Abstract
Background
High flow nasal cannulae (HFNC) are small, thin, tapered binasal tubes that deliver oxygen or blended oxygen/air at gas flows of more than 1 L/min. HFNC are increasingly being used as a form of non‐invasive respiratory support for preterm infants.
Objectives
To compare the safety and efficacy of HFNC with other forms of non‐invasive respiratory support in preterm infants.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 1), MEDLINE via PubMed (1966 to 1 January 2016), EMBASE (1980 to 1 January 2016), and CINAHL (1982 to 1 January 2016). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
Randomised or quasi‐randomised trials comparing HFNC with other non‐invasive forms of respiratory support in preterm infants immediately after birth or following extubation.
Data collection and analysis
The authors extracted and analysed data, and calculated risk ratio, risk difference and number needed to treat for an additional beneficial outcome.
Main results
We identified 15 studies for inclusion in the review. The studies differed in the interventions compared (nasal continuous positive airway pressure (CPAP), nasal intermittent positive pressure ventilation (NIPPV), non‐humidified HFNC, models for delivering HFNC), the gas flows used and the indications for respiratory support (primary support from soon after birth, post‐extubation support, weaning from CPAP support). When used as primary respiratory support after birth compared to CPAP (4 studies, 439 infants), there were no differences in the primary outcomes of death (typical risk ratio (RR) 0.36, 95% CI 0.01 to 8.73; 4 studies, 439 infants) or chronic lung disease (CLD) (typical RR 2.07, 95% CI 0.64 to 6.64; 4 studies, 439 infants). HFNC use resulted in longer duration of respiratory support, but there were no differences in other secondary outcomes. One study (75 infants) showed no differences between HFNC and NIPPV as primary support. Following extubation (total 6 studies, 934 infants), there were no differences between HFNC and CPAP in the primary outcomes of death (typical RR 0.77, 95% CI 0.43 to 1.36; 5 studies, 896 infants) or CLD (typical RR 0.96, 95% CI 0.78 to 1.18; 5 studies, 893 infants). There was no difference in the rate of treatment failure (typical RR 1.21, 95% CI 0.95 to 1.55; 5 studies, 786 infants) or reintubation (typical RR 0.91, 95% CI 0.68 to 1.20; 6 studies, 934 infants). Infants randomised to HFNC had reduced nasal trauma (typical RR 0.64, 95% CI 0.51 to 0.79; typical risk difference (RD) −0.14, 95% CI −0.20 to −0.08; 4 studies, 645 infants). There was a small reduction in the rate of pneumothorax (typical RR 0.35, 95% CI 0.11 to 1.06; typical RD −0.02, 95% CI −0.03 to −0.00; 5 studies 896 infants) in infants treated with HFNC. Subgroup analysis found no difference in the rate of the primary outcomes between HFNC and CPAP in preterm infants in different gestational age subgroups, though there were only small numbers of extremely preterm and late preterm infants. One trial (28 infants) found similar rates of reintubation for humidified and non‐humidified HFNC, and two other trials (100 infants) found no difference between different models of equipment used to deliver humidified HFNC. For infants weaning from non‐invasive respiratory support (CPAP), two studies (149 infants) found that preterm infants randomised to HFNC had a reduced duration of hospitalisation compared with infants who remained on CPAP.
Authors' conclusions
HFNC has similar rates of efficacy to other forms of non‐invasive respiratory support in preterm infants for preventing treatment failure, death and CLD. Most evidence is available for the use of HFNC as post‐extubation support. Following extubation, HFNC is associated with less nasal trauma, and may be associated with reduced pneumothorax compared with nasal CPAP. Further adequately powered randomised controlled trials should be undertaken in preterm infants comparing HFNC with other forms of primary non‐invasive support after birth and for weaning from non‐invasive support. Further evidence is also required for evaluating the safety and efficacy of HFNC in extremely preterm and mildly preterm subgroups, and for comparing different HFNC devices.
Plain language summary
Nasal cannula for breathing support in premature babies
Review question: In preterm infants, is the use of high flow nasal cannulae (HFNC) as effective as other non‐invasive methods of respiratory support in preventing chronic lung injury and death?
Background: There are a variety of ways in which non‐invasive breathing support can be provided to preterm infants with irregular breathing (apnoea) or lung disease. These include supplemental oxygen given into the incubator, via a head‐box or via a nasal cannula; continuous positive airways pressure (CPAP) given via nasal prongs or mask; and nasal intermittent positive pressure ventilation (NIPPV) where, in addition to CPAP, inflations of a higher pressure are given intermittently. High flow nasal cannulae (HFNC) deliver oxygen or a mixture of oxygen and air via small, thin tubes that sit just inside the nostrils. HFNC have recently been introduced as another potential form of non‐invasive support.
Study characteristics: This review found 15 randomised studies that compared HFNC with other non‐invasive ways of supporting babies' breathing. The studies differed in the interventions that were compared, the gas flows used and the reasons for respiratory support.
Results: When HFNC was used as first‐line respiratory support after birth compared to CPAP (4 studies, 439 infants), there were no differences in the rates of death or chronic lung disease (CLD). HFNC use resulted in longer duration of respiratory support, but there were no differences in other outcomes. One study (75 infants) showed no differences between HFNC and NIPPV as breathing support after birth. When HFNC were used after a period of mechanical ventilation (total 6 studies, 934 infants), there were no differences between HFNC and CPAP in the rates of death or CLD. There was no difference in the rate of treatment failure or reintubation. Infants randomised to HFNC had less trauma to the infant's nose. There was a small reduction in the rate of pneumothorax in infants treated with HFNC. We found no difference between the effect of HFNC compared with CPAP in preterm infants in different gestational age subgroups, though there were only small numbers of extremely preterm and late preterm infants. One trial (28 infants) found similar rates of reintubation for humidified and non‐humidified HFNC, and two other trials (100 infants) found no difference between different models of equipment used to deliver humidified HFNC. For infants weaning from non‐invasive respiratory support (CPAP), two studies (149 infants) found that preterm infants randomised to HFNC had a reduced duration of hospitalisation compared with infants who remained on CPAP.
Conclusions: HFNC use has similar rates of efficacy to other forms of non‐invasive respiratory support in preterm infants for preventing treatment failure, death and CLD. Most evidence is available for the use of HFNC as post‐extubation support. Following extubation, use of HFNC is associated with less nasal trauma, and may be associated with reduced pneumothorax compared with nasal CPAP. Further adequately powered randomised controlled trials should be undertaken in preterm infants comparing HFNC with other forms of primary non‐invasive support after birth and for weaning from non‐invasive support. Further evidence is also required for evaluating the safety and efficacy of HFNC in extremely preterm and mildly preterm subgroups, and for comparing different HFNC devices.
Background
There are a variety of ways in which respiratory support can be provided to preterm infants with apnoea or parenchymal lung disease non‐invasively, i.e. without an endotracheal tube . These include supplemental oxygen given into the incubator, via a head‐box or via a nasal cannula; continuous positive airways pressure (CPAP) given via nasal prongs or mask; and nasal intermittent positive pressure ventilation (NIPPV) where, in addition to CPAP, inflations of a higher pressure are given intermittently.
Nasal cannulae are small, thin, tapered tubes (usually less than 1 cm in length) that sit just inside each nostril without occluding them (Frey 2003). Oxygen delivered by 'low flow' nasal cannulae (LFNC) typically refers to the use of flow rates of less than or equal to 1 litre per minute (L/min). Usually the gas used is unblended (i.e. 100% oxygen), and is neither heated nor humidified. LFNC are commonly used in convalescing preterm infants, often with chronic lung disease (Walsh 2005). Use of LFNC does not appear to provide significant support to pulmonary function (apart from the provision of oxygen) (Hensey 2013; O'Donnell 2013).
In contrast, 'high‐flow' nasal cannulae (HFNC) deliver oxygen or blended oxygen and air at higher flow rates than LFNC. For the purposes of this review, HFNC delivery is defined as the use of gas flows greater than 1 L/min, although typically higher gas flows (e.g. 2 to 8 L/min) are used (Hough 2012; Manley 2012). Gas given via HFNC is routinely heated and humidified, as with CPAP. High gas flows in preterm infants may provide positive end‐expiratory pressure (PEEP) at similar levels to that commonly set with CPAP in clinical practice (Frey 2001; Sreenan 2001; Spence 2007; Wilkinson 2008; Lampland 2009). Washout of nasopharyngeal dead‐space has also been proposed as an important mechanism of action of HFNC (Dysart 2009; Frizzola 2011). In HFNC systems, circuit flow is adjusted according to clinical effect and, although a pressure relief valve is used in some circuits, the internal circuit pressure is not routinely measured. HFNC have been suggested as an alternative form of respiratory support for preterm infants with apnoea, respiratory distress syndrome or chronic lung disease. They appear to be easy to apply and maintain (Saslow 2006), and compared to CPAP they appear to be more comfortable for infants (Osman 2014), and are preferred by nurses (Roberts 2014) and parents (Klingenberg 2014).
Nasal CPAP is widely used in premature and term newborns and provides an effective, safe alternative to endotracheal intubation (Morley 2004). It has been shown to reduce extubation failure, treat apnoea and respiratory distress syndrome and, by minimising duration of mechanical ventilation, may reduce chronic lung disease (De Paoli 2003). The most effective and popular means of administering CPAP is by using short binasal prongs (Morley 2004). These prongs are designed to fit snugly into the infant's nostrils with minimal leakage. By contrast, nasal cannulae do not usually occlude the nostrils and have the potential for a large leak around them. Other methods of delivering CPAP to the nose that are in common use include single nasal prongs and nasal masks (De Paoli 2008). Oxygen administered by nasal CPAP is usually blended, humidified and heated. In contrast to HFNC, the pressure delivered by nasal CPAP circuits is directly measured and regulated.
Both CPAP and HFNC systems may have adverse effects in newborns. Binasal prongs used to deliver CPAP are associated with trauma to the nasal septum and distortion of the nares (Robertson 1996; Sreenan 2001). It has been thought that HFNC may cause less nasal injury (Saslow 2006), however the use of humidified, unheated HFNC has been associated with mucosal irritation, nasal obstruction or bleeding as well as a possible increase in the risk of nosocomial infection (Kopelman 2003a; Kopelman 2003b).
Concern has also been expressed about the possibility of lung overdistension and trauma from unmeasured and variable PEEP with HFNC (Finer 2005; Hegde 2013). One case associating HFNC with pneumocephalus, pneumo‐orbitis and scalp emphysema has been reported (Jasin 2008). Other possible risks associated with HFNC include gastric distension or perforation, as has been seen with CPAP (Garland 1985).
The purpose of this review is to compare HFNC with other methods of providing non‐invasive respiratory support in premature newborn infants.
Objectives
The objectives were as follows.
In preterm infants, to compare the efficacy and safety of HFNC with other non‐invasive methods of respiratory support including:
Ambient (head‐box or cot) oxygen;
Low flow nasal cannulae (LFNC);
Continuous positive airways pressure (CPAP), via nasal prongs or mask
Nasal intermittent positive pressure ventilation (NIPPV);
Alternative HFNC technique
Methods
Criteria for considering studies for this review
Types of studies
All randomised and quasi‐randomised studies (including crossover trials). Studies reported in abstract form were included in the 'Studies awaiting classification' category. Data from one unpublished study (published only in abstract form) were obtained from the authors to enable its inclusion in the review.
Types of participants
1. Preterm infants (< 37 weeks' gestational age) receiving respiratory support after birth, either prophylactically or for respiratory distress syndrome, without a prior period of intermittent positive pressure ventilation (IPPV).
2. Preterm infants (< 37 weeks' gestational age) receiving respiratory support following a period of intermittent positive pressure ventilation (IPPV).
Types of interventions
For the purposes of this review, we defined high flow nasal cannula oxygen as the delivery of oxygen or blended oxygen and air via nasal cannulae at gas flow rates greater than 1 L/min.
Alternative interventions included:
Head box oxygen;
Low flow nasal cannulae (gas flow rates less than or equal to 1 L/min);
Nasal CPAP;
NIPPV;
HFNC using an alternative technique (e.g. humidified versus non‐humidified, or different HFNC devices).
Types of outcome measures
Primary outcomes
Death (before hospital discharge) or chronic lung disease (as defined below);
Death;
Chronic lung disease. CLD was defined as a requirement for supplemental oxygen and/or respiratory support at 36 weeks' postmenstrual age (PMA) for infants born at less than 32 weeks' gestational age or at 28 days of age for infants born at 32 weeks' gestational age or later. Note: data from studies that reported an outcome of 'CLD' or 'bronchopulmonary dysplasia' ('BPD') without an accompanying definition were still included in this outcome.
Secondary outcomes
Treatment failure
Intubation (or re‐intubation) within 7 days of trial entry*. Note: studies that reported intubation (or re‐intubation) within a shorter period than 7 days (e.g. intubation < 72 hours) were included in the analysis of this outcome;
Treatment failure (as defined by the trial authors) within 7 days of trial entry*. Note: studies that reported treatment failure within a shorter period than 7 days (e.g. treatment failure < 72 hours) were included in the analysis of this outcome;
Intubation at any time point following trial entry*.
Respiratory support:
Duration of mechanical ventilation via an endotracheal tube (days, or post‐menstrual age (PMA) at end)*;
Duration of any form of respiratory support (mechanical ventilation, CPAP, high flow nasal cannulae, or oxygen) (days, or PMA at end);
Duration of hospitalisation (days, or PMA at end).
Complications:
Air leak syndromes (pneumothorax, pneumomediastinum, pneumopericardium or pulmonary interstitial emphysema (PIE)) reported either individually or as a composite outcome;
Nasal trauma (defined as erythema or erosion of the nasal mucosa, nares or septum). Note some studies reported this as a continuous outcome and were not able to be included in meta‐analysis;
Nosocomial sepsis (defined as positive blood or cerebrospinal fluid (CSF) cultures taken after five days of age). Note some studies used alternative definitions, or did not define sepsis. These were included in meta‐analysis;
Gastrointestinal perforation or severe necrotising enterocolitis (NEC) (stage II or more according to Bell's criteria (Bell 1978)). Note: some included studies only reported the incidence of NEC, and were included in the analysis of this outcome;
Weight gain prior to discharge from hospital;
Days to attain full feeds*.
Neurosensory outcomes:
Retinopathy of prematurity (ROP): any stage and stage 3 or greater;
Long‐term neurodevelopmental outcome (rates of cerebral palsy on physician assessment, developmental delay i.e. IQ 2 standard deviations less than the mean on validated assessment tools such as Bayley's Mental Developmental Index), blindness, hearing impairment requiring amplification.
Outcome measures that were not in the original review, that were modified or included after review of the available data, are marked with an asterisk (*).
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register). We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 1) in The Cochrane Library; MEDLINE via PubMed (1996 to 1 January 2016); EMBASE (1980 to 1 January 2016); CINAHL (1982 to 1 January 2016) using the following search terms: (oxygen OR positive pressure) AND (nasal cannula* OR nasal prong), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). No language restrictions were applied. We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International Trials Registry and Platform www.who.int/ictrp/en/; and the ISRCTN Registry). In addition, the published abstracts of the Society for Pediatric Research and the European Society for Paediatric Research were searched (2000 to 2014).
Data collection and analysis
The standard methods of the Cochrane Neonatal Review Group were employed.
Selection of studies
We included all randomised and quasi‐randomised controlled trials fulfilling the selection criteria described in the previous section. The authors reviewed the results of the search and separately selected the studies for inclusion. The review authors resolved any disagreement by discussion.
Data extraction and management
At least two review authors independently performed trial searches, assessments of methodology and extraction of data; and compared and resolved any differences found at each stage. For each trial, we collected information regarding blinding of randomisation, the intervention and outcome measurements as well as completeness of follow‐up. For crossover trials, data from the first period only were used.
Where any queries arose or where additional data were required, the study authors were contacted.
Assessment of risk of bias in included studies
We assessed the methodological quality of the studies using the following criteria: allocation concealment (blinding of randomisation), blinding of intervention, completeness of follow‐up, and blinding of outcome measurement or assessment. For each criterion, the assessment was one of the following: yes; no; can't tell. The review authors separately assessed each study and any disagreement was resolved by discussion. This information was added to the 'Characteristics of included studies' table.
The authors evaluated the following issues and entered them into the 'Risk of bias' table.
1) Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
‐ adequate (any truly random process e.g. random number table; computerised random number generator);
‐ inadequate (any non‐random process e.g. odd or even date of birth; hospital or clinic record number);
‐ unclear.
(2) Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
‐ adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
‐ inadequate (open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth);
‐ unclear.
(3) Blinding (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorised the methods as:
‐ adequate, inadequate or unclear for participants;
‐ adequate, inadequate or unclear for personnel;
‐ adequate, inadequate or unclear for outcome assessors.
We classified objective outcomes (for example death, chronic lung disease) in the absence of blinding as unclear for performance bias. We classified subjective outcomes (for example nasal mucosal injury) in the absence of blinding as high risk for bias.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis where possible. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total number of randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
‐ adequate (< 20% missing data);
‐ inadequate (≥ 20% missing data):
‐ unclear.
(5) Selective reporting bias. Are reports of the study free of any suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
‐ adequate (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
‐ inadequate (where not all the study’s pre‐specified outcomes were reported; one or more of the reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
‐ unclear.
(6) Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias, as:
‐ yes; no; or unclear.
If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Measures of treatment effect
We extracted categorical data (for example number dying or with chronic lung disease) for each intervention group, and calculated risk ratio (RR), risk difference (RD) and number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) as appropriate. We obtained means and standard deviations for continuous data (for example number of days of respiratory support, or duration of oxygen dependency); and we calculated the 95% confidence interval (CI) for each measure of effect.
Assessment of heterogeneity
We estimated heterogeneity using the I² statistic.
Data synthesis
We applied the fixed‐effect model for meta‐analysis. We obtained means and standard deviations for continuous data (for example number of days of respiratory support, or duration of oxygen treatment) and performed analysis using the weighted mean difference (WMD). We calculated the 95% CI for each measure of effect.
Subgroup analysis and investigation of heterogeneity
Where there was sufficient data we performed subgroup analysis by gestational age (GA) at birth for the primary outcome (death or CLD) and its components, and for treatment failure:
GA > 32 weeks*
GA 28 to 32 weeks*
GA < 28 weeks*
Subgroups that were modified since the original protocol/review are marked with an asterisk (*).
