Abstract
The PII protein is encoded by a unique glnB gene in Synechococcus sp. strain PCC 7942. Its expression has been analyzed in the wild type and in NtcA-null mutant cells grown under different conditions of nitrogen and carbon supply. RNA-DNA hybridization experiments revealed the presence of one transcript species 680 nucleotides long, whatever the nutrient conditions tested. A second transcript species, 620 nucleotides long, absent in the NtcA null mutant, was observed in wild-type cells that were nitrogen starved for 2 h under both high and low CO2 and in the presence of nitrate under a high CO2 concentration. Primer extension analysis indicated that the two transcript species are generated from two tandem promoters, a ς70 Escherichia coli-type promoter and an NtcA-dependent promoter, located 120 and 53 nucleotides, respectively, from the glnB initiation codon. The NtcA-dependent promoter is up-regulated under the conditions mentioned above, while the ς70 E. coli-type promoter displays constitutive levels of transcripts in the NtcA null mutant and slightly different levels in the wild-type cells, depending on the nitrogen and carbon supplies. In general, a good correlation between the amounts of the two transcript species and that of the PII protein was observed, as revealed by immunodetection with specific antibodies. The phosphorylation level of PII in the wild type is inversely correlated with nitrogen availability and directly correlated with higher CO2 concentration. This regulation is correspondingly less stringent in the NtcA null mutant cells. In contrast, the dephosphorylation of PII is NtcA independent.
In cyanobacteria, nitrogen assimilation is a genuine photosynthetic process that requires ATP and reducing equivalents generated in the light. Both nitrate and nitrite are reduced to ammonium in the presence of photosynthetically reduced ferredoxin as the physiological electron donor. Ammonium is incorporated, through the glutamine synthetase-glutamate synthase pathway, into glutamate to yield glutamine by an ATP-dependent ligation reaction catalyzed by glutamine synthetase, and glutamate synthase transfers the amido group of glutamine to 2-oxoglutarate to regenerate glutamate in the presence of reduced ferredoxin. Nitrogen assimilation is tightly regulated in response to environmental cues. Nitrate and nitrite are taken up and reduced only in the absence of ammonium and under CO2 fixation conditions, and the level of glutamine synthetase protein is severely reduced in the presence of ammonium (12).
In the unicellular Synechococcus sp. strain PCC 7942, which does not fix molecular nitrogen, the signal transduction protein PII (the glnB gene product) is a key element in the coordination of nitrogen and carbon metabolism (15). This protein is a homotrimer of 36 kDa whose isomeric forms carry either zero, one, two, or three phosphorylated seryl residues (Ser49), depending on the carbon and nitrogen supply of the cells (13, 14), in contrast to the Escherichia coli PII, which is uridylylated at a tyrosyl residue (Tyr51) (41). The highest degree of PII phosphorylation is observed in cells incubated under a high CO2 concentration in the presence of nitrate or under nitrogen-limiting conditions, while the protein is mainly dephosphorylated under low CO2 in the presence of ammonium (14, 15). In vitro phosphorylation experiments revealed that both the PII kinase and phosphatase activities depend on 2-oxoglutarate and ATP but not on glutamine or glutamate (16, 23). The modification of the Synechococcus sp. strain PCC 7942 PII protein is facilitated by the binding of ATP and 2-oxoglutarate (13, 16). Since the intracellular concentration of ATP is high under physiological conditions, it has been proposed that PII primarily functions as a sensor of 2-oxoglutarate (13). This metabolite not only serves as a source of carbon skeleton for nitrogen assimilation but would also be of particular importance as a small-molecule effector in the control of this metabolic process in Synechococcus sp. strain PCC 7942.
NtcA is a global nitrogen regulator that is widespread and highly conserved in cyanobacteria (12, 20). This DNA-binding protein, which belongs to the CRP family of bacterial transcriptional effectors, activates the expression of a number of genes in the absence of ammonium by recognizing the target consensus nucleotide sequence GTAN8TAC in their promoter regions (30). In Synechococcus sp. strain PCC 7942, NtcA positively regulates its own expression and activates the transcription of the nir operon (encoding nitrite reductase, the ABC-type permease complex, and nitrate reductase) (12, 30, 39), the nirB ntcB gene cluster (which encodes, respectively, a protein required for expression of nitrite reductase activity and a transcriptional effector of the bacterial LysR-type family that activates the nir operon in the presence of nitrite) (1, 26, 44), the glnA gene (encoding glutamine synthetase) (6, 7, 30), and the cynBDS operon (encoding two proteins likely to be involved in the active transport of cyanate and cyanase) (22). In the N2-fixing filamentous heterocystous cyanobacterium Anabaena sp. strain PCC 7120, NtcA acts as an activator for the expression of genes for the assimilation of nitrogen sources alternative to ammonium and as an activator for heterocyst development (18, 19, 49). It has also been proposed that it could behave as a repressor for the rbcL gene (encoding the large ribulose-1,5-bisphosphate carboxylase–oxygenase subunit) and the gor gene (encoding glutathione reductase) (24, 40). In the facultative photoheterotroph Synechocystis sp. strain PCC 6803, glnB appears to be regulated by both electron transport and nitrogen availability, and the increased level of glnB under nitrogen starvation might be under the control of NtcA (21). In the marine unicellular N2-fixing Cyanothece sp. strain BH68K, NtcA is involved in nitrogen assimilation rather than nitrogen fixation, and the expression of the ntcA gene may be under the control of the circadian rhythm (4).
