Abstract
Background
Pregnant individuals have been receiving COVID-19 vaccines following pre-authorisation clinical trials in non-pregnant people. This study aimed to determine the frequency and nature of significant health events among pregnant females after COVID-19 vaccination, compared with unvaccinated pregnant controls and vaccinated non-pregnant individuals.
Methods
We did an observational cohort study, set in seven Canadian provinces and territories including Ontario, Quebec, British Columbia, Alberta, Nova Scotia, Yukon, and Prince Edward Island. Eligibility criteria for vaccinated individuals were a first dose of a COVID-19 vaccine within the previous 7 days; an active email address and telephone number; ability to communicate in English or French; and residence in the aforementioned provinces or territories. Study participants were pregnant and non-pregnant females aged 15–49 years. Individuals were able to participate as controls if they were unvaccinated and fulfilled the other criteria. Data were collected primarily by self-reported survey after both vaccine doses, with telephone follow-up for those reporting any medically attended event. Participants reported significant health events (new or worsening of a health event sufficient to cause work or school absenteeism, medical consultation, or prevent daily activities) occurring within 7 days of vaccination or within the past 7 days for unvaccinated individuals. We employed multivariable logistic regression to examine significant health events associated with mRNA vaccines, adjusting for age group, previous SARS-CoV-2 infection, and trimester, as appropriate.
Findings
As of Nov 4, 2021, 191 360 women aged 15–49 years with known pregnancy status had completed the first vaccine dose survey and 94 937 had completed the second dose survey. 180 388 received one dose and 94 262 received a second dose of an mRNA vaccine, with 5597 pregnant participants receiving dose one and 3108 receiving dose two, and 174 765 non-pregnant participants receiving dose one and 91 131 receiving dose two. Of 6179 included unvaccinated control participants, 339 were pregnant and 5840 were not pregnant. Overall, 226 (4·0%) of 5597 vaccinated pregnant females reported a significant health event after dose one of an mRNA vaccine, and 227 (7·3%) of 3108 after dose two, compared with 11 (3·2%) of 339 pregnant unvaccinated females. Pregnant vaccinated females had an increased odds of a significant health event within 7 days of the vaccine after dose two of mRNA-1273 (adjusted odds ratio [aOR] 4·4 [95% CI 2·4–8·3]) compared with pregnant unvaccinated controls within the past 7 days, but not after dose one of mRNA-1273 or any dose of BNT162b2. Pregnant vaccinated females had decreased odds of a significant health event compared with non-pregnant vaccinated females after both dose one (aOR 0·63 [95% CI 0·55–0·72]) and dose two (aOR 0·62 [0·54–0·71]) of any mRNA vaccination. There were no significant differences in any analyses when restricted to events which led to medical attention.
Interpretation
COVID-19 mRNA vaccines have a good safety profile in pregnancy. These data can be used to appropriately inform pregnant people regarding reactogenicity of COVID-19 vaccines during pregnancy, and should be considered alongside effectiveness and immunogenicity data to make appropriate recommendations about best use of COVID-19 vaccines in pregnancy.
Funding
Canadian Institutes of Health Research, Public Health Agency of Canada.
Introduction
The COVID-19 pandemic has disproportionately affected pregnant people, who are at higher risk of severe disease compared with similarly aged non-pregnant individuals. Pregnancy leads to an increased risk of hospital admission with COVID-19, intensive care unit admission, mechanical ventilation, and death, due to changes in cardiopulmonary and immunological physiology.1 Additionally, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection increases risk of adverse pregnancy outcomes such as hypertension, pre-eclampsia, impaired fetal growth, and preterm birth.2
COVID-19 vaccines have been available in Canada since December, 2020.3 Multiple expert groups published positive recommendations for use of COVID-19 vaccines in pregnancy early on in vaccine deployment, based largely on previously established safety of inactivated vaccines in pregnancy, several decades of use of vaccines in pregnancy (such as tetanus toxoid, influenza, and tetanus-diphtheria-pertussis), and reassuring data from the small number of incidental pregnancies occurring during pre-authorisation trials.4, 5 Data from large cohorts requires post-implementation studies, and data has been accruing on the safety of mRNA vaccines during pregnancy.6, 7, 8 These are primarily descriptive studies, relying on comparisons with historic rates of adverse events and mostly without a contemporaneous control group to enable comparison with background rates of adverse events following immunisation.
Research in context.
Evidence before this study
We searched the peer-reviewed (PubMed) and pre-print (MedRxiv) literature for studies published in English related to safety, reactogenicity, and tolerability of COVID-19 vaccines in pregnant people between Nov 1, 2021, and May 9, 2022, with no date restrictions, and using search terms “COVID-19”, “vaccine” and “pregnancy”, combined with “safety”, “reactogenicity”, “adverse events” or “tolerability”. In addition to identified primary research articles, we also further reviewed citations from review articles that had not been identified in our search. Of studies involving direct collection of data from participants, we found several observational studies that described rates of adverse events after first and second doses or booster doses of mRNA vaccines in pregnancy. Most studies included a pregnant cohort only (except two that reported on vaccine-specific adverse events instead of adverse pregnancy outcomes, with smaller cohorts than our study), with some studies also including a non-pregnant vaccinated group. There were some additional studies using data from administrative databases without individual participant verification. Most studies described all adverse events reported, with limited differentiation of significant/severe/serious adverse events. Most comparisons of adverse pregnancy outcomes in vaccinated compared with unvaccinated pregnant people utilised historical control data, with no studies including a contemporaneous pregnant, unvaccinated control group.
Added value of this study
Our study included a sample of 5625 pregnant, vaccinated females aged 15–49 years, compared with 185 735 non-pregnant vaccinated females and 339 pregnant unvaccinated controls of a similar age. All data were collected during the study period, using standardised surveys, enabling direct comparison between groups. We also actively follow-up individuals with severe adverse events to enable detailed descriptions. Our study focused on significant health events—new or worsening health events following vaccination sufficient to cause work or school absenteeism, medical consultation, or prevent daily activities—and included sensitivity analyses restricted to events leading to medical consultation. Our data provide reassuring evidence that COVID-19 mRNA vaccines are safe in pregnancy, with lower rates of significant adverse events following immunisation in pregnant people than non-pregnant vaccine recipients for both mRNA vaccines used in Canada, after dose one and dose two. Although rates of significant adverse events following immunisation were highest after dose two for mRNA-1273 recipients, both mRNA vaccines are highly immunogenic and effective in pregnancy. Given the increased rate of significant complications related to COVID-19 in pregnancy, high vaccine coverage in this group is important for protection of the pregnant person and young infant.
Implications of all the available evidence
These data can be used in combination with previously published data to provide accurate information to pregnant females receiving COVID-19 vaccines regarding the rates of significant health events occurring after vaccination, putting the information in the context of an unvaccinated control group, with similar rates of health events other than after a second dose of mRNA-1273. The data also provides reassurance that the rate of significant health events is lower in pregnant people than non-pregnant individuals after vaccination, with similar rates of medical consultation for all comparisons.
The Canadian National Vaccine Safety (CANVAS) Network was established during the 2009 influenza pandemic to provide rapid, real-time safety data during rollout of immunisation programmes, and has monitored the safety of seasonal influenza vaccines in Canada and other emergency campaigns, such as for a capsular group B meningococcal vaccine.9 CANVAS obtains information via surveys administered to vaccinated and unvaccinated individuals. The CANVAS Network has been monitoring COVID-19 vaccine safety in Canada since the start of the vaccine rollout.10 The focus of this study and other CANVAS studies has been significant adverse events occurring in the week following immunisation that affect work or schooling, or that require medical attention. Distinguishing components of the CANVAS approach are active follow-up of individuals with important health events and active enrolment of a control group to enable comparisons with unvaccinated individuals in a similar time frame. The aim of this analysis was to compare rates of health events in (1) vaccinated pregnant females and vaccinated non-pregnant females of the same age and (2) vaccinated and unvaccinated (control) pregnant females.
