Abstract
Calluna vulgaris, belonging to the Ericaceae family, is an invasive plant that has widely spread from Europe all across Asia, North America, Australia and New Zealand. Being able to survive in rigid soil and environmental conditions, it is nowadays considered to be of high nature-conservation value. Known for its nutritional and medicinal properties, C. vulgaris stands out for its varied physiochemical composition, spotlighting a wide range of biological activity. Among the most important bioactive compounds identified in C. vulgaris, the phenolic components found in different parts of this herbaceous plant are the main source of its diverse pro-health properties (antioxidant, anti-inflammatory, antimicrobial, chemoprotective, etc.). Nonetheless, this plant exhibits an excellent nectariferous potential for social insects such as honeybees; therefore, comparing the bioactive compounds observed in the plant and in the final product of the beehive, namely honey, will help us understand and find new insights into the health benefits provided by the consumption of C. vulgaris-related products. Thus, the main interest of this work is to review the nutritional profile, chemical composition and biological activities of the C. vulgaris plant and its related honey in order to encourage the future exploration and use of this health-promoting plant in novel foods, pharmacological products and apitherapy.
Keywords: Calluna vulgaris, bioactive compounds, biological activities, therapeutic value, apitherapy, heather honey, antioxidant potential, invasive plant, health benefits
1. Introduction
Being the primary source of food, pharmaceutical products and organic material resources, plants play an outstanding role in the conservation and sustainability of ecosystem services [1]. Throughout these services, plants have a positive influence on the human population and mostly on human wellbeing [2,3,4]. As a consequence, recent decades have witnessed a continuous interest of the scientific field towards medicinal plants, natural products [5,6,7] and apitherapy [8,9] that can provide important resources for sustainable development [10,11]. Because of the importance given to plants for preventing and curing various diseases, their role has grown, and more attention has been given to the secondary metabolites and their nutritional and health benefits [12,13,14,15,16].
In this regard, Calluna vulgaris L. Hull (C. vulgaris), known also as Common Heather, Ling plant, Heather or Scotch Heather, is utilized for its pharmacological properties, along with other specific characteristics that guarantee its economic value [17].
Belonging to the Ericaceae family [18,19,20], C. vulgaris is native to Europe, but has become one of the most widespread plants all across Asia, North America, Australia and New Zealand [21,22].
Described as an oligotrophic, calcifuge species [23,24], this small evergreen perennial plant with woody, flexible stalks [25] is able to survive in rigid soils (acid soils, poor in minerals) [26,27,28] and severe environmental conditions (precipitations, temperature, altitudinal zonation, etc.) [29,30,31,32].
Measuring a maximum of 100 cm high and having a hemispherical shape, C. vulgaris is characterized by multiple-stemmed ascending branches, small and persistent leaves and purple flowers with single, long, inflorescence bunches [33,34] (Figure 1).
Figure 1.
C. vulgaris plant (Mihai Grama, beekeeper—personal collection).
The long period of blooming in C. vulgaris, namely from May to September–October, makes this invasive plant an excellent source of nectar for pollinators [35], especially bees, that are able to produce around 150–200 kg of honey per hectare [36]. This is a key aspect of the ecosystem services that C. vulgaris can provide, taking into consideration the environmental changes faced by both pollinators and plants [37], with critical implications for plant diversity, food and biomass production [38].
As it is a light-dependent plant, its ecological amplitude covers a wide range of areas, including pastures, peatlands, meadows and coniferous woodlands from hilly and mountainous regions [39,40], where the acidic soils can act as a reservoir for water and land-based carbon [41]. However, it exhibits a menacing character for native flora due to the phytotoxicity found in their roots [42] and their capacity to change the habitat and ambient conditions for plant regeneration [43,44].
Through the allelopathic character, namely the interaction of secondary metabolites of plants—of which one is invasive and the others are native [45,46]—perennial plants such as C. vulgaris are able to inhibit the germination and growth of native and surrounding plant species, thereby interrupting their regeneration ability [47,48,49]. This process occurs either when different parts of the plant are decomposed or through the rhizome and root exudations, and released into the soil [50]. In addition, the same phytotoxicity is exhibited in C. vulgaris tissues and litter that can restrain its own regeneration capacity [51].
Therefore, its invasive character cannot be contested, taking into account that, nowadays, biological invasions can represent a primary menace to biodiversity and nature conservation [52,53,54].
Although it tends to invade large surfaces, its spread is restricted by afforestation and the natural restoration of some native flora [39], or by the negative effects induced through the loss of traditional management [55,56,57,58], agricultural intensification [59], overgrazing, nitrogen deposition [60,61,62] and climate change [63,64].
Still, frequent burning and clipping are considered to be the main alteration factors for the habitats characterized by C. vulgaris species and for their related ecosystem services [58,65,66], as it produces a decrease in flower buds [55], a slow vegetative recovery [59], a lower flowering frequency [67] and thereby destroying the heather habitats and their associated fauna. Hence, this plant is nowadays considered to be of high nature-conservation value [68], being a protected habitat in the nature-conservation network of Natura 2000 [69] and assignable to the vulnerable conservation status [70].
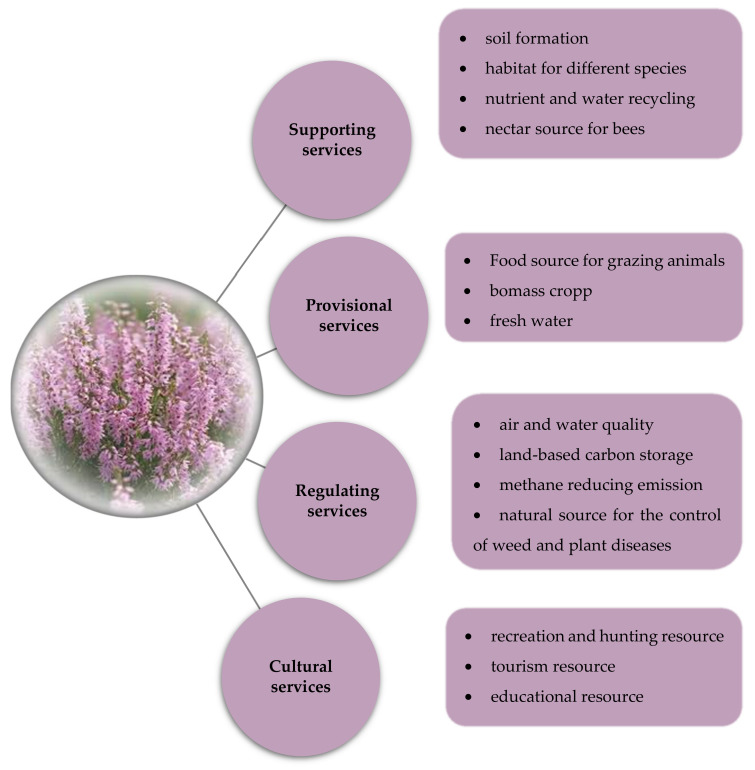
Reports in the literature have shown that this plant species is undervalued in terms of its economical, ecological, pharmacological and food potential, as it provides valuable ecosystem services for people and biodiversity that can support sustainable development [71,72,73]. In this respect, Figure 2 points out the key ecosystem services that C. vulgaris can provide for both biodiversity and human wellbeing.
Figure 2.
Ecosystem services provided by C. vulgaris (adapted from Bonn, 2010) [74].
Different authors have pointed out that C. vulgaris is a habitat for a variety of amphibians, reptiles and insects [75], but also for animals that can be represent a recreation and hunting resource [76,77,78]. Moreover, it represents a source of fodder for grazing animals [76,79], an excellent biomass energy crop [80,81], a methane-emission-reducing species [40,82] and a valuable tourism resource [75,83,84].
At the same time, C. vulgaris phytotoxicity can act as a competitive advantage in terms of an alternative natural source for the biological control of various weed species [85] or plant diseases [86]. This can be considered an ecofriendly strategy to reduce the use of chemicals in the agriculture sector and thus enhance the crop productivity [87,88]. This kind of bio-herbicide prepared from plants has no damaging effects on crop plants or people’s health [85,87]. For these reasons, the active management of C. vulgaris habitats is required in order to maintain its conservation status and its future as a potential mitigator of climate change [40,75,80].
Nevertheless, the potential of invasive plants with regard to their nutritional and bioactive properties has been understudied, in the face of their extensive expansion throughout the entire globe. Thus, the phytochemical composition of C. vulgaris has indicated the presence of bioactive compounds such as flavonoids, phenols, tannins, proanthocyanidins, caffeic acid derivatives, steroids, triterpenes, and hydroquinones [22,33,89,90]. These secondary metabolites, which are present in different parts of the plant, vary in composition and pharmacological effect. Hence, positive biological activities have been revealed, including antioxidant [28,89,91,92,93], antimicrobial [28,90,92,93,94,95], anti-inflammatory [95,96,97,98,99,100], neurotropic [101,102] and chemoprotective activities [103], just to mention a few.
Despite the fact that, recently, much of scientists’ focus has been on the chemical constituents and biological properties of C. vulgaris, there has not been any systematic review to point out all of the available constituents and the beneficial health properties of this invasive plant, or of its related honey. The prevention and treatment of various disorders using natural products, specifically honey-related products, is known as apitherapy [104,105,106]. This alternative medicinal treatment has been proved to have positive results on different in vivo and in vitro assays, as this overview will point out. Although apitherapy is not yet recognized as a complementary medicine, it represents a promising therapy with a large applicability to the medical field. In this regard, the findings revealed that heather honey exhibits similar properties to Manuka honey [107], and therefore can represent a valuable natural remedy that can be utilized for healing purposes.
In order to enhance the in-depth examination and full use of this plant, the aim of this manuscript is to systematically compile all of the current findings on the C. vulgaris plant and honey. Furthermore, the biological activities of the reported plant will be covered to allow us to explore its therapeutic potential, to highlight eventual gaps and to provide a scientific groundwork for future studies that may lead to the creation of functional products based on this plant.
Consequently, to carry out this review, the authors collected data from a wide range of databases such as Web of Science, Science Direct, PubMed, Scopus, and Google Scholar. The selection was unlimited and included up-to-date articles, in order to attain a deeper understanding of the subject.
2. Phytochemical Profile of C. vulgaris
The C. vulgaris chemical composition has been extensively studied in order to identify its main bioactive compounds and nutritive status. Hence, the studies carried out have revealed the presence of different classes of compounds, of which the phenolic compounds are the most noteworthy. Thus, phenolic acids (hydroxycinnamic acid, caffeic acid, coumarins), flavonoids (rutin, quercetin, isoquercetin, kaempferol, luteolin, apigenin), phenols and their glycosides (hydroquinone, arbutin, methylarbutin), tannins, triterpenes, anthocyanidins, terpenoids (lupeol, ursolic, oleanolic acids), organic acids, steroids, and essential oils and their subclasses are the most distinct compounds reported in C. vulgaris composition [22,33,90,92,108,109,110,111,112].
2.1. C. vulgaris Nutritional Value
C. vulgaris represents an integrant part of the pastoral resource throughout Europe and therefore an important grazing source for animals [113,114]. Its nutritive value has long been debated, but only a few studies have reported a complete nutritional profile of C. vulgaris [33,92]. As the flowers have been proved to contain the higher nutritive value, Table 1 presents the main macronutrients found in both C. vulgaris wild inflorescence and commercial samples.
Table 1.
Nutritional composition of C. vulgaris flowers: comparison between wild and commercial samples.
| Wild Samples [33] | Commercial Samples [90] | ||
|---|---|---|---|
| Nutritional Value (g/100 g) | Nutritional Value (mg per 100 g dw **) | ||
| Ash | 2.31 ± 0.09 | Ash | 4.06 ± 0.03 |
| Protein | 6.80 ± 0.27 | Protein | 8.40 ± 0.30 |
| Fat | 3.70 ± 0.10 | Fat | 4.42 ± 0.04 |
| Fiber | 38.96 ± 1.64 | Fiber | nm * |
| Carbohydrates | 36.21 ± 0.20 | Carbohydrates | 83.10 ± 0.30 |
| Fructose | nm * | Fructose | 2.92 ± 0.05 |
| Glucose | nm * | Glucose | 5.36 ± 0.08 |
* nm—not measured; ** dw—dried weight.
Thereby, the nutritional profile of wild C. vulgaris flowers indicates a great content of fiber (38.96 ± 1.64%) [33], while for the carbohydrates, the commercial samples suggested a higher content (83.1 ± 0.3%) [90] than the wildflower samples (36.21 ± 0.20%) [33]. In general, the proportion of macronutrients in the commercial flower samples is more significant than that in the wild samples; Table 1 points out a considerable difference in protein, fat and ash values. Similar studies have analyzed the nutritional value of various edible plants, suggesting that C. vulgaris contains higher amounts of carbohydrates and fiber and thus, greater nutritional value [115,116].
Other important constituents related to the nutritional value of C. vulgaris are the fatty acids, which are responsible for the energy intake of the analyzed plant. In this sense, Rodrigues et al., 2018 [33] revealed the presence of various fatty acids in the flowers of C. vulgaris, the most predominant being linolenic acid (34.71%), followed by linoleic (27.28%) and palmitic (20.79%) acids, while the less abundant was arachidonic acid (only 1.99%) [33]. These data are in accordance with the results presented by Olechnowicz-Stepien et al. in 1982 [117], but different from what Mandin et al. 2018 [90] suggested, namely that the greater proportion of fatty acids was represented by the polyunsaturated fatty acids (53%), followed by the presence of saturated fatty acids (39.9%), linoleic acid (21%), and palmitic acid (19.6%).
Despite the scarcity of literature regarding the macronutrient profile of C. vulgaris, the results shown above emphasize that this evergreen plant can be used as a food ingredient in future products, taking into consideration its high source of energy through the content of fiber and carbohydrates.
2.2. Bioactive Profile of C. vulgaris
These bioactive compounds are found in various parts of this perennial invasive plant (roots, flowers, shoots). Worth noting is that the chemical composition of the heather plant is dependent on a series of relevant factors, the most significant being: the climate, season, altitude, soil characteristics or growth stages [28,110,118,119,120,121,122]. In addition, being an invasive plant, C. vulgaris can adapt its structure to different environments and consequently, analyzing and comparing samples of the same plant collected from different regions, periods, altitudes or germination stages represents a real challenge.
In this regard, Table 2 summarizes the most relevant research works regarding the bioactive composition of C. vulgaris, underlining the territorial localization, the constituents found, the part of the plant analyzed, together with the identification methods and the results obtained, in order to obtain an overview on the phytochemical complexity of the C. vulgaris profile.
Table 2.
Phytochemical profile of C. vulgaris, structure, localization and identification methods.
