Abstract
This study was performed to investigate the role of neutrophil-to-lymphocyte ratio (NLR) in the diagnosis of adult onset Still disease (AOSD) and its performance to improve the sensitivity of the classifications criteria (Yamaguchi and Fautrel Classifications).
We conducted a multicenter prospective nationwide case-control study in Internal medicine, Rheumatology and Infectious disease departments, to include successively patients with suspected AOSD (2 or more major criteria of Yamaguchi or Fautrel classifications). All clinical and biological features were collected in a consensual and standardized clinical assessment at baseline and during follow-up. A receiving operating characteristic (ROC) curve was used to reassess the cutoff value of NLR. After determination of the cutoff value for NLR by ROC curve, 2 composite sets (Yamaguchi classification + NLR as a major criterion and Fautrel classification + NLR as a major criterion) were performed and evaluated.
One hundred sixty patients were included, 80 patients with AOSD and 60 controls with different diagnoses. Twenty patients with incomplete data were excluded. The cutoff value for NLR equals 4 (area under the curve, AUC: 0.82). The NLR was ≥ 4 in 93.7% (75/80) of AOSD patients with a sensitivity of 93.8% and specificity of 61.7%. The association of NLR as a major criterion with the classification of Yamaguchi or Fautrel improved their sensitivity, respectively for Fautrel (76.3% to 92.5%, P = .004) and Yamaguchi (78.8% to 90%, P = .05).
This study validates the NLR as a good simple biomarker of AOSD with a cutoff value of 4 and high sensitivity (93.8%). The addition of NLR (NLR ≥ 4) as a major criterion to the classifications (Yamaguchi and Fautrel) improved significantly their sensitivity and accuracy.
Keywords: Adult Onset Still Disease, classification criteria, diagnosis, glycosylated ferritin, neutrophil-to-lymphocyte ratio, typical rash
1. Introduction
Adult onset Still disease (AOSD) is a rare multigenic autoinflammatory disease. It is just another facet of childhood onset Still disease or systemic juvenile idiopathic arthritis, described a century ago by George Frederic Still. Usually, it affects young adults with a female predominance.[1–3]
The most common manifestations are high spiking fever, maculopapular rash, polyarthralgia, sore throat, lymphadenopathy, and serositis. The main laboratory findings are high ferritin with low glycosylated ferritin (GF) (≤20%), high C-reactive protein, leukocytosis with neutrophilia, and elevated liver enzymes. Nevertheless, the clinical expressions of AOSD can be heterogeneous especially in early stage of the disease, which can be a diagnostic challenge for physicians.[4–6] Moreover, delayed diagnosis can lead to life-threatening complications such reactive hemophagocytic lympho-histiocytosis (RHL) and myocarditis.[7,8]
Currently, the diagnosis of AOSD is based on classifications criteria, in the lack of specific clinical manifestation or biological biomarker. Several sets of classifications criteria have been proposed for AOSD. The most used are Yamaguchi and Fautrel classifications.[9,10] Thus, several studies searched specific biomarkers for AOSD. A preliminary study suggested that delta neutrophil index (DNI, the ratio of immature granulocytes) may discriminate AOSD from sepsis.[11] Moreover, beta-2 microglobulin was higher in AOSD patients and may be a diagnostic tool in AOSD.[12] These results should be confirmed.
The neutrophil-to-lymphocyte ratio (NLR) is an inflammatory biomarker that reflects systemic inflammation. The NLR is the absolute number of neutrophils divided by the absolute number of lymphocytes, measured in routine blood count.[13] It has been evaluated in several diseases and higher NLR was associated to poor prognosis in major cardiac events, ischemic stroke and cancer.[14] The neutrophil percentage ≥ 80% is a major criterion in Fautrel and Yamaguchi classifications.[9,10,15] The NLR could be a new simple biomarker of AOSD more sensitive than neutrophil count and should be useful in AOSD diagnosis as suggested by Seo et al.[13]
Herein, we aimed to validate the NLR as a diagnostic biomarker in AOSD. This is the first study to evaluate the effect of NLR on the sensitivity and the accuracy of the classifications criteria. Moreover, the role of other biomarkers in AOSD have been investigated (GF, DNI, and Beta-2 microglobulin).
2. Patients and methods
2.1. Study design
We conducted a multicenter prospective nationwide case-control study in internal medicine, rheumatology and infectious diseases departments. Seventeen tertiary centers (11 internal medicine, 5 rheumatology, 1 infectious diseases) participated in the study between December 2016 and December 2019, to include patients with suspected AOSD based on the presence of 2 or more major criteria of Yamaguchi classification and or Fautrel classification. The study protocol was approved by the Institutional Review Board of the University of Algiers 1.
2.2. Patients
Patients with suspected AOSD were successively included in the different centers. At inclusion, all clinical and biological features were collected in a consensual and standardized clinical assessment at baseline and during follow-up by the referring physician. The principal investigator (K.A.D) gathered subsequently all data in a unique database to conduct the statistical analysis.
We excluded patients who had <18 years old or those who did not consent. A written informed consent was obtained from each patient for the participation in the study.
The classification procedure was as follow: patients were classified as AOSD by the referring physician (step 1). AOSD patients classified by the principal investigator, should met also Yamaguchi and or Fautrel criteria (step 2). Patients were classified as controls if different diagnoses (autoimmune diseases, auto inflammatory diseases, infectious diseases, neoplastic diseases, and hypersensitivity conditions) were defined.
