Abstract
Background
Glioblastoma (GBM) has poor prognosis despite aggressive treatment. Dendritic cell (DC) vaccines are promising, but widespread clinical use has not been achieved, possibly reflecting manufacturing issues of antigen choice and DC potency. We previously optimized vaccine manufacture utilizing allogeneic human GBM tumor cell lysate and potent, mature autologous DCs. Here, we report a phase I study using this optimized DC vaccine in combination with standard therapy.
Methods
Following surgical resection and radiation with concurrent temozolomide (TMZ), newly diagnosed adult GBM patients received intradermal DC vaccines plus TMZ. Primary endpoints were safety and feasibility. Immune and treatment responses were recorded.
Results
Twenty-one patients were enrolled in this study. One progressed between leukapheresis and vaccine manufacture. Twenty patients received treatment per protocol. Vaccine doses (≥15) were generated following a single leukapheresis for each patient. No dose-limiting vaccine toxicities were encountered. One patient had symptomatic, histologically proven pseudoprogression. Median progression-free survival was 9.7 months. Median overall survival was 19 months. Overall survival was 25% at 2 years and 10% at 4 years. One patient remains progression-free 5 years after enrollment. Specific CD8 T-cell responses for the tumor-associated antigen gp100 were seen post-vaccination. Patients entered the trial with a leukocyte deficit compared to healthy donors which partly normalized over the course of therapy.
Conclusions
This vaccine platform is safe and highly feasible in combination with standard therapy for newly diagnosed patients. Imaging, histological, survival, and immunological data suggest a positive biological response to therapy that warrants further investigation.
Keywords: dendritic cell vaccine, glioblastoma, immunotherapy, temozolomide
Key Points.
Phase I trial results of a novel dendritic cell vaccine in new GBM are reported.
Autologous dendritic cell/allogeneic tumor lysate vaccines are safe and feasible.
Immunological and clinical outcomes suggest a biological response worth further investigation.
Importance of the Study.
Dendritic cell vaccines for glioblastoma are promising but face barriers, including manufacturing challenges in a broad patient population patient, poor yield of mature dendritic cells, and inability to test antigen-specific responses. We report results for a phase I clinical trial of a novel allogeneic tumor lysate/autologous dendritic cell vaccine in combination with standard therapy in newly diagnosed glioblastoma patients. Treatment was safe and highly feasible. We generated ≥15 vaccine doses for all patients enrolled. Treatment generated both clinical and immunological responses, including antigen-specific CD8 T-cell responses. Median overall survival was 19 months. Collectively, this suggests a biological response that warrants further investigation.
Glioblastoma (GBM) has median overall survival (OS) of 14.6 months1 despite surgery, radiation, and temozolomide (TMZ) chemotherapy. GBM vaccines have appeared beneficial in small clinical trials.2–4 However, larger, randomized studies have been less promising due to limited efficacy,5 dropout/poor enrollment due to manufacturing issues,6 or both.7 To address these issues, we previously developed a novel manufacturing strategy for a dendritic cell (DC) vaccine with scalable GBM antigen sources and improved DC maturation with potent in vitro T-cell stimulation.8 This platform relies on both a novel library of allogeneic human GBM cell lines as an antigen source and a novel method for DC culture that yields far more CD83+ mature DCs from GBM patients’ monocytes than standard techniques.
Antigen source is a key issue in tumor vaccines. Many prior GBM vaccines use bulk autologous patient tumor-derived antigens2,4 producing a large, personalized antigen library. However, tumor tissue requirements limit manufacturing yield and antigen-specific response testing in clinical trials is not feasible because each vaccine contains different antigens. Alternatively, some vaccines target specific GBM-associated antigens.3,5,9,10 These do not require tumor tissue and enable antigen-specific response testing. However, they are limited to tumors expressing specific antigens, sometimes require specific haplotypes,9,10 and are subject to immunoediting and variable antigen expression.3,5 We previously developed a hybrid antigen source for GBM vaccines utilizing a library of allogeneic human GBM cell lines grown in the presence of platelet lysate as a supplement, resulting in a mixture of stem-like and differentiated glioma cell phenotypes.8 This library contains defined antigens (eg, EGFRviii, erbB2, gp100, MAGE-A3, IL13Ra2) and presumably, a large library of additional antigens. It is not limited by quantity or haplotype.
DCs are professional antigen-presenting cells that have been widely used in experimental cancer vaccines11 but are not widely used in clinical practice. Myeloid DCs are expanded in vitro from CD14+ monocytes as immature DCs that subsequently undergo maturation. Mature DCs stimulate immune responses12,13 while immature DCs are immunosuppressive.14 Standard DC culture techniques were developed using healthy donor monocytes.15,16 However, GBM patients’ monocytes include many immunosuppressive variants such as myeloid-derived suppressor cells.17,18 Standard DC culture techniques yield large numbers of immunosuppressive immature DCs (up to 50% or more) when applied to GBM patients’ monocytes, but we developed a novel technique producing >90% mature DCs from the same cells.8
Our novel autologous mature DC/allogeneic tumor lysate vaccine platform allows production of many vaccine doses for every patient, provides a large library of potential antigens including several known tumor-associated antigens that could facilitate antigen-specific response testing, and utilizes far more mature (ie, potent) DCs from cancer patients’ monocytes than standard DC vaccines.8 In this study, we report initial phase I clinical trial results with this novel vaccine in combination with standard therapy for newly diagnosed GBM.