| Territorial Localization | Phytochemical Class | Compound | Part of the Plant | Identification Method | Results | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| UK | Phenolic acids | chlorogenic acid | Shoots | Chromatography | 3.2 g | [118] | ||||
| Tanins | quercetin 3-O-glucoside | Shoots | 2.4 g | |||||||
| quercetin 3-O-galactoside | Shoots | 2.4 g | ||||||||
| quercetin 3-O-arabinoside | Shoots | 1.3 g | ||||||||
| (+)-catechin | Shoots/ Roots |
1.5 g | ||||||||
| procyanidin D-1 | Shoots/Roots | 1.2 g | ||||||||
| 3, 5, 7, 8, 4′-pentahydroxyflavanone 8-O-glucoside (callunin) | Shoots | 1.1 g | ||||||||
| Turkey | Flavonoid | kaempferol-3-O-_-d-galactoside | Aerial part | Chromatography | 45.7 mg | [96] | ||||
| Poland | Triterpenoids | α -Amyrin | Flower and leaves | GC–MS/FID | Flower cuticular wax | Leaf cuticular wax | [111] | |||
| mg/g wax extract mass ± SD | ||||||||||
| 7.8 ± 0.6 | 8.2 ± 0.7 | |||||||||
| α -Amyrenone | 0.5 ± 0.1 | 3.6 ± 0.4 | ||||||||
| β -Amyrin | 6.9 ± 0.3 | 6.7 ± 0.5 | ||||||||
| Betulin | 0.4 ± 0.1 | 0.6 ± 0.1 | ||||||||
| Cycloartanol | 2.8 ± 0.2 | 4.1 ± 0.3 | ||||||||
| 24-Methylenecycloartanol | 3.1 ± 0.2 | 1.2 ± 0.1 | ||||||||
| Erythrodiol | 0.6 ± 0.1 | 2.4 ± 0.2 | ||||||||
| Friedelin | 16.8 ± 1.2 | 1.5 ± 0.1 | ||||||||
| 4-Epi-friedelin | 2.4 ± 0.2 | 1.5 ± 0.1 | ||||||||
| Friedelinol | 1.0 ± 0.1 | 1.4 ± 0.1 | ||||||||
| Germanicol | 0.2 ± 0.1 | 3.9 ± 0.3 | ||||||||
| Lupeol | 6.9 ± 0.5 | 7.1 ± 0.6 | ||||||||
| Oleanolic aldehyde | 0.6 ± 0.1 | 1.3 ± 0.1 | ||||||||
| Taraxasterol | 5.0 ± 0.1 | 3.3 ± 0.2 | ||||||||
| Taraxerone | 0.8 ± 0.1 | 1.4 ± 0.1 | ||||||||
| Ursolic aldehyde | 3.3 ± 0.2 | 5.2 ± 0.3 | ||||||||
| Uvaol | 2.5 ± 0.2 | 28.6 ± 2.2 | ||||||||
| Betulinic acid | n.d. | 9.4 ± 0.8 | ||||||||
| Oleanolic acid | 28.2 ± 1.6 | 125.1 ± 9.8 | ||||||||
| 3-Oxo-olean-12-en-28-oic acid | 7.1 ± 0.4 | 3.1 ± 0.3 | ||||||||
| 3-Oxo-ursan-12-en-28-oic acid | 1.1 ± 0.1 | 5.0 ± 0.3 | ||||||||
| Ursolic acid | 75.2 ± 4.1 | 398 ± 25.7 | ||||||||
| Campesterol | 1.0 ± 0.1. | 0.6 ± 0.1 | ||||||||
| Cholesterol | 2.3 ± 0.1 | 0.8 ± 0.1 | ||||||||
| Sitostanol | 3.0 ± 0.1 | 1.5 ± 0.1 | ||||||||
| Sitosterol | 8.1 ± 0.5 | 13.8 ± 1.1 | ||||||||
| Stigmasterol | 0.7 ± 0.1 | 0.3 ± 0.1 | ||||||||
| Stigmasta-3,5-dien-7-one | 4.4 ± 0.3 | 1.9 ± 0.2 | ||||||||
| Stigmastane-3,6-dione | 1.6 ± 0.1 | 1.4 ± 0.1 | ||||||||
| Esters | α -Amyrin | 1.0 ± 0.1 | 1.0 ± 0.1 | |||||||
| β -Amyrin | 0.4 ± 0.1 | 1.1 ± 0.2 | ||||||||
| Cycloartanol | 0.2 ± 0.1 | 0.7 ± 0.1 | ||||||||
| Lupeol | 0.8 ± 0.1 | 0.9 ± 0.1 | ||||||||
| Taraxasterol | 0.5 ± 0.1 | 0.5 ± 0.1 | ||||||||
| Oleanolic acid | 1.0 ± 0.1 | 0.6 ± 0.1 | ||||||||
| Ursolic acid | 2.5 ± 0.2 | n.d. | ||||||||
| Spain | Triterpenic acids | Oleanolic acid | Leaves | HPLC | 0.53-82.87 mg/g extract (max value obtained with the highest yield—7.31% and highest ethanol content—15%) | [123] | ||||
| Ursolic acid | 2.19–141.45 mg/g extract (max value obtained with the highest yield—7.31% and highest ethanol content—15%) | |||||||||
| Portugal | Vitamers | α-Tocopherol | Flowers | H-NMR and LC-UV-MS | 32.50 ± 0.48 mg/100 g | [33] | ||||
| β-Tocopherol | 0.39 ± 0.01 mg/100 g | |||||||||
| γ-Tocopherol | 1.19 ± 0.06 mg/100 g | |||||||||
| δ-Tocopherol | 0.36 ± 0.01 mg/100 g | |||||||||
| α-Tocotrienol | nd | |||||||||
| β-Tocotrienol | nd | |||||||||
| γ-Tocotrienol | 0.54 ± 0.03 mg/100 g | |||||||||
| δ-Tocotrienol | nd | |||||||||
| Tannins | Quinic acid | 27.07 ± 0.11 μg/g | ||||||||
| Proanthocyanidins trimmer | 25.05 ± 0.18 μg/g | |||||||||
| Proanthocyanidins tetramer | 8.87 ± 0.08 μg/g | |||||||||
| Flavonoid | 3,5,7,tetrahydroxy-4′-ethoxyflavanone 8-deoxyhexoside |
55.14 ± 0.11 μg/g | ||||||||
| Methoxy myricetin deoxyhexoside | 308.52 ± 0.48 μg/g | |||||||||
| Quercetin deoxyhexoside | 101.30 ± 0.34 μg/g | |||||||||
| Kaempferol deoxyhexoside | 2.11 ± 0.03 μg/g | |||||||||
| Myricetin deoxyhexoside | 6.39 ± 0.08 μg/g | |||||||||
| Quercetin | 140 ± 0.09 μg/g | |||||||||
| Kaempferol | 15.36 ± 0.11 μg/g | |||||||||
| Myricetin | 44.62 ± 0.12 μg/g | |||||||||
| Tocopherols | α-Tocopherol | Flowers | HPLC | 5.84 ± 0.07 mg/100 g dw | [90] | |||||
| β-Tocopherol | 0.25 ± 0.0001 mg/100 g dw | |||||||||
| γ-Tocopherol | 0.75 ± 0.03 mg/100 g dw | |||||||||
| δ-Tocopherol | 1.05 ± 0.08 mg/100 g dw | |||||||||
| Hydroxycinnamic acids | 5- O-Caffeoylquinic acid | HPLC-DAD- ESI/MS |
Ethyl acetate (mg/g extract) | Acetone (mg/g extract) |
Methanol (mg/g extract) |
Decoction (mg/g extract) |
Infusion (mg/g extract) |
|||
| n.d. | 0.20 ± 0.02 | 3.3 ± 0.1 | 3.1 ± 0.2 | 5.0 ± 0.2 | ||||||
| (+)-Catechin | 0.52 ± 0.09 | n.d. | n.d. | n.d. | n.d. | |||||
| Flavonoid | Myricetin-O-hexoside | n.d. | 2.12 ± 0.02 | 2.09 ± 0.01 | 4.50 ± 0.03 | 4.34 ± 0.02 | ||||
| 5-p-Coumaroylquinic acid | n.d. | n.d. | 0.10 ± 0.01 | 0.06 ± 0.01 | 0.150 ± 0.001 | |||||
| Myricetin-3- O-glucoside | 1.63 ± 0.07 | 4.96 ± 0.09 | 4.30 ± 0.09 | 5.80 ± 0.01 | 8.1 ± 0.1 | |||||
| Myricetin –O-rhamnoside | 1.66 ± 0.09 | 2.04 ± 0.06 | 2.81 ± 0.05 | 4.86 ± 0.06 | 5.8 ± 0.1 | |||||
| Quercetin-3- Oglucoside | 0.37 ± 0.03 | 2.20 ± 0.06 | 1.68 ± 0.004 | 1.65 ± 0.001 | 3.01 ± 0.08 | |||||
| Quercetin-O-hexoside | 0.355 ± 0.0003 | 0.8 ± 0.002 | 0.97 ± 0.07 | 1.22 ± 0.03 | 2.05 ± 0.09 | |||||
| Isorhamnetin-3- Oglucoside | 1.6 ± 0.3 | 11.25 ± 0.08 | 2.8 ± 0.1 | 2.33 ± 0.03 | 5.48 ± 0.08 | |||||
| Quercetin-O-hexosid | 0.298 ± 0.005 | 0.42 ± 0.03 | 0.350 ± 0.005 | 0.81 ± 0.001 | 0.77 ± 0.01 | |||||
| Kaempferol-O-rhamnoside | 0.36 ± 0.04 | n.d. | 0.39 ± 0.01 | 0.83 ± 0.003 | 0.81 ± 0.01 | |||||
| Isorhamnetin-O-rhamnoside | 0.428 ± 0.001 | 0.41 ± 0.01 | 0.53 ± 0.01 | 0.92 ± 0.01 | 1.12 ± 0.03 | |||||
| Rusia | Hydroxycinnamic acids | chlorogenic acid | Seeds | HPLC | 4.298 ± 0.301 mg/g | [124] | ||||
| caffeic acid | 0.0036 ± 0.0003 mg/g | |||||||||
| Flavonoids | kaempferol, | 0.036 ± 0.030 mg/g | ||||||||
| quercetin | 30.045 ± 2.003 mg/g | |||||||||
| rutin | 3.133 ± 0.219 mg/g | |||||||||
| myricetin | 2.116 ± 0.148 mg/g | |||||||||
| Tannins | epicatechin | 1.515 ± 0.106 mg/g | ||||||||
| catechin | 7.679 ± 0.538 mg/g | |||||||||
| Ukraine | Phenols | Arbutin | Aerial part | HPLC/spectro- photometry |
Water extraction (%, n = 5) |
Hydroethanolic Extract (%, n = 5) |
[102] | |||
| 1.25 ± 0.05 | 0.83 ± 0.04 | |||||||||
| Methylarbutin | 0.18 ± 0.03 | 0.23 ± 0.02 | ||||||||
| Hydroxy-cinnamic acids | Chlorogenic | HPLC/spectro- photometry |
1.25 ± 0.03 | 1.74 ± 0.03 | ||||||
| Caffeic | 0.02 ± 0.01 | 0.03 ± 0.01 | ||||||||
| Ferulic | 0.11 ± 0.02 | 0.12 ± 0.01 | ||||||||
| p-Coumaric | 0.03 ± 0.01 | 0.04 ± 0.01 | ||||||||
| Flavonoids | Rutin | HPLC/spectro- photometry |
0.65 ± 0.05 | 1.25 ± 0.05 | ||||||
| Hyperoside | 0.15 ± 0.05 | 0.20 ± 0.01 | ||||||||
| Quercetin-3-D-glucoside | 0.17 ± 0.03 | 0.29 ± 0.01 | ||||||||
| Luteolin | 0 | 0.05 ± 0.01 | ||||||||
| Apigenin | 0 | 0.04 ± 0.01 | ||||||||
| Kaempferol | 0.02 ± 0.01 | 0.05 ± 0.01 | ||||||||
| Tannins metabolites | Gallic acid | HPLC/spectro- photometry |
0.07 ± 0.01 | 0.13 ± 0.01 | ||||||
| (+)-Gallocatechin | 0.21 ± 0.01 | 0.94 ± 0.02 | ||||||||
| (−)-Epigallocatechin | 0.95 ± 0.05 | 1.36 ± 0.09 | ||||||||
| (+)-Catechin | 0.13 ± 0.03 | 0.21 ± 0.03 | ||||||||
| (−)-Epicatechin | 0.09 ± 0.01 | 0.26 ± 0.02 | ||||||||
| (−)-Catechin gallate | 0.11 ± 0.02 | 0.24 ± 0.01 | ||||||||
| (−)Epicatechin gallate | 0.05 ± 0.01 | 0.07 ± 0.01 | ||||||||
GC–MS/FID—gas chromatography–mass spectrometry with flame-ionization detection; HPLC—high-performance liquid chromatography; n.d.—not detected; dw—dry weight; H-NMR—proton nuclear magnetic resonance; LC–UV–MS—high-performance liquid chromatography coupled with diode array UV detection and mass spectrometry; GC–MS—chromatography–mass spectrometry; HPLC–DAD–ESI/MS—high-performance liquid chromatography coupled with a diode array detector and mass spectrometry using electrospray ionization.
Jalal et al., 1982 identified the presence of different tannins in both the shoots and roots of C. vulgaris [118]. In addition, the same authors observed that the composition and quantity of bioactive compounds may vary according to the season. Thus, in the fresh shoots of C. vulgaris was reported a significant variation in the phenolic compounds, with the lowest quantity in January and February—in the form of chlorogenic acid and quercetin glycosides—while from May to December, some other bioactive compounds appeared, specifically (+)-catechin, procyanidin D-l and callunin, and remained constant.
Another study that isolated kaempferol-3-O-β-D-galactoside from the aerial part of C. vulgaris was reported in Turkey [96]. Similarly, Deliorman-Orhan et al., 2009 [91] found that the major flavonoid present in the ethyl acetate fraction was kaempferol-3-O-β-D-galactoside (37.1 ± 0.89%) [91].
Another important group of bioactive compounds is represented by the triterpenoids, which are mostly found in the cuticular waxes of plants [125]. Regarding the C. vulgaris plant, Szakiel et al., 2012 [111] reported the presence of these compounds in both the flowers and leaves. The presence of triterpenoid compounds was higher in the leaf cuticular waxes compared to the flower cuticular waxes, with ursolic acid being the predominant triterpene compound of both the analyzed parts of C. vulgaris, showing 75.2 ± 4.1 mg/g wax extract in the flower part and 397.7 ± 25.7 mg/g wax extract in the leaf [111]. A representative value for the triterpenoid content is also reported for oleanolic acid (28.2 ± 1.6 mg/g wax extract in the flower, compared to 125.1 ± 9.8 mg/g wax extract in the leaves). Other compounds such as friedelin, lupeol, taraxasterol and taraxerone were also reported in this study, together with some new compounds (betulin, germanicol, 4-epi-friedelin, oleanolic and ursolic aldehydes), which have been isolated for the first time in C. vulgaris [111].
Similar results were obtained by Garcia-Risco et al., 2015, who extracted large amounts of triterpenes with supercritical CO2, more specifically oleanolic and ursolic acids. From the thirteen extractions, the highest concentration of triterpenic acids was obtained at 30 MPa pressure, 50 °C and at the highest ethanol content utilized (15%), with the highest yield (7.31%) resulting in a content of 141.45 mg/g ursolic acid, followed by 82.87 mg/g of oleanolic acid [123]. These results are higher than those reported previously by Pancost et al., 2002, who found a lower concentration of these two acids (40 mg/g dry matter of ursolic acid and 10 mg/g dry matter of oleanolic acid) [126].
The phytochemical analysis of C. vulgaris also showed the presence of vitamers, especially Vitamin E, composed mainly of tocopherols (α-, β-, γ-, δ-tocopherol) and tocotrienols (α-, β-, γ-, δ-tocotrienol) [33]. The importance of this vitamin for human health has been largely discussed; studies have shown its protective characteristics regarding the intracellular defense system and therefore its significance in preventing oxidative-stress-related diseases [127,128]. In this regard, Rodrigues et al., 2018 revealed that the principal vitamer found in the C. vulgaris flower extracts was α-tocopherol (32.5 ± 0.48 mg/100 g) [33]. Related reports found that the same vitamin, α-tocopherol, was the most predominant in the dried inflorescences of C. vulgaris (5.84 ± 0.07 mg per 100 g dw) [90] and in other invasive plant species [129]. Other important compounds found in the phytochemical analysis of Rodrigues et al., 2018 were quercetin, kaempferol and myricetin derivatives, the most predominant being methoxy myricetin deoxyhexoside, with a value of 308.52 ± 0.48 μg/g [33].
Madim et al., 2018 identified, using different organic and aqueous extracts, various bioactive compounds from the inflorescences of C. vulgaris [90]. Thus, of the twelve molecules described, considerable values were reported for myricetin-3-O-glucoside and myricetin-O-rhamnoside in all of the extracts used, whereas 5-O-caffeoylquinic acid had an increased concentration in the methanol, infusion and decoction extracts [90].
Important data on the phytochemical composition of Russian samples of C. vulgaris seeds were collected by Cherepanova et al., 2019 [124], who revealed that the main flavonoid present in the analyzed samples was quercetin (30.045 ± 2.003 mg/g), followed by the tannin metabolite catechin (7.679 ± 0.538 mg/g) and hydroxycinnamic acid as chlorogenic acid (4.298 ± 0.301 mg/g) [124].
Starchenko et al., 2020 [102] determined, through the spectrophotometric method, the presence of various phenolic metabolites in the composition of the aerial parts of C. vulgaris, of which the dominant ones were arbutin, chlorogenic acid, rutin, (−)-epigallocatechin and (+)-gallocatechin. The content of these compounds was higher in the water extraction (arbutin, rutin) compared with some other metabolites that were more present in the hydroethanolic extracts (70% ethanol), namely chlorogenic acid, rutin, hyperoside, quercetin-3-D-glucoside, (+)-gallocatechin and (−)-epigallocatechin [102] (Table 2). Despite the fact that arbutin was the prevalent compound found in C. vulgaris through the water extraction (1.25%), it was less present in the hydroethanolic extract of the plant (70% ethanol) (0.83%), or even undetected [130].
Overall, the presence of phenolic compounds identified in C. vulgaris has been reported in all parts of the plant, but their content may vary during different stages of plant growth or with different climatic factors, such as altitude or soil properties.
In this regard, Chepel et al., 2020 [28] monitored the changes of different phenolic compounds found in heather plants, especially in the leaves, stems, roots, rhizomes, flowers, and seeds, at different development stages (vegetative, floral budding, flowering, seed ripening) [28]. The authors pointed out that for the vegetative stage, the highest content of phenolic compounds (especially tannins, flavonoids and proanthocyanidins) was identified in roots (31.66 ± 0.53 mg/g), while the leaves had the lowest quantity of bioactive substances (14.52 ± 1.85 mg/g). Hence, the phenolic content changed with the development of the plant, the leaves gaining more phenolic compounds (flavonoids, anthocyanins, proanthocyanidins and hydroxycinnamic acid) in the floral budding stage (28.15 ± 0.76 mg/g) and the seed ripening stage (32.67 ± 0.12 mg/g), while, as expected, the flowers had the highest total phenolic content during the flowering stage (27.88 ± 0.18 mg/g), for all of the analyzed compounds (tannins, flavonoids, anthocyanins, proanthocyanidins and hydroxycinnamic acid) [28]. These results are sustained by another study on a plant of the same family species, namely Vaccinium ashei, which had the highest value of phenolic compounds in the leaves during harvesting time [131].
Moreover, because of its wide spread, C. vulgaris was analyzed for the altitudinal alteration of its bioactive metabolites [110,119]. Hence, the results of these studies showed that the amount of flavonols increases according to altitude.
These kinds of studies are very important, because they make a valuable contribution to the understanding of the changes that may occur in the phenolic compound accumulation in various parts of C. vulgaris at different growth stages, and therefore promote a better use of this plant.
2.3. C. vulgaris as a Bio-Indicator
It is well known that environmental contamination may have a negative impact on the ecosystem sustainability, and therefore it is very important to analyze the level of trace metals found in different habitats. C. vulgaris has been indicated to be sensitive towards metal tolerance and thus has been extensively used as a bio-indicator for the detection of different toxic heavy metals found in the soil or environment, due to the industrial and traffic emissions or the intensification of agriculture by the use of mineral fertilizers [132]. Furthermore, because of the soil characteristics that can ensure the development of C. vulgaris plant species, more specifically the high concentration of various constituents that are considered toxic to other species, the survival of native flora can be threatened [48,49]. In this regard, the study of Marks and Bannister (1978) [133] indicated a strong correlation between the constituents found in the soil and their presence in different plant organs of C. vulgaris, with lead (Pb) showing its highest concentrations in the woody parts of the analyzed plant. Similar results were published by Matanzas et al., 2021 [134], who investigated the soil and plants from an ancient mercury-mining area in Asturias, Spain and who revealed the ability of C. vulgaris to translocate Pb in its leaves [133].
Additional studies have revealed, by using different parts of C. vulgaris, the presence of several pollutant elements, including heavy metals. For instance, Mičieta and Murín (1997) [135] proved that in the polluted areas of Slovakia, C. vulgaris can be used as a bio-indicator for aluminum (Al), which has the highest observed frequency of unproductiveness of pollen grains in polluted environments (27.4 + 6.1%) [135]. Another important study compared the data from two different periods (1978–1988 and 2011) in the High Tatra mountains of Slovakia [26]. The results showed the presence of different concentrations of elements such as copper (Cu), molybdenum (Mo), zinc (Zn), manganese (Mn), iron (Fe), sulfur (S), fluorine (F), but also chromium (Cr), Pb and cadmium (Cd). These elements showed an increased concentration in 2011 compared to 1978–1988. In both of the analyzed periods, the results reported a positive correlation of Pb, Fe and Cu (r = 0.6320–0.9519) with rising altitude, while Mo, Mn, Cr, Cd and Zn showed no significant correlations with altitude. Regarding S, the highest concentration decreased from >109.8 mg/100 g in 1987–1988 to 87 mg/100 g in 2011 [26]. Similarly, Wojtuń et al., 2017 [136] analyzed the toxicity thresholds for some elements present in vascular plants from Poland, including C. vulgaris, revealing that metals such as Cu, Mn and nickel (Ni) were uptaken in the shoots of the plant, with the Mn concentration being the higher compared to the other elements [136]. On the contrary, the results found by Rajsz et al., 2021 [137] in the geothermal areas of Iceland underlined lower mass fractions of Mn (289–659 mg/kg) and titanium (Ti) (129–1180 mg/kg) compared to the results presented by Wojtun et al., 2017 for C. vulgaris [136], but higher concentrations of Cr, Cu, Fe and Ni. C. vulgaris has also been reported as an arsenic (As)-tolerant species, with the aerial parts of the plant revealing toxic concentrations of this element [138].
Therefore, it can be stated that it is useful to evaluate the presence of trace elements in plants mainly because it can offer an accurate image of the environmental pollution. However, as the acceptable concentrations of emissions of the elements presented above may exceed the normal toxicity amounts, they can represent a threat to human health due to their capacity to infiltrate the human food chain.
3. Biological Activities of C. vulgaris and Their Therapeutic Potential
C. vulgaris has long been used in traditional medicine due to the therapeutic potential of its phenolic compounds, whose presence is vital in exerting the plant’s biological activity. Furthermore, it is well known that even natural products that are C. vulgaris-derived, such as honey, contain high amounts of active compounds that exhibit numerous pharmacological benefits and that are commonly used in alternative medicine, or apitherapy [104,105].
Numerous studies have emphasized these beneficial effects by using extracts from different parts of the plant and testing their applicability in vivo and in vitro study models. Accordingly, as Table 3 points out, the C. vulgaris plant has manifested important pharmacological activities such as: antibacterial [28,90,92,93,95], anti-anxiety/antidepressant/neurotropic [101,102], antiviral [123], antiproliferative [139], antinociceptive [140], antioxidant [28,89,91,92,93], antihypertensive and analgesic [99], anti-inflammatory [95,97,98,99,100,140], hypouricemic [99], and chemopreventive and photoprotective activities [97,103,141].
Table 3.