Methods
Study design and participants
In this ongoing observational cohort study, we actively recruited participants from seven Canadian provinces and territories accounting for more than 75% of the national population including Ontario, Quebec, British Columbia, Alberta, Nova Scotia, Yukon, and Prince Edward Island.10 At the time of this analysis, the study was open to anyone eligible for a COVID-19 vaccine, which included individuals aged 12 years and older. Vaccinated individuals were eligible if they received the first dose of a COVID-19 vaccine within the previous 7 days; had an active email address and telephone number; were able to communicate in English or French; and reside in the aforementioned provinces or territories. Individuals were able to participate as controls if they were unvaccinated and fulfilled the other three criteria. Individuals were able to contribute to both vaccinated and control groups if they initially enrolled prior to vaccination and subsequently enrolled after vaccination. Individuals who enrolled as control participants and expected to be vaccinated within the next 6 months completed a retrospective control survey. All other control participants were followed prospectively. All participants provided informed consent electronically. Each study site obtained approval from Research Ethics Boards (British Columbia and Yukon: UBC Children's & Women's, Ref: H20-03704; Quebec: Centre Intégré univrsitaire de santé et de services sociaux de l’Estrie, Ref: MP-31-2021-4044; Nova Scotia and Prince Edward Island: Health PEI and IWK Health Research, Ref: 1026400; Alberta: Conjoint Health REB, University of Calgary, Ref: REB20-2177; Ontario: Unity Health Toronto, Ref: 20-334). The study protocol has previously been published.
Procedures
Recruitment methods for COVID-19 vaccinated participants included: (1) passive recruitment via information provided at immunisation clinics; (2) electronic invitation when an appointment was made for vaccination; and (3) electronic invitation after vaccination using names obtained from the relevant immunisation registry. Control participants were: (1) previous CANVAS study participants who indicated their agreement to be contacted for research; (2) users of the CANImmunize app; (3) enrolled via the study website and (4) recruited from volunteer databases. A detailed description of recruitment procedures has been previously published.10 Study enrolment commenced on Dec 22, 2020. Of relevance to this analysis, vaccinated participants were surveyed via email for the occurrence of adverse events following immunisation during the 7 days following each dose of COVID-19 vaccine. All participants were asked about injection site reactions, but only those that indicated they had a significant health event were requested to provide further details. Control participants were requested to note the occurrence of health problems in the previous 7 days, also via email. We did a telephone follow-up for those who reported any medically attended event. We sent up to two automatic reminders every 72 h for all non-responders to address loss to follow-up. Survey data were collected in a secure REDCap (Research Electronic Data Capture) database.11, 12 Data collected relevant to this analysis included age group, sex, gender, ethnicity, self-reported previous laboratory-confirmed SARS-CoV-2 infection, pregnancy and lactation status, general health status, COVID-19 vaccine brand administered, occurrence or worsening of health events, and requirement for medical attention and hospital admission related to the health event.
Outcomes
The primary endpoint was a significant health event, defined as a new or worsening health event sufficient to cause work or school absenteeism, medical consultation, or prevent daily activities in the previous 7 days (for controls) or the 7 days following vaccination for those that received a COVID-19 vaccine. The secondary endpoint was defined as any serious health event (an event resulting in emergency department visit or hospital admission) in the previous 7 days. Two types of exposures were analysed: (1) vaccination status among pregnant people, and (2) pregnancy status among vaccinated people.
For the purposes of this analysis, we included all females reporting a pregnancy on any survey, and non-pregnant females in the same age groups (15–49 years). When comparing health event rates among pregnant people, the same pregnant control group was used to compare with vaccinated pregnant people who received a first or second dose of a COVID-19 vaccine. For statistical modelling, the analysis was restricted to those who received any mRNA vaccines (BNT162b2 [Pfizer] and mRNA-1273 [Moderna]) because very few pregnant people reported receiving the ChAdox1-S [AstraZeneca] vaccine. For pregnant participants who reported experiencing a miscarriage following both dose one and dose two, only the first report was included to avoid duplicate counting from individuals reporting the same miscarriage twice, given the relatively short interval between vaccine doses.
Statistical analysis
We estimated rates of significant and serious health events including all symptoms following the first and second doses of COVID-19 vaccines. To examine associations between the outcomes and the exposures, two sets of univariable and multivariable logistic regression models were built for each type of exposure (vaccination status among pregnant people and pregnancy status among vaccinated people). When fitting multivariable models, we adjusted known or expected covariates such as age group, previous SARS-CoV-2 infection, and trimester of pregnancy, as appropriate. Three different vaccine groups were evaluated: (1) BNT162b2, (2) mRNA-1273, and (3) any mRNA vaccine. The estimated odds ratio (OR) and 95% CIs were reported. We did complete case analysis because no variable had more than 2% of missing data. We verified the absence of multicollinearity with variance inflation factor using a cut-off point of less than 5. To evaluate the robustness of findings, we did two sensitivity analyses. In the first, our primary end point (significant health event) was restricted to new or worsening health events also resulting in medical consultation in the previous 7 days. We repeated the same analysis after the first and second doses of COVID-19 vaccines. In the second analysis, we restricted the dataset of pregnant people to those who reported being in excellent health and compared this with the results from the primary analysis. Data cleaning was done in SAS version 9.4 (SAS Institute) and analysis was completed in R software version 4.1.1 (R foundation for Statistical Computing, Vienna, Austria).
Role of funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
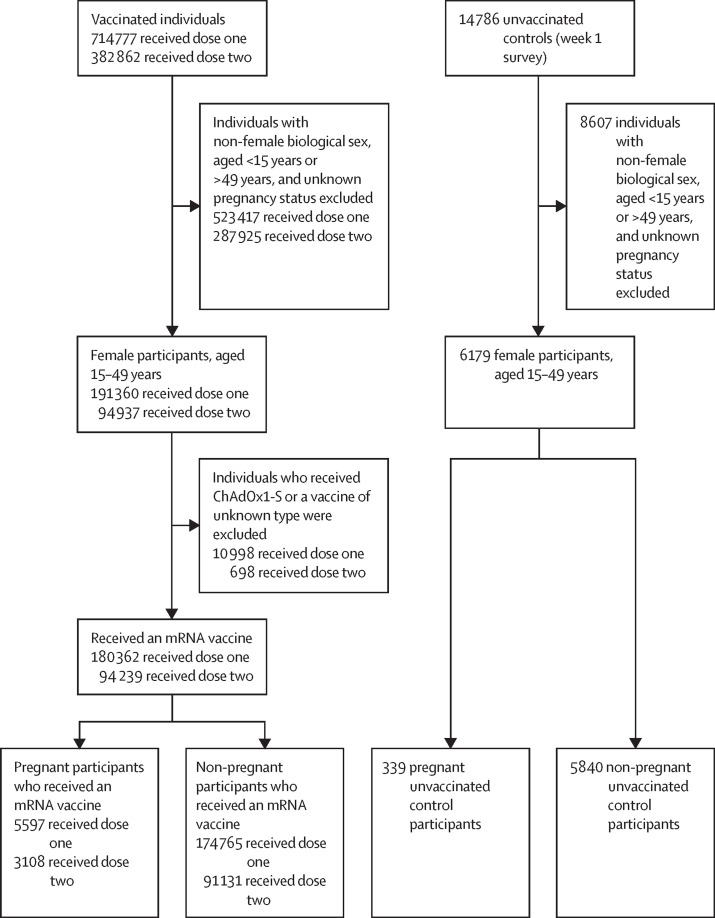
As of Nov 4, 2021, a total of 191 360 women aged 15–49 years with known pregnancy status had completed the first vaccine does survey and 94 937 had completed the second dose survey (figure 1 ). A total of 5625 (2·9%) of 191 360 and 3114 (3·3%) of 94 937 women reported being pregnant at the time of receiving the first and second doses of COVID-19 vaccines (appendix p 1). Of these, most pregnant participants had received BNT162b2 (3414 received dose one and 1892 received dose two) or mRNA-1273 (2183 received dose one and 1216 received dose two) COVID-19 vaccines and few individuals reported receiving ChAdOx1-S (25 received dose one and six received dose two). Among the enrolled unvaccinated control participants, a total of 6179 females aged 15–49 years had completed 7-day surveys. Of these, 339 (5·5%) reported being pregnant during the survey period (appendix p 1). Among both vaccinated and control groups, pregnant individuals in all three trimesters were well represented (table 1 ).
