evidence to determine whether nutrition intervention during cancer treatment improves QoL and nutritional status in people with UGI cancer [11,12,13,14,15]. Rigorous randomised controlled trials to determine the optimal timing, duration, and intensity of nutrition intervention during cancer treatment are needed [5]. The rapid growth in different approaches to delivering health interventions provides new service delivery opportunities that may be advantageous over more traditional approaches such as face-to-face and telephone follow-up. For example, in terms of accessibility, face-to-face services require both patient and dietitian to be available at the same time and in the same place which may be difficult during cancer treatment [7,16]. Telephone-based services may be more accessible, but they still require patients and therapists to be available at the same time. Mobile applications allow patients and clinicians to connect asynchronously and can also be structured to facilitate the application of behaviour change techniques such as goal setting and review, delivered at a time the patient wishes to access the information [17,18]. A recent review identified that asynchronous delivery modes can be used to elicit behaviour change to improve health outcomes in cancer patients compared with usual care or no intervention [19]. However, there has been no direct comparison of the same intervention delivered synchronously compared with asynchronously, and compared with usual care [19].
The objective of this trial was to deliver early and intensive dietitian-led nutrition counselling to people commencing treatment for UGI cancer, and assess the effects on quality-adjusted life years (QALY) lived compared with usual care.
2. Materials and Methods
2.1. Study Design
A three-arm randomised controlled trial was performed. Ethical approval was granted by the Monash Health Human Research Ethics Committee (14 October 2016 HREC/16/MonH/290). Site-specific authorisation was granted for all sites (Monash Health, Cabrini Health and Peninsula Health). Participants provided informed verbal consent to participate. The trial was registered prospectively on the Australian and New Zealand Clinical Trial Registry (Trial ID: ACTRN12617000152325 27 January 2017). The detailed protocol has been published [20].
2.2. Participants and Setting
Participants were newly diagnosed (<4 weeks) with UGI cancer and planned to commence surgical and/or medical (chemo- and/or radiotherapy) cancer treatment. Participants were recruited from health services (public and private) in southeast Victoria, Australia.
2.3. Eligibility
Patients who had received urgent surgical treatment prior to recruitment were eligible. Commencement of chemotherapy or radiotherapy prior to recruitment deemed the person ineligible.
2.4. Recruitment
Recruitment occurred between April 2017 and July 2019. Individuals were screened for eligibility by surgeons, ward dietitians, multidisciplinary team discussions, or screening of weekly outpatient upper gastrointestinal clinic list. Eligible participants were contacted either in person or via telephone and invited to participate.
2.5. Randomisation and Blinding
Randomisation was completed by an independent biostatistician. A permuted block randomisation with two group stratification (Malnutrition Screening Tool (MST) score of <3 or ≥3 [21]) was performed using computer-generated random numbers (STATA version 14, StataCorp LP, College Station, TX, USA). Researchers conducting recruitment, data collection, and data analysis were blinded to group allocation. Group allocation was concealed in opaque sealed envelopes and revealed to the participant by the research dietitian conducting the nutrition intervention.
2.6. Usual Dietetic Care and Control Group
Dietic care was referral-based, reliant on the physician identifying the need. Participants who attended a UGI outpatient service were screened for nutritional risk via MST [21], with a score of ≥3 triggering a referral to a general dietetic outpatient clinic, which required participants to wait up to several weeks to access the service [3]. Participants who had an inpatient admission, which could be up to 6 weeks after the time of diagnosis [22], were screened by nursing staff, and if nutritional risk was indicated they were referred to the dietetics service; or immediate referral if admission was for major upper gastrointestinal and hepatobiliary surgeries. The control group received usual care.
2.7. Intervention Groups
The intervention commenced as early as possible from the time of diagnosis and ran as a ‘centralised’ dietetics service for 18 weeks additional to usual care. The intervention was delivered by an experienced oncology dietitian as reported elsewhere [20]. Briefly, tailored nutritional recommendations were co-developed, based on medical history and nutrition impact symptoms. Goals were set and guidance was provided on how to perform the behaviour; goal achievement was monitored. Nutrition intervention was via telephone (synchronously) or an internet-enabled mobile app ‘myPace’ (random allocation). myPace was designed underpinned by behaviour change theory [23] and enables participants to self-monitor their goal attainment and body weight. For both groups, reviews were planned weekly or fortnightly (depending on need). If participants contacted the dietitian in addition to scheduled reviews or sought a private consultation with a dietitian external to the study, this was documented. Further information is reported in Supplementary File S1 according to the TIDiER checklist.
2.8. Community Involvement
Two community members contributed to the advisory committee over the duration of the study, from planning through to results discussion (2016–2020). Through reflecting and sharing their experience of cancer treatment, they contributed to procedures of recruitment, intervention provision, data collection procedures and interpretation of results.
2.9. Data Collection
Outcome data were collected by a blinded assessor at baseline, three, six, and twelve months via telephone or face-to-face.
2.10. Primary Outcome
QALY were calculated using the EuroQol 5D-5L instrument (EQ-5D-5L) [24] using the area under the curve calculation approach. Utility values were determined at each time point before converting to the QALY lived, using the approach reported by Norma et al. 2013 [25] (model D); a utility value of zero was given from the date of death onwards.
2.11. Secondary Outcomes
Cancer-specific quality of life was measured using the EORTC-C30 scale [26,27]. Date of death was recorded over the 12-month follow up period to examine survival. Nutritional status was measured using the short form version of the Patient-Generated Subjective Global Assessment (PG-SGASF); this is a change from the published protocol [20] as the physical examination component of the PG-SGA was not possible due to the majority of follow up data collection not being conducted in-person [28,29,30]. Participants reported on their weight and weight history, food intake history, nutrition impact symptoms, and activities of daily living and function. Changes in self-reported body weight were also assessed and cross-referenced with available medical records for accuracy.
2.12. Sample Size Calculation
Pilot data informed the sample size estimate [3], with a smaller standardised effect size estimate (0.70) for the present study, which estimated that n = 33 participants per group were required to attain 80% power for comparisons with the control group using a two-tailed alpha of 0.05. We inflated this to n = 37 per group to account for potential drop-outs.
2.13. Data Analysis
Analysis was performed in STATA version 14 (StataCorp LP, College Station, TX, USA). Participants who died were ascribed a score of zero from their recorded date of death. Multiple imputation was used to replace other missing individual data points for conducting comparisons in mean QALY per participant between groups [31,32,33]. Supplementary File S2 contains a detailed analysis to examine the patterns of missing data. The missing data were likely to be missing not at random; therefore, sensitivity analyses were conducted following multiple imputation, examining the potential impact on the base-case multiple imputation result. These sensitivity analyses systematically varied the magnitude of the imputed utility values by +0.1, −0.1, −0.25, and −0.5 (reflecting that half of the missing utility values were likely to be missing because they were low/participants were very unwell). Groups were compared using regression analyses adjusted for baseline EQ-5D utility values, age, gender, baseline PG-SGA short form scores, and cancer location (oesophageal, gastric, and pancreatic). QALY data from individual participants were censored at the last available measurement if the participant was lost to follow-up or withdrew from the study.
