Abstract
Objectives
Sibling loss is understudied in the bereavement and health literature. The present study considers whether experiencing the death of siblings in mid-to-late life is associated with subsequent dementia risk and how differential exposure to sibling losses by race/ethnicity may contribute to racial/ethnic disparities in dementia risk.
Methods
We use discrete-time hazard regression models, a formal mediation test, and a counterfactual simulation to reveal how sibling loss in mid-to-late life affects dementia incidence and whether unequal exposures by race/ethnicity mediate the racial/ethnic disparities in dementia. We analyze data from the Health and Retirement Study (2000–2016). The sample includes 13,589 respondents (10,670 non-Hispanic White, 1,761 non-Hispanic Black, and 1,158 Hispanic adults) aged 65 years and older in 2000 who show no evidence of dementia at baseline.
Results
Discrete-time hazard regression results show that sibling loss in mid-to-late life is associated with up to 54% higher risk for dementia. Sibling loss contributes to Black–White disparities in dementia risk. In addition, a simulation analysis shows that dementia rates would be 14% lower for Black adults if they experienced the lower rates of sibling loss experienced by White adults. This pattern was not observed among Hispanic adults.
Discussion
The death of a sibling in mid-to-late life is a stressor that is associated with increased dementia risk. Black adults are disadvantaged in that they are more likely than Whites to experience the death of siblings, and such losses contribute to the already substantial racial/ethnic disadvantage in dementia.
Keywords: Bereavement, Dementia, Minority aging (race/ethnicity), Stress
Dementia is a pernicious age-related disease, with adverse effects and burdens on individuals, families, and health care systems (James & Bennett, 2019). As people age, they also experience more frequent losses through the deaths of loved ones, and these deaths influence the health of individuals, including their dementia risk (Norton et al., 2016). Research on the link between bereavement and dementia risk has focused primarily on the loss of a spouse or child (Liu et al., 2020; Umberson et al., 2020; Zhang et al., 2021). However, the loss of siblings, particularly at older ages, merits greater attention. Given the importance of close relationships for cognitive health (Ertel et al., 2008; Livingston et al., 2020), and the lifelong relationships of siblings, often growing in interdependence at older ages (Stocker et al., 2020), it is important to understand whether the death of siblings in mid-to-late life affects the risk of dementia in later life. Our examination of sibling deaths is guided by a stress and life course perspective (Elder et al., 2003; Pearlin & Bierman, 2013), which suggests a process through which psychological impacts of grief and the loss of social support potentially increase dementia risk.
Moreover, this study examines how differential exposure to sibling death in mid-to-late life may contribute to racial/ethnic differences in dementia risk. The loss of close relationships, particularly through death, is unequally distributed in the population (Umberson et al., 2017). Black families are more likely to experience sibling deaths across the life course compared to White adults (Patterson et al., 2020). In addition, with few exceptions (Aneshensel, 2009), most studies of racial/ethnic differences in stress exposure compare the distribution of stressors among Black compared to White respondents and have not included Hispanic respondents. However, inclusion of Hispanic individuals when assessing racial/ethnic differences in the link between sibling deaths and dementia is essential because Hispanic populations now constitute the largest minority group in the United States, and they are disproportionately poor and experience high levels of stress (Williams & Sternthal, 2010) and dementia risk (Garcia et al., 2019).
In this spirit, we use nationally representative, longitudinal data from the Health and Retirement Study (HRS) to explore whether sibling deaths that occur in mid-to-late life affect subsequent dementia risk and how differential exposure to sibling death in mid-to-late life may contribute to racial/ethnic differences in dementia among White, Hispanic, and Black adults. Determining the relationship between sibling death in mid-to-late life and dementia and the impact of sibling deaths in the context of race and ethnicity is an important enterprise. First, sibling death is an understudied source of potential disadvantage in cognitive health. Failure to consider the impact of sibling loss in mid-to-late life may underestimate the consequences of family member deaths on cognitive health as an important stressor in older populations (Thoits, 2010). Second, research of this kind informs understanding of racial/ethnic disparities in health by elucidating how risk of family member deaths filters into the cognitive aging process in ways that may increase dementia risk.
Background
Sibling Deaths and Dementia
Sibling relationships involve a deep enduring closeness over the life course for most siblings (Cicirelli, 2009). Even siblings who have experienced a great deal of rivalry early in life tend to become interdependent after midlife (Stocker et al., 2020). Because siblings are typically of the same generation (Volkom, 2006), sibling relationships have the longest duration of any human relationship throughout life. Siblings share a common genetic and social heritage, a common cultural milieu and a sense of identity, and common early experiences within the family (Goetting, 1986). Siblings are permanent but flexible members of a mutual social support system during a life course (Furstenberg, 2020). For example, stressful life events (e.g., divorce or death of a parent) may cause siblings to cling rather tightly to the constancy and permanency a brother or sister can provide (White, 2001).
The integration of the stress process model with a life course perspective can provide important insights into how the death of siblings––the loss of a lifelong relationship and potential source of social support––affects cognitive health. Stress process frameworks have positioned exposures to stress as a key predictor of health disparities (Aneshensel, 2009; Pearlin & Bierman, 2013). Exposure to stressful life events, such as sibling loss in mid-to-later life, may undermine psychosocial resources and activate behavioral and psychosocial pathways that increase dementia risk (Bolton et al., 2016; Cicirelli, 2009; Eilegård et al., 2013; Sutin et al., 2020).
The death of a sibling in mid-to-later life is a unique stressor. Prior research on sibling bereavement from early childhood to old age has documented prolonged and intense grief reactions, often lasting for decades, in response to sibling loss (see Cicirelli, 2009 for a review). The death of a sibling yields a sense of existential incompleteness and may shift how one views life from a sense of time-since-birth to time-left-to-live (Perkins & Harris, 1990). Sibling loss may be a vivid reminder of personal mortality and sibling deaths may be emotionally unsettling as they make one realize the limitations and fragility of one’s existence (Perkins & Harris, 1990). Sibling loss is also unique in its potential to reoccur if one has multiple siblings, which may take a cumulative toll on dementia risk after midlife. Given that stressful experiences across the life course may leave a “trace of vulnerability” to the development of dementia (Livingston et al., 2020; Sotiropoulos et al., 2011), sibling loss may increase dementia risk.
The resulting biological dysregulation––the activated hypothalamic–pituitary–adrenal axis and sympathetic nervous system (Danese & McEwen, 2012)––due to death of siblings may increase dementia risk because the cortisol awakening response is associated with a diagnostic shift to mild cognitive impairment in later life (Peavy et al., 2012). Deleterious coping responses, such as smoking, increased alcohol consumption, and poor diet and exercise habits, which sometimes follow bereavement, also increase dementia risk (Lee et al., 2010). Studies of sibling bereavement have documented that bereaved siblings are at increased risk of low self-esteem, higher rates of mental disorders, and difficulties falling asleep as compared with nonbereaved counterparts (Bolton et al., 2016; Eilegård et al., 2013).
