Significance
Misalignment of the phase of circadian oscillators occurs upon shifts of schedule. The effects, such as occur in jet lag, have adverse effects on health. We lack understanding of factors that determine how rapidly optimal phase relationships of circadian oscillators are regained. We find that re-entrainment is accelerated in both duper mutant hamsters, which are Cryptochrome 1 (Cry1) null, and Cry1−/− mice after the light:dark cycle is shifted. The effect of the duper mutation to speed the circadian clock is not essential to its acceleration of re-entrainment. Duper shortens the lifespan of cardiomyopathic animals, but repeated phase shifts block this effect. Duper can elucidate pathways that determine the extent of deleterious effects of jet lag and shift work.
Keywords: circadian rhythms, Cryptochrome 1, entrainment, jet lag, cardiomyopathy
Abstract
The Cryptochrome 1 (Cry1)–deficient duper mutant hamster has a short free-running period in constant darkness (τDD) and shows large phase shifts in response to brief light pulses. We tested whether this measure of the lability of the circadian phase is a general characteristic of Cry1-null animals and whether it indicates resistance to jet lag. Upon advance of the light:dark (LD) cycle, both duper hamsters and Cry1−/− mice re-entrained locomotor rhythms three times as fast as wild types. However, accelerated re-entrainment was dissociated from the amplified phase-response curve (PRC): unlike duper hamsters, Cry1−/− mice show no amplification of the phase response to 15’ light pulses. Neither the amplified acute shifts nor the increased rate of re-entrainment in duper mutants is due to acceleration of the circadian clock: when mutants drank heavy water to lengthen the period, these aspects of the phenotype persisted. In light of the health consequences of circadian misalignment, we examined effects of duper and phase shifts on a hamster model of heart disease previously shown to be aggravated by repeated phase shifts. The mutation shortened the lifespan of cardiomyopathic hamsters relative to wild types, but this effect was eliminated when mutants experienced 8-h phase shifts every second week, to which they rapidly re-entrained. Our results reveal previously unsuspected roles of Cry1 in phase shifting and longevity in the face of heart disease. The duper mutant offers new opportunities to understand the basis of circadian disruption and jet lag.
Endogenous daily (circadian) oscillators govern metabolism and behavior in order to coordinate physiological functions. In mammals, light activates retinal projections that entrain a master pacemaker in the suprachiasmatic nucleus (SCN), which in turn sets the phase of subordinate oscillators elsewhere in the body in order to ensure appropriate timing relative to the environment (1, 2). Upon a shift of the light:dark (LD) schedule, rhythms of locomotor activity typically shift at a rate of 1 to 2 h/d until a stable phase relationship is regained. Misalignment occurs as the phase of oscillators in different organs shifts at different rates (3). Disruption of optimal phase relationships among circadian oscillators, as occurs in jet lag and shift work, has adverse consequences for health (4, 5).
The determinants of the latency with which normal phase is re-established after a shift of the LD cycle are unknown. The phase-response curve (PRC), an analytic tool which describes the shift of phase as a function of the phase at which the oscillation is perturbed, predicts both the entrained phase angle and the range of cycle lengths (T) to which the animal can entrain (6). In most mammals, a brief light pulse administered during the early or late subjective night typically elicits a phase delay or advance of 1 to 2 h, respectively. However, the value of the PRC as an indication of the latency to re-entrain to shifts of a full LD cycle is uncertain. A high amplitude PRC might indicate phase lability in multiple conditions. If so, strong resetting may predict a shortened duration of the misaligned state so that the adverse effects of phase shifts on health will be reduced. Alternatively, pacemaker period may influence re-entrainment latency. As the entrained phase angle varies, the portion of the subjective night struck by light when the LD cycle is advanced or delayed is altered and this may determine the speed and direction of phase shifts (7).
The study of mutants has led to the discovery of transcriptional-translational feedback loops (TTFLs), in which the protein products of core clock genes not only regulate their own transcription but also ensure rhythmicity of expression of thousands of other genes throughout the body (8). We discovered duper, a recessive mutation that shortens the circadian period (τDD) of Syrian hamsters to 22.8 h and amplifies the PRC (9–11). Using a forward genetic strategy, we crossed the duper allele into the cardiomyopathic Bio14.6 strain and used fast homozygosity mapping to determine that these mutants are deficient in Cryptochrome 1 (Cry1), a powerful repressor of transcriptional activation by BMAL1:CLOCK heterodimers at E-box motifs (12).
The effects of Cry1 knockout on free-running period and entrained phase angle have been described (13, 14), but the role of Cry1 in jet lag and its health consequences is unknown. We used duper mutants and Cry1-deficient mice to test the hypotheses that the amplification of the PRC or the shortening of period can account for acceleration of re-entrainment after shifts of the full photoperiod. We refer to the transient state following a shift during which locomotor rhythms have not yet regained a stable phase relationship to the LD cycle as “jet lag,” although the extent of misalignment of multiple circadian oscillators remains to be quantified. We find that Cry1 deficiency speeds re-entrainment in both mice and hamsters, but neither shortening of circadian period nor high PRC amplitude can account for reduced jet lag.
Frequent phase shifts shorten the lifespan of hamsters that suffer from cardiomyopathy due to deletion of delta sarcoglycan (dsg; 15, 16). We utilized the dsg-deficient Bio14.6 strain as the distant ecotype in our mapping studies (12). This also provided us with the opportunity to examine the impact of the duper mutation on longevity in cardiomyopathic hamsters. We asked whether acceleration of re-entrainment by duper might mitigate the effects of jet lag on cardiomyopathy. Our results indicate that Cry1-null hamsters are resistant to adverse effects of frequent phase shifts on the progress of heart disease.
Results
Duper Hamsters Rapidly Re-entrain to Shifted LD Cycles.
We subjected hamsters that were stably entrained in 14L:10D to an 8-h phase advance, allowed them to re-entrain, and then instituted an 8-h phase delay. Duper hamsters re-entrained at least threefold faster than wild types (Fig. 1), regardless of whether they were subjected to phase delays or advances. Re-entrainment of duper hamsters was equally fast for advances and delays (Fig. 1 A–C). Wild-type hamsters re-entrained more slowly to a phase delay than to a phase advance (Fig. 1C). The effect of genotype on the rate of re-entrainment did not differ between males and females (SI Appendix, Fig. S1). In most cases, wild-type hamsters shifted 1 to 2 h/d until the new steady state was reached; but in several instances, hamsters retained the phase of the previous 14L:10D cycle for several weeks before shifting (SI Appendix, Fig. S2). This never occurred in dupers.
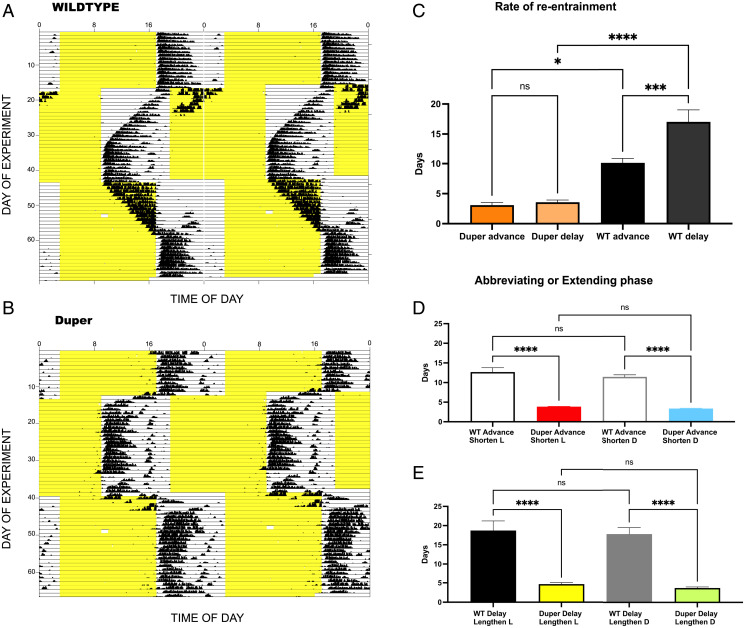
Fig. 1.
