Abstract
BACKGROUND:
Single-shot intrathecal morphine (ITM) is an effective strategy for postoperative analgesia, but there are limited data on its safety, efficacy, and relationship with functional recovery among patients undergoing pancreaticoduodenectomy.
STUDY DESIGN:
This was a retrospective review of patients undergoing pancreaticoduodenectomy from 2014 to 2020 as identified by the institutional NSQIP Hepato-pancreato-biliary database. Patients were categorized by having received no spinal analgesia, ITM, or ITM with transversus abdominus plane block (ITM+TAP). The primary outcomes were average daily pain scores from postoperative days (POD) 0 to 3, total morphine equivalents (MEQ) consumed over POD 0 to 3, and average daily inpatient MEQ from POD 4 to discharge. Secondary outcomes included the incidence of opioid related complications, length of stay, and functional recovery.
RESULTS:
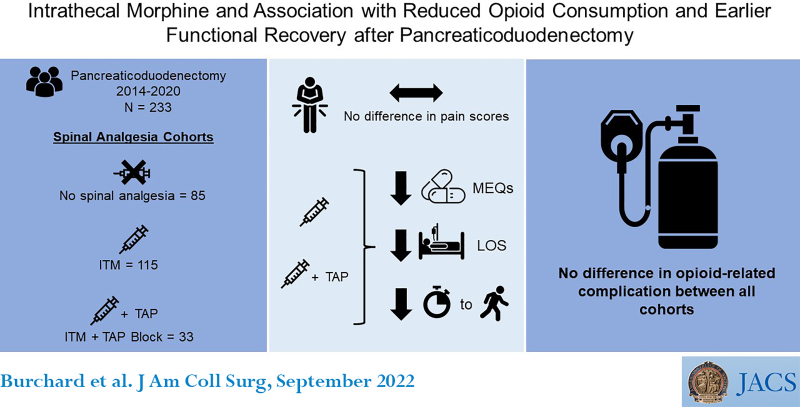
A total of 233 patients with a median age of 67 years were included. Of these, 36.5% received no spinal analgesia, 49.3% received ITM, and 14.2% received ITM+TAP. Average pain scores in POD 0 to 3 were similar by mode of spinal analgesia (none [2.8], ITM [2.6], ITM+TAP [2.3]). Total MEQ consumed from POD 0 to 3 were lower for patients who received ITM (121 mg) and ITM+TAP (132 mg), compared with no spinal analgesia (232 mg) (p < 0.0001). Average daily MEQ consumption from POD 4 to discharge was lower for ITM (18 mg) and ITM+TAP (13.1 mg) cohorts compared with no spinal analgesia (32.9 mg) (p = 0.0016). Days to functional recovery and length of stay were significantly reduced for ITM and ITM+TAP compared with no spinal analgesia. These findings remained consistent through multivariate analysis, and there were no differences in opioid-related complications among cohorts.
CONCLUSIONS:
ITM was associated with reduced early postoperative and total inpatient opioid utilization, days to functional recovery, and length of stay among patients undergoing pancreaticoduodenectomy. ITM is a safe and effective form of perioperative analgesia that may benefit patients undergoing pancreaticoduodenectomy.
This retrospective review of 233 patients undergoing pancreaticoduodenectomy found that single-shot intrathecal morphine significantly reduced opioid consumption, led to faster functional recovery, and decreased length of stay compared with no spinal analgesia, without differences in opioid-related complications.
Single-shot intrathecal morphine (ITM) is an emerging strategy for postoperative analgesia following major abdominal surgery. Traditional spinal analgesia protocols often rely on continuous thoracic epidural anesthesia due to its ability to provide adequate analgesia with few cardiopulmonary complications.1-3 However, epidural anesthesia is also associated with more frequent perioperative hypotension, technical failure, increased fluid administration, and greater length of stay (LOS). Therefore, ITM is an appealing alternative because it offers easier administration, strong efficacy at low doses, and reduced postoperative complications, and provides an alternative form of spinal analgesia for patients where an epidural catheter is contraindicated.4-10
The analgesic properties of ITM have recently been demonstrated in cardiac,11 gynecologic,9,12 spinal,13-15 orthopedic,16 urologic,17 colorectal,18 hepatopancreaticobiliary,10,19-23 and major abdominal surgery.7,24,25 With an estimated duration of action up to 24 hours,26 these studies have focused on the initial postoperative recovery period and often identified lower pain scores and, in some cases, decreased initial postoperative opioid requirements. However, data regarding the use of ITM among a large cohort of patients undergoing pancreaticoduodenectomy are limited.
In 2016, our institution started the use of ITM for patients undergoing pancreaticoduodenectomy. In this study, we sought to evaluate the effect of ITM with and without transversus abdominus plane (TAP) blocks on postoperative pain scores, opioid utilization, and functional recovery during the postoperative recovery period for patients undergoing pancreaticoduodenectomy.
METHODS
Patient selection
All patients who underwent pancreaticoduodenectomy by the Division of Surgical Oncology from January 2014 to September 2020 were reviewed. Patients who received epidural anesthesia or TAP blocks alone were excluded due to limited sample size. All patients who were extubated after the case and survived the index hospitalization were eligible. At our institution, all patients recovered in the postanesthesia care unit until meeting appropriate milestones for transfer. After transfer, those who received ITM were initially monitored with a “step-down” level of care on a general surgery floor, unless their clinical status required the intensive care unit. For patients without spinal analgesia, postoperative recovery location was determined by clinical status alone.
Demographic (age, sex, ethnicity), clinical (BMI, comorbidities, method of analgesia), operative (indication, duration, approach [open vs minimally invasive], vascular reconstruction), and outcomes (patient-reported pain scores [0 = none to 10 = severe], morphine equivalents [MEQ] consumed, functional recovery, LOS) were abstracted from the NSQIP Hepato-pancreato-biliary collaborative database. This study was approved by the institutional review board.
