Abstract
Purpose of the review:
To synthesize current evidence on the impact of cardiovascular disease among women living with HIV (WLWH) with a particular focus on disease prevalence, mechanisms and prevention.
Recent Findings:
HIV-related cardiovascular disease risk is 1.5 to 2-fold higher for women than for men. Mechanisms of enhanced risk are multifactorial and include reinforcing pathways between traditional risk factors, metabolic dysregulation, early reproductive aging and chronic immune activation. These pathways influence both the presentation of overt syndromes of myocardial infarction, stroke and heart failure, as well as subclinical pathology such as microvascular dysfunction and cardiac fibrosis. Cardiovascular disease therefore remains a consistent threat to healthy aging among WLWH.
Summary:
While no specific prevention strategies exist, patient-centered risk mitigation approaches that are adaptable to the needs of aging individuals are essential to combat disparities in cardiovascular outcomes among WLWH. Further research into the optimal prevention approach for CVD among WLWH, particularly for women living in under-resourced health systems, is needed.
Keywords: HIV, Cardiovascular disease, women
1. Introduction
The role of HIV as a unique driver of cardiovascular disease (CVD) has been a subject of ongoing inquiry over the past three decades. Early research demonstrated that HIV infection was an independent risk factor for incident CVD, and that risk could be partially mitigated with antiretroviral therapy (ART) (1–4). Yet, despite expanding access to ART, people living with HIV (PLWH) continue to face an increased burden of CVD driven by traditional cardiometabolic risk factors and persistent immune activation and inflammation (5,6).
Understanding CVD risk is especially important among subgroups with historic disparities in cardiovascular care, including women living with HIV (WLWH). Available data suggests that the risk of CVD is increased in WLWH, with WLWH having double the hazard of CVD compared to women without infection (7). This review will address evidence for differential risk among WLWH with regards to specific diagnoses and will explore pathophysiologic pathways that contribute to this risk. We will additionally address opportunities for improved preventive care specific to this unique population.
2. Epidemiology of Cardiovascular Disease in Women with HIV
Relative risk (RR) of CVD is increased in WLWH across a range of disease phenotypes relative to women without HIV. The relative risks of myocardial infarction, stroke, and heart failure in landmark studies which compared PLWH with HIV-negative controls and which reported sex-stratified HIV effects are shown in Figure 1. Across these studies, the magnitude of risk is 1.5 to 2-fold higher among WLWH, excluding one study (8) in which the HIV-associated risk was similar. The following sections will summarize existing epidemiologic data among subtypes of cardiovascular disease by sex and HIV serostatus.
Figure 1: Sex-stratified HIV-associated risks of myocardial infarction, stroke, and heart failure from epidemiologic studies.
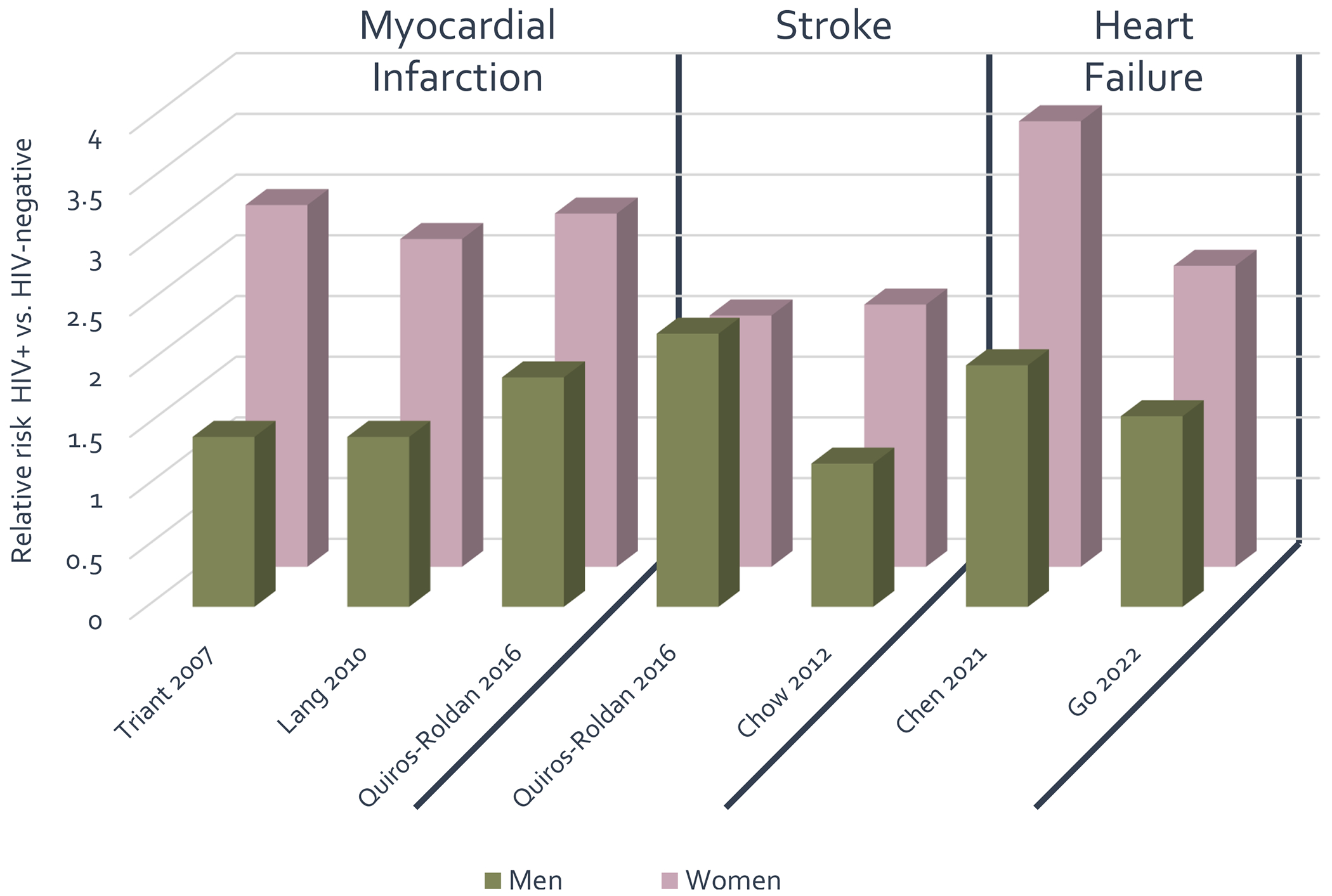
Relative risk of adverse cardiovascular outcomes stratified by sex as published in large-scale epidemiologic studies. Men living with HIV as compared to men without HIV infection are represented in the green bars, women living with HIV as compared to women without HIV infection are represented in purple bars.
Myocardial infarction
Multiple studies have established evidence for a differential risk of myocardial infarction (MI) among WLWH as compared to women without HIV. Triant et al demonstrated a relative risk for MI of 2.98 (95% CI 2.33 to 3.75) among WLWH in comparison to women in the general population after adjusting for traditional risk factors in a large US cohort followed from 1996 to 2004. The RR of MLWH in comparison to men without HIV was 1.40 (95% CI 1.16 to 1.67) in the same cohort (9). Similar documentation of increased risk of MI among WLWH in comparison to women in the general population were obtained in a national sample from France (10), a population-based study in northern Italy (8) and in women from the Veterans Aging Cohort Study in the US (11).
