Abstract
Objectives
The cognitive reserve hypothesis has been proposed as a key mechanism explaining the link between social networks and cognitive function but has rarely been empirically tested using neuroimaging data. This study examines whether social network attributes moderate the association between amygdalar volume and cognitive function.
Methods
Data were from the Social Networks in Alzheimer Disease study (N = 154) and Indiana Alzheimer’s Disease Research Center. Social networks were measured using the PhenX Social Network Battery. Regional data from magnetic resonance imaging (amygdalar volume [AV]) were analyzed using FreeSurfer software. Cognitive function was measured using the Montreal Cognitive Assessment (MoCA) and consensus diagnosis. Linear regression analyses were conducted to test the moderating role of social networks on the association between AV and cognitive function.
Results
Participants with greater ability to span multiple social roles and subgroups within their networks scored higher on the MoCA after adjusting for sociodemographic variables, depression, frequency of contact, and AV. Social networks moderated the association between AV and cognitive function.
Discussion
Among participants who engaged in diverse and loosely connected social networks, the expected adverse cognitive effects of brain volume in regions implicated in socioemotional processing were attenuated. These findings suggest that cognitive stimulation achieved through social interaction with a diverse array of social relationships across multiple contexts may help promote cognitive reserve.
Keywords: Alzheimer’s disease, Amygdala, Cognitive reserve, Social networks
Increasing longevity is associated with a rise in the prevalence of Alzheimer’s disease (AD). Approximately 5.8 million Americans aged 65 and older currently live with AD—a figure that will triple by 2050 (Alzheimer’s Association, 2019). Despite the prevalence of AD among the older population, prevention and treatments have been slow to emerge. Consequently, AD researchers are looking to social–environmental factors to identify candidates for clinical intervention (Chalfont et al., 2020). Improving our understanding of modifiable factors that can alter the clinical course of AD is critical for reducing the burden of the disease.
One promising area of research focuses on personal social networks, documenting an inverse association between various measures of frequency or quality of social interaction and risk for dementia in older adults (Crooks et al., 2008; Fratiglioni et al., 2004; Gow et al., 2013; Pillai & Verghese, 2009). For example, Barnes et al. (2004) found that adults older than age 65 at the 90th percentile for a number of social ties experienced a 39% reduction in the rate of cognitive decline over 5 years compared to those at the 10th percentile. Similar findings have been confirmed by several meta-analyses and systematic reviews (Evans et al., 2019; Kelly et al., 2017; Lara et al., 2019; Penninkilampi et al., 2018; Perry, McConnell, Coleman, et al., 2021). Yet minimal research considers multidimensional features of the personal networks in which older adults are embedded. This is an important omission as individuals who occupy loosely connected networks composed of a mix of relationship types are exposed to a broader range of social stimuli compared to those who occupy dense, homogenous networks (Ellwardt et al., 2015; Perry, McConnell, Peng, et al., 2021).
A prominent neurological theory explaining the link between social networks and cognitive function posits that exposure to diverse stimuli strengthens one’s cognitive reserve (i.e., the brain’s capacity to cope with neural damage; Donovan et al., 2016; Stern et al., 2020). According to the cognitive reserve hypothesis, cognitively stimulating activities (a) strengthen existing neural pathways that protect against neurodegeneration and (b) foster new neural pathways through which the brain can reroute to fulfill normal functioning (Stern, 2012). Indeed, individuals with high levels of cognitive reserve have been shown to function at cognitively normal (CN) levels in the presence of AD pathology (Esiri et al., 2001; Katzman et al., 1989), whereas individuals with limited cognitive reserve tend to exhibit a stronger relationship between brain volume and cognitive function.
Taken together, the literatures on social networks and cognitive reserve suggest that social networks may moderate the relationship between brain volume and cognitive function. In other words, individuals with lower brain volume (a possible sign of neurological atrophy) will perform well on cognitive assessments so long as they maintain personal networks that expose them to novel and diverse social stimuli. Yet most studies have been unable to properly test this hypothesis because few data sets contain the necessary combination of social network data and neuroimaging data (c.f. Bennett et al., 2006). We address this research gap by analyzing data from the Social Networks in Alzheimer Disease (SNAD) project, a study of older adults containing clinical cognitive assessments, quantitative neuroimaging phenotypes (QNPs), and a detailed personal social network inventory.
Social Networks and Cognitive Function
Social networks—and the social relationships embedded within them—have been long recognized to influence health and well-being across the life span (Roth, 2020; Smith & Christakis, 2008). A wide body of research demonstrates a consistent link between personal network characteristics and multiple health outcomes such as psychological distress and hypertension (Cornwell & Waite, 2012; Huxhold et al., 2020) Similarly, older adults with numerous social contacts tend to perform better on cognitive assessments than those with fewer contacts (Barnes et al., 2004; Perry, McConnell, Peng, et al., 2021). The prevailing message from epidemiologic and social science studies is that social networks and cognitive function are robustly associated.
Despite widespread research on social networks and cognitive function or decline, most existing studies rely on proxy measures (e.g., number of friends, frequency of social contact) that do not fully capture the complexities of social life that likely influence cognitive risk and resilience. These proxy measures limit the ability to identify which aspects of social networks are protective of AD pathology. An egocentric network approach, however, captures the social connections between a focal individual (i.e., ego) and their direct contacts (i.e., alters) as well as all possible connections between those contacts (Perry et al., 2018). This approach situates each person within a unique personal community whose composition and structure have health consequences.
In terms of cognitive demand, individuals who are embedded in diverse personal networks must routinely toggle between a combination of social roles and interactions when spanning multiple social contexts (Ellwardt et al., 2015; Mische & White, 1998). The mental processing associated with managing a complex set of relationships and roles, in turn, is hypothesized to strengthen cognitive reserve (Meyer et al., 2012; Wlodarski & Dunbar, 2016). Conversely, individuals who occupy dense, homogenous networks engage in less cognitive “exercise” because the majority of their alters are interconnected and share similar social roles (e.g., family members). These latter types of networks expose individuals to familiar situations that are less cognitively demanding. Based on these insights, we hypothesize that the ability to span multiple social roles and bridge different subgroups within one’s personal network will be positively associated with cognitive function (H1).
Neurodegeneration and Cognitive Reserve
AD is characterized by progressive neurodegeneration and the presence of toxic amyloid plaques and neurofibrillary tangles that initially appear in the amygdala and hippocampus. These plaques and tangles accumulate over time and overwhelm the brain thereby increasing the risk of cognitive decline. Despite the commonly observed debilitating effects of AD, prospective clinical studies indicate that a significant proportion of older adults with unimpaired neuropsychological test results meet full pathological criteria for AD (Nelson et al., 2021; Stern et al., 2020). In other words, extensive neuropathology does not invariably cause cognitive impairment.