The loss of social support and an increased likelihood of social isolation may also link sibling loss to cognitive health. Sibling loss may mean the loss of an important social contact and an exchange and support system (Furstenberg, 2020) because most adult siblings stay in each other’s lives and become more interdependent in later life (Stocker et al., 2020; White, 2001). For example, scholars have found that siblings in adulthood appear to maintain contact with one another between once a week and several times a week on average (Stocker et al., 2020). Given that greater contact with a range of social ties (including once a week or more contact with children) was associated with slower memory decline over 6 years (Ertel et al., 2008), the loss of siblings in mid-to-late life could reduce social connection and be a risk factor triggering dementia risk (Sutin et al., 2020).
Race/Ethnicity, Differential Exposure to Sibling Deaths, and Dementia
Exposure to sibling deaths is not randomly distributed throughout the population but rather some racial/ethnic groups have greater exposure than others. Racial disparities in life expectancy in the United States may mean that Black Americans experience more sibling deaths than White Americans from mid to later life. In particular, cumulative risk of sibling deaths among Black adults is 50% higher than White adults at age 60, and this disparity persists by age 90 (Umberson et al., 2017). Also, studies indicate that Hispanic adults are more likely than Whites to be more family-oriented and to have more siblings (Daw et al., 2016; Landale et al., 2006), which combined with lower average socioeconomic status (SES) among many Hispanic adults, may increase their risk of experiencing sibling deaths (Hummer & Hayward, 2015). On the other hand, despite their poorer average SES relative to White adults, mortality estimates are modestly favorable for Hispanic adults relative to White adults (Hummer & Hayward, 2015). This raises the possibility that Hispanic families are more similar to White families in risk of experiencing sibling death; research remains inconclusive regarding whether Hispanic adults experience higher exposure to sibling deaths compared to White adults.
In addition, Black and Hispanic adults in the United States are more likely to experience dementia than White adults (Garcia et al., 2019). Central to a stress and life course perspective is recognition of the extent to which exposure to stressors is unequally distributed in the population, and it suggests a pathway linking race/ethnicity to dementia disparities (Aneshensel, 2009; Pearlin & Bierman, 2013). Drawing on this framework, a large literature seeks to understand the social determinants of racial/ethnic cognitive health disparities by considering the stark racial and ethnic disparities in a host of stressors in mid-to-late life––in ways that are linked to the United States’ racialized social system (Brown et al., 2020; Glymour & Manly, 2008). For example, research documents Black–White and Hispanic–White disparities in exposure to stressful life events and chronic stressors that may contribute to cognitive impairment, such as financial instability and insecurity, discrimination, and neighborhood segregation (Williams, 2018).
Given the potential importance of the unequal concentration of sibling deaths among racial/ethnic minorities and racial/ethnic disparities in dementia risk, we apply the stress process model (Aneshensel, 2009; Pearlin & Bierman, 2013) to test sibling death exposure as a unique stressor affecting dementia and racial/ethnic disparities in dementia. Very little research has been conducted specifically on differential exposure to sibling deaths as a factor in racial/ethnic dementia disparities; however, prior research on the effects of various types of family member deaths on cognitive limitations provides us with important clues. Parents who lose a child prior to midlife have 50% greater odds of developing dementia compared with parents who do not lose a child, and this contributes to Black–White disparities in dementia because Black parents are twice as likely as White parents to have lost a child prior to midlife (Umberson et al., 2020). Widowhood is also significantly associated with a higher risk of dementia for both Black and White adults (Zhang et al., 2021), pointing to the importance of considering sibling deaths as another type of family member death that may contribute to dementia and racial/ethnic disparities in dementia.
Aims of the Present Study
Although scholars have provided insights into the impact of family member deaths on cognitive health, three critical gaps in the literature remain. First, most studies neglect to consider the impact of sibling deaths that occur in mid-to-late life as a factor influencing dementia and as a potential stressor explaining racial/ethnic disparities in dementia. Second, prior studies do not formally test differential exposure to family member deaths as contributing to racial/ethnic disparities in dementia. Third, most studies exclude Hispanic populations in the discussion of racial/ethnic disparities in dementia. This study uses nationally representative, longitudinal data to assess the impact of sibling deaths in mid-to-late life on dementia and its contributions to racial/ethnic disparities in dementia risk by addressing the following hypotheses:
Hypothesis 1: The death of one or more siblings after midlife will increase subsequent risk of dementia compared to those who have not experienced the death of a sibling.
Hypothesis 2a: Black and Hispanic adults will experience greater exposure to sibling deaths than White adults.
Hypothesis 2b: Black and Hispanic adults will have higher risk for dementia than White adults.
Hypothesis 2c: Unequal racial/ethnic differences in exposure to sibling deaths contribute to racial/ethnic disparities in dementia in late life.
Data and Method
Data
We rely on data from the HRS (2000–2016), an ongoing biannual survey launched in 1992 (Sonnega et al., 2014). The HRS provides a unique opportunity to address our research questions because of its large sample size, long-term follow-up, oversampling of racial/ethnic minorities, and verified measures of cognitive health. The present study begins with the 2000 wave as it was the first to collect detailed data on dementia. Because the focus of this article is on sibling loss, we exclude individuals who do not have siblings, consistent with prior research (Rostila et al., 2012, 2013). We also excluded respondents who had dementia at the baseline wave. About 10% of the interviews in our sample were conducted through proxies (spouses or children) for those who could not participate in the survey due to health issues (Langa et al., 2016). The final analytic sample contains 10,607 non-Hispanic White respondents, 1,761 non-Hispanic Black respondents, and 1,158 Hispanic respondents aged 65 and older in 2000 as well as respondents who became age-eligible from 2002 to 2016 (see Table 1 for baseline descriptive statistics by race/ethnicity).
Table 1.