A multidisciplinary expert group (senior internist and rheumatologist authors of this article) certified the final diagnosis for cases and controls if the criteria were not satisfied (step 3). Patients with incomplete data or insufficient follow-up were excluded.
Fautrel and Yamaguchi classifications were applied for AOSD patients and controls to test their discriminative performance. Each patient was followed up for at least 12 months.
Both groups were matched for sex, ethnic origin (all white north African) and clinical complications. The mean age in both groups was in the third decade.
2.3. Variables
Clinical variables were defined as present or absent: spiking fever (≥39°C, 38.3–38.9°C, hectic fever), joint symptoms (arthralgia, arthritis, number of affected joints), myalgia, skin rash (typical rash: transient macular or maculopapular nonpruritic rash, atypical cutaneous eruption: urticarial rash, persistent eruption, purpura, dermographism), lymphadenopathy, splenomegaly, hepatomegaly, abdominal pain, pharyngitis, pleuritis, pericarditis, myocarditis, neurological involvement, ophthalmological involvement, renal involvement, and digestive involvement.[2,8–10,15]
Biological variables were defined as normal or abnormal according to their predefined threshold: leukocytosis (≥10,000/mm), neutrophilia (≥80%, ≥ 75%, mean neutrophil count), lymphopenia (lymphocytes < 1500), NLR (normal range: 0.78 to 3.53),[16] mean hemoglobin, and platelets.
We have also reassessed the cutoff value of NLR in AOSD through the receiver operating characteristic curve (ROC). After determination of the cutoff value for NLR by ROC curve, 2 composite sets (Yamaguchi classification + NLR as a major criterion and Fautrel classification + NLR as a major criterion) were applied to each patient.
The Delta neutrophil index (DNI) is the immature granulocytes fraction provided by a blood cell analyzer (ADVIA 2120). DNI was determined by subtracting the fraction of mature polymorphic neutrophils from the sum of myeloperoxidase-reactive cells: DNI = [the neutrophil sub fraction + the eosinophil sub fraction] − [the polymorphic neutrophils sub fraction]. This result was confirmed by blood smear. A DNI > 2% was considered abnormal and may be a predictor factor of severe sepsis. DNI may discriminate AOSD from sepsis.[11]
We have also tested liver enzymes (alanine aminotransferase, aspartate aminotransferase, lactates deshydrogenase, gamma glutamyl transferase), Beta-2 microglobuline (0.9–2.6 mg/L), C-reactive protein (<6 mg/L), mean erythrocyte sedimentation rate, negative ANA (<1/80), negative rheumatoid factor (FR), negative anticyclic citrullinated peptide (anti-CCP), negative antiphospholipids, Increased serum ferritin (upper normal value and upper than 5-fold the normal value) and low GF (≤20% and ≤25%). The GF was not measurable or interpretable when the ferritin rate was low or in normal range.[10]
2.4. Statistical analysis
The calculation of the study size was based on the inclusion of all cases and controls during the 3-years study period, as AOSD is a rare disease.
Descriptive statistics included percentages, means, and standard deviations. Comparative analysis of clinical and biological variables between AOSD patients and controls was performed in univariate way then in multivariate with multiple logistic regression. The searches for associations between the different variables were performed using Pearson chi-square test for qualitative variables, when the conditions for applying the test were not met Yates’ correction was applied. The Fisher exact test was used for small samples.
Comparison of means was made with Student t test for the quantitative variables. Moreover, the comparison of several means was performed by analysis of variance (ANOVA test).
The results were expressed in odds ratio with their 95% confidence interval. They were statistically significant if P was <.05.
The cutoff value of NLR was determined by the receiver operating characteristic curve (ROC). The sensitivity, specificity, positive predictive value, negative predictive value and accuracy were calculated for Yamaguchi classification, Fautrel classification, Yamaguchi + NLR, Fautrel + NLR. Data analysis was performed with SPSS software (version 23).
3. Results
3.1. Population characteristics
We included 160 patients, of which 80 patients with a definite AOSD patients (63 classified according to Yamaguchi criteria, 61 classified according to Fautrel criteria and 17 classified according to expert adjudication) and 60 control patients. 20 patients with incomplete data or insufficient follow-up were excluded. Patients with AOSD were followed up from 12 months (75 patients) to 36 months (35 patients).
The control group included: autoimmune and auto inflammatory diseases (55%), infectious diseases (23.3%), neoplastic diseases (10%) and hypersensitivity conditions (11.7%). In this group, the autoimmune and autoinflammatory diseases were: systemic lupus erythematosus (n = 10), systemic vasculitis (n = 8) including granulomatosis with polyangeitis (n = 4), eosinophilic granulomatosis with polyangeitis (n = 3) and giant cells arteritis (n = 1), rheumatoid arthritis (n = 4), Behçet disease (n = 4) Polymyositis (n = 2), familial Mediterranean fever (n = 2), and Sweet syndrome (n = 2), while infectious diseases were: sepsis (n = 10), tuberculosis (n = 2), HIV (n = 1), leptospirosis (n = 1) and neoplastic diseases were: Hodgkin lymphoma (n = 2), non-Hodgkin lymphoma (n = 2), prostate cancer (n = 1), bladder cancer (n = 1), and finally the hypersensitivity conditions were chronic urticaria (n = 6) and salazopyrin toxicity (n = 1).