Analysis of the phenotype of MP2, a PII null mutant of the obligate photoautotroph Synechococcus sp. strain PCC 7942, revealed that nitrate utilization no longer depended on CO2 fixation (15). Moreover, in contrast to the wild-type cells, in which ammonium exerts a rapid and reversible inhibition of nitrate and nitrite uptake, no inhibition was observed in this mutant. It was thus concluded that the unphosphorylated form of PII is involved in the short-term inhibition by ammonium of nitrate and nitrite uptake (27). In this mutant, the synthesis of nitrate and nitrite reductases and glutamine synthetase was still subject to control by ammonium, suggesting that there is no direct interaction between PII and the activity of NtcA in the regulation of nitrogen assimilation (15). However, other relationships between PII and NtcA were not excluded.
Here we present results demonstrating that NtcA regulates PII synthesis at the transcriptional level and is required for a full control of the phosphorylation state of PII in response to nitrogen and carbon availability in Synechococcus sp. strain PCC 7942.
MATERIALS AND METHODS
Strains and culture conditions.
Wild-type and mutant cells of Synechococcus sp. strain PCC 7942 were grown in liquid BG110 medium (43) containing 0.4 mM Na2CO3 and supplemented with 10 mM HEPES, pH 8.0. Either NaNO3 (17.6 mM) or NH4Cl (5 mM) was used as the N source. The NtcA− mutant, constructed by using plasmid pMAV58 according to the method of Vega-Palas et al. (48), was grown in ammonium-containing BG110 medium with chloramphenicol (7 μg ml−1). Precultures grown in the presence of ammonium were incubated for 3 to 4 days (optical density at 750 nm [OD750], approximately 0.6) at 30°C in air and illuminated with fluorescent lamps (OSRAM L18W/25 universal white) providing a photosynthetic photon flux density of 50 μmol m−2 s−1 measured with a LI-COR LI-185B quantum radiometer-photometer equipped with an LI-190SB quantum sensor. Experimental cultures were incubated at 35°C under the same photosynthetic photon flux density in a culture medium containing ammonium and supplemented with NaHCO3 (10 mM) and with constant bubbling with air–3% (vol/vol) CO2. Cells from mid-exponential-phase cultures (OD750, approximately 0.4) were collected by centrifugation at 5,000 × g for 10 min at 25°C. The cell pellets were washed twice with BG110 and resuspended at the same cell density in BG110 medium containing either ammonium, nitrate, or no nitrogen source. After 2 h of incubation of the cells either in air without bubbling or bubbled with air–3% (vol/vol) CO2, samples were collected for analysis.
Nucleic acid methods.
Standard methods were used for E. coli plasmid DNA isolation. Restriction endonucleases (New England Biolabs or Pharmacia) and other DNA-modifying enzymes (New England Biolabs or Amersham) were used according to the manufacturers’ recommendations.
Extraction of total RNA, gel electrophoreses, blottings, and hybridizations were performed as described previously (27). DNA probes were labelled with [α-32P]dATP (110 TBq mmol−1) by using a Megaprime random-labelling kit (Amersham). A probe internal to glnB (241 bp) was obtained by PCR amplification of the corresponding fragment from plasmid pPM119 (45) with specific primers to which an EcoRI site was added at the 5′ extremity of the coding strand (5′ CGGAATTCCGTTCAAACTGGAC 3′) and a BamHI site was added at the 5′ extremity of the complementary strand (5′ CGGGATCCCGTCACCAATTTCGC 3′). The PCR product was cloned in the vector pTZ18R for further use in the DNA-binding assay. Plasmid DNA was extracted with the Nucleobond AX kit (Macherey-Nagel, Düren, Germany). A 0.6-kb KpnI-XhoI fragment containing the rnpB gene, encoding the RNA subunit of RNase P from Synechococcus sp. strain PCC 7942 (a gift from A. Vioque [3]), was used as a probe to quantify the amount of RNA loaded and transferred to the filters and to standardize the measurements.
Primer extension was performed as described by Liotenberg et al. (28) with 60 μg of total RNA and the 21-nucleotide-long primer 5′ GACTTCGTCCAGTTTGAACGG 3′. DNA sequencing was performed by using the sequencing dideoxynucleotide chain termination method (T7 sequencing kit; Pharmacia) with 35S-dATP (37 TBq mmol−1; Amersham) as the labelled nucleotide and the same primer mentioned above.
The relative transcript levels were quantified by scanning photoactivatable screens on a Molecular Dynamics 445SI PhosphoImager. All quantifications, data display, and analysis were performed with Molecular Dynamics Image Quant software.
Gel retardation assays.
Preparation of cell extracts from E. coli DH5α containing the expression vector pTrc99A and from isopropyl-β-d-thiogalactopyranoside (IPTG)-induced cells of the NtcA-overproducing strain DH5α(pCSI26), gel retardation assays, and labelling of the probes were performed as described previously (30). The DNA probes were a 440-bp NheI-AvaI fragment of the glnB promoter region from plasmid pPM119; the PCR product corresponding to a fragment internal to the glnB gene, obtained as described above in “Nucleic acid methods”; and a 350-bp EcoRI-XhoI fragment of the glnA promoter region from pCSI38 (30). These probes were labelled with [α-32P]dCTP (110 TBq/mmol; Amersham). The protein-DNA complex was visualized with an Instant Imager (Packard).
