Abstract
Endoscopic resection is an effective treatment for subepithelial tumors arising from the muscularis propria layer of the stomach. However, the invasion pattern revealed by the pathological examination of tumor specimens is often not consistent with the findings of preprocedural endoscopic ultrasounds (EUS).
We compared the real growing patterns of tumors, as evaluated on histopathological examination, with their EUS images, and analyzed the outcomes of endoscopic resections in relation to the EUS findings.
From January 2006 to June 2015, 32 patients underwent endoscopic resection for gastric tumors originating from the muscularis propria at our hospital.
We divided the patients into 3 groups according to the location of the tumor as diagnosed using pre procedural EUS: submucosa (group I, n = 5), muscularis propria (group II, n = 14), and tumors extending into the outer cavity (group III, n = 13).
Histopathological examination revealed 15 patients with gastrointestinal stromal tumors (GISTs), 14 with leiomyomas, and 3 with schwannomas. Accuracy of EUS in evaluating tumor invasion was 56%. Some tumors in groups I and II was removed by endoscopic submucosal dissection only. Muscular dissection was needed in 10 patients (71%) in group II and 9 patients (69%) in group III. Four patients (31%) in group III were found to have subserosal tumors. The complete resection rate was 88% (23 patients) among patients who underwent endoscopic submucosal dissection and endoscopic muscular dissection, and 67% (4 patients) among patients who underwent endoscopic subserosal dissection (ESSD). The tumor was completely removed in 12 patients (86%) in group II and 10 patients (77%) in group III.
EUS accurately predicts the layer of the subepithelial tumor in the stomach; however, the pattern of invasion of surrounding structures is difficult to evaluate using EUS.
Keywords: stomach, neoplasm, EUS, resection
1. Introduction
Most subepithelial tumors in the stomach are asymptomatic and, therefore, not clinically significant. However, neuroendocrine tumors, lymphomas, and gastrointestinal stromal tumors (GISTs) have malignant potential and are managed by definitive treatment. Methods used to obtain specimen for a histological diagnosis include bite-on-bite biopsy, endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA), endoscopic mucosal resection, and endoscopic submucosal dissection (ESD).[1] Of these methods, bite-on-bite biopsy and EUS-FNA are less invasive; however, they cannot accurately measure the tumor’s mitotic count.[2]
Endoscopic resection is used to treat malignant or precancerous lesions in the stomach and to remove subepithelial tumors arising from the muscularis propria of the stomach.[3] Endoscopic ultrasonography is the most accurate noninvasive diagnostic modality for the evaluation of lesions, including the layer in which they are located. When gastric subepithelial tumors are found to originate in the muscularis propria and extend mostly into the submucosal layer, endoscopic resection is indicated. However, the pathological evaluation of endoscopic resection specimen show that the pattern of tumor invasion is often different from the preoperative EUS diagnosis.[3] For example, tumors may penetrate the muscularis propria and invade the subserosal layer.[3]
In this study, we divided patients with gastric subepithelial tumors originating in the muscularis propria into 3 groups according to their pre procedural EUS findings, compared the EUS diagnoses to the real growing patterns detected on histopathological examination, and analyzed the results of the endoscopic resections.
2. Material and Methods
2.1. Selection of subjects
From January 2006 to June 2015, 32 patients underwent endoscopic resection for subepithelial gastric tumors at Presbyterian Medical Center. All patients were preoperatively diagnosed with a tumor originating in the muscularis propria using EUS. We retrospectively obtained the patient characteristics, preoperative and postoperative diagnoses, resection method, complete resection data, treatment time, perforation data, and resection outcomes from the patients’ medical records.
The patients were divided into 3 groups according to the preoperative EUS results. Group I included patients with tumors located mostly in the submucosal layer with thin connections to the muscularis propria on EUS.[4] The tumors of patients in group II originated in the muscularis propria but did not protrude into the outer cavity. Group III included patients with tumors extending into the outer cavity. Endoscopic resection was performed when <50% of the tumor was growing outward.
This study was approved by the Institutional Review Board of Presbyterian Medical Center. Informed consent was given for all patients.
