Abstract
Background:
Diabetes mellitus is a spectrum of metabolic disorders characterized by hyperglycemia and shows a growing global public health problem in the elderly. Resveratrol presents antiaging, anti-inflammatory, antitumor antioxidant, and cardioprotective activities. The purpose of this study was to investigate the ameliorative effects of resveratrol on blood glucose, insulin metabolism, lipid profile, renal function, inflammation, and nutrient sensing systems in the elderly patients with type 2 diabetes mellitus.
Methods:
The study is a single-blind, parallel-group, randomized controlled clinical trial consisting of a 6-month treatment period. A total of 472 elderly patients with type 2 diabetes mellitus were enrolled, and included participants will be randomized into 2 groups: resveratrol (n = 242) and placebo (n = 230). The clinical efficacy and changes in clinical parameters in each group will be measured at the indicated time. Clinical parameters included blood glucose, insulin resistance index, blood lipid index, proinflammatory cytokines, renal function, and nutrient sensing systems.
Results:
Resveratrol treatment greatly improved glucose metabolism, insulin tolerance, and insulin metabolism compared to placebo. Resveratrol relieved symptoms through enhancing nutrient sensing systems, which in turn reduced production and activity of glucose-6-phosphatase. Compared with placebo, resveratrol treatment significantly decreased proinflammatory cytokines glycated hemoglobin/hemoglobin A1c, interleukin-6, tumor necrosis factor-alpha, and interleukin-1beta in the elderly diabetes. Resveratrol treatment decreased blood glucose parameters, improved the lipid profile (total cholesterol, low-density lipoprotein, high-density lipoprotein, and triglycerides), and renal function compared to placebo.
Conclusion:
In conclusion, resveratrol treatment improves inflammation, renal function, blood glucose parameters, inflammation, insulin resistance, and nutrient sensing systems in the elderly patients with type 2 diabetes mellitus, indicating resveratrol may be a potential therapeutic drug for the treatment of the elderly patients with type 2 diabetes mellitus.
Keywords: blood glucose, inflammation, insulin resistance, nutrient sensing systems, renal function, resveratrol, type 2 diabetes mellitus
1. Introduction
Diabetes mellitus is a common complication associated with disorders of glucose and insulin metabolism.[1] Diabetes mellitus is a spectrum of metabolic disorders characterized by hyperglycemia.[2] Currently, there are 382 million people living with diabetes mellitus, and the total number is increasing in the world.[3] Diabetes mellitus is associated with aging-related cardiovascular diseases and kidney diseases due to high glucose levels.[4] The elderly diabetes is a common chronic disease and is usually frequently associated with metabolic disorders, insulin deficiency, and various diabetic complications.[5] The increasing global elderly diabetes has been a global healthy problem due to limited efficacy with existing therapies.[6] It is urgent to discover curative efficient therapies for the prevention and improvement of the elderly diabetes.
Resveratrol, a natural polyphenolic compound, has much therapeutic potential with its antioxidant, antiaging, antitumorigenic, anti-inflammatory, antiapoptotic, cardioprotective, and neuroprotective effects.[7–9] Increasing studies have recently reported the therapeutic role of resveratrol in the treatment of diabetes in various animal models.[10–12] Resveratrol can prevent cognitive deficits by attenuating oxidative damage and inflammation in rat model of diabetes.[13] Resveratrol may be beneficial in treating the diabetes mellitus. A study has showed that resveratrol has antihyperglycemic effects in diabetic patients, which is associated with its improvement of blood glucose parameters, inflammation, and insulin resistance.[14] As resveratrol treatment exerts beneficial effects in humans and may be helpful in preventing and treating metabolic diseases such as obesity and diabetes mellitus, resveratrol has been valued and widely used in the clinical treatment of diabetes mellitus in recent years. However, the therapeutic effect of resveratrol and the important improvement in hepatic insulin signaling and reduced inflammatory response have not been reported on its potential effect on the elderly diabetes patients. Although resveratrol is considered to have potent therapeutic effects in experimental diabetes mellitus animals, few reports study its efficacy on the elderly diabetes patients. In addition, whether resveratrol is safe for the elderly diabetes patients remains unknown.
Therefore, the present study aimed to investigate the therapeutic potential of resveratrol in the elderly diabetes patients. We examined the effects of resveratrol on blood glucose parameters, insulin resistance, nutrient sensing systems, and renal function in the elderly diabetes patients. The study also explored the effects of resveratrol on glucose uptake, inflammation, insulin signaling pathway, and insulin metabolism. We also aim to investigate the metabolism and safety of resveratrol in the elderly diabetes patients.
2. Materials and Methods
2.1. Study design
A randomized parallel placebo-controlled clinical trial among the elderly diabetes patients was designed. A total of 472 elderly patients with type 2 diabetes mellitus were enrolled at the First Hospital of Harbin. Patients were randomly assigned into 2 groups and receive either 500 mg/d of resveratrol (n = 242) (99% pure, Biotivia, Bioceuticals International, SRL, Verona, Italy) or placebo (n = 230) for 6 months. The study was approved by the Ethics Committee (approval number: MDJMU.201905191X1) of the First Hospital of Harbin (Harbin, China).