As posited by the cognitive reserve hypothesis, individual variations in enriching experiences across the life course provide different degrees of reserve against neurodegeneration (Chapko et al., 2018; Stern, 2012). The benefits of cognitive reserve are attributable to two mechanisms: (a) the development of greater cognitive capacity and efficiency prior to neurodegeneration and (b) a greater ability of the brain to compensate for pathological disruptions to preexisting networks when neurodegeneration emerges. Given that exposure to stimulating social environments is hypothesized to increase cognitive reserve (Wlodarski & Dunbar, 2016), we anticipate that older adults whose personal networks provide access to diverse social roles and groups will fare better in the presence of possible AD neuropathology (i.e., low brain volume) compared to older adults whose networks are narrower and more homogenous. Therefore, we hypothesize that the ability to span multiple social roles and subgroups within one’s personal network will moderate the relationship between brain volume and cognitive function (H2).
Method
Study Sample
Data are from the SNAD project, which leverages studies being conducted at the Indiana Alzheimer Disease Research Center (IADRC). The IADRC is one of 34 NIH-designated AD research centers that provide shared research resources and that recruit, clinically characterize, and longitudinally follow up individuals with and at risk of AD and related disorders. Three groups of participants from the IADRC cohort were recruited for the proposed study: (a) CN older adults, (b) adults with mild cognitive impairment (MCI), and (c) adults with early-stage AD dementia. Clinical diagnoses were made at the IADRC via consensus panel using the most recent diagnostic criteria and all available information from participants’ current and prior assessments. Weekly consensus conferences were held by the Clinical Core team, which included neurologists, neuropsychologists, psychiatrists, clinical trainees, and other investigators and staff involved in the cohort study. IADRC participants with advanced AD (i.e., Montreal Cognitive Assessment [MoCA] score <10) were excluded from SNAD eligibility due to incapability of sitting through a lengthy and cognitively burdensome interview. Participants with other types of dementia (e.g., frontotemporal lobar degeneration) were also ineligible for SNAD because there were too few to test the robustness of findings in distinct pathology groups, and these groups may have unique etiologies.
Between March 2015 and May 2019, all eligible IADRC participants were approached to voluntarily complete the SNAD protocol (89% response rate). For the current analysis, we used baseline SNAD data collected from 278 participants via face-to-face interview using computer-assisted personal interviewing in a private office during a routine IADRC clinic visit. A total of 124 participants who did not have neuroimaging data were excluded, resulting in a final analytic sample of 154. The excluded participants were largely missing data due to the study design, wherein neuroimaging data were collected less frequently than social network assessments (biannually). Nonetheless, we conducted a comparison of those with and without missing data on sociodemographic and social network variables and identified only one modest but significant difference (years of education; see Supplementary Table A1).
The sample was purposively skewed toward the mildly affected because a major goal of the IADRC is to improve the early detection of neurodegenerative disorders at the transition from normal cognition to prodromal stages of disease. Within the CN group, 10% were aged 80 or older, 70% had a positive family history of AD or related dementia, and 39% were at elevated genetic risk for AD based on APOE genotype. These characteristics increase the likelihood of observing low brain volume and clinical impairment—a unique and important feature of this sample.
Measures
Cognitive function
The primary dependent variable employed here is the MoCA (Nasreddine et al., 2005). The MoCA assesses global cognitive function across multiple domains, including attention, memory, visuospatial ability, abstraction, delayed recall, and orientation to time and place. Higher scores indicate better cognitive function. The MoCA was embedded in a more comprehensive assessment administered by an expert team of neurologists, neuropsychologists, and psychometricians and used for diagnostic classification at the IADRC. The MoCA scores were z-standardized (mean = 0; SD = 1) to aid interpretation of the magnitude of associations. We also used clinical diagnosis (CN, MCI, or Alzheimer’s dementia) as an alternative outcome. Diagnosis was determined through a consensus panel.
Social networks
In the present study, network data were collected using an expanded PhenX Social Network Battery (SNB) tailored to older adults. The PhenX Toolkit provides high-quality, standard measures for inclusion in health and human genetics research. It was developed by a panel of researchers from the National Human Genome Research Institute (PhenX Toolkit, 1991). The PhenX SNB took approximately 20–30 min to complete. This interviewer-administered survey elicited names of a participant’s alters who were activated in the past 6 months for discussions about important matters and health matters using items called name generators (Perry et al., 2018). These name generators targeted discussants (people respondents actively sought out for advice and discussion) and regulators (people who hassled the respondent and encouraged behavior change) to maximize measurement of potential social mechanisms. There was no limit on the number of alters named. After names were provided, a series of questions were asked about each alter. These included sociodemographic and relationship characteristics (e.g., relationship type, frequency of contact). In addition, we elicited basic information about ties between alters to compute structural measures of the network.
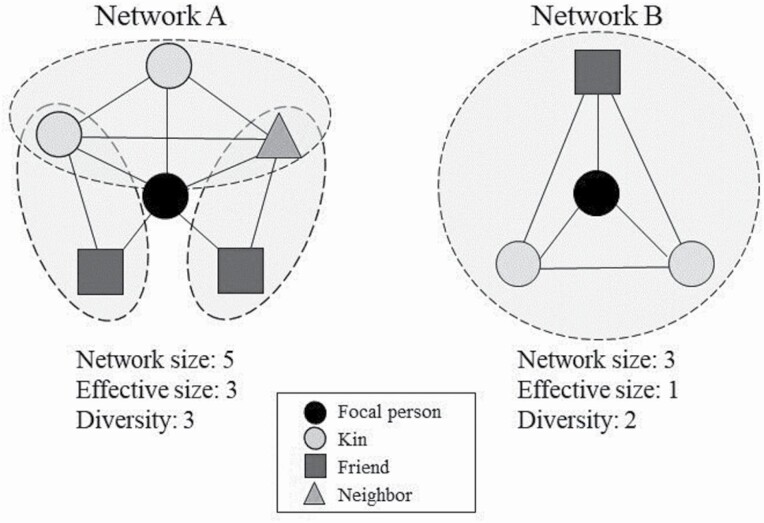
The following network-level attributes were constructed using data from the SNB. Figure 1 depicts two social networks with different values for structural characteristics used in this analysis. Network size was computed using the number of unique network members elicited in response to any name generator. Network diversity assessed participation in multiple types of social relationships (Cohen et al., 1997). This measure—designated with different shapes in Figure 1—assesses the total number of social roles that a participant occupies within their personal network (e.g., spouse, parent, child, friend, coworker, neighbor). Diversity scores ranged from 0 (for the one participant with no network ties) to 9. Effective size was calculated by subtracting network size from the mean number of ties that each alter had to all other alters. This quantifies the extent to which a participant engages in nonredundant social groups (i.e., dashed lines in Figure 1) that may provide access to distinct social experiences (Perry et al., 2018). Together, network diversity and effective size signify the degree to which participants are exposed to multiple social roles and subgroups within their personal networks.
Figure 1.
Two examples of effective size and diversity.
As part of a supplementary analysis, we also considered the density of each participant’s personal network. Network density was computed using the valued strength of ties between each pair of alters in a participant’s network, which ranged from 0 (Don’t know each other) to 3 (Very close). The valued strength between all alter pairs was summed and divided by the total possible number of alter–alter ties to generate the density measure. Higher values signified a larger degree of interconnectivity within each personal network. Density and effective size measure similar concepts, but the former does not directly account for number of alters in the network.