Weighted Baseline Descriptive Statistics by Race/Ethnicity, HRS 2000 (N = 13,589)
| Total | Non-Hispanic White | Non-Hispanic Black | Hispanic | |||||
|---|---|---|---|---|---|---|---|---|
| N = 13,589 | N = 10,670 | N = 1,761 | N = 1,158 | |||||
| Mean/% | SD | Mean/% | SD | Mean/% | SD | Mean/% | SD | |
| Dementiaa | 4.24 | 3.66 | 8.43 | 6.88 | ||||
| Sibling loss | ||||||||
| No loss | 47.27 | 50.85 | 25.91 | 28.94 | ||||
| One loss | 29.90 | 29.49 | 33.70 | 30.32 | ||||
| Multiple losses | 22.82 | 19.66 | 40.39 | 40.74 | ||||
| Race/ethnicity | ||||||||
| White (reference) | 84.84 | |||||||
| Black | 8.45 | |||||||
| Hispanic | 6.71 | |||||||
| Controls | ||||||||
| Female | 56.37 | 55.95 | 59.06 | 58.28 | ||||
| Born in South | 30.29 | 26.28 | 78.22 | 20.75 | ||||
| Childhood adversity | 1.01 | 0.01 | 0.88 | 0.01 | 1.58 | 0.03 | 1.99 | 0.04 |
| Born in the United States | 92.51 | 95.84 | 94.05 | 48.42 | ||||
| Education | ||||||||
| Less than high school | 23.57 | 18.84 | 41.77 | 60.48 | ||||
| High school | 35.65 | 37.54 | 29.22 | 19.80 | ||||
| Some college or more | 40.78 | 43.62 | 29.01 | 19.72 | ||||
| Married at baseline | 66.71 | 69.03 | 48.26 | 60.68 | ||||
| Experienced other family losses | 76.06 | 75.05 | 80.81 | 82.90 | ||||
| Total numbers of siblings | 3.28 | 0.02 | 2.97 | 0.02 | 4.71 | 0.09 | 5.41 | 0.14 |
| Age | 69.73 | 0.06 | 69.94 | 0.07 | 68.63 | 0.14 | 68.47 | 0.18 |
| Biosocial factors | ||||||||
| Logged household income | 10.15 | 0.01 | 10.27 | 0.01 | 9.64 | 0.03 | 9.32 | 0.06 |
| CES-D score | 1.36 | 0.02 | 1.28 | 0.02 | 1.67 | 0.06 | 1.95 | 0.09 |
| Behavioral health | ||||||||
| Alcohol consumption | ||||||||
| Nondrinker | 68.15 | 66.32 | 81.45 | 74.61 | ||||
| Moderate drinker | 22.85 | 24.12 | 13.19 | 18.87 | ||||
| Heavy drinker | 9.00 | 9.56 | 5.36 | 6.52 | ||||
| Smoking | ||||||||
| Never smoked | 41.05 | 40.73 | 39.89 | 46.46 | ||||
| Former smoker | 46.42 | 46.83 | 44.69 | 43.44 | ||||
| Current smoker | 12.54 | 12.44 | 15.42 | 10.10 | ||||
| Vigorous physical activity | 36.14 | 37.83 | 27.66 | 25.52 | ||||
| Biological health | ||||||||
| BMI | 27.58 | 0.06 | 27.34 | 0.06 | 29.40 | 0.19 | 28.31 | 0.19 |
| Poor self-rated health | 2.77 | 0.01 | 2.69 | 0.01 | 3.12 | 0.03 | 3.25 | 0.04 |
| Cardiometabolic conditions | 0.95 | 0.01 | 0.94 | 0.01 | 1.01 | 0.04 | 1.00 | 0.05 |
Notes: All means/proportions are statistically significant by race/ethnicity at p < .05 level except for the following variables: For Black respondents, the numbers of chronic diseases and the percentage of those who never smoked are not statistically significant from Whites. For Hispanic respondents, the percentage who are current smokers is the only variable not statistically significant from Whites. BMI = body mass index; CES-D = Center for Epidemiological Studies—Depression; HRS = Health and Retirement Study; SD = standard deviation.
aAll dementia cases in the baseline survey were excluded. The reported percentages of dementia were calculated based on person-period files (N = 67,759), reflecting dementia onset across the course of the study.
Measures
Dementia
We use the Langa–Weir classification of cognitive function among individuals aged 65 years and older, which has been used widely in dementia research with the HRS (Crimmins et al., 2011; Liu et al., 2020; Umberson et al., 2020). Scholars verified this dementia classification by using clinical diagnoses and survey scores from an HRS subsample in the Aging, Demographics, and Memory Study (Crimmins et al., 2011). This measurement contains proxy and self-reports to assess dementia because people who experience dementia may struggle with completing measures of cognitive status. First, we measure proxy-reported dementia based on a rating of respondent’s current memory from excellent to poor (0–4); assessments of limitations of five instrumental activities of daily living including using the phone, managing money, taking a medication, preparing hot meals, and shopping for groceries (0–5); and the interviewer’s assessment of difficulty in completing the interview due to cognitive limitation (0–2), which sum to 11. We code respondents with a proxy-dementia score of 6 or higher as having dementia (Crimmins et al., 2011). Second, we measure self-reported dementia status using a summary score of immediate word recall (0–10), delayed word recall (0–10), serial subtraction of 7s (0–5), and backward counting from 20 (0–2). The score ranges from 0 to 27. The Langa–Weir classification of cognitive function treats respondents who scored 6 or lower as having dementia.
Race/ethnicity
We examine non-Hispanic White (reference group), non-Hispanic Black, and Hispanic respondents.
Sibling loss in mid-to-late life
We examine sibling deaths occurring when respondents were aged 50 and older. Respondents were asked how many living brothers and sisters they had at each wave (1992–2016). We construct a time-varying sibling loss variable if the number of living siblings decreases in a subsequent wave. We treat sibling losses categorically, with dichotomous variables for no loss (reference), one loss, and multiple losses, following previous research (Rostila et al., 2012). Any nonmissing count (including zero) is carried backward to fill missing values at prior waves. All measures concerning death of a sibling were obtained prospectively, across HRS waves.
Sociodemographic controls
Other covariates measured at baseline include gender (1 = women, 0 = men), birthplace in the South (1 = South, 0 = otherwise), whether the respondent was born in the United States (1 = born in United States, 0 = born elsewhere), education (less than high school [reference], high school, some college, and college and above). We also include a time-varying categorical indicator of marital status (married [reference], separated/divorced, widowed, never married) and a binary measure for other family member deaths. We code as 1 if respondents experienced the death of a mother, father, spouse, or child by the respondent’s baseline wave. We control for the total number of siblings at the baseline wave because racial/ethnic minorities are more likely than White adults to have more siblings (Daw et al., 2016; Landale et al., 2006). To adjust for shared familial confounding shared by siblings, we create an indicator of childhood SES adversity in early life, which sums dichotomized measures of mother’s and father’s education (1 = less than 8 years), and income (1 = their childhood family was poor).