Quantification of the PII protein and determination of its modification state.
Cell extracts were prepared from cultures grown to an OD750 of 0.4, and aliquots corresponding to 10 μg of total protein were separated by polyacrylamide gel electrophoresis under either denaturing or nondenaturing conditions as described by Forchhammer and Tandeau de Marsac (14). The protein content of cell extracts was estimated by the method of Lowry modified as described previously (32), with bovine serum albumin as a standard. The PII protein was revealed by immunoblotting with a PII-specific antiserum in an enhanced chemoluminescence detection system (ECL kit; Amersham). Quantification was done with the National Institutes of Health Image program.
RESULTS
Control of glnB expression by NtcA.
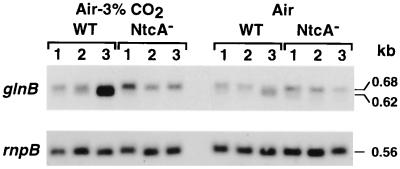
Examination of the nucleotide sequence of the glnB gene of Synechococcus sp. strain PCC 7942 revealed the presence of a consensus DNA-binding site for the transcriptional effector NtcA (Fig. 1) located upstream of the glnB initiation codon between nucleotides 88 and 102. Experiments were designed to analyze the conditions under which the expression of the glnB gene of Synechococcus sp. strain PCC 7942 was regulated and to determine the corresponding start sites for transcription. RNA-DNA hybridizations, using a probe internal to the glnB gene, and primer extension analysis were performed. Total RNA was extracted from ammonium-grown cultures of the wild type and the NtcA null mutant after transfer of the cells for 2 h to a medium containing either ammonium, nitrate, or no combined nitrogen and under either air or air enriched with 3% (vol/vol) CO2. One or two transcript species of slightly different sizes (0.68 and 0.62 kb) were observed, depending on the nutrient conditions and the strain (Fig. 2). A particularly high level of transcripts was found in the wild-type cells incubated under nitrogen limitation and a high CO2 concentration; their abundance was low under each of the other conditions tested.
FIG. 1.
Alignment of NtcA recognition sequences of different genes from Synechococcus sp. strain PCC 7942 (30).
FIG. 2.
RNA-DNA hybridization of total RNA from cells of wild-type Synechococcus sp. strain PCC 7942 (WT) and the NtcA-deficient mutant (NtcA−) in response to the nature of the nitrogen source and the availability of CO2. Ammonium-grown cells were transferred for 2 h to BG-110 medium containing ammonium (lanes 1), nitrate (lanes 2), or no nitrogen source (lanes 3), under either air or air–3% (vol/vol) CO2. The same RNA blots were hybridized with a DNA probe internal to the glnB gene encoding the PII protein and with a DNA probe of the rnpB gene encoding the RNA subunit of RNase P to provide an estimate of the RNA loading.
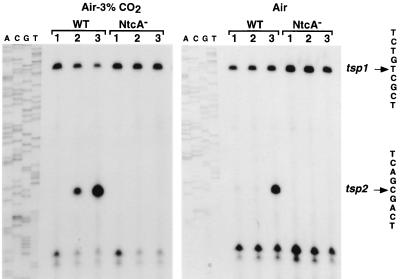
Primer extension analysis revealed four extension products (Fig. 3). Two of them, which might correspond to the transcription start points designated tsp1 and tsp2, varied in abundance with the conditions of nitrogen and carbon availability in both the wild-type and the NtcA null mutant cells. The two additional small extension products most likely result from earlier pauses caused by a GC-rich stretch starting immediately downstream from tsp2. The transcription start point tsp1, situated 120 nucleotides from the glnB initiation codon, was found with RNA from both the wild-type and NtcA null mutant cells, whatever the conditions tested. The transcript species corresponding to tsp1 was preceded on the DNA sequence by a ς70-like promoter (−10 TAAAAT; −35 TTGCCT). The second transcription start point, tsp2, localized 53 nucleotides from glnB, corresponded to extension products whose abundance increased under nitrogen limitation and high CO2 concentration in the wild-type cells. This was accompanied by a decreased intensity of the extension products corresponding to tsp1. The transcription start point tsp2 was preceded on the DNA sequence by a −10 box, TACCAA, and a perfect consensus NtcA-binding sequence, GTAN8TAC (30), between nucleotides −35 and −50. No extension products corresponding to tsp2 were detectable in the RNA from the NtcA− mutant cells, whatever the conditions tested. These results indicated that NtcA controls the expression of the glnB gene at tsp2.
FIG. 3.
Primer extension with the glnB gene. Total RNA (60 μg) from wild-type Synechococcus sp. strain PCC 7942 (WT) and NtcA-deficient mutant (NtcA−) cells was annealed with an oligonucleotide specific to the glnB gene and extended with avian myeloblastosis virus reverse transcriptase as indicated in Materials and Methods. The cells were incubated as described in the legend to Fig. 2. Lanes A, C, G, and T contain a dideoxy sequencing ladder of the same DNA region used as a size control of the extension products. The sequences around the 5′ ends are listed on the right. tsp1 and tsp2 are putative transcription start points.
Binding of NtcA to the glnB promoter region.