2.2. Endoscopy and EUS
Endoscopic findings were evaluated for shape, location, and size of the tumor. The location of the lesion was classified as anterior wall, posterior wall, lesser curvature, greater curvature, or fundus. A radial-type endoscopic ultrasound (GF-UMQ200, Olympus Optical, Japan) or catheter probe-type endoscopic ultrasound (UM-2R; Olympus Optical, Japan) was used. The layer, shape, size, and echogenicity of the lesions was noted. Tumors that were found to be 3 cm or more with an irregular surface, echogenic foci, heterogeneous echotexture, or malignant lymph nodes were considered to be malignant GISTs and were excluded from the study.
2.3. Resection method
Patients were sedated with midazolam, fentanyl, and propofol. Blood pressure, pulse, and oxygen saturation were monitored throughout the procedure. A soft cap was fixed to the tip of the endoscope to improve visibility during the submucosal dissection.
The lesion was marked using the tip of the snare, and normal saline solution was injected into the submucosal or subserosal layer. A round incision was made outside the marked area using a needle knife. An IT-knife (insulated-tip diathermic knife), IT knife-2, or hook knife was used for ESD, endoscopic muscular dissection (EMD), or endoscopic subserosal dissection (ESSD). An Erbe ICC-200 electrosurgical unit (Erbe; Tübingen, Germany) was used, and provided coagulation current (forced coagulation 60 watts) for marking, endoCut mode (effect 2, 60 watts) for incisions, and coagulation current (forced coagulation, 80 watts) for dissections. According to the degree of penetration of muscularis propria layer, the need of dissection for tumor was different such as ESD only, ESD combined with EMD, or 3 methods(ESD, EMD, ESSD). For tumor with subserosal growing, ESD was followed by EMD. After then, ESSD was done after making subserosal cushion.
Bleeding was controlled using coagulation current (soft coagulation, 60 watts) and clips (Olympus Optical; Tokyo, Japan) for large vessels. Deep damage to the muscle layer found during the procedure or due to perforation was immediately closed using a clip.
2.4. Management after endoscopic resection
No oral intake was permitted until post procedural day 1. Vital signs were checked at 4-hour intervals and a complete blood count and chest X-ray were performed 4 hours after the procedure and the next morning to check for perforation. Upper gastrointestinal endoscopy was performed within 24 hours of the procedure to check for bleeding or other changes in the stomach. Proton pump inhibitor was given, intravenously. If no evidence of perforation or bleeding was found, an oral diet was started. Emergency endoscopic examinations were performed if patients complained of black stool or hemorrhage.
2.5. Histologic evaluation and complete resection
The resected tissue was fixed with a pin on a flat, thin plate and placed into a formalin solution. The tumor cell type, cellularity, nuclear atypia, immunohistochemical staining (c-kit, CD 34, smooth muscle actin, and S-100), and mitotic count were evaluated histologically.
An en bloc resection was defined as the resected tissue, including the lesion, removed as 1 piece. Resections yielding 2 or more specimen were classified as piecemeal resections. A complete resection was defined as the lesion removed in an en bloc pattern with no residual lesion observed endoscopically after the resection.
2.6. Measurement of the results
We analyzed the general characteristics of the patients, locations of the lesions, pathologic diagnoses, complete resection rate, procedure times, procedure-related complications, and follow-up results. No statistical analysis was performed, as the sample size was low. A perforation was diagnosed when space in the abdominal cavity was observed during the procedure or when an air shadow was seen in the peritoneal cavity on the post procedural radiograph. Bleeding was defined as when the patient required a blood transfusion or if bleeding occurred after the procedure.
3. Results
3.1. General characteristics
Of the 32 patients included in this study, 5 were in group 1, 14 were in group II, and 13 were in group III. A histopathological diagnosis was possible in all patients and 15 patients (47%) were diagnosed with GISTs, 14 patients (44%) with leiomyomas, and 3 patients (9%) with schwannomas. GIST was considered to have very low or low malignant potential with a mitotic count of 5/50 HPF or less and a size of 3 cm or less, indicating no need for additional surgical treatment (Table 1).
Table 1.