Methods
Patients
All patients were enrolled and treated at a single institution. Adults (≥18 years) with histological GBM diagnosis who had undergone maximal safe resection followed by external beam radiation (59.4 Gy in 30 fractions) with concurrent TMZ chemotherapy (75 mg/m2) were eligible for the study. Enrollment was limited to HLA-A0201 haplotype for the first 10 patients. Adequate performance status (European Cooperative Oncology Group [ECOG] performance score 0-2), organ function, and absolute lymphocyte count (≥1500/µL) were required. Prior malignancy within 5 years (except non-melanoma skin cancer) or prior immunotherapy were exclusion criteria. The study was conducted under US Food and Drug Administration Investigational New Drug Application No. 15223, Institutional Review Board 13-000808, and is registered at clinicaltrials.gov (NCT01957956). Written informed consent was obtained from all patients.
Vaccine Manufacture
Vaccine manufacture was performed under current Good Manufacturing Practices (cGMP) conditions in the Mayo Immune Progenitor and Cell Therapy Laboratory as previously described.8 Enrolled patients underwent leukapheresis to acquire leukocytes for vaccine manufacture. CD14+ cells were purified by immunomagnetic selection (Miltenyi Biotec, Germany) and used to generate highly purified (>90%) CD83+ mature DCs. GBM patient-derived donor (allogeneic) primary tumor cultures were used as a source of tumor lysate8,19 DCs were pulsed with 0.1 mg/mL of tumor culture lysate (15 mg) and incubated for 18 ± 6 hours.
Clinical Trial Design
Beginning 4-6 weeks after completing radiation with concurrent TMZ, patients underwent up to 12 treatment cycles (28 days/cycle). Leukapheresis was performed on day 0). In cycle 1, patients received only oral TMZ (days 1-5) at 150 mg/m2/day during vaccine manufacture. Cycles 2 and 3 consisted of oral TMZ (days 1-5) at 200 mg/m2/day plus DC vaccine on days 1, 3, and 5. Cycles 4-6 were oral TMZ (days 1-5) at 200 mg/m2/day plus DC vaccine on day 1. Cycles 7-12 were DC vaccine alone on day 1. Vaccine (2 × 107 ± 0.5 × 107 tumor lysate-pulsed mature autologous DC) was administered intradermally. Patients were monitored via scheduled clinic visits and laboratory studies before each cycle plus MRI scans every second cycle and additional monitoring as clinically indicated. Patients remained on treatment until disease progression or the treatment protocol was completed.
Toxicity Assessment
Toxicities were assessed per the NCI Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Dose-limiting toxicities (DLTs) were defined as any toxicities attributable (definitely, probably, or possible) to the experimental agent (DC vaccine) that were ≥grade 3, any ≥grade 2 bronchial obstruction/pneumonitis/wheezing, or any ≥grade 2 toxicity that did not resolve to <grade 2 within 2 weeks.
Clinical Response Assessment for Treatment Evaluation
The Response Assessment in Neuro-Oncology (RANO) criteria were used to determine tumor progression in the first 6 patients felt to have progressed. However, following the publication of the Immunological RANO (iRANO) criteria, these criteria requiring confirmation of radiographic progression on subsequent imaging for patients meeting the RANO criteria within 6 months of initiating immunotherapy were adopted for all remaining patients.
Antigen-specific Response Testing
Peripheral blood mononuclear cells (PBMC, purified from whole blood by density gradient centrifugation) from baseline and at cycle 4 were obtained from HLA-A202+ patients. Flow cytometry was performed using human-specific, APC-CY7-conjugated anti-CD8 antibody (BD Pharmingen, clone SK1) and dextramer staining for nonsense control or gp100 (Immudex, gp100 peptide sequence IMDQVPFSV, control peptide sequence ILGFVFTLTV). Briefly, 1-3 × 106 PBMC were incubated with 10 µL of dextramer for 10 minutes followed by the addition of 2 µL of anti-CD8 antibody for an additional 20 minutes in the dark. After a brief wash, cells were resuspended in PBS containing 5% fetal bovine serum and analyzed (BD LSR flow cytometer with UV [325 nm], violet [407 nm], blue [488 nm], and red [633 nm] lasers). Final data analysis was performed with FlowJo software (Tree Star Technologies).
Immunophenotyping
Whole blood samples were stained directly with antibodies without additional manipulations. A quantitative flow cytometry approach to count circulating leukocytes was used with an 8-/10-color combination analysis tube related to quantitative BD TruCount tubes (BD Biosciences, San Jose, CA, USA) to accurately determine cells/µL of blood. This allows the determination of >100 unique phenotypes. Daily machine quality control (QC), individual antibody validation, and additional QC measures were performed. This comprehensive approach facilitates bioinformatics analysis.20–22 Both absolute (cells/µL) and relative (percentage of a related or parent population) frequency were determined. Identical immune analysis in 64 age-matched healthy volunteers (HV) provided a comparison.
Statistical Methods
This pilot study was meant to evaluate the safety and feasibility of vaccination with allogeneic tumor lysate-pulsed mature autologous DC in combination with standard TMZ chemotherapy in newly diagnosed GBM patients. Twenty evaluable patients were adequate to provide preliminary safety and feasibility data. Comparison of progression-free survival (PFS) and OS in O6-methylguanine-DNA methyltransferase (MGMT) promoter methylated and unmethylated patients was performed using the Kaplan-Meier test. For immunological assays, comparison between groups was performed using Student’s t test or chi-square test. GraphPad Prism (version 7.00 for Mac, GraphPad Software, La Jolla, CA, USA, www.graphpad.com) or software R (R Core Team [2019]. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/) were used.