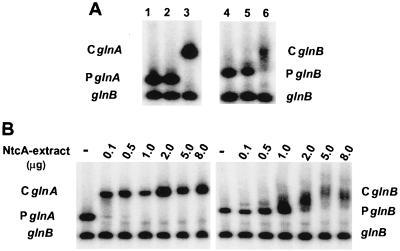
Mobility shift assays of electrophoretically resolved DNA fragments carrying the upstream region of glnB were performed with cell extracts of an NtcA-overproducing E. coli strain. This strain harbors plasmid pCSI26, which carries the ntcA gene downstream from the synthetic IPTG-inducible promoter trc and thus overexpresses NtcA after IPTG induction (30). As a positive control, experiments were performed in parallel with the promoter region of the glnA gene (PglnA). The DNA fragment containing the promoter region of the glnB gene (PglnB) was retarded by the NtcA-containing extract from E. coli carrying pCSI26 but not by the extract from E. coli cells harboring pTrc99A, the vector used to construct pCSI26 (Fig. 4A). The DNA fragment corresponding to an internal part of the glnB gene did not display any mobility shift and did not compete with either PglnB or PglnA (Fig. 4A). These results confirmed that the NtcA protein might bind specifically to DNA upstream from the glnB gene. At least a 50-fold-higher concentration of the cell extract was required, however, to obtain an NtcA-promoted shift of PglnB than to obtain a shift of PglnA (Fig. 4B), and the retarded band was fuzzier, whatever the concentration of the cell extracts tested (Fig. 4A and B).
FIG. 4.
Gel retardation of DNA fragments from the glnB and glnA promoter regions by cell extracts of an NtcA-overproducing E. coli strain. (A) glnA promoter region, PglnA (lanes 1 to 3), and glnB promoter region, PglnB (lanes 4 to 6), incubated with a DNA fragment internal to the glnB gene as the competitor DNA. Lanes 1 and 4, no NtcA-containing extract added; lanes 2 and 5, 5 μg of extract from cells of E. coli DH5α(pTrc99A) added; lanes 3 and 6, 5 μg of NtcA-containing extract from IPTG-induced cells of E. coli DH5α(pCSI26) added. CglnA and CglnB are complexes formed after incubation of the DNA fragments carrying PglnA and PglnB, respectively, with the NtcA-containing extracts. (B) Same conditions as in panel A, lanes 1 and 4, with various amounts (0 [−] to 8.0 μg) of extract from IPTG-induced cells of E. coli DH5α(pCSI26) added.
Immunological detection of PII in the wild type and in an NtcA null mutant.
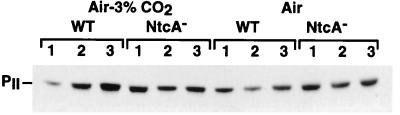
The amount of PII protein was estimated by immunoblotting with specific PII antibodies. In general, the total amount of the protein was found to be in good correlation with the levels of the transcripts in both the wild-type and the NtcA− cells incubated under the different nutrient conditions tested (Fig. 5). This indicated that, in addition to a basal level of expression of the glnB gene, which is NtcA independent, there is control by NtcA that occurs at a transcriptional level.
FIG. 5.
Immunoblot analysis of the PII protein in cells of wild-type Synechococcus sp. strain PCC 7942 (WT) and of the NtcA-deficient mutant (NtcA−) in response to the nature of the nitrogen source and CO2 availability. The cells were incubated as described in the legend to Fig. 2.
Modulation of phosphorylation levels of PII isoforms by NtcA.
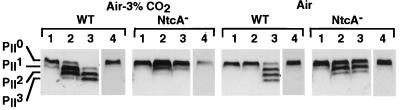
In the wild-type cells, the relative abundance of the four PII isoforms, PII0, PII1, PII2, and PII3, which possess either zero, one, two or three phosphorylated monomers, respectively, varied with both the nitrogen supply and the concentration of CO2 (Fig. 6). In the presence of ammonium, whatever the CO2 level, or in the presence of nitrate under low CO2, PII was mainly unphosphorylated. Three to four isoforms were observed in the presence of nitrate under high CO2 and in nitrogen-limiting conditions under both high and low CO2. In the NtcA null mutant, whatever the availability of CO2, PII remained unphosphorylated in cells incubated in the presence of ammonium, as in the wild-type cells. In contrast, only two (under high CO2) to three (under low CO2) isoforms were observed when the cells were incubated with nitrate or under nitrogen limitation. As expected, the same results were obtained under both nitrogen conditions, since the NtcA-deficient mutant is impaired in the expression of a number of genes involved in nitrogen assimilation (48). Addition of ammonium to the nitrogen-starved cells led to a complete dephosphorylation of PII in the NtcA null mutant, as in the wild type. Therefore, NtcA is not required for the dephosphorylation of PII but only for the accumulation of phosphorylated forms in response to both the nitrogen and carbon sources.
FIG. 6.
In vivo phosphorylation of the PII protein in cells of wild-type Synechococcus sp. strain PCC 7942 (WT) and the NtcA-deficient mutant (NtcA−) in response to the nature of the nitrogen source and CO2 availability. Lanes 1 to 3, the cells were incubated as described in the legend to Fig. 2. Lanes 4, cells starved for nitrogen as in lanes 3 were further incubated for 2 h following addition of ammonium to the culture medium. PII0, PII1, PII2, and PII3 correspond to the four isoforms of the trimeric PII protein containing zero, one, two, or three phosphorylated monomers.