In contrast, the risk of MI in WLWH as directly compared to MLWH varies by time period and population studied (12) and has shown additional interaction by race in at least one study (13). Additional data on adjudicated MI from the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) suggests that MI subtypes differ by sex. Crane et al evaluated incident MI among 28,741 PLWH between 2000 and 2019 at 6 clinical sites in CNICS and demonstrated WLWH represented 27% of PLWH presenting with type II MI in comparison to only 15% of PLWH presenting with type I MI (p=0.001) (14*). It should be noted that these data utilized the third universal definition of MI, and therefore some cases may be re-classified as “myocardial injury” under the fourth universal definition currently in use (15). Despite this limitation, this analysis suggests that different approaches to risk management may be warranted among WLWH to account for differences in MI pathophysiology.
More recently, there has been interest in whether the differential risk of MI may be changing for PLWH, particularly as CVD mortality in the general population appears to be declining (16). A recent analysis of two large cohorts in Massachusetts and California (US) demonstrated that, even as MI incidence rates have declined overall, they have remained stable in PLWH (17) such that the RR for PLWH is actually increasing. While this analysis did not evaluate the trend specifically in WLWH, such a trend has the potential to widen an already-existing disparity.
Stroke
Initial data for an increased risk of stroke among WLWH comes from a 2012 study in Boston, US comparing stroke rates in 4,308 PLWH with over 32,000 controls matched on age, sex and race. In a subgroup analysis by sex, the association between HIV and stroke was insignificant for men, but strongly significant for WLWH compared to women without HIV (adjusted incident rate ratio [IRR]1.76, 95% CI 1.24 – 2.52). Notably, stroke risk was attenuated by a longer duration on ART and increased with increasing HIV viral load (18).
An age-sex interaction was noted in a separate cohort of treatment-naïve PLWH comparing WLWH to MLWH between 1998 and 2011. The overall HR for stroke was 1.96 (95% CI 1.04 – 3.67) in a model adjusted for clinical characteristics. WLWH had a comparatively higher risk of stroke at younger ages (<50 years) whereas older age conferred a higher risk in MLWH (19).
Heart Failure
Multiple studies have corroborated an increased risk of heart failure, defined broadly, among PLWH relative to the general population (7). A recent metanalysis, including data from over 8 million participants, found a RR for heart failure of 1.81 (95% CI 1.51 −2.15) among PLWH compared to those without infection, robust to both fixed and random effects models. The RR specifically in WLWH compared to HIV-negative women was 3.67 (95% CI 1.66 – 8.07) (20**). These results were corroborated in a recent large cohort from Kaiser Healthcare (US) where the adjusted hazard ratio for heart failure was 1.57 (95% CI 1.41 – 1.75) among MLWH but 2.48 (95% CI 1.92 – 3.03) among WLWH compared to uninfected controls. In the overall cohort, the association between HIV and heart failure was only partially attenuated after adjusting for interim acute coronary events, suggesting that the mechanism of heart failure is not entirely driven by an increased rate of coronary disease (21*).
Studies directly comparing WLWH to MLWH have been restricted to single centers, but have demonstrated a similar prevalence of heart failure overall (22,23).
With regards to heart failure subtypes, the Veterans Aging Cohort Study demonstrated a 61% increased risk for heart failure with reduced ejection fraction (HFrEF) and a 21% increased risk of heart failure with preserved ejection fraction (HFpEF) among a predominantly male cohort of PLWH compared to controls (24). A single-center study from Boston, US found WLWH had a four-fold increased incidence rate of heart failure compared to women without infection, but no statistically significant difference in the breakdown of heart failure by subtype. Heart failure with preserved ejection fraction was more common in both groups (71% of WLWH vs 63% of women without HIV, p=0.44). WLWH had an increased hazard for heart failure hospitalization, higher rates of cumulative and cardiovascular mortality, and a lower likelihood of optimal therapy for heart failure with reduced ejection fraction (40% vs 83%, p=0.01) (25).
3. Mechanisms of Cardiovascular Disease
Mechanisms of CVD among WLWH span two categories – those that are related to the pathophysiology of chronic HIV and those that are related to sex-specific risk (Figure 2). In this section, we explore how these mechanisms, including traditional cardiovascular risk factors, HIV-associated metabolic dysregulation, early reproductive aging, and chronic inflammation, each constitute specific pathways that drive CVD among WLWH. We then discuss how they result in subclinical disease, particularly atherosclerosis and microvascular dysfunction, citing evidence from imaging and functional studies.
Figure 2: Intersecting Risk Pathways for Cardiovascular Disease Among Women Living with HIV.
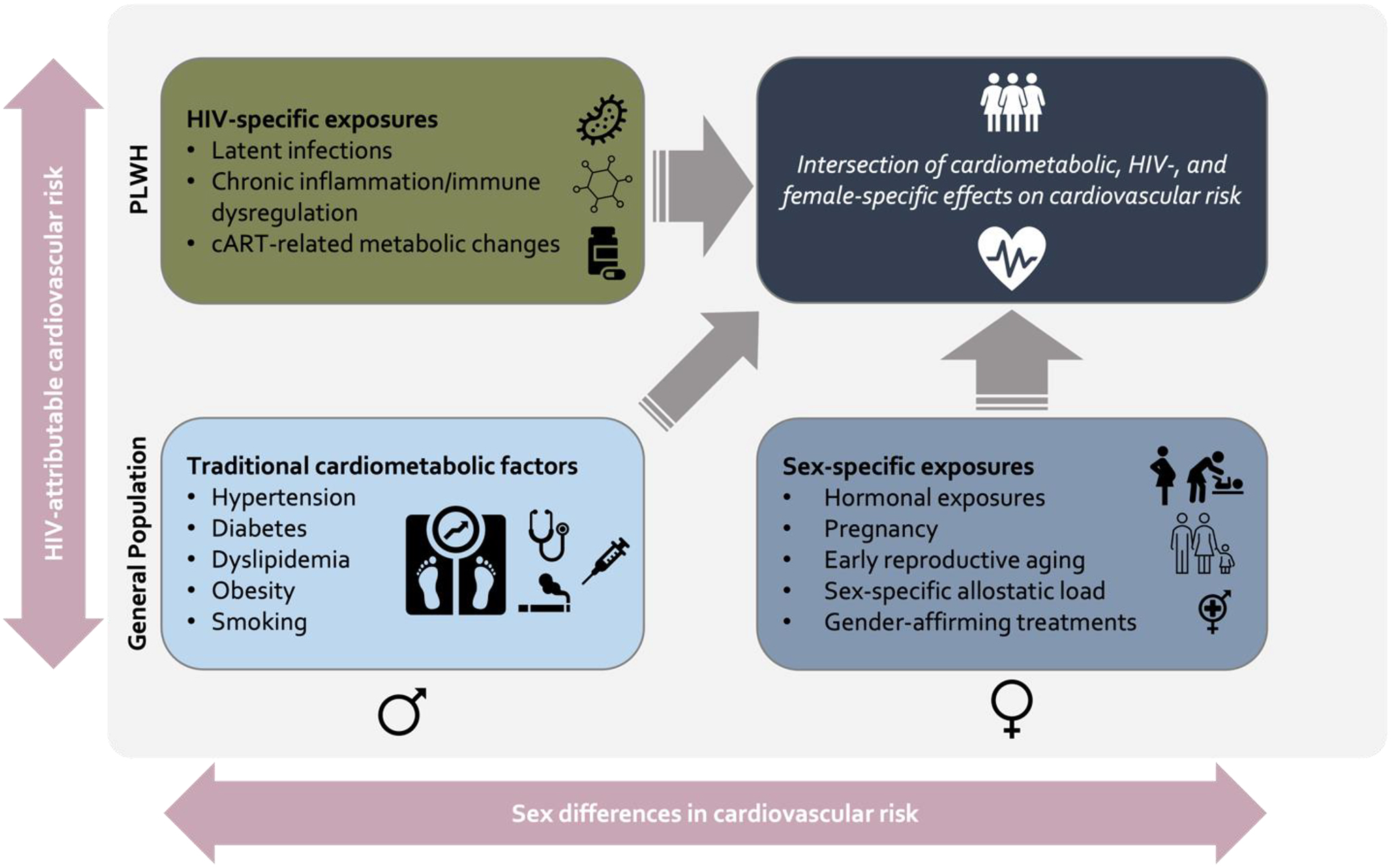
Women living with HIV have two axes on which cardiovascular risk is built – risk according to sex and risk according to HIV serostatus. Along these two axes, specific risk categories intersect to inform the overt presentation of cardiovascular disease. Abbreviations: cART = combination antiretroviral therapy.