Duper hamsters rapidly re-entrain to shifted LD cycles. (A and B) Representative double-plotted actograms of wheel-running activity of wild type (Top) and duper (Bottom) hamsters subjected to abrupt 8-h shifts of the 14L:10D schedule. Yellow shading indicates the time of lights on. (C) Mean (± SEM) latency to establish steady phase angle after an 8-h advance or delay of the 14L:10D cycle. Wild-type hamsters (n = 6) took significantly longer than duper mutants (n = 7) to re-entrain to an 8-h phase advance or an 8-h phase delay (F3,16 = 34.82, ****P < 0.0001). Wild-type hamsters took longer to re-entrain to a phase delay than a phase advance (***P < 0.002). (D and E) Genotype, but not direction or time of day of the phase shift, determines the latency to re-entrain locomotor activity rhythms. Duper hamsters completed a phase advance more quickly than wild types regardless of whether the shift was instituted by shortening the dark phase (n = 42 dupers, n = 24 wild types) or shortening the light phase (n = 91 dupers, n = 38 wild types). Similarly, dupers completed a phase delay more quickly than wild types regardless of whether the dark phase or the light phase was lengthened (n = 35 wild types, n = 70 dupers). Within genotype, latency to re-entrain did not depend upon whether the dark phase or the light phase was altered. Means were compared with multiple-comparisons ANOVA.
Phase shifts may be triggered by abbreviating or extending the light phase or the dark phase. The change initially experienced by the animal may determine whether the first phase response is to delay or advance and at what rate. In a second experiment, we compared responses of wild-type and duper hamsters to shifts instituted in each of these manners. Re-entrainment took about 12 d in wild types regardless of whether phase advances were accomplished by shortening the dark phase or shortening the light phase. Duper mutants required only about 4 d in both protocols (Fig. 1 D and E). In both genotypes, advances progressed steadily through the dark phase. Mutants showed larger shifts in the first few days, while wild types advanced steadily at a rate of 1 to 2 h/d. In the corresponding experiment on phase delays, wild-type hamsters took about 18 d to re-entrain regardless of whether the shift was achieved by lengthening the dark phase or by lengthening the light phase. Two of the wild-type hamsters in which delays were instituted by lengthening the dark phase did not begin to shift until 2 wk after the delay. In duper hamsters, phase delays elicited shifts within 3 to 4 d regardless of whether the dark phase or the light phase was lengthened. In about 10% of the mutants, entrainment to phase delays occurred antidromically, i.e., through an advance of onsets through the dark phase (SI Appendix, Fig. S3). This lengthened the latency to achieve a stable phase to about 7 d, which was still shorter than the typical latency of wild types.
Differences in the Rate of Re-entrainment Are Not Due to Masking.
The pacemaker of duper and wild-type hamsters might shift equally quickly upon changes in the LD cycle, but differences in the rate of re-entrainment of locomotor activity might occur because the mutation accelerates the rate at which subordinate oscillators regain their phase relationship to the central clock (3). We sought to investigate this possibility and the extent of masking: the circadian clock of wild-type hamsters may shift more quickly than is evident from the behavioral record, but light exposure could directly suppress wheel running.
We subjected animals to an 8-h phase advance of the 14L:10D cycle and transferred them to DD during the dark phase on the second day after the shift. Running onset of wild types had advanced slightly by the second day of the new LD schedule and free ran with a period of 24.1 ± 0.04 h (mean ± SEM). Extrapolation of the linear regression fitted to onsets in DD indicated a mean phase advance of 2.6 ± 0.4 h. In contrast, duper mutants had nearly completed their 8-h shift by the second day of the advanced cycle, and their free run in DD began from that phase with a period of 22.6 ± 0.1 h (SI Appendix, Fig. S4 C and D). Extrapolation of the linear regression line fitted to activity onsets in DD to the preshift LD cycle indicated an advance of 5.4 ± 0.5 h in duper mutants (P < 0.001 vs. wild type). Taken together, these results indicate that the duper mutation dramatically reduces the latency with which hamsters re-entrain to an 8-h phase advance or delay. Although masking occurs, particularly in animals experiencing phase delays, it cannot explain the difference in the latency with which wild-type and duper hamsters respond to shifts of the 14L:10D cycle.
Contribution of Parametric Effects of Light.
A long-standing issue in circadian biology concerns whether entrainment can be understood using “phase only” models in which transitions at dawn and dusk elicit shifts, as opposed to models in which light continuously alters the angular velocity of the circadian pacemaker (17). We found previously that parametric effects of light differ between wild-type and duper hamsters: in violation of “Aschoff’s rule,” mutants experience a progressive shortening of the period of locomotor rhythms as the intensity of constant light (LL) is increased (11). We sought to determine whether the duper mutation also accelerates re-entrainment in hamsters maintained in skeleton photoperiods. This allows us to ask whether parametric effects of light contribute to the duper pattern of entrainment and phase shifting.
Wild types confined their locomotor activity to the short dark interval, i.e., they initially regarded the 10-h interval as “night.” They adopted a negative phase angle, with activity onsets lagging the onset of darkness by 1.34 ± 0.42 h (SI Appendix, Fig. S5). Upon an 8-h advance of the skeleton photoperiod, wild types delayed their activity; they became active in the 12-h interval, again showing a negative phase angle (−1.56 ± 0.19 h). Entrainment to the new skeleton photoperiod was achieved in 4.8 ± 0.6 d. When the full photoperiod was restored, the 14-h light phase coincided with the animals’ active phase. Wild-type hamsters took 34.2 ± 3.4 d to regain entrainment and adopted a less negative phase angle (−0.1 ± 0.2 h) to the full photoperiod than to the skeleton.
Duper hamsters maintained a positive phase angle in the skeleton photoperiod, becoming active 1.14 ± 0.25 h before lights off in the “dusk” light pulse. Like the wild-type hamsters, dupers delayed activity onset so that wheel revolutions were confined to the long (12-h interval) “day” when the skeleton photoperiod was advanced by 8 h (SI Appendix, Fig. S5). As was the case in full photoperiods, dupers re-entrained more quickly than wild types: mutants shifted with a mean of 1.54 ± 0.25 d, significantly faster than wild types (P < 0.001). As was the case for wild types, the light phase of the full 14L:10D photoperiod restored after the shifted skeleton regime coincided with the active phase of the locomotor rhythm. In contrast to wild types, however, duper animals quickly adopted a nocturnal activity pattern, entraining within 4.16 ± 0.31 d.
Cry1 Deletion Speeds the Rate of Re-entrainment in Mice.
Results of our sequencing efforts revealed that duper mutant hamsters are Cry1-null (12). Effects of Cry1 deletion on latency to shift entrained phase have not been reported in mice. In experiments comparable to our work on duper hamsters, we examined the response of Cry1−/− and wild-type mice to an abrupt 6-h advance of the LD cycle. We found that Cry1-deficient mice re-entrained about three times as fast as wild type to a 6-h advance (Fig. 2 A and B). After all animals had established steady-state entrainment, we repeated the 6-h phase advance and instituted constant darkness (DD) on the second cycle. This allowed us to assess masking and to determine pacemaker phase by extrapolation of the free run to the preshift LD cycle. Similar to our finding in duper versus wild-type hamsters, the circadian clock shifted more than twice as far in Cry1−/− than wild-type mice over the course of the first 2 d after the shift (P < 0.05). In order to test further the importance of PRC amplitude on latency to shift entrained phase, we exposed wild-type and Cry1−/− mice to 15’ light pulses in DD (Fig. 2E). Unlike duper mutant hamsters, Cry1−/− mice show no amplification of the PRC (Fig. 2F). Thus, Cry1−/− mice are similar to duper hamsters in that they achieve re-entrainment faster than their wild-type counterparts, but they differ from dupers in that they do not have an amplified PRC.
Fig. 2.
Cry1−/− mice show little jet lag after a 6-h phase advance. (A and B) Representative double-plotted actograms of wheel-running activity of Cry1−/− (Top) and wild-type (Bottom) mice subjected to a 6-h shift of the 12L:12D cycle. After mice re-entrained to the new LD schedule, they were subjected to a second 6-h phase advance and released into DD 2 days later. The red line fitted to free-running onsets was extrapolated to preshift onset in order to assess phase shift over the first 2 d after the second 6-h phase advance. Upon release into DD 2d after the second 6-h phase advance, mean free-running onset was advanced by 0.77 ± 0.29 h in wild-type and 1.65 ± 0.26 h in Cry1−/− mice (t16.27 = 2.238, Welch's t-test, *P < 0.05). (C) Free-running period was calculated while animals were in DD. Cry1−/− mice had a shorter period than wild types (t25 = 10.98, ****P < 0.0001). (D) Wild-type mice (n = 12) took approximately three times longer than Cry1−/− mice (n = 15) to re-entrain after the first 6-h phase advance (t25 = 6.812, ****P < 0.0001). (E) Phase response of Cry1−/− mice (orange circles) and wild-type controls (black squares) to 15’ light pulses in DD. (F) Phase responses were binned to 2-h intervals of circadian time during subjective night and represented as boxplots. There were no significant differences between the two genotypes in shift amplitude at any of the circadian times examined.
Does the Shortened Period Underlie Rapid Phase Shifts of Duper Hamsters?