2.2. Inclusion and exclusion criteria
Participants provided blood samples to ensure that they were the elderly patients with type 2 diabetes mellitus. All patients were more than 60 years old. Patients on insulin treatment were included as they were on stable doses, and the dosage was not changed during the period. Participants with cancer, psychiatric illness, hepatic disease, severe heart disease, long-term chronic renal failure, allergy to resveratrol, a history of significant head injury, and dementing illness were excluded from this study.
2.3. Biochemical assessment
A total of 10-mL blood samples in each patient were collected at months 0, 2, 4, and 6. Serum was collected using centrifugation at 10,000 × g at 4°C for 10 minutes. Serum insulin level was measured according to the manufacturer’s instructions (infinitumbiotech, 1935 Cordell Court, El Cajon, CA). Commercial kits were used to measure fasting serum triglycerides, high-density lipoprotein, low-density lipoprotein, and total cholesterol concentrations (Pars Azmun, Tehran, Iran). Serum insulin values were assessed using an enzyme-linked immunosorbent assay kit (Monobind, CA) according to Manufacturer’s instrument. Serum was prepared, and levels of tumor necrosis factor-alpha (TNF-α; DTA00D, R&D System), interleukin-1beta (IL-1β; DLB50, R&D System), and interleukin-6 (IL-6; D6050, R&D System, Emeryville, CA) were analyzed in the elderly patients with diabetes patients using enzyme-linked immunosorbent assay kits according to the manufacturer’s instrument. Activities of serum glutamic pyruvic transaminase, glutamic oxaloacetic transaminase, and alkaline phosphatase were determined by using a commercial kit according to the manufacturer’s instructions (Pars Azmun Inc., Tehran, Iran). Insulin sensitivity was measured by using the metabolic clearance rate of glucose as described previously. The glucose-6-phosphatase activity in serum was determined previously.[15] The AMP-activated kinase (AMPK) activity was measured using AMPK Phosphorylation Assay Kit as reported previously.[16] The activity of sirtuins (SIRTs) was accessed using SIRT Activity Kit (No. P-4037, Amyjet Scientific Co., Ltd, Wuhan, China) according to the manufacturer’s instrument. The activity of mammalian/mechanistic target of rapamycin (mTOR) was determined by mTOR Kinase Activity Assay Kit (No.CBA055, Merck-Calbiochem, Darmstadt, Germany) according to the manufacturer’s instrument.
2.4. Statistical analyses
Data are expressed as mean ± standard deviation or n (%). All analyses were analyzed using predictive analytics software Statistics GradPack 18 (SPSS Inc., Chicago, IL) and GraphPad Prism software, version 8.0.2 (GraphPad Software, Inc., San Diego, CA). Data were compared among the two groups using the Kruskal-Wallis test or chi-square test. The paired t-test was used for assessing the side effects in each group at months 0, 2, 4, and 6. A P value of <.05 was accepted as statistically significant.
3. Results
3.1. Characteristics of the elderly patients with type 2 diabetes mellitus
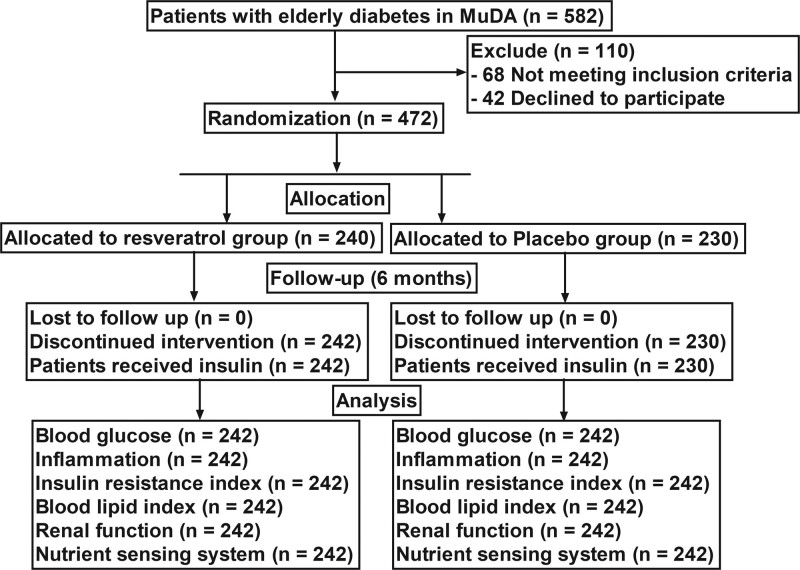
A total of 472 elderly patients (248 males and 224 females) with type 2 diabetes mellitus were enrolled, randomly assigned into 2 groups and received treatment with resveratrol (n = 242) and placebo (n = 230). The mean age (mean ± standard deviation) in resveratrol and placebo groups was 65.2 ± 4.2 and 66.0 ± 5.0 years, respectively. The consort diagram of the study is shown in Figure 1. All patients in 2 groups only received insulin (insulin detemir) at least 2 times per day during the investigation. All patients did not consume other agents. No statistically significant differences were observed in the baseline characteristic of the elderly diabetes patients with type 2 diabetes mellitus at the baseline (Table 1).
Figure 1.
Flow diagram of the study. MuDA = the First Hospital of Harbin.
Table 1.