Quantitative neuroimaging phenotypes
QNPs were derived from magnetic resonance imaging (MRI) performed by the Neuroimaging Core of the IADRC. All MRI scans were completed on an advanced research-dedicated Siemens PRISMA 3T magnet. T1-weighted MPRAGE structural scans were used to quantitatively characterize brain structure. QNPs were extracted from MRI data using FreeSurfer 6 (Fischl, 2012) and were merged with social network and clinical assessments for analysis.
We assessed brain region volume, which provides a direct quantitative index of brain atrophy with strong correlations to cognitive function and demonstrated diagnostic validity (Risacher & Saykin, 2013). Research has established that subtle structural abnormalities are highly predictive of future conversion from a CN state to MCI (i.e., prodromal or preclinical phase of AD) and from MCI to dementia (Apostolova et al., 2006; Davatzikos et al., 2008; Risacher et al., 2009). For final analyses, brain region volume was z-standardized (mean = 0; SD = 1) and multiplied by −1 to simplify interpretation and convey atrophy in tables and figures. We also adjust for intracranial volume to allow for differences in head size in the final analyses.
In the current analysis, we focused on mean bilateral volume in the amygdala (Klein-Koerkamp et al., 2014; Poulin et al., 2011). This brain region is ideal for two reasons. First, the amygdala is among the earliest regions of the brain affected by AD, allowing us to detect neuropathology in our sample that is largely asymptomatic or has MCI (Poulin et al., 2011; Wachinger et al., 2016). Second, the amygdala is implicated in emotional processing, social behavior, and decision making, thus we expect to see an association with social network characteristics in this area (Benarroch, 2015; Felix-Ortiz & Tye, 2014). A larger amygdala is thought to have evolved in primates to accommodate the increasingly complex nature of social life, and amygdalar volume has been linked to social network size and complexity in humans (Bickart et al., 2011). As part of a supplementary analysis, we substitute hippocampal for amygdalar volume as the hippocampus is also among the earliest regions of the brain to be affected by AD (Wachinger et al., 2016). Although variables like social network size, number of social roles, social engagement, and social support have been linked to brain volume across multiple brain regions (Blumen & Verghese, 2019; Cotton et al., 2020; Kwak et al., 2018), as the social information processing centers of the brain, the amygdala and hippocampus are a natural starting point for this line of research on dementia.
Covariates
We adjusted for the following measures in final analyses to address potential confounding factors: sex (female = 1; male = 0), race (White = 1; non-White = 0), age (years), and education (years). These measures all potentially confound the relationship between network characteristics and cognitive function. Additionally, we included the proportion of alters in the network with whom the participant sees or talks to “very often” to account for the extent of exposure to different network structures. Finally, we included the 15-item Geriatric Depression Scale (Sheikh & Yesavage, 1986) to control for depression. Postestimate diagnostics indicated this variable was highly right-skewed. We therefore used the natural log transformation of this variable in the final analysis to achieve homoskedasticity of errors.
Data Analysis
We first summarized our sample using descriptive statistics. Next, we employed linear regression models predicting standardized global cognitive function as measured by the MoCA. Baseline models examined direct associations between social network attributes, clinical measures, and cognitive function, adjusting for gender, race, age, education, depressive symptoms, and amygdalar volume (Models 1–3). Interaction models assessed whether the network attributes (i.e., diversity, effective size) moderated the relationship between brain volume (i.e., bilateral mean amygdalar volume, reverse-coded to indicate atrophy) and cognitive function (Models 4–5). We adjusted for network size in the models with diversity but not those with effective size because network size is a key component of effective size. In addition to the regression models, we provided figures of predicted marginal effects to aid interpretation of the direction and magnitude of amygdala volume associations with cognitive function across different types of networks. To avoid discarding cases with missing data, all models were estimated using full information maximum likelihood—a method that uses all available information provided without omitting any cases. All analyses were conducted using Stata 16 (StataCorp, 2019). Finally, we conducted multiple sensitivity analyses: (a) using network density in place of the other two network attributes, (b) using hippocampal atrophy as an alternative measure of neurodegeneration, (c) predicting clinical diagnosis instead of MoCA, and (d) replicating main models after eliminating participants with AD. Results from these models are presented in Supplementary Materials and were consistent with those presented in the main analyses.
Results
Descriptive Statistics
Table 1 provides the descriptive statistics for the full analysis sample and by diagnosis. Overall, 64% of participants were female (72% CN; 47% MCI; 42% AD). The mean age was 71.18 years (SD = 8.26), with a slightly higher mean age among those with cognitive impairment (70.40 CN; 72.89 MCI; 72.92 AD). Mean education was 16.51 years (SD = 2.65) overall (16.58 CN; 16.64 MCI; 15.58 AD). Although network size ranged from 0 to 17, 83% of participants named between 2 and 7 alters. The mean network size was 5.12 (SD = 2.69) for the full analysis sample (5.52 CN; 4.78 MCI; 2.67 AD). These networks consisted of more than three different social relationship types (mean = 3.58, SD = 1.53) as indicated by the network diversity measure (3.83 CN; 3.33 MCI; 2.00 AD). The mean effective size was 2.01 (SD = 1.60), indicating that the average participant shared network ties to roughly two nonredundant social groups (2.18 CN; 1.85 MCI; 1.03 AD). Mean cognitive function (assessed using the MoCA) was 24.23 (SD = 4.11, range = 10–30), though this varied, on average and as expected, by diagnosis (25.73 CN; 22.06 MCI; 17.58 AD).
Table 1.