Sensitivity analysis
We had planned to perform a sensitivity analysis for quality of methods used.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies
We identified 15 studies for inclusion. The studies by Campbell 2006 (40 infants), Woodhead 2006 (30 infants), Miller 2010 (40 infants), Abdel Hady 2011 (60 infants), Collins 2013 (132 infants), Manley 2013 (303 infants), Yoder 2013 (351 infants), Mostafa‐Gharehbaghi 2014 (85 infants), Sadeghnia 2014 (60 infants), Badiee 2015 (89 infants), and Kugelman 2015 (76 infants) were available as full journal publications. The study by Nair 2005 (67 infants) was published as an abstract; additional unpublished data were provided by the authors enabling its inclusion in this review. Iranpour 2011 (70 infants), Ciuffini 2014 (177 infants), and Liu 2014 (150 infants) were published in English in abstract form and in full text in Persian, Italian and Chinese respectively. The authors of Iranpour 2011, Collins 2013, Manley 2013, Yoder 2013, Mostafa‐Gharehbaghi 2014, Liu 2014, and Kugelman 2015 kindly provided additional data. Several randomised controlled trials of HFNC versus other means of non‐invasive support are currently in progress, have been completed but are not yet published, or are awaiting further assessment (NCT01939067; ACTRN12615000077561; IRCT2014012716376N1; Lawrence 2012; Chen 2015; Febre 2015; Tang 2015; NCT02055339; ACTRN12610000677000; ISRCTN66716753; ACTRN12613000303741; JPRN‐UMIN000013906; NCT01270581).
1. HFNC versus CPAP for primary respiratory support after birth
Nair 2005 was a single‐centre study that enrolled 67 preterm infants of 27 to 34 weeks' gestational age (GA) with respiratory distress in the first six hours after birth. Infants in this study had a mean GA of 32 weeks and birth weight of 1700 grams and were randomised to HFNC (mean flow rate 5 to 6 L/min) or CPAP (5 to 6 cmH2O). The primary outcome was respiratory failure requiring intubation, based on prespecified criteria.
Yoder 2013 was a multi‐centre study that enrolled 432 term and preterm infants of more than 28 weeks' GA who were planned to receive non‐invasive respiratory support either as primary support after birth or post‐extubation. Of these, 351 infants were preterm, with 125 preterm infants in the primary support arm, and 226 in the post‐extubation arm. Infants were randomised to HFNC (3 to 5 L/min) or nasal CPAP (5 to 6 cmH2O). The primary outcome was need for intubation within 72 hours of commencing the allocated treatment, based on prespecified criteria.
Iranpour 2011 was a single‐centre study, published in Persian, that enrolled 70 preterm infants of 30 to 35 weeks' gestation at 24 hours of age who had ongoing features of respiratory distress and oxygen requirement. Infants were randomised to HFNC (gas flow 1.5 to 3 L/min based on Sreenan 2001) or to continuing nasal CPAP (6 cmH2O). Infants who met prespecified criteria (before or after randomisation) received surfactant via an INSURE (Intubation, Surfactant administration, Extubation) technique.
Ciuffini 2014 was a report of interim results from a single‐centre study, published in Italian, that enrolled 177 of a planned 316 preterm infants, 29 to 36 weeks' GA, with mild to moderate respiratory distress after birth. Infants in the study had mean GA of 33 weeks and birth weight of 1900 grams. Infants were randomised to receive HFNC (4 to 6 L/min) or nasal CPAP (4 to 6 cmH2O). The primary outcome was the need for intubation within 72 hours of life, based on prespecified criteria.
2. HFNC versus NIPPV for primary respiratory support after birth
Kugelman 2015 was a single‐centre study that enrolled 76 preterm infants of less than 35 weeks' GA, with birth weight exceeding 1000 grams, who required primary non‐invasive respiratory support. Infants in the study had mean GA of 33 weeks and birth weight of 1800 grams. Infants were treated with either HFNC (starting gas flow 1 L/min, increased up to 5 L/min as required) or synchronised NIPPV (positive inflation pressure 14 to 22 cmH2O, positive end‐expiratory pressure 6 cmH2O, rate 12 to 30 inflations per minute). The primary outcome was treatment failure according to prespecified criteria.
3. HFNC versus CPAP to prevent extubation failure
Campbell 2006 was a single‐centre study that enrolled 40 intubated preterm infants (birth weight ≤ 1250 grams). Infants in this study had a mean GA of 27 weeks and birth weight of 1000 grams. Infants were randomised to humidified, unheated HFNC (mean gas flow 1.6 L/min) or variable flow CPAP (5 to 6 cmH2O) after extubation. The primary outcome was need for reintubation, based on prespecified criteria.
Collins 2013 was a single‐centre study that enrolled 132 intubated very preterm infants (< 32 weeks gestation at birth). Infants in the study had mean GA of 28 weeks and birth weight of 1100 grams. Infants were randomised to receive either HFNC (8 L/min) or nasal CPAP (8 cmH2O) after extubation. The primary outcome was extubation failure in the first seven days after extubation, based on prespecified criteria.
Manley 2013 was a multi‐centre, non‐inferiority study that enrolled 303 intubated very preterm infants (< 32 weeks' gestation at birth). Infants in the study had mean GA of 27 weeks and birth weight of 1000 grams. Infants were randomised to receive either HFNC (5 to 6 L/min) or CPAP (7 cmH2O) after extubation. The primary outcome was treatment failure within seven days of randomisation, based on prespecified criteria.
Yoder 2013 (see above) included 226 preterm infants enrolled post‐extubation.
Liu 2014 was a multi‐centre study, published in Chinese, that enrolled a total of 155 infants (< 7 days old), of which 150 were preterm. Infants in the study had a mean GA of 35.5 weeks, and birth weight of 2500 grams. Infants were randomised to either HFNC (gas flow 3 to 8 L/min depending on infant weight) or nasal CPAP (pressure as set pre‐extubation) after extubation. The primary outcomes were extubation failure (reintubation within seven days), BPD or death in hospital.
Mostafa‐Gharehbaghi 2014 was a single‐centre study that enrolled 123 preterm infants with GA of 30 to 34 weeks and birth weight of 1250 to 2000 grams. Infants in the study had mean GA of 32 weeks and birth weight of 1900 grams. Infants were initially stabilised with nasal CPAP and treated with intubation and surfactant in the NICU (INSURE technique). Infants were extubated after INSURE to either HFNC (6 L/min) or nasal CPAP (5 to 6 cmH2O). The primary outcome was re‐intubation within three days of surfactant administration, according to prespecified criteria.
4. Humidified HFNC versus non‐humidified HFNC to prevent extubation failure
Woodhead 2006 was a single‐centre study that enrolled 30 preterm infants. Infants in the study had a mean GA of 32 weeks and birth weight of 1700 grams. Infants were randomised to humidified HFNC (VapothermTM) (mean gas flow 3.1 L/min) or non‐humidified HFNC (mean gas flow 1.8 L/min) following extubation. This was a randomised crossover trial; results from only the first study period were used for analysis. The primary outcome was failure of extubation (defined either by the need for reintubation or a switch to the alternative modality of HFNC).
5. Alternative HFNC models to prevent extubation failure
Miller 2010 was a single‐centre pilot study that enrolled 40 preterm infants of 26 to 29 weeks' GA who had been intubated in the first 72 hours of life. The infants in the study had mean GA of 28 weeks and birth weight of 1100 grams. Infants were randomised to one of two different brands of equipment (Fischer and PaykelTM versus VapothermTM) for delivery of humidified HFNC at 6 L/min. The primary outcome was the need for reintubation within 72 hours of extubation, based on prespecified criteria.
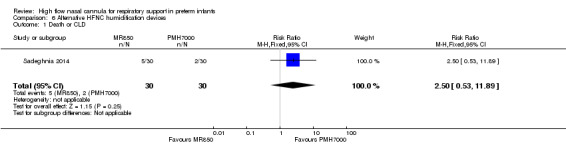
Sadeghnia 2014 was a single centre study that enrolled 60 preterm infants (1000 to 1500 grams) who had previously received surfactant, and were stable on CPAP 4 cmH2O with supplemental oxygen requirement of less than 30%, but required supplemental oxygen when CPAP discontinued. Infants were randomised to HFNC at a gas flow based on Sreenan 2001 using two different humidifiers (MR850 vs PMH7000). The primary outcome (specified at study registration) was humidity of gas delivered.
6. HFNC for weaning from CPAP
Abdel Hady 2011 was a single‐centre study that enrolled preterm infants whose GA was 28 weeks and above, and who were stable on low levels of non‐invasive respiratory support (CPAP 5 cmH2O and supplemental oxygen ≤ 30%). Infants in the study had mean GA of 31 weeks and birth weight of 1600 grams. Infants were randomised to HFNC (2 L/min) or to remain on CPAP until no longer requiring supplemental oxygen. The primary outcome was the duration of supplemental oxygen and respiratory support.
Badiee 2015 was also a single centre study. It enrolled infants of 28 to 36 weeks' GA who were stable on CPAP 5cmH2O and less than 30% supplemental oxygen. Infants had a mean GA at birth of 31 weeks. They were randomised to HFNC (2 L/min) or to remain on CPAP. The primary outcome was the duration of supplemental oxygen.
Risk of bias in included studies
Blinding of treatment allocation was not attempted in any of the studies. There were preset criteria for treatment failure/intubation in all of the studies except Woodhead 2006. Treatment with the alternate intervention was not permitted in the first 72 hours in Yoder 2013 or Liu 2014; it was permitted in Abdel Hady 2011, Collins 2013, Manley 2013, Mostafa‐Gharehbaghi 2014, and Kugelman 2015.
Abdel Hady 2011, Collins 2013, Manley 2013, and Kugelman 2015 separately reported the incidence of treatment failure as well as intubation. In these studies, a number of infants in the HFNC group meeting treatment failure criteria were treated with 'rescue' CPAP or NIPPV and not subsequently intubated.
In some of the studies alterations to flow rates or the level of non‐invasive support were left to the discretion of treating clinicians (Woodhead 2006; Miller 2010; Collins 2013; Manley 2013; Ciuffini 2014; Kugelman 2015). This may have contributed to a difference in HFNC gas flows between the two arms of the study in Woodhead 2006.
Frequency of blood gas analysis and recording of apnoea frequency and severity were potentially open to bias. Lack of blinding was a potential source of bias for subjective outcomes such as the presence of nasal mucosal injury or abdominal distension. Only Woodhead 2006 reported blinded assessment of the nasal mucosa.
Secondary outcomes were retrieved from medical records in all studies and were potentially open to bias. One patient in the study by Miller 2010 was excluded from the analysis after developing sepsis and dying during the study, though this patient should have been included as requiring reintubation.
Allocation concealment was not clear in the studies by Woodhead 2006, Miller 2010, and Sadeghnia 2014.
Trial registration was not evident (or occurred after trial completion) for Nair 2005, Campbell 2006, Woodhead 2006, Miller 2010, Abdel Hady 2011, Iranpour 2011, Collins 2013, Ciuffini 2014, Liu 2014, Sadeghnia 2014 and Badiee 2015, raising the potential for selective reporting of outcomes. Ciuffini 2014 is a report of preliminary data from their trial prior to achieving the planned sample size.
Effects of interventions
Comparison 1. HFNC versus CPAP for primary respiratory support after birth
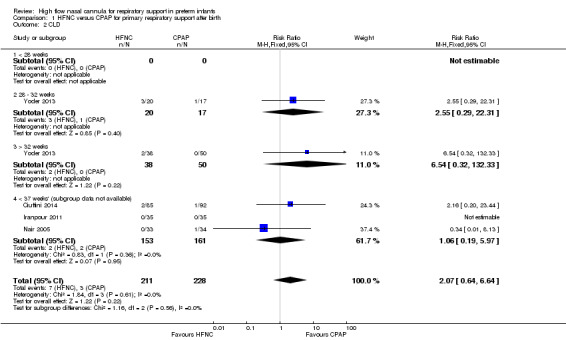
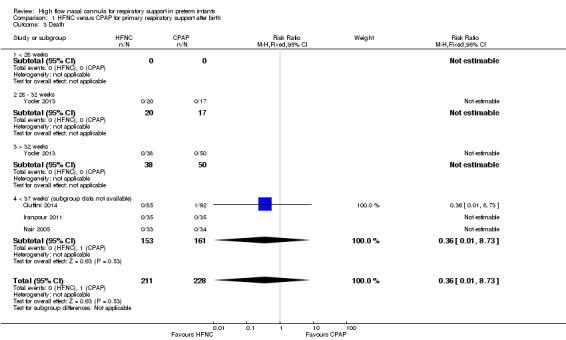
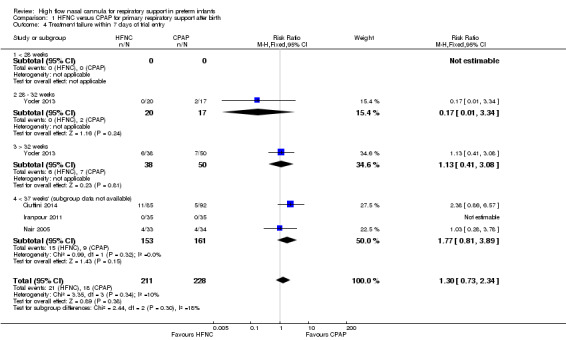
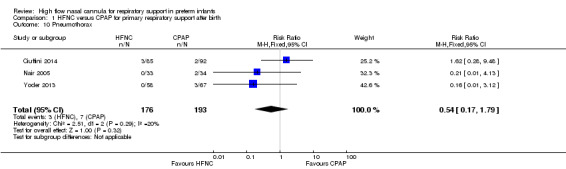
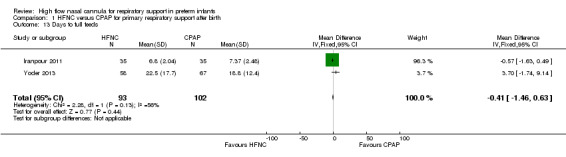
Four studies were available for this comparison (total 439 infants) (Nair 2005; Iranpour 2011; Yoder 2013; Ciuffini 2014). Rates of treatment failure (and need for intubation) within seven days of trial entry were similar between HFNC and CPAP (Figure 1). There were no differences in the rates of death (typical risk ratio (RR) 0.36, 95% confidence interval (CI) 0.01 to 8.73; 4 studies, 439 infants) or chronic lung disease (typical RR 2.07, 95% CI 0.64 to 6.64; 4 studies, 439 infants). The use of HFNC as primary support resulted in a longer duration of receiving respiratory support in one study (Yoder 2013). Other secondary outcomes (including nasal trauma, durations of supplemental oxygen and hospitalisation, pneumothorax, and sepsis) were similar between groups.
1.
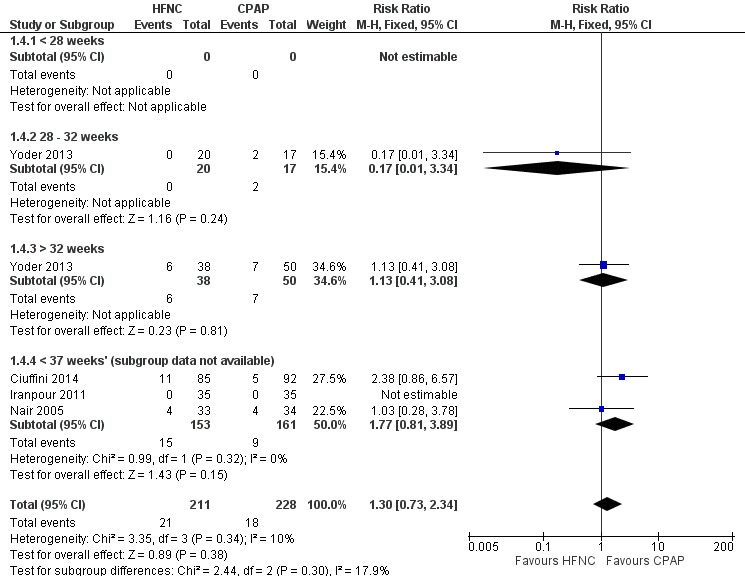
Forest plot of comparison: 1 HFNC versus CPAP soon after birth for treatment or prophylaxis of RDS, outcome: 1.4 Treatment failure within 7 days of trial entry.
Data on primary outcomes for gestational age subgroups were not available in Nair 2005, Iranpour 2011, or Ciuffini 2014. There were no differences in the primary outcomes or in treatment failure within GA subgroups from one study (Yoder 2013); however, there were only very small numbers of infants included in these subgroups and no extremely preterm infants (< 28 weeks' GA).
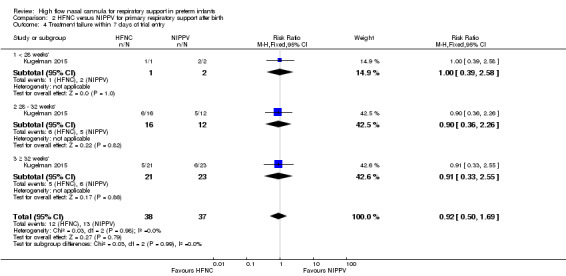
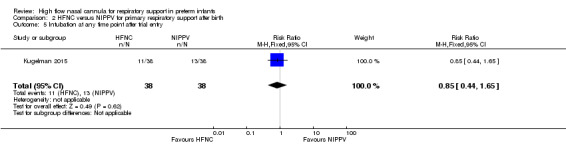
Comparison 2. HFNC versus NIPPV for primary respiratory support after birth
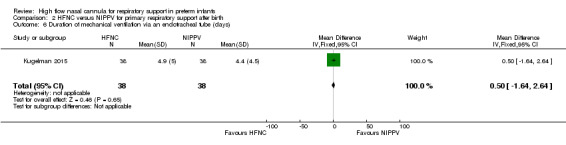
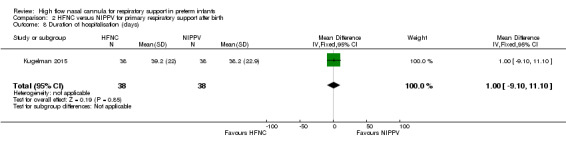
One study was available for this comparison (total 76 infants) (Kugelman 2015). There was no difference between HFNC and NIPPV in rates of treatment failure, death or CLD. Infants randomised to HFNC spent a longer period of time receiving non‐invasive respiratory support (median 4 days vs median 2 days, P < 0.01).