General characteristics according to group.
| Resveratrol | Placebo | P value | |
|---|---|---|---|
| Age (yr) | 65.2 ± 4.2 | 66.0 ± 5.0 | .88 |
| Number, n (%) | 230 (48.7) | 242 (51.3) | .90 |
| Males, n (%) | 128 (51.6) | 120 (48.4) | .85 |
| Females, n (%) | 110 (49.1) | 114 (50.9) | .96 |
| BMI (kg/m2) | 23.80 ± 3.85 | 24.08 ± 4.13 | .94 |
| Systolic blood pressure (mm Hg) | 138.2 ± 12.4 | 136.7 ± 14.2 | .97 |
| Diastolic blood pressure (mm Hg) | 84.5 ± 8.6 | 85.2 ± 9.2 | .93 |
| Hypertension, n (%) | 15 (6.5) | 13 (5.4) | .88 |
| HbA1c (%) | 9.40 ± 1.12 | 9.32 ± 1.05 | .96 |
| eGFR (mL/min/1.73 m2) | 78.2 ± 21.6 | 78.2 ± 21.6 | .80 |
| Total cholesterol (mg/dL) | 162.6 ± 36.2 | 157.0 ± 34.1 | .89 |
| LDL cholesterol (mg/dL) | 118.6 ± 14.25 | 116.8 ± 12.38 | .82 |
| TG (mg/dL) | 158.5 ± 13.48 | 162.3 ± 15.42 | .76 |
| HDL (mg/dL) | 44.35 ± 7.63 | 45.80 ± 8.08 | .95 |
| HOMA-IR | 14.08 ± 4.82 | 13.98 ± 4.54 | .87 |
| Fasting insulin (µU/mL) | 15.14 ± 4.06 | 14.98 ± 3.89 | .90 |
| Insulin AUC0-180 | 330.6 ± 34.52 | 335.8 ± 38.98 | .91 |
| Insulin secretion (pmol/kg/min) | 8.26 ± 2.54 | 8.34 ± 3.26 | .92 |
| hs-CRP (mg/dL) | 5.96 ± 1.56 | 5.84 ± 1.46 | .90 |
| SGOT (IU/L) | 19.67 ± 5.24 | 20.12 ± 6.05 | .86 |
| SGPT (IU/L) | 18.06 ± 5.11 | 17.88 ± 4.76 | .75 |
| Albumin (g/dL) | 5.10 ± 0.60 | 5.32 ± 0.70 | .93 |
| ALP (IU/L) | 432.42 ± 126.65 | 445.35 ± 148.62 | .82 |
| BUN (mg/dL) | 30.52 ± 7.86 | 29.30 ± 7.23 | .86 |
| Cr (mg/dL) | 0.75 ± 0.22 | 0.73 ± 0.32 | .98 |
| Insulin type | Insulin detemir | Insulin detemir | - |
3.2. Effect of resveratrol on proinflammatory cytokines in the elderly patients with type 2 diabetes mellitus
There was no statistically significant decrease in levels of glycated hemoglobin/hemoglobin A1c, C-reactive protein, IL-6, TNF-α, and IL-1β at the baseline between two groups. After 6-month treatment, resveratrol treatment significantly decreased levels of glycated hemoglobin/hemoglobin A1c, C-reactive protein, IL-6, TNF-α, and IL-1β compared to placebo for the elderly patients with type 2 diabetes mellitus. There were significant differences in the levels of homeostasis model assessment of insulin resistance and homeostasis model assessment-beta between 2 groups during the study (Table 2).
Table 2.
Effect of resveratrol on proinflammatory cytokines in the elderly patients with type 2 diabetes mellitus.
| Resveratrol | Placebo | P value | |
|---|---|---|---|
| HbA1c | 7.13 ± 1.23 | 8.46 ± 1.45 | .034 |
| hs-CRP (mg/dL) | 4.22 ± 0.85 | 5.87 ± 1.05 | .038 |
| IL-6 (pg/mL) | 2.38 ± 0.70 | 1.78 ± 0.58 | .043 |
| TNF-α | 8.80 ± 4.40 | 17.96 ± 7.50 | .003 |
| IL-1β | 6.54 ± 2.68 | 14.42 ± 4.18 | .002 |
| HOMA-IR | 5.54 ± 2.66 | 13.20 ± 14.51 | .005 |
| HOMA-β | 52.38 ± 7.87 | 73.6 ± 10.2 | .004 |
3.3. Effect of resveratrol on blood glucose parameters and insulin resistance in the elderly patients with type 2 diabetes mellitus
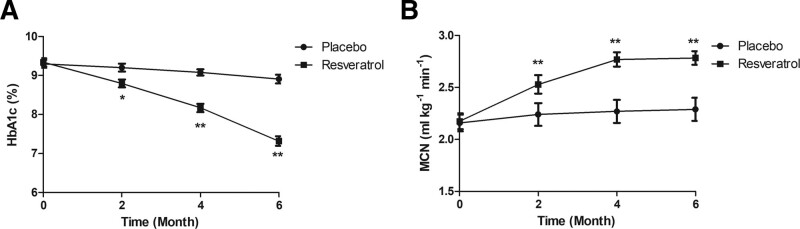
Blood glucose parameters and insulin resistance were compared between 2 groups. There were no significant differences in blood glucose parameters and insulin resistance observed between 2 groups at the baseline. As shown in Table 3, resveratrol treatment decreased fasting plasma glucose, 3-hour glucose area under the curve compared to placebo. As shown in Figure 2A, the glycosylated hemoglobin values were improved by resveratrol for the elderly diabetes patients compared to placebo. Resveratrol treatment also improves insulin resistance determined by metabolic clearance rate of glucose for the elderly diabetes patients compared to placebo (Fig. 2B).