3.2. Clinical and laboratory findings in AOSD patients
The mean age of AOSD patients was 33.76 ± 13 years and 61.2% were female. The most frequent clinical features were: fever (100%), arthralgia (93.7%), rash (87.5%), deterioration of general condition (83.7%), and sore throat (82.5%).
The association fever, arthralgia, and rash were present at the diagnosis in 65 (81%) patients. The GF with a cutoff value ≤ 25% was more frequent than the cutoff value ≤ 20% respectively (89.3%, 78.7%).
3.3. Univariate analysis
AOSD patients were younger than controls (P = .01). Several manifestations were significantly more frequent in AOSD patients: high spiking fever ≥ 39C° (P < 10−6, OR = 24), typical rash (P = 3 × 10−5, OR = 14) and particularly the transient and macular aspect of the rash (OR = 24, OR = 41) (Table 1).
Table 1.
Univariate analysis of the main characteristics for AOSD patients and controls.
| Variables | AOSD patients, n = 80 % | Controls, n = 60 % | P | Odds ratio [CI 95%] |
|---|---|---|---|---|
| Age | 33.76 ± 13 | 39.5 ± 14 | 0.01 | |
| Sex-ratio M/F | 0.63 | 0.46 | – | |
| Deterioration of general condition | 67/80 (83.7) | 32/60 (53.3) | 9 × 10−5 | 4.5 [1.9–10.6] |
| Fever | 80/80 (100) | 46/60 (76.7) | 5 × 10−6 | – |
| T, 39°C–40°C | 76/80 (95) | 28/46 (60.8) | <10−6 | 12.2 [3.5–47.1] |
| Hectic fever | 58/80 (72.5) | 5/46 (10.8) | <10−6 | 21.6 [6.9–71,9] |
| Rash | 70/80 (87.5) | 26/60 (43.3) | <10−6 | 9.5 [3.7–23.2] |
| Typical rash | 38/70 (54.3) | 2/26 (7.7) | <3 × 10−5 | 14.2 [2.9–94.7] |
| Atypical rash | 32/70 (45.7) | 24/26 (92.3) | 3 × 10−5 | 0.1 [0.01–0.34] |
| Macular rash | 59/70 (84.3) | 3/26 (11.5) | <10−6 | 41.12 [9.3–210.3] |
| Transient rash | 64/70 (91.4) | 8/26 (30.7) | <10−6 | 24 [6.5–95.5] |
| Sore throat | 66/80 (82.5) | 10/60 (16.6) | <10−6 | 23.6 [8.9–64.3] |
| Arthralgia | 75/80 (93.8) | 53/60 (88.3) | .2 | 1.9 [0.5–7.7] |
| Myalgia | 55/80 (68.8) | 27/60 (45) | .004 | 2.7 [1.3–5.7] |
| Arthritis | 54/80 (67.5) | 15/60 (25) | 10−5 | 6.2 [2.8–14.2] |
| N° pain joints | 11.6 ± 8.7 | 5.62 ± 4.5 | 10−5 | – |
| N° swollen joints | 4.9 ± 4.2 | 3.3 ± 2.5 | .1 | – |
| Liver dysfunction | 57/80 (71.2) | 27/60 (45) | .001 | 3 [1.4–6.5] |
| Lymphadenopathy | 16/80 (20) | 6/60 (10) | .1 | 2.5 [0.8–6.9] |
| Splenomegaly | 11/80 (13.8) | 2/60 (3.3) | .03 | 4.6 [0.9–31.5] |
| Pleuritis | 10/80 (12.5) | 3/60 (05) | .1 | 2.7 [0.6–13.1] |
| Pericarditis | 14/80 (17.5) | 5/60 (8.3) | .1 | 2.3 [0.7–7.9] |
The DNI and serum Beta-2 microglobulin were not discriminative for AOSD (P = .8, P = .4). The GF was unmeasurable in 41 controls who had a normal or low serum ferritin. A low GF was more frequent in AOSD patients than controls, particularly when it was <25% (GF ≤ 20%: OR: 25.6, GF ≤ 25% OR: 58.8) (Table 2).
Table 2.
Univariate analysis of the main laboratory findings for AOSD patients and controls.
