Abstract
Background:
The role of antioxidant intervention in polycystic ovary syndrome (PCOS) patients has been increasingly investigated in recent years. In order to further clarify whether antioxidant therapy is beneficial for PCOS patients and the emphasis of its effects, this study provides a systematic review and meta-analysis of randomized controlled trials examining the effect of antioxidant intervention on PCOS.
Methods:
Enrolled study designs related to antioxidant interventions and PCOS, published from 1999 to 2020, were searched from EMBASE, PubMed, and Web of Science databases to sort out proven studies on antioxidant interventions and PCOS. Data were reported as weighted mean difference (WMD) or standard mean difference with associated confidence intervals of 95%. The analysis was conducted using Stata version 16.0.
Results:
Twenty-three studies were included in total. Antioxidant intervention had a positive impact on homeostasis model assessment of insulin resistance (WMD = –0.37, P = .011) and Triglycerides (WMD = –25.51, P < .001). And antioxidant intervention did not improve testosterone levels significantly (WMD = –0.20, P = .2611). Subgroup analysis showed that except for the D-chiro-inosito subgroup, no difference in body mass index was observed between the intervention group and the control group.
Conclusions:
This meta-analysis demonstrates the efficacy of antioxidant intervention in patients with PCOS, demonstrating that antioxidant intervention has a significant effect on insulin resistance and lipid metabolism improvement. However, antioxidant intervention therapy has no discernible impact on testosterone levels or body mass index. Omega-3 may be a more effective antioxidant intervention for PCOS. In addition, this meta-analysis provides important reference opinions and treatment recommendations for PCOS.
Keywords: antioxidant intervention, meta-analysis, polycystic ovary syndrome
1. Introduction
Polycystic ovary syndrome (PCOS) is a prevalent gynecological endocrine diseases that affects 5% to 20% of women of reproductive age globally.[1–3] In addition to infertility and abnormal menstruation,[4–6] patients also face economic burdens and long-term health risks.[7,8] Numerous studies have demonstrated that PCOS patients tend to have insulin resistance.[1,3,9–11]
In addition, PCOS patients exhibit oxidative stress.[12–14] In patients with PCOS, oxidative stress is closely associated with metabolic disorders, ovulation disorders, and difficulties in embryo transfer.[2,4,15,16] This may be the reason why PCOS patients have an abnormal metabolic state and reduced fertility. Consequently, numerous studies are devoted to the treatment of polycystic ovaries by improving oxidative stress and have achieved a degree of curative effect.[17,18] Antioxidants are a group of substances that help to capture and neutralize free radicals, thereby eliminating their damaging effects on the body. It may be helpful to treat PCOS with antioxidants or drugs that promote the antioxidant process in the body. Normally, the damaging effects of reactive oxygen species can be offset by a sophisticated antioxidant system, which involves enzymatic antioxidants, such as superoxide catalase, dismutase, paraoxonase, and peroxidase, as well as nonenzymatic antioxidant substances, such as thiols, glutathione, vitamin E, vitamin C, selenium, vitamin A, thioredoxin, and zinc.[19]
Several findings suggest that antioxidant intervention may improve insulin resistance and lipid metabolism in polycystic ovary syndrome. Nonetheless, there is no exhaustive and objective assessment of the effects of various antioxidant treatments on PCOS patients. To further investigate the viability of antioxidant interventions in the treatment of PCOS, we selected for meta-analysis all relevant studies involving 7 types of clinically common antioxidants.
2. Materials and Methods
2.1. Search strategy
A comprehensive literature search was conducted using PubMed, Web of Science, and EMBASE from their inception to March 2021 to identify all potentially relevant articles. All search methods utilized a systematic strategy in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols.
The following terms were searched for: ((Coenzyme Q10) or (Inositol) or (Vitamin E) or (Selenium) or (Omega-3) or (Melatonin) or (zine)) and ((Polycystic Ovary Syndrome) or (Polycystic Ovarian) or (Syndrome, Polycystic Ovary) or (Ovary Syndrome, Polycystic)).
2.2. Selection criteria
Two reviewers independently performed a literature search, assessed potentially eligible studies for inclusion, and extracted data. Disagreements were resolved by negotiation with a third reviewer when necessary. If necessary, we contacted the authors of the original studies for extra info.
The primary criteria for inclusion are as follows:
Randomized controlled clinical trials. In the intervention group, patients received antioxidant treatment. In the control group, they got placebos or placebos plus the same drugs.
Study population: patients with PCOS. Two of the 3 diagnostic criteria for PCOS (oligoovulation and/or anovulation, clinical and/or biochemical signs of hyperandrogenemia, polycystic ovary) were recommended as the diagnostic criteria for PCOS.[20]
Aged 18 to 40 years.
Studies that report weighted mean difference (WMD) or standard mean difference (SMD) with corresponding 95% confidence intervals (CIs) or provide alternative methods to calculate or obtain these values.
2.3. Data extraction
Two researchers independently extracted data from eligible studies using this form and discussed discrepancies. Author, year of publication, age of patient, sample size, treatment method, WMD (95% CI) or SMD (95% CI), and variables controlled for matching or used in multivariable models were among the data collected. We entered the information into the software Review Manager (RevMan 5.3). According to the Cochrane scale’s quality standard, the Cochrane score was utilized to assess the quality of these selected studies. Two reviewers reconciled their differences through conversation. Disagreements were resolved, if necessary, through consultation with a third reviewer.
2.4. Data analysis
Six variables were extracted from each study as mean ± standard deviation. Our study utilized Stata version 16.0 to analyze data. P values are 2-sided, and a P value of < .05 was regarded as the statistical significance threshold. In addition, the heterogeneity of these 5 studies was evaluated. In this meta-analysis, we assessed the heterogeneity between the included studies using the I2 statistic, with I2 ≥ 50% indicating significant heterogeneity.[21] For studies with I² ≥ 50%, we calculated using the random-effects model and for studies with I² < 50%, we used the fixed-effects model. With the 95% CI, the WMD or SMD for continuous variables was used to explain outcomes. We have also conducted in-depth research on subgroup (or regression) analysis and sensitivity analysis for some data with significant heterogeneity. In addition, the bias was described using Egger test method.