Traditional risk factor burden
Traditional risk factor burden is expected to increase overall as PLWH age. An appreciation of the unique contributions to risk among WLWH is therefore essential for prevention of adverse outcomes. This issue was recently addressed in a sample of 3086 persons enrolled in the HIV Outpatient Study at a variety of sites in the US between 2010 and 2017. Cardiometabolic risk factors were used to calculate “excess heart age,” a summary measurement of accumulated risk factors representing the increased risk for adverse cardiovascular events over the lifetime. The highest excess heart age was calculated among WLWH aged 50–59 years (excess heart age 16.1 years, 95% CI 14.8 – 18.0) which exceeded the excess risk for MLWH in all age groups. The overall estimate of excess heart age was also increased in WLWH across all ages (13.1 years) in comparison to estimates for the general US female population obtained from the Behavioral Risk Factor Surveillance Survey (5.4 years) (26*).
In a nationally representative sample from the US followed between 2009 and 2012, WLWH aged 50–64 were more likely than MWLH to have obesity, hypertension and high total cholesterol. WLWH over the age of 65 were also more likely to have diabetes. WLWH across the age spectrum were less likely than MLWH to have completed high school, have private insurance or live above the federal poverty line (27). These data raise concern for sociodemographic disparities among WLWH relative to MLWH as a driver for increased cardiovascular risk among women in the US.
These trends may not be specific to countries with well-resourced health systems. Several studies from multiple countries in sub-Saharan Africa have also identified a higher prevalence of obesity, high cholesterol and the metabolic syndrome among WLWH relative to MLWH (28–32) though the distribution of most risk factors (particularly smoking) varies by population (33). Taken together, these studies highlight the necessity of population-specific risk factor assessments so that appropriate prevention strategies may be developed at the local level.
Metabolic Dysregulation
The accumulation of data from multiple regions documenting a heightened prevalence of obesity, weight change and associated markers of cardiovascular risk has spurred research on metabolic dysregulation in WLWH. The pathophysiology is likely mediated by multiple pathways, including immune activation, interaction with sex hormones and ART-induced metabolic changes (34). Obesity has been linked to higher levels of inflammatory markers associated with CVD among PLWH (35). WLWH with a high visceral fat burden have also been shown to have higher systolic blood pressure and hemoglobin A1c, as well as higher 24-hour urinary aldosterone secretion (36). These findings suggest an inappropriate activation of the renin-angiotensin-aldosterone system related to HIV infection, which has been tied to cardiac fibrosis and remodeling of the coronary vasculature in physiologic studies (37).
Large-scale data on the role of ART in promoting obesity and weight gain comes from both the ACCORD study and the Women’s Interagency Health Study, which both demonstrated a shift into higher weight categories among WLWH after several years of treatment with ART (38,39). The REPRIEVE study linked higher rates of obesity and increased BMI to integrase inhibitor use, with positive interaction by female sex (40). Additional studies have linked tenofovir alafenamide (TAF) to obesity (41) and adverse changes in lipid profile (42). The role of ART-induced changes in subsequent CVD risk will remain a source of ongoing investigation.
Early reproductive aging
Changes in metabolic function and overall cardiovascular health may be additionally influenced by a phenomenon of early reproductive aging in WLWH. In the general population, earlier age at menopause has been correlated with an increase in cardiovascular risk, including the risk for MI, stroke and heart failure (43–45). Studies of early menopause have been facilitated by the widespread adoption of anti-Müllerian hormone (AMH), which declines steadily prior to menopause onset, allowing reliable assessment of gonadal age in WLWH (46). Analysis of the Women’s Interagency Health Study found that age-adjusted AMH levels were 16% lower in WLWH with well-controlled disease in comparison to uninfected controls, and 26% lower in WLWH with detectable viremia, suggesting both that HIV is related to early menopause and that inadequate treatment may further drive the process of early reproductive aging. Interestingly, AMH levels were correlated with CD4+ lymphocyte count regardless of actual HIV serostatus, suggesting that CD4 cells may play a role in the granulosa cells of the ovarian follicle that produce AMH (47).
With regards to CVD risk, WLWH with undetectable AMH levels have been shown to have a higher burden of coronary plaque on coronary artery computed tomography than WLWH with normal AMH levels even after adjustment for age, cardiovascular risk factors and markers of immune activation (48). Among 1449 WLWH enrolled in the REPRIEVE trial, progressive increases in waist circumference were associated with a spectrum of menopausal age – from premenopausal with detectable AMH to pre-menopausal with undetectable AMH to post-menopausal. These data support a connection between early reproductive aging, impaired metabolic function and subsequent risk for CVD (49*). Early reproductive aging was additionally correlated with residence in sub-Saharan Africa and Latin America, making this the first study to describe geographic disparities in reduced ovarian reserve. In high-resourced settings, WLWH have been shown to receive hormonal therapy for early menopause at inappropriately low rates, suggesting that this condition may be underrecognized and its subsequent risks undertreated regardless of world region (50).
Chronic inflammation and immune dysregulation
Chronic HIV is characterized by dysregulated immune activity even after treatment with ART. Markers of inflammation (e.g., interleukin [IL]-6, IL-4, IL-1ß, CRP, tumor necrosis factor-α1 [TNF-α1], TNF-α2) and monocyte activation (e.g., soluble CD163 [sCD163] and sCD14) are frequently elevated in virally suppressed PWH and are independently associated with atherosclerosis, cardiovascular events and mortality (51–56) Increases in systemic levels of monocyte activation and expression of blood IL-32 isoforms have been correlated with coronary plaque, carotid atherosclerosis and myocardial fibrosis in WLWH relative to women without HIV infection (57–59). In addition, elevated tissue factor-positive microparticle (MP-TF) activity, a marker of potential clotting risk, was associated with coronary plaque specifically in WLWH in the Women’s Interagency HIV Study (60). Relative increases in inflammatory markers have been tied to obesity in a recent Kenyan cohort, suggesting that there may be additive risk associated with an abnormal body weight among WLWH (61).