| Variables | AOSD patients, n = 80 % | Controls, n = 60 % | P | Odds ratio [CI 95%] |
|---|---|---|---|---|
| Leukocytosis | 67/80 (83.7) | 20/60 (33.3) | <10−6 | 10.3 [4.3–25] |
| Neutrophils ≥ 75% | 68/80 (85) | 16/60 (26.6) | <10−6 | 15.6 [6.3–39.8] |
| Neutrophils ≥ 80% | 51/80 (63.7) | 10/60 (16.6) | <10−6 | 8.8 [3.6–21.8] |
| Mean neutrophils % | 80 ± 7.6 | 67.56 ± 11.61 | <10−6 | – |
| Lymphopenia % | 15 (18.7) | 16 (26.7) | .3 | |
| Mean NLR | 10 ± 10.24 | 4.48 ± 4.55 | 10−4 | – |
| DNI > 2% | 3/80 (3.75) | 1/60 (1.7) | .8 | – |
| Mean hemoglobin, g/dL | 9.6 ± 1.7 | 10.96 ± 2 | 4 × 10−4 | – |
| Mean ESR rate, mm | 106 ± 22 | 74.1 ± 42.9 | <10−6 | – |
| Mean CRP, mg/L | 136.8 ± 87.57 | 86.68 ± 87.47 | .001 | – |
| Beta-2 microglobulin (> N) | 6/22 (27.2) | 2/16 (12.5) | .4 | 2.6 [0.4–22.6] |
| Mean beta-2 microglobulin | 2.04 ± 0.99 | 1.56 ± 0.72 | .1 | – |
| Serum ferritin > N | 70/80 (87.5) | 19/60 (31.7) | <10−6 | 15.11 [5.9–39.4] |
| Serum ferritin ≥ 5 N | 59/80 (73.7) | 4/60 (7.8) | <10−6 | 39.3 [11.7–146] |
| 25.9 [2.6–630.9] | ||||
| Glycosylated ferritin ≤ 25% | 42/47 (89.2) | 1/08 (12.5) | <10−6 | 58.8 [5.1–1585] |
| Negative ANA | 72/80 (90) | 46/60 (76.7) | .03 | 2.7 [0.97–8.1] |
| Negative anti-CCP | 80/80 (100) | 54/60 (90) | .01 | – |
| Negative FR | 80/80 (100) | 54/60 (90) | .01 | – |
3.4. The NLR: cutoff value, sensitivity, and specificity
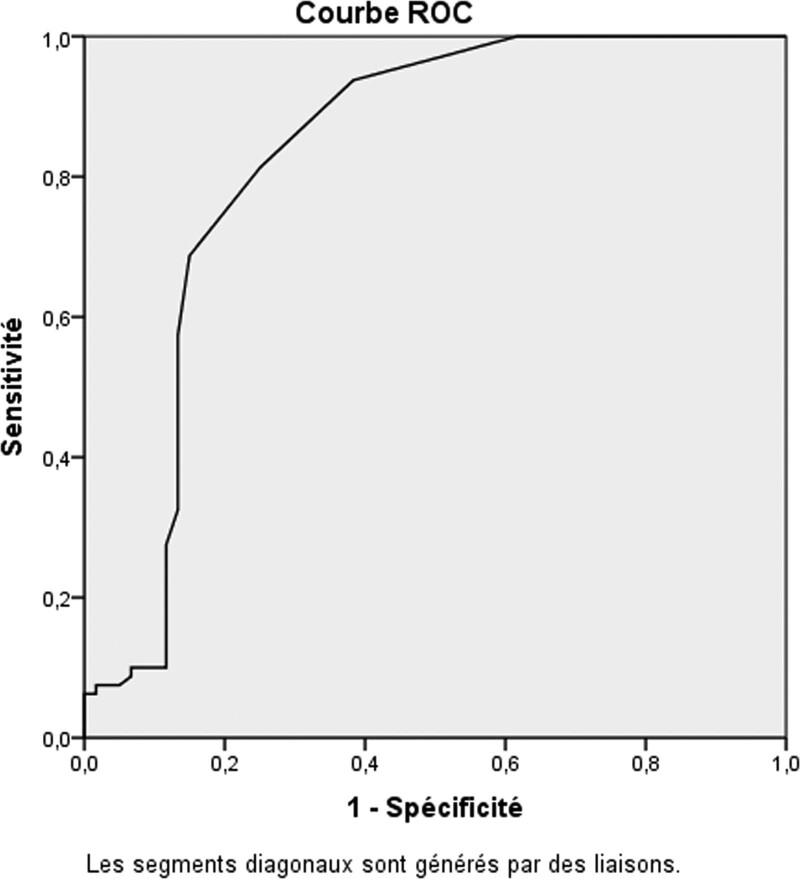
NLR was significantly associated to AOSD compared to controls respectively (mean NLR: 10 ± 10.24, 4.48 ± 4.55, P = 10−4). The optimal cutoff value that best distinguished AOSD from controls was determined at the maximum value which was estimated by sensitivity + 1- specificity in the ROC curve. Among the NLR thresholds (values), the cutoff value of 4, showed the greatest sensitivity (93.8%, [95% CI: 85.4–97.7]), specificity (61.7%, [95% CI: 48.2–73.6]), and AUC value 0.82 [95% CI: 0.74–0.90] as a diagnostic biomarker for AOSD (Fig. 1).
Figure 1.
Receiving operating characteristic curve for the neutrophil-to-lymphocyte ratio in adult onset Still disease patients and controls. Area under the curve for the neutrophil-to-lymphocyte ratio was 0.82 [95% CI: 0.74–0.90] with a cutoff value of 4 (normal range: 0.78 to 3.53). CI = confidence interval.
NLR ≥ 4 was more frequent in AOSD patients compared to controls (93.7%, 38.3%, P < 10–6, OR: 24.1 [95% CI: 7.8–79.7]). Furthermore, NLR was high in 24 AOSD patients despite a neutrophil percentage <80%.
The clinical complication that can affect NLR value was RHL. Nevertheless, the 2 groups were matched for this complication and the percentage of RHL was low (6 patients, 7.5%) in the AOSD group, which can not affect the AUC or ROC curve. Moreover, NLR remained high in patients with RHL because of the low rate of lymphocytes.
3.5. Multivariate analysis
Six variables were independently associated to AOSD: typical rash (P = .007), fever ≥ 39°C (P = .003), pharyngitis (P = .002), arthritis (P = .003), NLR (NLR ≥ 4) (P = .002), and GF ≤ 20 % (P = .019) (Table 3).
Table 3.