3. Results
3.1. Literature search and study characteristics
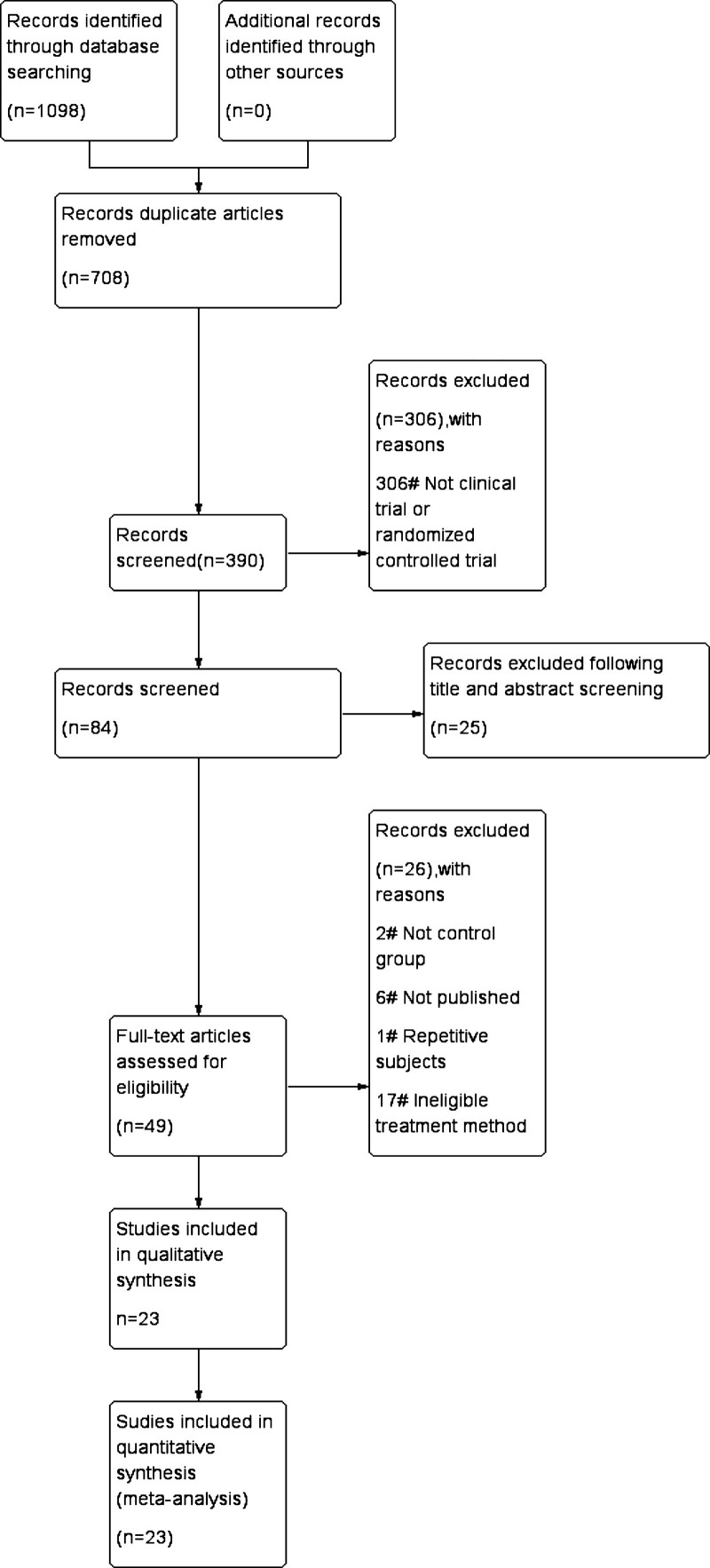
Figure 1 is a summary of the research selection process. The literature search yielded 1098 distinct references, of which 708 were considered duplicates. Three hundred six of these articles were omitted because they were of the incorrect inappropriate article type. Following title and abstract screening, 35 of these articles were excluded. The remaining 49, 26 were excluded due to sample characteristics (such as the absence of a control group) or a lack of pertinent data (e.g., not published). In total, 23 studies met the criteria for data extraction and inclusion in this meta-analysis. Table 1 provides a summary of their defining characteristics.
Figure 1.
Flow diagram of study selection.
Table 1.
The characteristics of included studies in this meta-analysis.
| Author | year | Study type | Intervention | Sample | Duration of intervention |
|---|---|---|---|---|---|
| Izadi, et al[22] | 2019 | RCT | 200 mg CoQ10 daily + vitamin E placebo\400 IU vitamin E daily + CoQ10 placebo\200 mg CoQ10 + 400 IU vitamin E daily\CoQ10 placebo + vitamin E placebo | 22\22\21\21 | 8 wk |
| Jamilian et al[23] | 2018 | RCT | 1000 mg omega-3 fatty acids plus 400 IU vitamin E supplements\placebo | 20\20 | 12 wk |
| Mirmasoumi et al[24] | 2018 | RCT | 1000 mg flaxseed oil omega-3 fatty acids\placebo | 30\30 | 12 wk |
| Ebrahimi et al[25] | 2017 | RCT | 1000 mg omega-3 fatty acids from flaxseed oil containing 400 mg α-linolenic acid plus 400 IU vitamin E supplements\placebo | 34\34 | 12 wk |
| Rahmani et al[26] | 2017 | RCT | 1000 mg omega-3 fatty acids from flaxseed oil containing 400 mg α-linolenic acid plus 400 IU vitamin E supplements\placebo | 34\34 | 12 wk |
| Rahmani et al[27] | 2018 | RCT | 100 mg CoQ10\placebo | 20\20 | 12 wk |
| Samimi et al[28] | 2017 | RCT | 100 mg CoQ10 supplements\placebo | 30\30 | 12 wk |
| Shabani et al[29] | 2019 | RCT | 10 mg melatonin\placebo | 29\29 | 12 wk |
| Jamilian et al[30] | 2018 | RCT | 8 × 109 CFU/d probiotic plus 200μg/d selenium supplements\placebo | 30\30 | 12 wk |
| Mohammad Hosseinzadeh et al[31] | 2016 | RCT | Daily administration of 200 g selenium\placebo | 26\27 | 12 wk |
| Jamilian et al[32] | 2015 | RCT | 200 µg/d selenium supplements\placebo | 35\35 | 8 wk |
| Zadeh Modarres et al[33] | 2018 | RCT | 200-μg selenium\placebo per day | 20\20 | 8 wk |
| Heidar et al[34] | 2020 | RCT | 200 μg/d of selenium\palcebo | 18\18 | 8 wk |
| Rashidi et al[35] | 2020 | RCT | 200 μg/d selenium\placebo | 34\32 | 12 wk |
| Fruzzetti et al[36] | 2017 | RCT | Myo-inositol 4 g plus folic acid 400 mcg/d\placebo | 24\30 | 6 mo |