Results
Patient, Surgery, and Tumor Characteristics
Twenty-one patients were enrolled in this study. Mean age was 60.5 years (range 28-77 years). Nine patients were female (42.9%). ECOG performance score was 0 in 12/21 (57%), 1 in 8/21 (38%), and 2 in 1/21 (4.8%). Tumors were multifocal in 5/21 (24%) and bilateral in 4/21 (19%). Ten patients (48%) had subtotal resection, whereas 11 (52%) had gross total resection. MGMT promoter methylation assay results were available in 20 patients. Six (30%) showed MGMT promoter methylation. Isocitrate dehydrogenase (IDH1/IDH2) mutation information was available in all patients. One patient’s tumor (4.8%) harbored an IDH1 (R132H) mutation. One patient (4.8%) was receiving corticosteroids at study entry. Patient information is summarized in Table 1.
Table 1.
Patient Demographics and Tumor Characteristics
| Patient | Age | Gender | HLA-A2 | Multifocal | Bilateral | Extent of Resection | MGMT Promoter Methylation | IDH1/IDH2-Mutant |
|---|---|---|---|---|---|---|---|---|
| 1 | 60 | M | Pos | No | No | GTR | Neg | Neg |
| 2 | 63 | M | Pos | Yes | No | STR | Yes | Neg |
| 3 | 65 | M | Pos | No | No | GTR | Yes | Neg |
| 4 | 68 | M | Pos | No | No | GTR | Neg | Neg |
| 5 | 64 | F | Pos | No | No | GTR | Yes | Neg |
| 6 | 78 | M | Pos | Yes | No | STR | Neg | Neg |
| 7 | 28 | M | Pos | Yes | Yes | STR | Yes | Pos |
| 8 | 48 | M | Pos | No | No | GTR | Neg | Neg |
| 9 | 73 | M | Pos | No | No | GTR | Neg | Neg |
| 10 | 71 | M | Pos | No | No | STR | Neg | Neg |
| 11 | 47 | F | Neg | No | Yes | STR | Neg | Neg |
| 12 | 57 | F | Neg | Yes | Yes | STR | Yes | Neg |
| 13 | 61 | M | Yes | No | No | GTR | Neg | Neg |
| 14 | 62 | F | Neg | No | No | GTR | Neg | Neg |
| 15 | 69 | F | Neg | Yes | Yes | STR | Neg | Neg |
| 16 | 66 | F | Neg | No | No | STR | Neg | Neg |
| 17 | 70 | M | ND | No | No | GTR | Neg | Neg |
| 18 | 52 | F | Yes | No | No | GTR | Neg | Neg |
| 19 | 49 | M | ND | No | No | GTR | Neg | Neg |
| 20 | 61 | F | ND | No | No | GTR | Yes | Neg |
Abbreviations: GTR, gross total resection; ND, not done; Neg, negative; Pos, positive; STR, subtotal resection.
Feasibility and Adverse Events
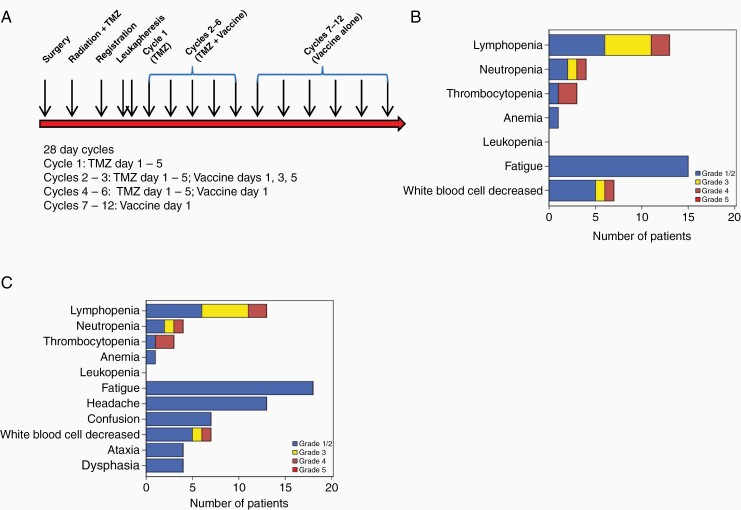
Treatment as outlined in schematic form in Figure 1A was highly feasible. At least 15 vaccine doses were generated for all patients enrolled (21/21) and 20/21 (95%) proceeded with therapy per protocol. One patient experienced histologically verified progression after vaccine manufacture but before receiving vaccine. No DLTs were observed. Toxicity at least possibly attributable to the overall therapy (vaccine and TMZ) included grade 3-4 lymphopenia in 7/20 (35%), neutropenia in 2/20 (10%), thrombocytopenia in 2/20 (10%), and global leukopenia in 2/20 (10%) (Figure 1B). These are in keeping with expected TMZ-mediated myelosuppression. Fatigue (grade 1-2) possibly attributable to either vaccine or TMZ was seen in 15 patients (75%). Any grade 2 fatigue absent at baseline resolved quickly and was not counted as a DLT. Additional adverse events included grade 1-2 headache in 13/20 (65%), confusion in 7/20 (35%), ataxia in 4/20 (20%), and dysphasia in 4/20 (20%) (Figure 1C). These neurological adverse events typically occurred in association with disease progression.
Figure 1.
Feasibility and safety. (A) Study schema. TMZ, temozolomide. (B) Adverse events at least possibly attributed to the treatment (vaccine plus temozolomide). (C) Adverse events regardless of attribution.