Major pharmacological properties of the phytochemicals present in C. vulgaris and their associated disorders.
| Pharmacological Properties | Main Compound | Associated Disorder | Model Used | References |
|---|---|---|---|---|
| Antibacterial | phenolic compounds | Urinary tract pathogens | In vitro | [95] |
| Vaginal microbiota | [90,92] | |||
| Human pathogen microorganisms | [28] | |||
| [93] | ||||
| Antidepressant | quercitin | In vitro | [101] | |
| Anti-anxiety | arbutin, chlorogenic acid, rutin, hyperoside, quercetin-3-D-glucoside, (+)-gallocatechin and (−)-epigallocatechin |
Depression | In vivo (rats and mice) | [102] |
| Neurotropic | ||||
| Anxiolytic | ||||
| Stress-protective | ||||
| Antiviral | oleanolic and ursolic acid | Hepatitis C | In vitro | [123] |
| Antiproliferative | ursolic acid | Leukemia | In vitro | [139] |
| Antioxidant | kaempferol-3-O-β-D-galactoside | Oxidative- stress-related disorders |
In vitro | [91] |
| chlorogenic acid | [89] | |||
| phenolic compounds | Human pathogen microorganisms | In vitro | [28,93] | |
| Antihypertensive | chlorogenic acid | Gout | In vivo (rats) | [99] |
| Analgesic | ||||
| Hypouricemic | ||||
| Anti-inflammatory | kaempferol-3-O--d-galactoside | Gastritis, ulcer | In vivo (mice) | [140] |
| phenols and flavonoids | Urinary tract pathogens | In vitro | [95] | |
| phenols and flavonoids | Alzheimer | In vitro | [98] | |
| chlorogenic acid | Gout | In vivo (rats) | [99] | |
| Chemoprotective | hyperoside, quercitrin, quercetin, kaempferol | Skin burns and skin cancer | In vivo (mice) | [141] |
| In vitro | [97,103] |
All these valuable properties have been associated with different biologically active compounds and therefore could have promising effects on a wide range of related disorders, as will be presented in what follows.
The antibacterial properties of C. vulgaris were investigated in disorders related to the urinary tract pathogens where strains of Escherichia coli (E. coli), Enterococcus faecalis (E. fecalis) and Proteus vulgaris were found to be sensitive to the plant extracts [95]. Similarly, Mandim et al., 2018, 2019 [90,92] highlighted the capacity of C. vulgaris inflorescence extracts (acetone, methanol, decoction and infusion) to inhibit twelve pathogenic bacteria strains that are thought to be responsible for urinary tract infections. Among the tested strains, the most susceptible were the Gram-positive bacteria (E. faecalis, Listeria monocytogenes, methicillin-resistant Staphylococcus aureus, S. aureus, methicillin-susceptible Staphylococcus aureus), which exhibited the lowest minimal inhibitory concentration (MIC) values in the decoction and infusion plant extracts [90,92]. The same authors also investigated the antibacterial activity of the same tested plant extracts in vaginal microbial strains (Gardnerella vaginalis and Neisseria gonorrhoeae) illustrating that the MIC value for the Lactobacillus strains was higher (20 mg/mL) than the values for the two tested vaginal microbial strains (Gardnerella vaginalis had a value of 5 mg/mL and Neisseria gonorrhoeae had a value of 2.5 mg/mL) [90,92]. This indicates that C. vulgaris can inhibit the tested pathogens without affecting the normal balance of vaginal microbiota. More recent studies have proved that the antibacterial capacity of C. vulgaris may vary according to the plant organ from which the extract was taken and the growth phases. In this regard, Chepel et al., 2020 [28] suggested that both the Gram-negative bacteria E.coli and the Gram-positive bacteria Bacillus subtilis (B. subtilis) are more susceptible to leaf and stem extracts (during vegetative and floral budding stages), while the root and flower extracts manifested a weak antibacterial potential against the two tested bacteria (at the stages of floral budding, flowering and seed ripening against B. subtilis and at the stage of seed ripening against E. coli) [28]. The inhibition capacity of C. vulgaris against human pathogen microorganisms was also revealed by Varga et al., 2021 [93], who proved that different extracts of the plant could inhibit the growth of two two Gram-positive and three Gram-negative strains [93]. Thus, the chloroform, ethyl acetate, butanol and water extracts were more effective against S. aureus and methicillin-resistant S. aureus, the butanol and water fractions inhibited E. coli strains, while E. coli ESBL and Klebsiella pneumoniae ESBL were more sensitive to the water extract [93].
The depression-associated disorders were studied by Saaby et al., 2009 [101], who found out that the main biologically active compound responsible for the nerve-calming effects of the heather plant was quercetin, which was able to inhibit monoamine oxidase A (MAO-A) in the ethyl acetate phase (the half-maximal inhibitory concentration of quercetin for MAO-A was 18 ± 0.2 μM) [101]. It is worth mentioning that MAO-A is the main compound responsible for the alteration of important neurotransmitters, such as serotonin, dopamine and norepinephrine [142], which are vital for nerve cells and normal brain functioning, thus the inhibition of MAO-A may have crucial effects in preventing depression [143,144]. Similar antidepressant, anti-anxiety, neurotropic and anxiolytic effects were observed by Starchenko et al., 2020 [102] using an in vivo study model on rats and mice. The hydroethanolic dry extracts of C. vulgaris (ethanol 70%) were administrated to the above-mentioned study models before testing their behavior using various compartmental tests [102]. Thus, the anti-anxiety effect was demonstrated by the fact that the administered dry extract of C. vulgaris determined the orienting–research behavior of the tested animals, while the antidepressant effect was investigated by the animals’ capacity to increase the time of active swimming and reduce the immobility time [102]. Moreover, the anxiolytic effect of the plant extracts was determined by analyzing the number of approaches of the animals to the cube and the time spent on cube examination. All of these neurotropic effects are strongly correlated with the presence of phenolic compounds such as: arbutin, chlorogenic acid, rutin, hyperoside, quercetin-3-D-glucoside, (+)-gallocatechin and (−)-epigallocatechin in the composition of the used extracts of C. vulgaris [102].
Other pharmacological effects of C. vulgaris include antiviral properties indicated by the presence of oleanolic and ursolic acid, which showed a high Hepatitis C virus inhibition in the ethyl acetate fractions [123]. In addition, the same ursolic acid isolated from heather flowers also showed antiproliferative activity, as assessed by Simon et al., 1992 [139]. The authors revealed that this compound has an increased role in inhibiting the lipoxygenase activity and thus the proliferation of HL60 leukemic cells in a dose-dependent manner.
Worth mentioning is the antioxidant capacity of C. vulgaris, as several studies underlined that the phenolic compounds are the main direct links to this property. For instance, Deliorman-Orhan et al., 2009 [91] tested the scavenging activity of the kaempferol-3-O-β-D-galactoside flavonoid against some reactive oxygen radical species, showing 90.1% and 93.5% inhibition of 1,1-diphenyl-2-pirylhydrazyl (DPPH) radicals at 200 and 1000 μg/mL, respectively, while the inhibition of O−2 was lower—70.4% and 79.8%—using the same concentrations of the flavonoid [91]. On the other hand, the research of Dróżdż et al., 2016 [89] showed that the antioxidant capacity of the wild heather plant is lower than the cultivated one, with the total reducing capacity of C. vulgaris extracts ranging from 75.7 mg GA/g (for wild plants) to 89.1 mg GA/g for cultivated plants [89].
As studies have revealed, the antioxidant activity is correlated to the content of the phytocomponents present in the analyzed samples. Thus, as the research of Chepel et al., 2020 [28] showed, the highest degree of correlation for the antioxidant activity using the FRAP (ferric-reducing antioxidant power) assay was exhibited for total phenolic content (r = 0.77, p ≤ 0.01), while for the DPPH assay, the correlation for the phenolic compounds was (r = 0.65, p ≤ 0.01) [28]. Similar results were published by Varga et al., 2021, who demonstrated the same positive correlation between the antioxidant activity and the bioactive compounds [93].
The antigout potential of C. vulgaris is manifested through the antihypertensive, analgesic, hypouricemic and anti-inflammatory effects, as Vostinaru et al., 2018 [99] evaluated in their study. The authors tested these effects in vivo, using an ethanolic extract from the C. vularis plant that was applied to different rat model experiments. Accordingly, the ethanolic extract of the plant, of which the major component was chlorogenic acid, confirmed the above-mentioned effects at the dose of 500 mg/kg plant extract [99].
As for the anti-inflammatory potential, C. vulgaris showed beneficial effects on various disorders such as gastritis, ulcers [140], urinary tract pathogens [95], and Alzheimer disease [98]. For instance, Orhan et al., 2007 indicated that kaempferol-3-O--d-galactoside was the main bioactive compound related to the anti-inflammatory effect of C. vulgaris in a mice experiment model [140]. Instead, Ghareeb et al., 2014 validated that C. vulgaris has the ability to inhibit acetylcholinesterase and thus could be integrated into the treatment of dementia in patients with Alzheimer’s disease [98].
Likewise, the chemoprotective effect of the reviewed plant was evidenced in different studies that emphasized that the C. vulgaris extract can be used for skin burns and skin cancer prevention, as part of a hydrogel before UVB exposure [141], or as an inhibitor agent of UVB-induced cell death [97]. Similarly, Virag et al., 2015 [103] underlined the photoprotective effects of heather by suggesting that it acts as a suppressive to the production of reactive oxygen species because of its compounds, namely hyperoside, quercitrin, quercetin and kaempferol [103].
As highlighted above, the increasing interest in plants, and especially C. vulgaris, as natural sources with pharmacological effects is manifested in a plethora of studies that confirm the valuable biological effects of this plant. All of these studies aim to provide new data for the potential medicinal use of C. vulgaris in the treatment of numerous human disorders.
4. C. vulgaris—From Plant to Honey
Because of their important constituents, plants, in general, represent an important source of food for various insects. Honeybees in particular are capable of producing numerous products that are beneficial to human health [145], the most important such product being honey [146].
As was described in the previous sections, the C. vulgaris plant contains a large spectrum of secondary metabolites that are present in all of the analyzed parts of the plant. When collecting the nectar from the flowers, the bees transfer the bioactive substances present in their nectary glands to the honey, together with all of the specific compounds contained by the plant [147,148], creating an outstanding product with countless benefits for human health.
C. vulgaris is considered to be of major importance for apiarists, both because of the long blooming period and the large quantity of nectar production [35,149,150]. Therefore, bees can produce substantial honey quantities, compared to other nectariferous plants [36].
Moreover, the structure of the C. vulgaris plant has anthers that contain breaches that make possible the extraction of pollen and nectar by various insects, especially honeybees [151]. Consequently, the rich source of pollen and nectar contained by this plant could explain the foraging behavior of honeybees [152,153].
It is well known that honey composition is highly related to and influenced by the botanical and geographical origin of plants [154,155,156], which has a great impact on some characteristics such as flavor, texture and color [157,158]. Therefore, comparing the bioactive compounds observed in the heather plant and in its related honey is of major importance due to the attention that natural products have gained lately, and the unlimited opportunities for their use as therapeutic agents. In addition, the clinical observations that will be pointed out in this chapter have a considerable contribution to future exploration studies in which the C. vulgaris plant and honey can be part of health-promoting therapies such as apitherapy.
4.1. Heather Honey and Its Physical–Chemical Parameters
Being a “natural sweet substance produced by Apis mellifera bees” [159], especially from the nectar of plants, blossom honeys such as heather honey contain various constituents (sugars, enzymes, amino acids, organic acids, carotenoids, minerals, vitamins, volatile compounds, flavonoids and phenolic acids) that are recognized for their nutritional and medicinal profiles [160,161,162]. Moreover, because sometimes bees collect most of the nectar from a specific plant, the honey produced is called monofloral honey and it acquires the same individual characteristics as the main plant [163,164]. Some of these characteristics are related to the color, fragrance or aroma, in addition to the pharmacological properties, which are attributes that are considered to be the main motivation factors of consumer choice [165,166,167,168,169,170,171].
Therefore, monofloral honeys such as heather honey have witnessed an increased demand over the last decades, expanding their market value [171,172,173,174,175], but at the same time giving rise to fraudulent mislabeling [156,176,177].
Besides the chemical parameters, the honey structure may be influenced by other external conditions such as the climate, environment or beekeeper knowledge about the good practices that should be maintained in order to keep the honey quality unspoiled [178]. Therefore, various methods were developed to prevent honey discrimination, mostly by analyzing the floral and the geographic origin [9,155,158,179,180,181,182]. These methods are extremely important for determining the quality and authenticity indicators of honey, especially when honey is used for therapeutic purposes.
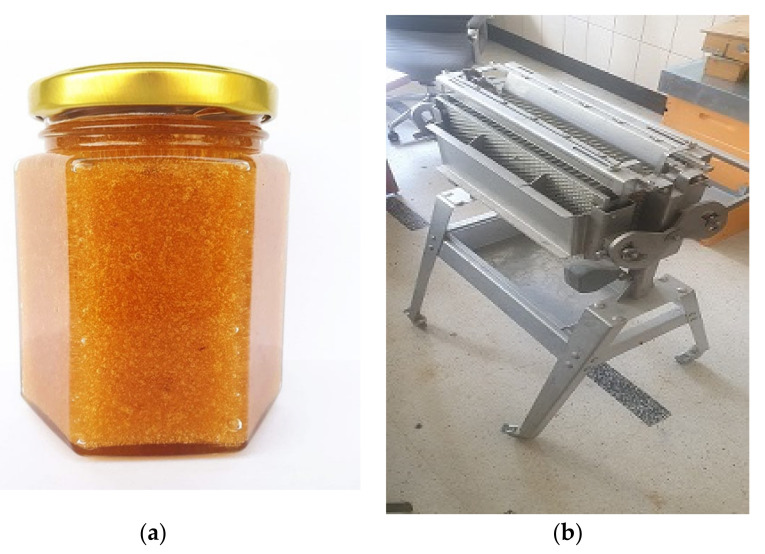
Among all the monofloral honey varieties, heather honey has atypical and unique physical–chemical properties. One of the most distinctive characteristics of this type of honey is called thixotropy [25,150,183,184,185,186]. This physical property is a consequence of the high content of colloidal proteins [187,188] that makes it very difficult to extract. As a consequence, its consistency changes when mechanically stressed, whereas it has a gelatinous appearance in the combs [189]. The extraction process is performed with the help of a special instrument (Figure 3), similar to a brush that penetrates the honey combs cells and enhances the fluidity of the honey, allowing it to flow [150]. One of the reasons for its higher price compared to the other honey varieties present on the market is the challenge in extracting this type of honey [190].
Figure 3.
(a) Heather honey (Săbăduş Ioan, beekeepr—personal collection), and (b) heather honey extractor (Beekeeping and Sericulture Department, Faculty of Animal Science and Biotechnology, UASVM Cluj-Napoca, Romania).
When analyzing the physical–chemical parameters of heather honey, regardless of the geographical origin, it can be stated that it is a distinctive type of honey, with unique features that are mostly related to: the electrical conductivity, free acidity, color, moisture content, free sugar composition, and 5-hydroxymethylfurfural (HMF) content [25,191,192], as described in Table 4.
Table 4.
Physicochemical characteristics of heather honey from different geographical origins.
| Geographical Origin |
EC (mS/cm) |
Free acidity (%) |
Pfund (mm) |
Moisture (%) |
Total Sugars (Brix%) |
HMF (mg/kg) |
References |
|---|---|---|---|---|---|---|---|
| Romania | 0.67 ± 0.17 | 17.93 ± 3.64 | n.m. | 20.66 ± 1.02 | 77.77 ± 7.82 | 11.23 ± 4.97 | [25] |
| 0.66 ± 0.07 | 16.90 ± 2.21 | n.m. | 20.52 ± 0.37 | 78.47 ± 4.26 | 10.00 ± 3.02 | [193] | |
| Poland | 0.62 | 22.10 | 69.00 | 19.10 | 73.70 | 4.80 | [192] |
| Turkey | n.m | 4.40 ± 0.03 | n.m. | 17.98 | 69.0 ± 0.07 | 94.66 ± 1.36 | [194] |
| Portugal | 0.71 ± 0.08 | 30.89 ± 5.58 | n.m. | 17.59 ± 0.37 | 72.16 ± 2.43 | 7.00 ± 6.68 | [172] |
| 0.66 ± 10.30 | 14.8 ± 0.76 | n.m. | 24.20 ± 0.00 | 73.8 ± 0.10 | 12.19 ± 0.74 | [195] | |
| 0.71 ± 0.00 | 30.33 ± 1.53 | 333 ± 0.00 | 16.47 ± 0.06 | 82.03 ± 0.06 | n.m. | [191] | |
| Spain | 0.777 ± 0.11 | n.m. | 116.00 ± 15.00 | n.m. | n.m. | n.m. | [196] |
| Ireland | 0.603 ± 0.09 | n.m. | 119 ± 40.85 | 23.33 ± 1.58 | 71.46 ± 5.94 | n.m. | [186] |
| Algeria | 0.750 ± 0.20 | 34.7 ± 10.20 | 122.00 ± 7.00 | 18.40 ± 0.60 | n.m. | 4.90 ± 0.80 | [197] |
| Estonia | 0.400 ± 0.20 | 24.3 ± 8.20 | n.m. | 18.80 ± 1.40 | 74.2 ± 2.70 | 7.70 ± 3.00 | [198] |
EC—electrical conductivity; HMF—hydroxymethylfurfural; n.m.—not measured; results reported are the average or average ± standard deviation values.
The EC of honey is strongly related to its botanical origin and therefore represents an important quality control for honey authentication [199]. This parameter is influenced by the concentration of organic acids, minerals, ash content and proteins present in the honey’s composition [199,200,201], and its proportion depends on the plant’s geographical origin [202]. In addition, both the ripening and storage time of honey can influence the EC quantity [203]. In general, heather honey possesses one of the highest ECs compared to the other honey varieties, with the only exception being chestnut honey, which surpasses all nectar honeys in terms of this feature [189,192]. The highest electrical conductivity has been observed in Spanish heather honey by Escuredo et al., 2019 [196] with a value of 0.777 ± 0.11 mS/cm, compared to the lowest identified in Estonia (0.4 ± 0.2 mS/cm). The Council Directive 2001/110/EC [159] related to honey specifies that the electrical conductivity of heather honey can be situated beyond the 0.8 mS/cm limit, as encountered in the above-mentioned studies in Table 4.
Mineral content can also be an essential feature in the determination of the electrical conductivity and thus of the botanical origin of honeys [204]. For instance, Nozal et al., 2005 [205] reported that it can be differentiated between several honey varieties such as: heather, rosemary, thyme, lavender, and honeydew honeys by establishing the amount of certain minerals, namely magnesium (Mg), calcium (Ca), aluminium (Al), iron (Fe), manganese (Mn), zinc (Zn), boron (B), copper (Cu), cobalt (Co), chromium (Cr), nickel (Ni), cadmium (Cd) and phosphorus (P) [205]. Similarly, Dezmirean et al., 2010 [25] revealed that the highest mineral content present in Romanian C. vulgaris honey was potassium (K). These results are similar to those obtained by Moise et al., 2013 [193] for the same Romanian honey variety, but from different geographical areas, as well as to those of Ghorab et al., 2021 [197]. These outcomes underline the fact that heather honey represents an excellent source of minerals for everyone that consumes it. In addition, vitamins are also present in the chemical composition of heather honey, particularly vitamin C, which has been proved to be the most predominant in Portuguese honey samples [191,206].
The free acidity is associated with the content of organic acids present in the honey samples, with gluconic acid being the major organic acid found in this honey variety [25]. Table 4 reports a wide range of acidity values for the tested heather honey samples and as it may be seen, all the samples contained levels of free acidity below the level imposed by the European regulations, namely 50 mL of acid equivalent per 1000 g [207]. The observed values ranged from 4.40 ± 0.03% free acidity in Turkey [194] to 34.7 ± 10.2% free acidity for the Algerian heather honey samples [197]. These data indicate that the tested honey samples do not undergo any fermentation process.
