Abstract
The transition element molybdenum (Mo) is an essential micronutrient for plants, animals, and microorganisms, where it forms part of the active center of Mo enzymes. To gain biological activity in the cell, Mo has to be complexed by a pterin scaffold to form the molybdenum cofactor (Moco). Mo enzymes and Moco are found in all kingdoms of life, where they perform vital transformations in the metabolism of nitrogen, sulfur, and carbon compounds. In this review, I recall the history of Moco in a personal view, starting with the genetics of Moco in the 1960s and 1970s, followed by Moco biochemistry and the description of its chemical structure in the 1980s. When I review the elucidation of Moco biosynthesis in the 1990s and the early 2000s, I do it mainly for eukaryotes, as I worked with plants, human cells, and filamentous fungi. Finally, I briefly touch upon human Moco deficiency and whether there is life without Moco.
Keywords: molybdenum, molybdenum cofactor biosynthesis, molybdopterin, nitrate reductase, gephyrin
1. Introduction
1.1. Molybdenum
It has long been known that the transition element molybdenum (Mo), with its rich redox chemistry, is an essential micronutrient for plants, animals, and microorganisms where it forms part of the active center of enzymes. It is the only fifth-row element with biological function and occurs in soil and the oceans as an oxide or sulfide compound [1]. Mo is a trace element, i.e., the organism needs it only in minute amounts, but the unavailability of Mo is detrimental to the organism. However, even if Mo is available for the cell, it is biologically inactive until it becomes complexed to form the molybdenum cofactor. Nature has developed two very different cofactors to position Mo within the active center of Mo enzymes. One type of molybdenum cofactor is the iron–molybdenum cofactor (FeMoco) which is unique to a single enzyme, bacterial nitrogenase (FeMoco is reviewed in detail by Ribbe et al. in this Special Issue). The other type is the pterin-based molybdenum cofactor (Moco), which occurs in all kingdoms of life. Both cofactors have in common that the metal atom is coordinated by sulfur—likely due to the sulfur-rich environment in the ancient ocean. Calculations of molecular evolution show that both cofactors were already present in the last universal common ancestor LUCA [2,3], indicating their essential role for life. Mo is abundant in the oceans in the form of the molybdate anion (about 100 nM [4]). In soils, the oxidation state of Mo varies from II to VI, but only the soluble molybdate anion is available for eukaryotes and bacteria.
1.2. Molybdenum Enzymes
Mo enzymes and Moco are found in all kingdoms of life, where they perform key transformations in the metabolism of nitrogen, sulfur, and carbon compounds [1]. They have a very rich redox chemistry and each reaction, either reduction or oxidation, is also characterized by the transfer of two electrons, forcing the Mo atom to vary its oxidation state between IV and VI. More than 50 Mo-containing enzymes are known, most of them of bacterial origin, although only a handful of Mo enzymes were found among eukaryotes. In brief, these eukaryotic enzymes are (1) sulfite oxidase, which catalyzes the final step in the degradation of sulfur-containing amino acids and is involved in detoxifying excess sulfite; (2) xanthine dehydrogenase, which is involved in purine catabolism and reactive oxygen production; (3) aldehyde oxidase, which oxidizes a variety of aldehydes and is essential for the biosynthesis of the stress hormone abscisic acid in plants; (4) nitrate reductase in autotrophic organisms, which catalyzes the crucial step in inorganic nitrogen assimilation; and (5) mitochondrial amidoxime reductase which has a detoxifying function. The structures and functions of all five enzymes are reviewed by Hille et al. (2011) [5]. In bacteria, well-studied Mo enzymes are formate dehydrogenase, nitrate reductase, DMSO-reductase, xanthine dehydrogenase, and aldehyde oxidase. In extremophile bacteria and archeae, Mo is replaced by the transition element tungsten [6].
In this review, I focus on Mo; details for tungsten can be found in the comprehensive review by Bevers et al. [7]. I started as a plant researcher; therefore, in my review, I focus on the history of eukaryotic Moco research, mainly in plants and humans, because these organisms are drivers in the field. Details for the history of bacterial Moco research are given by Leimkühler et al. [8].
2. History of Moco
2.1. 1900–1954: Mo Research Started with Cow Milk
As early as 1889, it was observed that xanthine could be converted to uric acid in tissue extract [9]. In 1902, Schardinger [10] found that a protein fraction of fresh cow milk could reduce methylene blue in the presence of formaldehyde. This protein fraction was also able to oxidize other aldehydes and became known as Schardinger’s enzyme. In 1922, Morgan et al. [11] identified the reducing substance as hypoxanthine and named the enzyme xanthine oxidase. In 1949, Westerfeld and Richert [12] found that for xanthine oxidase activity in addition to FAD, an unknown compound was necessary that was primarily found in liver extracts, and named it “liver residue factor” [12]. Two years later, these researchers renamed this factor “xanthine oxidase factor” and identified it as Mo [13]. In the same year, DeRenzo et al. [14] also identified Mo as the xanthine oxidase factor. Shortly after, Mo was also found in aldehyde oxidase [15] and nitrate reductase [16], and later in sulfite oxidase [17]. However, the occurrence of Mo in living organisms had already been described earlier—in 1930, Bortels [18] described Mo as catalytically crucial for the biological nitrogen fixation in the bacterium Azotobacter, and in 1932, Ter Meulen [19] chemically identified Mo in plants (especially rich in legumes) and mammals (especially rich in the liver).
2.2. The 1960s: Moco Was Postulated by Geneticists
In algae, fungi, and higher plants, the Mo enzyme nitrate reductase (NR) catalyzes the key step in inorganic nitrogen assimilation, and NR-deficient mutants were soon described. With the genetic analysis of these mutants, the genetic elucidation of Mo metabolism had begun, namely, in the filamentous fungus Aspergillus nidulans by Pateman et al. [20]. Cove and Pateman [21] had isolated NR-deficient mutants which revealed a novel phenotype: the simultaneous loss of the two Mo-dependent enzymes NR and xanthine dehydrogenase. Mo was the only link between these two otherwise very different enzymes; thus, it was suggested that both enzymes could share a common Mo-related cofactor, named molybdenum cofactor. Pateman et al. [20] described five genetic loci, each exhibiting that novel phenotype, and designated them with the mnemonic ‘Cnx’ (cofactor for nitrate reductase and xanthine dehydrogenase). Thus, it became evident that for this common cofactor, five genes were responsible, whereas for nitrate reductase, only one locus was coding.
One decade later, Cnx-like mutants were described for the plant Nicotiana tabacum [22]; two decades later, Cnx-like mutants were published for the fly Drosophila melanogaster [23]; and three decades later, Cnx-like mutants were defined for other higher plants, among them, the model plant Arabidopsis thaliana [24].
In parallel to the achievements of fungal biochemical genetics, also in Escherichia coli, a series of mutants had been isolated that showed the growth of mutagenized cells in the presence of high chlorate concentrations. Mutants selected for chlorate resistance (chl) do not reduce chlorate to toxic chlorite because they have lost their chlorate reductase activity, which appears to be a non-physiological catalytic activity related to NR [25,26]. We now know that the chlorate-resistant phenotype reflects the lack of NR activity, either due to a mutation in the corresponding structural genes or to a loss of Moco. However, at that time, it was surprising that many independent genes were involved in the loss of NR. This observation had already been made by Aspergillus researchers a few years earlier. Later, it turned out that the chlorate-resistant loci chlA, chlB, chlD, chlE, and chlG were all essential for Moco biosynthesis. Stewart and MacGregor [27] isolated an extensive series of Mu-phage insertion mutants for all these loci. In 1992, the Moco-specific chl loci were renamed mo loci [28].
2.3. The 1970s: The ‘Nit–1 Assay’ and the Discovery of Moco
In the filamentous fungus Neurospora crassa, Sorger and Giles [29] isolated a mutant named nit-1, similar to the Aspergillus Cnx mutants, exhibiting a pleiotropic loss of NR and xanthine dehydrogenase activities. In 1970, Nason and coworkers [30,31,32] provided the first biochemical evidence for the existence of a cofactor common to Mo enzymes. In crude extracts of nit-1 mutant cells, the inactive apoprotein of NR could be fully reconstituted by adding a low-molecular-weight fraction derived from denatured preparations of purified Mo enzymes of mammalian, plant, or bacterial origin. This fraction included a Mo-containing cofactor that could be integrated into inactive apoNR of the fungal nit-1 mutant lacking the cofactor. Thus, it was suggested that a ubiquitous and universal Moco of identical structure could associate with different apoenzymes to form Mo holoenzymes. However, there was one exception: nitrogenase, another Mo-containing enzyme, did not release a nit-1 positive cofactor after denaturing the enzyme [33]. Based on these data and functional analyses [34], it was shown that the dissociable cofactor of nitrogenase, named the FeMo cofactor, is different from Moco and unique for nitrogenase (see the review by Ribbe in this Special Issue).
The observed complementation of nit-1 apoNR by Moco from different Mo enzyme sources served as a basis to develop a very sensitive biological in vitro assay, referred to as the ‘nit-1 assay’, for determining the presence of biologically active Moco, which still serves as a tool for Moco analysis. Nason et al. [35] found that the reconstituting activity released from diverse Mo–enzymes was very labile, with a lifetime of a few minutes. This was the first indication of the cofactor’s labile nature in its isolated form. Subsequently, several attempts were undertaken to stabilize the cofactor and to optimize the transfer of Moco to nit-1 apoNR.
2.3.1. Release of Moco from Mo Enzymes under Stabilizing Conditions
Different methods of cofactor release from Mo enzymes (acidification [32]; heat treatment [36]; detergent and organic solvent treatment [37,38]; phosphate extraction [39]) were utilized to minimize the loss of cofactor due to its high sensitivity to air oxidation. Most of these in vitro complementations are dependent on an excess of molybdate (1–10 mM) in the reaction mixture [40,41,42,43], indicating a strong tendency of isolated Moco to lose Mo. Furthermore, it had been shown through the nit-1 assay that different sulfhydryl protecting agents stabilize the isolated cofactor [36,41], with glutathione exhibiting the best results, whereas sulfhydryl-reactive inhibitors such as p–hydroxymercuribenzoate abolished the reconstituting activity of the cofactor [42,44], thus indicating the involvement of free sulfhydryl groups in the nit-1 reconstitution process.
2.3.2. Moco Triggers the Dimerization of NR Monomers
Nason and coworkers [31] also observed another attractive property of Moco. In extracts of the Neurospora nit-1 mutant, inactive apo-NR occurs in monomeric form, sedimenting with 4.5S in sucrose density gradients. After adding Moco released from Mo enzymes to the nit-1 extract for in vitro complementation, NR gained activity and sedimented as a dimer with 7.9S. Moco triggered a clear conformational change in the NR monomer so that it could dimerize.
Although an absolute quantification of Moco by the nit-1 assay was hard to achieve, it has been beneficial for qualitative and semi-quantitative detection of cofactor activity. Later, this in vitro system was improved: Neurospora crassa Moco-free apo-NR had been cloned, tagged (twin-Strep and His), and the purified apo-NR could be used to quantify Moco [45]. This study also showed that Moco insertion into apo-NR is independent of the insertion of the two other prosthetic groups, FAD and heme. The formation of active holo-NR is an autonomous process intrinsically tied to NR dimerization. There is now a chemical assay to quantify Moco [46]. However, this assay based on the chemical detection of the pterin moiety of Moco does not allow for the detection of biologically active Moco. Therefore, the nit-1 assay is still the most straightforward way to detect biologically active Moco in crude extracts of diverse biological sources.
2.4. The 1980s: The Elucidation of the Chemical Nature of Moco
2.4.1. Moco Degradation Products Were Crucial for Uncovering Its Chemical Nature
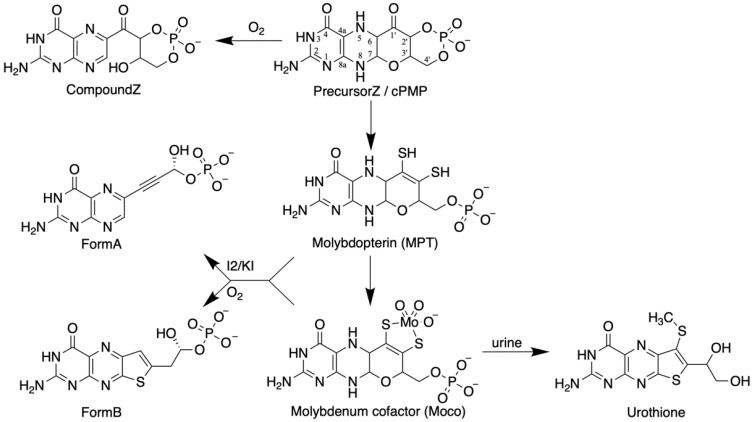
The elucidation of the chemical nature of Moco is based on the work of J. Johnson and K.V. Rajagopalan (Figure 1). Spectroscopic analyses of Moco isolated from chicken and rat livers yielded a pterin component [37]. Due to the labile nature of Moco and its high sensitivity to air oxidation, all further studies were conducted using degradation or oxidation products of the cofactor isolated from the liver. By oxidizing Moco under acidic conditions, two fluorescent pterin derivatives were found (FormA and FormB, Figure 1), which were crucial to solving the structure of Moco. In short, surprisingly, FormA contained a four-carbon atom side chain at its C6, which is uncommon for pterins because most of them have three carbons in their side chain [47]. However, no suitable ligand groups were found as candidates for binding Mo in FormA. Due to the sensitivity of Moco to sulfhydryl reagents and its increased stability in the presence of reducing reagents, the presence of sulfur atoms in the cofactor was assumed. Here, FormB led the way because it contained a sulfur atom. At that point, it must be mentioned that as early as 1940, Koschara [48] described a non-fluorescent sulfur-containing pterin named urothione in human urine. Goto et al. [49] determined the structure of urothione, which turned out to be a sulfur-containing pterin compound (Figure 1). The Rajagopalan group finally showed that the intense oxidative treatment of FormB and urothione yielded the same compound; thus, the conclusion was very suggestive that urothione may be the natural metabolic degradation product of Moco. Conclusive evidence for this came from the urine analysis from patients with Moco deficiency devoid of urothione [50]. The coordination of Mo via an enedithiolate function at the C1’ and the C2’ position in the four-carbon side chain of the cofactor was demonstrated so that the structure of Moco was suggested to be as shown in Figure 1 [50]. All details of this chemical detective story were described by Leimkühler et al. [8]. Finally, crystal structures of Mo enzymes confirmed this core structure [51] and showed a third pyrano ring between the OH-group at C3’ of the side chain and the pterin C7 atom in its 5,6 dihydro state. Once the pyrano ring is closed, a fully reduced hydrogenated pterin is formed. Due to the unique nature of the pterin in Moco, the metal-free form of the cofactor is called molybdopterin, or metal-binding pterin (MPT). The latter reflects that this pterin scaffold cannot only coordinate Mo, but also tungsten.