Method of analgesia
Patients were categorized by the type of spinal analgesia administered preoperatively as follows: none, ITM alone, and ITM with TAP block (ITM+TAP). ITM was administered at a dose of 0.15−0.3 mg before induction, per the attending anesthesiologist discretion. Bilateral TAP blocks were administered with bupivacaine at the conclusion of the case by the operating surgeon under direct visualization or the attending anesthesiologist via ultrasound guidance. Multimodal analgesia and patient-controlled analgesia were routinely used, and patients were transitioned to oral analgesics per protocol or surgeon preference.
Definitions and equations
Patients with a history of chronic opioid use or an active opioid prescription (including buprenorphine/naloxone) within 6 months of their operation were considered chronic opioid users. Substance use disorder was defined as any recreational drug use disorder history. MEQ were calculated using published conversion factors as follows: MEQ = (strength per unit × quantity × morphine milligram equivalent conversion factor).27 MEQ were assessed daily from postoperative day (POD) 0 to 3 and subsequently averaged from POD 4 to discharge by dividing the cumulative POD 4 to discharge MEQ by postoperative LOS. Operative duration included time from surgical incision to abdominal wall closure. Functional recovery was defined by the number of days until ambulation beyond 100 feet. Discharge was defined as the release of the patient from hospitalization to either home or rehabilitation facility.
Outcomes
The primary outcomes were average daily pain scores from POD 0 to 3, total MEQ consumed over POD 0 to 3, and average daily inpatient MEQ from POD 4 to discharge. Secondary outcomes of interest included the incidence of opioid related complications, LOS, and days to functional recovery. Any of the following were considered possible opioid-related complications: naloxone administration, postoperative respiratory failure, ileus, or delayed gastric emptying within 30 days of surgery.
Statistical analysis
The features of the cohorts were compared using the chi-square test for categorical variables and Kruskal–Wallis chi-square test where applicable. Bivariate associations among features of the cohorts with postoperative outcomes were evaluated using the Kruskal–Wallis test. Post hoc pairwise comparisons were carried out using Dunn’s test28 with the Benjamini–Hochberg29 adjustment for multiple comparisons. Multivariable regression models were used to estimate the independent, adjusted association of patient and disease characteristics with continuous outcomes, while minimizing the impact of response outliers and high leverage points.30 Model predictors included indicators for spinal analgesia plus any factor with a significant bivariate association (p < 0.10) with the outcome. Statistical analyses were conducted using SAS (v14.2) except for the post hoc pairwise tests, which used the R package, Dunn.test (v1.3.5).
RESULTS
A total of 233 patients were eligible (Supplemental Digital Content 1, http://links.lww.com/JACS/A92). The median age was 67 years, the majority (51.5%) were male, and 24.9% were considered chronic opioid users (Table 1). Most (81.6%) underwent open pancreaticoduodenectomy, the median operative duration was 378 minutes, and 84.3% of cases were performed for malignant pathology. The median LOS for the cohort was 7 days, and the median time to functional recovery (days to ambulation beyond 100 feet) was POD 2.
Table 1.
Demographics and Operative Characteristics
| Characteristic | Total n = 233 |
Method of spinal analgesia | p Value* | ||
|---|---|---|---|---|---|
| None n = 85 |
ITM n = 115 |
ITM+TAP n = 33 |
|||
| Age, y, median (IQR) | 67 (59, 73) | 66 (57, 72) | 67 (59, 73) | 68 (59, 74) | 0.7478 |
| Sex, m, n (%) | 120 (51.5) | 41 (48.2) | 62 (53.9) | 17 (51.5) | 0.7294 |
| White, n (%) | 211 (90.6) | 80 (94.1) | 98 (85.2) | 33 (100) | 0.0140† |
| BMI, kg/m2, median (IQR) | 27 (23, 31) | 27 (24, 30) | 27 (23, 32) | 26 (26, 32) | 0.5757 |
| COPD, n (%) | 12 (5.2) | 3 (3.5) | 6 (5.2) | 3 (9.1) | 0.5151 |
| Anxiety, n (%) | 31 (13.3) | 9 (10.6) | 19 (16.5) | 3 (9.1) | 0.3528 |
| Depression, n (%) | 64 (27.5) | 26 (30.6) | 30 (26.1) | 8 (24.0) | 0.7055 |
| Substance use disorder, n (%) | 11 (4.7) | 4 (4.7) | 5 (4.4) | 2 (6.1) | >0.999 |
| Alcohol use disorder, n (%) | 32 (13.7) | 16 (18.8) | 15 (13.0) | 1 (3.0) | 0.0782 |
| Chronic opioid use, n (%) | 58 (24.9) | 24 (28.2) | 27 (23.5) | 7 (21.2) | 0.6472 |
| Malignant pathology, n (%) | 193 (84.3) | 68 (81.9) | 95 (83.3) | 30 (93.8) | 0.2739 |
| Operative duration, min, median (IQR) | 378 (286, 475) | 349 (269, 446) | 397 (292, 495) | 381 (349, 461) | 0.0471† |
| Open approach, n (%) | 190 (81.6) | 69 (81.2) | 91 (79.1) | 30 (90.9) | 0.3048 |
| Year of operation, n (%) | <0.0001† | ||||
| 2014 | 34 (14.6) | 34 (40.0) | 0 | 0 | |
| 2015 | 18 (7.7) | 18 (21.2) | 0 | 0 | |
| 2016 | 44 (18.9) | 20 (23.5) | 22 (19.1) | 2 (6.1) | |
| 2017 | 38 (16.3) | 4 (4.7) | 24 (20.9) | 10 (30.3) | |
| 2018 | 32 (13.7) | 3 (3.5) | 20 (17.4) | 9 (27.3) | |
| 2019 | 39 (16.7) | 2 (2.4) | 28 (24.4) | 9 (27.3) | |
| 2020 | 28 (12.0) | 4 (4.7) | 21 (18.3) | 3 (9.1) | |
Analgesia cohorts are compared using chi-square test for categorical characteristics or Wilcoxon rank sum test for continuous characteristics.