The acceleration of re-entrainment in dupers was correlated not only with their high amplitude PRC but also with their short period and their positive phase angle of entrainment. In order to determine whether shortened circadian period was essential to other aspects of the phenotype, we took advantage of the effect of heavy water to lengthen τDD (37).
We first examined the effects of deuterium oxide (D2O) on the response of free-running male mutant hamsters to acute light pulses. During the pre-experimental baseline, when the duper hamsters used in this experiment drank tap water, they had a τDD of 23.24 ± 0.06 h. Light pulses given at about CT17.4 (range, CT17.2 to 17.8) produced a phase shift of 10.35 ± 1.55 h. When D2O was added to the drinking water, these hamsters consumed 21.7 ± 4.8 mL/d, and τDD lengthened to 24.51± 0.13 h within 9 d (Fig. 3A). Administration of a 15’ light pulse at CT17.3 (range, CT16.9 to 17.7) elicited a shift of 9.68 ± 2.48 h when the animals drank D2O. Thus, the amplitude of the phase shift elicited by the light pulse was not significantly altered by the slowing of the free-running circadian clock (Fig. 3 C–E).
Fig. 3.
The effect of the duper mutation to shorten the free-running period does not underlie the phase-shifting phenotype. (A) Mean (± SEM) free-running period of duper hamsters consuming tap water (H2O) (blue, n = 5) or 25% D2O adulterated water (black, n = 5). D2O significantly lengthened the free-running period (t4 = 3.911, ****P < 0.0001). (B) Phase angle of entrainment estimated as time difference in hours between activity onset and lights off in duper hamsters consuming tap water (blue, n = 6) and D2O adulterated water (black, n = 6). Heavy water significantly shortened the phase angle of entrainment (F2,15 = 3.736, *P < 0.05). Upon reinstitution of pure water (post-D2O, cyan, n = 6), phase angle reverted to pre-D2O values (F2,15 = 2.963, P > 0.05). (C and D) Representative actograms plotted modulo τDD of a duper hamster when consuming tap water (C) and D2O adulterated water (D) while maintained in DD. The asterisk indicates 15’ light pulse at CT17.5. Lines fit to activity onsets were used to estimate the free-running period and calculate the phase shift that resulted from the light pulse. (E) Phase-shift amplitude (mean ± SEM, in circadian hours) in response to a 15’ light pulse presented at CT17.5 in duper hamsters consuming tap water (H2O, blue bar, n = 5) or water adulterated with D2O (gray bar, n = 5). (F) Latency to re-entrain to 8-h phase advance of the 14L:10D cycle in duper hamsters before (blue bar, n = 6), during (black bar, n = 6), or after (cyan bar, n = 6) consumption of drinking water adulterated with D2O. Although heavy water slowed the circadian clock of duper hamsters, it did not alter either the amplitude of phase shifts in response to light pulses in the mid subjective night or the rate of re-entrainment after an 8-h shift of the 14L:10D cycle.
We next determined the effects of heavy water to influence entrainment of duper hamsters maintained in 14L:10D. During the baseline condition in which they drank tap water, locomotor activity onset preceded lights out by 1.71 ± 0.16 h. When these animals were given D2O to drink in 14L:10D, they stabilized entrainment, with mean activity onset occurring 0.1 ± 0.4 h before lights off. This phase angle was significantly smaller than that of the same animals when they drank tap water (Fig. 3B).
We then investigated the effect of deuteriation upon phase shifts in a full LD cycle. Prior to this experiment, we confirmed that the seven male duper hamsters used in this experiment had a τDD of 22.93 ± 0.17 h. After they achieved stable entrainment upon return to 14L:10D, the light cycle was shifted. Under the baseline (tap water) condition, these animals completed an 8-h phase advance with a latency of 2.8 ± 0.3 d (Fig. 3F). D2O was then added to their drinking water and the phase angle was allowed to stabilize before the light cycle was again advanced. Activity onset re-entrained with a latency of 4.43 ± 0.57 d, which did not differ significantly from the rate of re-entrainment when they drank tap water. In order to confirm that heavy water had slowed the circadian clock, these hamsters were released into DD from the advanced cycle while still drinking D2O. τDD was 24.11 ± 0.05 h. Hamsters were then returned to 14L:10D and tap water was reinstituted. When they were again subjected to an 8-h phase advance of the light cycle, these hamsters took 3.86 ± 0.55 d to re-entrain. Thus, neither the amplitude of the response to acute light pulses (Fig. 3 C–E) nor the latency to re-entrain upon an 8-h phase advance of the LD cycle (Fig. 3F) differed between the tap water and D2O treatments (Fig. 3F) despite the difference in free-running period (Fig. 3A).
We used five female duper hamsters in an additional experiment in which light pulses were administered earlier in the subjective night. Deuteriation lengthened τDD to 24.1 ± 0.7 h, and the light pulse was given at 15.56 ± 0.13 h. The resulting phase shifts averaged 9.67 ± 0.10 h. As with the male hamsters given light pulses later in the subjective night, this result in duper females confirms that lengthening the period to the wild-type range does not alter the effect of the mutation to amplify phase shifts.
The Duper Mutation and Heart Disease.
We utilized the Bio14.6 hamster strain as the distant ecotype for fast homozygosity mapping of the duper allele (12). The documented effects of circadian disruption on heart disease (15) afforded the opportunity to use the dsg deficiency to probe the effect of duper on cardiomyopathy and longevity. First, we examined survivorship in hamsters descended from a Bio14.6 × duper cross that were maintained in 14L:10D without phase shifts. We found that duper hamsters that were not dsg deficient (dsg+/+) lived as long as Lakeview-background (LVG) hamsters (Fig. 4A, P > 0.79, Gehan-Breslow-Wilcoxon test) but were ∼25% lighter than LVG wild-type hamsters (P < 0.0001; Fig. 4B). In contrast, duper dsg−/− hamsters succumbed to heart disease at a significantly younger age than did wild-type dsg−/− controls under unchanging 14L:10D (median age of death, 224 d for duper and 335 d for wild type; P = 0.0002, Gehan-Breslow-Wilcoxon test; Fig. 4C).
Fig. 4.
Phase shifts of the 14L:10D cycle eliminate the effect of the duper mutation to shorten the lifespan of cardiomyopathic (dsg−/−) hamsters. (A) Kaplan–Meier survival curve of unshifted duper dsg+/+ and LVG wild-type dsg+/+ hamsters demonstrates that the duper mutation does not impact longevity in noncardiomyopathic hamsters (P > 0.79, Gehan-Breslow-Wilcoxon test, duper n = 17, wild-type n = 8). Animals that are still living (three duper dsg+/+ and three LVG wild-type dsg+/+) are represented by black ticks. (B) Body weight measured every 50 d reveals that duper dsg+/+ are an average 25 ± 0.01% lighter than LVG wild-type dsg+/+ (****P < 0.0001, mixed-effects model) (C) Kaplan–Meier survival curve of unshifted duper dsg−/− and wild-type dsg−/− hamsters. Cardiomyopathic duper hamsters have a significantly reduced lifespan compared with their wild-type counterparts (****P < 0.0001, Gehan-Breslow-Wilcoxon test, duper n = 33, wild-type n = 35). (D) Kaplan–Meier survival curve of duper dsg−/− and Bio 14.6 wild-type dsg−/− hamsters subjected to alternating 8-h advances and delays at 2-wk intervals. Unlike unshifted hamsters of the same genotype, lifespans of duper and wild-type cardiomyopathic hamsters do not differ (P > 0.48, Gehan-Breslow-Wilcoxon test, duper n = 34, wild-type n = 41). (E) Rate of re-entrainment of 8-h shifted hamsters. Duper dsg−/− hamsters re-entrain faster than wild-type dsg−/− after both 8-h advances (****P < 0.0001) and delays (****P < 0.0001). Wild-type dsg−/− hamsters re-entrain faster after 8-h delays than wild-type dsg+/+ hamsters (****P < 0.0001) but not after 8-h advances (P > 0.07). Duper dsg−/− hamsters re-entrain faster after 8-h advances than after 8-h delays (****P < 0.0001), although they do not differ from duper dsg+/+ hamsters (P ≥ 0.23). (F) %EF calculated from M-mode echocardiography of unshifted duper and wild-type dsg−/− hamsters. Despite the difference in survivorship, %EF declines at a similar rate for both duper and wild-type hamsters, apart from the 210-d time point where the %EF of unshifted duper dsg−/− hamsters is lower than that of wild-type dsg−/− (*P = 0.04, Šídák multiple-comparisons test). (G) EF of duper and wild-type dsg−/− hamsters subjected to alternating 8-h advances and delays. %EF values do not differ between duper and wild type at all timepoints (P > 0.99, Šídák multiple-comparisons test).