Figure 1.
Structures of PrecursorZ, molybdopterin (MPT), Moco, and their derivatives. PrecursorZ is cyclic pyranopterin monophosphate (cPMP), and CompoundZ is its air-oxidized product. MPT and Moco are both 5,6,7,8-tetrahydropyranopterins. FormB is the air-oxidized degradation product of MPT and Moco, whereas FormA is the iodine-oxidized derivative. FormA and FormB are shown in their phosphorylated forms. Urothione is the degradation product of Moco in humans, which is excreted in the urine.
Later, it was found that in bacteria, a dinucleotide derivative of Moco exists where the MPT moiety of Moco is associated with the nucleotide GMP or CMP, and in most Mo enzymes occurred in the bis-form where two MPT dinucleotides coordinated one Mo atom (details are given in the review by Tiedemann et al. [52] in this Special Issue).
2.4.2. What Is the Task of the Pterin Moiety of Moco?
The fusion of a pterin with a pyrano ring, as identified for Moco and its direct precursor, the metal-free MPT, is unique in nature. It may have been evolved to serve the following possible tasks: (a) positioning the catalytic metal correctly within the active center of a given Mo enzyme, and depending on the class of Mo enzyme, Mo can have different ligands [1]; (b) another possible role of the pterin moiety could be modulation of the redox behavior of the Mo atom; (c) pterin might also participate in the electron transfer to or from Mo via delocalized electrons within the pterin; (d) with its possible reduction states and different structural conformations, the pterin could also be necessary to channel electrons to or from other prosthetic groups [1]. Moco is not located on the surface of the protein. Rather, X-ray crystallographic analyses of Mo enzymes revealed that the cofactor is buried deeply within the interior of the enzyme and a tunnel-like structure makes it accessible to both its appropriate substrates and interacting prosthetic groups [53,54,55].
2.4.3. Chemical Model Compounds Helped to Study Moco
Two lines of research converged after the chemical nature of Moco was determined: dithiolene chemistry and pterin chemistry. The research field of metal 1,2-dithiolene chemistry started in the 1960s [56]. After it became clear that Moco is a unique Mo–dithiolene–pterin compound, the synthesis and study of Mo–dithiolene complexes began with the aim of reproducing the general features of the Mo coordination sphere. The detailed chemical and spectroscopic analyses of these synthetic analogs of catalytic centers in Mo enzymes gave insight into Moco’s broad redox capabilities [57]. The Mo–dithiolene group, however, is only one part of Moco; the other component is its pterin scaffold. Pterin chemistry is a historic research field deriving from the analysis of natural products. Pterins are ubiquitous in nature. They have been identified as pigments in the wings of butterflies, which was name-giving: the Greek word for wing is ‘pteron’. They are C1 transfer cofactors (folic acid and tetrahydrofolates), redox cofactors (biopterin and neopterin), and even toxins in mollusks. Pterins are of great medical relevance; therefore, they are extensively studied in chemistry and biochemistry, life sciences, medicine, pharmacology, and the pharmaceutical industry. Chemical synthesis of pterins began in the 1940s (see the review by Basu and Burgmayer [58]). Additionally, soon after the identification of Moco as a pterin derivative, pterin-inspired model chemistry started with the synthesis of pterin-substituted dithiolenes [58]. There are ongoing attempts for the total chemical synthesis of Moco [59,60]. However, it has not yet been achieved, although the chemical synthesis of Moco’s precursor, cPMP, has been successful [61]. For a recent review, see Colston and Basu [62] in this Special Issue. In parallel to these achievements, another very active field of research has developed to reproduce the reactions catalyzed by Mo enzymes in vitro. Bio-inspired chemistry has emerged in this field and is also used as a basis for the design of biocatalysts to address contemporary issues.
2.5. The 1970s and 1980s: Biochemical Analyses of Moco Mutants in Bacteria, Fungi, and Plants Gave the First Insights into Moco Biosynthesis
The detailed biochemical characterization of NR-deficient mutants contributed significantly to our understanding of the biochemistry and genetics of Moco biosynthesis in bacteria, fungi, and plants. All of them exhibited no NR activity; thus, the question arose as to how to find other criteria for differentiating between them?
2.5.1. Repair by High Molybdate
It is reasonable to assume that the multitude of mutant loci in each model organism are involved in Moco metabolism; a first and easy experimental step was to grow the different NR-deficient mutants on a high-molybdate medium (1 mM): among the Aspergillus nidulans Cnx mutants, one locus turned out to be partially molybdate-repairable [63]; in the plant Nicotiana tabacum; one mutant type was also partially molybdate-repairable [40]. Among newly described Neurospora crassa nit loci [64], again, mutants in one locus were molybdate-repairable [40]. Additionally, among the E. coli chl mutants, two groups were molybdate-repairable, although the situation is more complex (see below). Thus, it was assumed that the gene products of the molybdate-repairable locus should be involved in transferring or inserting Mo into MPT. Subsequent molecular analyses proved this assumption to be correct.
2.5.2. What Was Missing in E. coli Chlorate Mutants?
E. coli NR was intensively studied in the 1960s and 1970s, and after the chlorate-resistant mutants had been published [25,65], which all lacked NR activity, it became of interest to find out what was missing in these mutants. How could criteria other than high molybdate repair be found for differentiating between them?
At first, cell-free extracts of chl mutants were mixed and analyzed for the reconstitution of NR activity. Later, protein fractions were purified, containing the reconstituting activities and resulting in the initial characterization of ‘Protein FA’ [66] and ‘Protein PA’ [67], which turned out to be encoded by chl loci. Using the Neurospora nit-1 assay (described above), the extracts of the mutants chlA, chlB, chlC, chlD, chlE, and chlG were analyzed in combination with additional molybdate in the assay, providing an initial suggestion about the sequence of activities of the chl gene products. Two loci were repairable by high molybdate (chlD and chlG), and two were assumed to act early in Moco biosynthesis (chlA and chlE) because they showed no nit-1 complementing activity. chlB was assumed to be involved in the insertion of Moco in apoNR [68,69]. Using the nit-1 assay, Miller and Miller and Amy [69] found that in chlG mutants, a demolybdo form of the cofactor (MPT) was present, which incorporated Mo into MPT only in the presence of high molybdate (10 mM). An additional factor named factor X was identified by in vitro complementation, essential for the formation of the active NR complex in E. coli. Two decades later, this enigmatic factor turned out to be the chaperone narJ, the first of a long list of specific chaperones assisting in the folding and acquisition of metal cofactors, including Moco.
2.5.3. Plant Mutants Disclose Further Details about Moco Biosynthesis
In the 1970s, in our laboratory, cell cultures of the model plant Nicotiana tabacum were mutagenized and selected for chlorate resistance [70]; I was assigned the task of biochemically analyzing them during my Ph.D. research [22,71,72]. All of them had lost NR activity, and some of the selected cell lines exhibited no xanthine dehydrogenase activity [22], thus representing putative Moco mutants. We chose to name them with the initialism ‘Cnx’ [72], which stands for cofactor for nitrate reductase and xanthine dehydrogenase, similar to the Aspergillus geneticists’ suggestion [20]. I used a number of different criteria to further biochemically classify these Cnx mutants:
By measuring intracellular amounts of molybdate, it could be ruled out that plant Moco mutants are defective in molybdate uptake [41,73];
Running the extracts of plant Cnx mutants on sucrose density gradients and measuring the diaphorase activity of NR (i.e., their cytochrome c reductase activity which is independent of Moco) identified one mutant type that had dimeric NR [72];
Additionally, when this mutant was subjected to the nit-1 assay, it was positive, i.e., it could synthesize MPT [40];
As this mutant type regained NR activity when grown on high molybdate [40] or when molybdate was added to crude extracts of the cells [74,75], it was assumed to be impaired in molybdate insertion into MPT, which should be a late step in Moco biosynthesis;
Other mutants were negative in the nit-1 assay (i.e., were unable to synthesize MPT), but they could be repaired in vitro by adding Moco released from bovine xanthine oxidase [74]. Those mutants were classified to be defective in the early steps of Moco synthesis;
As another differentiating criterion, I used Moco fluorescence—cell extracts of the Cnx mutants were heat-treated [36,76] to release Moco, and a fluorescence spectrum was recorded. Again, only the molybdate-repairable mutant type described above was positive [74].
In summary, six independent Cnx mutants (CnxA–CnxF) were identified in the model plant Nicotiana plumbaginifolia [77,78]. Additionally, in other plants (e.g., barley, pea, Arabidopsis thaliana (reviewed by Crawford [24]) and in the alga Chlamydomonas reinhardtii (cf. review [79]), Moco mutants were isolated.
2.6. 1992: The First Model for the Moco Biosynthesis Pathway in E. coli
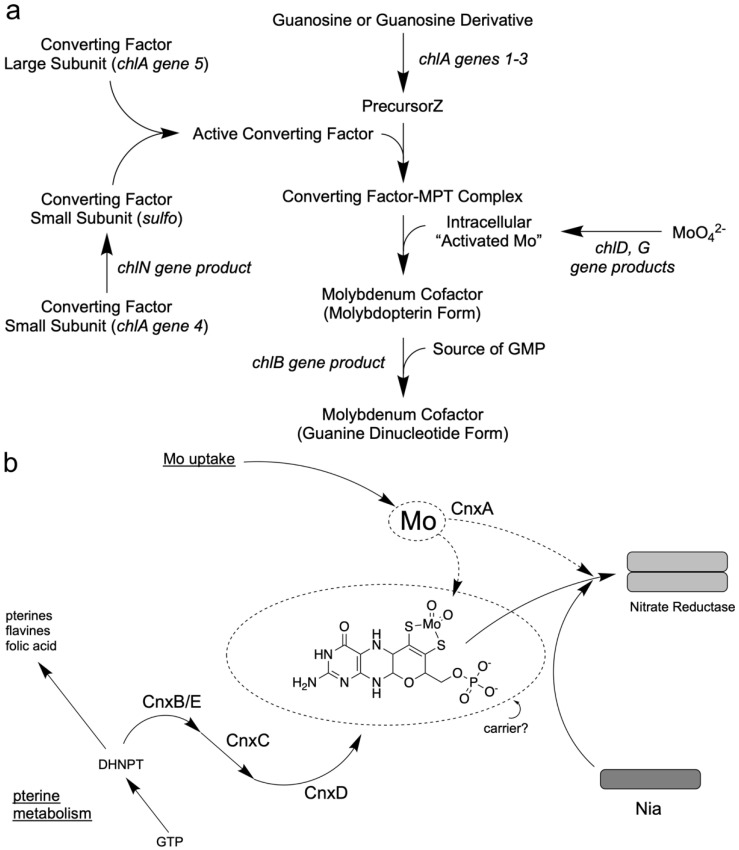
Comprehensive analyses of the E. coli chl mutants involving genetic and biochemical studies by several laboratories led to a working model for Moco biosynthesis. In the above section, “What was missing in E. coli chlorate mutants?” I referred to the proteins “Association factor FA” [66] and “Protein PA” [67] which were isolated and initially characterized from E. coli cell extracts by French groups and that were products of the chl genes. In 1990, Hinton and Dean [80] presented an initial model where they included all previous biochemical and genetical efforts undertaken to reveal insights into Moco biosynthesis in E. coli. However, only the 1992 review by Rajagopalan [81] put all these putative protein functions together and presented a pathway model where the single steps in Moco biosynthesis were indicated. They suggested for the first stage of Moco biosynthesis that GTP could be transformed into a sulfur-free pterin compound, the precursorZ (Figure 1), already possessing the Moco-typical four-carbon side chain, as shown in Figure 2a. Detailed labeling studies with E. coli [82] revealed evidence for a third unique route in pteridine synthesis, which starts from GTP with a novel and complex reaction sequence and results in the formation of precursorZ [83] as the first stable intermediate of Moco biosynthesis. PrecursorZ was suggested to be the substrate for the so-called “Converting Factor”, which transforms it to MPT. In contrast, the “Association factor FA” (a gene product of chlB) was situated at the end of the pathway subsequent to Moco formation (Figure 2a). Later, molecular analyses of cloned chl genes and the detailed biochemical analyses of the encoded proteins proved this working model to be correct for E. coli. Confusingly, the gene products of the chl genes became renamed in 1992 [28]; meanwhile, this new nomenclature has been accepted in the community. For more historical details about bacterial Moco biosynthesis, see the review by Leimkühler et al. [8].
Figure 2.
Models for the Moco biosynthesis pathway. (a) Model for the Moco biosynthesis pathway in E. coli, published by the Rajagopalan group in 1992. The proteins are named according to their functions. Modified after [81]. (b) Model for the Moco biosynthesis pathway in plants, published by Mendel in 1992 [84]. The proteins are named in the Cnx nomenclature. CnxA is the molybdate insertase. DHNPT stands for dihydro neopterin triphosphate. Nia is the designation for the nitrate reductase monomer. Modified after [84].
2.7. 1992: A First Model for the Moco Biosynthesis Pathway in Plants
In 1992, I was appointed Professor of Botany at the Technical University of Braunschweig, and I formed a group to unravel Moco biosynthesis in plants on the molecular level. Initially, I summarized the comprehensive analyses of Moco mutants in plants involving genetic and biochemical studies outlined above, thus leading to the first working model of Moco biosynthesis [84]. The pterin-based structure of Moco and the presence of an alkyl side chain raised the possibility that its biosynthetic pathway could share steps or intermediates common to the biosynthesis of other pteridines. Several pteridines have three-carbon side chains, although Moco is unique in having a four-carbon atom side chain that forms the pyrano ring. For the plants, I had the following assumption (Figure 2b) [84]: because the pterin ring system also forms part of other essential compounds of metabolism (i.e., folic acid, flavines, and pteridines), the mutational block of its synthesis would be lethal for the cell. Two major pathways are known for synthesizing pterins and flavins involving the initial conversion of GTP by the enzymes cyclohydrolase I and II [85,86]. Therefore, I assumed that the products of the Cnx genes are more likely involved in the formation of the four-carbon side chain and the insertion of important sulfur atoms and the Mo atom (Figure 2b). For plants, I put the first common intermediate DHNPT (dihydro neopterin triphosphate) of the pterin pathway as a branchpoint where Moco biosynthesis could leave the common pterin pathway, and ordered the different Cnx proteins according to the biochemical characteristics found in their mutants. I positioned CnxA as putative Mo insertase as the last step. Later, the latter proved right and the former proved wrong (see above). Additionally, the Moco carrier protein’s existence should be considered because Moco was an unstable compound [84].