Survival was assessed using Cox proportional hazards analysis, with adjustment for age, gender, baseline PG-SGA short form scores, and cancer location. Other secondary outcomes were compared between groups using linear mixed model analyses, adjusting for baseline values of the secondary outcome and age, gender, baseline PG-SGA short form scores, and cancer location.
3. Results
3.1. Participants
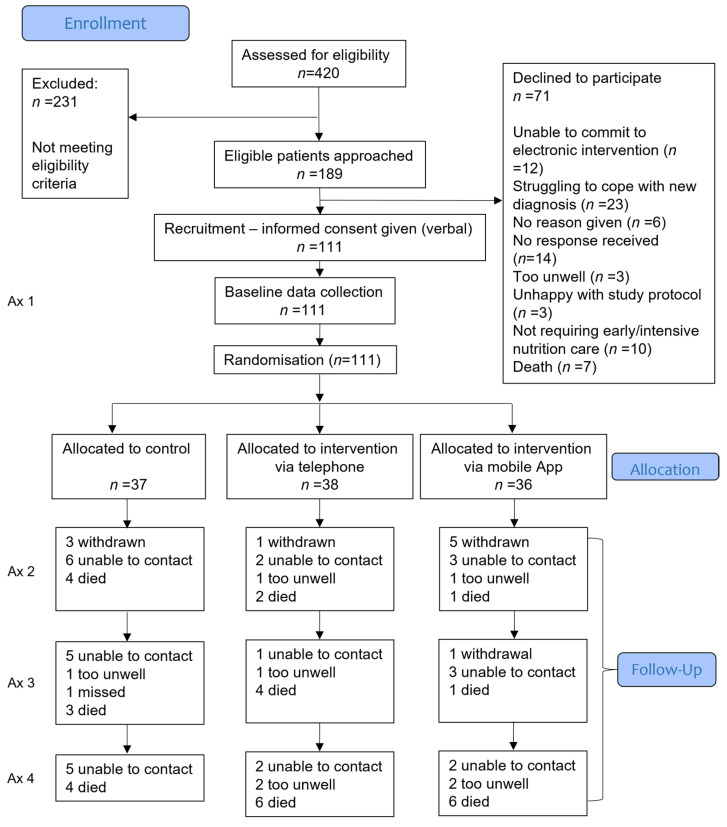
Of the 189 people identified as eligible, 111 consented to be randomised between April 2017 and July 2019 (Figure 1). Follow-up data collection was performed in July 2020. Participant characteristics are presented in Table 1.
Figure 1.
Flowchart of participants over the 12-month follow-up. Multiple imputation was used to replace missing individual data points (other than due to death) for conducting comparisons in mean QALY per participant between groups. Secondary data were analysed without imputation for missing data. Ax, assessment.
Table 1.
Participant demographics at randomisation.
| Control (n = 37) |
Telephone (n = 38) |
Mobile App (n = 36) |
|
|---|---|---|---|
| Age—mean (sd) | 63.2 (9.9) | 67.5 (10.3) | 66.6 (9.7) |
| Gender—n (%) | |||
| Male | 23 (62) | 25 (66) | 26 (72) |
| Female | 14 (38) | 13 (34) | 10 (28) |
| Tumour location—n (%) | |||
| Oesophageal | 13 (35) | 16 (42) | 17 (47) |
| Gastric | 8 (22) | 4 (11) | 9 (25) |
| Pancreatic | 16 (43) | 18 (47) | 10 (28) |
| Clinical stage of cancer—n (%) | |||
| Resectable | 16 (43) | 15 (39) | 18 (44) |
| Borderline resectable | 2 (5) | 1 (3) | 3 (5) |
| Locally advanced | 12 (32) | 12 (32) | 9 (30) |
| Metastatic | 7 (19) | 10 (26) | 6 (21) |
| Height—mean (sd) | 168.9 (10.7) | 170.7 (8.9) | 171.6 (9.3) |
| Weight—mean (sd) | 75.0 (20.0) | 71.9 (12.7) | 76.4 (14.7) |
| EQ-5D-5L—median (IQR) | |||
| Mobility | 1 (1, 2) | 1 (1, 1) | 1 (1, 2) |
| Personal care | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) |
| Usual activities | 1 (1, 3) | 1 (1, 1) | 1 (1, 3) |
| Pain or discomfort | 2 (1, 3) | 2 (1, 2) | 2 (1, 3) |
| Anxiety or Depression | 2 (1, 2) | 1.5 (1, 3) | 2 (1, 2) |
| EQ-5D-5L-utility score—mean (sd) | 0.68 (0.19) | 0.71 (0.23) | 0.65 (0.20) |
| EQ-5D-5L visual analogue scale mean (sd) | 66.16 (20.27) | 65.04 (22.9) | 62.08 (22.01) |
| First language—n (%) | |||
| English | 33 (89) | 33 (89) | 30 (86) |
| Familiarity with technology n “yes” (%) | |||
| Do you use email? | 33 (89%) | 29 (76) | 29 (81) |
| Do you have a smartphone? | 30 (81%) | 32 (84) | 30 (83) |
| Do you have a tablet device? | 16 (43%) | 25 (66)) | 21 (58) |
| Do you feel confident to communicate with your health professional using electronic messages from your smartphone or tablet device? | 33 (89%) | 31 (82) | 26 (72) |
| Do you regularly (at least once per day) use your smartphone or tablet device for purposes other than receiving or making phone calls? | 30 (81%) | 25 (66) | 26 (72) |
| PG-SGASF score—mean (sd) | 8.4 (6.5) | 8.5 (6.2) | 8.5 (6.5) |
| EORTC QLQ-C30 score—mean (sd) | |||
| Global health | 59.32 (25.72) | 63.41 (26.17) | 61.22 (24.60) |
| Physical functioning | 79.28 (22.21) | 81.23 (20.08) | 77.22 (25.67) |
| Role functioning | 63.51 (36.61) | 67.54 (34.43) | 65.28 (35.04) |
| Emotional functioning | 70.49 (21.02) | 72.15 (21.26) | 73.38 (25.34) |
| Cognitive functioning | 83.33 (21.52) | 85.09 (18.90) | 76.85 (23.66) |
| Social functioning | 72.52 (29.71) | 71.49 (30.49) | 74.07 (32.96) |
| Fatigue | 38.74 (25.48) | 35.38 (29.21) | 42.90 (31.44) |
| Nausea and vomiting | 10.81 (21.59) | 11.84 (20.47) | 11.11 (18.26) |
| Pain | 27.93 (27.51) | 25.44 (29.70) | 29.63 (33.36) |
| Dyspnoea | 8.10 (18.27) | 13.16 (23.94) | 12.96 (18.30) |
| Insomnia | 46.85 (36.39) | 28.95 (29.17) | 31.48 (29.76) |
| Appetite loss | 26.13 (30.60) | 35.09 (34.61) | 28.70 (35.77) |
| Constipation | 19.82 (29.89) | 17.54 (28.72) | 22.22 (31.87) |
| Diarrhoea | 10.81 (23.64) | 7.02 (22.13) | 4.63 (19.76) |
| Financial difficulties | 10.81 (22.30) | 15.79 (29.75) | 16.66 (40.19) |
EQ-5D-5L, EuroQol 5D-5L instrument; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Question—Core 30; PG-SGA, Patient-Generated Subjective Global Assessment; sd standard deviation.