Table 3.
Effect of resveratrol on blood glucose parameters and insulin resistance in the elderly patients with type 2 diabetes mellitus.
| Resveratrol | Placebo | P value | |
|---|---|---|---|
| Fasting plasma glucose (mg/dL) | 114.0 ± 10.2 | 101.2 ± 12.2 | .023 |
| Fasting blood sugar (mg/dL) | 173.6 ± 16.5 | 159.8 ± 13.8 | .036 |
| Glucose AUC0-180 | 505.7 ± 89.5 | 478.6 ± 75.0 | .048 |
| Fasting insulin (µU/mL) | 17.3 ± 4.2 | 16.4 ± 4.5 | .043 |
| Insulin AUC0-180 | 324.0 ± 28.6 | 364.5 ± 40.2 | .041 |
| Insulin secretion (pmol/kg/min) | 9.6 ± 2.0 | 8.4 ± 1.8 | .021 |
Figure 2.
Insulin sensitivity and glucose metabolism in the elderly patients with type 2 diabetes mellitus. (A) The percent of glycosylated hemoglobin in the elderly diabetes patients between resveratrol and placebo groups. (B) Insulin sensitivity was assessed by a hyperinsulinemic euglycemic clamp. HbA1c = glycated hemoglobin/hemoglobin A1c, MCR = metabolic clearance rate.
3.4. Effect of resveratrol on renal function in the elderly patients with type 2 diabetes mellitus
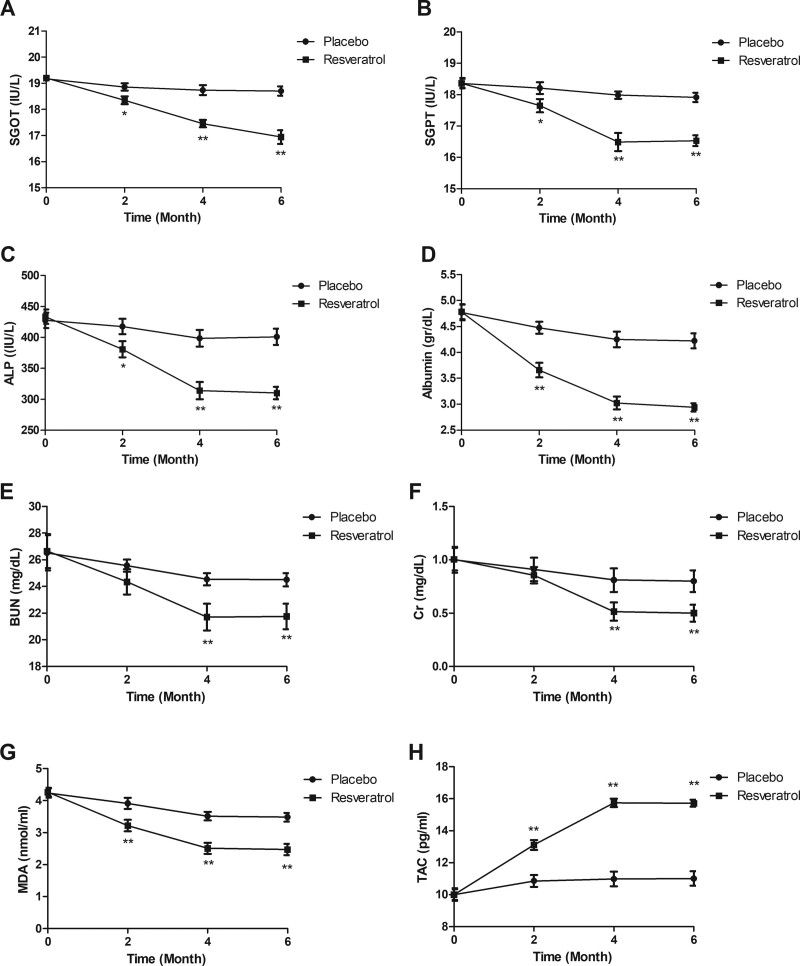
The results of renal function were compared for the elderly diabetes patients at 0, 2, 4 and 6 months between two groups. Renal function tests showed that resveratrol treatment improves levels of, glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase, alkaline phosphatase, Albumin, blood urea nitrogen, and creatinine compared to placebo (Fig. 3A–F). Resveratrol treatment decreased the levels of malondialdehyde and total antioxidant capacity compared to placebo (Fig. 3G and H).
Figure 3.
Effect of resveratrol on renal function in the elderly patients with type 2 diabetes mellitus. Serums levels of SGOT (A), SGPT (B), ALP (C), albumin (D), BUN (E), Cr (F), MDA (G), and TAC (H) in the elderly patients with type 2 diabetes mellitus between resveratrol and placebo groups. ALP = alkaline phosphatase, BUN = blood urea nitrogen, Cr = creatinine, MDA = malondialdehyde, SGOT = glutamic oxaloacetic transaminase, SGPT = serum glutamic pyruvic transaminase, TAC = total antioxidant capacity.