To examine effects of repeated phase shifts, we subjected hamsters to alternating 8-h advances and delays of the 14L:10D schedule at 2-wk intervals. Consistent with our earlier findings (Fig. 1), we observed that dupers descended from duper × Bio14.6 hamsters re-entrained much more rapidly than the corresponding wild-type animals after both advances and delays regardless of dsg deficiency (P < 0.0001, Fig. 4D and Table 1). Wild-type dsg+/+ hamsters re-entrained slightly faster to delays than advances (P = 0.03), but duper dsg+/+ hamsters entrained equally quickly to advances and delays. The latency to re-entrain remained consistent for all experimental groups over the course of repeated shifts. However, the phase angle of entrainment of duper dsg+/+ hamsters decreased with age (Table 1). No such effect of age was seen in the wild-type dsg+/+ hamsters that achieved steady-state entrainment within 2 wk after each shift, although many repeatedly failed to re-entrain within the 14-d window. As we observed in earlier experiments (SI Appendix, Fig. S3), every duper mutant exhibited antidromic re-entrainment after an 8-h delay at least once during the series of repeated shifts.
Table 1.
Re-entrainment rate (Mean ± SEM) and entrained phase angle (Ψ) of duper and wt hamsters with and without dsg-deficiency*
| duper dsg−/− | n | wt dsg−/− | n | duper dsg+/+ | n | wt dsg+/+ | n | |
|---|---|---|---|---|---|---|---|---|
| Shift latency, days, 8h advance | 4.8 ± 0.1**** | 34 | 13.3 ± 0.2 | 38 | 3.9 ± 0.1**** | 16 | 12.5 ± 0.4 | 9 |
| Shift latency, days, 8h delay | 5.3 ± 0.3**** | 34 | 11.2 ± 0.3 | 38 | 4.8 ± 0.4**** | 16 | 13.8 ± 0.1 | 9 |
| Entrained phase angle, Ψ, 8h advance | ||||||||
| 60–199 days | 0.80 ± 0.07 | 34 | −0.30 ± 0.06 | 38 | 1.09 ± 0.11 | 16 | −0.51 ± 0.04 | 9 |
| 200–339 days | 0.70 ± 0.12 | 25 | −0.45 ± 0.07 | 24 | 0.54 ± 0.13 | 16 | −0.39 ± 0.04 | 9 |
| 340–479 days | 0.97 ± 0.27 | 5 | −0.56 ± 0.07 | 14 | 0.30 ± 0.11 | 15 | −0.37 ± 0.05 | 6 |
| 480+ days | nd | 0 | nd | 0 | 0.07 ± 0.10 | 14 | −0.38 ± 0.06 | 6 |
| Entrained phase angle, Ψ, 8h delay | ||||||||
| 60–199 days | 0.73 ± 0.09 | 34 | −0.32 ± 0.03 | 38 | 1.02 ± 0.12 | 16 | −0.36 ± 0.11 | 9 |
| 200–339 days | 0.43 ± 0.18 | 25 | −0.34 ± 0.04 | 24 | 1.02 ± 0.29 | 16 | −0.85 ± 0.74 | 9 |
| 340–479 days | 1.10 ± 0.46 | 5 | −0.45 ± 0.06 | 14 | 0.91 ± 0.49 | 15 | −0.33 ± 0.09 | 6 |
| 480+ days | nd | 0 | nd | 0 | −0.07 ± 0.39 | 14 | nd | 6 |
| Entrained phase angle, Ψ, unshifted | ||||||||
| 60–199 days | 0.65 ± 0.12 | 34 | −0.39 ± 0.02 | 40 | 1.17 ± 0.18 | 17 | −0.45 ± 0.05 | 8 |
| 200–339 days | 0.21 ± 0.25 | 6 | −0.44 ± 0.08 | 16 | 0.25 ± 0.12 | 15 | −0.52 ± 0.04 | 8 |
| 340–479 days | nd | 0 | −0.45 ± 0.05 | 1 | −0.26 ± 0.13 | 15 | nd | 8 |
| 480+ days | nd | 0 | nd | 0 | −0.49 ± 0.13 | 15 | nd | 6 |
*Rates of re-entrainment for wild types that did not completely entrain within 2 weeks of transfer were assigned a value of 14 days. Both dsg+/+ and dsg−/− duper hamsters re-entrained more quickly than their wt counterparts after both advances and delays of the LD cycle (****P < 0.0001), but dsg condition had no effect on the rate of re-entrainment. Entrained phase angle was derived after activity onset stabilized and could not be calculated for many wt animals, which did not complete re-entrainment within two weeks after shifts of the LD cycle. Although animals did not change the rate at which they re-entrained to repeated phase shifts over the course of the experiment, the duper dsg+/+ hamsters decreased phase angle with age (****P < 0.0001) but wt dsg+/+ did not. Shorter survival time of duper dsg−/− animals prevented statistical assessment of effect of genotype on decrease of phase angle at older ages.
The repeated shift protocol eliminated the effect of the duper mutation on survival of cardiomyopathic hamsters: longevity did not differ between wild-type and duper dsg−/− hamsters that were subjected to 8-h phase shifts every other week (Fig. 4E, P > 0.48, Gehan-Breslow-Wilcoxon test). The lifespan of shifted duper dsg−/− hamsters tended to lengthen relative to the unshifted duper dsg−/− (P = 0.06, Gehan-Breslow-Wilcoxon test), while the opposite occurred in shifted relative to unshifted wild types (P > 0.25, Gehan-Breslow-Wilcoxon test). The ejection fraction (EF), a volumetric measure of blood expelled from the left ventricle during contraction expressed as a percentage, is often used as an indicator of heart health (18) (SI Appendix, Fig. S6). The EF of unshifted duper dsg−/− hamsters was lower than their unshifted wild-type dsg−/− counterparts only at the 210-d time point (P < 0.05, Fig. 4F). Despite the difference in survivorship between the unshifted duper dsg−/− and wild-type dsg−/− hamsters, no other significant effects of circadian genotype or shift condition on EF of dsg-deficient hamsters were observed (Fig. 4 F and G).
Discussion
In light of the discovery that the duper mutant is Cry1 null (12), our examination of phase-shifting responses and longevity of these hamsters indicates previously unsuspected functions of this core clock gene. Taken together with our experiments on Cry1-deficient mice and effects of manipulating τDD by deuteriation in hamsters, the results indicate that neither the amplification of the PRC nor the acceleration of the circadian clock can account for the effects of duper to speed re-entrainment upon shifts of the LD cycle. Nevertheless, the rapid re-entrainment of duper mutants is associated with reduction of the impact of jet lag on longevity in cardiomyopathic animals.
The short free-running period of duper mutant hamsters is consistent with earlier findings that τDD is 1 h shorter in Cry1-deficient mice than in wild types. CRY1 is a strong repressor of transcriptional activation by BMAL1:CLOCK (19–21). In its absence, or upon deletion of its cytosolic stabilizer FBXL21, the negative arm of the TTFL is curtailed (22–24). Conversely, mutations in the C terminus that stabilize CRY1, or mutations of its degradative ligase FBXL3, lengthen circadian period (25, 26). Thus, shortening of τDD is expected in CRY1-deficient hamsters, much as for the destabilization of PER2 in the tau mutant hamster.
Our finding that Cry1−/− mice, like duper hamsters, re-entrain more quickly than wild-type upon advances of the LD cycle suggests that CRY1 may stabilize the circadian phase and thus prolong misalignment (Fig. 2). The threefold reduction in re-entrainment rate in Cry1−/− relative to wild-type mice was comparable to the change in the phase shift latency of duper relative to wild-type hamsters. Although mutations of core clock genes may have similar effects in mice and hamsters (27), differences in genetic background among strains of mice can profoundly affect their impact (28). Thus, the similarity between C57/BL6 mice and duper hamsters in the effect of Cry1 deficiency on the phase-shifting phenotype is remarkable.
The mechanism by which Cry1 slows re-entrainment is unknown. While CRY1 participates in a large complex to displace BMAL1:CLOCK from the E-box in the early night, and by itself to block transcriptional activation in the late subjective night, its role in light-induced phase shifts is not clear (21). Cry expression is thought not to be directly regulated by light (29). In mice subjected to a jet lag protocol, changes in the phase of Cry1 expression lag those in Per (30). Given that CRY1 facilitates the translocation of PER1/2 and casein kinase 1d (31, 32), one might expect Cry1-null animals to be less responsive to light. To the extent that CRY1 might serve as a “brake” on phase shifts, however, the more rapid re-entrainment in Cry1-deficient mice and hamsters is consistent with earlier findings.