Figure 1.
Study profile
Flow chart depicting the analytical samples (dose one and dose two) for examining adverse events following immunisation associated with COVID-19 vaccine. Non-pregnant controls were not included in the regression analysis.
Table 1.
Baseline demographics and health events in study populations
| Pregnant control group (n=339) |
Dose 1 |
Dose 2 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any mRNA |
BNT162b2 |
mRNA-1273 |
Any mRNA |
BNT162b2 |
mRNA-1273 |
||||||||
| Not pregnant (n=174 765) | Pregnant (n=5597) | Not pregnant (n=107 121) | Pregnant (n=3414) | Not pregnant (67 644) | Pregnant (n=2183) | Not pregnant (91 131) | Pregnant (n=3108) | Not pregnant (n=53 077) | Pregnant (n=1892) | Not pregnant (38 054) | Pregnant (n=1216) | ||
| Health events | |||||||||||||
| Significant health event | 11 (3·2%) | 10950 (6·3%) | 226 (4·0%) | 6501 (6·1%) | 137 (4·0%) | 4449 (6·6%) | 89 (4·1%) | 10 254 (11·3%) | 227 (7·3%) | 4121 (7·8%) | 80 (4·2%) | 6133 (16·1%) | 147 (12·1%) |
| Severe health event | 4 (1·2%) | 2439 (1·4%) | 78 (1·4%) | 1392 (1·3%) | 45 (1·3%) | 1047 (1·5%) | 33 (1·5%) | 1257 (1·4%) | 47 (1·5%) | 663 (1·2%) | 22 (1·2%) | 594 (1·6%) | 25 (2·1%) |
| Serious health event | 2 (0·6%) | 733 (0·4%) | 31 (0·6%) | 421 (0·4%) | 18 (0·5%) | 312 (0·5%) | 13 (0·6%) | 343 (0·4%) | 19 (0·6%) | 192 (0·4%) | 8 (0·4%) | 151 (0·4%) | 11 (0·9%) |
| Trimester* | |||||||||||||
| 1st trimester | 6 (1·8%) | .. | 81 (1·4%) | .. | 47 (1·4%) | .. | 34 (1·6%) | .. | 67 (2·2%) | .. | 23 (1·2%) | .. | 44 (3·6%) |
| 2nd trimester | 3 (0·9%) | .. | 96 (1·7%) | .. | 56 (1·6%) | .. | 40 (1·8%) | .. | 86 (2·8%) | .. | 29 (1·5%) | .. | 57 (4·7%) |
| 3rd trimester | 2 (0·6%) | .. | 48 (0·9%) | .. | 34 (1·0%) | .. | 14 (0·6%) | .. | 74 (2·4%) | .. | 28 (1·5%) | .. | 46 (3·8%) |
| Unknown | .. | .. | 1 (<0·1%) | .. | .. | .. | 1 (<0·1%) | .. | .. | .. | .. .. | .. | .. |
| Age groups, years | |||||||||||||
| 15–29 | 51 (15·0%) | 59 263 (33·9%) | 1417 (25·3%) | 41 221 (38·5%) | 796 (23·3%) | 18 042 (26·7%) | 621 (28·4%) | 26 324 (28·9%) | 716 (23·0%) | 17 008 (32·0%) | 422 (22·3%) | 9316 (24·5%) | 294 (24·2%) |
| 30–49 | 288 (85·0%) | 115 502 (66·1%) | 4180 (74·7%) | 65 900 (61·5%) | 2618 (76·7%) | 49 602 (73·3%) | 1562 (71·6%) | 64 807 (71·1%) | 2392 (77·0%) | 36 069 (68·0%) | 1470 (77·7%) | 28 738 (75·5%) | 922 (75·8%) |
| Gender | |||||||||||||
| Woman | 338 (99·7%) | 170 674 (97·7%) | 5579 (99·7%) | 104 279 (97·3%) | 3403 (99·7%) | 66 395 (98·2%) | 2176 (99·7%) | 89 176 (97·9%) | 3091 (99·5%) | 51 914 (97·8%) | 1881 (99·4%) | 37 262 (97·9%) | 1210 (99·5%) |
| Man | 0 | 819 (0·5%) | 3 (0·1%) | 561 (0·5%) | 1 (<0·1%) | 258 (0·4%) | 2 (0·1%) | 341 (0·4%) | 1 (<0·1%) | 215 (0·4%) | 1 (0·1%) | 126 (0·3%) | 0 |
| Non-binary | 1 (0·3%) | 2380 (1·4%) | 12 (0·2%) | 1698 (1·6%) | 9 (0·3%) | 682 (1·0%) | 3 (0·1%) | 1254 (1·4%) | 13 (0·4%) | 737 (1·4%) | 8 (0·4%) | 517 (1·4%) | 5 (0·4%) |
| Two-spirit | 0 | 148 (0·1%) | 0 | 96 (0·1%) | 0 | 52 (0·1%) | 0 | 49 (0·1%) | 0 | 33 (0·1%) | 0 | 16 (<0·1%) | 0 |
| Other | 0 | 139 (0·1%) | 1 (<0·1%) | 101 (0·1%) | 0 | 38 (0·1%) | 1 (<0·1%) | 69 (0·1%) | 0 | 40 (0·1%) | 0 | 29 (0·1%) | 0 |
| Unknown | 0 | 605 (0·3%) | 2 (<0·1%) | 386 (0·4%) | 1 (<0·1%) | 219 (0·3%) | 1 (<0·1%) | 242 (0·3%) | 3 (0·1%) | 138 (0·3%) | 2 (0·1%) | 104 (0·3%) | 1 (0·1%) |
| Ethnicity | |||||||||||||
| White | 22 (6·5%) | 47 539 (27·2%) | 1818 (32·5%) | 29 421 (27·5%) | 1166 (34·2%) | 18 118 (26·8%) | 652 (29·9%) | 50 779 (55·7%) | 1778 (57·2%) | 25 645 (48·3%) | 957 (50·6%) | 25 134 (66·0%) | 821 (67·5%) |
| Black | 2 (0·6%) | 1155 (0·7%) | 18 (0·3%) | 802 (0·7%) | 12 (0·4%) | 353 (0·5%) | 6 (0·3%) | 1169 (1·3%) | 22 (0·7%) | 692 (1·3%) | 14 (0·7%) | 477 (1·3%) | 8 (0·7%) |
| East Asian | 0 | 3367 (1·9%) | 117 (2·1%) | 2427 (2·3%) | 87 (2·5%) | 940 (1·4%) | 30 (1·4%) | 3471 (3·8%) | 106 (3·4%) | 2213 (4·2%) | 72 (3·8%) | 1258 (3·3%) | 34 (2·8%) |
| South Asian | 0 | 1977 (1·1%) | 95 (1·7%) | 1502 (1·4%) | 81 (2·4%) | 475 (0·7%) | 14 (0·6%) | 2041 (2·2%) | 104 (3·3%) | 1325 (2·5%) | 83 (4·4%) | 716 (1·9%) | 21 (1·7%) |
| South-East Asian | 1 (0·3%) | 1552 (0·9%) | 47 (0·8%) | 1121 (1·0%) | 36 (1·1%) | 431 (0·6%) | 11 (0·5%) | 1587 (1·7%) | 45 (1·4%) | 995 (1·9%) | 27 (1·4%) | 592 (1·6%) | 18 (1·5%) |
| Indigenous | 0 | 699 (0·4%) | 22 (0·4%) | 476 (0·4%) | 18 (0·5%) | 223 (0·3%) | 4 (0·2%) | 703 (0·8%) | 21 (0·7%) | 436 (0·8%) | 13 (0·7%) | 267 (0·7%) | 8 (0·7%) |
| Middle Eastern | 0 | 1352 (0·8%) | 44 (0·8%) | 821 (0·8%) | 30 (0·9%) | 531 (0·8%) | 14 (0·6%) | 1361 (1·5%) | 44 (1·4%) | 751 (1·4%) | 25 (1·3%) | 610 (1·6%) | 19 (1·6%) |
| Latino | 0 | 1392 (0·8%) | 48 (0·9%) | 807 (0·8%) | 23 (0·7%) | 585 (0·9%) | 25 (1·1%) | 1460 (1·6%) | 50 (1·6%) | 736 (1·4%) | 19 (1·0%) | 724 (1·9%) | 31 (2·5%) |
| Mixed | 0 | 2714 (1·6%) | 92 (1·6%) | 1888 (1·8%) | 68 (2·0%) | 826 (1·2%) | 24 (1·1%) | 2800 (3·1%) | 88 (2·8%) | 1619 (3·1%) | 53 (2·8%) | 1181 (3·1%) | 35 (2·9%) |
| Unknown or other | 314 (92·6%) | 113 018 (64·7%) | 3296 (58·9%) | 67 856 (63·3%) | 1893 (55·4%) | 45 162 (66·8%) | 1403 (64·3%) | 25 758 (28·3%) | 850 (27·3%) | 18 665 (35·2%) | 629 (33·2%) | 7095 (18·6%) | 221 (18·2%) |
| Health status | |||||||||||||
| Excellent, very good, or good | 333 (98·2%) | 151 446 (86·7%) | 5029 (89·9%) | 91 266 (85·2%) | 2961 (86·7%) | 60 180 (89·0%) | 2068 (94·7%) | 77 911 (85·5%) | 2747 (88·4%) | 44 362 (83·6%) | 1617 (85·5%) | 33 549 (88·2%) | 1130 (92·9%) |