Multivariate analysis with multiple logistic regression.
| Criteria | Adjusted odds ratio | Confidence interval 95% | P |
|---|---|---|---|
| Typical rash | 24.01 | 2.35–245.30 | .007 |
| Fever ≥ 39c° | 17.34 | 2.6–113.37 | .003 |
| NLR ≥ 4 | 11.10 | 2.35–52.3 | .002 |
| Pharyngitis | 10.23 | 2.36–44.34 | .002 |
| Arthritis | 9.01 | 2.07–39.142 | .003 |
| Glycosylated ferritin ≤ 20% | 1.59 | 1.08–2.35 | .019 |
3.6. Discriminative performance of Yamaguchi set, Fautrel set, and composite sets (Yamaguchi + NLR, Fautrel + NLR)
Some major criteria were modified which affected the sensitivity of the classifications. Atypical rash was present in 32, neutrophil percentage was <80% in 29 and GF was higher than 20% in 10. Hence, 17 AOSD diagnoses were missed by Yamaguchi set and 19 AOSD diagnoses were missed by Fautrel set. Nevertheless, NLR was high (NLR ≥ 4) in 24 AOSD patients despite a neutrophil percentage <80%. Thus, the addition of NLR as a major criterion to these classifications reclassified as AOSD 9 missed AOSD patients by Yamaguchi criteria and 13 missed AOSD patients by Fautrel criteria while none of controls were reclassified as AOSD.
The association of NLR as a major criterion to Fautrel classification and Yamaguchi classification improved significantly their sensitivity respectively for Fautrel (76.3% to 92.5%, P = .004) and Yamaguchi (78.8% to 90%, P = .05). However, the specificity of Fautrel and Yamaguchi classifications was high respectively 96.7% and 100%.
These composite sets improved also the accuracy of the classifications, respectively, for Fautrel classification (85% to 94.3%) and Yamaguchi classification (87.8% to 94.3%). Two patients in Fautrel classification were falsely classified as AOSD (1 HIV, 1 meningitis), while the application of the exclusion criteria in Yamaguchi classification implicated that none of controls was classified as AOSD (Table 4).
Table 4.
Evaluation of AOSD classifications criteria.
| Classification criteria | AOSD, n = 80 | Controls, n = 60 | Sensitivity % | Specificity % | PPV % | NPP % | Accuracy % |
|---|---|---|---|---|---|---|---|
| Yamaguchi | 63 | 0 | 78.8 [67.9–86.8] | 100 [92.5–100] | 100 [92.8–100] | 77.9 [66.7–86.3] | 87.8 |
| Yamaguchi and NLR ≥ 4 | 72 | 0 | 90 [80.7–95.3] | 100 [92.5–100] | 100 [93.7–100] | 88.2 [77.6–94.4] | 94.3 |
| Fautrel | 61 | 2 | 76.3 [65.2–84.8] | 96.7 [87.5–99.4] | 96.8 [88–99.4] | 75.3 [64–84.1] | 85 |
| Fautrel and NLR ≥ 4 | 74 | 2 | 92.5 | 96.7 | 97.3 | 90.6 | 94.3 |
| [83.8–96.9] | [87.5–99.4] | [90–99.5] | [80.1–96.1] |
4. Discussion
This prospective study validates the NLR (NLR ≥ 4) as a good diagnostic biomarker of AOSD with high sensitivity (93.8%). This is the first study to investigate the effect of NLR on the sensitivity and the specificity of the classifications criteria. The addition of NLR to the classifications (Yamaguchi and Fautrel classifications) as a major criterion improved significantly their sensitivity with maintaining high specificity.
The NLR has been recently reported as a new biomarker of AOSD more sensitive than neutrophils count, with a cutoff value of 3.08 to differentiate AOSD from controls.[13] Nevertheless, this cutoff value was near from the normal range, recently estimated from 0.78 to 3.53 with a mean of 1.65 ± 1.96.[16] Hence, the cutoff value of NLR was reassessed in this study.
Crispin et al published an interesting study reporting the mean neutrophils and lymphocytes counts in AOSD patients.[17] Thus, mean NLR in this study equals 10.9, which is frankly high.
NLR is a simple inexpensive biomarker, routinely measured in blood count. It reflects systemic inflammation with intervention of the innate immune system (neutrophil count) and adaptive immunity (lymphocyte count). NLR was evaluated in several diseases and was associated with poor prognosis in infectious diseases, cardiac events and cancer.[14]
In this cohort, NLR was high (NLR ≥ 4) in 24 AOSD patients despite a neutrophil percentage <80 %. The addition of NLR as a major criterion to Yamaguchi and Fautrel criteria reclassified as AOSD 9 missed AOSD patients by Yamaguchi criteria and 13 missed AOSD patients by Fautrel criteria while none of controls were reclassified as AOSD. Furthermore, the 2 classifications (Fautrel and Yamaguchi) had a high specificity, which was helpful to rule out confusable diseases.
Some studies reported also modifications in major criteria such as neutrophil percentage <80% in 30% to 40% of cases and GF higher than 20% in 30% to 40% of cases.[6,10,18] Moreover, atypical rash was often reported in recent studies.[19–21]
Lebrun et al reported that GF (≤20%) showed a good specificity and improved the sensitivity of Yamaguchi criteria thanks to a composite set (98.2%).[6] Several studies including our showed that ferritin ≥ 5-fold upper the limit of the normal and GF ≤ 20% had a good diagnostic value.[6,10] In our study, GF with a cutoff value of ≤ 25% (89.2%) was more frequent than the cutoff value of ≤ 20% (78.7%) and may be considered for AOSD diagnosis.