2.8. The 1990s: Moco Biosynthesis Was Uncovered in Pregenomic Times
In 1997, the E. coli genome was sequenced [87] followed by the human genome [88] and the Arabidopsis thaliana genome [89] in the year 2000. However, the molecular analysis of Moco biosynthesis in E. coli, plants, and humans started earlier than this, which I call the “pregenomic times”. Neither sequence databases nor internet-based search tools were available at that time. So how did we do it? Piece by piece, partial gene sequences were published for the E. coli chl genes in combination with fine structure mapping studies. As early as 1987, Reiss et al. [90] cloned a genomic fragment, indicating that there might be at least seven complementation regions (putative genes) that were able to complement mutants in chlA, chlB, chlE, and chlG. One year later, Nohno et al. [91] cloned and sequenced two open reading frames for the chlE locus. In 1993, Rivers et al. [92] presented the molecular analysis of the complete chlA locus, which was renamed as the moaA operon. These efforts formed the basis for characterizing the corresponding proteins encoded by these genes.
The MPT structure of Moco is conserved in all organisms. Hence, it was tempting to conclude that perhaps part of the biosynthetic pathway for Moco could also be similar in all organisms other than E. coli, perhaps even in higher organisms such as humans and plants. The first attempts to clone eukaryotic Moco genes using the E. coli genes as hybridization probes failed. Today, we know that the homologies on the DNA level are not sufficient for this approach. Hence, the following problem arose: How to clone a gene for which there is only a corresponding mutant? Furthermore, it had to be considered that the gene product of a given Moco gene is not Moco itself, but rather a protein involved at some step in Moco biosynthesis. We have successfully used three approaches to clone eukaryotic genes involved in Moco biosynthesis: (1) the functional complementation of E. coli Moco mutants; (2) the generation of Moco-defective plant mutants by tagging; and (3) exploiting protein–protein interactions using the yeast two-hybrid system. Most of these genes showed only negligible homologies to the E. coli genes on the DNA level. However, on the amino acid level, significant homologies (30–40% identity) did emerge between bacterial and eukaryotic proteins. It turned out that all E. coli Moco proteins (except MoaB and MobAB) have counterparts in plants, fungi, and humans, as shown in Figure 3. However, in addition to these homologies to the bacterial proteins, there are also differences typical for eukaryotes.
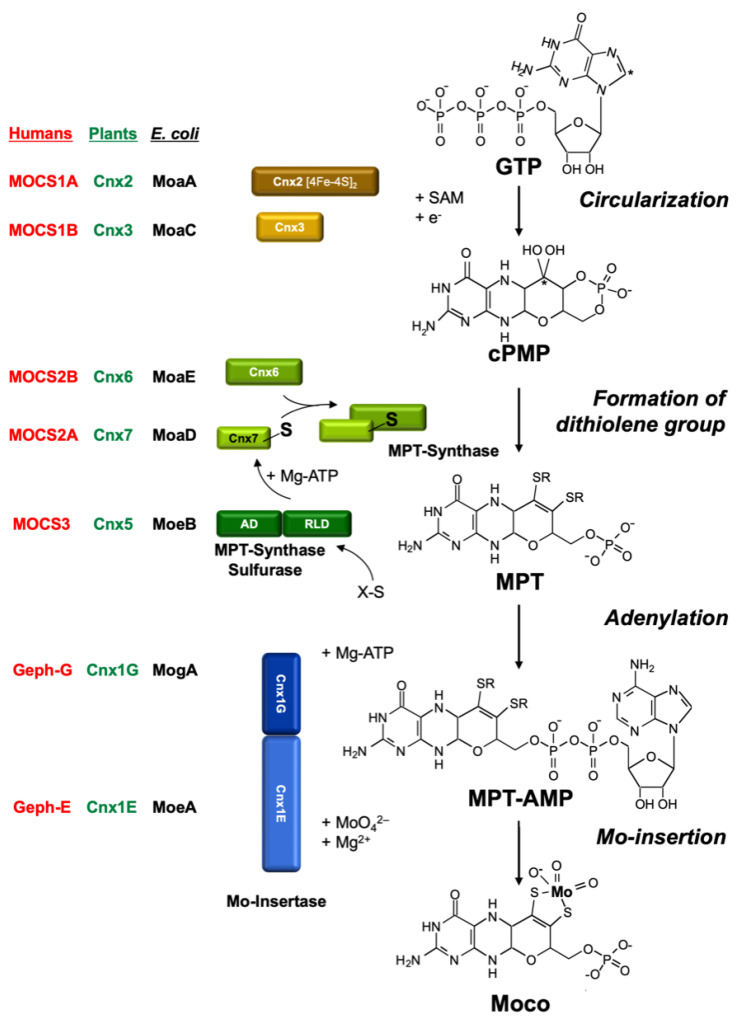
Figure 3.
Pathway of Moco biosynthesis in eukaryotes. The pathway can be divided into four steps, each being characterized by its main features as given in italics on the right side. The names for the proteins from the plant Arabidopsis thaliana (green), humans (red), and E. coli (black) catalyzing the respective steps are given. In GTP, the C8 atom of the purine is labeled with a star. This carbon is inserted between the 2′ and 3′ ribose carbon atoms, thus forming the new C1′ position in the four-carbon side chain of the pterin (labeled with a star in cPMP). The in vivo sulfur (X-S) source for Cnx5 and MOCS3 is probably cysteine. Steps three and four in eukaryotes are catalyzed by the individual domains of Cnx1 (G and E) or Gephyrin (G and E). Functional properties such as [Fe–S] clusters in Cnx2 and Mocs1A, the use of S-adenosyl methionine (SAM), adenylation, and sulfuration of the small subunit of MPT synthase (Cnx7 and Mocs2B, respectively) are indicated. In Cnx5, MoeBD denotes the MoeB-like domain and RLD the rhodanese-like domain. Modified after Mendel and Kruse [93].
Most of our initial knowledge about eukaryotic Moco biosynthesis is derived from plant studies. This may be surprising; however, in pre-genomic times, the detailed characterization of NR-deficient mutants (using callus cultures and in vitro plants) substantially contributed to our understanding of the genetics and biochemistry of Moco biosynthesis (summarized by Müller and Mendel [78]). These NR-deficient mutants fell into two groups: those with a defect in the NR apoprotein, and others which were defective in Moco. The identification of six genetic complementation groups among Moco-deficient plant mutants in the 1980s (using crossing methods and protoplast fusions) and the ongoing molecular gene analysis in E. coli in the 1990s also laid the basis for cloning plant and human genes. In 1995, the first eukaryotic Moco biosynthesis genes were cloned and published for the plant Arabidopsis thaliana, followed by the first human gene in 1998. subsequently, I will reflect on the history of this molecular and biochemical endeavor in the order of the three steps of Moco biosynthesis. For comparison, Figure 3 gives the names of proteins involved in Moco biosynthesis in plants, humans, and E. coli, and the pathway of Moco biosynthesis is shown.
2.9. Molecular and Biochemical Analysis of Moco-Biosynthesis in Plants and Humans—The Early Years
2.9.1. Step 1: Conversion of GTP to PrecursorZ (cPMP)
PLANTS: In 1995, the genes for the Arabidopsis thaliana proteins Cnx1, Cnx2, and Cnx3 (Figure 3) were cloned by functional complementation of the E. coli Moco mutants mogA, moaA, and moaC [94,95], respectively, which indicates that the functions of these proteins have been strongly conserved during evolution. Cnx2 and Cnx3 are involved in the first step of Moco biosynthesis, whereas Cnx1 catalyzes the last step. Nevertheless, a consortium for the nomenclature of plant genes [96] assigned Cnx1 as the number 1 as the function of this protein was best characterized in the preceding years. No biochemical data were available for Cnx2 and Cnx3 at that time.
More than one decade later, my postdoc Florian Bittner and his team discovered that step 1 of plant Moco biosynthesis occurs in the mitochondria, where GTP is available as a substrate for precursorZ synthesis and where Fe–S clusters are synthesized as the essential prosthetic group for Cnx2 [97]. Both proteins Cnx2 and Cnx3 contain a mitochondrial targeting sequence. After its synthesis in mitochondria, PrecursorZ is exported to the cytosol, where all further reactions occur. From now on, I will refer to PrecursorZ with the name “cyclic pyranopterin monophosphate (cPMP)”, which we assigned to this compound after having characterized it in greater detail [98,99] as a geminal diol. This geminal diol served as a matter of discussion that Günter and I later reviewed in detail [100].
Florian Bittner used a mutant of the transporter protein Atm3, which is localized in the inner membrane of mitochondria. However, when the Atm3 exporter is mutated, cPMP accumulates within mitochondria, and the cytosol becomes depleted; consequently, Moco levels and Mo enzyme activities decrease in the cell. The human analog to Atm3 is designated ABCB7.
HUMANS: As the gene sequences for the plant proteins Cnx2 and Cnx3 were known, they were taken as probes to identify the corresponding human genes, because we assumed that Moco biosynthesis is a highly conserved pathway, which turned out to be correct. Surprisingly, these two proteins are encoded by only one gene (mocs1) [101]. The corresponding transcript is bicistronic, with two consecutive reading frames separated by a stop codon. The first reading frame encodes for MOCS1A and the second one for MOCS1B. Initially, we tried to name the human genes “mcs”, for “Moco synthesis”, but this gene symbol was already assigned for a muscle gene, so we chose “mocs”. No biochemical data were available for the MOCS1 proteins at that time.
2.9.2. Step 2: Conversion of PrecursorZ (cPMP) to MPT
PLANTS: In the 1990s, the E. coli MPT synthase was characterized to consist of a small and a large subunit where the small subunit carries a sulfur atom at its highly conserved C-terminus, which is transferred to cPMP. This series of papers is summarized by Leimkühler et al. [8]. However, there was no way to clone eukaryotic cDNAs encoding for MPT synthases using the approach of functional reconstitution in E. coli, as had worked successfully for the plant genes Cnx1 [94], Cnx2, and Cnx3 [95]. Therefore, BLAST N searches were performed in the Expressed Sequence Tag Database (GenBank) to pull the corresponding plant and human genes. Among eukaryotes (Figure 3), genes for MPT synthases were identified in plants, humans [102], and fungi [103]. Interestingly, they showed the same high degree of conservation in the C terminus of the small subunit with a typical double-glycine motif. In plants, the cloning of the small subunit of MPT synthase was achieved in a yeast two-hybrid screen using the assumed protein–protein interaction of the small subunit with the large subunit, exactly as we described this for the small subunit of the E. coli MPT synthase [104]. The result indicates that, in plants, both MPT synthase subunits interact to form MPT synthase. We named the large subunit of MPT synthase Cnx6 and the small subunit Cnx7.
HUMANS: Assuming that Moco biosynthesis is a highly conserved pathway, we used the amino acid sequence of the E. coli MoaE protein and performed a BLAST N search in the Expressed Sequence Tag Database (GenBank), and pulled the corresponding human gene that we named mocs2 [102]. This human gene became even more exciting when we found out that it encodes for two proteins, namely the small and the large subunit of MPT synthase. On the bicistronic messenger RNA, the first reading frame codes for the small subunit MOCS2A and the second for the large subunit MOCS2B. This bicistronic gene disclosed another fascinating aspect because both reading frames overlap and exhibit a frameshift of +1 for mocs2B. Notably, we published this result in the honorable American Journal of Human Genetics, and I assume that we were the first plant biochemists to publish therein.
Bicistronicity ensures colinear expression and implicates the vicinity of the newly synthesized and interacting proteins. Such a micro-compartmentalization is certainly advantageous for low substrate concentrations, as in Moco biosynthesis. However, it remains enigmatic as to why in the human Moco biosynthetic pathway two times the extremely rare case of bicistronicity is found, whereas in higher plants, the corresponding genes are widely separated, and as in the case of step-1 proteins, are even located on different chromosomes. The plant cell needs the vicinity of newly synthesized and interacting proteins, because here, we encounter strong cytoplasmic streaming.
2.9.3. Where Does the Sulfur Come from?
PLANTS and HUMANS: For E. coli, MoeB activates the small subunit of MPT synthase MoaD to form an acyl adenylate at the C–terminus in plants and humans, which is subsequently converted to a thiocarboxylate under the participation of the sulfurtransferase IscS [105,106]. This was the focus of Silke Leimkühler when she was a postdoc in the group of Rajagopalan. We used the MoeB gene sequence as a probe to pull the corresponding plant and human genes and named the encoded proteins Cnx5 and MOCS3, respectively [107]. To our surprise, the plant and human genes had N- and C- terminal extensions. No significant homologies were found for the N-terminal extension and no motifs emerged during database searches. However, the C-terminal extension forms a rhodanese-like domain encoded by a single exon. Rhodaneses are ubiquitous enzymes catalyzing the transfer of a sulfur atom. Thus, we assumed that this two-domain protein combines two functions: the adenylation of Cnx7/MOCS2A by its MoeB-like domain, and subsequent sulfur transfer by its rhodanese domain to form the thiocarboxylate at the C-terminus of Cnx7/MOCS2A. Experimental evidence for this was given by Silke Leimkühler when she joined my lab in 2001 as an independent group leader. She showed that the rhodanese domain of human MOCS3 transfers a sulfur atom to MOCS2A [108,109], and that this thiocarboxylation is of functional importance for humans [110]. Silke left my lab in 2004 to take up a professor position at the University of Potsdam. In summary, evolution combined the two functions “activation” and “sulfur transfer” catalyzed by two separate proteins in E. coli in one protein in eukaryotes.