| Fair, poor, or unknown | 6 (1·8%) | 23 319 (13·3%) | 568 (10·1%) | 15 855 (14·8%) | 453 (13·3%) | 7464 (11·0%) | 115 (5·3%) | 13 220 (14·5%) | 361 (11·6%) | 8715 (16·4%) | 275 (14·5%) | 4505 (11·8%) | 86 (7·1%) |
| Province | |||||||||||||
| Alberta | 37 (10·9%) | 28 987 (16·6%) | 778 (13·9%) | 22 129 (20·7%) | 650 (19·0%) | 6858 (10·1%) | 128 (5·9%) | 17 260 (18·9%) | 544 (17·5%) | 12 052 (22·7%) | 422 (22·3%) | 5208 (13·7%) | 122 (10·0%) |
| Ontario | 178 (52·5%) | 44 184 (25·3%) | 2080 (37·2%) | 36 571 (34·1%) | 1636 (47·9%) | 7613 (11·3%) | 444 (20·3%) | 32 324 (35·5%) | 1318 (42·4%) | 20 445 (38·5%) | 888 (46·9%) | 11 879 (31·2%) | 430 (35·4%) |
| Quebec | 4 (1·2%) | 65 501 (37·5%) | 1464 (26·2%) | 21 446 (20·0%) | 174 (5·1%) | 44 055 (65·1%) | 1290 (59·1%) | 27 621 (30·3%) | 664 (21·4%) | 11 609 (21·9%) | 188 (9·9%) | 16 012 (42·1%) | 476 (39·1%) |
| British Columbia and Yukon | 85 (25·1%) | 31 287 (17·9%) | 1132 (20·2%) | 23 197 (21·7%) | 834 (24·4%) | 8090 (12·0%) | 298 (13·7%) | 10 393 (11·4%) | 484 (15·6%) | 7437 (14·0%) | 341 (18·0%) | 2956 (7·8%) | 143 (11·8%) |
| Prince Edward Island and Nova Scotia | 35 (10·3%) | 4806 (2·7%) | 143 (2·6%) | 3778 (3·5%) | 120 (3·5%) | 1028 (1·5%) | 23 (1·1%) | 3533 (3·9%) | 98 (3·2%) | 1534 (2·9%) | 53 (2·8%) | 1999 (5·3%) | 45 (3·7%) |
| History of SARS-COV-2 infection | |||||||||||||
| No | 331 (97·6%) | 167 100/174 516 (95·8%) | 5434/5592 (97·2%) | 102 013/106 965 (95·4%) | 3310/3409 (97·1%) | 65 087/67 551 (96·4%) | 2124 (97·3%) | 87 891/91 072 (96·5%) | 3009/3106 (96·9%) | 50 993/53 048 (96·1%) | 1821/1890 (96·3%) | 36 898/38 024 (97·0%) | 1188 (97·7%) |
| Yes | 8 (2·4%) | 7416/174 516 (4·2%) | 158/5592 (2·8%) | 4952/106 965 (4·6%) | 99/3409 (2·9%) | 2464/67 551 (3·6%) | 59 (2·7%) | 3181/91 072 (3·5%) | 97/3106 (3·1%) | 2055/53 048 (3·9%) | 69/1890 (3·7%) | 1126/38 024 (3·0%) | 28 (2·3%) |
Data are n (%) or n/N (%). Significant health event indicates a new or worsening health event sufficient to cause work or school absenteeism, medical consultation, or prevent daily activities within 7 days after vaccination for vaccinated participants and in the previous 7 days for control groups. Severe health event is a subgroup of significant health events and indicates a new or worsening a health event resulting in medical consultation within 7 days after vaccination for vaccinated participants and in the previous 7 days for control groups. Serious health event indicates any event resulting in emergency department visit or admission to hospital within 7 days after vaccination for vaccinated participants and in the previous 7 days for control groups.
Breakdown of trimester data applies only to significant health events.
Most respondents in both control and vaccinated groups were aged 30–49 (ranging from 65 900 [61·5%] of 107 121 in not-pregnant individuals receiving dose one of BNT162b2 to 288 [85·0%] of 339 in the pregnant control group) and reported being in excellent/very good/good health (ranging from 44 362 [83·6%] of 53 077 in not-pregnant individuals receiving dose two of BNT162b2 to 333 [98·2%] of 339 in the pregnant control group; table 1). The ethnic origin of more than half of participants in both vaccinated and control groups was unknown because the question regarding ethnicity was introduced only in the dose two survey questionnaires and was optional. Among those who reported their ethnicity, the majority were White, followed by East Asian, and South Asian. Prevalence of previous SARS-CoV-2 infection varied between 2·3% and 4·6% (table 1).
Overall, 226 (4·0%) of 5597 mRNA-vaccinated pregnant females reported a significant health event within 7 days after dose one of an mRNA vaccine, and 227 (7·3%) of 3108 after dose two (table 1). The rates were similar after dose one for both mRNA vaccines (137 [4·0%] of 3414 for BNT162b2 and 89 [4·1%] of 2183 for mRNA-1273), but higher for mRNA-1273 (147 [12·1%] of 1216) than BNT162b2 (80 [4·2%] of 1892) after dose two. The most common significant health events after dose two of mRNA-1273 in pregnant females were feeling unwell or malaise or myalgia (139 [11·4%] of 1216), headache or migraine (103 [8·5%] of 1216), and respiratory tract infection (68 [5·6%] of 1216; table 2 ). Among pregnant and vaccinated participants who reported significant health events, most of them recognised their symptoms within 24 h following vaccination (overall 130 [57·5%] of 226 after dose one and 187 [82·4%] of 227 after dose two of an mRNA vaccine) and the majority (124 [54·9%] of 226 after dose one and 168 [74·0%] of 227) resolved within 3 days (appendix p 2). In comparison, 11 (3·2%) of 339 pregnant unvaccinated participants (controls) reported similar events in the 7 days before survey completion (table 1). Of these, two (18·2%) reported their symptoms as resolved at least 24 h before the control survey and nine (81·8%) reported their events as on-going for at least 6 days (appendix 2). The reported serious health events were rare (between eight [0·4%] of 1892 and 11 [0·9%] of 1216 across various pregnant groups; table 1) and occurred at similar rates in vaccinated pregnant individuals and unvaccinated controls, and after dose one and dose two for all vaccine types (table 1; appendix p 3).