DISCUSSION
In this study, a role for the global nitrogen regulator NtcA in the control of the transcription of the glnB gene, which encodes the signal transduction PII protein, in response to changes in the availability of nitrogen and carbon in the environment has been demonstrated in the unicellular cyanobacterium Synechococcus sp. strain PCC 7942.
At least two distinct promoter-like structures have been identified that control the expression of the unique glnB gene in Synechococcus sp. strain PCC 7942, depending on the nitrogen supply and CO2 concentration. The first, located upstream from tsp1, is of the common ς70 E. coli type. It yields constitutive levels of glnB mRNA in both wild-type and NtcA null mutant cells. The second, located upstream from tsp2, is activated by the global nitrogen regulator NtcA. The putative NtcA-binding site from the Synechococcus glnB promoter exactly matches the consensus sequence (GTAN8TAC), as is the case with other genes from this organism, such as glnA, ntcA, and the nir operon (Fig. 1). However, there is obviously a different binding affinity for the glnB and the glnA promoter regions (Fig. 4). Previous in vitro studies have shown that NtcA from the filamentous heterocystous cyanobacterium Anabaena sp. strain PCC 7120 displays different affinities, depending on the target genes (40). A similar type of control could occur in Synechococcus sp. strain PCC 7942.
The abundance of the NtcA-dependent transcripts is inversely correlated with the availability of nitrogen and increases with higher CO2 concentrations (Fig. 3). This positive effect of CO2 could be exerted by CO2 itself or by a metabolite derived from its fixation. A corresponding decrease in the level of the transcripts that initiate at tsp1 is particularly evident in cells incubated with high CO2. This would result if the binding of NtcA to the tsp2 transcript species prevents the RNA polymerase from initiating transcription from the more distal tsp1 promoter region, and it is not due to a specific recognition of the tsp1 promoter region by NtcA, since no NtcA consensus sequence is present in that region.
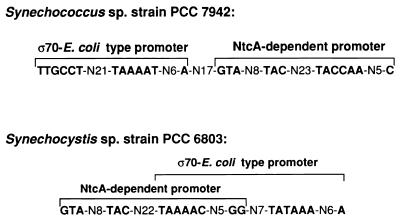
In Synechocystis sp. strain PCC 6803, nitrogen availability and electron transport have been shown to control the expression of the glnA and glnB genes (21). In this cyanobacterium, one putative promoter of the glnB gene is constitutively expressed at a very low level in the absence of nitrogen and in the presence of ammonium and/or nitrate. The other promoter is functional only in cells starved for nitrogen (21). Therefore, the glnB control system in Synechocystis sp. strain PCC 6803, as well as those of other genes (e.g., icd, encoding NADP+-isocitrate dehydrogenase, and glnA and glnN, encoding glutamine synthetases I and III) (38, 42), differs from the one that operates in Synechococcus sp. strain PCC 7942 for glnA (6, 30) and glnB (Fig. 3), in which the inducible promoter is active in cells grown both in the absence of nitrogen and in the presence of nitrate. Moreover, the physical organizations of the promoter regions in these two strains differ. In contrast to Synechocystis sp. strain PCC 6803, in Synechococcus sp. strain PCC 7942 the two promoter sequences do not overlap and the constitutively expressed promoter is upstream of the nitrogen-regulated one (Fig. 7).
FIG. 7.
Comparison of the physical organization of the promoter regions of the glnB genes from Synechococcus sp. strain PCC 7942 and Synechocystis sp. strain PCC 6803.
Two types of promoters for the genes encoding PII and PII-like proteins that recognize either ς70 or a nitrogen-regulated RNA polymerase are commonly found in bacteria. Their physical organization and their levels of expression may vary, depending on the organism, but in most cases nitrogen control is exerted from a ς54-dependent promoter through the NtrC protein (2, 5, 9–11, 17, 25, 29, 34, 36, 37, 46, 47, 50, 51).
In this study, we did not look at the kinetics of phosphorylation and dephosphorylation of PII in the wild-type and mutant strains in response to nitrogen shifts. Therefore, we do not know whether the accumulation of the phosphorylated forms of PII depends on a kinase or a phosphatase activity. Nevertheless, the dephosphorylation of PII in the presence of ammonium appears to be NtcA independent, while the accumulation of the phosphorylated forms is at least in part NtcA dependent in Synechococcus sp. strain PCC 7942 (Fig. 6, compare lanes 3). The transcriptional activator NtcA might therefore modulate the phosphorylation state of PII in response to the intracellular N-C balance by directly or indirectly controlling the synthesis, activity, and/or stability of the serine kinase-phosphatase enzyme system, which posttranslationally modifies PII.
At present, in Synechococcus sp. strain PCC 7942, we do not know whether NtcA is a single protein or a complex of one or more proteins or if it needs to be liganded to some effector(s) or posttranslationally modified to be active or to modulate its activity, depending on environmental conditions. The effect of NtcA on the activation of transcription of the glnB gene in Synechococcus sp. strain PCC 7942 and on the increased phosphorylation state of the protein could result from a higher level of expression of the corresponding ntcA gene and/or from a greater affinity of this regulator for its target sites under a high CO2 concentration. Whether this is due to an increased electron transport activity or to the presence of a specific CO2 fixation product(s) remains to be elucidated. The fact that the concentration of 2-oxoglutarate varies greatly in cyanobacterial cells, depending on the supply of carbon and nitrogen (8, 33, 35, 38), and that this is a very important metabolite involved in the regulation of nitrogen assimilation pathways in cyanobacteria and other prokaryotes (15, 35, 31) favors the hypothesis that this compound could play a key role in the regulatory system that coordinates the corresponding metabolic processes via PII and NtcA.