Duper hamsters and Cry1−/− mice are similar in free-running period, entrained phase angle, and accelerated shifts upon changes of the LD cycle. Their circadian phenotype differs from wild types, in which the longer period is correlated with a rate of re-entrainment of 1 to 2 h/d after shifts of the LD cycle. Although the period of the free-running clock of dupers is quite stable (33), circadian phase can shift by 8 to 12 h after a 15’ light pulse (10, 11). Thus, we hypothesized that the phase lability revealed by PRC amplitude of dupers might account for more rapid re-entrainment of Cry1-deficient animals in full photoperiods. Our finding that while dupers have a high amplitude PRC, Cry1−/− mice do not, forces us to reject this hypothesis. Our results (Fig. 2 E and F) confirm the report of Spoelstra et al. (34), who found that Cry1 deletion has little effect on PRC amplitude in mice. This finding may have discouraged investigation of effects of Cry1 deletion on the latency with which animals re-entrain after shifts of a full photoperiod. It remains possible that the mutation of Cry1 accounts for rapid re-entrainment in duper, but that some other sequence change is responsible for the amplification of the PRC. Alternatively, Cry1 may play a greater role in maintaining phase stability in hamsters than in mice. For example, other TTFL components and modulators (35, 36) may have a stronger influence in mice such that the PRC functions more normally in the absence of Cry1 than is the case in hamsters.
Upon finding that the amplified PRC does not explain the reduction of jet lag in dupers, we asked whether the shortening of period does. As a consequence of their short period, dupers and Cry1−/− mice entrain to the LD cycle with a positive phase angle (10, 13, 14). As a result, changes in the LD cycle that are achieved by advancing or delaying the time of lights out cause different portions of the subjective night to be illuminated in dupers than in wild types. In this way, the mutation may result in a change in the re-entrainment rate or direction of the phase shift. However, the effects of the duper mutation on the rate of re-entrainment were similar regardless of the way in which advances or delays were instituted (Fig. 1 D and E). Furthermore, we find that slowing of the duper clock by deuteriation failed to eliminate effects of the mutation on the rate of re-entrainment, even though the entrained phase angle reverted to wild-type values (Fig. 3). Thus we must reject the hypothesis that changes in period are essential to the effect of the duper mutation to shorten the latency to re-entrain.
The finding that deuteriation lengthens τDD in dupers without altering acute phase shifts in DD is consistent with results obtained in wild-type mice (37). Studies of circadian mutants may allow us to dissociate some circadian properties (period and phase angle) from others (latency to re-entrain and PRC amplitude). Some mutations or deletions of core clock genes alter both free-running period and the PRC (38, 39), but others may be more selective. It remains to be determined whether mutations that have similar effects on the circadian phenotype target common components of the TTFL. The comparison of phenotypes of dupers and tau heterozygotes is of interest in this regard because both affect the negative arm of the TTFL, the former by deleting CRY1 and the latter by exaggerating the action of casein kinase 1e. Nevertheless, tau and duper mutants differ in the variability of the PRC and the limits of the range of entrainment (10, 11, 40–45). These differences in mechanism may underlie the discrepancies between effects of period-shortening mutations on the health of hamsters lacking a preexisting cardiomyopathy: dupers live as long as wild types in T24, while tau heterozygotes die earlier from heart and kidney disease (45). Unfortunately, the re-entrainment rate of tau hamsters subjected to advances or delays of 14L:10D cycles was not examined before the line was discontinued.
Although the effects of Cry1 deficiency on cell-autonomous circadian function clearly contribute to the duper phenotype, our results do not exclude the possibility that the mutation changes SCN network function. Mathematical simulations indicate that a reduction in coupling of SCN oscillators by one-third can account for several aspects of the duper phenotype (46). Tau and duper hamsters are both less likely than wild types to split in LL (11, 47). Given that the left and right SCN enter an antiphase relationship in this condition (48), coupling between the bilateral SCNs may be altered by mutations that affect the negative limb of the TTFL. The duper mutation may alter the strength of coupling between cells of the pacemaker, perhaps as an effect of Cry1 deficiency, and this could amplify the PRC (49–52) and thus increase the range of entrainment. Changes in coupling that occur upon exposure of mice to LL reduce the capacity of Cry1-deficient SCN neurons to oscillate (53). The species difference in the effect of Cry1 deletion on the PRC suggests that its impact on cell-autonomous circadian function may have greater consequences for network organization in hamsters than in mice.
LL intensity alters circadian period, indicating parametric influences on the pacemaker that may participate in entrainment. Although effects of light intensity on period are altered in duper (11), mutants re-entrain more quickly in both the skeleton and full photoperiods (SI Appendix, Fig. S5). Thus nonparametric signals are of primary importance in re-entrainment. The response to skeleton photoperiods clarifies our observation that duper increases the frequency of antidromic entrainment to phase delays: because the shorter free-running period results in a more positive entrained phase angle in the mutants, 8-h shifts likely result in more extensive illumination of the late subjective night and thus the advance portion of the PRC (7). Furthermore, the effect of the duper mutation to increase the amplitude of the PRC magnifies the advance shift. It is possible that other short period mutations will have a similar effect, to the extent that they both result in a positive phase angle and amplification of the PRC.
The impact of altered cell-autonomous function on pacemaker organization may depend upon the identity of the cell types affected. Effects of clock gene mutations on different cell populations within the SCN may each contribute to changes in period (54–57). Cry1 deficiency may act in vasoactive intestinal peptide (VIP) cells to alter coupling but in arginine vasopressin (AVP) cells to affect circadian output. Light-induced activation and Per1 expression in the VIP cell population is altered in duper (58). Deletion of VIP in mice weakens coupling, ultimately resulting in arrhythmicity, but changes in period and acceleration of phase shifts are inconsistent and do not match the duper phenotype (51). Although vasopressin receptor–deficient mice show reduced jet lag, they differ from duper in that circadian period is unaffected (59). Individual differences in the rate of re-entrainment of mice correlated with a lower variability of circadian phase in the vasopressin-rich SCN shell, suggesting that stronger coupling accelerates phase shifts (60). Nevertheless, variation of cell phases in mid-SCN was greater in animals that re-entrained more quickly to a 6-h advance. It will be useful to examine effects of duper on peptidergic cell types and oscillator coupling more directly.
Dupers entrained rapidly to both advances and delays of 14L:10D. This is consistent with the symmetry of duper’s effect on the PRC. Dupers achieve the lion’s share of the shift within the first cycle, while wild types only gradually approach the appropriate phase angle. Two pulse experiments (11, 61, 62) and bioluminescence studies (5, 63) suggest that phase shifts of the pacemaker occur more rapidly than those of subordinate oscillators. We asked whether the slower re-entrainment of wild types arises not from a change in the rate at which the pacemaker can shift, but in how rapidly driven systems controlling locomotor activity align with the SCN. Upon release into DD 2 d after a phase shift, free runs began from the previous phase of running onset, which was much farther advanced in dupers than in wild type (SI Appendix, Fig. S4). This contrasts with previous findings in rats (64). Thus, duper accelerates the rate at which the pacemaker acutely shifts, rather than speeding the alignment of downstream oscillators with the central clock. Our experiments in Cry1−/− mice produced the same result (Fig. 2 A and B). Wild-type hamsters entrained more rapidly to advances than delays. While this is not unprecedented (65, 66), studies in mice and rats find re-entrainment to phase advances to proceed more slowly and to be more injurious to health (67–69).
The impact upon health of jet lag and circadian mutations justifies emphasis on the importance of internal temporal coordination. It is believed that the adverse effects of circadian disruption are attributable to misalignment, as circadian oscillators between (and perhaps within) organs shift at different rates. This appealing hypothesis has been difficult to test, as acute disruption caused by shifts of the LD cycle has been invariably associated with protracted misalignment. The rapid re-entrainment of dupers may provide a tool to dissociate effects of the shift per se from those of long-lasting desynchrony. Use of bioluminescent reporters of clock gene expression will help to quantify effects of the duper mutation on the phases of cell-autonomous oscillators, the central pacemaker, and peripheral oscillators.
Deleterious effects of repeated phase shifts on dilated cardiomyopathy were first shown in hamsters deficient in dsg (15, 16). We deliberately chose a mild challenge (shifts of only 8 h imposed only every 2 wk) to devise a more realistic model for human jet lag. It is likely that the impact of phase shifts depends upon multiple factors, including the number of hours shifted, the frequency of the challenge, and the severity of the cardiomyopathy that they amplify. Duper dsg+/+ hamsters experienced no shortening of lifespan when maintained in unchanging 14L:10D or when repeatedly shifted. When challenged with dsg deletion, however, dupers succumb much sooner than wild type when they are maintained in stable LD cycles. It is unclear whether this susceptibility is related to the alteration of circadian function imposed by the mutation. Cryptochromes have multiple effects on metabolism that may be independent of their influence on circadian function (70–74), and this may explain the reduced body weight of duper hamsters (Fig. 4B). Metabolic effects of Cry1 deletion, rather than or in addition to speeding of the clock or amplification of the PRC, may contribute to the reduction of lifespan despite the failure of EF to decline faster in duper than the wild type in either stable or changing LD cycles.