3.5. Effect of resveratrol on blood lipid profile in the elderly patients with type 2 diabetes mellitus
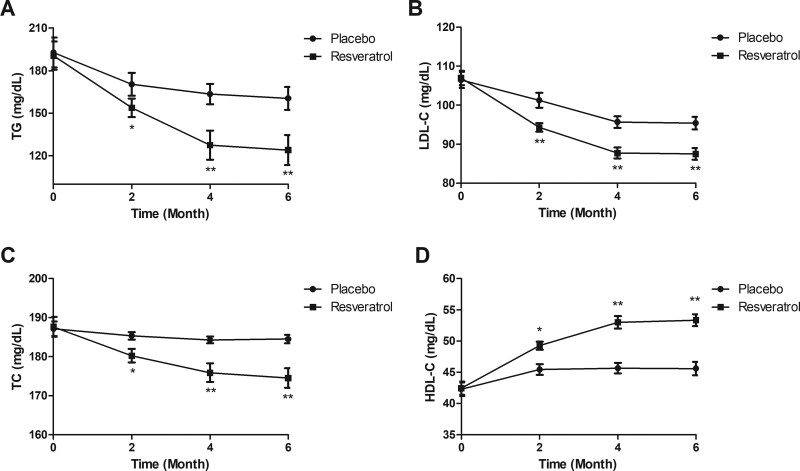
Management of resveratrol resulted in a significant reduction in serum triglycerides, low-density lipoprotein, and total cholesterol compared to placebo group for the elderly diabetes patients (Fig. 4A–C). Levels of high-density lipoprotein were increased by resveratrol compared to placebo group for the elderly diabetes patients (Fig. 4D). Statistical analysis demonstrated that resveratrol improved metabolism of blood lipid (P < .05 at month 2, P < .01 at months 4 and 6).
Figure 4.
Changes of blood lipid metabolism in the elderly patients with type 2 diabetes mellitus. Serum levels TG (A), LDL (B), and total cholesterol (C), and HDL (D) in the elderly patients with type 2 diabetes mellitus received resveratrol or placebo. HDL = high-density lipoprotein, HDL-C = high density lipoprotein-cholesterol, LDL = low-density lipoprotein, LDL-C = low density lipoprotein-cholesterol, TC = total cholesterol, TG = triglycerides.
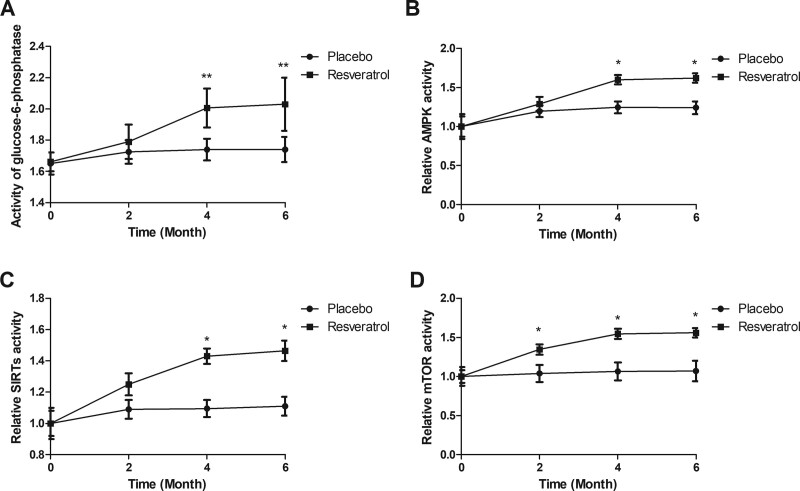
3.6. Effect of resveratrol on nutrient sensing systems in the elderly patients with type 2 diabetes mellitus
Nutrient sensing systems were compared between resveratrol and placebo groups. Resveratrol relieved symptoms of the elderly patients with type 2 diabetes mellitus. Resveratrol reduced production and activity of glucose-6-phosphatase in the elderly patients with type 2 diabetes mellitus compared to placebo (Fig. 5A). Results demonstrated that resveratrol activated nutrient sensing systems including AMPK, SIRTs, and mTOR (Fig. 5B–D).
Figure 5.
Effect of resveratrol on nutrient sensing systems in the elderly patients with type 2 diabetes mellitus. (A) Activity of glucose-6-phosphatase in the elderly patients with type 2 diabetes mellitus after treatment with resveratrol or placebo. Resveratrol-activated nutrient sensing system molecules AMPK (B), SIRTs (C), and mTOR (D). AMPK = AMP-activated kinase, mTOR = mammalian/mechanistic target of rapamycin, SIRTs = sirtuins.