The low level of GF is due to the deficiency in glycosylation process of the ferritin that cannot follow a high ferritin production.[22] However, GF was not available in most centers and can be low in other diseases such as RHL.[23–25] In addition, GF rate raised in remission period.[26] We confirmed these findings in our study.
Delta neutrophil index and Beta-2 microglobulin were not specific for AOSD and did not discriminate AOSD patients from controls in our cohort.
The most specific clinical manifestation of AOSD is erythematous or pink macular, transient and recurrent rash concomitant with the fever. Moreover, fever and arthralgia are the most frequent manifestations. In several studies including our, the association “rash, fever and arthralgia” was present in <80% of patients.[2,9,15] The severity of AOSD can be attributed to its life-threatening complications with organ damage, particularly, RHL, coagulation disorder, myocarditis, heart failure, tomponade, pulmonary arterial hypertension, acute respiratory distress syndrome, pancreatitis, and fulminant Hepatitis.[7,8] In our cohort, the complications occurred in 11 patients, particularly 6 RHL.
Despite a better knowledge of the clinical picture of AOSD, the diagnostic delay remains long, especially in atypical presentations. The classifications criteria are used to classify patients in clinical research into homogeneous groups.[27–31] Their use is also based on the experience of the physician who should rule out confusable diseases.[6,9,17,32,33]
Thus, the diagnosis can be difficult in early stage of the disease and needs exclusion of infections, malignancies and other immune-mediated inflammatory diseases. In the present study, NLR improved the diagnostic approach and the accuracy of Fautrel and Yamaguchi classifications respectively (94.3%, 100%).
Yamaguchi classification was the most sensitive (96.2%) and the most used in clinical research, but the exclusion procedure was costly, needed time and missed AOSD diagnoses through the requirement of negative antinuclear antibodies and other serologies.[6,17,27]
Therefore, in typical picture of the disease, particularly: typical rash, high spiking fever, arthralgia and pharyngitis lasting 2 weeks or longer with a high ferritin, low GF, high CRP, high neutrophil count and high NLR, the diagnosis of AOSD should be considered to allow early healthcare.[1,10,13,15,29]
The main limit of our study was the number of controls, we wished to include 1 AOSD case for 1 control. However, 20 patients were excluded for insufficient data and follow-up. Moreover, NLR can be high in other diseases, particularly infectious diseases and needs to be interpreted carefully.
This prospective study validates the NLR as a good diagnostic biomarker of AOSD with a cutoff value of 4 and high sensitivity (93.8%). An update of the classification criteria for AOSD may be proposed including the NLR and the GF to improve the diagnostic approach and the clinical research in this rare systemic disease.
5. Key messages
NLR (≥ 4) is a good simple biomarker of AOSD with a high sensitivity and may replace the neutrophil percentage (≥80%) in the classifications criteria (Yamaguchi and Fautrel classifications).[9,10,13]
Although, NLR is not very specific, its addition as a major criterion to Yamaguchi and Fautrel classifications improved significantly their sensitivity and their accuracy with maintaining high specificity for these composite sets[9,10,13]
This study confirms the performance of Yamaguchi and Fautrel classifications and the GF (≤20%) in the diagnosis of AOSD.[6,9,10]
Acknowledgments
We thank all physicians who collaborate in the realization of this study. We thank also Mrs Kasdi Faiza for laboratory analysis and Mrs Sara Daghor for computer assistance.
Author contributions
Karima Daghor Abbaci : wrote the paper, collected the data, concieved and designed the study
Nadia Ait Hamadouche : performed the statistical analysis
Fifi Otmani : revised critically the article
Chafia Dahou Makhloufi : revised critically the article
Farida Mechid: contributed to the data collection
Mohamed Makrelouf : revised critically the article
Amel Otmane : performed the laboratory analysis
Nourredine Smail : performed the statistical analysis
Malika Boucelma : contributed to the data collection
Fatma Zohra Aissat: contributed to the data collection
Salima Lefkir-Teffiani: contributed to the data collection
Bilel Bengana: contributed to the data collection
Nadia Boukheris: contributed to the data collection
Amar Tebaibia: contributed to the data collection
Baya Taharbouchet: contributed to the data collection
Soraya Ayoub: contributed to the data collection
Brahim Benziane: contributed to the data collection
Nadia Oumnia: contributed to the data collection
Chafika Haouichet: contributed to the data collection
Fella Hanni: contributed to the data collection
Nazim Laraba: contributed to the data collection
Djennete Hakem : contributed to the data collection
Nacera Benfenatki : revised critically the article
Abdelkrim Berrah : concieved and designed the analysis, revised critically the article
Abbreviations:
- ANA =
- antinuclear antibodies
- Anti-CCP =
- anticyclic citrullinated peptide
- AOSD =
- adult onset Still disease
- AUC =
- area under the curve
- CI =
- confidence interval
- CRP =
- C-reactive protein
- DNI =
- delta neutrophil index
- ER =
- erythrocyte sedimentation rate
- GF =
- glycosylated ferritin
- HIV =
- human immunodeficiency virus
- NLR =
- neutrophil-to-lymphocyte ratio
- NPV =
- negative predictive value
- OR =
- odds ratio
- PPV =
- positive predictive value
- RHL =
- reactive hemophagocytic lympho-histiocytosis
- RF =
- rheumatoid factor
- ROC =
- receiver operating characteristic
How to cite this article: Daghor Abbaci K, Hamadouche NA, Otmani F, Makhloufi CD, Mechid F, Makrelouf M, Otmane A, Smail N, Boucelma M, Aissat FZ, Lefkir-Teffiani S, Bengana B, Boukheris N, Tebaibia A, Taharbouchet B, Ayoub S, Benziane B, Oumnia N, Haouichet C, Hanni F, Laraba N, Hakem D, Benfenatki N, Berrah A. Validation of the neutrophil-to-lymphocyte ratio as a new simple biomarker of adult onset Still disease: A STROBE-Compliant prospective observational study. Medicine 2022;101:32(e29970).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AOSD = adult onset Still disease, CI = confidence interval.