Nevertheless, alternating advances and delays at 2-wk intervals eliminated the effect of duper to accelerate the demise of dsg−/− hamsters. It is not clear why repeated shifts improved the condition of duper hamsters. One possibility is that the discrepancy between their endogenous period and the T24 cycle resulted in internal misalignment despite the ability of dupers to entrain their locomotor rhythms, causing abnormalities in heart function (75). If so, disruption by phase shifts on alternate weeks may have mitigated such adverse effects. Identification of the duper allele (12) will facilitate identification of mechanisms that control circadian responses to phase shifts and suggest approaches to amelioration of jet lag and its consequences for health.
Materials and Methods
Animals.
Syrian hamsters (Mesocricetus auratus) were born and raised in a 14L:10D cycle and allowed ad libitum access to food and water throughout the study. Wild-type hamsters of the LVG strain were obtained from Charles River Laboratories or bred in our laboratory from that stock. Duper hamsters used in studies to assess latency of re-entrainment upon shifts of phase of the LD cycle were derived from the original super duper animals crossed to Lakeview wild types as previously described (13). They descended from animals that were confirmed by restriction digest mapping of their genomic DNA (13) to lack the tau mutation and in which the free-running period (τDD) was ∼23 h. We confirmed that duper hamsters used in this study had a shorter free-running period than the wild type (23.19 ± 0.06 vs. 24.0 ± 0.02h).
To assess the effect of the duper mutation on cardiomyopathy, we crossed duper homozygotes with Bio14.6 hamsters (BioBreeder) as described in the companion article (12). F2 offspring were assessed for dsg deletion using PCR genotyping (see SI Appendix, Methods). To identify duper homozygotes, hamsters were behaviorally phenotyped to determine τDD and latency to re-entrain after 8-h phase shifts of the 14L:10D cycle.
C57Bl6/J mice (strain 000664) were purchased from Jackson Laboratories and bred in our laboratory. Cry1−/− mice were generously provided by Dr. Aziz Sancar (North Carolina State University, Chapel Hill, N.C.) and were also bred in our laboratory to generate subjects for these experiments.
All experiments were approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Amherst.
Duper Phenotyping and Activity Monitoring.
To record the rhythm of locomotor activity, adult hamsters (3 to 11 mo old; 35 males) were individually housed in activity wheel–equipped cages under 14L:10D. Throughout these experiments, light intensity during the day was ∼250 lx at cage level. Locomotor activity was recorded and analyzed using ClockLab software (Actimetrics) as previously described (9–11). As the duper sequence was not yet known, animals homozygous for duper were identified phenotypically before the onset of these experiments. τDD was assessed by maintaining animals in DD for ∼10 d. Hamsters were exposed to 15’ light during the subjective night and returned to DD to assess phase shifts.
Phase Advances and Delays.
To assess latency to re-entrain upon shifts of the LD cycle, hamsters were maintained in 14L:10D for at least 3 wk before being subjected to an 8-h phase advance, which was achieved by abbreviating the dark phase to 2 h. Entrainment to the shifted LD cycle was determined to have occurred when the slope of a linear least-squares regression line fit to activity onsets paralleled the line fit to lights off on the actogram. After stable re-entrainment had occurred, and no sooner than 3 wk after the advance shift, an abrupt 8-h phase delay was instituted by lengthening the light phase in order to return the hamsters to the previous LD schedule. The latency to reattain stable entrainment of locomotor activity was assessed. In no instance was a shift made less than 2 wk after the previous transfer. To evaluate the influence of masking on changes in the phase of locomotor activity onset upon shifts of the light schedule, wild-type hamsters were subjected to another 8-h phase advance or delay of the 14L:10D cycle but were released into DD on the second or fifth day after the shift. We evaluated whether the advance or delay of locomotor activity would continue in DD or whether the wild-type free run would continue from the phase achieved on day 2 of the shift.
To assess latency to re-entrain upon shifts of the LD cycle, mice were maintained in 12L:12D for at least 2 wk before being subjected to a 6-h phase advance, which was achieved by abbreviating the dark phase to 6 h. Entrainment to the shifted LD cycle was determined to have occurred when the slope of a linear least-squares regression line fit to activity onsets paralleled the line fit to lights off on the actogram. To evaluate the influence of masking on changes in the phase of locomotor activity onset upon shifts of the light schedule, wild-type animals were subjected to a second 6-h phase advance as before but were released into DD on the third day after the shift. We evaluated whether the advance or delay of locomotor activity would continue in DD or whether the wild-type free run would continue from the phase achieved on day 2 of the shift. While in DD, mice were subjected to 15’ light pulses at different time points of the subjective night in order to construct a PRC.
To assess the effect of repeated phase shifts of the 14L:10D cycle on survivorship in cardiomyopathic hamsters, we subjected 34 duper dsg−/−, 41 wild-type dsg−/−, 16 duper dsg+/+, and 9 wild-type dsg+/+ males to alternating 8-h advances and delays at 2-wk intervals. All hamsters had continuous ad libitum access to running wheels, food, and water. Hamsters were transferred between two rooms on 14L:10D schedules that were 8 h out of phase. Animals were moved when lights were on in both rooms. Control hamsters (33 duper dsg−/−, 35 wild-type dsg−/−, 17 duper dsg+/+, and 8 wild-type dsg+/+ males, also maintained in running-wheel cages) remained in 14L:10D but experienced no phase shifts. All duper and dsg−/− animals were descendants of the duper × Bio14.6 cross described above. Lakeview wild types were purchased from Charles River. Heart condition was assessed by echocardiography at intervals of ∼50 d beginning at 60 d of age (see below), at which time body weight was recorded. Hamsters that developed severe heart failure tended to gain weight rapidly as pulmonary edema developed. Such animals were euthanized when judged to be moribund upon veterinary consultation, and the day of sacrifice was recorded in order to calculate longevity.
D2O Experiments.
The first experiment examined the effect of free-running period on acute responses of free-running mutant hamsters to light pulses. Six duper hamsters were maintained in DD for ∼10 d before assessment of phase shifts in response to a 15’ light pulse at about CT17.5. Approximately 7 wk after return to 14L:10D, they were again transferred to DD and D2O (Sigma-Aldrich, 25% vol/vol) was added to the drinking water. The free-running period stabilized at ∼24 h within 5 d. On day 9 of DD, a 15’ light pulse was given at CT17.5. The amplitude of the shift was assessed over the next 12 d, during which the animals continued to drink 25% heavy water. The phase shift during deuteriation was compared with the previous response to the light pulse exhibited while drinking unadulterated water.
The next experiment examined the relationship of free-running period to the rate of re-entrainment upon phase shifts of a 14L:10D cycle. The duper phenotype of seven adult female duper hamsters was first confirmed by assessment of free-running period and amplitude of phase shifts to 15’ light pulses while drinking tap water. These animals were then allowed to achieve stable entrainment in 14L:10D, and their latency to re-entrain after an 8-h phase delay of the LD light cycle was determined. Seven weeks after the last manipulation of the light cycle, the animals were given 25% D2O as their sole source of drinking water. After an interval of 9 d during which the phase angle of entrainment stabilized, these hamsters were subjected to another 8-h phase advance by acutely shortening the dark phase to 2 h. Thirteen days after the phase advance, these hamsters were transferred to DD in order to confirm that τDD had lengthened to the wild-type range. A 15’ light pulse was given at CT15; after an additional 3 d in DD, D2O was removed and the animals were given tap water to drink. After 8 additional days in DD, the hamsters were returned to LD. Stable entrainment was evident within 10 d, at which point the light cycle was again advanced by 8 h so that the time course of re-entrainment could be compared with that previously shown before and during deuteriation.
Echocardiography.
Echocardiograms were performed with a Telemed 0.5- to 12.0-mHz ultrasound probe and recorded using EchoWave II software. Ultrasound recordings of repeatedly shifted and unshifted males took place at intervals of ∼50 d, beginning at 60 d old until death or 360 d of age. Hamsters were weighed and subsequently anesthetized with 3% isoflurane inhalant in oxygen, immobilized and positioned on an angled platform, and maintained at 1 to 2% isoflurane for the duration of the procedure (see SI Appendix, Methods). Echocardiograms were used to later calculate EF by taking two sets of M-mode measurements at diastole (d) and systole (s), respectively: interventricular septum, left ventricular diameter (LVID), and left ventricular posterior wall thickness. Heart rate was generated from averaging two heartbeats in M-mode. The image with the highest LVIDd value was chosen to calculate the left ventricular volume at end-diastole and end-systole, LVEDv and LVESv, respectively, and then derive %EF according to the following formula: (LVEDv − LVESv)/LVEDv × 100.