Studies have also suggested that inflammatory markers may be elevated in WLWH relative to MLWH, though the exact patterns depend on the population and biomarkers studied (62–64). Females generally mount a more robust initial immune response to HIV with lower viral loads (65) but then demonstrate accelerated disease progression compared with men (66). Persistent maladaptive patterns of immune activation have therefore been postulated as one potential contributor to the increased susceptibility to CVD identified in WLWH (67,68).
Immunological differences between sexes may arise from combined effects of genetic differences, sex hormone balance and environmental influences. For example, although one of the two copies of the X-chromosome are usually inactivated, X-linked genes such as Toll-like receptor 7 (TLR7), which senses viral RNA and elicits strong type-1 interferon production by dendritic cells, can escape from inactivation in some proportion of cells (69). Estrogen and androgens also alter the immune response via critical regulation of gene expression in macrophages, monocytes, dendritic cells and lymphocytes. Early reproductive aging may therefore trigger immune system changes as one mechanism for modulating CVD risk among older WLWH (70,71). Likewise, sex hormone fluctuations also have an impact in the microbiome composition of the gut, which could have consequences for gut permeability, microbial translocation and inflammation as women age (72,73). In a cross-sectional analysis of US women, there was an overall difference in the gut microbiome composition between pre- and post-menopausal women with HIV, but not in women without HIV (74). Further research is warranted to determine potential mechanisms of these microbiome changes and their implications for CVD among WLWH.
Subclinical atherosclerosis
The recognized mechanistic differences in CVD pathogenesis have led to a substantial literature on subclinical atherosclerosis among PLWH over the past decade. Investigators have used non-invasive imaging modalities—particularly carotid ultrasound and coronary CT angiography (CCTA)—to investigate HIV effects and explore potential mechanisms of disease. Recent meta-analyses have synthesized the data on coronary artery disease (75) and carotid artery disease (76) in studies comparing PLWH with HIV-negative persons.
Soares et al demonstrated a similar prevalence of coronary artery calcification and total coronary plaque among PLWH compared to people without HIV despite younger age and fewer traditional risk factors. Notably, PLWH had higher non-calcified coronary plaque burden. In meta-regressions, coronary plaque prevalence was lower in studies with higher percentage of women, but the association between male sex and disease appeared weaker when specifically assessing the relationship among PLWH (75). In a study that directly compared ART-treated men and women (77), male sex was strongly associated with total and high-risk plaque features. Additionally, sex was not a significant predictor of peri-coronary fat attenuation index, a marker of tissue inflammation and subsequent coronary events (78).
Subclinical carotid artery disease appears to be modestly higher in PLWH, with an absolute estimated difference of +0.27mm (95% CI 0.04–0.49) higher carotid intima-media thickness (CIMT)] compared to HIV-negative persons in a meta-analysis of 17 cross-sectional studies (76). CIMT was generally higher in males, although there was substantial heterogeneity making the validity of the meta-regression uncertain.
Two recent studies from sub-Saharan Africa, where >2/3 of all WLWH reside, provide a broader picture of subclinical atherosclerosis in PLWH outside of high-income countries (79,80). In these studies, neither HIV nor sex was a predictor of CIMT progression over 4 years in Uganda (80). In a CCTA study of coronary disease in Uganda, WLWH had 4x higher odds of plaque compared to MLWH, while men without HIV had 3x higher odds of plaque than women without HIV in models that adjusted for ASCVD risk and age (p for interaction 0.02) (79*). Why sex effects may vary across different countries and contexts has not been studied in detail.
Microvascular dysfunction and reduced coronary flow reserve
Disease in the coronary microcirculation has been linked both to abnormalities in cardiac structure and function, and to an increased risk of cardiovascular mortality in the general population (81,82). Preliminary studies among PLWH suggest that excess risk may be conferred by HIV infection. A pair of studies published by Iantoro et al utilized cardiac MRI with isometric handgrip exercise to assess two parameters of cardiac endothelial function (coronary blood flow and coronary artery surface area change) among PLWH in comparison to HIV-negative controls matched by cardiovascular risk factors. PLWH had marked reductions in cardiac endothelial function as compared to HIV negative controls. These studies additionally demonstrated that both IL-6 levels and epicardial adipose tissue were inversely related to cardiac endothelial function among PLWH, suggesting that local inflammation and fat deposition may contribute to the pathophysiology of this condition (83,84). A similar study, also utilizing cardiac MRI and isometric handgrip exercise, additionally demonstrated PLWH have a higher level of circulating PCSK-9 levels despite matching with controls on LDL level, additionally highlighting the association with deranged lipid metabolism in HIV infection (85). These findings have not yet been assessed in studies designed to evaluate interactions by sex.
In contrast to the above findings, Knudsen et al did not find a difference between PLWH and HIV-negative controls using rubidium PET-CT with adenosine stress to assess myocardial blood flow, an alternative method for assessing microvascular dysfunction (86). However, a follow up analysis of 94 PLWH with an analogous protocol demonstrated significantly lower myocardial flow reserve among WLWH as compared to MLWH (>45% women vs 23% men) despite younger age and a lower overall burden of traditional ASCVD risk factors (87). Taken together, these findings suggest that microvascular dysfunction may play an important role in the pathophysiology of cardiac disease among WLWH, and highlight the importance of ongoing sex-stratified investigations to clarify optimal treatment strategies.
4. Primary Prevention Strategies
Disparities in the prevalence of cardiovascular disease have led to a growing interest in appropriate prevention strategies for WLWH. To date, consensus statements have largely followed guidelines for the general population. There are currently no firm recommendations for the use of HIV-specific risk calculators, nor are there recommendations for advanced imaging in PLWH to refine risk prediction tools. However, recently updated guidelines from the European AIDS Clinical Society recommend new, strict targets for LDL lowering, including reducing LDL level to <55mg/dL (1.4 mmol/L), among PLWH in the highest risk category for ASCVD (88). For prevention in WLWH, US guidelines specifically recommend close attention to risk-modifying factors including early menopause and adverse pregnancy outcomes such as pre-eclampsia (89).
Concerns regarding underestimation of CVD risk and under-prescribing of cardiovascular therapies in WLWH (90,91) need to be balanced with the risk of progressive frailty as WLWH age (92). Female sex has been associated with an increased risk of frailty across world regions among PLWH in the REPREIVE trial (93*). The optimal approach to intensive risk factor management when risk is most modifiable, followed by appropriate de-escalation in response to age, has not been evaluated among WLWH.
5. Conclusions and Future Directions
Cardiovascular disease represents a modifiable threat to the long-term health of women aging with HIV. While current management mirrors the general population, ongoing research is investigating therapies specifically targeted to PLWH. These include the REPREIVE trial – Randomized Trial to Prevent Vascular Events in HIV – an ongoing study of pitavastatin to reduce cardiovascular risk among PLWH (94) and the CANA trial – Effect of IL-1ß inhibition on Inflammation and Cardiovascular risk – a study of the monoclonal antibody canakinumab to reduced inflammatory markers in PLWH at risk for cardiovascular disease (ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02272946). Insights from this ongoing work will continue to shed light on CVD management among PLWH and will provide opportunity to further improve care for WLWH.