Clinical, Imaging, and Histological Response
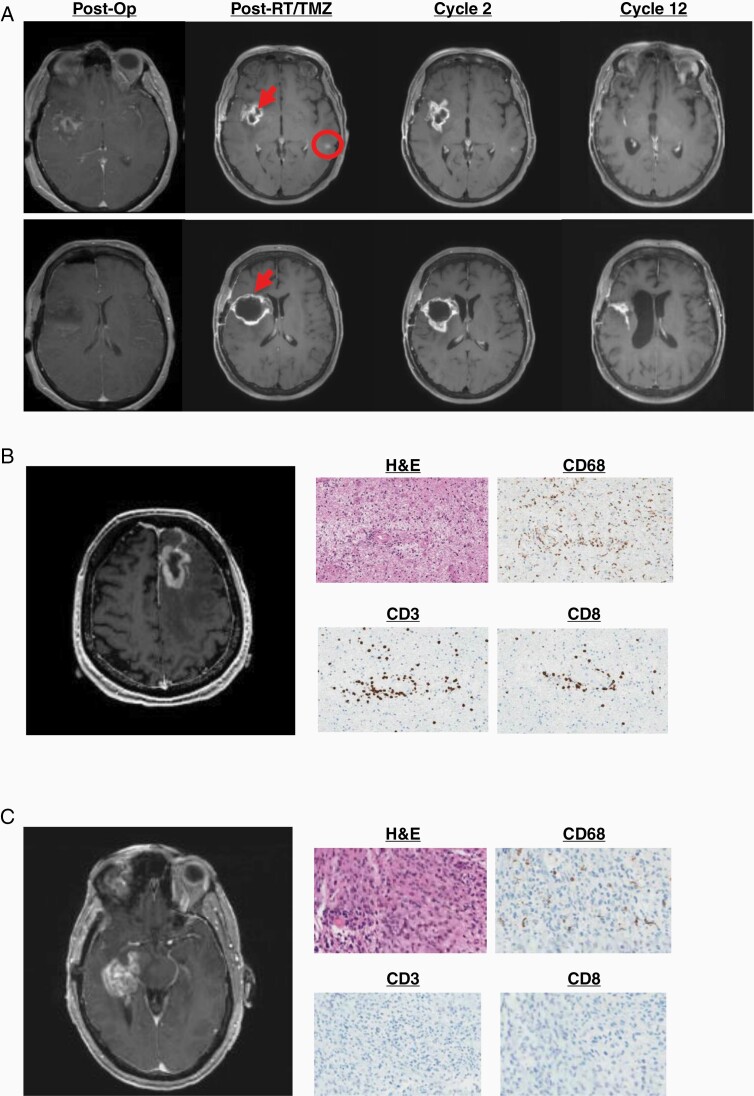
With one objective imaging response (Figure 2A), overall response rate was 5%. Clinical benefit was 40% (stable disease for at least 12 months [7/20] or complete response or partial response at any time [1/20] divided by the total number of evaluable patients). In total, 10/20 evaluable patients had subsequent tissue diagnosis at imaging progression after receiving vaccine. Of these, 5 (50%) had viable recurrent tumor without significant inflammation or treatment-related changes. Four (40%) had a mixture of viable recurrent/residual tumor and inflammation but were interpreted as predominantly tumor. These patients were taken off treatment. One patient had predominantly treatment-related inflammation and necrosis and subsequently resumed study treatment. In all 5 patients with significant inflammatory cell infiltrates, this was predominantly represented by CD3+ or CD4+ T cells as well as CD68+ or CD163+ macrophages with smaller contributions from CD8+ T cells and scant or absent CD20+ B cells. Representative imaging, histological, and immunohistochemical findings at imaging recurrence are shown in Figure 2B and C.
Figure 2.
Clinical response to vaccination. (A) Objective response in a 57-year-old woman. New enhancement developed around the resection cavity (arrows) and in the contralateral hemisphere (circle) after radiation with concurrent temozolomide. While both regions could potentially represent treatment-related inflammatory pseudoprogression, the enhancement in the contralateral hemisphere is outside the radiation field and is therefore more likely to represent true progression. Both regions resolved with therapy. (B) New enhancement at cycle 4 in a 55-year-old man. Biopsy showed necrosis with marked inflammatory infiltrate. (C) New enhancement at cycle 7 in a 60-year-old man. Biopsy showed viable tumor without inflammatory infiltrate.