Descriptive Statistics
| All (n = 154) | Cognitively normal (n = 106) | MCI (n = 36) | Dementia (n = 12) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | |
| MoCA | 24.23 (4.11) | 10–30 | 25.73 (3.08) | 11–30 | 22.06 (3.28) | 15–27 | 17.58 (4.93) | 10–25 |
| Age | 71.18 (8.26) | 46–95 | 70.40 (8.05) | 46–93 | 72.89 (8.09) | 50–87 | 72.92 (10.17) | 63–95 |
| Female | 0.64 | 0.72 | 0.47 | 0.42 | ||||
| Education (years) | 16.51 (2.65) | 7–21 | 16.58 (2.59) | 12–21 | 16.64 (2.50) | 12–20 | 15.58 (3.50) | 7–18 |
| White | 0.77 | 0.75 | 0.83 | 0.75 | ||||
| GDS (logged) | 0.52 (0.70) | −0.01 to 2.48 | 0.38 (0.60) | −0.01 to 2.20 | 0.68 (0.74) | −0.01 to 2.40 | 1.25 (0.94) | 0–2.48 |
| Amygdala atrophy (z) | 0.00 (1.00) | −2.40 to 2.76 | 0.18 (0.88) | −2.00 to 2.76 | −0.44 (1.26) | −2.40 to 2.76 | −0.37 (1.08) | −1.51 to 1.69 |
| Hippocampus atrophy (z) | 0.00 (1.00) | −2.47 to 2.46 | 0.12 (0.85) | −2.47 to 1.68 | −0.53 (1.06) | −1.78 to 2.36 | −0.67 (1.20) | −1.37 to 2.46 |
| Intracranial volume (z) | −0.05 (1.01) | −2.72 to 2.98 | −0.08 (0.92) | −2.72 to 2.90 | 0.19 (1.09) | −1.99 to 2.98 | 0.03 (1.26) | −1.63 to 2.28 |
| Network size | 5.12 (2.69) | 0–17 | 5.52 (2.67) | 1–17 | 4.78 (2.54) | 1–14 | 2.67 (1.87) | 0–6 |
| Diversity | 3.58 (1.52) | 0–9 | 3.83 (1.48) | 1–9 | 3.33 (1.45) | 1–7 | 2.00 (1.10) | 0–4 |
| Effective size | 2.01 (1.60) | 0.00–10.00 | 2.18 (1.59) | 1.00–9.12 | 1.85 (1.73) | 1.00–10.00 | 1.03 (0.55) | 0.00–2.33 |
| Prop. frequent contact | 0.72 (0.25) | 0.13–1.00 | 0.68 (0.25) | 0.17–1.00 | 0.77 (0.25) | 0.13. 1.00 | 0.87 (0.19) | 0.50–1.00 |
Note: MoCA = Montreal Cognitive Assessment; MCI = mild cognitive impairment; GDS = 15-item Geriatric Depression Scale.
Multivariate Analyses
Table 2 presents the findings from the linear regression predicting cognitive function. Model 1 indicates that female participants (β = 0.37, SE = 0.15), participants with more education (β = 0.08, SE = 0.02), and White participants (β = 0.45, SE = 0.15) all had significantly higher cognitive function compared to each of their counterparts. Geriatric Depression Scale scores were negatively associated with cognitive function (β = −0.48, SE = 0.09). Amygdalar atrophy was also negatively associated with cognitive function (β = −0.19, SE = 0.08). For every SD increase in amygdalar atrophy, participants scored 0.21 SD lower on the MoCA.
Table 2.
Linear Regression Models Predicting Montreal Cognitive Assessment (z-standardized)
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| β (SE) | β (SE) | β (SE) | β (SE) | β (SE) | |
| Sociodemographics | |||||
| Age | −0.02 (0.01) | −0.02 (0.01) | −0.02* (0.01) | −0.02 (0.01) | −0.02* (0.01) |
| Woman | 0.39* (0.15) | 0.33* (0.14) | 0.36** (0.14) | 0.31* (0.14) | 0.30* (0.15) |
| Education (years) | 0.08*** (0.02) | 0.07** (0.02) | 0.08*** (0.02) | 0.07*** (0.02) | 0.08*** (0.02) |
| White | 0.46** (0.15) | 0.43** (0.14) | 0.55*** (0.13) | 0.42** (0.14) | 0.42** (0.14) |
| Clinical measures | |||||
| GDS (logged) | −0.50*** (0.09) | −0.45*** (0.08) | −0.29*** (0.08) | −0.46*** (0.08) | −0.51*** (0.08) |
| Intracranial volume (z) | 0.12** (0.07) | 0.08 (0.08) | 0.06 (0.07) | 0.07 (0.08) | 0.05 (0.08) |
| Amygdalar atrophy (z) | −0.21* (0.08) | −0.19** (0.07) | −0.19** (0.07) | −0.45*** (0.13) | −0.43*** (0.11) |
| Network attributes | |||||
| Size | −0.03 (0.03) | −0.023 (0.03) | |||
| Prop. frequent contact | −0.61* (0.25) | −0.59** (0.22) | −0.48 (0.25) | −0.55* (0.25) | |
| Diversity | 0.12* (0.05) | 0.13* (0.05) | |||
| Effective size | 0.01 (0.04) | 0.03 (0.04) | |||
| Interactions | |||||
| Amygdala × Diversity | 0.08* (0.04) | ||||
| Amygdala × Effective size | 0.14* (0.05) | ||||
| Intercept | 0.23 (0.81) | −0.38 (0.84) | 0.51 (0.75) | −0.23 (0.82) | 0.79 (0.80) |
| Adjusted R 2 | 0.43 | 0.48 | 0.44 | 0.49 | 0.49 |
| N | 154 | 154 | 154 | 154 | 154 |
Notes: GDS = 15-item Geriatric Depression Scale. Models 3 and 5 do not control for network size because network size is a key component used to calculate effective size.
*p < .05, **p < .01, ***p < .001.
Models 2 and 3 introduce the network attributes into the regression. Model 2 shows that network diversity is positively associated with cognitive function such that every additional social relationship type corresponds to a 0.12 SD increase on the MoCA. Effective size, however, showed no significant association with cognitive function (Model 3, β = 0.01, SE = 0.04, p = .82). The findings from these models provide partial support to H1, which states that the ability to span multiple social roles and subgroups within one’s personal network will be positively associated with cognitive function.
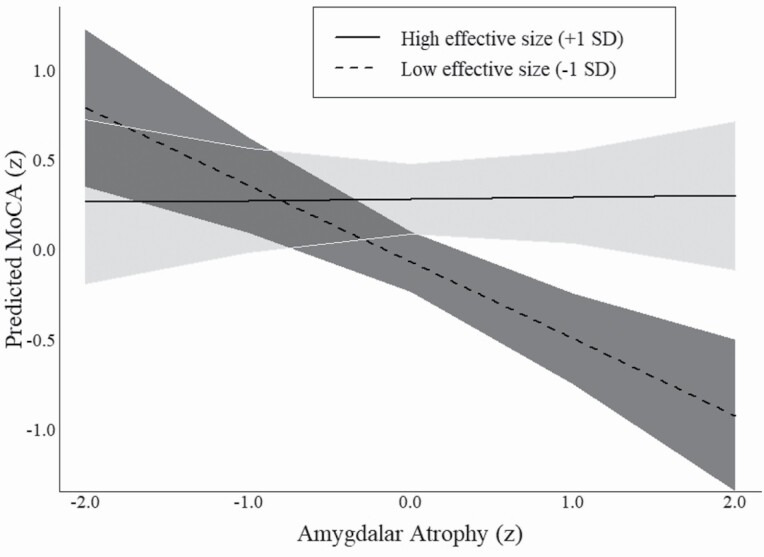
Models 4 and 5 test the interactions between amygdalar volume and network attributes to assess whether network diversity and effective size moderate the (negative) relationship between brain volume and cognitive function (H2). The significant interaction term in Model 4 (β = 0.08, SE = 0.04) indicates that the relationship between amygdalar atrophy and cognitive function is weaker for participants with diverse networks (i.e., multiple social relationship types) compared to those with less diverse networks. Figure 2 uses the marginal effects from this model to visualize the interaction. As shown, there is a clear negative association between amygdalar atrophy and MoCA scores when network diversity was lower than average (i.e., −1 SD). This follows the theoretically expected relationship between lower brain volume and poorer clinical cognitive performance (i.e., low MoCA score). However, Figure 2 also shows that participants with high levels of network diversity (i.e., +1 SD) failed to demonstrate any relationship between amygdalar atrophy and MoCA scores. In other words, access to social roles via one’s personal network appears to attenuate the association between brain volume and cognitive impairment.