Key points:
Cardiovascular disease represents a modifiable threat to the long-term health of women aging with HIV.
Available data suggest that WLWH face a unique burden of multiple cardiovascular outcomes, including myocardial infarction, stroke and heart failure.
Pathophysiologic pathways for the development of cardiovascular disease intersect along the axes of sex- and HIV-specific risks and include the overlap of traditional risk factor burden, metabolic derangement, early reproductive aging and chronic immune dysregulation.
Current prevention strategies build predominantly on risk factor modification tailored to an individual’s age and comorbidities.
Research focused on the development of new risk-mitigation tools is needed and should intentionally enroll WLWH with a specific focus on the interaction between sex and CVD.
Financial support and sponsorship:
This work was supported in part by the following grants/awards – NIH training grant T32 HL007828 to Dr. Shakil, NIH project grants 1R01HL146267 and K24AI157882 to Dr. Zanni, NIH project grants 1K01HL147723 and P30 AI027757 to TMT.
Disclosures:
CTL has received research funding from Medtronic Foundation and Gilead Sciences and has served on an advisory board for Esperion Pharmaceuticals. Dr. Zanni is a Principal Investigator on an Investigator-Initiated Research Grant from Gilead to her institution (Massachusetts General Hospital).
Reference List
- 1.Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, et al. Coronary Heart Disease in HIV-Infected Individuals: JAIDS J Acquir Immune Defic Syndr. 2003. Aug;33(4):506–12. [DOI] [PubMed] [Google Scholar]
- 2.Tabib A, Leroux C, Mornex JF, Loire R. Accelerated coronary atherosclerosis and arteriosclerosis in young human-immunodeficiency-virus-positive patients. Coron Artery Dis. 2000. Feb;11(1):41–6. [DOI] [PubMed] [Google Scholar]
- 3.Freiberg MS, Chang CCH, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV Infection and the Risk of Acute Myocardial Infarction. JAMA Intern Med. 2013. Apr 22;173(8):614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips AN, Carr A, Neuhaus J, Visnegarwala F, Prineas R, Burman WJ, et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antivir Ther. 2008;13(2):177–87. [DOI] [PubMed] [Google Scholar]
- 5.Hunt PW, Lee SA, Siedner MJ. Immunologic Biomarkers, Morbidity, and Mortality in Treated HIV Infection. J Infect Dis. 2016. Oct 1;214 Suppl 2:S44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Losina E, Hyle EP, Borre ED, Linas BP, Sax PE, Weinstein MC, et al. Projecting 10-year, 20-year, and Lifetime Risks of Cardiovascular Disease in Persons Living With Human Immunodeficiency Virus in the United States. Clin Infect Dis Off Publ Infect Dis Soc Am. 2017. Oct 15;65(8):1266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alonso A, Barnes AE, Guest JL, Shah A, Shao IY, Marconi V. HIV Infection and Incidence of Cardiovascular Diseases: An Analysis of a Large Healthcare Database. J Am Heart Assoc. 2019. Jul 16;8(14):e012241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quiros-Roldan E, Raffetti E, Focà E, Brianese N, Ferraresi A, Paraninfo G, et al. Incidence of cardiovascular events in HIV-positive patients compared to general population over the last decade: a population-based study from 2000 to 2012. AIDS Care. 2016. Dec;28(12):1551–8. [DOI] [PubMed] [Google Scholar]
- 9.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007. Jul;92(7):2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang S, Mary-Krause M, Cotte L, Gilquin J, Partisani M, Simon A, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS Lond Engl. 2010. May 15;24(8):1228–30. [DOI] [PubMed] [Google Scholar]
- 11.Womack JA, Chang CCH, So-Armah KA, Alcorn C, Baker JV, Brown ST, et al. HIV infection and cardiovascular disease in women. J Am Heart Assoc. 2014. Oct 16;3(5):e001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baldé A, Lang S, Wagner A, Ferrières J, Montaye M, Tattevin P, et al. Trends in the risk of myocardial infarction among HIV-1-infected individuals relative to the general population in France: Impact of gender and immune status. PloS One. 2019;14(1):e0210253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feinstein MJ, Nance RM, Drozd DR, Ning H, Delaney JA, Heckbert SR, et al. Assessing and Refining Myocardial Infarction Risk Estimation Among Patients With Human Immunodeficiency Virus: A Study by the Centers for AIDS Research Network of Integrated Clinical Systems. JAMA Cardiol. 2017. Feb 1;2(2):155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *14.Crane HM, Nance RM, Whitney BM, Heckbert SR, Budoff M, High K, et al. Brief Report: Differences in Types of Myocardial Infarctions Among People Aging With HIV. J Acquir Immune Defic Syndr 1999. 2021. Feb 1;86(2):208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]; This clinical study highlights differences in type of MI presentation between WLWH and MLWH. WLWH are more likely to present with type II MI, emphasizing that classic atherosclerotic plaque rupture is not the sole mechanism by which HIV-associated myocardial infarction impacts women. This study underlines the need for risk assessment tools that do not rely on the paradigm of traditional atherosclerotic disease development.
- 15.Thygesen K What’s new in the Fourth Universal Definition of Myocardial infarction? Eur Heart J. 2018. Nov 7;39(42):3757–8. [DOI] [PubMed] [Google Scholar]
- 16.Amini M, Zayeri F, Salehi M. Trend analysis of cardiovascular disease mortality, incidence, and mortality-to-incidence ratio: results from global burden of disease study 2017. BMC Public Health. 2021. Feb 25;21(1):401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverberg MJ, Lyass A, Hurley L, Ehrbar R. Trends in Myocardial Infarction Risk by HIV Status in Two US Healthcare Systems. CROI; 2022. Feb. [Google Scholar]
- 18.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of Ischemic Stroke Incidence in HIV-Infected and Non-HIV-Infected Patients in a U.S. Health Care System. J Acquir Immune Defic Syndr 1999. 2012. Aug 1;60(4):351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chow FC, Wilson MR, Wu K, Ellis RJ, Bosch RJ, Linas BP. Stroke incidence is highest in women and non-Hispanic blacks living with HIV in the AIDS Clinical Trials Group Longitudinal Linked Randomized Trials cohort. AIDS Lond Engl. 2018. Jun 1;32(9):1125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **20.Chen Y, Gao Y, Zhou Y, Li X, Wang H, Polonsky TS, et al. Human Immunodeficiency Virus Infection and Incident Heart Failure: A Meta-Analysis of Prospective Studies. J Acquir Immune Defic Syndr 1999. 2021. May 1;87(1):741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study is the largest metanalysis assessing risk for heart failure among PLWH to date. The authors highlight both the increased risk of heart failure among PLWH as compared to the general population, and additionally highlight the sharp increase in relative risk when female-only participants are evaluated.