Progression-Free Survival and Overall Survival
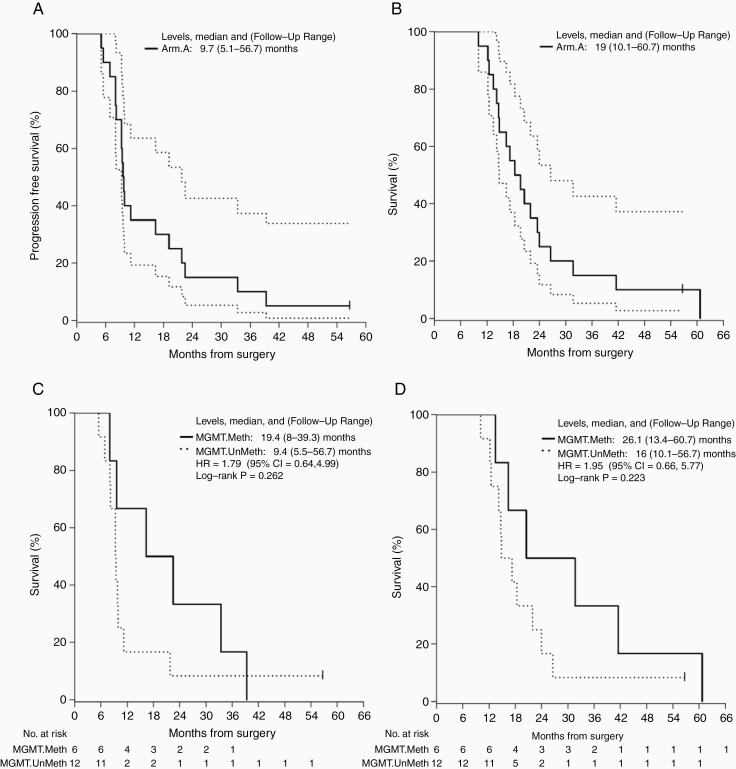
Median PFS and 95% confidence interval (95% CI) was 9.7 months (9.3-21.8 months) (Figure 3A), though PFS is a challenging endpoint in the context of immunotherapy due to confounding inflammatory pseudoprogression. One patient remains progression-free at last follow-up (60.7 months). Of note, this patient’s tumor was IDH wild type and did not exhibit MGMT promoter methylation. Median OS and 95% CI was 19 months (14.8-26.6 months) (Figure 3B). MGMT promoter methylation was associated with survival. Median PFS and follow-up range in MGMT methylated patients was 19.4 months (8.0, 39.3 months) vs 9.4 months (5.5, 56.7 months) in unmethylated patients (Figure 3C; HR = 1.8 [95% CI: 0.6, 5.0, favoring MGMT methylated patients]; log-rank P = .26). Similarly, median OS was prolonged in MGMT methylated patients at 26.5 months (13.4, 60.7 months) vs 16.0 months (10.1, 56.7 months) for unmethylated patients (Figure 3D; HR = 1.9 [95% CI: 0.7, 5.8, favoring MGMT-methylated patients]; log-rank P = .22).
Figure 3.
Survival. (A) Progression-free survival. Median PFS = 9.7 (5.1-56.7) months. (B) Overall survival. Median OS = 19 (10.1-60.7) months. (C) Progression-free survival by MGMT methylation. Median methylated = 19.4 (8-39.3) months vs median unmethylated = 9.4 (5.5-56.7) months. Log-rank P = .262. (D) Overall survival by MGMT methylation. Median methylated = 26.1 (13.4-60.7) months vs 16 (10.1-56.7) months. Log-rank P = .223.
Immune Responses Following Treatment
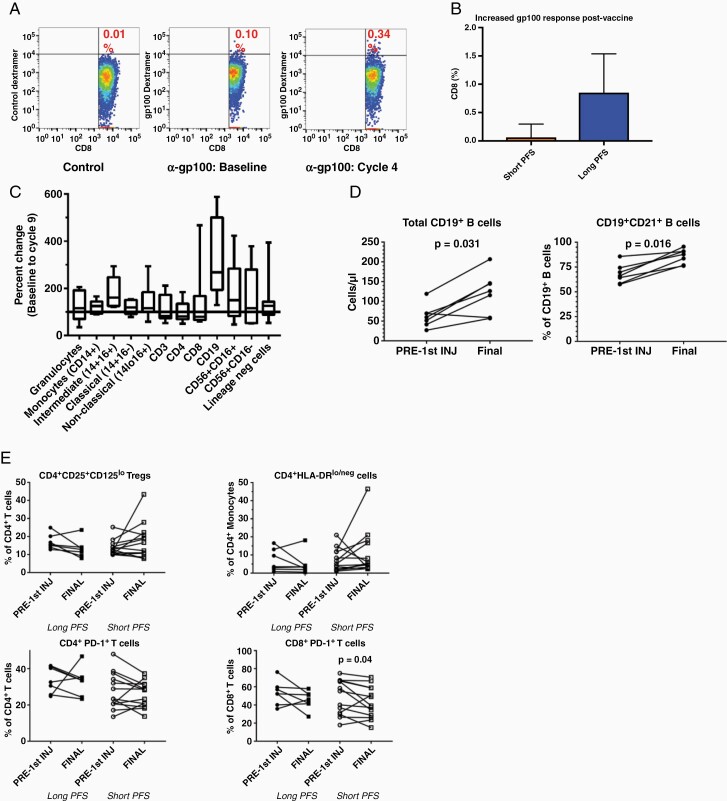
The allogeneic tumor lysate used to pulse autologous DCs in this trial contains several defined tumor-associated antigens8 including gp100. Dextramer staining and flow cytometry show increased gp100-specific CD8 T-cell post-vaccination in HLA-A202+ patients (Figure 4A). There was a trend to increased gp100-specific CD8 T-cell post-vaccination in HLA-A202+ patients with PFS >2 years compared to those with PFS < 2 years, but this was not statistically significant (Figure 4B). Overall increases in multiple leukocyte populations occurred between enrollment and cycle 9 (Figure 4C). This was most prominent for B cells (Figure 4D). Importantly, there were no significant alterations in immunosuppressive populations, such as CD4+/CD25+/CD125lo regulatory T cells, CD14+/HLA-DRlo/neg monocytic myeloid-derived suppressor cells, or PD-1+ CD4 or CD8 cells in either long or short PFS patients (Figure 4E).
Figure 4.
Specific immune responses to vaccination. (A) Representative dot plots showing the percentage of gp100-specific CD8+ cells compared to control dextramer staining pre- and post-vaccination in a patient with long progression-free survival (PFS). (B) Trend to increased gp100-specific CD8 T-cell post-vaccination in patients with prolonged PFS compared with short PFS. (C) Immunophenotyping demonstrates overall increases in multiple leukocyte populations between enrollment and cycle 9. (D) Increases were most prominent for B cells between enrollment (pre-first injection) and cycle 9 (final). (E) There were no significant increases or decreases in CD4+/CD25+/CD125lo regulatory T cells, CD14+/HLA-DRlo/neg monocytic myeloid-derived suppressor cells, or PD-1+ CD4 or CD8 cells between enrollment and cycle 9 in either long or short PFS patients.