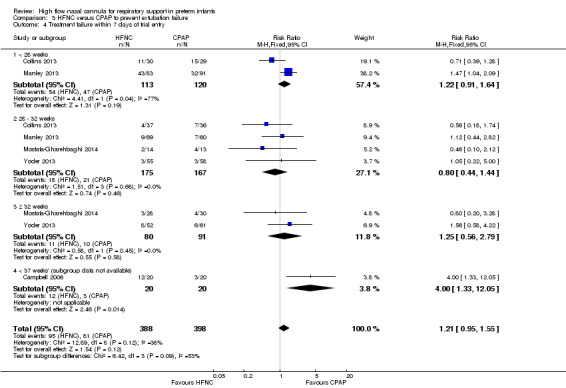
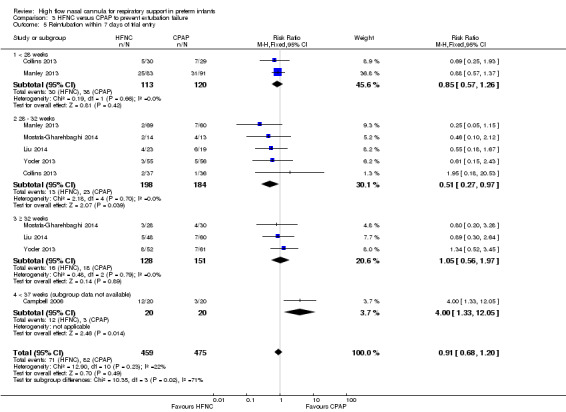
Comparison 3. HFNC versus CPAP to prevent extubation failure
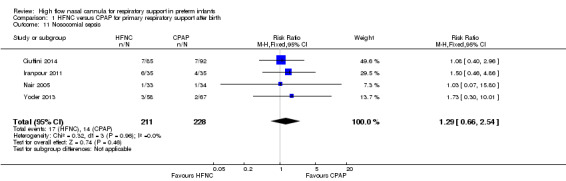
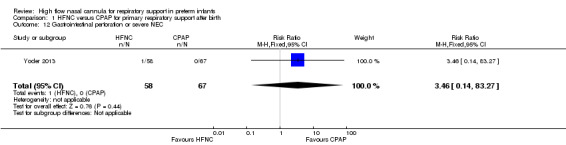
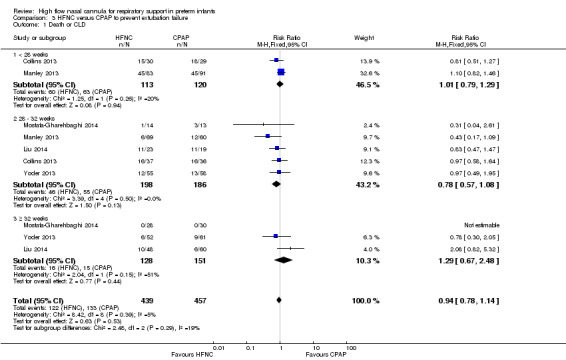
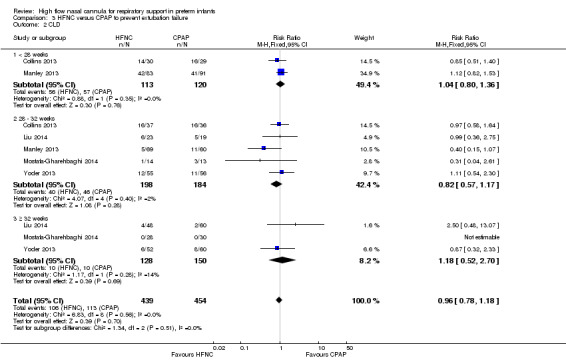
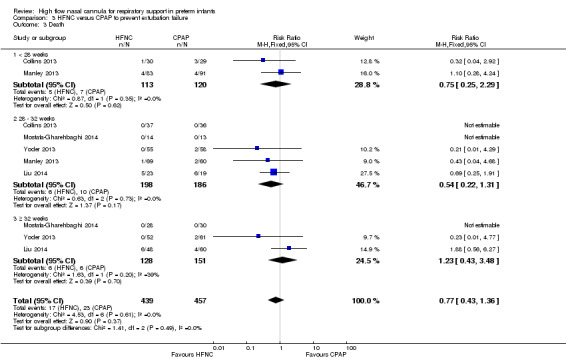
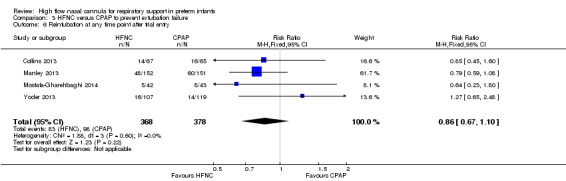
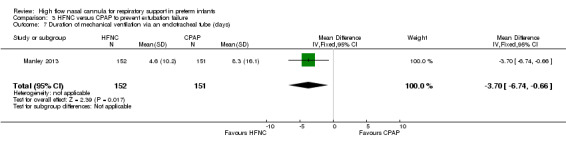
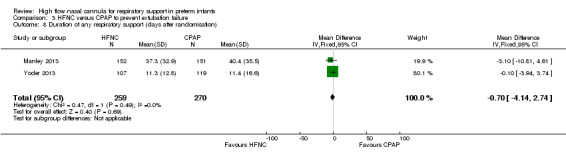
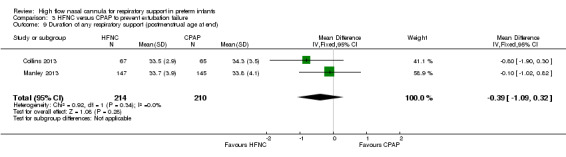
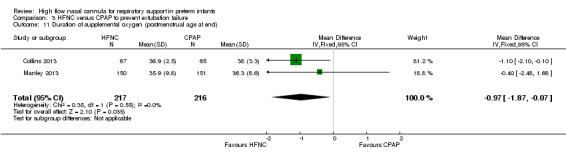
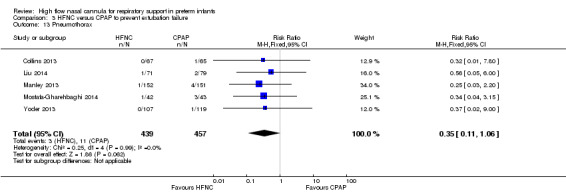
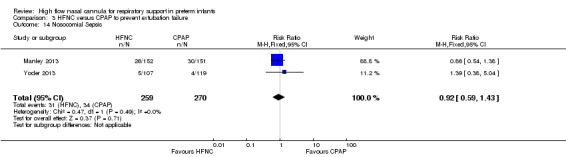
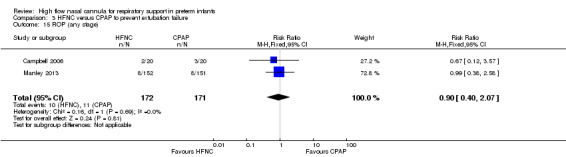
Six studies were available for this comparison (total 934 infants) (Campbell 2006; Collins 2013; Manley 2013; Yoder 2013; Liu 2014; Mostafa‐Gharehbaghi 2014). Following extubation, there were no differences between HFNC and CPAP in the primary outcomes of death (typical RR 0.77, 95% CI 0.43 to 1.36; 5 studies, 896 infants) (Figure 2); or CLD (typical RR 0.96, 95% CI 0.78 to 1.18; 5 studies, 893 infants) (Figure 3). There was no difference in the rate of treatment failure (typical RR 1.21, 95% CI 0.95 to 1.55; 5 studies, 786 infants) (Figure 4); or reintubation (typical RR 0.91, 95% CI 0.68 to 1.20; 6 studies, 934 infants) (Figure 5). Infants randomised to HFNC had reduced nasal trauma (typical RR 0.64, 95% CI 0.51 to 0.79; typical risk difference (RD) −0.14, 95% CI −0.20 to −0.08; 4 studies, 645 infants) (Figure 6). There was a small reduction in the rate of pneumothorax (typical RR 0.35, 95% CI 0.11 to 1.06; typical RD −0.02, 95% CI −0.03 to −0.00; 5 studies, 896 infants) (Figure 7) in infants treated with HFNC. There was also an apparent small reduction in the rate of gastrointestinal perforation or severe NEC (typical RR 0.52, 95% CI 0.24 to 1.11; typical RD −0.02, 95% CI −0.05 to −0.00; 5 studies, 840 infants), though this did not reach statistical significance. There was no significant difference in the incidence of intraventricular haemorrhage, sepsis or ROP between groups.
2.
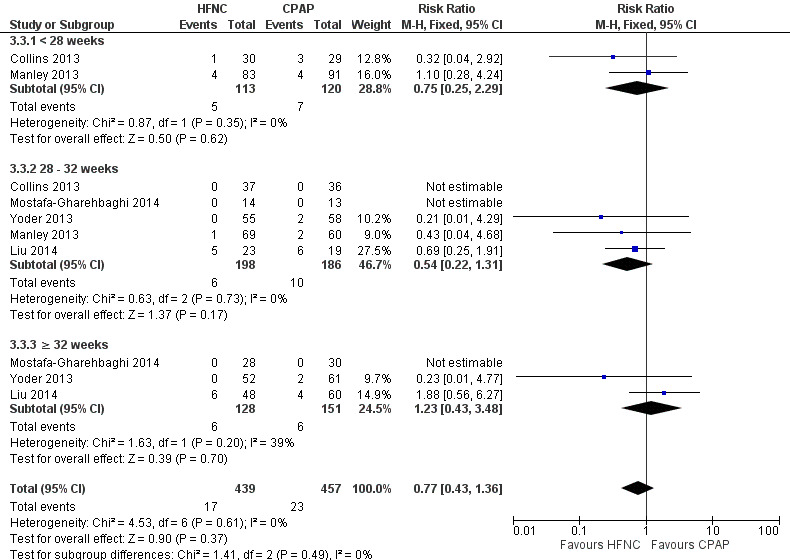
Forest plot of comparison: 3 HFNC versus CPAP to prevent extubation failure, outcome: 3.3 Death.
3.
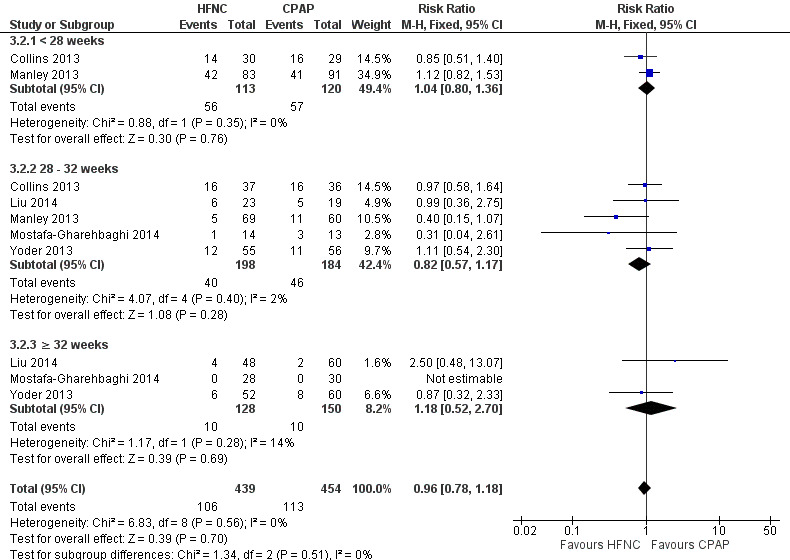
Forest plot of comparison: 3 HFNC versus CPAP to prevent extubation failure, outcome: 3.2 CLD.
4.
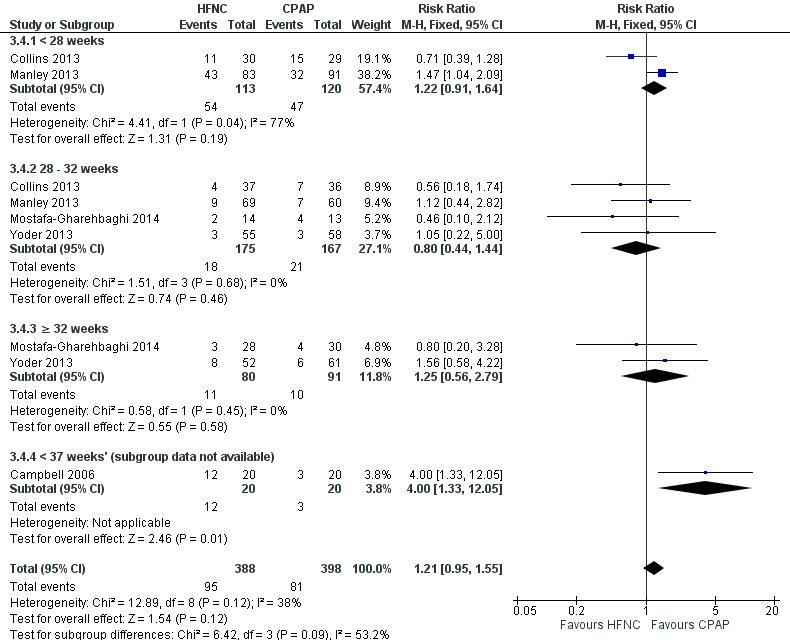
Forest plot of comparison: 3 High Flow Nasal Cannula versus CPAP to prevent extubation failure, outcome: Treatment failure.
5.
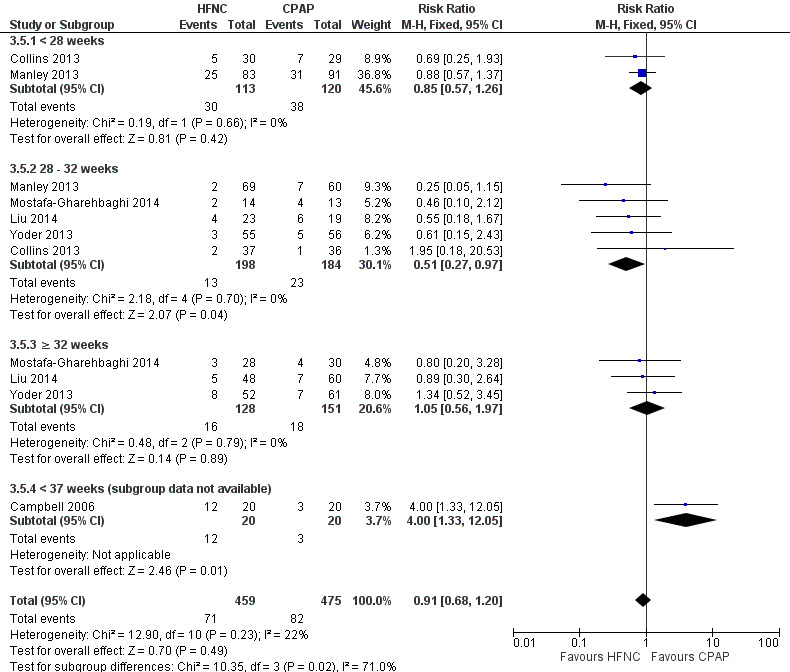
Forest plot of comparison: 3 HFNC versus CPAP to prevent extubation failure within 7 days, outcome: 3.5 Reintubation within 7 days of trial entry.
6.
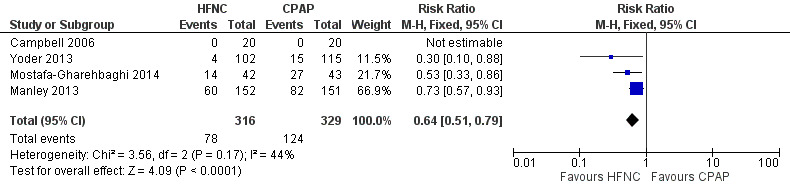
Forest plot of comparison: 3 HFNC versus CPAP to prevent extubation failure, outcome: Nasal trauma.
7.
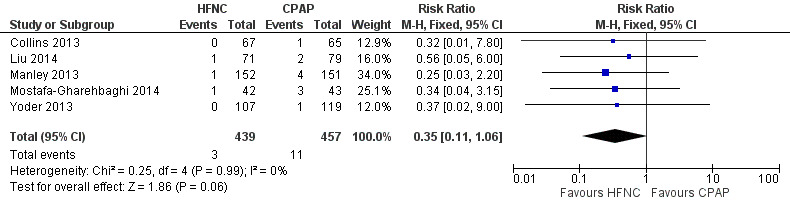
Forest plot of comparison: 3 HFNC versus CPAP to prevent extubation failure, outcome: Pneumothorax.
Data on gestational age subgroups were available for five studies (Collins 2013; Manley 2013; Yoder 2013; Liu 2014; Mostafa‐Gharehbaghi 2014). There was no difference between HFNC and CPAP in the rate of death, CLD, or treatment failure in different subgroups. However, in infants from 28 to 32 weeks' gestation (the GA subgroup with the most data available) HFNC was associated with a significantly reduced rate of re‐intubation (typical RR 0.51, 95% CI 0.27 to 0.97; typical RD −0.06, 95% CI −0.12 to −0.00; 5 studies, 382 infants) (Figure 5). In several of these studies 'rescue' treatment with CPAP/NIPPV was used for infants randomised to HFNC meeting treatment failure criteria. One small study that did not have subgroup data available found a higher rate of reintubation in infants randomised to HFNC (Campbell 2006).
Comparison 4. Humidified HFNC versus non‐humidified HFNC to prevent extubation failure
One study was available for this comparison (Woodhead 2006). There was no significant difference in need for intubation during the first 24 hours of the study (prior to crossover) (0/15 infants treated with humidified HFNC compared to 2/15 infants treated with non‐humidified HFNC). Nasal mucosal scores were not available for the first study period, however the authors noted more infants in the humidified HFNC group had nasal mucosa with a normal appearance.
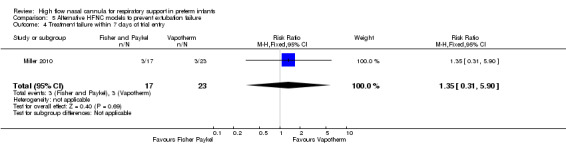
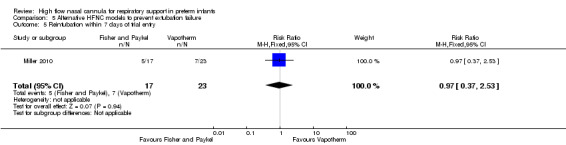
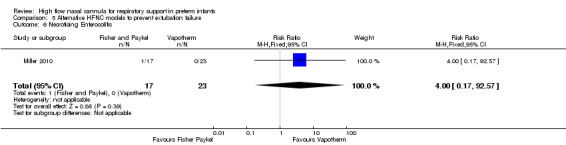
Comparison 5. Alternative HFNC models to prevent extubation failure
One study compared different HFNC models (Miller 2010). There was no significant difference in the need for reintubation within 72 hours of extubation (3/17 infants treated with Fisher and Paykel, 3/22 infants treated with Vapotherm). There was one death in the Vapotherm group, and one infant in the Fisher and Paykel group developed necrotising enterocolitis. Rates of chronic lung disease were similar between the two groups. Other secondary outcomes were not different or not reported.
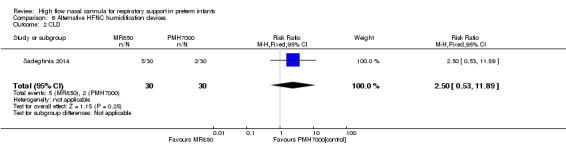
One study compared different humidification devices for delivery of HFNC (Sadeghnia 2014). There was no significant difference in the need for mechanical ventilation, rate of CLD, or other secondary outcomes.