Regarding the honey color, it is associated with the content of the mineral elements, phenolic compounds and pollen/nectar botanical origin [165,198]. Heather honey has an interesting color, similar to maple syrup, graded from a light to dark amber color [188]. On the other hand, the smell is quite strong and the flavor is bittersweet [189,208,209]. As described in Table 4, the darkest heather honey samples were identified in Portugal (333 ± 0.00 mm Pfund) [191], while the lightest samples were from Poland (69 mm Pfund) [192].
The moisture content in honey’s composition is considered to be one of the most significant criteria for its quality, as it can affect the storage conditions and thus determine honey fermentation [210,211,212]. Despite the limits established by the European regulations regarding the maximum moisture limit allowed for commercial honeys—20% (g/100g)—[159], in heather honey this parameter is exceeded, reaching up to 23% of its composition [150]. A similar exceedance was reported in Italy for citrus honey (20.56%) [213]. The studies presented in Table 4 do not indicate a moisture level exceeding 21%, except the average ± standard deviation values of 24.20 ± 0.00% identified in Portugal [196] and 23.33 ± 1.58% in Ireland [188]. Besides this, Dezmirean et al., 2010 [25] observed in the Romanian samplings an average moisture value of 20.66 ± 1.02%, while the lowest value was found in Portugal 16.47 ± 0.06% [191].
As for the carbohydrates, honey contains sugars that represent 95% of the dry matter [212], especially in the form of fructose and glucose [147], which ensures honey’s nutritive value. The concentration of sugars present in honey varieties depends on the botanical origin of the flowers and it can be negatively influenced by the conditions and the time of storage [148]. Hence, the sugar content in honey is one of the most important elements in identifying honey quality.
Regarding heather honey composition, it reveals a high content of fructose over glucose [189,208]. This proportion between fructose and glucose is usually employed in the classification of monofloral honeys [214]. In the above-mentioned studies, the amount of total sugar was relatively similar for all the analyzed heather honey samples, with a slight difference in favor of Portugal, which had a Brix value of 82.03 ± 0.06% [191]. The exception was for Spain [196] and Algeria [197], which did not measure this parameter. However, all the samples met the European quality requirements (above 60 g/100 g).
The last analyzed feature is represented by the 5-hydroxymethylfurfural (HMF) content that is produced during the acid-catalyzed dehydration of sugars [195]. This chemical reaction is due to the honey temperature [215] and storage conditions [162,216].
The 5-HMF reaction is considered to be strongly related to the higher free acidity values [189]. 5-HMF is a parameter that establishes the honey freshness [198,216,217,218,219] or the possible adulteration of honey with inverted sugars [162], its content being established by the European legislation, namely the Codex Alimentarius Standard Commission (5-HMF in honey at 40 mg/kg and 80 mg/kg for tropical origin honeys) [220].
Among the samples listed in Table 4, the only sample that exceeded these limits was the heather honey from Turkey, which had a 5-HMF content of 94.66 ± 1.36 mg/kg [194], which can be explained by the fact that the honey was heated or inappropriately stored.
4.2. Bioactive Polyphenols and Volatile Compounds Specific to Heather Honey
4.2.1. Polyphenols
The biologically active compounds found in honey are the substances that are related to the beneficial properties exhibited on human health, with the antioxidant, anti-inflammatory and antibacterial effects [221,222,223] being the most common. The majority of these substances are transferred from the plant into the honey through the nectar and thus their composition is correlated with the geographical and botanical origin of the plant source [212,224,225,226]. Nevertheless, the composition and amount of these bioactive substances can be affected by other external factors such as the season, temperature, storage conditions, harvesting period, etc. [148,212].
Depending on their chemical structure, these biologically active compounds can be classified into phenolic acids and flavonoids [224,227], that in turn have other sub-divisions and different chemical structures [214,228].
Regarding the specific biologically active compounds found in heather honey and that can be considered as authentication tool markers, studies have revealed the presence of cis/trans-abscisic acids in samples of Portuguese heather honey [229,230]. Accordingly, Ferreres et al., 1996 pointed out the presence of various flavonoids, finding that myricetin, myricetin-3-methylether, myricetin- 30-methylether, and tricetin are the uppermost compounds that are related to the heather honey floral origin [229,230]. The results are similar to those described by Jasicka-Misiak et al., 2012 [231], who found, besides abscisic acid, many more phenolic compounds in two Polish monofloral honeys, which can be considered potential markers for the heather honey. In this regard, the most worth mentioning are: 3-hydroxybenzoic acid, 4-hydroxybenzoic acid, chlorogenic acid, syringic acid, vanillic acid, ellagic acid, rosmarinic acid and myricetin.
Furthermore, Can et al., 2015 [217] reported notable values in Turkish heather honey for p-coumaric acid (2.98 ± 1.60 µg/100 g) and quercitin (21.05 ± 0.512 µg/100 g), while, compared to the Polish heather honey samples, apigening, chlorogenic, vanillic and ferulic acids were not detected [231]. On the contrary, Halagarda et al., 2020 [232] identified higher values for p-coumaric acid (407 ± 8 µg/100 g), cystin (108 ± 7 µg/100 g), galangin (154 ± 34 µg/100 g), apigening (45 ± 1 µg/100 g) and quercetin (31 ± 3 µg/100 g) in the samples of heather honey from Poland. Similar results have been reported for Latvian heather honey in terms of the levels of p-coumaric acid (2519 ± 738 μg/kg) and quercetin (198 ± 86 μg/kg) [233]. Even so, there were dissimilarities in the other phenolic compounds such as luteolin (higher values in Latvian samples), chrysin, galangin, and apigenin (higher values in Polish heather honey samples).
Rodrigues da Silva et al., 2021 [234] identified 17 phenolic compounds in different Portuguese honey heather samples; the hydroxybenzoic acids were the most predominant phenolic acids, while the catechin derivative was the main flavonoid present in the analyzed honey.
Another phenolic compound that can be considered a marker for heather honey is 3-hydroxyphenylacetic acid, which was identified in the study of Vazquez et al., 2021 [226]. This compound had a value ranging from 54 to 242 μg/g for Galician heather honey, being the most predominant phenolic compound in all of the 91 analyzed samples, and thus a specific authenticity marker for this honey variety.
As it can be seen, there are still differences between the compositions of bioactive compounds, differences that may be explained by the influence of the geographical origin of the plant source and thus of the analyzed honey samples [216].
4.2.2. Volatile Compounds
The volatile compounds present in different honey varieties are aromatic substances that are acquired from the nectar obtained by the bees from the foraged flower [158,235]. However, their composition can vary depending on some chemical reactions that take place during the honey thermal processing, extraction or storage period [212,236]. These aromatic substances can include fragrances, aromas and pharmacological properties, which may have an important impact on consumer choice [237]. In addition, these substances can be used as botanical source indicators [212,238].
The most important volatile aromatic compounds that were observed in various honey varieties include: aldehydes, acids, ketones, esters, alcohols, linear hydrocarbons, and cyclic compounds [182]. In the case of heather honey, studies have described the presence of different volatile compounds that could be correlated with the botanical origin of C. vulgaris. For instance, guaiacol, p-cresol, propylanisole and p-anisaldehyde were reported for various Spanish monofloral honey varieties, including citrus, rosemary, eucalyptus, lavender, thyme and heather honeys [239], while isophorone and 2-methylbutyric acid were reported only for heather honey [209].
Another finding was reported by Plutowska et al., 2011, where the presence of several volatile compounds, namely: 3,4,5-trimethylphenol, phenylacetic acid, benzoic acid, b-damascenone and isophorone (3,5,5-trimethyl-2-cyclohexen-1-one) were observed in the chemical composition of heather honey from Poland [240].
Moreover, Karabagias et al., 2018 indicated that the total volatile compounds found in Portuguese tested honeys were mostly assigned to the heather honey variety, of which the most predominant compounds were heptane, benzaldehyde, benzeneacetaldehyde, cis-linalool oxide, and hotrienol [191].
4.3. Biological Activities of Heather Honey and Its Potential Use in Apitherapy
As studies have revealed, the biological activities of honey are in general associated with the presence of phenolic acids and flavonoids, which exhibit a great influence on the wellbeing of humankind [162,170]. The most notable such beneficial properties are the antioxidant, anti-inflammatory and antimicrobial effects, which are commonly used in alternative medicine, including apitherapy [208].
The use of bee products as therapeutic agents, known as apitherapy, is not a recent practice, as natural products and mostly honey have been used for preventing or treating various disorders or for beauty benefits [241]. The therapeutic benefits of honey have been employed by ancient civilizations whose knowledge has been widespread for thousand years up until present times, when the compositions of diverse honey varieties are being widely investigated for their medical potential [217,218,223,242,243].
Heather honey, like its source plant C. vulgaris, possesses plenty of biologically active compounds that are responsible for the pharmacological activities that this type of honey exhibits, as will be presented below.
4.3.1. Antioxidant Activity
The antioxidant activity of honey depends on the composition of different classes of secondary metabolites that prevent or inhibit various oxidative mechanisms in living organisms [244]. Such metabolites are the phenolic compounds, whose antioxidant capacity is related to the plant species, harvest season, climatic conditions and storage [131,216,217,218]. As will be presented in the following lines, the antioxidant activity varies between the geographical areas of the analyzed honey samples. This fact suggests that the geographical origin with different soil and climatic parameters has a huge influence on the antioxidant property of honey in general, and particularly of heather honey.
Concerning the evaluation of the antioxidant activity of heather honey, the most employed assays are the ferric-reducing antioxidant capacity (FRAP), the radical-scavenging activity (DPPH) and the radical-cation-based assays (ABTS).
For instance, Wilczyńska, 2010 [245] evaluated the antioxidant capacity of thirty-two Polish honey samples, of which the three samples of heather honey showed the highest DPPH-radical-scavenging activity (100%), while in the ABTS test, this property varied from 21.42 to 31.51%. In this study, the correlation between the antioxidant capacity of heather honey and the phenolic content was a positive one (r = 0.74 for TPC/DPPH, r = 0.55 for TPC/ABTS•+, p = 0.05), proving once again that the phenolic compounds are the substances that most influence this property [245]. The radical-scavenging activity measured in terms of DPPH for heather honey presented in this study is higher than the values obtained by Moise et al., 2013 for the Romanian heather honey samples, ranging from 49 to 61% [193], and for the Portuguese samples (varying from 33.2 ± 0.1% in 2012 to 31.0 ± 1.3% in 2016) [246].
Other Polish heather honey samples were analyzed by Kuś et al., 2014 in comparison with other five honey varieties such as black locust, rapeseed, lime, goldenrod and buckwheat, revealing that, besides buckwheat, heather honey exhibited the highest antioxidant potential in both the DPPH (0.6 ± 0.1 mmol TEAC/kg) and FRAP assays (2.1 ± 0.5 mmol Fe2+/kg) [247]. Both the content of polyphenols and the color parameters were significantly correlated with the antioxidant activity of heather honey, as studies have indicated that the darker the color of the honey, the more polyphenols it contains [248]. Moreover, the studies on Turkish monofloral honeys, including heather, evidenced the same existing correlation between the phenolic content and the antioxidant activity of various honey samples [216]. In this case study, the heather antioxidant capacity was exceeded by chestnut and oak honeys in terms of FRAP values, while for the DPPH assay, heather honey had one of the lowest values compared with the other thirteen analyzed honey assortments [216]. On the other hand, from the Portuguese eucalyptus, chestnut and heather honey samples, heather had the highest phenolic content and thus the highest antioxidant property, measured in terms of DPPH-scavenging activity (83.75 ± 0.01%) [191].
Comparatively, the study performed in the Galicia region of Spain on six different honey types showed a higher antioxidant capacity for honeydew (420–1017) and chestnut honeys (140–54) expressed as micromoles of Trolox equivalents (TRE) per 100 g of honey, while heather honey contained 132–392 μmol TRE/100g [226].
Another assay employed by Starowicz et al., 2021 for the evaluation of the antioxidant potential of heather honey was the photochemiluminescence technique (ACW) [249]. In this study, the highest value for the superoxide-anion-radical-scavenging capacity in the ACW system was reported for buckwheat (289.8 ± 14.1 μmol Trolox/g) and heather (119.9 ± 2.51 μmol Trolox/g) honeys, while the lowest value was identified in acacia honey sample. As for the FRAP assay, it provided an average value of 47.13 μmol Trolox/g for buckwheat honey and 31.58 μmol Trolox/g for heather honey, in contrast to rapeseed honey, which had the lowest identified FRAP value [249]. These findings are in agreement with the study of Kivima et al., 2021 that found an average level value of water-soluble antioxidants (ACW), for the Estonian tested honey samples, of 115.2 ± 67.9 mg AAE/100g. These samples contained substances that were specific to honeydew (cinnamic acid) and heather honey (myricetin), thus the correlation with these two honey varieties. In addition, the same study showed that heather honey was associated with the lowest lightness parameter, which suggested once again its darker color and thus the interdependence with the antioxidant property [198].
One interesting study was performed by Rodrigues da Silva et al., 2021, who tested the antioxidant capacity of C. vulgaris honey from Portugal and its effect on the oxidative stress manifested in human erythrocytes. Thus, the study showed that heather honey could scavenge the reactive oxygen species (ROS) and reactive nitrogen species (RNS) through different assays such as: superoxide radical (O2−), hydrogen peroxide (H2O2), hypochlorous acid (HOCl), singlet oxygen (1O2), nitric oxide (NO) and peroxynitrite anion (ONOO−). From all of these assays, the highest antioxidant capacity of heather honey in the ROS was 95 ± 2 μg/mL (IC50) for the HOCl assay, whereas for the RNS the highest scavenging activity was against the ONOO− assay with a value ranging between 29 ± 2 and 32 ± 1 μg/mL. Moreover, the authors proved that the C. vulgaris honey extract was able to inhibit hemoglobin oxidation (158 ± 2 μg/mL), lipid peroxidation (36 ± 1 μg/mL) and hemolysis (19 ± 1), in a dose-dependent manner (IC50) [217].
All of these mentioned studies and results demonstrate that heather honey has a strong antioxidant potential that can be further evaluated in vitro and in vivo model studies, which will have a great impact on the medical field, especially in treating oxidative-stress-associated disorders.
4.3.2. Antibacterial Activity
Another important biological property of honey that exhibits valuable therapeutic effects is the antibacterial capacity. This property is mainly associated with the geographical and botanical source of plants, bee species or the processes that honey goes through before it arrives at the customer [250]. Some of the most important factors that have a significant contribution to the antibacterial activity of honey are: the high osmolality and sugar content, low pH, viscosity, water activity, polyphenols and methylglyoxal (MGO) content, hydrogen peroxide activity, and the bee immune-enhancing peptide [251,252,253,254]. All of these features can influence honey composition and the synergic action of its compounds. Moreover, because antibiotics can no longer inhibit all of the bacterial strains [255], natural products such as honey have regained the interest of the research community.
In this direction, heather honey has been proved to have remarkable antimicrobial capacity. Serving as an example, Feás et al., 2013 tested the inhibition activity of Portuguese heather honey against four bacteria: Bacillus cereus (B. cereus), S. aureus, E. coli, and Pseudomonas aeruginosa (P. aeruginosa), and four yeast species: Candida famata (C. famata), Candida neoformans (C. neoformans), Candida krusei (C. krusei), and Candida albicans (C. albicans). The results showed that heather honey manifested growth-inhibition effects for all of the tested strains, especially for the Gram-positive bacteria (B. cereus—MIC = 1.51 ± 0.90%; S. aureus—MIC = 4.73 ± 2.09%). As for the yeast species, the most resistant to the honey effect were C. albicans (MIC = 23.33 ± 10.31%) and C. famata (MIC = 22.38 ± 9.14%), while C. krusei was the most sensitive (MIC = 14.33 ± 5.68%) [256].
In addition, comparable results were published by Dezmirean et al., 2015, who showed that heather honey samples from Romania had the highest inhibition effects (20μL honey solution, 80%) for the same Gram-positive bacteria B. cereus (MIC average = 2.1%). On the contrary, Salmonella typhi had the highest MIC (7.4%). The same authors also measured the inhibition diameter of heather honey against the tested microorganism, revealing that Gram-negative bacteria such as Salmonella enteritidis had the highest diameter (11 mm), while Salmonella typhi had the lower inhibition diameter zone (9.3 mm) [252].
A most recent comparative study between the antimicrobial and antifungal effects of Manuka and Portuguese heather honey varieties against single biofilms of Candida tropicalis (C. tropicalis) and mixed biofilms with P. aeruginosa was employed by Fernandes et al., 2020 [257]. These effects were tested using different honey concentrations. Thus, a cell reduction of the two tested bacteria was obtained with a honey concentration above 25% (w/v), with heather honey showing a higher antifungal effect for single-species biofilms of C. tropicalis, while Manuka honey also exhibited this activity in the mixed biofilms with P. aeruginosa. As for the antimicrobial effect, Manuka honey again had the highest inhibition for both single and mixed biofilms at a concentration of honey of 50% (w/v). However, the combination of honey with antifungals and antibiotics showed no inhibition result [257].
Going further, Shirlaw et al., 2020 tried to evaluate the antibiofilm and antivirulence potential of some constituents of the above-mentioned types of honey, namely Manuka and heather, against several Gram-positive and Gram-negative species biofilms, including P. aeruginosa. The results confirmed that at a minimal inhibition concentration (0.25 mg/mL), both the Manuka and heather honey types inhibited the biofilm formation in the majority of the tested strains, except for the S. aureus strain, which increased with the Manuka honey, and the E. faecalis bacteria, which was stimulated by the heather honey. However, both types of honey inhibited the biofilm formation in P. aeruginosa mainly because of the presence of oleanolic acid (associated with heather honey), benzoic acid (present in both honeys) and MGO (characteristic of Manuka honey) [107].
Overall, the antimicrobial activity of honey, and particularly of heather honey, demonstrates that bee products could play a key role in alternative therapies. Therefore, understanding the factors and mechanisms involved in the antimicrobial activity of honey is essential for the development of new products and biomaterials that could enhance its health-promoting applications. Moreover, it suggests that heather honey could be employed as an antifungal agent, especially in skin disorders, keeping in mind that in order to assess its contribution to the medical field (particularly apitherapy), future clinical studies on the impact that heather honey has on treating different disorders are still needed.
5. Conclusions and Future Perspectives
The scientific interest regarding C. vulgaris plant has emphasized the large spectrum of ecosystem services that it can provide, in terms of sustainable economic and ecological development. Moreover, this review reported the nutritional and phytochemical profile of C. vulgaris and particularly the phenolic components found in all of the parts of this herbaceous plant, trying to form a connection between the variety of pharmacological activities and its main bioactive compounds. The promising pro-health benefits of the C. vulgaris plant were also pointed out, focusing on its antibacterial, anti-anxiety, anti-inflammatory, antioxidant and chemoprotective effects, just to name the most important ones.
As this plant exhibits excellent nectariferous potential for honeybees because of the long blooming period, our review brings forth a natural, functional and undervalued product, namely heather honey. Thus, we also evaluated the specific bioactive compounds of heather honey and their related medicinal properties, in order to demonstrate once again the high quality of this sort of honey.
Despite the promising information on the chemical and pharmacological composition of heather plant, the studies regarding its related honey as well as its potential beneficial activities are still scarce. That is why we consider that this review may contribute to a starting point for future studies regarding the health benefits provided by the consumption of C. vulgaris-related products, notably heather honey, and for the introduction of this natural product into novel pharmacological products. In this regard, evidence-based pre-clinical and clinical trials are necessary in order to explore the potential applicability of heather honey for medical use and apitherapy. To the best of our knowledge, this is the first review that brings together C. vulgaris plant and honey and their therapeutic potential.