2.10. Step 3: Formation of Active Moco—The Insertion of MO into MP
In plants, after synthesis of the MPT moiety, the chemical backbone is built for binding and coordinating the Mo atom. Mo is taken up into the cell in the form of molybdate, which is facilitated by high-affinity transport systems (reviewed by Minner-Meinen et al. [111] in this Special Issue). As described above in the paragraph “High molybdate repair”, molybdate-repairable mutants have been known for a long time: Aspergillus CnxE [63], Neurospora nit–9ABC [112,113], and tobacco CnxA [40]. The gene defective in the molybdate-repairable Arabidopsis thaliana mutants is Cnx1. My Ph.D. student, Birgit Stallmeyer, isolated it by functional complementation of the molybdate-repairable E. coli mogA mutants [94]. Surprisingly, the encoded protein Cnx1 was much larger than E. coli MogA. It consists of two domains (Figure 3) where the N-terminus (we named it E-domain) is homologous to E. coli MoeA and the C-terminus (we named it G-domain) is homologous to E. coli MogA. The high functional homology allows Cnx1 or its separately expressed G domain to replace the bacterial MogA function very efficiently [114]. In contrast, the N–terminus of Cnx1 was not able to take over the task of its homologous bacterial protein MoeA.
A protein that acts as Mo insertase should bind both substrates, Mo and MPT, to convert MPT into active Moco. Günter Schwarz, who was a Ph.D. student in my group at that time, purified the two domains of Cnx1 separately and showed a high-affinity interaction between MPT and Cnx1G (Kd = 100 nM); additionally, the Cnx1G domain was able to bind MPT (Kd = 1 µM) [115]. These data suggest a common binding fold for MPT in both domains of Cnx1. After graduation in 1998, Günter stayed in my group and started his own team. To identify functionally important residues within Cnx1G, he performed a mutagenesis screening followed by selection for loss of function using E. coli mogA complementation [116], thus prompting the assumption that MPT binding is not the only function of Cnx1G. Rather, it should activate MPT in an as-yet unknown way [116]. Four years later, with the discovery of MPT–AMP bound and generated by Cnx1G [117], this assumption proved to be correct. Günter also assumed that the E-domain of Cnx1 is essential for activating Mo and inserting it into MPT, thus forming active Moco [116]. Here, our key question was whether molybdate serves as a donor for insertion of Mo into MPT or whether molybdate has to undergo intracellular processing prior to insertion. Günter left my group in 2004 and took up a professor position at the University of Cologne. However, in 2006, we believed that molybdate had to be activated (by ATP) to be ready for insertion in MPT [118]. For a decade, we did not touch Cnx1; then, my former Ph.D. student, Tobias Kruse, who meanwhile had set up his own team in my group, restarted Cnx1 research. His team determined the atomic structure of the E-domain [119] and revised our old model by showing that no activation of molybdate was necessary for insertion into MPT [120,121]. Details are given in Tobias Kruse’s review in this Special Issue.
In 1999, Birgit Stallmeyer was about to graduate in my group. Previously she had cloned and characterized the gene for Cnx1 in humans [94]. While Günter Schwarz continued to elucidate the biochemistry of Cnx1, Birgit became interested in studying human Moco biosynthesis, and she not only identified the bicistronic mocs2 gene [102], but also found the mammalian counterpart to the plant Cnx1 Mo-insertase, the protein Gephyrin. Gephyrin was previously described as a neuroreceptor anchor protein linking glycine receptors in the postsynaptic membrane to the subcellular cytoskeleton [122]. Gephyrin is thought to be an instructive molecule for the formation of glycinergic synapses, and its expression was shown to be essential for the postsynaptic aggregation of glycine receptors [123]. Based on the homology of Gephyrin to Cnx1, we suggested an additional function of Gephyrin in Moco biosynthesis. Birgit showed that recombinant Gephyrin binds MPT with high affinity, heterologous Gephyrin expression could restore Moco biosynthesis in E. coli, plants, and the murine cell line L929, and the inhibition of Gephyrin expression by antisense RNA expression in cultured murine cells significantly reduced their Moco content [124]. In addition, Gephyrin knockout mice did not only show the expected absence of synaptic glycine receptor clustering, but also developed symptoms identical to those of Moco deficiency [125] where no SO activity could be detected. It is obvious that Gephyrin combines two different functions, such as (1) biosynthetic activity in Moco formation and (2) a structural role in receptor clustering. The latter function is evolutionarily younger, and hence must have been recruited from the older in primary metabolism. Notably, in Gephyrin, the orientation of the two domains is reversed—it has the E-domain on its N-terminus and not the G-domain as in Cnx1. The same orientation was seen in the Gephyrin-homologous protein Cinnamon in the fly Drosophila melanogaster [126], and this orientation was also described in the corresponding fungal protein Neurospora crassa Nit-9 [127]. There were two independent evolutionary events, one for the animal lineage in the Gephyrin orientation and one for the plant lineage in the Cnx1 orientation. Interestingly, the Gephyrin linker between the E- and G-domains is much longer than in Cnx1 (134 vs. 11 amino acids). After starting his group at the University of Cologne, Günter Schwarz focused on Gephyrin research. We now know that the long linker in Gephyrin represents a separate domain involved in the modulation of signaling pathways [128]. On another note, there must have been evolutionary pressure to fuse monofunctional bacterial proteins to multifunctional eukaryotic proteins, perhaps to facilitate a better substrate-product channeling [128].
2.11. Cytoskeleton Binding
Gephyrin fulfills its anchoring function for neuroreceptors by linking them via its E-domain to actin filaments of the subcellular cytoskeleton [123]. Gephyrin and its plant homolog Cnx1 exhibit functional properties that are distinct from Moco biosynthesis. Based on the observed cytoskeleton binding of Gephyrin, in vitro experiments demonstrated binding to actin filaments for Cnx1 [129], and I wanted to demonstrate this in the living cell. Robert Hänsch, who conducted his Ph.D. in our group, stayed with me and founded his own team. He set up live-cell imaging using confocal laser scanning microscopy, and later, he confirmed the cytoskeleton binding of Cnx1 in the living cell [130]. In 2000, Günter Schwarz’s finding of cytoskeleton anchoring prompted speculations on whether this might be of functional importance [129]. One reason we saw was the anchoring and stabilization of a putative multienzyme protein complex for Moco-biosynthesis. The other reason was the spatial positioning of Cnx1 next to a putative molybdate importer for sequestering incoming molybdate into molybdate insertase Cnx1. Figure 4 presents our early model published in 2000 [129]. Years later, both assumptions were proven by live-cell imaging in Robert Hänsch’s team [130,131]. In this old model, we assumed that the G-domain was inserting molybdate because mutants in the G-domain could be repaired in vivo by high levels of molybdate. We had not yet discovered MPT–AMP on the G-domain (which was only in 2004)—nowadays, we know that it is the E-domain that catalyzes the insertion of molybdate into MPT supplied by the G-domain in the form of MPT-AMP. Robert Hänsch’s team found that Cnx5, Cnx6/7, and Cnx1 (catalyzing steps 2 and 3) undergo tight protein–protein interactions in the cytosol of living plant cells [132], thus supporting the idea of channeling the fragile Moco intermediates in a protected way within a transient multiprotein complex. Furthermore, Cnx1 also makes protein–protein contact with the molybdate transporter in the living cell. Details are summarized in the paper by Minner-Meinen et al. [111] in this Special Issue.
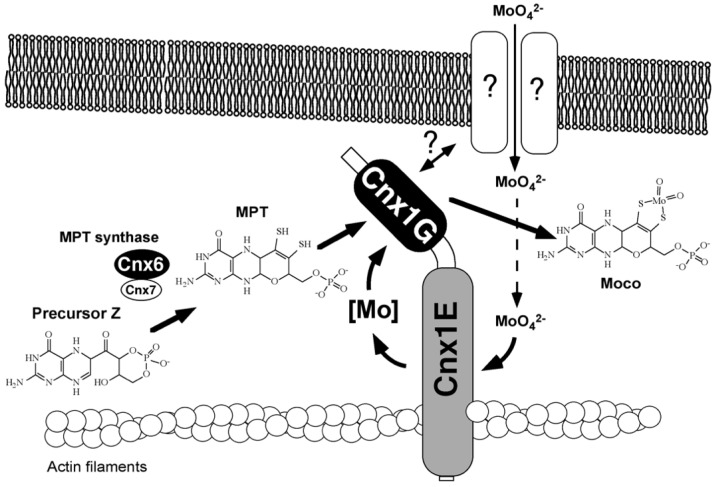
Figure 4.
Model for the Moco biosynthesis pathway in plants, published by Schwarz et al. in the year 2000 [129]. Cnx1 is shown to be bound to an actin filament. An unidentified molybdate transport system was proposed that interacts with Cnx1 to facilitate molybdate channeling to the E domain, given that a mutation in this protein results in a molybdate-repairable phenotype. The conversion of precursorZ cPMP) to MPT by the MPT synthase (Cnx6 and Cnx7) is shown. MPT is highly sensitive to oxidation; therefore, it was suggested that the rapid conversion of precursorZ to Moco should occur in a multienzyme complex anchored by Cnx1 on the cytoskeleton. Modified after Schwarz et al. [129].
2.12. Bacteria Have Additional forms of Moco
As first detected by Krüger and Meyer in 1986 [133,134], bacteria can carry various substituents at the C4′ atom of the side chain of Moco. Johnson et al. [135] showed that the Moco from DMSO reductase from Rhodobacter sphaeroides consists of an MPT guanine dinucleotide where a GMP is bound to the C4′ atom via a pyrophosphate bond. Other prokaryotic variants of the cofactor containing CMP, AMP, or IMP linked to the MPT were also identified [81]. These dinucleotide forms were only found in prokaryotes. Bacteria may even contain both Moco forms, because it was found that XDH from Rhodobacter capsulatus and Pseudomonas putida contains the MPT form of the cofactor, whereas the other Mo enzymes (NR, DMSO reductase, and chinoline oxidoreductase) contain the dinucleotide form [8,136]. Another difference between bacterial and eukaryotic Moco biosynthesis is the formation of bis-MPT-based cofactors in bacteria where two dithiolenes of two MPT molecules coordinate one Mo (or W) atom. Details of the role of these nucleotide attachments to MPT are discussed by Tiedemann et al. [52] in this Special Issue. For a comprehensive review of Moco biosynthesis and insertion into Mo enzymes, see Magalon and Mendel [137].
2.13. Terminal Sulfuration of Moco
As a final step of maturation, the eukaryotic xanthine dehydrogenase and aldehyde oxidase as enzymes with a mono-oxo Mo center require the addition of a terminal inorganic sulfide to the Mo site. This sulfur ligand does not originate from the apoprotein, nor does it come from the Moco moiety. For rats and flies, the early work of Wahl and Rajagopalan [42] demonstrated that, in vitro, this sulfur can be spontaneously lost or can be removed from the enzymes by cyanide treatment, generating an inactive enzyme. The reaction, however, is reversible, and the enzyme can be reactivated by sulfide treatment under reducing conditions. In vivo, this terminal sulfur has to be added by a separate enzymatic reaction catalyzed by a Moco sulfurase (Figure 5). Moco sulfurase activities were earlier described for Drosophila melanogaster Ma–l [138], the fungus A. nidulans HxB [139], cattle [140], humans [141], and plants (ABA3 [142]). The N-terminus of these proteins shares significant homologies with the bacterial sulfur transferase NifS. Mutants defective in this Moco sulfurase were lacking both xanthine dehydrogenase and aldehyde oxidase activities, but retained their activities of enzymes with a dioxo Mo center, such as NR in plants [139,141] and SO in cattle and insects [138]. In both the fly ma–l and the fungal hxB genes, intragenic complementation was observed [143], demonstrating that the N-terminal NifS-like domain and the C-terminal domain function independently within the oligomeric sulfurase protein. In 2001, my Ph.D. student Florian Bittner cloned and characterized the plant Moco sulfurase ABA3 from Arabidopsis thaliana [144]. He characterized the N-terminal domain as cysteine desulfurase. He showed in a defined in vitro system that purified ABA3 was able to activate aldehyde oxidase, using L-cysteine as a sulfur donor. Thus, we suggested that the C-terminal domain recognizes the target enzyme and catalyzes a trans-sulfuration reaction to introduce sulfur into Moco. As a postdoc, Florian set up his own team in my group and determined the mechanism of ABA3-catalyzed Moco sulfuration (as summarized in [145]).
Figure 5.
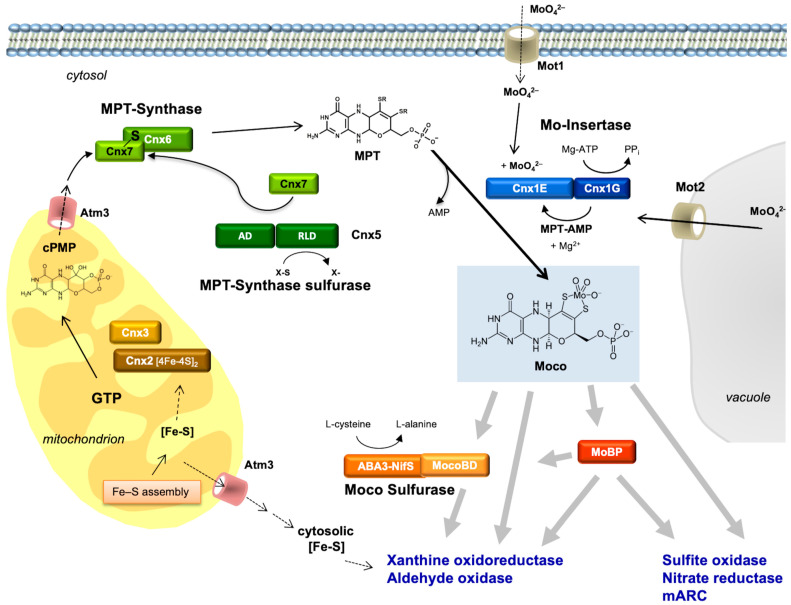
Present model for the biosynthesis, distribution, and maturation of Moco in plant cells. Biosynthesis starts with the conversion of GTP to cPMP in the mitochondria. The dependence of Cnx2 on [Fe–S] is indicated. The transporter Atm3 is assumed to be involved in the export of cPMP to the cytosol [97], where MPT-synthase, consisting of Cnx6 and Cnx7, is sulfurated by Cnx5, with the primary sulfur donor of Cnx5 (X-S) being unknown. The individual reactions of the Mo insertase Cnx1 and its products (Moco, pyrophosphate, and AMP) are indicated. Molybdate for this reaction is supplied by the vacuolar exporter Mot2 (a Mot2-mutant leads to a shortage of molybdate in the cytosol and the accumulation of molybdate in the vacuole) [146]. Later, Mot2 was renamed as Mot1.2 [111]. The Mot1-transporter is the cellular Mo-importer and resides in the plasma membrane [111]. Mature Moco can be either bound to a Moco-binding protein (MoBP) or directly to the Mo enzymes. The Moco sulfurase ABA3 generates a protein-bound persulfide, which is the source of the terminal sulfur ligand of Moco enzymes in the XDH/AO family. Similarly to Cnx2, xanthine dehydrogenase and aldehyde oxidase also depend on [Fe–S] from mitochondria. Modified after Mendel and Kruse [93].