Comparison 6. HFNC for weaning from CPAP
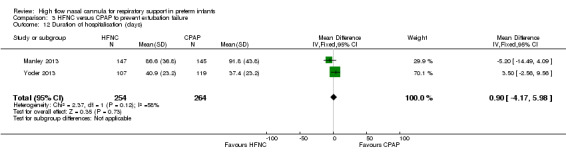
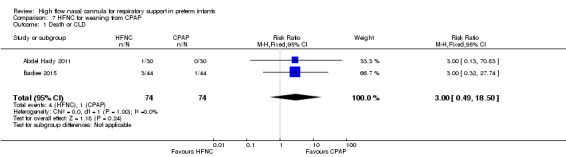
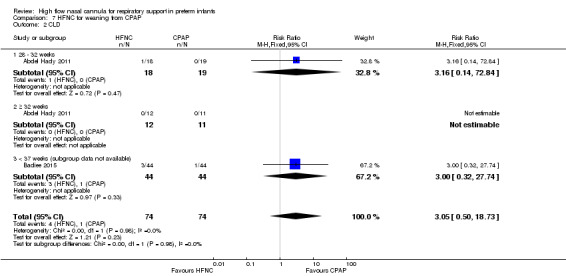
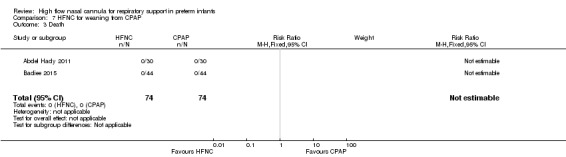
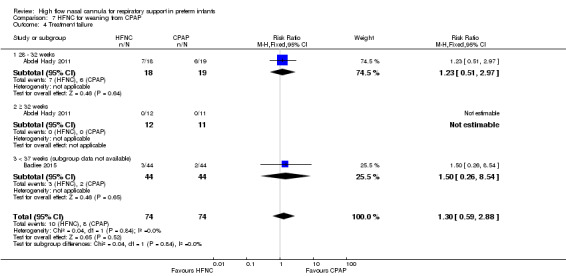
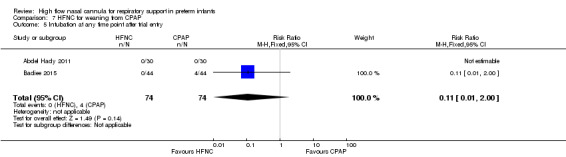
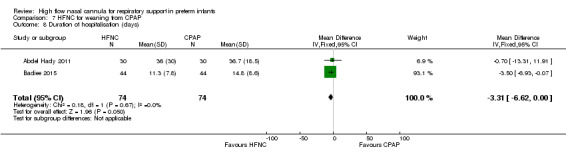
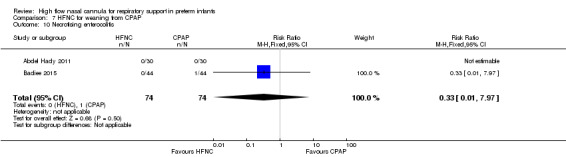
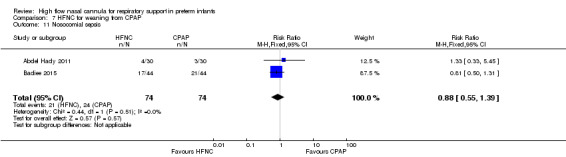
Two studies compared the use of HFNC versus continued CPAP for weaning preterm infants who were stable on low levels of CPAP (Abdel Hady 2011; Badiee 2015). In one of these studies (Abdel Hady 2011), infants randomised to HFNC had a longer total duration of oxygen therapy (median 14 days vs median 5 days P < 0.001) and a longer period of respiratory support (median 18 days vs median 10.5 days, P < 0.05). Infants in the HFNC group had a slightly longer duration of respiratory support prior to randomisation (median 8.5 days, IQR 7 to 14.25) compared with the CPAP group (median 5.5 days, IQR 3 to 13, P = 0.07). In the second study (Badiee 2015), infants randomised to HFNC had a shorter duration of oxygen therapy (21 hours vs 50 hours); however, infants in this group commenced weaning at an earlier gestational age (32.2 weeks vs 33.6 weeks). Four babies in the CPAP group required intubation in one study, while no infants randomised to HFNC weaning required intubation. There was no difference in weaning failure, nor in major morbidities (sepsis, IVH, BPD). There was a small overall reduction in length of hospitalisation in infants receiving HFNC (typical RD −3.3 days, 95% CI −6.6 to 0.0 days; 2 studies, 149 infants).
Discussion
This review identified 15 randomised trials including a total of 1725 premature infants that compared respiratory support with high flow nasal cannulae (HFNC) with other forms of non‐invasive respiratory support in preterm infants. There were no studies comparing HFNC with ambient oxygen or low flow nasal cannulae (LFNC). Subgroup analysis by GA was only possible for the comparison of HFNC with continuous positive airway pressure (CPAP) for preventing extubation failure.
The 15 studies varied in study quality. None of the studies were blinded and bias may have occurred, particularly where there were no established criteria for treatment failure/reintubation, or where rescue treatment with other forms of respiratory support was permitted. We did not perform a sensitivity analysis for study quality.
HFNC for primary respiratory support after birth
For preterm infants needing primary respiratory support after birth, there were no differences in the rates of primary or secondary outcomes between HFNC and CPAP, or HFNC and nasal intermittent positive pressure ventilation (NIPPV). Four studies compared HFNC with CPAP, while only one study compared HFNC with NIPPV. Studies varied in the HFNC gas flows used and in the degree of prematurity of infants. Subgroup meta‐analysis was not possible, as data for GA subgroups were available for only one study (Yoder 2013)
HFNC for respiratory support after extubation
Eight studies evaluated HFNC as respiratory support post‐extubation. Overall, there was no difference in the rates of death or CLD in 934 preterm infants treated with HFNC or CPAP. There were no differences in the rates of treatment failure or reintubation. Infants treated with HFNC had a small reduction in the rate of pneumothorax (NNTB 50), and there was an apparent (though not significant) reduction in the rate of necrotising enterocolitis (NNTB 50). Subgroup analysis revealed a small reduction in the rate of reintubation in infants of 28 to 32 weeks' gestation treated with HFNC (NNTB 17). However, subgroup data were not available for all studies, and there were relatively few extremely preterm infants (< 28 weeks' GA) included in the studies we identified.
HFNC for weaning from CPAP
Two small studies assessed the use of HFNC for stable preterm infants weaning off respiratory support. Those studies found a small reduction in length of hospitalisation, but no difference in weaning failure or major morbidities..
Duration of support
Three studies included in this review reported a longer duration of weaning from respiratory support in the HFNC group. Abdel Hady 2011 found that infants randomised to HFNC (compared with those remaining on CPAP) received a longer total duration of respiratory support and a longer period of oxygen. Infants randomised to HFNC had a longer period of respiratory support prior to enrolment than those infants randomised to CPAP, which may have contributed to the difference. Kugelman 2015 found longer median duration of respiratory support compared with NIPPV. Yoder 2013 identified a longer duration of respiratory support compared with CPAP for preterm infants receiving HFNC as primary support (but not post‐extubation).
The significance of this finding is unclear. Other studies found no difference in the duration of respiratory support between HFNC and CPAP (Manley 2013; Collins 2013). Badiee 2015 found a lower duration of oxygen in infants weaned from CPAP using HFNC (2 L/min). Meta‐analysis was not possible, because of the different interventions compared and different methods for quantifying and reporting duration of support. It is possible that the lower flow rates of HFNC used in Abdel Hady 2011 (2 L/min) and Kugelman 2015 (starting flow rate 1 L/min) contributed to slower weaning.
Nasal trauma
Nasal trauma was less common in HFNC‐treated infants than infants treated with alternative means of respiratory support in seven of the studies included in this review (Nair 2005; Woodhead 2006; Iranpour 2011; Collins 2013; Manley 2013; Yoder 2013; Mostafa‐Gharehbaghi 2014); but no difference was seen in two ( Liu 2014; Kugelman 2015). Studies varied widely in the tools used to assess the severity of nasal injury. Only one study attempted to blind assessment of nasal mucosa (Woodhead 2006).
Different forms of HFNC
There was no evidence from one small study of benefit from humidification of HFNC (Woodhead 2006). During the second half of the crossover trial infants treated with non‐humidified HFNC had a higher rate of being switched to humidified HFNC because of perceived treatment failure. This may have related to the higher flow rates used in infants receiving humidified HFNC. There were higher (more abnormal) scores for nasal mucosal injury in infants treated with non‐humidified HFNC.
There was no difference in effectiveness between two different models of equipment used to deliver HFNC in two other small studies (Miller 2010; Sadeghnia 2014). None of the included studies examined the effect of different flow rates or cannula sizes.
Neurodevelopmental outcomes
No included studies reported long‐term neurodevelopmental outcomes.
Authors' conclusions
Implications for practice.
HFNC has similar rates of efficacy to other forms of non‐invasive respiratory support in preterm infants for preventing treatment failure, death or BPD. Most evidence is available for the use of HFNC as post‐extubation support. Following extubation, HFNC is associated with lower rates of pneumothorax and nasal trauma compared with nasal CPAP.
Implications for research.
Further adequately powered randomised controlled trials should be undertaken in preterm infants comparing HFNC with other non‐invasive supports as primary respiratory support, particularly in extremely preterm and late preterm infants, and comparing different HFNC devices. Further studies are needed to clarify possible benefits of HFNC post‐extubation in subgroups of preterm infants, or in reducing pulmonary or gastrointestinal complications. Although the evidence for HFNC use is strongest as post‐extubation support, there are currently inadequate data on its use in extremely preterm infants.
What's new
| Date | Event | Description |
|---|---|---|
| 1 January 2016 | New citation required and conclusions have changed | Updated search January 2016. |
| 1 January 2016 | New search has been performed | This updates the review "High flow nasal cannula for respiratory support in preterm infants". (Wilkinson 2011). |
History
Protocol first published: Issue 1, 2007 Review first published: Issue 5, 2011
| Date | Event | Description |
|---|---|---|
| 14 February 2012 | Amended | Correction to denominator in Comparison 2. Figures reordered. |
Acknowledgements
Colleen Ovelman and Yolanda Brosseau provided invaluable assistance with literature searches. We are grateful to authors who provided additional data from their studies, in particular Dr Ma Li and Dr Cuiqing Liu, Dr Ramin Iranpour, Dr Bradley Yoder, Professor Hesham Abdel‐Hady, Dr Amir Kugelman, Dr Clare Collins, Dr Gharehbaghi, and Dr Nair. Dr Wei Ling Lean (The Royal Women's Hospital, Melbourne) kindly assisted with translation of one of the papers..
Appendices
Appendix 1. Standard search methodology
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomised [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])) EMBASE: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomised controlled trial or controlled clinical trial or randomised or placebo or clinical trials as topic or randomly or trial or clinical trial) CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomised controlled trial OR controlled clinical trial OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) The Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Data and analyses
Comparison 1. HFNC versus CPAP for primary respiratory support after birth.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or CLD | 4 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.56, 4.94] |
| 1.1 < 28 weeks | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 28 ‐ 32 weeks | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [0.29, 22.31] |
| 1.3 ≥ 32 weeks | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.54 [0.32, 132.33] |
| 1.4 < 37 weeks' (subgroup data not available) | 3 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.15, 3.77] |
| 2 CLD | 4 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.64, 6.64] |
| 2.1 < 28 weeks | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 28 ‐ 32 weeks | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [0.29, 22.31] |
| 2.3 > 32 weeks | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.54 [0.32, 132.33] |
| 2.4 < 37 weeks' (subgroup data not available) | 3 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.19, 5.97] |
| 3 Death | 4 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.73] |
| 3.1 < 28 weeks | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 28 ‐ 32 weeks | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 > 32 weeks | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 < 37 weeks' (subgroup data not available) | 3 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.73] |
| 4 Treatment failure within 7 days of trial entry | 4 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.73, 2.34] |
| 4.1 < 28 weeks | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 28 ‐ 32 weeks | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.34] |
| 4.3 > 32 weeks | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.41, 3.08] |
| 4.4 < 37 weeks' (subgroup data not available) | 3 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.81, 3.89] |
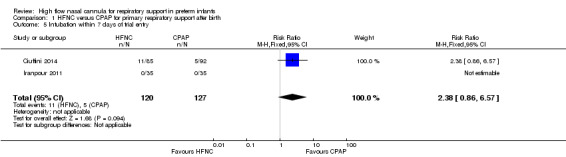
| 5 Intubation within 7 days of trial entry | 2 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [0.86, 6.57] |
| 6 Intubation at any time point after trial entry | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.49, 2.71] |
| 7 Duration of any respiratory support (days) | 2 | 192 | Mean Difference (IV, Fixed, 95% CI) | 0.97 [0.08, 1.86] |
| 8 Duration of supplemental oxygen (days) | 1 | 125 | Mean Difference (IV, Fixed, 95% CI) | 3.70 [‐0.66, 8.06] |
| 9 Duration of hospitalisation (days) | 3 | 262 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐0.55, 1.56] |
| 10 Pneumothorax | 3 | 369 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.17, 1.79] |
| 11 Nosocomial sepsis | 4 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.66, 2.54] |
| 12 Gastrointestinal perforation or severe NEC | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.46 [0.14, 83.27] |
| 13 Days to full feeds | 2 | 195 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐1.46, 0.63] |
| 14 Nasal trauma | 3 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.34, 1.15] |
1.1. Analysis.
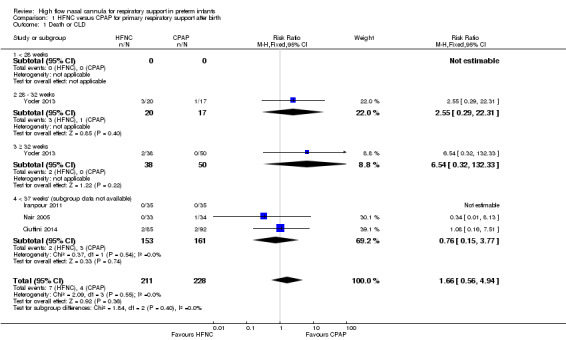
Comparison 1 HFNC versus CPAP for primary respiratory support after birth, Outcome 1 Death or CLD.
1.2. Analysis.
Comparison 1 HFNC versus CPAP for primary respiratory support after birth, Outcome 2 CLD.
1.3. Analysis.
Comparison 1 HFNC versus CPAP for primary respiratory support after birth, Outcome 3 Death.
1.4. Analysis.
Comparison 1 HFNC versus CPAP for primary respiratory support after birth, Outcome 4 Treatment failure within 7 days of trial entry.
1.5. Analysis.
Comparison 1 HFNC versus CPAP for primary respiratory support after birth, Outcome 5 Intubation within 7 days of trial entry.
1.6. Analysis.
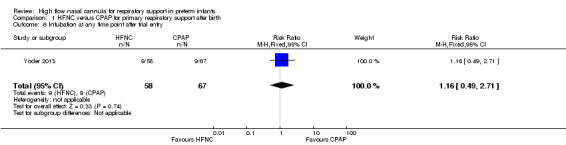
Comparison 1 HFNC versus CPAP for primary respiratory support after birth, Outcome 6 Intubation at any time point after trial entry.
1.7. Analysis.
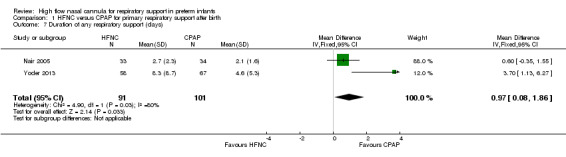
Comparison 1 HFNC versus CPAP for primary respiratory support after birth, Outcome 7 Duration of any respiratory support (days).
1.8. Analysis.
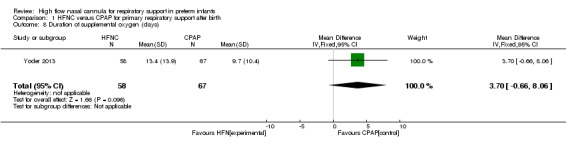
Comparison 1 HFNC versus CPAP for primary respiratory support after birth, Outcome 8 Duration of supplemental oxygen (days).
1.9. Analysis.
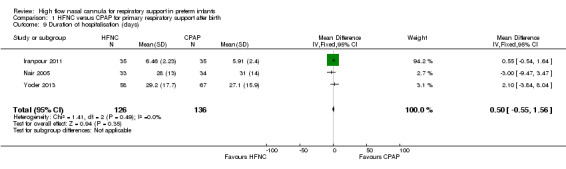
Comparison 1 HFNC versus CPAP for primary respiratory support after birth, Outcome 9 Duration of hospitalisation (days).
1.10. Analysis.
Comparison 1 HFNC versus CPAP for primary respiratory support after birth, Outcome 10 Pneumothorax.
1.11. Analysis.
Comparison 1 HFNC versus CPAP for primary respiratory support after birth, Outcome 11 Nosocomial sepsis.
1.12. Analysis.
Comparison 1 HFNC versus CPAP for primary respiratory support after birth, Outcome 12 Gastrointestinal perforation or severe NEC.
1.13. Analysis.
Comparison 1 HFNC versus CPAP for primary respiratory support after birth, Outcome 13 Days to full feeds.
1.14. Analysis.
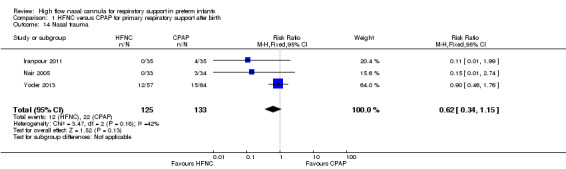
Comparison 1 HFNC versus CPAP for primary respiratory support after birth, Outcome 14 Nasal trauma.
Comparison 2. HFNC versus NIPPV for primary respiratory support after birth.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or CLD | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.30, 4.32] |
| 1.1 < 28 weeks' | 1 | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.38, 6.00] |
| 1.2 28 ‐ 32 weeks' | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.05, 10.82] |
| 1.3 ≥ 32 weeks' | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 CLD | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.30, 4.32] |
| 2.1 < 28 weeks' | 1 | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.38, 6.00] |
| 2.2 28 ‐ 32 weeks' | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.05, 10.82] |
| 2.3 ≥ 32 weeks' | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Death | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 < 28 weeks' | 1 | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 28 ‐ 32 weeks' | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 ≥ 32 weeks' | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Treatment failure within 7 days of trial entry | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.50, 1.69] |
| 4.1 < 28 weeks' | 1 | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.58] |
| 4.2 28 ‐ 32 weeks' | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.9 [0.36, 2.26] |
| 4.3 ≥ 32 weeks' | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.33, 2.55] |
| 5 Intubation at any time point after trial entry | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.44, 1.65] |
| 6 Duration of mechanical ventilation via an endotracheal tube (days) | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐1.64, 2.64] |
| 7 Duration of any respiratory support (days) | Other data | No numeric data | ||
| 8 Duration of hospitalisation (days) | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐9.10, 11.10] |
| 9 Pneumothorax | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 100.80] |
| 10 Nosocomial Sepsis | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.32, 5.56] |
| 11 Gastrointestinal perforation or severe NEC | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 100.80] |
| 12 Days to full feeds | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐1.21, 4.61] |
| 13 Nasal trauma | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
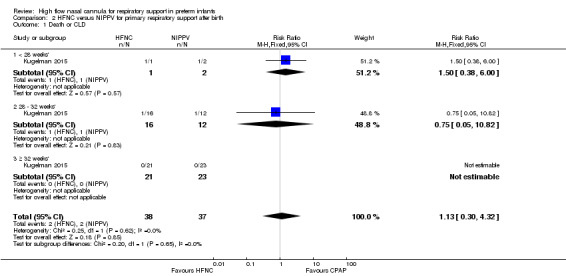
Comparison 2 HFNC versus NIPPV for primary respiratory support after birth, Outcome 1 Death or CLD.