- *21.Go AS, Reynolds K, Avula HR, Towner WJ, Hechter RC, Horberg MA, et al. Human Immunodeficiency Virus Infection and Variation in Heart Failure Risk by Age, Sex, and Ethnicity: The HIV HEART Study. Mayo Clin Proc. 2022. Mar;97(3):465–79. [DOI] [PMC free article] [PubMed] [Google Scholar]; This additional, recently published large database analysis mirrors the findings of Chen et al highlighted above. The study additionally demonstrates that excess HF risk among PLWH is only partially attenuated by adjusting for incident acute coronary syndromes, suggesting that HF risk is not solely driven by an increased burden of coronary events.
- 22.Feinstein MJ, Steverson AB, Ning H, Pawlowski AE, Schneider D, Ahmad FS, et al. Adjudicated Heart Failure in HIV-Infected and Uninfected Men and Women. J Am Heart Assoc. 2018. Nov 6;7(21):e009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sliwa K, Carrington MJ, Becker A, Thienemann F, Ntsekhe M, Stewart S. Contribution of the human immunodeficiency virus/acquired immunodeficiency syndrome epidemic to de novo presentations of heart disease in the Heart of Soweto Study cohort. Eur Heart J. 2012. Apr;33(7):866–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freiberg MS, Chang CCH, Skanderson M, Patterson OV, DuVall SL, Brandt CA, et al. Association Between HIV Infection and the Risk of Heart Failure With Reduced Ejection Fraction and Preserved Ejection Fraction in the Antiretroviral Therapy Era: Results From the Veterans Aging Cohort Study. JAMA Cardiol. 2017. May 1;2(5):536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janjua SA, Triant VA, Addison D, Szilveszter B, Regan S, Staziaki PV, et al. HIV Infection and Heart Failure Outcomes in Women. J Am Coll Cardiol. 2017. Jan 3;69(1):107–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *26.Thompson-Paul AM, Palella FJ, Rayeed N, Ritchey MD, Lichtenstein KA, Patel D, et al. Excess heart age in adult outpatients in routine HIV care. AIDS Lond Engl. 2019. Oct 1;33(12):1935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors utilize a novel concept for modeling long-term cardiovascular risk, termed “excess heart age” to compare risk factor burden in WLWH to MLWH and to the general population. Their approach emphasizes the excess risk associated with female sex across the age spectrum for PLWH.
- 27.Frazier EL, Sutton MY, Tie Y, Fagan J, Fanfair RN. Differences by Sex in Cardiovascular Comorbid Conditions Among Older Adults (Aged 50–64 or ≥65 Years) Receiving Care for Human Immunodeficiency Virus. Clin Infect Dis Off Publ Infect Dis Soc Am. 2019. Nov 27;69(12):2091–100. [DOI] [PubMed] [Google Scholar]
- 28.Bloomfield GS, Hogan JW, Keter A, Sang E, Carter EJ, Velazquez EJ, et al. Hypertension and obesity as cardiovascular risk factors among HIV seropositive patients in Western Kenya. PloS One. 2011;6(7):e22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaziano TA, Abrahams-Gessel S, Gomez-Olive FX, Wade A, Crowther NJ, Alam S, et al. Cardiometabolic risk in a population of older adults with multiple co-morbidities in rural south africa: the HAALSI (Health and Aging in Africa: longitudinal studies of INDEPTH communities) study. BMC Public Health. 2017. Feb 17;17(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houle B, Gaziano TA, Angotti N, Mojola SA, Kabudula CW, Tollman SM, et al. Hypertension incidence among middle-aged and older adults: findings from a 5-year prospective study in rural South Africa, 2010–2015. BMJ Open. 2021. Dec 7;11(12):e049621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ketelaar EJ, Vos AG, Godijk NG, Scheuermaier K, Devillé W, Tempelman H, et al. Ideal Cardiovascular Health Index and Its Determinants in a Rural South African Population. Glob Heart. 15(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamooya BM, Mulenga LB, Masenga SK, Fwemba I, Chirwa L, Siwingwa M, et al. Metabolic syndrome in Zambian adults with human immunodeficiency virus on antiretroviral therapy: Prevalence and associated factors. Medicine (Baltimore). 2021. Apr 9;100(14):e25236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kazooba P, Kasamba I, Mayanja BN, Lutaakome J, Namakoola I, Salome T, et al. Cardiometabolic risk among HIV-POSITIVE Ugandan adults: prevalence, predictors and effect of long-term antiretroviral therapy. Pan Afr Med J. 2017;27:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godfrey C, Bremer A, Alba D, Apovian C, Koethe JR, Koliwad S, et al. Obesity and Fat Metabolism in Human Immunodeficiency Virus-Infected Individuals: Immunopathogenic Mechanisms and Clinical Implications. J Infect Dis. 2019. Jul 2;220(3):420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conley LJ, Bush TJ, Rupert AW, Sereti I, Patel P, Brooks JT, et al. Obesity is associated with greater inflammation and monocyte activation among HIV-infected adults receiving antiretroviral therapy. AIDS Lond Engl. 2015. Oct 23;29(16):2201–7. [DOI] [PubMed] [Google Scholar]
- 36.Lo J, Looby SED, Wei J, Adler GK, Grinspoon SK. Increased aldosterone among HIV-infected women with visceral fat accumulation. AIDS Lond Engl. 2009. Nov 13;23(17):2366–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srinivasa S, Thomas TS, Feldpausch MN, Adler GK, Grinspoon SK. Coronary Vasculature and Myocardial Structure in HIV: Physiologic Insights From the Renin-Angiotensin-Aldosterone System. J Clin Endocrinol Metab. 2021. Nov 19;106(12):3398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koethe JR, Jenkins CA, Lau B, Shepherd BE, Justice AC, Tate JP, et al. Rising Obesity Prevalence and Weight Gain Among Adults Starting Antiretroviral Therapy in the United States and Canada. AIDS Res Hum Retroviruses. 2016. Jan;32(1):50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma A, Bynum SA, Schneider MF, Cox C, Tien PC, Hershow RC, et al. Changes in Body Mass Index Following HAART Initiation among HIV-Infected Women in the Women’s Interagency HIV Study. J AIDS Clin Res. 2014;5:1000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kileel EM, Lo J, Malvestutto C, Fitch KV, Zanni MV, Fichtenbaum CJ, et al. Assessment of Obesity and Cardiometabolic Status by Integrase Inhibitor Use in REPRIEVE: A Propensity-Weighted Analysis of a Multinational Primary Cardiovascular Prevention Cohort of People With Human Immunodeficiency Virus. Open Forum Infect Dis. 2021. Dec;8(12):ofab537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCann K, Shah S, Hindley L, Hill A, Qavi A, Simmons B, et al. Implications of weight gain with newer anti-retrovirals: 10-year predictions of cardiovascular disease and diabetes. AIDS Lond Engl. 2021. Aug 1;35(10):1657–65. [DOI] [PubMed] [Google Scholar]