ANA = antinuclear antibodies, Anti-CCP = anticyclic citrullinated peptide, AOSD = adult onset Still disease, CI = confidence interval, CRP = C-reactive protein, DNI = delta neutrophil index, ERS = erythrocyte sedimentation rate, NLR = neutrophil-to-lymphocyte ratio, RF = rheumatoid factor.
NLR = neutrophil-to-lymphocyte ratio.
AOSD = adult onset Still disease, NLR = neutrophil-to-lymphocyte ratio, NPV = negative predictive value, PPV = positive predictive value.
Contributor Information
Nadia Ait Hamadouche, Email: oumnianadia2005@yahoo.fr.
Fifi Otmani, Email: otmanififidz@yahoo.fr.
Chafia Dahou Makhloufi, Email: dahou-makhloufi@hotmail.com.
Farida Mechid, Email: fairouza33@gmail.com.
Mohamed Makrelouf, Email: mmakrelouf@gmail.com.
Amel Otmane, Email: bioamel03_dz@yahoo.fr.
Nourredine Smail, Email: smail_nourredine@yahoo.fr.
Malika Boucelma, Email: mboucelma@yahoo.fr.
Fatma Zohra Aissat, Email: aissatfzl@yahoo.fr.
Salima Lefkir-Teffiani, Email: s.lefkir-tafiani@sarhumatologie-dz.org.
Bilel Bengana, Email: newbilal@live.fr.
Nadia Boukheris, Email: oumnianadia2005@yahoo.fr.
Amar Tebaibia, Email: tebaib@hotmail.com.
Baya Taharbouchet, Email: taharbouchet_b@hotmail.fr.
Soraya Ayoub, Email: ayoubsorayadz@gmail.com.
Brahim Benziane, Email: drbrabenz@gmail.com.
Nadia Oumnia, Email: oumnianadia2005@yahoo.fr.
Chafika Haouichet, Email: chaouichat@yahoo.fr.
Fella Hanni, Email: hannifella@hotmail.com.
Nazim Laraba, Email: nazilaraba@gmail.com.
Djennete Hakem, Email: hakemdj@yahoo.fr.
Nacera Benfenatki, Email: eternalhind@hotmail.com.
Abdelkrim Berrah, Email: berrahbeo@yahoo.fr.
References
- [1].Mitrovic S, Fautrel B. New markers for adult-onset Still’s disease. Joint Bone Spine. 2018;85:285–93. [DOI] [PubMed] [Google Scholar]
- [2].Pouchot J, Sampalis JS, Beaudet F, et al. Adult Still’s disease: manifestations, disease course, and outcome in 62 patients. Medicine (Baltim). 1991;70:118–36. [PubMed] [Google Scholar]
- [3].Maria T, Alain Le Q, Christian J, et al. Adult onset Still’s disease (AOSD) in the era of biologic therapies: dichotomous view for cytokine and clinical expressions. Autoimmun Rev. 2014;13:1149–59. [DOI] [PubMed] [Google Scholar]
- [4].Criado PR, Carvalho JF, Ayabe LA, et al. Urticaria and dermographism in patients with adult-onset Still’s disease. Rheumatol Int. 2012;32:2551–5. [DOI] [PubMed] [Google Scholar]
- [5].Lee J JY, Hsu CK, Liu MF, et al. Evanescent and persistent pruritic eruptions of adult-onset Still disease: a clinical and pathologic study of 36 patients. Semin Arthritis Rheum. 2012;42:317–26. [DOI] [PubMed] [Google Scholar]
- [6].Lebrun D, Mestrallet S, Dehoux M, et al. Validation of the Fautrel classification criteria for adult-onset Still’s disease. Semin Arthritis Rheum. 2017;47:578–85. [DOI] [PubMed] [Google Scholar]
- [7].Ruscitti P, Cipriani P, Masedu F, et al. Adult onset Still’s disease: evaluation of prognostic tools and validation of the systemic score by analysis of 100 cases from three center. Medicine. 2016;14:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Néel A, Wahbi A, Tessoulin B, et al. Diagnostic and management of life-threatening adult-onset still disease: a French nationwide multicenter study and systematic literature review. Critical Care. 2018;22:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yamaguchi M, Ohta A, Tsunematsu T, et al. Preliminary criteria for classification of adult Still’s disease. J Rheumatol. 1992;19:424–30. [PubMed] [Google Scholar]
- [10].Fautrel B, Zing E, Golmard JL, et al. Proposal for a newset of classification criteria for adult-onset Still disease. Med (Baltimore). 2002;81:194–200. [DOI] [PubMed] [Google Scholar]
- [11].Park HJ, Ha YJ, Pyo JY, et al. Delta neutrophil index as an early marker for differential diagnosis of adult-onset Still’s disease and sepsis. Yonsei Med J. 2014;55:753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wakabayashi K, Inokuma S, Matsubara E, et al. Serum β2-microglobulin level is a useful indicator of disease activity and hemophagocytic syndrome complication in systemic lupus erythematosus and adult-onset Still’s disease. Clin Rheumatol. 2013;32:999–1005. [DOI] [PubMed] [Google Scholar]