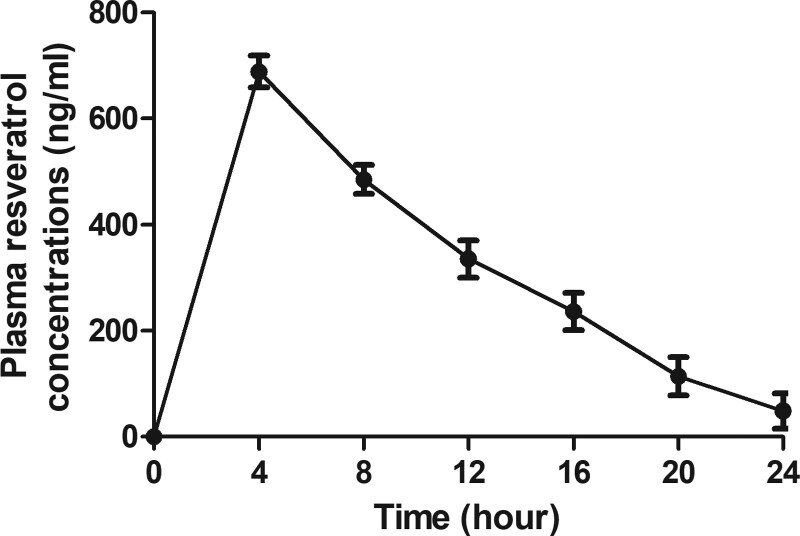
3.7. Safety of resveratrol in the elderly patients with type 2 diabetes mellitus
The safety and tolerability of resveratrol were reported in the elderly patients with type 2 diabetes mellitus. The results in Figure 6 demonstrated that the maximal plasma concentration of resveratrol was 688 ± 84 ng/mL. Adverse events in resveratrol group are described in Table 4. The most common side effects of resveratrol were diarrhea and fatigue. The rates of adverse events were low, and there were no statistically significant differences in adverse events reported from participants in either resveratrol group compared to the placebo group.
Figure 6.
Metabolism of resveratrol in the elderly patients with type 2 diabetes mellitus. The plasma concentration of resveratrol in 24 hours.
Table 4.
Adverse event incidence of resveratrol treatment in the elderly patients with type 2 diabetes mellitus.
| Resveratrol | Placebo | P value | |
|---|---|---|---|
| Diarrhea | 28 (11.6) | 2 (0.9) | .001 |
| Constipation | 6 (2.5) | 3 (1.3) | .068 |
| Muscle cramps/pain | 12 (5.0) | 6 (2.6) | .052 |
| Fatigue | 30 (12.4) | 3 (1.3) | .005 |
| Memory loss | 4 (1.7) | 1 (0.4) | .058 |
| Allergies/upper respiratory infection | 4 (1.7) | 1 (0.4) | .070 |
| Difficulty swallowing | 3 (1.2) | 1 (0.4) | .060 |
| Rash | 5 (2.1) | 2 (0.9) | .052 |
| Headache | 4 (1.7) | 1 (0.4) | .066 |
4. Discussion
Diabetes mellitus is a chronic disorder that is characterized by insulin resistance and hyperglycemia and may lead to metabolic syndrome.[17] The elderly patients with diabetes frequently lead to f abnormal glucose metabolism and diabetic complications including nephropathy in these patients.[18] Resveratrol has antidiabetic properties, and few reports investigate its safety and efficacy in the treatment of elderly patients with diabetes. In this study, we investigated the therapeutic efficacy of resveratrol in the elderly patients with type 2 diabetes mellitus. Outcomes in the current study found that resveratrol shows beneficial effects in improvement of abnormal glucose metabolism, insulin resistance, and diabetic complications. Notably, we indicated that resveratrol enhanced nutrient sensing systems and improved renal function, as well as reduced production and activity of glucose-6-phosphatase in the elderly patients with type 2 diabetes mellitus. Our findings suggest that resveratrol is well tolerated at doses of 700 mg/d in the elderly patients with type 2 diabetes mellitus.
A study has identified the efficacy and safety of resveratrol in the treatment of adults with type 2 diabetes mellitus.[19] Elderly patients with diabetes who have been administered a dietary supplementation of resveratrol (150 mg/d) for 30 days had no changes in fasting and postprandial incretin hormone plasma levels, but suppression on postprandial glucagon responses was documented.[20] Bhatt et al[21] showed that oral hypoglycemic drugs plus resveratrol 250 mg/d present more benefits than oral hypoglycemic drugs in patients with type 2 diabetes after 3-month period. Brasnyo et al[22] reported that resveratrol 2 × 5 mg for 4 weeks improves insulin sensitivity, reduces oxidative stress, and activates the Akt pathway in type 2 diabetic patients. In this study, we observed that resveratrol reduced production and activity of glucose-6-phosphatase, and activated nutrient sensing systems including AMPK, SIRTs, and mTOR in the elderly patients with type 2 diabetes mellitus compared to placebo. Poulsen et al[23] demonstrated that high-dose resveratrol (500 mg/d) has failed to show any significant improvement in insulin resistance. Here, we used the same dose and prolonged duration of resveratrol in the elderly patients with type 2 diabetes mellitus. The results in this study showed that resveratrol (500 mg/d) significantly decreased levels of inflammation, and improved blood glucose parameters and insulin resistance in the elderly patients with type 2 diabetes mellitus.