| Nestler et al[37] | 1999 | RCT | The oral administration of 1200 mg of D-chiro-inositol\placebo once daily | 22\22 | 6 wk |
| Iuorno et al[38] | 2002 | RCT | Initiated with either 600 mg of D-chiro-inositol\placebo once daily | 10\10 | 6–8 wk |
| Jamilian et al[39] | 2018 | RCT | 50,000 IU vitamin D every 2 wk plus 2000 mg/day omega-3 fatty acid from fish oil\placebo | 30\30 | 12 wk |
| Shokrpour et al[40] | 2019 | RCT | 250 mg/d magnesium plus 400 mg/d vitamin E supplements\placebo | 30\30 | 12 wk |
| Jamilian et al[41] | 2019 | RCT | Participants were randomly divided into 2 groups to receive 250 mg/d magnesium plus 400 mg/d vitamin E supplements\placebo | 30\30 | 12 wk |
| Hager et al[42] | 2019 | RCT | Assigned to either the “multinutrient supplementation group” (1 unlabeled soft capsule containing omega-3 fatty acids and 1 unlabeled tablet containing folic acid, selenium, vitamin E, catechin, glycyrrhizin, and Co Q10) or the “control group” (2 unlabeled soft capsules containing 200 μg folic acid each) | 30\30 | 3 mo |
| Maktabi et al[43] | 2018 | RCT | Treated with 100 mg magnesium, 4 mg zinc, 400 mg calcium plus 200 IU vitamin D supplements (n = 30)\placebo (n = 30) twice a day | 30\30 | 12 wk |
| Foroozanfard et al[44] | 2015 | RCT | 220 mg zinc sulfate (containing 50 mg zinc) supplements\placebo per day | 26\26 | 8 wk |
3.2. Risk of bias in all studies
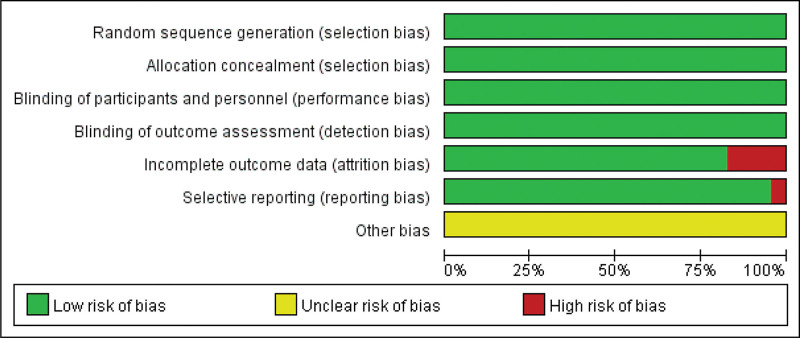
The risk of bias was selected for each randomized and prospective nonrandomized clinical study which was selected, the risk of bias was assessed according to the criteria described in the Cochrane Reviewers Handbook.[27] The summary of the risk of bias is shown in Figure 2. All included studies were at low risk of bias level in terms of “selection bias”, “performance bias” and “detection bias.” Few of the included studies were at high risk of bias level in terms of “attrition bias” and “reporting bias.” The included studies were at unclear risk of bias level in terms of “Other bias.”
Figure 2.
Summary of risk for each included study. Green means low risk of bias; yellow means unclear risk of bias; red means high risk of bias.
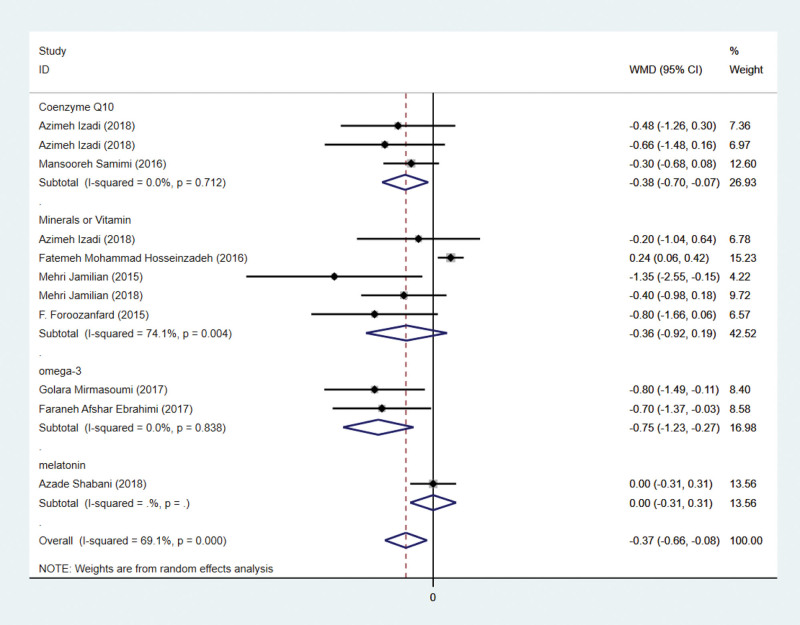
3.3. Effects on HOMA-IR
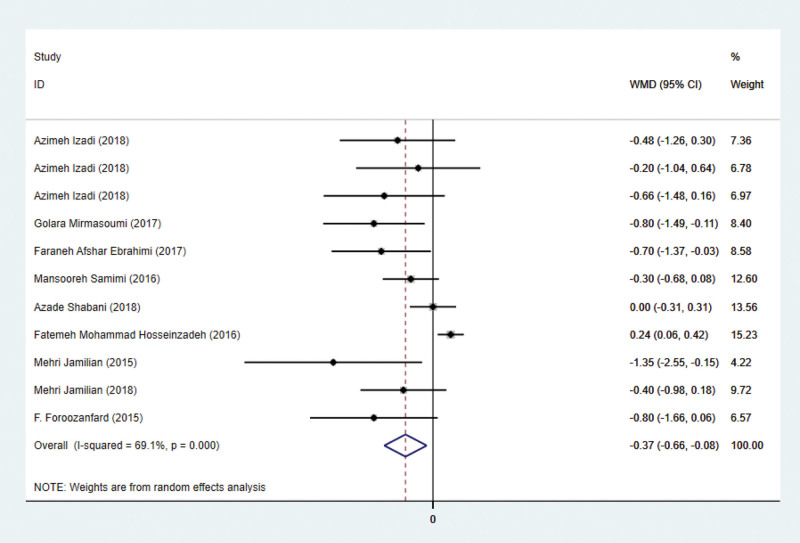
Nine studies have addressed HOMA-IR indicators. HOMA-IR has been reported in eleven studies involving 609 subjects. There are 305 patients in the intervention group and 304 in the control group. Using a random-effects model, the WMD was 0.37 (95% CI –0.66 to –0.08, P = .011) lower in the intervention group than in the control group. Significant heterogeneity was observed (P = .000, I² = 69.1%; Fig. 3). This suggests that antioxidant treatment can reduce HOMA-IR significantly.