Biosocial factors
Consistent with prior research (Liu et al., 2020; Umberson et al., 2020), we control a constellation of factors that may be associated with bereavement and dementia. We measure household income, a time-varying variable, by the total household income from the year before each interview wave. We divide the values by the square root of household size and log-transformed it (Zhang et al., 2016). We measure distress with an 8-item Center for Epidemiological Studies—Depression (CES-D) scale. Higher scores indicate higher levels of psychological distress (ranging from 0 to 8). We code smoking into three categories: never smoked (reference), former smoker, and current smoker. We code alcohol consumption into three categories: nondrinker, moderate drinker (reference; those who drank on average fewer than 7 alcoholic beverages per week during the past 3 months), and heavy drinker (8 beverages or more per week). We code exercise as a dummy variable comparing respondents who participated in vigorous physical activity or exercised three times a week or more over the past 12 months (reference) with those who did not. Because siblings share genetic risk for many health outcomes, including dementia (Rostila et al., 2012), we include body mass index (BMI; weight divided by the square of height), poor self-rated health (1 = excellent to 5 = poor), and the number of ever-diagnosed cardiometabolic conditions, including heart disease, stroke, diabetes, and hypertension.
Analytic Strategy
First, we report descriptive statistics to assess racial/ethnic disparities in the measures of dementia, exposures to sibling deaths, and other characteristics, using t tests (two-tailed) to formally estimate racial/ethnic differences. Then, we use multinomial logistic regression analyses to test the association between race/ethnicity and exposures to sibling deaths (no death, one death, multiple deaths). Next, we estimate discrete-time hazard models to compare dementia risk by sibling deaths and race/ethnicity. We create person-wave record files and use a logit model for the discrete-time event history analysis. A respondent contributes an observation for each wave up to the onset of dementia or censoring (i.e., loss to follow-up or death). The estimates reflect the effects of sibling losses on new onsets of dementia because the analytic sample is restricted to those who had no dementia at baseline. We specify the discrete-time hazard model as:
where h(tij) indicates the discrete-hazard of the onset of dementia for person i at wave j; h0(tij) indicates the discrete-hazard of baseline dementia status for person i at wave j; represents the vector of multiple intercepts for HRS 2000–2016, one per period; indicates the set of time-invariant covariates; and indicates the vector of time-varying covariates including sibling loss. B1 and B2 are corresponding coefficient vectors.
We estimate a series of models to understand the association between sibling deaths, race/ethnicity, and dementia. Model 1 controls for basic sociodemographic covariates (e.g., age, gender, education) and it provides evidence for dementia risk by exposures to sibling deaths. Model 2 shows the association between race/ethnicity and dementia risk, independent from sibling deaths with sociodemographic controls. Model 3 builds on Model 2 by including sibling deaths. In this way, we pay particular attention to whether inclusion of sibling deaths in Model 3 reduces racial/ethnic disparities in dementia risk. To formally test mediation, whether the sibling deaths measures help to explain the race/ethnicity gaps in dementia in discrete-time hazard models, we calculate the proportion explained by sibling deaths using the Karlson–Holm–Breen test (Breen et al., 2013). Model 4 adds time-varying biosocial factors (e.g., CES-D scale, smoking, and BMI) to Model 3.
Finally, we estimate the contribution of sibling loss differences to racial/ethnic differences in dementia risk by simulating what the dementia risk of Black and Hispanic adults would have been had they experienced White adults’ distribution of sibling losses during the study. We assign White respondents’ sibling loss exposure to Black and Hispanic respondents to calculate the counterfactual rates of dementia risk using propensity score reweighting (see Supplementary Appendix A for detailed explanation).
Those whose dementia risk is most adversely affected by sibling death exposure are more likely to die and thus drop out of the sample, and this may be more likely for racial/ethnic minorities than Whites. We create inverse probability-of-censoring weights to account for attrition (Ding et al., 2019). All descriptive statistics and model estimates are weighted to adjust for survey design and right censoring due to attrition. We analyze all models using Stata 16.1.
Results
Table 1 shows descriptive statistics for all analyzed variables in the baseline 2000 HRS sample. The results show that among people aged 65 and older, White respondents had the lowest proportion of dementia during the study (3.7%), followed by Hispanic respondents (6.9%), and Black respondents (8.4%). Black and Hispanic respondents are significantly more likely to develop dementia than Whites. White respondents are significantly less likely to experience sibling loss than others. Only 49% of White respondents experienced sibling deaths, followed by Hispanic respondents (72%) and Black respondents (74%).
Results in Table 2 document that racial/ethnic minorities are more likely than White adults to experience sibling deaths in late life. Compared to White adults, Black adults have 65% higher odds for experiencing one loss and 132% higher odds for multiple losses relative to nonbereavement (Model 1). Hispanic adults have 55% and 129% higher odds than White adults with respect to experiencing one sibling loss and multiple losses, respectively, relative to nonbereavement. However, in Model 2, after adjusting for sociodemographic variables, the results for Hispanic adults become nonsignificant.
Table 2.
Estimated Odds Ratios of Sibling Deaths From Multinomial Logistic Models by Race/Ethnicity, HRS 2000–2016 (N = 67,759)
| Odds ratio | ||||
|---|---|---|---|---|
| No loss vs one loss | No loss vs multiple loss | |||
| Model 1 | Model 2 | Model 1 | Model 2 | |
| Race/ethnicity (ref. White) | ||||
| Black | 1.65*** (0.07) | 1.37*** (0.05) | 2.32*** (0.14) | 1.45*** (0.11) |
| Hispanic | 1.55*** (0.08) | 1.09 (0.06) | 2.29*** (0.13) | 0.96 (0.08) |
| Sociodemographic controls | ||||
| Female | 1.07 (0.04) | 0.87* (0.05) | ||
| Born in the South | 1.17*** (0.05) | 1.30*** (0.10) | ||
| Child SES adversity | 1.16** (0.06) | 1.26*** (0.08) | ||
| Born in United States | 0.96 (0.06) | 0.97 (0.10) | ||
| Education (ref. Less than HS) | ||||
| High school | 0.81** (0.06) | 0.62*** (0.07) | ||
| Some college or more | 0.74*** (0.03) | 0.60*** (0.05) | ||
| Marital status (ref. Married) | ||||
| Separated/divorced | 1.02 (0.05) | 0.93 (0.08) | ||
| Widowed | 0.93 (0.06) | 0.98 (0.08) | ||
| Never married | 1.03 (0.07) | 1.36*** (0.13) | ||
| Experienced other family death at baseline | 1.01 (0.11) | 1.60** (0.27) | ||
| Number of siblings | 1.16*** (0.03) | 1.47*** (0.04) | ||
| Age | 1.09*** (0.01) | 1.18*** (0.01) | ||
| Constant | 0.41*** (0.01) | 0.00*** (0.00) | 0.15*** (0.00) | 0.00*** (0.00) |
| Akaike Information Criterion | 132,946 | 116,836 | 132,946 | 116,836 |
Notes: Robust standard errors in parentheses. The coefficients are odds ratios. HRS = Health and Retirement Study; HS = high school; SES = socioeconomic status.