2.14. Moco Carrier Proteins
Free Moco is sensitive to oxidation; therefore, it was assumed that Moco permanently occurs as protein-bound in the cell. We assumed that either apo-Mo enzymes connect directly to Cnx1-E to receive Moco, or Moco has to become bound to a carrier protein that protects and stores Moco until further use, thus providing a way to buffer the supply and demand of Moco. Therefore, a cellular Moco distribution system should meet two demands: (i) it should bind Moco subsequent to its synthesis, and (ii) it should allocate Moco from the Moco donor Cnx1E to the Moco-dependent enzymes. In 1992, a Moco carrier protein (CrMCP) was identified in the green alga Chlamydomonas reinhardtii, which was found to tightly bind Moco, thus protecting it against oxidation [147]. We showed that without any denaturing procedure, the subsequent transfer of Moco from CrMCP to apo-nitrate reductase from Neurospora crassa was possible. These properties of Chlamydomonas MCP made it a promising candidate for being part of a cellular Moco delivery system. In 2006, Günter Schwarz’s team solved the crystal structure of CrMCP. It is a homotetramer with each 16 kDa monomer binding one molecule of Moco and possessing a typical Rossmann fold [148]. However, this crystal structure was empty, i.e., it had no co-structure with Moco. One decade later, in 2016, Jörn Krausze, a crystallographer who joined my group’s team of Tobias Kruse, tried hard to co-crystallize CrMCP with Moco; however, was also unsuccessful. We could solve the co-structure of the MCP with Moco after switching from Chlamydomonas to another green algae (Volvox carterii). Details are given in the review by Tobias Kruse in this Special Issue. Tobias Kruse not only investigated Mo insertion into MPT (see chapter above), but in 2010, during his Ph.D. time in my group, discovered a novel protein family in the plant Arabidopsis thaliana consisting of eight members that can all bind Moco, which are therefore named Moco-binding proteins (MoBPs) [149]. These proteins bind Moco less tightly than Chlamydomonas MCP and are therefore no good candidates to serve as Moco storage proteins. Rather, they seem to be involved in the cellular distribution of Moco.
In summary, these carrier proteins only occur in some bacteria, algae, and in higher plants, but not in fungi and mammals. None of them was identified via a selectable loss-of-function mutant phenotype. Hence, their function is not mandatory for Mo enzyme maturation. Instead, they may contribute to cellular Moco allocation.
2.15. Moco Deficiency in Humans Is Lethal
Moco deficiency is a rare recessive inborn error of metabolism first described by Duran in 1978 [150], resulting in a complete loss of activities of all Mo enzymes, which is lethal for the patient. After cloning all human Moco biosynthesis genes, Günter Schwarz and I decided to develop a Moco replacement therapy for this devastating disease. Moco itself and MPT were exceedingly unstable, but cPMP was sufficiently stable [98]. Therefore, we joined forces with the geneticist Jochen Reiss from Göttingen University, who had performed molecular patient analysis and had shown that type A patients exhibit a mutation in mocs1 and are unable to synthesize cPMP. These patients are most prevalent. Type B patients accumulate cPMP due to a mutation either in mocs2 or mocs3 (resulting in the loss of MPT synthase activity). Type C patients are extremely rare and carry a mutation in gephyrin [151]. These genetic analyses confirmed prior biochemical characterizations of mutant cell lines by Vivian Shih. In 1989, she made a breakthrough observation that became the compass for our own research strategy. Vivian Shih used cell cultures of numerous Moco-deficient patients and biochemically defined two groups of patients. Group A cells were unable to synthesize cPMP; group B cells could not convert cPMP into MPT. Most excitingly, she co-cultured cells from groups A and B and observed the reappearance of sulfite oxidase activity. She concluded that “a relatively stable diffusible molybdopterin precursor produced by group B cells and utilized by group A cells to synthesize active molybdenum cofactor is identified, and evidence is presented which suggests that this diffusible intermediate is identical to a molybdopterin precursor…” [152]. Thus, cPMP was excreted from one type of cells and taken up by the other type. This was the first and most important indication that replacement therapy with cPMP should be feasible. However, in my laboratory, Günter Schwarz’s team developed a mouse model for step 1 Moco deficiency resembling the patient phenotype. We successfully cured these mice with an intrahepatic injection of cPMP, restoring Mo enzyme activity and lifespan extension [153]. cPMP was purified from an overproducing E. coli strain that they had developed. After Günter left my group, he focused on the clinical translation of this therapy. His first patient therapy was reported in 2010 [154]. For details of this endeavor, see his review in this Special Issue. In 2021, the FDA approved cPMP therapy, and chemically synthesized cPMP is marketed under the name Nulibry.
2.16. Is There Life without Moco?
All higher organisms possess Mo enzymes. However, genome-wide database analyses revealed a significant number of bacteria and unicellular lower eukaryotes (yeasts, fungi, and protists) that do not need Mo. This suggests that Mo utilization is an ancient trait and was once common to almost all species in the three domains of life. As a matter of fact, Mo utilization was already present in LUCA, the last universal common ancestor of life [3]. Large-scale comparative genome analyses revealed that among eukaryotes, about 30% of all species had lost Mo utilization. They were mainly unicellular yeasts, fungi, and protists [155]. It turned out that there was a high correlation between the loss of Mo utilization and habitat and lifestyle [156]. The majority of obligate intracellular parasites and symbionts lost the ability to use Mo and exhibited condensed (i.e., shortened) genomes, which are associated with their intracellular parasitic life and the evolutionary pressure on genome size—nevertheless, these organisms may still use Mo-dependent enzyme functions of their host. All multi-cellular eukaryotes are dependent on Mo [157]. Model yeasts such as Saccharomyces cerevisiae and Schizosaccharomyces pombe played no role in Mo research, because they belong to those organisms that do not contain Mo enzymes. In contrast, other yeasts such as Pichia pastoris do need Moco. However, there is an indication that Saccharomyces cerevisiae should have possessed Moco biosynthesis, because a MoeB-homologous protein sequence occurs in the highly conserved ubiquitin-activating enzyme E1 (Uba4) in eukaryotes using the same adenylation mechanism as in MoeB. This finding suggests that protein E1 derived from the ancestral gene moeB [158] which, in Saccharomyces cerevisiae, was lost along with all other members of Mo metabolism during evolution.
3. Conclusions
Moco is an ancient invention of nature. The structure of MPT as the organic moiety of Moco is highly conserved. In bacteria, a nucleotide is attached to MPT and MPT can form dimers. Moco occurs ubiquitously—except for a number of bacteria, fungi, and unicellular yeasts that have lost Mo metabolism. During the evolution from prokaryotes to eukaryotes, the pterin core structure of Moco has not changed. Accordingly, its biosynthetic pathway is conserved, and diverse genome projects have revealed a similar set of Moco genes in bacteria, archaea, fungi, flies, plants, and mammals. However, there are also some notable differences between prokaryotes and eukaryotes. During evolution, the genes of single monofunctional prokaryotic proteins have been fused in some cases to form two-domain multifunctional eukaryotic proteins, as in Arabidopsis Cnx1 and animal Gephyrin (comprising E. coli MogA and MoeA). Furthermore, the dinucleotide form of Moco has not been found in eukaryotes. Another difference resides in the complex morphology and size of a eukaryotic cell with its several compartments, thus making adaptations of the Moco biosynthetic pathway inevitable, as in the case of the cytoskeleton binding.
For all Moco-using organisms, Moco is essential, and a defective Moco has detrimental or lethal consequences due to the pleiotropic loss of all Mo–enzymes. To this end, the single steps of Moco biosynthesis were uncovered in great detail, but the regulation of this pathway, as well as the steps how Moco is allocated and inserted into the apoproteins, are fascinating topics of ongoing research in several laboratories working with mammals, plants, fungi, nematodes, algae, and bacteria.
In 1974, I started studying Moco, and in 2021, I retired from my administrative and teaching duties, and I continue conducting Moco research. In collaboration with the young professor Kurt Warnhoff (USA), we study the unique case of the nematode C. elegans, which is able to take up protein-bound Moco from its diet and distribute it through its body until Moco reaches sulfite oxidase, where it becomes inserted into this essential enzyme [159,160]. Kurt discovered this phenomenon, and I contribute our biochemical knowledge to his project. Novel but still hidden mechanisms of Moco biology remain to be uncovered in this exciting emeritus endeavor into uncharted land.
Acknowledgments
I am very grateful to all my postdocs, Ph.D. students, and undergraduate students for their dedicated and enthusiastic work through the years. My thanks also go to the many colleagues in our community for their inspiring discussions and experimental collaborations. I apologize to all those colleagues whose work I have not mentioned in this personal review. My thanks also go to Kevin Oliphant for help with the figures.
Conflicts of Interest
The author declares no conflict of interest.
Funding Statement
I gratefully acknowledge the continuous financial support from the Deutsche Forschungsgemeinschaft through the years 1994–2022 (Me1266).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hille R., Hall J., Basu P. The mononuclear molybdenum enzymes. Chem. Rev. 2014;114:3963–4038. doi: 10.1021/cr400443z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoepp-Cothenet B., van Lis R., Philippot P., Magalon A., Russell M.J., Nitschke W. The ineluctable requirement for the trans-iron elements molybdenum and/or tungsten in the origin of life. Sci. Rep. 2012;2:263. doi: 10.1038/srep00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss M.C., Preiner M., Xavier J.C., Zimorski V., Martin W.F. The last universal common ancestor between ancient Earth chemistry and the onset of genetics. PLoS Genet. 2018;14:e1007518. doi: 10.1371/journal.pgen.1007518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pau R.N., Lawson D.M. Transport, homeostasis, regulation, and binding of molybdate and tungstate to proteins. Met. Ions Biol. Syst. 2002;39:31–74. [PubMed] [Google Scholar]
- 5.Hille R., Nishino T., Bittner F. Molybdenum enzymes in higher organisms. Coord. Chem. Rev. 2011;255:1179–1205. doi: 10.1016/j.ccr.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magalon A., Fedor J.G., Walburger A., Weiner J.H. Molybdenum enzymes in bacteria and their maturation. Coord. Chem. Rev. 2011;255:1159–1178. doi: 10.1016/j.ccr.2010.12.031. [DOI] [Google Scholar]
- 7.Bevers L.E., Hagedoorn P.-L., Hagen W.R. The bioinorganic chemistry of tungsten. Coord. Chem. Rev. 2009;253:269–290. doi: 10.1016/j.ccr.2008.01.017. [DOI] [Google Scholar]
- 8.Leimkuhler S., Wuebbens M.M., Rajagopalan K.V. The History of the Discovery of the Molybdenum Cofactor and Novel Aspects of its Biosynthesis in Bacteria. Coord. Chem. Rev. 2011;255:1129–1144. doi: 10.1016/j.ccr.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spitzer W. Überführung von Nucleïnbasen in Harnsäure durch die sauerstoffübertragende Wirkung von Gewebsauszügen. Pflüg. Arch. 1899;76:192–203. doi: 10.1007/BF01662075. [DOI] [Google Scholar]
- 10.Schardinger F. Über das Verhalten der Kuhmilch gegen Methylenblau und seine Verwendung zur Unterscheidung von ungekochter und gekochter Milch. Z. Unters. Nahr. Genussm. 1902;5:1113–1121. doi: 10.1007/BF02506750. [DOI] [Google Scholar]
- 11.Morgan E.J., Stewart C.P., Hopkins F.G. On the anaerobic and aerobic oxidation of xanthin and hypoxanthin by tissues and by milk. Proc. R. Soc. Lond. B. 1922;94:109–131. [Google Scholar]
- 12.Richert D.A., Westerfeld W.W. A bioassay procedure for the dietary factor related to xanthine oxidase. J. Biol. Chem. 1951;192:49–56. doi: 10.1016/S0021-9258(18)55905-6. [DOI] [PubMed] [Google Scholar]