Figure 3.
Forest plots of antioxidant intervention on HOMA-IR in patients with PCOS. HOMA-IR = Homeostasis Model Assessment of Insulin Resistance, PCOS = polycystic ovary syndrome.
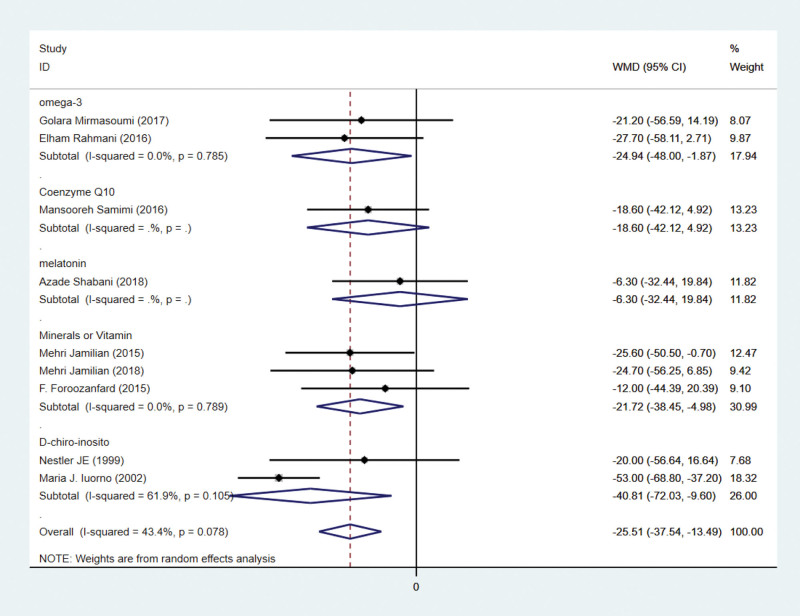
3.4. Effects on triglycerides
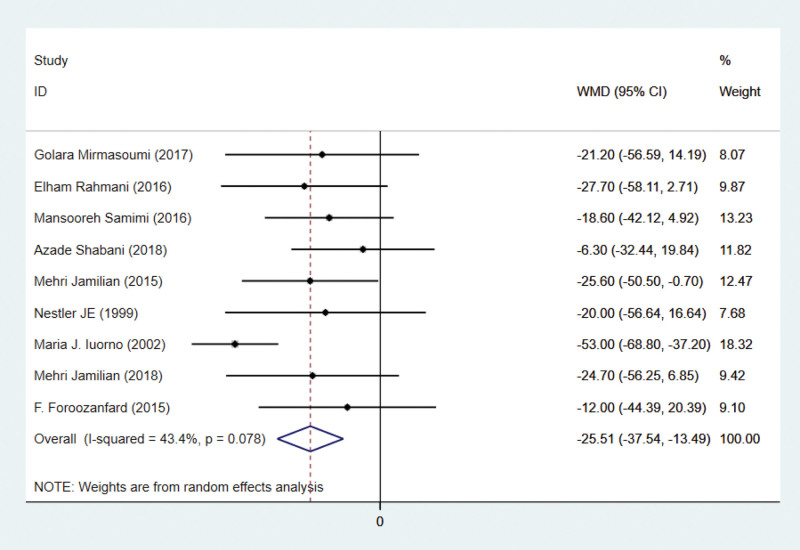
There are 9 studies on triglyceride indicators. Triglycerides were reported in 9 studies involving 492 subjects. There are 246 patients in the intervention group and 246 patients in the control group. Using a random-effects model, the WMD was 25.51 mg/dL (95% CI –37.54 to –13.49, P < .001) lower in the intervention group than in the control group. Heterogeneity was deemed insignificant (P = .078, I² = 43.4%; Fig. 4). This indicates that antioxidant therapy can significantly lower triglycerides.
Figure 4.
Forest plots of antioxidant intervention on triglycerides in patients with PCOS. CI = confidence interval, PCOS = polycystic ovary syndrome.
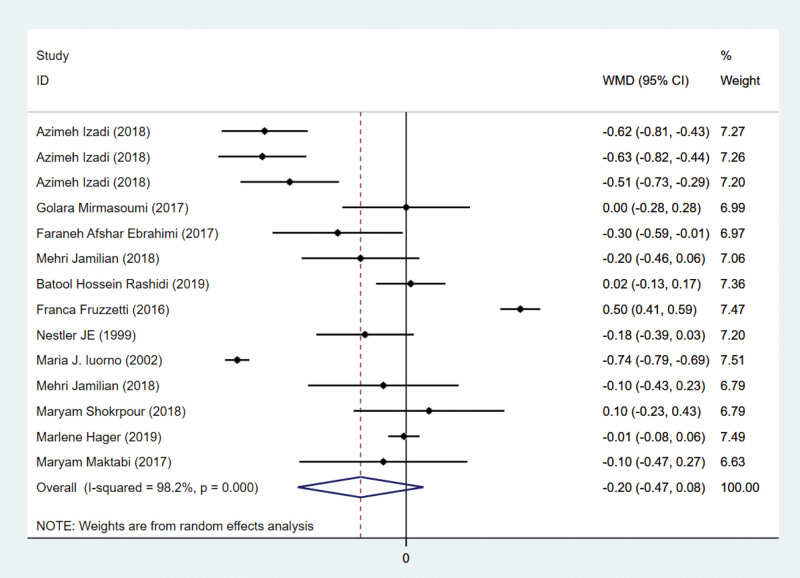
3.5. Effects on testosterone
Twelve studies have examined testosterone indicators. In 9 studies with 740 subjects, testosterone was reported. Three hundred sixty-nine patients are assigned to the intervention group and 371 to the control group. The current meta-analysis revealed that there was no difference in testosterone levels between intervention and control groups (WMD = –0.20 ng/mL, 95% CI –0.47 to 0.08, P = .2611). Figure 5 demonstrates that heterogeneity was considered significant (P < .001, I² = 98.2%), so a random-effects model was employed.
Figure 5.
Forest plots of antioxidant intervention on testosterone in patients with PCOS. CI = confidence interval, PCOS = polycystic ovary syndrome.