Author Contributions
Conceptualization, A.-A.C., A.-B.C., O.B. and D.S.D.; methodology, A.-A.C., G.-M.B., Ş.D. and D.S.D.; software, A.-A.C., A.-B.C., C.L., I.C.H. and Ş.D; validation, A.-A.C., G.-M.B., A.R.M., O.B., Ş.D., A.-B.C. and D.S.D.; formal analysis, A.-A.C., O.B. and D.S.D.; resources, A.-A.C., A.R.M., A.-B.C., O.B., I.C.H. and G.-M.B.; data curation, A.-A.C., Ş.D., O.B. and D.S.D.; writing—original draft preparation, A.-A.C., O.B., A.-B.C. and D.S.D.; writing—review and editing, A.-A.C., G.-M.B., Ş.D., O.B., A.R.M., A.-B.C., I.C.H. and D.S.D.; visualization, A.-A.C., Ş.D., A.-B.C., G.-M.B., O.B., A.R.M., C.L., I.C.H. and D.S.D.; supervision, A.-A.C., G.-M.B., O.B. and D.S.D. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ecosystems and Human Well-Being: Synthesis. Island Press; Washington, DC, USA: 2005. Millennium Ecosystem Assessment; pp. 1–125. [Google Scholar]
- 2.Haines-Young R.H., Potschin-Young M.B. The Links between Biodiversity, Ecosystem Services and Human Well-Being. In: Raffaelli D.G., Frid C.L.J., editors. Ecosystem Ecology: A New Synthesis. Cambridge University Press; Cambridge, UK: 2010. pp. 110–139. [DOI] [Google Scholar]
- 3.Traka M.H., Mithen R.F. Plant Science and Human Nutrition: Challenges in Assessing Health-Promoting Properties of Phytochemicals. Plant Cell. 2011;23:2483–2497. doi: 10.1105/tpc.111.087916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaplin-Kramer R., Sharp R.P., Weil C., Bennett E.M., Pascual U., Arkema K.K., Brauman K.A., Bryant B.P., Guerry A.D., Haddad N.M., et al. Global Modeling of Nature’s Contributions to People. Science. 2019;366:255–258. doi: 10.1126/science.aaw3372. [DOI] [PubMed] [Google Scholar]
- 5.Chen S.L., Yu H., Luo H.M., Wu Q., Li C.F., Steinmetz A. Conservation and Sustainable Use of Medicinal Plants: Problems, Progress, and Prospects. Chin. Med. 2016;11:37. doi: 10.1186/s13020-016-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar Rai P., Singh J.S. Invasive Alien Plant Species: Their Impact on Environment, Ecosystem Services and Human Health. Ecol. Indic. 2020;111:106020. doi: 10.1016/j.ecolind.2019.106020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atazadegan M.A., Bagherniya M., Askari G., Tasbandi A., Sahebkar A. The Effects of Medicinal Plants and Bioactive Natural Compounds on Homocysteine. Molecules. 2021;26:3081. doi: 10.3390/molecules26113081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ara S.A., Ashraf S., Arora V., Rampure P. Use of Apitherapy as a Novel Practice in the Management of Oral Diseases: A Review of Literature. J. Contemp. Dent. 2013;3:25–31. doi: 10.5005/jp-journals-10031-1030. [DOI] [Google Scholar]
- 9.Salehnezhad S. Characterization of Apitherapy Honeys for Medical Applications. Biomed. J. Sci. Tech. Res. 2019;22:16666–16671. doi: 10.26717/BJSTR.2019.22.003751. [DOI] [Google Scholar]
- 10.Chemat F., Abert-Vian M., Fabiano-Tixier A.S., Strube J., Uhlenbrock L., Gunjevic V., Cravotto G. Green Extraction of Natural Products. Origins, Current Status, and Future Challenges. TrAC Trends Anal. Chem. 2019;118:248–263. doi: 10.1016/j.trac.2019.05.037. [DOI] [Google Scholar]
- 11.Hussain M.J., Abbas Y., Nazli N., Fatima S., Drouet S., Hano C., Abbasi B.H. Root Cultures, a Boon for the Production of Valuable Compounds: A Comparative Review. Plants. 2022;11:439. doi: 10.3390/plants11030439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahidi F., Yeo J.D. Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. Int. J. Mol. Sci. 2018;19:1573. doi: 10.3390/ijms19061573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mena P., Angelino D. Plant Food, Nutrition, and Human Health. Nutrients. 2020;12:2157. doi: 10.3390/nu12072157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sitarek P., Merecz-Sadowska A., Kowalczyk T., Wieczfinska J., Zajdel R., Śliwiński T. Potential Synergistic Action of Bioactive Compounds from Plant Extracts against Skin Infecting Microorganisms. Int. J. Mol. Sci. 2020;21:5105. doi: 10.3390/ijms21145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haruna A., Yahaya S.M. Recent Advances in the Chemistry of Bioactive Compounds from Plants and Soil Microbes: A Review. Chem. Afr. 2021;4:231–248. doi: 10.1007/s42250-020-00213-9. [DOI] [Google Scholar]
- 16.Kausar F., Kim K.H., Farooqi H.M.U., Farooqi M.A., Kaleem M., Waqar R., Khalil A.A.K., Khuda F., Rahim C.S.A., Hyun K., et al. Evaluation of Antimicrobial and Anticancer Activities of Selected Medicinal Plants of Himalayas, Pakistan. Plants. 2022;11:48. doi: 10.3390/plants11010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen D., Bilz M., Leaman D.J., Miller R.M., Timoshyna A., Window J. European Red List of Medicinal Plants. Publication Office of the European Union; Luxembourg: 2016. [DOI] [Google Scholar]
- 18.Tutin T.G., Heywood V.H., Barges N.A., Moore D.M., Valentine D.H., Waters S.M., Webb D.A., editors. Cambridge University Press; Cambridge, UK: 1973. Flora Europaea, Vol. 3: Diapensiaceae to Myoporaceae; p. 541. [DOI] [Google Scholar]
- 19.Chapman H.M., Bannister P. The Spread of Heather, Calluna vulgaris (L.) Hull, into Indigenous Plant Communities of Tongariro National ParkNo Title. N. Z. J. Ecol. 1990;14:7–16. [Google Scholar]
- 20.Dróżdż P., Pyrzynska K. Screening of Ionic Liquids for Extraction of Flavonoids from Heather. Nat. Prod. Res. 2019;33:148–151. doi: 10.1080/14786419.2018.1437441. [DOI] [PubMed] [Google Scholar]
- 21.Ghedira K., Goetz P. Common Heather: Calluna vulgaris (L.) Hull. or Calluna Vulgáris Salisb. (Ericaceae) Phytotherapie. 2013;11:52–55. doi: 10.1007/s10298-013-0759-9. [DOI] [Google Scholar]
- 22.Dursunoğlu B., Yuca H., Gözcü S., Yilmaz B., Güvenalp Z. Qualitative and Quantitative Determination of the Effective Components of the Plants in Different Herbal Slimming Products in Turkey by HPLC. Turk. J. Chem. 2019;43:825–833. doi: 10.3906/kim-1711-5. [DOI] [Google Scholar]
- 23.Gimingham C.H. Biological Flora of the British Isles. Calluna Salisb. A Monotypic Genus Calluna vulgaris (L.) Hull. J. Ecol. 1960;48:455. doi: 10.2307/2257528. [DOI] [Google Scholar]
- 24.Fühner C., Runge M. Are Fe and P Availabilities Involved in Determining the Occurrence and Distribution of Calluna vulgaris (L.) Hull in Semi-Arid Grasslands on Calcareous Soils? Plant Soil. 2009;316:161–176. doi: 10.1007/s11104-008-9768-0. [DOI] [Google Scholar]
- 25.Dezmirean D., Mărghitaş L.A., Bobiş O., Stan L., Bonta V., Maghear O., Dinita G., Morar O. Physical-Chemical Attributes and Mineral Content of Heather Honey (Calluna vulgaris L.) Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 2010;67:163–168. [Google Scholar]
- 26.Šoltés R., Gregušková E., Šoltésová A. Bioindication of Chemical Elements Deposition in the High Tatra Mts (Slovakia) Based on Calluna vulgaris (L.) Hull: Comparative Levels after the Improvement of Emissions. Carpathian J. Earth Environ. Sci. 2014;9:5–14. [Google Scholar]
- 27.Delerue F., Gonzalez M., Achat D.L., Puzos L., Augusto L. Competition along Productivity Gradients: News from Heathlands. Oecologia. 2018;187:219–231. doi: 10.1007/s00442-018-4120-8. [DOI] [PubMed] [Google Scholar]
- 28.Chepel V., Lisun V., Skrypnik L. Changes in the Content of Some Groups of Phenolic Compounds and Biological Activity of Extracts of Various Parts of Heather (Calluna vulgaris (L.) Hull) at Different Growth Stages. Plants. 2020;9:926. doi: 10.3390/plants9080926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiarucci A., Calderisi M., Casini F., Bonini I. Vegetation at the Limits for Vegetation: Vascular Plants, Bryophytes and Lichens in a Geothermal Field. Folia Geobot. 2008;43:19–33. doi: 10.1007/s12224-008-9002-0. [DOI] [Google Scholar]
- 30.Pippucci A., Lorenzi R., Spanò C., Sorce C. Stress-Induced Changes to the Flora in a Geothermal Field in Central Italy. Acta Physiol. Plant. 2015;37:198. doi: 10.1007/s11738-015-1953-1. [DOI] [Google Scholar]
- 31.Arabuli G.J., Connor S.E., Kvavadze E.V. In the Colchis Mire Fl Ora: Weeds or Relicts? Acta Palaeobot. 2007;47:469–478. [Google Scholar]
- 32.Spindelböck J.P., Cook Z., Daws M.I., Heegaard E., Måren I.E., Vandvik V. Conditional Cold Avoidance Drives Between-Population Variation in Germination Behaviour in Calluna vulgaris. Ann. Bot. 2013;112:801–810. doi: 10.1093/aob/mct142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodrigues F., Moreira T., Pinto D., Pimentel F.B., Costa A.S.G., Nunes M.A., Gonçalves Albuquerque T.S., Costaa H., Palmeira-de-Oliveira A., Oliveira A.I., et al. The Phytochemical and Bioactivity Profiles of Wild Calluna vulgaris L. Flowers. Food Res. Int. 2018;111:724–731. doi: 10.1016/j.foodres.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Schellenberg J., Bergmeier E. The Calluna Life Cycle Concept Revisited: Implications for Heathland Management. Biodivers. Conserv. 2022;31:119–141. doi: 10.1007/s10531-021-02325-1. [DOI] [Google Scholar]
- 35.Escuredo O., Míguez M., Fernández-González M., Carmen Seijo M. Nutritional Value and Antioxidant Activity of Honeys Produced in a European Atlantic Area. Food Chem. 2013;138:851–856. doi: 10.1016/j.foodchem.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 36.Cirnu I.V. Flora Meliferă. Ceres; Bucuresti, Romania: 1980. [Google Scholar]
- 37.Forup M.L., Henson K.S.E., Craze P.G., Memmott J. The Restoration of Ecological Interactions: Plant-Pollinator Networks on Ancient and Restored Heathlands. J. Appl. Ecol. 2008;45:742–752. doi: 10.1111/j.1365-2664.2007.01390.x. [DOI] [Google Scholar]
- 38.Forest I., Calcagno V., Hector A., Connolly J., Stanley Harpole W., Reich P.B., Scherer-Lorenzen M., Schmit B., Tilman D., Van Ruijven J., et al. High Plant Diversity Is Needed to Maintain Ecosystem Services. Nature. 2011;477:199–202. doi: 10.1038/nature10282. [DOI] [PubMed] [Google Scholar]
- 39.Beldeanu E.C. Specii de Interes Sanogen Din Fondul Forestier. Blog la WordPress; Braşov, Romania: 2004. [Google Scholar]
- 40.Pérez-Barbería F.J., Mayes R.W., Giráldez J., Sánchez-Pérez D. Ericaceous Species Reduce Methane Emissions in Sheep and Red Deer: Respiration Chamber Measurements and Predictions at the Scale of European Heathlands. Sci. Total Environ. 2020;714:136738. doi: 10.1016/j.scitotenv.2020.136738. [DOI] [PubMed] [Google Scholar]
- 41.Rezanezhad F., Price J.S., Quinton W.L., Lennartz B., Milojevic T., Van Cappellen P. Structure of Peat Soils and Implications for Water Storage, Flow and Solute Transport: A Review Update for Geochemists. Chem. Geol. 2016;429:75–84. doi: 10.1016/j.chemgeo.2016.03.010. [DOI] [Google Scholar]
- 42.Effah E., Barett P.D., Peterson P.G., Wargent J.J., Potter M.A., Holopainen J.K., Mccormick A.C. Herbivory and Attenuated UV Radiation A Ff Ect Volatile Emissions of the Invasive Weed. Molecules. 2020;25:3200. doi: 10.3390/molecules25143200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keesing V.F. Ph.D. Thesis. Massey University; Palmerston, New Zealand: 1995. Impacts of Invasion on Community Structure: Habitat and Invertebrate Assemblage Responses to Calluna vulgaris (L.) Hull Invasion, in Tongariro National Park, New Zealand. [Google Scholar]
- 44.Rogers G.M., Leathwick J.R. North Island Seral Tussock Grasslands. 3. the Influence of Heather (Calluna vulgaris) on Rates of Change from Tussock Grassland to Shrubland. N. Z. J. Bot. 1996;34:473–487. doi: 10.1080/0028825X.1996.10410128. [DOI] [Google Scholar]
- 45.Rice E.L. Allelopathy. 2nd ed. Academic Press; Orlando, FL, USA: 1984. [Google Scholar]
- 46.Callaway R.M., Aschehoug E.T. Invasive Plants versus Their New and Old Neighbors: A Mechanism for Exotic Invasion. Science. 2000;290:521–523. doi: 10.1126/science.290.5491.521. [DOI] [PubMed] [Google Scholar]
- 47.Ballester A., Vieitez A.M., Vieitez E. Allelopathic Potential of Erica Vagans, Calluna vulgaris, and Daboecia Cantabrica. J. Chem. Ecol. 1982;8:851–857. doi: 10.1007/BF00994785. [DOI] [PubMed] [Google Scholar]
- 48.Orr S.P., Rudgers J.A., Clay K. Invasive Plants Can Inhibit Native Tree Seedlings: Testing Potential Allelopathic Mechanisms. Plant Ecol. 2005;181:153–165. doi: 10.1007/s11258-005-5698-6. [DOI] [Google Scholar]
- 49.Bais H.P., Weir T.L., Perry L.G., Gilroy S., Vivanco J.M. The Role of Root Exudates in Rhizosphere Interactions with Plants and Other Organisms. Annu. Rev. Plant Biol. 2006;57:233–266. doi: 10.1146/annurev.arplant.57.032905.105159. [DOI] [PubMed] [Google Scholar]
- 50.Kato-Noguchi H. Phytotoxic Substances Involved in Teak Allelopathy and Agroforestry. Appl. Sci. 2021;11:3314. doi: 10.3390/app11083314. [DOI] [Google Scholar]
- 51.Bonanomi G., Legg C., Mazzoleni S. Autoinhibition of Germination and Seedling Establishment by Leachate of Calluna vulgaris Leaves and Litter. Community Ecol. 2005;6:203–208. doi: 10.1556/ComEc.6.2005.2.8. [DOI] [Google Scholar]
- 52.Pyšek P., Lambdon P.W., Arianoutsou M., Kühn I., Pino J., Winter M. Handbook of Alien Species in Europe. Springer; Berlin/Heidelberg, Germany: 2009. Alien Vascular Plants of Europe; pp. 43–61. [DOI] [Google Scholar]
- 53.Christopoulou A., Christopoulou A., Fyllas N.M., Dimitrakopoulos P.G., Arianoutsou M. How Effective Are the Protected Areas of the Natura 2000 Network in Halting Biological Invasions? A Case Study in Greece. Plants. 2021;10:2113. doi: 10.3390/plants10102113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Almeida T., Forey E., Chauvat M. Alien Invasive Plant Effect on Soil Fauna Is Habitat Dependent. Diversity. 2022;14:61. doi: 10.3390/d14020061. [DOI] [Google Scholar]
- 55.Grant S.A., Hunter R.F. The Effects of Frequency and Season of Clipping on the Morphology, Productivity and Chemical Composition of Calluna vulgaris (L) Hull. New Phytol. 1966;65:125–133. doi: 10.1111/j.1469-8137.1966.tb06345.x. [DOI] [Google Scholar]
- 56.Holden J., Shotbolt L., Bonn A., Burt T.P., Chapman P.J., Dougill A.J., Fraser E.D.G., Hubacek K., Irvine B., Kirkby M.J., et al. Environmental Change in Moorland Landscapes. Earth-Sci. Rev. 2007;82:75–100. doi: 10.1016/j.earscirev.2007.01.003. [DOI] [Google Scholar]
- 57.Velle L.G., Vandvik V. Succession after Prescribed Burning in Coastal Calluna Heathlands along a 340-Km Latitudinal Gradient. J. Veg. Sci. 2014;25:546–558. doi: 10.1111/jvs.12100. [DOI] [Google Scholar]
- 58.Sanderson R., Newton S., Selvidge J. Effects of Vegetation Cutting on Invertebrate Communities of High Conservation Value Calluna Upland Peatlands. Insect Conserv. Divers. 2020;13:239–249. doi: 10.1111/icad.12384. [DOI] [Google Scholar]
- 59.Calvo L., Alonso I., Fernàndez A.J., De Luis E. Short-Term Study of Effects of Fertilisation and Cutting Treatments on the Vegetation Dynamics of Mountain Heathlands in Spain. Plant Ecol. 2005;179:181–191. doi: 10.1007/s11258-004-7511-3. [DOI] [Google Scholar]
- 60.Heil G., Bobbink R. In: Impact of Atmospheric Nitrogen Deposition on Dry Heathlands. Aerts R., Heil G.W., editors. Springer; Dordrecht, The Netherlands: 1993. pp. 181–200. [Google Scholar]
- 61.Britton A.J., Fisher J.M. Growth Responses of Low-Alpine Dwarf-Shrub Heath Species to Nitrogen Deposition and Management. Environ. Pollut. 2008;153:564–573. doi: 10.1016/j.envpol.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 62.Damgaard C. Is the Degree of Spatial Aggregation of Calluna vulgaris Regulated by Nitrogen Deposition or Precipitation? Acta Oecol. 2019;97:1–5. doi: 10.1016/j.actao.2019.04.002. [DOI] [Google Scholar]
- 63.Fagúndez J. Heathlands Confronting Global Change: Drivers of Biodiversity Loss from Past to Future Scenarios. Ann. Bot. 2013;111:151–172. doi: 10.1093/aob/mcs257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giejsztowt J., Classen A.T., Deslippe J.R. Climate Change and Invasion May Synergistically Affect Native Plant Reproduction. Ecology. 2019;101:e02913. doi: 10.1002/ecy.2913. [DOI] [PubMed] [Google Scholar]
- 65.Davies G.M., Legg C.J., Smith A.A., MacDonald A.J. Rate of Spread of Fires in Calluna vulgaris-Dominated Moorlands. J. Appl. Ecol. 2009;46:1054–1063. doi: 10.1111/j.1365-2664.2009.01681.x. [DOI] [Google Scholar]
- 66.Log T., Gjedrem A.M. A Fire Revealing Coastal Norway’s Wildland—Urban Interface Challenges and Possible Low-Cost Sustainable Solutions. Int. J. Environ. Res. Public Health. 2022;19:3038. doi: 10.3390/ijerph19053038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moss R., Welch D., Rothery P. Effects of Grazing by Mountain Hares and Red Deer on the Production and Chemical Composition of Heather. [(accessed on 25 March 2022)];J. Appl. Ecol. 2010 18:487–496. doi: 10.2307/2402410. Available online: http://www.jstor.org/stable/2402410. [DOI] [Google Scholar]
- 68.Global Invasive Species Database (2022) Species Profile: Calluna vulgaris. 2022. [(accessed on 25 March 2022)]. Available online: http://www.iucngisd.org/gisd/species.php?sc=1623.