Figure 2.
Marginal effect of brain atrophy on cognitive function by network diversity. Note: Predicted values are derived using average marginal effects from Table 2, Model 4. MoCA = Montreal Cognitive Assessment.
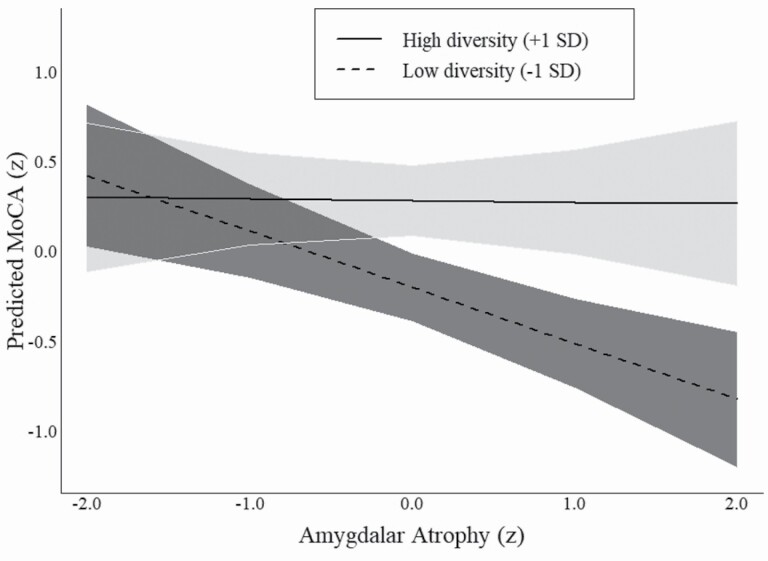
Model 5 tests the interaction between amygdalar atrophy and effective size. Similar to the interaction term from the previous model, this interaction shows that the association between atrophy and cognitive function is moderated by effective size (β = 0.14, SE = 0.05). Figure 3, which displays the marginal effects from Model 5, shows that participants whose networks expose them to fewer nonredundant social groups (i.e., −1 SD below the mean effective size) exhibited a strong negative association between amygdalar atrophy and cognitive function. In substantive terms, a participant with a healthy amygdala (e.g., 2 SD above the mean) and whose effective size was 1 SD below the mean was expected to score 0.79 SD above the mean on the MoCA (95% CI = 0.35–1.23). By contrast, a participant with the same effective size but whose amygdalar volume was 2 SD below the mean (i.e., an unhealthy amygdala) was expected to score 0.92 SD below the mean on the MoCA (95% CI = −1.35, −0.50). Participants whose networks had an effective size that was 1 SD above the mean were expected to score similar on the MoCA regardless of their levels of amygdalar atrophy (as shown by the solid line in Figure 3). Supplementary models that use network density instead of effective size replicate these findings (Supplementary Table A1). Collectively, these findings support H2.
Figure 3.
Marginal effect of brain atrophy on cognitive function by effective size. Note: Predicted values are derived using average marginal effects from Table 2, Model 5. MoCA = Montreal Cognitive Assessment.
Sensitivity Analyses
We conducted additional analyses to ensure the robustness of our findings by estimating a parallel set of models. First, we ran a linear regression in which we interacted network density by amygdalar atrophy (Supplementary Table A2). The findings from this model showed that participants who had a more interconnected network (i.e., high density) exhibited a negative relationship between amygdalar atrophy and MoCA, whereas participants who had a more loosely connected network (i.e., low density) were expected to score similar on the MoCA across levels of atrophy. Second, we reestimated the linear regression models from Table 2 and Supplementary Table A2 by substituting hippocampal atrophy for amygdalar atrophy (Supplementary Table A3). The results from these models were substantively consistent with the results from the models used in the main analyses. Third, we ran a series of logistic regression models using clinical diagnosis as the outcome (Supplementary Table A4). These modeled the odds of being diagnosed as either MCI or AD compared to CN (MCI and AD were combined due to small cell sizes). The results from these models suggested that the significant interactions between network attributes and brain atrophy from previous models also held when using clinical diagnosis as an outcome rather than cognitive performance. That is, we found a stronger relationship between amygdalar atrophy and diagnosis of clinical cognitive pathology for those with less network diversity and lower effective size compared to those with higher values. Finally, we reestimated the original linear models from Table 2 but omitted participants who were clinically diagnosed with AD (Supplementary Table A5). The findings from these latter models were consistent with findings from the models that included participants with dementia.
Discussion
This study examined the moderating role of social networks in the association between low amygdalar volume—a potential indicator of neurodegeneration—and cognitive function in a sample of older adults at risk for AD. We found that individuals who span multiple social roles and subgroups within their personal networks demonstrated better global cognitive function (H1). More importantly, we found that these network attributes moderated the association between cognitive function and probably underlying neuropathology in the regions of the brain associated with emotional processing, social behavior, and decision making (H2).
These findings are broadly consistent with the cognitive reserve hypothesis of AD resilience. A viable explanation is that interacting with a diverse and expansive set of social ties engages the brain in ways that attenuate the impact of brain atrophy on cognitive decline (Ellwardt et al., 2015; Mische & White, 1998; Wlodarski & Dunbar, 2016). It is possible that older adults in our study with low cognitive reserve (i.e., minimal social network diversity and low effective size) experienced a steep trajectory of cognitive decline as AD neuropathology increased. In contrast, participants with high cognitive reserve may have exhibited greater resilience and a delayed trajectory of decline, resulting in a null relationship between amygdalar volume and cognitive function. If these interpretations are correct, we anticipate that older adults with diverse networks that allow them to engage in multiple nonredundant social groups—if followed longitudinally—would maintain relatively higher levels of cognitive function before eventually experiencing delayed but rapid decline. Unfortunately, this trajectory is currently unobservable in our cross-sectional analyses.
Social scientists argue that because people who are embedded in the same social groups likely share similar characteristics, interests, and activities, those who bridge two otherwise unconnected networks have a social advantage (Burt, 1992; Cornwell, 2009). We extend this theory to the case of cognitive aging and integrated with theories of cognitive reserve. Specifically, having access to novel and diverse social stimuli and processing complex social information may work to establish cognitive reserve over the life course, creating conditions for greater resilience to AD pathology. Meaningful contact with heterogeneous interaction partners—such as that which occurs in monthly supper clubs or community volunteering—likely provides access not only to novel ideas, information, and social activities, but also to diverse visual and auditory stimuli (Mische & White, 1998). Interactions within these types of diverse contexts produce eustress (i.e., good stress), requiring greater cognitive effort to interpret social cues and exercise other social cognitive skills (Meyer et al., 2012; Wlodarski & Dunbar, 2016). Social networks that force individuals to span multiple contexts are therefore more cognitively enriching than familiar, repetitive, and comfortable exchanges with immediate family members and other interconnected confidantes. This explanation is consistent with related research suggesting that the volume of specific brain regions (e.g., amygdala, hippocampus) are associated with social network size and density in humans (Blumen & Verghese, 2019; Noonan et al., 2018).