Statistically significant.
IQR, interquartile range; ITM, intrathecal morphine; TAP, transversus abdominus plane block.
Overall, 36.5% of patients received no spinal analgesia, 49.3% received ITM, and 14.2% received ITM+TAP (Table 1). The distribution of chronic opioid users was similar across all cohorts (p = 0.65). Patients who received ITM and ITM+TAP had longer operative duration compared with those who did not receive spinal anesthesia (p = 0.047). The majority (73%) of TAP blocks were performed by the operating surgeon (data not shown).
Postoperative outcomes bivariate analysis
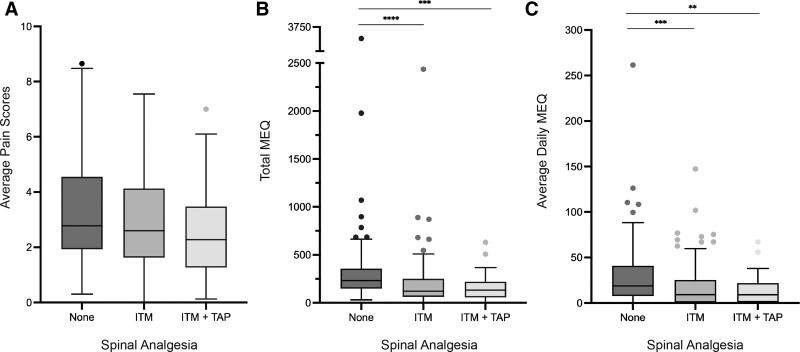
In bivariate analysis, average pain scores on POD 0 to 3 were similar by method of analgesia (no spinal [2.8], ITM [2.6], ITM+TAP [2.3]) (Table 2). Despite similar pain scores, the total MEQ consumed over POD 0 to 3 varied significantly by the mode of spinal analgesia (none [232 mg], ITM [121 mg], ITM+TAP [132 mg], p < 0.0001) (Table 2, Fig. 1A). Similarly, the average daily MEQ consumed from POD 4 to discharge varied by the type of spinal anesthesia (none [32.9 mg], ITM [18 mg], ITM+TAP [13.1 mg], p = 0.0016) (Table 2, Fig. 1B). After POD 3, no additional opioids were required in 23.5% of ITM and 24.2% of ITM+TAP patients, compared with just 2.3% of those with no spinal analgesia (Supplemental Digital Content 2, http://links.lww.com/JACS/A93). Patients younger than 60 years and those with a history of depression, substance or alcohol use disorder, or chronic opioid use had higher average pain scores throughout POD 0 to 3, greater MEQ consumption on POD 0 to 3, and higher average daily MEQ consumption from POD 4 to discharge (Table 2).
Table 2.
Unadjusted Associations with Postoperative Outcomes
| Baseline factor | n | Average pain score POD 0 to 3 |
MEQ consumed POD 0 to 3 |
MEQ consumed/d POD 4 to discharge |
Functional recovery (POD) | LOS, d | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | p Value | Median (IQR) | p Value | Median (IQR) | p Value | Median (IQR) | p Value | Median (IQR) | p Value | ||
| Overall | 233 | 2.7 (1.6, 4.3) | 166 (90, 283) | 21.7 (3.8, 50.3) | 2 (1, 3) | 7 (6, 11) | |||||
| Spinal analgesia | 0.19 | <0.0001* | 0.0016* | 0.0001* | <0.0001* | ||||||
| None | 85 | 2.8 (2.0, 4.5) | 232 (149, 353) | 32.9 (10.7, 59.9) | 2 (1, 4) | 9 (7, 14) | |||||
| ITM | 115 | 2.6 (1.6, 4.1) | 121 (62, 250) | 18.0 (0.4, 38.7) | 1 (1, 3) | 7 (5, 10) | |||||
| ITM+TAP | 33 | 2.3 (1.3, 3.4) | 132 (61, 212) | 13.1 (2.7, 35.8) | 1 (1, 2) | 6 (5, 9) | |||||
| Age at operation | <0.0001* | <0.0001* | <0.0001* | 0.9419 | 0.9142 | ||||||
| <59 y | 67 | 4.2 (2.5, 5.4) | 268 (164, 505) | 46.8 (19.5, 67.5) | 2 (1, 4) | 7 (5, 12) | |||||
| 60–70 y | 80 | 2.6 (1.7, 3.8) | 166 (104, 256) | 21.1 (2.9, 45.8) | 2 (1, 3) | 7 (6, 10) | |||||
| ≥70 y | 86 | 2.2 (1.3, 3.0) | 104 (55, 197) | 12.0 (1.3, 28.2) | 2 (1, 3) | 7 (6, 13) | |||||
| Sex | 0.38 | 0.0060* | 0.93 | <0.0001* | 0.9477 | ||||||
| Male | 120 | 2.8 (1.6, 4.6) | 202 (104, 356) | 22.3 (3.8, 46.1) | 1 (1, 2) | 7 (6, 11) | |||||
| Female | 113 | 2.5 (1.6, 4.0) | 137 (85, 234) | 20.7 (3.3, 53.6) | 2 (1, 4) | 7 (6, 11) | |||||
| Ethnicity | 0.052 | 0.93 | 0.018* | 0.9258 | 0.4625 | ||||||
| White | 211 | 2.6 (1.6, 4.2) | 168 (89, 290) | 20.2 (2.7, 46.8) | 2 (1, 3) | 7 (6, 11) | |||||