Characteristics of patients and lesions.
| General characteristics | |
|---|---|
| Age (yr) | 60.9 |
| Sex (M/F) | 14/18 |
| Lesion sizes (mm, mean ± SD) | 17 ± 7.2 |
| Location | |
| Cardia | 8 |
| Anterior | 3 |
| posterior | 7 |
| Lesser | 9 |
| Greater | 5 |
| Pathologic diagnoses | |
| Leiomyona | 14 |
| GIST | 15 |
| Schwannoma | 3 |
3.2. Histologic characteristics
The most common pathological findings of each group were GIST (3 patients) in group I, leiomyoma (9 patients) in group II, and GIST (9 patients) in group III.
Accuracy of EUS in evaluating tumor penetration depth was 56%. ESD only was mainly conducted in groups I and II. Muscular dissection was performed in 10 patients (71%) in group II and 9 patients (69%) in group III (Fig. 1). No patients in group I had lesions connected to the subserosal layer; however, a subserosal location was detected in 4 patients (31%) in group III (Table 2).
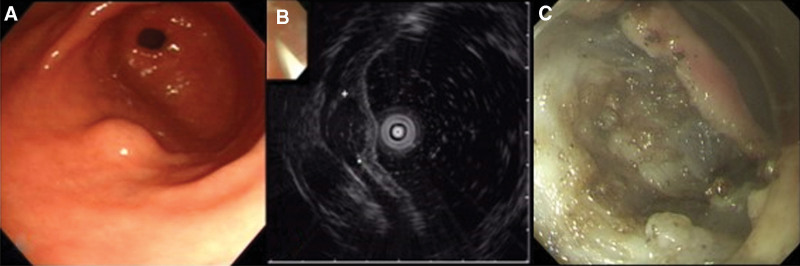
Figure 1.
Group I tumor was removed by ESD and EMD. Pathologic diagnosis was GIST. (A). Endoscopic finding (B). EUS image. (C). Ulceration after endoscopic removal shows the deep injury of inner circular muscle layer. ESD = endoscopic submucosal dissection, EMD = endoscopic muscular dissection, EUS = endoscopic ultrasounds, GIST = gastrointestinal stromal tumor
Table 2.
Correlation between endosonographic findings and tumor invasion depth.
| Outcomes | Group I (n = 5) | Group II (n = 14) | Group III (n = 13) |
|---|---|---|---|
| Location | |||
| Cardia | 0 | 2 | 6 |
| Anterior | 1 | 0 | 2 |
| Posterior | 0 | 6 | 2 |
| Lesser | 1 | 6 | 1 |
| Greater | 3 | 0 | 2 |
| Pathologic diagnoses | |||
| Leiomyoma | 2 | 9 | 3 |
| GIST | 3 | 3 | 9 |
| Schwannoma | 0 | 2 | 1 |
| Endoscopic resections | |||
| ESD | 4 | 2 | 0 |
| ESD and EMD | 1 | 10 | 9 |
| ESD, EMD, and ESSD | 0 | 2 | 4 |
| Accuracy of EUS (%) | 80 | 71 | 31 |
3.3. Outcomes of endoscopic resection
The average procedure time was 60 ± 62 minutes in group I, 36 ± 23 minutes in group II, and 43 ± 36 minutes in group III (Table 3; Fig. 2).
Table 3.
Endoscopic resection outcomes according to endosonographic findings.
| Outcomes | Group | ||
|---|---|---|---|
| I | II | III | |
| Lesion sizes (mm, mean ± SD) | 18 ± 7.5 | 15 ± 6.8 | 18 ± 7.4 |
| Procedure time (mm, mean ± SD) | 60 ± 62 | 36 ± 23 | 43 ± 36 |
| Complete resection rate | 5 (100%) | 12 (86%) | 10 (77%) |
| Perforation | 1 (20%) | 1 (7%) | 2 (15%) |
| Follow-up (mo) | 49 | 33 | 38 |
| Local recurrence | 0 | 1 (7%) | 1 (8%) |
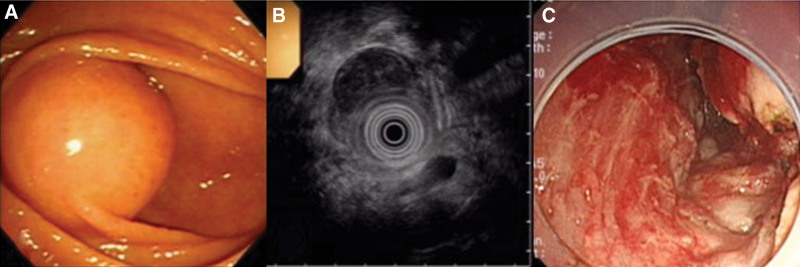
Figure 2.