An alternative explanation is that individuals with more robust neural structure and function may be resistant to cognitive decline and therefore also able to maintain social relationships and activities with a more diverse social network (Cornwell, 2009). In this case, the observed relationships might be surrogate rather than causal. In all likelihood, associations between social networks and cognitive outcomes are beneficial and self-reinforcing, such that those with a higher level of initial social engagement are more resilient to cognitive decline and also more capable of maintaining social functioning despite underlying neuropathology.
Strengths and Limitations
The present study has a unique strength that contributes to the existing literature on social networks and cognitive function. By gathering contemporaneous data on participants’ cognitive function, QNPs, and social networks, we were able to properly test a key element of the cognitive reserve hypothesis by examining whether social networks moderate the relationship between brain atrophy and cognitive function.
At the same time, our study has several limitations. First, our data were cross-sectional. Until longitudinal observations become available, we are unable to examine relationships over time between reductions in brain volume and declines in cognitive function. We instead inferred trajectories by observing individuals at different levels of amygdalar volume and cognitive function. Therefore, any conclusions about causality are inappropriate. Second, we relied on data from a clinical sample. Although this limits our ability to generalize to the broader population, it was the only way to ensure both neuroimaging data and network data on a sufficient number of older adults. Third, although our focus on the amygdala and hippocampus stems from a large body of research implicating atrophy in these regions in MCI and early-stage AD (Poulin et al., 2011; Wachinger et al., 2016), recent studies have suggested that atrophy in other regions, such as the prefrontal cortex, may also be indicative of AD (Boublay et al., 2020; Yu et al., 2021). Moreover, there is research suggesting that the neurological linkages to social networks and social engagement are well distributed across different regions of the brain (Eisenberger & Cole, 2012; Kwak et al., 2018). Future research should examine the moderating role of social networks on the effects of other brain regions and neurological processes, as this might reveal important and underexplored etiological pathways (Yu et al., 2021).
Although it has many advantages, the social network approach used here has some potential drawbacks. First, self-reported social networks are subject to recall error. Such errors may theoretically be heightened among older adults and especially those with lower levels of cognitive function. However, a previous study using SNAD data showed that when compared against network perceptions from a study partner, focal participants with early-stage AD or MCI were no more likely to omit specific alters from their personal networks compared to CN participants (Roth et al., 2021). Second, we used a relatively brief social network inventory that largely captures relatively strong and contemporaneous ties. Moreover, we were unable to compare results using social network measures to other more traditional measures of social engagement, such as participation in leisure activities. Notwithstanding, measurement of multiple social network dimensions, and especially structural characteristics, is an important contribution to existing research in this area. Future research would further benefit from the use of a more expansive network inventory that elicits information about weaker, inactive, or sporadic network ties, and from direct comparisons between network measures and traditional indicators of social engagement or activity.
Conclusions
Our results are consistent with a cognitive reserve explanation for the well-established protective role of social networks and interactions in cognitive decline (Evans et al., 2019; Kelly et al., 2017). Findings suggest that access to novel social stimuli and cognitively diverse social networks may attenuate the effects of underlying neurodegeneration on global cognitive function. Additional research using longitudinal data is critical for establishing stronger evidence. More broadly, our research suggests that leveraging a social network approach provides unique insight into the specific mechanisms underlying the association between social interactions and cognitive outcomes. Increasing our understanding of social processes in the neurology of aging is likely to help identify novel targets for intervention to reduce the burden of AD and age-related cognitive decline on individuals, families, and the health care system.
Supplementary Material
Acknowledgments
We thank faculty and staff at the Indiana Alzheimer Disease Center, the Indiana Consortium for Mental Health Services Research, and the Indiana University Network Science Institute for their contributions to project conceptualization and data collection. These include Evan Finley, Hope Sheean, William McConnell, Max Coleman, Bernice Pescosolido, Erin Pullen, Kate Eddens, Alex Capshew, Tugce Duran, Mary Austrom, Sujuan Gao, and Frederick Unverzagt.
Contributor Information
Brea L Perry, Department of Sociology, Indiana University, Bloomington, Indiana, USA; Indiana University Network Science Institute, Indiana University, Bloomington, Indiana, USA.
Adam R Roth, Department of Sociology, Indiana University, Bloomington, Indiana, USA; Indiana University Network Science Institute, Indiana University, Bloomington, Indiana, USA.
Siyun Peng, Department of Sociology, Indiana University, Bloomington, Indiana, USA.
Shannon L Risacher, Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, Indiana, USA; Indiana Alzheimer Disease Research Center, Indiana University School of Medicine, Indianapolis, Indiana, USA.
Andrew J Saykin, Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, Indiana, USA; Indiana Alzheimer Disease Research Center, Indiana University School of Medicine, Indianapolis, Indiana, USA.
Liana G Apostolova, Indiana Alzheimer Disease Research Center, Indiana University School of Medicine, Indianapolis, Indiana, USA; Department of Neurology, Indiana University School of Medicine, Indianapolis, Indiana, USA.
Funding
This work was supported by the National Institute on Aging (5R01AG057739-02, 5P30AG010133-24) and by an Indiana University Collaborative Research Grant through the Vice President for Research. This project also received support from the Indiana Clinical and Translational Sciences Institute funded in part by award number UL1TR002529 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
None declared.
Author Contributions
B. L. Perry participated in funding, concept design, data analysis, interpretation, writing of the manuscript, and approval of the manuscript. A. R. Roth participated in concept design, data analysis, and writing of the manuscript. S. Peng participated in concept design and data analysis. S. L. Risacher participated in data analysis. A. J. Saykin and L. G. Apostolova participated in funding and interpretation.