3.6. Effects on BMI
Nineteen studies have addressed BMI indicators. BMI was reported in 9 studies involving 1086 subjects. Five hundred forty-one patients are assigned to the intervention group and 545 to the control group. The results of the meta-analysis showed that the BMI index increased in the antioxidant intervention group compared with the control group. However, this current meta-analysis showed no difference in BMI was witnessed between the intervention group and control group except the D-chiro-inosito subgroup. Heterogeneity was considered nonsignificant (Fig. 6).
Figure 6.
Forest plots of antioxidant intervention on BMI in patients with PCOS. BMI = body mass index, CI = confidence interval, PCOS = polycystic ovary syndrome.
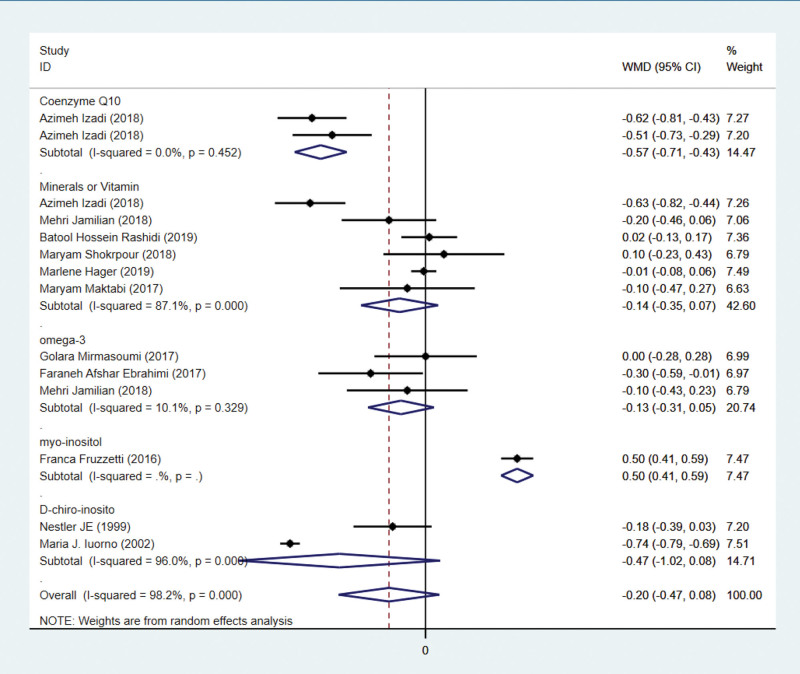
3.7. Subgroup analysis of antioxidant intervention on HOMA-IR
By subgroup analysis, it was seen that although the antioxidant effect had an overall effect on HOMA-IR in PCOS patients, minerals or vitamin and melatonin did not have a significant effect on reducing HOMA-IR in patients with polycystic ovary syndrome. In contrast, both coenzyme Q10 and omega-3 had a significant effect on the reduction of HOMA-IR.
3.8. Subgroup analysis of antioxidant intervention on triglycerides
By subgroup analysis, it was seen that although the antioxidant effect had an overall effect on triglycerides in PCOS patients, coenzyme Q10 and melatonin did not have a significant effect on reducing triglycerides in patients with polycystic ovary syndrome. In contrast, both minerals or vitamin, D-chiro-inosito and omega-3 had a significant effect on the reduction of triglycerides.
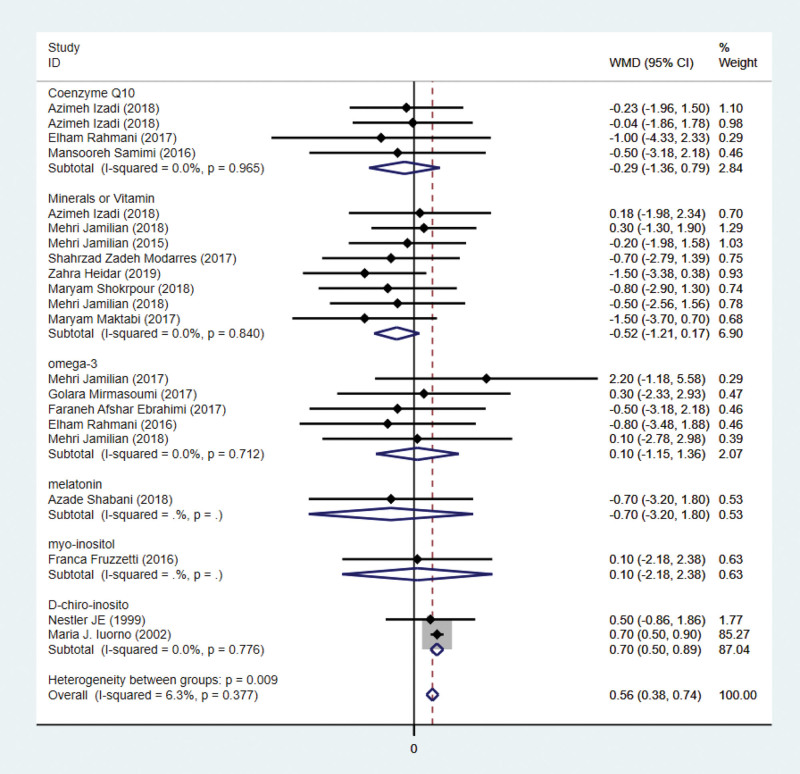
3.9. Subgroup analysis of antioxidant intervention on testosterone
By subgroup analysis, it was seen that although the antioxidant effect had no effect on testosterone in PCOS patients. Minerals or vitamin, D-chiro-inosito and omega-3 did not have a significant effect on reducing testosterone in patients with polycystic ovary syndrome. In contrast, both coenzyme Q10 had a significant effect on the reduction of testosterone. Myo-inositol had a significant effect on the elevation of testosterone.
4. Discussion
In conclusion, the antioxidant intervention significantly improved insulin resistance and abnormal lipid metabolism in PCOS patients, but had no significant effect on testosterone, although the effect of the BMI analysis needs to be further investigated due to bias.
We have therefore conducted an appropriate subgroup analysis. The results of the subgroup analysis will have a profound effect on our ability to further differentiate the effects of different antioxidants on PCOS.