***p < .001. **p < .01. *p < .05.
In Table 3, Model 1 reveals that experiencing sibling loss is associated with heightened dementia risk for aging adults. Experiencing one sibling loss is associated with 46% higher odds (p < .001), and multiple loss is associated with 54% higher odds (p < .001) of dementia than those who did not experience any sibling loss, net of sociodemographic covariates. These results provide support for the hypothesis that sibling loss increases dementia risk for aging adults.
Table 3.
Estimated Odds Ratios of Dementia Onset From Discrete-Time Hazard Models, HRS 2000–2016
| Dementia incidence | ||||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Sibling loss (ref. No loss) | ||||
| One loss | 1.46*** (0.14) | 1.44*** (0.14) | 1.46*** (0.13) | |
| Multiple losses | 1.54*** (0.19) | 1.53*** (0.19) | 1.57*** (0.17) | |
| Race/ethnicity (ref. White) | ||||
| Black | 1.58*** (0.13) | 1.55*** (0.13) | 1.70*** (0.15) | |
| Hispanic | 1.51*** (0.15) | 1.52*** (0.15) | 1.49*** (0.17) | |
| Sociodemographic controls | ||||
| Female | 0.97 (0.06) | 0.97 (0.06) | 0.98 (0.06) | 0.93 (0.06) |
| Born in the South | 1.82*** (0.13) | 1.67*** (0.15) | 1.63*** (0.14) | 1.51*** (0.13) |
| Child SES adversity | 1.03 (0.04) | 1.02 (0.04) | 1.00 (0.04) | 0.98 (0.03) |
| Born in United States | 0.67*** (0.07) | 0.82 (0.09) | 0.83 (0.09) | 0.83 (0.10) |
| Education (ref. Less than HS) | ||||
| High school | 0.52*** (0.04) | 0.53*** (0.05) | 0.54*** (0.05) | 0.56*** (0.05) |
| Some college or more | 0.36*** (0.03) | 0.37*** (0.03) | 0.38*** (0.03) | 0.43*** (0.04) |
| Marital status (ref. Married) | ||||
| Separated/divorced | 1.45* (0.21) | 1.37* (0.21) | 1.37* (0.21) | 1.29 (0.22) |
| Widowed | 1.19* (0.08) | 1.15 (0.09) | 1.16* (0.08) | 1.15* (0.08) |
| Never married | 1.27 (0.18) | 1.20 (0.17) | 1.19 (0.17) | 1.18 (0.18) |
| Experienced other family death at baseline | 1.04 (0.09) | 1.04 (0.10) | 1.03 (0.09) | 1.04 (0.09) |
| Number of siblings | 1.00 (0.02) | 1.00 (0.02) | 0.99 (0.02) | 0.98 (0.01) |
| Age | 1.10*** (0.01) | 1.11*** (0.01) | 1.10*** (0.01) | 1.09*** (0.01) |
| Biosocial factors | ||||
| Logged household income | 1.09 (0.10) | |||
| Psychological distress | 1.03* (0.02) | |||
| Alcohol consumption (ref. Moderate drinker) | ||||
| Nondrinker | 1.71*** (0.15) | |||
| Heavy drinker | 0.99 (0.14) | |||
| Smoking (ref. Current smoker) | ||||
| Nonsmoker | 1.09 (0.08) | |||
| Former smoker | 1.02 (0.12) | |||
| Vigorous physical activity | 0.64*** (0.06) | |||
| BMI | 0.96*** (0.01) | |||
| Poor self-rated health | 1.40*** (0.05) | |||
| Cardiometabolic conditions | 1.15*** (0.03) | |||
| Constant | 0.00*** (0.00) | 0.00*** (0.00) | 0.00*** (0.00) | 0.00*** (0.00) |
| Person-years | 67,759 | 67,759 | 67,759 | 67,759 |
| Akaike Information Criterion | 27,718 | 27,735 | 27,629 | 26,482 |
Notes: Robust standard errors in parentheses. The coefficients are odds ratios. BMI = body mass index; HRS = Health and Retirement Study; HS = high school; SES = socioeconomic status.
***p < .001. **p < .01. *p < .05.
We next consider whether racial/ethnic differences in dementia risk exist (Model 2). Black and Hispanic adults have 58% (p < .001) and 51% (p < .001) higher odds of dementia risk, net of sociodemographic covariates, respectively, than White adults. Including the measures of sibling deaths in Model 3 reduces the Black–White disparity seen in Model 2. After the inclusion of sibling deaths, Model 3 shows that Black and Hispanic adults are almost 1.55 times (p < .001), and 1.52 times (p < .001), respectively, more likely than White adults to develop dementia over the study period (p < .001). The formal mediation test (not shown) indicates that accounting for sibling deaths reduces the Black–White disparity in dementia risk by 5.5% (p < .001). The mediation test for Hispanic adults is not statistically significant, consistent with results from Table 2. Model 4 shows that the results from Model 3 are robust to the inclusion of biosocial factors. In short, sibling loss and race/ethnicity have independent effects on dementia risk, and sibling loss contributes to Black–White disparities in dementia risk.
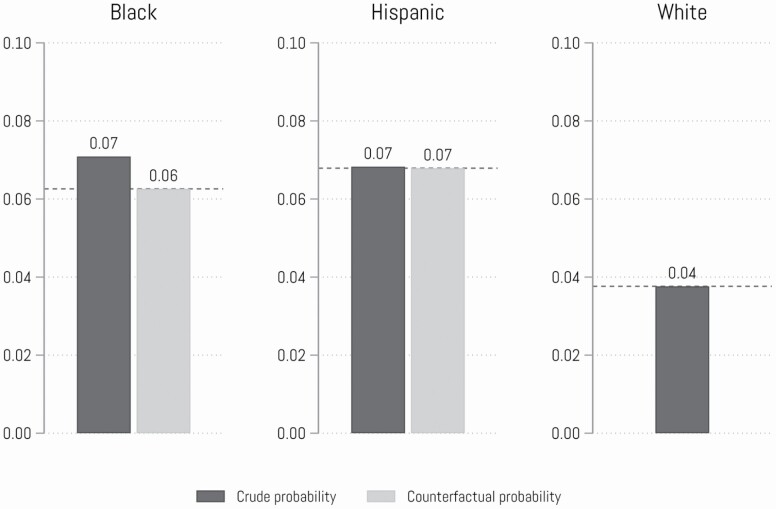
Next, we explore a counterfactual simulation analysis by standardizing the sibling loss distribution between racial/ethnic groups (Figure 1). We ask, what would the dementia risk of Black and Hispanic adults look like if they experienced the same sibling loss exposure as White adults? Figure 1 adjusts for control variables, showing that if Black adults experienced the same amount of sibling loss as White adults, net of controls, their dementia incidence would be 14% lower. In contrast, dementia incidence among Hispanic adults did not substantially change if they experienced the same amount of sibling loss as White adults, net of covariates.