Table 2.
Injection site reactions and details of significant health events reported in the 7 days following COVID-19 vaccination in vaccinated pregnant females and unvaccinated controls
| Pregnant control group (n=339) |
Dose 1 |
Dose 2 |
|||||
|---|---|---|---|---|---|---|---|
| Any mRNA (n=5597) | BNT162b2 (n=3414) | mRNA-1273 (n=2183) | Any mRNA (n=3108) | BNT162b2 (n=1892) | mRNA-1273 (n=1216) | ||
| Health events | |||||||
| Injection site reactions | NA | 4515 (80·7%) | 2631 (77·1%) | 1884 (86·3%) | 2440 (78·5%) | 1430 (75·6%) | 1010 (83·1%) |
| Significant health events* | 11 (3·2%) | 226 (4·0%) | 137 (4·0%) | 89 (4·1%) | 227 (7·3%) | 80 (4·2%) | 147 (12·1%) |
| General | |||||||
| Feeling unwell, malaise, or myalgia | 3 (0·9%) | 168 (3·0%) | 104 (3·0%) | 64 (2·9%) | 205 (6·6%) | 66 (3·5%) | 139 (11·4%) |
| Fever | 1 (0·3%) | 34 (0·6%) | 21 (0·6%) | 13 (0·6%) | 73 (2·3%) | 18 (1·0%) | 55 (4·5%) |
| Arthritis or joint pain | 2 (0·6%) | 31 (0·6%) | 16 (0·5%) | 15 (0·7%) | 57 (1·8%) | 19 (1·0%) | 38 (3·1%) |
| Jaundice | 0 | 1 (<0·1%) | 0 | 1 (<0·1%) | 0 | 0 | 0 |
| Neurological | |||||||
| Headache or migraine | 2 (0·6%) | 128 (2·3%) | 82 (2·4%) | 46 (2·1%) | 144 (4·6) | 41 (2·2%) | 103 (8·5%) |
| Dizziness or vertigo | 1 (0·3%) | 49 (0·9%) | 30 (0·9%) | 19 (0·9%) | 41 (1·3%) | 16 (0·8%) | 25 (2·1%) |
| Paresthesia | 0 | 24 (0·4%) | 16 (0·5%) | 8 (0·4%) | 27 (0·9%) | 12 (0·6%) | 15 (1·2%) |
| Fainting | 0 | 8 (0·1%) | 4 (0·1%) | 4 (0·2%) | 2 (0·1%) | 0 | 2 (0·2%) |
| Inability to walk | 0 | 7 (0·1%) | 2 (0·1%) | 5 (0·2%) | 8 (0·3%) | 2 (0·1%) | 6 (0·5%) |
| Loss of taste or smell | 0 | 10 (0·2%) | 9 (0·3%) | 1 (<0·1%) | 4 (0·1%) | 1 (0·1%) | 3 (0·2%) |
| Loss of vision | 0 | 4 (0·1%) | 2 (0·1%) | 2 (0·1%) | 1 (<0·1%) | 0 | 1 (0·1%) |
| Sudden unilateral facial weakness or paralysis | 0 | 1 (<0·1%) | 0 | 1 (<0·1%) | 3 (0·1%) | 2 (0·1%) | 1 (0·1%) |
| Seizure or convulsions | 0 | 1 (<0·1%) | 0 | 1 (<0·5%) | 0 | 0 | 0 |
| Other neurological symptoms† | 0 | 9 (0·2%) | 5 (0·1%) | 4 (0·2%) | 3 (0·1%) | 1 (0·1%) | 2 (0·2%) |
| Infection | |||||||
| Respiratory tract infection | 3 (0·9%) | 91 (1·6%) | 61 (1·8%) | 30 (1·4%) | 98 (3·2%) | 30 (1·6%) | 68 (5·6%) |
| Shingles | 0 | 1 (<0·1%) | 0 | 1 (<0·1%) | 1 (<0·1%) | 0 | 1 (0·1%) |
| Urinary tract infection symptoms | 0 | 6 (0·1%) | 2 (0·1%) | 4 (0·2%) | 2 (0·1%) | 0 | 2 (0·2%) |
| Cardio-respiratory | |||||||
| Chest pain or palpitations | 1 (0·3%) | 38 (0·7%) | 24 (0·7%) | 14 (0·6%) | 26 (0·8%) | 7 (0·4%) | 19 (1·6%) |
| Difficulty in breathing | 3 (0·9%) | 29 (0·5%) | 17 (0·5%) | 12 (0·5%) | 17 (0·5%) | 5 (0·3%) | 12 (1·0%) |
| Cough | 2 (0·6%) | 27 (0·5%) | 19 (0·6%) | 8 (0·4%) | 15 (0·5%) | 8 (0·4%) | 7 (0·6%) |
| Chest tightness | 0 | 20 (0·4%) | 13 (0·4%) | 7 (0·3%) | 17 (0·5%) | 6 (0·3%) | 11 (0·9%) |
| Allergic reactions | |||||||
| Hives | 1 (0·3%) | 14 (0·3%) | 8 (0·2%) | 6 (0·3%) | 10 (0·3%) | 5 (0·3%) | 5 (0·4%) |
| Itchy eyes | 0 | 10 (0·2%) | 5 (0·1%) | 5 (0·2%) | 8 (0·3%) | 2 (0·1%) | 6 (0·5%) |
| Throat swelling | 0 | 6 (0·1%) | 3 (0·1%) | 3 (0·1%) | 2 (0·1%) | 1 (0·1%) | 1 (0·1%) |
| Redness of both eyes | 0 | 3 (0·1%) | 1 (<0·1%) | 2 (0·1%) | 2 (0·1%) | 0 | 2 (0·2%) |
| Facial swelling | 0 | 2 (<0·1%) | 1 (<0·1%) | 1 (<0·1%) | 3 (0·1%) | 0 | 3 (0·2%) |
| Eyelid swelling | 0 | 2 (<0·1%) | 1 (<0·1%) | 1 (<0·1%) | 1 (<0·1%) | 0 | 1 (0·1%) |
| Anaphylaxis | 0 | 1 (<0·1%) | 0 | 1 (<0·1%) | 0 | 0 | 0 |
| Coagulation symptoms‡ | |||||||
| Total number | 0 | 6 (0·1%) | 3 (0·1%) | 3 (0·1%) | 4 (0·1%) | 1 (0·1%) | 3 (0·2%) |
Data are n (%).
All participants were asked about injection site reactions; only those who indicated a significant health event were further asked to provide details and participants were able to select multiple symptoms.
Weakness or paralysis of the arms or legs, confusion, change in personality or behaviour, or difficulty with urination or defecation.
Symptoms of blood clot or bleeding, including swelling, pain in legs, or bruising or pinpoint dark rash
Miscarriage or stillbirth was the most frequently reported adverse pregnancy outcome and it was reported at similar rates between control (seven [2·1%] of 339) and vaccinated groups within seven days after dose one of any mRNA vaccine (83 [1·5%] of 5597; table 3 ). Almost all pregnancy losses (73 [88%] of 83; occurred during the first trimester. There were an additional 175 (5·6%) of 3114 individuals who reported experiencing miscarriage or stillbirth between the first COVID-19 vaccine dose and completion of the second (dose two) survey (up to 10 days after dose two), although precise timing of these events relative to vaccination was not collected. Other adverse pregnancy outcomes such as vaginal bleeding, abnormal fetal heart rate, and reduced fetal movement were rarely reported within 7 days following any mRNA vaccination (table 3).