| Other | 22 | 4.0 (2.2, 5.2) | 165 (105, 247) | 36.4 (18.8, 67.1) | 2 (1, 3) | 7.5 (7, 11) | |||||
| Anxiety | 0.19 | 0.29 | 0.63 | 0.6305 | 0.4802 | ||||||
| Yes | 31 | 3.3 (2.0, 4.9) | 208 (98, 306) | 19.5 (12.5, 50.3) | 2 (1, 3) | 8 (6, 11) | |||||
| No | 202 | 2.6 (1.6, 4.2) | 165 (85, 280) | 22.3 (2.7, 50.5) | 2 (1, 4) | 7 (6, 11) | |||||
| Depression | 0.0056* | 0.022* | 0.0075* | 0.4191 | 0.5612 | ||||||
| Yes | 64 | 3.2 (2.1, 5.1) | 224 (111, 341) | 27.4 (12.6, 60.0) | 2 (1, 3) | 8 (6, 11) | |||||
| No | 169 | 2.5 (1.5, 3.9) | 152 (84, 250) | 18.6 (2.7, 38.6) | 2 (1, 3) | 7 (6, 12) | |||||
| Substance use disorder | 0.022* | 0.0014* | 0.0018* | 0.6646 | 0.1278 | ||||||
| Yes | 11 | 4.8 (3.2, 5.6) | 507 (166, 662) | 60.0 (30.1, 75.9) | 2 (1, 2) | 6 (5, 8) | |||||
| No | 222 | 2.6 (1.6, 4.1) | 156 (89, 279) | 20.6 (2.7, 46.1) | 2 (1, 3) | 7 (6, 11) | |||||
| Alcohol use disorder | 0.34 | 0.012* | 0.0009* | 0.6608 | 0.6594 | ||||||
| Yes | 32 | 2.8 (1.6, 5.2) | 248 (119, 457) | 51.9 (18.8, 71.7) | 2 (1, 3) | 7 (6, 10) | |||||
| No | 201 | 2.7 (1.6, 4.1) | 160 (89, 276) | 18.9 (2.7, 41.9) | 2 (1, 3) | 7 (6, 11) | |||||
| Chronic opioid use | <0.0001* | <0.0001* | <0.0001* | 0.3498 | 0.0421* | ||||||
| Yes | 58 | 3.8 (2.5, 5.6) | 246 (165, 499) | 46.1 (19.5, 85.4) | 2 (1, 4) | 9 (6, 12) | |||||
| No | 175 | 2.4 (1.5, 3.8) | 137 (81, 246) | 16.1 (2.0, 37.7) | 2 (1, 3) | 7 (5, 10) | |||||
| Approach | 0.11 | 0.53 | 0.80 | 0.0610 | 0.3251 | ||||||
| Open | 190 | 2.7 (1.7, 4.4) | 166 (86, 276) | 22.5 (3.8, 50.3) | 2 (1,4) | 7 (6, 11) | |||||
| MIS | 43 | 2.3 (1.3, 3.6) | 168 (105, 323) | 20.2 (2.4, 52.2) | 1 (1, 2) | 7 (5, 10.5) | |||||
| Operative duration | 0.3078 | 0.0467* | 0.031* | 0.1275 | 0.0487* | ||||||
| <293 min | — | 2.8 (1.8, 4.3) | 151 (61, 242) | 12.7 (0.1, 38.7) | 1 (1, 3) | 7 (5, 10) | |||||
| 293–385 min | — | 3.1 (1.4, 5.0) | 191 (107, 412) | 29.0 (3.8, 62.6) | 2 (1, 3) | 7 (5, 10) | |||||
| 385–480 min | — | 2.4 (1.6, 3.4) | 132 (86, 237) | 18.0 (5.6, 37.5) | 2 (1, 4) | 8 (6, 12) | |||||
| >480 min | — | 2.8 (1.6, 4.2) | 192 (106, 280) | 25.4 (12.7, 42.7) | 2 (1, 4) | 8 (6, 14) | |||||
Differences in continuous outcomes, described with median (IQR), were assessed with the Kruskal–Wallis chi-square test (p value].
Statistically significant.
IQR, interquartile range; ITM, intrathecal morphine; LOS, length of stay; MEQ, morphine equivalents; MIS, minimally invasive surgery; POD, postoperative day; TAP, transversus abdominus plane block.
Figure 1.
Tukey plots of median and interquartile range for (A) average pain score from postoperative day 0 to 3, (B) total morphine equivalents (MEQ) consumed from postoperative day 0 to 3, and (C) average daily MEQ from postoperative day 4 to discharge. **p < 0.01, ***p < 0.001, ****p < 0.0001. ITM, intrathecal morphine; TAP, transversus abdominus plane block.
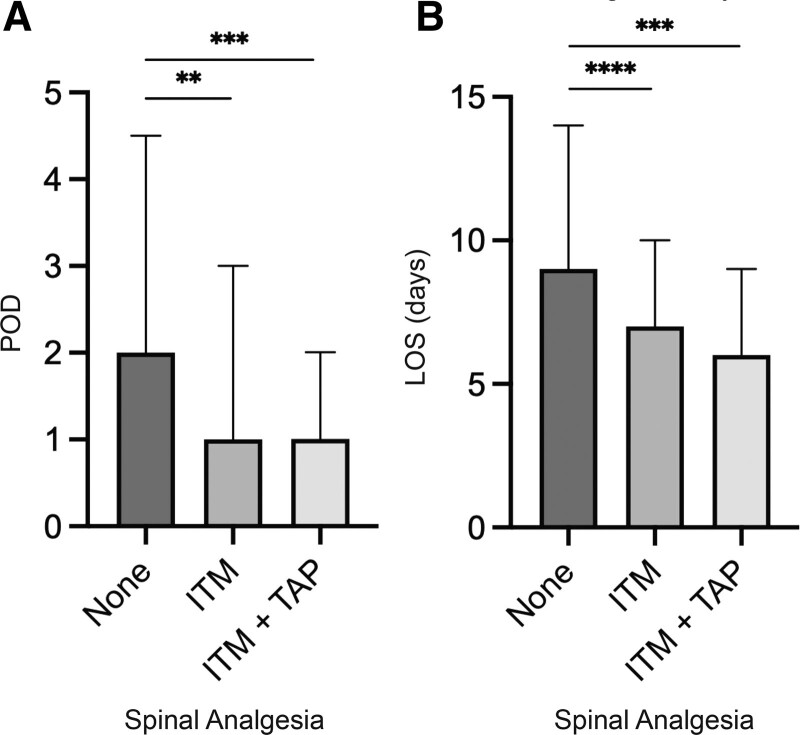
Increased days to functional recovery was more common among females and varied significantly among modes of spinal analgesia (none [2], ITM [1], ITM+TAP [1], p = 0.0001) (Table 2, Fig. 2A). LOS varied significantly by mode of spinal analgesia (none [9], ITM [7], ITM+TAP [6], p < 0.0001) (Table 2, Fig. 2B), and was increased for chronic opioid users and operative duration greater than 385 minutes.