Figure 1.
Counterfactual probabilities of dementia risk by race/ethnicity. Note: We calculated the propensity score from sociodemographic covariates used in Model 2 in Table 2 and applied an inverse probability treatment weighting (see Supplementary Appendix A for detail).
Sensitivity Checks
We assessed the sensitivity of our results to several different specifications of key variables. The results were substantively unchanged across these various specifications, providing additional assurance in the robustness of the findings. First, we estimated cognitive impairment with no dementia rather than dementia risk. Second, we conducted the analyses using only proxy reports, which are reliable in the HRS (Crimmins et al., 2011). The overall pattern of results and basic conclusions were consistent across measures and methods (available upon request). In another set of additional analyses (not shown), we tested whether the association between sibling loss and dementia is sensitive to the timing of bereavement. The results did not differ by the timing of sibling loss.
Discussion
Although sibling relationships typically persist across a large span of the life course, lasting longer than most social relationships (Cicirelli, 2009), there is a dearth of research documenting the health implications of the death of a sibling in adulthood. The death of a sibling is often meaningful and stressful, challenging notions of one’s own mortality and disrupting a potentially important source of social support, contact, and connection (Rostila et al., 2012; Volkom, 2006). Moreover, little attention has been directed to how sibling deaths may affect racial/ethnic disparities in dementia. Racial/ethnic minorities, in particular Black adults, are more likely to be exposed to sibling deaths than White adults. The unequal exposure to sibling deaths by race and ethnicity potentially contributes to patterns of dementia risk. We analyzed longitudinal, nationally representative data to consider whether exposure to sibling deaths might influence dementia risk and whether unequal exposure to these traumatic events mediates the association between race/ethnicity and dementia.
Our first major finding supports the hypothesis that people who lose one or more siblings have a higher risk of dementia compared to people who do not lose a sibling. We found that the death of a sibling contributes to dementia risk, and losing more than one sibling adds to this risk. The stress and distress evoked by the death of a sibling may activate social and biological processes that undermine health (Danese & McEwen, 2012; Peavy et al., 2012). Higher levels of stress are linked to lower cognitive scores and greater risk for various forms of dementia (Leng et al., 2013). Moreover, the stress of sibling death may increase depressive symptoms (Greenberg et al., 2014) and prompt unhealthy behaviors as coping mechanisms, which also contribute to increased dementia risk (Cicirelli, 2009).
Our second major finding concerns tests for differential exposure to sibling deaths by race/ethnicity. Although research documents that Black adults are exposed to more family member deaths (Umberson et al., 2017) and that exposure to these deaths can increase dementia risk (Umberson et al., 2020; Zhang et al., 2021), these two literatures have not been integrated to test the role of differential exposures to sibling loss as contributing to racial/ethnic cognitive health disparities in later life. Our results support the differential exposure hypothesis related to Black–White dementia disparities, suggesting that greater exposure to sibling death among Black adults compared to White adults contributes to racial disparities in dementia risk. The findings indicate that older Black adults are much more likely to lose a sibling than are White adults and the death of a sibling contributes to Black–White disparities in developing dementia. These findings are consistent with findings from a study of dementia risk associated with having experienced a child’s death (Umberson et al., 2020). The findings from our counterfactual simulation analysis––applying the sibling death exposure rates of White adults to Black adults––revealed that Black adults’ risk for dementia would be 14% lower if they experienced the lower exposure rates to sibling death that White adults experience, net of covariates. Greater systemic racial discrimination across the life course may contribute to unequal exposure to family member deaths and disparities in dementia risk for Black Americans (Umberson, 2017).
Although Hispanic adults also experience greater exposure to sibling deaths than White adults, Hispanic adults do not experience the same risk as Black adults. To advance understanding of this finding, we estimated a multinomial logistic regression predicting sibling deaths in a stepwise fashion (not shown). We find that the total number of siblings explains the association between the Hispanic category and sibling deaths. Results from the Karlson–Holm–Breen analysis suggest that the mediating effect of the total number of siblings is statistically significant (p < .001). This implies that the higher exposure of sibling deaths among the Hispanic population is due to the relatively large number of siblings on average. Future research should further navigate heterogeneity in the effects of bereavement among racial/ethnic minorities.
This study has several limitations. First, the measure of dementia is based on cognitive tests and proxy reports rather than clinical diagnosis. Due to data limitations, namely the lack of magnetic resonance imaging and clinical evaluations, we cannot determine the type of dementia for any group. Second, genetic selectivity may be a threat to causal inference. However, prior research has found that the association between sibling loss and health conditions was not confounded by an unobserved trait, such as genetic similarities between siblings (Rostila et al., 2012), and our findings are robust to controls for several factors that might reflect shared genetic risk and increase dementia risk (e.g., shared childhood family environments and midlife health conditions). Third, HRS questions about sibling relationships do not ask about relationship quality among siblings. Increased closeness of the bond to a sister (by both men and women) was related to lower levels of depression, whereas feelings of conflict or indifference in sibling relationships were related to increased depression (Cicirelli, 2009). Future research should consider how quality of sibling relationships influences the impact for sibling death on cognitive function. Finally, the HRS does not have reliable measures of sibling loss prior to midlife, such as deaths that occurred during childhood and young adulthood. The life course timing of loss may be especially important in that prior research shows stress has stronger and more lasting effects if it occurs during certain sensitive periods of development (Glymour & Manly, 2008). Focusing only on sibling deaths in mid-to-late life likely underestimates the total influences of sibling loss and racial/ethnic disparities in dementia. However, sibling deaths are much more common in mid-to-late life than early life, and it may be particularly important due to older adults’ possible vulnerability to bereavement effects on health. Future research should further consider the timing of family deaths on cognitive health.
Conclusion
A growing body of evidence underscores the importance of sibling relationships for health and well-being throughout life (Rostila et al., 2012, 2013). The current study extends this literature by considering how the death of siblings in mid-to-late life may affect dementia risk and contribute to dementia disparities by race/ethnicity. Based on longitudinal data drawn from a nationally representative sample of U.S. older adults, the results suggest that the death of a sibling increases dementia risk and contributes to disparities in dementia risk associated with race/ethnicity. Considering the substantial adverse impact of sibling loss for dementia risk found in this study, and the substantially greater exposure to sibling deaths for Black adults, future research should recognize the importance of sibling relationships throughout life and begin to address the underlying mechanisms through which the loss of a sibling may accelerate biological and brain aging across diverse populations.