3.2. Dietetic Contact
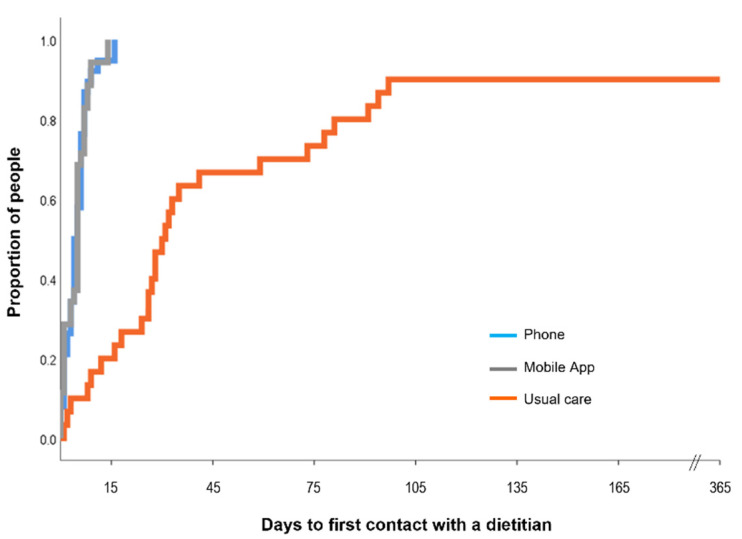
First contact with a dietitian was markedly earlier in the intervention groups (Figure 2). The frequency of contact with a usual care dietitian at each follow-up was similar across the three groups (Table 2), demonstrating that intervention groups had earlier and more intensive nutrition intervention compared with usual care participants.
Figure 2.
Time from randomisation to first contact with a dietitian. The first contact with a dietitian was significantly delayed in the usual care group compared with the intervention groups (telephone mean (SD) 5 (4) days, range 0–16 days n = 38; mobile app 5 (4) days, range 0–14 days, n = 33 noting that three participants withdrew prior to first contact) compared with the control group (70 (104) days, range 1–365 days, n = 30; n = 7 reported no data about contact with a dietitian). Data were censored at the end of the follow up period of 365 days.
Table 2.
Frequency of contact with a dietitian as part of usual care *.
| Control | Telephone | Mobile App | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 Months (n = 26) |
6 Months (n = 20) |
12 Months (n = 18) |
3 Months (n = 32) |
6 Months (n = 28) |
12 Months (n = 21) |
3 Months (n = 26) |
6 Months (n = 24) |
12 Months (n = 17) |
|
| Dietitian contact prior to this follow-up—n “Yes” (%) | 23 (88) | 12 (60) | 11 (61) | 21 (66) | 15 (54) | 6 (29) | 16 (61) | 16 (67) | 8 (47) |
| Median number of contacts with dietitian (range) | 2.5 (0–13) | 2.5 (0–26) | 1 (0–15) | 2 (0–14) | 1.5 (0–23) | 0 (0–2) | 2 (0–7) | 2 (0–17) | 0 (0–5) |
* Public or private hospital as an outpatient or inpatient, or contact with a consultant dietitian in the community.
3.3. Numbers Analysed and Missing Data
For the primary analysis of QALY, all participants (n = 111) were included. For secondary outcomes, the numbers analysed are reported in the relevant tables and figures. All participants were analysed according to the group to which they were randomised.
3.4. Primary Outcome—Quality-Adjusted Life Years (QALY)
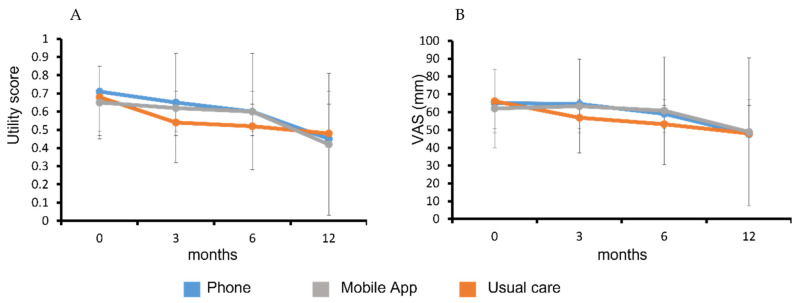
There was a declining health status in all study groups over the 12-month follow-up period (Figure 3) assessed by the EQ-5D-5L utility and VAS scores. The average QALY for each group is reported are Table 3. There were no significant differences in QALY between the intervention groups (−0.02 (−0.13, 0.08), p = 0.712) or compared with the control group, with adjustment for covariates (Table 4).
Figure 3.
Change in health status of participants from baseline to 12-month follow-up. EQ-5D-5L utility score (A) and visual analogue scale of perceived health on day of assessment (B). Data are presented as mean (SD).
Table 3.
Summative outcomes by group at each follow-up.