Figure 2.
Bar graphs of median and interquartile range for (A) postoperative day (POD) of functional recovery, and (B) length of stay (LOS) by type of spinal analgesia. **p < 0.01, ***p < 0.001, ****p < 0.0001. ITM, intrathecal morphine; TAP, transversus abdominus plane block.
Postoperative outcomes multivariable regression
Total MEQ consumed from POD 0 to 3 was significantly lower for the patients who received ITM (mean difference –81.4, p < 0.0001) or ITM+TAP (mean difference –91.2, p = 0.0002) compared with no spinal analgesia (Table 3). Additionally, the average daily MEQ consumption from POD 4 to discharge was lower for ITM (mean difference –12.0, p = 0.0006) and ITM+TAP (mean difference –8.8, p = 0.072) cohorts compared with no spinal analgesia (Table 3). Patients who received either ITM or ITM+TAP had similar total MEQ from POD 0 to 3 (p = 0.67) and daily MEQ from POD 4 to discharge (p = 0.50).
Table 3.
Robust Regression Estimation of Adjusted Association with Outcomes of Interest
| Independent predictor* | Average pain score POD 0 to 3 |
MEQ total POD 0 to 3 |
Average daily MEQ POD 4 to discharge | Functional recovery (POD) | LOS | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate (SE) | p Value | Estimate (SE) | p Value | Estimate (SE) | p Value | Estimate (SE) | p Value | Estimate (SE) | p Value | |
| Age, y† | –0.05 (0.01) | <0.0001‡ | –4.5 (0.8) | <0.0001‡ | –0.7 (0.2) | <0.0001‡ | — | — | 0.03 (0.02) | 0.1303 |
| Male vs female | — | — | 56.4 (15.9) | 0.0004‡ | — | — | –0.77 (0.17) | <0.0001‡ | — | — |
| White vs non-White | –0.46 (0.39) | 0.24 | — | — | –11.2 (5.6) | 0.044‡ | — | — | — | — |
| Depression, yes vs no | 0.63 (0.25) | 0.012‡ | 43.9 (17.9) | 0.014‡ | 10.5 (3.6) | 0.0032‡ | — | — | — | — |
| Substance use disorder, yes vs no | 0.76 (0.53) | 0.16 | 107.2 (45.2) | 0.018‡ | 11.6 (7.8) | 0.14 | — | — | — | — |
| Alcohol use disorder, yes vs no |
— | — | –21.6 (24.8) | 0.38 | 12.6 (4.7) | 0.0079‡ | — | — | — | — |
| Chronic opioid use, yes vs no | 0.76 (0.28) | 0.0059‡ | 61.7 (19.3) | 0.0014‡ | 11.8 (4.0) | 0.0030‡ | — | — | 1.20 (0.45) | 0.0072‡ |
| Open vs robotic approach | 0.47 (0.29) | 0.010 | — | — | — | — | 0.28 (0.21) | 0.1780 | — | — |
| Operative duration§, min | — | — | 0.07 (0.07) | 0.28 | 0.02 (0.01) | 0.17 | — | — | 0.004 (0.002) | 0.0285‡ |
| ITM vs none (reference group) | –0.07 (0.24) | 0.77 | –81.4 (17.4) | <0.0001‡ | –12.0 (3.5) | 0.0006‡ | –0.57 (0.18) | 0.0018‡ | –1.90 (0.41) | <0.0001‡ |
| ITM+TAP vs none | –0.27 (0.34) | 0.43 | –91.2 (24.5) | 0.0002‡ | –8.8 (4.9) | 0.072 | –0.90 (0.25) | 0.0003‡ | –2.18 (0.58) | 0.0002‡ |
| ITM+TAP vs ITM‖ | –0.20 (0.33) | 0.55 | –9.9 (23.0) | 0.67 | 3.1 (4.7) | 0.50 | –0.33 (0.24) | 0.1591 | –0.09 (0.55) | 0.8698 |
Regression model for each outcome includes all factors significant in univariate analysis, and otherwise shown as —.
Continuous factor age is centered at the overall mean (66 y).
Statistically significant.
Continuous factor surgical duration is centered at the overall mean (387 min).
Additional test is a linear contrast of model parameters.
ITM, intrathecal morphine; LOS, length of stay; MEQ, morphine equivalents; POD, postoperative day; TAP, transversus abdominus plane block.
Young age, depression, and chronic opioid use were independent predictors of higher average pain scores POD 0 to 3, total MEQ POD 0 to 3, and average daily MEQ from POD 4 to discharge. Additionally, male sex and substance use disorder predicted increased total MEQ POD 0 to 3, while alcohol use disorder predicted higher average daily MEQ from POD 4 to discharge (Table 3).
Days to functional recovery were significantly reduced for patients receiving ITM (mean difference –0.57, p = 0.0018) or ITM+TAP (mean difference –0.9, p = 0.0003) compared with no spinal analgesia (Table 3). Similarly, LOS was significantly reduced for ITM (mean difference –1.97, p < 0.0001) and ITM+TAP (mean difference –2.25, p = 0.0001) cohorts compared with no spinal analgesia (Table 3). Patients who received either ITM or ITM+TAP had similar days to functional recovery (p = 0.16) and LOS (p = 0.61).