- 69.European Commission . Interpretation Manual of European Union Habitats. European Commission; Brussels, Belgium: 2013. [(accessed on 26 March 2022)]. Available online: https://ec.europa.eu/environment/nature/legislation/habitatsdirective/docs/Int_Manual_EU28.pdf. [Google Scholar]
- 70.Directorate-General for Environment (European Commission) Tsiripidis I., Piernik A., Janssen J.A.M., Tahvanainen T., Molina J.A., Giusso del Galdo G., Gardfjell H., Dimopoulos P., Šumberová K., et al. European Red List of Habitats. Part 2, Terrestrial and Freshwater Habitats. Publications Office of the European Union; Luxembourg: 2017. [(accessed on 26 March 2022)]. Available online: https://data.europa.eu/doi/10.2779/091372. [Google Scholar]
- 71.Gimingham C.H. Ecology of Heathlands. Chapman and Hall; London, UK: 1972. [Google Scholar]
- 72.Prøsch-Danielsen L., Simonsen A. Palaeoecological Investigations towards the Reconstruction of the History of Forest Clearances and Coastal Heathlands in Southwestern Norway. Veg. Hist. Archaeobot. 2000;9:189–204. doi: 10.1007/BF01294634. [DOI] [Google Scholar]
- 73.Metallinou M.M. Emergence of and Learning Processes in a Civic Group Resuming Prescribed Burning in Norway. Sustainability. 2020;12:5668. doi: 10.3390/su12145668. [DOI] [Google Scholar]
- 74.Bonn A., Allott T., Hubacek K., Stewart J., editors. Drivers of Environmental Change in Uplands. Routledge; London, UK: 2009. Ecosystem Services: A New Rationale for Conservation of Upland Environments; pp. 448–474. [Google Scholar]
- 75.Gjedrem A.M., Log T. Study of Heathland Succession, Prescribed Burning, and Future Perspectives at Kringsjå, Norway. Land. 2020;9:485. doi: 10.3390/land9120485. [DOI] [Google Scholar]
- 76.Cordingley J.E. Ph.D. Thesis. Bournemouth University; Poole, UK: 2012. Ecosystem Service Provision in Dynamic Heath Landscapes. [Google Scholar]
- 77.Pérez-Barbería F.J., Hooper R.J., Gordon I.J. Long-Term Density-Dependent Changes in Habitat Selection in Red Deer (Cervus elaphus) Oecologia. 2013;173:837–847. doi: 10.1007/s00442-013-2686-8. [DOI] [PubMed] [Google Scholar]
- 78.Milligan G., Rose R.J., O’Reilly J., Marrs R.H. Effects of Rotational Prescribed Burning and Sheep Grazing on Moorland Plant Communities: Results from a 60-Year Intervention Experiment. Land Degrad. Dev. 2018;29:1397–1412. doi: 10.1002/ldr.2953. [DOI] [Google Scholar]
- 79.Milner C., Gwynne D. The Soay Sheep and Their Food Supply. In: Jewell P.A., Milner C., Morton Boyd J., editors. Island Survivors: The Ecology of the Soay Sheep of St. Kilda. Athlone Press; London, UK: 1974. pp. 273–325. [Google Scholar]
- 80.Worrall F., Clay G.D. The Potential Use of Heather, Calluna vulgaris, as a Bioenergy Crop. Biomass Bioenergy. 2014;64:140–151. doi: 10.1016/j.biombioe.2014.03.007. [DOI] [Google Scholar]
- 81.Encinar J.M., González J.F., Nogales-Delgado S. Thermogravimetry of the Steam Gasification of Calluna vulgaris: Kinetic Study. Catalysts. 2021;11:657. doi: 10.3390/catal11060657. [DOI] [Google Scholar]
- 82.Jayanegara A., Goel G., Makkar H.P.S., Becker K. Divergence between Purified Hydrolysable and Condensed Tannin Effects on Methane Emission, Rumen Fermentation and Microbial Population In Vitro. Anim. Feed Sci. Technol. 2015;209:60–68. doi: 10.1016/j.anifeedsci.2015.08.002. [DOI] [Google Scholar]
- 83.Thompson D.B.A., Hester A.J., Usher M.B. Heaths and Moorland, Cultural Landscapes. HMSO; Edinburgh, UK: 1995. [Google Scholar]
- 84.Boustras G., Boukas N. Forest Fires’ Impact on Tourism Development: A Comparative Study of Greece and Cyprus. Manag. Environ. Qual. 2013;24:498–511. doi: 10.1108/MEQ-09-2012-0058. [DOI] [Google Scholar]
- 85.Soltys D., Krasuska U., Bogatek R., Gniazdowska A. Allelochemicals as Bioherbicides—Present and Perspectives. In: Price A.J., Kelton J.A., editors. Herbicides—Current Research and Case Studies in Use. IntechOpen; London, UK: 2013. [Google Scholar]
- 86.Farooq M., Jabran K., Cheema Z.A., Wahid A., Siddique K.H. The Role of Allelopathy in Agricultural Pest Management. Pest Manag. Sci. 2011;67:493–506. doi: 10.1002/ps.2091. [DOI] [PubMed] [Google Scholar]
- 87.Lamberth C. Naturally Occurring Amino Acid Derivatives with Herbicidal, Fungicidal or Insecticidal Activity. Amino Acids. 2016;48:929–940. doi: 10.1007/s00726-016-2176-5. [DOI] [PubMed] [Google Scholar]
- 88.Naeem M., Cheema Z.A., Ihsan M.Z., Hussain Y., Mazari A., Abbas H.T. Allelopathic Effects of Different Plant Aqueous Extracts on Yield and Weeds of Wheat. Planta Daninha. 2018;36 doi: 10.1590/s0100-83582018360100094. [DOI] [Google Scholar]
- 89.Dróżdż P., Sentkowska A., Pyrzyńska K. Biophenols and Antioxidant Activity in Wild and Cultivated Heather. Nat. Prod. Res. 2016;31:1181–1184. doi: 10.1080/14786419.2016.1222389. [DOI] [PubMed] [Google Scholar]
- 90.Mandim F., Barros L., Calhelha R.C., Abreu R.M.V., Pinela J., Alves M.J., Heleno S., Santos P.F., Ferreira I.C.F.R. Calluna vulgaris (L.) Hull: Chemical Characterization, Evaluation of Its Bioactive Properties and Effect on the Vaginal Microbiota. Food Funct. 2018;10:78–89. doi: 10.1039/C8FO01910J. [DOI] [PubMed] [Google Scholar]
- 91.Deliorman-Orhan D., Şenol S., Kartal M., Orhan I. Assessment of Antiradical Potential of Calluna vulgaris (L.) Hull and Its Major Flavonoid. J. Sci. Food Agric. 2009;89:809–814. doi: 10.1002/jsfa.3517. [DOI] [Google Scholar]
- 92.Mandim F., Barros L., Heleno S.A., Pires T.C.S.P., Dias M.I., Alves M.J., Santos P.F., Ferreira I.C.F.R. Phenolic Profile and Effects of Acetone Fractions Obtained from the Inflorescences of: Calluna vulgaris (L.) Hull on Vaginal Pathogenic and Non-Pathogenic Bacteria. Food Funct. 2019;10:2399–2407. doi: 10.1039/C9FO00415G. [DOI] [PubMed] [Google Scholar]
- 93.Varga E., Becsek E., Bartha S.G., Stranczinger S., Mihalovits F., Papp N. Determination of Polyphenols and in Vitro Antimicrobial and Antioxidant Activity of Calluna vulgaris (L.) Hull. Biol. Futur. 2021;72:251–256. doi: 10.1007/s42977-020-00059-9. [DOI] [PubMed] [Google Scholar]
- 94.Kutsyk R.V., Kurovets L.M. Synergism of the Antimicrobial Action of Extracts of Plants of the Ericaceae Juss. Family and Cefazolin: Features of the Effect against S. Haemolyticus with Dierent Mechanisms of Resistance to β-Lactam Antibiotics. Biomed. Andanthropol. 2010;15:167–172. [Google Scholar]
- 95.Vučić D.M., Petković M.R., Rodić-Grabovac B.B., Stefanović O.D., Vasić S.M., Čomić L.R. In Vitro Activity of Heather [Calluna vulgaris (L.) Hull] Extracts on Selected Urinary Tract Pathogens. Bosn. J. Basic Med. Sci. 2014;14:234–238. doi: 10.17305/bjbms.2014.4.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kupeli E., Orhan I., Yesilada E. Evaluation of Some Plants Used in Turkish Folk Medicine for Their Anti-Inflammatory and Antinociceptive Activities. Pharm. Biol. 2007;45:547–555. doi: 10.1080/13880200701498895. [DOI] [Google Scholar]
- 97.Olteanu D., Baldea I., Clichici S., Bolfa P., Cenariu M., Schrepler-Perde M., Alupei M., Muresan A., Filip A. In Vitro Studies on the Mechanisms Involved in Chemoprevention Using Calluna vulgaris on Vascular Endothelial Cells Exposed to UVB. J. Photochem. Photobiol. B Biol. 2014;136:54–61. doi: 10.1016/j.jphotobiol.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 98.Ghareeb D.A., ElAhwany A.M.D., El-Mallawany S.M., Saif A.A. In Vitro Screening for Anti-Acetylcholiesterase, Anti-Oxidant, Anti-Glucosidase, Anti-Inflammatory and Anti-Bacterial Effect of Three Traditional Medicinal Plants. Biotechnol. Biotechnol. Equip. 2014;28:1155–1164. doi: 10.1080/13102818.2014.969877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vostinaru O., Dinte E., Soran M.L., Lung I., Opris O., Mogosan C. Evaluation of the Anti-Gout Potential of Calluna vulgaris L. (Ericaceae) in Rats. Rec. Nat. Prod. 2018;12:432–444. doi: 10.25135/rnp.71.18.03.253. [DOI] [Google Scholar]
- 100.Villanueva-Bermejo D., Vázquez E., Villalva M., Santoyo S., Fornari T., Reglero G., García-Risco M.R. Simultaneous Supercritical Fluid Extraction of Heather (Calluna vulgaris L.) and Marigold (Calendula Officinalis, L.) and Anti-Inflammatory Activity of the Extracts. Appl. Sci. 2019;9:2245. doi: 10.3390/app9112245. [DOI] [Google Scholar]
- 101.Saaby L., Rasmussen H.B., Jäger A.K. MAO-A Inhibitory Activity of Quercetin from Calluna vulgaris (L.) Hull. J. Ethnopharmacol. 2009;121:178–181. doi: 10.1016/j.jep.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 102.Starchenko G., Hrytsyk A., Raal A., Koshovyi O. Phytochemical Profile and Pharmacological Activities of Water and Hydroethanolic Dry Extracts of Calluna vulgaris (L.) Hull. Herb. Plants. 2020;9:751. doi: 10.3390/plants9060751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Virág P., Brie I.C., Burz C.C., Tatomir C.B., Hodor T., Orasan R., Decean H.P. Modulation of the UV-B-Induced Oxidative Stress and Apoptosis in HaCaT Cell Line with Calluna vulgaris Extract. Not. Bot. Horti Agrobot. Cluj-Napoca. 2015;43:313–319. doi: 10.15835/nbha43210000. [DOI] [Google Scholar]
- 104.Hellner M., Winter D., Von Georgi R., Münstedt K. Apitherapy: Usage and Experience in German Beekeepers. Evid.-Based Complement. Altern. Med. 2008;5:475–479. doi: 10.1093/ecam/nem052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stângaciu S., Mărghitaş L.A., Dezmirean D.S., Bonta V., Mărgăoan R., Bobiş O. Quality Parameters Needed for Bee Products Used in Apitherapy. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 2015;72:66–71. doi: 10.15835/buasvmcn-asb:10561. [DOI] [Google Scholar]
- 106.Ali A.M., Kunugi H. Apitherapy for Age-Related Skeletal Muscle Dysfunction (Sarcopenia): A Review on the Effects of Royal Jelly, Propolis, and Bee Pollen. Foods. 2020;9:1362. doi: 10.3390/foods9101362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shirlaw O., Billah Z., Attar B., Hughes L., Qasaymeh R.M., Seidel V., Efthimiou G. Antibiofilm Activity of Heather and Manuka Honeys and Antivirulence Potential of Some of Their Constituents on the DsbA1 Enzyme of Pseudomonas Aeruginosa. Antibiotics. 2020;9:911. doi: 10.3390/antibiotics9120911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Allais D.P., Simon A., Bennini B., Chulia A.J., Kaouadji M., Christiane D. Flavone and Flavonol Glycosides from Calluna vulgaris. Phytochemistry. 1991;30:3099–3101. doi: 10.1016/S0031-9422(00)98261-X. [DOI] [Google Scholar]
- 109.Allais D.P., Chulia A.J., Kaouadji M., Simon A., Delage C. 3-Desoxycallunin and 2″-Acetylcallunin, Two Minor 2,3-Dihydroflavonoid Glucosides from Calluna vulgaris. Phytochemistry. 1995;39:427–430. doi: 10.1016/0031-9422(94)00910-L. [DOI] [Google Scholar]
- 110.Monschein M., Iglesias Neira J., Kunert O., Bucar F. Phytochemistry of Heather (Calluna vulgaris (L.) Hull) and Its Altitudinal Alteration. Phytochem. Rev. 2010;9:205–215. doi: 10.1007/s11101-009-9153-5. [DOI] [Google Scholar]
- 111.Szakiel A., Nizyński B., Pączkowski C. Triterpenoid Profile of Flower and Leaf Cuticular Waxes of Heather Calluna vulgaris. Nat. Prod. Res. 2012;27:1404–1407. doi: 10.1080/14786419.2012.742083. [DOI] [PubMed] [Google Scholar]
- 112.Dróżdż P., Sentkowska A., Pyrzynska K. Flavonoid Content and Antioxidant Properties in Different Extracts of Calluna vulgaris (L.) Flowers. J. Agric. Sci. Technol. A. 2017;7:39–44. doi: 10.17265/2161-6256/2017.01.006. [DOI] [Google Scholar]
- 113.Osoro K., Oliván M., Celaya R., Martínez A. The Effect of Calluna vulgaris Cover on the Performance and Intake of Ewes Grazing Hill Pastures in Northern Spain. Grass Forage Sci. 2000;55:300–308. doi: 10.1046/j.1365-2494.2000.00226.x. [DOI] [Google Scholar]
- 114.Celaya R., Benavides R., García U., Ferreira L.M.M., Ferre I., Martínez A., Ortega-Mora L.M., Osoro K. Grazing Behaviour and Performance of Lactating Suckler Cows, Ewes and Goats on Partially Improved Heathlands. Animal. 2008;2:1818–1831. doi: 10.1017/S1751731108003224. [DOI] [PubMed] [Google Scholar]
- 115.Santos J., Herrero M., Mendiola J.A., Oliva-Teles M.T., Ibáñez E., Delerue-Matos C., Oliveira M.B.P.P. Fresh-Cut Aromatic Herbs: Nutritional Quality Stability during Shelf-Life. LWT Food Sci. Technol. 2014;59:101–107. doi: 10.1016/j.lwt.2014.05.019. [DOI] [Google Scholar]
- 116.Rodrigues F., Santos J., Pimentel F.B., Braga N., Palmeira-de-Oliveira A., Oliveira M.B. Promising New Applications of Castanea Sativa Shell: Nutritional Composition, Antioxidant Activity, Amino Acids and Vitamin E Profile. Food Funct. 2015;6:2854–2860. doi: 10.1039/C5FO00571J. [DOI] [PubMed] [Google Scholar]
- 117.Olechnowicz-Stepien W., Rzadkowska-Dodalska H., Grimshaw J. Investigation on Lipid Fraction Compounds of Heather Flowers (Calluna vulgaris L.) Pol. J. Chem. 1982;56:153–157. [Google Scholar]
- 118.Jalal M.A.F., Read D.J., Haslam E. Phenolic Composition and Its Seasonal Variation in Calluna vulgaris. Phytochemistry. 1982;21:1397–1401. doi: 10.1016/0031-9422(82)80150-7. [DOI] [Google Scholar]
- 119.Rieger G., Müller M., Guttenberger H., Bucar F. Influence of Altitudinal Variation on the Content of Phenolic Compounds in Wild Populations of Calluna vulgaris, Sambucus Nigra, and Vaccinium Myrtillus. J. Agric. Food Chem. 2008;56:9080–9086. doi: 10.1021/jf801104e. [DOI] [PubMed] [Google Scholar]
- 120.Yao X.H., Zhang Z.B., Song P., Hao J.Y., Zhang D.Y., Zhang Y.F. Different Harvest Seasons Modify Bioactive Compounds and Antioxidant Activities of Pyrola Incarnata. Ind. Crop. Prod. 2016;94:405–412. doi: 10.1016/j.indcrop.2016.08.033. [DOI] [Google Scholar]
- 121.Yang L., Wen K.S., Ruan X., Zhao Y.X., Wei F., Wang Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules. 2018;23:762. doi: 10.3390/molecules23040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ribeiro D.A., de Macêdo D.G., Boligon A.A., Menezes I.R.A., de Almeida Souza M.M., da Costa J.G.M. Influence of Seasonality on the Phenolic Composition of Secondatia Floribunda, A. DC (Apocynaceae) during Its Phenological Cycle. Acta Physiol. Plant. 2019;41:185. doi: 10.1007/s11738-019-2975-x. [DOI] [Google Scholar]
- 123.García-Risco M.R., Vázquez E., Sheldon J., Steinmann E., Riebesehl N., Fornari T., Reglero G. Supercritical Fluid Extraction of Heather (Calluna vulgaris) and Evaluation of Anti-Hepatitis C Virus Activity of the Extracts. Virus Res. 2015;198:9–14. doi: 10.1016/j.virusres.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 124.Cherepanova O., Dudkina N., Kalinina T., Vysokova O., Krivopalov S., Glukhareva T. The Evaluation of Influence of Synthesized Compounds Belonging to Thiadiazolines Family on the Growth and Biochemical Composition of Callus Culture of Calluna vulgaris (L.) Hull. AIP Conf. Proc. 2019;2063:030003. doi: 10.1063/1.5087311. [DOI] [Google Scholar]
- 125.Todorova I.T., Batovska D.I., Parushev S.P., Djakova G.J., Popov S.S. Surface Metabolic Composition of Berries Collected from 13 Bulgarian Grapevine Plants. Nat. Prod. Res. 2010;24:935–941. doi: 10.1080/14786410802469413. [DOI] [PubMed] [Google Scholar]
- 126.Pancost R.D., Baas M., van Geel B., Damste J.S.S. Biomarkers as Proxies for Plant Inputs to Peats: An Example from a Sub-Boreal Ombrotrophic Bog. Org.Geochem. 2002;33:675–690. doi: 10.1016/S0146-6380(02)00048-7. [DOI] [Google Scholar]
- 127.Gama P., Casal S., Oliveira B., Ferreira M.A. Development of an Hplc/Detector Method for Monitoring Tocopherols and Tocotrienols in Edible Oils. J. Liq. Chromatogr. Relat. Technol. 2007;23:3011–3022. doi: 10.1081/JLC-100101839. [DOI] [Google Scholar]
- 128.Kim T.S., Decker E.A., Lee J. Antioxidant Capacities of α-Tocopherol, Trolox, Ascorbic Acid, and Ascorbyl Palmitate in Riboflavin Photosensitized Oil-in-Water Emulsions. Food Chem. 2012;133:68–75. doi: 10.1016/j.foodchem.2011.12.069. [DOI] [Google Scholar]
- 129.Pinela J., Barros L., Carvalho A.M., Ferreira I.C.F.R. Influence of the Drying Method in the Antioxidant Potential and Chemical Composition of Four Shrubby Flowering Plants from the Tribe Genisteae (Fabaceae) Food Chem. Toxicol. 2011;49:2983–2989. doi: 10.1016/j.fct.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 130.Pavlović R.D., Lakušić B., Došlov-Kokoruš Z., Kovačević N. Arbutin Content and Antioxidant Activity of Some Ericaceae Species. Pharmazie. 2009;64:656–659. doi: 10.1691/ph.2009.9551. [DOI] [PubMed] [Google Scholar]