2.2. Analysis.
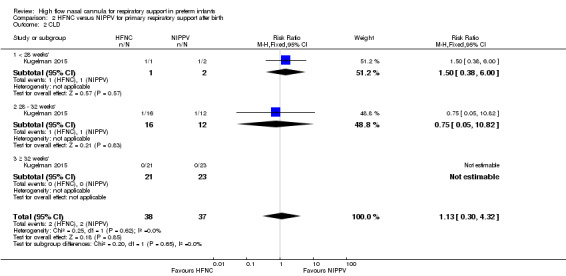
Comparison 2 HFNC versus NIPPV for primary respiratory support after birth, Outcome 2 CLD.
2.3. Analysis.
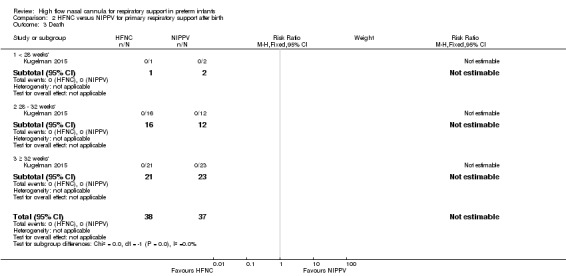
Comparison 2 HFNC versus NIPPV for primary respiratory support after birth, Outcome 3 Death.
2.4. Analysis.
Comparison 2 HFNC versus NIPPV for primary respiratory support after birth, Outcome 4 Treatment failure within 7 days of trial entry.
2.5. Analysis.
Comparison 2 HFNC versus NIPPV for primary respiratory support after birth, Outcome 5 Intubation at any time point after trial entry.
2.6. Analysis.
Comparison 2 HFNC versus NIPPV for primary respiratory support after birth, Outcome 6 Duration of mechanical ventilation via an endotracheal tube (days).
2.7. Analysis.
Comparison 2 HFNC versus NIPPV for primary respiratory support after birth, Outcome 7 Duration of any respiratory support (days).
| Duration of any respiratory support (days) | ||
|---|---|---|
| Study | HFNC (median, Interquartile range) | CPAP (median, IQR) |
| Kugelman 2015 | 4, 1.0‐15.0 | 2, 0.3‐6.5 |
2.8. Analysis.
Comparison 2 HFNC versus NIPPV for primary respiratory support after birth, Outcome 8 Duration of hospitalisation (days).
2.9. Analysis.
Comparison 2 HFNC versus NIPPV for primary respiratory support after birth, Outcome 9 Pneumothorax.
2.10. Analysis.
Comparison 2 HFNC versus NIPPV for primary respiratory support after birth, Outcome 10 Nosocomial Sepsis.
2.11. Analysis.
Comparison 2 HFNC versus NIPPV for primary respiratory support after birth, Outcome 11 Gastrointestinal perforation or severe NEC.
2.12. Analysis.
Comparison 2 HFNC versus NIPPV for primary respiratory support after birth, Outcome 12 Days to full feeds.
2.13. Analysis.
Comparison 2 HFNC versus NIPPV for primary respiratory support after birth, Outcome 13 Nasal trauma.
Comparison 3. HFNC versus CPAP to prevent extubation failure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or CLD | 5 | 896 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.78, 1.14] |
| 1.1 < 28 weeks | 2 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.29] |
| 1.2 28 ‐ 32 weeks | 5 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.57, 1.08] |
| 1.3 ≥ 32 weeks | 3 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.67, 2.48] |
| 2 CLD | 5 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.78, 1.18] |
| 2.1 < 28 weeks | 2 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.80, 1.36] |
| 2.2 28 ‐ 32 weeks | 5 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.57, 1.17] |
| 2.3 ≥ 32 weeks | 3 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.52, 2.70] |
| 3 Death | 5 | 896 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.43, 1.36] |
| 3.1 < 28 weeks | 2 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.25, 2.29] |
| 3.2 28 ‐ 32 weeks | 5 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.22, 1.31] |
| 3.3 ≥ 32 weeks | 3 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.43, 3.48] |
| 4 Treatment failure within 7 days of trial entry | 5 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.95, 1.55] |
| 4.1 < 28 weeks | 2 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.91, 1.64] |
| 4.2 28 ‐ 32 weeks | 4 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.44, 1.44] |
| 4.3 ≥ 32 weeks | 2 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.56, 2.79] |
| 4.4 < 37 weeks' (subgroup data not available) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [1.33, 12.05] |
| 5 Reintubation within 7 days of trial entry | 6 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.68, 1.20] |
| 5.1 < 28 weeks | 2 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.57, 1.26] |
| 5.2 28 ‐ 32 weeks | 5 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.27, 0.97] |
| 5.3 ≥ 32 weeks | 3 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.56, 1.97] |
| 5.4 < 37 weeks (subgroup data not available) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [1.33, 12.05] |
| 6 Reintubation at any time point after trial entry | 4 | 746 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.67, 1.10] |
| 7 Duration of mechanical ventilation via an endotracheal tube (days) | 1 | 303 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐6.74, ‐0.66] |
| 8 Duration of any respiratory support (days after randomisation) | 2 | 529 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐4.14, 2.74] |
| 9 Duration of any respiratory support (postmenstrual age at end) | 2 | 424 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.09, 0.32] |
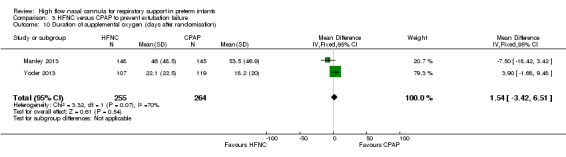
| 10 Duration of supplemental oxygen (days after randomisation) | 2 | 519 | Mean Difference (IV, Fixed, 95% CI) | 1.54 [‐3.42, 6.51] |
| 11 Duration of supplemental oxygen (postmenstrual age at end) | 2 | 433 | Mean Difference (IV, Fixed, 95% CI) | ‐0.97 [‐1.87, ‐0.07] |
| 12 Duration of hospitalisation (days) | 2 | 518 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐4.17, 5.98] |
| 13 Pneumothorax | 5 | 896 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.11, 1.06] |
| 14 Nosocomial Sepsis | 2 | 529 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.59, 1.43] |
| 15 ROP (any stage) | 2 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.40, 2.07] |
| 16 Gastrointestinal perforation or severe NEC | 5 | 840 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.24, 1.11] |
| 17 Days to full feeds | 3 | 387 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [0.35, 0.85] |
| 18 Weight gain prior to discharge from hospital (grams) | 2 | 518 | Mean Difference (IV, Fixed, 95% CI) | 66.32 [‐45.63, 178.27] |
| 19 Nasal trauma | 4 | 645 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.51, 0.79] |
3.1. Analysis.
Comparison 3 HFNC versus CPAP to prevent extubation failure, Outcome 1 Death or CLD.
3.2. Analysis.
Comparison 3 HFNC versus CPAP to prevent extubation failure, Outcome 2 CLD.
3.3. Analysis.
Comparison 3 HFNC versus CPAP to prevent extubation failure, Outcome 3 Death.
3.4. Analysis.
Comparison 3 HFNC versus CPAP to prevent extubation failure, Outcome 4 Treatment failure within 7 days of trial entry.
3.5. Analysis.
Comparison 3 HFNC versus CPAP to prevent extubation failure, Outcome 5 Reintubation within 7 days of trial entry.
3.6. Analysis.
Comparison 3 HFNC versus CPAP to prevent extubation failure, Outcome 6 Reintubation at any time point after trial entry.
3.7. Analysis.
Comparison 3 HFNC versus CPAP to prevent extubation failure, Outcome 7 Duration of mechanical ventilation via an endotracheal tube (days).
3.8. Analysis.
Comparison 3 HFNC versus CPAP to prevent extubation failure, Outcome 8 Duration of any respiratory support (days after randomisation).
3.9. Analysis.
Comparison 3 HFNC versus CPAP to prevent extubation failure, Outcome 9 Duration of any respiratory support (postmenstrual age at end).
3.10. Analysis.
Comparison 3 HFNC versus CPAP to prevent extubation failure, Outcome 10 Duration of supplemental oxygen (days after randomisation).
3.11. Analysis.
Comparison 3 HFNC versus CPAP to prevent extubation failure, Outcome 11 Duration of supplemental oxygen (postmenstrual age at end).
3.12. Analysis.
Comparison 3 HFNC versus CPAP to prevent extubation failure, Outcome 12 Duration of hospitalisation (days).
3.13. Analysis.
Comparison 3 HFNC versus CPAP to prevent extubation failure, Outcome 13 Pneumothorax.
3.14. Analysis.
Comparison 3 HFNC versus CPAP to prevent extubation failure, Outcome 14 Nosocomial Sepsis.
3.15. Analysis.
Comparison 3 HFNC versus CPAP to prevent extubation failure, Outcome 15 ROP (any stage).
3.16. Analysis.
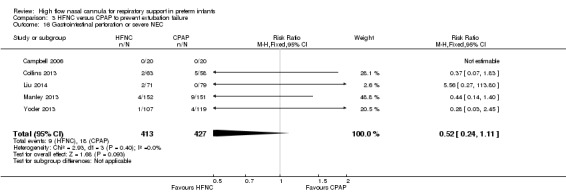
Comparison 3 HFNC versus CPAP to prevent extubation failure, Outcome 16 Gastrointestinal perforation or severe NEC.
3.17. Analysis.
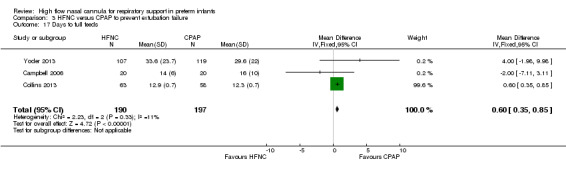
Comparison 3 HFNC versus CPAP to prevent extubation failure, Outcome 17 Days to full feeds.
3.18. Analysis.
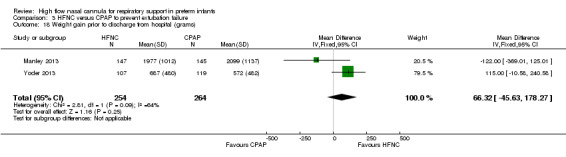
Comparison 3 HFNC versus CPAP to prevent extubation failure, Outcome 18 Weight gain prior to discharge from hospital (grams).
3.19. Analysis.
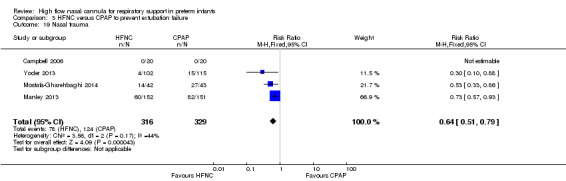
Comparison 3 HFNC versus CPAP to prevent extubation failure, Outcome 19 Nasal trauma.
Comparison 4. Humidified HFNC versus non‐humidified HFNC to prevent extubation failure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reintubation within 7 days of trial entry | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.34] |
4.1. Analysis.
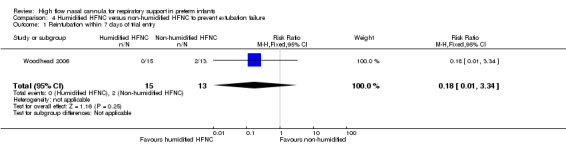
Comparison 4 Humidified HFNC versus non‐humidified HFNC to prevent extubation failure, Outcome 1 Reintubation within 7 days of trial entry.
Comparison 5. Alternative HFNC models to prevent extubation failure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or CLD | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 CLD | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.29, 2.58] |
| 3 Death | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.02, 10.29] |
| 4 Treatment failure within 7 days of trial entry | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.31, 5.90] |
| 5 Reintubation within 7 days of trial entry | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.37, 2.53] |
| 6 Necrotising Enterocolitis | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.17, 92.57] |
5.1. Analysis.
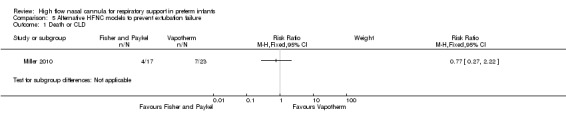
Comparison 5 Alternative HFNC models to prevent extubation failure, Outcome 1 Death or CLD.
5.2. Analysis.
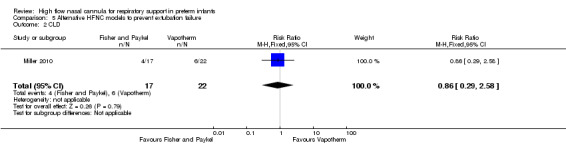
Comparison 5 Alternative HFNC models to prevent extubation failure, Outcome 2 CLD.
5.3. Analysis.
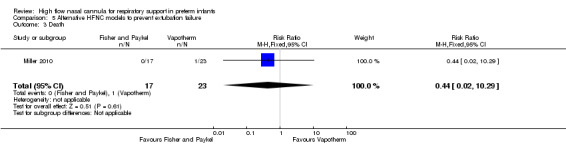
Comparison 5 Alternative HFNC models to prevent extubation failure, Outcome 3 Death.
5.4. Analysis.
Comparison 5 Alternative HFNC models to prevent extubation failure, Outcome 4 Treatment failure within 7 days of trial entry.
5.5. Analysis.
Comparison 5 Alternative HFNC models to prevent extubation failure, Outcome 5 Reintubation within 7 days of trial entry.
5.6. Analysis.
Comparison 5 Alternative HFNC models to prevent extubation failure, Outcome 6 Necrotising Enterocolitis.
Comparison 6. Alternative HFNC humidification devices.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or CLD | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.53, 11.89] |
| 2 CLD | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.53, 11.89] |
| 3 Treatment failure | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.23] |
| 4 Intubation at any time point after trial entry | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.26] |
| 5 Nasal trauma | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.34] |
6.1. Analysis.
Comparison 6 Alternative HFNC humidification devices, Outcome 1 Death or CLD.
6.2. Analysis.
Comparison 6 Alternative HFNC humidification devices, Outcome 2 CLD.
6.3. Analysis.
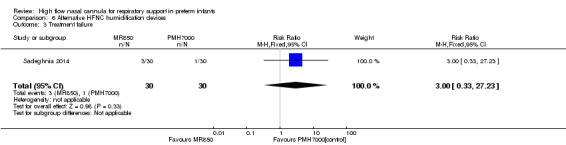
Comparison 6 Alternative HFNC humidification devices, Outcome 3 Treatment failure.
6.4. Analysis.
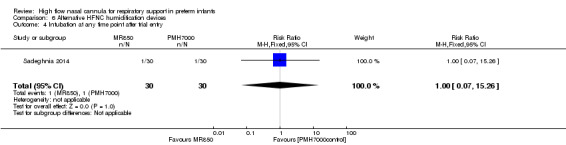
Comparison 6 Alternative HFNC humidification devices, Outcome 4 Intubation at any time point after trial entry.
6.5. Analysis.
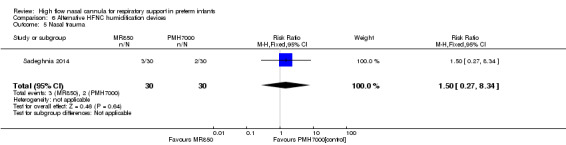
Comparison 6 Alternative HFNC humidification devices, Outcome 5 Nasal trauma.
Comparison 7. HFNC for weaning from CPAP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or CLD | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.49, 18.50] |
| 2 CLD | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.50, 18.73] |
| 2.1 28 ‐ 32 weeks | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.14, 72.84] |
| 2.2 ≥ 32 weeks | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 < 37 weeks (subgroup data not available) | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.32, 27.74] |
| 3 Death | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Treatment failure | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.59, 2.88] |
| 4.1 28 ‐ 32 weeks | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.51, 2.97] |
| 4.2 ≥ 32 weeks | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 < 37 weeks (subgroup data not available) | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.26, 8.54] |
| 5 Intubation at any time point after trial entry | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.00] |
| 6 Duration of any respiratory support (days) | Other data | No numeric data | ||
| 7 Duration of oxygen supplementation (days) | Other data | No numeric data | ||
| 8 Duration of hospitalisation (days) | 2 | 148 | Mean Difference (IV, Fixed, 95% CI) | ‐3.31 [‐6.62, 0.00] |
| 9 Pneumothorax | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Necrotising enterocolitis | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.97] |
| 11 Nosocomial sepsis | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.55, 1.39] |
7.1. Analysis.
Comparison 7 HFNC for weaning from CPAP, Outcome 1 Death or CLD.
7.2. Analysis.
Comparison 7 HFNC for weaning from CPAP, Outcome 2 CLD.
7.3. Analysis.
Comparison 7 HFNC for weaning from CPAP, Outcome 3 Death.
7.4. Analysis.
Comparison 7 HFNC for weaning from CPAP, Outcome 4 Treatment failure.
7.5. Analysis.
Comparison 7 HFNC for weaning from CPAP, Outcome 5 Intubation at any time point after trial entry.
7.6. Analysis.
Comparison 7 HFNC for weaning from CPAP, Outcome 6 Duration of any respiratory support (days).
| Duration of any respiratory support (days) | ||
|---|---|---|
| Study | HFNC | CPAP |
| Abdel Hady 2011 | median 18 days (IQR 11.5‐29) | median 10.5 (IQR 4‐21) |
7.7. Analysis.
Comparison 7 HFNC for weaning from CPAP, Outcome 7 Duration of oxygen supplementation (days).
| Duration of oxygen supplementation (days) | ||
|---|---|---|
| Study | HFNC | CPAP |
| Abdel Hady 2011 | median (interquartile range): 14 (7.5–19.25) | median (interquartile range): 5 (1–8) |
| Badiee 2015 | mean 20.6 +/‐16.8 hours | mean 49.5 +/‐ 25.3 hours |
7.8. Analysis.
Comparison 7 HFNC for weaning from CPAP, Outcome 8 Duration of hospitalisation (days).
7.9. Analysis.
Comparison 7 HFNC for weaning from CPAP, Outcome 9 Pneumothorax.
7.10. Analysis.
Comparison 7 HFNC for weaning from CPAP, Outcome 10 Necrotising enterocolitis.