Group I tumor was removed by ESD. Pathologic diagnosis was GIST. (A) Endoscopic finding. (B) EUS image(C). Ulceration after ESD. ESD = endoscopic submucosal dissection, EUS = endoscopic ultrasounds, GIST = gastrointestinal stromal tumor
The complete resection rate was 88% (23 patients) among patients who underwent ESD or EMD and 67% (4 patients) among patients who underwent ESSD. In group II, 12 patients (86%) had a complete resection, as did 10 patients (77%) in group III (Fig. 3). All 3 patients with a schwannoma had incomplete resection due to unclear tumor boundaries that severely infiltrated into the surrounding tissue with fibrosis. All patients with GIST or leiomyomas in group I (5/5, 100%) and group II (12/12, 100%) had complete resections, while 83% (10/12) of patients with GIST or leiomyomas in group III had complete resections. Overall, complete resection was achieved in 13 patients (87%) with GIST and 14 patients (100%) with leiomyoma.
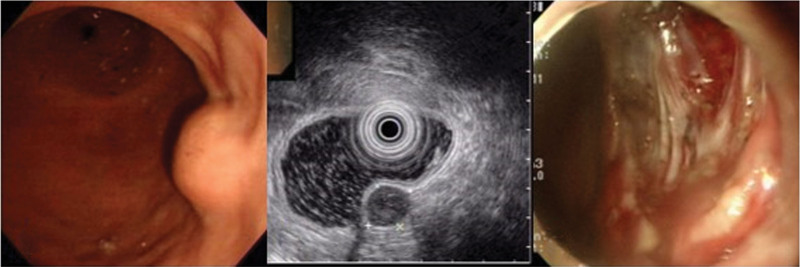
Figure 3.
Group III tumor was resected by ESD, EMD, and ESSD. Pathologic diagnosis was GIST. (A) Endoscopic finding. (B) EUS image. (C) Ulceration shows the subserosal layer through defect of the muscularis propria. ESD = endoscopic submucosal dissection, EMD = endoscopic muscular dissection, ESSD = endoscopic subserosal dissection, EUS = endoscopic ultrasounds, GIST = gastrointestinal stromal tumor.
3.4. Complications
In total, 4 patients experienced perforation which occurred in 1 patient (20%) in group I, 1 patient (7%) in group II, and 2 patients (15%) in group III. Two patients (33%) who underwent ESSD experienced it. Three cases of perforation were discovered during the procedure and were successfully treated using a metallic clip. One patient’s perforation was detected radiologically after the procedure with no signs of peritoneal irritation. This patient was successfully treated conservatively. All 4 patients who experienced perforation recovered without surgical treatment.
No patient required a blood transfusion and no patients died due to causes related to the procedure.
3.5. Recurrence
All patients underwent endoscopic examination 3 months after the procedure and were observed at 1-year intervals thereafter. The mean follow-up period was 38 ± 31 months, and local recurrence occurred in 2 patients with incomplete EMD resections. One patient was found to have recurrent schwannoma 45 months after the initial procedure and is currently being monitored. One patient was found to have recurrent GIST 25 months after the initial procedure and was surgically treated.