- 42.Plum PE, Maes N, Sauvage AS, Frippiat F, Meuris C, Uurlings F, et al. Impact of switch from tenofovir disoproxil fumarate-based regimens to tenofovir alafenamide-based regimens on lipid profile, weight gain and cardiovascular risk score in people living with HIV. BMC Infect Dis. 2021. Sep 6;21(1):910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Appiah D, Schreiner PJ, Demerath EW, Loehr LR, Chang PP, Folsom AR. Association of Age at Menopause With Incident Heart Failure: A Prospective Cohort Study and Meta-Analysis. J Am Heart Assoc. 2016. Jul 28;5(8):e003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D. Early menopause predicts future coronary heart disease and stroke: the Multi-Ethnic Study of Atherosclerosis. Menopause N Y N. 2012. Oct;19(10):1081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshida Y, Chen Z, Baudier RL, Krousel-Wood M, Anderson AH, Fonseca VA, et al. Early Menopause and Cardiovascular Disease Risk in Women With or Without Type 2 Diabetes: A Pooled Analysis of 9,374 Postmenopausal Women. Diabetes Care. 2021. Nov;44(11):2564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scherzer R, Greenblatt RM, Merhi ZO, Kassaye S, Lambert-Messerlian G, Maki PM, et al. Use of antimüllerian hormone to predict the menopausal transition in HIV-infected women. Am J Obstet Gynecol. 2017. Jan;216(1):46.e1–46.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scherzer R, Bacchetti P, Messerlian G, Goderre J, Maki PM, Seifer DB, et al. Impact of CD4+ lymphocytes and HIV infection on Anti-Müllerian Hormone levels in a large cohort of HIV-infected and HIV-uninfected women. Am J Reprod Immunol N Y N 1989. 2015. Mar;73(3):273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Looby SE, Fitch KV, Srinivasa S, Lo J, Rafferty D, Martin A, et al. Reduced ovarian reserve relates to monocyte activation and subclinical coronary atherosclerotic plaque in women with HIV. AIDS Lond Engl. 2016. Jan 28;30(3):383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *49.Zanni MV, Currier JS, Kantor A, Smeaton L, Rivard C, Taron J, et al. Correlates and Timing of Reproductive Aging Transitions in a Global Cohort of Midlife Women With Human Immunodeficiency Virus: Insights From the REPRIEVE Trial. J Infect Dis. 2020. Jul 9;222(Suppl 1):S20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study utilizes an extremely diverse patient sample to correlate evidence of metabolic change (as measured by waist circumference) with early reproductive aging (assessed across a spectrum using both menstruation pattern and AMH) and highlights the excess risk in WLWH. Additionally, this study is the first to demonstrate a geographic disparity in early reproductive aging with women living in sub-Saharan Africa and Latin America/Caribbean at increased risk relative to other world regions.
- 50.Bullington BW, Edmonds A, Ramirez C, Rahangdale L, Neal-Perry G, Konkle-Parker D, et al. Premature and early menopause among US women with or at risk for HIV. Menopause N Y N. 2022. Mar 25; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bahrami H, Budoff M, Haberlen SA, Rezaeian P, Ketlogetswe K, Tracy R, et al. Inflammatory Markers Associated With Subclinical Coronary Artery Disease: The Multicenter AIDS Cohort Study. J Am Heart Assoc. 5(6):e003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nordell AD, McKenna M, Borges ÁH, Duprez D, Neuhaus J, Neaton JD, et al. Severity of Cardiovascular Disease Outcomes Among Patients With HIV Is Related to Markers of Inflammation and Coagulation. J Am Heart Assoc. 3(3):e000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanna DB, Lin J, Post WS, Hodis HN, Xue X, Anastos K, et al. Association of Macrophage Inflammation Biomarkers With Progression of Subclinical Carotid Artery Atherosclerosis in HIV-Infected Women and Men. J Infect Dis. 2017. May 1;215(9):1352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ, Kingsley LA, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis. 2015. Apr 15;211(8):1219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoel H, Ueland T, Knudsen A, Kjær A, Michelsen AE, Sagen EL, et al. Soluble Markers of Interleukin 1 Activation as Predictors of First-Time Myocardial Infarction in HIV-Infected Individuals. J Infect Dis. 2020. Feb 3;221(4):506–9. [DOI] [PubMed] [Google Scholar]
- 56.Wanjalla CN, Temu TM, Mashayekhi M, Warren CM, Shepherd BE, Gangula R, et al. IL-17A is associated with flow-mediated dilation and IL-4 with carotid plaque in persons with HIV. AIDS Lond Engl. 2022. Feb 14; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis. 2013. Dec 1;208(11):1737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.El-Far M, Hanna DB, Durand M, Larouche-Anctil E, Sylla M, Chartrand-Lefebvre C, et al. Brief Report: Subclinical Carotid Artery Atherosclerosis Is Associated With Increased Expression of Peripheral Blood IL-32 Isoforms Among Women Living With HIV. J Acquir Immune Defic Syndr 1999. 2021. Oct 1;88(2):186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zanni MV, Awadalla M, Toribio M, Robinson J, Stone LA, Cagliero D, et al. Immune Correlates of Diffuse Myocardial Fibrosis and Diastolic Dysfunction Among Aging Women With Human Immunodeficiency Virus. J Infect Dis. 2020. Mar 28;221(8):1315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin J, Xue X, Anastos K, Cohen MH, Gange SJ, Lazar JM, et al. Elevated Microparticle Tissue Factor Activity Is Associated With Carotid Artery Plaque in HIV-Infected Women. J Acquir Immune Defic Syndr 1999. 2019. May 1;81(1):36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Temu TM, Wagoner J, Masyuko S, O’Connor A, Zifodya JS, Macharia P, et al. Central obesity is a contributor to systemic inflammation and monocyte activation in virally suppressed adults with chronic HIV in Kenya. AIDS Lond Engl. 2021. Sep 1;35(11):1723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siedner MJ, Zanni M, Tracy RP, Kwon DS, Tsai AC, Kakuhire B, et al. Increased Systemic Inflammation and Gut Permeability Among Women With Treated HIV Infection in Rural Uganda. J Infect Dis. 2018. Aug 14;218(6):922–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Looby SE, Kantor A, Burdo TH, Currier JS, Fichtenbaum CJ, Overton ET, et al. Factors Associated with Systemic Immune Activation Indices in a Global Primary Cardiovascular Disease Prevention Cohort of People with HIV on Antiretroviral Therapy. Clin Infect Dis. 2022. Mar 2;ciac166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Santinelli L, Ceccarelli G, Borrazzo C, Innocenti GP, Frasca F, Cavallari EN, et al. Sex-related differences in markers of immune activation in virologically suppressed HIV-infected patients. Biol Sex Differ. 2020. May 1;11(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Addo MM, Altfeld M. Sex-Based Differences in HIV Type 1 Pathogenesis. J Infect Dis. 2014. Jul 15;209(Suppl 3):S86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scully EP. Sex Differences in HIV Infection. Curr HIV/AIDS Rep. 2018. Apr;15(2):136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raghavan A, Rimmelin DE, Fitch KV, Zanni MV. Sex Differences in Select Non-communicable HIV-Associated Comorbidities: Exploring the Role of Systemic Immune Activation/Inflammation. Curr HIV/AIDS Rep. 2017. Dec;14(6):220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mathad JS, Gupte N, Balagopal A, Asmuth D, Hakim J, Santos B, et al. Sex-Related Differences in Inflammatory and Immune Activation Markers Before and After Combined Antiretroviral Therapy Initiation. J Acquir Immune Defic Syndr 1999. 2016. Oct 1;73(2):123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016. Oct;16(10):626–38. [DOI] [PubMed] [Google Scholar]