Table 3.
Reported pregnancy outcomes in the 7 days following a first dose COVID-19 vaccination in vaccinated pregnant women and unvaccinated pregnant controls
| Control (n=339) | Any mRNA (n=5597) | BNT162b2 (n=3414) | mRNA-1273 (n=2183) | ||
|---|---|---|---|---|---|
| Miscarriage or still birth | |||||
| Overall | 7 (2·1%) | 83 (1·5%) | 51 (1·5%) | 32 (1·5%) | |
| 1st trimester | 7/7 (100·0%) | 73/83 (88·0%) | 47/51 (92·2%) | 26/32 (81·3%) | |
| 2nd trimester | 0 | 7/83 (8·4%) | 4/51 (7·8%) | 3/32 (9·4%) | |
| 3rd trimester | 0 | 1/83 (1·2%) | 0 | 1/32 (3·1%) | |
| Unknown | 0 | 2/83 (2·4%) | 0 | 2/32 (6·3%) | |
| Adverse pregnancy outcomes | |||||
| Any adverse outcome | 2 (0·6%) | 50 (0·9%) | 31 (0·9%) | 19 (0·9%) | |
| Preterm labour | 0 | 3 (0·1%) | 1 (<0·1%) | 2 (0·1%) | |
| High blood pressure | 0 | 6 (0·1%) | 4 (0·1%) | 2 (0·1%) | |
| Vaginal spotting or vaginal bleeding | 1 (0·3%) | 21 (0·4%) | 10 (0·3%) | 11 (0·5%) | |
| Abnormal fetal heart rate | 0 | 2 (<0·1%) | 1 (<0·1%) | 1 (<0·1%) | |
| Other pregnancy complications* | 1 (0·3%) | 30 (0·5%) | 17 (0·5%) | 13 (0·6%) | |
Data are n (%) or n/N (%). Participants were able to report multiple outcomes.
Included lower abdominal pain, reduced fetal movement, cramp, and vomiting.
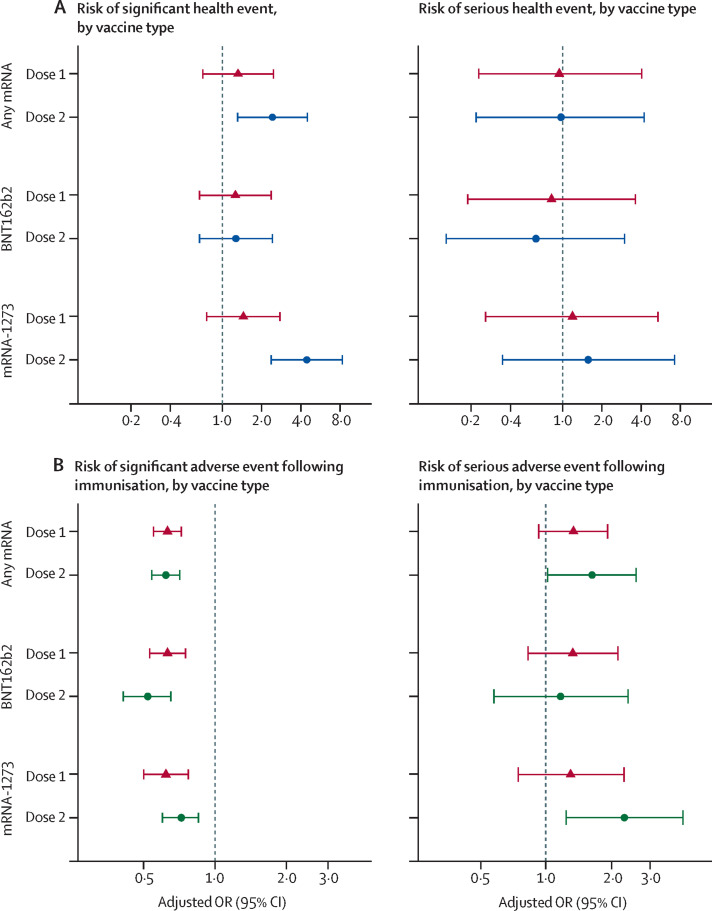
In the multivariable analysis adjusting for age group, previous SARS-CoV-2 infection, and trimester, we observed an increased risk of significant health events within 7 days after the second dose of any mRNA vaccine (adjusted OR [aOR] 2·4 [95% CI 1·3–4·5]) or the second dose of mRNA-1273 COVID-19 vaccination (aOR 4·4 [2·4–8·3]) among pregnant vaccinated individuals, compared with pregnant unvaccinated controls (figure 2A ). The first dose of any mRNA vaccine (mRNA-1273 or BNT162b2) and either dose of BNT162b2 were not associated with increased risk of significant health events. Similarly, we found no significant association between vaccination status and serious health events in pregnant people (figure 2A).
Figure 2.
Multivariable logistic regression analyses comparing significant and serious health events
(A) Comparison between vaccinated and unvaccinated pregnant females. Red triangles and blue circles represent point estimates of adjusted ORs for vaccine doses one and two, with 95% CIs. The reference group is unvaccinated pregnant people. ORs are adjusted for age group, trimester, and previous SARS-CoV-2 infection. Seven (0·1%) of 5936 pregnant vaccinated plus pregnant unvaccinated cases were not included in the multivariable analysis due to missing data. (B) Comparison between pregnant and non-pregnant vaccinated females. Red triangles and green circles represent point estimates of adjusted ORs for vaccine doses one and two, with 95% CIs. The reference group is vaccinated non-pregnant people. ORs are adjusted for age group and previous SARS-CoV-2 infection. 254 (0·1%) of the 180 362 vaccinated cases were not included in the multivariable analysis due to missing data. The x-axis of each graph is a log scale. OR=odds ratio.
In the sensitivity analysis, both univariable and multivariable models among pregnant individuals with excellent health status revealed similar findings to those in the main analysis. The increase in significant health events after dose two of any mRNA vaccine and mRNA-1273 was no longer observed when we restricted our outcome variable to events requiring medical consultation (any mRNA vaccine: aOR 1·26 [95% CI 0·45–3·52] and mRNA-1273 vaccine: aOR 1·81 [0·62–5·28]; appendix p 4).
Injection site reactions such as redness, pain, or swelling were reported in between 1430 (75·6%) of 1892 (dose two of BNT162b2) and 1884 (86·3%) of 2183 (dose one of mRNA-1273) vaccine recipients. The most frequently reported significant health events within 7 days of each vaccine dose were feeling unwell or malaise or myalgia (between 64 [2·9%] of 2183 for dose one of mRNA-1273 and 139 [11·4%] of 1216 for dose two of mRNA-1273) and headache (between 46 [2·1%] of 2183 for dose one of mRNA-1273 and 103 [8·5%] of 1216 for dose two of mRNA-1273]; table 2). When comparing vaccinated pregnant and vaccinated non-pregnant people, significant adverse events following immunisation rates (ie, excluding injection site reactions) were consistently lower among pregnant people across all mRNA vaccine types and doses (table 1). Overall, 226 (4·0%) of 5597 pregnant people reported a significant adverse event following immunisation after dose one and 227 (7·3%) of 3108 reported one after dose two, compared with 10 950 (6·3%) of 174 765 after dose one and 10 254 (11·3%) of 91 131 after dose two for non-pregnant people. Similar differences were seen for both BNT162b2 and mRNA-1273 after both dose one and dose two. The reported rates of serious health events were low (<1%) and similar in vaccinated pregnant people compared with vaccinated non-pregnant controls (table 1).