- 13.Richert D.A., Westerfeld W.W. Isolation and identification of the xanthine oxidase factor as molybdenum. J. Biol. Chem. 1953;203:915–923. doi: 10.1016/S0021-9258(19)52361-4. [DOI] [PubMed] [Google Scholar]
- 14.Renzo E.C.D., Kaleita E., Heytler P., Oleson J.J., Hutchings B.L., Williams J.H. The Nature of the Xanthine Oxidase Factor. J. Am. Chem. Soc. 1953;75:753. doi: 10.1021/ja01099a515. [DOI] [Google Scholar]
- 15.Mahler H.R., Mackler B., Green D.E. Studies on metallo-flavoproteins. III. Aldehydeoxidase: A molybdoflavoprotein. J. Biol. Chem. 1954;210:465–480. doi: 10.1016/S0021-9258(18)65471-7. [DOI] [PubMed] [Google Scholar]
- 16.Nicholas D.J.D., Nason A.J. Molybdenum and nitrate reductase. II. Molybdenum as a constituent of nitrate reductase. J. Biol. Chem. 1954;207:353–360. doi: 10.1016/S0021-9258(18)71275-1. [DOI] [PubMed] [Google Scholar]
- 17.Cohen H.J., Betcher-Lange S., Kessler D.L., Rajagopalan K.V. Hepatic sulfite oxidase. Congruency in mitochondria of prosthetic groups and activity. J. Biol. Chem. 1972;247:7759–7766. doi: 10.1016/S0021-9258(19)44588-2. [DOI] [PubMed] [Google Scholar]
- 18.Bortels H. Molybdän als Katalysator bei der biologischen Stickstoffbindung. Arch. Mikrobiol. 1930;1:333–342. doi: 10.1007/BF00510471. [DOI] [Google Scholar]
- 19.Ter Meulen H. Distribution of molybdenum. Nature. 1932;130:966. doi: 10.1038/130966b0. [DOI] [Google Scholar]
- 20.Pateman J.A., Cove D.J., Rever B.M., Roberts D.B. A common cofactor for nitrate reductase and xanthine dehydrogenase wich also regulates the synthesis of nitrate reductase. Nature. 1964;201:58–60. doi: 10.1038/201058a0. [DOI] [PubMed] [Google Scholar]
- 21.Cove D.J., Pateman J.A. Independently segregating genetic loci concerned with nitrate reductase activity in Aspergillus nidulans. Nature. 1963;198:262–263. doi: 10.1038/198262a0. [DOI] [PubMed] [Google Scholar]
- 22.Mendel R.R., Müller A.J. A common genetic determinant of xanthine dehydrogenase and nitrate reductase in Nicotiana tabacum. Biochem. Physiol. Pflanz. 1976;170:538–541. doi: 10.1016/S0015-3796(17)30254-8. [DOI] [Google Scholar]
- 23.Warner C.K., Finnerty V. Molybdenum hydroxylases in Drosophila. II Molybdenum cofactor in xanthine dehydrogenase, aldehyde oxidase and pyridoxal oxidase. Mol. Gen. Genet. 1981;184:92–96. doi: 10.1007/BF00271201. [DOI] [PubMed] [Google Scholar]
- 24.Crawford N.M. Study of chlorate resistant mutants of Arabidopsis: Insights into nitrate assimilation and ion metabolism of plants. In: Setlow J.K., editor. Genetic Engineering, Principles and Methods. Volume 14. Plenum Press; New York, NY, USA: 1992. pp. 89–98. [Google Scholar]
- 25.Puig J., Azoulay E., Pichinoty F. Etude genetique dune mutation a effet pleiotrope chez l’Escherichia coli K12. Compt. Rend. Hebd. Sean. Acad. Sci. Ser. D. 1967;264:1507. [PubMed] [Google Scholar]
- 26.Glaser J.H., DeMoss J.A. Comparison of nitrate reductase mutants of Escherichia coli selected by alternative procedures. Mol. Gen. Genet. 1972;116:1–10. doi: 10.1007/BF00334254. [DOI] [PubMed] [Google Scholar]
- 27.Stewart V., MacGregor C.H. Nitrate reductase in Escherichia coli K-12: Involvement of chlC, chlE, and chlG loci. J. Bacteriol. 1982;151:788–799. doi: 10.1128/jb.151.2.788-799.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shanmugam K.T., Stewart V., Gunsalus R.P., Boxer D.H., Cole J.A., Chippaux M., DeMoss J.A., Giordano G., Lin E.C., Rajagopalan K.V. Proposed nomenclature for the genes involved in molybdenum metabolism in Escherichia coli and Salmonella typhimurium. Mol. Microbiol. 1992;6:3452–3454. doi: 10.1111/j.1365-2958.1992.tb02215.x. [DOI] [PubMed] [Google Scholar]
- 29.Sorger G.J., Giles N.H. Genetic control of nitrate reductase in Neurospora crassa. Genetics. 1965;52:777–788. doi: 10.1093/genetics/52.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ketchum P.A., Cambier H.Y., Frazier W.A., 3rd, Madansky C.H., Nason A. In vitro assembly of Neurospora assimilatory nitrate reductase from protein subunits of a Neurospora mutant and the xanthine oxidizing or aldehyde oxidase systems of higher animals. Proc. Natl. Acad. Sci. USA. 1970;66:1016–1023. doi: 10.1073/pnas.66.3.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nason A., Antoine A.D., Ketchum P.A., Frazier W.A., 3rd, Lee D.K. Formation of assimilatory nitrate reductase by in vitro inter-cistronic complementation in Neurospora crassa. Proc. Natl. Acad. Sci. USA. 1970;65:137–144. doi: 10.1073/pnas.65.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nason A., Lee K.Y., Pan S.S., Ketchum P.A., Lamberti A., DeVries J. In vitro formation of assimilatory reduced nicotinamide adenine dinucleotide phosphate: Nitrate reductase from a Neurospora mutant and a component of molybdenum-enzymes. Proc. Natl. Acad. Sci. USA. 1971;68:3242–3246. doi: 10.1073/pnas.68.12.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pienkos P.T., Shah V.K., Brill W.J. Molybdenum cofactors from molybdoenzymes and in vitro reconstitution of nitrogenase and nitrate reductase. Proc. Natl. Acad. Sci. USA. 1977;74:5468–5471. doi: 10.1073/pnas.74.12.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah V.K., Brill W.J. Isolation of an iron-molybdenum cofactor from nitrogenase. Proc. Natl. Acad. Sci. USA. 1977;74:3249–3253. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nason A., Lee K.Y., Pan S.S., Ericson R.H. Evidence for a molybdenum cofactor common to all molybdenum enzymes based on the in vitro assembly of assimilatory NADPH-nitrate reductase using the Neurospora mutant nit-1. J. Less Common Met. 1974;36:449–459. doi: 10.1016/0022-5088(74)90129-5. [DOI] [Google Scholar]
- 36.Mendel R.R. Release of molybdenum co-factor from nitrate reductase and xanthine oxidase by heat treatment. Phytochemistry. 1983;22:817–819. doi: 10.1016/0031-9422(83)85004-3. [DOI] [Google Scholar]
- 37.Johnson J.L., Hainline B.E., Rajagopalan K.V. Characterization of the molybdenum cofactor of sulfite oxidase, xanthine, oxidase, and nitrate reductase. Identification of a pteridine as a structural component. J. Biol. Chem. 1980;255:1783–1786. doi: 10.1016/S0021-9258(19)85945-8. [DOI] [PubMed] [Google Scholar]
- 38.Hawkes T.R., Bray R.C. Quantitative transfer of the molybdenum cofactor from xanthine oxidase and from sulphite oxidase to the deficient enzyme of the nit-1 mutant of Neurospora crassa to yield active nitrate reductase. Biochem. J. 1984;219:481–493. doi: 10.1042/bj2190481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Spanning R.J.M., Wansell-Bettenhaussen C.W., Oltmann L.F., Stouthamer A.H. Extraction and purification of molybdenum cofactor from milk xanthine oxidase. Eur. J. Biochem. 1987;169:349–352. doi: 10.1111/j.1432-1033.1987.tb13618.x. [DOI] [PubMed] [Google Scholar]
- 40.Mendel R.R., Alikulov Z.A., Lvov N.P., Müller A.J. Presence of the molybdenum-cofactor in nitrate reductase-deficient mutant cell lines of Nicotiana tabacum. Mol. Gen. Genet. 1981;181:395–399. doi: 10.1007/BF00425618. [DOI] [Google Scholar]
- 41.Mendel R.R., Buchanan R.J., Wray J.L. Characterization of a new type of molybdenum cofactor-mutant in cell cultures of Nicotiana tabacum. Mol. Gen. Genet. 1984;195:186–189. doi: 10.1007/BF00332744. [DOI] [Google Scholar]
- 42.Wahl R.C., Hageman R.V., Rajagopalan K.V. The relationship of Mo, molybdopterin, and the cyanolyzable sulfur in the Mo cofactor. Arch. Biochem. Biophys. 1984;230:264–273. doi: 10.1016/0003-9861(84)90107-3. [DOI] [PubMed] [Google Scholar]
- 43.Hagemann R.V., Rajagopalan K.V. Assay and detection of the molybdenum cofactor. Methods Enzymol. 1986;122:399–412. doi: 10.1016/0076-6879(86)22200-4. [DOI] [PubMed] [Google Scholar]
- 44.Alikulov Z.A., Mendel R.R. Molybdenum cofactor from tobacco cell cultures and milk xanthine oxidase: Involvement of sulfhydryl groups in dimerization activity of cofactor. Biochem. Physiol. Pflanz. 1984;179:693–705. doi: 10.1016/S0015-3796(84)80026-8. [DOI] [Google Scholar]
- 45.Ringel P., Krausze J., van den Heuvel J., Curth U., Pierik A.J., Herzog S., Mendel R.R., Kruse T. Biochemical characterization of molybdenum cofactor-free nitrate reductase from Neurospora crassa. J. Biol. Chem. 2013;288:14657–14671. doi: 10.1074/jbc.M113.457960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hercher T.W., Krausze J., Hoffmeister S., Zwerschke D., Lindel T., Blankenfeldt W., Mendel R.R., Kruse T. Insights into the Cnx1E catalyzed MPT-AMP hydrolysis. Biosci. Rep. 2020;40:BSR20191806. doi: 10.1042/BSR20191806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajagopalan K.V., Johnson J.L., Hainline B.E. The pterin of the molybdenum cofactor. Fed. Proc. 1982;41:2608–2612. doi: 10.1016/S0021-9258(19)50001-1. [DOI] [PubMed] [Google Scholar]
- 48.Koschara W. Urothion, a yellow sulfur-rich pigment from human urine. Hoppe-Seylers Z. Physiol. Chem. 1940;263:78. doi: 10.1515/bchm2.1940.263.1-2.78. [DOI] [Google Scholar]
- 49.Goto M., Sakurai A., Ota K., Yamakami H. Structure of urothion. J. Biochem. 1969;65:611–620. doi: 10.1093/oxfordjournals.jbchem.a129054. [DOI] [PubMed] [Google Scholar]
- 50.Johnson J.L., Rajagopalan K.V. Structural and metabolic relationship between the molybdenum cofactor and urothione. Proc. Natl. Acad. Sci. USA. 1982;79:6856–6860. doi: 10.1073/pnas.79.22.6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romao M.J., Archer M., Moura I., Moura J.J., LeGall J., Engh R., Schneider M., Hof P., Huber R. Crystal structure of the xanthine oxidase-related aldehyde oxido-reductase from D. gigas. Science. 1995;270:1170–1176. doi: 10.1126/science.270.5239.1170. [DOI] [PubMed] [Google Scholar]
- 52.Tiedemann K., Iobbi-Nivol C., Leimkuhler S. The Role of the Nucleotides in the Insertion of the bis-Molybdopterin Guanine Dinucleotide Cofactor into apo-Molybdoenzymes. Molecules. 2022;27:2993. doi: 10.3390/molecules27092993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kisker C., Schindelin H., Rees D.C. Molybdenum-cofactor-containing enzymes: Structure and mechanism. Annu. Rev. Biochem. 1997;66:233–267. doi: 10.1146/annurev.biochem.66.1.233. [DOI] [PubMed] [Google Scholar]
- 54.Kisker C., Schindelin H., Pacheco A., Wehbi W.A., Garrett R.M., Rajagopalan K.V., Enemark J.H., Rees D.C. Molecular basis of sulfite oxidase deficiency from the structure of sulfite oxidase. Cell. 1997;91:973–983. doi: 10.1016/S0092-8674(00)80488-2. [DOI] [PubMed] [Google Scholar]
- 55.Fischer K., Barbier G.G., Hecht H.-J., Mendel R.R., Campbell W.H., Schwarz G. Crystal Structure of the Yeast Nitrate Reductase Molybdenum Domain Provides Insight into Eukaryotic Nitrate Assimilation. Plant Cell. 2005;17:1167–1179. doi: 10.1105/tpc.104.029694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCleverty J.A. Metal 1,2-Dithiolene and Related Complexes. Prog. Inorg. Chem. 1968;10:49. [Google Scholar]
- 57.Holm R.H., Solomon E.I., Majumdar A., Tenderholdt A. Comparative molecular chemistry of molybdenum and tungsten and its relation to hydroxylase and oxotransferase enzymes. Coord. Chem. Rev. 2011;255:993–1015. doi: 10.1016/j.ccr.2010.10.017. [DOI] [Google Scholar]
- 58.Basu P., Burgmayer S.J. Pterin chemistry and its relationship to the molybdenum cofactor. Coord. Chem. Rev. 2011;255:1016–1038. doi: 10.1016/j.ccr.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bradshaw B., Dinsmore A., Ajana W., Collison D., Garner C.D., Joule J.A. Synthesis of the organic ligand of the molybdenum cofactor, in protected form. J. Chem. Soc. Perk. Trans. 2001;1:24. [Google Scholar]
- 60.Gisewhite D.R., Nagelski A.L., Cummins D.C., Yap G.P.A., Burgmayer S.J.N. Modeling Pyran Formation in the Molybdenum Cofactor: Protonation of Quinoxalyl-Dithiolene Promoting Pyran Cyclization. Inorg. Chem. 2019;58:5134–5144. doi: 10.1021/acs.inorgchem.9b00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clinch K., Watt D.K., Dixon R.A., Baars S.M., Gainsford G.J., Tiwari A., Schwarz G., Saotome Y., Storek M., Belaidi A.A., et al. Synthesis of cyclic pyranopterin monophosphate, a biosynthetic intermediate in the molybdenum cofactor pathway. J. Med. Chem. 2013;56:1730–1738. doi: 10.1021/jm301855r. [DOI] [PubMed] [Google Scholar]
- 62.Colston K.J., Basu P. Synthesis, Redox and Spectroscopic Properties of Pterin of Molybdenum Cofactors. Molecules. 2022;27:3324. doi: 10.3390/molecules27103324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cove D.J. Genetic studies of nitrate assimilation in Aspergillus nidulans. Biol. Rev. 1979;54:291–327. doi: 10.1111/j.1469-185X.1979.tb01014.x. [DOI] [PubMed] [Google Scholar]
- 64.Tomsett A.B., Garrett R.H. The isolation and characterization of mutants defective in nitrate assimilation in Neurospora crassa. Genetics. 1980;95:649–660. doi: 10.1093/genetics/95.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glaser J.H., DeMoss J.A. Phenotypic restoration by molybdate of nitrate reductase activity in chlD mutants of Escherichia coli. J. Bacteriol. 1971;108:854–860. doi: 10.1128/jb.108.2.854-860.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Riviere C., Giordano G., Pommier J., Azoulay E. Membrane reconstitution in chl-r mutants of Escherichia coli K 12. Biochim. Biophys. Acta-Biomembr. 1975;389:219–235. doi: 10.1016/0005-2736(75)90317-X. [DOI] [PubMed] [Google Scholar]