- 131.Cezarotto V.S., Giacomelli S.R., Vendruscolo M.H., Vestena A.S., Cezarotto C.S., Da Cruz R.C., Maurer L.H., Ferreira L.M., Emanuelli T., Cruz L. Influence of Harvest Season and Cultivar on the Variation of Phenolic Compounds Composition and Antioxidant Properties in Vaccinium Ashei Leaves. Molecules. 2017;22:1603. doi: 10.3390/molecules22101603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Prins A.H., Berdowski J.J.M., Latuhihin M.J. Effect of NH4-Fertilization on the Maintenance of a Calluna vulgaris Vegetation. Acta Bot. Neerl. 1991;40:269–279. doi: 10.1111/j.1438-8677.1991.tb01558.x. [DOI] [Google Scholar]
- 133.Marks R.H., Bannister P. The Adaptation of Calluna vulgaris (L.) Hull To Contrasting Soil Types. New Phytol. 1978;81:753–761. doi: 10.1111/j.1469-8137.1978.tb01650.x. [DOI] [Google Scholar]
- 134.Matanzas N., Afif E., Díaz T.E., Gallego J.L.R. Screening of Pioneer Metallophyte Plant Species with Phytoremediation Potential at a Severely Contaminated Hg and as Mining Site. Environments. 2021;8:63. doi: 10.3390/environments8070063. [DOI] [Google Scholar]
- 135.Mičieta K., Murín G. Wild Plant Species in Bioindication of Polluted Environment. Ekológia. 1997;16:193–202. [Google Scholar]
- 136.Wojtuń B., Samecka-Cymerman A., Żołnierz L., Rajsz A., Kempers A.J. Vascular Plants as Ecological Indicators of Metals in Alpine Vegetation (Karkonosze, SW Poland) Environ. Sci. Pollut. Res. 2017;24:20093–20103. doi: 10.1007/s11356-017-9608-y. [DOI] [PubMed] [Google Scholar]
- 137.Rajsz A., Wojtuń B., Samecka-Cymerman A., Wąsowicz P., Mróz L., Rudecki A., Kempers A.J. Metals in Calluna vulgaris, Empetrum Nigrum, Festuca Vivipara and Thymus Praecox Ssp. Arcticus in the Geothermal Areas of Iceland. Environ. Sci. Pollut. Res. 2021;28:67224–67233. doi: 10.1007/s11356-021-15046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pichtel J., Salt C.A. Vegetative Growth and Trace Metal Accumulation on Metalliferous Wastes. J. Environ. Qual. 1998;27:618–624. doi: 10.2134/jeq1998.00472425002700030020x. [DOI] [Google Scholar]
- 139.Simon A., Najid A., Chulia A.J., Delage C., Rigaud M. Inhibition of Lipoxygenase Activity and HL60 Leukemic Cell Proliferation by Ursolic Acid Isolated from Heather Flowers (Calluna vulgaris) Biochim. Biophys. Acta/Lipids Lipid Metab. 1992;1125:68–72. doi: 10.1016/0005-2760(92)90157-Q. [DOI] [PubMed] [Google Scholar]
- 140.Orhan I., Küpeli E., Terzioǧlu S., Yesilada E. Bioassay-Guided Isolation of Kaempferol-3-O-β-d-Galactoside with Anti-Inflammatory and Antinociceptive Activity from the Aerial Part of Calluna vulgaris L. J. Ethnopharmacol. 2007;114:32–37. doi: 10.1016/j.jep.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 141.Olteanu E.D., Filip G.A., Clichici S., Daicoviciu D., Achim M., Postescu I.D., Bolfǎ P., Bolojan L., Vlase L., Muresan A. Photochemoprotective Effect of Calluna vulgaris Extract on Skin Exposed to Multiple Doses of Ultraviolet b in SKH-1 Hairless Mice. J. Environ. Pathol. Toxicol. Oncol. 2012;31:233–243. doi: 10.1615/JEnvironPatholToxicolOncol.v31.i3.50. [DOI] [PubMed] [Google Scholar]
- 142.Billett E.E. Monoamine Oxidase (MAO) in Human Peripheral Tissues. Neurotoxicology. 2004;25:139–148. doi: 10.1016/S0161-813X(03)00094-9. [DOI] [PubMed] [Google Scholar]
- 143.Kumar B., Sheetal , Mantha A.K., Kumar V. Recent Developments on the Structure-Activity Relationship Studies of MAO Inhibitors and Their Role in Different Neurological Disorders. RSC Adv. 2016;6:42660–42683. doi: 10.1039/C6RA00302H. [DOI] [Google Scholar]
- 144.Carradori S., Secci D., Petzer J.P. MAO Inhibitors and Their Wider Applications: A Patent Review. Expert Opin. Ther. Pat. 2018;28:211–226. doi: 10.1080/13543776.2018.1427735. [DOI] [PubMed] [Google Scholar]
- 145.Dezmirean D.S., Paşca C., Moise A.R., Bobiş O. Plant Sources Responsible for the Chemical Composition and Main Bioactive Properties of Poplar-Type Propolis. Plants. 2021;10:22. doi: 10.3390/plants10010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rana S., Mishra M., Yadav D., Subramani S.K., Katare C., Prasad G. Medicinal Uses of Honey: A Review on Its Benefits to Human Health. Prog. Nutr. 2018;20:5–14. [Google Scholar]
- 147.Da Silva P.M., Gauche C., Gonzaga L.V., Costa A.C.O., Fett R. Honey: Chemical Composition, Stability and Authenticity. Food Chem. 2016;196:309–323. doi: 10.1016/j.foodchem.2015.09.051. [DOI] [PubMed] [Google Scholar]
- 148.Santos-Buelga C., González-Paramás A.M. Bee Products—Chemical and Biological Properties. Volume 306. Springer International Publishing; Basel, Switzerland: 2017. [DOI] [Google Scholar]
- 149.Beekman M., Ratnieks F.L.W. Long-Range Foraging by the Honey-Bee, Apis Mellifera, L. Funct. Ecol. 2000;14:490–496. doi: 10.1046/j.1365-2435.2000.00443.x. [DOI] [Google Scholar]
- 150.Lehébel-Péron A., Sidawy P., Dounias E., Schatz B. Attuning Local and Scientific Knowledge in the Context of Global Change: The Case of Heather Honey Production in Southern France. J. Rural Stud. 2016;44:132–142. doi: 10.1016/j.jrurstud.2016.01.005. [DOI] [Google Scholar]
- 151.Descamps C., Moquet L., Migon M., Jacquemart A.L., Brunet J. Diversity of the Insect Visitors on Calluna vulgaris (Ericaceae) in Southern France Heathlands. J. Insect Sci. 2015;15:130. doi: 10.1093/jisesa/iev116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vanderplanck M., Moerman R., Rasmont P., Lognay G., Wathelet B., Wattiez R., Michez D. How Does Pollen Chemistry Impact Development and Feeding Behaviour of Polylectic Bees? PLoS ONE. 2014;9:e86209. doi: 10.1371/journal.pone.0086209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Moquet L., Mayer C., Michez DWathelet B., Jacquemart A.L. Early Spring Floral Foraging Resources for Pollinators in Wet Heathlands in Belgium. J. Insect Conserv. 2015;19:837–848. doi: 10.1007/s10841-015-9802-5. [DOI] [Google Scholar]
- 154.Schievano E., Morelato E., Facchin C., Mammi S., Chimiche S., Padova U., Marzolo V. Characterization of Markers of Botanical Origin and Other Compounds Extracted from Unifloral Honeys. J. Agric. Food Chem. 2013;61:1747–1755. doi: 10.1021/jf302798d. [DOI] [PubMed] [Google Scholar]
- 155.Homrani M., Escuredo O., Rodríguez-Flores M.S., Fatiha D., Mohammed B., Homrani A., Seijo M.C. Botanical Origin, Pollen Profile, and Physicochemical Properties of Algerian Honey from Different Bioclimatic Areas. Foods. 2020;9:938. doi: 10.3390/foods9070938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Suhandy D., Yulia M. The Use of UV Spectroscopy and SIMCA for the Authentication of Indonesian Honeys According to Botanical, Entomological and Geographical Origins. Molecules. 2021;26:915. doi: 10.3390/molecules26040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Manyi-Loh C.E., Ndip R.N., Clarke A.M. Volatile Compounds in Honey: A Review on Their Involvement in Aroma, Botanical Origin Determination and Potential Biomedical Activities. Int. J. Mol. Sci. 2011;12:9514–9532. doi: 10.3390/ijms12129514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rodopoulou M.A., Tananaki C., Dimou M., Liolios V., Kanelis D., Goras G., Thrasyvoulou A. The Determination of the Botanical Origin in Honeys with Over-Represented Pollen: Combination of Melissopalynological, Sensory and Physicochemical Analysis. J. Sci. Food Agric. 2018;98:2705–2712. doi: 10.1002/jsfa.8764. [DOI] [PubMed] [Google Scholar]
- 159.EU Council Directive 2001/110/CE Concerning Honey. Off. J. Eur. Communities. 2002;L10:47–52. [Google Scholar]
- 160.Pita-Calvo C., Vázquez M. Differences between Honeydew and Blossom Honeys: A Review. Trends Food Sci. Technol. 2016;59:79–87. doi: 10.1016/j.tifs.2016.11.015. [DOI] [Google Scholar]
- 161.Baglio E. The Industry of Honey. An Introduction. In: Baglio E., editor. Chemistry and Technology of Honey Production. Springer International Publishing; Cham, Switzerland: 2018. pp. 1–14. [Google Scholar]
- 162.Cianciosi D., Forbes-Hernández T.Y., Afrin S., Gasparrini M., Reboredo-Rodriguez P., Manna P.P., Zhang J., Lamas L.B., Flórez S.M., Toyos P.A., et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules. 2018;23:2322. doi: 10.3390/molecules23092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.De la Fuente E., Martínez-Castro I. Characterization of Spanish Unifloral Honeys by Solid Phase Microextraction and Gas Chromatography-Mass Spectrometry. J. Sep. Sci. 2005;28:1093–1100. doi: 10.1002/jssc.200500018. [DOI] [PubMed] [Google Scholar]
- 164.El-Haskoury R., Kriaa W., Lyoussi B., Makni M. Ceratonia Siliqua Honeys from Morocco: Physicochemical Properties, Mineral Contents, and Antioxidant Activities. J. Food Drug Anal. 2018;26:67–73. doi: 10.1016/j.jfda.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tuberoso C.I.G., Jerković I., Sarais G., Congiu F., Marijanović Z., Kuś P. Color Evaluation of Seventeen European Unifloral Honey Types by Means of Spectrophotometrically Determined CIE L Ã C Ã Ab h Ab Chromaticity Coordinates. Food Chem. 2014;145:284–291. doi: 10.1016/j.foodchem.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 166.Küster-Boluda I., Vidal-Capilla I. Consumer Attitudes in the Election of Functional Foods. Span. J. Mark. ESIC. 2017;21((Suppl. 1)):65–79. doi: 10.1016/j.sjme.2017.05.002. [DOI] [Google Scholar]
- 167.Testa R., Asciuto A., Schifani G., Schimmenti E., Migliore G. Quality Determinants and Effect of Therapeutic Properties in Honey Consumption. An Exploratory Study on Italian Consumers. Agriculture. 2019;9:174. doi: 10.3390/agriculture9080174. [DOI] [Google Scholar]
- 168.Saraiva A., Carrascosa C., Raheem D., Ramos F., Raposo A. Natural Sweeteners: The Relevance of Food Naturalness for Consumers, Food Security Aspects, Sustainability and Health Impacts. Int. J. Environ. Res. Public Health. 2020;17:6285. doi: 10.3390/ijerph17176285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zafar M., Latafat T., Zehra A., Farooqui Y. Therapeutic Properties of Honey: A Review of Literature. Res. Rev. A J. Pharmacol. 2020;10:41–49. [Google Scholar]
- 170.Guimarães I., Baptista-Silva S., Pintado M., Oliveira A.L. Polyphenols: A Promising Avenue in Therapeutic Solutions for Wound Care. Appl. Sci. 2021;11:1230. doi: 10.3390/app11031230. [DOI] [Google Scholar]
- 171.Wojciechowska-Solis J., Barska A. Exploring the Preferences of Consumers’ Organic Products in Aspects of Sustainable Consumption: The Case of the Polish Consumer. Agriculture. 2021;11:138. doi: 10.3390/agriculture11020138. [DOI] [Google Scholar]
- 172.Pires J., Estevinho M.L., Feás X., Cantalapiedra J., Iglesias A. Pollen Spectrum and Physico-Chemical Attributes of Heather (Erica Sp.) Honeys of North Portugal. J. Sci. Food Agric. 2009;89:1862–1870. doi: 10.1002/jsfa.3663. [DOI] [Google Scholar]
- 173.Ciucure C.T., Geană E.I. Phenolic Compounds Profile and Biochemical Properties of Honeys in Relationship to the Honey Floral Sources. Phytochem. Anal. 2019;30:481–492. doi: 10.1002/pca.2831. [DOI] [PubMed] [Google Scholar]
- 174.Vapa-Tankosić J., Ignjatijević S., Kiurski J., Milenković J., Milojević I. Analysis of Consumers’willingness to Pay for Organic and Local Honey in Serbia. Sustainability. 2020;12:4686. doi: 10.3390/su12114686. [DOI] [Google Scholar]
- 175.Chalupová M., Rojík S., Kotouĉková H., Kauerová L. Food Labels (Quality, Origin, and Sustainability): The Experience of Czech Producers. Sustainability. 2021;13:318. doi: 10.3390/su13010318. [DOI] [Google Scholar]
- 176.Soares S., Amaral J.S., Oliveira M.B.P.P., Mafra I. A Comprehensive Review on the Main Honey Authentication Issues: Production and Origin. Compr. Rev. Food Sci. Food Saf. 2017;16:1072–1100. doi: 10.1111/1541-4337.12278. [DOI] [PubMed] [Google Scholar]
- 177.Pauliuc D., Dranca F., Oroian M. Antioxidant Activity, Total Phenolic Content, Individual Phenolics and Physicochemical Parameters Suitability for Romanian Honey Authentication. Foods. 2020;9:306. doi: 10.3390/foods9030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Escuredo O., Carmen Seijo M. Honey: Chemical Composition, Stability and Authenticity. Foods. 2019;8:577. doi: 10.3390/foods8110577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Oroian M., Ropciuc S., Paduret S., Sanduleac E.T. Authentication of Romanian Honeys Based on Physicochemical Properties, Texture and Chemometric. J. Food Sci. Technol. 2017;54:4240–4250. doi: 10.1007/s13197-017-2893-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fechner D.C., Hidalgo M.J., Ruiz Díaz J.D., Gil R.A., Pellerano R.G. Geographical Origin Authentication of Honey Produced in Argentina. Food Biosci. 2019;33:100483. doi: 10.1016/j.fbio.2019.100483. [DOI] [Google Scholar]
- 181.Mădaş M.N., Mărghitaş L.A., Dezmirean D.S., Bobiş O., Abbas O., Danthine S., Francis F., Haubruge E., Nguyen B.K. Labeling Regulations and Quality Control of Honey Origin: A Review. Food Rev. Int. 2019;36:215–240. doi: 10.1080/87559129.2019.1636063. [DOI] [Google Scholar]
- 182.Parri E., Santinami G., Domenici V. Front-Face Fluorescence of Honey of Different Botanic Origin: A Case Study from Tuscany (Italy) Appl. Sci. 2020;10:1776. doi: 10.3390/app10051776. [DOI] [Google Scholar]
- 183.Blair G.W.S. Heather Honey. J. Phys. Chem. 1935;111:213–220. doi: 10.1021/j150362a006. [DOI] [Google Scholar]
- 184.Louveaux J. Essai de Caracterisation Des Miels de Callune (Calluna vulgaris Salisb.) Ann. Abeille. 1966;9:351–358. [Google Scholar]
- 185.Osés S.M., Ruiz M.O., Pascual-Maté A., Bocos A., Fernández-Muiño M.Á., Sancho M.T. Ling Heather Honey Authentication by Thixotropic Parameters. Food Bioprocess Technol. 2017;10:973–979. doi: 10.1007/s11947-017-1875-6. [DOI] [Google Scholar]
- 186.Kavanagh S., Gunnoo J., Marques Passos T., Stout J.C., White B. Physicochemical Properties and Phenolic Content of Honey from Different Floral Origins and from Rural versus Urban Landscapes. Food Chem. 2019;272:66–75. doi: 10.1016/j.foodchem.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 187.Samanalieva J., Senge B. Analytical and Rheological Investigations into Selected Unifloral German Honey. Eur. Food Res. Technol. 2009;229:107–113. doi: 10.1007/s00217-009-1031-2. [DOI] [Google Scholar]
- 188.Moise A., Mărghitaş L.A., Dezmirean D.S., Bobiş O., Maghear O. Theoretical Study Regarding the Heather Honey (Calluna vulgaris) Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 2011;68:233–237. [Google Scholar]
- 189.Persano Oddo L., Piro R. Main European Unifloral Honeys: Descriptive Sheets. Apidologie. 2004;35:38–81. doi: 10.1051/apido:2004049. [DOI] [Google Scholar]
- 190.Přidal A., Trávníček P., Kudělka J., Nedomová Š., Ondrušíková S., Trost D., Kumbár V. A Rheological Analysis of Biomaterial Behaviour as a Tool to Detect the Dilution of Heather Honey. Materials. 2021;14:2472. doi: 10.3390/ma14102472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Karabagias I.K., Maia M., Karabagias V.K., Gatzias I., Badeka A.V. Characterization of Eucalyptus, Chestnut and Heather Honeys from Portugal Using Multi-Parameter Analysis and Chemo-Calculus. Foods. 2018;7:194. doi: 10.3390/foods7120194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Waś E., Rybak-Chmielewska H., Szczesna T., Kachaniuk K., Teper D. Characteristics of Polish Unifloral Honeys. III. Heather Honey (Calluna vulgaris L.) [Charakterystyka Polskich Miodów Odmianowych. III. Miód Wrzosowy (Calluna vulgaris L.)] J. Apic. Sci. 2011;55:129–136. [Google Scholar]
- 193.Moise A., Liviu A.M., Dezmirean D., Bobis O. Nutraceutical Properties of Romanian Heather Honey. Nutr. Food Sci. 2013;43:218–227. doi: 10.1108/00346651311327864. [DOI] [Google Scholar]
- 194.Özcan M.M., Al Juhaimi F., Uslu N., Ghafoor K., Babiker E.F. A Traditional Food: Sunflower (Helianthus annuus, L.) and Heather [Calluna vulgaris (L.) Hull] Honeys. Indian J. Tradit. Knowl. 2017;16:78–82. [Google Scholar]