| Control (n = 37) | Telephone (n = 38) | Mobile App (n = 36) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 Months | 6 Months | 12 Months | 3 Months | 6 Months | 12 Months | 3 Months | 6 Months | 12 Months | |
| QALY—mean (sd) | - | - | 0.55 (0.28) | - | - | 0.57 (0.28) | - | - | 0.59 (0.23) |
| Mortality prior to this follow-up—n (cumulative %) | 4 (11%) | 3 (19%) | 4 (30%) | 2 (5%) | 4 (16%) | 6 (33%) | 1 (3%) | 1 (6%) | 6 (22%) |
| n (% relative to baseline) # | n = 30 (81%) | n = 28 (76%) | n = 30 (81%) | n = 33 (87%) | n = 34 (89%) | n = 34 (89%) | n = 28 (78%) | n = 29 (80%) | n = 28 (78%) |
| EQ-5D-5L utility score—mean (sd) | 0.54 (0.37) | 0.52 (0.35) | 0.48 (0.42) | 0.65 (0.29) | 0.60 (0.35) | 0.45 (0.41) | 0.62 (0.30) | 0.60 (0.32) | 0.42 (0.39) |
| EQ-5D-5L visual analogue scale—mean (sd) | 56.8 (29.9) | 53.2 (35.6) | 48.1 (41.5) | 64.5 (24.1) | 59.1 (32.8) | 47.6 (41.4) | 63.3 (26.4) | 60.8 (30.2) | 48.9 (41.6) |
| n (% relative to baseline) * | n = 26 (70%) | n = 20 (54%) | n = 18 (49%) | n = 32 (84%) | n = 28 (74%) | n = 21 (55%) | n = 26 (72%) | n = 25 (69%) | n = 17 (47%) |
| EQ-5D-5L—median (IQR) | |||||||||
| Mobility | 1.5 (1, 2) | 1 (1, 2) | 1 (1, 1) | 1 (1, 2.5) | 1 (1, 2.5) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 2 (1, 2) |
| Personal care | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 2) |
| Usual activities | 2 (1, 3) | 1 (1, 2) | 1 (1, 1) | 1.5 (1, 3) | 1.5 (1, 2) | 1 (1, 2) | 1.5 (1, 3) | 1 (1, 2) | 2 (1, 2) |
| Pain or discomfort | 2 (1, 2) | 2 (1, 2) | 1.5 (1, 2) | 2 (1, 3) | 1 (1, 3) | 2 (1, 2) | 1 (1, 2) | 2 (1, 2) | 1 (1, 2) |
| Anxiety or depression | 2 (1, 2) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) |
| Weight (kg)—mean (sd) | 75.6 (20.3) | 75.6 (17.5) | 73.2 (18.4) | 71.7 (11.8) | 70.2 (11.7) | 68.6 (13.3) | 71.7 (15.6) | 68.7 (14.1) | 68.5 (14.1) |
| PG-SGASF score—mean (sd) | 7.5 (5.0) | 4.6 (3.6) | 4.1 (4.1) | 7.8 (5.7) | 6.2 (5.1) | 4.3 (4.7) | 8.4 (6.1) a | 7.2 (4.0) | 4.9 (3.6) |
| EORTC QLQ-C30 score—mean (sd) | |||||||||
| Global health | 54.3 (25.1) | 69.8 (12.2) | 72.7 (15.9) | 66.4 (19.7) b | 68.0 (28.13) | 74.8 (23.8) | 62.3 (24.5) | 59.25 (21.10) | 73.5 (20.5) |
| Physical functioning | 70.8 (26.0) | 81.3 (14.1) | 86.7 (15.5) | 74.0 (18.5) | 75.95 (21.72) | 80.6 (20.0) | 73.8 (26.8) | 73.33 (17.32) | 82.7 (16.3) |
| Role functioning | 48.7 (31.9) | 71.7 (25.4) | 78.7 (25.4) | 62.0 (32.9) | 68.45 (32.18) | 75.4 (34.0) | 59.6 (35.3) | 54.67 (25.24) | 77.4 (16.7) |
| Emotional functioning | 72.8 (24.8) | 80.8 (16.7) | 85.6 (11.4) | 82.6 (18.7) | 80.65 (22.23) | 84.1 (15.1) | 76.3 (22.40) | 73.33 (22.31) | 83.8 (17.8) |
| Cognitive functioning | 72.4 (26.6) | 80.0 (17.6) | 82.4 (16.6) | 82.8 (19.0) | 83.93 (21.98) | 85.7 (20.6) | 78.7 (24.8) | 80 (20.41) | 82.4 (21.6) |
| Social functioning | 58.3 (41.7) | 80.0 (20.7) | 82.4 (27.7) | 71.5 (30.2) | 76.79 (25.0) | 84.1 (26.1) | 74.0 (32.3) | 66.6 (26.8) | 87.2 (21.7) |
| Fatigue | 54.7 (26.6) | 37.2 (22.0) | 25.3 (22.5) | 42.9 (23.5) | 39.28 (23.7) | 33.3 (24.3) | 45.7 (26.2) | 45.8 (26.3) | 34.0 (26.5) |
| Nausea and vomiting | 14.1 (16.1) | 8.3 (14.8) | 11.1 (21.4) | 7.8 (15.8) | 9.5 (12.4) | 7.1 (11.3) | 9.6 (14.8) | 6.0 (9.5) | 8.8 (19.6) |
| Pain | 29.5 (32.1) | 29.2 (24.7) | 22.2 (23.6) | 22.4 (30.1) | 25.0 (30.6) | 22.2 (22.0) | 20.5 (29.9) | 16.0 (21.2) | 18.6 (15.5) |
| Dyspnoea | 19.2 (30.1) | 11.7 (19.6) | 14.8 (23.5) | 21.9 (24.8) | 19.0 (24.7) | 23.8 (28.2) | 21.8 (23.0) | 18.7 (23.7) | 15.7 (23.9) |
| Insomnia | 35.9 (35.2) | 28.3 (29.2) | 25.9 (21.6) | 29.2 (37.6) | 22.6 (27.3) | 25.0 (30.3) | 34.6 (40.5) | 32.0 (31.1) | 31.4 (34.3) |
| Appetite loss | 38.5 (33.6) | 20 (27.4) | 14.8 (23.5) | 32.3 (28.7) | 23.8 (29.9) | 20.6 (26.8) | 35.9 (38.8) | 26.7 (25.5) | 25.5 (32.3) |
| Constipation | 16.7 (30.2) | 11.7 (24.8) | 9.3 (22.3) | 12.5 (22.0) | 17.9 (23.1) | 12.7 (19.6) | 19.2 (34.2) | 13.3 (25.6) | 7.8 (18.7) |
| Diarrhoea | 23.1 (32.3) | 18.3 (22.9) | 22.2 (30.2) | 19.3 (29.5) | 20.2 (27.7) | 19.0 (29.0) | 18.7 (27.5) | 22.7 (31.5) | 15.7 (24.0) |
| Financial difficulties | 21.8 (33.9) | 13.3 (29.4) | 13.0 (23.3) | 8.6 (19.2) | 9.5 (23.8) | 17.5 (34.3) | 19.3 (34.0) | 20.7 (33.8) | 19.6 (37.4) |
# Sample size decreased due to withdrawal or lost to follow up. * Sample size decreased due to participant death, withdrawal, or lost to follow up. a n = 25; b n = 31; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire—Core 30; PG-SGASF, Patient-Generated Subjective Global Assessment Short Form.
Table 4.