ACKNOWLEDGMENTS
We are grateful to M. Herdman for critical reading of the manuscript. We also thank J. Houmard, J. Gomez Ochoa de Alda, and I. Luque for helpful discussions and A. Vioque for kindly providing the plasmid containing the rnpB gene from Synechococcus sp. strain PCC 7942.
This work was supported by the Institut Pasteur and the Centre National de la Recherche Scientifique (URA 1129), by grant PB 95-1267 from the Dirreción General Ensenanza Superior, and, in part, by a joint Picasso program from the Ministère des Affaires Etrangères (France) and the Ministerio de Educación y Ciencia (Spain).
REFERENCES
- 1.Aichi M, Omata T. Involvement of NtcB, a LysR family transcription factor, in nitrite activation of the nitrate assimilation operon in the cyanobacterium Synechococcus sp. strain PCC 7942. J Bacteriol. 1997;179:4671–4675. doi: 10.1128/jb.179.15.4671-4675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amar M, Patriarca E J, Manco G, Bernard P, Riccio A, Lamberti A, Defez R, Iaccarino M. Regulation of nitrogen metabolism is altered in a glnB mutant strain of Rhizobium leguminosarum. Mol Microbiol. 1994;11:685–693. doi: 10.1111/j.1365-2958.1994.tb00346.x. [DOI] [PubMed] [Google Scholar]
- 3.Banta A B, Haas E S, Brown J W, Pace N R. Sequence of the ribonuclease P RNA gene from the cyanobacterium Anacystis nidulans. Nucleic Acids Res. 1992;20:911. doi: 10.1093/nar/20.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley R L, Reddy K J. Cloning, sequencing, and regulation of the global nitrogen regulator gene ntcA in the unicellular diazotrophic cyanobacterium Cyanothece sp. strain BH68K. J Bacteriol. 1997;179:4407–4410. doi: 10.1128/jb.179.13.4407-4410.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiurazzi M, Iaccarino M. Transcriptional analysis of the glnB-glnA region of Rhizobium leguminosarum biovar viciae. Mol Microbiol. 1990;4:1727–1735. doi: 10.1111/j.1365-2958.1990.tb00550.x. [DOI] [PubMed] [Google Scholar]
- 6.Cohen-Kupiec R, Gurevitz M, Zilberstein A. Expression of glnA in the cyanobacterium Synechococcus sp. strain PCC 7942 is initiated from a single nif-like promoter under various nitrogen conditions. J Bacteriol. 1993;175:7727–7731. doi: 10.1128/jb.175.23.7727-7731.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen-Kupiec R, Zilberstein A, Gurevitz M. Characterization of cis elements that regulate the expression of glnA in Synechococcus sp. strain PCC 7942. J Bacteriol. 1995;177:2222–2226. doi: 10.1128/jb.177.8.2222-2226.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coronil T, Lara C, Guerrero M G. Shift in carbon flow and stimulation of amino-acid turnover induced by nitrate and ammonium assimilation in Anacystis nidulans. Planta. 1993;189:461–467. doi: 10.1007/BF00194446. [DOI] [PubMed] [Google Scholar]
- 9.de Zamaroczy M, Delorme F, Elmerich C. Characterization of three different nitrogen-regulated promoter regions for the expression of glnB and glnA in Azospirillum brasilense. Mol Gen Genet. 1990;224:421–430. doi: 10.1007/BF00262437. [DOI] [PubMed] [Google Scholar]
- 10.de Zamaroczy M, Paquelin A, Elmerich C. Functional organization of the glnB-glnA cluster of Azospirillum brasilense. J Bacteriol. 1993;175:2507–2515. doi: 10.1128/jb.175.9.2507-2515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Zamaroczy M, Paquelin A, Pletre G, Forchhammer K, Elmerich C. Coexistence of two structurally similar but functionally different PII proteins in Azospirillum brasilense. J Bacteriol. 1996;178:4143–4149. doi: 10.1128/jb.178.14.4143-4149.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flores E, Herrero A. Assimilatory nitrogen metabolism and its regulation. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 487–517. [Google Scholar]
- 13.Forchhammer K, Hedler A. Phosphoprotein PII from cyanobacteria. Analysis of functional conservation with the PII signal-transduction protein from Escherichia coli. Eur J Biochem. 1997;244:869–875. doi: 10.1111/j.1432-1033.1997.00869.x. [DOI] [PubMed] [Google Scholar]
- 14.Forchhammer K, Tandeau de Marsac N. The PII protein in the cyanobacterium Synechococcus sp. strain PCC 7942 is modified by serine phosphorylation and signals the cellular N-status. J Bacteriol. 1994;176:84–91. doi: 10.1128/jb.176.1.84-91.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forchhammer K, Tandeau de Marsac N. Functional analysis of the phosphoprotein PII (glnB gene product) in the cyanobacterium Synechococcus sp. strain PCC 7942. J Bacteriol. 1995;177:2033–2040. doi: 10.1128/jb.177.8.2033-2040.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forchhammer K, Tandeau de Marsac N. Phosphorylation of the PII protein (glnB gene product) in the cyanobacterium Synechococcus sp. strain PCC 7942: analysis of in vitro kinase activity. J Bacteriol. 