Immunophenotype and Survival
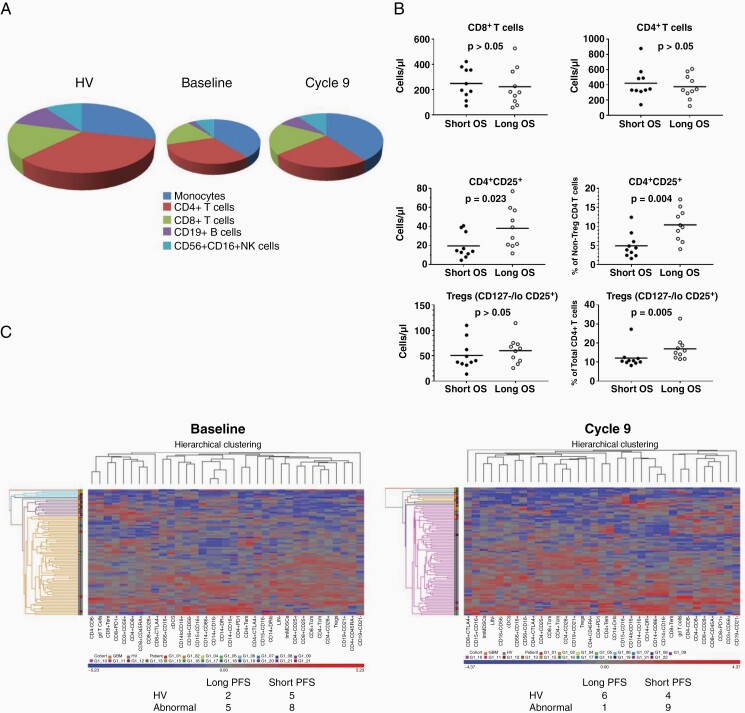
Whole blood immunophenotyping was performed for more than 100 leukocyte phenotypes at multiple time points. As previously described,17 overall leukocyte numbers were substantially reduced in patients at baseline compared with HV, particularly for T and B cells (Supplementary Table 1). This recovered partially by cycle 9 (Figure 5A). T-cell populations at baseline were similar between patients who ultimately had survival ≤median OS compared to patients who survived >median OS (Figure 5B). No differences were seen in total CD4 and CD8 counts, but activated (CD25+) CD4 T cells were increased, both as a percentage of total CD4s and as percentage of non-Treg CD4s at baseline in patients with long survival. This was not associated with an absolute increase in CD4 Treg numbers at baseline but was associated with a relative increase in Tregs as a percentage of overall CD4s. Unsupervised hierarchical clustering was performed comparing global immunophenotypes of patients who received study treatment (n = 20) vs HV (n = 64) and additional GBM patients (n = 20). This demonstrated that 5/7 (71%) trial patients with long PFS (>2 years) had an abnormal systemic immunophenotype at baseline (ie, overlapping with prior GBM patients). This was similar in patients with short PFS (≤2 years) who were abnormal in 61% (P = .6583; chi-square). However, immunophenotypes became more like those of HV (ie, immune normalization) at the end of the study in 6/7 long PFS patients (86%) compared with only 4/13 patients with short PFS (31%; P = .0191; chi-square).
Figure 5.
Immunophenotypes associated with long vs short survival compared to healthy volunteers. (A) Pie charts representing leukocyte total numbers (size of the pie chart) and relative distribution (monocytes, CD4 T cells, CD8 T cells, B cells, and NK cells) for healthy volunteers (HV) and patients enrolled in the clinical trial at baseline and at cycle 9. (B) Scatter plots showing T-cell populations at baseline between patients who ultimately had survival ≤median OS (short survival) compared to patients who survived >median OS. No differences were seen in total CD4 and CD8 counts, but activated (CD25+) CD4 T cells were increased, both as a percentage of total CD4s and as a percentage of non-Treg CD4s at baseline in patients with long survival. This was not associated with any absolute increase in CD4 Treg numbers at baseline but was associated with a relative increase in Tregs as a percentage of overall CD4s. (C) Unsupervised hierarchical clustering demonstrates that 5 of the 7 (71%) patients in the trial with long PFS (>1 year) have an abnormal systemic immunophenotype at baseline which is similar to patients with short PFS (≤1 year) who were abnormal in 61% (P = .6583). However, this normalized at the end of the study in 6 of the 7 long PFS patients (86%) compared with only 4 of the 13 patients with short PFS (31%; P = .0191).
Discussion
Though this small non-randomized study must be interpreted with caution, several important conclusions can be made regarding feasibility and safety. First, treatment with an optimized autologous DC/allogeneic tumor lysate vaccine as outlined here is highly feasible. We were able to generate ≥15 vaccine doses per patient enrolled and proceed with treatment per protocol in 20/21. This is in marked contrast to other GBM vaccine platforms utilizing the bulk tumor antigen sources dependent upon fresh autologous tumor.7 Furthermore, the vaccine appears safe in combination with standard adjuvant therapy (TMZ) in newly diagnosed GBM patients. No DLTs were observed.