References
- Alzheimer’s Association . (2019). 2019 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia, 15(3), 321–387. doi: 10.1016/j.jalz.2019.01.010 [DOI] [Google Scholar]
- Apostolova, L. G., Dutton, R. A., Dinov, I. D., Hayashi, K. M., Toga, A. W., Cummings, J. L., & Thompson, P. M. (2006). Conversion of mild cognitive impairment to Alzheimer’s disease predicted by hippocampal atrophy maps. Archives of Neurology, 63(5), 693–699. doi: 10.1001/archneur.63.5.693 [DOI] [PubMed] [Google Scholar]
- Barnes, L. L., Mendes de Leon, C. F., Wilson, R. S., Bienias, J. L., & Evans, D. A. (2004). Social resources and cognitive decline in a population of older African Americans and whites. Neurology, 63(12), 2322–2326. doi: 10.1212/01.wnl.0000147473.04043.b3 [DOI] [PubMed] [Google Scholar]
- Benarroch, E. E. (2015). The amygdala: Functional organization and involvement in neurologic disorders. Neurology, 84(3), 313–324. doi: 10.1212/WNL.0000000000001171 [DOI] [PubMed] [Google Scholar]
- Bennett, D. A., Schneider, J. A., Tang, Y., Arnold, S. E., & Wilson, R. S. (2006). The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: A longitudinal cohort study. The Lancet. Neurology, 5(5), 406–412. doi: 10.1016/S1474-4422(06)70417-3 [DOI] [PubMed] [Google Scholar]
- Bickart, K. C., Wright, C. I., Dautoff, R. J., Dickerson, B. C., & Barrett, L. F. (2011). Amygdala volume and social network size in humans. Nature Neuroscience, 14(2), 163–164. doi: 10.1038/nn.2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumen, H. M., & Verghese, J. (2019). Gray matter volume covariance networks associated with social networks in older adults. Social Neuroscience, 14(5), 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boublay, N., Bouet, R., Dorey, J. M., Padovan, C., Makaroff, Z., Fédérico, D., Gallice, I., Barrellon, M. O., Robert, P., Moreaud, O., Rouch, I., & Krolak-Salmon, P.; Alzheimer’s Disease Neuroimaging Initiative . (2020). Brain volume predicts behavioral and psychological symptoms in Alzheimer’s disease. Journal of Alzheimer’s Disease, 73(4), 1343–1353. doi: 10.3233/JAD-190612 [DOI] [PubMed] [Google Scholar]
- Burt, R. S. (1992). Structural holes: The social structure of competition. Harvard University Press. [Google Scholar]
- Chalfont, G., Milligan, C., & Simpson, J. (2020). A mixed methods systematic review of multimodal non-pharmacological interventions to improve cognition for people with dementia. Dementia (London, England), 19(4), 1086–1130. doi: 10.1177/1471301218795289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapko, D., McCormack, R., Black, C., Staff, R., & Murray, A. (2018). Life-course determinants of cognitive reserve (CR) in cognitive aging and dementia—A systematic literature review. Aging & Mental Health, 22(8), 915–926. doi: 10.1080/13607863.2017.1348471 [DOI] [PubMed] [Google Scholar]
- Cohen, S., Doyle, W. J., Skoner, D. P., Rabin, B. S., & Gwaltney, J. M. (1997). Social ties and susceptibility to the common cold. JAMA, 277(24), 1940–1944. doi: 10.1001/jama.1997.03540480040036 [DOI] [PubMed] [Google Scholar]
- Cornwell, B. (2009). Good health and the bridging of structural holes. Social Networks, 31(1), 92–103. doi: 10.1016/j.socnet.2008.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell, E. Y., & Waite, L. J. (2012). Social network resources and management of hypertension. Journal of Health and Social Behavior, 53(2), 215–231. doi: 10.1177/0022146512446832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton, K., Verghese, J., & Blumen, H. M. (2020). Gray matter volume covariance networks, social support, and cognition in older adults. The Journals of Gerontology: Series B, 75(6), 1219–1229. doi: 10.1177/1471301218795289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks, V. C., Lubben, J., Petitti, D. B., Little, D., & Chiu, V. (2008). Social network, cognitive function, and dementia incidence among elderly women. American Journal of Public Health, 98(7), 1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davatzikos, C., Fan, Y., Wu, X., Shen, D., & Resnick, S. M. (2008). Detection of prodromal Alzheimer’s disease via pattern classification of magnetic resonance imaging. Neurobiology of Aging, 29(4), 514–523. doi: 10.1016/j.neurobiolaging.2006.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan, N. J., Okereke, O. I., Vannini, P., Amariglio, R. E., Rentz, D. M., Marshall, G. A., Johnson, K. A., & Sperling, R. A. (2016). Association of higher cortical amyloid burden with loneliness in cognitively normal older adults. JAMA Psychiatry, 73(12), 1230–1237. doi: 10.1001/jamapsychiatry.2016.2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger, N. I., & Cole, S. W. (2012). Social neuroscience and health: Neurophysiological mechanisms linking social ties with physical health. Nature Neuroscience, 15(5), 669–674. doi: 10.1038/nn.3086 [DOI] [PubMed] [Google Scholar]
- Ellwardt, L., Van Tilburg, T. G., & Aartsen, M. J. (2015). The mix matters: Complex personal networks relate to higher cognitive functioning in old age. Social Science & Medicine (1982), 125, 107–115. doi: 10.1016/j.socscimed.2014.05.007 [DOI] [PubMed] [Google Scholar]
- Esiri, M. M., Matthews, F., Brayne, C., Ince, P. G., Matthews, F. E., Xuereb, J. H., Broome, J. C., McKenzie, J., Rossi, M., & McKeith, I. G. (2001). Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet, 357(9251), 169–175. doi: 10.1016/S0140-6736(00)03589-3 [DOI] [PubMed] [Google Scholar]
- Evans, I. E. M., Martyr, A., Collins, R., Brayne, C., & Clare, L. (2019). Social isolation and cognitive function in later life: A systematic review and meta-analysis. Journal of Alzheimer’s Disease, 70(s1), S119–S144. doi: 10.3233/JAD-180501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz, A. C., & Tye, K. M. (2014). Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. The Journal of Neuroscience, 34(2), 586–595. doi: 10.1523/JNEUROSCI.4257-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. (2012). FreeSurfer. Neuroimage, 62(2), 774–781. doi: 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratiglioni, L., Paillard-Borg, S., & Winblad, B. (2004). An active and socially integrated lifestyle in late life might protect against dementia. The Lancet Neurology, 3(6), 343–353. [DOI] [PubMed] [Google Scholar]
- Gow, A. J., Corley, J., Starr, J. M., & Deary, I. J. (2013). Which social network or support factors are associated with cognitive abilities in old age? Gerontology, 59(5), 454–463. [DOI] [PubMed] [Google Scholar]
- Huxhold, O., Fiori, K. L., Webster, N. J., & Antonucci, T. C. (2020). The strength of weaker ties: An underexplored resource for maintaining emotional well-being in later life. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 75(7), 1433–1442. doi: 10.1093/geronb/gbaa019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman, R., Aronson, M., Fuld, P., Kawas, C., Brown, T., Morgenstern, H., Frishman, W., Gidez, L., Eder, H., & Ooi, W. L. (1989). Development of dementing illnesses in an 80-year-old volunteer cohort. Annals of Neurology, 25(4), 317–324. doi: 10.1002/ana.410250402 [DOI] [PubMed] [Google Scholar]