- 67.Grillet L., Giordano G. Identification and purification of a protein involved in the activation of Nitrate reductase in the soluble fraction of a chlA mutant of Escherichia coli K12. Biochim. Biophys. Acta-Protein Struct. Mol. Enzymol. 1983;749:115–124. doi: 10.1016/0167-4838(83)90158-9. [DOI] [PubMed] [Google Scholar]
- 68.Amy N.K. Identification of the molybdenum cofactor in chlorate-resistant mutants of Escherichia coli. J. Bacteriol. 1981;148:274–282. doi: 10.1128/jb.148.1.274-282.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller J.B., Amy N.K. Molybdenum cofactor in chlorate-resistant and nitrate reductase-deficient insertion mutants of Escherichia coli. J. Bacteriol. 1983;155:793–801. doi: 10.1128/jb.155.2.793-801.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mueller A.J.G., Mendel R.R. Isolation and characterization of cell lines of Nicotiana tabacum lacking nitrate reductase. Mol. Gen. Genet. 1978;161:67–76. doi: 10.1007/BF00266616. [DOI] [Google Scholar]
- 71.Mendel R.R., Müller A.J. Reconstitution of NADH-nitrate reductase in vitro from nitrate reductase-deficient Nicotiana tabacum mutants. Mol. Gen. Genet. 1978;161:77–80. doi: 10.1007/BF00266617. [DOI] [Google Scholar]
- 72.Mendel R.R., Müller A. Nitrate reductase-deficient mutant cell lines of Nicotiana tabacum. Further biochemical characterization. Mol. Gen. Genet. 1979;177:145–153. doi: 10.1007/BF00267264. [DOI] [Google Scholar]
- 73.Mendel R.R., Kirk D.W., Wray J.L. Assay of molybdenum cofactor of barley. Phytochemistry. 1985;24:1631–1634. doi: 10.1016/S0031-9422(00)82524-8. [DOI] [Google Scholar]
- 74.Mendel R.R., Marton L., Müller A.J. Comparative biochemical charakterization of nitrate reductase/molybdenum cofactor loci cnxA, cnxB and cnxC of Nicotiana plumbaginifolia. Plant Sci. 1986;43:125–129. doi: 10.1016/0168-9452(86)90152-4. [DOI] [Google Scholar]
- 75.De Fries S.E., Dirks R., Mendel R.R., Schaart J.G., Feenstra W.J. Biochemical characterization of nitrate reductase deficient mutants of Nicotiana plumbaginifolia. Plant Sci. 1986;44:105–110. doi: 10.1016/0168-9452(86)90078-6. [DOI] [Google Scholar]
- 76.Mendel R.R., Alikulov Z.A., Müller A.J. Molybdenum cofactor in nitrate reductase-deficient tobacco mutants. III. Induction of cofactor synthesis by nitrate. Plant Sci. Lett. 1982;27:95–101. doi: 10.1016/0304-4211(82)90076-1. [DOI] [Google Scholar]
- 77.Gabard J., Pelsy F., Marion-Poll A., Caboche M., Saalbach I., Grafe R., Müller A.J. Genetic analysis of nitrate reductase deficient mutants of Nicotiana plumbaginifolia: Evidence for six complementation groups among 70 classified molybdenum cofactor deficient mutants. Mol. Gen. Genet. 1988;213:275–281. doi: 10.1007/BF00339583. [DOI] [Google Scholar]
- 78.Müller A.J., Mendel R.R. Biochemical and somatic cell genetics of nitrate reductase in Nicotiana. In: Wray J.L., Kinghorn J.R., editors. Molecular and Genetic Aspects of Nitrate Assimilation. Oxford University Press; Oxford, UK: 1989. pp. 166–185. [Google Scholar]
- 79.Fernandez E., Cardenas J. Genetics and regulatory aspects of nitrate assimilation in algae. In: Wray J.L., Kinghorn J.R., editors. Molecular and Genetic Aspects of Nitrate Assimilation. Oxford University Press; Oxford, UK: 1989. pp. 102–124. [Google Scholar]
- 80.Hinton S.M., Dean D. Biogenesis of molybdenum cofactors. Crit. Rev. Microbiol. 1990;17:169–188. doi: 10.3109/10408419009105724. [DOI] [PubMed] [Google Scholar]
- 81.Rajagopalan K.V., Johnson J.L. The pterin molybdenum cofactors. J. Biol. Chem. 1992;267:10199–10202. doi: 10.1016/S0021-9258(19)50001-1. [DOI] [PubMed] [Google Scholar]
- 82.Wuebbens M.M., Rajagopalan K.V. Investigation of the early steps of molybdopterin biosynthesis in Escherichia coli through the use of in vivo labeling studies. J. Biol. Chem. 1995;270:1082–1087. doi: 10.1074/jbc.270.3.1082. [DOI] [PubMed] [Google Scholar]
- 83.Wuebbens M.M., Rajagopalan K.V. Structural characterization of a molybdopterin precursor. J. Biol. Chem. 1993;268:13493–13498. doi: 10.1016/S0021-9258(19)38676-4. [DOI] [PubMed] [Google Scholar]
- 84.Mendel R.R. The plant molybdenum cofactor (MoCo)-its biochemical and molecular genetics. In: Gresshoff P.M., editor. Plant Biotechnology and Development-Current Topics in Plant Molecular Biology. Volume 1. CRC Press; Boca Raton, FL, USA: London, UK: 1992. pp. 11–16. [Google Scholar]
- 85.Brown G.M., Williamson J.M. Biosynthesis of riboflavin, folic acid, thiamine, and pantothenic acid. Adv. Enzymol. Relat. Areas Mol. Biol. 1982;53:345–381. doi: 10.1002/9780470122983.ch9. [DOI] [PubMed] [Google Scholar]
- 86.Bacher A. Biosynthesis of flavins. In: Müller F., editor. Chemistry and Biochemistry of Flavoenzymes. Volume 1. CRC-Press; Boca Raton, FL, USA: 1990. pp. 215–259. [Google Scholar]
- 87.Blattner F.R., Plunkett G., 3rd, Bloch C.A., Perna N.T., Burland V., Riley M., Collado-Vides J., Glasner J.D., Rode C.K., Mayhew G.F., et al. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 88.Venter J.C. The sequence of the human genome. Science. 2000;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 89.Copenhaver G.P., Arabidopsis Genome Initiative Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- 90.Reiss J., Kleinhofs A., Klingmuller W. Cloning of seven differently complementing DNA fragments with chl functions from Escherichia coli K12. Mol. Gen. Genet. 1987;206:352–355. doi: 10.1007/BF00333594. [DOI] [PubMed] [Google Scholar]
- 91.Nohno T., Kasai Y., Saito T. Cloning and sequencing of the Escherichia coli chlEN operon involved in molybdopterin biosynthesis. J. Bacteriol. 1988;170:4097–4102. doi: 10.1128/jb.170.9.4097-4102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rivers S.L., McNairn E., Blasco F., Giordano G., Boxer D.H. Molecular genetic analysis of the moa operon of Escherichia coli K-12 required for molybdenum cofactor biosynthesis. Mol. Microbiol. 1993;8:1071–1081. doi: 10.1111/j.1365-2958.1993.tb01652.x. [DOI] [PubMed] [Google Scholar]
- 93.Mendel R.R., Kruse T. Cell biology of molybdenum in plants and humans. Biochim. Biophys. Acta. 2012;1823:1568–1579. doi: 10.1016/j.bbamcr.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 94.Stallmeyer B., Nerlich A., Schiemann J., Brinkmann H., Mendel R.R. Molybdenum co-factor biosynthesis: The Arabidopsis thaliana cDNA cnx1 encodes a multifunctional two-domain protein homologous to a mammalian neuroprotein, the insect protein Cinnamon and three Escherichia coli proteins. Plant J. 1995;8:751–762. doi: 10.1046/j.1365-313X.1995.08050751.x. [DOI] [PubMed] [Google Scholar]
- 95.Hoff T., Schnorr K.M., Meyer C., Caboche M. Isolation of two Arabidopsis cDNAs involved in early steps of molybdenum cofactor biosynthesis by functional complementation of Escherichia coli mutants. J. Biol. Chem. 1995;270:6100–6107. doi: 10.1074/jbc.270.11.6100. [DOI] [PubMed] [Google Scholar]
- 96.Caboche M., Campbell W.H., Crawford N.M., Fernandez E., Kleinhofs A., Ida S., Mendel R.R., Amato T., Rothstein S., Wray J. Genes involved in nitrogen assimilation. Plant Mol. Biol. Rep. 1994;12:45–49. doi: 10.1007/BF02671569. [DOI] [Google Scholar]
- 97.Teschner J., Lachmann N., Selbach K., Geisler M., Balk J., Mendel R.R., Bittner F. A novel role for Arabidopsis mitochondrial ABC transporter ATM3 in molybdenum cofactor biosynthesis. Plant Cell. 2010;22:468–480. doi: 10.1105/tpc.109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Santamaria-Araujo J.A., Fischer B., Otte T., Nimtz M., Mendel R.R., Wray V., Schwarz G. The tetrahydropyranopterin structure of the sulfur-free and metal-free molybdenum cofactor precursor. J. Biol. Chem. 2004;279:15994–15999. doi: 10.1074/jbc.M311815200. [DOI] [PubMed] [Google Scholar]
- 99.Schwarz G. Molybdenum cofactor biosynthesis and deficiency. Cell. Mol. Life Sci. 2005;62:2792–2810. doi: 10.1007/s00018-005-5269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mendel R.R., Schwarz G. Molybdenum cofactor biosynthesis in plants and humans. Coord. Chem. Rev. 2011;255:1145–1158. doi: 10.1016/j.ccr.2011.01.054. [DOI] [Google Scholar]
- 101.Reiss J., Cohen N., Dorche C., Mandel H., Mendel R.R., Stallmeyer B., Zabot M.T., Dierks T. Mutations in a polycistronic nuclear gene associated with molybdenum cofactor deficiency. Nat. Genet. 1998;20:51–53. doi: 10.1038/1706. [DOI] [PubMed] [Google Scholar]
- 102.Stallmeyer B., Drugeon G., Reiss J., Haenni A.L., Mendel R.R. Human molybdopterin synthase gene: Identification of a bicistronic transcript with overlapping reading frames. Am. J. Hum. Genet. 1999;64:698–705. doi: 10.1086/302295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Unkles S.E., Heck I.S., Appleyard M.V., Kinghorn J.R. Eukaryotic molybdopterin synthase. Biochemical and molecular studies of Aspergillus nidulans cnxG and cnxH mutants. J. Biol. Chem. 1999;274:19286–19293. doi: 10.1074/jbc.274.27.19286. [DOI] [PubMed] [Google Scholar]
- 104.Gutzke G., Fischer B., Mendel R.R., Schwarz G. Thiocarboxylation of molybdopterin synthase provides evidence for the mechanism of dithiolene formation in metal-binding pterins. J. Biol. Chem. 2001;276:36268–36274. doi: 10.1074/jbc.M105321200. [DOI] [PubMed] [Google Scholar]
- 105.Leimkuehler S., Rajagopalan K.V. A sulfurtransferase is required in the transfer of cysteine sulfur in the in vitro synthesis of molybdopterin from precursor Z in Escherichia coli. J. Biol. Chem. 2001;276:22024–22031. doi: 10.1074/jbc.M102072200. [DOI] [PubMed] [Google Scholar]
- 106.Leimkuehler S., Wuebbens M.M., Rajagopalan K.V. Characterization of Escherichia coli MoeB and its involvement in the activation of molybdopterin synthase for the biosynthesis of the molybdenum cofactor. J. Biol. Chem. 2001;276:34695–34701. doi: 10.1074/jbc.M102787200. [DOI] [PubMed] [Google Scholar]
- 107.Mendel R.R., Schwarz G. Biosynthesis and molecular biology of the molybdenum cofactor (Moco) Met. Ions Biol. Syst. 2002;39:317–368. [PubMed] [Google Scholar]
- 108.Matthies A., Rajagopalan K.V., Mendel R.R., Leimkuhler S. Evidence for the physiological role of a rhodanese-like protein for the biosynthesis of the molybdenum cofactor in humans. Proc. Natl. Acad. Sci. USA. 2004;101:5946–5951. doi: 10.1073/pnas.0308191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Matthies A., Nimtz M., Leimkuhler S. Molybdenum Cofactor Biosynthesis in Humans: Identification of a Persulfide Group in the Rhodanese-like Domain of MOCS3 by Mass Spectrometry. Biochemistry. 2005;44:7912–7920. doi: 10.1021/bi0503448. [DOI] [PubMed] [Google Scholar]
- 110.Leimkuehler S., Freuer A., Araujo J.A., Rajagopalan K.V., Mendel R.R. Mechanistic studies of human molybdopterin synthase reaction and characterization of mutants identified in group B patients of molybdenum cofactor deficiency. J. Biol. Chem. 2003;278:26127–26134. doi: 10.1074/jbc.M303092200. [DOI] [PubMed] [Google Scholar]
- 111.Minner-Meinen R., Weber J.N., Kistner S., Meyfarth P., Saudhof M., van den Hout L., Schulze J., Mendel R.R., Hansch R., Kaufholdt D. Physiological Importance of Molybdate Transporter Family 1 in Feeding the Molybdenum Cofactor Biosynthesis Pathway in Arabidopsis thaliana. Molecules. 2022;27:3158. doi: 10.3390/molecules27103158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dunn-Coleman N.S. Biochemical characterization of the molybdenum cofactor mutants of Neurospora crassa: In vivo and in vitro reconstitution of NADPH-nitrate reductase activity. Curr. Genet. 1984;8:581–588. doi: 10.1007/BF00395703. [DOI] [PubMed] [Google Scholar]
- 113.Heck I.S., Ninnemann H. Molybdenum cofactor biosynthesis in Neurospora crassa: Biochemical characterization of pleiotropic molybdoenzyme mutants nit-7, nit-8, nit- 9A, B and C. Photochem. Photobiol. 1995;61:54–60. doi: 10.1111/j.1751-1097.1995.tb09242.x. [DOI] [PubMed] [Google Scholar]
- 114.Schwarz G., Boxer D.H., Mendel R.R. The plant protein Cnx1 binds molybdopterin and is involved in the last step of molybdenum cofactor biosynthesis. In: Pfleiderer W., Rokos H., editors. Chemistry and Biology of Pteridines and Folates. Blackwell Science; Berlin, Germany: 1997. pp. 697–702. [Google Scholar]
- 115.Schwarz G., Boxer D.H., Mendel R.R. Molybdenum cofactor biosynthesis. The plant protein Cnx1 binds molybdopterin with high affinity. J. Biol. Chem. 1997;272:26811–26814. doi: 10.1074/jbc.272.43.26811. [DOI] [PubMed] [Google Scholar]