7.11. Analysis.
Comparison 7 HFNC for weaning from CPAP, Outcome 11 Nosocomial sepsis.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdel Hady 2011.
| Methods | Randomised controlled trial | |
| Participants | 60 preterm infants ≥ 28 weeks stable on nasal CPAP 5 cmH2O and < 30% oxygen for at least 24 hours | |
| Interventions | Nasal cannula (2 L/min) weaning ‐ infants were switched to HFNC until infant requiring no supplemental oxygen then flow weaned No nasal cannula weaning ‐ infants kept on CPAP (binasal prongs) until infant requiring no supplemental oxygen |
|
| Outcomes | Duration of oxygen therapy; duration of respiratory support (from birth); length of hospitalisation; weaning success; need for intubation; complications. BPD not defined | |
| Notes | Underpowered due to overestimate of duration of oxygen in comparison group. Infants randomised to wean via HFNC were slightly older at the time of enrolment than those randomised to remain on CPAP | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | Opaque, sealed envelopes |
| Blinding (performance bias and detection bias) Need for intubation | Unclear risk | Pre‐set criteria for re‐instituting CPAP. No mention of whether criteria were met |
| Blinding (performance bias and detection bias) Nasal damage | Low risk | Nasal damage was not assessed in this study |
| Selective reporting (reporting bias) | Unclear risk | Study only registered after completion |
| Other bias | Low risk | |
Badiee 2015.
| Methods | Randomised controlled trial | |
| Participants | Preterm infants 28 to 36 weeks gestation, stable on CPAP 5 cmH2O, FiO2 < 30% | |
| Interventions | HFNC: infants were switched to HFNC (2 L/min), oxygen was weaned (SpO2 88% to 95%) until in air, then flow weaned by 0.5 L/min per hour until 0.5 L/min, then ceased CPAP: infants were continued on CPAP 5 cmH2O, oxygen was weaned (SpO2 88% to 95%) until in air for 6 hours, then ceased |
|
| Outcomes | Duration of supplemental oxygen; duration of respiratory support; duration of hospitalisation; failure of weaning | |
| Notes | Infants in the HFNC group had a lower corrected gestational age (i.e. were younger) at time of start of weaning. Difference between table and text in number of infants 'successfully weaning' vs 'failed weaning'. Difference between text and table in duration of hospitalisation (standard deviation). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) Need for intubation | Unclear risk | Criteria for weaning and treatment failure, but speed of weaning oxygen potentially influenced by non‐blinding |
| Blinding (performance bias and detection bias) Nasal damage | Unclear risk | Not assessed |
| Selective reporting (reporting bias) | Unclear risk | Study only registered at time of completion |
| Other bias | Low risk | |
Campbell 2006.
| Methods | Randomised controlled trial | |
| Participants | 40 intubated preterm infants < 1250 grams (mean 27 weeks' gestation, median 39/24 hours old at extubation) | |
| Interventions | Humidified, non‐heated HFNC (mean flow rate 1.6 L/min, n = 20) CPAP ‐ variable flow, short binasal prongs, 5 to 6 cmH2O pressure; n = 20 |
|
| Outcomes | Need for reintubation in first 7 days after extubation (criteria for intubation included uncompensated respiratory acidosis, FiO2 > 60%, severe or frequent apnoea) Nasal damage; NEC; CLD (oxygen at 36 weeks PMA); IVH; ROP; sepsis; change in oxygen use post‐extubation; episodes of apnoea or bradycardia; rate of weight gain |
|
| Notes | Higher caffeine use in HFNC group (14/20 compared to 9/20). Study funded by Physicians Services Incorporated Foundation, Toronto. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block randomisation with random number table |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding (performance bias and detection bias) Need for intubation | Unclear risk | Standardised criteria for reintubation (but not reported whether infants met these criteria equally in each group) |
| Blinding (performance bias and detection bias) Nasal damage | High risk | |
| Selective reporting (reporting bias) | Unclear risk | Study not registered |
| Other bias | Low risk | |
Ciuffini 2014.
| Methods | Randomised controlled trial | |
| Participants | 177 Inborn preterm infants 29 to 36 weeks' gestation with mild to moderate respiratory distress | |
| Interventions | High flow nasal cannula (flow rate 4 to 6 L/min) Nasal CPAP (4 to 6 cmH2O) |
|
| Outcomes | Need for intubation and mechanical ventilation (excluding intubation/surfactant administration/extubation); duration of respiratory support (ventilation, non‐invasive, oxygen dependency); surfactant treatment; days to reach full enteral feeds (120 mL/kg/day); duration of hospitalisation; pneumothorax; IVH; PDA; infections; NEC; BPD (not defined); ROP; mortality | |
| Notes | Preliminary results of the study published prior to completion. Total sample size planned 316. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed envelope |
| Blinding (performance bias and detection bias) Need for intubation | Unclear risk | Set criteria for intubation; however, did not report whether or not these criteria were met |
| Blinding (performance bias and detection bias) Nasal damage | Unclear risk | Nasal damage was not assessed in this study |
| Selective reporting (reporting bias) | Unclear risk | No comment about study registration. Unclear whether other outcomes measured/planned |
| Other bias | High risk | Publication of partial results prior to achieving set sample size |
Collins 2013.
| Methods | Randomised controlled trial | |
| Participants | 132 intubated preterm infants < 32 weeks, considered ready for extubation | |
| Interventions | Humdified heated HFNC (Vapotherm), 1.5 mm prongs, flow rate of 8 L/min initially, weaned by clinician preference to minimum 4 L/min NCPAP Hudson binasal prongs, 7 to 8 cmH2O initially, weaned to minimum 5 cmH2O |
|
| Outcomes | Treatment failure in the first 7 days after extubation (apnoea, respiratory acidosis, sustained increase in oxygen requirement (> 15%)); reintubation within the first 7 days after extubation; nasal trauma; duration of respiratory support or supplemental oxygen; BPD (oxygen or respiratory support at 36 weeks PMA); IVH; NEC; pneumothoraces; time to full feeds | |
| Notes | The trial was underpowered due to a lower than expected primary outcome rate in the control group. They calculated a sample size of 300 would have been required to show a reduction by 50% in the primary outcome with HHHFNC. Study strengthened by having treatment failure as primary outcome. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number sequence, stratified by gestational age |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes, variable block size |
| Blinding (performance bias and detection bias) Need for intubation | Unclear risk | Decisions to re‐intubate and weaning of respiratory support were made by treating physicians in knowledge of allocation |
| Blinding (performance bias and detection bias) Nasal damage | High risk | Nasal trauma scores were non‐blinded |
| Selective reporting (reporting bias) | Unclear risk | Study not registered. Unclear if other outcomes measured |
| Other bias | Low risk | |
Iranpour 2011.
| Methods | Randomised controlled trial | |
| Participants | 70 preterm infants (30 to 35 weeks' gestation) with respiratory distress needing non‐invasive respiratory support at 24 hours of age | |
| Interventions | HFNC (1 to 4 L/min) from 24 hours of age until no longer needing respiratory support Nasal CPAP 6 cmH2O |
|
| Outcomes | Treatment failure (intubation); death; duration of hospitalisation; failure of treatment; duration of respiratory distress; NEC; PDA; IVH; CLD (not defined); pneumothorax; pulmonary haemorrhage; apnoea; sepsis; duration of hospitalisation; duration to reach to full enteral feeding; nasal mucosal injury | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information about sequence generation not available |
| Blinding (performance bias and detection bias) Need for intubation | Unclear risk | No mention of set criteria for treatment failure/intubation |
| Blinding (performance bias and detection bias) Nasal damage | High risk | Non‐blinded |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
Kugelman 2015.
| Methods | Randomised controlled single centre trial | |
| Participants | 76 preterm infants < 35 weeks' gestation, > 1000 grams requiring primary respiratory support from birth | |
| Interventions | HFNC (1 to 5 L/min) Synchronised nasal intermittent positive pressure ventilation (NIPPV) via nasal prongs, 12 to 30 cycles per minute, inspiratory time of 0.3 sec, positive end expiratory pressure (PEEP) of 6 cmH2O, and peak inspiratory pressure (PIP) of 14 to 22 cmH2O |
|
| Outcomes | Treatment failure (increased respiratory distress plus respiratory acidosis (pH < 7.2, CO2 > 60 mmHg), FiO2 > 0.50 or recurrent significant apnoea); duration of respiratory support; clinical features; IVH; BPD (oxygen at 36 weeks PMA); time until full feeds; length of stay; air leak; nasal trauma; gastrointestinal perforation | |
| Notes | Pilot study, underpowered to detect clinically significant difference in outcomes between interventions. Time point for treatment failure not defined. Infants meeting failure of treatment criteria in the HFNC arm were able to receive NIPPV. Treatment failure reported separately from reintubation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) Need for intubation | Unclear risk | Criteria for failure of nasal support were described, and reported separately from intubation. |
| Blinding (performance bias and detection bias) Nasal damage | High risk | |
| Selective reporting (reporting bias) | Low risk | Trial registered. All outcomes recorded in protocol reported. |
| Other bias | Unclear risk | HFNC equipment supplied by Vapotherm. No external funding |
Liu 2014.
| Methods | Randomised controlled trial | |
| Participants | 255 intubated newborn infants, admitted to newborn intensive care < 7 days of life, and judged ready to be extubated | |
| Interventions | Humidified heated HFNC (Fisher and Paykel), starting flow 3 to 8 L/min depending on weight Nasal CPAP (Infant Flow/Stephanie) 6 to 10 L/min, end‐expiratory pressure the same as given during mechanical ventilation |
|
| Outcomes | Primary outcomes: Extubation failure (reintubation within 7 days); BPD (as defined in Jobe 2001); death; total invasive and non‐invasive ventilation time; time in oxygen before discharge; apnoea; nasal septal trauma; pneumothorax; abdominal distension; NEC; bowel perforation; time to achieve full feeds (≥ 120 mL/kg/day) |
|
| Notes | Crossover permitted after 72 hours; unclear how often this occurred. Intubation criteria (based on Yoder 2013); unclear if adhered to. Nasal septal injury and abdominal distension assessed by attending physician. Study funded by Hebei government. No overlap in patients between this study and Yoder 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description in paper |
| Allocation concealment (selection bias) | Low risk | Sealed envelope (not clear if block randomisation used. Stratified by centre) |
| Blinding (performance bias and detection bias) Need for intubation | Unclear risk | Unclear if intubation criteria adhered to |
| Blinding (performance bias and detection bias) Nasal damage | High risk | No pre‐set criteria for evaluating nasal trauma or abdominal distension |
| Selective reporting (reporting bias) | Unclear risk | Trial not registered. |
| Other bias | Low risk | |
Manley 2013.
| Methods | Randomised controlled non‐inferiority trial | |
| Participants | Preterm infants < 32 weeks gestation at birth, mechanically ventilated and ready for extubation | |
| Interventions | HFNC (starting at 5 to 6 L/min) via 'Optiflow' (Fisher and Paykel) CPAP 7 cmH2O (subsequently 5 to 8 cmH2O) via midline or binasal prongs |
|
| Outcomes | Treatment failure < 7 days; reintubation; death before hospital discharge; BPD (oxygen at 36 weeks PMA); pneumothorax after trial entry; total days of respiratory support; duration of oxygen supplementation; length of hospital admission; nasal trauma | |
| Notes | High rate of rescue treatment with CPAP in the HFNC group. Lower rates of treatment failure in infants treated with CPAP, but use of NIPPV available to infants treated with CPAP. Prespecified treatment failure criteria. Study strengthened by having treatment failure as primary outcome. Non‐inferiority design. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block‐randomisation sequence with random block sizes |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, sealed, opaque envelopes opened immediately before extubation |
| Blinding (performance bias and detection bias) Need for intubation | Low risk | Prespecified treatment failure criteria with separate reporting of treatment failure and intubation |
| Blinding (performance bias and detection bias) Nasal damage | High risk | Non‐blinded assessment |
| Selective reporting (reporting bias) | Low risk | Trial registered. (Mentions 'infant comfort' ‐ not reported) |
Miller 2010.
| Methods | Randomised controlled trial | |
| Participants | 40 preterm infants, 26 to 29 weeks' gestation, intubated in the first 72 hours of life | |
| Interventions | HFNC ‐ VapothermTM 6 L/min; n = 20 HFNC ‐ Fisher and PaykelTM 6 L/min; n = 20 Weaned by no more than 1 L/min per day |
|
| Outcomes | Extubation failure within 72 hours (re‐intubated if oxygen requirement persistently > 70%; CO2 on arterial blood gas of > 65 with pH of < 7.25; > 3 apnoea episodes requiring moderate stimulation in 12 hour or two apnoea episodes requiring vigorous stimulation in an 8 hour period); reintubation ≤ 7 days; duration of mechanical ventilation after initial extubation; incidence of CLD (oxygen requirement at 36 weeks corrected age); pneumothorax; hyperinflated on CXR; pulmonary haemorrhage; feeding intolerance (> 50% residuals) | |
| Notes | One infant randomised to HFNC (Vapotherm) was re‐intubated but then died during the study (due to sepsis) and was excluded from analysis. Study was jointly funded by the two manufacturers of HFNC equipment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not provided on sequence generation |
| Blinding (performance bias and detection bias) Need for intubation | Unclear risk | Not reported if prespecified criteria met |
| Blinding (performance bias and detection bias) Nasal damage | Low risk | Not assessed |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Other bias | Low risk | |
Mostafa‐Gharehbaghi 2014.
| Methods | Single centre randomised controlled trial | |
| Participants | 85 preterm infants, 30 to 34 weeks gestation, 1250 to 2000 grams, stabilised initially on CPAP, RDS and received surfactant (intubated, surfactant, extubated) for increased oxygen requirement | |
| Interventions | HFNC ‐ 6 L/min by short binasal cannula CPAP ‐ 5 to 6 cmH2O (Bubble CPAP) |
|
| Outcomes | Intubation and mechanical ventilation within 3 days after surfactant; oxygen dependency at 36 weeks' corrected age; pneumothorax; nasal mucosal injury; intraventricular haemorrhage |
|
| Notes | Note: high rate of rescue treatment with CPAP in infants randomised to HFNC. Analysis based on intention to treat. Nasal mucosal injury said to be blinded; however, assessed by nursing staff and clinicians caring for the infant. No record of whether prespecified intubation criteria were adhered to. Additional information and data provided by the authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "random number list" |
| Allocation concealment (selection bias) | Low risk | Opaque envelope |
| Blinding (performance bias and detection bias) Need for intubation | Unclear risk | Not clear if intubation criteria followed |
| Blinding (performance bias and detection bias) Nasal damage | High risk | Nasal mucosal injury assessed and charted by nurses and confirmed by physicians caring for the infant (non‐blinded) |
| Selective reporting (reporting bias) | Unclear risk | Study registered only at end of recruitment period. |
| Other bias | Low risk | |
Nair 2005.
| Methods | Randomised controlled trial | |
| Participants | 67 preterm infants with respiratory distress requiring CPAP in 1st 6 hours, 27 to 34 weeks' gestation (mean 32 weeks) | |
| Interventions | HFNC: VapothermTM 5 to 6 L/min; n = 33 CPAP: bubble CPAP, Hudson prongs, 5 to 6 cmH2O; n = 34 |
|
| Outcomes | Respiratory failure (leading to intubation) (pH ≤ 7.25 and PaCO2 ≥ 60 mmHg , or FiO2 > 0.70, or severe or frequent apnoea); nasal injury; BPD (as defined in Jobe 2001); mortality; length of hospitalisation; sepsis; pneumothorax | |
| Notes | Study finished prior to achieving target sample size due to recall of Vapotherm units A full study manuscript including results was obtained from the authors Vapotherm provided equipment for the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified into 27 to 30 weeks' and 31 to 34 weeks' gestation. Permuted block randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) Need for intubation | Unclear risk | Standardised criteria for respiratory failure, though frequency of blood gases and recording of apnoea not blinded |
| Blinding (performance bias and detection bias) Nasal damage | High risk | Assessment of nasal injury non‐blinded |
| Selective reporting (reporting bias) | Unclear risk | Not registered |
| Other bias | Unclear risk | Vapotherm provided equipment for the study. |
Sadeghnia 2014.
| Methods | Randomised controlled trial | |
| Participants | 60 preterm infants, 1000 to 1500 grams, on low level of CPAP support (4 cmH2O, < 30% supplemental oxygen) | |
| Interventions | HFNC with one brand of humidifier (MR850) HFNC with second brand of humidifier (PMH7000) |
|
| Outcomes | Primary outcome (specified at study registration): humidity of gas delivered; treatment failure (requiring CPAP); intubation; CLD (oxygen after day 28 of life); nasal trauma; duration of treatment | |
| Notes | Discrepancy in numbers of patients between abstract (35 patients in each group) and table (apparently 30 in each group). No comment on ethics approval |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Need for intubation | Unclear risk | Set criteria for intubation, and for weaning from HFNC; however, not described whether these were applied |
| Blinding (performance bias and detection bias) Nasal damage | High risk | Not blinded |
| Selective reporting (reporting bias) | Unclear risk | Study registered retrospectively after completed. |
| Other bias | Unclear risk | Discrepancy in patient numbers between abstract and table |
Woodhead 2006.
| Methods | Randomised crossover trial | |
| Participants | 30 infants admitted to neonatal intensive care unit, intubated, planned to extubate to HFNC | |
| Interventions | Randomised to one modality for 24 hours after extubation then switched to other modality Humidified HFNC ‐ VapothermTM (mean 3.1 L/min); n = 15 Non‐humidified HFNC (mean 1.8 L/min); n = 13 |
|
| Outcomes | Need for intubation (no pre‐specified criteria); nasal mucosa examination; pneumothorax or pneumomediastinum | |
| Notes | Data from the first crossover period only were included in the review Flow rates differed significantly between interventions Funding source unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table, stratified by weight |
| Blinding (performance bias and detection bias) Need for intubation | High risk | No set criteria for intubation |
| Blinding (performance bias and detection bias) Nasal damage | Low risk | 'Masking' of intervention; unclear how effective |
| Other bias | Low risk | |
Yoder 2013.