- Kelly, M. E., Duff, H., Kelly, S., McHugh Power, J. E., Brennan, S., Lawlor, B. A., & Loughrey, D. G. (2017). The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: A systematic review. Systematic Reviews, 6(1), 259. doi: 10.1186/s13643-017-0632-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Koerkamp, Y., Heckemann, R. A., Ramdeen, K. T., Moreaud, O., Keignart, S., Krainik, A., Hammers, A., Baciu, M., & Hot, P.; Alzheimer’s Disease Neuroimaging Initiative . (2014). Amygdalar atrophy in early Alzheimer’s disease. Current Alzheimer Research, 11(3), 239–252. doi: 10.2174/1567205011666140131123653 [DOI] [PubMed] [Google Scholar]
- Kwak, S., Joo, W., Youm, Y., & Chey, J. (2018). Social brain volume is associated with in-degree social network size among older adults. Proceedings of the Royal Society B: Biological Sciences, 285(1871), 20172708. doi: 10.1098/rspb.2017.2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara, E., Martín-María, N., De la Torre-Luque, A., Koyanagi, A., Vancampfort, D., Izquierdo, A., & Miret, M. (2019). Does loneliness contribute to mild cognitive impairment and dementia? A systematic review and meta-analysis of longitudinal studies. Ageing Research Reviews, 52, 7–16. doi: 10.1016/j.arr.2019.03.002 [DOI] [PubMed] [Google Scholar]
- Meyer, M. L., Spunt, R. P., Berkman, E. T., Taylor, S. E., & Lieberman, M. D. (2012). Evidence for social working memory from a parametric functional MRI study. Proceedings of the National Academy of Sciences of the United States of America, 109(6), 1883–1888. doi: 10.1073/pnas.1121077109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mische, A., & White, H. (1998). Between conversation and situation: Public switching dynamics across network domains. Social Research, 65, 695–724. http://www.jstor.org/stable/40971267 [Google Scholar]
- Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., Cummings, J. L., & Chertkow, H. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Nelson, M. E., Jester, D. J., Petkus, A. J., & Andel, R. (2021). Cognitive reserve, Alzheimer’s neuropathology, and risk of dementia: A systematic review and meta-analysis. Neuropsychology Review, 31(2), 233–250. doi: 10.1007/s11065-021-09478-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan, M. P., Mars, R. B., Sallet, J., Dunbar, R. I. M., & Fellows, L. K. (2018). The structural and functional brain networks that support human social networks. Behavioural Brain Research, 355, 12–23. doi: 10.1016/j.bbr.2018.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninkilampi, R., Casey, A. N., Singh, M. F., & Brodaty, H. (2018). The association between social engagement, loneliness, and risk of dementia: A systematic review and meta-analysis. Journal of Alzheimer’s Disease, 66(4), 1619–1633. doi: 10.3233/JAD-180439 [DOI] [PubMed] [Google Scholar]
- Perry, B. L., McConnell, W. R., Coleman, M. E., Roth, A. R., Peng, S., & Apostolova, L. G. (2021). Why the cognitive “fountain of youth” may be upstream: Pathways to dementia risk and resilience through social connectedness. Alzheimer’s & Dementia, XX(X), 1–8. doi: 10.1002/alz.12443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, B. L., McConnell, W. R., Peng, S., Roth, A., Coleman, M., Manchella, M., Roessler, M., Francis, H., Sheean, H., & Apostolova, L. (2021). Social networks and cognitive function: An evaluation of social bridging and bonding mechanisms. The Gerontologist, gnab112. doi: 10.1093/geront/gnab112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, B. L., Pescosolido, B. A., & Borgatti, S. P. (2018). Egocentric network analysis: Foundations, methods, and models. Cambridge University Press. doi: 10.1017/9781316443255 [DOI] [Google Scholar]
- PhenX Toolkit . (1991). https://www.phenxtoolkit.org/protocols/view/211101
- Pillai, J. A., & Verghese, J. (2009). Social networks and their role in preventing dementia. Indian Journal of Psychiatry, 51(Suppl. 1), S22–S28. https://pubmed.ncbi.nlm.nih.gov/21416012/ [PMC free article] [PubMed] [Google Scholar]
- Poulin, S. P., Dautoff, R., Morris, J. C., Barrett, L. F., & Dickerson, B. C.; Alzheimer’s Disease Neuroimaging Initiative . (2011). Amygdala atrophy is prominent in early Alzheimer’s disease and relates to symptom severity. Psychiatry Research, 194(1), 7–13. doi: 10.1016/j.pscychresns.2011.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risacher, S. L., & Saykin, A. J. (2013). Neuroimaging and other biomarkers for Alzheimer’s disease: The changing landscape of early detection. Annual Review of Clinical Psychology, 9, 621–648. doi: 10.1146/annurev-clinpsy-050212-185535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risacher, S. L., Saykin, A. J., West, J. D., Shen, L., Firpi, H. A., & McDonald, B. C.; Alzheimer’s Disease Neuroimaging Initiative (ADNI) . (2009). Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Current Alzheimer Research, 6(4), 347–361. doi: 10.2174/156720509788929273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth, A. R. (2020). Social networks and health in later life: A state of the literature. Sociology of Health & Illness, 42, 1642–1656. doi: 10.1111/1467-9566.13155 [DOI] [PubMed] [Google Scholar]
- Roth, A. R., Peng, S., Coleman, M. E., Finley, E., & Perry, B. (2021). Network recall among older adults with cognitive impairments. Social Networks, 64, 99–108. doi: 10.1016/j.socnet.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh, J. I., & Yesavage, J. A. (1986). Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontologist, 5, 165–173. doi: 10.1300/J018v05n01_09 [DOI] [Google Scholar]
- Smith, K. P., & Christakis, N. A. (2008). Social networks and health. Annual Review of Sociology, 34, 405–429. doi: 10.1146/annurev.soc.34.040507.134601 [DOI] [Google Scholar]
- StataCorp. (2019). Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC. [Google Scholar]
- Stern, Y. (2012). Cognitive reserve in ageing and Alzheimer’s disease. The Lancet. Neurology, 11(11), 1006–1012. doi: 10.1016/S1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, Y., Arenaza-Urquijo, E. M., Bartrés-Faz, D., Belleville, S., Cantilon, M., Chetelat, G., Ewers, M., Franzmeier, N., Kempermann, G., Kremen, W. S., Okonkwo, O., Scarmeas, N., Soldan, A., Udeh-Momoh, C., Valenzuela, M., Vemuri, P., & Vuoksimaa, E.; The Reserve, Resilience and Protective Factors PIA Empirical Definitions and Conceptual Frameworks Workgroup . (2020). Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s & Dementia, 16(9), 1305–1311. doi: 10.1016/j.jalz.2018.07.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachinger, C., Salat, D. H., Weiner, M., & Reuter, M.; Alzheimer’s Disease Neuroimaging Initiative . (2016). Whole-brain analysis reveals increased neuroanatomical asymmetries in dementia for hippocampus and amygdala. Brain, 139(Pt 12), 3253–3266. doi: 10.1093/brain/aww243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarski, R., & Dunbar, R. I. (2016). When BOLD is thicker than water: Processing social information about kin and friends at different levels of the social network. Social Cognitive and Affective Neuroscience, 11(12), 1952–1960. doi: 10.1093/scan/nsw101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, M., Sporns, O., & Saykin, A. J. (2021). The human connectome in Alzheimer disease—Relationship to biomarkers and genetics. Nature Reviews Neurology, 17, 1–19. doi: 10.1038/s41582-021-00529-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.