Supplementary Material
Contributor Information
Hyungmin Cha, Department of Sociology, Center on Aging and Population Sciences and Population Research Center, The University of Texas at Austin, Austin, Texas, USA.
Patricia A Thomas, Department of Sociology, Center on Aging and the Life Course, Purdue University, West Lafayette, Indiana, USA.
Debra Umberson, Department of Sociology, Center on Aging and Population Sciences and Population Research Center, The University of Texas at Austin, Austin, Texas, USA.
Funding
This research was supported by the National Institute on Aging, grants P30AG066614 and R01AG054624, and by the U.S. Eunice Kennedy Shriver National Institute of Child Health and Human Development, grant P2CHD042849.
Conflict of Interest
None declared.
Author Contributions
H. Cha developed the conceptual framework, conducted the analysis, and drafted the paper. P. A. Thomas assisted with writing, supervision of data analysis, and revising the article. D. Umberson contributed to the conceptual framework and writing.
References
- Aneshensel, C. S. (2009). Toward explaining mental health disparities. Journal of Health and Social Behavior, 50(4), 377–394. doi: 10.1177/002214650905000401 [DOI] [PubMed] [Google Scholar]
- Bolton, J. M., Au, W., Chateau, D., Walld, R., Leslie, W. D., Enns, J., Martens, P. J., Katz, L. Y., Logsetty, S., & Sareen, J. (2016). Bereavement after sibling death: A population-based longitudinal case-control study. World Psychiatry, 15(1), 59–66. doi: 10.1002/wps.20293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen, R., Karlson, K. B., & Holm, A. (2013). Total, direct, and indirect effects in Logit and Probit models. Sociological Methods & Research, 42(2), 164–191. doi: 10.1177/0049124113494572 [DOI] [Google Scholar]
- Brown, L. L., Mitchell, U. A., & Ailshire, J. A. (2020). Disentangling the stress process: Race/ethnic differences in the exposure and appraisal of chronic stressors among older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 75(3), 650–660. doi: 10.1093/geronb/gby072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicirelli, V. G. (2009). Sibling death and death fear in relation to depressive symptomatology in older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 64(1), 24–32. doi: 10.1093/geronb/gbn024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins, E. M., Kim, J. K., Langa, K. M., & Weir, D. R. (2011). Assessment of cognition using surveys and neuropsychological assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66(Suppl. 1), i162–i171. doi: 10.1093/geronb/gbr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese, A., & McEwen, B. S. (2012). Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior, 106(1), 29–39. doi: 10.1016/j.physbeh.2011.08.019 [DOI] [PubMed] [Google Scholar]
- Daw, J., Verdery, A. M., & Margolis, R. (2016). Kin count(s): Educational and racial differences in extended kinship in the United States. Population and Development Review, 42(3), 491–517. doi: 10.1111/j.1728-4457.2016.00150.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, X., Barban, N., Tropf, F. C., & Mills, M. C. (2019). The relationship between cognitive decline and a genetic predictor of educational attainment. Social Science & Medicine (1982), 239, 112549. doi: 10.1016/j.socscimed.2019.112549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilegård, A., Steineck, G., Nyberg, T., & Kreicbergs, U. (2013). Psychological health in siblings who lost a brother or sister to cancer 2 to 9 years earlier. Psycho-Oncology, 22(3), 683–691. doi: 10.1002/pon.3053 [DOI] [PubMed] [Google Scholar]
- Elder, G. H., Johnson, M. K., & Crosnoe, R. (2003). The emergence and development of life course theory. In Mortimer J. T. & Shanahan M. J. (Eds.), Handbook of the life course (pp. 3–19). Springer US. doi: 10.1007/978-0-306-48247-2_1 [DOI] [Google Scholar]
- Ertel, K. A., Glymour, M. M., & Berkman, L. F. (2008). Effects of social integration on preserving memory function in a nationally representative US elderly population. American Journal of Public Health, 98(7), 1215–1220. doi: 10.2105/AJPH.2007.113654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furstenberg, F. F. (2020). Kinship reconsidered: Research on a neglected topic. Journal of Marriage and the Family, 82(1), 364–382. doi: 10.1111/jomf.12628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, M. A., Downer, B., Chiu, C. T., Saenz, J. L., Rote, S., & Wong, R. (2019). Racial/ethnic and nativity differences in cognitive life expectancies among older adults in the United States. The Gerontologist, 59(2), 281–289. doi: 10.1093/geront/gnx142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glymour, M. M., & Manly, J. J. (2008). Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychology Review, 18(3), 223–254. doi: 10.1007/s11065-008-9064-z [DOI] [PubMed] [Google Scholar]
- Goetting, A. (1986). The developmental tasks of siblingship over the life cycle. Journal of Marriage and the Family, 48(4), 703–714. doi: 10.2307/352563 [DOI] [Google Scholar]
- Greenberg, M. S., Tanev, K., Marin, M. F., & Pitman, R. K. (2014). Stress, PTSD, and dementia. Alzheimer’s & Dementia, 10(3 Suppl.), S155–S165. doi: 10.1016/j.jalz.2014.04.008 [DOI] [PubMed] [Google Scholar]
- Hummer, R. A., & Hayward, M. D. (2015). Hispanic older adult health & longevity in the united states: Current patterns & concerns for the future. Daedalus, 144(2), 20–30. doi: 10.1162/DAED_a_00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, B. D., & Bennett, D. A. (2019). Causes and patterns of dementia: An update in the era of redefining Alzheimer’s disease. Annual Review of Public Health, 40, 65–84. doi: 10.1146/annurev-publhealth-040218-043758 [DOI] [PubMed] [Google Scholar]
- Landale, N. S., Oropesa, R. S., & Bradatan, C. (2006). Hispanic families in the United States: Family structure and process in an era of family change. In M. Tienda, & F. Mitchell, & National Research Council (US) Panel on Hispanics in the United States (Eds.), Hispanics and the future of America. National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK19902/ [PubMed] [Google Scholar]
- Langa, K. M., Weir, D. R., Kabeto, M., & Sonnega, A. (2016). Langa–Weir classification of cognitive function (1995 onward).https://hrsdata.isr.umich.edu/sites/default/files/documentation/data-descriptions/data_description_Langa_Weir_Classifications2016.pdf, 10.