In light of the insulin resistance of PCOS patients, we chose to analyze the HOMA-IR index, which is essential for measuring insulin resistance. This meta-analysis revealed that the intervention group had significantly lower HOMA-IR levels than the control group. This suggests that antioxidant therapy can significantly improve insulin resistance in PCOS patients. Certain evidence suggests that insulin resistance develops as a result of coordinated interactions between stress responses and various cellular stresses.[45] Insulin resistance is associated with a specific increase in mitochondrial hydroperoxides, as determined by analyzing the oligomeric status of comparator-specific peroxiredoxins.[46] Subgroup analysis revealed additional distinctions regulator within the group (Fig. 7). The coenzyme Q10 subgroup and the omega subgroup had significantly lower HOMA-IR levels than the placebo group. The P value of the HOMA-IR was not significantly different between the melatonin group and the nutrient element group. Previous research has demonstrated that coenzyme Q10 is a regulator of insulin and adiponectin receptors, phosphatidylinositol kinase 3, tyrosine kinase, and glucose transporters, suggesting that the antioxidant can enhance insulin sensitivity.[47] Omega-3 fatty acids may improve insulin sensitivity by inhibiting proinflammatory mediators and decreasing nuclear factor-kappa B activation, thereby reversing insulin resistance.[48]
Figure 7.
Subgroup analysis of antioxidant intervention on HOMA-IR in patients with PCOS. CI = confidence interval, HOMA-IR = Homeostasis Model Assessment of Insulin Resistance, PCOS = polycystic ovary syndrome.
In terms of lipid metabolism, this study revealed that the intervention group had significantly lower triglyceride levels than the control group. It indicates that the antioxidant treatment improves the lipid metabolism of PCOS patients significantly. Numerous previous studies have demonstrated this.[24,26] Through regulating endoplasmic reticulum stress-related genes and cellular defense regulation reactive oxygen species, antioxidant therapy can decrease oxidative stress.[49] However, we have subcategorized the various interventions. Subgroup analysis revealed that there was no significant difference in triglyceride levels between the melatonin and coenzyme Q10 subgroups (Fig. 8). It is noteworthy that the antioxidant effect on lipogenesis was comparable regardless of the antioxidant type. We cannot make accurate judgments because the number of research articles on the melatonin and coenzyme Q10 subgroups is insufficient.
Figure 8.
Subgroup analysis of antioxidant intervention on triglycerides in patients with PCOS. CI = confidence interval, PCOS = polycystic ovary syndrome.
Figure 5 indicates that antioxidant intervention had no significant effect on testosterone improvement. Due to the significant heterogeneity, a subgroup analysis was conducted. According to Figure 9, coenzyme Q10 subgroup treatment significantly increases testosterone levels. The number of articles on indicators is relatively low, so we adopt a conservative stance. The current meta-analysis revealed no difference in BMI between the intervention and control groups, with the exception of the D-chiro-inosito subgroup. We speculate that it may be related to the duration of medication and the patients’ lifestyles. Nevertheless, it cannot be ruled out that this might be related to the short duration of the medication and the insufficient intervention in patients’ lifestyle.
Figure 9.
Subgroup analysis of antioxidant intervention on testosterone in patients with PCOS. CI = confidence interval, PCOS = polycystic ovary syndrome.
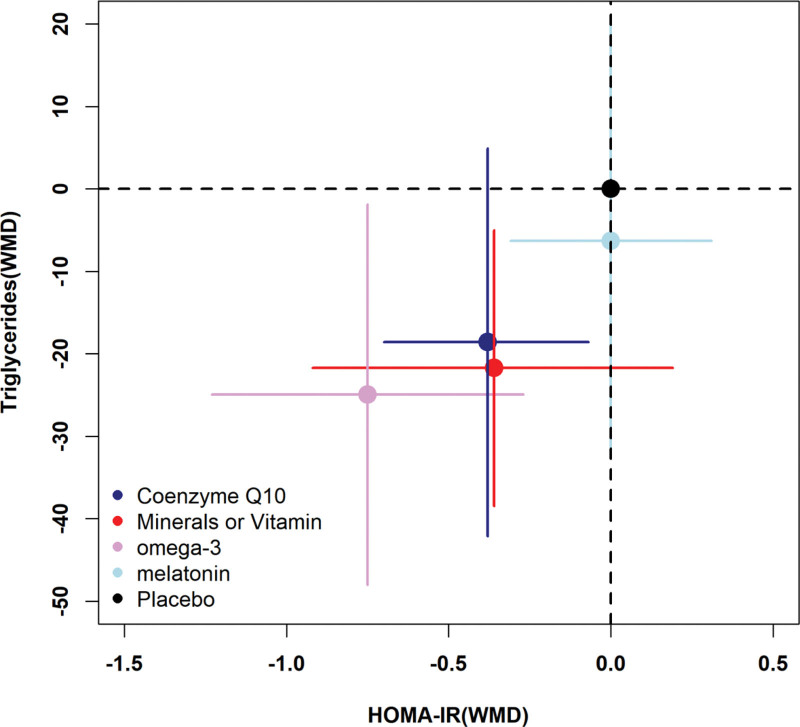
In conclusion, the HOMA-IR and triglyceride indicators of PCOS patients can be significantly improved by antioxidant treatment. Consequently, the results of each subgroup are depicted using a 2-dimensional plot. According to Figure 10, omega-3 has a greater effect on the improvement of HOMA-IR and lipid metabolism overall. Numerous studies have demonstrated that omega-3 significantly reduces serum triglyceride levels in PCOS patients.[50,51] It seems that an increase in adiponectin levels after supplementation with omega-3 fatty acids, which have antiatherosclerotic, anti-inflammatory, and antidiabetic effects, might improve insulin sensitivity.[52] In muscles, adiponectin stimulates AMPK, which leads to downstream oxidative pathways.[53] By activating the AMPK pathway, insulin control and blood lipid profiles are improved.[54]
Figure 10.
Two-dimensional plot on HOMA-IR and triglycerides. HOMA-IR = Homeostasis Model Assessment of Insulin Resistance.
When examining the results of this meta-analysis, certain limitations should be considered. The low quality obtained for HOMA-IR, triglycerides, and BMI was due to publication bias, as indicated by their Egger test P values of .000, .016, and .000.
Polycystic ovaries treatment requires a precise determination of the therapeutic effect of antioxidant intervention in PCOS patients despite our limitations. Different antioxidants have different effects on PCOS patients. As an antioxidant, omega-3 can reduce HOMA-IR, testosterone, and TG, and its potential therapeutic value for PCOS patients warrants further investigation. We propose that additional randomize controlled clinical trials of different antioxidant doses, medication duration, or drug combinations are required to demonstrate the therapeutic efficacy of antioxidant intervention in PCOS patients.