Interpreting clinical outcomes is challenging given the limitations of a small, non-randomized trial. Some patients had clear imaging and histological evidence of an inflammatory response (Figure 2). The overall clinical benefit rate of 40% for this combination of TMZ and DC vaccine is substantial, though only randomized trials would be able to determine the contribution of the vaccine. Similarly, median PFS and OS of 9.7 and 19.0 months compare favorably to historical median PFS and OS with radiation and TMZ alone (6.9 and 14.6 months, respectively1). A progression-free survivor tail of 10% at 36 months also compares favorably to historical PFS of 0.3% at 36 months with radiation and TMZ alone.1 Subgroup analysis based on the presence or absence of MGMT promoter methylation showed a trend to prolonged survival in patients with MGMT promoter methylation both for median PFS (19.4 vs 9.4 months) (Figure 3C) and OS (26.5 vs 16 months) (Figure 3D), as expected. Again, these compare favorably to historical PFS and OS rates in MGMT promoter methylated (10.3 and 21.7 months) and unmethylated patients (5.3 and 12.7 months).23 Finally, our longest survivor (progression-free at last follow-up 60.7 months) was MGMT promoter unmethylated and IDH wild type (ie, genetically unfavorable). Thus, while no definite conclusion about efficacy can be made based on this small, non-randomized study, clinical outcomes are sufficiently promising to warrant further investigation.
The human GBM cell lines used as our antigen source for this vaccine express the GBM-associated antigen gp100.8 gp100 expression has been reported in between 40% and 95% of GBM tumors but this is sometimes based on RNA expression alone and is clearly not universal.24,25 While gp100 may or may not represent an important tumor-associated antigen within our vaccine for a given patient, its presence facilitates antigen-specific response testing after vaccination regardless. Enrollment in this study was initially limited to HLA-A0201+ individuals to allow antigen-specific response testing. gp100-specific CD8 T cells were increased post-vaccination compared with pre-vaccination or nonspecific controls (Figure 4A) and trended toward increase in patients with long PFS (>1 year; n = 3) compared to short PFS (≤1 year; n = 7) (Figure 4B). Immunophenotyping showed leukocyte recovery between enrollment and cycle 9, most marked for CD19+ B cells (Figure 4C and D). Importantly, treatment was not associated with increased immunosuppressive regulatory T cells or myeloid-derived suppressor cells, nor did it result in increased “exhausted” (PD-1+) CD4 or CD8 T cells.
GBM patients in this study had significant pan-leukopenia at baseline compared to HV (Figure 5). This is our most striking immunological finding and occurred without exogenous corticosteroids in 95%. It affected multiple leukocyte populations but was most marked for CD4+ T cells and CD19+ B cells. There was relative proportional preservation of CD8+ T cells and NK cells and proportional expansion of monocytic cells. This is in keeping with our prior studies showing the expansion of immunosuppressive myeloid-derived suppressor cells in GBM patients’ blood.17,18 Baseline immunophenotyping features associated with prolonged OS included increased activated (CD25+) CD4 T cells and proportion of CD4+/CD127−/CD25lo regulatory T cells among total CD4 T cells. This suggests that patients with heightened baseline CD4 activation ultimately had prolonged survival. In contrast, the hierarchical clustering of global immunophenotype of study patients at baseline compared to HV or other GBM patients did not predict PFS. However, patients who ultimately had prolonged PFS (>1 year) clustered significantly more frequently with HV by cycle 9 than patients with shorter PFS (86% vs 31%; P = .0191; Figure 5C). This illustrates immune “normalization” over the course of therapy. How much this reflects response to treatment vs stopping myelosuppressive TMZ is uncertain. Earlier work in both malignant and nonmalignant diseases suggests that whole blood immunophenotyping has prognostic potential.20–22 However, further validation in GBM awaits analysis of larger datasets.
In summary, combined adjuvant TMZ and mature autologous DC/allogeneic tumor lysate vaccine treatment is feasible and safe in newly diagnosed GBM patients. It is associated with sufficiently promising PFS and OS compared to historical controls to warrant further investigation, though no definitive conclusion regarding efficacy can be made based on this small, non-randomized study. GBM patients enrolled in this study after completing radiation therapy with concurrent TMZ have marked leukopenia compared to healthy donors, particularly for CD4+ T cells. Assessing clinical outcome in an appropriately powered phase II study and determining safety and feasibility of combining this vaccine with additional immunotherapeuties such as immune checkpoint inhibitors will be important next steps. Furthermore, it may be possible to optimize the allogeneic cell lines used as a source of tumor antigens for autologous DCs in this study in future trials by expanding our library of cGMP-grade human GBM cell lines and matching antigenic/genomic expression patterns to those seen in individual patient’s tumors.
Supplementary Material
Contributor Information
Ian F Parney, Department of Neurological Surgery, Mayo Clinic, Rochester, Minnesota, USA; Department of Immunology, Mayo Clinic, Rochester, Minnesota, USA.
S Keith Anderson, Department of Quantitative Health Sciences, Mayo Clinic Cancer Center, Rochester, Minnesota, USA.
Michael P Gustafson, Nyberg Human Cell Therapy Lab, Division of Laboratory Medicine, Department of Laboratory Medicine and Pathology, Mayo Clinic Arizona, Phoenix, Arizona, USA.
Susan Steinmetz, Department of Neurology, Mayo Clinic, Rochester, Minnesota, USA.
Timothy E Peterson, Department of Neurological Surgery, Mayo Clinic, Rochester, Minnesota, USA.
Trynda N Kroneman, Division of Anatomic Pathology, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, Minnesota, USA.
Aditya Raghunathan, Division of Anatomic Pathology, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, Minnesota, USA.
Brian P O’Neill, Department of Neurology, Mayo Clinic, Rochester, Minnesota, USA.
Jan C Buckner, Department of Oncology, Mayo Clinic, Rochester, Minnesota, USA.
Mary Solseth, Immune Progenitor and Cellular Therapy (IMPACT) Laboratory, Division of Transfusion Medicine, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, Minnesota, USA.