The multivariable models revealed that pregnancy was associated with a decreased risk of significant health events for any mRNA vaccine for both dose one (any mRNA: aOR 0·63 [95% CI 0·55–0·72]; BNT162b2: aOR 0·63 [0·53–0·75]; mRNA-1273: aOR 0·62 [0·5–0·77]) and dose two (any mRNA: aOR 0·62 [0·54–0·71]; BNT162b2: aOR 0·52 [0·41–0·65]; mRNA-1273: aOR 0·72 [0·6–0·85]; figure 2B). However, for the secondary endpoint of serious adverse events following immunisation, we found the second dose of mRNA-1273 was associated with a higher risk of serious adverse events (aOR 2·3 [95% CI 1·2–4·2]) in pregnant individuals compared with non-pregnant individuals. The second dose of any mRNA vaccine was also associated with a higher risk of serious adverse events (aOR 1·6 [95% CI 1·0–2·6]. This association was not observed in individuals who received BNT162b2 for dose two or with any vaccine groups for dose one. However, a sensitivity analysis that restricted our primary end point (significant health event) to those who sought medical care 7 seven days following each vaccine dose yielded no significant association between any mRNA vaccine and pregnancy status among vaccinated individuals (appendix p 5).
Discussion
In our prospective study collecting real-time data from pregnant individuals who were both vaccinated and unvaccinated, we found that significant health events—new or worsening health events following vaccination sufficient to cause work or school absenteeism, medical consultation, or prevent daily activities—were lower in pregnant people than in age-matched non-pregnant vaccine recipients. Amongst pregnant individuals, significant adverse events following immunisation were higher in those who received the mRNA-1273 vaccine for their second dose than in unvaccinated pregnant people, with no difference observed for BNT162b2 after either dose. When restricted to events resulting in medical consultation, there was no difference between groups in any of the analyses. These data can be used to appropriately inform pregnant people regarding reactogenicity of COVID-19 vaccines during pregnancy and should be considered alongside effectiveness and immunogenicity data to make appropriate recommendations about best use of COVID-19 vaccines in pregnancy.
The largest study to date of COVID-19 vaccine reactogenicity in pregnancy, which directly collected data from study participants, reported on approximately 30 000 pregnant people in the USA who had received BNT162b2 or mRNA-1273, using data from the v-safe registry.8 Pregnant people in this study reported high rates of injection site pain (92% after dose two), fatigue (72%), headache (55%), myalgia (54%) and fever or chills (35–37%), with higher rates of adverse events after dose two than after dose one. The data from the v-safe registry suggested an increased rate of these events after mRNA-1273 compared with BNT162b2 after dose two, but no formal comparisons between vaccines was done. Notably, the study collected data on all adverse events following immunisation and specific information regarding severity was not reported, so it is not possible to directly compare results with our study, although if total adverse event rates are higher after dose two, and after mRNA-1273, then rates of significant adverse events would probably follow a similar pattern. A more recent study of 7800 pregnant people compared with 2900 non-pregnant individuals also reported higher rates of adverse events, in pregnant people, after dose two of a COVID-19 mRNA vaccine.13 Some adverse events occurred at lower rates in non-pregnant individuals, including fever. Specific data on significant adverse events following immunisation were not comprehensively reported, although 156 pregnant individuals (2·5%) sought medical care after their second vaccine dose, compared with only 1·5% in our cohort. A systematic review including Shimabukuro and colleague's study8 and multiple other smaller studies reported similarly higher rates of adverse events following immunisation after dose two of a COVID-19 vaccine, but no differences between pregnant and non-pregnant controls.6
Initial clinical trials of mRNA-1273 and BNT162b2 reported relatively high rates of adverse events following immunisation compared with most routinely administered vaccines, including higher rates for dose two than dose one.14, 15 Therefore, the fact that our analysis revealed similar patterns among pregnant individuals was unsurprising—although we have been able to specifically quantify the significant and serious adverse event rates in this population for each of the mRNA vaccines. Previous studies of influenza vaccines done using the same CANVAS methodology similarly reported higher rates of adverse events following immunisation among vaccinated than among unvaccinated control participants.9
The lower rate of significant adverse events following immunisation among pregnant people than among vaccinated non-pregnant individuals is important. Before the COVID-19 pandemic, influenza and tetanus-diphtheria-pertussis (Tdap) vaccines had been routinely recommended for pregnant people in many countries. Previous studies have mostly reported no significant differences in adverse events following immunisation between pregnant and non-pregnant individuals,16, 17, 18, 19 or higher rates in pregnancy—for example, one study reported increased moderate to severe injection-site pain in pregnant (17·9%) compared with non-pregnant (11·1%) women after Tdap vaccination.17 Several highly dynamic immunological adaptations occur during pregnancy, including a skew towards a T-helper-2 dominant state.20 Because mRNA vaccines have been specifically designed to elicit a Th1-biased immune response,21 it is possible that the Th2-bias during pregnancy is partly responsible for this lower rate of significant adverse events following immunisation.
Our study has several strengths and limitations. It is a multicentre study across Canada and thus includes broad representation of people within Canada, although most participants who reported ethnicity were White and these data therefore might not be fully generalisable to other populations. Overall, the pregnant people in our study had similar characteristics (age, residence, health status) to the pregnant population in Canada.22, 23, 24 Specifically, most of our participants were aged 30–49 years (74·7% versus 61·5% of pregnant people in Canada);24 most participants were from Ontario (37·2%), Quebec (26·2%), British Columbia/Yukon (20·2%), and Alberta (13·9%), regions which represent 37·9%, 22·8%, 11·8%, and 13·7% of live births in Canada in 2020;24 89·9% described their health as excellent, very good, or good—across Canada, severe maternal morbidity has been described in 1·3–1·6% of pregnancies22 and 27% of pregnant people have chronic conditions (although the severity is variable and some will have a good or better than good health status).23 Participants were enrolled from all trimesters in pregnancy and this was included as a co-variate in the multivariable models. An important advantage over many other similar studies is the contemporaneous recruitment of non-pregnant vaccinated individuals, enabling a robust direct comparison in significant adverse events following immunisation between pregnant and non-pregnant individuals. We focused on events occurring within the first 7 days following vaccination and thus acute and local reactions. Longer-term follow-up of this cohort is ongoing, and we will be able to comment on health events that occur over a longer time frame after vaccination once these data are available.
Furthermore, CANVAS is based on self-reports from study participants, without verification from medical records. This method of reporting is subjective and might be subject to recall bias, but has been shown to be reliable for short time periods, such as in this study.25, 26 The study relied on individuals having an email address and actively enrolling. Such individuals might differ in health-seeking behaviour from the rest of the population because they might represent a different socio-economic status, but at least for our comparisons between groups it would be expected that they would be similarly affected by any bias as recruitment required an email address. Study participants were asked their age within prespecified age groups, and we therefore do not know the specific age of each participant. Pregnant participants reported trimester of pregnancy rather than weeks’ gestation. Finally, our sample size precludes detection of very rare adverse events, which can be identified in the general population from the passive surveillance systems in place across Canada and in other countries.
Our data provide reassuring evidence that COVID-19 mRNA vaccines are safe in pregnancy, with lower rates of significant adverse events following immunisation in pregnant people than in non-pregnant vaccine recipients for both mRNA vaccines used in Canada, after dose one and dose two. Although rates of significant adverse events following immunisation were highest after dose two for mRNA-1273 recipients, both mRNA vaccines are highly immunogenic and effective in pregnancy.6 Given the increased rate of significant complications associated with COVID-19 in pregnancy, high vaccine coverage in this group is important for protection of the pregnant individual and young infant,27 via passive transplacental transfer of antigen-specific IgG antibody and protection via breast milk.28, 29 These data can be used to inform pregnant individuals of the expected adverse events following vaccination. Further studies of non-COVID-19 mRNA vaccines are required to identify if the reduced reactogenicity observed in pregnant people in this study is a feature of the mRNA vaccine platform, or of these specific vaccines. Further long-term data are awaited from this cohort following a 6-month follow-up. The number of pregnant individuals receiving the ChAdOx-S vaccine in our population was very low and data for this vaccine in countries where it has been more widely used are important to provide a more complete overview of the safety of COVID-19 vaccines in pregnancy.