It has been reported that the frequency of kidney diseases, hypertension, and the risk of cardiovascular disease are high in the elderly patients with diabetes.[24] Insulin, inflammation, and renal function are not significantly affected by resveratrol treatment; however, resveratrol decreases hyperglycemia in patients with type 1 diabetes.[25] Inflammation and oxidative stress are key mechanisms underlying the genesis of patients with type 1 diabetes.[26] Conversely, our results showed that resveratrol treatment not only decreased inflammation but also improved kidney function and insulin resistance in the elderly patients with type 2 diabetes mellitus A study found that resveratrol supplementation shows the anti-inflammatory effects and regulates energy expenditure through increased skeletal muscle SIRT1 and AMPK expression in patients with type 2 diabetes mellitus.[11] Resveratrol exhibits significant antidiabetic potential by attenuating hyperglycemia and enhancing insulin secretion in patients with diabetes mellitus.[27] Resveratrol supplementation is well tolerated and safe that it does not considerably impact liver fat content or cardiometabolic risk parameters in humans.[28] In this study, we found beneficial effects of resveratrol on blood glucose metabolic parameters, levels of insulin, and renal function in the elderly patients with type 2 diabetes mellitus.
Few studies have tested the side effects of resveratrol in an older adult population. Findings from this study showed that resveratrol has beneficial effects on metabolic parameters, which is well tolerant for the elderly patients with diabetes. Given that participants had a mean age of 66 years, this study included a comprehensive safety, which showed few adverse events during 180-day period. This study also identified the safety dose of resveratrol (500 mg/d), and our findings showed that resveratrol was well tolerated at doses of 300 mg/d, which is related to the effects of resveratrol on renal function in the elderly patients with type 2 diabetes mellitus. Additionally, patients receiving resveratrol (500 mg/d) showed no statistically significant differences in adverse events reported from participants compared to the placebo group.
5. Conclusions
Data in the current study demonstrate that resveratrol has greater improvement in blood glucose, lipid and insulin metabolism, inflammation, and renal function in the elderly patients with type 2 diabetes mellitus. Along with these improvements, the results of this study are expected to serve as the basis for the improvement of customized diabetes treatment programs that are effective and less side effects, according to each patient’s characteristics through safety result and tolerance analysis.
Future studies should perform to explore the other benefits of resveratrol, develop more function of resveratrol to sustain continued improvements in glucose metabolism and lipid metabolism, and establish the long-term safety and efficacy of varying doses of resveratrol in the elderly patients with diabetes.
Author contribution
YZ summarized experimental data, conducted data analysis, and wrote the article. NM designed this study and revised article. All authors read and approved the final article.
Abbreviations:
- ALP =
- alkaline phosphatase
- AMPK =
- AMP-activated kinase
- BUN =
- blood urea nitrogen
- Cr =
- creatinine
- CRP =
- C-reactive protein
- HbA1c =
- glycated hemoglobin/hemoglobin A1c
- HDL =
- high-density lipoprotein
- HOMA-IR =
- homeostasis model assessment of insulin resistance
- HOMA-β =
- homeostasis model assessment-beta
- IL-1β =
- interleukin-1beta
- IL-6 =
- interleukin-6
- LDL =
- low-density lipoprotein
- MCR =
- metabolic clearance rate
- MDA =
- malondialdehyde
- SGOT =
- glutamic oxaloacetic transaminase
- SGPT =
- serum glutamic pyruvic transaminase
- SIRTs =
- sirtuins
- TAC =
- total antioxidant capacity
- TC =
- total cholesterol
- TG =
- triglycerides
- TNF-α =
- tumor necrosis factor-alpha
How to cite this article: Zhang Y, Ma N. Effects of resveratrol therapy on glucose metabolism, insulin resistance, inflammation, and renal function in the elderly patients with type 2 diabetes mellitus: A randomized controlled clinical trial protocol: Medicine 2022;101:32(e30049).
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The study was approved by the Ethics Committee (approval number: MDJMU.201905191X1) of the First Hospital of Harbin (Harbin, China). Written informed consent was obtained from each subject.
YZ contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
ALP = alkaline phosphatase, AUC0-180 = area under the curve 0-180 min, BMI = body mass index, BUN = blood urea nitrogen, eGFR = estimated glomerular filtration rate, HDL = high-density lipoprotein, HOMA-IR = homeostasis model assessment of insulin resistance, HOMA-β = homeostasis model assessment-beta, hs-CRP = high-sensitivity C-reactive protein, LDL = low-density lipoprotein, SGOT = glutamic oxaloacetic transaminase, SGPT = serum glutamic pyruvic transaminase, TG = triglycerides, TNF-α = tumor necrosis factor-alpha.
HbA1c = glycated hemoglobin/hemoglobin A1c, HOMA-IR = homeostasis model assessment of insulin resistance, HOMA-β = homeostasis model assessment-beta, hs-CRP = high-sensitivity C-reactive protein, IL-1β = interleukin-1beta, IL-6 = interleukin-6, TNF-α = tumor necrosis factor-alpha.
AUC0-180 = area under the curve 0-180 min.