4. Discussion
Asymptomatic upper gastric subepithelial tumors were reported changes in 10% of the lesions, and 76% (19/25) of patients who underwent surgery or endoscopy were diagnosed with GIST.[5] GISTs in the stomach are the most common subepithelial tumor in the muscularis propria and have a better prognosis than GIST in other organs.[6,7] The European Society for Medical Oncology noted that small, histologically diagnosed GIST should be resected. Tumors that are 2 cm or less with a mitotic count of 5/50 HPF or less are classified as having very low malignant potential and rarely metastasize.[8,9]
EUS is the most useful, noninvasive diagnostic method for subepithelial tumors in the stomach. However, the accuracy of EUS is low. According to a study by Hwang et al, inaccurate diagnoses occurred most frequently in the examination of the third and fourth lesions.[10,11] However, EUS is the most accurate method for identifying the layer in which a lesion is located and evaluating the characteristics of the lesion.[12] In this study, all patients had tumors in the muscularis propria, with only 6 patients (four of whom were in group III) diagnosed as having subserosal involvement.
Since GIST mainly grows in the muscularis propria, tissue cannot be obtained via endoscopic biopsy. The accuracy of diagnoses made using EUS alone is <50%; however, the diagnostic accuracy made using invasive tests, such as EUS-FNA, is 90% or more. The prognosis is difficult to determine, as the mitotic count varies depending on the location within the tumor and may be altered by the fixed time of the tissue and the type of drug used.[13] Therefore, complete resection is needed to determine the prognosis and to treat GISTs. GISTs <5 cm with a low mitotic count have a low probability of metastasis and can be treated with local resection.[6]
Endoscopic ablation is largely divided into endoscopic enucleation and endoscopic full-thickness resection. Tumor nucleation is performed by exposing the submucosal layer using various methods through an endoscope (including ESD, EMD, ESSD, and endoscopic submucosal tunnel dissection) prior to removing the tumor nucleus using an electrosurgical knife. In this study, the lesions were removed using ESD, EMD, and ESSD.[14,15] Several studies have reported a complete excision rate of 64% to 97% that resulted in the successful treatment of GISTs.[16–19] In this study, the en bloc resection rate was 100%, and complete resection was achieved in 87% of patients with GISTs and 100% of patients with leiomyomas. The complete resection was 89% (17/19) among patients with tumors growing only in the lumen (groups I and II) and 77% (10/13) among patients with tumors growing outward (group III). We found that many patients initially classified as group III had lesions that grew only into the lumen of the stomach without subserosal invasion.
To obtain a pathologic complete resection, the lesion must be removed with full thickness. Feng et al performed endoscopic full-thickness resection, and reported that the endoscopic procedure may be related to peritoneal seeding of tumor cells.[20] In this study, all patients with tumors with subserosal invasion that did not have schwannomas were successfully treated with ESSD. Perforation occurred in 3 patients who underwent ESSD with tumors located in the greater curvature and the posterior wall, which may be due to thin subserosal tissue in these areas.
Neoplasms that grow in the muscularis propria of the stomach include GIST, leiomyoma, schwannoma, glomangioma, and ectopic pancreas. The degree of infiltration and fibrosis into surrounding tissues varies depending on the lesion. GIST and leiomyoma are clearly distinguished from surrounding tissues; however, other gastric lesions are tightly coupled with surrounding tissues, rendering enucleation difficult. Among tumors that grow outward, a significant number of benign schwannomas may be detected.[21,22] In this study, the en bloc resection rate of patients with GIST or leiomyoma was 100% and complete resection was possible in 93% (27/29) of patients with GIST or leiomyoma. Therefore, it is necessary to evaluate the gross appearance of lesions before and during the procedure in order to identify GIST and leiomyoma and to eliminate these lesions with the goal of complete resection.
Complications of endoscopic treatment of tumors growing in the muscularis propria include bleeding and perforation. Perforation is the most dangerous complication. It has been reported in 0% to 13% of patients undergoing endoscopic treatment of tumors, most of which can be medically treated; however, surgery is sometimes necessary.[16–19] If the pseudocapsule is damaged in a patient with GIST, there is a risk of dissemination of the tumor cells into the peritoneum, which may be associated with a high recurrence rate.[20] In our study, perforations occurred in 2 patients (11%) in group I and II, and 2 patients (15%) in group III. All 3 cases of perforation in patients with GIST in this study were found during the procedure and successfully closed using a metallic clip.