1995;177:5812–5817. doi: 10.1128/jb.177.20.5812-5817.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster-Hartnett D, Kranz R G. The Rhodobacter capsulatus glnB gene is regulated by NtrC at tandem rpoN-independent promoters. J Bacteriol. 1994;176:5171–5176. doi: 10.1128/jb.176.16.5171-5176.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frías J E, Flores E, Herrero A. Requirement of the regulatory protein NtcA for the expression of nitrogen assimilation and heterocyst development genes in the cyanobacterium Anabaena sp. PCC 7120. Mol Microbiol. 1994;14:823–832. doi: 10.1111/j.1365-2958.1994.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 19.Frías J E, Flores E, Herrero A. Nitrate assimilation gene cluster from the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1997;179:477–486. doi: 10.1128/jb.179.2.477-486.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frías J E, Mérida A, Herrero A, Martín-Nieto J, Flores E. General distribution of the nitrogen control gene ntcA in cyanobacteria. J Bacteriol. 1993;175:5710–5713. doi: 10.1128/jb.175.17.5710-5713.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Dominguez M, Florencio F J. Nitrogen availability and electron transport control the expression of glnB gene (encoding PII protein) in the cyanobacterium Synechocystis sp. PCC 6803. Plant Mol Biol. 1997;35:723–734. doi: 10.1023/a:1005846626187. [DOI] [PubMed] [Google Scholar]
- 22.Harano Y, Suzuki I, Maeda S-I, Kaneko T, Tabata S, Omata T. Identification and nitrogen regulation of the cyanase gene from the cyanobacteria Synechocystis sp. strain PCC 6803 and Synechococcus sp. strain PCC 7942. J Bacteriol. 1997;179:5744–5750. doi: 10.1128/jb.179.18.5744-5750.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irmler A, Sanner S, Dierks H, Forchhammer K. Dephosphorylation of the phosphoprotein PII in Synechococcus PCC 7942: identification of an ATP and 2-oxoglutarate-regulated phosphatase activity. Mol Microbiol. 1997;26:81–90. doi: 10.1046/j.1365-2958.1997.5521918.x. [DOI] [PubMed] [Google Scholar]
- 24.Jiang F, Mannervik B, Bergman B. Evidence for redox regulation of the transcription factor NtcA, acting both as an activator and a repressor, in the cyanobacterium Anabaena PCC 7120. Biochem J. 1997;327:513–517. doi: 10.1042/bj3270513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansson M, Nordlund S. Transcription of the glnB and glnA genes in the photosynthetic bacterium Rhodospirillum rubrum. Microbiology. 1996;142:1265–1272. doi: 10.1099/13500872-142-5-1265. [DOI] [PubMed] [Google Scholar]
- 26.Kikuchi H, Aichi M, Suzuki I, Omata T. Positive regulation by nitrite of the nitrate assimilation operon in the cyanobacteria Synechococcus sp. strain PCC 7942 and Plectonema boryanum. J Bacteriol. 1996;178:5822–5825. doi: 10.1128/jb.178.19.5822-5825.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee H M, Flores E, Herrero A, Houmard J, Tandeau de Marsac N. A role for the signal transduction protein PII in the control of nitrate/nitrite uptake in a cyanobacterium. FEBS Lett. 1998;427:291–295. doi: 10.1016/s0014-5793(98)00451-7. [DOI] [PubMed] [Google Scholar]
- 28.Liotenberg S, Campbell D, Rippka R, Houmard J, Tandeau de Marsac N. Effect of the nitrogen source on phycobiliprotein synthesis and cell reserves in a chromatically adapting filamentous cyanobacterium. Microbiology. 1996;142:611–622. doi: 10.1099/13500872-142-3-611. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Magasanik B. The glnB region of the Escherichia coli chromosome. J Bacteriol. 1993;175:7441–7449. doi: 10.1128/jb.175.22.7441-7449.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luque I, Flores E, Herrero A. Molecular mechanism for the operation of nitrogen control in cyanobacteria. EMBO J. 1994;13:2862–2869. doi: 10.1002/j.1460-2075.1994.tb06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magasanik B, Neidhardt F C. Regulation of carbon and nitrogen utilization. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 1318–1325. [Google Scholar]
- 32.Markwell M A, Haas S M, Bieber L L, Tolbert N E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 33.Marqués S, Mérida A, Candau P, Florencio F J. Light-mediated regulation of glutamine-synthetase activity in the unicellular cyanobacterium Synechococcus sp. PCC 6301. Planta. 1992;187:247–253. doi: 10.1007/BF00201947. [DOI] [PubMed] [Google Scholar]