The only independent predictor of longer days to functional recovery was female sex. The only independent predictors of increased LOS were chronic opioid use and longer operative duration.
Opioid-related complications
Overall, there were 5 incidents of naloxone administration (none with underlying COPD) and 9 cases of postoperative respiratory failure requiring reintubation (3 with underlying COPD). Among the 3 patients with COPD who developed postoperative respiratory failure, 1 had no spinal analgesia, 1 had ITM, and 1 had ITM+TAP. The rate of each complication was similar among the no spinal anesthesia, ITM, and ITM+TAP groups (all p > 0.45). The incidence of ileus (26.6%) and delayed gastric emptying (15.5%) was similar regardless of spinal anesthetics (Table 4).
Table 4.
Opioid-Related Complications
| Characteristic | Total n = 233 |
None n = 85 |
ITM N = 115 |
ITM+TAP n = 33 |
|---|---|---|---|---|
| Naloxone administration | 5 (2.2) | 3 (3.5) | 1 (0.9) | 1 (3.0) |
| Respiratory failure | 9 (3.9) | 3 (3.5) | 4 (3.5) | 2 (6.1) |
| Ileus | 62 (26.6) | 21 (24.7) | 33 (28.7) | 8 (24.2) |
| Delayed gastric emptying | 36 (15.5) | 12 (14.1) | 19 (16.5) | 5 (15.2) |
Data presented as n (%).
Analgesia cohorts are compared using chi-square test. An exact test is used when any expected cell size is <5.
ITM, intrathecal morphine; TAP, transversus abdominus plane block.
DISCUSSION
In this study, we evaluated the effect of ITM on postoperative pain scores and opioid utilization after pancreaticoduodenectomy. In the early postoperative period from days 0 to 3, there was no difference in average daily pain scores, yet patients who received ITM or ITM+TAP consumed significantly fewer opioids compared with those without spinal analgesia. This pattern persisted throughout the rest of hospitalization. Patients who received ITM or ITM+TAP also had significantly faster functional recovery and reduced LOS compared with those who did not receive spinal analgesia. The addition of TAP blocks to patients who received ITM did not appear to confer an additional analgesic or functional benefit to patients who received ITM alone. Overall, the rate of opioid-related or potentially related complications was low, but the distribution of these adverse events was similar across all modes of analgesia. In summary, these data demonstrated that spinal analgesia was safe and associated with less opioid consumption, similar pain scores, faster functional recovery (days to ambulation beyond 100 feet), and decreased LOS among patients undergoing pancreaticoduodenectomy.
While studies have evaluated the effect of ITM on postoperative pain control and opioid utilization, few have focused on pancreatic surgery and those undergoing pancreaticoduodenectomy, an operation in which inadequate analgesia can influence functional recovery and hospital LOS. Elsewhere, a retrospective review with 197 patients undergoing pancreaticoduodenectomy also showed lower opioid consumption on POD 0 with no difference in postoperative pain scores among pancreaticoduodenectomy patients who received ITM compared with those who received TAP or quadratus lumborum nerve blocks.19 This is consistent with our findings that pancreaticoduodenectomy patients who receive ITM require less opioids to achieve adequate pain control, which reflects findings across multiple surgical disciplines11,12,15,23 and demonstrates translation of this effect to pancreaticoduodenectomy. Compared to the previous studies of ITM in pancreaticoduodenectomy patients, we have additionally identified reduced days to functional recovery and LOS with the addition of ITM or ITM+TAP. This has potential economic implications through reduced inpatient hospital costs and warrants further investigation. We found no difference in potential side effects (delayed gastric emptying and ileus) and adverse events (naloxone administration and postoperative respiratory failure) after ITM administration, supporting the safety of this intervention.
Most previous studies of ITM have emphasized the first 24 to 72 hours postoperatively, given an estimated duration of action of 18 to 24 hours.26 This is the first study to extend the evaluation of MEQ consumed beyond the very early postoperative period. This was performed due to the observation that ITM in combination with thoracic epidurals had lasting effects for patients after hepatectomy.6 The mechanism behind these prolonged analgesic effects is not fully understood but may reflect preemptive analgesia on central nociceptive sensitization,31 tolerance to earlier mobilization, and/or transition to oral multimodal analgesia. These findings may also represent selection bias because patients are preoperatively counseled on the benefits of ITM, which can change expectation of perioperative pain management and affect opioid utilization postoperatively. Regardless, we observed significantly decreased average daily MEQ from POD 4 to discharge for patients receiving ITM and ITM+TAP compared with no spinal analgesia. This suggests that ITM can have lasting effects on postoperative opioid utilization in patients undergoing pancreaticoduodenectomy, even after the ITM effect has worn off. This was supported by the observation that nearly 25% of ITM patients did not require any additional opioids beyond POD 3 compared with just 2.3% of those without spinal analgesia. These data suggest that early postoperative regional analgesia sets the course for subsequent need of systemic opioids.
Similar to studies on cesarean deliveries, we observed comparable benefits with respect to postoperative pain scores and MEQ among all ITM patients with or without TAP.32-34 Costello and colleagues conducted a randomized controlled trial of ITM with or without ultrasound-guided ropivacaine TAP blocks after cesarean delivery and found no difference in pain scores or opioid consumption.32 Further supporting these findings, Singh and colleagues performed a randomized controlled trial of ITM in combination with high- or low-dose ropivacaine TAP blocks or placebo after cesarean delivery. Despite reduced pain scores at 12 hours postoperatively with high-dose ropivacaine, this effect did not last, and scores were no different at 24, 36, and 48 hours or at 6 and 12 weeks.34 This may be due to a reliable visceral analgesic effect of ITM compared with variable somatic analgesia of TAP blocks.19,35 Singh and colleagues also found no difference in postoperative opioid consumption, adverse events, quality of recovery, or satisfaction among cohorts. This is an important discovery because patients may not require the additional procedural time and sedation required to conduct TAP blocks after ITM administration. However, our small sample size of patients with ITM+TAP may limit our ability to detect clinically important differences after pancreaticoduodenectomy.