Pairwise comparisons between groups on primary and secondary outcomes.
| Control vs. Telephone | Mobile App vs. Telephone | Mobile App vs. Control | |
|---|---|---|---|
| QALY—coef (95% CI), p-value † | 0.04 (0.43, 2.3), p = 0.998 | −0.02 (−0.13, 0.08), p = 0.712 | −0.08 (−0.18, 0.02), p = 0.135 |
| Survival—HR (95% CI), p-value * | 0.999 (−0.45, 2.39), p = 0.923 | 0.61 (0.27, 1.74), p = 0.434 | 0.52 (0.23, 1.50), p = 0.265 |
| EORTC QLQ-C30 score #,† | |||
| Global health | −4.02 (−10.4, 2.4), p = 0.22 | −6.00 (−12.70, 0.75), p = 0.082 | −0.67 (−7.62, 6.28), p = 0.850 |
| Physical functioning | −2.75 (−9.63, 4.12), p = 0.433 | −3.20 (−10.03, 3.63), p = 0.359 | −2.31 (−8.29, 3.67), p = 0.448 |
| Role functioning | −6.11 (−16.78, 4.56), p = 0.262 | −6.31 (−16.16, 3.54), p = 0.210 | −0.12 (−9.95, 9.71), p = 0.980 |
| Emotional functioning | −0.88 (−8.08, 6.33), p = 0.812 | −7.07 (−14.37, 0.22), p = 0.057 | 4.95 (−1.88, 11.78), p = 0.155 |
| Cognitive functioning | −7.36 (−14.15, −0.57), p = 0.034 | −1.60 (−8.57, 5.37), p = 0.652 | −6.43 (−13.90, 1.04), p = 0.092 |
| Social functioning | −5.38 (−16.73, 6.00), p = 0.353 | −3.01, (−12.30, 6.28), p = 0.525 | −4.93 (−16.54, 6.68), p = 0.405 |
| Fatigue | 3.08 (−5.77, 11.93), p = 0.496 | 3.28 (−5.63, 12.19), p = 0.471 | 1.47 (−7.63, 10.58), p = 0.751 |
| Nausea and vomiting | 0.02 (−5.68, 5.72), p = 0.994 | −1.94 (−6.91, 3.04), p = 0.445 | 3.17 (−2.16, 8.50), p = 0.244 |
| Pain | 1.22 (−9.04, 11.47), p = 0.816 | −5.87 (−15.60, 3.85), p = 0.237 | 11.63 (1.20, 22.06), p = 0.029 |
| Dyspnoea | −0.76 (−9.66, 8.13), p = 0.867 | 1.26 (−7.14, 9.65), p = 0.769 | 1.67 (−6.67, 10.02), p = 0.694 |
| Insomnia | −1.94 (−14.94, 11.06), p = 770 | 5.21 (−8.13, 18.56), p = 0.444 | −2.06 (−15.12, 11.00), p = 0.757 |
| Appetite loss | 0.65 (−9.71, 11.00), p = 0.902 | 3.49 (−6.74, 13.72), p = 0.504 | −2.01 (−12.71, 8.70), p = 0.713 |
| Constipation | −2.44 (−12.35, 7.35), p = 0.625 | 2.75 (−7.11, 12.62), p = 0.584 | −0.35 (−12.25, 11.56), p = 0.955 |
| Diarrhoea | 4.84 (−7.16, 16.83), p = 0.429 | −0.61 (−12.53, 11.31), p = 0.920 | 3.94 (−8.47, 16.35), p = 0.534 |
| Financial difficulties | 6.00 (−4.96, 16.97), p = 0.283 | 8.54 (−1.37, 18.46), p = 0.091 | −4.0 (−16.00, 8.00), p = 0.514 |
| PG-SGASF score * | −0.87 (−2.69, 0.94), p = 0.346 | 0.57 (−1.42, 2.55), p = 0.575 | −1.20 (−2.98, 0.58), p = 0.186 |
| Weight † | −2.43 (−5.11, 0.25), p = 0.075 | −2.56 (−4.89, −0.23), p = 0.031 | 0.92 (−1.65, 3.50), p = 0.481 |
# Multiple imputation was used to replace missing individual data points for conducting comparisons in mean QALY per participant between groups. † Adjusted for baseline value of outcome measure, age, gender, baseline PG-SGASF, and cancer location. * Adjusted for age, gender, baseline PG-SGASF score, and cancer location. EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire—Core 30; HR, hazard ratio; PG-SGASF, Patient-Generated Subjective Global Assessment Short Form; QALY, quality-adjusted life years.
3.5. Secondary Outcomes
Assessments of quality of life using the cancer-specific EORTC QLQ-C30 were similar between groups for the global score (Table 4). Thirty-one participants died during the 12-month follow-up period (28% of all participants): eleven of the control group, twelve of the telephone group, and eight of the mobile app group. The adjusted hazard ratios were similar across the three groups (Table 4). The poorest survival was among participants with pancreatic cancer, where 18 of 44 participants died (41%); followed by oesophageal cancer, where 13 of 46 participants died (28%). None of the participants with gastric cancer died in the follow-up period. Weight loss over the 12-month follow-up was attenuated in the telephone group compared with the mobile app group (p = 0.031) and compared with the control, albeit not significantly (p = 0.075) (Table 4). Nutritional status was similar between groups (Table 4).
4. Discussion
This is the largest randomised-controlled trial (RCT) to date investigating the effect of a dietitian-led, individualised nutrition counselling intervention on quality-of-life outcomes in people newly diagnosed with UGI cancer. Using a three-arm design to directly compare two health service delivery modes with usual care our results showed: (1) quality-adjusted life years lived were not different between the intervention and usual care groups; (2) nutritional adequacy was not achieved with intensive remote dietetic counselling alone; (3) non-face-to-face service delivery modes enable the much earlier commencement of nutrition intervention and contact with a dietitian; however, there were disproportionately more withdrawals and missing data points from participants in the mobile app group relative to the telephone group. This finding, combined with the 12 participants who refused to participate in the study for concern of being allocated to the mobile app group, potentially indicates poorer acceptance of this mode of delivery in adults newly diagnosed with UGI cancer.