- Lee, Y., Back, J. H., Kim, J., Kim, S. H., Na, D. L., Cheong, H. K., Hong, C. H., & Kim, Y. G. (2010). Systematic review of health behavioral risks and cognitive health in older adults. International Psychogeriatrics, 22(2), 174–187. doi: 10.1017/S1041610209991189 [DOI] [PubMed] [Google Scholar]
- Leng, Y., Wainwright, N. W., Hayat, S., Stephan, B. C., Matthews, F. E., Luben, R., Surtees, P. G., Khaw, K. T., & Brayne, C. (2013). The association between social stress and global cognitive function in a population-based study: The European Prospective Investigation into Cancer (EPIC)-Norfolk study. Psychological Medicine, 43(3), 655–666. doi: 10.1017/S0033291712001316 [DOI] [PubMed] [Google Scholar]
- Liu, H., Zhang, Z., Choi, S., & Langa, K. M. (2020). Marital status and dementia: Evidence from the Health and Retirement Study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 75(8), 1783–1795. doi: 10.1093/geronb/gbz087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., Brayne, C., Burns, A., Cohen-Mansfield, J., Cooper, C., Costafreda, S. G., Dias, A., Fox, N., Gitlin, L. N., Howard, R., Kales, H. C., Kivimäki, M., Larson, E. B., Ogunniyi, A., … Mukadam, N. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (London, England), 396(10248), 413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton, M. C., Fauth, E., Clark, C. J., Hatch, D., Greene, D., Pfister, R., Tschanz, J. T., & Smith, K. R. (2016). Family member deaths across adulthood predict Alzheimer’s disease risk: The Cache County Study. International Journal of Geriatric Psychiatry, 31(3), 256–263. doi: 10.1002/gps.4319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, S. E., Verdery, A. M., & Daw, J. (2020). Linked lives and childhood experience of family death on educational attainment. Socius, 6, 2378023120975594. doi: 10.1177/2378023120975594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin, L. I., & Bierman, A. (2013). Current issues and future directions in research into the stress process. In Aneshensel C. S., Phelan J. C., & Bierman A. (Eds.), Handbook of the sociology of mental health (pp. 325–340). Springer Netherlands. doi: 10.1007/978-94-007-4276-5_16 [DOI] [Google Scholar]
- Peavy, G. M., Jacobson, M. W., Salmon, D. P., Gamst, A. C., Patterson, T. L., Goldman, S., Mills, P. J., Khandrika, S., & Galasko, D. (2012). The influence of chronic stress on dementia-related diagnostic change in older adults. Alzheimer Disease & Associated Disorders, 26(3), 260–266. doi: 10.1097/WAD.0b013e3182389a9c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins, H. W., & Harris, L. B. (1990). Familial bereavement and health in adult life course perspective. Journal of Marriage and the Family, 52(1), 233. doi: 10.2307/352853 [DOI] [Google Scholar]
- Rostila, M., Saarela, J., & Kawachi, I. (2012). The forgotten griever: A nationwide follow-up study of mortality subsequent to the death of a sibling. American Journal of Epidemiology, 176(4), 338–346. doi: 10.1093/aje/kws163 [DOI] [PubMed] [Google Scholar]
- Rostila, M., Saarela, J., & Kawachi, I. (2013). Mortality from myocardial infarction after the death of a sibling: A nationwide follow-up study from Sweden. Journal of the American Heart Association, 2(2), e000046. doi: 10.1161/JAHA.112.000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnega, A., Faul, J. D., Ofstedal, M. B., Langa, K. M., Phillips, J. W., & Weir, D. R. (2014). Cohort profile: The Health and Retirement Study (HRS). International Journal of Epidemiology, 43(2), 576–585. doi: 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulos, I., Catania, C., Pinto, L. G., Silva, R., Pollerberg, G. E., Takashima, A., Sousa, N., & Almeida, O. F. (2011). Stress acts cumulatively to precipitate Alzheimer’s disease-like tau pathology and cognitive deficits. The Journal of Neuroscience, 31(21), 7840–7847. doi: 10.1523/JNEUROSCI.0730-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker, C. M., Gilligan, M., Klopack, E. T., Conger, K. J., Lanthier, R. P., Neppl, T. K., O’Neal, C. W., & Wickrama, K. A. S. (2020). Sibling relationships in older adulthood: Links with loneliness and well-being. Journal of Family Psychology, 34(2), 175–185. doi: 10.1037/fam0000586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin, A. R., Stephan, Y., Luchetti, M., & Terracciano, A. (2020). Loneliness and risk of dementia. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 75(7), 1414–1422. doi: 10.1093/geronb/gby112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoits, P. A. (2010). Stress and health: Major findings and policy implications. Journal of Health and Social Behavior, 51(Suppl.), S41–S53. doi: 10.1177/0022146510383499 [DOI] [PubMed] [Google Scholar]
- Umberson, D. (2017). Black deaths matter: Race, relationship loss, and effects on survivors. Journal of Health and Social Behavior, 58(4), 405–420. doi: 10.1177/0022146517739317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umberson, D., Olson, J. S., Crosnoe, R., Liu, H., Pudrovska, T., & Donnelly, R. (2017). Death of family members as an overlooked source of racial disadvantage in the United States. Proceedings of the National Academy of Sciences of the United States of America, 114(5), 915–920. doi: 10.1073/pnas.1605599114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umberson, D., Donnelly, R., Xu, M., Farina, M., & Garcia, M. A. (2020). Death of a child prior to midlife, dementia risk, and racial disparities. The Journals of Gerontology. Series B: Psychological Sciences and Social Sciences, 75(9), 1983–1995. doi: 10.1093/geronb/gbz154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkom, M. V. (2006). Sibling relationships in middle and older adulthood: A review of the literature. Marriage & Family Review, 40(2–3), 151–170. doi: 10.1300/J002v40n02_08 [DOI] [Google Scholar]
- White, L. (2001). Sibling relationships over the life course: A panel analysis. Journal of Marriage and Family, 63(2), 555–568. doi: 10.1111/j.1741-3737.2001.00555.x [DOI] [Google Scholar]
- Williams, D. R. (2018). Stress and the mental health of populations of color: Advancing our understanding of race-related stressors. Journal of Health and Social Behavior, 59(4), 466–485. doi: 10.1177/0022146518814251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, D. R., & Sternthal, M. (2010). Understanding racial–ethnic disparities in health: Sociological contributions. Journal of Health and Social Behavior, 51(Suppl.), S15–S27. doi: 10.1177/0022146510383838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z., Hayward, M. D., & Yu, Y. L. (2016). Life course pathways to racial disparities in cognitive impairment among older Americans. Journal of Health and Social Behavior, 57(2), 184–199. doi: 10.1177/0022146516645925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z., Liu, H., & Choi, S. E. (2021). Marital loss and risk of dementia: Do race and gender matter? Social Science & Medicine (1982), 275, 113808. doi: 10.1016/j.socscimed.2021.113808 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.