Data sharing statement
De-identified data collected for the study (with data dictionary) might be made available upon approval by the study investigators, with relevant agreements (eg, data sharing agreement) and approvals (eg, relevant ethics approvals). Requests should be directed to the corresponding author in the first instance.
Declaration of interests
MS has been an investigator on projects funded by GlaxoSmithKline, Merck, Moderna, Pfizer, Sanofi-Pasteur, Seqirus, Symvivo, and VBI Vaccines. All funds have been paid to his institute, and he has not received any personal payments. OGV has been an investigator, coinvestigator, or expert panelist on projects funded by GlaxoSmithKline, Merck, Pfizer, and Seqirus, outside of the submitted work. JDK has been an investigator on projects funded by GlaxoSmithKline, Merck, Moderna, and Pfizer. All funds have been paid to his institute, and he has not received any personal payments. KAT has been an investigator on projects funded by GlaxoSmithKline. All funds have been paid to her institute, and she has not received any personal payments. JEI has been an investigator on projects funded by GlaxoSmithKline, and Sanofi-Pasteur. All funds have been paid to her institute, and she has not received any personal payments. AJM has been an investigator on projects funded by GlaxoSmithKline, Merck, Pfizer, Sanofi-Pasteur, and Seqirus, with funds paid to her institution, and has received honoraria for participation in advisory boards from Astra-Zeneca, GlaxoSmithKline, Medicago, Merck, Moderna, Pfizer, Sanofi-Pasteur, Seqirus, and for presentations from Astra-Zeneca, and Moderna. GDS has been an investigator on a project funded by Pfizer. All funds have been paid to his institute, and he has not received any personal payments. All other authors declare no competing interests.
Acknowledgments
Acknowledgements
This work was supported by the COVID-19 Vaccine Readiness funding from the Canadian Institutes of Health Research and the Public Health Agency of Canada CANVAS (grant number CVV-450980) and by funding from the Public Health Agency of Canada, through the Vaccine Surveillance Reference Group and the COVID-19 Immunity Task Force. MS is supported via salary awards from the BC Children's Hospital Foundation, the Canadian Child Health Clinician Scientist Program, and the Michael Smith Foundation for Health Research.
Contributors
JAB conceived and obtained funding for the study. MS, LV, OGV, JDK, MPM, KAT, JEI, AM, GDS, and JAB collected data and contributed to study design. KM contributed to study design. PS, HPS, and MI analysed the data. MS, PS, HPS, MI, and JAB accessed and verified the data. MS and PS drafted the original version of the manuscript. All authors had full access to the data, reviewed the manuscript, contributed to data interpretation, approved the final version and accept responsibility to submit for publication.
Supplementary Material
References
- 1.Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370 doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cojocaru L, Noe M, Pahlavan A, et al. Increased risk of severe COVID-19 disease in pregnancy in a multicenter propensity score-matched study. medRxiv. 2021 doi: 10.1101/2021.06.18.21258899. published online June 21. (preprint). [DOI] [PubMed] [Google Scholar]
- 3.National Advisory Committee on Immunization Recommendations on the use of COVID-19 vaccines. 2020. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines.html#a8
- 4.Poliquin V, Castillo E, Boucoiran I, et al. Society of Obstetricians and Gynecologists of Canada; Ottawa, ON, Canada: 2020. SOGC statement on COVID-19 vaccination in pregnancy. [Google Scholar]
- 5.The American College of Obstetricians and Gynecologists COVID-19 vaccination considerations for obstetric-gynecologic care. 2020. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care
- 6.Fu W, Sivajohan B, McClymont E, et al. Systematic review of the safety, immunogenicity, and effectiveness of COVID-19 vaccines in pregnant and lactating individuals and their infants. Int J Gynaecol Obstet. 2021;156:406–417. doi: 10.1002/ijgo.14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delara M, Sadarangani M. Immunization in pregnancy to protect pregnant people and their newborns against COVID-19. Expert Rev Vaccines. 2022:1–3. doi: 10.1080/14760584.2022.2031987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS) Vaccine. 2015;33:4398–4405. doi: 10.1016/j.vaccine.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bettinger JA, De Serres G, Valiquette L, et al. 2017/18 and 2018/19 seasonal influenza vaccine safety surveillance, Canadian National Vaccine Safety (CANVAS) Network. Euro Surveill. 2020;25:22. doi: 10.2807/1560-7917.ES.2020.25.22.1900470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bettinger JA, Sadarangani M, De Serres G, et al. The Canadian National Vaccine Safety Network: surveillance of adverse events following immunisation among individuals immunised with the COVID-19 vaccine, a cohort study in Canada. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-051254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kachikis A, Englund JA, Singleton M, Covelli I, Drake AL, Eckert LO. Short-term reactions among pregnant and lactating individuals in the first wave of the COVID-19 vaccine rollout. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munoz FM, Englund JA. Vaccines in pregnancy. Infect Dis Clin North Am. 2001;15:253–271. doi: 10.1016/s0891-5520(05)70278-6. [DOI] [PubMed] [Google Scholar]
- 17.Fortner KB, Swamy GK, Broder KR, et al. Reactogenicity and immunogenicity of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant and nonpregnant women. Vaccine. 2018;36:6354–6360. doi: 10.1016/j.vaccine.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munoz FM, Patel SM, Jackson LA, et al. Safety and immunogenicity of three seasonal inactivated influenza vaccines among pregnant women and antibody persistence in their infants. Vaccine. 2020;38:5355–5363. doi: 10.1016/j.vaccine.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan SG, Price OH, Regan AK. Burden, effectiveness and safety of influenza vaccines in elderly, paediatric and pregnant populations. Ther Adv Vaccines Immunother. 2019;7 doi: 10.1177/2515135519826481. 2515135519826481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abu-Raya B, Michalski C, Sadarangani M, Lavoie PM. Maternal immunological adaptation during normal pregnancy. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.575197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunology. 2021;21:475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Public Health Agency of Canada . Government of Canada; Ottawa, ON, Canada: 2019. Perinatal Health Indicators for Canada 2017. [Google Scholar]
- 23.Public Health Agency of Canada Pregnancy in Canada. 2020. https://www.canada.ca/en/public-health/services/publications/healthy-living/pregnancy-canada-infographic.html
- 24.Statistics Canada Births, 2020. 2021. https://www150.statcan.gc.ca/n1/daily-quotidien/210928/dq210928d-cansim-eng.htm
- 25.Short ME, Goetzel RZ, Pei X, et al. How accurate are self-reports? Analysis of self-reported health care utilization and absence when compared with administrative data. J Occup Environ Med. 2009;51:786–796. doi: 10.1097/JOM.0b013e3181a86671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clothier HJ, Selvaraj G, Easton ML, Lewis G, Crawford NW, Buttery JP. Consumer reporting of adverse events following immunization. Hum Vaccin Immunother. 2014;10:3726–3730. doi: 10.4161/hv.34369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halasa NB, Olson SM, Staat MA, et al. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants aged <6 months—17 States, July 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:264–270. doi: 10.15585/mmwr.mm7107e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang YJ, Murphy EA, Singh S, et al. Association of gestational age at coronavirus disease 2019 (COVID-19) vaccination, history of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection, and a vaccine booster dose with maternal and umbilical cord antibody levels at delivery. Obstet Gynecol. 2022;139:373–380. doi: 10.1097/AOG.0000000000004693. [DOI] [PubMed] [Google Scholar]
- 29.Shook LL, Atyeo CG, Yonker LM, et al. Durability of anti-spike antibodies in infants after maternal COVID-19 vaccination or natural infection. JAMA. 2022;327:1087–1089. doi: 10.1001/jama.2022.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data collected for the study (with data dictionary) might be made available upon approval by the study investigators, with relevant agreements (eg, data sharing agreement) and approvals (eg, relevant ethics approvals). Requests should be directed to the corresponding author in the first instance.