| Methods | Multi‐centre randomised controlled trial | |
| Participants | 432 preterm infants (> 28 weeks' gestation and > 1000 grams) being managed with non‐invasive respiratory support either as primary support after birth, or post‐extubation | |
| Interventions | HFNC (various devices) starting at 3 to 5 L/min (increased as required to maximum of 3 L/min above starting point) Nasal CPAP 5 to 6 cmH2O or equivalent to end expiratory pressure on ventilator (subsequently increased to maximum 8 cmH2O) |
|
| Outcomes | Need for intubation within 72 hours of treatment assignment; duration of respiratory support; oxygen; delayed intubation; significant apnoea; pulmonary air leaks; feed intolerance; abdominal distension; necrotising enterocolitis; intestinal perforation; late onset nosocomial infection; nasal mucosal injury; infant comfort; BPD (based on need for oxygen as assessed by an oxygen reduction test at 36 weeks PMA); death; oxygen requirement at discharge; time to full feeds |
|
| Notes | Study underpowered because lower incidence than expected of intubation in infants treated with CPAP No crossover permitted between interventions in the first 72 hours of the study More infants in the HFNC group crossed over to the alternative treatment after 72 hours. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Random number generation" |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding (performance bias and detection bias) Need for intubation | Low risk | Prespecified criteria for intubation (however, did not report compliance with criteria) |
| Blinding (performance bias and detection bias) Nasal damage | High risk | Subjective assessment, non‐blinded. |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes not reported in detail. Feeding intolerance not reported |
| Other bias | Low risk | |
BPD: bronchopulmonary dysplasia
CLD: chronic lung disease
CXR: chest x‐ray
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beltramo 2008 | This study was non‐randomised. It compared the need for reintubation in 20 consecutive infants with birth weight < 1750 grams needing respiratory support post‐extubation (the first 8 treated with CPAP, subsequent 12 treated with HFNC). |
| Boumecid 2007 | This crossover trial compared variable flow CPAP with constant‐flow CPAP and non‐humidified nasal cannula at 2 L/min. No outcomes of relevance to this review were reported. |
| Capasso 2005 | This study did not examine the use of nasal cannula for the target indication for this review (resuscitation at birth was studied). |
| Choi 2011 | This was a retrospective comparison of 35 infants treated with CPAP and 35 infants treated with HFNC. |
| Courtney 2001 | This crossover trial compared variable flow CPAP with constant flow CPAP, and a modified nasal cannula attached to a constant flow CPAP circuit. No outcomes of relevance to this review were reported. |
| de Jongh 2014 | This study was non‐randomised. It compared work of breathing on CPAP compared with HFNC (initial modality dependent on what infant was already receiving). No outcomes of relevance to this review were reported. |
| Fernandez‐Alvarez 2013 | This study was non‐randomised (it compared HFNC as a means of weaning from CPAP with low flow nasal cannula in a matched pair retrospective cohort study). |
| Holleman‐Duray 2007 | This study was non‐randomised (it compared HFNC in combination with an early extubation policy in comparison with historical controls). |
| Klingenberg 2014 | In this randomised crossover trial, patient comfort was compared between HFNC and CPAP. No outcomes relevant to this review were reported. |
| Lampland 2009 | This non‐randomised crossover study compared CPAP with HFNC. No outcomes of relevance to this review were reported. |
| Lavizzari 2014 | This crossover trial measured lung mechanics in infants while on nasal CPAP and HFNC at different flow rates. No outcomes relevant to this review were reported. |
| Mazmanyan 2013 | This non‐randomised study compared infants treated with HFNC with historical controls treated with head box or low flow nasal cannula oxygen. |
| Nasef 2015 | Preterm infants < 1500 grams were randomised in a crossover design to receive 2 hours of either Infant Flow CPAP (IF‐CPAP) at 5 to 6 cmH2O or HFNC with the flow rate adjusted to achieve an equivalent pharyngeal pressure. No outcomes of relevance to this review were reported. |
| Phadtare 2009 | This study was non‐randomised. Infants were given HFNC or CPAP depending on availability and physician preference, with an observational study of outcome. |
| Pyon 2008 | This crossover trial compared NCPAP with HFNC. No outcomes of relevance to this review were reported. |
| Saslow 2006 | This crossover trial compared CPAP with high flow nasal cannula. No outcomes of relevance to this review were reported. |
| Shoemaker 2007 | This non‐randomised study compared HFNC with historical controls treated with CPAP. |
| Sreenan 2001 | This crossover trial of CPAP and non‐humidified HFNC was non‐randomised. |
| Wilson 1996 | This study examined nasal cannula compared to nasopharyngeal catheters at flow rates < 1 L/min. |
Characteristics of studies awaiting assessment [ordered by study ID]
Chen 2015.
| Methods | Random assignment |
| Participants | VLBW infants admitted to the neonatal intensive care unit with RDS |
| Interventions | 66 VLBW infants admitted to the neonatal intensive care unit were diagnosed with RDS, and they were randomly assigned to HHHFNC group and NCPAP group after receiving treatment with porcine pulmonary surfactant and conventional treatment |
| Outcomes | Changes in clinical symptoms and the incidence of complications were observed in the two groups |
| Notes | Article in Chinese |
Febre 2015.
| Methods | Randomised controlled trial |
| Participants | 20 preterm and term infants 400 to 5000 grams, needing FiO2 > 30% |
| Interventions | HFNC: "Adaptive Dynamic Inspiratory Nasal Apparatus" 2 to 4 L/min, pop‐off valve if circuit pressure exceeds 10 cmH2O CPAP: Hudson prongs 4 to 8 cmH2O |
| Outcomes | Oxygen requirement, level of pressure or flow support, radiological changes, blood gas measurement, time to wean off protocol, and failure to wean or necessity for endotracheal intubation |
| Notes | Potential for allocation bias |
Lawrence 2012.
| Methods | ? randomised study |
| Participants | Infants gestational ages of 26 and 33 6/7 weeks and weighing between 750 and 2500 grams |
| Interventions | Patients stratified into one of two groups (NCPAP or HFNC) |
| Outcomes | Transpleural pressure, patent ductus arteriosus (PDA), necrotising enterocolitis (NEC), intraventricular haemorrhage (IVH), or air leaks |
| Notes |
Tang 2015.
| Methods | Single‐centre pilot, factorial design, 4‐arm randomised controlled trial |
| Participants | Infants born < 30 weeks' gestation |
| Interventions | 60 eligible infants who met stability criteria were randomised into four groups (Group 1 abrupt wean with HFNC; group 2 abrupt wean without HFNC; group 3 gradual wean with HFNC; group 4 gradual wean without HFNC). |
| Outcomes | Primary outcomes evaluated were chronic lung disease (CLD) at 36 weeks, duration of respiratory support, length of hospital stay and time to achieve full sucking feeds. |
| Notes | Registration Number: ACTRN12610001003066 |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12610000677000.
| Trial name or title | High Flow support versus Continuous Positive Airway Pressure (CPAP) support in non‐acute respiratory support for preterm infants from 30 weeks corrected gestation |
| Methods | RCT. Sample size 30 |
| Participants | Infants are eligible to enrol if: 1. They are at least 5 days old 2. They are at least 30 weeks' corrected gestation 3. They are less than 32 weeks' corrected gestation 4. They are CPAP‐dependent but not requiring greater than 5 cmH2O pressure or greater than 25% oxygen |
| Interventions | HFNC (minimum 4 L/min to maximum 6 L/min in 25% oxygen) Dose size determined by level of support needed (i.e. all will start on 6 L/min, then be reduced as needed) |
| Outcomes | CLD; failure of HFNC; stability of treatment |
| Starting date | 31/8/2010 |
| Contact information | Ashley McEwan, ashley.mcewan@hotmail.com |
| Notes | ACTRN12610000677000 |
ACTRN12613000303741.
| Trial name or title | High‐flow nasal cannulae as primary respiratory support in the treatment of early respiratory distress: The HIPSTER trial. |
| Methods | Multicentre, randomised, non‐inferiority trial. Sample size 750 infants. |
| Participants | Preterm infants at least 28 weeks' and < 37 weeks' gestation, admitted to a participating NICU, < 24 hours old with early respiratory distress requiring non‐invasive support |
| Interventions | HFNC (Fisher & Paykel ‘Optiflow’ or Vapotherm) vs. nasal CPAP (binasal prongs or mask) |
| Outcomes | Treatment failure within 72 hours of randomisation, based on pre‐specified failure criteria. |
| Starting date | December 2013 |
| Contact information | Calum Roberts, Newborn Research Centre, The Royal Women’s Hospital, Parkville. Email: calum.roberts@thewomens.org.au |
| Notes | Australian New Zealand Clinical Trials Registry: ACTRN12613000303741 |
ACTRN12615000077561.
| Trial name or title | Duration of respiratory support in preterm infants with a gestational age (GA) of < 30 weeks weaned from continuous positive airway pressure (CPAP): a randomised controlled trial comparing heated humidified high flow nasal cannula (HHHFNC) and CPAP. |
| Methods | RCT. Sample size 120 infants. |
| Participants | Preterm infants < 30 weeks' gestation having been on nasal CPAP for at least one week. |
| Interventions | HFNC (Fisher & Paykel) vs. bubble nasal CPAP (Fisher & Paykel). |
| Outcomes | Duration (in hours) of respiratory support from randomisation to achieving at least 72 hours free of respiratory support. |
| Starting date | March 2015. |
| Contact information | Joanne Clements, Middlemore Hospital, New Zealand Email: joanne.clements@middlemore.co.nz |
| Notes | Australian New Zealand Clinical Trials Registry: |
IRCT2014012716376N1.
| Trial name or title | Comparing two methods of cannula nasal with high flow and conventional FiO2 for successful weaning of preterm infants with respiratory distress from Nasal CPAP in Alzahra and Shahidbeheshti hospitals, Isfahan |
| Methods | RCT. Sample size 88 |
| Participants | Preterm infants with respiratory distress syndrome (RDS) and gestational age of 28 to 37 weeks' who require NCPAP; being stable on NCPAP at FiO2 = 0.30 for 6 hours; no clinical sign of RDS such as tachypnoea, severe apnoea, intercostals retraction and nasal flaring. |
| Interventions | Intervention 1: Intervention group (Humidified High Flow Nasal Cannula group) who wean from NCPAP at FiO2= 0.30 and receive 2 L/min O2 via cannula nasal. Intervention 2: Control group: who are connected to the NCPAP at FiO2= 0.21 to achieve a stable condition for 24 hours. |
| Outcomes | Apnoea. Timepoint: from the time on the Nasal Continuous Positive Airway Pressure (NCPAP) until weaning from O2. Method of measurement: Observation Duration of need for respiratory support. Timepoint: from the time on the Nasal Continuous Positive Airway Pressure (NCPAP) till weaning from O2. Method of measurement: Observation |
| Starting date | 20/04/2012 |
| Contact information | Dr. Alireza Eshghi ali_phd203@ yahoo.com |
| Notes | IRCT2014012716376N1 |
ISRCTN66716753.
| Trial name or title | Can High Flow Nasal Prongs therapy facilitate earlier establishment of full oral feeds in babies who are Nasal Continuous Positive Airway Pressure dependent at 32 weeks gestation? |
| Methods | RCT. Sample size 44 |
| Participants | Infants < 30 weeks and < 1500 grams requiring CPAP at 32 weeks corrected age with oxygen requirement of < 30% |
| Interventions | Group A: Continue on Nasal CPAP Group B: High Flow nasal prongs 7 L/min |
| Outcomes | Establishment of full oral feeding |
| Starting date | 2/2013 |
| Contact information | Dr. Jan Miletin jmiletin@coombe.ie |
| Notes | ISRCTN66716753 |
JPRN‐UMIN000013906.
| Trial name or title | A Randomized Controlled Trial to Compare High‐Flow Nasal Cannula with Nasal CPAP after Extubation in Preterm infants |
| Methods | RCT, 160 infants |
| Participants | 22 to 34 weeks' gestation |
| Interventions | HFNC CPAP |
| Outcomes | The incidence of re‐intubation within 7 days after extubation |
| Starting date | 2014 |
| Contact information | kusuda.satoshi@twmu.ac.jp |
| Notes | UMIN000013906 |
NCT01270581.
| Trial name or title | High Flow Nasal Cannula vs Bubble Nasal CPAP for the Treatment of Transient Tachypnea of the Newborn in Infants > 35 Weeks Gestation |
| Methods | RCT, estimated enrolment 66 infants |
| Participants | Infants > 35 weeks' gestation diagnosed with transient tachypnoea and admitted to the NICU within the first 24 hours of life |
| Interventions | HFNC versus bubble nasal CPAP |
| Outcomes | Duration of respiratory support |
| Starting date | July 2010 |
| Contact information | Andrea Weintraub, Mount Sinai School of Medicine, New York, andrea.weintraub@mssm.edu |
| Notes | ClinicalTrials.gov identifier: NCT01270581 |
NCT01939067.
| Trial name or title | Pulmonary Mechanics in Preterm Infants Treated With Heated Humidified High Flow Nasal Cannula as Compared to Nasal Continuous Positive Airway Pressure. |
| Methods | RCT. Sample size 150 infants. |
| Participants | Preterm infants 28 to 37 weeks' gestation, up to 72 hours old, requiring non‐invasive respiratory support |
| Interventions | HFNC vs. nasal CPAP |
| Outcomes | Pulmonary mechanics and chest wall asynchrony measures. |
| Starting date | June 2013 |
| Contact information | Soraya Abbasi, Children’s Hospital of Philadelphia, USA. Email: soraya.abbasi@uphs.upenn.edu |
| Notes | ClinicalTrials.gov Identifier: NCT01939067 |
NCT02055339.
| Trial name or title | Comparison of Nasal Continuous Positive Airway Pressure With Low Flow Oxygen Versus Heated, Humidified High Flow Nasal Cannula for Oral Feeding of the Premature Infant (CHOMP Trial): A Pilot Study |
| Methods | RCT. Sample size 40 infants. |
| Participants | Preterm infants born < 28 weeks' gestation who are now 34 weeks' corrected gestational age, dependent on non‐invasive respiratory support, and receiving nasogastric feeds. |
| Interventions | HFNC (Fisher & Paykel) vs. Infant Flow CPAP |
| Outcomes | Time to reach full oral feeds |
| Starting date | February 2014 |
| Contact information | Sandra Leibel, Mount Sinai Hospital, New York Email: sleibel@mtsinai.on.ca |
| Notes | ClinicalTrials.gov Identifier: NCT02055339 |
NICU: neonatal intensive care unit
Contributions of authors
The authors Wilkinson, Andersen and O'Donnell developed the protocol; DW and BM performed the literature search, data collection and analysis for this update. All authors collaborated in the writing of the review.
Sources of support
Internal sources
University of Oxford, UK.
University of Adelaide, Australia.
University of Melbourne, Australia.
External sources
-
NHMRC, Australia.
Fellowship ‐ DW, BM
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
Declarations of interest
Brett Manley was the first author of one of the trials included in this review. Analysis of that paper was performed by other review authors.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abdel Hady 2011 {published and unpublished data}
- Abdel‐Hady H, Shouman B, Aly H. Early weaning from CPAP to high flow nasal cannula in preterm infants is associated with prolonged oxygen requirement: A randomized controlled trial. Early Human Development 2011;87(3):205‐8. [PUBMED: 21276671] [DOI] [PubMed] [Google Scholar]
Badiee 2015 {published data only}
- Badiee Z, Eshghi A, Mohammadizadeh M. High flow nasal cannula as a method for rapid weaning from nasal continuous positive airway pressure. International Journal of Preventive Medicine 2015;6:33. [PUBMED: 25949783] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2006 {published data only}
- Campbell DM, Shah PS, Shah V, Kelly EN. Nasal continuous positive airway pressure from high flow cannula versus infant flow for preterm infants. Journal of Perinatology 2006;26(9):546‐9. [PUBMED: 16837929] [DOI] [PubMed] [Google Scholar]
Ciuffini 2014 {published data only}
- Ciuffini F, Pietrasanta C, Lavizzari A, Musumeci S, Gualdi C, Sortino S, et al. Comparison between two different modes of non‐invasive ventilatory support in preterm newborn infants with respiratory distress syndrome mild to moderate: preliminary data. La Pediatria Medica e Chirurgica: Medical and Surgical Pediatrics 2014;36(4):88. [PUBMED: 25573704] [DOI] [PubMed] [Google Scholar]
Collins 2013 {published and unpublished data}
- Collins CL, Barfield C, Davis PG, Horne RS. Randomized controlled trial to compare sleep and wake in preterm infants less than 32 weeks of gestation receiving two different modes of non‐invasive respiratory support. Early Human Development 2015;91(12):701‐4. [PUBMED: 26529175] [DOI] [PubMed] [Google Scholar]
- Collins CL, Barfield C, Horne RS, Davis PG. A comparison of nasal trauma in preterm infants extubated to either heated humidified high‐flow nasal cannulae or nasal continuous positive airway pressure. European Journal of Pediatrics 2014;173(2):181‐6. [PUBMED: 23955516] [DOI] [PubMed] [Google Scholar]
- Collins CL, Holberton JR, Barfield C, Davis PG. A Randomized Controlled Trial to Compare Heated Humidified High‐Flow Nasal Cannulae with Nasal Continuous Positive Airway Pressure Postextubation in Premature Infants. Journal of Pediatrics 2013;162(5):949‐54. [PUBMED: 23260098] [DOI] [PubMed] [Google Scholar]
- Ignacio L, Alfaleh K. A randomized controlled trial to compare heated humidified high‐flow nasal cannulae with nasal continuous positive airway pressure postextubation in premature infants. Journal of Neonatology 2013;2(2):75‐7. [PUBMED: 24049748] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Iranpour 2011 {published and unpublished data}
- Iranpour R, Sadeghnia A, Hesaraki M. High‐flow nasal cannula versus nasal continuous positive airway pressure in the management of respiratory distress syndrome. Journal of Isfahan Medical School 2011;29(143):1. [Google Scholar]
Kugelman 2015 {published and unpublished data}
- Kugelman A, Riskin A, Said W, Shoris I, Mor F, Bader D. A randomized pilot study comparing heated humidified high‐flow nasal cannulae with NIPPV for RDS. Pediatric Pulmonology 2015;50(6):576‐83. [PUBMED: 24619945] [DOI] [PubMed] [Google Scholar]
Liu 2014 {published and unpublished data}
- Liu C, Collaborative Group for the Multicenter Study on Heated Humidified High‐flow Nasal Cannula Ventilation. Efficacy and safety of heated humidified high·flow nasal cannula for prevention of extubation failure in neonates [应用加温湿化高流量鼻导管通气预防 新生儿拔管失败的临床研究]. Zhonghua Er Ke Za Zhi. Chinese Journal of Pediatrics 2014;52(4):271‐6. [PUBMED: 24915914] [PubMed] [Google Scholar]
Manley 2013 {published and unpublished data}
- Manley BJ, Owen LS, Doyle LW, Andersen CC, Cartwright DW, Pritchard MA, et al. High‐flow nasal cannulae in very preterm infants after extubation. New England Journal of Medicine 2013;369(15):1425‐33. [PUBMED: 24106935] [DOI] [PubMed] [Google Scholar]
Miller 2010 {published data only}
- Miller SM, Dowd SA. High‐flow nasal cannula and extubation success in the premature infant: a comparison of two modalities. Journal of Perinatology 2010;30(12):805‐8. [PUBMED: 20237485] [DOI] [PubMed] [Google Scholar]
Mostafa‐Gharehbaghi 2014 {published and unpublished data}
- Mostafa‐Gharehbaghi M, Mojabi H. Comparing the effectiveness of nasal continuous positive airway pressure (NCPAP) and high flow nasal cannula (HFNC) in prevention of post extubation assisted ventilation. Zahedan Journal of Research in Medical Sciences 2015;17(6):e984. [Google Scholar]
Nair 2005 {unpublished data only}
- Nair G, Karna P. Comparison of the effects of Vapotherm and nasal CPAP in respiratory distress. Pediatric Academic Societies Meeting; 2005 May 14‐17; Washington, DC; http://www.abstracts2view.com/pas/ (accessed May 2015):E‐PAS2005:57:2054.