- 34.Martin G B, Thomashow M F, Chelm B K. Bradyrhizobium japonicum glnB, a putative nitrogen-regulatory gene, is regulated by NtrC at tandem promoters. J Bacteriol. 1989;171:5638–5645. doi: 10.1128/jb.171.10.5638-5645.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mérida A, Candau P, Florencio F J. Regulation of glutamine synthetase activity in the unicellular cyanobacterium Synechococcus sp. strain PCC 6803 by the nitrogen source: effect of ammonium. J Bacteriol. 1991;173:4095–4100. doi: 10.1128/jb.173.13.4095-4100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michel-Reydellet N, Desnoues N, Elmerich C, Kaminski P A. Characterization of Azorhizobium caulinodans glnB and glnA genes: involvement of the PII protein in symbiotic nitrogen fixation. J Bacteriol. 1997;179:3580–3587. doi: 10.1128/jb.179.11.3580-3587.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreno S, Patriarca E J, Chiurazzi M, Meza R, Defez R, Lamberti A, Riccio A, Iaccarino M, Espin G. Phenotype of a Rhizobium leguminosarum ntrC mutant. Res. Microbiol. 1993;143:161–171. doi: 10.1016/0923-2508(92)90005-9. [DOI] [PubMed] [Google Scholar]
- 38.Muro-Pastor M I, Reyes J C, Florencio F J. The NADP+-isocitrate dehydrogenase gene (icd) is nitrogen regulated in cyanobacteria. J Bacteriol. 1996;178:4070–4076. doi: 10.1128/jb.178.14.4070-4076.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Omata T. Structure, function and regulation of the nitrate transport system of the cyanobacterium Synechococcus sp. PCC7942. Plant Cell Physiol. 1995;36:207–213. doi: 10.1093/oxfordjournals.pcp.a078751. [DOI] [PubMed] [Google Scholar]
- 40.Ramasubramanian T S, Wei T F, Golden J W. Two Anabaena sp. strain PCC 7120 DNA-binding factors interact with vegetative cell- and heterocyst-specific genes. J Bacteriol. 1994;176:1214–1223. doi: 10.1128/jb.176.5.1214-1223.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reitzer L. Ammonia assimilation and the biosynthesis of glutamine, glutamate, aspartate, asparagine, l-alanine, and d-alanine. In: Neidhardt F, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C: American Society for Microbiology; 1996. pp. 391–407. [Google Scholar]
- 42.Reyes J C, Muro-Pastor M I, Florencio F J. Transcription of glutamine synthetase genes (glnA and glnN) from the cyanobacterium Synechocystis sp. strain PCC 6803 is differently regulated in response to nitrogen availability. J Bacteriol. 1997;179:2678–2689. doi: 10.1128/jb.179.8.2678-2689.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rippka R, Herdman M. Catalogue & Taxonomic Handbook. I. Catalogue of Strains. Paris, France: Institut Pasteur; 1992. Pasteur culture collection of cyanobacterial strains in axenic culture; pp. 1–103. [Google Scholar]
- 44.Suzuki I, Horie N, Sugiyama T, Omata T. Identification and characterization of two nitrogen-regulated genes of the cyanobacterium Synechococcus sp. strain PCC 7942 required for maximum efficiency of nitrogen assimilation. J Bacteriol. 1995;177:290–296. doi: 10.1128/jb.177.2.290-296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsinoremas N F, Castets A M, Harrison M A, Allen J F, Tandeau de Marsac N. Photosynthetic electron transport controls nitrogen assimilation in cyanobacteria by means of posttranslational modification of the glnB gene product. Proc Natl Acad Sci USA. 1991;88:4565–4569. doi: 10.1073/pnas.88.11.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Heeswijk W C, Stegeman B, Hoving S, Molenaar D, Kahn D, Westerhoff H V. An additional PII in Escherichia coli: a new regulatory protein in the glutamine synthetase cascade. FEMS Microbiol Lett. 1995;132:153–157. doi: 10.1111/j.1574-6968.1995.tb07825.x. [DOI] [PubMed] [Google Scholar]
- 47.Van Heeswijk W C, Hoving S, Molenaar D, Stegeman B, Kahn D, Westerhoff H V. An alternative PII protein in the regulation of glutamine synthetase in Escherichia coli. Mol Microbiol. 1996;21:133–146. doi: 10.1046/j.1365-2958.1996.6281349.x. [DOI] [PubMed] [Google Scholar]
- 48.Vega-Palas M A, Flores E, Herrero A. NtcA, a global nitrogen regulator from the cyanobacterium Synechococcus that belongs to the Crp family of bacterial regulators. Mol Microbiol. 1992;6:1853–1859. doi: 10.1111/j.1365-2958.1992.tb01357.x. [DOI] [PubMed] [Google Scholar]
- 49.Wei T F, Ramasubramanian T S, Pu F, Golden J W. Anabaena sp. strain PCC 7120 bifA gene encoding a sequence-specific DNA-binding protein cloned by in vivo transcriptional interference selection. J Bacteriol. 1993;175:4025–4035. doi: 10.1128/jb.175.13.4025-4035.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wray L V, Jr, Atkinson M R, Fisher S H. The nitrogen-regulated Bacillus subtilis nrgAB operon encodes a membrane protein and a protein highly similar to the Escherichia coli glnB-encoded PII protein. J Bacteriol. 1994;176:108–114. doi: 10.1128/jb.176.1.108-114.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zinchenko V, Churin Y, Shestopalov V, Shestakov S. Nucleotide sequence and characterization of the Rhodobacter sphaeroides glnB and glnA genes. Microbiology. 1994;140:2143–2151. doi: 10.1099/13500872-140-8-2143. [DOI] [PubMed] [Google Scholar]