In the present study, we showed that intensive nutrition counselling commencing at diagnosis and continuing for 18 weeks had no marked impact on QoL over 12 months. Only a few published studies are available to understand the impact of nutrition counselling interventions on changes in QoL in people undergoing treatment for cancer, and the results are conflicting [15,34,35,36]. An RCT tested a three-month nutrition intervention plus three-month follow-up in cancer outpatients and found no significant difference in QoL or nutritional status between intervention (n = 30) and control groups (n = 28), despite achieving a higher protein and energy intake [36]; this study also had a high withdrawal rate. In contrast, a quasi-experimental trial of a two-month nutrition intervention for people with gastric or colon cancer (n = 53), commencing during an inpatient stay, found improved global QoL (EORTC QLQ-C30) and improvements in scores of physical functioning and role functioning compared with the control group (n = 50) [35]. Similarly, a 12-week RCT in people with gastrointestinal or head and neck cancer receiving radiotherapy found that intensive nutrition counselling (n = 29) mitigated weight loss and improved global QoL scores and physical function scale scores (EORTC QLQ-C30) compared with the control group (n = 31) [34]. These previous studies had shorter intervention periods and follow-up durations compared with the present study, and there were also differences in the cancer locations and treatment phase, which may impact on QoL scores. Weight loss despite nutrition intervention in our study suggests that nutritional adequacy was not achieved. A systematic review of nutrition interventions found that few studies have achieved nutritional adequacy to prevent weight loss in cancer therapy [15]. Nutrition impact symptoms are commonly reported in people with upper gastrointestinal cancers [37] and effective management is not achieved with dietetic care alone, because medication management is necessary and beyond the scope of dietetic practice. This highlights the importance of a multidisciplinary team approach to nutrition care during active cancer treatment. Moreover, energy requirements for people with cancer receiving anti-cancer treatment remains an understudied area [38]. Larger long-term studies are needed to determine whether attenuated weight loss during cancer treatment can be achieved and, in turn, improve QALY or survival outcomes.
A benefit of the telephone and mobile app delivery modes was that they enabled earlier commencement of nutrition intervention and access to nutrition services from patients’ own homes, reducing the traditional barriers of physical clinic space, geographical location, and transportation. The ability of carers to access evidence-based information about diet and symptom management is also important to recognise in these e-health modes. Our blanket referral approach removed reliance on the clinician identification of malnutrition risk [6,8], which is sub-optimal and delays the initiation of referral [6,8]. A cost–benefit analysis of the nutrition intervention in this study compared with usual care will inform whether a blanket referral approach is a useful process to minimise the delay in referral to a dietitian. Future studies should examine the use of e-health for the multidisciplinary care management of nutrition in UGI cancer treatment.
For successful delivery using electronic health services, digital infrastructure needs to be secure, and digital platforms should be easily operational and accepted by clinicians and patients. Seventeen percent of people who declined to participate (12/71) indicated that it was because they did not wish to be randomised to the mobile app group; throughout the study, the mobile app group had a greater number of withdrawals and missing data, particularly amongst those with poorer health status. The participant’s health status and digital literacy may be barriers to accepting or engaging with an asynchronous health service, due to lack of confidence to manage their own health [39]. Previous studies have reported that asynchronous digital platforms may be suboptimal when a person’s condition changes quickly, or important questions arise requiring decision support [40]. The prospect of learning new technology platforms at the same time as coming to terms with a diagnosis of cancer may be too overwhelming, which is supported by qualitative data from our participants who felt that their age and skill level were barriers to learning the mobile app platform after diagnosis [41]. Health service delivery needs to be patient-centred; therefore, more work needs to be undertaken to understand how digital health services should be designed to optimise the acceptance and engagement of patients, or even re-directed to enhance support to carers.
A strength of this study is that it directly compared synchronous telephone counselling with asynchronous mobile-app-delivered counselling of an individually tailored nutrition intervention in people with UGI cancer. It commenced prior to the COVID-19 pandemic, which triggered the rapid adoption of telehealth services. The high number of withdrawals from the mobile app group is a limitation, but also importantly demonstrates the preferences of people receiving health care. This study gives a better, more pragmatic representation of the likely uptake of this approach if used in a real-world setting than other designs where mobile apps are the only intervention option. The 12-month follow-up is a strength of this study compared with similar published literature.
The generalisability of standard care in the participating health services may be limited to other similar health services, and areas where the cancer care treatment pathways are less developed may have shown greater benefit from the nutrition intervention. Other limitations are that recruitment was limited to one area of Melbourne and English-speaking participants, which may not reflect the broader demographic of this patient population.
Implications for Practice
Participants in both intervention groups were receiving nutrition care following best practice guidelines and the commencement of nutrition intervention was earlier than achieved with standard care and more frequent; however, only participants in the telephone group showed some attenuation of weight loss. Escalation to supplementary enteral or parenteral nutrition support was possible in this study through the usual nutrition care pathway during the intervention period; however, our results suggest that greater use of this type of nutrition support may be necessary to achieve nutritional adequacy. There is evidence that there are gaps in dietetic service provision in Australia which may have delayed this action [42]. Interventions for people with UGI cancer who are at very high risk of nutritional decline may require a more clinical, prescriptive approach to nutrition support, rather than intensive nutrition counselling alone, to achieve nutritional adequacy and minimise symptoms [42,43].
5. Conclusions
Early and intensive nutrition intervention using behavioural-based nutrition counselling delivered at home, for people newly diagnosed with UGI cancer, did not change QALY or survival during a 12-month follow-up compared with usual care. Behavioural counselling alone was unable to restore body weight to pre-diagnosis levels. The optimal management of nutrition impact symptoms requires a multidisciplinary approach to optimise the medication management of symptoms and discuss options for enteral feeding. Dietetic services delivered using e-health methods enabled the earlier commencement of nutrition intervention compared with what was achieved with usual face-to-face care. High engagement was achieved with telephone delivery; however, asynchronous delivery using a mobile app had low acceptance for patients undergoing anticancer treatment.
Acknowledgments
Theresa Dodson is acknowledged for her assistance with the identification of eligible participants. Janne Williams and Betty Wilderman are acknowledged for their role as consumer representatives, and we sincerely thank them for their contribution to the advisory committee meetings. Andrew Sinclair is acknowledged for his role as Chair of the Advisory committee. Julie Barnett is acknowledged for the academic development, and White October for providing access to the mobile application myPace.
Supplementary Materials
The following Supporting Information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14153234/s1, File S1: TIDieR Checklist; File S2: Missing data report. References [44,45] are cited in the Supplementary File S2.
Author Contributions
Conceptualization, C.E.H., M.A.S., J.S., H.T. and T.P.H.; methodology, C.E.H., L.H., K.F., M.A.S., J.S., D.C., P.C., L.L., H.F., J.B., H.T. and T.P.H.; formal analysis, T.P.H.; investigation, K.F. and L.H.; resources, M.A.S. and J.S.; data curation, C.E.H. and L.H.; writing—original draft preparation, C.E.H.; writing—review and editing, all authors; project administration, C.E.H.; funding acquisition, T.P.H., H.T., C.E.H., H.F., J.B., M.A.S., P.C. and L.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Human Research Ethics Committee of Monash Health (14th October 2016 HREC/16/MonH/290). Site-specific authorisation was granted for all sites (Monash Health, Cabrini Health and Peninsula Health).
Informed Consent Statement
Informed verbal consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the requirement that proposed uses must have been by an ethics review board.