Sadeghnia 2014 {published data only}
- Sadeghnia A, Badiei Z, Talakesh H. A comparison of two interventions for HHHFNC in preterm infants weighing 1,000 to 1,500 g in the recovery period of newborn RDS. Advanced Biomedical Research 2014;3:172. [PUBMED: 25250286] [DOI] [PMC free article] [PubMed] [Google Scholar]
Woodhead 2006 {published data only}
- Woodhead DD, Lambert DK, Clark JM, Christensen RD. Comparing two methods of delivering high‐flow gas therapy by nasal cannula following endotracheal extubation: a prospective, randomized, masked, crossover trial. Journal of Perinatology 2006;26(8):481‐5. [PUBMED: 16724119] [DOI] [PubMed] [Google Scholar]
Yoder 2013 {published and unpublished data}
- Yoder BA, Stoddard RA, Li M, King J, Dirnberger DR, Abbasi S. Heated, humidified high‐flow nasal cannula versus nasal CPAP for respiratory support in neonates. Pediatrics 2013;131(5):e1482‐90. [PUBMED: 23610207] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Beltramo 2008 {published data only}
- Beltramo F, Romero R, Chandler B, Soliz A. Successful extubation in low birth weight infants: A comparison of continuous positive airway pressure (CPAP) versus Vapotherm. Pediatric Academic Society Meeting; 2008 May 3‐6; Honolulu (Hawaii); http://www.abstracts2view.com/pas/ (accessed May 2015):E‐PAS2008:63376.
Boumecid 2007 {published data only}
- Boumecid H, Rakza T, Abazine A, Klosowski S, Matran R, Storme L. Influence of three nasal continuous positive airway pressure devices on breathing pattern in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2007;92(4):F298‐300. [PUBMED: 17088340] [DOI] [PMC free article] [PubMed] [Google Scholar]
Capasso 2005 {published data only}
- Capasso L, Capasso A, Raimondi F, Vendemmia M, Araimo G, Paludetto R. A randomized trial comparing oxygen delivery on intermittent positive pressure with nasal cannulae versus facial mask in neonatal primary resuscitation. Acta Paediatrica 2005;94(2):197‐200. [PUBMED: 15981754] [DOI] [PubMed] [Google Scholar]
Choi 2011 {published data only}
- Choi BM, Lee EH, Park KH, Chung BH, Park HJ, Choi YO, et al. Comparing Usefulness of Humidified High‐Flow Nasal Cannula (HHFNC) and Nasal Continuous Positive Airway Pressure (NCPAP) for Neonatal Respiratory Diseases in Preterm Infants (Poster). Pediatric Research 2011;70:504. [Google Scholar]
Courtney 2001 {published data only}
- Courtney SE, Pyon KH, Saslow JG, Arnold GK, Pandit PB, Habib RH. Lung recruitment and breathing pattern during variable versus continuous flow nasal continuous positive airway pressure in premature infants: an evaluation of three devices. Pediatrics 2001;107(2):304‐8. [PUBMED: 11158463] [DOI] [PubMed] [Google Scholar]
de Jongh 2014 {published data only}
- Jongh BE, Locke R, Mackley A, Emberger J, Bostick D, Stefano J, et al. Work of breathing indices in infants with respiratory insufficiency receiving high‐flow nasal cannula and nasal continuous positive airway pressure. Journal of Perinatology 2014;34(1):27‐32. [PUBMED: 24071905] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandez‐Alvarez 2013 {published data only}
- Fernandez‐Alvarez JR, Gandhi RS, Amess P, Mahoney L, Watkins R, Rabe H. Heated humidified high‐flow nasal cannula versus low‐flow nasal cannula as weaning mode from nasal CPAP in infants ≤28 weeks of gestation. European Journal of Pediatrics 2014;173(1):93‐8. [PUBMED: 23942744] [DOI] [PubMed] [Google Scholar]
Holleman‐Duray 2007 {published data only}
- Holleman‐Duray D, Kaupie D, Weiss MG. Heated humidified high‐flow nasal cannula: use and a neonatal early extubation protocol. Journal of Perinatology 2007;27(12):776‐81. [PUBMED: 17855805] [DOI] [PubMed] [Google Scholar]
Klingenberg 2014 {published data only}
- Klingenberg C, Pettersen M, Hansen EA, Gustavsen LJ, Dahl IA, Leknessund A, et al. Patient comfort during treatment with heated humidified high flow nasal cannulae versus nasal continuous positive airway pressure: a randomised cross‐over trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(2):F134‐7. [PUBMED: 24225220] [DOI] [PubMed] [Google Scholar]
Lampland 2009 {published data only}
- Lampland AL, Plumm B, Meyers PA, Worwa CT, Mammel MC. Observational study of humidified high‐flow nasal cannula compared with nasal continuous positive airway pressure. The Journal of Pediatrics 2009;154(2):177‐82. [PUBMED: 18760803] [DOI] [PubMed] [Google Scholar]
Lavizzari 2014 {published data only}
- Lavizzari A, Veneroni C, Colnaghi M, Ciuffini F, Zannin E, Fumagalli M, et al. Respiratory mechanics during NCPAP and HHHFNC at equal distending pressures. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(4):F315‐20. [PUBMED: 24786469] [DOI] [PubMed] [Google Scholar]
Mazmanyan 2013 {published data only}
- Mazmanyan P, Darakchyan M. Humidified high flow nasal cannula for the treatment of respiratory distress in premature newborns > 34 weeks gestation. Journal of Perinatal Medicine 2013;41. [Google Scholar]
Nasef 2015 {published data only}
- Nasef N, El‐Gouhary E, Schurr P, Reilly M, Beck J, Dunn M, et al. High‐flow nasal cannulae are associated with increased diaphragm activation compared with nasal continuous positive airway pressure in preterm infants. Acta Paediatrica 2015;104(8):e337‐43. [PUBMED: 25759095] [DOI] [PubMed] [Google Scholar]
Phadtare 2009 {published data only}
- Joshi R, Rajhans A, Patil S, Dominic S, Phadtare R, Devaskar U. High flow oxygen in neonatal respiratory failure: Is it better than CPAP. Pediatric Academic Society. 2008; Vol. http://www.abstracts2view.com/pas/.
- Phadtare R, Joshi R, Rajhans A, Patil S, Dominic S, Devaskar U. High flow nasal cannula oxygen (Vapotherm) in premature infants with respiratory distress syndrome: is it better than the conventional nasal continuous positive airways pressure (CPAP)?. Perinatology 2009;11(1):1‐8. [Google Scholar]
Pyon 2008 {published data only}
- Pyon KH, Aghai ZH, Nakhla TA, Stahl GE, Saslow JG. High flow nasal cannula in preterm infants: Effects of high flow rates on work of breathing. Proceedings of the Pediatric Academic Societies Annual Meeting; 2008 May 3‐6; Honolulu (HI). 2008:E‐PAS2008:633763.13.
Saslow 2006 {published data only}
- Saslow JG, Aghai ZH, Nakhla TA, Hart JJ, Lawrysh R, Stahl GE, et al. Work of breathing using high‐flow nasal cannula in preterm infants. Journal of Perinatology 2006;26(8):476‐80. [PUBMED: 16688202] [DOI] [PubMed] [Google Scholar]
Shoemaker 2007 {published data only}
- Shoemaker MT, Pierce MR, Yoder BA, DiGeronimo RJ. High flow nasal cannula versus nasal CPAP for neonatal respiratory disease: a retrospective study. Journal of Perinatology 2007;27(2):85‐91. [PUBMED: 17262040] [DOI] [PubMed] [Google Scholar]
Sreenan 2001 {published data only}
- Sreenan C, Lemke RP, Hudson‐Mason A, Osiovich H. High‐flow nasal cannulae in the management of apnea of prematurity: a comparison with conventional nasal continuous positive airway pressure. Pediatrics 2001;107(5):1081‐3. [PUBMED: 11331690] [DOI] [PubMed] [Google Scholar]
Wilson 1996 {published data only}
- Wilson J, Arnold C, Connor R, Cusson R. Evaluation of oxygen delivery with the use of nasopharyngeal catheters and nasal cannulas. Neonatal Network 1996;15(4):15‐22. [PUBMED: 8716524] [PubMed] [Google Scholar]
References to studies awaiting assessment
Chen 2015 {published data only}
- Chen J, Gao WW, Xu F, Du LL, Zhang T, Ling X, Li WT. Comparison of clinical efficacy of heated humidified high flow nasal cannula versus nasal continuous positive airway pressure in treatment of respiratory distress syndrome in very low birth weight infants. Zhongguo Dang Dai Er Ke Za Zhi 2015;17(8):847‐51. [PUBMED: 26287351] [PubMed] [Google Scholar]
Febre 2015 {published data only}
- Febre A, Merritt TA, Terry M, Tong C, Goldstein M. Adaptive Dynamic Inspiratory Nasal Apparatus: Comparison to Traditional Nasal Continuous Airway Pressure (NCPAP). Newborn and Infant Nursing Reviews 2015;15(1):17‐20. [Google Scholar]
Lawrence 2012 {published data only}
- Lawrence JR, Martin GC. A Pilot Study To Evaluate the Safety and Efficacy of High Flow Nasal Cannula vs. Conventional NCPAP. Pediatric Academic Society. 2012; Vol. http://www.abstracts2view.com/pas/.
Tang 2015 {published data only}
- Lutz TL, Tang J, Osborn DA, Malcolm GA, Reid S, Oliver S. High flow nasal cannula for weaning preterm infants from continuous positive airway pressure. Pediatric Academic Society Meeting; 2013 May 4‐7; Washington, DC; http://www.abstracts2view.com/pas/ (accessed May 2015):E‐PAS2013:3800.38.
- Tang J, Reid S, Lutz T, Malcolm G, Oliver S, Osborn DA. Randomised controlled trial of weaning strategies for preterm infants on nasal continuous positive airway pressure. BMC Pediatrics 2015;15:147. [PUBMED: 26446072] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12610000677000 {published data only}
- ACTRN12610000677000. High flow support versus continuous positive airway pressure (cpap) support in non‐acute respiratory support for preterm infants from 30 weeks corrected gestation. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12610000677000.
ACTRN12613000303741 {published data only}
- ACTRN12613000303741. A multi‐centre, randomised, controlled, non‐inferiority trial comparing high flow nasal cannulae to nasal continuous positive airway pressure as primary respiratory support, in preterm infants of 28 weeks' gestation and above, with early respiratory distress or apnoea, assessing assigned treatment failure within 72 hours. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12613000303741.
ACTRN12615000077561 {published data only}
- ACTRN12615000077561. Weaning preterm infants with a gestational age (GA) of < 30 weeks from respiratory support: a comparison of duration of respiratory support with heated humidified high flow nasal cannula (HHHFNC) and continuous positive airway pressure (CPAP). http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12615000077561.
IRCT2014012716376N1 {published data only}
- IRCT2014012716376N1. Comparing two methods of cannula nasal with high flow and conventional FiO2 for successful weaning of preterm infants with respiratory distress from Nasal CPAP in Alzahra and Shahidbeheshti hospitals, Isfahan. http://www.irct.ir/index.php.
ISRCTN66716753 {published data only}
- ISRCTN66716753. High Flow Nasal Prongs (HFNP) therapy versus Nasal Continuous Positive Airway Pressure (NCPAP) in establishing full oral feeds in Very Low Birth Weight (VLBW) infants ‐ randomized controlled trial. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN66716753.
JPRN‐UMIN000013906 {published data only}
- JPRN‐UMIN000013906. A randomized controlled trial to compare high‐flow nasal cannula with nasal cpap after extubation in preterm infants. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000013906.
NCT01270581 {unpublished data only}
- High Flow Nasal Cannula vs Bubble Nasal CPAP for the Treatment of Transient Tachypnea of the Newborn in Infants > 35 Weeks Gestation. Ongoing study July 2010.
NCT01939067 {published data only}
- NCT01939067. Pulmonary mechanics in preterm infants treated with heated humidified high flow nasal cannula as compared to nasal continuous positive airway Pressure. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT01939067.
NCT02055339 {published data only}
- NCT02055339. Comparison of nasal continuous positive airway pressure with low flow oxygen versus heated, humidified high flow nasal cannula for oral feeding of the premature infant (chomp trial): a pilot study. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT02055339.
Additional references
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1‐7. [PUBMED: 413500] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Paoli 2003
- Paoli AG, Morley C, Davis PG. Nasal CPAP for neonates: what do we know in 2003?. Archives of Disease in Childhood. Fetal and Neonatal Edition 2003;88(3):F168‐72. [PUBMED: 12719386] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Paoli 2008
- Paoli AG, Davis PG, Faber B, Morley CJ. Devices and pressure sources for administration of nasal continuous positive airway pressure (NCPAP) in preterm neonates. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD002977.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dysart 2009
- Dysart K, Miller TL, Wolfson MR, Shaffer TH. Research in high flow therapy: mechanisms of action. Respiratory Medicine 2009;103(10):1400‐5. [PUBMED: 19467849] [DOI] [PubMed] [Google Scholar]
Finer 2005
- Finer NN. Nasal cannula use in the preterm infant: oxygen or pressure?. Pediatrics 2005;116(5):1216‐7. [PUBMED: 16264009] [DOI] [PubMed] [Google Scholar]
Frey 2001
- Frey B, McQuillan PJ, Shann F, Freezer N. Nasopharyngeal oxygen therapy produces positive end‐expiratory pressure in infants. European Journal of Pediatrics 2001;160(9):556‐60. [PUBMED: 11585079] [DOI] [PubMed] [Google Scholar]
Frey 2003
- Frey B, Shann F. Oxygen administration in infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2003;88(2):F84‐8. [PUBMED: 12598492] [DOI] [PMC free article] [PubMed] [Google Scholar]
Frizzola 2011
- Frizzola M, Miller TL, Rodriguez ME, Zhu Y, Rojas J, Hesek A, et al. High‐flow nasal cannula: Impact on oxygenation and ventilation in an acute lung injury model. Pediatric Pulmonology 2011;46(1):67‐74. [PUBMED: 21171186] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garland 1985
- Garland JS, Nelson DB, Rice T, Neu J. Increased risk of gastrointestinal perforations in neonates mechanically ventilated with either face mask or nasal prongs. Pediatrics 1985;76(3):406‐10. [PUBMED: 4034300] [PubMed] [Google Scholar]
Hegde 2013
- Hegde S, Prodhan P. Serious Air Leak Syndrome Complicating High‐Flow Nasal Cannula Therapy: A Report of 3 Cases. Pediatrics 2013;131(3):e939‐44. [PUBMED: 23382446] [DOI] [PubMed] [Google Scholar]
Hensey 2013
- Hensey CC, Hayden E, O'Donnell CPF. A randomised crossover study of low‐flow air or oxygen via nasal cannulae to prevent desaturation in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2013;98(5):F388‐91. [PUBMED: 23315286] [DOI] [PubMed] [Google Scholar]
Hough 2012
- Hough JL, Shearman AD, Jardine LA, Davies MW. Humidified high flow nasal cannulae: Current practice in Australasian nurseries, a survey. Journal of Paediatrics and Child Health 2012;48(2):106‐13. [PUBMED: 21470336] [DOI] [PubMed] [Google Scholar]
Jasin 2008
- Jasin LR, Kern S, Thompson S, Walter C, Rone JM, Yohannan MD. Subcutaneous scalp emphysema, pneumo‐orbitis and pneumocephalus in a neonate on high humidity high flow nasal cannula. Journal of Perinatology 2008;28(11):779‐81. [PUBMED: 18974751] [DOI] [PubMed] [Google Scholar]
Jobe 2001
- Jobe AH, Bancalari E. Bronchopulmonary Dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1723‐9. [PUBMED: 11401896] [DOI] [PubMed] [Google Scholar]
Kopelman 2003a
- Kopelman AE. Airway obstruction in two extremely low birthweight infants treated with oxygen cannulas. Journal of Perinatology 2003;23(2):164‐5. [PUBMED: 12673269] [DOI] [PubMed] [Google Scholar]
Kopelman 2003b
- Kopelman AE, Holbert D. Use of oxygen cannulas in extremely low birthweight infants is associated with mucosal trauma and bleeding, and possibly with coagulase‐negative staphylococcal sepsis. Journal of Perinatology 2003;23(2):94‐7. [PUBMED: 12673256] [DOI] [PubMed] [Google Scholar]
Manley 2012
- Manley BJ, Owen L, Doyle LW, Davis PG. High‐flow nasal cannulae and nasal continuous positive airway pressure use in non‐tertiary special care nurseries in Australia and New Zealand. Journal of Paediatrics and Child Health 2012;48(1):16‐21. [PUBMED: 21988616] [DOI] [PubMed] [Google Scholar]
Morley 2004
- Morley C, Davis P. Continuous positive airway pressure: current controversies. Current Opinion in Pediatrics 2004;16(2):141‐5. [PUBMED: 15021191] [DOI] [PubMed] [Google Scholar]
O'Donnell 2013
- O'Donnell SM, Curry SJ, Buggy NA, Moynihan MM, Sebkova S, Janota J, et al. The NOFLO trial: low‐flow nasal prongs therapy in weaning nasal continuous positive airway pressure in preterm infants. Journal of Pediatrics 2013;163(1):79‐83. [PUBMED: 23312683] [DOI] [PubMed] [Google Scholar]
Osman 2014
- Osman M, Elsharkawy A, Abdel‐Hady H. Assessment of pain during application of nasal continuous positive airway pressure and heated, humidified high‐flow nasal cannulae in preterm infants. Journal of Perinatology 2014 2015;35(4):263‐7. [PUBMED: 25429383] [DOI] [PubMed] [Google Scholar]
Roberts 2014
- Roberts CT, Manley BJ, Dawson JA, Davis PG. Nursing perceptions of high‐flow nasal cannulae treatment for very preterm infants. Journal of Paediatrics and Child Health 2014;50(10):806‐10. [PUBMED: 24943729] [DOI] [PubMed] [Google Scholar]
Robertson 1996
- Robertson NJ, McCarthy LS, Hamilton PA, Moss AL. Nasal deformities resulting from flow driver continuous positive airway pressure. Archives of Disease in Childhood. Fetal and Neonatal Edition 1996;75(3):F209‐12. [PUBMED: 8976689] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spence 2007
- Spence KL, Murphy D, Kilian C, McGonigle R, Kilani RA. High‐flow nasal cannula as a device to provide continuous positive airway pressure in infants. Journal of Perinatology 2007;27(12):772‐5. [PUBMED: 17762844] [DOI] [PubMed] [Google Scholar]
Walsh 2005
- Walsh M, Engle W, Laptook A, Kazzi SN, Buchter S, Rasmussen M, et al. Oxygen delivery through nasal cannulae to preterm infants: can practice be improved?. Pediatrics 2005;116(4):857‐61. [PUBMED: 16199694] [DOI] [PubMed] [Google Scholar]
Wilkinson 2008
- Wilkinson DJ, Andersen CC, Smith K, Holberton J. Pharyngeal pressure with high‐flow nasal cannulae in premature infants. Journal of Perinatology 2008;28(1):42‐7. [PUBMED: 17989697] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wilkinson 2011
- Wilkinson D, Andersen C, O'Donnell CPF, Paoli AG. High flow nasal cannula for respiratory support in preterm infants. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD006405.pub2] [DOI] [PubMed] [Google Scholar]