Skeleton Photoperiod Experiments.
We utilized skeleton photoperiods to evaluate the contribution of parametric cues to responses to phase shifts of the LD cycle and their possible involvement in effects of the duper mutation to accelerate re-entrainment (76). Hamsters (12 wild type, 14 duper) entrained to 14L:10D were transferred to a 1L:12D:1L:10D regimen in which the light exposure corresponded to the first and last hour of the full photocycle. After at least 2 wk of entrainment to the skeleton photoperiod, the 1-h light pulses were abruptly advanced by 8 h. After ∼2 wk, the 14L:10D regimen was restored by filling in the light phase between the dawn and dusk pulses.
Data Analysis and Statistical Tests.
The period and phase of locomotor rhythms were determined by linear regression fit of running-wheel onsets using Actimetrics software as previously described (10). The latency of behavioral phase shifts was evaluated by two-way ANOVA with genotype and direction of shift as main factors, with repeated measures to compare effects of advances versus delays within the same animals. Effects of deuteriation on the amplitude of phase shifts to 15’ light pulses, or to advances of the 14L:10D cycle, were evaluated by one-way ANOVA with repeated measures to compare responses of the same individuals while drinking H2O versus heavy water.
PRCs were evaluated by fitting a linear regression line to the onsets of activity that occurred in DD over the 10 d before and after exposure of mice or hamsters to a 15’ light pulse. Cages were removed from the dark room and subjected to fluorescent light of ∼250 lx. The shift was calculated as the difference in time of running onset after the light pulse, as extrapolated to the day of the perturbation, and expressed in circadian hours as determined by τDD.
Survivorship of cardiomyopathic and control duper and wild-type hamsters was assessed by log-rank and Kaplan–Meier statistics. Animals not yet deceased at the time of writing were censored at their present age. Survival curves were compared using the Gehan-Breslow-Wilcoxon test. Two-way ANOVA was performed to delineate any effect of circadian genotype from age alone. Data analyses and statistical tests were performed in Prism 6 (GraphPad).
Supplementary Material
Acknowledgments
We thank Dr. Timothy P. Fitzgibbons for instruction and advice on echocardiography, Dr. Aziz Sancar for Cry1−/− mice, and Ms. Amanda Hageman for meticulous animal care. This work is supported by National Heart, Lung and Blood Institute (grant 5R01HL138551 to E.L.B..
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2121883119/-/DCSupplemental.
Data Availability
Mouse and hamster behavioral values for phase shifts and free runs as well as hamster echocardiograms have been deposited in Figshare https://doi.org/10.6084/m9.figshare.20346645.v1 (77), https://doi.org/10.6084/m9.figshare.20343756.v1 (78), and DOI: 10.6084/m9.figshare.17118914 (79).
References
- 1.Welsh D. K., Takahashi J. S., Kay S. A., Suprachiasmatic nucleus: Cell autonomy and network properties. Annu. Rev. Physiol. 72, 551–577 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoo S.-H., et al. , PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. U.S.A. 101, 5339–5346 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamazaki S., et al. , Resetting central and peripheral circadian oscillators in transgenic rats. Science 288, 682–685 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Woller A., Gonze D., Circadian misalignment and metabolic disorders: A story of twisted clocks. Biology (Basel) 10, 207 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patke A., Young M. W., Axelrod S., Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 21, 67–84 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Daan S., Pittendrigh C. S., A functional analysis of circadian pacemakers in nocturnal rodents. II. The variability of phase response curves. J. Comp. Physiol. 106, 253–266 (1976). [Google Scholar]
- 7.Leloup J.-C., Goldbeter A., Critical phase shifts slow down circadian clock recovery: Implications for jet lag. J. Theor. Biol. 333, 47–57 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Koike N., et al. , Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 338, 349–354 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monecke S., Brewer J. M., Krug S., Bittman E. L., Duper: A mutation that shortens hamster circadian period. J. Biol. Rhythms 26, 283–292 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Krug S., McKinley Brewer J., Bois A. S., Bittman E. L., Effects of the duper mutation on circadian responses to light. J. Biol. Rhythms 26, 293–304 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Bittman E. L., Effects of the duper mutation on responses to light: Parametric and nonparametric responses, range of entrainment, and masking. J. Biol. Rhythms 29, 97–109 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Lee Y. Y., et al. , duper is a null mutation of Cryptochrome 1 in Syrian hamsters. Proc. Natl. Acad. Sci. U.S.A. 119, e2123560119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vitaterna M. H., et al. , Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc. Natl. Acad. Sci. U.S.A. 96, 12114–12119 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Horst G. T., et al. , Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398, 627–630 (1999). [DOI] [PubMed] [Google Scholar]
- 15.Penev P. D., Kolker D. E., Zee P. C., Turek F. W., Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. Am. J. Physiol. 275, H2334–H2337 (1998). [DOI] [PubMed] [Google Scholar]
- 16.Nigro V., et al. , Identification of the Syrian hamster cardiomyopathy gene. Hum. Mol. Genet. 6, 601–607 (1997). [DOI] [PubMed] [Google Scholar]
- 17.Comas M., Beersma D. G. M., Hut R. A., Daan S., Circadian phase resetting in response to light-dark and dark-light transitions. J. Biol. Rhythms 23, 425–434 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Konstam M. A., Kramer D. G., Patel A. R., Maron M. S., Udelson J. E., Left ventricular remodeling in heart failure: Current concepts in clinical significance and assessment. JACC Cardiovasc. Imaging 4, 98–108 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Griffin E. A. Jr., Staknis D., Weitz C. J., Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science 286, 768–771 (1999). [DOI] [PubMed] [Google Scholar]
- 20.Rosensweig C., Green C. B., Periodicity, repression, and the molecular architecture of the mammalian circadian clock. Eur. J. Neurosci. 51, 139–165 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao X., Yang Y., Selby C. P., Liu Z., Sancar A., Molecular mechanism of the repressive phase of the mammalian circadian clock. Proc. Natl. Acad. Sci. U.S.A. 118, e2021174118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godinho S. I. H., et al. , The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science 316, 897–900 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Siepka S. M., et al. , Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell 129, 1011–1023 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.E.A. Griffin Jr, D. Staknis , C.J. Weitz, Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science 286,768-71 (1999)..X [DOI] [PubMed]
- 25.Hirano A., et al. , FBXL21 regulates oscillation of the circadian clock through ubiquitination and stabilization of cryptochromes. Cell 152, 1106–1118 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Patke A., et al. , Mutation of the human circadian clock gene CRY1 in familial delayed sleep phase disorder. Cell 169, 203–215.e13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loudon A. S. I., et al. , The biology of the circadian Ck1epsilon tau mutation in mice and Syrian hamsters: A tale of two species. Cold Spring Harb. Symp. Quant. Biol. 72, 261–271 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Shimomura K., et al. , Usf1, a suppressor of the circadian Clock mutant, reveals the nature of the DNA-binding of the CLOCK:BMAL1 complex in mice. eLife 2, e00426 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maywood E. S., et al. , Analysis of core circadian feedback loop in suprachiasmatic nucleus of mCry1-luc transgenic reporter mouse. Proc. Natl. Acad. Sci. U.S.A. 110, 9547–9552 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy A. B., Field M. D., Maywood E. S., Hastings M. H., Differential resynchronisation of circadian clock gene expression within the suprachiasmatic nuclei of mice subjected to experimental jet lag. J. Neurosci. 22, 7326–7330 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aryal R. P., et al. , Macromolecular assemblies of the mammalian circadian clock. Mol. Cell 67, 770–782.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smyllie N. J., et al. , Cryptochrome proteins regulate the circadian intracellular behavior and localization of PER2 in mouse suprachiasmatic nucleus neurons. Proc. Natl. Acad. Sci. U.S.A. 119, e2113845119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bittman E. L., Does the precision of a biological clock depend upon its period? Effects of the duper and tau mutations in Syrian hamsters. PLoS One 7, e36119 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spoelstra K., Albrecht U., van der Horst G. T. J., Brauer V., Daan S., Phase responses to light pulses in mice lacking functional per or cry genes. J. Biol. Rhythms 19, 518–529 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Goriki A., et al. , A novel protein, CHRONO, functions as a core component of the mammalian circadian clock. PLoS Biol. 12, e1001839 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klemz S., et al. , Protein phosphatase 4 controls circadian clock dynamics by modulating CLOCK/BMAL1 activity. Genes Dev. 35, 1161–1174 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daan S., Pittendrigh C. S., A functional analysis of circadian pacemakers in nocturnal rodents. III. Heavy water and constant light: Homeostasis of frequency? J. Comp. Physiol. 106, 267–290 (1976). [Google Scholar]