References
- [1].Bekele BB, Manzar MD, Alqahtani M, et al. Diabetes mellitus, metabolic syndrome, and physical activity among Ethiopians: a systematic review. Diabetes Metab Syndr. 2021;15:257–6. [DOI] [PubMed] [Google Scholar]
- [2].Halushko O, Loskutov O, Kuchynska I, et al. The main causes of the complicated course of covid-19 in patients with diabetes mellitus and treatment (Review). Georgian Med News. 2020;307:114–20. [PubMed] [Google Scholar]
- [3].Hua F. New insights into diabetes mellitus and its complications: a narrative review. Ann Transl Med. 1689;8:2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lin W, Huang H, Wen J, et al. The predictive value of procalcitonin for early detection of infection in elderly type 2 diabetes mellitus. J Infect Chemother. 2020;26:343–8. [DOI] [PubMed] [Google Scholar]
- [5].Li W, Tong J, Li N, et al. A study on the relationships of inflammation, antioxidant ability and healing with blood glucose in elderly patients with type 2 diabetes mellitus after dental implantation. Panminerva Med. 2020;62:283–4. [DOI] [PubMed] [Google Scholar]
- [6].Silverii GA, Caldini E, Dicembrini I, et al. Deprescription in elderly patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2020;170:108498. [DOI] [PubMed] [Google Scholar]
- [7].Kelly G. A review of the sirtuin system, its clinical implications, and the potential role of dietary activators like resveratrol: part 1. Altern Med Rev. 2010;15:245–63. [PubMed] [Google Scholar]
- [8].Athar M, Back JH, Tang X, et al. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fulda S, Debatin KM. Resveratrol modulation of signal transduction in apoptosis and cell survival: a mini-review. Cancer Detect Prev. 2006;30:217–23. [DOI] [PubMed] [Google Scholar]
- [10].Nanjan MJ, Betz J. Resveratrol for the management of diabetes and its downstream pathologies. Eur Endocrinol. 2014;10:31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Goh KP, Lee HY, Lau DP, et al. Effects of resveratrol in patients with type 2 diabetes mellitus on skeletal muscle SIRT1 expression and energy expenditure. Int J Sport Nutr Exerc Metab. 2014;24:2–13. [DOI] [PubMed] [Google Scholar]
- [12].Timmers S, Hesselink MK, Schrauwen P. Therapeutic potential of resveratrol in obesity and type 2 diabetes: new avenues for health benefits? Ann N Y Acad Sci. 2013;1290:83–9. [DOI] [PubMed] [Google Scholar]
- [13].Gocmez SS, Sahin TD, Yazir Y, et al. Resveratrol prevents cognitive deficits by attenuating oxidative damage and inflammation in rat model of streptozotocin diabetes induced vascular dementia. Physiol Behav. 2019;201:198–207. [DOI] [PubMed] [Google Scholar]
- [14].Imamura H, Yamaguchi T, Nagayama D, et al. Resveratrol ameliorates arterial stiffness assessed by cardio-ankle vascular index in patients with type 2 diabetes mellitus. Int Heart J. 2017;58:577–83. [DOI] [PubMed] [Google Scholar]
- [15].Madsen A, Bjune JI, Bjorkhaug L, et al. The cAMP-dependent protein kinase downregulates glucose-6-phosphatase expression through RORalpha and SRC-2 coactivator transcriptional activity. Mol Cell Endocrinol. 2016;419:92–101. [DOI] [PubMed] [Google Scholar]
- [16].Theret M, Gsaier L., Ben Larbi S, et al. Analysis of muscle stem cell fate through modulation of AMPK activity. Methods Mol Biol. 2018;1732:539–49. [DOI] [PubMed] [Google Scholar]
- [17].El-Saiedi SA, Hafez MH, Sedky YM, et al. Novel biomarkers for subtle myocardial involvement in type I diabetes mellitus. Cardiovasc Endocrinol Metab. 2021;10:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Takamatsu K. Renal status in elderly patients with type 2 diabetes. Clin Exp Nephrol. 2020;24:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jeyaraman MM, Al-Yousif NSH, Singh Mann A, et al. Resveratrol for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2020;1:CD011919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Knop FK, Konings E, Timmers S, et al. Thirty days of resveratrol supplementation does not affect postprandial incretin hormone responses, but suppresses postprandial glucagon in obese subjects. Diabet Med. 2013;30:1214–8. [DOI] [PubMed] [Google Scholar]
- [21].Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res. 2012;32:537–41. [DOI] [PubMed] [Google Scholar]
- [22].Brasnyo P, Molnar GA, Mohas M, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr. 2011;106:383–9. [DOI] [PubMed] [Google Scholar]
- [23].Poulsen MM, Vestergaard PF, Clasen BF, et al. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62:1186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kobayashi K, Toyoda M, Hatori N, et al. Sodium-glucose cotransporter 2 inhibitor-induced reduction in the mean arterial pressure improved renal composite outcomes in type 2 diabetes mellitus patients with chronic kidney disease: a propensity score-matched model analysis in Japan. J Diabet Investig. 2021;12:1408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Movahed A, Raj P, Nabipour I, et al. Efficacy and safety of resveratrol in type 1 diabetes patients: a two-month preliminary exploratory trial. Nutrients. 2020;12:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Frias JP, Auerbach P, Bajaj HS, et al. Efficacy and safety of once-weekly semaglutide 2.0 mg versus 1.0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B trial. Lancet Diabetes Endocrinol. 2021;9:563–74. [DOI] [PubMed] [Google Scholar]
- [27].Hoseini A, Namazi G, Farrokhian A, et al. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct. 2019;10:6042–51. [DOI] [PubMed] [Google Scholar]
- [28].Kantartzis K, Fritsche L, Bombrich M, et al. Effects of resveratrol supplementation on liver fat content in overweight and insulin-resistant subjects: a randomized, double-blind, placebo-controlled clinical trial. Diabetes Obes Metab. 2018;20:1793–7. [DOI] [PubMed] [Google Scholar]