Our study has strengths and limitations that should be acknowledged. This is the largest study to evaluate the effect of ITM on postoperative pain scores and opioid utilization in patients undergoing pancreaticoduodenectomy. Additionally, this is the first study in this population to show an association between ITM and earlier functional recovery and reduced LOS. Finally, these patients with benign and malignant pathology have representative rates of substance use and mental health diagnoses, which increases its external validity. Due to its retrospective nature, certain selection biases cannot be overcome. For example, all ITM patients underwent pancreaticoduodenectomy from 2016 to 2020 compared with just 16.3% (n = 13) of patients without spinal analgesia. This coincides with the implementation of the divisional enhanced recovery after surgery program, which included ITM as an adjunctive measure for pain control. This enhanced recovery pathway has reduced LOS among patients undergoing pancreaticoduodenectomy, which is why this was not chosen as a primary outcome measure. Instead, we focused on pain control and MEQ used since all patients routinely received multimodal and patient-controlled analgesia throughout the study period. Finally, due to limited sample size, we were unable to compare ITM with alternative forms of spinal analgesia, which may be routinely used at some institutions. Regardless, recent data suggest that ITM may be equally effective, safer, and better tolerated than other analgesic techniques across a variety of surgical disciplines.4-9 Future studies should prospectively study ITM in combination with analgesic regimens for pancreatic surgery to provide further insight on outcomes such as operative duration, LOS, and hospital costs.
CONCLUSIONS
Compared with no spinal analgesia, ITM was associated with decreased early postoperative and total inpatient opioid utilization in patients undergoing pancreaticoduodenectomy, with no difference in patient-reported pain scores. Additionally, ITM and ITM+TAP were associated with decreased days to functional recovery and LOS, suggesting functional recovery benefit among patients undergoing pancreaticoduodenectomy. In this population, TAP blocks did not appear to confer additional benefits to ITM for postoperative pain control or opioid utilization. Opioid-related complications such as rates of ileus, delayed gastric emptying, postoperative respiratory failure, and naloxone administration were unchanged with the addition of ITM. ITM is therefore a safe and effective form of perioperative analgesia that may benefit patients undergoing pancreaticoduodenectomy.
Author Contributions
Study conception and design: Burchard, Melucci, Lynch, Moalem, Linehan
Acquisition of data: Lynch, Burchard
Analysis and interpretation of data: Burchard, Melucci, Strawderman, Loria, Schoeniger, Galka, Moalem, Linehan
Drafting of manuscript: Burchard, Melucci, Loria, Dave, Moalem, Linehan
Critical revision: Burchard, Loria, Melucci, Schoeniger, Galka, Moalem, Linehan
Acknowledgment:
The authors would like to acknowledge Dr Darren R Carpizo and Dr Danielle M Lindemuth for their contributions to the development and design of this manuscript.
Supplementary Material
Abbreviations and Acronyms
- ITM=
- intrathecal morphine
- LOS=
- length of stay
- MEQ=
- morphine equivalents
- POD=
- postoperative day
- TAP=
- transversus abdominus plane
Disclosure Information: Nothing to disclose.
Presented virtually at the American College of Surgeons 107th Annual Clinical Congress, October 2021.
Supplemental digital content is available for this article.
REFERENCES
- 1.Fotiadis RJ, Badvie S, Weston MD, Allen-Mersh TG. Epidural analgesia in gastrointestinal surgery. Br J Surg 2004;91:828–841. [DOI] [PubMed] [Google Scholar]
- 2.McLean SR, von Homeyer P, Cheng A, et al. Assessing the benefits of preoperative thoracic epidural placement for lung transplantation. J Cardiothorac Vasc Anesth 2018;32:2654–2661. [DOI] [PubMed] [Google Scholar]
- 3.Rigg JR, Jamrozik K, Myles PS, et al. MASTER Anaethesia Trial Study Group. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet 2002;359:1276–1282. [DOI] [PubMed] [Google Scholar]
- 4.Bujedo BM, Santos SG, Azpiazu AU. A review of epidural and intrathecal opioids used in the management of postoperative pain. J Opioid Manag 2012;8:177–192. [DOI] [PubMed] [Google Scholar]
- 5.De Pietri L, Siniscalchi A, Reggiani A, et al. The use of intrathecal morphine for postoperative pain relief after liver resection: a comparison with epidural analgesia. Anesth Analg 2006;102:1157–1163. [DOI] [PubMed] [Google Scholar]
- 6.Sakowska M, Docherty E, Linscott D, Connor S. A change in practice from epidural to intrathecal morphine analgesia for hepato-pancreato-biliary surgery. World J Surg 2009;33:1802–1808. [DOI] [PubMed] [Google Scholar]
- 7.Duncan MA, Savage J, Tucker AP. Prospective audit comparing intrathecal analgesia (incorporating midazolam) with epidural and intravenous analgesia after major open abdominal surgery. Anaesth Intensive Care 2007;35:558–562. [DOI] [PubMed] [Google Scholar]
- 8.Kasivisvanathan R, Abbassi-Ghadi N, Prout J, et al. A prospective cohort study of intrathecal versus epidural analgesia for patients undergoing hepatic resection. HPB (Oxford) 2014;16:768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjølhede P, Bergdahl O, Borendal Wodlin N, Nilsson L. Effect of intrathecal morphine and epidural analgesia on postoperative recovery after abdominal surgery for gynecologic malignancy: an open-label randomised trial. BMJ Open 2019;9:e024484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang JZJ, Weinberg L. A literature review of intrathecal morphine analgesia in patients undergoing major open hepato-pancreatic-biliary (HPB) surgery. Anesth Pain Med 2019;9:e94441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhawan R, Daubenspeck D, Wroblewski KE, et al. Intrathecal morphine for analgesia in minimally invasive cardiac surgery: a randomized, placebo-controlled, double-blinded clinical trial. Anesthesiology 2021;135:864–876. [DOI] [PubMed] [Google Scholar]