- [13].Seo JY, Suh CH, Jung JY, et al. The Neutrophil to lymphocyte ratio could be a good diagnostic marquer and predictor of relapses in adult onset Still’s disease. Medicine. 2017;96:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Song M, Graubard BI, Rabkin CS, et al. Neutrophil-to-lymphocyte ratio and mortality in United States general population. Nat Res. 2021;11:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gerfaud-Valentin M, Jamilloux I, Iwaz J, et al. Adult onset Still’s disease. Autoimmun Rev. 2014;13:708–22. [DOI] [PubMed] [Google Scholar]
- [16].Forget P, Khalifa C, Defour JP, et al. What is the normal value of the neutrophil-to-lymphocyte ratio. Bmc Res Notes. 2017;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Crispin J, Martinez-Banos D, Alcocer-Varela J. Adult-onset Still disease as the cause of fever of unknown origin. Medicine. 2005;84:331–7. [DOI] [PubMed] [Google Scholar]
- [18].Chen P-D, Yu S-L, Chen S, et al. Retrospective study of 61 patients with adult onset Still’s disease admitted with fever of unknown origin in China. Clin Rheumatol. 2012;31:175–81. [DOI] [PubMed] [Google Scholar]
- [19].Nagai Y, Hasegawa M, Okada E, et al. Clinical follow-up study of adult-onset Still’s disease. J Dermatol. 2012;39:898–901. [DOI] [PubMed] [Google Scholar]
- [20].Kikuchi N, Satoh M, Ohtsuka M, et al. Persistent pruritic papules and plaques associated with adult onset Still’s disease: report of six cases. J Dermatol. 2014;41:407–10. [DOI] [PubMed] [Google Scholar]
- [21].Larson AR, Laga AC, Granter SR. The spectrum of histopathologic fundings in cutameous lesions in patients with Still’s disease. Am J Clin Pathol. 2015;144:945–51. [DOI] [PubMed] [Google Scholar]
- [22].Feist E, Mitrovic S, Fautrel B. Mechanisms, biomarkers and targets for adult onset Still’s disease. Nat Rev Rheumatol. 2018;14:603–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Madeeha M, Fiore CT, Wilder E. Atypical cutaneous and histological presentation of adult-onset Still’s disease. PROC. 2019;32:422–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mitrovic S, Feist E, Fautrel B. Adult-onset still’s disease. Cimaz R, ed. In: Periodic and Non-Periodic Fevers. Rare Diseases of the Immune System (book series). Cham: Springer; 2020. [Google Scholar]
- [25].Hot A, Toh ML, Coppéré B, et al. Reactive hemophagocytic syndrome in adult-onset Still disease: clinical features and long-term outcome: a case-control study of 8 patients. Medicine (Baltim). 2010;89:37–46. [DOI] [PubMed] [Google Scholar]
- [26].Gerfaud-Valentin M, Maucort Boulch D, Hot A, et al. Adult onset Still’s disease, manifestations, treatment, outcome, prognostic factor in 57 patients. Medicine. 2014;93:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gabay C, Fautrel B, Rech B, et al. Open-label, multicentre, dose-escalating phase II clinical trial on the safety and efficacy of tadekinig alfa (IL-18BP) in adult-onset Still’s disease. Ann Rheum Dis. 2018;77:840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Colina M, Zucchini W, Ciancio G, et al. The evolution of adult-onset Still’s disease: an observational and comparative study in a cohort of 76 Italian patients. Semin Arthritis Rheum. 2011;41:279–85. [DOI] [PubMed] [Google Scholar]
- [29].Kong X-D, Xu D, Zhang W, et al. Clinical features and prognosis in adult-onset Still’s disease: a study of 104 cases. Clin Rheumatol. 2010;29:1015–9. [DOI] [PubMed] [Google Scholar]
- [30].Evensen KJ, Nossent HC. Epidemiological and outcomes of adult-onset Still’s disease in Northern Norway. Scand J Rheumatol. 2006;35:48–51. [DOI] [PubMed] [Google Scholar]
- [31].Asanuma YF, Mimura T, Tsuboi H, et al. Nationwide epidemiological survey of 169 patients with adult Still’s disease in Japan. Mod Rheumatol. 2015;25:393–400. [DOI] [PubMed] [Google Scholar]
- [32].Vanderschueren S, Hermans F, De Munter P, et al. Adult-onset Still’s disease: still a diagnosis of exclusion. A nested case–control study in patients with fever of unknown origin. Clin Exp Rheumatol. 2012;30:514–9. [PubMed] [Google Scholar]
- [33].Oznur A, Serdar O, Yasemin C, et al. An adult onset Still’s disease mimicking pneumonia. Rheumatol Int. 2012;32:2539–41. [DOI] [PubMed] [Google Scholar]