Allan B Dietz, Department of Immunology, Mayo Clinic, Rochester, Minnesota, USA; Immune Progenitor and Cellular Therapy (IMPACT) Laboratory, Division of Transfusion Medicine, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, Minnesota, USA.
Funding
This study was supported by the Ben and Catherine Ivy Foundation.
Conflict of interest statement. M.P.G. and A.B.D. are inventors of technology related to establishing cell lines used in this article. The technology has been licensed to Mill Creek Life Sciences; Mayo Clinic and the inventors have contractual rights to receive royalties from the licensing of this technology. In addition, Mayo Clinic and A.B.D. hold equity in the company to which the technology is licensed. This conflict is managed according to policies and procedures regarding conflict of interest at Mayo Clinic.
Authorship statement. Conception and supervision of the study: I.F.P. and A.B.D. Conduct of the trial: I.F.P., S.K.A., M.P.G., S.S., A.R., B.P.O., J.C.B., and A.B.D. Data generation and analysis: all authors; Manuscript writing: I.F.P. and A.B.D. Manuscript editing: all authors.
References
- 1. Stupp R, Mason WP, van den Bent MJ, et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2. Liau LM, Prins RM, Kiertscher SM, et al. . Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11(15):5515–5525. [DOI] [PubMed] [Google Scholar]
- 3. Sampson JH, Heimberger AB, Archer GE, et al. . Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(31):4722–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bloch O, Crane CA, Fuks Y, et al. . Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. Neuro Oncol. 2014;16(2):274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weller M, Butowski N, Tran DD, et al. . Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–1385. [DOI] [PubMed] [Google Scholar]
- 6. Liau LM, Ashkan K, Tran DD, et al. . First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018;16(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bloch O, Shi Q, Anderson SK, et al. . Alliance A071101: a phase II randomized trial comparing the efficacy of heat shock protein peptide complex-96 (Hsppc-96) vaccine given with bevacizumab versus bevacizumab alone in the treatment of surgically resectable recurrent glioblastoma. Neuro Oncol. 2017;19(Suppl_6):vi29. [Google Scholar]
- 8. Parney IF, Gustafson MP, Solseth M, et al. . Novel strategy for manufacturing autologous dendritic cell/allogeneic tumor lysate vaccines for glioblastoma. Neurooncol Adv. 2020;2(1):vdaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okada H, Kalinski P, Ueda R, et al. . Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with α-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29(3):330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pollack IF, Jakacki RI, Butterfield LH, et al. . Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. J Clin Oncol. 2014;32(19):2050–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garg AD, Coulie PG, Van den Eynde BJ, Agostinis P. Integrating next-generation dendritic cell vaccines into the current cancer immunotherapy landscape. Trends Immunol. 2017;38(8):577–593. [DOI] [PubMed] [Google Scholar]
- 12. Spisek R, Bretaudeau L, Barbieux I, Meflah K, Gregoire M. Standardized generation of fully mature p70 IL-12 secreting monocyte-derived dendritic cells for clinical use. Cancer Immunol Immunother. 2001;50(8):417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trepiakas R, Pedersen AE, Met O, et al. . Comparison of α-Type-1 polarizing and standard dendritic cell cytokine cocktail for maturation of therapeutic monocyte-derived dendritic cell preparations from cancer patients. Vaccine. 2008;26(23):2824–2832. [DOI] [PubMed] [Google Scholar]
- 14. Mbongue JC, Nieves HA, Torrez TW, Langridge WH. The role of dendritic cell maturation in the induction of insulin-dependent diabetes mellitus. Front Immunol. 2017;8:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kiertscher SM, Roth MD. Human CD14+ leukocytes acquire the phenotype and function of antigen-presenting dendritic cells when cultured in GM-CSF and IL-4. J Leukoc Biol. 1996;59(2):208–218. [DOI] [PubMed] [Google Scholar]
- 16. Czerniecki BJ, Cohen PA, Faries M, et al. . Diverse functional activity of CD83+ monocyte-derived dendritic cells and the implications for cancer vaccines. Crit Rev Immunol. 2001;21(1–3):157–178. [PubMed] [Google Scholar]
- 17. Gustafson MP, Lin Y, New KC, et al. . Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. 2010;10(1–3):631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rodrigues JC, Gonzalez GC, Zhang L, et al. . Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol. 2010;12(4):351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crespo-Diaz R, Behfar A, Butler GW, et al. . Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant. 2011;20(6):797–811. [DOI] [PubMed] [Google Scholar]
- 20. Gustafson MP, DiCostanzo AC, Wheatley CM, et al. . A systems biology approach to investigating the influence of exercise and fitness on the composition of leukocytes in peripheral blood. J Immunother Cancer. 2017;5(6):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gustafson MP, Lin Y, LaPlant B, et al. . Immune monitoring using the predictive power of immune profiles. J Immunother Cancer. 2013;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gustafson MP, Staff NP, Bornschlegl S, et al. . Comprehensive immune profiling reveals substantial immune system alterations in a subset of patients with amyotrophic lateral sclerosis. PLoS One. 2017;12(7):e0182002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hegi ME, Diserens AC, Gorlia T, et al. . MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 24. Syed ON, Mandigo CE, Killory BD, Canoll P, Bruce JN. Cancer-testis and melanocyte-differentiation antigen expression in malignant glioma and meningioma. J Clin Neurosci. 2012;19(7):1016–1021. [DOI] [PubMed] [Google Scholar]
- 25. Wen PY, Reardon DA, Armstrong TS, et al. . A randomized double-blind placebo-controlled phase II trial of dendritic cell vaccine ICT-107 in newly diagnosed patients with glioblastoma. Clin Cancer Res. 2019;25(19):5799–5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.