- 12.Seki H, Shiga T, Mihara T, et al. Effects of intrathecal opioids on cesarean section: a systematic review and Bayesian network meta-analysis of randomized controlled trials. J Anesth 2021;35:911–927. [DOI] [PubMed] [Google Scholar]
- 13.Pendi A, Acosta FL, Tuchman A, et al. Intrathecal morphine in spine surgery: a meta-analysis of randomized controlled trials. Spine (Phila Pa 1976) 2017;42:E740–E747. [DOI] [PubMed] [Google Scholar]
- 14.Dhaliwal P, Yavin D, Whittaker T, et al. Intrathecal morphine following lumbar fusion: a randomized, placebo-controlled trial. Neurosurgery 2019;85:189–198. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Guo X, Guo Z, Xu M. Preemptive analgesia with a single low dose of intrathecal morphine in multilevel posterior lumbar interbody fusion surgery: a double-blind, randomized, controlled trial. Spine J 2020;20:989–997. [DOI] [PubMed] [Google Scholar]
- 16.Kaczocha M, Azim S, Nicholson J, et al. Intrathecal morphine administration reduces postoperative pain and peripheral endocannabinoid levels in total knee arthroplasty patients: a randomized clinical trial. BMC Anesthesiol 2018;18:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koning MV, de Vlieger R, Teunissen AJW, et al. The effect of intrathecal bupivacaine/morphine on quality of recovery in robot-assisted radical prostatectomy: a randomised controlled trial. Anaesthesia 2020;75:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koning MV, Teunissen AJW, van der Harst E, et al. Intrathecal morphine for laparoscopic segmental colonic resection as part of an enhanced recovery protocol: a randomized controlled trial. Reg Anesth Pain Med 2018;43:166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boisen ML, McQuaid AJ, Esper SA, et al. Intrathecal morphine versus nerve blocks in an enhanced recovery pathway for pancreatic surgery. J Surg Res 2019;244:15–22. [DOI] [PubMed] [Google Scholar]
- 20.Roy JD, Massicotte L, Sassine MP, et al. A comparison of intrathecal morphine/fentanyl and patient-controlled analgesia with patient-controlled analgesia alone for analgesia after liver resection. Anesth Analg 2006;103:990–994. [DOI] [PubMed] [Google Scholar]
- 21.Koea JB, Young Y, Gunn K. Fast track liver resection: the effect of a comprehensive care package and analgesia with single dose intrathecal morphine with gabapentin or continuous epidural analgesia. HPB Surg 2009;2009:271986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko JS, Choi SJ, Gwak MS, et al. Intrathecal morphine combined with intravenous patient-controlled analgesia is an effective and safe method for immediate postoperative pain control in live liver donors. Liver Transpl 2009;15:381–389. [DOI] [PubMed] [Google Scholar]
- 23.Dichtwald S, Ben-Haim M, Papismedov L, et al. Intrathecal morphine versus intravenous opioid administration to impact postoperative analgesia in hepato-pancreatic surgery: a randomized controlled trial. J Anesth 2017;31:237–245. [DOI] [PubMed] [Google Scholar]
- 24.Meylan N, Elia N, Lysakowski C, Tramèr MR. Benefit and risk of intrathecal morphine without local anaesthetic in patients undergoing major surgery: meta-analysis of randomized trials. Br J Anaesth 2009;102:156–167. [DOI] [PubMed] [Google Scholar]
- 25.Devys JM, Mora A, Plaud B, et al. Intrathecal + PCA morphine improves analgesia during the first 24 hr after major abdominal surgery compared to PCA alone. Can J Anaesth 2003;50:355–361. [DOI] [PubMed] [Google Scholar]
- 26.Mugabure Bujedo B. A clinical approach to neuraxial morphine for the treatment of postoperative pain. Pain Res Treat 2012;2012:612145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Center for Injury Prevention and Control. CDC Compilation of Benzodiazepines, Muscle Relaxants, Stimulants, Zolpidem, and Opioid Analgesics with Oral Morphine Milligram Equivalent Conversion Factors, 2018 Version; 2018. Atlanta, GA: Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/drugoverdose/resources/data.html. Accessed December 2, 2021. [Google Scholar]
- 28.Dunn O. Multiple comparisons using rank sums. Technometrics 1964;6:241–252. [Google Scholar]
- 29.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995;57:289–300. [Google Scholar]
- 30.Chun Y, Weixin Y. Robust linear regression: a review and comparison. Commun Stat Simul Comput 2017;46: 6261–6282. [Google Scholar]
- 31.Kissin I. Preemptive analgesia. Anesthesiology 2000;93:1138–1143. [DOI] [PubMed] [Google Scholar]
- 32.Costello JF, Moore AR, Wieczorek PM, et al. The transversus abdominis plane block, when used as part of a multimodal regimen inclusive of intrathecal morphine, does not improve analgesia after cesarean delivery. Reg Anesth Pain Med 2009;34:586–589. [DOI] [PubMed] [Google Scholar]
- 33.Wang P, Chen X, Chang Y, et al. Analgesic efficacy of ultrasound-guided transversus abdominis plane block after cesarean delivery: a systematic review and meta-analysis. J Obstet Gynaecol Res 2021;47:2954–2968. [DOI] [PubMed] [Google Scholar]
- 34.Singh S, Dhir S, Marmai K, et al. Efficacy of ultrasound-guided transversus abdominis plane blocks for post-cesarean delivery analgesia: a double-blind, dose-comparison, placebo-controlled randomized trial. Int J Obstet Anesth 2013;22:188–193. [DOI] [PubMed] [Google Scholar]
- 35.Carney J, Finnerty O, Rauf J, et al. Studies on the spread of local anaesthetic solution in transversus abdominis plane blocks. Anaesthesia 2011;66:1023–1030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.