- 70.Shepherd R, Cheung AS, Pang K, Saffery R, Novakovic B. Sexual Dimorphism in Innate Immunity: The Role of Sex Hormones and Epigenetics. Front Immunol. 2020;11:604000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taneja V Sex Hormones Determine Immune Response. Front Immunol. 2018;9:1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mayneris-Perxachs J, Arnoriaga-Rodríguez M, Luque-Córdoba D, Priego-Capote F, Pérez-Brocal V, Moya A, et al. Gut microbiota steroid sexual dimorphism and its impact on gonadal steroids: influences of obesity and menopausal status. Microbiome. 2020. Sep 20;8(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao H, Chen J, Li X, Sun Q, Qin P, Wang Q. Compositional and functional features of the female premenopausal and postmenopausal gut microbiota. FEBS Lett. 2019. Sep;593(18):2655–64. [DOI] [PubMed] [Google Scholar]
- 74.Peters BA, Xue X, Wang Z, Usyk M, Santoro N, Sharma A, et al. Menopausal status and observed differences in the gut microbiome in women with and without HIV infection. Menopause N Y N. 2021. Jan 11;28(5):491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soares C, Samara A, Yuyun MF, Echouffo-Tcheugui JB, Masri A, Samara A, et al. Coronary Artery Calcification and Plaque Characteristics in People Living With HIV: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2021. Oct 5;10(19):e019291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Msoka TF, Van Guilder GP, van Furth M, Smulders Y, Meek SJ, Bartlett JA, et al. The effect of HIV infection, antiretroviral therapy on carotid intima-media thickness: A systematic review and meta-analysis. Life Sci. 2019. Oct 15;235:116851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Foldyna B, Fourman LT, Lu MT, Mueller ME, Szilveszter B, Neilan TG, et al. Sex Differences in Subclinical Coronary Atherosclerotic Plaque Among Individuals With HIV on Antiretroviral Therapy. JAIDS J Acquir Immune Defic Syndr. 2018. Aug 1;78(4):421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jeudy J, Patel P, George N, Burrowes S, Husson J, Chua J, et al. Assessment of coronary inflammation in antiretroviral treated people with HIV infection and active HIV/hepatitis C virus co-infection. AIDS Lond Engl. 2022. Mar 1;36(3):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *79.Longenecker CT, Bogorodskaya M, Margevicius S, Nazzinda R, Bittencourt MS, Erem G, et al. Sex modifies the association between HIV and coronary artery disease among older adults in Uganda. J Int AIDS Soc. 2022. Jan;25(1):e25868. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors describe the first study to utilize advanced imaging techniques (coronary computed tomography angiography, CCTA) in a sub-Saharan African population with the goal of evaluating sex-specific interaction for sub-clinical coronary artery disease. HIV serostatus changed the direction of association between female sex and plaque risk.
- 80.Siedner MJ, Bibangambah P, Kim J, Lankowski A, Chang JL, Yang IT, et al. Treated HIV Infection and Progression of Carotid Atherosclerosis in Rural Uganda: A Prospective Observational Cohort Study. J Am Heart Assoc Cardiovasc Cerebrovasc Dis. 2021. Jun 5;10(12):e019994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gdowski MA, Murthy VL, Doering M, Monroy-Gonzalez AG, Slart R, Brown DL. Association of Isolated Coronary Microvascular Dysfunction With Mortality and Major Adverse Cardiac Events: A Systematic Review and Meta-Analysis of Aggregate Data. J Am Heart Assoc. 2020. May 5;9(9):e014954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kelshiker MA, Seligman H, Howard JP, Rahman H, Foley M, Nowbar AN, et al. Coronary flow reserve and cardiovascular outcomes: a systematic review and meta-analysis. Eur Heart J. 2022. Apr 19;43(16):1582–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iantorno M, Schär M, Soleimanifard S, Brown TT, Moore R, Barditch-Crovo P, et al. Coronary artery endothelial dysfunction is present in HIV-positive individuals without significant coronary artery disease. AIDS Lond Engl. 2017. Jun 1;31(9):1281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iantorno M, Soleimanifard S, Schär M, Brown TT, Bonanno G, Barditch-Crovo P, et al. Regional coronary endothelial dysfunction is related to the degree of local epicardial fat in people with HIV. Atherosclerosis. 2018. Nov;278:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leucker TM, Weiss RG, Schär M, Bonanno G, Mathews L, Jones SR, et al. Coronary Endothelial Dysfunction Is Associated With Elevated Serum PCSK9 Levels in People With HIV Independent of Low-Density Lipoprotein Cholesterol. J Am Heart Assoc. 2018. Oct 2;7(19):e009996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Knudsen A, Christensen TE, Ghotbi AA, Hasbak P, Lebech AM, Kjær A, et al. Normal Myocardial Flow Reserve in HIV-Infected Patients on Stable Antiretroviral Therapy. Medicine (Baltimore). 2015. Oct 30;94(43):e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Knudsen A, Thorsteinsson K, Christensen TE, Hasbak P, Ripa RS, Panum I, et al. Cardiac Microvascular Dysfunction in Women Living With HIV Is Associated With Cytomegalovirus Immunoglobulin G. Open Forum Infect Dis. 2018. Sep;5(9):ofy205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ryom L, De Miguel R, Cotter AG, Podlekareva D, Beguelin C, Waalewijn H, et al. Major revision version 11.0 of the European AIDS Clinical Society Guidelines 2021. HIV Med. 2022. Mar 25; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feinstein MJ, Hsue PY, Benjamin LA, Bloomfield GS, Currier JS, Freiberg MS, et al. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation. 2019. Jul 9;140(2):e98–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hatleberg CI, Ryom L, El-Sadr W, Mocroft A, Reiss P, De Wit S, et al. Gender differences in the use of cardiovascular interventions in HIV-positive persons; the D:A:D Study. J Int AIDS Soc. 2018. Mar;21(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shahmanesh M, Schultze A, Burns F, Kirk O, Lundgren J, Mussini C, et al. The cardiovascular risk management for people living with HIV in Europe: how well are we doing? AIDS Lond Engl. 2016. Oct 23;30(16):2505–18. [DOI] [PubMed] [Google Scholar]
- 92.Kuniholm MH, Vásquez E, Appleton AA, Kingsley L, Palella FJ, Budoff M, et al. Cardiovascular risk score associations with frailty in men and women with or at risk for HIV. AIDS Lond Engl. 2022. Feb 1;36(2):237–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *93.Erlandson KM, Fitch KV, McCallum SA, Ribaudo HJ, Overton ET, Zanni MV, et al. Geographical Differences in the Self-Reported Functional Impairment of People with HIV and Associations with Cardiometabolic Risk. Clin Infect Dis Off Publ Infect Dis Soc Am. 2022. Feb 15;ciac098. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this secondary analysis from REPRIEVE trial data, cardiometabolic risk was associated with patient-reported functional impairment. Metrics of impairment varied by world region studied and were overall increased in WLWH relative to MWLH.
- 94.Grinspoon SK, Fitch KV, Overton ET, Fichtenbaum CJ, Zanni MV, Aberg JA, et al. Rationale and design of the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE). Am Heart J. 2019. Jun;212:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