5. Conclusion
This meta-analysis demonstrates the efficacy of antioxidant intervention in patients with PCOS, demonstrating that antioxidant intervention has a significant effect on insulin resistance and lipid metabolism improvement. Omega-3 may be a more effective antioxidant intervention for PCOS.
Author contributions
Junde Zhao: Data collection or management, Data analysis, Manuscript writing
Xiaohui Sui: Data collection or management, Data analysis
Qingyu shi: Data analysis, Manuscript writing
Dan Su: Data analysis, Manuscript writing
Zhiheng Lin: project development
Abbreviations:
- BMI =
- body mass index
- CI =
- confidence interval
- PCOS =
- polycystic ovary syndrome
- RCT =
- randomized controlled trial
- SMD =
- standard mean difference
- WMD =
- weighted mean difference.
JZ and XS contributed to the work equally.
How to cite this article: Zhao J, Sui X, Shi Q, Su D, Lin Z, Effects of antioxidant intervention in patients with polycystic ovarian syndrome: A systematic review and meta-analysis. Medicine 2022;101:32(e30006).
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
This article does not contain any studies with human participants or animals performed by any of the authors.
All the data in this paper support the results of this study.
Informed consent was obtained from all individual participants included in the study. All authors read and approved the final manuscript.
CoQ10 = coenzyme Q10, RCT = randomized controlled trial, CFU = colony-forming unit.
Contributor Information
Junde Zhao, Email: zhaojunde2020@163.com.
Xiaohui Sui, Email: suixiaohui1998@163.com.
Qingyu Shi, Email: qingyu_shi@126.com.
Dan Su, Email: 411147665@qq.com.
References
- [1].Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057. [DOI] [PubMed] [Google Scholar]
- [2].Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14:270–84. [DOI] [PubMed] [Google Scholar]
- [3].Polak K, Czyzyk A, Simoncini T, et al. New markers of insulin resistance in polycystic ovary syndrome. J Endocrinol Invest. 2017;40:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fauser BC. Reproductive endocrinology: revisiting ovulation induction in PCOS. Nat Rev Endocrinol. 2014;10:704–5. [DOI] [PubMed] [Google Scholar]
- [5].Balen AH, Morley LC, Misso M, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. 2016;22:687–708. [DOI] [PubMed] [Google Scholar]
- [6].Skiba MA, Islam RM, Bell RJ, et al. Understanding variation in prevalence estimates of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2018;24:694–709. [DOI] [PubMed] [Google Scholar]
- [7].Azziz R, Marin C, Hoq L, et al. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. 2005;90:4650–8. [DOI] [PubMed] [Google Scholar]
- [8].Brutocao C, Zaiem F, Alsawas M, et al. Psychiatric disorders in women with polycystic ovary syndrome: a systematic review and meta-analysis. Endocrine. 2018;62:318–25. [DOI] [PubMed] [Google Scholar]
- [9].Harborne L, Fleming R, Lyall H, et al. Descriptive review of the evidence for the use of metformin in polycystic ovary syndrome. Lancet. 2003;361:1894–901. [DOI] [PubMed] [Google Scholar]
- [10].Jayasena CN, Franks S. The management of patients with polycystic ovary syndrome. Nat Rev Endocrinol. 2014;10:624–36. [DOI] [PubMed] [Google Scholar]
- [11].Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Murri M, Luque-Ramírez M, Insenser M, et al. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Hum Reprod Update. 2013;19:268–88. [DOI] [PubMed] [Google Scholar]
- [13].Barrea L, Marzullo P, Muscogiuri G, et al. Source and amount of carbohydrate in the diet and inflammation in women with polycystic ovary syndrome. Nutr Res Rev. 2018;31:291–301. [DOI] [PubMed] [Google Scholar]
- [14].Lu J, Wang Z, Cao J, et al. A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2018;16:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang J, Bao Y, Zhou X, et al. Polycystic ovary syndrome and mitochondrial dysfunction. Reprod Biol Endocrinol. 2019;17:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shukla P, Mukherjee S. Mitochondrial dysfunction: an emerging link in the pathophysiology of polycystic ovary syndrome. Mitochondrion. 2020;52:24–39. [DOI] [PubMed] [Google Scholar]
- [17].Razavi M, Jamilian M, Kashan ZF, et al. Selenium supplementation and the effects on reproductive outcomes, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome. Horm Metab Res. 2016;48:185–90. [DOI] [PubMed] [Google Scholar]
- [18].Afshar Ebrahimi F, Foroozanfard F, Aghadavod E, et al. The effects of magnesium and zinc co-supplementation on biomarkers of inflammation and oxidative stress, and gene expression related to inflammation in polycystic ovary syndrome: a randomized controlled clinical trial. Biol Trace Elem Res. 2018;184:300–7. [DOI] [PubMed] [Google Scholar]
- [19].Ding Y, Jiang Z, Xia B, et al. Mitochondria-targeted antioxidant therapy for an animal model of PCOS-IR. Int J Mol Med. 2019;43:316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41–7. [DOI] [PubMed] [Google Scholar]
- [21].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Izadi A, Ebrahimi S, Shirazi S, et al. Hormonal and metabolic effects of coenzyme Q10 and/or vitamin E in patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104:319–27. [DOI] [PubMed] [Google Scholar]
- [23].Jamilian M, Shojaei A, Samimi M, et al. The effects of omega-3 and vitamin E co-supplementation on parameters of mental health and gene expression related to insulin and inflammation in subjects with polycystic ovary syndrome. J Affect Disord. 2018;229:41–7. [DOI] [PubMed] [Google Scholar]
- [24].Mirmasoumi G, Fazilati M, Foroozanfard F, et al. The effects of flaxseed oil omega-3 fatty acids supplementation on metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Exp Clin Endocrinol Diabetes. 2018;126:222–8. [DOI] [PubMed] [Google Scholar]
- [25].Ebrahimi FA, Samimi M, Foroozanfard F, et al. The effects of omega-3 fatty acids and vitamin E co-supplementation on indices of insulin resistance and hormonal parameters in patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Exp Clin Endocrinol Diabetes. 2017;125:353–9. [DOI] [PubMed] [Google Scholar]
- [26].Rahmani E, Samimi M, Ebrahimi FA, et al. The effects of omega-3 fatty acids and vitamin E co-supplementation on gene expression of lipoprotein(a) and oxidized low-density lipoprotein, lipid profiles and biomarkers of oxidative stress in patients with polycystic ovary syndrome. Mol Cell Endocrinol. 2017;439:247–55. [DOI] [PubMed] [Google Scholar]