Conflicts of Interest
All authors have completed the ICMJE uniform disclosure form. L.H., K.F., M.-A.S., J.S., D.C., P.C. and L.L. were all employees of the Health Service that was the predominant site of recruitment. D.C., P.C. and L.L. referred patients with a histological diagnosis of included cancers, but were not involved with screening, obtaining informed consent, or randomisation. T.H. has provided expert witness testimony on the topic of the prevention of falls in hospitals for Minter Ellison Law Firm and K&L Gates Law Firm within the past 36 months.
Funding Statement
This research was funded by the Victorian Cancer Agency project identification number HSR15007. The Victorian Cancer Agency is based at 50 Lonsdale St, Melbourne, victorian.canceragency@dhhs.vic.gov.au. C.E.H was funded by a National Health and Medical Research Council TRIP fellowship GNT1168483. K.F and L.H were supported by an Australian Government RTP Scholarship. The funding agencies had no role in the development of the study design, nor any involvement in the collection, management, analysis, or interpretation of data. The decision to submit the report for publication was made independent of the funding organisation.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Guo Z.Q., Yu J.M., Li W., Fu Z.M., Lin Y., Shi Y.Y., Hu W., Ba Y., Li S.Y., Li Z.N., et al. Survey and analysis of the nutritional status in hospitalized patients with malignant gastric tumors and its influence on the quality of life. Supportive Care Cancer Off. J. Multinatl. Assoc. Supportive Care Cancer. 2020;28:373–380. doi: 10.1007/s00520-019-04803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silvers M.A., Savva J., Huggins C.E., Truby H., Haines T. Potential benefits of early nutritional intervention in adults with upper gastrointestinal cancer: A pilot randomised trial. Supportive Care Cancer. 2014;22:3035–3044. doi: 10.1007/s00520-014-2311-3. [DOI] [PubMed] [Google Scholar]
- 4.Dewys W.D., Begg C., Lavin P.T., Band P.R., Bennett J.M., Bertino J.R., Cohen M.H., Douglass H.O., Jr., Engstrom P.F., Ezdinli E.Z., et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am. J. Med. 1980;69:491–497. doi: 10.1016/S0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 5.Arends J., Bachmann P., Baracos V., Barthelemy N., Bertz H., Bozzetti F., Fearon K., Hütterer E., Isenring E., Kaasa S., et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017;36:11–48. doi: 10.1016/j.clnu.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Lorton C.M., Griffin O., Higgins K., Roulston F., Stewart G., Gough N., Barnes E., Aktas A., Walsh T.D. Late referral of cancer patients with malnutrition to dietitians: A prospective study of clinical practice. Supportive Care Cancer. 2020;28:2351–2360. doi: 10.1007/s00520-019-05042-2. [DOI] [PubMed] [Google Scholar]
- 7.Trujillo E.B., Claghorn K., Dixon S.W., Hill E.B., Braun A., Lipinski E., Platek M.E., Vergo M.T., Spees C. Inadequate Nutrition Coverage in Outpatient Cancer Centers: Results of a National Survey. J. Oncol. 2019;2019:7462940. doi: 10.1155/2019/7462940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldwin C., McGough C., Norman A.R., Frost G.S., Cunningham D.C., Andreyev H.J. Failure of dietetic referral in patients with gastrointestinal cancer and weight loss. Eur. J. Cancer. 2006;42:2504–2509. doi: 10.1016/j.ejca.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Roeland E.J., Dunne R.F. The Impact of Early Referrals to Dietitians for Patients with Esophagogastric Cancer. J. Natl. Compr. Cancer Netw. JNCCN. 2021;19:235–238. doi: 10.6004/jnccn.2021.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lis C.G., Gupta D., Lammersfeld C.A., Markman M., Vashi P.G. Role of nutritional status in predicting quality of life outcomes in cancer--a systematic review of the epidemiological literature. Nutr. J. 2012;11:27. doi: 10.1186/1475-2891-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldwin C., Spiro A., Ahern R., Emery P.W. Oral nutritional interventions in malnourished patients with cancer: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2012;104:371–385. doi: 10.1093/jnci/djr556. [DOI] [PubMed] [Google Scholar]
- 12.Deftereos I., Kiss N., Isenring E., Carter V.M., Yeung J.M. A systematic review of the effect of preoperative nutrition support on nutritional status and treatment outcomes in upper gastrointestinal cancer resection. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2020;46:1423–1434. doi: 10.1016/j.ejso.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Halfdanarson T.R., Thordardottir E., West C.P., Jatoi A. Does dietary counseling improve quality of life in cancer patients? A systematic review and meta-analysis. J. Supportive Oncol. 2008;6:234–237. [PubMed] [Google Scholar]
- 14.Zhang F., Jin Y., Qiang W. The effects of dietary advice on malnutrition in Cancer patients: A systematic review and meta-analysis. Supportive Care in Cancer. 2020;28:1579–1585. doi: 10.1007/s00520-019-05222-0. [DOI] [PubMed] [Google Scholar]
- 15.Hamaker M.E., Oosterlaan F., van Huis L.H., Thielen N., Vondeling A., van den Bos F. Nutritional status and interventions for patients with cancer—A systematic review. J. Geriatr. Oncol. 2021;12:6–21. doi: 10.1016/j.jgo.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Spelten E.R., Hardman R.N., Pike K.E., Yuen E.Y.N., Wilson C. Best practice in the implementation of telehealth-based supportive cancer care: Using research evidence and discipline-based guidance. Patient Educ. Couns. 2021;104:2682–2699. doi: 10.1016/j.pec.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths F., Lindenmeyer A., Powell J., Lowe P., Thorogood M. Why are health care interventions delivered over the internet? A systematic review of the published literature. J. Med. Internet Res. 2006;8:e10. doi: 10.2196/jmir.8.2.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen O.T., Alishahi Tabriz A., Huo J., Hanna K., Shea C.M., Turner K. Impact of Asynchronous Electronic Communication–Based Visits on Clinical Outcomes and Health Care Delivery: Systematic Review. J. Med. Internet Res. 2021;23:e27531. doi: 10.2196/27531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furness K., Sarkies M.N., Huggins C.E., Croagh D., Haines T.P. Impact of the Method of Delivering Electronic Health Behavior Change Interventions in Survivors of Cancer on Engagement, Health Behaviors, and Health Outcomes: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2020;22:e16112. doi: 10.2196/16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanna L., Huggins C.E., Furness K., Silvers M.A., Savva J., Frawley H., Croagh D., Cashin P., Low L., Bauer J., et al. Effect of early and intensive nutrition care, delivered via telephone or mobile application, on quality of life in people with upper gastrointestinal cancer: Study protocol of a randomised controlled trial. BMC Cancer. 2018;18:707. doi: 10.1186/s12885-018-4595-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson M., Capra S., Bauer J., Banks M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition. 1999;15:458–464. doi: 10.1016/S0899-9007(99)00084-2. [DOI] [PubMed] [Google Scholar]