- 116.Kuper J., Palmer T., Mendel R.R., Schwarz G. Mutations in the molybdenum cofactor biosynthetic protein Cnx1G from Arabidopsis thaliana define functions for molybdopterin binding, Mo-insertion and molybdenum cofactor stabilization. Proc. Natl. Acad. Sci. USA. 2000;97:6475–6480. doi: 10.1073/pnas.110568497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kuper J., Llamas A., Hecht H.J., Mendel R.R., Schwarz G. Structure of molybdopterin-bound Cnx1G domain links molybdenum and copper metabolism. Nature. 2004;430:803–806. doi: 10.1038/nature02681. [DOI] [PubMed] [Google Scholar]
- 118.Llamas A., Otte T., Multhaup G., Mendel R.R., Schwarz G. The Mechanism of nucleotide-assisted molybdenum insertion into molybdopterin. A novel route toward metal cofactor assembly. J. Biol. Chem. 2006;281:18343–18350. doi: 10.1074/jbc.M601415200. [DOI] [PubMed] [Google Scholar]
- 119.Krausze J., Probst C., Curth U., Reichelt J., Saha S., Schafflick D., Heinz D.W., Mendel R.R., Kruse T. Dimerization of the plant molybdenum insertase Cnx1E is required for synthesis of the molybdenum cofactor. Biochem. J. 2017;474:163–178. doi: 10.1042/BCJ20160846. [DOI] [PubMed] [Google Scholar]
- 120.Krausze J., Hercher T.W., Zwerschke D., Kirk M.L., Blankenfeldt W., Mendel R.R., Kruse T. The functional principle of eukaryotic molybdenum insertases. Biochem. J. 2018;475:1739–1753. doi: 10.1042/BCJ20170935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Probst C., Yang J., Krausze J., Hercher T.W., Richers C.P., Spatzal T., Khadanand K.C., Giles L.J., Rees D.C., Mendel R.R., et al. Mechanism of molybdate insertion into pterin-based molybdenum cofactors. Nat. Chem. 2021;13:758–765. doi: 10.1038/s41557-021-00714-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kirsch J., Wolters I., Triller A., Betz H. Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature. 1993;366:745–748. doi: 10.1038/366745a0. [DOI] [PubMed] [Google Scholar]
- 123.Kirsch J., Betz H. The postsynaptic localization of the glycine receptor-associated protein gephyrin is regulated by the cytoskeleton. J. Neurosci. 1995;15:4148–4156. doi: 10.1523/JNEUROSCI.15-06-04148.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stallmeyer B., Schwarz G., Schulze J., Nerlich A., Reiss J., Kirsch J., Mendel R.R. The neurotransmitter receptor-anchoring protein gephyrin reconstitutes molybdenum cofactor biosynthesis in bacteria, plants, and mammalian cells. Proc. Natl. Acad. Sci. USA. 1999;96:1333–1338. doi: 10.1073/pnas.96.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Feng G., Tintrup H., Kirsch J., Nichol M.C., Kuhse J., Betz H., Sanes J.R. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science. 1998;282:1321–1324. doi: 10.1126/science.282.5392.1321. [DOI] [PubMed] [Google Scholar]
- 126.Kamdar K.P., Primus J.P., Shelton M.E., Archangeli L.L., Wittle A.E., Finnerty V. Structure of the molybdenum cofactor genes in Drosophila. Biochem. Soc. Trans. 1997;25:778–783. doi: 10.1042/bst0250778. [DOI] [PubMed] [Google Scholar]
- 127.Probst C., Ringel P., Boysen V., Wirsing L., Alexander M.M., Mendel R.R., Kruse T. Genetic characterization of the Neurospora crassa molybdenum cofactor biosynthesis. Fungal Genet. Biol. 2014;66:69–78. doi: 10.1016/j.fgb.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 128.Mayr S.J., Mendel R.R., Schwarz G. Molybdenum cofactor biology, evolution and deficiency. Biochim. Biophys. Acta Mol. Cell Res. 2021;1868:118883. doi: 10.1016/j.bbamcr.2020.118883. [DOI] [PubMed] [Google Scholar]
- 129.Schwarz G., Schulze J., Bittner F., Eilers T., Kuper J., Bollmann G., Nerlich A., Brinkmann H., Mendel R.R. The molybdenum cofactor biosynthetic protein Cnx1 complements molybdate-repairable mutants, transfers molybdenum to the metal binding pterin, and is associated with the cytoskeleton. Plant Cell. 2000;12:2455–2472. doi: 10.1105/tpc.12.12.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kaufholdt D., Bikker B.C.R., Burkart V., Dudek C.A., von Pein L., Rothkegel M., Mendel R.R., Hänsch R. The molybdenum cofactor biosynthesis complex interacts with actin filaments via molybdenum insertase Cnx1 as anchor protein in Arabidopsis thaliana. Plant Sci. 2016;244:8–18. doi: 10.1016/j.plantsci.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 131.Kaufholdt D., Gehl C., Geisler M., Jeske O., Voedisch S., Ratke C., Bollhoner B., Mendel R.R., Hansch R. Visualization and quantification of protein interactions in the biosynthetic pathway of molybdenum cofactor in Arabidopsis thaliana. J. Exp. Bot. 2013;64:2005–2016. doi: 10.1093/jxb/ert064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kaufholdt D., Baillie C.K., Meyer M.H., Schwich O.D., Timmerer U.L., Tobias L., van Thiel D., Hansch R., Mendel R.R. Identification of a protein-protein interaction network downstream of molybdenum cofactor biosynthesis in Arabidopsis thaliana. J. Plant Physiol. 2016;207:42–50. doi: 10.1016/j.jplph.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 133.Krüger B., Meyer O. The pterin (bactopterin) of carbon monoxide dehydrogenase from Pseudomonas carboxydoflava. Eur. J. Biochem. 1986;157:121–128. doi: 10.1111/j.1432-1033.1986.tb09647.x. [DOI] [PubMed] [Google Scholar]
- 134.Krüger B., Meyer O. Structural elements of bactopterin from Pseudomonas carboxydoflava carbon monoxide dehydrogenase. Biochim. Biophys. Acta. 1987;912:357–364. doi: 10.1016/0167-4838(87)90040-9. [DOI] [PubMed] [Google Scholar]
- 135.Johnson J.L., Bastian N.R., Rajagopalan K.V. Molybdopterin guanine dinucleotide: A modified form of molybdopterin identified in the molybdenum cofactor of dimethyl sulfoxide reductase from Rhodobacter sphaeroides forma specialis denitrificans. Proc. Natl. Acad. Sci. USA. 1990;87:3190–3194. doi: 10.1073/pnas.87.8.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Leimkühler S., Kern M., Solomon P.S., McEwan A.G., Schwarz G., Mendel R.R., Klipp W. Xanthine dehydrogenase from the phototrophic purple bacterium Rhodobacter capsulatus is more similar to its eukaryotic counterparts than to prokaryotic molybdenum enzymes. Mol. Microbiol. 1990;27:853–869. doi: 10.1046/j.1365-2958.1998.00733.x. [DOI] [PubMed] [Google Scholar]
- 137.Magalon A., Mendel R.R. Biosynthesis and insertion of the molybdenum cofactor. EcoSal Plus. 2015;6:6. doi: 10.1128/ecosalplus.ESP-0006-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wahl R.C., Warner C.K., Finnerty V., Rajagopalan K.V. Drosophila melanogaster ma-l mutants are defective in the sulfuration of desulfo Mo hydroxylases. J. Biol. Chem. 1982;257:3958–3962. doi: 10.1016/S0021-9258(18)34876-2. [DOI] [PubMed] [Google Scholar]
- 139.Scazzocchio C., Holl F.B., Foguelman A.I. The genetic control of molybdoflavoproteins in Aspergillus nidulans. Allopurinol-resistant mutants constitutive for xanthine-dehydrogenase. Eur. J. Biochem. 1973;36:428–445. doi: 10.1111/j.1432-1033.1973.tb02928.x. [DOI] [PubMed] [Google Scholar]
- 140.Watanabe T., Ihara N., Itoh T., Fujita T., Sugimoto Y. Deletion mutation in Drosophila ma-l homologous, putative molybdopterin cofactor sulfurase gene is associated with bovine xanthinuria type II. J. Biol. Chem. 2000;275:21789–21792. doi: 10.1074/jbc.C000230200. [DOI] [PubMed] [Google Scholar]
- 141.Ichida K., Matsumura T., Sakuma R., Hosoya T., Nishino T. Mutation of human molybdenum cofactor sulfurase gene is responsible for classical xanthinuria type II. Biochem. Biophys. Res. Commun. 2001;282:1194–1200. doi: 10.1006/bbrc.2001.4719. [DOI] [PubMed] [Google Scholar]
- 142.Leon-Klooserziel K.M., Gil M.A., Ruijs G.J., Jacobsen S.E., Olszewski N.E., Schwartz S.H., Zeevaart J.A.D., Koorneef M. Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J. 1996;10:655–661. doi: 10.1046/j.1365-313X.1996.10040655.x. [DOI] [PubMed] [Google Scholar]
- 143.Amrani L., Primus J., Glatigny A., Arcangeli L., Scazzocchio C., Finnerty V. Comparison of the sequences of the Aspergillus nidulans hxB and Drosophila melanogaster ma-l genes with nifS from Azotobacter vinelandii suggests a mechanism for the insertion of the terminal sulphur atom in the molybdopterin cofactor. Mol. Microbiol. 2000;38:114–125. doi: 10.1046/j.1365-2958.2000.02119.x. [DOI] [PubMed] [Google Scholar]
- 144.Bittner F., Oreb M., Mendel R.R. ABA3 is a molybdenum cofactor sulfurase required for activation of aldehyde oxidase and xanthine dehydrogenase in Arabidopsis thaliana. J. Biol. Chem. 2001;276:40381–40384. doi: 10.1074/jbc.C100472200. [DOI] [PubMed] [Google Scholar]
- 145.Lehrke M., Rump S., Heidenreich T., Wissing J., Mendel R.R., Bittner F. Identification of persulfide-binding and disulfide-forming cysteines in the NifS-like domain of the molybdenum cofactor sulfurase ABA3 by cysteine scanning mutagenesis. Biochem. J. 2012;441:823–832. doi: 10.1042/BJ20111170. [DOI] [PubMed] [Google Scholar]
- 146.Gasber A., Klaumann S., Trentmann O., Trampczynska A., Clemens S., Schneider S., Sauer N., Feifer I., Bittner F., Mendel R.R., et al. Identification of an Arabidopsis solute carrier critical for intracellular transport and inter-organ allocation of molybdate. Plant Biol. 2011;13:710–718. doi: 10.1111/j.1438-8677.2011.00448.x. [DOI] [PubMed] [Google Scholar]
- 147.Witte C.P., Igeno M.I., Mendel R., Schwarz G., Fernandez E. The Chlamydomonas reinhardtii MoCo carrier protein is multimeric and stabilizes molybdopterin cofactor in a molybdate charged form. FEBS Lett. 1998;431:205–209. doi: 10.1016/S0014-5793(98)00756-X. [DOI] [PubMed] [Google Scholar]
- 148.Fischer K., Llamas A., Tejada-Jimenez M., Schader N., Kuper J., Ataya F.S., Galvan A., Mendel R.R., Fernandez E., Schwarz G. Function and structure of the molybdenum cofactor carrier protein MCP from Chlamydomonas reinhardtii. J. Biol. Chem. 2006;281:30186–30194. doi: 10.1074/jbc.M603919200. [DOI] [PubMed] [Google Scholar]
- 149.Kruse T., Gehl C., Geisler M., Lehrke M., Ringel P., Hallier S., Hansch R., Mendel R.R. Identification and biochemical characterization of molybdenum cofactor-binding proteins from Arabidopsis thaliana. J. Biol. Chem. 2010;285:6623–6635. doi: 10.1074/jbc.M109.060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Duran M., Beemer F.A., van de Heiden C., Korteland J., de Bree P.K., Brink M., Wadman S.K., Lombeck I. Combined deficiency of xanthine oxidase and sulphite oxidase: A defect of molybdenum metabolism or transport? J. Inherit. Metab. Dis. 1978;1:175–178. doi: 10.1007/BF01805591. [DOI] [PubMed] [Google Scholar]
- 151.Reiss J., Gross-Hardt S., Christensen E., Schmidt P., Mendel R.R., Schwarz G. A mutation in the gene for the neurotransmitter receptor-clustering protein gephyrin causes a novel form of molybdenum cofactor deficiency. Am. J. Hum. Genet. 2001;68:208–213. doi: 10.1086/316941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Johnson J.L., Wuebbens M.M., Mandell R., Shih V.E. Molybdenum cofactor biosynthesis in humans. Identification of two complementation groups of cofactor-deficient patients and preliminary characterization of a diffusible molybdopterin precursor. J. Clin. Investig. 1989;83:897–903. doi: 10.1172/JCI113974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schwarz G., Santamaria-Araujo J.A., Wolf S., Lee H.J., Adham I.M., Grone H.J., Schwegler H., Sass J.O., Otte T., Hanzelmann P., et al. Rescue of lethal molybdenum cofactor deficiency by a biosynthetic precursor from Escherichia coli. Hum. Mol. Genet. 2004;13:1249–1255. doi: 10.1093/hmg/ddh136. [DOI] [PubMed] [Google Scholar]
- 154.Veldman A., Santamaria-Araujo J.A., Sollazzo S., Pitt J., Gianello R., Yaplito-Lee J., Wong F., Ramsden C.A., Reiss J., Cook I., et al. Successful treatment of molybdenum cofactor deficiency type A with cPMP. Pediatrics. 2010;125:e1249–e1254. doi: 10.1542/peds.2009-2192. [DOI] [PubMed] [Google Scholar]
- 155.Zhang Y., Ying H., Xu Y. Comparative genomics and metagenomics of the metallomes. Metallomics. 2019;11:1026–1043. doi: 10.1039/c9mt00023b. [DOI] [PubMed] [Google Scholar]
- 156.Zhang Y., Rump S., Gladyshev V.N. Comparative Genomics and Evolution of Molybdenum Utilization. Coord. Chem. Rev. 2011;255:1206–1217. doi: 10.1016/j.ccr.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang Y., Gladyshev V.N. Molybdoproteomes and evolution of molybdenum utilization. J. Mol. Biol. 2008;379:881–899. doi: 10.1016/j.jmb.2008.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lake M.W., Wuebbens M.M., Rajagopalan K.V., Schindelin H. Mechanism of ubiquitin activation revealed by the structure of a bacterial MoeB-MoaD complex. Nature. 2001;414:325–329. doi: 10.1038/35104586. [DOI] [PubMed] [Google Scholar]
- 159.Warnhoff K., Ruvkun G. Molybdenum cofactor transfer from bacteria to nematode mediates sulfite detoxification. Nat. Chem. Biol. 2019;15:480–488. doi: 10.1038/s41589-019-0249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Warnhoff K., Hercher T.W., Mendel R.R., Ruvkun G. Protein-bound molybdenum cofactor is bioavailable and rescues molybdenum cofactor-deficient C. elegans. Genes Dev. 2021;35:212–217. doi: 10.1101/gad.345579.120. [DOI] [PMC free article] [PubMed] [Google Scholar]