- 38.Dallmann R., DeBruyne J. P., Weaver D. R., Photic resetting and entrainment in CLOCK-deficient mice. J. Biol. Rhythms 26, 390–401 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pendergast J. S., Friday R. C., Yamazaki S., Photic entrainment of period mutant mice is predicted from their phase response curves. J. Neurosci. 30, 12179–12184 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osiel S., Golombek D. A., Ralph M. R., Conservation of locomotor behavior in the golden hamster: Effects of light cycle and a circadian period mutation. Physiol. Behav. 65, 123–131 (1998). [DOI] [PubMed] [Google Scholar]
- 41.Shimomura K., Menaker M., Light-induced phase shifts in tau mutant hamsters. J. Biol. Rhythms 9, 97–110 (1994). [DOI] [PubMed] [Google Scholar]
- 42.Shimomura K., et al. , Circadian behavior and plasticity of light-induced c-fos expression in SCN of tau mutant hamsters. J. Biol. Rhythms 13, 305–314 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Grosse J., Loudon A. S. I., Hastings M. H., Behavioural and cellular responses to light of the circadian system of tau mutant and wild-type Syrian hamsters. Neuroscience 65, 587–597 (1995). [DOI] [PubMed] [Google Scholar]
- 44.Martino T. A., et al. , Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1675–R1683 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Hurd M. W., Ralph M. R., The significance of circadian organization for longevity in the golden hamster. J. Biol. Rhythms 13, 430–436 (1998). [DOI] [PubMed] [Google Scholar]
- 46.Manoogian E. N. C., Leise T. L., Bittman E. L., Phase resetting in duper hamsters: Specificity to photic zeitgebers and circadian phase. J. Biol. Rhythms 30, 129–143 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bittman E. L., Costello M. K., Brewer J. M., Circadian organization of tau mutant hamsters: Aftereffects and splitting. J. Biol. Rhythms 22, 425–431 (2007). [DOI] [PubMed] [Google Scholar]
- 48.de la Iglesia H. O., Meyer J., Carpino A. Jr., Schwartz W. J., Antiphase oscillation of the left and right suprachiasmatic nuclei. Science 290, 799–801 (2000). [DOI] [PubMed] [Google Scholar]
- 49.Beersma D. G. M., van Bunnik B. A. D., Hut R. A., Daan S., Emergence of circadian and photoperiodic system level properties from interactions among pacemaker cells. J. Biol. Rhythms 23, 362–373 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Abraham U., et al. , Coupling governs entrainment range of circadian clocks. Mol. Syst. Biol. 6, 438 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.An S., et al. , A neuropeptide speeds circadian entrainment by reducing intercellular synchrony. Proc. Natl. Acad. Sci. U.S.A. 110, E4355–E4361 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.VanderLeest H. T., et al. , Phase shifting capacity of the circadian pacemaker determined by the SCN neuronal network organization. PLoS ONE 4, e4976 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evans J. A., Pan H., Liu A. C., Welsh D. K., Cry1-/- circadian rhythmicity depends on SCN intercellular coupling. J. Biol. Rhythms 27, 443–452 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smyllie N. J., Chesham J. E., Hamnett R., Maywood E. S., Hastings M. H., Temporally chimeric mice reveal flexibility of circadian period-setting in the suprachiasmatic nucleus. Proc. Natl. Acad. Sci. U.S.A. 113, 3657–3662 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patton A. P., et al. , The VIP-VPAC2 neuropeptidergic axis is a cellular pacemaking hub of the suprachiasmatic nucleus circadian circuit. Nat. Commun. 11, 3394 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mieda M., Okamoto H., Sakurai T., Manipulating the cellular circadian period of arginine vasopressin neurons alters the behavioral circadian period. Curr. Biol. 26, 2535–2542 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Lee I. T., et al. , Neuromedin s-producing neurons act as essential pacemakers in the suprachiasmatic nucleus to couple clock neurons and dictate circadian rhythms. Neuron 85, 1086–1102 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manoogian E. N. C., Kumar A., Obed D., Bergan J., Bittman E. L., Suprachiasmatic function in a circadian period mutant: Duper alters light-induced activation of vasoactive intestinal peptide cells and PERIOD1 immunostaining. Eur. J. Neurosci. 48, 3319–3334 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamaguchi Y., et al. , Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science 342, 85–90 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Evans J. A., et al. , Shell neurons of the master circadian clock coordinate the phase of tissue clocks throughout the brain and body. BMC Biol. 13, 43 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma V. K., Chandrashekaran M. K., Probing the circadian oscillator of a mammal by two-pulse perturbations. Chronobiol. Int. 17, 129–136 (2000). [DOI] [PubMed] [Google Scholar]
- 62.Best J. D., Maywood E. S., Smith K. L., Hastings M. H., Rapid resetting of the mammalian circadian clock. J. Neurosci. 19, 828–835 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sellix M. T., et al. , Aging differentially affects the re-entrainment response of central and peripheral circadian oscillators. J. Neurosci. 32, 16193–16202 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takamure M., Murakami N., Takahashi K., Kuroda H., Etoh T., Rapid reentrainment of the circadian clock itself, but not the measurable activity rhythms to a new light-dark cycle in the rat. Physiol. Behav. 50, 443–449 (1991). [DOI] [PubMed] [Google Scholar]
- 65.Wever R. A., Phase shifts of human circadian rhythms due to shifts of artificial Zeitgebers. Chronobiologia 7, 303–327 (1980). [PubMed] [Google Scholar]
- 66.Monk T. H., Buysse D. J., Carrier J., Kupfer D. J., Inducing jet-lag in older people: Directional asymmetry. J. Sleep Res. 9, 101–116 (2000). [DOI] [PubMed] [Google Scholar]
- 67.Davidson A. J., et al. , Chronic jet-lag increases mortality in aged mice. Curr. Biol. 16, R914–R916 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yan L., Structural and functional changes in the suprachiasmatic nucleus following chronic circadian rhythm perturbation. Neuroscience 183, 99–107 (2011). [DOI] [PubMed] [Google Scholar]
- 69.Kott J., Leach G., Yan L., Direction-dependent effects of chronic “jet-lag” on hippocampal neurogenesis. Neurosci. Lett. 515, 177–180 (2012). [DOI] [PubMed] [Google Scholar]
- 70.Zhang E. E., et al. , Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat. Med. 16, 1152–1156 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kriebs A., et al. , Circadian repressors CRY1 and CRY2 broadly interact with nuclear receptors and modulate transcriptional activity. Proc. Natl. Acad. Sci. U.S.A. 114, 8776–8781 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jang H., et al. , SREBP1c-CRY1 signalling represses hepatic glucose production by promoting FOXO1 degradation during refeeding. Nat. Commun. 7, 12180 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jordan S. D., et al. , CRY1/2 selectively repress PPARd and limit exercise capacity. Cell Metab. 26, 243–255.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cal-Kayitmazbatir S., et al. , CRY1-CBS binding regulates circadian clock function and metabolism. FEBS J. 288, 614–639 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.West A. C., et al. , Misalignment with the external light environment drives metabolic and cardiac dysfunction. Nat. Commun. 8, 417 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taylor S. R., Webb A. B., Smith K. S., Petzold L. R., Doyle F. J. III, Velocity response curves support the role of continuous entrainment in circadian clocks. J. Biol. Rhythms 25, 138–149 (2010). [DOI] [PubMed] [Google Scholar]
- 77.E. Bittman, Running data for Fig 1, 2, and 3. Figshare. https://figshare.com/articles/dataset/Running_data_for_Fig_1_2_and_3/20346645/1. Deposited 21 July 2022. [Google Scholar]
- 78.E. Bittman, Fig4 Heart Data. Figshare. https://figshare.com/articles/dataset/Fig4_Heart_Data/20343756/1. Deposited 20 July 2022. [Google Scholar]
- 79.E. Bittman, Entrainment data Duper vs WT.xlsx. Figshare. https://figshare.com/articles/dataset/Entrainment_data_Duper_vs_WT_xlsx/17118914. Deposited 12 March 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Mouse and hamster behavioral values for phase shifts and free runs as well as hamster echocardiograms have been deposited in Figshare https://doi.org/10.6084/m9.figshare.20346645.v1 (77), https://doi.org/10.6084/m9.figshare.20343756.v1 (78), and DOI: 10.6084/m9.figshare.17118914 (79).