- 195.Silva L.R., Sousa A., Taveira M. Characterization of Portuguese Honey from Castelo Branco Region According to Their Pollen Spectrum, Physicochemical Characteristics and Mineral Contents. J. Food Sci. Technol. 2017;54:2551–2561. doi: 10.1007/s13197-017-2700-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Escuredo O., Rodríguez-Flores M.S., Rojo-Martínez S., Seijo M.C. Contribution to the Chromatic Characterization of Unifloral Honeys from Galicia (NW Spain) Foods. 2019;8:233. doi: 10.3390/foods8070233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ghorab A., Rodríguez-Flores M.S., Nakib R., Escuredo O., Haderbache L., Bekdouche F., Seijo M.C. Sensorial, Melissopalynological and Physico-Chemical Characteristics of Honey from Babors Kabylia’s Region (Algeria) Foods. 2021;10:225. doi: 10.3390/foods10020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kivima E., Tanilas K., Martverk K., Rosenvald S., Timberg L., Laos K. The Composition, Physicochemical Properties, Antioxidant Activity, and Sensory Properties of Estonian Honeys. Foods. 2021;10:511. doi: 10.3390/foods10030511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Acquarone C., Buera P., Elizalde B. Food Chemistry Pattern of PH and Electrical Conductivity upon Honey Dilution as a Complementary Tool for Discriminating Geographical Origin of Honeys. Food Chem. 2007;101:695–703. doi: 10.1016/j.foodchem.2006.01.058. [DOI] [Google Scholar]
- 200.Karabagias I.K., Badeka A.V., Kontakos S., Karabournioti S., Kontominas M.G. Botanical Discrimination of Greek Unifloral Honeys with Physico-Chemical and Chemometric Analyses. Food Chem. 2014;165:181–190. doi: 10.1016/j.foodchem.2014.05.033. [DOI] [PubMed] [Google Scholar]
- 201.Boussaid A., Chouaibi M., Rezig L., Hellal R., Donsì F., Ferrari G., Hamdi S. Physicochemical and Bioactive Properties of Six Honey Samples from Various Floral Origins from Tunisia. Arab. J. Chem. 2018;11:265–274. doi: 10.1016/j.arabjc.2014.08.011. [DOI] [Google Scholar]
- 202.León-Ruiz V., Vera S., González-Porto A.V., Andrés M.P.S. Vitamin C and Sugar Levels as Simple Markers for Discriminating Spanish Honey Sources. J. Food Sci. 2011;76:356–361. doi: 10.1111/j.1750-3841.2011.02041.x. [DOI] [PubMed] [Google Scholar]
- 203.Baloš M.Z., Popov N., Vidakovic S., Ljubojević Pelić D., Pelić M., Mihalijev Z., Jakšić S. Electrical Conductivity and Acidity of Honey. Arch. Vet. Med. 2019;11:91–101. doi: 10.46784/e-avm.v11i1.20. [DOI] [Google Scholar]
- 204.Bogdanov S., Ruoff K., Persano Oddo L. Physico-Chemical Methods for the Characterisation of Unifloral Honeys: A Review. Apidologie. 2004;35:4–17. doi: 10.1051/apido:2004047. [DOI] [Google Scholar]
- 205.Nalda M.J.N., Yagüe J.L.B., Diego Calva J.C., Gómez M.T.M. Classifying Honeys from the Soria Province of Spain via Multivariate Analysis. Anal. Bioanal. Chem. 2005;382:311–319. doi: 10.1007/s00216-005-3161-0. [DOI] [PubMed] [Google Scholar]
- 206.Ferreira I.C.F.R., Aires E., Barreira J.C.M., Estevinho L.M. Antioxidant Activity of Portuguese Honey Samples: Different Contributions of the Entire Honey and Phenolic Extract. Food Chem. 2009;114:1438–1443. doi: 10.1016/j.foodchem.2008.11.028. [DOI] [Google Scholar]
- 207.Codex Alimentarius Commission . Revised Codex Standard for Honey, Codex Stan 12-1981, Rev. 1 (1987), Rev. 2 (2001) Food and Agriculture Organization (FAO); Rome, Italy: 2001. pp. 1–7. [Google Scholar]
- 208.Witczak M., Juszczak L., Gałkowska D. Non-Newtonian Behaviour of Heather Honey. J. Food Eng. 2011;104:532–537. doi: 10.1016/j.jfoodeng.2011.01.013. [DOI] [Google Scholar]
- 209.Seisonen S., Kivima E., Vene K. Characterisation of the Aroma Profiles of Different Honeys and Corresponding Flowers Using Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry/Olfactometry. Food Chem. 2015;169:34–40. doi: 10.1016/j.foodchem.2014.07.125. [DOI] [PubMed] [Google Scholar]
- 210.Cereser Camara V., Laux D. Moisture Content in Honey Determination with a Shear Ultrasonic Reflectometer. J. Food Eng. 2010;96:93–96. doi: 10.1016/j.jfoodeng.2009.06.049. [DOI] [Google Scholar]
- 211.Iglesias A., Feás X., Rodrigues S., Seijas J.A., Vázquez-Tato M.P., Dias L.G., Estevinho L.M. Comprehensive Study of Honey with Protected Denomination of Origin and Contribution to the Enhancement of Legal Specifications. Molecules. 2012;17:8561–8577. doi: 10.3390/molecules17078561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Machado De-Melo A.A., Almeida-Muradian L.B., de Sancho M.T., Pascual-Maté A. Composición y Propiedades de La Miel de Apis Mellifera: Una Revisión. J. Apic. Res. 2017;57:5–37. doi: 10.1080/00218839.2017.1338444. [DOI] [Google Scholar]
- 213.Terio V., Bozzo G., Ceci E., Savarino A.E., Barrasso R., Di Pinto A., Mottola A., Marchetti P., Tantillo G., Bonerba E. Methylglyoxal (MGO) in Italian Honey. Appl. Sci. 2021;11:831. doi: 10.3390/app11020831. [DOI] [Google Scholar]
- 214.Zhu M., Zhao H., Wang Q., Wu F., Cao W. A Novel Chinese Honey from Amorpha Fruticosa, L.: Nutritional Composition and Antioxidant Capacity In Vitro. Molecules. 2020;25:5211. doi: 10.3390/molecules25215211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Haouam L., Dailly H., Bruneau E., Tahar A. The Quality of Honeys Influenced by the Traditional Heating Method. J. Microbiol. Biotechnol. Food Sci. 2019;8:1276–1280. doi: 10.15414/jmbfs.2019.8.6.1276-1280. [DOI] [Google Scholar]
- 216.Mouhoubi-Tafinine Z., Ouchemoukh S., Bachir Bey M., Louaileche H., Tamendjari A. Effect of Storage on Hydroxymethylfurfural (HMF) and Color of Some Algerian Honey. Int. Food Res. J. 2018;25:1044–1050. [Google Scholar]
- 217.Can Z., Yildiz O., Sahin H., Akyuz Turumtay E., Silici S., Kolayli S. An Investigation of Turkish Honeys: Their Physico-Chemical Properties, Antioxidant Capacities and Phenolic Profiles. Food Chem. 2015;180:133–141. doi: 10.1016/j.foodchem.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 218.Nascimento K.S., do Gasparotto Sattler J.A., Lauer Macedo L.F., Serna González C.V., Pereira de Melo I.L., da Silva Araújo E., Granato D., Sattler A., de Almeida-Muradian L.B. Phenolic Compounds, Antioxidant Capacity and Physicochemical Properties of Brazilian Apis Mellifera Honeys. LWT Food Sci. Technol. 2018;91:85–94. doi: 10.1016/j.lwt.2018.01.016. [DOI] [Google Scholar]
- 219.Escuredo O., Rodríguez-Flores M.S., Meno L., Seijo M.C. Prediction of Physicochemical Properties in Honeys with Portable Near-Infrared (MicroNIR) Spectroscopy Combined with Multivariate Data Processing. Foods. 2021;10:317. doi: 10.3390/foods10020317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shapla U.M., Solayman M., Alam N., Khalil M.I., Gan S.H. 5-Hydroxymethylfurfural (HMF) Levels in Honey and Other Food Products: Effects on Bees and Human Health. Chem. Cent. J. 2018;12:35. doi: 10.1186/s13065-018-0408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sousa J.M., de Souza E.L., Marques G., Meireles B., de Magalhães Cordeiro Â.T., Gullón B., Pintado M.M., Magnani M. Polyphenolic Profile and Antioxidant and Antibacterial Activities of Monofloral Honeys Produced by Meliponini in the Brazilian Semiarid Region. Food Res. Int. 2016;84:61–68. doi: 10.1016/j.foodres.2016.03.012. [DOI] [Google Scholar]
- 222.Miguel M.G., Antunes M.D., Faleiro M.L. Honey as a Complementary Medicine. Integr. Med. Insights. 2017;12:1–15. doi: 10.1177/1178633717702869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kamal D.A.M., Ibrahim S.F., Kamal H., Kashim M.I.A.M., Mokhtar M.H. Physicochemical and Medicinal Properties of Tualang, Gelam and Kelulut Honeys: A Comprehensive Review. Nutrients. 2021;13:197. doi: 10.3390/nu13010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ávila S., Beux M.R., Ribani R.H., Zambiazi R.C. Stingless Bee Honey: Quality Parameters, Bioactive Compounds, Health-Promotion Properties and Modification Detection Strategies. Trends Food Sci. Technol. 2019;81:37–50. doi: 10.1016/j.tifs.2018.09.002. [DOI] [Google Scholar]
- 225.Puścion-Jakubik A., Borawska M.H., Socha K. Modern Methods for Assessing the Quality of Bee Honey and Botanical Origin Identification. Foods. 2020;9:1028. doi: 10.3390/foods9081028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Vazquez L., Armada D., Celeiro M., Dagnac T., Llompart M. Evaluating the Presence and Contents of Phytochemicals in Honey Samples: Phenolic Compounds as Indicators to Identify Their Botanical Origin. Foods. 2021;10:2616. doi: 10.3390/foods10112616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Matović K., Ćirić J., Kaljević V., Nedić N., Jevtić G., Vasković N., Baltić M. Physicochemical Parameters and Microbiological Status of Honey Produced in an Urban Environment in Serbia. Environ. Sci. Pollut. Res. 2018;25:14148–14157. doi: 10.1007/s11356-018-1659-1. [DOI] [PubMed] [Google Scholar]
- 228.Cheung Y., Meenu M., Yu X., Xu B. Phenolic Acids and Flavonoids Profiles of Commercial Honey from Different Floral Sources and Geographic Sources. Int. J. Food Prop. 2019;22:290–308. doi: 10.1080/10942912.2019.1579835. [DOI] [Google Scholar]
- 229.Ferreres F., Andrade P., Tomás-Barberán F.A. Natural Occurrence of Abscisic Acid in Heather Honey and Floral Nectar. J. Agric. Food Chem. 1996;44:2053–2056. doi: 10.1021/jf9507553. [DOI] [Google Scholar]
- 230.Ferreres F., Andrada P., Gil M.I., Tomas-Barberan F.A. Floral Nectar Phenolics as Biochemical Markers for the Botanical Origin of Heather Honey. Z. Leb. Unters. Forsch. 1996;202:40–44. doi: 10.1007/BF01229682. [DOI] [Google Scholar]
- 231.Jasicka-Misiak I., Poliwoda A., Dereń M., Kafarski P. Phenolic Compounds and Abscisic Acid as Potential Markers for the Floral Origin of Two Polish Unifloral Honeys. Food Chem. 2012;131:1149–1156. doi: 10.1016/j.foodchem.2011.09.083. [DOI] [Google Scholar]
- 232.Halagarda M., Groth S., Popek S., Rohn S., Pedan V. Antioxidant Activity and Phenolic Profile of Selected Organic and Conventional Honeys from Poland. Antioxidants. 2020;9:44. doi: 10.3390/antiox9010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Labsvards K.D., Rudovica V., Kluga R., Rusko J., Busa L., Bertins M., Eglite I., Naumenko J., Salajeva M., Viksna A. Determination of Floral Origin Markers of Latvian Honey by Using Irms, Uhplc-Hrms, And1 h-Nmr. Foods. 2022;11:42. doi: 10.3390/foods11010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rodrigues da Silva L., Campos Chisté R., Fernandes E. Chemical and Antioxidant Characterization of the Portuguese Heather Honey from Calluna vulgaris. Separations. 2021;8:177. doi: 10.3390/separations8100177. [DOI] [Google Scholar]
- 235.Costa A.C.V., Garruti D.S., Madruga M.S. The Power of Odour Volatiles from Unifloral Melipona Honey Evaluated by Gas Chromatography–Olfactometry Osme Techniques. J. Sci. Food Agric. 2019;99:4493–4497. doi: 10.1002/jsfa.9647. [DOI] [PubMed] [Google Scholar]
- 236.Jerković I., Kuś P.M. Terpenes in Honey: Occurrence, Origin and Their Role as Chemical Biomarkers. RSC Adv. 2014;4:31710–31728. doi: 10.1039/C4RA04791E. [DOI] [Google Scholar]
- 237.Patrignani M., Fagúndez G.A., Tananaki C., Thrasyvoulou A., Lupano C.E. Volatile Compounds of Argentinean Honeys: Correlation with Floral and Geographical Origin. Food Chem. 2018;246:32–40. doi: 10.1016/j.foodchem.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 238.Demir Kanbur E., Yuksek T., Atamov V., Ozcelik A.E. A Comparison of the Physicochemical Properties of Chestnut and Highland Honey: The Case of Senoz Valley in the Rize Province of Turkey. Food Chem. 2021;345:128864. doi: 10.1016/j.foodchem.2020.128864. [DOI] [PubMed] [Google Scholar]
- 239.Castro-Vázquez L., Díaz-Maroto M.C., González-Viñas M.A., Pérez-Coello M.S. Differentiation of Monofloral Citrus, Rosemary, Eucalyptus, Lavender, Thyme and Heather Honeys Based on Volatile Composition and Sensory Descriptive Analysis. Food Chem. 2009;112:1022–1030. doi: 10.1016/j.foodchem.2008.06.036. [DOI] [Google Scholar]
- 240.Plutowska B., Chmiel T., Dymerski T., Wardencki W. A Headspace Solid-Phase Microextraction Method Development and Its Application in the Determination of Volatiles in Honeys by Gas Chromatography. Food Chem. 2011;126:1288–1298. doi: 10.1016/j.foodchem.2010.11.079. [DOI] [Google Scholar]
- 241.Cucu A.-A., Baci G.-M., Moise A.R., Dezsi Ş., Marc B.D., Stângaciu Ş., Dezmirean D.S. Towards a Better Understanding of Nutritional and Therapeutic Effects of Honey and Their Applications in Apitherapy. Appl. Sci. 2021;11:4190. doi: 10.3390/app11094190. [DOI] [Google Scholar]
- 242.Đogo Mračević S., Krstić M., Lolić A., Ražić S. Comparative Study of the Chemical Composition and Biological Potential of Honey from Different Regions of Serbia. Microchem. J. 2019;152:104420. doi: 10.1016/j.microc.2019.104420. [DOI] [Google Scholar]
- 243.Bobis O., Moise A.R., Ballesteros I., Reyes E.S., Durán S.S., Sánchez-Sánchez J., Cruz-Quintana S., Giampieri F., Battino M., Alvarez-Suarez J.M. Eucalyptus Honey: Quality Parameters, Chemical Composition and Health-Promoting Properties. Food Chem. 2020;325:126870. doi: 10.1016/j.foodchem.2020.126870. [DOI] [PubMed] [Google Scholar]
- 244.Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., Altavilla D., Bitto A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017;2017:8416763. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wilczyńska A. Phenolic Content and Antioxidant Activity of Different Types of Polish Honey—A Short Report. Pol. J. Food Nutr. Sci. 2010;60:309–313. [Google Scholar]
- 246.Soares S., Pinto D., Rodrigues F., Alves R.C., Oliveira M.B.P.P. Portuguese Honeys from Different Geographical and Botanical Origins: A 4-Year Stability Study Regarding Quality Parameters and Antioxidant Activity. Molecules. 2017;22:1338. doi: 10.3390/molecules22081338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kuś P.M., Congiu F., Teper D., Sroka Z., Jerković I., Tuberoso C.I.G. Antioxidant Activity, Color Characteristics, Total Phenol Content and General HPLC Fingerprints of Six Polish Unifloral Honey Types. LWT Food Sci. Technol. 2014;55:124–130. doi: 10.1016/j.lwt.2013.09.016. [DOI] [Google Scholar]
- 248.Bertoncelj J., Doberšek U., Jamnik M., Golob T. Evaluation of the Phenolic Content, Antioxidant Activity and Colour of Slovenian Honey. Food Chem. 2007;105:822–828. doi: 10.1016/j.foodchem.2007.01.060. [DOI] [Google Scholar]
- 249.Starowicz M., Ostaszyk A., Zieliński H. The Relationship between the Browning Index, Total Phenolics, Color, and Antioxidant Activity of Polish-Originated Honey Samples. Foods. 2021;10:967. doi: 10.3390/foods10050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Baci G.-M., Cucu A.-A., Moise A.R., Dezmirean D.S. Applicability of Honey on Silkworms (Bombyx Mori) and Quality Improvement of Its Biomaterials. Appl. Sci. 2021;11:4613. doi: 10.3390/app11104613. [DOI] [Google Scholar]
- 251.Mandal M.D., Mandal S. Honey: Its Medicinal Property and Antibacterial Activity. Asian Pac. J. Trop. Biomed. 2011;1:154–160. doi: 10.1016/S2221-1691(11)60016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Dezmirean D.S., Mărghitaş L.A., Fiţ N., Chirilă F., Gherman B., Mărgăoan R., Aurori A., Bobiş O. Antibacterial Effect of Heather Honey (Calluna vulgaris) against Different Microorganisms of Clinical Importance. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 2015;72:72–77. doi: 10.15835/buasvmcn-asb:10562. [DOI] [Google Scholar]
- 253.Nolan V.C., Harrison J., Cox J.A.G. Dissecting the Antimicrobial Composition of Honey. Antibiotics. 2019;8:251. doi: 10.3390/antibiotics8040251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Proaño A., Coello D., Villacrés-Granda I., Ballesteros I., Debut A., Vizuete K., Brenciani A., Álvarez-Suarez J.M. The Osmotic Action of Sugar Combined with Hydrogen Peroxide and Bee-Derived Antibacterial Peptide Defensin-1 Is Crucial for the Antibiofilm Activity of Eucalyptus Honey. LWT. 2021;136:110379. doi: 10.1016/j.lwt.2020.110379. [DOI] [Google Scholar]
- 255.Mama M., Teshome T., Detamo J. Antibacterial Activity of Honey against Methicillin-Resistant Staphylococcus Aureus: A Laboratory-Based Experimental Study. Int. J. Microbiol. 2019;2019:7686130. doi: 10.1155/2019/7686130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Feás X., Iglesias A., Rodrigues S., Estevinho L.M. Effect of Erica Sp. Honey against Microorganisms of Clinical Importance: Study of the Factors Underlying This Biological Activity. Molecules. 2013;18:4233–4246. doi: 10.3390/molecules18044233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Fernandes L., Oliveira A., Henriques M., Rodrigues M.E. Honey as a Strategy to Fight Candida Tropicalis in Mixed-Biofilms with Pseudomonas Aeruginosa. Antibiotics. 2020;9:43. doi: 10.3390/antibiotics9020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.