- 22.Cancer Council Victoria and Department of Health Victoria . Optimal Care Pathway for People with Oesophagogastric Cancer. 2nd ed. Cancer Council Victoria; Melbourne, VIC, Australia: 2021. [Google Scholar]
- 23.Barnett J., Harricharan M., Fletcher D., Gilchrist B., Coughlan J. myPace: An Integrative Health Platform for Supporting Weight Loss and Maintenance Behaviors. IEEE J. Biomed. Health Inform. 2015;19:109–116. doi: 10.1109/JBHI.2014.2366832. [DOI] [PubMed] [Google Scholar]
- 24.Herdman M., Gudex C., Lloyd A., Janssen M., Kind P., Parkin D., Bonsel G., Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norman R., Cronin P., Viney R. A pilot discrete choice experiment to explore preferences for EQ-5D-5L health states. Appl. Health Econ. Health Policy. 2013;11:287–298. doi: 10.1007/s40258-013-0035-z. [DOI] [PubMed] [Google Scholar]
- 26.Cankurtaran E.S., Ozalp E., Soygur H., Ozer S., Akbiyik D.I., Bottomley A. Understanding the reliability and validity of the EORTC QLQ-C30 in Turkish cancer patients. Eur. J. Cancer Care. 2008;17:98–104. doi: 10.1111/j.1365-2354.2007.00827.x. [DOI] [PubMed] [Google Scholar]
- 27.Michels F.A., Latorre Mdo R., Maciel Mdo S. Validity, reliability and understanding of the EORTC-C30 and EORTC-BR23, quality of life questionnaires specific for breast cancer. Rev. Bras. Epidemiol. Braz. J. Epidemiol. 2013;16:352–363. doi: 10.1590/S1415-790X2013000200011. [DOI] [PubMed] [Google Scholar]
- 28.Bauer J., Capra S., Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur. J. Clin. Nutr. 2002;56:779–785. doi: 10.1038/sj.ejcn.1601412. [DOI] [PubMed] [Google Scholar]
- 29.Jager-Wittenaar H., Ottery F.D. Assessing nutritional status in cancer: Role of the Patient-Generated Subjective Global Assessment. Curr. Opin. Clin. Nutr. Metab. Care. 2017;20:322–329. doi: 10.1097/MCO.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 30.Ottery F.D. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition. 1996;12:S15–S19. doi: 10.1016/0899-9007(95)00067-4. [DOI] [PubMed] [Google Scholar]
- 31.Dong Y., Peng C.Y. Principled missing data methods for researchers. SpringerPlus. 2013;2:222. doi: 10.1186/2193-1801-2-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faria R., Gomes M., Epstein D., White I.R. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEconomics. 2014;32:1157–1170. doi: 10.1007/s40273-014-0193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Ginkel J.R., Linting M., Rippe R.C.A., van der Voort A. Rebutting Existing Misconceptions About Multiple Imputation as a Method for Handling Missing Data. J. Personal. Assess. 2020;102:297–308. doi: 10.1080/00223891.2018.1530680. [DOI] [PubMed] [Google Scholar]
- 34.Isenring E.A., Capra S., Bauer J.D. Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. Br. J. Cancer. 2004;91:447–452. doi: 10.1038/sj.bjc.6601962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen L.T., Dang A.K., Duong P.T., Phan H.B.T., Pham C.T.T., Nguyen A.T.L., Le H.T. Nutrition intervention is beneficial to the quality of life of patients with gastrointestinal cancer undergoing chemotherapy in Vietnam. Cancer Med. 2021;10:1668–1680. doi: 10.1002/cam4.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uster A., Ruefenacht U., Ruehlin M., Pless M., Siano M., Haefner M., Imoberdorf R., Ballmer P.E. Influence of a nutritional intervention on dietary intake and quality of life in cancer patients: A randomized controlled trial. Nutrition. 2013;29:1342–1349. doi: 10.1016/j.nut.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Deftereos I., Yeung J.M.C., Arslan J., Carter V.M., Isenring E., Kiss N., Group oboTNPPS Assessment of Nutritional Status and Nutrition Impact Symptoms in Patients Undergoing Resection for Upper Gastrointestinal Cancer: Results from the Multi-Centre NOURISH Point Prevalence Study. Nutrients. 2021;13:3349. doi: 10.3390/nu13103349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arends J., Strasser F., Gonella S., Solheim T.S., Madeddu C., Ravasco P., Buonaccorso L., de van der Schueren M.A.E., Baldwin C., Chasen M., et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines. ESMO Open. 2021;6:100092. doi: 10.1016/j.esmoop.2021.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ole N., Dorthe F., Louise K., Astrid K., Roy B., Lars K., Richard H.O. The e-health literacy framework: A conceptual framework for characterizing e-health users and their interaction with e-health systems. Knowl. Manag. E-Learn. 2015;7:522–540. [Google Scholar]
- 40.Cooley M.E., Nayak M.M., Abrahm J.L., Braun I.M., Rabin M.S., Brzozowski J., Lathan C., Berry D.L. Patient and caregiver perspectives on decision support for symptom and quality of life management during cancer treatment: Implications for eHealth. Psycho-Oncol. 2017;26:1105–1112. doi: 10.1002/pon.4442. [DOI] [PubMed] [Google Scholar]
- 41.Furness K., Huggins C.E., Truby H., Croagh D., Haines T.P. Attitudes of Australian Patients Undergoing Treatment for Upper Gastrointestinal Cancers to Different Models of Nutrition Care Delivery: Qualitative Investigation. JMIR Form. Res. 2021;5:e23979. doi: 10.2196/23979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steer B.L.J. In: Cancer Malnutrition Point Prevalence Study 2018 Summary Report. VCMC, editor. Peter MacCallum Cancer Centre; Melbourne, VIC, Australia: 2020. [Google Scholar]
- 43.de Oliveira Faria S., Simões Lima G.A., Lopes Carvalho A., Nader Marta G., Howell D., Eluf-Neto J. Clinically significant changes in health-related quality of life in head and neck cancer patients following intensive nutritional care during radiotherapy. Eur. J. Oncol. Nurs. 2022;56:102065. doi: 10.1016/j.ejon.2021.102065. [DOI] [PubMed] [Google Scholar]
- 44.Demirtas H., Hedeker D. An imputation strategy for incomplete longitudinal ordinal data. Stat. Med. 2008;27:4086–4093. doi: 10.1002/sim.3239. [DOI] [PubMed] [Google Scholar]
- 45.Lee K.J., Carlin J.B. Multiple imputation for missing data: Fully conditional specification versus multivariate normal imputation. Am. J. Epidemiol. 2010;171:624–632. doi: 10.1093/aje/kwp425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the requirement that proposed uses must have been by an ethics review board.