After the endoscopic removal of GISTs, it is difficult to obtain a pathologic complete resection. En bloc resection without macroscopic remnant lesions may be an effective treatment method as the tumor cells remaining on the dissection surface are severely damaged due to the electric cut current. Schimidt et al reported a recurrence of 5.8% after endoscopic resection of GIST.[23] In this study, a local recurrence was found in 1 patient with GIST (1/29, 3.4% of patients with GIST or leiomyoma) and was surgically treated.
This study is the first to compare the clinical outcomes of endoscopic resection and the degree of tumor infiltration according to the growth pattern diagnosed on preprocedural EUS. There are some limitations. First, the data were analyzed by examining medical records and images retrospectively and were subject to any errors in the case records. In addition, most of the patients in group I underwent resection during the first half of this study, before the surgeons gained experience with the procedure. This may account for the longer average procedure time for patients in group I. Second, the sample size was small and statistical analysis was not possible. Endoscopic resection of subepithelial tumors growing in the stomach requires a high level of technique and the technical difficulty varies greatly depending on the location of the lesion. Therefore, a large-scale study is necessary. Third, 1 surgeon performed all of the procedures included in this study, thus it is likely that the preferred patient or lesion of the operator is included in the study, which may bias the results.
Until now, endoscopic resection of GISTs or leiomyomas in the stomach has been performed only for tumors that grow inward with a narrow connection to the mucularis propria. In this study, preprocedural EUS predicted the layer of the tumor accurately; however, it was limited in the evaluation of the pattern of invasion. We found that tumors that were diagnosed as growing outward on EUS rarely penetrated the muscularis propria and invaded the subserosal layer upon pathological examination. EUS has the limitations to evaluate the invasion patterns of tumors using EUS. We think that some tumors diagnosed as growing outward can be treated by endoscopic resection.
Acknowledgments
I would like to express my sincere gratitude to all doctors and nurses in gastrointestinal endoscopy center in Presbyterian Medical Center, Jeonju city, South Korea.
Author contributions
Jinwoong Cho played major role in study design, data acquisition, analysis, and interpretation as well as making article. Mina Yang, Jihyun Han, Mirim Choi, Jaesun Song, and Youngjae Lee had a role in study conception and data acquisition.
Abbreviations:
- EMD =
- endoscopic muscular dissection
- EMR =
- endoscopic mucosal resection
- ESD =
- endoscopic submucosal dissection
- ESSD =
- endoscopic subserosal dissection
- ESTD =
- endoscopic submucosal tunnel dissection
- EUS =
- endoscopic ultrasounds
- EUS-FNA =
- endoscopic ultrasonography-guided fine needle aspiration
- GISTs =
- gastrointestinal stromal tumors
- IRB =
- institutional review board
- SMA =
- smooth muscle actin.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Cho J, Han J, Choi M, Song J, Yang M, Lee Y. Correlation between endoscopic resection outcomes and endosonographic findings in gastric tumors with muscularis propria origin. Medicine 2022;101:32(e29947).
SD = standard deviation.
ESD = endoscopic submucosal dissection, EMD = endoscopic muscular dissection, ESSD = endoscopic subserosal dissection, EUS = endoscopic ultrasounds, GIST = gastrointestinal stromal tumor.
Values are presented as n (%).
SD = standard deviation.
Contributor Information
Jihyun Han, Email: hanjihyun2003@daum.net.
Mirim Choi, Email: kianumr@daum.net.
Jaesun Song, Email: jesusjunior@daum.net.
Mina Yang, Email: newtongil@hotmail.com.
Youngjae Lee, Email: yz9017@daum.net.