- [27].Rahmani E, Jamilian M, Samimi M, et al. The effects of coenzyme Q10 supplementation on gene expression related to insulin, lipid and inflammation in patients with polycystic ovary syndrome. Gynecol Endocrinol. 2018;34:217–22. [DOI] [PubMed] [Google Scholar]
- [28].Samimi M, Zarezade Mehrizi M, Foroozanfard F, et al. The effects of coenzyme Q10 supplementation on glucose metabolism and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol (Oxf). 2017;86:560–6. [DOI] [PubMed] [Google Scholar]
- [29].Shabani A, Foroozanfard F, Kavossian E, et al. Effects of melatonin administration on mental health parameters, metabolic and genetic profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. J Affect Disord. 2019;250:51–6. [DOI] [PubMed] [Google Scholar]
- [30].Jamilian M, Mansury S, Bahmani F, et al. The effects of probiotic and selenium co-supplementation on parameters of mental health, hormonal profiles, and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. J Ovarian Res. 2018;11:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mohammad Hosseinzadeh F, Hosseinzadeh-Attar MJ, Yekaninejad MS, et al. Effects of selenium supplementation on glucose homeostasis and free androgen index in women with polycystic ovary syndrome: a randomized, double blinded, placebo controlled clinical trial. J Trace Elem Med Biol. 2016;34:56–61. [DOI] [PubMed] [Google Scholar]
- [32].Jamilian M, Razavi M, Fakhrie Kashan Z, et al. Metabolic response to selenium supplementation in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol (Oxf). 2015;82:885–91. [DOI] [PubMed] [Google Scholar]
- [33].Zadeh Modarres S, Heidar Z, Foroozanfard F, et al. The effects of selenium supplementation on gene expression related to insulin and lipid in infertile polycystic ovary syndrome women candidate for in vitro fertilization: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res. 2018;183:218–25. [DOI] [PubMed] [Google Scholar]
- [34].Heidar Z, Hamzepour N, Zadeh Modarres S, et al. The effects of selenium supplementation on clinical symptoms and gene expression related to inflammation and vascular endothelial growth factor in infertile women candidate for in vitro fertilization. Biol Trace Elem Res. 2020;193:319–25. [DOI] [PubMed] [Google Scholar]
- [35].Rashidi BH, Mohammad Hosseinzadeh F, Alipoor E, et al. Effects of selenium supplementation on asymmetric dimethylarginine and cardiometabolic risk factors in patients with polycystic ovary syndrome. Biol Trace Elem Res. 2020;196:430–7. [DOI] [PubMed] [Google Scholar]
- [36].Fruzzetti F, Perini D, Russo M, et al. Comparison of two insulin sensitizers, metformin and myo-inositol, in women with polycystic ovary syndrome (PCOS). Gynecol Endocrinol. 2017;33:39–42. [DOI] [PubMed] [Google Scholar]
- [37].Nestler JE, Jakubowicz DJ, Reamer P, et al. Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome. N Engl J Med. 1999;340:1314–20. [DOI] [PubMed] [Google Scholar]
- [38].Iuorno MJ, Jakubowicz DJ, Baillargeon JP, et al. Effects of d-chiro-inositol in lean women with the polycystic ovary syndrome. Endocr Pract. 2002;8:417–23. [DOI] [PubMed] [Google Scholar]
- [39].Jamilian M, Samimi M, Mirhosseini N, et al. The influences of vitamin D and omega-3 co-supplementation on clinical, metabolic and genetic parameters in women with polycystic ovary syndrome. J Affect Disord. 2018;238:32–8. [DOI] [PubMed] [Google Scholar]
- [40].Shokrpour M, Asemi Z. The effects of magnesium and vitamin E co-supplementation on hormonal status and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. Biol Trace Elem Res. 2019;191:54–60. [DOI] [PubMed] [Google Scholar]
- [41].Jamilian M, Sabzevar NK, Asemi Z. The effect of magnesium and vitamin E co-supplementation on glycemic control and markers of cardio-metabolic risk in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Horm Metab Res. 2019;51:100–5. [DOI] [PubMed] [Google Scholar]
- [42].Hager M, Nouri K, Imhof M, et al. The impact of a standardized micronutrient supplementation on PCOS-typical parameters: a randomized controlled trial. Arch Gynecol Obstet. 2019;300:455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Maktabi M, Jamilian M, Asemi Z. Magnesium-zinc-calcium-vitamin D co-supplementation improves hormonal profiles, biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res. 2018;182:21–8. [DOI] [PubMed] [Google Scholar]
- [44].Foroozanfard F, Jamilian M, Jafari Z, et al. Effects of zinc supplementation on markers of insulin resistance and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Exp Clin Endocrinol Diabetes. 2015;123:215–20. [DOI] [PubMed] [Google Scholar]
- [45].Onyango AN. Cellular stresses and stress responses in the pathogenesis of insulin resistance. Oxid Med Cell Longev. 2018;2018:4321714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fazakerley DJ, Minard AY, Krycer JR, et al. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. J Biol Chem. 2018;293:7315–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Singh RB, Niaz MA, Rastogi SS, et al. Effect of hydrosoluble coenzyme Q10 on blood pressures and insulin resistance in hypertensive patients with coronary artery disease. J Hum Hypertens. 1999;13:203–8. [DOI] [PubMed] [Google Scholar]
- [48].Bellenger J, Bellenger S, Bataille A, et al. High pancreatic n-3 fatty acids prevent STZ-induced diabetes in fat-1 mice: inflammatory pathway inhibition. Diabetes. 2011;60:1090–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yang JP, Shin JH, Seo SH, et al. Effects of antioxidants in reducing accumulation of fat in hepatocyte. Int J Mol Sci. 2018;19:2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Oh R. Practical applications of fish oil (Omega-3 fatty acids) in primary care. J Am Board Fam Pract. 2005;18:28–36. [DOI] [PubMed] [Google Scholar]
- [51].Woodman RJ, Mori TA, Burke V, et al. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am J Clin Nutr. 2002;76:1007–15. [DOI] [PubMed] [Google Scholar]
- [52].Wu JH, Cahill LE, Mozaffarian D. Effect of fish oil on circulating adiponectin: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2013;98:2451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. [DOI] [PubMed] [Google Scholar]
- [54].Altavilla D, Deodato B, Campo GM, et al. IRFI 042, a novel dual vitamin E-like antioxidant, inhibits activation of nuclear factor-kappaB and reduces the inflammatory response in myocardial ischemia-reperfusion injury. Cardiovasc Res. 2000;47:515–28. [DOI] [PubMed] [Google Scholar]