References
- [1].Khashab MA, Pasricha PJ. Conquering the third space: challenges and opportunities for diagnostic and therapeutic endoscopy. Gastrointest Endosc. 2013;77:146–8. [DOI] [PubMed] [Google Scholar]
- [2].Hwang JH, Rulyak SD, Kimmey MB. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology. 2006;130:2217–28. [DOI] [PubMed] [Google Scholar]
- [3].Schmidt A, Bauder M, Riecken B, et al. Endoscopic resection of subepithelial tumors. World J Gastrointest Endosc. 2014;6:592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chun SY, Kim KO, Park DS, et al. Endoscopic submucosal dissection as a treatment for gastric subepithelial tumors that originate from the muscularis proprialayer: a preliminary analysis of appropriate indications. Surg Endosc. 2013;27:3271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim MY, Jung HY, Choi KD, et al. Natural history of asymptomatic small gastric subepithelial tumors. J Clin Gastroenterol. 2011;45:330–6. [DOI] [PubMed] [Google Scholar]
- [6].Miettinen M, Lasota J. Gastrointestinal stromal tumor. Semin Diagn Pathol. 2006;23:70–83. [DOI] [PubMed] [Google Scholar]
- [7].Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411–9. [DOI] [PubMed] [Google Scholar]
- [8].Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–65. [DOI] [PubMed] [Google Scholar]
- [9].Meesters BL, Pauwels PA, Pijnenburg AM, et al. Metastasis in a benign duodenal stromal tumour. Eur J Surg Oncol. 1998;24:334–5. [DOI] [PubMed] [Google Scholar]
- [10].Hwang JH, Saunders MD, Rulyak SJ, et al. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc. 2005;62:202–8. [DOI] [PubMed] [Google Scholar]
- [11].Karaca C, Turner BG, Cizginer S, et al. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc. 2010;71:722–7. [DOI] [PubMed] [Google Scholar]
- [12].Lachter J, Bishara N, Rahimi E, et al. EUS clarifies the natural history and ideal management of GISTs. Hepatogastroenterology. 2008;55:1653–6. [PubMed] [Google Scholar]
- [13].Trupiano JK1, Stewart RE, Misick C, et al. Gastric stromal tumors: a clinicopathologic study of 77 cases with correlation of features with nonaggressive and aggressive clinical behaviors. Am J Surg Pathol. 2002;26:705–14. [DOI] [PubMed] [Google Scholar]
- [14].Cho JW, Lee YJ, Kim JW, et al. A modified new method beyond endoscopic muscularis dissection for an exophytic gastric tumor. VideoGIE. 2018;3:177–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu F, Zhang S, Ren W, et al. The fourth space surgery: endoscopic subserosal dissection for upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc. 2018;32:2575–82. [DOI] [PubMed] [Google Scholar]
- [16].Lee IL, Lin PY, Tung SY, et al. Endoscopic submucosal dissection for the treatment of intraluminal gastric subepithelial tumors originating from the muscularis propria layer. Endoscopy. 2006;38:1024–8. [DOI] [PubMed] [Google Scholar]
- [17].Mönkemüller K, Peter S, Toshniwal J, et al. Multipurpose use of the “”bear claw’’ (over-the-scope-clip system) to treat endoluminal gastrointestinal disorders. Dig Endosc. 2014;26:350–7. [DOI] [PubMed] [Google Scholar]
- [18].von Renteln D, Rösch T, Kratt T, et al. Endoscopic full-thickness resection of submucosal gastric tumors. Dig Dis Sci. 2012;57:1298–303. [DOI] [PubMed] [Google Scholar]
- [19].von Renteln D, Kratt T, Rösch T, et al. SchachschalG. Endoscopic full-thickness resection in the colon by using a clip-and-cut technique: an animal study. Gastrointest Endosc. 2011;74:1108–14. [DOI] [PubMed] [Google Scholar]
- [20].Kim HH. Endoscopic treatment for gastrointestinal stromal tumor: advantages and hurdles. World J Gastrointestinal Endosc. 2015;7:192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bruneton JN, Drouillard J, Roux P, et al. Neurogenic tumors of the stomach. Report of 18 cases and review of the literature. Rofo. 1983;139:192–8. [DOI] [PubMed] [Google Scholar]
- [22].Voltaggio L, Murray R, Lasota J, et al. Gastric schwannoma: a clinicopathologic study of 51 cases and critical review of the literature. Hum Pathol. 2012;43:650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schmidt A, Damm M, Caca K. Endoscopic full-thickness resection using a novel over-the-scope device. Gastroenterology. 2014;147:740–2. [DOI] [PubMed] [Google Scholar]